Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales High Court (Patents Court) Decisions
You are here: BAILII >> Databases >> England and Wales High Court (Patents Court) Decisions >> Fisher & Paykel Healthcare Ltd v Flexicare Medical Lt & Anor [2020] EWHC 3282 (Pat) (08 December 2020)
URL: http://www.bailii.org/ew/cases/EWHC/Patents/2020/3282.html
Cite as: [2020] EWHC 3282 (Pat)
[New search] [Printable PDF version] [Help]
Neutral Citation Number: [2020] EWHC 3282 (Pat)
Case No: HP-2019-000016
IN THE HIGH COURT OF JUSTICE
BUSINESS AND PROPERTY COURTS OF ENGLAND AND WALES
Royal Courts of Justice
Strand, London, WC2A 2LL
Date: 08/12/2020
Before :
MR JUSTICE MEADE
- - - - - - - - - - - - - - - - - - - - -
Between :
|
|
FISHER & PAYKEL HEALTHCARE LIMITED |
Claimant |
|
|
- and - |
|
|
|
(1) FLEXICARE MEDICAL LIMITED (2) FLEXICARE (GROUP) LIMITED |
Defendants |
- - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - -
Benet Brandreth QC and David Ivison (instructed by Bird & Bird LLP) for the Claimant
Charlotte May QC and Tom Alkin (instructed by DAC Beachcroft LLP) for the Defendants
Hearing dates: November 3, 4 and 9 2020
- - - - - - - - - - - - - - - - - - - - -
Judgment Approved
Covid 19 Protocol: This judgment is to be handed down by the judge remotely by circulation to the parties' representatives by email and release to BAILII. The date for hand-down is deemed to be 8th December, 2020.
Mr Justice Meade:
The common general knowledge. 10
A typical breathing circuit 11
Knowledge of breathable materials. 17
Disputed and potentially disputed issues of common general knowledge. 17
Safety issues and the standards. 18
Issues of Claim interpretation. 20
Location of the breathable material 20
"Singular exhalation flow passage". 21
"Drying the humidified gases". 22
Lack of technical contribution and insufficiency. 36
Introduction
1. In this action, the Claimant ("F&P") sues for infringement of European Patent (UK) 2 025 359 B1 ("the Patent").
2. The Patent concerns the expiratory limbs of breathing circuits, and in particular the use of breathable materials to reduce or eliminate condensation.
3. The Defendants (together, "Flexicare") have admitted that their products fall within the Patent's claims but say that the Patent is invalid and counterclaim for its revocation.
4. Mr Benet Brandreth QC appeared for F&P with Mr David Ivison and Ms Charlotte May QC appeared for Flexicare with Mr Tom Alkin. I am grateful for their submissions and for the summary of agreed common general knowledge ("CGK") provided after the oral hearing, to which I refer below.
5. Owing to the COVID pandemic the trial was conducted entirely remotely. This worked well and there were no significant difficulties. I am grateful for the support I received from the parties in terms of providing bundles and the like.
6. The pandemic also meant that the Claimant's witness Mr Pierro could not travel from California. This was accommodated by conducting the oral evidence starting at 2pm each day and continuing until 6.30 or 7.30 pm. The parties and witnesses are to be commended for their flexibility, but special thanks are due to the Court staff for dealing with this on top of everything else.
The issues
7. The issues for me to decide are as follows:
i) Whether the Patent is anticipated by European Application 0 535 379 A1("Psaros"). This depends on claim scope and proper interpretation of the disclosure of Psaros, there being no dispute about the facts.
ii) Whether the Patent is obvious over:
a) Psaros; or
b) International Patent Application WO 88/01903 A1 ("Kertzman"); or
c) Japanese Patent Application 2000-24111 ("Inoue").
iii) Whether the Patent is obvious for lack of a technical contribution or insufficient for lack of enablement. These were run as squeezes and as I will explain below they did their job and thereupon faded away. I will address them only briefly below, therefore.
8. The issues are to be determined by reference to unconditionally amended claims, in which the feature of dependent claim 3 of the granted claims was added into claim 1. There is no opposition to the amendment other than that it does not cure the alleged invalidity.
The witnesses
9. The parties each submitted expert reports from two witnesses, a product design engineer and a materials scientist. The materials scientists were mainly concerned with infringement and the significance of their evidence faded away so that in the end they were not cross-examined. So I heard oral evidence from Mr Brian Pierro for F&P and Dr Paul Dixon for Flexicare. Somewhat unusually those two gentlemen work for the same company called Vyaire, although nothing turned on this.
10. There was no attack of significance on the qualifications of either witness. Minor points were made in relation to Dr Dixon's experience which I deal with below but which were not material in my view.
Mr Pierro
11. Flexicare limited its comments on Mr Pierro to the observation that his views on obviousness were influenced too much by his perception of the technical challenges that would have to be overcome to construct an expiratory limb, pointing out that he made frequent references to them even though he accepted that they could in fact be overcome by routine means in the light of the CGK.
12. This was, rightly, not made as a personal criticism of Mr Pierro, who I thought was a very good and fair witness. It is however a relevant point as directed against the nature of F&P's case as it developed. I return to this in relation to Flexicare's obviousness/insufficiency squeeze below. For reasons given there, the point has some force and I have taken it into account, both generally and in assessing Mr Pierro's written and oral evidence.
Dr Dixon
13. There were, by contrast, significant problems with Dr Dixon as a witness.
14. As to his qualifications and experience, F&P submitted that he was not a good approximation to the notional addressee because his degree was in mathematics and because he only worked on medical devices for a year before the priority date.
15. These were not important issues. In this field there was no one undergraduate discipline that was universal or necessary, and Dr Dixon's pre- and post-priority date experience gave him the necessary knowledge of medical devices.
16. The difficulty, rather, was the way that Dr Dixon let in hindsight in the preparation of his written evidence and in his oral evidence.
17. I will say straight away that I would reject any assertion that Dr Dixon lacked honesty, and I do not understand any to have been made. He was direct and open in his oral evidence and in general accepted the points put to him about the preparation of his reports. However, I do think that the way his evidence was put together and a general lack of care and thought about how he should avoid hindsight means that his views on obviousness have to be treated with a good deal of scepticism. I will explain why.
18. After the nature of the likely attack on Dr Dixon became apparent, I asked the parties to provide submissions in their closing submissions about the right legal approach. They did so, and there was for practical purposes complete agreement. They both referred to the judgment of Arnold J as he then was in MedImmune v. Novartis [2011] EWHC 1669 (Pat) at [99] to [114]. In addition, F&P referred to Akebia Therapeutics v. Fibrogen [2020] EWHC 866 (Pat) at [36], a decision of Arnold LJ sitting as a trial judge, and Flexicare referred to HTC v. Gemalto [2013] EWHC Pat at [273] where Birss J commented on MedImmune.
19. As can be seen from those judgments, in recent years there has been a heightened focus on the way in which expert evidence in patent cases is prepared and especially the sequence in which experts are shown documents. The main driver behind this is that if experts are shown the patent in suit before they have said what the CGK is or, more importantly, before they have said what they consider to be obvious over the prior art, then there is a risk of hindsight.
20. It is therefore preferable for experts to be asked about the CGK, then shown the prior art, and only then shown the patent in suit (MedImmune). But this is a counsel of perfection since the expert may well already know what the invention of the patent in suit is, for example from the patentee's commercial embodiment of it (HTC, Akebia).
21. Where the expert already knows the invention there may yet be value in sequencing the documents that he or she reviews to focus the mind on avoiding hindsight, but the opportunity to give a completely untainted view of the prior art does not exist; the expert has to discipline themself carefully to avoid hindsight. If they do so well then there is no reason why they cannot give cogent evidence on obviousness, but in such a situation I think it must be important for the expert to identify how they knew about the invention and when, and to reflect carefully on how that might influence them. This is really where the problem with Dr Dixon arose and it is an issue of the assessment of his evidence, not a disagreement between the parties on the applicable principles.
22. The sequencing of an expert's involvement can cause other practical problems. It is not usually sensible to ask an expert "what was the common general knowledge in your field?" without some guidance, as it will lead to far more information being given than is relevant (Akebia). So it was sensible and really inevitable to ask Dr Dixon to address himself to expiratory limbs and to rainout when he was originally asked about the case. But this led to a criticism that he had, in his reports, talked about the CGK of the skilled team as identified by reference to the Patent, when in fact he was not supposed to have seen the Patent at that stage of the preparation of his evidence. It turned out that his report was miswritten in this respect, for which he apologised, but in fact I do not think that this was an area in which he was at fault. He had to be told at least in general terms what the topic was so as to get his views, and of course in telling him that the solicitors had to have in mind what the Patent was about. That is just a practical reality.
23. Where Dr Dixon did fall short, however, was in relation to his prior knowledge of the Patent and the alleged invention. He did, rightly, acknowledge in his first report that he had seen the Patent before, probably in 2012 when he was working on humidity management in breathing circuits, and he said that he only remembered this when shown the Patent after he saw the prior art.
24. However, his exposure to the Patent's alleged invention was considerably more extensive and long-standing than that, since he had seen F&P's Evaqua products from 2003 and knew the relevant features of them pertinent to this case. In addition, in his own patent filing in 2011 he had identified the use of breathable materials as a solution to rainout (in addition to water traps and heating), and, as he had to accept, this can only have been a reference to the F&P products. None of this was in his report in chief and it, and its significance, had to be drawn out of him in cross-examination. His explanations were not convincing and changed under pressure. For example, he said that when he saw the Patent in 2012 he thought it was simply all about manufacturing methods, but that did not make sense in the light of his own patent.
