Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales High Court (Patents Court) Decisions
You are here: BAILII >> Databases >> England and Wales High Court (Patents Court) Decisions >> Abbott Diabetes Care Inc & Ors v Dexcom Inc & Ors [2024] EWHC 1664 (Pat) (28 June 2024)
URL: http://www.bailii.org/ew/cases/EWHC/Patents/2024/1664.html
Cite as: [2024] EWHC 1664 (Pat)
[New search] [Printable PDF version] [Help]
Neutral Citation Number: [2024] EWHC 1664 (Pat)
Case Nos: HP-2021-000025 & HP-2021-000026
IN THE HIGH COURT OF JUSTICE
BUSINESS & PROPERTY COURTS OF ENGLAND AND WALES
INTELLECTUAL PROPERTY (ChD)
PATENTS COURT
Rolls Building, 7 Fetter Lane,
London, EC4A 1NL
Date: 28 June 2024
Before :
MR JUSTICE MELLOR
- - - - - - - - - - - - - - - - - - - - -
Between :
(1) ABBOTT DIABETES CARE INC.
(2) ABBOTT LABORATORIES VASCULAR ENTERPRISES LP
(3) ABBOTT IRELAND
(4) ABBOTT DIABETES CARE LIMITED
(5) ABBOTT DIAGNOSTICS GMBH
(6) ABBOTT LABORATORIES LIMITED
Claimants in HP-2021-000025
and
(2) DEXCOM INTERNATIONAL LIMITED
(4) DEXCOM (UK) DISTRIBUTION LIMITED
Defendants in HP-2021-000025
and
ABBOTT LABORATORIES LIMITED
Claimant/Part 20 Defendant in HP-2021-000026
and
DEXCOM INCORPORATED
Defendant/Part 20 Claimant in HP-2021-000026
and
DEXCOM INTERNATIONAL LIMITED
Part 20 Claimant in HP-2021-000026
and
(1) ABBOTT DIABETES CARE LIMITED
(2) ABBOTT DIABETES CARE INC.
- - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - -
Tom Mitcheson KC, Isabel Jamal and Tim Austen (instructed by Taylor Wessing LLP) for the Abbott parties
Iain Purvis KC and Christopher Hall (instructed by Bird & Bird LLP) for the Dexcom parties
Hearing dates: 24th-28th April, 4th-5th May 2023
- - - - - - - - - - - - - - - - - - - - -
APPROVED JUDGMENT
Remote hand-down: This judgment will be handed down remotely by circulation to the parties or their representatives by email and release to The National Archives. A copy of the judgment in final form as handed down should be available on The National Archives website shortly thereafter but can otherwise be obtained on request by email to the Judicial Office (press.enquiries@judiciary.uk).
- - - - - - - - - - - - - - - - - - - - -
Mr Justice Mellor : This Judgment is structured as follows.
Limitations of his expertise. 8
The influence of his experience at Roche. 9
Dr Schoemaker’s view of what was obvious. 10
Professor Pantelis Georgiou. 11
Who is EP044 addressed to?. 22
Prejudices, preferences, attitudes and barriers. 22
Barriers to obviousness and insufficiency. 23
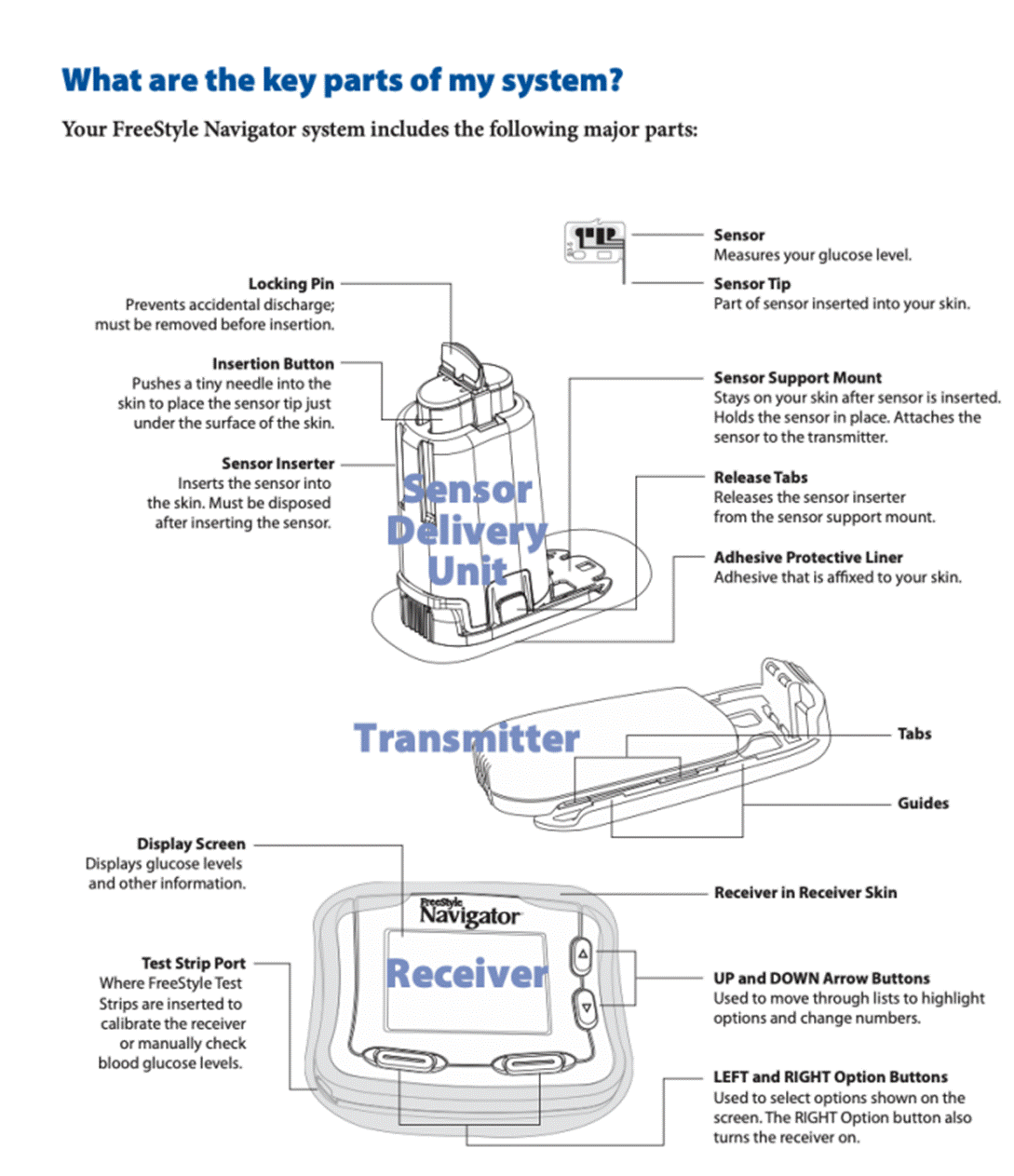
What the Patent assumes to be CGK.. 24
Insertion of needles and the effect of the wound on a sensor. 25
Interaction between members of the Skilled Team.. 25
The issues of construction. 38
1.4 ‘an activation switch’ and 1.6 ‘when the activation switch is triggered’ 39
The influence of claim 6 on construction. 43
‘configured to automatically retract’ 45
INFRINGEMENT - The Dexcom G7. 46
THE PRIOR ART - DISCLOSURE.. 49
What does [0029] disclose to the Skilled Team?. 61
The relevance of commercial considerations to the question of obviousness. 65
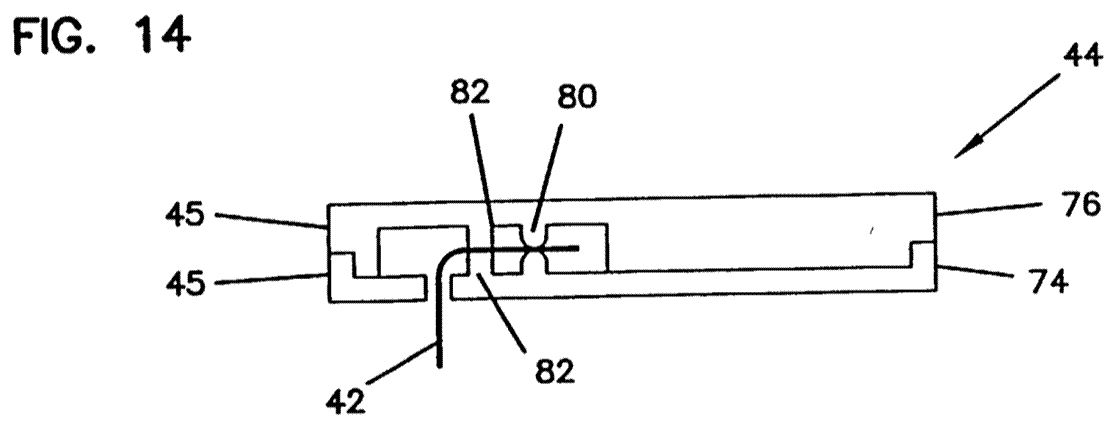
Motivation and problems associated with the prior art 67
Obvious to try and multiple paths. 68
Hindsight and a step by step approach. 68
Dexcom’s implicit reliance on obviousness over the CGK.. 70
The ‘established’ architecture. 72
Why was it not done before?. 73
Issues with manufacture and sterilisation. 79
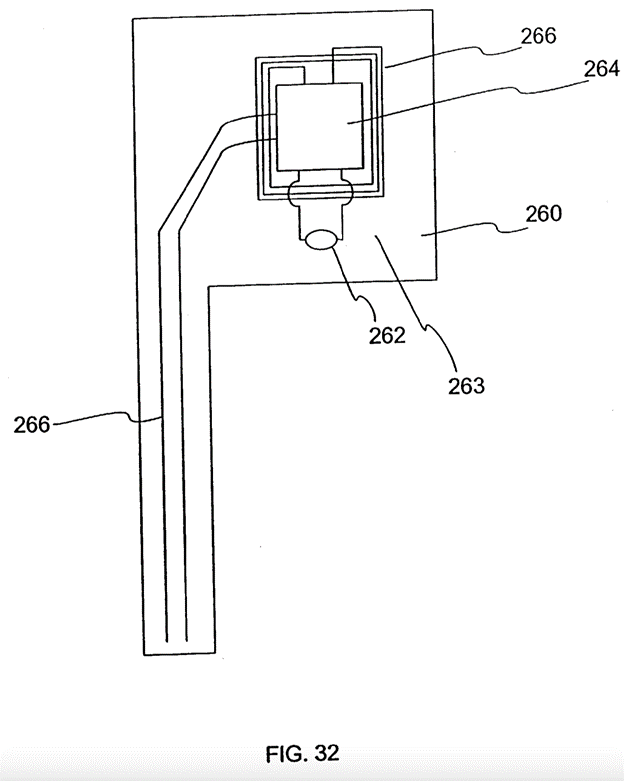
Step 1A - Decide to take forward the Fig 32 embodiment. 83
Step 1B was the alternative of having the electronics and the sensor on different substrates. 84
Step 2 - putting Fig 32 in a housing. 84
Step 3A - Modifying Fig 14 to house Fig 32. 84
Step 3B - Make a new housing for Figure 32 with only part of the sensor exposed. 84
Step 4 - Decide to use Figure 33 as the starting point for the new insertion device. 85
Step 5 - Change the dimensions of Figure 33 to accommodate Figure 32. 85
Step 6 - Remove the mounting unit. 88
Step 7 - Adjust the force to be applied to Figure 33. 88
Step 8 - Establish how to adhere the housed Figure 32 to the skin. 88
Step 9 - Establish how to ensure a safe and reliable insertion without a mounting device. 88
Step 10 - Decide to implement automatic retraction in the new insertion device. 89
Conclusions regarding Heller. 90
INTRODUCTION
1. This is my judgment from Trial B in these proceedings, one of three trials concerning various patents owned by Abbott and by Dexcom which have application in the field of Continuous Glucose Monitoring (CGM) devices. This trial concerns the alleged infringement and validity of EP 3 730 044 B1 (EP044 or the Patent). Originally there were four patents scheduled to be the subject of this trial, including EP418 which is the parent of EP044. For various reasons which it is unnecessary to relate, the original four patents have fallen away, leaving only EP044.
2. EP044 is entitled ‘Compact on-body physiological monitoring device’ with a priority date of 3 February 2009. The First Claimant is the registered proprietor, and the other claimants are said to be exclusive licensees.
3. By the priority date, CGM systems were known. There were three well-known systems on the market:
i) The Abbott Freestyle Navigator;
ii) The Dexcom STS-7;
iii) The Medtronic Guardian Real-Time CGM System.
4. I have to describe each of those systems in greater detail below, but what they had in common was:
i) They were authorised for ‘adjunctive’ use only, which meant that their glucose measurements had to be confirmed by a finger-prick test before any therapeutic decision could be taken.
ii) They were required to be calibrated using finger-prick tests to match the sensor signal to blood glucose concentration.
iii) They comprised four essential components: a sensor, sensor electronics unit, an applicator device and a reader device.
iv) In each system, the sensor was inserted into the skin with an applicator/insertion device. Once inserted, the user then added a separate sensor electronics unit to the sensor. Blood glucose readings were taken from the sensor electronics unit by the reader device which displayed the readings to the user.
6. In the trial, these prior art systems were described as ‘two-part’ or ‘non-integrated’ systems, by way of distinction from the Patent which describes and claims an integrated system, where the sensor and the sensor electronics unit form an integrated unit before the sensor is inserted into the skin.
7. As Abbott were keen to emphasise, the claims in the Patent are concerned with more than just integration of the sensor and sensor electronics unit, and this is a topic to which I must return later.
8. In fact, EP044 is essentially concerned with a system and method of applying the integrated sensor and sensor electronics unit to the user.
9. The alleged infringement is the Dexcom G7 product which was launched in October 2022. As is often the case, Dexcom’s arguments against infringement resolve to issues of construction of the claims of EP044. The Dexcom G7 is Dexcom’s answer to and competition for Abbott’s flagship integrated CGM product, the Abbott Freestyle Libre. Other patents in this litigation covered various aspects of these products, but EP044 is evidently seen by Abbott as protecting their flagship product. Equally, the Dexcom G7 is Dexcom’s flagship product. The two sides put considerable resources and energy into their cases.
10. Abbott were keen to emphasise the number of invalidity attacks which Dexcom had pleaded over the course of this case but by the time of trial, Dexcom alleged invalidity of EP044 on the following grounds:
i) Obviousness over prior art citations known as Heller, Ethelfeld and Fennell.
ii) An added matter attack against claim 5.
iii) Two insufficiency attacks which were explained as squeezes on obviousness.
11. The most significant issues at trial concerned:
i) The identity of the Skilled Addressee/Team of the Patent;
ii) The roles of different members of the Skilled Team;
iii) Their CGK;
iv) The obviousness arguments.
12. Dexcom submitted that a CGM system was and is a complex system which required a multi-disciplinary team comprised of individuals with a wide range of expertise including chemistry, electro-chemistry, biochemistry, software engineering, materials science, electronic engineering, mechanical engineering, product design, clinical studies, wireless communications, biocompatibility, sterilisation, user experience and more to design and implement. To that list, Abbott added process engineering, product management and data analysis/algorithm development but, above all, project management. Indeed, Abbott emphasised the need for this type of team to be headed by a project leader with experience in the overall design of the CGM product and with responsibility for the development of the entire system.
13. It would, rather obviously, be difficult to accommodate expert evidence covering all those disciplines. It would also be unnecessary since it is possible to discern from the Patent itself the principal disciplines to which it is directed.
14. At an earlier stage when the other patents were in issue, the parties were given permission to adduce expert evidence limited to the fields of (a) electrical engineering (b) close proximity wireless communications and (c) design (including mechanical engineering, as required) of medical devices. As Dexcom submitted, the reduction in the claims in issue limited the need for communications expertise.
15. In the event, the parties took different approaches on the expert evidence they called, and these different approaches gave rise to or heavily influence almost all of the issues which I have to decide.
THE EXPERT WITNESSES
16. At times during the trial I thought the expert evidence in this case was like two ships passing in the night. A lot of energy appeared to be being devoted to attempts to establish the views of which expert more closely approximated to that of the Skilled Addressee of the Patent. Furthermore, due to their differing experiences and expertise, an important issue was the extent to which one expert was in a position to disagree with opinions expressed by the opposing expert(s).
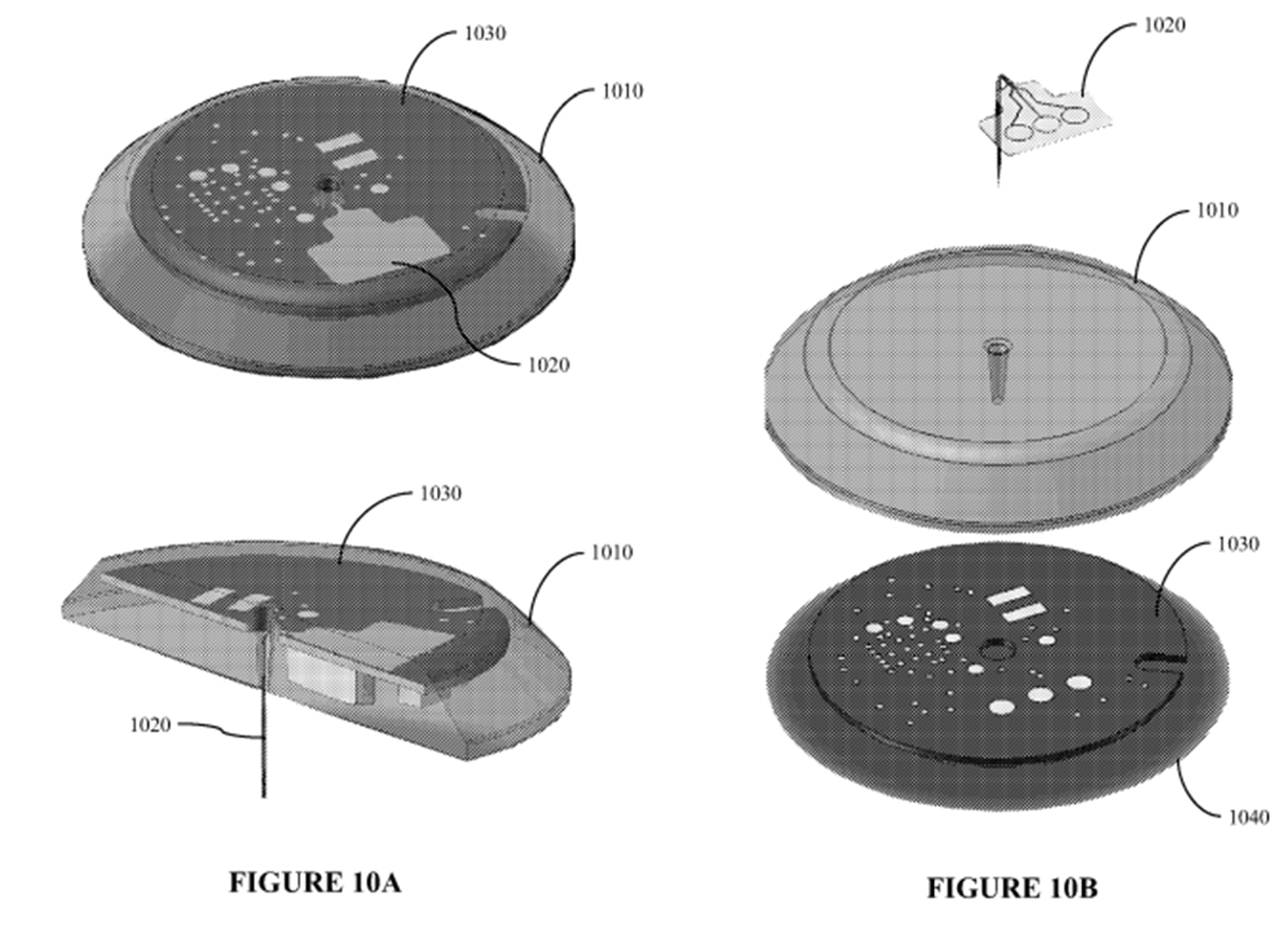
17. As both sides submitted, the principal issues arose between the evidence of Dr Schoemaker and Mr Varde, and aspects of the evidence given by each of them was the subject of criticism by the opposing party.
18. I address these criticisms below. First, however, I must express my gratitude to all three experts for their assistance. Between them I consider they provided me with the education required to decide the disputed issues in this case.
Dr Michael Schoemaker
19. Abbott chose to call Dr Michael Schoemaker as their sole expert. They characterised him as a medical device and diagnostics development specialist in the field of diabetes care, with a particular focus on CGM systems. Throughout his career, he worked as part of multi-disciplinary teams developing CGM systems. He worked at Boehringer and then Roche from 1996-2020. He was Head of the CGM Technology Program at Roche 2001-2003, worked at Disetronic as a System Project Leader from 2003-2005, as Project Leader in Research & Technology from 2005-2007 and then System Project Lead on CGM product development from 2007-2017.
20. Abbott submitted that Dr Schoemaker had extensive first-hand experience working as the project lead in the very teams to which the Patent is addressed, including at the Priority Date. Dr Schoemaker’s particular specialty in the teams in which he worked was as a sensor specialist.
21. A key submission made by Abbott in closing argument was that Dr Schoemaker was ‘clearly comfortable dealing with all the issues in this case at the level at which they have arisen’. The clear import was that Dr Schoemaker had sufficient expertise to opine on all the issues in the case.
22. In closing, Dexcom agreed that Dr Schoemaker was clearly seeking to the best of his ability to assist the Court. Their criticisms were directed to two main points: first, limitations in his expertise and second, the influence on certain matters arising from his specific experience at Roche. There was also a third short point that Dr Schoemaker had a strange idea of what was obvious.
Limitations of his expertise
23. Dexcom submitted that Dr Schoemaker’s expertise in mechanical and electronic engineering was limited to aspects he had absorbed from engineers in his team during his time as a system project leader. I agree. As he put it in his first report at ¶2.3: “In this role [as a system project leader], I gained a proficient working knowledge of all essential components of a CGM system”. However, he readily accepted in XX that he did not have a working knowledge of the ‘toolkit of concepts’ available to a mechanical engineer (such as configuration of housings, springs, insertion/retraction mechanisms, caps, adhesives, needle design, etc.), nor was he able to say how easy it would be for them to overcome an actual design problem using those concepts [T1/6215 - 6620]. The same went for the electronic engineer [T1/6621 - 6719].
24. Dr Schoemaker claimed the ability to understand what his engineers would tell him about their thinking or where they saw problems, which is consistent with his position as the project leader at Roche. Thus, at T1/63:
17 Q. But, what you would not claim is a working knowledge of the
18 whole toolkit of concepts available, say, to a mechanical
19 engineer from their own specific expertise or how easy it
20 would be for them to overcome some design problem using those
21 concepts?
22 A. No, I would not, but the mechanical engineer would be able to
23 explain to me, so I would be in a good position to understand
24 what the electronic engineer or the mechanical engineer are
25 thinking and where they see problems.
25. As Dexcom submitted, this begs the question of the extent to which Dr Schoemaker was able to opine on the questions of obviousness in his Reports when he had not spoken to a mechanical engineer or an electronic engineer about their thinking in relation to the specific issues arising on the prior art.
26. There is, of course, a difference between understanding what a mechanical engineer has explained to you as team leader and being able to think like the mechanical engineer.
27. Dr Schoemaker was very aware of the CGK devices which were on the market at the priority date as a result of his contemporaneous work at Roche developing a new product, but even there it seemed that he left the details of engineering to the engineers. Asked about whether the Navigator used two springs for its automatic insertion/retraction system, he did not know (see T1/76):
4 A. I did not go that much into detail. So that was the job of
5 the mechanical engineer to do that, yes, because I was not
6 responsible for developing an insertion device. That was the
7 job of the mechanical engineer.
2 Q. But, it does not show you, or describe how to actually achieve
3 that, does it?
4 A. No, but I would assume that as part of the common general
5 knowledge of the mechanical engineer, he will find out a way
6 how that works, because there are devices like the Abbott
7 FreeStyle Navigator insertion device which used spring-loaded
8 retraction.
29. On other occasions, for example what had been flagged as the ‘key battleground’ of the Fig.33 redesign on Heller, he simply had to defer to Mr Varde’s evidence because this kind of thing was a matter for the mechanical engineer [T2/14617-1476]. The same occurred with his evidence on how Fig.13 would work in conjunction with Fig.8 of Ethelfeld. In his second report he had drawn an implementation which showed the needle being pushed to one side by the descending spring (see [328] below). But no mechanical engineer would have done that, something he did not challenge at T2/1855-17.
5 Q. But what you have illustrated as causing a problem with that,
6 in your Option 2, is caused by the fact that the needle has
7 been positioned right at the outer edge of the spring; yes?
8 A. Yes.
9 Q. That is what is causing it to be pushed at an angle, as I
10 understand it?
11 A. Yes, now I got your point; yes.
12 Q. Right, good. What I am going to suggest to you is that a
13 skilled mechanical engineer probably would not make it like
14 that. He would ensure that the pressure that was coming down
15 from the bi-stable spring on to the needle was properly
16 vertical, not angled; right?
17 A. Yes.
30. The same went for the specific issues arising in the case concerned with electronics. He did agree however that a good mechanical and electronic engineer would always be interested in improving ease of use (e.g. a one part, rather than two-part system) [T1/9322-9413].
31. Dr Schoemaker was plainly well-placed to assist on matters concerning sensor chemistry, and the identity of those in the Skilled Team, but that leads me to Dexcom’s second point.
The influence of his experience at Roche
32. Dexcom pointed to three aspects which they submitted meant Dr Schoemaker’s particular experiences at Roche cannot be relied upon as typical of a Skilled Team working in this field. The first was his evidence that the practical issues that arose in the course of development at Roche were not of any direct relevance to those in this case [T1/6817-25].
33. The second was his rather intense focus on improving sensor accuracy, a point I have to discuss further below. A recurring theme in his written reports was the lack of motivation for the Skilled Team to move forward with any new product design unless or until sensor accuracy had first been improved. This formed a large part of his Reply Report, commenting on Mr Varde and Prof. Georgiou’s evidence. Despite the number of references he made to this in his Report, however, Dr Schoemaker strangely denied in XX that he had taken it into account on the question of obviousness [T1/9315-21].
34. The third concerned Roche’s priority to protect its market-leading position in the rival Blood Glucose Monitoring (‘BGM’ also ‘SMBG’) technology. This emerged in answer to a question I raised at the end of Dr Schoemaker’s evidence at [T2/218]:
19 Q. I was struck by the fact that your CGM project at Roche did
20 not succeed in getting a product on the market, despite the
21 size of your market share in the blood glucose market as a
22 whole. Was that because it was difficult to catch up with the
23 development of the companies already in the market?
24 A. I would see two reasons for that, actually. One reason was
25 what you can see in other industries as well. We are a market
2 leader in a specific market, selling a specific product and
3 that finally leads to certain mindset in the organisation that
4 makes it difficult to accept new emerging technologies. That
5 happened to Kodak, that happened to Nokia and there are many
6 examples of that.
7 Q. Yes.
8 A. The other reason I can see is, especially at the beginning of
9 continuous glucose monitoring technology, but also around
10 2009, the situation within Roche Diabetes Care was everything
11 you did and everything you want to do, you want to develop,
12 you had to measure against the extremely successful market of
13 selling SMBG devices. That made it difficult as well inside
14 such an organisation to come up with an innovative technology.
Dr Schoemaker’s view of what was obvious
35. Dexcom drew attention to the contrast between Dr Schoemaker’s written evidence, in which he asserted that there was only one obvious way, and his oral evidence in which he readily conceded that there were other obvious ways. They submitted that contrast may have been the result of him misunderstanding that something which was ‘obvious’ needed to be “very well-known in the market”, an answer he gave in this passage of cross-examination on Heller [T2/1738-19]:
8 Q. Okay. Your understanding of obviousness is that it has to be
9 the only obvious solution, is it?
10 A. No.
11 Q. No.
12 A. No.
13 Q. They are both obvious, are they not?
14 A. My definition of "obvious" is, it is what is very well-known
15 in the market…
36. Overall, in view of the content of the Patent and of the Prior Art, I agree with Dexcom that Abbott’s choice of Dr Schoemaker as their sole expert witness in this case was a strange one.
37. In the evidence there were several examples which indicated to me that Dr Schoemaker was not able to adopt the viewpoint of the mechanical engineer in the Skilled Team and that this underpinned many of his opinions that something was not obvious. These examples arise in the context of the prior art. The general point was illustrated by his approach to the figures in the patents examined in this case. The figures are clearly schematic: they illustrate the concept in question. They are not detailed design drawings. Yet in several instances, Dr Shoemaker approached figures in a very literal way, as if they were detailed design drawings. The mechanical engineer in the Skilled Team would not interpret figures in the same way.
38. I have kept all these points in mind.
Professor Pantelis Georgiou
39. Dexcom called Professor Pantelis Georgiou to give expert evidence on matters relating to electronics.
40. Professor Georgiou is a Professor of Biomedical Electronics at Imperial College, London, and Head of the Institute of Bio-inspired Metabolic and Infection Technology Laboratory in the Centre for Bio-Inspired Technology at Imperial. At the Priority Date, Professor Georgiou had no experience in the design or development of a CGM device, although he had experience of using a CGM in the context of his research.
