Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales High Court (Patents Court) Decisions
You are here: BAILII >> Databases >> England and Wales High Court (Patents Court) Decisions >> Gilead Sciences Inc & Anor v NuCana PLC [2023] EWHC 611 (Pat) (21 March 2023)
URL: http://www.bailii.org/ew/cases/EWHC/Patents/2023/611.html
Cite as: [2023] EWHC 611 (Pat)
[New search] [Printable PDF version] [Help]
Neutral Citation Number: [2023] EWHC 611 (Pat)
Case No: HP-2021-000007
IN THE HIGH COURT OF JUSTICE
BUSINESS AND PROPERTY COURTS OF ENGLAND AND WALES
INTELLECTUAL PROPERTY LIST (ChD)
PATENTS COURT
The Rolls Building
7 Rolls Buildings
Fetter Lane
London EC4A 1NL
Date: Tuesday 21 March 2023
Before:
The Hon. Mr. Justice MEADE
- - - - - - - - - - - - - - - - - - - - -
Between:
|
|
(1) GILEAD SCIENCES Inc. (a company registered in the US State of Delaware) (2) GILEAD SCIENCES LIMITED |
Claimants/Part 20 Defendants |
|
|
- and –
|
|
|
|
NUCANA PLC |
Defendant/Part 20 Claimant |
- - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - -
MICHAEL TAPPIN KC, TOM MOODY-STUART KC and JAMES WHYTE (instructed by Bird & Bird LLP) for the Claimants/Part 20 Defendants
PIERS ACLAND KC, TOM MITCHESON KC and ANDREW LOMAS (instructed by Powell Gilbert LLP) for the Defendant/Part 20 Claimant
Hearing dates: 20 and 23-27 January and 1-3 February 2023
- - - - - - - - - - - - - - - - - - - - -
JUDGMENT APPROVED
Remote hand-down: This judgment will be handed down remotely by circulation to the parties or their representatives by email and release to The National Archives. A copy of the judgment in final form as handed down should be available on The National Archives website shortly thereafter but can otherwise be obtained on request by email to the Judicial Office (press.enquiries@judiciary.uk).
Mr Justice Meade:
Relevance of Idenix v Gilead. 9
Professor Micklefield - Gilead’s medicinal chemistry expert 10
Doctor Galmarini - Gilead’s oncology expert 10
Professor Seley-Radtke - NuCana’s medicinal chemistry expert (non-synthesis) 11
Professor Smyth - NuCana’s oncology expert 12
Professor Davies - NuCana’s synthetic chemistry expert 12
Prof Davies’ instructions for the EPO.. 14
Approach to CGK - the law.. 17
Nucleotides and nucleosides. 18
Overcoming resistance/intracellular drug delivery issues with nucleoside analogues 21
Disputed CGK –issues other than synthetic chemistry. 23
Mechanisms of action and metabolism of NAs in cancer 23
2’ position of the sugar, 5 position of the pyrimidine base. 25
Prof McGuigan’s ProTide work. 31
Potential utility as anti-cancer agents. 32
Disputed CGK - synthetic chemistry. 34
Teaching of the specification. 35
Quality of the experiments in the Patents. 37
Claim Scope and infringement 41
Domestic law about selection from lists. 47
The preliminary opinion of the TBA on EP190. 53
Relevant disclosure of the PCT. 54
Relevant disclosure of EP190 as proposed to be amended. 57
Conclusion on EP190 added matter 60
Industrial applicability and plausibility. 61
Law on capable of industrial application. 61
Ab initio plausibility or implausibility. 64
Technical contribution - NuCana’s case. 65
Disclosure of the contributions. 67
Cytotoxicity - plausibility facts and assessment 68
Two aspects of Dr Galmarini’s evidence. 69
Lack of Industrial Application. 74
“Sufficiency”/“existence in fact”. 75
Improved intracellular delivery - plausibility facts and assessment 79
Undue burden insufficiency. 80
The standard for undue burden. 82
The role of primary and secondary evidence. 83
Fluorination Schemes Document 87
Choice of successful fluorination conditions used by Concept 88
Secondary evidence: real teams. 92
Failure of the glyceraldehyde work. 95
Mr Clark and Prof Pankiewicz. 96
Secondary evidence: CRO experiments. 98
Conclusions. 102
Introduction
- In this action the Claimants (together, “Gilead”) seek the revocation of two patents owned by the Defendant, NuCana. They are European Patent (UK) 2 955 190 B1 (“EP190”) and European Patent (UK) 3 904 365 B1 (“EP365”). I will refer to them together as “the Patents”. They are from the same family and although their specifications differ, the differences mostly do not matter and I pick up those points of significance as they arise below. Where I refer to specification paragraph numbers without expressly saying which of the Patents, then I mean EP190.
- EP190 and EP365 also differ in their claims, with EP365 being narrower.
- The Patents have the same unchallenged priority date of 21 July 2003. As I explain in more detail below, EP190 is under opposition in the EPO with a Technical Board of Appeal (“TBA”) hearing due on 24 March this year. EP365 was only granted on 7 September 2022.
- NuCana counterclaims for infringement by Gilead’s commercially very successful drug compound sofosbuvir, which is the active ingredient, or one of the active ingredients in its Sovaldi, Harvoni, Vosevi and Epclusa products, which are anti-virals.
- NuCana had its own product covered by the claims of the Patents, Acelarin, intended as a cancer drug. It has run into difficulties with clinical trials. That is not of any direct relevance to the issues before me although Gilead sought to emphasise that the Patents teach that the compounds disclosed are cytotoxic, which is desirable in a cancer drug but, Gilead submits, positively to be avoided in an anti-viral.
- There is no real challenge to infringement if the claims of the Patents are valid and Gilead took the role of claimant at the trial.
- The Patents arise from the work of the late Prof Chris McGuigan of Cardiff University. It will help an understanding of this judgment if I explain at this early stage and at a very high level the technology involved.
- For decades it has been known that cancer can be treated with drugs based on nucleoside analogues (also referred to as “NAs”, I will use the full term and the abbreviation interchangeably in this judgment and both are used extensively in the papers in the case). These are compounds which are similar to natural nucleosides but which are modified so that, by one or more mechanisms, they interfere with cells’ normal replication and kill them or stop them from growing. This process affects healthy as well as cancerous cells but cancer cells replicate much faster so are hit harder.
- A common general knowledge (“CGK”) nucleoside analogue drug which had been successfully used in treating cancer was gemcitabine:
- A problem with nucleoside analogues arose with getting them into cells. The reasons for this are relatively complex and are discussed in more detail below. At this introductory level what is significant is that Prof McGuigan’s work was concerned with making prodrugs, referred to as ProTides, to try to overcome this problem. The ProTide approach involves phosphoramidate modifications (again, explained below) to nucleoside analogues.
- The claims of the Patents are product claims; they are defined by a Markush formula (the permitted substituents vary from claim to claim) and cover ProTide nucleoside analogues as just described in which the nucleoside moiety is gemcitabine or gemcitabine-like. Sofosbuvir falls within the Markush group:
- In prosecution and in the opposition proceedings NuCana has successively narrowed its patent claims. At this trial it seeks to amend EP190 in two proposed amended forms (one unconditional and one conditional, the latter being a small extra limitation over the former), and EP365 has claims that are narrower still. NuCana floated a further set of amendments to EP190 in late December 2022, shortly before trial, but abandoned it on the first day of trial.
- Gilead attacks the Patents on the following grounds:
- At trial Gilead also sought to run a “classical” obviousness case over Shepard which I deal with (and reject) below.
- The only infringement point was an argument by Gilead that although the claims are product claims they can only be infringed by compounds which have the plausible technical contribution relied on. This was only faintly run and to the extent still live was abandoned in Gilead’s written closing so I deal with it very briefly below. Other than this point and a point about the meaning of a particular substituent in the specification there were no issues of claim construction.
- The lack of plausibility case and to a lesser extent the undue burden case each involved arguments over the skilled team and the CGK.
- The undue burden insufficiency attack is essentially completely separate from the other attacks (although one witness was in common, Prof Micklefield for Gilead) and so the hearing before me was effectively two trials in one. The undue burden dispute included:
- With the undue burden case being so distinct from the rest of the case, each side used separate Counsel for it. For Gilead Mr Tappin KC took the lead and handled all the oral advocacy except for the undue burden case, which Mr Moody-Stuart KC handled. For NuCana Mr Acland KC did the oral advocacy on the undue burden case and Mr Mitcheson KC on all the other issues.
- There was a very large amount of detail in the trial, especially on the plausibility/technical effect in fact case and on the secondary evidence on undue burden. The factual disputes, it turned out, were quite narrow and I was greatly assisted by post-trial submissions organising the materials which led to some very useful agreed summary documents, for which I am extremely grateful.
- As well as in the EPO, there are proceedings in Germany which are for infringement. As to the latter, NuCana prevailed on infringement but there is an appeal and the German Court did not think there was sufficient doubt over validity to stay the proceedings. An injunction was granted but has not been enforced by NuCana yet.
- As I have mentioned above, the TBA will hear an appeal in relation to EP190 on 24 March 2023; the Opposition Division (“OD”) upheld EP190 with amended claims corresponding to the unconditional amendment put forward by NuCana at this trial.
- I reiterate comments that I and other judges of the Patents Court have made recently to the effect that the parties are under an obligation to keep the Court informed about the progress of EPO proceedings and especially if the TBA is likely to give a final ruling close in time to a UK trial. In the present case I think the parties could have done more in that direction. In the end it may well be that even if they had flagged the closeness of this trial to the TBA hearing it would have been undesirable or impractical to change the UK trial date, but it would have been desirable for the Court to have been able to make an informed choice. As matters have turned out, I have had the TBA’s preliminary opinion but not their actual decision (which may of course be different).
- NuCana submitted that I should not give this judgment until after the TBA hearing so that I can take the decision into account. Gilead submitted that I should give this judgment in any event, and even put in some witness statements in support of that contention, saying that NuCana had tried to slow things down.
- Neither side said I was obliged to follow either course and in my view it is a matter for my own judgment. I think the most important thing is that I should fully prepare my judgment while the trial is still reasonably fresh in my mind. Were I to wait for the TBA I would, in order to get a real benefit, have to wait not only for its decision but for the reasons as well, which could be some significant time later. Additionally, whatever the TBA decides will be in relation to EP190 only and not EP365. It might be that the decision on EP190 turns out to be damaging to EP365 (on added matter in particular), or helpful for it but given the way this litigation has been run by both sides, I do not expect with any confidence that either will give up on EP365 in the light of whatever decision is made on EP190.
- I have not been taken to the materials before the EPO in any detail, but from what I have seen it is apparent that on many of the issues I will have received different evidence from it. For one thing, I have heard cross-examination, and for another, I have had information about the CRO work and how it was set up that I do not think the TBA will have had. So if there are differences between our conclusions in due course that could well be a reason. On added matter one would hope that our conclusions would be the same, since it is a question primarily of disclosure of documents. I have had the opportunity to consider the OD’s decision and the TBA’s preliminary opinion. While I have not been able to consider the TBA’s final decision, they will at least be able to see mine.
- In Idenix v Gilead [2014] EWHC 3916 (Pat) Arnold J, as he then was, had to consider and decide essentially the same issue that arises in this trial in relation to alleged undue burden in synthesising the 2MU2FD compounds. Arnold J concluded that the patent in suit there was invalid for undue burden insufficiency.
- That trial concerned a different patent, albeit with a similar priority date, and NuCana was not a party. However, that trial covered a lot of the same ground as this, because reliance was placed on the Pharmasset and Idenix work in similar ways.
- In its Grounds of Invalidity Gilead expressly relied on the decision in Idenix v Gilead. Gilead was not as clear as it might have been about whether it was relying on the result of that action or simply saying that it was advancing the same case as it had there. NuCana objected that Gilead could not rely on the result because of the rule in Hollington v Hewthorn [1943] KB 587 and the point was resolved at the PTR where Gilead said that it was indeed simply saying that it would advance the same case, but that this trial had to be determined on its own merits with the materials before me.
- In those circumstances, and as I said to the parties during the trial, I have tried to avoid studying the decision of Arnold J in relation to the facts that he found on the undue burden argument. It seemed to me that it would involve the risk of my being influenced by a decision based on materials that were not before me and in which NuCana had no say.
- It has not been possible for me entirely to avoid Arnold J’s judgment. I knew the result because I read it at the time (and likewise the decision of the Court of Appeal); the parties have cited it and the Court of Appeal’s judgment in arguing points of law in this case; Prof Davies was instructed by reference to Arnold J’s judgment when assisting NuCana to design its EPO experiments; Prof Micklefield was given the judgment too as part of his “briefing bundle”.
- In addition, NuCana made a point in its closing submissions of explaining how the materials before Arnold J differed from those that I have had. One difference pointed out is that Dr Griffon was cross-examined in detail in that case but did not give evidence before me.
- Against that background, I emphasise that my task is to decide the undue burden issue on the materials before me, although I am aware that I have reached the same overall conclusion as Arnold J. It would be wrong to take Arnold J’s judgment and to seek to resolve this case by seeing how it differs, and that is not what I have done.
- The only witnesses from whom I heard oral evidence were the experts. All of the factual matters concerning work done by Idenix, Pharmasset and the CROs were proved by documentary materials.