25. Overall, I thought that the mere reference in his report to having seen the Patent was inadequate because he had not thought about his previous knowledge of the alleged invention properly, and this lack of care meant that he had not really turned his mind to how it might have influenced his thinking on the issue of obviousness.
26. I thought there was significant hindsight in Dr Dixon's approach for two other reasons, which are related to each other and to what I say below about the skilled addressee and the prior art.
27. The first reason was that he came to each piece of prior art with an expectation that it might contain a solution to rainout in the expiratory limb of breathing circuits.
28. The second reason was that he thought the skilled addressee would reach exactly the same end point (a device according to claim 1) from each of the prior art citations by obvious steps despite their being, as he accepted, very different devices for very different purposes. I asked Counsel for Flexicare in closing to consider this and her response was that the reasons for going from each piece of prior art toward the alleged invention, and the steps to be taken, were very different in each case. I did not find this a satisfying explanation and if anything it reinforced the concern.
29. Finally, and separately from hindsight, it is well known that what really matters with expert witnesses in patent cases is not their conclusions but their reasons: See Technip France SA's Patent [2004] RPC 46 at [12]-[16]. Dr Dixon's evidence was very thin on each piece of prior art in relation to the reasons for the notional skilled addressee getting to the idea of claim 1. Again, this fortified my concern about Dr Dixon reaching the same goal from very different starting points: had he fully articulated different and independent reasons for each citation that would have been one thing, but he simply did not.
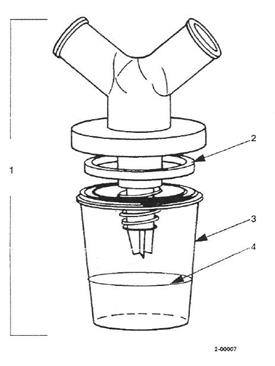
The Skilled Addressee
30. There was no dispute about the basic principles that the notional skilled addressee is a person or team with a practical interest in putting the invention into effect.
31. The parties agreed that the skilled addressee would be a team consisting of a design engineer and a materials scientist. Although it is often misleading or even wrong to ask which member of the skilled team would be the "leader", in the circumstances of the present case the parties were right to identify that most of the work would be done by the design engineer, and the materials scientist would be called upon for certain matters of detail relating to the selection of the precise characteristics of the materials to be used. Since none of the issues at trial turned on this detailed input, there was no oral evidence about it.
32. Since the materials scientist faded in this way, I will refer to the Skilled Design Engineer using the abbreviation "SDE" when I mean the notional skilled addressee. This is what Flexicare did, but I make it clear that my adopting of the abbreviation is purely for convenience, without implying any decision either way about the disputed aspects of the attributes of the skilled addressee, and without ignoring the fact that the materials scientist would be standing by if needed. I also refer below to the "skilled addressee" when talking about the concept generally.
33. The parties also agreed that the SDE would be working in the field of breathing circuits, or similar medical devices. He or she would have a relevant degree or degrees and practical experience thereafter. There were some minor issues about the length of experience of the notional product designer which I found unimportant. I also mention as a minor point that Dr Dixon said in relation to Kertzman that the SDE might know what process drying was because some such engineers might in real life have experience of chemical engineering. That was a confusion between the characteristics of just some real-life engineers and the notional SDE who would not know about chemical engineering in that way.
34. There are two issues that I think I must address in relation to the skilled addressee. The issues relate not to their notional qualifications but to the proper legal approach.
35. One of the things the SDE would know about would be expiratory limbs of breathing circuits, and one of the problems with them was rainout, as I explain below in relation to CGK. Building on this, many of Counsel for Flexicare's questions in cross-examining Mr Pierro were along the lines that the SDE was a person working on the expiratory limb of breathing circuits and wanting to find a different and better solution to rainout than those that existed already; she pointed to paragraph 18 of his first report in this respect.
36. I felt that this tended to inject into the questioning on each prior art citation the unstated premises that the SDE would, in coming to the document, have most specifically that thing (the expiratory limb) and that problem (rainout) in mind, along with the assumption of abandoning the existing solutions and the possibility that an alternative and/or better solution existed and might be found in the document.
37. This state of mind cannot just be taken for granted, since although the skilled addressee is deemed to read the prior art with care and interest (see the cases cited in Terrell, 19th Ed at 12-103 to 12-105), he or she does not have any expectation in advance of doing so that it will be useful, either generally or for something specific. I asked the parties to provide me with legal submissions on this point and they both referred to the following passage in the judgment of Laddie J in Inhale v. Quadrant [2002] R.P.C. 21 at [47], cited in Terrell at 12-106:
"[The skilled person] comes to the prior art without any preconceptions and, in particular, without any expectation that it offers him a solution to any problem he has in mind. Some pieces of prior art will be much more interesting than others. A document directed at solving the particular problem at issue will be seized upon by the skilled addressee. Its very contents may suggest that it is a worthwhile starting point for further development. But the same may not be the case where a document comes, say, from a distant and unrelated field. For example, in theory a notional skilled person engaged in trying to improve the operation of an internal combustion engine is assumed to know, have read and assimilated the contents of all published material including those, say, in the baking field. It may be that a document in the latter field discloses something which, if applied to the internal combustion art, would produce a marked improvement in performance. However, the person skilled in the art is not deemed to read the baking document in the knowledge, or even with a suspicion, that it is of significance to the problems he has to deal with. It may be that it is written in such a way that, although he understands it, the skilled person will dismiss it as irrelevant to his work. The more distant a prior art document is from the field of technology covered by the patent, the greater the chance that an intelligent but uninventive person skilled in the art will fail to make the jump to the solution found by the patentee."
38. This passage and this principle in it are more often referred to in relation to obviousness than in relation to the skilled addressee, and I have borne them in mind in that context as well. I mention the principle now because I thought there was a risk of it being broken, and hindsight coming in, when Counsel for Flexicare first defined the SDE in narrow terms during her cross-examination of Mr Pierro. I also mention that Counsel for Flexicare also cited HTC v. Gemalto (supra, at [267]) on this issue, but I do not think it establishes any different principle, although it is an instance where on the facts no hindsight arose from defining the CGK.
39. As an example of the issue (and I return to this below), Kertzman is not about expiratory limbs of breathing circuits but about much smaller gas sampling lines used for different purposes, and it may not be legitimate merely by defining the SDE narrowly as a person specifically interested in better solving rainout in expiratory limbs to create a notional expectation of being able to use Kertzman in that way, if none would arise in reality. Nor can one legitimately frame the skilled addressee so as to box them in to abandoning existing solutions to a known problem. It may well be a part of the obviousness assessment that the skilled addressee would want to, or would consider, improving existing solutions (in this case, heaters and water traps).
40. This approach was, in my view, symptomatic of the hindsight in Flexicare's obviousness case as a whole as I explain further below.
41. I note that paragraph 18 of Mr Pierro's report in the part relied on by Counsel for Flexicare during cross-examination and in Flexicare's written closing submissions is directed specifically to his view of the SDE after seeing the Patent. He expressed a broader notion earlier in the same paragraph as to the SDE in general.
42. The second general issue relates to the ability of the skilled addressee to implement the Patent, and treating it consistently with their expectation of success in considering implementation of the prior art.
43. Flexicare anticipated that it might be said by F&P that the SDE would be concerned that there was not a reasonable expectation of implementing the prior art, or that the expectation was low enough to deter attempting it, and so work against obviousness. I can see why Flexicare was concerned about that, as much of Mr Pierro's evidence was directed to perceived difficulties in the detailed implementation of the prior art.
44. As a general matter, it is often possible to deduce the attributes which the skilled addressee must possess from the assumptions that the patent in suit makes about his abilities (Horne v. Reliance [2000] FSR 90 at [11]). So, for example, the fact that the Patent expects its skilled addressee to be able to make a co-extruded tube with breathable material with very little guidance implies that the skilled addressee could undertake the same or a similar task starting from the prior art.
45. More concretely, Flexicare ran a squeeze that if the expectation of success from the prior art would be absent or too low then the Patent was insufficient because it provided no more teaching than the prior art, and argued in relation to inventive step that it was not legitimate for F&P to rely on a perceived problem in implementing the prior art (a "lion in the path") unless the Patent showed how to overcome it. Flexicare cited the dictum of Floyd LJ in Koninklijke Philips NV v Asustek Computer Corp [2019] EWCA Civ 2230 at [73]:
"The principle is that you cannot have a patent for doing something which the skilled person would regard as old or obvious but difficult or impossible to do, if it remains equally difficult or impossible to do when you have read the patent. To put it another way, the perceived problem must be solved by the patent."
46. I accept this statement of principle and its potential application to the present case.
47. In response, F&P said that it was not relying on perceived problems of implementing the prior art or some modification of it, but rather was only saying that what the Patent involved was unusual and would be unfamiliar to the SDE; while it could be made with confidence using CGK if the idea occurred to the SDE, the unfamiliarity meant that it would not be prone to occur to him or her in the first place. Counsel for F&P cited Lord Justice Jacob in Unilever v Chefaro [1994] R.P.C. 567 at 587: that (paraphrasing) it is not so much that the challenges would put people off trying, but rather in the absence of firm knowledge and experience, the conception of modifying the prior art as claimed would not come readily to mind. I accept this principle as well and think it is relevant. The SDE would not be familiar with breathable tubes of the dimensions and physical characteristics (e.g. resistance to crushing, ability to "drape") required for the claimed products of the Patent.