41. At trial, Professor Georgiou was cross-examined for a relatively short period of time and his evidence was largely uncontroversial. Abbott evidently did not like some of the things he said in cross-examination, submitting that his written evidence came across as measured, but his oral evidence less so. Abbott submitted that on a number of occasions he sought to stray beyond his electronic engineering expertise and that he somewhat surprisingly sought to disagree with points put which were entirely consistent with what he had written. In the light of this, Abbott submitted that where there is any conflict, his written evidence should be preferred to his oral.
42. In my judgment, Abbott’s criticisms were misplaced. On the supposed conflict between his written and oral evidence, the example given related to the respective roles of the mechanical and electronics engineer in deciding on form factor. In his written evidence, Prof Georgiou said the form factor was, in large part, within the remit not of the electronics engineer but the product designer. The passage of cross-examination quoted was [T3/43819-43915] but, in my view, his answers there were entirely consistent with his written evidence. The product designer would decide the form factor but the electronics engineer would contribute. I formed the distinct impression that Counsel was trying to create conflict where none existed.
Mr Andrew Varde
43. Mr Varde is a mechanical engineer and the founder of a consultancy which advises on the development and launch of medical devices. His experience up to the Priority Date was in the development of a range of medical devices including feminine hygiene products, respiratory care, suture, urinary, ventilation and drug delivery and blood sampling products. He has not personally been involved in the development of CGM devices, either before or since the Priority Date.
44. Abbott accepted that when giving his oral evidence, Mr Varde was doing his best to assist the Court but ‘within the confines of the situation into which he had been placed’. Abbott stressed his lack of CGM design experience. They submitted that his only basis for his evidence of the CGK was his assessment of the User Guides for the pre-2009 CGM devices, which he read for the first time for the purposes of this case [T2/22418-2254]. Indeed, he accepted that prior to being provided with the User Guides by Bird & Bird he did not know that Medtronic, Dexcom and Abbott were the three main players in the CGM field in 2009 [T2/2255-11].
45. When assessing Mr Varde’s evidence, I have kept Abbott’s criticisms in mind. However, it seemed that one of their principal points was that without experience in CGM design, Mr Varde was uninfluenced by Abbott’s mindset point - that designers in the CGM market were beset with the mindset of the ‘two-part’ or ‘non-integrated’ systems which I mentioned earlier. I have to assess this mindset argument later.
46. Another principal point made by Abbott was hindsight. Abbott submitted that this can be a particular problem in the world of mechanical patents which rely on well-established laws of physics and where many genuine inventions can be characterised as a “mere” combination of plastic, springs and levers (or needles) (see Varde T2/2646-2654). This was a reference to part of Mr Varde’s answer where he said: ‘At the end of the day it is plastic and springs and needles…’.
47. However, his answer needs to be read in context of his earlier and later answers - see the whole passage from T2/2632-2665 - in which a number of points were under discussion. I have added underlining to draw attention to particular points:
A. It is my position that it is likely that a design engineer
3 would have experience of designing a range of medical devices
4 and my view is that, given that CGM, and I think given that
5 CGM was still a relatively small part of the overall diabetes
6 market, there would be a large number of engineers who would
7 be working on, let us say, the more traditional mechanisms
8 that were involved in diabetes and would have knowledge of
9 that that is applicable, in terms of springs and plastic and
10 needles and so on to the mechanics of working on CGMs. A
11 number of the mechanisms of those devices, at least have an
12 analogue, at least have some comparability to the insertion
13 mechanisms for a CGM device. So you have springs storing and
14 creating energy. You have plastic components that need to be
15 designed and operate with each other and you have needles of
16 different designs that need to go in and be retracted.
17 Q. As I understand it, your position is that the design engineer
18 in the notional skilled team in this case would have
19 experience designing non-CGM articles, but would not have
20 experience designing CGM articles; is that right?
21 A. Yes. They may well have experience designing CGM as well by
22 that moment. What I am saying is that they are likely to
23 understand, have an understanding of the other devices that
24 were widely used in the diabetes market.
25 Q. They may have an understanding, but are you saying they would
2 have had experience designing them, because that is a
3 different thing, is it not?
4 A. Potentially they would have had experience designing them,
5 yes.
6 Q. Even if they were aware of these other types of devices,
7 Mr. Varde, I suggest there are significant differences in the
8 way they operate because of their different purposes, as
9 Dr. Schoemaker explained, such as a device intended to cause
10 bleeding to provide blood for a BGM, as compared to a device
11 which you are trying to design in this case, which prevents
12 bleeding so as not to foul the sensor; correct?
13 A. Well, I do not think so, because one needs to look at it in
14 terms of the elements of the design that you are considering.
15 If you break a product down into its constituent elements, if
16 you have a mechanism that is designed to accurately move,
17 advance a component and retract it, that is a mechanism that
18 can be transferred to another environment where that same
19 movement is required. So, the way I see it, and the way I
20 have put it in my report here, is essentially it is that
21 breaking down of the elemental parts. At the end of the day,
22 it is plastic and springs and needles and there will be some
23 specific elements that require specific understanding or
24 require specific input from other experts as to what the
25 particular features are or what the particular considerations
2 that might be required are. But there are a number of
3 mechanisms that are transferable from one type of device to
4 another.
5 Q. I suggest that these different types of devices would not be
6 much help to the design engineer who is seeking to design a
7 CGM, because the devices are themselves different in their
8 purpose?
9 A. It depends what you call a CGM. Are you talking about the
10 whole system or are you talking about just the on-body unit?
11 Q. I am talking about the design team for a new CGM product, so
12 you may be considering only the sub-team that you have
13 identified with the design engineer, but it is a team who is
14 seeking to design a CGM product overall.
15 A. Yes, and the design engineer is designing the mechanical
16 aspects of that. And what I am saying is that with regard to
17 the mechanical aspects of a CGM, there are other devices
18 within diabetes that are performing very similar actions, I
19 think is the way I would describe it, very similar actions.
20 Q. Right, but as far as the sensor is concerned, the insertion of
21 the sensor, the conditions in the skin in which the sensor
22 finds itself once it has been inserted and the retraction of
23 the needle leaving the sensor behind, those are all unique,
24 are they not, to the CGM field?
25 A. Those would be specific features that would need to be handled
2 appropriately as part of the design of the CGM system.
3 Q. With input from the sensor designer and other members of the
4 team?
5 A. Yes.
48. In this passage, Mr Varde gave, in my view, an entirely rational explanation for his view that the Skilled Mechanical Engineer may well have experience designing CGM devices (although not essential) but would also have an understanding or experience of other devices which were widely used in the diabetes market but which embodied similar actions (insertion and retraction in particular). Mr Varde was focussing on the role of the mechanical engineer, but readily acknowledged the need for input from other members of the team. In that context, he was saying that there were other devices in the diabetes market which had mechanisms analogue to the insertion mechanisms in CGM devices.
THE SKILLED TEAM
50. Two matters were identified as still in dispute, but they are best resolved in the light of at least the agreed CGK and along with the other CGK disputes which were identified.
COMMON GENERAL KNOWLEDGE
Agreed CGK
51. Pursuant to an Order made at the PTR, the parties filed an agreed summary of the CGK, along with a list of disputed issues. The following is based on that agreed summary with a few edits of my own.
Diabetes
52. CGM systems are predominantly aimed at users diagnosed with diabetes. It is therefore worth starting with an outline of the key characterises of the condition, all of which the parties agree would have been CGK for the relevant skilled person or team at the Priority Date.
53. Diabetes is a metabolic disorder whereby an individual’s blood glucose levels are naturally too high. In effect, the glucose that is broken down from carbohydrates is not absorbed by the user (or is absorbed insufficiently), meaning it cannot be stored or metabolised to later generate energy. There are two main types of diabetes. Type 1 diabetes occurs when the pancreas is unable to produce insulin. Insulin is a hormone released by the pancreas which acts to reduce the levels of glucose in the blood by increasing the rate of conversion of blood glucose and larger molecules (such as carbohydrates) stored in bodily tissue. Type 2 diabetes, on the other hand, occurs when, although some insulin is made, it is insufficient or does not work effectively. Both types of diabetes can lead to chronic high blood sugar which, in turn, can result in a number of symptoms including an increased need to urinate, fatigue, thirst, weight loss, blurred vision and slow-healing wounds. It can also cause significant complications for the eyes, heart, feet and/or kidneys. If left untreated, diabetes is a life-threatening condition.
54. As a result, and by the Priority Date, it was CGK that individuals diagnosed with diabetes could (and generally would) manage their condition by monitoring their own blood glucose levels. This allowed them to keep their levels within an acceptable range and avoid unnecessary complications. Traditionally, and certainly prior to the advent of CGM devices, this was done by way of Blood Glucose Monitoring (‘BGM’) devices. In short, blood glucose measurements would be taken by the user who would prick their finger in order to obtain a blood sample. They would then apply the sample to the end of a disposable test strip which was inserted into a reader device to obtain a precise blood glucose measurement. Notwithstanding the advent of CGM, BGM devices remain in widespread use, particularly for patients with type-2 diabetes.
55. It was CGK that BGM testing had advantages and disadvantages. A well-known advantage was its accuracy, but a significant disadvantage was the discomfort or pain caused to users, particularly those who were required to check their blood glucose levels on various occasions throughout the day. Another disadvantage was that finger-prick testing provided only a handful of discrete data points throughout the day, so users did not know how their blood glucose levels fluctuated between measurements. Finger-prick testing also placed the responsibility on the user to adhere to a strict testing schedule (which, for those with Type-1 diabetes, would be a lifelong commitment). For those reasons, finger-prick testing was rarely ever performed as often as would ideally be necessary, and therefore was seldom used to its fullest potential.
56. As a result, several players in the industry shifted their focus towards developing a technology which would reduce (or ideally replace) the need for finger-prick testing. The aim was to create a system which continuously monitored blood glucose levels but was less painful and required minimal effort from users.
CGM at the priority date
57. CGM systems had been available on the market since 1999 in various forms but remained at a relatively early stage of development. Notwithstanding, anyone interested in developing a new CGM device would have made it a priority to learn about the features of those devices. What follows is an overview of their relevant features, along with known advantages and disadvantages.
i) The Medtronic MiniMed CGMS (1999) was the first CGM-type device on the market. It comprised a sensor and a reader device but did not provide real-time glucose measurements. Instead, it collected and stored the measurements so that they could be retrospectively reviewed by a clinician (but only once a certain period of time had lapsed). The device used a minimally invasive transcutaneous sensor that measured glucose levels in the interstitial fluid. In 1999, when the device was first released, it had no applicator device to guide the sensor introducer needle, which would have to be inserted manually by the user. By 2000, however, the FDA had approved an auto-inserter for the device which allowed for automatic insertion of both the needle and the sensor (by pressing a button) but required manual retraction of the needle.
ii) The Cygnus GlucoWatch Biographer (2001) was a non-invasive transdermal electrochemical sensor. In contrast to the MiniMed CGMS’s minimally invasive method, the GlucoWatch used a method known as reverse iontophoresis and provided close to real-time results. Effectively, a low-level electrical current would be applied to the user’s skin allowing glucose to be extracted onto a pad. The glucose levels were then measured through the device’s electronics. The GlucoWatch display was provided in the same unit as the sensor, rather than being in a separate reader, which meant no data transmission was needed. Further, because nothing was inserted into the body, no application device was required. The device did, however, suffer from significant shortcomings. For example, accuracy could be impacted by a build-up of sweat underneath the watch. Further, because the GlucoWatch’s sensor took readings from the interstitial fluid (rather than from blood), it suffered from a time lag (compared to BGM testing). Finally, the device only lasted 12 hours on average between charges.
iii) The Medtronic Guardian TGMS (2004) was a telemetered glucose monitoring system. It was indicated for continuous or periodic monitoring of interstitial glucose values and both hypoglycaemia and hyperglycaemia alerts would be displayed in real-time. Glucose measurements, however, could only be displayed retrospectively. In terms of mechanics, the system used the same type of transcutaneous sensor as had been used in the MiniMed CGMS. Insertion was also provided using the same auto-inserter feature as the MiniMed GCMS.
58. Three devices would have been of particular interest to the skilled person or team considering EP-044 at the Priority Date:
i) Abbott Freestyle Navigator I;
ii) Dexcom STS-7; and
iii) Medtronic Guardian Realtime.
59. These three devices would have been of most relevance given that, by the Priority Date, they had emerged as the main CGM devices in the market. The other devices (above) had either been withdrawn, were commercially unsuccessful and/or had significant technical shortcomings.
60. These three systems had important differences but, in terms of general design, were broadly similar. Thus, it was CGK that each system comprised a transcutaneous electrochemical sensor, a sensor electronics unit, an applicator device (or inserter) and a reader device that would receive and display glucose data. The devices also shared a common architecture, whereby a sensor would be inserted into the skin with an applicator/ insertion device, and a separate sensor electronics unit would be manually added as an additional step for the user. For that reason, these (along with the other pre-Priority Date CGM systems) were referred to by the experts as ‘non-integrated systems’.
61. For completeness, an image depicting these four components (and how they interact) in the Freestyle Navigator, by way of example, is reproduced here:
Glucose sensors
62. The Skilled Team would have been able to understand how the sensor worked from a teardown of the on-market CGM devices.
63. The Freestyle Navigator, the STS-7 and the Guardian Realtime all used transcutaneous electrochemical sensors. At the Priority Date, these were considered the most reliable form of CGM sensor, although they had a very limited lifespan (3 days for the Guardian Realtime, 5 days for the Freestyle Navigator and 7 days for the Dexcom STS-7).
64. The transcutaneous electrochemical sensors worked by electrolysis. They comprised two (sometimes three) electrodes covered by coating(s) and were inserted into the skin so that they came into contact with the interstitial fluid at one end. Once a potential difference was applied to the sensor, the electrodes generated the electrochemical reaction necessary to detect glucose levels. The sensor did not need to engage with the user’s bloodstream because the glucose concentration in the interstitial fluid broadly reflected overall blood glucose concentration.
65. Creation of the sensor involved deposition of a delicate enzyme layer. Known methods for doing so included screen-printing onto a flat substrate, and dip coating. It was also CGK that sensors would need to be sterilised. At the Priority Date, known methods of medical sterilisation included ethylene oxide (‘ETO’), steam, gamma ray and electron beam (or e-beam). It would have been CGK that these techniques existed, although the skilled person would have recognised that some may not have been compatible with the sensor chemistry and/or electronics.
Sensor Electronics
66. The sensor electronics served to process the analog signal received from the sensor into a digital form. The electrical current reading obtained was an analogue signal that would need to be converted into digital readings before they could be further processed and transmitted wirelessly. The digital signals could be filtered to reduce noise, translated into accurate glucose concentration values or transmitted as raw data. It was therefore CGK that at least some processing would be required within the sensor electronics to enable wireless transmission.
67. The sensor electronics unit generally contained a power supply (to power the sensor), component(s) for processing the sensor signal, and a transmitter for wireless transmission to the reader device. The skilled person/team would have sought to understand the power requirements necessary for processing, the size and type of battery required and therefore the dimensions of the on-body unit. Each of three main CGM systems used a battery as the form of power supply, but did so in different ways:
i) The Abbott Freestyle Navigator I used a non-rechargeable battery that lasted for 30 days and needed to be replaced by the user at the end of its lifetime;
ii) The Dexcom STS-7 used a non-replaceable, non-rechargeable battery which was integrated into the sensor electronics. At the end of the battery’s lifetime (around 3 months), both the battery and the sensor electronics would be disposed of together; and
iii) The Medtronic Guardian Real-Time used a non-replaceable but rechargeable battery which was also integrated into the sensor electronics. Similar to the STS-7, both the battery and the sensor electronics would be disposed of at the end of the battery’s lifetime (around 14 days per charge, rechargeable on average for 1 year).
68. At the Priority Date, all CGM systems using a transcutaneous sensor (including the Freestyle Navigator, the STS-7 and the Guardian Real-Time) used an assembly whereby the sensor electronics unit and the sensor would be provided to the user as separate components. The user would, in effect, have to manually connect the sensor electronics unit to the sensor after the sensor had already been inserted. The reason for this would have been CGK to the skilled person. The sensor had a much shorter lifetime (between 3-5 days) compared to the sensor electronics unit (one year). It therefore needed to be replaced more frequently. However, the electronic components were significantly more expensive than the sensor. The cost, therefore, to the user of a system who threw away the electronics once every few days would have been multiple times that of the 2-part system. As such, it made both practical and commercial sense for the non-integrated systems to use disposable sensors and reusable electronics, to avoid the costs associated with disposing of expensive electronics every 5-7 days.
69. On a related note, in general companies did not yet invest in bespoke sensor electronics components, such as an ASIC, due to significant upfront costs. Instead, companies typically relied on sourcing electronic components off-the-shelf. This contributed to the manufacturing costs of the sensor electronics units being several times that of the sensor - indeed, in cross-examination, the figure of 20x was used. This was also partly due to the fact that CGM devices were not yet in wide use in the market, even though the potential market was acknowledged to be huge.
Insertion devices and mechanisms
70. An applicator device was used to insert the sensor into the body. At the Priority Date, the design and functionality of the applicator devices varied, but they typically had the same basic components including a housing, a needle, some sort of insertion mechanism and a retraction mechanism. The applicator could insert or retract either manually or automatically and the three main CGM devices varied in this respect:
i) The Abbott Freestyle Navigator I used an automatic insertion and removal process which was activated by a single push button;
ii) The Dexcom STS-7 used a manual insertion and removal process; and
iii) The Medtronic Guardian Real-Time used an automatic insertion which was activated by a single push button, and a manual removal process.
71. Both the Abbott Freestyle Navigator and Dexcom STS-7 insertion devices also made use of a mounting unit onto which the sensor electronics unit was attached. The unit would be adhered to the user’s skin and allowed the sensor electronics unit to be coupled to the sensor as precisely as possible whilst minimising trauma to the tissue. Notwithstanding, the user still needed to manually attach the sensor electronics unit to the mounting unit after insertion to connect it to the sensor.
72. Medtronic did not make use of a mounting unit. Instead, the user would couple the sensor electronics to the sensor through a connector on the sensor base (which contained an adhesive).
73. It may help at this juncture to summarise and compare the key features of the CGM devices:
|
Device |
Release |
Insertion |
Sensor lifetime |
Transmitter lifetime |
Other points |
|
MiniMed CGMS |
June 1999 Oct 2000 |
No insertion device at first.
From Oct 2000, it came with a re-usable automatic insertion and manual retraction device. |
|
[no transmitter] |
Wired directly to the reader device |
|
Medtronic Guardian TGMS |
Jan 2004 |
Re-Usable inserter; automatic insertion; manual retraction |
3 days |
|
Wired to a transmitter; radiofrequency transmission to a reader |
|
Medtronic Guardian RT (i.e., Realtime) |
Jul 2005 |
Re-usable inserter; automatic insertion; manual retraction |
3 days |
1 year |
Initially wired to an on-body transmitter, with radiofrequency transmission to a receiver
Activation switch |
|
Dexcom STS |
Mar 2006 |
Disposable inserter, manual insertion & retraction |
3 days |
3 months |
Sensor and electronics arranged on a mounting unit attached to the skin |
|
Medtronic Guardian RT with MiniLink Real-Time Transmitter |
Feb 2007 |
Re-usable inserter; automatic insertion; manual retraction |
3 days |
14 days per charge; rechargeable for ~ 1 year |
Sensor attached to the skin via adhesive. No mounting unit used.
Activation switch. |
|
Dexcom STS-7 |
May 2007 |
Disposable inserter; manual insertion; manual retraction |
7 days |
3 months |
Sensor and electronics arranged on a mounting unit |
|
Abbott Freestyle Navigator |
Mar 2008 |
Disposable inserter; Automatic insertion & retraction |
5 days |
30 days |
Sensor and electronics arranged on a mounting unit |
74. None of the three CGM systems (or in fact any) at the Priority Date were considered accurate or reliable enough to receive regulatory approval to be a ‘replacement’ for Blood Glucose Measurements (‘BGM’) through finger-prick testing. In fact, prior to the Priority Date, all of the CGM devices on the market were authorised for adjunctive use only, meaning their glucose measurement had to be confirmed by a finger-prick test before any therapeutic decision (e.g., the administration of insulin) could be taken. In addition, all known CGM systems required calibration through finger-prick measurements, which the user would have to do once upon insertion (following an initial ‘warm up period’), and then periodically thereafter.
CGK Disputes
75. Ultimately, although the specific functionality of the on-market CGM devices was agreed to be CGK, the reasons given by respective experts as to why the devices worked in a particular way, and their opinion as to what aspects the skilled person or team might have wished to improve upon were not agreed.
76. The two disputes which relate to the Skilled Team were expressed as follows:
i) First, whether the patent is addressed to a team developing a CGM product as a whole, or to a Design/Mechanical Engineer and Electronic Engineer, and how decisions would be made by the relevant team (Varde 1 §5.15, §5.16, §5.20 and §5.21, Georgiou 1 §3.2, Schoemaker 1 §5.4 and §5.9, Varde 2 §3.1 and §3.4, Georgiou 2 §2.4, Schoemaker 2 §2.3).
ii) Second, whether the relevant skilled person/team (either the CGM product team or the Design/Mechanical Engineer with the Electronic Engineer) would have had direct experience working in CGM (Varde 1 §5.14; Schoemaker 2 §§2.9-2.11, §3.26).
77. As for the CGK disputes, I can summarise them as follows:
i) The weight which the skilled person/team placed upon the sensor and associated concepts such as accuracy, lifetime, the need for a replacement claim, etc. (Schoemaker 1 §§6.16, 6.22, Schoemaker 2 §§3.2-3.4), and on improving ease of use and insertion, form factor and power management (Varde 1 §§7.7-7.8, 7.16, 8.3, Georgiou 1 §§4.21, 4.34-4.36, Varde 2 §§4.3-4.5).
ii) Whether “Non-CGM Devices” were part of the CGK (Varde 1 §§7.43-7.64; Schoemaker 2 §§3.25-3.27).
iii) The extent to which the existing CGM devices followed an architecture that could be considered as “accepted”, or whether the use of a 2-part device was seen by to the skilled person/team as an ‘overarching drawback’.
iv) Whether the cost of components was a barrier to technical development of an integrated wearable, or whether there would be significant cost reductions linked to the volumes and timescales involved in manufacturing an integrated wearable on a commercial scale.
v) The relevance of pain and injury and the extent to which wounds caused by insertion of a needle (and the healing of such wounds) interfered with sensor accuracy.
vi) Dr Schoemaker’s reasoning as to why the CGK was in the state it was, and Mr Varde’s opinions about the extent to which the skilled person appreciated there to be disadvantages with the state of the art.
78. A number of these disputes were linked to the potential issue as to which party’s expert(s) more closely approximates to the hypothetical Skilled Team. It is well-known that such arguments are not helpful. As Jacob LJ made clear in Technip France SA’s Patent [2004] RPC 46 at [12]-[15], what matters in an expert witness is not their opinions per se but their reasons.
79. In this case, closely related to that issue are the additional issues of what prejudices, preferences and attitudes are to be ascribed to the Skilled Team (or not) and whether the Team has knowledge of the ‘non-CGM devices’.
80. I address all these disputes under the following headings, but first I should remind myself of the applicable principles.
Who is EP044 addressed to?
81. In view of the measure of agreement as to the Skilled Team (see [49] above), the issue is not a binary one between Dr Schoemaker’s project leader and those with specific expertise - the Electronic Engineer and the Mechanical Design Engineer - it is, as Dexcom submitted, better framed in terms of the degree of influence that any given member of the team might have on the other members.
82. On this issue, Dexcom drew attention to two well-known principles:
i) The first, as stated in Terrell at 8-36 is that ‘The purpose of assembling a team of different specialists is, of course, that each member should bring his or her individual skill and general knowledge.’
ii) The second is the dictum of Arnold J. in Generics (UK) v Warner-Lambert [2016] RPC 3 in which he addressed the role of a team leader (a point which was not disturbed on appeal):
‘[118] …each member of the team is assumed to play his (or her) own part. Depending on the facts of the case, that may involve one member taking the lead. Taking the lead is not the same thing as directing the other member as if the other member were a subordinate.’
Prejudices, preferences, attitudes and barriers
83. It is well-established that the Skilled Team will share common prejudices and conservatism which prevail in the art concerned: see Jacob LJ in Technip France SA’s Patent [2004] RPC 46 at [10].
84. In Glaxo Group Ltd’s Patent [2004] RPC 43, Pumfrey J explained the difference between permissible and impermissible prejudices under which the skilled person may labour:
‘[30] Such a prejudice may be a commercial one (“this device won’t sell”) or it may be a technical one (“this won’t work and it is not worth bothering with”). A 20-year monopoly is conferred for overcoming a prejudice of the second kind, but not for overcoming a commercial prejudice (see Hallen Co v Brabantia (UK) Ltd [1989] RPC 307 (Aldous J.)). A technical prejudice must be general: it is not enough that some persons actually engaged in the art at the material time labour under a particular prejudice if a substantial number of others do not. A prejudice which is insufficiently widespread for it properly to be regarded as commonly shared will not, in my view, be attributed to the notional skilled person.’
Barriers to obviousness and insufficiency
85. A particular prejudice which Dr Schoemaker addressed in his evidence was the Skilled Team’s alleged preoccupation with sensor improvements. This gave rise to three issues: first, whether it was factually correct; second, whether it was merely a commercial prejudice and third, whether it was a problem solved or even recognised by EP044.
86. On that third issue, Dexcom drew attention to this passage in the judgment of Richard Meade QC (as he then was) in Fisher & Paykel v Flexicare [2020] EWHC 3282 (Pat) at [44]-[47]:
‘44. As a general matter, it is often possible to deduce the attributes which the skilled addressee must possess from the assumptions that the patent in suit makes about his abilities (Horne v. Reliance [2000] FSR 90 at [11]). So, for example, the fact that the Patent expects its skilled addressee to be able to make a co-extruded tube with breathable material with very little guidance implies that the skilled addressee could undertake the same or a similar task starting from the prior art.
45. More concretely, Flexicare ran a squeeze that if the expectation of success from the prior art would be absent or too low then the Patent was insufficient because it provided no more teaching than the prior art, and argued in relation to inventive step that it was not legitimate for F&P to rely on a perceived problem in implementing the prior art (a "lion in the path") unless the Patent showed how to overcome it. Flexicare cited the dictum of Floyd LJ in Koninklijke Philips NV v Asustek Computer Corp [2019] EWCA Civ 2230 at [73]:
"The principle is that you cannot have a patent for doing something which the skilled person would regard as old or obvious but difficult or impossible to do, if it remains equally difficult or impossible to do when you have read the patent. To put it another way, the perceived problem must be solved by the patent."
46. I accept this statement of principle and its potential application to the present case.
47. In response, F&P said that it was not relying on perceived problems of implementing the prior art or some modification of it, but rather was only saying that what the Patent involved was unusual and would be unfamiliar to the SDE; while it could be made with confidence using CGK if the idea occurred to the SDE, the unfamiliarity meant that it would not be prone to occur to him or her in the first place. Counsel for F&P cited Lord Justice Jacob in Unilever v Chefaro [1994] R.P.C. 567 at 587: that (paraphrasing) it is not so much that the challenges would put people off trying, but rather in the absence of firm knowledge and experience, the conception of modifying the prior art as claimed would not come readily to mind. I accept this principle as well and think it is relevant. The SDE would not be familiar with breathable tubes of the dimensions and physical characteristics (e.g. resistance to crushing, ability to "drape") required for the claimed products of the Patent.’
Analysis
87. In the light of those principles, following the evidence and various challenges made in cross-examination, the position was clear and I can summarise it as follows:
i) The Patent is addressed specifically to the Design Engineer and the Electronic Engineer working within a wider CGM team.
ii) It was common ground that the hypothetical Skilled Team would require a leader of some description. However, I reject any suggestion (which appeared to be being made in some of the cross-examination of Mr Varde) that the leader would be a sensor specialist who would not seek to pull the team together towards the design of a new CGM device, but would act more like a dictator, impervious to ideas from team members. Each member was bringing their own ideas for different areas of the CGM device, and developments would go on in parallel.
iii) Dr Schoemaker’s personal prejudices etc from his time at Roche are not relevant, since they were clearly not shared by all relevant skilled persons in the field. A number were also commercial in nature and similarly not relevant to the Skilled Team.
iv) As Dexcom pointed out, the Skilled Team must be taken to possess a high level of ability and knowledge. This is evident from what the Patent assumes about the CGK of the Skilled Team, a point which I address further below.
v) In particular, the idea that the Skilled Team would have no knowledge of non-CGM devices did not survive cross-examination. Dr Schoemaker accepted the Team would know of non-CGM devices in the field and how they operated, even if they might not have experience in developing such devices.
vi) The Design Engineer and Electronics Engineer did not require prior experience in developing CGM devices, although at least some members of the Team had that experience.
88. That leaves the following specific topics.
What the Patent assumes to be CGK
89. Dexcom correctly pointed out that the Patent is entirely silent on a number of matters critical to the making of the claimed device including:
i) How to make a working sensor. The Patent contains no teaching of the deposition of enzyme layers or any information about electrochemistry, or any information about sterilisation of the device and the possible effects of sterilisation on the sensor materials.
ii) Needle design, or how it is to be enabled to be engaged with the sensor.
iii) Many of the features of the insertion and retraction mechanisms. EP044 discloses the existence of a spring but no details of how it is engaged or activated and leaves it entirely to the mechanical engineer on the Team to design a working spring-loaded retraction system.
iv) How to implement various types of activation switch.