- Prof Micklefield is Professor of Chemical Biology at The University of Manchester Department of Chemistry and The Manchester Institute of Biotechnology. He covered all the medicinal chemistry issues in the case.
- On the undue burden side of the case NuCana accepted that Prof Micklefield was a good and careful witness. I agree. In relation to the other issues, NuCana submitted that Prof Micklefield’s evidence was to be given less weight and was “hampered” by his lack of knowledge of nucleoside analogue medicinal chemistry. I cover this when I deal with the skilled team, below. NuCana said that this led to his having a “glass half empty” approach. I do not agree with that and do not think NuCana gave any convincing examples of it.
- Overall I find that on all the issues in the case Prof Micklefield was an excellent witness on whose evidence I can safely rely.
- Dr Galmarini received his MD in 1991, and then worked in clinical oncology. In 1999 he took up a fellowship in molecular oncology at the Léon Bérard Cancer Centre in Lyon, France, working on nucleoside analogues; during his time there he obtained his PhD. In 2005 he became Associate Professor at the Lyon Sud Hospital, where he continued working on NAs. Since 2008 he has had roles in pharmaceutical companies.
- NuCana said that it was not suggesting that Dr Galmarini was not trying to assist the Court, but that his views were out of step with the contemporaneous literature (particular emphasis was placed on one of his own articles, which I address separately below). NuCana said that this may have been a result of his being relatively junior at the priority date, still completing his PhD, but in fact he already had quite some years in clinical practice by then, as the summary in the preceding paragraph illustrates.
- In some instances I have rejected Dr Galmarini’s view of the CGK, and I cover these where they arise below. In particular, his view of the level of activity required for a compound to be considered promising for use in cancer therapy was more stringent than the CGK. But I do not think this was because of any personal shortcoming as a witness; identifying CGK correctly is difficult and witnesses who give entirely honest and straightforward evidence sometimes turn out to be incorrect. Often (as here) the position the court arrives at is in between what two experts have said.
- I also reject NuCana’s submission that there was a problem with Dr Galmarini’s evidence suffering from “a complete absence of supporting material” on CGK. That is simply incorrect.
- NuCana also criticised Dr Galmarini for a mistake he made in his initial evidence to the effect that glucose and fructose had recorded IC50s yet were plainly not cytotoxic because they are widely included in foods. He had misread the documents, which did not in fact relate to glucose/fructose. NuCana very sensibly accepted that the initial error was understandable, but that Dr Galmarini made the situation “much worse” by maintaining the underlying argument, when he was unable to give any other example in place of glucose/fructose. I agree that Dr Galmarini was significantly too stubborn about this when it was obvious that he was wrong, but I did not think that was symptomatic of any wider pattern or attitude.
- I therefore find that overall Dr Galmarini was a good witness. The criticisms identified above do not undermine that.
- In addition to Prof Micklefield and Dr Galmarini, Gilead put in an expert report from Prof Matthias Götte on the subject of virology. Virology as a possible technical contribution, and hence as a relevant discipline for expert evidence, has dropped out of the case, so in the circumstances I need not go into nor need I say any more about Prof Götte’s evidence.
- Prof Seley-Radtke is a Full Professor in the Department of Chemistry and Biochemistry at the University of Maryland, Baltimore County (UMBC).
- Prof Seley-Radtke has been extremely closely involved with nucleoside analogues for many years. Her very deep knowledge and experience were the basis for Gilead’s main criticism of her, which was that she applied the wrong standard for CGK, and treated as CGK any paper which was from a major group in the field or could be found in a literature search. I think this was indeed a problem with her evidence, although Gilead’s criticisms overstated it. However, it is not a criticism of her personally and in general I have been able to assess whether specific publications and items of information were or were not CGK without too much difficulty. In other instances during her oral evidence when she was finding it difficult to justify something as CGK based on the papers in the case she made references to “the wider literature” as a source for CGK. I found these sorts of answers unconvincing, and have taken that into account, though it is perfectly possible that she had read, but in the moment could not cite, materials that she thought supported her position.
- Overall my conclusion is that Prof Seley-Radtke was a good and helpful witness but I do have to take into account her approach to CGK as just mentioned.
- Prof Smyth is the Emeritus Professor of Medical Oncology at the University of Edinburgh. He has had a long and distinguished career and in particular from 1978 to 2008 he was the inaugural Chair of Medical Oncology at the University of Edinburgh. His research has had a focus on the development of new anti-cancer drugs.
- Gilead rightly did not criticise Prof Smyth’s qualifications but said there were a number of matters which individually were minor but collectively “gave some cause for concern”. The matters alleged were:
- Overall I agree that Prof Smyth’s written evidence could have been prepared with a little more care so as to reflect his views more accurately, and that in fact his reports supported NuCana’s arguments more than could be justified. This left him in a somewhat uncomfortable position during his oral evidence when he changed position. But at the same time Gilead’s criticisms are somewhat overdone and no doubt they would have made a criticism if Prof Smyth had not modified his position when effectively challenged. I do not accept the submission that the aggregate effect of these points was to cause “concern” and certainly not such as to lead me to question Prof Smyth’s integrity or independence and I thought he gave his oral evidence very fairly and straightforwardly.
- Prof Davies is the Waynflete Professor Emeritus at the University of Oxford and an Extraordinary Lecturer at New College, Oxford. He is a synthetic organic chemist of the greatest distinction. As he said in his first EPO declaration “I am not an ordinarily skilled person in organic synthesis. In fact, I am one of the leading experts in the world in organic synthesis”. This lack of any false modesty was realistic and refreshing.
- Prof Davies was criticised on a number of fronts by Gilead.
- It was argued for Gilead that Prof Davies was wrong to say (as he did) that he had experience in assessing the ability of synthetic chemists, because that was not a relevant discipline of the notional skilled team in this case. I reject the criticism. I am sure Prof Davies does have such experience and it was of real if indirect relevance in this case because of the need for the experts to assess whether the real world teams under consideration, especially Idenix and Pharmasset, succeeded or failed because of their level of skill. Although his comment about his experience was therefore on a topic which it was sensible to address (if not strictly necessary), the standards by which he judges others are clearly extraordinarily high, and not pitched at the level of the ordinary skilled addressee.
- More substantively, Counsel for Gilead attacked the way in which Prof Davies dealt with his material instructions in these proceedings in his reports. He acknowledged in his first report that he had given evidence in the EPO and indeed annexed his declarations there. The problem, though, was that it was in the context of those proceedings, and not this action, that he was first instructed in relation to undue burden. His instructions in the EPO were therefore potentially very important, and to emphasise the issue he was instructed by different people in the EPO and in these proceedings. In the EPO he was instructed by HGF (in particular Dr Jonathan Atkinson) and NuCana's US advisers, KIPS (in particular Ms Sherry Knowles). I deal with Prof Davies' EPO instructions in more detail in a subsection below, but in my view there was a significant and avoidable lack of transparency about Prof Davies' instructions which reduces the extent to which I can rely on his evidence about what the skilled synthetic chemist could and would do from the CGK. There was too much unacknowledged input from NuCana's advisers, the scope of which even now is significantly unclear, despite the provision of quite a lot of contemporaneous documents.
- I make it clear however that this is not a criticism of Prof Davies' bona fides. I do not consider there was any ill intent in the way in which he described his instructions.
- As I have said, Prof Davies has expertise, knowledge and experience far beyond that of the notional skilled person. In itself that does not mean that he could not put himself in the position of such a person, and whether and to what extent he did so is the key question. This point is frequently made in relation to eminent experts (more frequently in the context of obviousness) and often the Court finds that they have managed perfectly well to give their evidence from the right perspective.
- I also acknowledge that Prof Davies is a very experienced expert witness. He is fully familiar with the concept of the ordinary skilled person, and I accept his evidence that where he did not expressly call out the concept in his written evidence, he had it in mind. That does not mean that he managed to apply it, though.
- Taking his written and oral evidence as a whole, I formed the clear impression that Prof Davies did not succeed in putting himself in the position of the ordinary skilled person. His starting point was, in substance, how he himself would have approached the exercise of synthesising the compounds in question. This was well illustrated, for example, by his view that a literature search would not be done, or at least not in any great detail, prior to starting work, and that the person tasked with the synthesis would initiate some experiments very soon after being assigned, and deal with problems as they occurred. No doubt Prof Davies could come up with a range of strategies from his own personal knowledge without a literature search, and would be content to leave detailed issues until later, having a justified confidence that he would have the ingenuity to solve them. That does not reflect the position of the ordinary skilled person. I was also struck by the degree of difference between his predictions as to the ease of synthesis and the time needed, and the real world experience of the actual teams, even of those that succeeded.
- For this reason, I found Prof Micklefield significantly the more reliable guide to what the ordinary skilled chemist would think and do. I repeat that it is not a criticism of Prof Davies’s integrity or independence.
- Prof Davies does not have personal experience of synthesising nucleoside analogues but as I say in relation to the skilled team, I was not persuaded that that had any significance in itself.
- Prof Davies was instructed for the purposes of the EPO proceedings in early 2019 and interacted, as I have said, with Dr Atkinson of HGF and Ms Knowles of KIPS, from the US.
- Although of course Prof Davies gave oral evidence, neither Dr Atkinson nor Ms Knowles did. I was told by Counsel for NuCana without contradiction from Gilead that Dr Atkinson’s medical condition meant he would have been unable to give evidence, but that there was no positive reason why Ms Knowles could not. Gilead did not attach importance to their not being called, and in general I think it would be an undesirable trend for those who instruct experts in patent litigation themselves to have to give evidence, not least because usually there will be a good documentary record to refer to if necessary. In the present case I think it was foreseeably likely that the way in which Prof Davies was instructed would be important and need to be proved, and it ought also to have been apparent that there were material gaps in the paper trail, not least because on at least one occasion Prof Davies was invited not to commit matters to paper but to pass on his views in person at a meeting (this is a common practice in US litigation and I do not suggest it was nefarious). Nonetheless I do not draw any inference from Ms Knowles not giving evidence and propose simply to do the best I can with the materials available and with the help of Prof Davies’ evidence.
- At the outset of his instruction Prof Davies was given Gilead’s EPO Opposition Statement which informed him of the points run by Gilead on undue burden and of the routes tried and failed by Idenix. This posed an obvious risk of hindsight. One can understand rational reasons why it may have been done, not least because NuCana may have wanted Prof Davies’ input to assess the strength of its position, but to the extent that the intention was to have him replicate what the ordinary skilled person could do armed only with the Patent and the CGK, that was bound to be undermined by telling him how not to do it (as it were) and where the difficulties would be said to lie. Similarly, he was later given the judgment of Arnold J in Idenix v Gilead which accentuates the problems.
- Gilead also made a point of the fact that Prof Davies was given the Patent (EP190). I fail to see the force of this, since the issue was whether the Patent was enabling (although of course it contains no information about how to synthesise the 2MU2FD compounds).
- It was suggested to Prof Davies that at this early stage of his instruction for the EPO he was not told about the concept of the ordinary skilled person, was not told about the obligations of CPR Part 35, and was rather told that his task was to meet Gilead’s arguments.
- As to the first of those points, Prof Davies said that from his previous experience as an expert he knew about the concept of the ordinary skilled person, which, as I have said above, I accept, and that he would have been thinking in those terms even if not expressly told to do so, which I do not accept. There was in fact some hint of the relevant legal standard in HGF’s initial email of 20 February 2019 (“a skilled chemist should have been able …”) but the sense one gets from the documents and that I had from Prof Davies’ oral evidence was simply of him providing high level ideas from his own knowledge and experience without thought as to whether they were routine, to support the notion of the synthesis being possible. An example is his proposal of his own speciality SuperQuat technique, and he did not undertake the exercise of himself checking that what he was proposing was supported by the literature in its details.
- As to the second of those points (CPR Part 35), Prof Davies was not being instructed for High Court proceedings and I am sure he understood that he should act honestly and fairly, informed by his previous extensive experience as an expert. Such a general understanding is not a complete substitute for the more specific obligations of CPR Part 35, in particular in relation to the overriding duty to the Court, but I do not think it was wrong not to remind him of CPR Part 35, I do not think not doing so made a difference, and he was of course reminded of it by Powell Gilbert in due course.
- As to the third of those points (meeting Gilead’s arguments) I do think there is force in it. HGF and KIPS were engaged in knocking down those arguments and Prof Davies joined them in the exercise.
- Moving on in time, Ms Knowles was responsible for turning Prof Davies’ high level suggestions into the instructions for the CROs. This meant that it was she and not he who went to look for supporting CGK references. That was far from optimal. If Prof Davies was in due course to give evidence that the ordinary skilled person could carry out the syntheses correctly and without any hindsight, then this is an exercise he should have carried out himself, or overseen more closely (I note that he did “sign off” the schemes as being based on materials available in 2003/4 on 1 April 2019 but it does not appear that his review was a close one).
- Relatedly, two of the synthetic routes taken forward by NuCana in its litigation strategy (protected and unprotected uridine, one of which featured in Prof Davies’ first report in these proceedings) appear to have come from Ms Knowles; in other words she was not just filling in gaps in points of detail. Prof Davies was a little inconsistent on whether they came from her or not but on balance I find that they did.