48. Counsel for F&P's retreat on this (for such it was) meant that F&P did not rely on those parts of Mr Pierro's evidence relying purely on perceived problems of implementation. There were quite a lot of these, and on important issues as well, such as obviousness over Kertzman. I have borne this in mind, along with Counsel for Flexicare's very reasonable point that one must assess Mr Pierro's oral evidence to understand whether at any given point he was considering a perceived problem (no longer relied on by F&P) or only unfamiliarity with the type of work required (maintained by F&P). It would be wrong to casually treat the one as the other, and I have aimed to avoid doing so.
49. The upshot is that Flexicare's squeeze did its job but I must take care in assessing F&P's evidence carefully to exclude the matters no longer relied on.
The common general knowledge
50. There was no dispute about the relevant legal standard: CGK is that which is generally known and generally regarded as a good basis for further action by the bulk of those in the particular art.
Agreed CGK
51. In the present case there was no primer and nor was there anything in the nature of a textbook that the parties could direct the Court to for its pre-reading as a source of agreed CGK. At the same time, the parties' respective opening skeleton arguments contained statements that there was much agreement about the CGK , but they had not got together to provide a joint list or a summary for the Court of what was agreed and what was not.
52. At my request the parties conferred during the evidence and during the time allowed for writing closings and provided a joint summary of the CGK. This was extremely useful and has saved a lot of time in the preparation of this judgment. I am very grateful.
53. Because this was a relatively simple case the absence of a primer, textbook or agreed CGK summary during my reading and the oral evidence was not too much of a problem (although I was in an unnecessary state of uncertainty about some details when the evidence began) but in a more complex case it would have been. It is to be hoped parties will ensure that at least one of these is available by trial in future cases.
54. The rest of this section on agreed CGK is taken, with only very slight editing and some small deletions, directly from the parties' agreed CGK document. I have also tried to render it all into the present tense: the parties' document used almost entirely the present but also in some few instances the past (of which I make no criticism) and it was therefore easier when preparing this judgment to change to the present tense, but of course one must bear in mind that what is being referred to is CGK at the priority date.
Assisted breathing
55. Patients may require assistance with breathing for a number of reasons. This includes treatment of respiratory distress, respiratory care where the patient is incapable of natural breathing and assistance with breathing while under anaesthesia.
A typical breathing circuit
56. A typical breathing circuit is shown below with `V' being the breathing machine, `1' being the inspiratory limb, `2' the expiratory limb and `3' the connector with the patient interface:
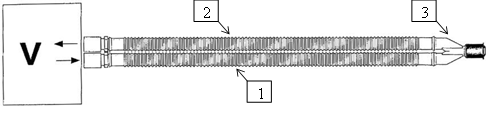
57. The system shown in the above is a "dual limb" breathing system. In it, gas (which may simply be air, or may for example incorporate anaesthetic agents) is:
i) pneumatically pumped from the breathing machine into the patient's lungs (so-called "inspiratory flow"); and then
ii) exhaled by the patient and returned to the breathing machine (so-called "expiratory flow").
58. The ventilator works by pumping air (sometimes with supplemental oxygen or anaesthetic gases) into a patient's lungs under pressure controlled by pressure valves within the ventilator.
59. Most lung ventilators for hospital use have flow meters/sensors and pressure sensors to measure the flow rate and pressure, respectively, of the breathing gas being provided to the patient and to detect if there is a problem, as well as an integrity alarm which would sound if the breathing circuit were occluded or leaking (situations which can have serious consequences for the patient).
60. Gas sampling lines are utilised in respiratory or anaesthesia applications, where they are used to analyse the composition of the patient's exhaled gas, particularly its CO2 content. These measurements are commonly performed by a side stream capnometer (a device that numerically calculates and displays CO2 content) that extracts a small amount of air from the expiratory limb of the breathing circuit (typically at a rate of approximately 1ml per second). Gas sampling lines have a narrow diameter in order to reduce the overall volume of the line, allowing the gas to be rapidly delivered to the capnometer.
61. Filters are also commonly placed at the end of the expiratory limb of a ventilator to trap small airborne particles, including viruses and bacteria, as the air passes through.
Breathing tubes
62. Breathing tubes need to be flexible and lightweight to allow for optimal positioning around the patient while avoiding kinking and the tube dragging on the airway device. The tubing should also be capable of resisting crushing, and the tube should have low compliance to maximise the tidal volume.
63. In the United Kingdom, the construction and performance of breathing tubes is governed by a number of standards. A tube is difficult to commercialise unless it complies with these standards. Chief among these in the UK was European Standard BS EN 12342:1998. This sets standards for:
i) A breathing tube's resistance to flow.
ii) The `compliance' of breathing tubes. When the pressure of gas in a tube is increased, more gas is required to fill the same volume (pneumatic compliance). If the increased pressure also causes the tube to distend, this causes a secondary increase in the amount of gas required to fill the vessel or tube (elastic compliance).
iii) The leakage of breathing tubes. Breathing tubes must not allow gases to leak beyond certain thresholds, which are set by reference to how the breathing circuit is supplied to the end user.
64. Broadly speaking, three types of tubing are used in breathing circuits:
i) Tubing with a smooth inner surface and smooth outer surface. Both experts describe this as `smoothbore', although Dr Dixon also uses the term to also describe the tubing with a smooth inner surface and helically-ribbed surface, which is described at iii) below.
ii) Corrugated tubing. Corrugated tubing can be bent more easily and will naturally resist crushing.
iii) `Reinforced' smoothbore or `spiral' tubing, which has a smooth inner surface and ribbing on the outside. Dr Dixon describes both this type and the tubing having a smooth inner and smooth outer surface as `smoothbore' tubing. Mr Pierro describes this type as `spiral' tubing in order to avoid confusion. This spiral ribbing adds reinforcement to the exterior of the tubing, allowing it to bend without kinking, while retaining the benefit of the smooth internal surface to allow for laminar flow, promoting low resistance to flow.
65. There were a variety of commonly-used constructions and associated manufacturing processes that could be used for making tubes.
66. Typically, the tubes carrying inspiratory flow and expiratory flow were separate ("dual-limb" or "biaxial"), as shown in the figure 2 above, but systems in which one tube is located inside the other ("coaxial") were also known and mainly for use in anaesthesia or for neonatal patients.
67. In a dual-limb circuit, the size of a circuit's inspiratory and expiratory limbs is determined by the intended patient and therapy. The limbs of a circuit for:
i) an adult circuit would normally have an inner diameter of approximately 22mm and, in respiratory care, a length of up to about 1.5-1.8 meters (5-6 ft) or longer for specific applications;
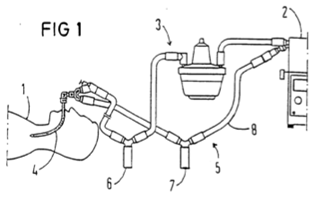
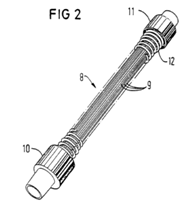
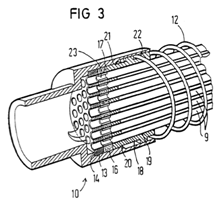
ii) a neonatal circuit, intended for use with a neonate (i.e. a newborn, typically in an incubator), would normally have an inner diameter of approximately 10mm, and, in respiratory care, a length of up to about 1.2-1.5 meters (4-5 ft) or longer for specific applications; and
iii) a paediatric circuit, intended for patients between neonatal and adult stages, would normally have an inner diameter of approximately 15mm, and, in respiratory care, a length of up to 1.5 meters (5 ft) or longer for specific applications.
Tube materials
68. A range of different polymers are used for the construction of breathing tubing. Common materials included polyethylene, polypropylene, and ethylene vinyl acetate (EVA).
Humidification
69. `Absolute humidity' is a measure of the mass of water vapour in each litre of air. The maximum absolute humidity of air increases with temperature.
70. `Relative humidity' is absolute humidity expressed as a percentage of the maximum absolute humidity for air at that temperature (i.e. 100% relative humidity means that the gas is fully saturated at that temperature).
71. The `dew point' for gas with a specified absolute humidity is the temperature at which the gas is at 100% relative humidity and, at which, any additional vapour must condense as liquid water.
72. Typically, the air in a hospital room has a relative humidity in the range of 30-40%. This corresponds to an absolute humidity of 6-8mg of water per litre. When a person breathes normally, air enters their upper airway through their nose or mouth, passes over the soft palate, past the epiglottis, through the vocal cords, down the trachea and into the lungs. The membranes in the upper airway have a very good blood supply and add moisture and heat to the air that has been inspired so that, by the time it reaches the bronchi, the air is at 37°C and 100% relative humidity (corresponding to 44 mg of water per litre). This is also the equilibrium state for the air in the lungs, as this air is in close contact with the wet surface of the lung tissues. As the air is expired, the process is reversed so that moisture is redeposited into the soft tissue in the upper airway and so, when air is exhaled by a person breathing normally, it is at a somewhat lower temperature of typically 33-35°C, but with 100% relative humidity at that temperature. This results in the absolute humidity being reduced from 44mg to around 35-39 mg per litre.