90. In accordance with the dictum of Pumfrey J. in Horne Engineering, the Patent assumes that all of this is within the CGK of (members of) the Skilled Team. With this point in mind, it can be seen that what the parties managed to agree on the CGK omitted some very important knowledge and practical skills which the mechanical engineer in particular would bring to the Skilled Team. These would be essential to enable the Team to implement the Patent, so they must be attributed to the Skilled Team when reading each piece of prior art cited in this case.
91. Dexcom pointed out that this case is not the sort of case mentioned in [47] of Fisher & Paykel. I agree.
Insertion of needles and the effect of the wound on a sensor
92. This is a particular aspect of the preceding topic, but it has a particular relevance to one piece of prior art - Ethelfeld. To the extent necessary, it is best discussed in that context.
Prejudices and motivation
93. Abbott contended there were two key prejudices or influences on the Skilled Team. The first concerned sensor accuracy and the second, the ‘established’ two-part architecture. These cannot be analysed in isolation since both were said to affect the motivation for the Skilled Team to take any particular idea forward. Both are therefore best dealt with in the context of the obviousness arguments.
Interaction between members of the Skilled Team
94. Although I have now made findings as to the constitution and characteristics of the Skilled Team and their CGK, I must continue with some closely related points made by Abbott which were designed to impact on the evidence of Mr Varde and Professor Georgiou.
95. Following the evidence, Abbott submitted there was no dispute as to the characteristics of the notional Skilled Team. It was agreed that the Team would have a leader with experience in the development of a CGM device. Prof Georgiou had explained that the team leader would typically be someone with experience of designing a CGM device in the past (Georgiou 1 [D1/1/9] §3.3), and Mr Varde agreed (T2/22721-2283). Mr Varde also agreed that the design engineer would work very closely with and seek advice from other members of the team including those with CGM experience - but that he had not had the benefit of such advice in compiling his evidence (T2/2309-18). Mr Varde went on to accept that the team leader with CGM experience would be able to educate the design engineer as to the preferences and prejudices of those working in the CGM field (T2/2284-11). In relation to the team leader, he continued at T2/228-229:
21 Q. All right, but they would know about the well-established and
22 proven ways of tackling a particular problem so as to avoid,
23 as you put it, having to reinvent the wheel?
24 A. Yes.
25 Q. They would also know about pre-existing problems and they
2 would know be the FDA complaint data that you talk about?
3 A. Yes.
4 Q. They would manage the development process and facilitate the
5 decision making between the different disciplines in order to
6 arrive at the best overall outcome?
7 A. Yes.
8 Q. They would not necessarily implement something suggested by a
9 sub-team; it would depend on the overall balance of factors
10 required?
11 A. Yes.
12 Q. And as Dr. Schoemaker explains, system design decisions would
13 be made at a team level, would they not?
14 A. Yes, they would.
15 Q. There would be communication between the different sub-teams
16 within that?
17 A. Yes, absolutely.
18 Q. That is because CGM is a complex product and decisions
19 relating to one aspect of the product may well affect other
20 aspects?
21 A. Yes.
96. The purpose of Abbott’s emphasis on these matters was to highlight alleged inadequacies in the way Dexcom’s experts had been instructed.
97. In fact, in closing, Abbott submitted that Mr Varde and Professor Georgiou had not been instructed properly in two important respects.
98. The first (which I deal with here) concerned Abbott’s argument that no attempt had been made on Dexcom’s side to ensure the experts considered matters in the context of the Skilled Team as a whole. The second (which I deal with later) was whether Dexcom’s experts had been instructed properly as to the appropriate level at which to assess obviousness.
99. Abbott accepted that relevant evidence was admissible from the notional design engineer and electronics engineer, but submitted that, for such evidence to be of assistance to the Court, it had to be given from the perspective of such a person having donned the relevant mantle as of February 2009. Further, given that the design of the CGM in this case would be the job of a multidisciplinary team led by someone with CGM experience, Abbott submitted that some attempt needed to be made to replicate the overall team and decision-making process to determine whether an idea conceived in one of the sub-teams would actually be taken forward or dismissed.
100. In this regard, Abbott relied on Convatec Limited v Smith & Nephew Healthcare Limited [2012] RPC 9 for the point that what had to be established was whether the invention was obvious to the Skilled Team as a whole. In that case, the patentee’s evidence was that the prior art had technical challenges which would have dissuaded the wound care scientist on the Skilled Team, whereas the defendant relied solely on evidence from a cellulose chemist who did not consider that that member of the team would have been dissuaded on the basis of those issues. Birss J (as he then was) held that the claim would only be obvious if it was obvious “to the relevant team as a whole” (at [142]) and so held that the invention would not be obvious (at [143]). The Court of Appeal agreed with the Judge’s analysis: Convatec Limited v Smith & Nephew Healthcare Limited [2013] RPC 7 at [88]-[90]. In this case, so Abbott submitted, it is equally important to consider the prior art and the question of obviousness from the perspective of the whole of the relevant team, which would have included someone with CGM experience and a sensor developer.
101. In this vein, Abbott submitted that experts need to be instructed such that their reasoning can be judged in the context of the point of view of the notional Skilled Team. This means that they should be aware of all the material that is likely to comprise the CGK. They should also consider the input of others on the Skilled Team and whether there are priorities or prejudices which need to be considered as part of the overall decision-making progress.
102. Abbott submitted that Dexcom’s approach failed in both of these respects. They submitted that Mr Varde did not have any relevant personal experience of a CGM team and was plainly not supplied with the necessary material to ‘don the CGK mantle’. All he was given was the user guides of the commercial products. He had not had an opportunity to strip the products down, nor was he supplied with any of the papers, review articles or conference materials which would have formed part of the CGK of the notional team in 2009 (T2/22515-22613). For example, he was not even aware when he wrote his first report that sensors contained enzymes (T2/24919-21). Prof Georgiou had a little more personal experience, but it was completely unclear what, if any, additional materials he had over and above the User Guides. Indeed, it appears that he himself found the User Guides online rather than being provided them by Bird & Bird. He also was reading them for the first time in 2022 (T3/39911-13).
103. Abbott also submitted that no attempt was made to put either expert’s evidence in the context of the Skilled Team as a whole. Instead, it is said they gave their evidence in a vacuum and did not attempt to consider what other members of the team might say. Mr Varde acknowledged that he had only assessed the prior art from the perspective of the design engineer and not the team as a whole (T2/2714-11). This allegedly post-hoc, deductive approach was said to be apparent from what he explained at T2/23716-19: “I think I can make a reasonable deduction from, as to, from a user perspective how a device could be improved. That, I think, is pretty much the guard rails in which I have operated in my report.”
104. As regards Prof Georgiou’s evidence, Abbott likewise alleged it was confined to that of the electronics engineer, primarily directed at power management and shelf life (D1/1/§§5.3-5.4 & T3/4089-23). Yet he confirmed that there would be a team in 2009, with the members interacting with each other to reach agreement and achieve a common goal under the direction of a team leader (T3/39417-39620).
105. Abbott also pointed out that neither of Dexcom’s experts was even permitted to read the other’s written reports, let alone consider the impact of any suggestions on the team as a whole. As a result, so Abbott said, the Court has only been given two partial snapshots by Dexcom of how two sub-sections of the Skilled Team would react to any of the prior art. The Court has, so it is said, evidence on behalf of a design engineer and an electronics engineer, but this amounts to listening to only one or two voices in a multi-person discussion which would also have input from the sensor expert, manufacturing engineer, production engineer and the head of the project with experience in the design of CGM products overall. It also ignores the realities inherent in the design of a complex medical device, the primary purpose of which is to provide accurate sensing of glucose levels. The culmination of all these points was Abbott’s contention that, even at its highest, Dexcom’s evidence is incapable of supporting a finding of obviousness in this case.
106. By contrast, Abbott maintained that Dr Schoemaker was instructed and able to cover the input of the other relevant experts at the level required to deal with the issues arising in this case. As a result, it was said that his reasoning is of far more assistance than that which could be provided by either Mr Varde or Prof Georgiou as a result of the way in which they have been instructed.
Analysis
107. Speaking in general terms, there were shortcomings in the expert evidence led by each side. Dr Schoemaker did not have the ability or expertise to be able to comment meaningfully on what the notional design engineer would have done. I reject the suggestion that he was able to ‘cover the input of the other relevant experts at the level required to deal with the issues arising in this case’. At the same time, there is some force in the criticisms made by Abbott, but only some, since they were overdone.
108. I agree that in cases involving a team, it is better if the experts in different disciplines at least see what the other is saying. In some cases, it can be necessary for them to confer (see e.g. Alcon Eye Care UK Limited v AMO Development LLC [2022] EWHC 955 (Pat) at [233]-[235]). But even where each expert is kept siloed, the issue remains as to whether that has had any detrimental effect on the force of their reasoning. Beyond Dr Schoemaker’s points about sensor accuracy and the ‘established architecture’, no examples were given where a team leader (such as Dr Schoemaker) would have had any reason to overrule or reject suggestions made by the mechanical or electronics engineers in the team. The closest one got to such a situation was the arrangement of the insertion needle in Ethelfeld, but, as I explain below, that situation arose because of the overly literal approach taken by Dr Schoemaker to schematic drawings, whereas Mr Varde produced two practical solutions. That conflict would not have arisen in the notional Skilled Team or in any real-life team because the team leader would have listened to the mechanical engineer’s suggestions and they would have agreed on one of the practical solutions.
109. Furthermore, many of the criticisms which I have summarised above were expressed at a very general level and I have to examine later whether they apply at the more detailed level of the disclosures in the prior art. In particular, I reject the submission that Dexcom’s evidence is incapable of supporting a finding of obviousness.
THE PATENT
110. In the introductory Background section, EP044 explains:
‘[0001] The detection of the level of glucose or other analytes, such as lactate, oxygen or the like, in certain individuals is vitally important to their health. For example, the monitoring of glucose is particularly important to individuals with diabetes. Diabetics may need to monitor glucose levels to determine when insulin is needed to reduce glucose levels in their bodies or when additional glucose is needed to raise the level of glucose in their bodies.
[0002] Devices have been developed for continuous or automatic monitoring of analytes, such as glucose, in bodily fluid such as in the blood stream or in interstitial fluid. Some of these analyte measuring devices are configured so that at least a portion of the devices are positioned below a skin surface of a user, e.g., in a blood vessel or in the subcutaneous tissue of a user.
…
[0004] Ease of insertion and use, including minimal user intervention and on-body size and height (or thickness) of such transcutaneous or percutaneous medical devices that are worn on the body are important in usability, wearability, and comfort during the device usage. Moreover, for many of such medical devices that require a battery or a similar power source to perform the device specific operations, power management as well as shelf life is important.’
111. The ‘Summary’ section sets out some general points about various embodiments disclosed in the remainder of the specification. There are only two points which it is necessary to mention. The first concerns the sensor life contemplated in [0005]:
‘Sensing time period may be determined by the analyte sensor life, for example, including, but not limited to about three days or more, about five days or more, or about seven days or more or about fourteen days or more.’
112. The second concerns the rather oblique introduction of perhaps a key part of the invention in [0008] where reference is made to:
‘…the on-body patch device including the analyte sensor and the data processing and communication components provided in a compact, low profile housing and placed on the skin surface of the user.’
113. A similar phrase is used in the description of several of the drawings, including in particular, Figs 10-18, ‘..the on-body patch device including sensor and sensor electronics assembly in accordance with embodiments of the present invention’.
114. A key point comes in [0011], where the last sentence explains:
‘Embodiments include an on-body assembly including a transcutaneously positioned analyte sensor and sensor electronics in a compact low profile integrated assembly and coupled to an insertion device for deployment.’ (my emphasis).
115. Figs 1-7 & 9 are either block or circuit diagrams of various components in the overall system. Figs 13-18 are concerned with various power supply switch mechanisms. I need not set out these figures, but I will mention particular paragraphs relating to those figures to which Abbott drew particular attention.
116. [0033] states that “In aspects of the present disclosure, the sensor and the data processing unit (sensor electronics) may be configured as a single integrated sensor and sensor electronics assembly (110)” which may be “configured as an on body patch device”.
117. Figure 2 (and the description at [0050]-[0051]) shows an “on body patch device 211 including sensor electronics coupled to an analyte sensor 250 [that] is positioned on a skin surface 210 of a patient or supervisor” and describes the introducer mechanism (i.e. the insertion device, one example of which is shown in Figures 12A-12G) as being “fully or partially automated, or that it may be fully or partially manual.”
118. [0051] also refers to the fact that the on-body patch device may include an introducer needle to guide the sensor during the insertion. [0051] identifies a further aspect, in which:
“the placement of the on body patch device 211 on the skin layer 210 includes the initial piercing of the skin layer 210 with a force applied on the on body patch device 211 in conjunction with the on-body patch device 211 placement on the skin layer 210, effectively driving the sensor 250 (and/or the introducer) through the skin layer 210”.
119. Further, [0051] describes the fact that:
“within the scope of the present disclosure, a mechanism (such as a spring for example) may be provided within the on-body patch device or alternatively in the introducer in cooperation with the on body patch device to withdraw the introducer needle after the sensor has been positioned in fluid contact with the body fluid.”
120. [0089] refers to one aspect where the on-body patch device may include a single integrated housing or body assembly that includes the analyte sensor, electronics and an adhesive patch. It goes on to state:
“Such configuration provides for fewer parts that require manipulation by the patient or user, leading to improved ease of use, and further, with an over moulded assembly, may be configured to provide the desired water tight seal during the course of the wear, preventing moisture or other contaminants from entering into the on-body patch device housing. Such single body configurations may additionally provide ease of manufacturing with the fewer components that require assembly.”
121. Figs 10-12 are the key figures for explaining the ‘integrated analyte monitoring assembly’ the subject of the claims of EP044.
122. I can start with Figs 10A and 10B:
123. Figure 10A shows a cross-sectional and perspective view of the integrated sensor and sensor electronics, whereas Fig 10B is a somewhat exploded view. The sensor is labelled 1020 and comprises the thin needle attached to what appears to be a small circuit board carrying three connectors.
124. [0110] explains the arrangement and its advantages:
‘[0110] Referring back to FIG. 10A, in one embodiment, the analyte sensor 1020 is assembled (e.g., provided to the user) with the sensor electronics 1030 and provided within the housing 1010. Furthermore an adhesive (single sided or two sided) layer 1040 (FIG. 10C) may be provided on a lower surface of the housing 1010 to provide secure positioning of the housing 1010 on the skin surface during and after sensor deployment. As discussed in further detail below, the integrated sensor and sensor electronics assembly/on-body patch device 110 [sc.1010] may be positioned (e.g., during manufacture to provide to the user) within the housing of an insertion device, avoiding the need for a user to align, position, or otherwise connect or couple the sensor and sensor electronics to the insertion device prior to the insertion of the sensor and turning on the sensor electronics. Accordingly, potential misuse, misalignment of the sensor relative to the introducer of the insertion device, or errors and difficulties in use of the integrated assembly by the user may be avoided.’ (emphasis added)
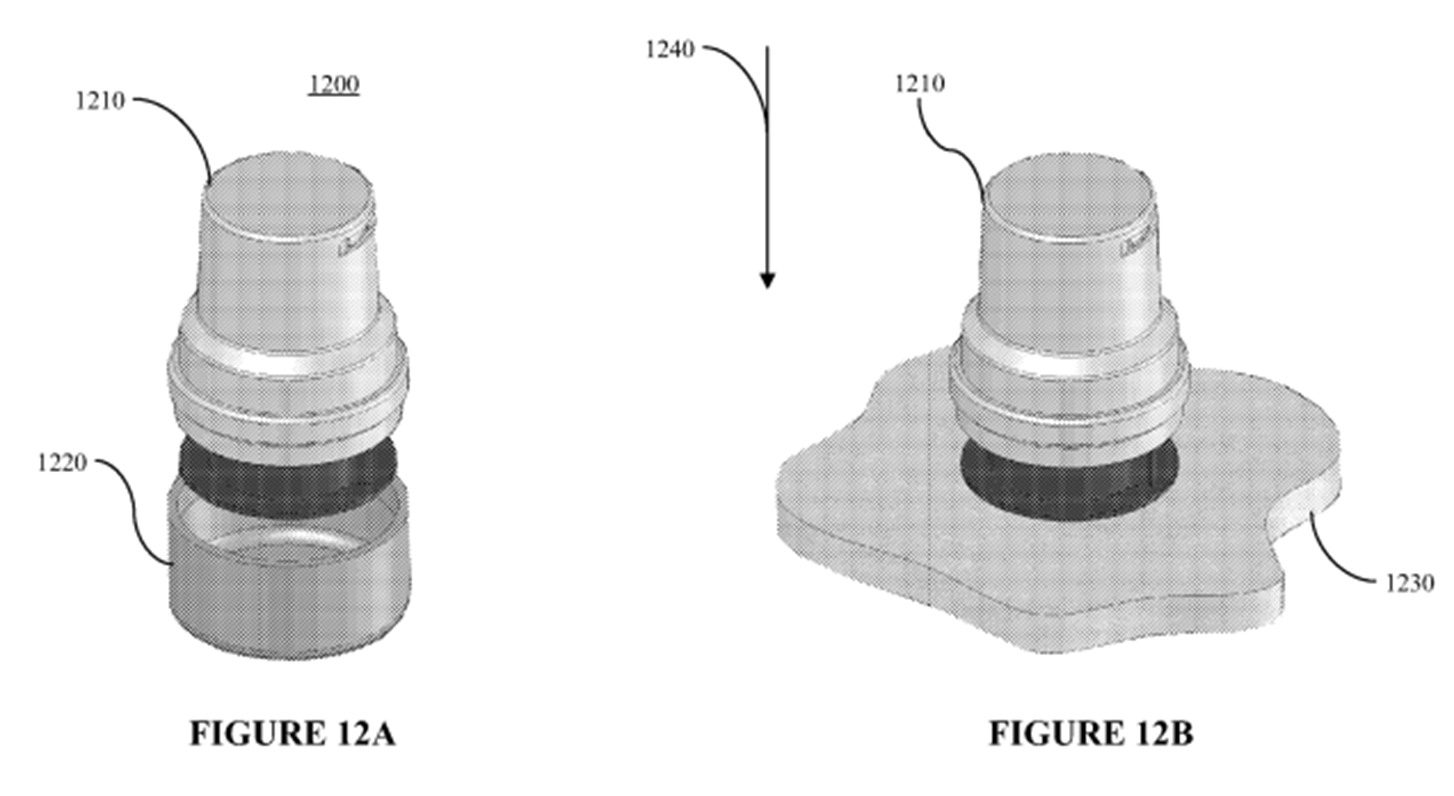
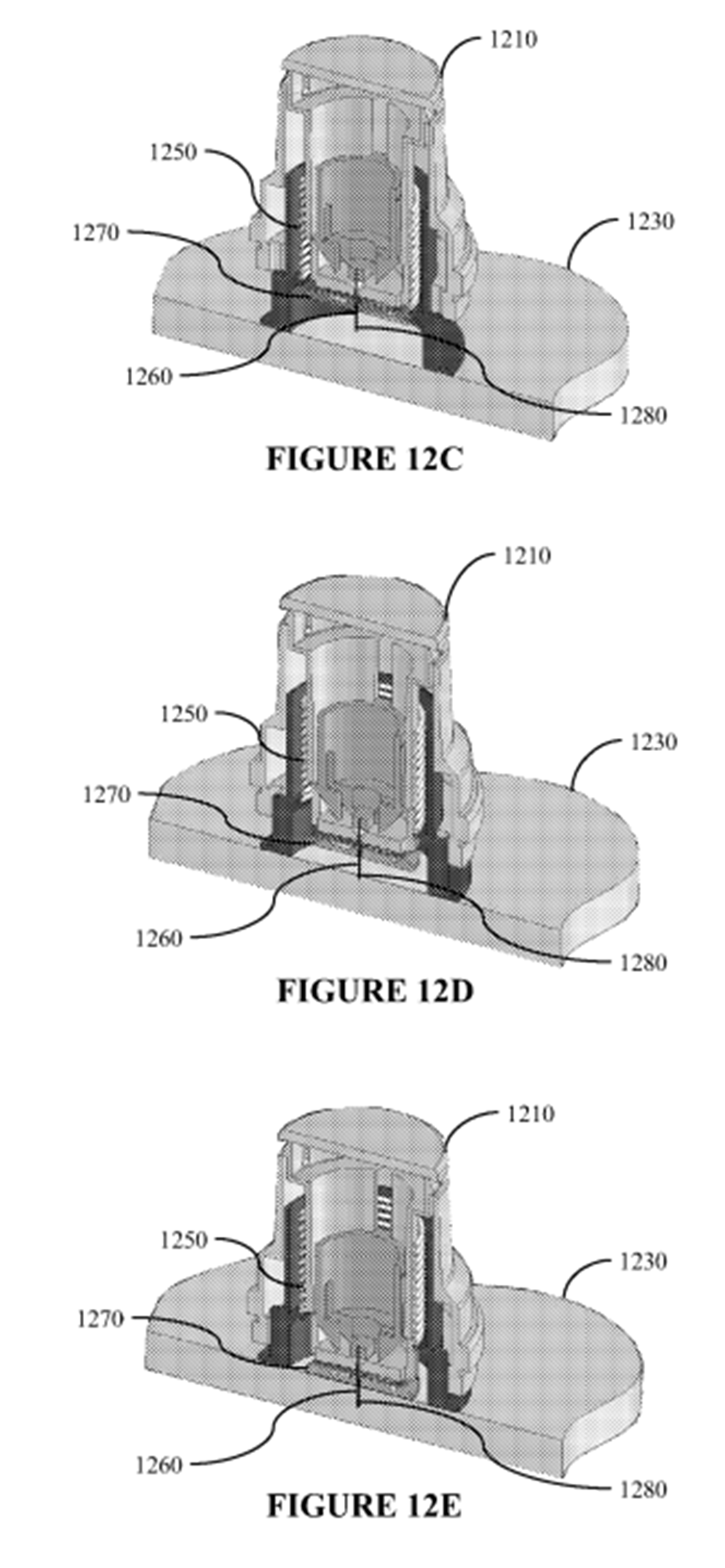
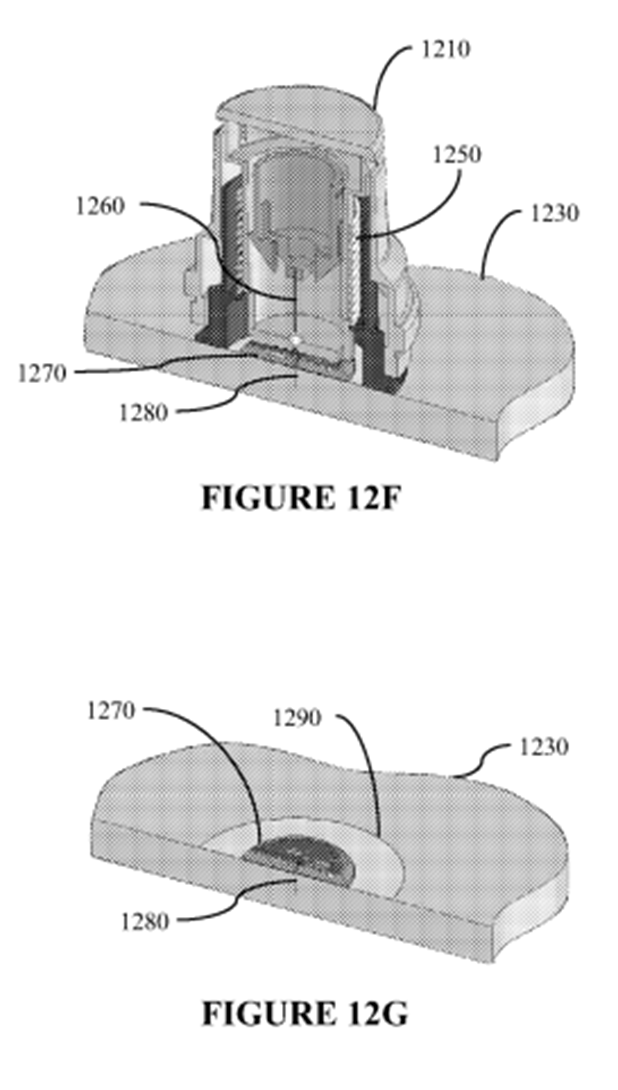
126. In these figures:
i) 1200 is the insertion device
ii) 1210 is the housing or body. It is not immediately clear whether the designation ‘the housing’ comprises just the light coloured part at the top or whether it also includes the dark coloured portion lower down. It may not matter because the figures show relative movement between the darker lower portion which is placed on the user’s skin and the light-coloured upper part which is labelled 1210.
iii) 1220 is the cap in Fig 12A which is removed before the insertion device is placed on the skin 1230 of the user in Fig 12B.
iv) The arrow 1240 in Fig 12B shows the direction of ‘a force, e.g. a manual force’ is applied to the top end of the housing which brings the integrated sensor and sensor electronics assembly into contact with the skin surface 1230.
‘[0117] …..Furthermore, the force applied as discussed above also may be configured to move the introducer (not shown) within the housing in the same direction as shown by arrow 1240 to pierce the skin surface 1230 and position the sensor in fluid contact with an analyte of the user.’
v) Figs 12C-12F are schematic drawings of cross-sectional perspectives of the operation of the insertion device and Fig 12G shows the sensor and sensor electronics assembly in place on the user’s skin, in which:
vi) 1250 is a bias spring.
vii) 1260 is the introducer needle.
viii) 1270 is the sensor electronics assembly.
ix) 1280 is the sensor.
x) Not labelled is the cylindrical internal component with a conical shape at its base (which for convenience I will call the needle carriage) which drives the introducer needle 1260 into the skin and then retracts back into the housing in Fig 12F, carrying the introducer needle with it.
127. The way this embodiment works is explained as follows:
‘[0119] As shown in these figures, in response to the force applied on the insertion device housing 1210, the introducer 1260 is driven in a direction substantially perpendicular to the skin surface 1230, and along with the movement of the introducer 1260, the sensor 1280 and the sensor electronics assembly 1270 are moved in the same direction. When the bottom surface of the sensor electronics assembly 1270 comes into contact with the skin surface 1230, the bottom surface is maintained in an adhered relationship with the skin surface 1230 by, for example, the adhesive layer 1290 (FIG. 12G). Moreover, also shown in the Figures is a bias spring 1250 which, in one embodiment, is configured to retract the introducer needle from the insertion position to a retracted position which is an opposite direction from the direction indicated by arrow 1240 (FIG. 12B).
[0120] Referring back to the Figure, it can be seen that the introducer needle 1260 is substantially and entirely retained within the insertion device housing 1210 after sensor insertion, and thereafter, when the insertion device 1200 is removed from the skin surface 1230, the sensor electronics assembly 1270 is retained on the skin surface 1230, while the position of the sensor 1280 is maintained in fluid contact with the analyte of the user under the skin layer 1230.’
128. To the unskilled eye, the arrangement of the introducer needle 1260 and the sensor 1280 may be a little confusing. The Skilled Addressee would know the typical arrangement of introducer needle and sensor.
129. These figures are not particularly clear. As Dr Shoemaker agreed in cross-examination, in these figures the bias spring 1250 is not shown as moving i.e. either being compressed or expanding, even though it can be seen that the upper part of the housing moves down over most of the darker lower part as the introducer needle 1260 and the sensor 1280 moves down and then into the skin. Furthermore, even when the introducer needle has been retracted into the housing and out of the skin, leaving the sensor 1280 in place in the subcutaneous tissue in the skin, in Fig 12F the upper lighter part of the housing is still shown in the same lowest position as in Fig 12E. This implies (to the mechanical engineer) that the relative movement between the upper light-coloured housing and the dark lower part compresses the spring which then must disconnect from the upper light-coloured housing in order to drive the retraction of the needle carriage back into the upper light-coloured housing. In this regard, the Skilled Addressee of the Patent would understand these figures as schematic and not purporting to show the precise retraction mechanism which is (as [0119] explains) powered by the bias spring.
130. As Mr Varde pointed out, Figures 12C to 12G make clear that the act of the user applying a force during the insertion process causes a spring within the insertion device housing to compress. This is the bias spring 1250. This process is described more explicitly in [0151] which I set out below.
131. As I mentioned Figs 13-18 illustrate various ‘activation switch mechanisms’. They are introduced in [0121]:
‘[0121] Prior to activation of the integrated sensor and sensor electronics assembly for use, there may be a period of time from the manufacturing that the assembly may be in sleep or idle mode. With a power supply such as a battery integrated within the assembly, for reasons including cost optimization and prolonging shelf life, embodiments of the present disclosure include systems that are activated merely by positioning the sensor and electronics unit on a skin surface as described above, i.e., no additional action need be required of the user other than applying a force to housing 1210. As such, insertion of the sensor causes activation of the electronics unit. In certain embodiments, activation switch configurations are included which may be configured to be triggered, for example, by the insertion device activation, thereby turning on the integrated sensor and sensor electronics assembly into an active mode.’
133. Then [0135] states:
“In accordance with various embodiments of the present disclosure, sensor electronics activation switch configurations are provided that may be triggered or activated automatically or semi automatically in response to the activation of the insertion device described above, or alternatively may be separately activated by the user by, for example, depressing upon a portion of the housing or switch provided on the housing of the sensor electronics assembly while improving post manufacturing shelf life of the device prior to use or activation.”