- My conclusion is that Prof Davies’ instructions in the EPO were such as to nudge him towards putting forward synthetic routes that were more likely to succeed, more in matters of detail than in overall structure, and led him to have an approach which was some distance from that which would have been taken by the ordinary skilled chemist. In addition, significant details of the routes put forward came not from him but from KIPS. This was not in any way dishonest on his part or on the part of NuCana’s advisers, and it may well have been a function of time pressure as much as anything else. It does not disqualify Prof Davies’ evidence but I will take it into account when considering whether and to what extent his evidence represents what the ordinary skilled chemist would have done armed with the Patents and the CGK.
- There was broad agreement that the skilled team would include a medicinal chemist and a biologist/clinician. NuCana ended up calling three experts to Gilead's two because it had two medicinal chemists: Prof Davies for the undue burden synthesis issues and Prof Seley-Radtke for all other aspects of medicinal chemistry. There is nothing inherently wrong with this, and there are quite often good practical reasons for assigning a self-contained and experiment-heavy part of a case to a dedicated expert. However, it should not be allowed to undermine the fact that there were only two disciplines involved here, and I consider that the skilled medicinal chemist would be expected to know about both synthesis of molecules for testing, and about SAR analysis and development. Also, the division of labour that NuCana used should not be allowed to obscure the fact that Professor Seley-Radtke had herself done work on synthesis relevant to the undue burden argument, and found it difficult, as expressed in one of her publications.
- Such dispute over the skilled team as there was concerned the knowledge that the skilled medicinal chemist would have of nucleoside analogues. Gilead said that they would have and need knowledge of synthesising nucleoside analogues; the reason for this was to found a submission that Prof Micklefield had the advantage over Prof Davies. NuCana said that they would not need any special knowledge for synthesis but that when it came to SAR-type work there was an established field in relation to nucleoside analogues; it submitted that Prof Seley-Radtke was in this field and Prof Micklefield was not.
- In relation to synthesis, I agree and find that making nucleoside analogues presented difficulties, but I was not at all persuaded that those difficulties were of a special, let alone unique kind such that a general grounding in organic synthesis would not be adequate.
- In relation to SAR work, I find that there was a community of nucleotide analogue researchers, mainly in academia, with an identifiable body of literature and meetings and the like. But at the same time many of the skills and knowledge in medicinal chemistry are clearly transportable from one target to another, and the nucleoside analogue area was not so different that it was inaccessible to generalists. Also, I accept Gilead’s submission that the skilled medicinal team might be in industry and not academia in which case they would not have the extremely deep knowledge that Prof Seley-Radtke envisaged. In any event, in my view Gilead had an answer to this point on the facts, because although Prof Micklefield was not a dedicated specialist in this area, he had some knowledge and experience and I was satisfied that he had been able to put himself into the position of someone with the specific interest relevant to the issues in this case. If anything, there was a problem with Prof Seley-Radtke being excessively immersed in the area, as I addressed above.
- The other observation I would make in relation to the skilled team is that the delineation between the disciplines is not a rigid one, and the team would work in a flexible and collaborative way. One effect of this was that Dr Galmarini covered more ground (at the interface between the clinical and medicinal chemistry disciplines) than did Prof Smyth, certainly in the oral evidence. This did not itself affect the weight to be given to the evidence of any of the experts.
- I use “skilled team” and “skilled person” interchangeably below while keeping in mind that it is a team in this field, albeit that the oncologist does not have a role to play on the undue burden side of the case. When dealing with the undue burden issues I also refer to the “skilled chemist” (as the parties did) in the same sense.
- I will deal with the agreed CGK first.
- There was no dispute about this: see Terrell on the Law of Patents, 19th Ed. at 8-61 to 8-65. It is well recognised that information that the skilled person would find as a matter of routine when embarking on a task is not CGK as such but can be taken into account in assessing (classical) obviousness. I do not see why it should be any different for plausibility/sufficiency (the main issue for me) and the parties did not argue otherwise. Gilead argued, as I have mentioned above, that Prof Seley-Radtke was too prone to treat as CGK anything that was in a publication in the literature, and I have accepted the argument, but it was not a dispute about the legal principles.
- The parties produced a Statement of Agreed CGK (“SACGK”). It forms Annex A to this judgment. I found it very helpful and thank the parties for it. It is long and detailed and much of its contents, for example the early sections on the fundamentals of organic chemistry, will not be necessary for the understanding of this judgment for readers who already have some familiarity with that. Those sections include essentially all the agreed CGK relevant to the undue burden case (readers unfamiliar with the area would be best served by reading all of pages 3 to 16 with particular emphasis on the materials about stereochemistry).
- On the other hand, the material about nucleosides and nucleotides, nucleoside analogues for treating cancer, their mechanisms of action and their metabolic pathways, are probably less well known but are important to understanding the arguments and my decisions on plausibility/industrial application/technical contribution.
- To make this judgment understandable but also self-contained for readers who do not want to study the whole of the SACGK I therefore summarise the key aspects on that side of the case in the following section. Readers wanting more detail can of course refer to the SACGK; my account here starts at paragraph 55 of that document. The fact that something from the SACGK is not contained in this summary does not alter the fact that it is agreed to be CGK.
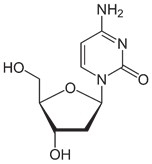
- Nucleic acid is made by linking together individual subunits called nucleotides, which consist of a pentose sugar, a heterocyclic base, and a phosphate moiety:
- A nucleoside does not have the phosphate part:
- Numbers with a prime (e.g. 2’) are conventionally used to refer to a position on the sugar part of a nucleoside (pentose above, in which the 2’ position is at the bottom right). Where the sugar is not attached to a nucleobase, the same atom is referred to without a prime.
- Nucleic acid chains form by the successive addition of nucleotides in triphosphate form. In an RNA or DNA chain there is only one phosphate between each nucleoside; the substrate for the polymerase enzyme that adds each nucleotide is the triphosphate. This is explained in more detail in the SACGK at paragraphs 60 to 65, with illustrations.
- Nucleoside analogues mimic the natural building blocks of DNA and RNA but are modified so as to interfere with replication. A number were known in 2003, and details are given in the SACGK at paragraphs 74 to 97. The ones that were mentioned most in the evidence and are of most significance were gemcitabine, AraC, 5-FU (also referred to as 5-fluorouracil) which is a nucleobase analogue and capecitabine, which is a prodrug of 5-FU.
- Given its importance to the case, I will give some more details of gemcitabine here.
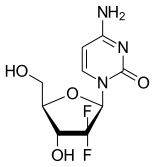
- Gemcitabine is also referred to as dFdC and is an analogue of its natural nucleoside counterpart deoxycytidine with two fluorine atoms substituted for the two hydrogen atoms in the 2’-position of the deoxyribose sugar.
- The two fluorine atoms at the 2’ position are important to a number of the issues in the case.
- Following clinical trials, gemcitabine was approved in 1995 for use in the treatment of pancreatic cancer and in 1998 for non-small cell lung cancer. It also demonstrated activity in breast cancer and was regarded as a promising new drug for solid tumours.
- After initial phosphorylation of gemcitabine (dFdC) into gemcitabine monophosphate (dFdC-MP) by deoxycytidine kinase (dCK), dFdC-MP is further phosphorylated by kinases to the diphosphate (dFdC-DP) and then triphosphate (dFdC-TP). dFdC-TP is the active form of gemcitabine that is a substrate for DNA polymerase (a more general explanation of phosphorylation is given below).
- Following the interaction of dFdC-TP with DNA polymerase, gemcitabine is incorporated into the growing DNA chain. Once incorporated, an additional natural nucleotide is added before chain termination occurs, a process known as “masked DNA chain termination”.
- In 2003 it was known that nucleoside analogues exert their anticancer effect in different ways, including by acting on specific enzymes (such as ribonucleoside reductase or thymidylate synthase), acting as a substrate for DNA polymerase and competing with natural nucleosides. When anticancer nucleoside analogues act as substrates for DNA polymerase and are incorporated into the growing DNA chain, they cause DNA chain termination. This means that further nucleosides cannot be incorporated into the growing DNA chain. This prevents the cell from completing the process of DNA replication and activates apoptosis (killing the cell) or causing growth inhibition (stopping or disrupting replication). The SACGK contains a diagram of this, but I do not think it is necessary to reproduce it here.
- Although not stated in the SACGK, it was not in dispute at trial that it was CGK that nucleoside analogues could take effect by more than one pathway.
- The active, therapeutic form of a nucleoside analogue is usually the triphosphate, since the triphosphate forms of nucleosides are the substrates of DNA polymerase.
- Natural nucleosides are generally hydrophilic molecules and do not readily permeate the lipophilic cell membrane. Their cellular uptake therefore primarily occurs via specialised nucleoside transporter proteins situated in the cell membrane (again, there is a diagram in the SACGK). Nucleoside analogues, being similar in structure to the natural nucleosides, are taken up into the cell by the same mechanism.
- Once the nucleoside analogue is inside the cell, activation into its therapeutically active form must take place through a series of phosphorylation steps mediated by cellular kinase enzymes.
- The first step of the phosphorylation process can involve the enzyme dCK, which transforms the nucleoside analogue into its monophosphate form. In almost all cases, initial monophosphorylation was the rate-limiting and therefore critical step for the activation of nucleoside analogues. Cells that were deficient in dCK were often resistant to the cytotoxicity of nucleoside analogues.
- A second phosphorylation step, mediated by another kinase, can convert the nucleoside monophosphate to a nucleoside diphosphate.
- The nucleoside diphosphate may be converted into a nucleoside triphosphate by yet another kinase. The triphosphate form of all nucleoside analogues is the active molecule that interacts with cellular DNA polymerase to be incorporated into DNA and that subsequently inhibits further DNA synthesis.
- There is more detail about this, including in particular some qualifications about the first phosphorylation step, and a diagram, in the SACGK at paragraphs 102-105.
- If it became apparent from testing (or was already known) that a nucleoside analogue’s metabolic pathway was irregular, or that the nucleoside analogue was susceptible to resistance issues or issues with drug delivery, then part of the goal for the skilled person would be to find an approach to overcome this. The desired consequence would be that anticancer activity (at least against resistant cells if not for all cancer cells) would be improved over the known nucleoside analogue.
- A well-known problem with otherwise potentially effective nucleoside analogue drugs was reduced intracellular concentration of the active nucleoside analogue triphosphate, which was caused by the existence of, or upregulation of, various resistance pathways. Of these pathways, the major ones affecting active anticancer NAs were poor or reduced uptake of the nucleoside analogue (caused by low expression or downregulation of nucleoside transporters) and poor activation of the nucleoside analogue (caused by low expression or downregulation of dCK).
- As mentioned above, the initial phosphorylation step to generate the nucleoside monophosphate was known to be rate-limiting for the majority of NAs. However, it was also well known that, in order to cross the cell membrane without first being dephosphorylated, the monophosphate form of a nucleoside analogue could not be administered directly without masking it in some way. This was because an unmasked phosphate is negatively charged and hydrophilic and therefore unable to permeate the hydrophobic cell membrane.
- One strategy to attempt to overcome this known to the skilled person was to use a protective group that allowed a NA monophosphate to be delivered into the cell in a masked form, hiding the negative charge and improving cellular uptake. The protective group would then be removed inside the cell to reveal the pre-activated nucleoside analogue monophosphate underneath. This is known as a “masked phosphate prodrug” approach.
- A key step in the development of a new nucleoside analogue is measuring its activity in vitro.
- In vitro cytotoxicity and antiproliferative activity assays are conducted in a variety of cancer cell lines. A cell line is a defined population of cells that can be maintained in culture for an extended period of time. Often, cell lines of breast cancer, pancreatic cancer, colon cancer, lung cancer, prostate cancer, leukaemias and lymphomas were used in cytotoxicity assays to study the effect of nucleoside analogues.
- The activity of a drug is usually described by some or a combination of the parameters listed below.
- Details of how to conduct the assays are given in the SACGK at paragraphs 117 to 127 but did not prove important at trial.
- There is a difference between killing cells and preventing them from replicating (“cytostatic”). “Cytotoxicity” more strictly refers to the former but sometimes is used to embrace both.
- A well-known in vitro assay was the MTT assay. Details are given in paragraphs 129 and 130 of the SACGK but were not referred to much at trial. The MTT assay does not distinguish between cytotoxic and cytostatic effects.
- It is possible to compare across assays but care is needed; details of what has to be taken into account are given in paragraphs 131 to 132 of the SACGK.
- There were also various in vivo tests, typically done in immune-compromised mice which had human cancer cells implanted subcutaneously. Tumour growth was compared for mice which received the compound under test and mice which received a control. Details are given in paragraphs 133 to 138 of the SACGK.
- I will now address the disputes over CGK other than those specific to the synthetic chemistry/undue burden issues, which are addressed separately below.
- As mentioned above, there was agreement that it was CGK that NAs exerted their anticancer effects by a variety of means. However, some points of detail arose about this, and about the related question of whether certain metabolites remained cytotoxic.
- The first was that, as Dr Galmarini said in his written evidence and Prof Seley-Radtke accepted in oral evidence, changes to an NA’s structure could lead to a change in its mechanism of action (assuming that it stayed cytotoxic at all), and that mechanisms had to be assessed by experiment. I find that as a general matter this somewhat increased the difficulty of making any predictions about the effect of changes, and in understanding such effects if observed. However, this is a relatively minor part of the picture because Prof Seley-Radtke said that while a change in structure could lead to a change in mechanism it was unlikely, and Dr Galmarini did not say that it was at all common.
- The second point related to Ara U and the third related to dFdUMP. They require a bit more explanation.