73. When a patient is on a ventilator, they will often have an endotracheal tube or a laryngeal mask inserted. An endotracheal tube, commonly used in emergency and intensive care ventilation scenarios, is placed through the vocal cords, with the tip in the trachea. A laryngeal mask is placed into the larynx and incorporates a cuff which seals to the sides of the airway adjacent to the vocal cords. In both cases, the passages in the nose and most of the upper airway are bypassed, and the heating and humidification for inhaled air which occurs in normal breathing no longer takes place.
74. Further, in a hospital, air and oxygen are commonly supplied from central gas supplies. Gas in the air pipeline is often quite cold (as pipelines run in unheated parts of the hospital campus) and has a relative humidity of less than 1%. Oxygen supplied in hospitals is even drier. It has a relative humidity of a fraction of 1%.
75. Several different methods have been employed to improve the humidification and warming of air so that it reaches a ventilated patient at approximately the same temperature and humidity as it would do if the air had passed through the patient's own upper airway.
76. One such method is active humidification. Use of active humidification is standard practice in paediatric care and common in adult care within the hospital environment. The method works by feeding the gas flowing along the inspiratory limb of a breathing circuit through a humidification chamber. This is a vessel containing continuously heated water. As the air passes through the humidification chamber, it is warmed and humidified. The temperature in the humidification chamber can be adjusted to control the amount of humidification and also the temperature of the air.
77. In order to reduce the cooling of the humidified air as it passes from the humidifier to the patient, it is common to use a loop of electrical heating wire along the inside of the inspiratory limb. Power to the heating wire is typically supplied by the humidifier. Probes monitor the temperature of the gas being delivered to the patient and the temperature in the humidifier, allowing control of the power to the humidifier so that the air reaching the patient can be kept at approximately 37°C.
78. An alternative to active humidification is to use a heat and moisture exchanger ("HME"). In an HME, both inspired and expired air passes through a chamber with a hydrophilic foam or mesh material. As air is expired and passes through the HME, moisture and heat are left behind in the mesh. As the air to be inspired then passes through in the opposite direction, some part of this moisture and heat is picked up by the incoming air. An HME therefore works a little bit like the upper airway in normal breathing.
79. HMEs do not create heat or moisture, both of which are created and supplied by the patient. Instead they act as a "loss-limiter", slowing the rate at which a patient's lungs are dried. As a result, they are most commonly used with patients undergoing operations and for short term immediate post-operative recovery in adults. HMEs are not suitable for neonatal use because they contain quite a large volume of air relative to the size of an infant's lungs. As a result, an infant might end up rebreathing expired air contained within the HME.
Condensation or `rainout'
80. One problem that arises in assisted breathing is condensation in the breathing circuit. In active humidification, warm, humidified air coming out of the humidifier will typically cool as it passes along the inspiratory limb. Warm exhaled air with a relative humidity of 100% will typically cool as it passes along the expiratory limb. As soon as it cools below its dew point, water will condense out. In respiratory therapy, such condensation is known as `rainout'.
81. Rainout causes a number of well-known problems in a breathing circuit. The most immediate problem is that water will tend to collect at the lowest point in each of the limbs. This reduces the internal cross-sectional area of the breathing tube, which can affect the pressure of air moving through the circuit. In extreme circumstances, the pooled water can completely occlude the breathing tube.
82. Other problems associated with rainout are the possibility of a patient inspiring liquid water, and the possibility of liquid water entering the filters, sensors and valves within the ventilator, impairing their function.
83. If a patient should inhale even a relatively small amount of liquid water, this can damage their lungs. Where a patient already has impaired lung function, any further reduction in lung function because of liquid water within the lungs is a serious problem.
84. If water is allowed to condense on a particulate filter at the end of the expiratory limb, it can block the filter and make it harder for the patient to exhale. Liquid water within the filters and valves of the ventilator can also impair the ventilator's ability to control the pressure and flow provided to the patient's lungs.
85. High humidity levels in the sampled breathing gases and/or liquid water could damage the CO2 monitoring device.
86. Where water is present in the gas sample being measured for CO2, this creates an error in the carbon dioxide measurement. This latter problem is commonly solved either by the capnometer heating the air flowing to the sensor or by using Nafion tubing on the side stream gas sampling tube to remove water vapour from the gas before it reaches the carbon dioxide sensor.
87. Prolonged presence of water within a breathing circuit can also act as a site for moulds or bacteria to grow, which may then be re-inhaled by the patient and damage their lungs. In the case of a patient with an infectious disease, liquid water within the breathing circuit can act as a reservoir for exhaled bacteria or viruses and, if this water is released into the environment, it can create an infection risk for staff or other patients nearby.
88. It is possible to manage rainout by the use of water traps in the inspiratory and expiratory limbs of breathing circuits.
89. An example water trap is depicted below.
90. Although water traps are good at removing water that has condensed and drained into them, they do not remove condensed water that has collected elsewhere in the circuit. They also do not reduce the relative humidity of the gas and so do not eliminate the possibility of residual water vapour condensing downstream in filters, flow sensors or valves in the ventilator. A further problem is that the rainout may carry pathogens, hence the liquid collected from water traps has to be treated as hazardous waste. In addition, any operation that opens a connection to the breathing circuit carries the risk of introducing pathogens into the circuit.
91. In respiratory care applications, it is common to heat the inspiratory limb of the breathing circuit in order to try to maintain the temperature within the tube. Where the inspiratory limb is heated, the expiratory limb may also be heated. As explained above, the inspiratory limb is heated to optimise the humidity and temperature of gas being delivered to the patient.
92. However, heating wires are not a complete solution to the problem of rainout. The resulting heating can be uneven and heating the expiratory limb tends to shift the formation of condensation downstream to the ventilator. This is where the sensors and valves tend to be located, meaning that problems caused by water condensing in these components remain.
Knowledge of breathable materials
93. There are selectively water permeable polymers. An example of such a selectively water permeable polymer that the SDE would be aware of the use of in drying tubes is Nafion.
94. Such selectively water permeable polymers were used to dry expired gases in a gas sampling tube for monitoring patient CO2 levels as part of a respiratory breathing circuit, since at least 1994.
Disputed and potentially disputed issues of common general knowledge
95. There were three issues of potential or actual dispute and they seem to me to be of minor significance if any.
Expiratory flow rates
96. It was agreed between the experts that expiratory flow rates vary across patient populations, for example being higher for adults than infants. They can reach 120 litres/minute, but that is a worst case, peak rate. Average rates are a lot lower, perhaps 5-10 litres/minute.
97. Dr Dixon explained that it is the average rate that matters for calculating drying requirements. This makes obvious sense and in the event that it was disputed (I am not sure that it was) I accept his evidence and Flexicare's argument to that effect.
98. I accept that the peak rate would be a relevant consideration for choosing a breathing tube material and dimensions, but so far as it matters I accept Dr Dixon's evidence that it had a relatively modest effect on the actual pressure in the expiratory limb.
99. The relevance of this point was, I think, in relation to an alleged perceived difficulty of making a breathing tube which, in addition to all the other requirements, could withstand peak flow. Since F&P retreated from that kind of argument, the relevance dwindled, although it was still relied on in closing in relation to obviousness over Inoue.
Process drying
100. Kertzman refers to process drying, as I explain below. Dr Dixon suggested that the SDE would have some understanding from his or her CGK of chemical engineering to interpret this. I have dealt with this above in relation to Dr Dixon's evidence. It was not CGK. I do not think Flexicare relied on it in any event and it did not form any part of its closing submissions.
Safety issues and the standards
101. F&P made submissions in closing about the interaction between safety issues, the published standards, and the construction of breathing tubes. These are all related: resistance to kink or crushing was important for breathing tube design because it would be relevant to providing a safe product, and the purpose of the standards was to ensure products were safe. This would be CGK, as would the relevant safety considerations and standards. F&P's closing submissions in this regard were written in case there was a dispute, but I do not perceive that there was, in the end.
The patent
102. The Patent is entitled "Components for breathing circuits".
103. The priority date (entitlement to which is not challenged) is 10 May 2000. The filing date is 9 May 2001.
104. The Patent is a divisional and this explains, at least partially, why it was not granted until 2013. It was unsuccessfully opposed by parties other than Flexicare on the basis of prior art including that cited in this action, although, for example, Inoue was mentioned but not really pursued.
105. The evidence and arguments before me are different from those considered by the EPO and so I get no real assistance from the Opposition Division's decision, which I was only shown because I asked for it, although I note that the Division seems to have assumed the same interpretation of the disputed features of claim 1 that I have.
106. Consistent with its being a divisional, there are parts of the Patent's specification which are not covered by the claims. For example, the catheter mount of figure 13 is not the subject of any specific claims and the coaxial arrangement of figures 4 and 5 is outside the claims, a point to which I will return below since that embodiment is for practical purposes identical to the Inoue prior art. As often happens, the specification has not been well tidied up in these respects so that, for example, [0016] refers to figures 4 and 5 as "a further aspect of the present invention". I do not think anything turns on this, however.
107. The Patent contains a short explanation of the problems of rainout and the known solutions of heaters and water traps, at [0002].
108. There are some simple figures of, for example, how to co-extrude the necessary breathable tubes and their overall configuration at a high level (e.g. figures 1 and 3), and further figures going into a lot more detail of how to make appropriate breathable tubes by winding multiple strips of material onto a former (e.g. figures 7 to 12).
109. The Patent contains a statement of what it is said to achieve at [0003], as follows:
"[0003] It is an object of the present invention to provide a component, with particular application to the expiratory limb of a breathing circuit, which will go at least some way towards improving on the above or which will at least provide the public and the medical profession with a useful choice".