134. [0146] repeats the point made earlier about the insertion device: (emphasis added):
‘[0146] In accordance with embodiments of the present disclosure, the integrated sensor and sensor electronics assembly may be positioned on the skin surface of the user using an insertion device. For example, automated or semi-automated, spring biased and/or manual insertion device may be provided to deploy the sensor and the sensor electronics such that the implantable portion of the sensor is positioned in fluid contact with the analyte of the user such as the interstitial fluid, while the housing of the sensor electronics is securely positioned and adhered to the skin surface. In embodiments of the present disclosure, the sensor electronics device (for example, a transmitter unit of an analyte monitoring system) may be switched to an operational state or condition (from an inactive, shelf mode) upon deployment of the integrated assembly by the insertion device.’
135. [0148] contains the sole reference in the specification to sterilisation. The brevity of this confirms that the Patent assumes that the Skilled Team’s CGK includes how to tackle any issues regarding sterilisation:
[0148] In one aspect, the integrated sensor and sensor electronics assembly and the insertion device may be sterilized and packaged as one single device and provided to the user. Furthermore, during manufacturing, the insertion device assembly may be terminal packaged providing cost savings and avoiding the use of, for example, costly thermoformed tray or foil seal. In addition, the inserter device may include an end cap that is rotatably coupled to the insertion device body, and which provides a safe and sterile environment (and avoid the use of desiccants for the sensor) for the sensor provided within the insertion device along with the integrated assembly. Also, the insertion device sealed with the end cap may be configured to retain the sensor within the housing from significant movement during shipping such that the sensor position relative to the integrated assembly and the insertion device is maintained from manufacturing, assembly and shipping, until the device is ready for use by the user.
136. [0151] provides more detail of the insertion device illustrated (schematically in Fig12) and [0152] explains its advantages. Dexcom contended this was the only enabling disclosure of an insertion/retraction mechanism and drew attention to the last sentence of [0151] (and the underlined words in particular) as relevant to an issue of construction I have to consider later:
‘[0151] In a further embodiment, the insertion device may be configured for manual deployment with spring biased or automatic retraction of the introducer. That is, sensor insertion, the user may apply a predetermined amount of pressure upon the housing of the insertion device to insert the introducer and the sensor, the applied pressure sufficient to pierce through the skin layer of the user, and the device housing configured such that the applied pressure or the distance traveled by the introducer is predetermined (for example, by the use of a stopper or a protrusion within the inner wall of the insertion device that effectively stops of blocks further downward movement of the introducer towards the skin piercing direction after the introducer has reached a predetermined distance. In one aspect, the applied pressure may be configured to also press down upon a spring or a bias mechanism provided within the housing of the insertion device such that, when the applied pressure is released, the introducer is automatically retracted to its original predeployment position within the housing of the insertion device, by the return force from the spring or bias mechanism.
[0152] In this manner, consistent and repeatable insertion depth for the placement of the analyte sensor may be achieved. Furthermore, the insertion device housing (for example, a plastic or a combination of plastic and metal housing) may not be under the stress of spring tension since the bias spring provided for retraction of the introducer is, in the predeployment state, unbiased and in a relaxed state.’
137. In the usual way, additional paragraphs describe various further details. As Dexcom pointed out [0190] discloses a specific embodiment which appears to form the basis for claim 1. In addition, I mention in particular:
‘[0196] The movement of the introducer from the first position to the second position may be in response to a manual force applied on the housing. (emphasis added).’
138. Before I proceed further, I should mention particular features of the Patent which were strongly relied upon by Abbott. The following five points are contentions made by Abbott.
139. First, that the claimed invention of the Patent is, in broad outline, to an “integrated analyte monitoring assembly”, in which (1) the sensor and sensor electronics are brought together into one “sensor electronics assembly” which includes a power supply and activation switch, (2) the sensor electronics assembly is retained entirely within a housing of an insertion device prior to the insertion of the sensor, and (3) the assembly is configured to retract the needle automatically after insertion of the sensor.
140. Second, that the Patent provides, for the first time, a fully developed system in which all the elements required for continuous glucose monitoring are, before the insertion of the sensor, built into an “all in one assembly”. That all in one assembly can then be applied to the user’s skin, the sensor then be inserted, the needle automatically retract safely inside the housing of the insertion device, and - when the insertion device is removed - the sensor and sensor electronics are already securely in place without the user having to do anything more. Such an assembly is materially different from, and provides a clear benefit over, the standard architecture that had been adopted in the existing CGM products at the Priority Date, all of which required the user to insert the sensor into their skin using an insertion device, remove the insertion device, and then manually attach the sensor electronics to the sensor.
141. Third, that the invention provides important advantages over the prior art CGM systems. It provides a system that is much easier to use, as the user does not have the issue of having to try to align and then connect the sensor electronics to the sensor when it is already in their skin. This avoids the risk of misuse or misalignment of the sensor and the electronics, that in turn can cause errors or difficulties in the use of the CGM. These benefits are identified explicitly by the Patent at [0089] and [0110], but are also manifest when one compares the products which implement the invention, including the Dexcom G7, with the prior art CGM systems.
142. Fourth, that the integrated system claimed in the Patent also has the benefit of allowing battery saving (which in turn prolongs shelf life and reduces costs) because it includes an activation switch which ensures that, although the sensor and the electronics are already contained in the all in one assembly before insertion of the sensor, the sensor electronics are not activated until the switch is triggered, which would only be done at or after sensor insertion (as reflected in the Patent at [0121]). Further, the system ensures that the retraction of the needle is automatic and that the needle ends up safely within the housing of the insertion device before the insertion device is removed, which increases the system’s ease of use and safety.
143. Fifth and finally, Abbott submitted that it is important to keep in mind (given Dexcom’s approach to the prior art) that the invention is not simply a high-level idea of integration or its potential benefits. The Patent discloses not only the concept of integration, but also how to implement it, and provides a fully thought through system that achieves all the aforesaid benefits of integration and ease of use in a practical, power efficient and safe way.
144. I keep these points in mind when I come to the issues of obviousness, but they seem to me to be aimed at a case of obviousness over CGK.
The Claims in issue
145. At the start of trial, the claims in issue were claims 1, 2-4, 5, 7 and 13. By the time of closings, this list had been whittled down somewhat to claims 1, 2, 3, 5 and 7. The parties agreed the following breakdown of the integers of those claims. The construction issues which remain live concern the expressions in italics in claim 1 which I will deal with in turn.
|
An integrated analyte monitoring assembly, comprising: | |
|
1.1 |
a sensor electronics assembly including: |
|
1.2 |
an analyte sensor; and |
|
1.3 |
sensor electronics including a power supply and comprising: |
|
1.4 |
an activation switch operatively coupled to the power supply and the analyte sensor; and |
|
1.5 |
a controller unit having one or more programming instructions stored therein for execution, |
|
1.6 |
the controller unit in electrical contact with the analyte sensor and the activation switch and configured to process one or more signals received from the analyte sensor when the activation switch is triggered; and |
|
1.7 |
an insertion device including: |
|
1.8 |
a housing; |
|
1.9 |
an introducer needle coupled to the housing configured to move between a first position and a second position; and |
|
1.10 |
a bias mechanism operatively coupled to the housing and configured to automatically retract the introducer needle from the second position to a retracted position entirely within the insertion device housing, |
|
1.11 |
wherein the sensor electronics assembly is configured for communication with a remote device |
|
1.12 |
and is retained entirely within the housing of the insertion device prior to the introducer needle movement from the first position to the second position. |
2 |
The integrated analyte monitoring assembly of claim 1, further comprising a cap configured to mate with an open end of the housing of the insertion device, to seal the sensor electronics assembly therein, optionally wherein the cap is configured to rotatably couple to the end of the housing |
|
3 |
The integrated analyte monitoring assembly of claim 2, wherein when the cap is coupled to the housing prior to deployment, the interior space of the housing is maintained in a substantially contaminant free and/or sterile environment |
|
4 |
The integrated analyte monitoring assembly of claim 2 or 3, wherein the insertion device and cap are configured to be coupled during manufacture and sterilized and packaged together |
|
5 |
The integrated analyte monitoring assembly of any preceding claim, wherein |
|
5.1 |
the sensor electronics assembly is engaged with the introducer needle such that the sensor electronics assembly is moved in the same direction as the introducer needle during its movement from the first position to the second position, and further, wherein |
|
5.2 |
the introducer needle is configured to disengage from the sensor electronics assembly during its movement from the second position to the retracted position. |
7 |
The integrated analyte monitoring assembly of any preceding claim, wherein the insertion device is configured to retain the introducer needle within the insertion device housing after the bias mechanism has automatically retracted the introducer needle from the second position to the retracted position entirely within the insertion device housing |
The issues of construction
‘Finally, and most importantly, over-meticulousness is not to be equated to carefulness. Care in working out what the patentee was aiming at when he chose the words he used is absolutely necessary.’
1.4 ‘an activation switch’ and 1.6 ‘when the activation switch is triggered’
147. Keeping the interpretation of ‘an activation switch’ in issue was designed by Dexcom to ‘keep the patentee honest’. Dexcom submitted that Abbott and Dr Schoemaker went for the widest construction of ‘activation switch’ and the widest construction of ‘triggered’ in integer 1.6. Dexcom agreed that this was the better construction. They did so because it meant that the activation mechanisms of Heller and Fennell were each ‘an activation switch’ within the meaning of claim 1. I agree with the wide construction.
1.9 ‘an introducer needle coupled to the housing configured to move between a first position and a second position;’
1.10 ‘a bias mechanism operatively coupled to the housing and configured to automatically retract the introducer needle from the second position to a retracted position entirely within the insertion device housing,’
148. The most contentious construction issue was concerned with the interpretation of ‘coupled to the housing’ in integer 1.9. It is related to the interpretation of ‘operatively coupled to the housing’ in integer 1.10 in the bias mechanism. In closing an additional issue arose as to the meaning and scope of ‘automatically retract the introducer needle’, which I address below.
149. Dexcom identified the main issue as relevant to infringement and this is one of those cases where an understanding of the alleged infringement brings the construction issue into sharp focus. Nonetheless I focus on the teaching in the Patent first.
Dexcom’s arguments
150. Throughout, Dexcom argued for a narrow interpretation of ‘coupled to the housing’. In closing, the argument was put in this way:
‘…properly understood, the coupling of the housing with the introducer needle has its normal meaning of the two components being physically yoked together so that they move together, at least during the movement between first and second position which is the purpose of this feature. It is hard to see what other purpose the word ‘coupled’ can have here, or why otherwise the patentee would have used the word at all.’
151. On this interpretation, Dexcom submitted the G7 does not infringe. They suggested the G7 works in a completely different way.
Abbott’s arguments
152. Abbott argued for an extremely broad construction of ‘coupled’ in integer 1.9:
‘… “coupled” simply requires that the needle is (either directly or indirectly) in contact with the housing so as to enable either manual or automatic insertion of the needle. There is no additional requirement that there be physical joining between the needle and the housing.’
153. In this regard, Abbott emphasised how Dexcom’s argument had changed in the course of the trial. At the start of trial, Dexcom’s argument was founded on some evidence given by Mr Varde in his first report regarding integer 1.9. He interpreted ‘coupled to the housing’ narrowly because he considered the claim was limited to manual insertion. This was reflected in Dexcom’s Opening Skeleton, where the argument was based on the particular embodiment shown in Fig.12 which involves manual insertion. Dexcom argued that in that arrangement the introducer needle and the housing are coupled together so that they ‘move in lockstep during insertion’.
154. In Abbott’s oral Opening, attention was drawn to [0146] and claim 6 which explicitly contemplate using a spring based (non-manual) insertion mechanism. Furthermore, so Abbott argued, [0117] and [0196] make it clear that manual insertion is an option rather than a requirement. Confronted with these passages in cross-examination, Mr Varde accepted that the Patent (i.e. the specification) described automatic insertion as well as manual.
155. Consequently, in their Closing, Dexcom put their argument slightly differently, even though the end result - a narrow construction - was the same.
156. So in Closing, Dexcom sought to contrast the ‘coupled to the housing’ in integer 1.9 with the ‘operatively coupled to the housing’ in integer 1.10. Once again, the argument focussed on what was shown in Fig.12:
‘59. …The drawings at Fig. 12 explain and illustrate the contrasting wording. The first movement is achieved by the coupling between housing and introducer needle so that they move together. This means that a force (whether manual or not) pushing the housing downwards also pushes the needle downwards. But the second movement is automated. The operative coupling of the spring to the housing (in which its potential energy is built up and stored by being pushed against the shoulder of the housing, in a manner not shown in the drawings) is what causes the automatic retraction of the introducer needle back into the housing.
60. The whole point of the contrasting wording is to explain the distinct movements and how they are achieved. By coupling the housing to the needle the patentee enables the downward movement of the needle to be achieved by the application of downward force to the housing (whether manual or automated - see [0151]) so that one will carry the other downwards towards the skin. The patentee conceives of this occurring by using a two-part housing, but it could equally be achieved by a one-part housing.
61. But the reverse movement needs to retract the needle within the housing so that it comes out of the skin and any necessary adjustment of the assembly on the skin surface can be achieved (see [0149]). This means that the movement has to occur relative to the housing. Hence the needle needs to be engaged with a bias mechanism which is only ‘operatively’ coupled to the housing so that it can push against the housing and thus move the needle back into the housing.’
157. As before, Abbott’s answer was that all of this was founded on the particular embodiment illustrated in Fig.12 which involves manual insertion. The manual insertion illustrated does require the housing and the introducer needle to be ‘physically yoked together’, but that is only the ‘purpose’ of this feature for that manual insertion arrangement. As I mentioned above, Abbott clearly demonstrated (and Mr Varde accepted) that the specification is not limited to manual insertion and makes specific mention of automated (and semi-automated) insertion mechanisms.
158. Therefore, Abbott argued, at the correct level of generality, the purpose of the coupling between the housing and the introducer needle is so that the introducer needle moves between the first position and the second position and this leads to their wide interpretation set out at paragraph 152 above.
159. Dexcom were critical of this, suggesting that it robbed ‘coupled’ of any meaning, merely that the needle and the housing had to be in contact in some way.
160. However, as Abbott pointed out, ‘coupled’ is one of the broadest terms one can use in a mechanical patent to characterise the relationship between two components.
161. Although there was a superficial initial attraction to Abbott’s arguments, I came to realise there was a problem. At the same time as arguing for a very wide construction of ‘coupled’, Abbott did not really address two related points:
i) First, the meaning to be given to ‘operatively coupled’ (although Dexcom discerned that Abbott were arguing the phrase meant simply ‘coupled so they work together’).
ii) Second, the juxtaposition of the use of ‘coupled’ in connection with the insertion step and ‘operatively coupled’ for the retraction step.
162. Integer 1.10 is explicit in requiring automatic retraction of the needle and this occurs by the configuration of the bias mechanism being ‘operatively coupled’ to the housing. Automatic retraction means there must be relative movement between the needle and the housing and this relative movement is caused by the bias mechanism. Although the bias mechanism is not explicitly limited to a spring, a spring is a convenient way to envisage the bias mechanism. For the retraction to be automatic, that implies the use of the potential energy in a compressed spring, which causes the needle to be retracted to the ‘retracted position entirely within the insertion device housing’.
163. Turning to the juxtaposition of these two terms, although it is clear that the insertion and retraction mechanisms (and the drivers for them) can be separate, the Skilled Team, exercising appropriate ‘care’ (cf Pumfrey J.) would, in my judgment be struck by the use of the term ‘coupled’ for insertion and ‘operatively coupled’ for the automatic retraction.
164. Automatic insertion of the needle would require relative movement between the needle and the housing and, in my judgment, the Skilled Team would expect the use of the term ‘operatively coupled’ to characterise the relationship between the needle and the housing in such an embodiment, with the relative movement being caused by some inserting bias mechanism. Yet the insertion step in integer 1.9 requires the needle to be coupled to the housing configured to move (i.e. so that it moves) between a first position and a second position. This suggests (a) no relative movement between needle and housing and (b) that it is the movement of the housing which causes insertion of the sensor and needle.
165. In passing, I note that these concepts are illustrated in Fig 12, and in that context it is clear that the housing is the upper light-coloured part (to which the force is applied) and not the darker lower part which is placed on the skin, but I remind myself that I must not assume the claim is restricted to the embodiment shown in Fig.12.
166. Furthermore, since the phrase ‘operatively coupled’ adds the qualifier to ‘coupled’, the Skilled Team would assume the former term to have a different meaning to ‘coupled’ on its own. In the context of claim 1, ‘operatively coupled’ means that the bias mechanism need only be ‘coupled’ to the housing in order to be able to operate i.e. to perform its function - to cause the retraction of the needle.
167. I have considered whether this analysis involves meticulous verbal analysis but in my judgment it does not. This is purposive construction - giving purpose to the expressions used in a carefully worded claim.
168. Meticulous verbal analysis (MVA) is the antithesis of purposive construction. The claimed lintel in Catnic called for ‘a second rigid support member extending vertically from or from near the rear edge of the first horizontal plate…’. The MVA in that case was the literal or precise interpretation of ‘vertically’ which affected the allegation of infringement regarding the defendant’s lintel in which the relevant support member was at 6 degrees to the vertical. As Lord Diplock explained the reduction in the load bearing capacity was proportional to the cosine of the angle of inclination from the vertical. For the 6 degree variant, the reduction was 0.6%, the import being that the support member could still do its job as part of the lintel and had no material effect on the way the invention worked, so strict compliance was not required.
169. Although I am contrasting ‘coupled’ with ‘operatively coupled’ that is a necessary step to give purpose to these expressions. These are words of the patentee’s own choosing. Furthermore, it is possible to identify a concrete reason for the use of each of (i) ‘operatively coupled’ for the automatic retraction step and (ii) ‘coupled’ for the insertion step.
170. The consequence is that although the specification describes automatic (and semi-automatic) insertion, those are not claimed. Accordingly, I agree with Dexcom that claim 1 is limited to manual insertion in which the force on and movement of the housing is the cause of the insertion of the needle.
171. After I had reached this conclusion, I was provided with a machine translation of the Judgment of Munich Regional Court 1 on whether the Dexcom G7 infringed the German designation of EP044. Although the judgment appears to contain relatively little discussion of the meaning of ‘operatively coupled’, the Court was clear that the insertion step does not involve independent movement of the needle relative to the housing. Whilst the movement of needle and housing need not be synchronous, the Court ruled that they must be related so some sort of connection between them is required. It is reassuring that another EPC Court has arrived at the same construction.
172. I should add that the German court identified three reasons why the G7 did not infringe EP(DE)044. The first two can easily be identified: the court held that (1) the G7 contained no activation switch; and (2) the automatic insertion mechanism of the G7 meant that the insertion needle was not coupled to the housing. The third reason the Court gave was that which I refer to as integer 1.10 was not fulfilled by the G7. On construction, the Court’s reasoning (at least in translation) is not easy to follow:
‘The term "coupling" is to be understood, as in the context of [integer 1.9], as meaning that the movement of the pretensioning mechanism must be related to the movement of the housing, although it is not necessary for the pretensioning mechanism to be triggered directly by the housing itself through its movement from the first to the second position. There is no indication in the patent claim for this restrictive understanding advocated by the defendant (statement of defense, p. 35). In particular, according to [integer 1.9], it is not the housing but the insertion needle that moves from a first to the second position’
173. However, when it came to consider whether the G7 fulfilled this integer, the Court’s reasoning sheds some light on what they meant on construction.
‘[Integer 1.10] is also not realized in the contested embodiment because the pretensioning mechanism is not coupled to the housing ready for operation. The triggering of the pre-tensioning mechanism is not related to the movement of the housing, but solely to the application process of the sensor unit. This process is based on the movement of the insertion needle, which in turn is not coupled to the housing, so that there is also no indirect coupling of the pretensioning mechanism with the housing.’
174. With respect, this appears to miss the point. Even if the needle is not coupled to the housing for the insertion step, that, it seems to me, does not prevent the bias mechanism (i.e. the pre-tensioning mechanism) being operatively coupled to the housing for the retraction step. After all, it is only required to be operatively coupled for the retraction step. Outside that step, it does not matter how, if at all, they are coupled.
The influence of claim 6 on construction
175. Finally, on this point, Dexcom referred to a point made by Abbott in their opening based on their reading of claim 6 and [0146]. Abbott’s suggestion, as Dexcom understood it, was that claim 6 should be read in such a way as to disclose the idea of a spring biased insertion force distinct from a force on the housing. The import being that if claim 6 covered both, then necessarily claim 1 must be wide enough to cover automatic insertion.
176. Claim 6 reads as follows:
6. The integrated analyte monitoring assembly of any preceding claim, wherein the insertion device (1200) is configured such that movement of the introducer needle (1260) from the first position to the second position is in response to at least one of a manual force applied on the housing (1210) and a spring biased force.
177. I quoted [0146] at paragraph 132 above, but it is worth repeating the key phrase here: the insertion device may be ‘automated or semi-automated, spring biased and/or manual’
178. Dexcom developed an argument that I should be very cautious with Abbott’s suggestion for a number of reasons:
i) First, Dexcom disputed that claim 6 does make that disclosure. This point was not developed further, but it is difficult to see that the spring biased force mentioned in the claim is doing anything other than driving the insertion movement. The claim is not concerned with retraction.
ii) Second, Dexcom’s main point was that, even if it did make that disclosure, it would add matter since there is no basis for this in the Application as filed.
iii) This was explained as follows. The basis for the ‘manual force applied to the housing’ is claim 43 in the Application as filed. Yet the reference to a ‘spring biased force’ comes from claim 44 of the Application, but that is said to relate to the retraction mechanism of claim 37, not to an insertion force.
iv) Dexcom say this is the reason why Abbott has never relied on claim 6 as being infringed.
179. In the Application as filed, claim 37 is similar to granted claim 1. For present purposes, it suffices to note that it includes the same wording as in integers 1.9 and 1.10, so the only bias mechanism relates to the retraction step. Both claims 43 and 44 are dependent on claim 37:
‘43. The device of claim 37 wherein the movement of the introducer from the first position to the second position is in response to a manual force applied on the housing.
44. The device of claim 37 wherein the bias mechanism includes a spring.’
180. It is true that claim 44 concerns the retraction step and therefore claim 44 cannot justify the feature of claim 6 where the movement of the needle from the first position to the second (i.e. in the insertion step) is in response to a spring biased force. [0167] in the Application as filed is the equivalent of [0146] in the Patent.
181. In these circumstances, it seems that claim 6 does add matter - the idea that the movement of the needle from the first position to the second position is in response to a spring biased force.
182. Returning to the possible influence of claim 6 on claim 1, these considerations do not deter me from the construction of ‘coupled’ and ‘operatively coupled’ as explained above. Abbott did not repeat the submission in closing.
‘configured to automatically retract’
183. This issue arose in the context of Ethelfeld. Abbott argued that the retraction must occur without further intervention by the user and in such a way that it cannot be prevented once the process has started. In support of this argument, Abbott relied on some evidence from Mr Varde in cross-examination about the Dexcom G7. Although the question put was whether the retraction motion was ‘automatic in the sense of the patent’ and secured an affirmative answer, it is clear that Mr Varde answered by reference to the mechanism in the G7 and was not focussed at all on the precise limit of the meaning of ‘automatically’. On this basis, Abbott argued there was no dispute over the construction of this phrase.
184. Abbott also referred to some evidence which Mr Varde had given in his CGK section regarding the meaning of automated and semi-automated. In essence, Mr Varde’s point was that the distinction between manual and automated insertion mechanisms lies in the degree of control the user has over the insertion process, and in the case of automatic insertion, the user has no control once the mechanism is initiated.
185. In closing, Dexcom expressed surprise that Abbott were contending that an Ethelfeld-type device, where the retraction step is initiated by the user releasing pressure, did not satisfy this integer. This was because the last sentence of [0151], which describes the preferred embodiment, explicitly describes the retraction step being initiated by the user releasing pressure on the housing.
186. With Ethelfeld in mind, the issue is a fine one and these are the two alternative meanings:
i) First, that retraction occurs automatically without any further user input as soon as insertion has been completed; or
ii) Once insertion has been completed, the retraction process is automatic, but it may be initiated by some action of the user (such as, for example, as releasing pressure).
187. The issue was not really explored in closings, but setting out the alternative meanings helps to identify the real issue. The first meaning involves automatic initiation so that, once initiated, the retraction is automatic. The second meaning does not have automatic initiation but, once initiated, the retraction is automatic in the sense the user has no control once the retraction is initiated.
188. Particularly in view of the final sentence of [0151], I have no doubt that ‘automatically’ in this element of the claim is concerned with the retraction process and not with how that is initiated. I also bear in mind that, assuming Ethelfeld was not prior art, and someone built that type of device, it is highly likely that Abbott would point to [0151], argue that there is no reason to limit the meaning of this phrase and would succeed in an allegation that the device infringed due to its retraction being automatic.
INFRINGEMENT - The Dexcom G7
189. There was no dispute as to the Dexcom G7 product or how it worked. Although large swathes of the PPD which described the G7 system were designated confidential, certainly at the level at which I summarise the G7 in this section of the Judgment, I am unable to understand how the information can attract confidence, bearing in mind the G7 is on the market and anyone can take the product apart to examine the individual components and work out how they operate. I should add there are a few paragraphs in the PPD which contain information which could not be gleaned from inspection of the G7 product, but it is not necessary to refer to any of that information.
190. If I had resolved all the issues of construction in favour of Abbott, Dexcom did not dispute that the G7 infringed. However, my decision on integer 1.9 means that the G7 does not infringe.
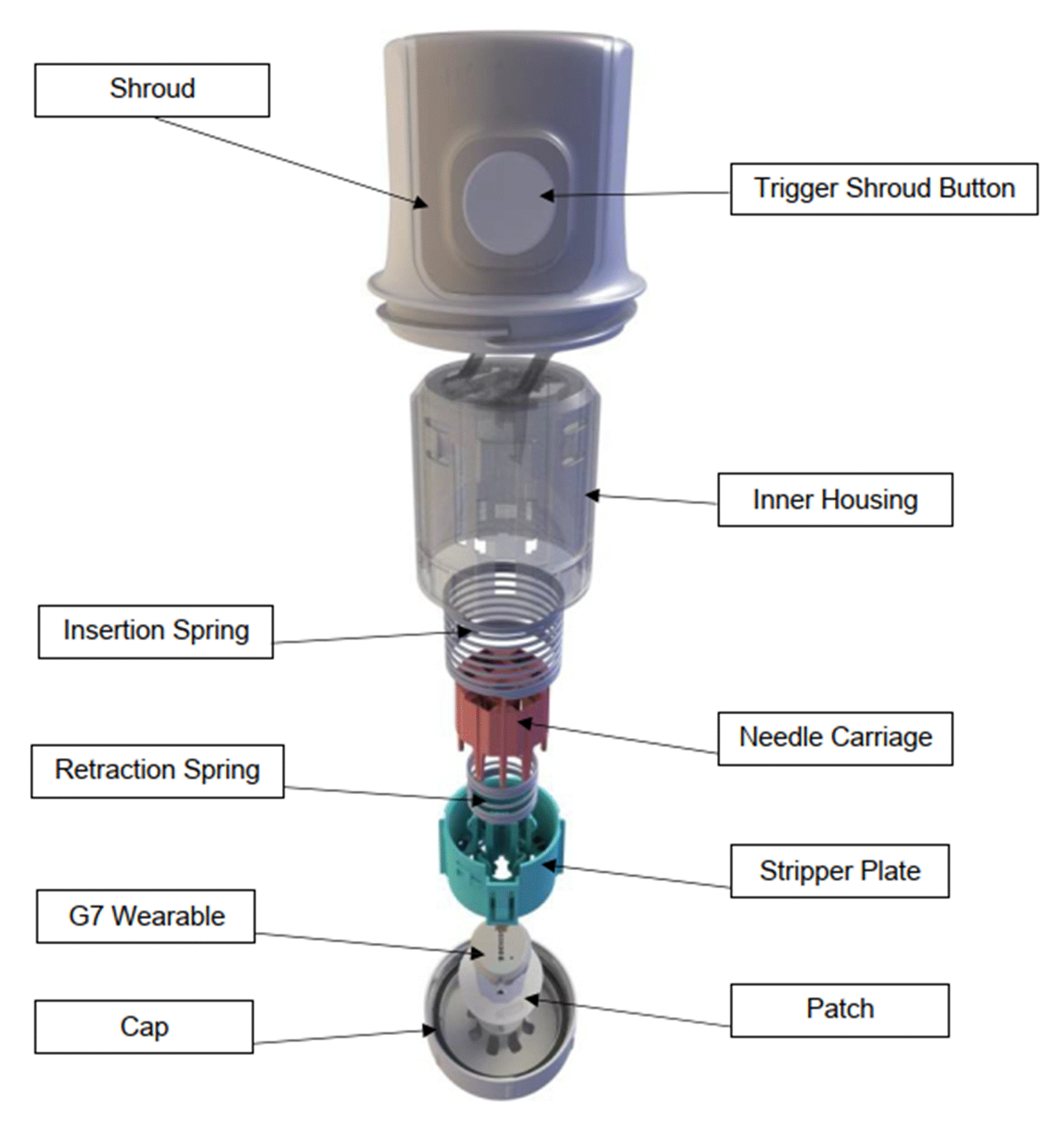
192. To deploy the G7 wearable onto the user’s skin, the user must first remove the cap and then apply the exposed end of the applicator to their skin. At this point the Stripper Plate is locked in place. To activate the insertion mechanism, the user presses on the Trigger Shroud Button. This presses the Trigger Shroud Button Pin against the Trigger Snap on the Stripper Plate.