- AraU is a metabolite of the NA AraC, also known as cytarabine, which was in clinical use. Dr Galmarini said that it was CGK that AraU was not cytotoxic, based in part on his own publication from 2002 (Galmarini et al, ‘Nucleoside analogues and nucleobases in cancer treatment’ (2002)).
- Having originally herself said that the contents of Galmarini (2002) were CGK, Prof Seley-Radtke in her second report cited two papers from 1979 (Muller and Zahn, ‘Metabolism of 1-beta-D-Arabinofuranosyluracil in Mouse L5178Y Cells’ (1979) and 1985 (Yang, ‘Effect of Uracil Arabinoside on Metabolism and Cytotoxicity of Cytosine Arabinoside in L5178Y Murine Leukemia’ (1985)) which she said were to the effect that AraU was cytotoxic. When these were put to Dr Galmarini he questioned the force of their conclusions and said that they were not CGK.
- I find that the state of the CGK was that in Galmarini (2002): that AraU was inactive. The strength of that conclusion may possibly have been open to some modest doubt for a researcher who had spent significant effort looking into the matter and found the earlier papers (not cited in Galmarini (2002)). Galmarini (2002) was a review article with a very relevant title published close to the priority date; the earlier papers had nothing about them to make it likely that they represented the CGK and this was, as Gilead said, an example of Prof Seley-Radtke misunderstanding the standard for CGK.
- The AraU point was only a minor one in any event, though, because it just provided an additional example where changing the base on an NA abolished activity, and there were other examples in the case as I identify below.
- This was of more significance to the substantive issues in the case than AraU because it was specifically pleaded by Gilead that phosphoramidates of dFdU would be expected to be inactive but are within the scope of the claims. Gilead relied in its pleadings on three papers as representing the relevant CGK: two by Dr Galmarini (Galmarini et al ‘Pyrimidine nucleoside analogues in cancer treatment’ (2003), Galmarini et al ‘Nucleoside Analogues: mechanisms of drug resistance and reversal strategies’ (2001)) and one by Plunkett, ‘Gemcitabine: Metabolism, Mechanisms of Action and Self-Potentiation’ (1995).
- It is important to be precise here because, as NuCana pointed out, dFdU is not within the scope of the claims, being a bare nucleoside. The claims cover ProTide versions with a phosphoramidate group which are designed to get the monophosphorylated form into the cell. So one cannot just assume that because dFdU is inactive (which was not disputed to be the CGK), so is dFdUMP.
- Prof Seley-Radtke pointed out in her written evidence that Plunkett explained that dFdU was inactive because it was not a substrate for phosphorylation, whereas dFdUMP was.
- In his first report, Dr Galmarini had in fact said that there was a possibility (though he said a small one) that the use of a prodrug could cause dFdUMP to be more cytotoxic than dFdU. So there was a degree of common ground there.
- Prof Seley-Radtke also said in her first report that there was a basis for thinking that dFdUMP would be active because it was a TS inhibitor, and that was a known mechanism for the action of NAs. Her evidence in this respect was based on a 1999 textbook, Anticancer Drug Development guide, and a review article by Bergman (Bergman, ‘Determinants of resistance to 2’,2’-difluorodeoxycytidine (gemcitabine)’ (2002)). Further, Prof Seley-Radtke relied on another Plunkett paper from 1992 (Xu and Plunkett (1992), ‘Modulation of deoxycytidylate deaminase in intact human leukemia cells') which said that dUMP could be processed to the tri-phosphate of dTMP and incorporated into DNA leading to chain termination.
- Counsel for NuCana made some progress with Dr Galmarini on these points, at least to the extent of his accepting that these matters were possibilities. He resisted the proposition that they were CGK, but in my view at least the TS inhibitor possibility would have been found by routine research since it is in a textbook and in a review article. Gilead’s answer was that the CGK as to the mechanisms of action of gemcitabine did not include TS inhibition and therefore that NuCana’s position was illogical. But I do not think the position was as clear as that (as Bergman shows) or the logic so simple.
- So those are my findings about the CGK on the dFdUMP issue, and on the basis of them my conclusion is that having in mind the low standard required for plausibility, Gilead has not shown that it was implausible that dFdUMP would have some cytotoxic activity. This is not itself a conclusion about CGK, but it is a convenient point to state it. This is a very narrow victory for NuCana however; it does not cut across any of Gilead’s points about the general reasons to think that across the scope of the claims there would be likely to be many compounds with no real activity.
- I now come to a collection of related points about the CGK as it concerned the effect of positional changes in nucleoside analogues of the kind that this case concerns.
- Prof Micklefield was referred to a 2002 publication, a review article by Verma and others (‘Functional Tuning of Nucleic Acids by Chemical Modifications: Tailored Oligonucleotides as Drugs, Devices and Diagnostics’). He accepted on the basis of it that it was CGK that some modifications at the 2’ position could be tolerated by polymerases. But this was a very general proposition and was a long way from saying that it was CGK that predictions could be made in any particular situation. In addition, NuCana’s use of the document lacked cogency given that the document was only put in for cross-examination and was not spoken to by Prof Seley-Radtke. A similar point was made by NuCana in relation to the 5 position but suffered from the same problems.
- Of much greater importance to the arguments before me was a 1983 paper by Watanabe and others (Watanabe et al, ‘Synthesis of Antiviral Nucleosides’ (1983)) about an SAR study of modified nucleosides, with the intended purpose of antiviral activity. This was an important part of the basis put forward by Prof Seley-Radtke in her report for both the 2’ position on the sugar and the 5 position on the pyrimidine base.
- There was a dispute over whether Watanabe was CGK. Prof Seley-Radtke said that Prof Watanabe was a major figure in the field, which I accept, but that is not necessarily determinative of whether the publication was CGK. Watanabe was, Gilead pointed out, not cited in a 2000 review by Pankiewicz or in Prof Seley-Radtke’s own 2018 review papers despite a very large number of references being given. On the other hand, Prof Micklefield said that it would be found on a literature search for relevant nucleoside SAR work in 2003. This, coupled with the obvious relevance of the title, the fact that it was in a very reputable journal (J Med Chem) and the prominence of Prof Watanabe lead me to conclude that it would be found by routine means by a skilled researcher looking to put the invention of the Patents into effect.
- Watanabe shows in Table 1 various modifications at the 2’ position of the sugar and the 5 position of a pyrimidine base (X and R respectively):
- The ID50 column is a measure of cytotoxicity. However, the assay was done in normal and not cancerous cells which reduces the significance of the results, and there is no information about the mechanism of action of the compounds which makes it hard to extend any predictions which could be made from it to NAs which might be hypothesised to act like gemcitabine.
- Even putting these matters to one side it does not seem to me that Watanabe helps NuCana. As Gilead pointed out, for the 2A-2C compounds which have Br at the 5 position, F and Br at the 2’ position give cytotoxic activity but when Cl is used the activity is lost. Similar points can be made by reference to the inactive compounds 5A and 8B. So what is actually shown is that activity cannot be correlated just with the 2’ position or the 5 position but that they are interdependent and small changes make big differences (to the extent of a total lack of activity). There is really no predictability and what the skilled person would expect is that some combinations of substituents would preserve activity while others would abolish it, such combinations being unknown and uncertain until tested.
- Prof Seley-Radtke accepted that Watanabe showed that small changes could make a big difference including in relation to combinations of 2’ and 5 position substitutions but tried to limit her answers to the specific context, saying that in many other instances it was not the case. This was an example of her unsupported reliance on “the broader literature” that I have mentioned in my assessment of her as a witness and it was particularly striking in this case that she herself had chosen to put Watanabe forward in support of a fairly general proposition but then had to try to run it down. She did not point to specific other literature for examples to a different effect and none was put to her in re-examination.
- Prof Seley-Radtke relied on some other points/publications in relation to substitutions at the 5 position. These were not really relied on much by NuCana in closing so I will be brief:
- Gilead relied on one of Prof Seley-Radtke’s own publications, a 2018 review (‘The evolution of nucleoside analogue antivirals: A review for chemists and non-chemists’ (2018), published in two parts), which said that changes at the 5 position “can potentially alter the sterics, the electronic environment and even the hydrogen bonding interactions between the enzyme binding site and the nucleoside analogue”. She agreed that this would be CGK in 2003. I agree that this emphasises the complexity of the situation and the ways that substitutions at that position could affect activity.
- Similarly, Prof Seley-Radtke made some individual points about the 2’ position in her written evidence:
- A further point in relation to the 2’ position which I did find important was what Prof Seley-Radtke had referred to as “sugar pucker”. The point is that fluorine greatly influences the conformation of the sugar because of its high electronegativity. Prof Seley-Radtke agreed that this was CGK and it was set out in her own review paper and in Prof Pankiewicz’s 2000 review (‘Fluorinated nucleosides’ (2000)).
- Also in her review, Prof Seley-Radtke had commented in relation to gemcitabine that its two fluorines at the 2’ position had been found to have “pulled the ring carbon ‘out’ of the plane rather than a north or south conformation”. Again, Prof Seley-Radtke confirmed that this was CGK in 2003. The obvious implication of this is that changing away from two fluorines at that position will have a major effect on the conformation of the molecule; Prof Seley-Radtke gave some answers which I thought were very speculative in answer to this, and I think they were not to any greater effect than that it was just possible that a sufficiently electronegative substituent would not cause the change. The upshot is that the skilled person would think as a matter of CGK that having anything other than F at position X in Formula I when Y was F (expressing the point in terms of the Patents) would be a major change with unpredictable effects on activity.
- In her first report, Prof Seley-Radtke put forward what she said were seven examples of cytotoxic compounds in which the furanose oxygen of the sugar was replaced with a carbon.
- Of these, only one (C-Cyd, discussed in a 1991 paper by De Clercq et al (‘Broad Spectrum Antiviral and Cytocidal Activity of Cyclopentenylcytosine’(1991)) was put to Prof Micklefield. He said that no prediction as to the effect of changing the oxygen could be made because the oxygen was understood to have a big effect on the stereoelectronics of the ribose ring. Prof Seley-Radtke said the same in her review to which I have referred above, and confirmed that it was CGK.
- There is therefore positive basis in the CGK to think that this change might well affect cytotoxicity. It is true that C and O are classical isosteres (see below) but that does not overcome the specific effect of the change in this particular context.
- I was not satisfied that Prof Seley-Radtke’s other references were CGK and in any event my comment above about the 2’ and 5 positions apply: they do not allow SAR-type analysis but are just a disparate collection. However, the position was worse than that from NuCana’s point of view because a number of them included small/bioisosteric changes which reduced or eliminated activity. By way of example (there were others):
- I have referred to De Clercq as an example where changing the base changed a cytotoxic compound into an inactive one, and the same can be said of Watanabe (compound 4B v compound 8B). I have accepted NuCana’s submission that Watanabe would have been found by the skilled person by routine means.
- So in this instance too the skilled person would have reason based on the CGK to think that the change in question would have an unpredictable effect which might well reduce or remove activity. AraU was another example, and I have stated my findings about the CGK on that above.
- NuCana objected that the base point was not pleaded. I do not accept this. Gilead’s pleading was perhaps rather general and did not call out the base as an aspect of lack of plausibility specifically, but the point was touched on in Prof Seley-Radtke’s report in relation to plausibility (though not the SAR analysis) and was covered in NuCana’s opening.
- NuCana argued that predictions could be made that (bio)isosteres would behave in the same way, and that the effect of possibilities allowed for in Formula I could therefore be predicted (this did not apply to all of them, in particular X = Br is not an isostere of the other options allowed there).
- It was not in dispute that isosterism and bioisosterism were CGK concepts. The dispute was around the detailed nature of the concepts, and in particular whether and to what extent they permitted predictions to be made.
- I was referred to the following parts of Burger’s Medicinal Chemistry and Drug Discovery (2003 Edn.), both sides relying on the book:
- Gilead referred me to the following in Patrick’s textbook ‘An Introduction to Medicinal Chemistry’ (2001):
- I was referred by NuCana to the following passages in Silverman’s ‘The Organic Chemistry of Drug Design and Drug Action’ (1992):
- There was general agreement about what were regarded as classical isosteres, for example from Silverman Table 2.2. It is not necessary to set this out, but it should be noted that category 1a included CH3, NH2, OH, F and Cl but not Br (this is relevant to position X in Formula I of the Patents).
- I accept that the textbooks referred to above are sources of CGK. The question is what one draws from them. My assessment of the CGK is that:
- The closest Prof Seley-Radtke really came in her oral evidence to supporting there being predictability was to say that it could “inspire your particular design on your scaffold. Obviously you are given enough prior results in similar systems or other systems. If you see a consistent effect you are going to be able to predict, ‘Well, mine probably will too.’ But in order to actually know, you are going to make it and test it.” I understood this to mean that if the skilled person had already seen isosteric changes having consistent effects in a number of similar contexts to their own, they would have more confidence that the equivalent change would work for them. This is a facet of ongoing SAR work. Even there, she recognised that testing was necessary. As I go into when I come to the facts of this case, below, the situation with the Patents is not one where there was a body of meaningful SAR work to go on, only disparate individual results.
- There was a dispute about whether Prof McGuigan’s work on ProTides was CGK, although it was common ground that prodrug approaches in general were. The dispute does not have much practical importance, if any, since the work is clearly flagged in the specification of the Patents and so enters the picture for the assessment of plausibility (it is also referred to in Shepard). For what it is worth I find that it was widely published, sufficient to make it CGK.