110. "The above" is the problem of rainout in [0002] to which I have already referred. Thus the Patent does not claim that it has enabled doing away with water traps or heaters in all instances. Also, [0057] refers to the possibility of using a heater with the breathing tube disclosed.
111. The Patent recognises as prior art, but does not pass comment on, Psaros, at [0008].
Claims in issue
112. Claim 1, broken into integers, is as follows (integer 1G is the feature brought into claim 1 from granted claim 3 by F&P's amendment):
|
Integer |
Claim Wording |
|
1A |
A flexible breathing tube |
|
1B |
which is an expiratory limb of a breathing circuit, and |
|
1C |
is adapted to be located between a patient and a ventilator |
|
|
the tube comprising |
|
1D |
an inlet |
|
1E |
an outlet, and |
|
1F |
a singular exhalation flow passage between said inlet and said outlet defined by an enclosing wall |
|
1G |
wherein said enclosing wall bounds the singular exhalation flow passage and ambient air |
|
1H |
wherein at least a region of said enclosing wall is of a material that allows the passage of water vapour by diffusion without allowing the passage of liquid water or respiratory gases thereby providing a water vapour flow path from said exhalation flow passage to ambient air through said material |
|
1I |
wherein the region or regions is or are distributed over the length of the tube |
|
1J |
such that the tube allows said diffusion of water vapour from the expiratory limb of the breathing circuit along said singular exhalation flow passage |
|
1K |
thereby drying the humidified gases during their flow through the expiratory limb |
Issues of Claim interpretation
113. In its opening skeleton, F&P suggested that it would be wrong to construe claim 1 to cover Psaros if a non-anticipated interpretation was available, because Psaros is acknowledged prior art. I invited Counsel for F&P to provide the relevant authorities on the right legal approach to this argument, especially given that claim 1 is not in the usual two-part pre-characterising and characterising form. The argument was dropped in closing.
114. Otherwise, there was no dispute about the relevant principles of claim interpretation, and no issues of claim scope having regard to equivalence arose.
"Flexible breathing tube"
115. This is integer 1A.
116. I interpret this to mean that the tube in question is flexible enough to "drape", i.e. to lie across gaps between the ventilator and the patient, including over the patient's body and objects such as bed rails, while still doing its job in allowing gas flow and without undue kinking.
117. This, I believe, makes sense both contextually and practically, in terms of the necessary function.
118. As I have said above, the SDE would be aware of the relevant standards. But that does not mean that the claim feature requires compliance with any specific standard, and in the end I do not think that either side argued that.
Location of the breathable material
119. This issue relates to several features of claim 1 (primarily, as I see it, 1B and 1I) and the bone of contention is whether the claim requires breathable material all the way along the tube.
120. The point mainly matters on anticipation by Psaros. F&P argues that as shown the breathable material in Psaros is only along part of the path from patient to ventilator, and Flexicare replies that the claim only requires breathable material somewhere over the length of the tube, and/or that the bundle of tubes in Psaros is a breathing tube with breathable material all along its length, and that the claim only requires the breathing tube of integer 1A to be part of the expiratory limb. I mention this to identify why and where the point matters; I have noted above that the argument about construing the claim with specific reference to Psaros was dropped.
121. I prefer F&P's position:
i) The flexible breathing tube has to be the expiratory limb (integer 1B); the conventional language of "an expiratory limb comprising a breathing tube" not being used.
ii) Integer 1I requires that the region or regions (of breathable material) are distributed "over the length" of the (breathing) tube. At a purely textual level this might mean either somewhere along the length, or all along the length, but the former would mean that the integer would be redundant, as the combined effect of integers 1D to 1H is already that the regions have to be somewhere along the length of the tube.
122. This view of the ordinary contextual meaning is also consistent with the figures and the methods of manufacture, which all result in expiratory circuits consisting of tubes with breathable fabric all along their lengths, but one cannot put too much weight on this since a patent's claims may well be broader than the preferred embodiments. [0051] also provides further support.
123. I note that the Patent at [0018] refers to the possibilities of longitudinal sections being formed from breathable material, or "isolated regions" being formed from it. This might provide some modest support for Flexicare's position, but there is nothing to say that the "isolated regions" option would meet this aspect of the claim and on the contrary the paragraph immediately goes on to stress that what is preferred is continuous manufacturing means which would lead to breathable material all the way along the tube.
124. As I have said, I reach this conclusion without relying on the fact that Psaros is acknowledged prior art.
"Singular exhalation flow passage"
125. This is feature 1F and the issue is whether the claim is truly limited to just one flow passage in the flexible breathing tube. Again, the significance is in relation to Psaros where a bundle of tubes is alleged to fall within the claim so as to anticipate.
126. The feature has to be seen in the context of the fact that, as I have already mentioned, the tube of integer 1A is the expiratory limb,
127. I think it is clear that if the word "singular" were omitted, the claim might allow a divided tube and hence multiple flow passages, because the word "comprising" following integer 1C would require the tube to have a flow passage (and the other things mentioned) but permit additional features on top, including more flow passages and some kind of divider(s) to form and separate them.
128. However, I think that the word "singular" would be understood clearly to change this. Its natural meaning is exactly and only one. If it did not serve to exclude a situation with multiple flow passages then it seems to me that it would be redundant.
129. Counsel for Flexicare argued that "singular" served to exclude a single and (I think) undivided tube with more than one flow passage. I found this unclear as I did not understand what such a thing would physically look like, and despite asking I did not get any assistance on it. If what was meant was that within one undivided lumen different parts of the airflow which were not physically separated from one another by a barrier could be regarded as different flow passages then I reject the submission as being at a quite different level of complication from that with which the Patent is concerned, and unreal.
130. Flexicare also made the submission, based on Dr Dixon's evidence, that the claim does not allow a bifurcated tube but does allow two non-bifurcated tubes in parallel. That is hair-splitting at best and I think in any event it was just another way of putting the same argument.
131. As with the previous issue, my understanding from the text is reinforced by the figures and methods of manufacture, and again I would have reached the same conclusion without regard to Psaros' acknowledgement in the Patent.
"Drying the humidified gases"
132. Flexicare submitted that there is no requirement in integer 1K as to how much drying is achieved and that any amount will therefore do. I refer to my comments above about paragraphs [0002], [0003] and [0057] and agree that there is no quantification of how much is needed, but I nonetheless hold that some useful degree of drying is required. That is the clear object of the teaching and embodiments.
133. Counsel for Flexicare pointed out that the alleged infringement and F&P's own commercial product still have heating (why this is was not explained on either side), so it must be the case that drying by means of the breathable fabric to an extent which still requires heating is within the claim. I agree with this and have already said as much based on [0002], [0003] and [0057].
134. In principle, therefore, Flexicare could have run a prior art attack directed to showing a device with more limited drying from breathable material in the tube and with a heater was obvious (or old).
135. I do not think this matters, however, since all the prior art attacks were directed to producing a useful degree of drying, and the motivation relied upon was achieving enough drying to get rid of heaters and/or water traps.
136. I note that the claim clearly includes (but is not limited to) an expiratory limb in which the drying by means of the breathable material is enough to avoid the need for a heater or a water trap. It was not alleged that the SDE could not make such a thing.
137. Overall, therefore, integer 1K has a part to play in the assessment of inventive contribution and inventive step, on the footing that useful drying is what is required, not any greater level than that. That is the advantage achieved across the scope of claim 1.
Integers 1G and 1H
138. It was common ground that, at least in combination, these require that the breathable regions are at the interface between the expiratory flow path and ambient air. Consequently the claim does not cover a coaxial arrangement where the breathable material is at the interface between the inner and outer tubes with one carrying the patient's exhalation and the other carrying e.g. anaesthetic gas. That means that it does not cover Inoue, or the embodiment shown in Figure 4 of the Patent.
139. For myself, I think this result was probably achieved by integer 1H anyway so that the amendment to include 1G in claim 1 was unnecessary, but there is no need to decide that.
Validity
Anticipation
140. Anticipation is alleged only by Psaros. There was no dispute over the applicable legal principles: there has to be clear and unambiguous disclosure of all the features of the claim.
Teaching of Psaros
141. Psaros is a patent application by Siemens published in 1993.
142. Psaros is directly and unambiguously directed to the same problem as the Patent. It specifically calls out the problem of rainout in columns 1 and 2 and talks about the solutions of water traps and heaters, and their problems.
143. At column 2 lines 38-40 it says that "a simple, compact, efficient and energy-sparing dehumidifying device would be highly desirable."
144. Psaros' solution can be seen in the following three figures:
145. Figure 1 gives an overview and figures 2 and 3 show more detail, figure 3 being a cut away view.
146. In summary, there is a bundle of tubes of breathable material surrounded by a loosely-wrapped spiral harness; this wrapped bundle is the "dehumidifying device", and it is identified in the drawings by reference numeral 8. The length of the tubes is said (column 6 lines 1-7) to be 300mm, this being "sufficient" for drying, but could be longer if desired.
147. It is somewhat unclear from figure 1 whether the dehumidifying device runs all the way from the ventilator 2 to the water trap 7. On the one hand the whole thing is identified by numeral 8 and the connectors at the ventilator 2 and water trap 7 resemble the connectors 10 and 11 in figure 2. On the other hand it seems unlikely that that length would only be 300mm. In any event, the expiratory limb runs all the way from the ventilator to the patient and the left-hand section (in figure 1) between the water trap and the patient is clearly not formed from dehumidifying device 8. Only part of the expiratory line is formed from dehumidifying device 8. This is made even clearer in column 5 lines 49 to 53.