193. The operation of the Trigger Shroud Button can be discerned from this sectioned image:
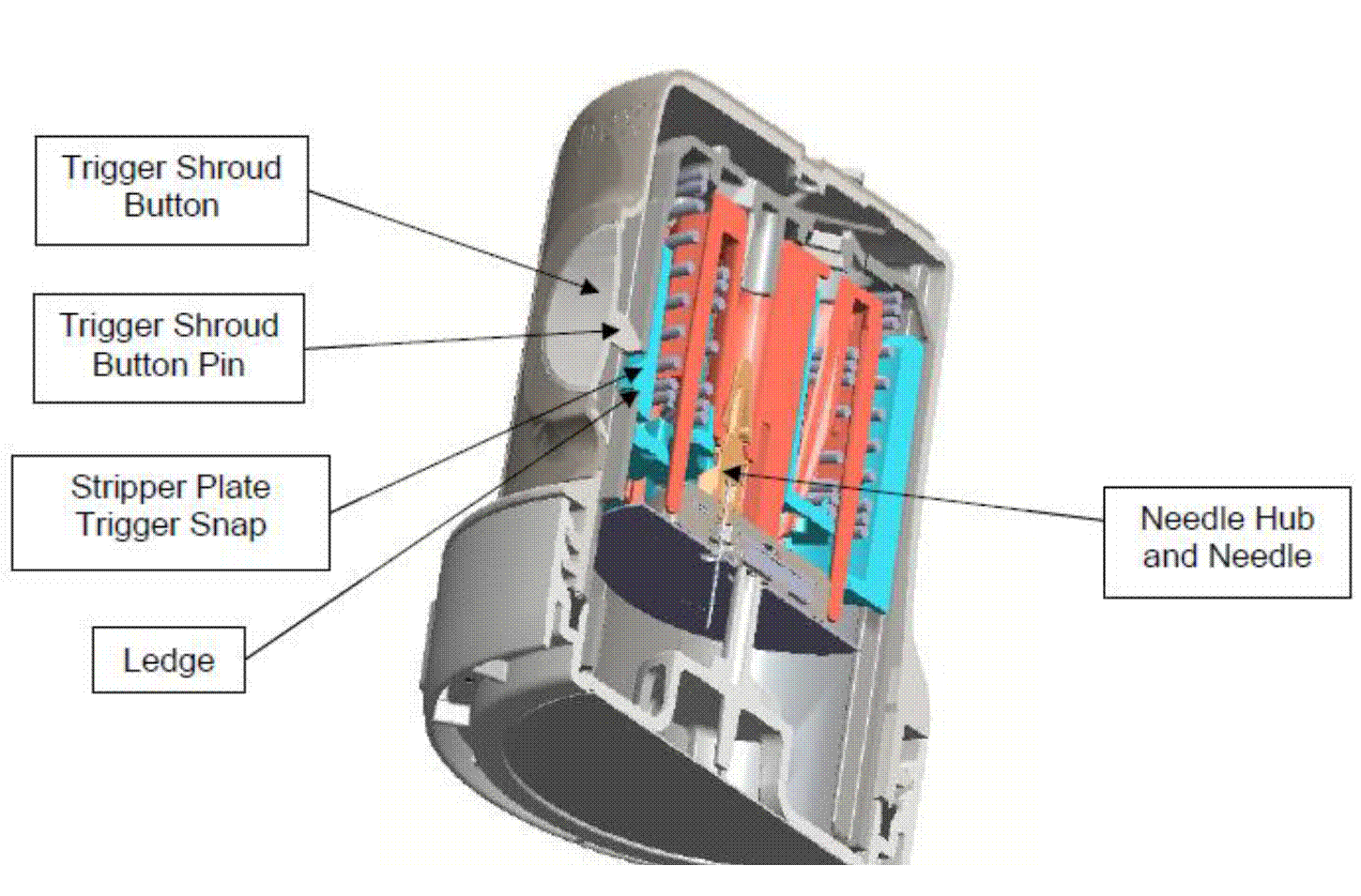
194. A further cross-section shows the assembly and the arrangement of the Insertion and Retraction Springs:
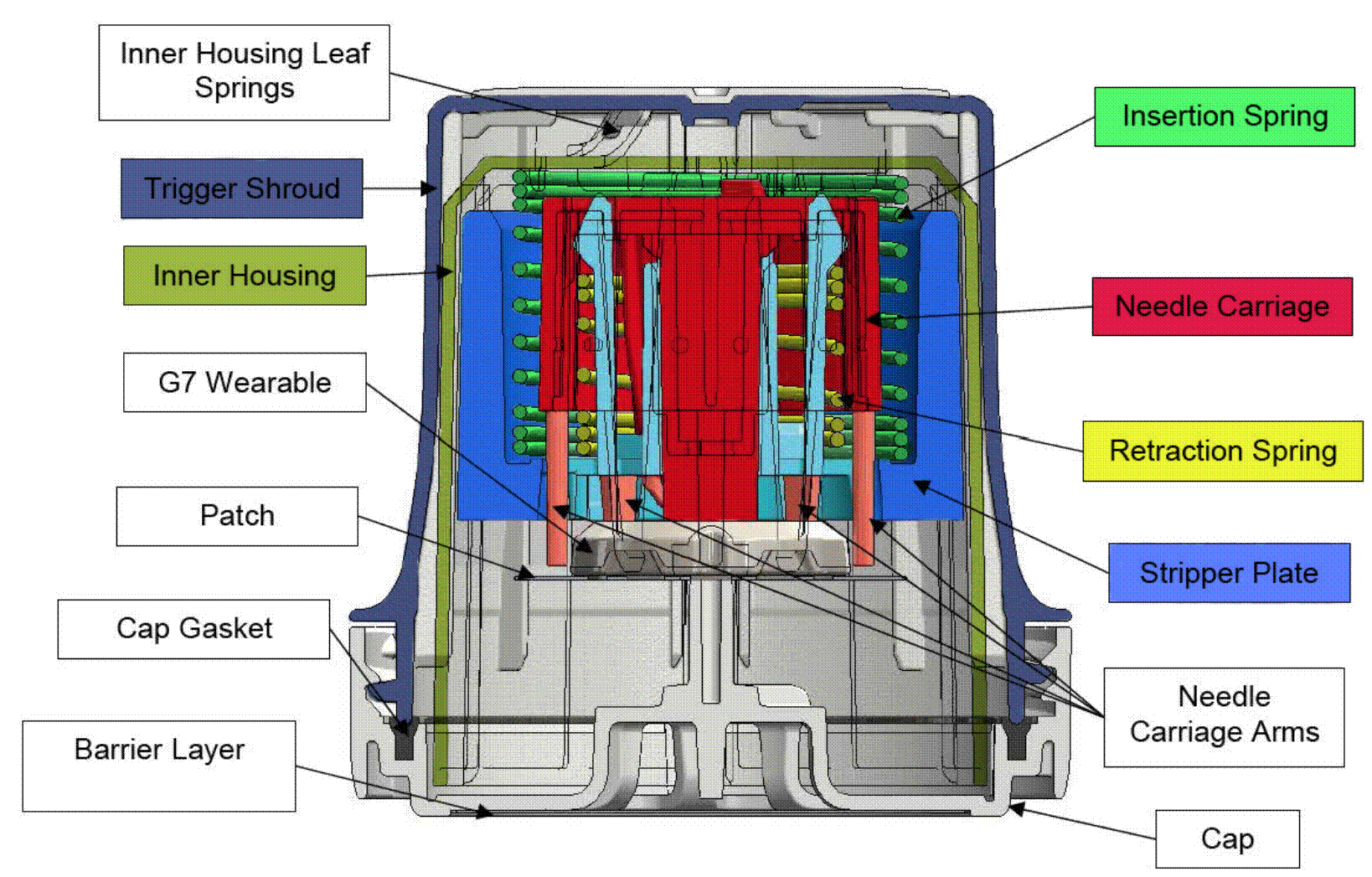
195. The pressing of the Trigger Shroud Button Pin against the Trigger Snap on the Stripper Plate releases the Stripper Plate whereupon the compressed Insertion Spring is released, which drives the Stripper Plate towards the user’s skin. As a result, the combination of the Stripper Plate, the Needle carriage, Needle and G7 Wearable all move towards the user’s skin and the Needle is inserted into the skin.
196. When the G7 Wearable hits the skin, a reaction force pushes back on the G7 Wearable which prevents the Needle Carriage from travelling further towards the user’s skin but the Insertion Spring continues to drive the Stripper Plate towards the skin. This further movement causes the Needle Carriage and the Stripper Plate to disengage. As they disengage, the force from the Needle Carriage on the Stripper Plate Retraction Arms is removed. Thus the compressed Retraction Spring extends, pushing the Stripper Plate Retraction Arms inwards. The Needle Carriage, Needle and Needle Hub are moved away from the User’s Skin by the extension of the Retraction Spring, whilst the Stripper Plate holds the G7 Wearable against the User’s skin so the G7 Wearable remains on the skin. The user lifts the G7 Applicator away from their skin, leaving the G7 Wearable in place. The cap can then be replaced to create a closed container for the used Needle.
198. Abbott’s argument as to why the G7 fulfilled integer 1.9 shows just how broad their construction of ‘coupled’ was. The argument went as follows:
i) The insertion spring presses against the inner housing at the top.
ii) The spring pushes the stripper plate down when the trigger is released.
iii) The stripper plate is frictionally engaged with the needle carriage and is pressed against it.
iv) The needle carriage is attached to the needle.
v) And the argument concluded thus:
‘There is therefore indirect physical contact between the needle and the housing, and each of the elements between the needle and the housing are engaged with each other (in the sense of either being attached to each other or pushing against each other).’
199. Bearing in mind that the starting point (see integer 1.12) is that the sensor electronics assembly (which itself is part of the integrated assembly on which the insertion device operates) is entirely within the housing of the insertion device, Abbott’s argument was effectively that anything which puts the assembly into the second position (i.e. on the user’s skin) means the needle is coupled to the housing. Taken out of context, this could have been a possible interpretation of ‘coupled’. However, in the context of the claim as a whole and integer 1.10 in particular, such a wide construction is not possible.
THE PRIOR ART - DISCLOSURE
200. It was evident that Dexcom considered Heller to represent their best case on obviousness, followed by Ethelfeld and Fennell. I consider they were correct.
201. However, I must bear in mind that in his written evidence, Mr Varde said that EP044 was obvious over each of Heller, Ethelfeld and Fennell. In accordance with his duties as an expert witness, he did change certain views when challenged in cross-examination and on certain other points, his reasoning became more nuanced. However, particularly in view of Abbott’s allegation that his views (and Dexcom’s case) were driven and infected by hindsight, it is prudent to consider Mr Varde’s evidence on Heller for example, with an assessment of whether or to what extent his evidence on the other prior art was infected by hindsight.
202. Of course, the allegation of hindsight can infect not just what is said to be obvious over the prior art, but the view taken of what the prior art actually discloses. Abbott made that accusation in this case. Since there were some substantial disputes over what was disclosed to the Skilled Team by each piece of prior art, I will start by discussing what each piece of prior art disclosed to the Skilled Team at the priority date.
Heller - disclosure
203. Heller is US Patent Application No. US 2003/0100821 published on 29 May 2003 and entitled ‘Analyte monitoring device and methods of use’. The document is long, with considerable detail in some areas and a lot of it is not relevant to the issues in this case.
204. It was common ground that Heller describes two configurations of analyte monitoring system, which were referred to at trial as ‘the Main Configuration’ and ‘the Fig.32 Configuration’. Dexcom’s case involves the Skilled Team deciding to take forward something of a combination of the two whereas Abbott submitted that these were ‘two mutually incompatible configurations’. Therefore it is important to be precise about what would be disclosed to relevant members of the Skilled Team by Heller when the document was read with the CGK in mind.
205. Although the invention is stated generally as being directed to devices and methods for the in vivo monitoring of an analyte, the monitoring of glucose is the main focus, using a subcutaneously implantable sensor.
206. The summary of the invention, at [0007]-[0009], expanded upon in [0075], describes that such devices include an analyte sensor and sensor electronics with a power supply (termed a “sensor control unit”). The sensor is formed on a substrate (see [0077]). The sensor control unit transmits sensor data to a receiver display unit. The analyte sensor and the sensor control unit are coupled, which may be via contact pads ([0007]) or in an “assembly”:
‘[0008] Another embodiment of the invention is a sensor assembly that includes the sensor control unit described above. The sensor assembly also includes a sensor having at least one working electrode and at least one contact pad coupled to the working electrode or electrodes. The sensor may also include optional components, such as, for example, a counter electrode, a counter/reference electrode, a reference electrode, and a temperature probe. Other components and options for the Sensor are described below.’
207. [0010] discloses that the sensor is inserted using a needle (termed an “inserter” in [0010], and an “insertion device” elsewhere in Heller) and an “insertion gun” which has a driving mechanism for inserting the needle and a retraction mechanism for removing it.
208. The Detailed Description starts at [0060] with a series of definitions. There is a lot of detail about various forms of sensor from [0077]-[0197] by reference to Figs 2-10. By way of example Fig.2 shows the sensor 42. Fig 2 and other important figures were gathered by Dexcom on this page of their closing with the various parts identified.
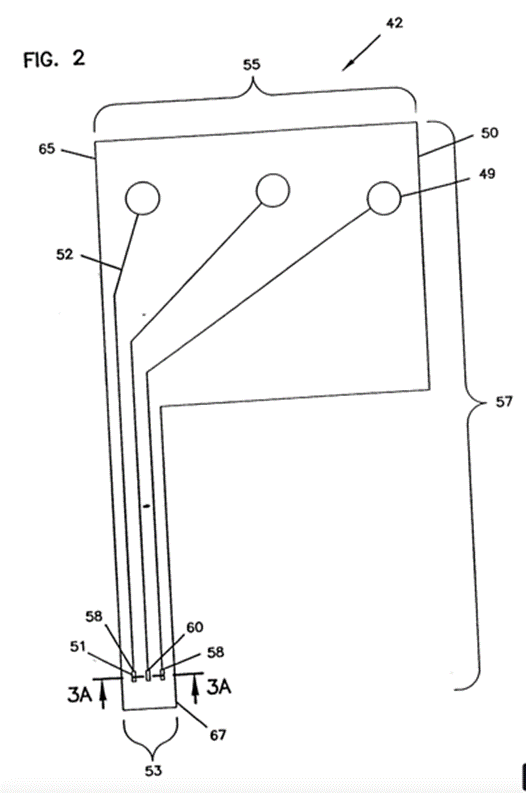
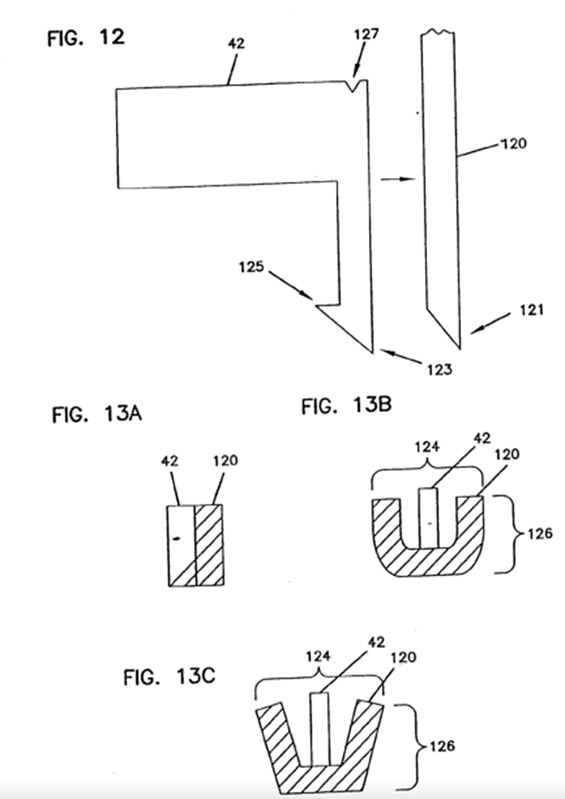
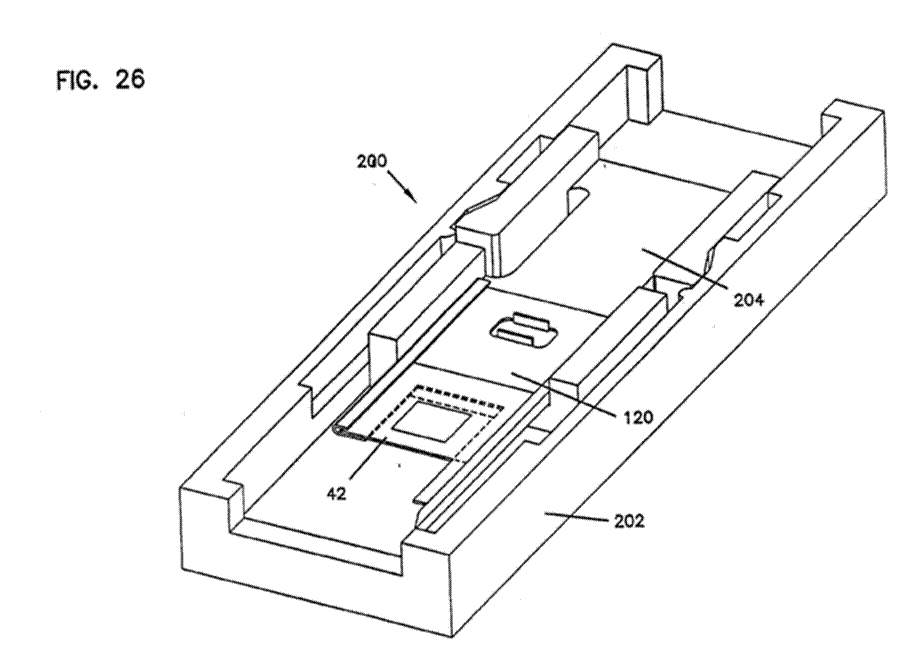
42/272: Sensor
45: Housing
50: Substrate
52: Conductive traces
58,60: Sensor electrodes
76: Cover
77/276: Mounting unit
120/270: Insertion needle
200/274: Insertion gun
204/278: Carrier
![]()
![]()
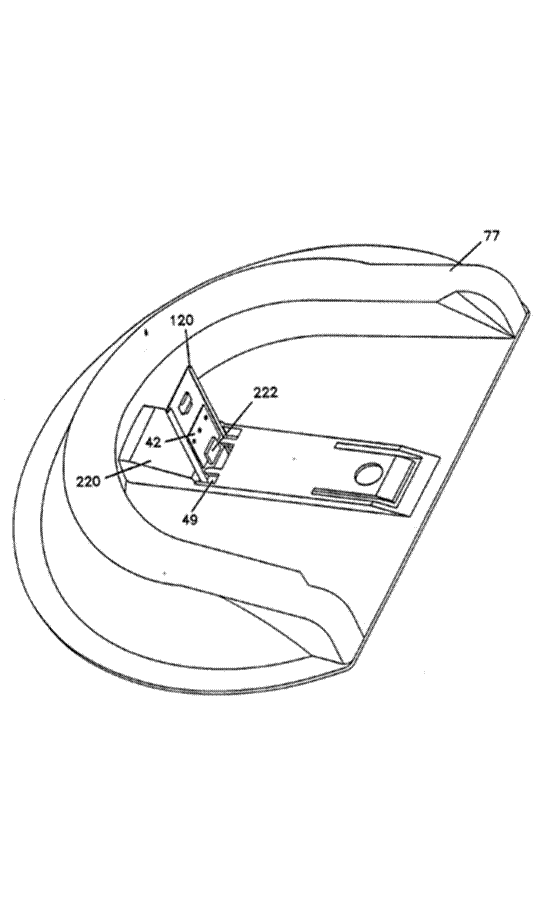
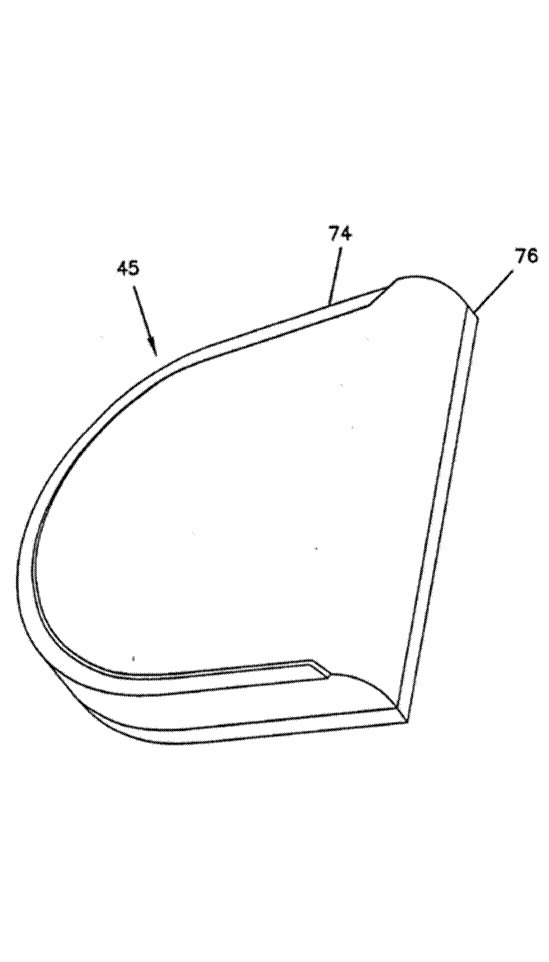
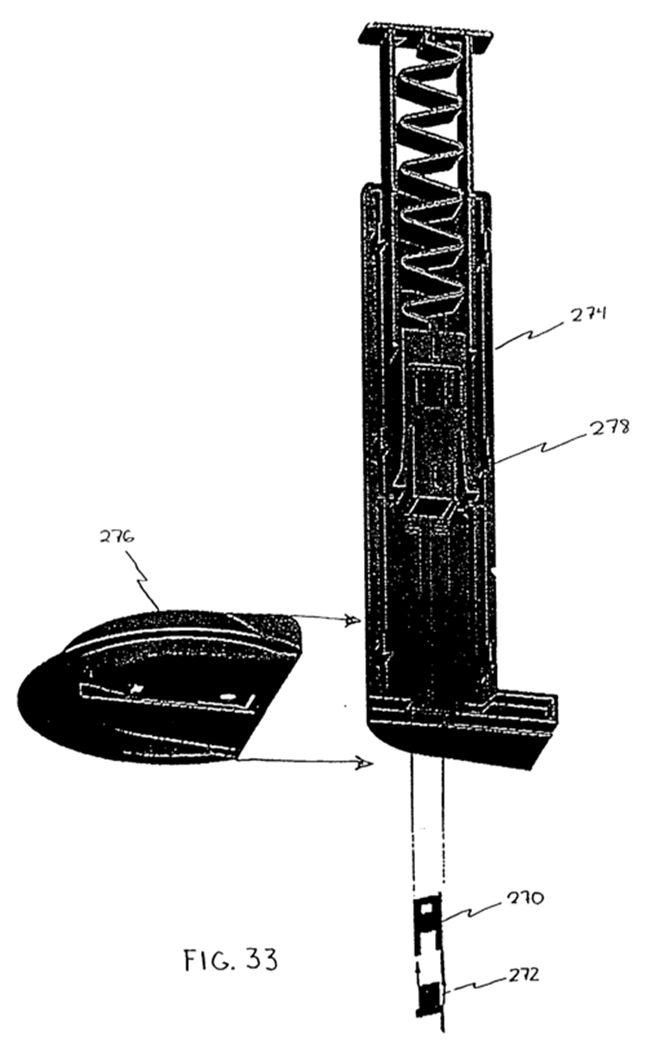
209. The Insertion Device is described at [0201]-[0211] by reference to Figs 12 and 13A-C, which illustrate various forms of insertion device (i.e. a shaped needle, with a U-shaped cross-section) 120 in conjunction with the implantable sensor and an ‘insertion gun’, one example of which is shown in Fig 26, labelled 200 (see [0206]). A carrier 204 on the insertion gun drives the sensor and, optionally, the insertion device 120 into the skin of the patient using a force of various means which may be manual or mechanical, including a spring, or a burst of compressed gas. [0207] discloses that ‘the insertion gun 200 may contain a mechanism which pulls the insertion device 120 out of the skin of the patient. Such a mechanism may use a spring…. to remove the insertion device 120’.
210. The next section concerns the On-Skin Sensor Control Unit [0212]-[0256] and involves discussion of a number of figures: Figs 14-16, 27A, 28A, 32, 17, 27B, 28B, 28C, 28D, 28E, 12, 26, 19A-D, 18A, 18B, 19E, 19F and 33.
211. There are repeated references back to the sensor 42 throughout the specification, even without explicit reference to Fig 2. One example is in Fig 14 which shows a cross-sectional view of the on-skin sensor control unit shown in Figs 15 (top view) and 16 (view from underneath) and Dexcom presented an annotated version of Fig 14:
|
|
In which: 42: sensor 44: on-skin sensor control unit 45: housing 74: base 76: cover 80: conductive contacts 82: support
|
212. As Mr Varde pointed out, in the Fig 14 arrangement (a) the sensor 42 is bent through 90 degrees which has the effect that the substrate lies parallel to the skin, which allows for the on-skin sensor control unit 44 to have a neat form factor, and (b) the housing as shown has two parts - the base 74 and the cover 76, which may be coupled together [0221] and taken apart and fitted together occasionally, when a battery or sensor is replaced.
213. However, the specification discloses various embodiments/arrangements of the on-skin sensor control unit in which a number of options are considered. The housing may be a single piece [0219] or two piece (as above). It can have a port 78 through which the sensor 42 can be directed to access the conductive contacts 80, but it would appear from [0225] that even the conductive contacts are optional (note the use of the word ‘Often’ in the quote below).
214. [0225] expressly discloses the use of and advantages associated with an activation switch to wake the device from a ‘sleep mode’ in an embodiment where the on-skin sensor control unit is a disposable unit complete with battery:
[0225] The on-skin sensor control unit 44 optionally remains in a sleep mode prior to use to conserve the battery’s power. The on-skin sensor control unit 44 detects that it is being used and activates itself. Detection of use may be through a number of mechanisms. These include, for example, detection of a change in resistance across the electrical contacts, actuation of a switch upon mating the on-skin sensor control unit with a mounting unit 77 (see Figs.27A and 28A). The on-skin sensor control unit 44 is typically replaced when it no longer operates within threshold limits, for example if the battery or other power source does not generate sufficient power. Often this embodiment of the on-skin sensor control unit 44 has conductive contacts 80 on the exterior of the housing 45. Once the sensor 42 is implanted in the patient, the sensor control unit 44 is placed over the sensor 42 with the conductive contacts 80 in contact with the contact pads 49 of the sensor 42.
215. Thus, one of the arrangements contemplated in [0225] is what may be termed the traditional two-part wearable where the control unit is mated with a mounting unit 77 which has already inserted the sensor into the skin.
216. In [0229]-[0230], an alternate embodiment is described where the transmitter 98 is disposed on the sensor substrate 50. It is said that:
‘This arrangement provides the advantage of relieving the user of the analyte monitoring device from having to electrically connect the transmitter 98 to the sensor 42. This is advantageous because the mechanics involved in forming the aforementioned electrical connection may be difficult for a user to accomplish.’
217. [0230] describes Fig 32:
‘[0230] FIG. 32 depicts one possible embodiment of a transmitter 263 disposed upon a substrate 260. As can be seen from FIG. 32, substrate 260 has a conductive trace 268 disposed upon it, a portion of which is chemically enabled to form an electrochemical sensor. The substrate 260 may be flexible, thereby enhancing patient comfort. Such flexibility also reduces the risk of the substrate 260 shattering upon impact, potentially embedding a shard of the substrate within the user. Thus, flexibility enhances user safety. The transmitter 263 is comprised of an integrated circuit 264 designed to generate a transmission signal representative of the analyte level of the bodily fluid. …’
218. In view of a submission made by Abbott, I should point out that the Skilled Team reading this paragraph would understand that the suggested flexibility related primarily to the portion of the substrate which would pass through the skin (i.e. the descending arm in Fig 32), and not necessarily to the portion carrying the electronics.
219. An annotated Fig 32 looks like this:
|
|
In which: 260: substrate 262: battery 263: transmitter 264: integrated circuit 266 (top right): antenna 266 (bottom left) but should be 268: conductive trace
|
220. I should also quote [0231] and [0232]:
[0231] It is important that transmitter 263 is protected from corrosive or contaminating influences. To this end, in one embodiment, transmitter 263 is encapsulated in a protective non-conductive coating, such as an epoxy.
[0232] A patient using the aforementioned embodiment wherein the transmitter 263 is disposed upon the substrate 260, may make use of the device by simply inserting the implantable portion of the sensor transcutaneously and fixing the unit to the skin. The sensor need not be connected by the patient to an on-skin sensor control unit (such as on-skin sensor control unit 44 in FIG. 17). Thus, the entire device becomes disposable, meaning that a user of the device is able to purchase the device as a single unit and dispose of it as such, after a period of use that may range from one to fourteen days, or more.