- In relation to technical contribution, as I explain below, NuCana makes two alternative cases which involve different standards for cytotoxicity:
- There was a dispute about what the CGK was as to the attitude of the skilled person to assessing the potential of a compound as an anti-cancer agent.
- The “any measurable value” standard was NuCana’s primary case and Prof Seley-Radtke confirmed that that was the standard she had been applying in her reports when she referred to “cytotoxicity”. Prof Smyth initially said the same, but as his cross-examination progressed it became apparent that in fact he thought the level of cytotoxicity that had to be achieved in an in vitro assay for a compound to be considered to be worth taking forward was highly context-dependent, and he used the word “meaningful” cytotoxicity to encapsulate this.
- Dr Galmarini’s written evidence was that while there was no single value of IC50 that could be applied across the board, consistent values of 100 µM and above would be considered to show that a compound had no potential use as an anti-cancer agent, that 1 µM would be a reasonable cut-off, that ideally one would look for 10 nM or so for a highly promising compound, and that 1-10 µM would have been seen as borderline.
- Dr Galmarini also said that any compound could achieve some measurable value in some assay if the dose were high enough and the conditions of the assay lenient enough. Despite the error he made in relation to glucose and fructose, this point was not really contradicted and makes sense.
- In any event, I can dismiss the “any measurable value” standard quickly. At a purely semantic level it might conceivably be defended as a loose definition, but there was no evidence at all that it was used as a matter of CGK to assess whether compounds had potential as anti-cancer agents. Prof Smyth did not defend it in that sense but instead went for the more nuanced “meaningful” cytotoxicity, and Prof Seley-Radtke did not really defend it either, despite having said it was the standard she had applied in her reports.
- Having dismissed that standard, I think there was in truth common ground at least between Prof Smyth and Dr Galmarini that the CGK was that:
- Nonetheless, the parties continued to argue the case with a very numerical flavour to their contentions. One reason for this was that although the experts agreed that there was no single numerical cut off, it was an important part of the CGK that the NCI in the US carried out compound screening for cytotoxicity against a large panel of cell lines, and tested at concentrations up to 100µM. So 100µM was a very real value for the skilled person in this field, but not because it was a definite cut off for anti-cancer utility.
- I hold that the NCI screening approach was CGK and there was no real dispute about that. I also hold that it was CGK that the upper limit tested was 100µM and that the NCI went that high not because it thought that all compounds screened that achieved an IC50 in that range were potential anti-cancer agents, but because it was desired to get broad data for a wide range of compounds for analysis and further research.
- Gilead sought to meet the NCI approach by pointing to a 2017 publication by Hendriks and others written on behalf of the PAMM group of EORTC (‘Pharmacologically directed strategies in academic anticancer drug discovery based on the European NCI compounds initiative' (2017)), and in which Prof Smyth had been closely involved. Although the document was well post-priority it related to work which had started in 1993. It advocated a selection criterion of 1µM, along with other parameters, as a basis for deciding whether to take compounds forward, potentially into in vivo studies in mice. I was not satisfied that this was CGK and the exercise was done for a different purpose than the NCI’s work. It was also unclear to what extent resource constraints had led to the selection of the criterion.
- I think I need to go on to consider the parties’ numerical contentions, but I propose to do so only relatively briefly. I also have to bear in mind that the legal question I will have to answer is whether or not there is plausibility across the scope of the claims of the Patents, which is not the question the art was asking itself when discussing cytotoxicity values.
- First, I find that Dr Galmarini’s 1 µM was set too low as the cut off to decide whether to take compounds forward into an in vivo study. There was no CGK literature which said that, and for at least capecitabine, antagonist G (which entered clinical trials), cisplatin and oxaliplatin there were higher values, although for antagonist G the position is more complex because it had some lower IC50 results as well. There may well have been workers who applied such a cut-off if they were only willing to take forward very strong candidates but it was not the CGK and it was not regarded as a limit above which a compound would not be regarded as plausibly being useful as an anti-cancer agent.
- Second, as the IC50 went up towards and into the tens of µMs the number of known useful compounds dwindled sharply. It was put to Prof Smyth in cross-examination that there were no examples of compounds with results in that range being taken forward. NuCana said that was wrong because of the case of antagonist G. Even if I were to accept that Gilead’s absolute proposition was incorrect for that reason (which is not clear because of the variety of EC50s reported for antagonist G) or because of NB-1011 which I cover below, they are very isolated instances. As a matter of CGK the skilled team would think that IC50s at that sort of level were very unlikely to represent any real potential for anti-cancer use.
- Third, references in publications merely to “cytotoxicity” or “moderate” inhibition or sensitivity were often just ways of describing results in assays and were not judgments about clinical potential at all.
- Fourth, relatedly, some results were described as being of interest for further study. An example is Temburnikar, ‘Antiproliferative activities of halogenated thieno[3,2-d]pyrimidines’ (2014) (where an IC50 of 157 µM was reported and described as “modest”). But the skilled team would not equate that with potential as an anti-cancer agent. It just meant that something of interest had been seen that merited more research.
- Fifth, there were some special cases. An example was capecitabine which had an IC50 of over 1000µM in some cell lines in some reports. But capecitabine has to be activated by a liver enzyme and the very high values were found in cells which lacked the relevant enzyme (and a similar point applied to cyclophosphamide).
- Sixth, the skilled team would usually be looking for positive results in a range of cell lines. A result in a single cell line would not give rise to optimism on its own, except if what was being explored was drug resistance and only one resistant cell line was available. A similar consideration applies to the case of NB-1011, which is the phosphoramidate of BVdU and the focus of the Shepard prior art. It was of interest for use in TS-overexpressing cells and was taken forward for that reason despite an IC50 of 65µM in just one cell line; Prof Smyth agreed that one could not generalise to other compounds with similar IC50s in different circumstances. I am unclear if it was alleged that NB-1011 was said to be CGK in itself but whether or not it was, it is a good illustration of the CGK in relation to single cell line results and the importance of context.
- The CGK issues on synthetic chemistry were relatively few and tended to get folded into the rest of the argument. I summarise them in high level terms here and where necessary return to them in more detail where they arise.
- This is perhaps more an issue about the characteristics of the skilled chemist but it is convenient to cover it here. Prof Micklefield said that the skilled chemist would do a careful literature search before starting down the path of a synthesis of the complexity that arises in this case. Prof Davies said that the skilled chemist would not do so. Prof Micklefield’s evidence was more convincing - I do not see, for example, how else the skilled chemist could proceed with the fluorination step which had no direct precedent - and matches what the real teams did. I agree that it was CGK to do a literature search.
- This was put forward by NuCana as being a point on CGK. I think it is a point about the skilled chemist and I have dealt with it there. In any event I conclude that it does not matter to the result.
- This is not a point about CGK in the true sense but about what would routinely be found on a literature search. The parties generally agreed that Singh and Shreeve (‘Recent Advances in Nucleophilic Fluorination Reactions of Organic Compounds Using Deoxofluor and DAST’ (2002), (“Singh”)) and Wachtmeister et al (‘Synthesis of 4-Substituted Carbocyclic 2,3-Dideoxy-3-C-hydroxymethyl Nucleoside Analogues as Potential Anti-viral Agents’ (1999), (“Wachtmeister”)) would both be found so the issue is really what the skilled chemist would draw from them, and I address that where it arises, below.
- There were two disputes under this heading:
- As I have already said, the priority date is 21 July 2003.
- As I have also already said, I will give references to paragraph numbers in the EP190 specification.
- At [0001] the specification says that the invention relates to nucleotide derivatives and the treatment of cancer, and at [0002] it refers to three examples including gemcitabine, and says that they are activated to their 5’ phosphate form. But, it then explains at [0003], the phosphate forms have bad membrane permeability, in response to which prodrugs had been developed.
- The specification then explains that the inventors have worked extensively on a phosphoramidate prodrug approach, mainly in the antiviral field, and references are given to publications from Prof McGuigan’s groups. There is reference to improved intracellular delivery.
- At [0009] the specification refers to a publication called Lackey which relates to the use of the McGuigan approach to the anti-viral nucleoside BVdU. The resulting compound is said to have had significant anti-cancer activity as well. Lackey, it is common ground, is basically the same work as is relied on by Gilead in the Shepard prior art (which is itself mentioned at [0012]).
- [0011] says that “surprisingly” it has been found that other related derivatives are significantly more potent in the treatment of cancer.
- The specification then introduces formula I:
- And details of the choices at each position follow. They are subject to change as part of NuCana’s amendment application so I will not set out the granted or amended versions, which can instead be seen when I deal with the claims.
- There follow some paragraphs of definitions, and then starting at [0050] there is a description of how modifying the ester moiety in compound (7) (from the Lackey work) had showed increased potency with respect to some cancer cell lines. The passage from there to [0057] discusses some comparisons and inferences that the authors say may be drawn, and then [0058] makes a similar claim to [0011] - improvement in anti-cancer potency for pro-tided BVdU derivatives. [0059] and [0062] reiterate the use against cancer with the latter giving some examples of types of cancer for application.
- [0073] introduces a method for preparing a compound of formula I, starting with a compound of formula (III). Although the method is explicitly said not to be part of the present invention, formula (III) is relevant to the undue burden case because it is particular instances of these intermediates that Gilead contends cannot be made without undue effort.
- There is then a very long section about syntheses which is not relevant to the issues between the parties. The next part that is relevant begins at [0297], which introduces Table I, setting out the EC50 in three cell lines of a large number of compounds. However, only three are within the claims of the Patents: the last 3 in the Table, CPF31, CPF40, CPF41, which follow immediately after “G”, which is gemcitabine. All the other compounds are BVdU derivatives.
- Some details of the experimental methods are given in [0298]. The experimental protocols and particularly what is said to be a lack of details given are attacked by Gilead and I deal with that below. In paragraph [0298] the authors refer to “cytotoxity” [sic] and Gilead relies on this as being the only place in the Patent where cytotoxicity is referenced, in its attack on NuCana’s arguments on technical contribution.
- Details of in vivo experiments in mice are then given at [0300]. They are said to compare gemcitabine with CPF31. Again, Gilead attacks the rigour of what is presented and I deal with that below.
- Although it forms part of the assessment of plausibility rather than simply being an identification of what the Patents say, I find it convenient to deal here with the quality of the experimental data in the Patents, which Gilead submitted did not allow reliable conclusions to be drawn.
- Gilead made a number of criticisms of the in vitro data in the Patent.
- First, it said that [0298] showed that the times over which the test compounds were added to the cells were variable and that that meant that comparisons could not be made between the results, in particular to see whether the compounds in the claims were better or worse than gemcitabine. Dr Galmarini accepted, however, that it was likely that consistent times were used, albeit that he said that the failure to say what they were meant that the experiments could not be specifically reproduced. Gilead relied on the fact that Dr Galmarini used the word “speculation” in this evidence, but read as a whole he meant that one could not be certain of consistent exposure times because it was not stated, but it was likely.
- Second, Gilead said that the data in Table I had no standard deviations. This is true and it is exacerbated by the fact that elsewhere in the Patents (at [0056]), albeit in a different assay to that of the Table, results which are eightfold different are said to be the same within experimental error. However, Prof Smyth rejected the proposition that this made the Table I data worthless.
- Third, Gilead said that the data in Table I are EC50 values but the maximal effect is not stated. Prof Smyth accepted that this is undesirable.
- Fourth, Gilead pointed out that the EC50 for gemcitabine in the HT115 colon cell line, 606µM, was much higher than would be expected. Dr Galmarini said that this was an indicator that something had gone wrong with the assay or that the cells had developed resistance and that the result was a “red flag”. Prof Smyth’s written evidence was that the skilled team would not think there was either a problem or resistance but in his oral evidence he said that the skilled team would take the data as presented but would want to see further information before relying on the result.
- Fifth, Gilead said that if one left the HT115 results out of account, because of the previous point, then the compounds of the claims were only better than gemcitabine in one instance (CPF31 in the prostate cell line). This is true. If one adds in the HT115 results then naturally all three claimed compounds are better, because the gemcitabine EC50 is so high. Prof Smyth’s oral evidence gave me the impression that he was applying a standard that the compounds should be regarded as working if they demonstrated cytotoxicity and that improvement over gemcitabine was not the metric he was applying and could not be inferred from the Table.
- NuCana made the general points that (a) the standard for plausibility is significantly lower than that for a peer reviewed paper, and (b) that it could be inferred that the work had been done competently even if details were not given, in particular because of the reputation of Prof McGuigan’s group. The first point is certainly correct and Dr Galmarini accepted it. He also accepted the second point to a considerable degree and I think that there is sense to an assumption where the Patent is silent that the workers did not do something that would be foolish or bad practice, but assuming that the methods were sound cannot help with the limited amount of data and statistical presentation of it.
- The in vivo data is from mouse xenograft experiments done only with CPF31 and gemcitabine.
- Gilead pointed out that Figure 2 did not compare CPF31 with gemcitabine but only with control, that it had no error bars and that it gave p-values of 0.094 and 0.096 so did not apply the conventional value of 0.05 as an indication of statistical significance. I understood Prof Smyth to accept that one could not compare across from Figure 1 to Figure 2 without having error bars or standard deviations and so no comparison of CPF31 with gemcitabine could be made.
- Figure 3 is not relevant; it is unclear what was done and anyway it relates only to side effects, as Prof Smyth accepted.