148. Given my conclusions about the interpretation of claim 1, Psaros does not anticipate because:
i) The dehumidifying device 8 is a breathing tube but is just part of the expiratory limb whereas the claim requires a tube that is the expiratory limb.
ii) Alternatively, if the whole tube from patient to ventilator is to be regarded as the breathing tube of the claim then the breathable regions of device 8 are along only part and not distributed all along the length as I have held is required.
iii) There are in any event multiple flow passages in drying device 8, not just a "singular" flow passage.
149. I would however have found (had I been wrong about those points) that the requirement for a "flexible" tube was met, since the tube between the patient and the ventilator is shown to drape in figure 1.
150. As to points i) and ii) I would further mention that Counsel for Flexicare cross-examined on the basis that Psaros did not expressly limit its design to one in which the dehumidifying device was only part of the expiratory limb. To the extent that that is relied on by Flexicare in relation to anticipation then I reject it. The fact that something unmentioned is not expressly ruled out does not mean that it is disclosed, still less to the standard required for anticipation.
Obviousness
The law
151. There was no significant dispute about the high-level applicable principles. Both parties referred to the recent decision of the Supreme Court in Actavis v. ICOS, per Lord Hodge at [52]-[73]. Thus:
i) There is a single statutory question: whether the invention is obvious, having regard to the state of the art at the priority date.
ii) In some cases the Pozzoli [2007] EWCA Civ 588 approach is helpful, and subject to a point I address below, both parties argued their positions by reference to it.
iii) The Supreme Court endorsed the statement of Kitchin J (as he then was) in Generics (UK) Ltd v H Lundbeck A/S [2007] EWHC 1040 (Pat) at [72]:
"The question of obviousness must be considered on the facts of each case. The court must consider the weight to be attached to any particular factor in the light of all the relevant circumstances. These may include such matters as the motive to find a solution to the problem the patent addresses, the number and extent of the possible avenues of research, the effort involved in pursuing them and the expectation of success."
152. Flexicare referred in closing to the law on "obvious to try", and expectation of success. I do not need to say more about this given F&P's retreat on perceived technical difficulties of implementation that I have discussed above.
153. As to Pozzoli, while it is a useful approach the second question, the identification of the inventive concept, can be difficult, and it is not mandatory to undertake it. The Court may instead use the claim features themselves. See MedImmune v. Novartis [2012] EWCA Civ 1234 at [86], referring to Lord Hoffmann in Conor v. Angiotech [2008] UKHL 49 at [19].
154. I do not find this a case where it is useful to try to define the inventive concept separately from the claim. It can be helpful to do so in some cases to allow focus on what really matters and strip away what has been called unnecessary verbiage, but at the same time, as the cases cited above make clear, it can lead to unproductive arguments about paraphrasing the claim.
155. As it happens, the parties characterised the inventive concept in very different terms, in particular in that Flexicare left out integer 1K as being unnecessary verbiage on the basis that any degree of drying would meet the claim.
156. I cannot see why characterising a feature as verbiage or not is in itself a useful debate to have (again, see MedImmune), but the point about drying being unimportant is one that I think I should touch on: although Kertzman and Psaros are both certainly about drying expiratory gases so that the SDE would have it firmly in mind, it is a point of serious dispute in relation to Inoue that (F&P says) the focus is on humidifying the anaesthetic gases being breathed in, albeit that drying will also be taking place. So leaving drying out of account would rather sell the pass.
157. F&P reminded me that hindsight must be avoided and is especially a danger with simple inventions. I accept that and I think it is an important point in the present case, where the alleged invention is a very simple one and the obviousness case over Psaros and Inoue involves further simplification. Flexicare did not specifically comment on this principle, since its main point (which I have rejected) was to argue on the facts that Dr Dixon did not exercise hindsight in the circumstances of this case.
158. F&P also relied on long-felt want, citing Jacob LJ in Technip France SA's Patent [2004] EWCA Civ 384, [2004] RPC 46 at [122]:
"The question `why was it not done before' is always a powerful consideration when considering obviousness, particularly when all the components of a combination have been long and widely known."
159. Flexicare did not dissent from this being a relevant principle at a broad level, but sought to meet it by pointing out that other cases (e.g. Brugger v. Medicaid [1996] RPC 635 at 654-5) establish that there can be explanations as to "why not", such as lack of demand, the expense not being merited, and so on. Thence Flexicare sought to meet the point on the facts, and I address that below.
Obviousness over Kertzman
160. Kertzman concerns a breathable tube reinforced with braided netting.
161. The principal application disclosed is sampling of breath expired by a patient (page 2).
162. There is also reference to "process drying" in industry (page 2 and page 5), and it is said (page 5) that the braided netting allows this to be done at higher pressures. But neither expert really seemed to know concretely what was meant by process drying.
163. The document also contains some experimental data testing drying with the claimed tubes under various conditions. Dr Dixon said that this sort of information was not usually published.
164. Nafion is referred to specifically, though not by name, in the reference to a US Patent 3,735,558, as being the breathable material used.
165. Specific parts of the document make clear that the tubes under consideration are of just a couple of millimetres in diameter, for example the reference to Leur fittings at pages 6-7 and the dimensions in the table at page 11A. These are consistent with the known sampling tubes.
166. There are also various statements about the benefits of the braided netting in terms of protecting the breathable membrane and preventing kinking and the like.
167. For Pozzoli purposes, I have already dealt with questions 1 and 2 (skilled addressee/SDE and CGK) above.
168. For question 3 and the assessment in question 4, the differences between Kertzman and claim 1 are that Kertzman's sampling tube is not a breathing tube, is not an expiratory limb of a breathing circuit, and is not adapted to be located between a patient and a ventilator. So at least features 1A to 1C are missing (with a knock-on consequence for the later features insofar as they refer to expiratory limbs). The parties disagreed about precisely which integers were missing, just as they did about how to characterise the inventive concept, but it does not matter because the issue is whether it was obvious to modify Kertzman's sampling tube so as to use it as the expiratory limb of a breathing circuit.
169. It is not said by Flexicare that there is any active pointer in Kertzman to using the physical structure of the tubes that it teaches in the context of the expiratory limb of a breathing circuit (or indeed in any other context than sampling tubes and the unclear "process drying"). Nor is there any pointer to making the tubes much larger, as they would have to be for use in the expiratory limb.
170. Apart from the different size, sampling tubes also served less critical functions than breathing circuits (in that they did not support breathing, but only monitoring), and operated at different flow rates and pressures, with the flow caused in a different way, by negative pressure (it was put to Mr Pierro without specific examples that there were sampling tubes that did not use negative pressure, but he had not heard of any). These are individually relatively minor matters but they all go to reduce the likelihood that the SDE would think in the first place of the idea of taking Kertzman's teaching across to breathing circuits, through lack of familiarity with the idea of a tube made of breathable material of the necessary diameter, length, resistance to crushing damage and other features necessary to a breathing tube.
171. Further, although Mr Pierro accepted that the SDE would be able to adapt Kertzman for use in the expiratory limb of a breathing circuit if tasked with doing so, he was clearly strongly of the view that the braiding taught by Kertzman for its sampling tubes would not be appropriate for such a task. This also indirectly supports the notion that without being pointed to such a change of use, the SDE would not think of it.
172. In her cross-examination Counsel for Flexicare sought to address the lack of a positive pointer to use much larger tubes, or to apply the teaching beyond sampling tubes, by pointing out that the teaching does not limit the size of the tubes or limit their use to sampling. As I said in relation to anticipation by Psaros, I do not think this can help Flexicare; the absence of a prohibition is not at all the same as the presence of active encouragement or suggestion. This lack-of-limitation approach ran through a lot of the cross-examination. Counsel for Flexicare also sought to establish that the bigger sizes of tubes taught by Kertzman are not so far in size from the smaller breathing tubes known in the CGK. But even comparing the biggest sampling tube in Kertzman with the smallest neonatal breathing tube there is nearly an order of magnitude difference.
173. Turning to Flexicare's evidence in support of obviousness, I accept F&P's submission in relation to Dr Dixon's evidence that it was long on the question of how one would go about adapting the sampling tubes of Kertzman to make them into expiratory limbs, and very short on the question of why the SDE would think of doing that in the first place. As I have said, he thought that the inclusion of hard data would be interesting, but even if that were so the data simply showed that the tubes were good sampling tubes, and there is no reason why their being good sampling tubes at the small sizes used would call to mind the possibility of making them much bigger, and physically quite different, and using them for something else.
174. I also bear in mind my general finding of the way in which Dr Dixon's evidence was tainted by hindsight as I have explained above.
175. I found Mr Pierro's evidence (written as well as oral) more convincing. I do not overlook passages in his oral evidence relied on by Flexicare which superficially could be said to support the proposition that the SDE would see a potential solution to rainout in breathing circuits in Kertzman.
176. I think, however, that these were very largely because the basis of the questions to him injected hindsight in the way I have identified in relation to the skilled team: by defining the SDE very narrowly so as to have a tight focus on breathing circuits, and at the same time notionally putting him or her in the frame of mind to consider rainout specifically when reading Kertzman. Examples may be found at T1/114-5 and T1/142-145 (and I note in relation to the latter that the questions related to what could be done).