221. In the final few paragraphs of this section, [0254]-[0256] discuss how the sensor control unit 44 can be prepared and used. A mounting unit 77 is applied to the skin. An insertion gun 200 carrying the sensor 42 and the insertion device 120 is positioned against the mounting unit 77. The insertion gun is activated and a portion of the sensor (and, optionally, a portion of the insertion device 120) is driven through the skin into the subcutaneous tissue. The insertion gun withdraws the insertion device, leaving the portion of the sensor inserted through the skin. Then the housing of the on-skin control unit is then coupled to the mounting unit. Optionally, establishing contact between the contact pads 49 on the sensor 42 and the conductive contacts on the on-skin sensor control unit activates it to begin operation.
222. [0255] then says that the insertion device, sensor, insertion gun and mounting unit can be manufactured, marketed or sold as a unit or ‘insertion kit’. Fig 33 is then described. Various options are then discussed as to how an insertion kit can be presented to the customer/user - ‘pre-loaded’ with the components already assembled together. [0256] then discusses the state of the insertion spring. The insertion kit can be packaged with the spring ‘cocked’ but preferably with a ‘safety, a barrier to prevent the release of the stored potential energy, with the barrier removed when insertion is to take place. An example of a barrier is a pin (not shown in Fig 33) that prevents the spring from expanding once compressed. Or the insertion kit can be packaged in the ‘pre-loaded’ configuration but without the spring being cocked, cocking of the spring taking place just prior to use. [0256] concludes by stating the insertion kit can be sterilised prior to packaging with examples given of gamma radiation or an e-beam.
223. The next section concerns the On-Skin Control Unit Electronics in [0257]-[0326] by reference to Figs 18A, 18B, 20A, 20B, 29 and 30. [0258] explains that the on-skin sensor control unit 44 also includes at least a portion of the electronic components that operate the sensor 42 and the analyte monitoring device system 40, with a block diagram of one embodiment in Fig 18A., which I need not set out, but which shows a transmitter 98 and an aerial 93.
224. The final two sections concern the Receiver Display Unit [0327]-[0362] illustrated in Figs.22, 30, 29, 23, 24 and 1, and ‘Integration with a Drug Administration System’ at [0363]-[0370] by reference to a block diagram in Fig 25.
225. To summarise, the first embodiment disclosed in Heller is a two-part system in which the sensor is adhered to the skin using a mounting unit, through which it is inserted (and the needle retracted) using spring-loaded mechanisms embodied in an insertion gun. The user then applies the electronics/transmission unit (Heller’s ‘sensor control unit’) and a cover to complete the assembly.
226. Heller describes and depicts the various parts: the sensor (Fig.2 and [0077]), suitable insertion needles (Figs.12-13 and [0202]-[0204]), an insertion gun (Fig.26 and [0205]-[0207]), the mounting unit (Fig.28B & [0237]) and the cover (Fig.28C & [0237] - note that the sensor control unit beneath the cover is not shown). Fig. 33 (described in [0255]-[0256], as I have said) shows this in a single unit. Note that whilst an automatic retraction mechanism is not shown in the figures, it is expressly taught in [0207], as I have mentioned.
227. The alternative arrangement is described at the end of [0227] and in [0229]-[0232], an example of which is depicted in Fig. 32. This arrangement is of the same ‘flag’ shape as the sensor shown in Fig.2, but (with the addition of the electronics) would now have a depth of a few millimetres (a point from Mr Varde’s evidence which Dr Schoemaker accepted in XX [T2/14322-14411-15]). It has the sensor, transmitting/processing electronics, and the battery, all disposed on the same substrate, rather than in two separable parts.
228. In connection with the integrated device illustrated in Fig 32, [0230] explains that the conductive trace forms the sensor. [0232] explains that the entire device is disposable ‘meaning that a user of the device is able to purchase the device as a single unit and dispose of it as such, after a period of use that may range from 1-14 days, or more’. It also explains that with such an embodiment the user can simply insert the implantable portion transcutaneously and fix the unit to the skin. Methods of insertion are discussed in Heller and include methods similar to the CGK, in which the sensor is placed within an insertion gun which inserts a needle having a U-shaped cross-section and automatically retracts it using (e.g.) a spring (see [0203], [0206], [0207]). Heller also discloses the use of mounting devices to assist with fixation to the skin.
229. A principal dispute as to what Heller disclosed to the Skilled Team concerned how the Fig 32 sensor would be inserted into the skin. Abbott contended there was no disclosure at all in Heller of an insertion device for the Fig 32 sensor. Abbott also contended that ‘To the extent that insertion is discussed at all, Heller simply states that the patient can use the embodiment ‘by simply inserting the implantable portion of the sensor transcutaneously and fixing the unit to the skin’ ([0232])’ and further contended that ‘There is no guidance or teaching of what kind of insertion device should be used or what should be contained in the insertion device, nor how the sensor could be inserted or the needle retracted using such a device.’
230. In my judgment, this dispute was based on a wholly impractical reading of Heller. It was supported by a sketch X1 which Dr Schoemaker drew in cross-examination to illustrate his point that the insertion needle would or might be obstructed by the electronics or any encapsulation of them, whether in epoxy or a housing. Since this is a point which goes to obviousness I consider it further at [371] below. Dr Schoemaker did not appear to have considered the idea of a housing or encapsulation which covered the electronics but left room for the insertion needle to perform its function. By contrast, Mr Varde did not consider there would be any difficulty in adapting the inserter of Fig.33 to work with an integrated unit, and Dexcom submitted that this would have been the view of any experienced mechanical engineer. I agree entirely. This incident was one of a number of examples which demonstrated that Dr Schoemaker was not able or prepared to think like the mechanical design engineer of ordinary skill in this field.
Ethelfeld - disclosure
231. Ethelfeld generally relates to skin-mountable units containing ‘sharps’ for penetrating the skin, and ‘handling units’ (also described as “inserters” - see e.g. [0064]) to aid in inserting those sharps. The background to the invention refers to drug delivery devices / infusion pumps ([0004]), and to transcutaneous glucose monitors / sensors ([0015]-[0018]). Such devices and sensors have elements which penetrate the skin, which elements Ethelfeld refers to as “transcutaneous devices”. Ethelfeld teaches the use of an insertion device to aid insertion:
[0026] …The transcutaneous device has a first position in which the distal end is retracted relative to the skin-engaging portion and a second position in which the distal end projects relative to the skin-engaging portion. The second unit [i.e. the handling unit / inserter] comprises actuatable driving means adapted to move the transcutaneous device from the first position to the second position when the driving means is actuated with the second unit attached to the first unit [i.e. the skin-mountable unit].
[0028] The transcutaneous device may be in the form of a … sensor device with a pointed insertion needle may provide a pointed transcutaneous device, the insertion needle being retractable after insertion of the blunt portion of the transcutaneous device.
232. There then follows various embodiments of inserters disclosed in the context of drug delivery systems. Dexcom drew attention to the ‘bi-stable spring’ arrangement shown in Figure 8, described from [0072]:
410 - inserter
411 - circumferential flange
413 - seal member
420/430 - actuation members
441, 442, 443 - central and leg portions of a bi-stable spring
455 - base portion
456 - upper housing portion
460 - hollow infusion needle
233. The operation of this is explained in [0075]. The user places the device on their skin and pinches the actuation members together which forces the curved portion of the bi-stable spring downwards thus applying force to the upper housing portion, closing the housing and inserting the needle into the skin. [0075] then says:
[0075] … As the second bi-stable state is only semi-stable, the spring will return to its initial position as the user reduces the compression force on the actuation members, however this will not influence the infusion device. When the needle thus has been introduced the inserter can be removed.
234. It is important to note that, in the Fig 8 embodiment, the infusion needle is inserted into the skin and the insertion device is then removed (end of [0075]), so the infusion device is left on the user’s skin.
235. The sensor device embodiment is discussed in more detail from [0088] and shown in Figs 13A-C:
|
|
In which: 750: sensor device 755: lower base portion 756: upper housing portion 757: hinge means 758: opening 760: sensor 761: signal receiving means 770: insertion needle 771: gripping member
|
236. [0088] says that the sensor device has “the same general hinged configuration” as the needle device of the earlier embodiment, and that the structures relating to the inserter have been removed from the drawings for clarity.
237. [0089] discloses that the sensor element is “a relatively flexible needle-formed sensor 760” which is fixedly attached to a housing.
238. In terms of sensor electronics, [0089] discloses that the sensor element:
[0089] … is in communication with signal receiving means 761. The signal receiving means may be contact means for connecting the sensor device to external processor means for evaluating the signals, transmitting means for wireless transmission to an external processor, or a processor arranged within the housing.
239. Dexcom submitted that the Skilled Team would immediately appreciate that such signal receiving means require a power supply and that it was common ground that the “transmitting means for wireless transmission to an external processor” would include electronics for processing signals received from the analyte sensor. Dexcom also contended that it did not appear to be in dispute that the device shown in Figs 13A-C is an ‘integrated’ assembly of the sensor and sensor electronics within the meaning of claim 1 of EP’044.
240. As for insertion of the sensor, [0089] goes on to say that:
[0089] … The needle-sensor is supported by an insertion needle 770, the support preventing deformation of the needle-sensor during insertion. The insertion needle is slidably received in the upper housing portion and comprises a gripping member 771 allowing the insertion needle to be withdrawn by the user after insertion has taken place.
241. [0090] specifically addresses Figures 13A-13C, noting that:
[0090] … Fig. 13C shows the situation in which the needle-sensor has been inserted and the insertion needle has been withdrawn. In this embodiment the insertion needle is adapted to be withdrawn by the user, however, the driving means and the gripping means may be designed to engage each other such that the insertion needle is removed from the sensor device together with the inserter.
242. Abbott were keen to stress that no further detail is provided as to how that design would work, but Dexcom pointed to the earlier discussion in [0075] of the operation of the bistable spring arrangement.
243. Abbott emphasised two points about the disclosure of Ethelfeld:
i) First, that Ethelfeld discloses a device which is intended to be a very low-cost, simple device and that achieves insertion with minimal complexity and minimal componentry, a point with which Mr Varde agreed [T2/31021-24]. At [0021] Ethelfeld says it is a device “which is convenient to handle and use, and which can be manufactured cost effectively.”
ii) Second, that the central feature of Ethelfeld is a hinged skin-mountable device like a stapler, with a cannula or needle sensor (or additionally with an introducer needle) on the upper portion, designed to be forced into the skin by a spring coupled to the handling unit above. The insertion profile is therefore rotational, according to the hinged device.
244. The culmination of Abbott’s submissions on the disclosure of Ethelfeld was that both the general scheme of Ethelfeld and the specific embodiments described therein would not be seen by the Skilled Team as suitable for the insertion of a delicate glucose sensor for a CGM product, and this would lead the Skilled Team to reject Ethelfeld in favour of a handling device with linear needle insertion. This is a point which I pick up under obviousness below.
Fennell - disclosure
245. Fennell is another patent application relating to analyte monitoring systems. The systems are described in [0024]-[0026] and shown in Fig.1 to include an analyte sensor (101), electronics in the form of a “transmitter unit” (102) which is “coupleable” to the sensor, and a primary receiver unit (104) which communicates with the transmitter unit over a bi-directional communications link (103). The material part of Fig.1 is reproduced below:
246. The sensor unit is disclosed in [0078] as having a lifetime of “3, 5, 7 days or other predetermined time periods”.
247. It is true, as Abbott submitted, that Fennell is very heavily focussed on the electronics aspects of analyte monitoring systems. None of the figures show any physical representation of a physical system. Nonetheless, possible physical configurations are discussed in [0029], in which I have underlined the two key sentences:
[0029] In one embodiment of the present invention, the sensor unit (101) is physically positioned in or on the body of a user whose analyte level is being monitored. The sensor unit (101) may be configured to continuously sample the analyte level of the user and convert the sampled analyte level into a corresponding data signal for transmission by the transmitter unit (102). In certain embodiments, the transmitter unit (102) may be physically coupled to the sensor unit (101) so that both devices are integrated in a single housing and positioned on the user’s body. The transmitter unit (102) may perform data processing such as filtering and encoding on data signals and/or other functions, each of which corresponds to a sampled analyte level of the user.
248. Mr Varde and Prof Georgiou took the view that this discloses a sensor and a transmitter unit integrated in a single housing. Dr Schoemaker considered this is disclosure of a 2-part device of the sort known from the CGK. I return to this key issue below.
249. Beyond that, the transmitter unit is disclosed as having a CPU (see [0037]), and [0074] explains that the transmitter unit may have a power supply such as a battery, in which case the transmitter can be shipped in a low power ‘sleep mode’ and activated or powered up. Various methods are disclosed:
i) [0073]-[0074] specifically disclose (in conjunction with the block diagram in Fig 6) such activation occurring in the context of a 2-part device when the user connects the sensor and transmitter units together. See also [0091].
ii) [0077] discloses another activation switch occurring in the context of a device involving a mounting unit when the transmitter unit is placed upon the mounting unit.
iii) [0084]-[0085] disclose the use of an RF on/off command to turn the transmitter unit on or off.
250. Finally, the use of an insertion device is taught in [0077] (albeit in the context of a 2-part device) for positioning the sensor unit on the skin.
What does [0029] disclose to the Skilled Team?
251. Although this is the question on which the parties focussed their attention and submissions, as will appear below, this may not be the correct question to consider.
252. In his written reports, Dr Schoemaker was clear that ‘Fennell only discloses a system with the familiar CGM architecture at the time of a sensor inserted first with transmitter unit being coupled subsequently’ [Schoemaker 1, 11.61 based on his earlier 8.51, it based on his reading of [0029]].
253. Abbott were keen to emphasise that [0029] is a single paragraph out of 118 in the document. Likewise in cross-examination, Dr Schoemaker said:
25 A. My point, I am sorry, my point here is we do have these two
2 sentences in the entire document.
3 Q. Sure.
4 A. That is all. Fennell is not talking very much about the
5 physical components of the system at all, because Ethelfeld [sic] is
6 about the data communication, it is not about the system
7 architecture, it is not about the architecture of the physical
8 components. These two sentences are the only indication into
9 the direction of an integrated system and they can easily,
10 easily be read in a different way.
254. However, in cross-examination, Dr Schoemaker agreed that [0029] was not a disclosure of the standard or familiar architecture, in this passage at T2/204:
19 What you say, at 8.51, is: "In the absence of any clear
20 indication otherwise, the Skilled Team would read paragraph
21 [0029] as describing the standard architecture of CGM systems
22 ..." at the prior art date. If we can just go back to the
23 text, first of all, you see it says, in the second sentence,
24 that the sensor unit may be configured to continuously sample
25 the analyte level and convert the sampled analyte level into a
2 corresponding data signal for transmission, so what is being
3 taught there is that the sensor unit actually has some
4 processing capacity, is it not?
5 A. Yes.
6 Q. That was not the standard architecture of the time; correct?
7 A. That was the standard architecture of the time, of course.
8 Q. The sensor unit itself did not process the signals.
9 A. Ah, the, not the sensor electronics, the sensor unit.
10 Q. Yes, the distinction here is between the sensor unit and the
11 transmitter unit, yes?
12 A. Yes.
13 Q. It is right, is it not, that they are not describing here the
14 standard architecture?
15 A. Yes.
16 Q. If they are not describing the standard architecture, the
17 reasoning that you give in 8.51 is undermined, is it not?
18 That the skilled person actually would think that something
19 different is being disclosed in this paragraph.
20 A. (Pause for reading) Yes.
255. There is no dispute that the discussion in the later paragraphs [0073]-[0080] relates to an arrangement where the sensor unit 610 is separate from the transmitter unit 620. It is not entirely clear what is comprised in the sensor unit, although it is clear it is more than just the sensor, even though it is supplied with power only when connected to the transmitter unit. In both Figs 2 and 6, the battery supplying the power is shown as being part of the transmitter unit.
256. Thus, in ‘one aspect’, described in [0074], the power supply/battery is included in the transmitter unit. Fennell discusses a number of features of this arrangement, including that battery power is preserved during the post-manufacturing sleep mode prior to connection of the sensor unit.
257. In a ‘further aspect’ [0076]-[0077]:
‘… the power supply for the transmitter unit 620 may be provided within the housing of the mounting unit such that transmitter unit 620 may be configured to power on or activated upon placement of the transmitter unit 620 on the mounting unit and in electrical contact with the sensor unit 610’.
258. Fennell continues as follows in [0077] and [0078]:
‘[0077] …. For example, the sensor unit 610 may be provided pre-configured or integrated with the mounting unit and the insertion device such that, the user may position the sensor unit 610 on the skin layer of the user using the insertion device coupled to the mounting unit. Thereafter, upon transcutaneous positioning of the sensor unit 610, the insertion device may be discarded or removed from the mounting unit, leaving behind the transcutaneously positioned sensor unit 610 and the mounting unit on the skin surface of the user.
‘[0078] Thereafter, when the transmitter unit 620 is positioned on, over or within the mounting unit, the battery or power supply provided within the mounting unit is configured to electrically couple to the transmitter unit 620 and/or the sensor unit 610. Given that the sensor unit 610 and the mounting unit are provided as replaceable components for replacement every 3, 5, 7 days or other predetermined time periods, the user is conveniently not burdened with verifying the status of the power supply providing power to the transmitter unit 620 during use. That is, with the power supply or battery replaced with each replacement of the sensor unit 610, a new power supply or battery will be provided with the new mounting unit for use with the transmitter unit 620.
259. [0079] and [0080] then describe a facility where the transmitter unit can be configured to detect when the sensor unit is removed from the transmitter unit so that a ‘last gasp’ transmission of data can be undertaken before the transmitter unit is powered down to await a replacement sensor unit.
260. It is notable in the detailed description in relation to Figure 6 that Fennell discusses these two locations for the power supply, but both involve a mounting unit. As described in [0077], quoted above, the sensor unit may be integrated with the mounting unit and the insertion device. The sensor is inserted into the skin, the insertion device is removed and then the transmitter unit is coupled to the mounting unit and power is supplied. In all these arrangements involving a mounting unit, the transmitter unit may be physically coupled to the sensor unit so that both devices are integrated in a single housing and positioned on the users’ body.
261. Nowhere is there any description of the insertion of a sensor which is part of a ‘sensor electronics assembly’ within the meaning of claim 1. If Fennell really was describing this new arrangement, I would have expected it to have been fully described in the specification, but it isn’t. In these circumstances, it seems to me that Fennell uses the word ‘integrated’ in [0029] in a different sense to the Patent and the argument based on the second sentence of [0029] reads too much significance into it.
262. All the experts and the submissions focussed on [0029], but that focuses on the wrong question. The correct question is what does Fennell disclose? When [0029] is properly read in the context of [0073]-[0080], I consider the disclosure is tolerably clear: it is of the CGK arrangement where the sensor is inserted and then connected to the transmitter unit.
263. Both Dr Schoemaker and Mr Varde fairly acknowledged that [0029] could be read in the opposite way to the way each had interpreted that paragraph. Since the experts focussed on [0029], it is understandable that they drew different conclusions from it. I have found against Mr Varde’s interpretation by viewing [0029] in the light of the later paragraphs. It appears that his attention was not drawn to those later paragraphs.
264. What remains is Abbott’s general accusation of hindsight on Mr Varde’s part. I am inclined to discount the influence of hindsight. After all, Mr Varde’s interpretation was based on the normal meaning of ‘integrated’. To a mechanical engineer if one speaks of an integrated sensor unit and transmitter unit, one expects a single component. I have found, in effect, that Fennell uses that word with a rather special meaning of ‘integrated into a single housing once physically coupled together’.
265. Even if, contrary to my view, a degree of hindsight was involved in Mr Varde’s interpretation, it was slight and not anywhere near as serious as the allegation advanced by Abbott.
266. In view of my conclusion as to what Fennell discloses to the Skilled Team and since I did not understand Dexcom to advance any case of obviousness on this basis, I could end my consideration of Fennell here. What I take forward is my assessment of the limited extent, if any, to which hindsight contributed to Mr Varde’s view of [0029].
Obviousness - the law
267. Abbott drew my attention to some familiar principles which bear on the issue of obviousness. Although familiar, they are worth setting out so that I have them firmly in mind. Abbott’s submissions have led me to consider the prior art in reverse order, for reasons which I explain below.
268. It is often convenient to assess obviousness using the structured approach set out by the Court of Appeal in Pozzoli v BDMO SA [2007] RPC 37 at [23] and restated as follows:
“(1) (a) Identify the notional “person skilled in the art”;
(b) Identify the relevant common general knowledge of that person;
(2) Identify the inventive concept of the claim in question or if that cannot readily be done, construe it;
(3) Identify what, if any, differences exist between the matter cited as forming part of the ‘state of the art’ and the inventive concept of the claim or the claim as construed;
(4) Viewed without any knowledge of the alleged invention as claimed, do those differences constitute steps which would have been obvious to the person skilled in the art or do they require any degree of invention?”
269. I was also reminded of the well-known statement of Kitchin J in Generics (UK) Ltd v H Lundbeck [2007] RPC 32 at [72] which was endorsed by Lord Hoffmann in Conor v Angiotech [2008] RPC 28 at [42]:
“The question of obviousness must be considered on the facts of each case. The court must consider the weight to be attached to any particular factor in the light of all the relevant circumstances. These may include such matters as the motive to find a solution to the problem the patent addresses, the number and extent of the possible avenues of research, the effort involved in pursuing them and the expectation of success.”
“I confess that I view with suspicion arguments to the effect that a new combination, bringing with it new and important consequences in the shape of practical machines, is not an invention, because, when it has once been established, it is easy to show how it might be arrived at by starting from something known, and taking a series of apparently easy steps. This ex post facto analysis of invention is unfair to the inventors, and in my opinion it is not countenanced by English Patent Law.”
271. In Jarden Consumer Solutions v SEB SA [2014] EWHC 445 (Pat), Arnold J (as he then was) said this at [102]-[105]:
“102. The skilled person is deemed to read the prior art properly, and in that sense with interest, but without assuming that it will provide him with any assistance in solving the problem which confronts him. In some cases he may conclude that it is not a useful starting point for development: see Terrell on the Law of Patents (17th ed) §12–27 to 12–30.
103. As Kitchin LJ and Sir Robin Jacob said in their joint judgment in Gedeon Richter plc v Bayer Pharma AG [2012] EWCA Civ 235, [2013] Bus LR D17 at [61], “it is trite law that … the older (from the priority date of a patent under attack) a piece of prior art said to render a patent obvious, the harder it is to show obviousness”.
104. It is relevant, although not conclusive, to consider whether the skilled person would have a motive to take the step in question: see Terrell §12–74 to 12–7640.
105. In assessing whether a claimed invention is obvious, it is always important, although difficult, to avoid hindsight. The fact that, after the event, it is easy to see how the invention could be arrived at by starting from an item of prior art and taking a series of apparently simple steps does not necessarily show that it was obvious at the time: British Westinghouse Electric & Manufacturing Co Ltd v Braulik (1910) 27 RPC 209 at 230 (Fletcher Moulton LJ), Non-Drip Measure Co Ltd v Strangers Ltd (1943) 60 RPC 135 at 142 (Lord Russell) and Technograph Printed Circuits Ltd v Mills & Rockley (Electronics) Ltd [1972] RPC 346 at 362 (Lord Diplock).”
272. Abbott were also keen to stress that an allegation of obviousness needs to be considered based on the teaching of each prior art reference. Each reference can be read in light of the CGK, but the “inconvenient details” of what the document or prior use is actually teaching the skilled person cannot be ignored. That is why (so Abbott submitted) Floyd J explained that an attack on CGK alone can be favoured by those seeking to revoke patents, because that attack is then not saddled with what a particular document is actually telling the skilled person to do. See the warnings made by Floyd J (as he then was) in ratiopharm v Napp [2008] EWHC 3070 §§154-159 which were approved by the Court of Appeal in Nokia v IPcom [2013] RPC 5 at §§128-129. These concerns were quoted with approval by Birss J (as he then was) in Accord v Medac [2016] EWHC 24 at §§119-124 where the learned judge again emphasised the dangers of an approach based on the CGK alone.
273. Abbott also made a related point, based on their perception that Dexcom’s case relied heavily on CGK in various respects. Abbott contended that, in Dexcom’s written expert evidence, there were instances where features of the CGM system described in the prior art were abandoned in favour of features from the CGK which facilitated reaching the desired target.
274. In their written Closing, Abbott addressed and stressed the following additional topics, in the light of Dexcom’s written opening and what they detected from the approach taken in the cross-examination of Dr Shoemaker.
The relevance of commercial considerations to the question of obviousness
275. Abbott pointed to Dexcom’s reliance on Brugger v Medic-Aid [1996] RPC 635 as establishing that purely commercial considerations are irrelevant to the question of obviousness (Dexcom’s opening skeleton §§102-103), and as seeking to side-step all of Dr Schoemaker’s evidence as to the focus in the industry on sensor accuracy. Abbott submitted this approach was misguided for a number of reasons.
276. Firstly, Abbott submitted that the evidential premise of Dexcom’s case on commercial factors is wrong. The relevance of sensor accuracy is not a purely commercial factor, as it is relevant to the technical issues concerning the Skilled Team. Dr Schoemaker explained that the concerns about sensor accuracy would feed into the Skilled Team’s concern not to manufacture, sterilise or insert the sensor in a way that would reduce the accuracy of the sensor. See T1/1047-24. Those, so Abbott submitted, are technical matters, not commercial matters.
277. Secondly, in any event, Abbott submitted that the legal picture regarding commercial factors is more nuanced than Dexcom suggests. Whilst something which is otherwise obvious does not become inventive purely because there is a commercial obstacle to doing it, that principle needs to be considered together with the principle that a commercially driven mindset can be a relevant aspect of the skilled person’s common general knowledge, particularly in circumstances where substantial changes to the prior art are required to reach the invention. As Floyd LJ stated in Koninklijke Philips NV v Asustek Computer Corp [2019] EWCA Civ 2230 at [0112]-[118], concluding (emphasis added):
‘118. These passages show that a commercially driven mindset can be a relevant aspect of the skilled person’s common general knowledge. Thus, what the skilled person does in the light of a given prior disclosure has to be decided with that mindset in mind. If the technical differences from the prior art to the invention are trivial, then the mindset may not matter, but if more substantial changes are involved, the court may conclude that the reluctant and prejudiced skilled person would not make them. If the court reaches the conclusion that the claimed invention would be arrived at by the skilled person, there is no further hurdle to be crossed concerned with whether the invention would be perceived as likely to lead to sufficient commercial success to make its manufacture worthwhile.’
278. Third, Abbott made the argument that the interrelationship between the number of changes that would need to be made to the prior art and the question of whether the commercial mindset or prejudice is relevant to the question of obviousness is also highlighted in one of the passages from Dyson [2002] RPC 22 at [95], cited in Philips at [116] which states:
“However, at the end of the day, the prior art would have required substantial changes to bring the Dyson claims within it (see judgment, para 156). The “mindset” in favour of bags was, as the judge held relevant to the skilled addressee's “active repertoire of skill” (judgment, para 45) and the enthusiasm and ease with which he could have been able to make those changes, without showing the imagination which he is presumed not to have (see judgment, paras 156, 157)”.
279. Fourth, Abbott submitted that in the present case, where, even on Dexcom’s case, significant changes are required to be made to the prior art to reach the invention, the mindset of the Skilled Team embarking on such an exercise is clearly relevant. Dr Schoemaker, the only expert who can truly speak to the mindset of those working in the field of CGM product development in 2009, has explained the factors that would deter the team from embarking on the project of reconfiguring the insertion devices that are taught in the prior art (in the case of Ethelfeld and Heller), or the insertion devices known from the commercial products in the case of Fennell.
280. I acknowledge and accept the points made by Abbott from Philips but, in my view, it is relevant also to note what Floyd LJ observed at [133] when discussing the facts:
‘If a particular prejudice is to prevent the skilled person from having a technically obvious idea at all, it needs to be a strong one. Sedley LJ in Dyson said that the relevant skilled person was “functionally deaf and blind” to the relevant development.’
281. In my view, the same consideration applies (possibly with even greater force) when one side is saying a prejudice would put the Skilled Team off taking forward something explicitly disclosed in the prior art.
Motivation and problems associated with the prior art
282. As Abbott submitted, it is well established that the motivation of the skilled person to take any particular step is relevant to the question of obviousness, and that the absence of a motivation to take any particular step makes the argument of obviousness more difficult (Actavis v ICOS [2019] UKSC 15 at [70]).
283. Next, Abbott submitted that in considering motivation the problems relating to the prior art are of course relevant. Dexcom relies upon the principle that a problem not overcome by the patent cannot be relied on as a barrier to obviousness, by reference in particular to Fisher & Paykel at [44]-[47] (which I set out in [86] above. However the point made in Fisher at [47] (by reference to Unilever) is that, even if the challenges would not be so significant that they would put people off pursuing the prior art, “in the absence of firm knowledge and experience, the conception of modifying the prior art as claimed would not come readily to mind” (see the quote cited at §36 of Dexcom’s skeleton). Abbott contended that that point very much applies to the present case, because there was an absence of firm knowledge and experience of inserting integrated devices, and so the conception of modifying the insertion devices taught in the prior art (in the case of Heller and Ethelfeld) or the commercially available insertion devices (relied on in the case of Fennell) so as to insert the sensor and the sensor electronics would not, without hindsight, come readily to mind.