- On Figure 4, Prof Smyth had said in his second report that firm conclusions could not be drawn from it but that the difference between the CPF31 group and the gemcitabine group looked promising.
- NuCana maintained that there was no sufficient reason to doubt the comparison in Figure 4, but did not specifically cross-examine Dr Galmarini on his written evidence that no conclusion could be drawn from any of the in vivo work.
- Many of Gilead’s points have significant force but their impact is reduced by the fact that the standard for plausibility is a low one. Others of Gilead’s points are picking holes where the Patent falls short of the standard of giving detail that would be expected of a scientific paper.
- Weighing these points up, I find that the in vitro data makes it plausible that the three exemplified compounds within the claims have some degree of cytotoxicity in the breast and prostate cell lines. The colon cell line results cannot be given any weight. Not knowing the maximal effect for the EC50 means that the level of potency cannot be assessed. The in vitro data does not allow any comparison to be made with gemcitabine, even allowing for the undemanding standard applied.
- On the in vivo data I find that it is plausible that CPF31 has greater activity than gemcitabine in the one cell line used. There is a concrete difference in Figure 4 and while there are real issues with the experiment and the presentation of the data, the plausibility threshold is crossed.
- My findings mean that the experimental data renders certain effects plausible, but they are on a weak evidence base and, more importantly, across a very narrow scope (three compounds having some cytotoxic activity in some unknown degree, one being more active than gemcitabine in one cell line). I reject Prof Smyth’s assertions that conclusions can be drawn any more broadly, at least from the perspective of the skilled oncologist. The issue of drawing inferences across the much broader scope of the claims of the Patents is one for the medicinal chemist and must take account of the very narrow scope of the data in the Patent.
- The specification of EP365 is much shorter than that of EP190. The following points of difference were drawn to my attention:
- All the relevant claims of both Patents are compound claims defined by Markush groups, i.e. “A chemical compound having Formula I:”. The differences between the claims lie in changes to formula I, which is as follows:
- In its opening skeleton, NuCana provided a very helpful chart which allows the reader to see at a glance the differences between the claims. At my request an agreed version was prepared:
- This also includes a comparison with the disclosure of the PCT and indicates the support in the PCT relied on by NuCana for the proposed amended claims of EP190 and the claims of EP365 (in the format pageline).
- It should be noted that “alkyl” does not have the same meaning in each document. In the PCT it is defined at p.5 lines 22-26, in EP190 (as proposed to be amended) it is undefined, and in EP’365 it is defined at [0014]-[0015].
- Claim 3 of EP365 was not separately in issue; it is just a claim to a pharmaceutical composition containing a compound of the earlier claims. For EP190 only claim 1 needs to be considered.
- The claims are all product claims. Sofosbuvir falls within the structural definitions given so dealing in it would be an infringement unless the claims were somehow implicitly limited by reference to their use. It appeared that Gilead might have been going to argue that they were so limited but in its opening submissions it accepted that on the basis of Fibrogen v Akebia, supra that the claims were to be construed as being to the products per se.
- On that basis sofosbuvir would infringe if the claims of the Patents were valid, but they are not.
- I will deal first with added matter since that is “internal” to the Patents and less evidence dependent.
- Then I will deal with industrial applicability/plausibility.
- Next I will deal with obviousness over Shepard.
- Lastly I will deal with the undue burden case.
- Claim 1 of EP190 both as unconditionally and conditionally proposed to be amended, and claims 1 and 2 of EP365 are all alleged by Gilead to be bad for added matter.
- As I have said above the form of claim 1 of EP190 as unconditionally proposed to be amended is the claim upheld by the OD.
- Gilead’s essential complaint behind the added matter points is that NuCana has impermissibly cut down the Markush group of formula I by making deletions from the respective lists for the various substituent groups. Gilead says that this leads to the disclosure of classes of compounds not taught in the relevant original application.
- Some of Gilead’s complaints were run against the claims of EP190 as granted and some only in relation to the proposed amendments. Since NuCana accepts that EP190 has to be revoked if the amendment is not permitted, this distinction does not matter and procedurally Gilead consolidated all its points on EP190 into the Statement of Objections to the amendments.
- In terms of the prosecution history of EP190 and EP365, each was granted on a divisional application from an original PCT, WO 2005/012357 (“the PCT”). Since the divisional applications were not relevantly different from the PCT, the parties argued added matter by comparing the disclosures of EP190 and EP365 with that of the PCT.
- The reasons why defining the claims of the Patents by reference to narrower (compared with the PCT) classes of compounds may be beneficial to NuCana are:
- NuCana disputes the legal relevance of the reasons for, or benefits of, the amendments.
- There is a lot of detail surrounding these issues. It will help if I illustrate the crucial point and say what the parties’ main points are.
- In the PCT the substituents at positions X and Y (the 2’ position of the sugar) were to be independently chosen from the group comprising H, F, Cl, Br, I, OH and Me (methyl).
- By contrast, in claim 1 of EP190 as unconditionally proposed to be amended, Y has to be F and X is to be chosen from F, Cl, Br or Me.
- The conditionally amended form of EP190 and claim 1 of EP365 remove Cl as an option for X, and claim 2 of EP365 removes Br, leaving only F or Me.
- Gilead says that this adds matter by disclosing new, narrower classes of compounds. It also says that this amounts to putting forward a “different invention”.
- NuCana on the other hand says that it is allowed to make deletions from multiple independent lists without adding matter, providing that it is merely restricting what the claims cover. It says that it does not matter what the reasons for or benefits of the deletions were. It accepts that there has to be some limit on doing this, but that that limit only comes if a patentee tries to cut back to a tiny number of compounds, possibly only just one. It says that the notion of a “different invention” is irrelevant and that the only test is the “gold standard” (a term from EPO case law) of disclosure. It says that there is adequate disclosure of each possibility in any list and that with multiple lists there is adequate disclosure to make any choice from any list in combination with any other choice from any other list. Subject only to the limitation against dropping down to one or a very small number of compounds, it says that a patentee is allowed to revisit an initial disclosure and cut down multiple lists by deletion, even if guided by knowledge as to what works and what does not that it obtained only later.
- Which side is right depends mainly on the proper legal approach to deletions from multiple lists. Both domestic and EPO case law are relied on.
- The basic law about added matter is now well settled in this jurisdiction. A commonly cited authority is the decision of the Court of Appeal in Nokia v IPCom [2012] EWCA Civ 567. This contains nested citations of earlier cases and of EPO case law, and it is worth my setting out a significant section in view of the issues in this case:
- The Court of Appeal in Nokia went on to consider the law applicable to claim broadening and intermediate generalisations, neither of which is relevant to the present case.
- Apart from its clear statements of the basic approach to apply, I find the judgment helpful for the encapsulation of the reasons for the rule, expressed by reference to G1/93 and the decision of Richard Arnold QC, as he then was: adding matter not disclosed in the application could give the applicant an unwarranted advantage and damage the legal security of third parties. An aspect of the potential unwarranted advantage would be to circumvent the first-to-file rule.
- In the light of the arguments in this case I will expand briefly on those reasons.
- As to the legal security of third parties, that matters because although patent claims can change and indeed be broadened in prosecution, third parties ought to be able to read a patent application and form a view as to the maximum extent of valid protection that will be achievable. If they (rightly) conclude that a feature is essential then they ought to be able to conclude that they will not infringe a valid claim that grants later if they do not use that feature. There is no perfect certainty because whether a feature is essential is a matter of judgment, but the principle is clear.
- NuCana argues that selecting down from multiple lists does not cut across this kind of legal security because all the possibilities to be covered in the claims now in issue were covered by the original Formula I. I agree with this but it is a distraction and an incomplete answer, for at least two reasons.
- The first and probably more minor reason is that making a selection from multiple lists for which there is no basis, if permitted, could cut across the expectation of third parties who had concluded both that a broad class disclosed in an earlier application was invalid (for insufficiency or over the prior art), and also that there was no narrower class disclosed to which the applicant for the patent could fall back, or perhaps that any narrower fall back would not be infringed.
- The second reason is circumvention of the first-to-file rule. An applicant might file an application with a very broad Markush group without knowing or having any idea which of the compounds covered worked. If the applicant were allowed later in prosecution freely to cut down the Markush group by reducing the options in multiple lists with later knowledge of which compounds did work, it could keep the original application’s date of filing for an invention which was in fact not disclosed in it (indeed one would say that the selection-type invention had not even been made at the time of filing). This would of course be unfair on a third party who had in fact, earlier than the applicant but after the original application, worked out which compounds worked.
- Whether and when selection from multiple lists can add matter has been considered in the UK. It was reviewed by Arnold J (as he then was) in Merck v Shionogi [2016] EWHC 2989 (Pat) at [288]-[293] in the context of the Court of Appeal’s decision in Nokia v IPCom, the Case Law of the Boards of Appeal of the European Patent Office (then the 8th Edition, but I do not think there has been any significant change relevant to this case) and to the earlier decision of Henry Carr J in GlaxoSmithKline v Wyeth [2016] EWHC 1045 (Pat).
- Although Arnold J’s citation from the EPO Case Law did not cover all the same EPO decisions as were cited to me, in my view the gist was exactly the same as the conclusions that I reach below about the EPO case law.
- In Merck, Arnold J went on to conclude that there was no added matter. The EPO reached a different conclusion on the same patent in T1150/15 (see below), but that does not mean there was any difference of approach.
- A similar issue was considered by Arnold J in Idenix v Gilead. At [609]-[610] Arnold J found in relation to some proposed amended claims that deleting options from the possibilities for R1 and R2 (it does not matter what they were) disclosed a new sub-class of compounds, not previously disclosed. He also accepted a submission that this was made worse by the fact that it was done to remove from the granted claims compounds which, the patentee’s own expert had said, were not plausibly effective.
- The Court of Appeal said ([2016] EWCA Civ 1089 at [206]-[210]) that Arnold J was right for the reasons he had given.
- NuCana submitted that Idenix was distinguishable on this point because it was the patentee’s own expert who said that there was a lack of plausibility. I reject that. It cannot make any difference what the evidential basis was, what mattered was that it was relevant to take into account that the amendment had the effect of providing for the first time a class for which plausibility was present.
- There is extensive case law in the EPO about selections from multiple lists and (more specifically relevant to the present case) deletions from lists.
- The current statement in the EPO Case Law book (10th Edition, 2022) is as follows (emphasis as in the original):
- As I have already said, the equivalent section of the 8th Edition was considered by Arnold J in Merck where one of the conclusions which he drew was that the degree of narrowing was important. I agree. There will be a particularly severe problem if the patentee tries to narrow down to just one previously undisclosed compound, but that is not the only time there will be added matter (see below). It is a question of degree.
- In the present case both sides took me to individual decisions of the TBA and indeed of the Enlarged Board (there is no Enlarged Board case directly on point, they were cited in relation to broader concepts). I consider them individually below to the extent I consider it helpful, but because the EPO Boards of Appeal do not apply a strict doctrine of precedent and because some decisions are more closely reasoned than others, the distillation in the Case Law book is always extremely valuable. In the present case I identify from it the following principles:
- I turn to consider the individual EPO cases relied on by the parties before me.
- This is certainly a seminal case and the first citation given in the Case Law book. NuCana relied on it in particular, and it was the citation given by the OD as justifying the EP190 claims as unconditionally proposed to be amended.
- In relation to identifying points of principle, it clearly says that:
- These support the propositions I have listed above, because of course they are the origin of them. It is notable that the “generate another invention” touchstone was thus in the case law from this early stage. It is rather inconsistent of NuCana to rely on the case while saying (see below) that the “another invention” concept is not good in law.
- It appears that the amendment which was allowed was for the purpose of establishing novelty. In information provided to me after trial (in response to my request during closing submissions to be told the reason for the narrowing) NuCana submitted that other amendments were made in prosecution to remove from the claims compounds which the examiner had said did not work, but this was not the basis of the TBA’s reasoning so is irrelevant to establishing legal principles.
- NuCana relied on this case as well. It is more briefly reasoned than T615/95, but referred back to it, in apparent agreement. As it happens, it does not mention “another invention” but that is plainly not because of a disagreement with the legal reasoning in the decision in T615/95.
- I was told by the parties after trial (again in response to a question from me) that the amendments were made in prosecution at least in part to support inventive step (and novelty), but this was not considered one way or another by the TBA so again does not help with identifying the applicable legal principles.
- The decision in the case was also significantly influenced in my view by the fact that the patentee did have a specific basis for some of the choices from the lists (see 2.1), a factor not shared by the present case.
- In any event, I do not get any additional or different principles of law from this case.
- Gilead relied on this decision because it emphasises the relevance of whether the deletions “generate another invention” (see the last paragraph of 2.4.1) and for the statement in the second paragraph of 2.4.2 contrasting simple exclusion of embodiments with a limitation which “results in improvements, or even additional effects, for which the original disclosure provides no basis”.
- NuCana met this on two fronts. First, it said that it was doubtful whether the “another invention” limb was good in law. I deal with this further below. Second, it said that the case was distinguishable on the facts. I do not think comparing facts is useful or legitimate; it is enough to observe that the TBA thought it was relevant that the limitation of the claim was relied on to improve the patentee’s position over the prior art.
- I think the case provides support for Gilead’s case that if a limitation gives improvements or additional effects, that can be an important tell-tale sign of “another invention”.