177. In closing I asked Counsel for Flexicare to work through this cross-examination in detail and she did so. She focused attention heavily on the question and answer at T1/144 lines 2-5; that is entirely understandable since in itself and taken alone it may be said to be an acknowledgement of Flexicare's case, which is why I asked for submissions on it. But it was built, among other things, on the narrow definition of the SDE back at T1/114 and e.g. its link at T1/115 to just the first paragraph of Kertzman (which has no specific context for sampling tubes and has a much more general and abstract tone than the document as a whole, which is about sampling tubes and the rather unclear process drying).
178. I paid close attention to this passage of evidence when it was given. It struck me as presenting the danger of hindsight. I have read it again more than once since, including during the oral closings when I was assisted by both parties' submissions. The cross-examination was very skilfully done, and, as I say, one can entirely understand the reliance on the specific answer mentioned above, but the overall effect and sequencing mean that I do not think it was an answer to the key question of what would be obvious from Kertzman without knowledge of the invention of the Patent and without an advance expectation that Kertzman would have a useful solution to the problem of rainout in expiratory limbs.
179. I also do not think Mr Pierro was ever really pressed on what the SDE would make of Kertzman if they came to it with no preconception of whether it was or might be relevant to breathing circuits and no expectation that it would be useful for solving the problem.
180. I bear in mind that Counsel for Flexicare argued that the narrow focus of the SDE in her question was justified because F&P had not identified any potential routes for progress for the SDE other than aiming to get rid of water traps and heaters. But, as Counsel for F&P pointed out, this was not accepted. Why would the SDE not be interested, for example, in improving water traps further?
181. I also have to look at the oral evidence as a whole. Elsewhere, Mr Pierro resisted the suggestion of obviousness, and in saying this I have borne in mind Counsel for Flexicare's valid point that I must keep an eye on whether the answers were only about the difficulties of implementation. There is some force in it, but I still think Mr Pierro was not accepting obviousness, and was explaining why differences between sampling tubes and breathing tubes supported his view. Overall, I find that it would not be obvious to the SDE, without hindsight, to use the teaching of Kerzman in the context of the expiratory limb of breathing tubes.
182. There is also the question of long felt want. Kertzman dates from the late 1980s. I believe it was accepted by Dr Dixon and I anyway find that sampling tubes of this general kind were CGK well before the priority date of the Patent, including in relation to the fact that they used breathable materials to dry the gas being sampled.
183. Further, the problems of rainout in breathing circuits and the unsatisfactory solutions of water traps and heating in seeking to address it were also CGK and had been for a long time before the priority date. Flexicare did not try to argue that the appreciation of these problems was recent at the priority date.
184. It is therefore a powerful real-world point against obviousness that despite this, no one actually thought to make the expiratory limb of a breathing circuit out of breathable materials so as to dry the exhaled gases prior to the Patent. The point cannot be answered by suggesting that Kertzman was obscure, since it was essentially the CGK. Nor was I impressed by Flexicare's reliance on there being concrete data in Kertzman since, as I have said, it just demonstrated that the sampling tubes disclosed, of the generally known construction, would work well as sampling tubes.
185. Counsel for Flexicare sought to argue that F&P had not properly raised the point, or that the problems of rainout and the deficiencies of water traps and heaters were not sufficiently motivating. I reject this: it was Flexicare's own case that those problems were well known and they were said to be the drivers for looking for improvements. Flexicare itself established the "want" (which was common ground) and F&P simply pointed out that that want had been around for a long time.
186. Other points raised by Flexicare on the facts were that the UK market in breathing circuits was dominated by SMEs who only considered product design every few years, that there were high barriers to entry, that costs were significant, that expensive designs were not attractive and that manufacturers would not make new designs unless there was a high confidence in their likely success. I did not find any of these convincing since there still would have been ample time for attempts to be made, even by SMEs with modest resources, after materials like Nafion in sampling tubes became well known, and the issues with water traps and heaters were clearly regarded as significant.
187. Finally, Counsel for Flexicare relied on the fact that the parties' commercial products are quite expensive and have not completely replaced devices with water traps and heaters. This can have no bearing on the position in 2000.
188. Overall, even if I had thought the question was more evenly balanced on the primary evidence on obviousness, this "why not earlier" point, which I agree is secondary and must be kept in its place, would have persuaded me to reject the attack.
189. I therefore reject the attack of obviousness over Kertzman.
Obviousness over Psaros
190. I refer to what I have already said about Psaros in the context of anticipation, to identify its teaching. As with Kertzman, for Pozzoli questions 1 and 2 the obviousness attack has to be assessed in the light of my findings about the SDE and the CGK.
191. There is no evidence that Psaros was ever commercialised. Siemens being a big company I expect that it would have been possible to identify a commercial product based on it, had there been one.
192. Psaros explains the thinking behind the bundle of tubes in the following passage at column 3 line 32 to column 4 line 21:
Despite the fact that these materials have been
prior art for a long time, even in the
ventilator/respirator field, and despite the fact that
the problem of condensation in the flow meter has
been known even longer, no solution according to
the present invention has ever been presented or
proposed.
To the contrary, professionals in the field, as
the above has shown, have made major efforts to
make external devices for heating expired gas or
designed different kinds of water traps to collect
condensed water after expired gas was cooled.
Even from the technical point of view, great
insight and creativity is needed to achieve this
elegant solution to a problem which has been around
for such a long time in the field.
Since each individual tube has an internal diameter
which is less than the internal diameter of
the expiratory line, a more effective contact surface
between gas and ambient air is obtained. Drying
then takes place more rapidly, and tubes can be
shorter without any impairment of results.
An advantageous refinement of the dehumidifying
device is obtained according to the invention
by arraying the tubes in a bundle between the
respective tube-to-expiratory line attachment areas.
193. As well as explaining the thinking behind the specific construction, this reinforces the longstanding nature of the problem and the lack of solutions other than water traps and heaters. The first three of the quoted paragraphs are exactly F&P's case over Kertzman in relation to long felt want.
194. As I have construed claim 1, the differences between Psaros and it for the purposes of Pozzoli question 3 are that there are multiple flow passages within dehumidifying device 8, not just one, and the breathable material is not all the way along the expiratory limb but only within dehumidifying device 8.
195. Correspondingly, Flexicare's obviousness case is that it would be obvious to (a) switch to a single tube, which (b) incorporated breathable material all along its length. The motivation to do so was argued to be a desire to simplify.
196. This is not an attractive argument and I reject it. The authors of Psaros say that they have put a lot of work into solving a longstanding problem, and they propose a very specific solution for reasons which they spell out. The bundle of multiple breathable tubes is provided to achieve drying in a compact device, and the bundle is provided to get the right drying while at the same time protecting the individual tubes, a judgment having been made that despite the inner tubes not being directly exposed to air, the drying would suffice. The result is a sort of cartridge which can be removed easily for e.g. cleaning (column 5, first two paragraphs).
197. While in hindsight it can be said that going to a single tube with breathable material all along its length would be a simplification, that is not the question. I do not think that, without knowledge of the Patent, it would occur to the SDE at all - it would be to abandon the whole philosophy of Psaros and to move to a device that was not compact and did not provide the protection referred to, unless possibly the spiral binding were used all along the expiratory limb, which in itself would seem unattractive. It would also involve complex considerations of whether appropriate drying could be maintained.
198. Even if it occurred to the SDE to consider a single tube with breathable material all along its length as one possibility among others, I think the same factors would mean that it would not be obvious to choose and to pursue. I think the SDE would consider that the authors of Psaros must have had good reasons to go with the more complex approach.
199. As with Kertzman, though to a lesser extent, Mr Pierro gave some answers in cross-examination which may be said to support Flexicare's case, and I do not overlook them. In particular, he accepted that the SDE would consider simplifying Psaros in view of its high complexity (T1/176), but he did not thereafter accept that it would be obvious to go for a single tube as the simplest construction (ibid), and later on (e.g. at T1/178) it seemed to me that the most that he was saying was that the SDE could do that. He did say at T1/179-180 that the SDE would think of extending a single tube for the whole length of the expiratory limb, but that was on the assumption, put at T1/179 of having made the decision to use a single tube, an assumption which I do not think he had accepted (and in any event the questioning had a Technograph illegitimate step-wise feel to it, I thought). And at T1/180 he said, which supports my finding above, that if the skilled person got that far, they would reject it ("it might get crossed out quickly"). In addition, when propositions were put to him in terms of "obvious" or "routine" he did not accept them.
200. As for Dr Dixon's oral evidence, he maintained his overall view as to obviousness, but I thought the cross-examination revealed that he had to accept that what he was proposing involved abandoning Psaros' approach entirely, and also that he had not really previously taken on board the consistent teaching in Psaros that the dehumidifying device should be short, and only part of the expiratory line. Further, he had not thought through the knock-on effects that would result from the changes he advocated. For example, going to a single tube would be thought by the reader to remove the protection said to result from a bundle of tubes, and it would be expected that some means were necessary to make up for that. As ever, I must also bear in mind the hindsight that Dr Dixon brought to bear.
201. Finally, Psaros again raises the question of "why not done before"? It does so in a different way from Kertzman, because there the question was why a CGK idea for sampling tubes had not been deployed in relation to breathing circuits. With Psaros the issue is why, despite working on breathing circuits and with all the necessary components to hand, and focusing on the same shortcomings of the known solutions to rainout that Flexicare now relies on, the workers went in a very different direction to the Patent. I find this a significant point in F&P's favour and I do not think Flexicare had a good answer to it, or to why following Psaros no one simplified it down until the Patent (although on this the evidence that Psaros reached a wide audience is thin). However, the case for obviousness from Psaros is so weak anyway that I do not think F&P needed it.