284. Further, so Abbott submitted, there are some challenges that apply to the prior art, which would put the Skilled Team off from taking them forward (for example the unnecessary trauma and resulting potential damage to sensor accuracy caused by Ethelfeld) which are particular to the prior art and would not apply to the Patent. In respect of those types of issues, they are relevant to the question of obviousness even though the Patent does not need to teach how to overcome them.
Obvious to try and multiple paths
285. The question of what is obvious to try is only one of many factors to be taken into consideration (Actavis v ICOS [2019] UKSC 15 at [65]-[66]). Further, the burden and cost of the research programme, and the existence of alternative or multiple paths of research are both relevant, although the weight to be attached to those factors will vary depending on the particular circumstances (ibid at [67]-[69]). Abbott submitted that in the present case each of these factors points away from the invention of the Patent being obvious.
Hindsight and a step by step approach
286. I have already set out Abbott’s submissions in their opening skeleton on hindsight at [270]-[271] above. Abbott also drew attention to the reminder of the dangers of hindsight in the Supreme Court’s summary in Actavis v ICOS at [72]. Abbott submitted that, whilst an invention can be obvious based on a step by step approach, this is only provided that the steps are not driven by hindsight. Further, as the Court of Appeal indicated in Optis v Apple [2023] EWCA Civ 438 at [77], it is not the precise number of steps that matter, but rather whether “the gap between the prior art and patent is being deconstructed in such a way as to build in hindsight”. See also Floyd LJ in Mishan v Hozelock [2020] EWCA Civ 871 at [89]-[98]. Abbott submitted that Dexcom’s obviousness case over each piece of prior art suffers from this problem.
287. In support of this Abbott pointed to the inconsistency as between the suggestion that the invention was inherently obvious in 2009 and the 8 pieces of prior art which Dexcom has cited at various times in this litigation - referring to what Abbott called ‘the rainbow pleading’ at B1/5/p.50-57.
288. When considering what steps would be obvious in light of the prior art combined with the CGK, Abbott stressed that it is important to keep in mind that merely because some piece of information falls within the definition of CGK, it is not necessarily obvious to combine that piece of CGK with the piece of prior art (see Terrell 19th edition 12-46). They submitted that this is relevant to the present case because, for many of the steps that the Skilled Team would have to take over the prior art to reach the invention, Dexcom simply argues that the skilled design engineer would be able to do that based on their CGK, but ignores the fact that it would not be obvious to apply that CGK to the particular device taught in the prior art. See for example Dexcom’s case on the application of automatic retraction to the Ethelfeld insertion device (addressed below).
289. Further Abbott referred in opening to the dangers of hindsight in the context of an attack based on the CGK alone - as I summarised in [272] above. To that they added a reference to Conversant v Huawei [2019] EWHC 1687 where Arnold J (as he then was) made it clear that, if a case of obviousness over the CGK is not alleged, then it cannot be obvious to start from a piece of prior art, only to then throw away the teaching of the prior art and simply take forward the “high level idea” that would have been common general knowledge in any event. As Arnold J put it, “at best this is an approach based on hindsight”. The same can be said for an approach which essentially starts with a blank sheet of paper. See also Yeda at JA1/14/[62].
290. Finally, when considering the question of hindsight, Abbott reminded me of what I stated in InterDigital v Lenovo [2023] EWHC 172 at [202], “if you know the end point you are trying to reach, it is easy to direct the questioning with a laser-like focus to lead to that end point” and that (as the witness in that case demonstrated) “the Skilled Person proceeding without hindsight would not have such a focus”.
291. I will bear all these points in mind. However, to a degree they have been made in a rather scattergun approach. Certain points have greater application on certain of the art cited in this case.
Could/Would
292. Abbott also made a more specific criticism and this was their second point on inadequate instruction of Dexcom’s experts, that they had not been instructed properly as to the appropriate level at which to assess obviousness. Abbott submitted that Mr Varde’s evidence was replete with suggestions about steps which he considered were “logical”, not obvious, and which were “possible” or “could” be taken, not “would” be taken. Abbott contended that his design engineer was in constant problem-solving mode and he seemed to think it was enough for obviousness for the design engineer simply to come up with an idea, regardless of whether that was taken forward into development - relying on T3/3178-15:
8 Q. Even if there was only a risk of damaging the sensor readings,
9 it would not be taken forward by the team as a whole, for that
10 reason.
11 A. When you say taken forward, do you mean into development?
12 Q. Yes.
13 A. I would argue that whether something goes forward into
14 development does not really change whether it is obvious to
15 the design engineer that they could do it.
293. Abbott made similar criticisms of Prof Georgiou, submitting that he equally referred to steps that “could” be taken. He also seemed to think that the appropriate way for ideas to be discussed was by “brainstorming” (T3/40715-4088). Further, he appeared to consider that every detail of every paper published in any of the diabetes journals would have been CGK, which is plainly wrong (Exhibit PG-9 [D2.2/9] & T3/4047-4052).
294. Thus, Abbott submitted that given that both Mr Varde and Prof Georgiou were speaking from a silo-ed perspective, they were not at all in a position to say what steps "would" be taken by the Skilled Team as a whole. Abbott drew attention to some specific examples in context, but their overall submission was that it is clear that Dexcom’s experts were not applying the correct standard.
296. Abbott submitted that the risk of hindsight was particularly acute in the present case because both Dexcom experts were aware of the Abbott Libre 1 before embarking on their engagement in this case (and before they had attempted to recreate the CGK from the User Guides). So they knew of a commercially successful endpoint within the Patent before they even started. In contrast, as a result of his contemporaneous experience, Abbott submitted that Dr Schoemaker was able to anchor his evidence in 2009 and was accordingly insulated from such hindsight analysis.
297. Once again, all of Abbott’s points were made generally but their force can only be assessed in the context of each particular piece of prior art.
Obviousness - the facts
298. It is helpful to set the scene. The Skilled Team, comprising a collection of unimaginative individuals who have the CGK relating to their discipline, is interested in designing and developing a new CGM system and device. They have regard to the existing CGK devices on the market. So, per Pozzoli, I have identified the Skilled Team and their CGK and it is now necessary to identify the differences between what each piece of prior art discloses and the invention and then to ask Pozzoli question 4.
299. However, in advance of discussing the individual pieces of prior art, Abbott were keen to make an overarching point which I deal with here.
Dexcom’s implicit reliance on obviousness over the CGK
300. Abbott were very keen to characterise Dexcom’s case as being obviousness over the CGK, as follows.
301. Abbott contended that although Dexcom has not pleaded nor sought explicitly to introduce a case of obviousness over the CGK, much of Dexcom’s case implicitly relies on such an unpleaded argument. For example:
i) Dexcom’s case as to motivation to stick with the painful and unattractive insertion device in Ethelfeld, and/or press on with Figure 32 of Heller despite the problems with it, relies on the idea that the invention was inherently obvious from the CGK alone.
ii) In respect of Fennell, even on Dexcom’s case, Fennell only teaches the high level idea of integration of the sensor and transmitter from a vague reference in one paragraph, so the rest of Dexcom’s obviousness case over that citation is a pure CGK case.
302. Furthermore, as explained in more detail below, Abbott contended that whenever Dexcom’s experts were confronted with the myriad issues that the Skilled Team would need to address when seeking to take forward the specific teaching of Heller and Ethelfeld, their response was to suggest that the Skilled Team would simply move away from the teaching of those documents, and just take forward the high level idea of integration of the sensor and sensor electronics, and then develop an insertion device to insert an integrated wearable from their CGK knowledge and skill set.
303. Abbott invited me to treat Dexcom’s case with extreme scepticism - particularly as it had also recently abandoned its related collocation case. Abbott drew attention to the passages in their opening skeleton (§§118-119), where they reminded me that allegations of obviousness over the CGK alone, especially where the starting point has not been pleaded, must be treated with caution because they are not “obviously encumbered with inconvenient details of the kind found in documentary disclosures, such as misleading directions or distracting context” - the warnings made by Floyd J (as he then was) in ratiopharm v Napp [2008] EWHC 3070 §§154-159 which were approved by the Court of Appeal in Nokia v IPcom [2013] RPC 5 at §§128-129 & 143. These concerns were quoted with approval by Birss J (as he then was) in Accord v Medac [2016] EWHC 24 at §§119-124 where the learned judge again emphasised the dangers of an approach based on the CGK alone. As regards obviousness, the question needs to be considered based on the teaching of each prior art reference. Each reference can be read in light of the CGK, but the “inconvenient details” of what the document or prior use is actually teaching (or not teaching) the skilled person cannot be ignored.
304. Furthermore, Abbott emphasised what actually happened in the market both before and after the priority date and contended those events highlighted why any obviousness over the CGK case must fail.
305. In this regard, Abbott drew attention to the fact that Dexcom’s CEO Mr Sayer described the applicator used on the STS as “kind of scary” (CXX/4/16). Yet, so Abbott contended, Dexcom did not do from the CGK what it now says would have been obvious. Instead, the products it developed following the STS were the G4 and G5 (with the same form factor and insertion/retraction mechanism), and then the G6 launched in 2018, which incorporated automatic insertion and retraction only, and not the allegedly obvious integration step. This only came with the G7, only launched in 2022, the ease of use of which has been promoted by Dexcom as its major advance over the G6 (see the advert at CXX/6 and the 510(K) Summary at CXX/13/122 - “The G7 CGM System primarily improves upon the user experience of the predicate G6 CGM System by providing a fully enclosed miniaturized wearable with pre-connected sensor that is applied to the body in a single button press.”
306. So far as Mr Varde’s evidence was concerned, Abbott relied on the following extracts:
i) First, his agreement that the insertion process for the G7 as shown in the instruction video at CXX/17 was very easy and beneficial compared to the STS/G4/G5 (CXX/5 & T2/24415-22).
ii) Second, his agreement with the specific benefit of integrating the sensor electronics assembly with an insertion device prior to insertion (T2/24514-18).
iii) Third, his acceptance that Dexcom’s conduct (in not developing an integrated system until the G7) was consistent with Dr Schoemaker’s evidence that people in the field in 2009 did not see the two-part system as an overarching drawback (T2/24113-18).
307. Abbott also pointed to the fact that Medtronic, even now, has not developed an integrated system.
308. Abbott’s final point was that, given Dexcom was effectively running a case of obviousness over the CGK, it ought to have realised that the absence of any explanation in evidence as to why it did not do what it now says was the obvious step for notional teams in the field is damning of its invalidity case.
309. These points naturally lead me to consideration of the mindset aspects of this case.
The ‘mindset’ issues
310. As I mentioned above, Abbott relied heavily on Dr Schoemaker being the only expert who was in a position to speak to the mindset of those working in the field of CGM product development in 2009.
311. Although it is convenient to refer to these issues as ‘mindset’ issues by way of shorthand, Abbott took a more nuanced approach (hence their reliance on Philips – see [277] above) which I address below. First, I must explain how the issues were framed by Abbott.
Sensor accuracy.
312. Abbott contended that every CGM developer at the Priority Date was aspiring to have a CGM system with a ‘replacement claim’ i.e. being able to claim that their CGM system eliminated finger-prick testing for the purposes of confirmation before therapeutic decision-making. At the Priority Date, no product had received approval to be sold with such a claim. As such, it was said that CGM system developers were particularly motivated to improve the accuracy and signal stability of the sensor.
313. In fact, at times Abbott appeared to go further, suggesting that the Skilled Team would not bother to develop a new CGM device unless or until they had developed a sensor which delivered the ability to make a replacement claim.
The ‘established’ architecture.
314. One of Abbott’s overarching arguments was that the two-part device was ubiquitous and accordingly, the Skilled Team would consider the two-part device to be the standard approach or the ‘established architecture’. They also submitted that the Skilled Team would be aware of the manufacturing and costs benefits of that architecture, see [5] above. So a key feature of the existing devices was a requirement for the user to attach manually a sensor electronics unit to the sensor after it had been inserted into the skin, the insertion often being effected using a mounting unit to which the sensor electronics were then attached.
Abbott’s arguments
315. Abbott acknowledged (as they had to) that neither of these issues were addressed or solved by the Patent. Nonetheless they submitted that a commercially driven mindset can be a relevant aspect of the skilled person’s common general knowledge, particularly in circumstances where substantial changes to the prior art are required to reach the invention. As that formulation recognises, it boils down to the weight to be given to an alleged commercially driven mindset in the overall obviousness assessment.
316. Having said that, I am inclined to give both these factors relatively little weight. In terms of sensor accuracy, it is clear that companies were continuing to develop CGM systems and were not waiting for sensor accuracy to provide the ability for a replacement claim. In terms of the so-called established architecture, it was common ground that the Skilled Team knew all about the reasons for the existing two-part architecture, such that, once the cost of sensor electronics reduced, an integrated unit would be taken forward. Neither of these alleged prejudices were at all strong. The commercial considerations did not render the idea of an integrated unit inventive from a technical standpoint. However, as I have already indicated, these matters must be considered in the light of each piece of prior art and particularly in the light of what changes have to be made to the prior art in Dexcom’s obviousness cases.
Why was it not done before?
317. These considerations also provide the answer to another of Abbott’s arguments. They relied on the fact that Dexcom did not release their first integrated G7 until 2022 and argued, if the Patent was obvious, why had Dexcom not launched an integrated device long before.
318. There are two answers to this rhetorical question. The first is cost. It is clear to me that the cost of the electronics had come down sufficiently to make it feasible for Dexcom to develop an integrated device in the G7. The second is that Dexcom had its previous system in the market. If they had been entering the CGM market for the first time they might well have launched an integrated unit earlier. In this regard, Abbott were critical that Dexcom had adduced no evidence as to their position on these matters, but in my judgment, there was ample evidence from the experts to enable me to reach the conclusions I have just stated. No doubt Abbott would have loved delving into Dexcom’s internal documentation, at least some of which would have had to have been produced had Dexcom pleaded its position explicitly, but it is likely that the whole exercise would have been disproportionate.
Fennell
319. If I were to assume Dexcom’s construction of [0029], that still leaves the Skilled Team with the disclosure, at a high level of generality, of the idea of an integrated wearable. From that starting point, I understood Abbott to contend that the Skilled Team are essentially starting with a blank sheet of paper. This again is an exaggeration because at the very least the Skilled Team have the existing CGK products to work from, but it is true that the case is really based on the CGK using the idea of an integrated wearable. In those circumstances, Abbott’s reminders of the dangers of a CGK case have application.
320. I do not believe it is necessary to go any further with Fennell, because, if I am wrong about Heller, the case based on Fennell cannot possibly succeed. However, I must keep in mind Abbott’s accusation that Mr Varde nonetheless stated his view that claim 1 was obvious over Fennell, and that could only have been reached using hindsight.
Ethelfeld
321. I can deal relatively briefly with Ethelfeld, because the case of obviousness based on Ethelfeld underwent some development in the course of cross-examination (and also because I am anticipating what I have concluded on Heller, below). I can start with the case as put to Dr Schoemaker in cross-examination which was as set out by Mr Varde in his written evidence. In summary, this was a combination of Fig 8 and 13 - the bistable spring arrangement of Fig 8 with the introducer needle/sensor device embodiment in Fig.13.
322. Based on this starting point, Abbott submitted in their written closing that Dexcom’s case involved the following steps which I summarise here:
i) Step 1: Decide to take Ethelfeld forward at all
ii) Step 2B: Use of a curved needle
iii) Step 3: Choosing the fourth embodiment (Figure 8) with the bistable spring as the starting point.
iv) Step 4: Combine the bistable spring with the Figure 13 inserter device.
v) Step 5: Re-configure the bistable spring with the introducer needle and the sensor to improve the geometry.
vi) Step 6: Decide to pursue an automatic retraction mechanism.
vii) Step 7: Re-configure the handing unit, introducer needle and bistable spring for retraction.
viii) Step 8: Incorporate an activation switch.
ix) Step 9: Add a cap prior to deployment
x) Step 10: Re-configure the device so the sensor electronics is engaged with the introducer needle and is moved in the same direction as the introducer needle.
323. Dr Schoemaker and Abbott made much of Step 1 where their point was that the Skilled Team would not take Ethelfeld forward at all because of the trauma which would be caused by the hinged insertion of a straight needle, a point which Abbott illustrated with this schematic which was put to Mr Varde.
324. This schematic was undoubtedly exaggerated. In his second report, Mr Varde had approached this point from the point of view of the skilled mechanical engineer. As he pointed out, ‘The level of trauma using Ethelfeld would depend on the radius of rotation and the depth of insertion, i.e. the larger the radius of insertion and shallower the depth of insertion, the lesser the trauma.’
325. Adopting a 5mm insertion depth, which was based on the Abbott Navigator Freestyle system he calculated the ‘needle drag’ for a pivoting insertion mechanism:
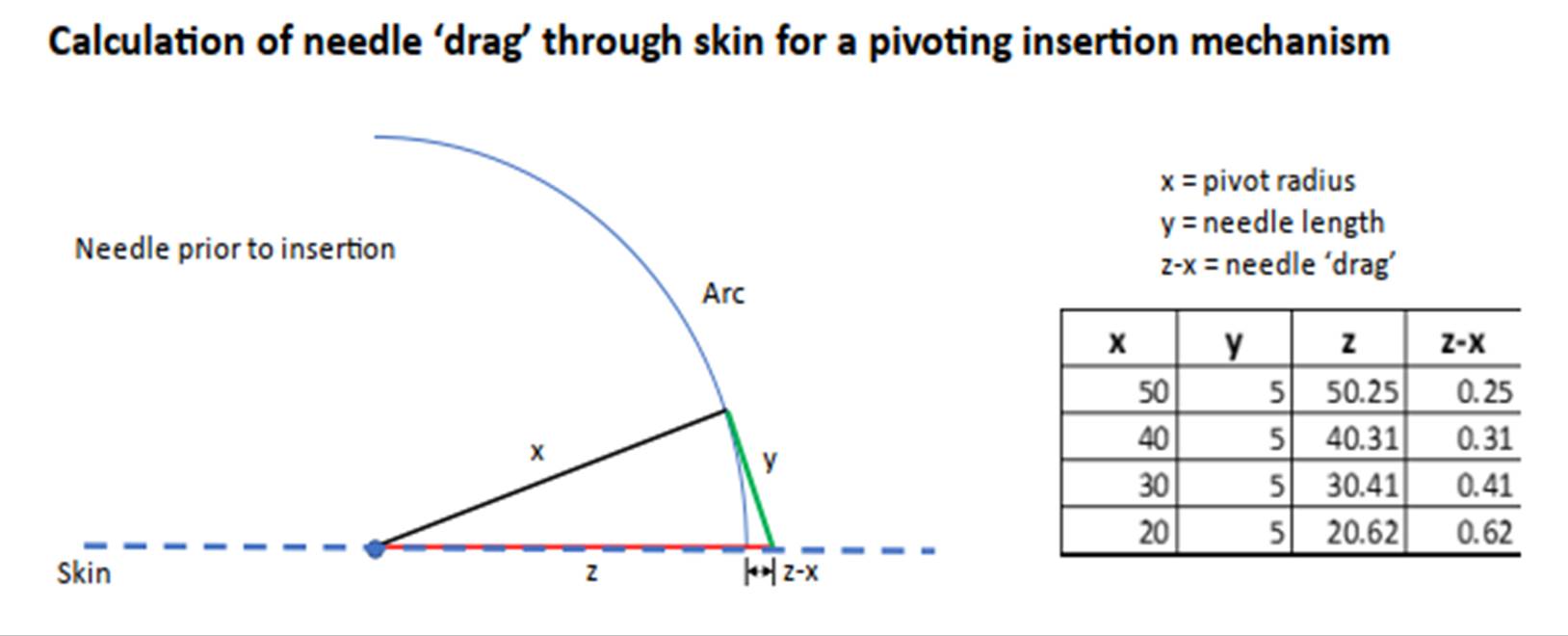
326. Based on his experience with various automatic injecting devices, Mr Varde added two observations: first, that skin has a degree of elasticity and 0.25mm of ‘needle drag’ would be unlikely to lead to any tearing of the skin and second that any insertion mechanism would require a degree of lateral freedom of movement to prevent the mechanism from jamming. He considered that values of x and y as in the first line of his table were eminently sensible in the context of an Ethelfeld device. Mr Varde also gave two other options to minimise the problem - one was to remove the hinge and use a latch arrangement and the other was the use of an arcuate needle. Dr Schoemaker was prepared to accept that a curved needle would resolve his issue over trauma, but a curved needle created an additional problem for the retraction step.
327. On this trauma issue, I am satisfied that Dr Schoemaker and Abbott exaggerated the scale of the problem, largely because Dr Schoemaker was not able to analyse the scale of the problem accurately nor to envisage the solutions which Mr Varde presented. I do not believe that this would have put the Skilled Team off Ethelfeld altogether.
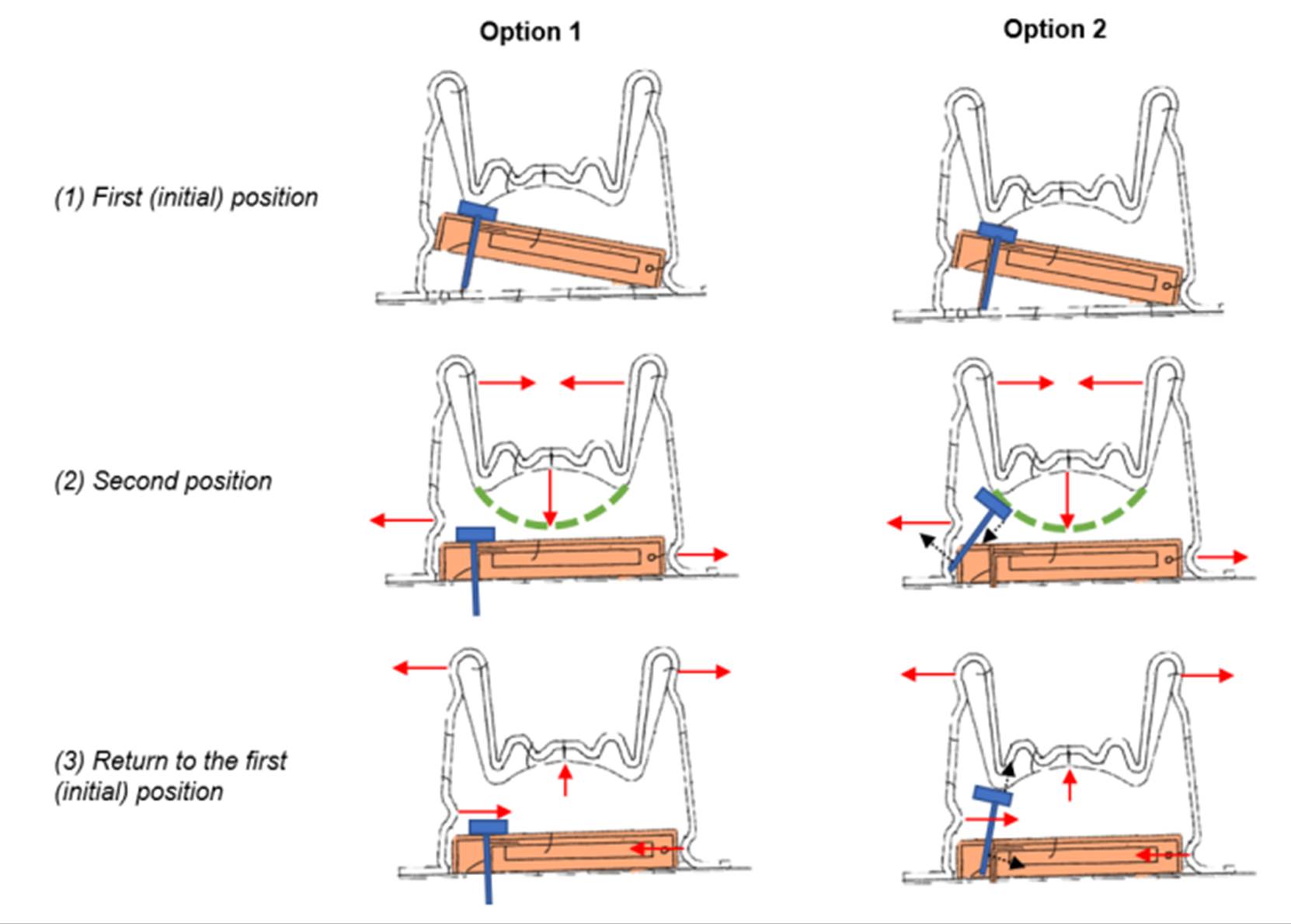
329. These diagrams are based on a very literal view of the combination of Figs 8 and 13. The Skilled Mechanical Engineer would not take such a literal view, so the problems identified by Dr Schoemaker were, as before, either exaggerated or might not arise in any practical implementation.
330. On Mr Varde’s side, he appeared confident that the skilled mechanical engineer would be able to achieve a working design, but, as Abbott submitted, he had not carried through his analysis in his written evidence to a design which could be shown to work.
331. This state of affairs emerged clearly in the course of Mr Varde’s cross-examination, where he was being asked about the problems that Dr Schoemaker had identified over the retraction step:
‘There are two options that one
3 can use with the Figure 8 embodiment in order to correct for
4 that very real problem that Dr. Schoemaker has highlighted.
5 The first is to essentially create a housing that shifts the
6 position of the sensor unit so that the insertion needle is
7 directly underneath the centre of the bi-stable spring. The
8 second option is to take essentially the teaching in Ethelfeld
9 and actually rotate the actuation means through 90°. You end
10 up with a kind of T-shaped device with the sensor here and the
11 actuating means at one end, and that would allow you to then,
12 again, centre the bi-stable spring directly over the, over the
13 thing, so it is a reconfiguration of the teaching.
14 Q. What you are saying is that in order to get automatic
15 retraction of the needle in this fourth embodiment, you would
16 need to carry out multiple and substantial modifications?
17 A. Well, those modifications are within the CGK of a qualified
18 mechanical engineer.
19 Q. But they are multiple and substantial; yes?
20 A. I do not think so. It depends, you know, none of, well, this
21 patent does not provide information at the level of tolerances
22 and configuration. It provides figurative information about
23 how mechanisms operate and how they might work. I think it is
24 well within the skill set of a skilled engineer to be able to
25 configure those disclosures in a manner which allows them to
achieve the aim that they are trying to achieve.
3 Q. Mr. Varde, you have submitted two written reports on the
4 subject of Ethelfeld; you have not come up with these major
5 reconfigurations in either of those reports, have you?
6 A. Because it is obvious, it is obvious to the skilled engineer
7 to, as to it would be possible to do.
332. Overall, despite Mr Varde’s confidence in the abilities of the Skilled Mechanical Engineer I find myself unpersuaded that it was obvious to move from the combination of Figs 8 & 13 to a developed design which would fall in claim 1. Even if I am wrong about that, the developed design would have a curved needle and I am not persuaded that it would then be obvious to develop a design which would land in claim 5.
333. In this regard, and in the absence of Mr Varde presenting a developed design based on the disclosure of Ethelfeld in his reports, there was some force in Abbott’s suggestion that Mr Varde was in problem-solving mode, and he was tackling each problem as it arose, and saying the solution to each problem was obvious. The correct question I have to address is whether all the steps required to get from the disclosure of the prior art to something which falls within the claim is obvious to the unimaginative man/team of ordinary skill in the art. These are relatively fine distinctions in the context of this case, where the Skilled Mechanical Engineer must have the ability to implement a schematic design as, for example, suggested in the combination of Figs 8 & 13 of Ethelfeld. I bear in mind that Mr Varde might well be correct, but my point is that he did not do enough to satisfy me.
334. I should also mention that I have not reached these conclusions based on Dr Schoemaker’s analysis which, as I have indicated, was not based on what a Skilled Mechanical Engineer would derive from the prior art or on what he or she would implement in a design based on it. Instead, and this is a point which has particular force in mechanical cases, it is, in my view, necessary for the party saying a claim is obvious to present an obviousness case which provides the Court with a clear idea of what is said to be the end result of the process.
Heller
335. I have discussed what Heller discloses above.
Dexcom’s case
336. Dexcom’s case in closing was simple: Heller discloses all the features of claim 1, with various options for putting them together. A device with all those features in combination is a natural result of following the teaching of Heller and could readily have been achieved at the priority date by the Skilled Team necessary to implement the Patent. Thus, on Dexcom’s case, no substantial changes were required which meant there was ample motivation for the Skilled Team to take Heller forward.
337. Dexcom submitted that Heller describes with precision and detail a CGK Navigator-type insertion device, including details of the needle, the preferred shape for the sensor, the electronics, an insertion and retraction mechanism with spring loaded insertion and retraction etc., and automated activation or the use of an activation switch. It also discloses that the sensor (whilst keeping the same general shape and configuration) can be combined with the electronics unit including the transmitter and the battery in the manufacturing process on the same substrate. It should be noted that the disclosure of the use of an integrated sensor and electronics unit is not simply a passing reference in Heller. It is in fact the subject of the invention which is actually claimed.