- This is the TBA decision on the same patent as considered (earlier) by Arnold J in the Merck decision I refer to above. Of course, Arnold J did not have the benefit of the TBA decision but in any event I agree with Gilead that the difference in conclusion was not due to divergent legal standards but to the assessment of the facts.
- I do not think the decision establishes any new or different principle. It is true, as Gilead submits, that the TBA commented on mixing preferred choices with choices for which no preference was expressed, saying that that did not necessarily provide basis for the combination, but the reasoning was quite fact-specific. At a more general level it is of course relevant to added matter whether a particular feature is expressed to be preferred, and I consider the impact of that when I come to the facts.
- NuCana relied on this decision to argue that taking account of whether there is a “different invention” is not part of the law of added matter generally, and that the Enlarged Board put in place, or reaffirmed a “gold” standard based (purely) on disclosure.
- I reject this. G2/10 is one of a number of decisions of the Enlarged Board dealing with disclaimers. The question referred related to disclaimers of embodiments of the invention and whether and when such disclaimers would constitute added matter. A particular issue the Board had to grapple with was consistency with its earlier decision in G1/93 about undisclosed limiting features. It also had to consider whether its earlier decision G1/03 had been to the effect that a disclaimer could a priori not add matter.
- In this context, the Board decided (4.3, last paragraph) that “neither decision G1/93 nor decision G1/03 intended to modify the general definition of the requirements of Article 123(2) EPC established in opinion G3/89 and G11/91, which definition has become the generally accepted, one could also say the ‘gold’ standard, for assessing any amendment for its compliance with Article 123(2).”
- What the Board meant by the general definition in G3/89 can be seen at the start of 4.3:
- This simply says that all amendments are subject to Article 123(2) and are limited by the matter disclosed (explicitly or implicitly) in the application as filed.
- The Board also said that G1/93 was dealing with the special situation of the relationship between Article 123(2) and Article 123(3) and the “inescapable trap” problem.
- I do not see anything inconsistent in G2/10 with the notion that when asking whether an amendment adds matter, which is the fundamental question, it will be relevant to ask whether it presents a different invention, and that part of that inquiry may be whether it provides a new technical contribution. One is not inquiring whether there is a new technical contribution instead of asking whether there is added matter, but simply recognising it as a likely symptom of there being added matter.
- Furthermore, there is no sign in the EPO’s case law of its thinking that G2/10 meant that the existing law about deleting from lists was wrong. The Case Law book cites decisions from before and after G2/10 and they are all to the same effect.
- I also noted above that in Idenix v Gilead Arnold J accepted an argument that the provision of a technical contribution across the scope of the claim for the first time was relevant to added matter, and the Court of Appeal upheld him. The G2/10 argument was not made, however.
- NuCana submitted that the fact that a patentee makes an amendment because it desires to cut the claims back to embodiments that are enabled, or to give a better argument for inventive step over some prior art citation, does not mean that there is added matter. I agree with this. As NuCana also argued, those are two entirely normal and very common reasons for amendment and there is nothing wrong with either of them. However, the issue I am considering is nothing to do with motive, but about substantive permissibility. If there is basis for an amendment it will not be refused because the patentee wants to solve an insufficiency problem, and if there is no basis for the amendment then it will not be allowed whatever the motive.
- Given my reasoning above, the effect of an amendment, such as to allow a new argument on inventive step (as distinct from the motive for it) may also be relevant to added matter. But again, that is not the same as motive, although no doubt the patentee’s motive will have been to obtain the effect in question.
- Gilead relies on the preliminary opinion of the TBA, paragraph 6.3, which was as follows:
- NuCana submitted that the decision of the OD in its favour is of equal weight to this. I disagree. They are just not comparable because (a) what the OD said was its actual decision and the TBA’s statement is preliminary and may change at the hearing, but (b) the TBA is an appellate body whose decision, were it final, would be more persuasive than a final decision of the OD.
- Although the TBA’s opinion would not be binding on me even if it were its final decision, it is still a considered expression of its current views and it is right that I should give careful thought to it. It fully accords with my views. Restricting Y to a single meaning would not, on its own, add matter according to the EPO’s approach. But combining that restriction with a more limited set of options for X amounts to “singling out” a smaller class of compounds. And it is relevant that the effect of doing so is to provide a technical contribution over Shepard (although my decision would have been the same without that factor).
- I will need to consider the disclosure of the PCT. Then I will need to identify the disclosure of EP190 and EP365. Then I need to compare them and consider whether there is added matter.
- There is no material dispute about this. That being so, the following description is taken largely from Gilead’s skeleton, with submissions removed.
- After a preamble corresponding to [0001]-[0011] of EP190 ([0001]-[0003] & [0005]-[0012] of EP365), the PCT states (p.3 line 12 - p.4 line 10):
- At p.5 lines 22-23 the PCT explains that:
- Suitable and preferable R, R’ and R” groups are discussed at p.7 line 16 - p.9 line 12 (and there are dependent claims relating to such groups). This passage is relevant to EP365 but I will not set it out in full because in the light of the arguments made the details do not matter; Gilead accepts that the groupings for R, R’ and R’’ are contained in it, though not, it says, in combination.
- At p.9 line 14 the PCT explains that preferably Q is O. Then it says (p.9 lines 16-21):
- It then says (p.9 lines 23-33):
- At p.10 lines 2-12 it says:
- After considering Ar, at p.11 line 5 the PCT turns to Z. At p.11 lines 15-22 it says that:
- When it comes to the in vitro testing, the same compounds as in the Patent are made and tested, nearly all being phosphoramidated derivatives of BVdU. These are compounds in which n is 1 and X and Y are both H (see p.9 line 18) and indeed compounds in which n is 1, Z’ is O, Q is O, X and Y are both H, and Z is -CH=CHBr (see the last passage quoted in the preceding paragraph).
- Again as in the Patents, the other three compounds which are made and tested are phosphoramidated derivatives of gemcitabine (as well as gemcitabine itself). These are compounds in which n is 0 and X and Y are both F (see p.9 line 20 and p.10 line 2) and indeed compounds in which n is 0, X and Y are both F, Q is O and Z is H (see p.10 lines 2-4).
- As to the claims of the PCT, claim 1 corresponds to formula I as set out at p.3 line 12 - p.4 line 10. Claims 16-19 are as follows:
- Thus the PCT discloses four combinations of n, X and Y: (1) n is 1, X and Y are both H, (2) n is 0, X and Y are both F, (3) n is 0, X is OH and Y is H and (4) n is 0, X is H and Y is OH.
- It will also be relevant to consider the statements of the utility of the compounds disclosed, for example at page 14, second, third and fifth paragraphs.
- The key points are the definition of formula I and the corresponding aspects of the claims.
- The statements of utility of the compounds disclosed remain textually the same as in the application but Gilead asserts that they are different in substance because they refer to different classes of compounds. For example paragraph [0059] refers to formula I as did the third paragraph on page 14 of the PCT, and formula I is to be amended.
- There is a different definition of “alkyl” at [0015] with related changes at [0017], [0023], [0044], [0047] and [0049], and the statement that X is preferably selected from F, H and OH is deleted at [0038].
- The unconditionally amended form of EP190 differs from the conditionally amended form only in that the latter also has X = Cl deleted.
- There is no dispute about the differences in formula I or the claims (which can be seen from the agreed table set out above). I agree with Gilead that in the context of EP190 the statements of utility would be understood to refer to amended formula I, so that is an additional difference.
- It is also clear that the definition of alkyl is changed, but I intend to deal with that separately below since it is also the subject of a clarity challenge and a more minor point.
- In my view there plainly is. I will focus on X and Y.
- Compared with the PCT, the class of compounds of Formula I is much narrower. Y has to be F and X has to be F, Cl, Br or Me (except Cl is excised in the conditional amendment). By contrast the PCT allowed X and Y each to be any of H, F, Cl, Br, I, OH and Me.
- The new class is significantly different and the skilled person would not think that there had been a mere reduction still leaving the same generic class differing only in size.
- The problem is significantly exacerbated by the fact that the narrowing does not correspond to the statements of which possibilities are preferred: Y’s having to be F was one of the preferences expressed, but at the same time it was said that X ought also preferably to be F, H or OH, and the proposed amended claim keeps F but discards H and OH and replaces them with F, Cl, Br and Me, a mixture of those preferred and those not said to be preferred. There are also more detailed preferences for X and Y in the PCT depending on the base, but the amended claims do not correspond to them, either.
- When EP190 says that there are benefits to the compounds disclosed it is talking about a different class of compounds from those the PCT identified. There was no such claim for the class in the PCT.
- NuCana’s only real answer to these points (it particularly struggled with the point about the statements of preference) was that a patentee is always allowed to cut down multiple lists without adding matter because all the possibilities in all the lists are disclosed, the only exception being that it is not permissible to cut all the way down to a single compound or a very small number. I think the difficulties in its position drove it to such an extreme contention, but it plainly is not the law. None of the domestic or EPO cases says that.
- As I have outlined above, I think it is relevant that the effect of the amendments is to define a new class that does not cover inactive compounds or compounds that cannot be made, and that avoids an obviousness attack from Shepard. These are all symptoms of there being a different invention put forward in the amended patent. This point reinforces my conclusion but is not essential to it; the primary reason for my conclusion is that there simply is added matter in the new class of compounds put forward.
- NuCana had a couple of other points that I should mention:
- The PCT contained an unconventional definition of “alkyl” at page 5. In particular the skilled person would not normally think that an alkyl could be unsaturated. In the amendments to EP190 it is proposed to delete this and just leave “alkyl” to take its conventional meaning.
- Gilead says that this adds matter because it is a further narrowing given that R, R’, R’’ and Z are all allowed to be alkyl. Gilead also says that “alkyl” lacks clarity.
- I agree that the removal of the definition constitutes a further narrowing. It may be said to exacerbate the problem when taken in conjunction with the added matter that I have found in relation to X and Y. Clearly it cannot make the position any better for NuCana, but if I had been wrong about X and Y and NuCana had been allowed to make those changes, I do not think the alkyl point would have meant that there was still some added matter. The skilled person’s reaction to it would have been that the patentee had defined alkyl oddly but that its usual sense was still clearly meant to be comprehended within the artificial one.
- In addition and as I have said, NuCana’s ultimate argument on added matter was that a patentee is always allowed to select down within multiple lists, and had that succeeded then it would have also been able to select within the possibilities for “alkyl”.
- On clarity, the issue is simply whether “alkyl” was a sufficiently clearly understood concept in chemistry of this kind. Unsurprisingly Prof Seley-Radtke said it was - it has an IUPAC definition - and Prof Micklefield agreed. Gilead tried to argue that the IUPAC definition was different from that which the UKIPO had stated (see below) but I found the point very unconvincing.
- So I reject the clarity objection.
- It is convenient to mention at this point that Gilead argued that the definition of alkyl in the specification contains no limit on the number or nature of the substituents, which may themselves be substituted. This is true as a matter of definition. Gilead went on to argue that this permitted multiply substituted compounds which obviously would not work. Prof Seley-Radtke gave this short shrift and rightly so, in my view. The skilled person seeks success and just would not bother with obviously silly structures, or expect them to work. So this point is of no relevance to sufficiency and to be fair Gilead did not rely on it very much in closing.
- Both the unconditionally and the conditionally amended forms of EP190 suffer from added matter.
- By a letter of 14 November 2022 the UKIPO reported that the examiner did not think there was any added matter. I am grateful for the UKIPO’s attention and note that I have reached a different conclusion. Because the UKIPO’s views are set out very briefly (which I do not criticise in the slightest) I cannot tell why this is. One possible reason is the much fuller argument that I have had (Gilead’s Statement of Objections was considered by the examiner but is of course very brief indeed). Nor did the UKIPO have the chance to consider the TBA’s provisional view with its reasons.
- The UKIPO rejected Gilead’s point about “alkyl” and provided brief reasoning which is generally consistent with my own.
- EP365 cannot be any better than EP190 because its choices for X and Y are either the same as the conditionally amended form of EP190 (for claim 1 of EP365) or even narrower (for claim 2 of EP365 where X has to be F or Me, and there is also an addition in the text of EP 365 at [0020] reflecting this).
- It therefore follows that my conclusions on EP190 mean that EP365 is also invalid for added matter. That being so I will deal only very briefly with the additional points on added matter raised by Gilead.
- The other matters raised by Gilead are that compared with formula I in the PCT:
- Gilead submits that EP365’s groupings other than for Z can be found in the PCT, but that Z cannot, in that there is no disclosure of specifically acyclic C1-6 alkyl. On this sub-point I agree with NuCana that it falls away with the deletion of the artificial definition of “alkyl” and Gilead did not make anything of it.
- However, even if each grouping were individually disclosed there is no disclosure of them in combination with each other or with the relevant choices for X and Y. So to that extent EP365 is worse than EP190, but again the overriding points are the clear added matter in relation to X and Y and the fact that if NuCana’s main legal submission were correct, it would be entitled to make all the choices in combination anyway.
- These two points are very closely related.
- This requirement arises under Arts 52 and 57 of the EPC (and ss. 1(c) and 4(1) of the Patents Act 1977).