202. Taking all these things together, I reject the obviousness case over Psaros.
Obviousness over Inoue
203. Inoue is a patent application published in Japanese in January 2000. The fact that it is in Japanese is not relevant - it formed part of the state of the art and the skilled addressee is deemed to read it with interest.
204. The proximity in time of the publication of Inoue to the priority date of the Patent means that the "why not done before?" point does not run in F&P's favour. This is a material difference from the other citations and I bear it firmly in mind.
205. Inoue discloses a coaxial two-tube arrangement for use in anaesthesia. One of the tubes carries the anaesthetic gas, and the other carries the humid air breathed out by the patient. Breathable sections are provided in the wall of the inner tube to allow the humid exhalations to humidify the drier anaesthetic gases.
206. This can be seen in the following figure, taken from F&P's skeleton, with the breathable sections in the inner tube coloured for visibility (in this arrangement the exhalation is in the outer tube, but it is taught that this can be inverted):
207. This is said to be an advance over a previous arrangement in which an "artificial nose" (another name for an HME) was provided at the patient end of the circuit to allow humidification of the anaesthetic gases, but which provided a dead volume (leading to the patient re-inhaling some of their own exhalation) and a resistance to flow.
208. Inoue contains a modest amount of data in figure 6 showing that an arrangement with "stirring walls" gave better drying than one without. I did not find this of relevance although it shows that trial devices were built.
209. I have addressed Pozzoli questions 1 and 2 above in relation to the other prior art. In terms of Pozzoli question 3 because Inoue has the breathable material at the interface between the anaesthetic gas and the exhaled air, it lacks feature 1G and 1H of claim 1.
210. Flexicare's obviousness case is that it would have been obvious to modify Inoue into a biaxial arrangement, using its inner tube as the expiratory limb of a breathing circuit and providing a separate tube for inhalation. The motivation to do so is said to be to solve the problems with rainout, which I have explained above.
211. F&P objects that this would be quite different from Inoue, in that the goal of Inoue is the passive humidification of the inhaled gases. Although that is in point of fact necessarily accompanied by drying of the exhalation, Inoue does not mention that because it is not the goal. Switching to a biaxial arrangement would mean that there was no humidification of the inhaled gases at all and the object of the document would be lost.
212. I agree with F&P on all of this.
213. It also bears articulating that Inoue is about an arrangement specifically for anaesthesia. That is where coaxial arrangements were used in the CGK because (Dr Dixon explained to me and I accept) a single tube was less likely to get in the way of a surgeon operating. So it would be very unlikely to modify Inoue to a biaxial arrangement for use in anaesthesia, and I do not think that was Flexicare's case. Rather it was to take Inoue's arrangement and both to modify it into a biaxial arrangement and at the same time transfer it to use in breathing circuits for longer-term use than anaesthesia, for example ventilated patients in intensive care. Inoue contains a general statement in [0023] that its embodiments are not confined in use to anaesthesia, but not coupled with any pointer to modification.
214. Flexicare pointed out that figures 4 and 5 of the Patent are materially the same as Inoue. This is true, and I have mentioned it above. It accepted that claim 1 (in particular with the amendment but, I hold, in any event) does not cover figures 4 and 5, or Inoue, but it said (opening skeleton, paragraph 11) that:
"at its heart, the inventive contribution of the Patent is the idea of using a breathable material in the construction of an expiratory tube in order to dry the humidified gases flowing through it. That idea is disclosed in Inoue. It is very difficult to see how the retrograde step of eliminating simultaneous humidification can be said to represent an invention over that disclosure."
215. I do not think this captures the invention of claim 1 accurately since it simply omits the requirement of integers 1G and 1H. It also assumes, wrongly, that the invention represented by claim 1 is the same as the invention of figures 4 and 5. They are different. Nor is it fair to say that the idea even as characterised above is disclosed in Inoue, which does not talk about drying of exhalation, but about humidification of what is being inhaled. In fact of course, as I have already said, those two things physically must accompany one another, but it is also a question of perceiving that that is both true and useful, and seeing something in just a subtly different way can perfectly well be an inventive step if it leads to a new product, as is the case here. I also reject the characterisation of the change from Inoue as a retrograde step of eliminating simultaneous humidification. For reasons I have just touched on, the proper analysis is more subtle than that: the SDE would have to see that there was another process going on (drying) that could be leveraged in a different (non-anaesthesia) context to get a useful result (reduction of rainout) in a simpler set-up. Yes, humidification would be lost, but that would be because something else was gained.
216. It is really only because the step from Inoue to the invention of the Patent lies in a simplification that Flexicare can assert that it is a retrograde move. This is exactly the sort of reason why one has to be careful to separate simplicity from obviousness. It is misleading to say that when one simplifies one loses features, that losing features must be bad, and that making things worse cannot be inventive.
217. Turning to the oral evidence, I thought that Flexicare made very little, if any, real progress with Mr Pierro:
i) He accepted that the desired and critical humidification would be accompanied by drying and that the SDE would understand that, but it was not really put to him and he certainly did not accept that the skilled person would without hindsight identify that as something useful as opposed to a mere incident of the main goal.
ii) He accepted that it would be nice to simplify if possible, but not specifically to get rid of the coaxial arrangement.
iii) He resisted the idea that the inhaled gases were similar to ambient air, in the context of the arrangement with exhalation in the inner tube.
iv) He accepted that drying of the exhalation would be achieved if the outer wall was removed, but he said that that removal was contrary to Inoue's intent.
v) He characterised the splitting into a biaxial arrangement as "creative".
vi) In the context of anaesthesia he identified the desirability of a coaxial arrangement.
218. As with his cross-examination on the other prior art, the questions to him were freighted with the expectation that Inoue might have a solution to rainout in the expiratory limb of breathing circuits. I do not think he ever accepted that Inoue would be perceived without hindsight as useful to that purpose, or that without hindsight the idea of moving away from a coaxial arrangement is one that would occur to the SDE.
219. As to Dr Dixon's oral evidence:
i) He accepted that the coaxial arrangement in Inoue was crucial to it, was the basis for its claim to inventiveness, and, coupled with the goal of humidifying inhaled gases, was what would be of interest to the SDE. These were damaging admissions for Flexicare's case, for reasons I have explained above.
ii) He also accepted that in Inoue the humidity of the exhaled gases is not regarded as a problem, but a benefit, because it allows the humidification of the inhaled gases.
iii) He accepted that in the anaesthetic context of Inoue the coaxial arrangement was a significant benefit.
iv) He accepted there was nothing intrinsically undesirable about a coaxial arrangement.
v) He accepted that the change to a biaxial arrangement would lose the benefit of passive humidification of the inhaled gases.
vi) He accepted that removing the outer wall would leave the inner wall exposed and possibly needing protection, with associated added complication.
220. Taking this all into account, I reject the allegation of obviousness over Inoue.
Lack of technical contribution and insufficiency
221. These related arguments were run only briefly in Flexicare's closing.
222. On lack of technical contribution, Flexicare's submissions were as follows:
223. The alleged inventive contribution of the Patent is the use of a `breathable' material to dry the humidified gases that flow through the expiratory limb of a breathing circuit. But as we have seen from the prior art, that is not inventive at all: the need to dry exhaled gases was already known - there is no dispute that the problem that the Patent aims to solve was CGK; and the use of "breathable" material to achieve drying is disclosed by the prior art.
224. As an attempt to overcome this fundamental problem and to try to imbibe superficial novelty into the claim, the drafter has packed it full of verbiage. But the words do not add anything of technical value and the claim does not provide a technical advantage over the prior art.
223. This is circular. The argument would not arise if it was in fact obvious to make something within the claims, i.e. if the conventional obviousness attacks over the specific cited art had succeeded. On the assumption that they failed, however, then it was inventive to make the products within the claims of the Patent and the question on lack of technical contribution would be whether they achieved any useful purpose. Compared with Kertzman they do because they solve a problem in a different application; compared with Psaros they do because they provide a simpler solution; compared with Inoue they do because they allow a different and simpler product to be used for a different purpose. And in a general sense without specific reference to any particular art they provide real products which provide a simple solution to a real problem.
224. As to insufficiency, this was really a squeeze which did its job on the obviousness side of the case, as I have already said several times. Right at the end of Flexicare's closing skeleton (paragraphs 226-227) it was perhaps asserted in addition to the squeeze ("Moreover" in paragraph 227) that if F&P relied on uncertainty as to drying levels that could be achieved from modifications of the prior art, then the Patent would be insufficient because it does not contain data on drying levels and leaves it to the empirical efforts of the SDE. I was unable to see how this added to the squeeze and I have dealt with the interplay between the SDE's abilities from the prior art and from the Patent above anyway.
225. I therefore reject these two attacks, save that the squeeze worked to the extent indicated.
Conclusions
226. My conclusions are:
i) The Patent is valid in the form as unconditionally proposed to be amended.
ii) Amendment of the Patent is allowable.
iii) Flexicare has infringed the Patent.
227. I will hear Counsel as to the form of Order that I should make. I will adjourn consideration of those matters and of any application for permission to appeal to a later hearing, and time for any Appellant's Notice shall not run in the meantime. I ask the parties to submit an agreed order accordingly.