338. Dexcom acknowledged that Heller does not explicitly teach the use of the disclosed spring loaded CGK-type insertion mechanism with the integrated sensor/electronics assembly (simply referring in [0232] to ‘insertion’ by the patient) but submitted it was common ground between the experts that the Skilled Team would expect to use that mechanism, adapted as necessary [T2/1453-1468]. Similarly for activation, Dexcom submitted it was common ground that in an integrated device an activation switch was necessary (Schoemaker 1 ¶12.5 [C1/1/79]; Georgiou 2 ¶7.4 [D1/5/232]), and that either of the activation mechanisms specifically disclosed in Heller in relation to the 2-part device could readily be used for the integrated device (Schoemaker 1 ¶11.18 [C1/1/69] and XX [T2/15918-24]; Georgiou 1 ¶¶8.10-8.12 [D1/1/69] & XX [T3/40924 - 41219] and [T3/41720 - 42011]).
Abbott’s case
339. Abbott’s principal point was that Dexcom’s case on Heller was classic ex post facto hindsight, involving multiple redesign steps which involved moving away from the teaching of Heller in material ways. Abbott submitted that, cumulatively, all the steps constituted a significant research project that the Skilled Team would either not be motivated to embark upon in the first place or, if embarked upon, it was not clear that the Skilled Team would end up within the claims of the Patent.
340. Abbott managed to develop no less than 12 steps which they said Dexcom’s case required to get from Heller to claim 1, although there were alternative choices on two of the 12 steps.
341. Before dealing with Abbott’s steps, I must discuss some overarching points which Abbott took.
Issues with manufacture and sterilisation
342. There was no challenge to Dexcom’s evidence that the Fig.32 embodiment has a sensor, that the integrated circuit of the transmitter is ‘sensor electronics’, that there is a power supply, and that the embodiment is a sensor electronics assembly.
343. Dr Schoemaker suggested two potential problems of implementation in making the Fig.32 sensor for a CGM device: manufacture, and sterilisation.
344. On manufacturing, he said in his first report that one would effectively have to choose whether to manufacture the sensor end first, or the electronics end first. If the choice was to manufacture the sensor first, it would not have been obvious to the Skilled Team how then to lay down the electronics at the other end of the substrate in a manner that avoided impacting the enzyme on the sensor. Alternatively, if the Skilled Team laid down the electronics and conductive traces etc. first, then subsequently applying the sensor membrane layers by dip-coating would endanger the electronics at the other end.
345. Prof. Georgiou had two answers to this in reply. He pointed out that encapsulating the electronics would protect them during the dip-coating process, and he posited an obvious alternative whereby the sensor and transmitter are manufactured on separate substrates, which are then stuck together back-to-back. Mr Varde also relied on the ‘back-to-back’ approach, noting that the Design Engineer was not attracted to the [0229] embodiment by the single substrate disclosure, but rather by the idea of the single integrated unit and the advantages that flowed from it, as taught by Heller.
346. In cross-examination, Dr Schoemaker in the end accepted Prof. Georgiou’s evidence on appropriate methods of manufacture, subject only to matters of expense, culminating in the following at T3/167:
15 Q. But if you wanted to achieve the advantages which uniquely
16 have been taught by Heller, in terms of ease of use which are
17 reflected in the Figure 32 device, you are not disputing that
18 it could not be done, so far as the manufacturing techniques
19 are concerned, by the methods, for example, dealt with by
20 Professor Georgiou in his second report.
21 A. Still difficult. As I said, you can do it, but it adds
22 additional challenges, it adds additional risks and the result
23 out of that will be, once we have found a solution, the result
24 will be that the manufacturing costs are expensive.
347. In this regard, Dexcom pointed out that we have no idea how the embodiment in the Patent would be manufactured, or how expensive this would be. The Patent also teaches the use of a single flexible substrate (see e.g. [0141], [0144]), so, as Dexcom submitted, it is hard to see how or why Heller should be any more difficult or expensive.
348. Prof. Georgiou maintained his evidence on dip-coating - see e.g. T3/423:
25 Q. You and Dr. Schoemaker have both commented on the
2 manufacturing process for the Figure 32 embodiment in Heller
3 and I think you agree with him that the electronic engineer
4 would not first apply the membrane layers and then apply the
5 electronics; correct?
6 A. It depends what -- I mean, I do not have expertise in the
7 manufacturing of sensors, but I would assume that a good place
8 to start would be to put the electronics on first, and then do
9 some form of dip-coating for the sensor.
10 Q. Yes, but even that might raise its own problems, because
11 attaching the transmitter before applying the electrode and
12 conductive traces would upset the flat surface; yes?
13 A. The conductive traces go on first, on the planar device. So
14 first you put on the conductive traces, which includes the
15 traces for the sensor, also the place where the ASIC is going
16 to land in the connections. Then you epoxy down the ASIC, do
17 the glob topping and then you would functionalise the ends
18 with something which allow the sensor chemistry to be
19 deposited.
20 Q. In either case, you are suggesting you encapsulate the
21 electronics before the dip-coating; yes?
22 A. That would be a sensible thing to do.
349. Mr Varde was challenged on his view of the attraction of the idea disclosed in [0229] - see [T3/33613 - 33919] and I agree this challenge was not successful. Prof. Georgiou also maintained his evidence on the ‘back-to-back’ manufacturing process [T3/42323 - 42518], as did Mr Varde on his evidence to the same effect [T3/34010 - 34123].
350. Turning to sterilisation, Dr Schoemaker’s position in his first report was as follows:
11.17 A further challenge the Skilled Team would have faced would have been how to sterilise the Figure 32 Configuration in 2009. Based on their experience of the sterilisation methods of other medical devices as of the Priority Date, the Skilled Team would assume that different sterilisation techniques would be required for the sensor and transmitter parts because chemical sterilisation (e.g. ethylene oxide) would not be readily compatible with the sensor chemistry while sterilisation by irradiation would not be readily compatible with the electronics of the transmitter.
351. In XX he said that the CGK was to use e-beam radiation to sterilise the sensor (rather than ETO) but that this could not be used for the electronics. He even suggested that this was a technical reason why the CGK used a 2-part solution. See T1/82:
6 […] So the Skilled Team would
7 have to find a solution for that and that was one of the
8 technical reasons in 2009 why we had the separation between
9 the sensor, which was sterilised using e-beam and the
10 electronics which did not have to be sterilised in that
11 two-part concept.
352. As Dexcom pointed out, the difficulty here is that this is another ‘problem’ not solved by the Patent. Indeed, as I mentioned above, the single mention of sterilisation in the Patent assumes the Skilled Team knows how to achieve it:
[0148] In one aspect, the integrated sensor and sensor electronics assembly and the insertion device may be sterilized and packaged as one single device and provided to the user.
353. When this point was put to him, Dr Schoemaker’s oral evidence was elusive.
i) On Day 1 he said that an integrated product could be sterilised at the priority date by the Skilled Team using CGK techniques with optimisation, tests and efforts [T1/8123 - 8525], (presumably using ethylene oxide as is done in e.g. the G7 product). This culminated at T1/85:
21 Q. You are agreeing that the skilled person, the skilled team
22 would reasonably expect to be able to achieve the necessary
23 sterilisation ----
24 A. After putting -- I am sorry. After putting efforts into that;
25 yes.
ii) On Day 2 Dr Schoemaker sought to qualify that evidence in an extended passage [T2/1624 - 16520]. It is best to take this in stages (with emphasis added). At T2/163 he began:
5 The patent does not specify the point in time that the
6 integration takes place. In 2009, the skilled team would be
7 able to implement the patent, even if the skilled team would
8 not have found a solution how to sterilise the sensor and the
9 sensor electronics together, because the solution to that
10 would be what Libre 1 and Libre 2 were doing; the user couples
11 the sensor and the sensor electronics prior to insertion. By
12 doing that, the sensor electronics does not have to be
13 sterilised. By looking at Figure 32, there is no other
14 option. If you have Figure 32, you have to sterilise both in
15 the same process.
iii) As Dexcom contended, that evidence was equivocal. Dr Schoemaker did not say that the Skilled Team would not have been able to sterilise the integrated unit.
iv) Then at T2/164 he explained that in 2009 the Skilled Team would have tried sterilisation with ethylene oxide, that this does in fact work, but that there was ‘no guarantee’ in 2009 that it would work:
9 Q. Well, ethylene oxide ----
10 A. In 2009, it was part of the general common knowledge that this
11 might, as a matter of fact, this might not work. There was no
12 guarantee that the skilled team would be able to find a
13 sterilisation process which is suitable to sterilise the
14 sensor and the sensor electronics in 2009.
15 Q. Sorry, I am just trying to have a quick look at the
16 transcript, what you said a little earlier. (Pause) Right. I
17 think what you are saying is we now know that ethylene oxide
18 does work; right? You can sterilise the whole thing in one
19 go; yes?
20 A. We know that since 2022.
21 Q. Ethylene oxide would have been what you would have tried, if
22 you had wanted to try it, in 2009; correct?
23 A. Correct.
v) The point here is that the Skilled Team may not have known that ethylene oxide sterilisation would work, but they would have had sufficient expectation of success to try it, and it does in fact work. Dr Schoemaker then went on to explain what happened at Roche.
24 Q. If you had tried it, you would have found that it could be
25 optimised to a sufficient degree to work. That we now know,
2 right?
3 A. We do not know. I cannot say that. What I can say is that
4 we, at Roche, around the same time, must have been around
5 2008/2009, we have tried ethylene oxide, to sterilise the
6 sensor with ethylene oxide and we found that the enzyme
7 activity was suffering too much from that, so the enzyme
8 activity went down, thereby increasing the risk that the
9 lifetime of the sensor will be reduced and that was the reason
10 why we went away from sterilising the sensor with ethylene
11 oxide.
354. Overall, I accept Dexcom’s submission that Dr Schoemaker’s evidence taken as a whole was that the Skilled Team would at the priority date have had the ability and resources to sterilise a combined sensor/electronics assembly with ETO and a reasonable expectation of success in doing so.
356. The final point is that the problem proposed by Dr Schoemaker in his written evidence would not arise with the back-to-back manufacturing method, whereupon the sensor and electronics can be sterilised separately before being sandwiched together. That was a point made by Mr Varde in reply. As I noted above, the suggestion that the back-to-back method was obvious was maintained during cross-examination and is a suggestion which I have accepted.
357. I can now revert to consider the steps which Abbott contended were required to get from the disclosure in Heller to claim 1, although it will be seen I have already dealt with some of the points. I should also add that I have not found it necessary to set out the lengthy submissions made by Abbott in relation to each of these steps. The allegation of hindsight was made repeatedly, but, in view of what Heller actually disclosed, it is an allegation which has very little force.
358. As is sometimes the case, the patentee greatly exaggerated the number of steps required. Furthermore, the identification of all these steps indicates to me that Abbott did not approach matters from the correct viewpoint - that of the unimaginative skilled design engineer fulfilling their role in the Skilled Team. Nonetheless, I analyse each suggested step and, importantly, whether it was obvious to take forward the combination of all of them.
Step 1A - Decide to take forward the Fig 32 embodiment.
359. Dexcom disputed this was a step at all, since Fig 32 is what is disclosed. Abbott sought to justify this as a step in view of their ‘issues with manufacture’. Abbott submitted further that even if the decision was to take the Fig 32 embodiment forward, the Skilled Team would need to carry out the following additional steps in order to be able to make Fig 32, all of which they contended would require significant development work:
i) Ensure a careful disposition of delicate sensor membrane and enzyme layer(s).
ii) Ensure a sufficiently low temperature during all manufacturing processes to ensure that the enzyme layer(s) are unaffected.
iii) If applying the electronics first, finding alternative means of manufacturing the sensor which works despite the substrate not being flat (in light of the fact that standard methods such as screen printing would not be suitable, and dip coating would not be easily reproducible and may affect the electronics).
iv) Find a solution to the sterilisation issue which is compatible for both sensor and the electronics which is challenging because of how close together the electronics and the sensor would be on the substrate, and because chemical sterilisation will impact the chemistry of the sensor and irradiation sterilisation would affect the electronics.
360. I have already dealt with these points. There is, of course, a short answer to all these points. The Patent assumes the Skilled Team has all the necessary skills and does not provide any assistance on any of these points.
Step 1B was the alternative of having the electronics and the sensor on different substrates.
361. Abbott submitted this was not pursuing Fig 32 at all, and that this suggestion of using different substrates was pure hindsight. I disagree, for the reasons already discussed. In my view this is part of routine product development by the unimaginative design engineer fulfilling his role in the Skilled Team.
Step 2 - putting Fig 32 in a housing.
362. Abbott interpreted this as requiring a ‘rigid’ housing, which would go against the benefit taught in Heller of the substrate being flexible, and the use of an epoxy coating to protect the substrate (at [0231]). In my judgment, how to enclose the Fig 32-type sensor and electronics was routine design.
Step 3A - Modifying Fig 14 to house Fig 32.
Step 3B - Make a new housing for Figure 32 with only part of the sensor exposed.
363. These alternatives illustrate, in my judgment, Abbott’s impractical approach. The skilled mechanical engineer would understand the figures in Heller are schematic. Any implementation would require that engineer to make real-life practical routine design choices, which include these supposed ‘steps’.
Step 4 - Decide to use Figure 33 as the starting point for the new insertion device
364. I do not consider this to be a step at all. It is a choice from what Heller actually discloses, by way of insertion device. In this regard, in my judgment, it would be clear to the Skilled Team, and the mechanical engineer in that Team, that Heller presents them with a series of options from which they can make their selection without requiring any inventive capacity.
Step 5 - Change the dimensions of Figure 33 to accommodate Figure 32.
365. This is routine development and a further illustration of Abbott’s deeply impractical and overly literal approach.
366. Mr Varde was cross-examined to the effect that the modifications necessary to the Fig. 33 device would be ‘substantial’, but his evidence was that those modifications were all routine and obvious to the mechanical engineer [T3/34919 - 36020]. By way of example, I can quote this passage at T3/353:
5 Q. Can I suggest, just as your evidence was in relation to
6 Figure 26, what you are suggesting, in fact, is a substantial
7 modification of Figure 33, because Figure 33 is taught with a
8 mounting unit and for a two-part system, as we have discussed.
9 A. Again, all of the modifications that would be required, I
10 think, are within a design engineer's CGK. The removal of a
11 mounting unit is essentially the removal of a mating
12 component, so it is a simplification of the configuration, of
13 the overall configuration. Then there are geometric changes
14 to make and the means to hold the sensor and deliver.
15 Q. However, it is the same order of magnitude of changes as you
16 have described in relation to Figure 26, it is a substantial
17 modification?
18 A. It is a case of mechanical engineering using CGK.
19 Q. Yes, and in Figure 26, you describe that as a substantial
20 modification. I am suggesting to you it is the same order of
21 changes you have to make to Figure 33.
22 A. Yes, there are several changes you need to make to it, yes.
367. Dr Schoemaker’s written evidence was that there would be several implementation difficulties [Schoemaker 1 ¶11.18 [C1/1/69] and ¶¶11.7-11.11 [C1/1/66]].
i) A particular problem was that if the mounting unit shown in Fig. 33 were removed, that would cause difficulties with reliability, consistency, minimising trauma, and adherence to the skin [Schoemaker 1 ¶¶11.8(a), (e) and (f)]. Mr Varde made the point in response that you would not necessarily have to abandon the mounting unit. [1]
ii) Other purported practical difficulties related to reconfiguring the Fig. 33 device to interact with the larger integrated sensor unit [11.8(b), (c), (d)].
iii) Dr Schoemaker also suggested difficulties with activation [11.9-11.10], which I deal with below.
368. To address these points, Dr Schoemaker’s cross-examination began with the Fig.2 embodiment. He accepted that the Skilled Team would use the Fig.2 sensor with one of the Fig. 12/13 needles, in the insertion gun of [0206] with spring-loaded insertion and retraction, aided by a mounting unit and a cover [T2/13518 - 1412]. He considered that all this was within the Skilled Team’s CGK, and they would have no difficulty implementing any of it.
369. Moving onto the Fig. 33 embodiment, he agreed that this was of a similar shape to the Fig. 2 sensor, but with an additional depth of a few millimetres, and with a protective housing (e.g. the encapsulating coating of [0231]) over the electronics. He agreed that the starting point for insertion and retraction would be to use the same spring mechanisms applicable to Fig.2. See T2/146:
25 Q. I think you agree, the skilled person, the starting point for
2 the skilled team, so far as an inserter for this device is
3 concerned, would be something like the same mechanism that had
4 been proposed in Figure 33, to insert the Figure 2 device;
5 right?
6 A. With regard to the insertion and retraction mechanisms, yes,
7 you can use the same insertion and retraction mechanisms and
8 springs, if you want to.
370. He also agreed that any changes to the size of the Fig.33 device were obvious to make [T2/14319 - 14713]. However, he suggested in XX that having the housing over the electronics would lead to difficulties in engaging the sensor with (and disengaging it from) the insertion needle.
372. With a housing built in this way, it would interfere with the insertion needle engaging with the ‘flagpole’. However, Dr Schoemaker was then asked to consider a smaller housing surrounding only the electronic components shown in Fig. 32, with some space around it, which he accepted would lack aesthetic appeal but would work (T2/155):
21 Q. I am sorry, if you leave enough space between the housing and
22 the edge of the substrate, you have space to push the needle
23 down, do you not?
24 A. Then you will have a very, very odd device. You will have a
25 device, the sensor substrate which is housed only partially
2 and on one side of the housing the sensor substrate sticks
3 out.
4 Q. Yes. That is something that could be made and there is no
5 reason why it would not work; right?
6 A. It will be a very strange design. It will be a partly housed
7 sensor and the skilled team would not be thinking that this is
8 a very attractive solution.
9 Q. You have housed the electronic components, which are what you
10 want to protect; right? That is all that really matters. You
11 have something that works; you agree?
12 A. From the insertion principle, it might work, yes, but it will
13 look, definitely it will look very odd if you have something
14 like that sticking out of your body. It will not be an
15 attractive product.
373. I am sure that the Skilled Team would have regard to the aesthetics of the sensor unit on the user’s body and would package the unit appropriately. In particular, there seems to be no reason why the Skilled Team would have to leave the flag type sensor sticking out of any housing or encapsulation of the sensor unit. This point did not seem to be pressed with Mr Varde. On adhesion, Dr Schoemaker accepted that the integrated unit could be kept in place simply by adhering the housing to the skin or using a plaster (T2/15723-1588). Or it could be used with the mounting unit. In this respect, Heller clearly explains in [0227] that the Fig.32 embodiment can be used with any of the methods for fixing described in relation to the Fig.2 embodiment, which includes the use of a mounting unit. Dr Schoemaker readily accepted that the Skilled Team could therefore use it if they wanted to [T2/15616 - 15917] -see e.g. T2/158:
18 Q. Yes. We are now talking about the Figure 32 embodiment that
19 we have discussed.
20 A. Okay.
21 Q. One way of implementing Heller with that configuration,
22 following the teaching of [0227], would be to apply it through
23 a mounting unit; correct?
24 A. Yes. That would be an option; yes, correct.
25 Q. The mounting unit which was adhered to the skin could have a
2 cover, as described in [0237] that we have already looked at;
3 yes?
4 A. Correct.
5 Q. Again, that would be an obvious way of following the teaching
6 of Heller, with this embodiment?
7 A. Yes, but that would, in my opinion, that would give away the
8 advantage of having an integrated device, because the nice
9 thing of having an integrated device is that you have fewer
10 parts. By adding a body mount to it is just adding another
11 part. So that gives away, in my opinion, that gives away the
12 benefits of having an integrated device.
13 Q. That is not the case, is it, if the mounting unit is mounted
14 on the inserter, which is Heller's suggestion? You have the
15 fully integrated sensor inserter mounting unit device which
16 Heller describes?
17 A. Yes, you can do that, yes.
374. As Dexcom submitted, both Mr Varde and Dr Schoemaker therefore arrived at the same place - a modified version of the Fig.33 device that would automatically insert the Fig.32 integrated sensor / transmitter unit with a housing. The unit could be adhered to the skin using the housing with or without using a mounting unit. It satisfied integers 1.7-1.10 of claim 1.
Step 6 - Remove the mounting unit.
375. This is not a necessary step but, as Dexcom submitted, it is obvious either way - either with or without the mounting unit.
Step 7 - Adjust the force to be applied to Figure 33.
376. Routine implementation.
Step 8 - Establish how to adhere the housed Figure 32 to the skin.
377. This is not a step at all. Heller clearly discloses the use of an adhesive patch.
Step 9 - Establish how to ensure a safe and reliable insertion without a mounting device
378. This is routine implementation.
Step 10 - Decide to implement automatic retraction in the new insertion device.
379. Again, an option disclosed in Heller and the choice of it was routine.
Step 11 - Decide to use an activation switch in the insertion device which is operatively coupled to the sensor and the sensor electronics
380. Dr Schoemaker said the Skilled Team would need to consider activation of the Fig.32 configuration. See for example T2/159:
18 Q. Can we talk about activation? It is common ground -- I think,
19 I think we discussed this yesterday -- that in any device
20 where you have integrated the sensor and the sensor controls
21 in a single unit which includes a battery, you have to have
22 some form of activation so that you can switch it on when you
23 are using it; yes?
24 A. Yes.
381. Electronic switches that activated electronics upon insertion of the sensor were already known as part of the common general knowledge from the Guardian Realtime CGM device (see paragraph 73 above), and I proceed on the basis that, as was assumed by Dr Schoemaker, that ‘activation switch’ includes any method of activating the circuitry (T1/1254-21).
382. Dexcom submitted that Heller actually teaches two methods of activating the battery out of a sleep mode in [0225] - the first involves detecting for a change of resistance between two contacts, and the second involves a physical switch in combination with a mounting unit. Dr Schoemaker accepted that both of those methods were obvious for use with the integrated Fig.32 embodiment [T2/15918 -1623]. So, in respect of the ‘change in resistance’ detection method, for example, there is this passage from T2/16118:
18 Q. Right. I mean, this may be outside your area of expertise
19 but, so far as an electronic engineer is concerned, he would
20 be aware, if discussing the Figure 32 device, that all he
21 needs is something in the circuit that can respond to a change
22 in resistance in the circuit; yes?
23 A. I agree with that; I just wanted to point out that this is not
24 the teaching within Heller.
25 Q. All right. But, it would be an obvious way of activating the
2 circuitry in the Figure 32 embodiment?
3 A. Yes.
383. Prof. Georgiou gave evidence that it was obvious to adopt both methods of activation in Heller, and also that a simple ‘pull tab’ could be used [Georgiou 1 ¶¶8.10-8.12]. I agree that this evidence was not disturbed under cross-examination: see [T3/40924 - 41219] and [T3/41720 - 42011]. It was suggested to Prof. Georgiou that a specific battery solution taught in [0233] rendered the need for an activation switch obsolete in the Fig.32 embodiment, but Prof. Georgiou dismissed this suggestion: see, e.g. T3/419:
15 Q. Look at paragraph [0233], this is the solution of the user
16 attaching the re-useable battery and that is how Heller
17 teaches power in the Figure 32 device; yes?
18 A. Yes.
19 Q. That does not involve an activation switch, as we have
20 discussed.
21 A. No, but you need an activation mechanism because otherwise the
22 device would lose power.
23 Q. But Heller does not link the sleep mode in [0225] with the
24 integrated device described later on where the battery is
25 referred to; yes?
2 A. No, but it would be an obvious thing to do, having read that,
3 and knowing at the time there was a need to conserve power in
4 these devices, also because of shelf life concerns.
5 Q. But you would need to devise an alternative sleep mode to the
6 one that is actually described in Heller, because you do not
7 have the insertion step as we have looked at in [0225];
8 correct?
9 A. Yes, but there are ways around it. I suggested one which
10 could be used to detect the change in resistance between two
11 electrical contacts.
Step 12 - Adding a cap.
384. I agree that this is a step. Heller describes packaging of the insertion kit in general terms, along with sterilisation, although it does describe a safety or barrier to prevent a cocked insertion spring being released (and the insertion needle being driven out) before it is intended to be used.
385. Once the sensor has been inserted using the insertion device/needle, Heller’s teaching is that the insertion device/needle is withdrawn. Little is said about it probably because the needle appears to be withdrawn into the insertion gun so that there is no sharp sticking out.
386. Although the sterilised state of the insertion device and sensor could be maintained by placing a film over the device, I consider that an equally obvious alternative would be to add a cap which would have the added advantage of preventing anyone being stuck with any inadvertent release of the insertion device/needle.
387. As regards the additional integers in claims 3, 4, 5 & 7, I did not understand Abbott to contend that any of these conferred inventiveness if I concluded that claims 1 and 2 were obvious. In any event, I find that it was obvious to have the cap attached prior to deployment (claim 3) i.e. attached during manufacture, and sterilised and packaged together (claim 4). Based on Heller, the integers in claims 5 and 7 were satisfied in any event.
Conclusions regarding Heller
388. I can now revert to the rival submissions regarding Heller. Stepping back from the detail, many of the arguments made by Abbott on obviousness as a matter of generality had little force when it came to Heller because, as Dexcom submitted, Heller disclosed not just the idea of an integrated device but considered details of how to implement and deliver such a device.
389. Similarly, Abbott’s accusations that the whole obviousness analysis was driven by hindsight and that Mr Varde was constantly in problem-solving mode have, in my judgment, very little force in relation to Heller. I take into account (a) Mr Varde’s view of [0029] in Fennell; (b) his analysis from that starting point to claim 1, (c) his analysis of Ethelfeld and, of course, (d) his approach to Heller but, in the light of the disclosure of Heller, I acquit Mr Varde of the use of hindsight. In my judgment he was properly focussed on how the unimaginative skilled mechanical engineer would implement what Heller disclosed.
390. In this regard, it is relevant that Abbott characterised any change from what was shown in a schematic (and literally interpreted) figure as driven by and indicative of hindsight. This was unrealistic. I acknowledge Abbott’s point (see [295] above, that hindsight can be a particular problem in mechanical cases. I also acknowledge Abbott’s point on the passage from Mr Varde’s cross-examination to which they drew particular attention at T2/2646-2654.
391. As appears from my analysis of the ‘steps’ which Abbott said were required to get from Heller to claim 1, most of them were not ‘steps’ at all, but were inevitable in any practical implementation of the Fig 32/Fig 33 teaching in Heller. In these circumstances, the differences between Heller and claim 1 were minimal and merely required some necessary but routine design implementation choices.
392. As regards the integers added in claims 2, 3, 4, 5 and 7, none appeared to confer any independent validity over Heller. Accordingly, I find claims 1, 2, 3, 4, 5 and 7 to have been obvious over Heller.
INSUFFICIENCY
393. At various points there were three insufficiency squeezes identified. They concerned (a) automatic insertion, (b) the activation switch and (c) sterilisation of a combined sensor and sensor electronics unit. (a) does not arise due to my decision on construction. So far as (b) and (c) are concerned, the squeezes have done their job, albeit largely because the Patent assumes that the Skilled Team has the ability to implement both from their CGK. In these circumstances, there is no reason to discuss these any further.
ADDED MATTER - claim 5
394. Abbott maintained that claim 5 was independently valid and infringed whilst acknowledging in opening that it is of limited value in the commercial dispute. I understood the significance of this attack to concern Abbott’s attempt to distinguish over Ethelfeld. Since I have rejected the obviousness attack based on Ethelfeld, but also found that claim 5 is invalid over Heller, I propose to deal with this point briefly.
395. The relevant legal principles (from Bonzel) are not in dispute.
396. Dexcom’s argument is that claim 5 discloses that the introducer needle engages with and disengages from the sensor electronics assembly but there is no such disclosure in the Application as filed.
397. In closing, Abbott contended that the Application discloses that the introducer needle is engaged with or disengaged from the sensor electronics assembly in two ways, but Mr Mitcheson indicated he was content to rely on the first way he identified.
398. He pointed to the Fig 12 embodiment and [0140] and [0141] of the Application. Fig. 12 of the Application is identical to Fig.12 in the Patent which I set out above at [125]. [0140] and [0141] in the Application are identical to [0119] and [0120] in the Patent, which I set out at [127] above.
399. In their closing, Dexcom contended there is nothing in [0140] or [0141] about the relationship between the retracted position of the needle and its engagement or disengagement from the sensor electronics assembly. Further, Dexcom submitted that the Application does not even teach that the needle is ever engaged with the sensor electronics assembly save to the specific extent that [0216] discloses that it is engaged with and disengaged from the sensor. Dexcom say that the only relationship taught in [0140]-[0141] is between the retracted position of the needle and its position within the insertion device housing. It says since the sensor electronics assembly is also within the insertion device housing at this point, this establishes nothing.
400. In my judgment, these paragraphs in the Application, read together with Fig. 12, clearly disclose that:
i) First, the sensor electronics assembly is engaged with the introducer needle and remains so during the movement from the first position to the second position with the introducer needle and the sensor electronics assembly moving together in the same direction.
ii) Second, the introducer needle disengages from the sensor electronics assembly (which is left on the user’s skin) during the retraction of the introducer needle from the second position to the retracted position, and therefore is clearly configured to do so.
401. It seems to me that Dexcom’s argument relies on the selective quotation from [0140] and [0141] set out in their Closing Skeleton but also requires one to ignore what is shown in Fig.12. I reject the added matter attack.
CONCLUSION
402. For all the reasons set out above, I find:
i) The Dexcom G7 does not infringe EP044.
ii) EP044 is invalid for obviousness over Heller, but not invalid over Ethelfeld or Fennell.
iii) The added matter attack on claim 5 fails.
Postscript
403. I must apologise to the parties for the long delay in delivering this judgment, much of which was caused by my heavy involvement in the case management of various actions involving Dr Craig Wright and the trial in COPA v Wright. It is not a situation which I will allow to arise again.