- As to case law, both sides referred to HGS v Lilly [2011] UKSC 51 (“HGS”). Gilead particularly relied on [107]:
- Gilead submitted that the effect of (i) and (ii) above was that the compounds claimed must plausibly (to a standard which is undemanding, as Gilead accepted) have a practical application and industrial use which is “derivable directly” from the description of the patent, read with the CGK. In the context of this case, its argument was that even if the compounds of the claims of the Patents had some minimal cytotoxicity, there would nevertheless be a lack of industrial applicability if that cytotoxicity could not be put to some practical use.
- NuCana relied on the same paragraph of HGS. It submitted that the claimed compounds, by virtue of having improved intracellular delivery and being cytotoxic by possession of a range of biological mechanisms, had practical application as research tools “to probe and further understand those mechanisms” and/or for “potential use in therapy”.
- It did not seem to me that there was a dispute between the parties that there must be a practical application. NuCana was not submitting that “bare” cytotoxicity with no conceivable practical importance would be enough. Had it done so, I would have rejected the submission. Some practical use shown to the low level of plausibility is clearly required by HGS.
- However, there were two points of dispute about what would amount to a “practical application”.
- The first is that NuCana relies as a technical contribution on the class of compounds of Formula I plausibly having the potential to be used in the treatment of cancer. HGS says that the test for plausibility is an undemanding one. It does not endorse the doubly-remote approach (just plausibly having potential) that is implicit in NuCana’s position. So I reject NuCana’s argument in this respect. It seemed to me to amount to saying that the specification just has to render it plausible that the effect might or might not exist, which is meaningless.
- The second is that NuCana relies on parts of the Supreme Court’s decision in support of its “research tool” technical contribution contention. This is an argument that, in summary, the Patents provide a class of compounds whose study would be useful to understand mechanisms of action and structure-activity relationships and the like, as opposed to themselves being useful to do something. NuCana argued that in addition to [107], quoted above, [103], [104], [109] and [123] supported this argument. I reject this. What is said in those paragraphs is, again, essentially about the low standard for plausibility. They also touch on the fact that a limited understanding of how the compounds in question might have their effect did not mean that there was no plausibility to their having that effect. Isolated phrases like “important to the pharmaceutical industry” in paragraph 109 (xiii) conceivably have some resonance with NuCana’s argument, but in substance there is nothing to support the extent of what it contends for under the “research tool” rubric, and on the contrary [107] is explicit that there has to be some practical utility of the invention.
- There was not very much dispute about the applicable law. I summarised the relevant principles and key cases recently in Sandoz & Teva v. Bristol-Myers Squibb [2022] EWHC 822 (“Sandoz”). There, I referred to the three-step test from Fibrogen v. Akebia [2021] EWCA Civ 1279 at [53]:
- In [56] and [57] of Fibrogen, the Court of Appeal explained that where claims are to compounds as such, defined by structure e.g. by means of a Markush formula, the Court has to interpret the specification to identify what utility they are said to have.
- I have said already that the standard for plausibility is a low one and depends on context. It has been stated in a number of slightly different ways in the case law, but for present purposes I think the Supreme Court’s formulation in Warner-Lambert at [37] is the one I should adopt, encapsulated as: “something that would cause the skilled person to think that there was a reasonable prospect that the assertion would prove to be true”. I do not think any nuance about the expression of the standard would make any difference to the result of this case anyway. NuCana said that Birss LJ’s “reasonable prediction” in Fibrogen was better and cut through a linguistic debate in earlier cases, including Warner-Lambert, but in fact Birss LJ said at [59] that the standard had been set in Warner-Lambert.
- NuCana also submitted/accepted that:
- As to the first of those points, Gilead submitted on the facts that the specification of the Patents does not disclose either 100µM as the cut-off for cytotoxicity, or “any measurable value” as the relevant standard. Wrapped up in this but not expressly stated was the contention that as a matter of law a patentee can only rely on a useful characteristic disclosed in a specification where not only its general nature (cytotoxicity) but its specific level (100µM, or any measurable value) is identified.
- I reject this. I do not think Gilead identified any cases which state such a proposition and it would be an unfair burden on patentees. Many patents do state numerical values to be achieved, often as being “preferable”, but there is no reason why they have to do so. In many instances a patentee may have identified a useful property in general terms but not had time to quantify it across the scope of the claim. In the present case, NuCana has relied on the 100µM value on the insistence of Gilead when it pressed for a number, but that is not its main case. The real issue between the parties is what “cytotoxicity” would mean to the skilled reader in context and with regard to the CGK. Unsurprisingly, the result I reach below is that the reader would think it depends on a widely varying context. I note that in Sandoz I referenced the principle that a patentee can rely on a technical contribution of a different but closely related nature to that disclosed in the specification, and a strict numerical limit of the kind for which Gilead argued seems to be to be conceptually inconsistent with that, too.
- After the decision of the Supreme Court in Warner-Lambert, in case T 0016/18 Sumitomo, the TBA referred questions about plausibility to the Enlarged Board of Appeal (reference G2/21). I discussed it in Sandoz at [70]-[72]. As I said there, the TBA discussed in its referring decision ab initio plausibility, a requirement that the specification must render the relevant effect plausible, and ab initio implausibility, which means that there is or may be an insufficiency only if there are positive reasons to doubt that the effect in question will exist across the scope of the claims.
- In Sandoz I said that the existence of the reference did not affect my reasoning because I am bound by Warner-Lambert, which (as the TBA said) propounds an ab initio plausibility test.
- I am of course still bound by Warner-Lambert, but since Sandoz the Enlarged Board has held its oral proceedings in G2/21 and a decision will be given in due course. Since it is possible that the Enlarged Board will adopt an ab initio implausibility test, and since that could at least possibly lead to a change in the case law in this jurisdiction in due course, it seemed to me that it might be sensible to make findings of fact to that standard as well as to the standard from Warner-Lambert that binds me. Gilead and NuCana both agreed that I should do so. It presents a minor practical problem since there is no definitive standard set out in an EPO decision for ab initio implausibility, but in my view it is workable for me to make a decision with regard to the statement of that test as summarised in T 0016/18 at paragraph 13.5. In any event I can narrate my findings and given their content as set out below I do not think nuance will matter, since I conclude there is clear, positive reason to think that the effect(s) asserted will not exist across the scope of the claims.
- A very important part of working out what it means to say that the invention works is to identify the technical contribution.
- NuCana’s case as to the technical contribution of the Patents has changed over time. It was the subject of a Request for Further Information (“RFI”) by Gilead, and NuCana’s Response was amended twice.
- NuCana’s original Response included the assertion that the technical contribution included increased therapeutic potency for cancer or as an anti-viral agent. This was abandoned by amendment.
- As of October 2022, as set out in its Re-Amended Response to Gilead’s RFI, NuCana’s case in this respect was in three parts (it developed further in points of detail which I will address below):
- NuCana’s written opening submissions said that its primary case would focus on the third of those, cytotoxic activity, but that the first and second were “formally maintained”.
- As matters developed, the first alleged technical contribution, a new class of phosphoramidate nucleosides simpliciter, was not really pursued by NuCana, and rightly so. Mere structures without more are not a technical contribution even if they are new and on its own this technical contribution could not have avoided Agrevo obviousness.
- In its written closing, NuCana accepted that the second alleged technical contribution, improved intracellular delivery, would not provide a “standalone” technical contribution. This was said to be “because although the skilled person expected the phosphoramidate approach to work by increasing intracellular concentration of the nucleoside monophosphate, the only way to tell this from the specification of the Patents is to look at the cytotoxicity data referred to therein.”
- Therefore, NuCana said, “whilst … the skilled person reading the Patents would have considered that there was a technical contribution in the sense that the ProTides were disclosed as being able to deliver the nucleoside monophosphate into the cell, thereby maintaining or improving intracellular concentration, this is demonstrated by measuring cytotoxicity. To this extent, both contributions go hand in hand - the observed cytotoxicity is only possible because levels of intracellular concentration are achieved.”
- Based on that, NuCana said that while not separable, the intracellular delivery contribution bolstered the cytotoxicity (and vice versa).
- I accept the logic of this (although Gilead attacked the factual basis for it, which I will deal with below). In practical terms the result was that the evidence and argument focused very heavily on the third alleged contribution, cytotoxicity, and for the same reason that will be the focus of this judgment. It also meant that if NuCana lost on the issue of whether there was a relevant contribution in terms of cytotoxicity, intracellular delivery would not help it.
- I turn therefore to the third alleged contribution, cytotoxicity. As I mentioned above, this was developed further in the run up to trial, by an expansion of NuCana’s Re-Amended Response to the RFI as follows:
- This purported to add potential as therapeutic agents (actual therapy having been abandoned) and non-therapeutic applications, including as “research tools”.
- NuCana’s evidential support for this expansion was provided in, in particular, paragraphs 57-61 of Prof Seley-Radtke’s second report, all of which it is necessary to set out here:
- In this context, there was a discussion on the first day of trial as to the proper scope of NuCana’s case. The upshot was that it was allowed to rely on those parts of Prof Seley-Radtke’s second report but (a) not in relation to any clinical context other than cancer, and (b) provided that it committed to what it meant by “cytotoxicity”. As to the latter, it provided the following statement:
- Gilead said that the specification did not disclose improved intracellular delivery because it was only mentioned expressly in relation to the prior art. In my view the skilled team would understand the disclosure to be that the ProTide phosphoramidate idea was for the purpose of intracellular delivery and that the compounds of formula I took the same structural approach with the same goal.
- Gilead also said there was no adequate disclosure of cytotoxicity; that the limited references in the context of the experimental work was not good enough. Relatedly it said that the real disclosure as to technical effect was of use in cancer treatment, achieved by cytotoxic effect, not freestanding cytotoxicity. I agree with Gilead on this and the skilled team would think that the experimental data where reference to cytotoxicity is made was there for the purpose of showing the feasibility of treating cancer. But in view of the law on plausibility addressed above (that patentees can rely on less ambitious contributions and that contributions related to those expressly disclosed can be relied on) I do not think that this rules cytotoxicity out of consideration.
- In dealing with the Patents I have set out what the experimental information in them directly shows and concluded that the in vitro data make plausible, for the three compounds of the claims that are compared with gemcitabine, that there is a cytotoxic effect at a level which cannot be quantified, in two particular cell lines. I also concluded that the in vivo data make plausible that a single compound has a greater cytotoxic effect than gemcitabine in the animal model used.
- The following matters are also relevant facts based on my assessment of the Patents and the CGK:
- NuCana relied on (bio)isosterism to meet these matters. I have found that as a matter of CGK it was not regarded as predictive.
- NuCana also relied particularly heavily, and repeatedly, on two aspects of the evidence Dr Galmarini gave under cross-examination.
- The first related to a review article from 2006 on which he was an author (Jordheim et al, Recent Developments to Improve the Efficacy of Cytotoxic Nucleoside Analogues (2006)). It contained, in particular, the following:
- The patent referred to is the PCT.
- NuCana relied on this as Dr Galmarini having said that CPF31 was very promising both in terms of efficacy and lower toxicity, and that the compounds of the PCT/Patent in general should be efficient in resistant cell lines (by virtue of the reference to “these compounds”).
- Dr Galmarini said that although he was a named author on the paper he did not review the PCT. I find that a bit surprising since it was one of only three documents marked in the References section as being of special interest, but accept it. In any event, though, I think NuCana was reading much more into the above passage than can be justified. The text only singles out CPF31 as being of specific merit, and the text explicitly says that there are no data on resistant cell lines. Most importantly, the text certainly is not addressing itself to the legally relevant question of whether substantially all of the compounds of Formula I would plausibly be thought to have anticancer activity, or cytotoxic activity. “These compounds” just means CPF31 and possibly some unspecified related compounds.
- The second matter in Dr Galmarini’s cross-examination heavily relied on by NuCana related really to “existence in fact”, which I address below. NuCana relied on the following from T3/492-5:
- I think in context that Dr Galmarini was saying in the last answer that all of this was speculation and when he said “assume” he meant “postulate” or “guess”. I certainly do not think he was accepting that for all or even most of the compounds in the case where cytotoxicity had been assessed but no result under 100µM was found, it was plausible that there would be some other cell line that would give a better result.
- Against this background, my assessment is that the skilled team would positively think that a significant number of compounds within the claims of the Patents would not have either “meaningful” cytotoxic activity or activity at the level of an IC50 of 100µM. The skilled team would also positively expect that a significant number of compounds within the claims of the Patents would have cytotoxic activity in one of those senses.
- However, the skilled team would be completely unable to predict what or how many compounds would be in either of those two categories.
- I have borne the whole picture in mind in making these assessments. There are however two main reasons for my conclusions:
- My conclusion is that the skilled team would not think it was plausible that meaningful cytotoxic activity would be preserved across the range of possibilities encompassed by the claims of the Patents at the 2’ and 5 positions (even without considering the other parameters of formula I, which only make matters worse). On the contrary, the skilled person would think that the effect of the substituents in combination was unpredictable, but would expect based on the general understanding that small changes could make big differences, and that there were highly likely to be combinations of substituents at those positions which would not work. The skilled person would be unable to predict how many such non-working combinations there might be, but would not expect them to be rare one-offs.
- Even if I had regarded the in vivo and in vitro work in the Patents more favourably and thought that they provided really solid evidence for the compounds tested (as opposed to merely passing the plausibility threshold), they are a very narrow base from which no understanding of SAR can be drawn. So my conclusion would not have been any different in that case.
- Similarly, even if I had found the CGK to be that (bio)isosterism had some general predictive power as NuCana alleged, it would not have made any difference because the concrete CGK that the specific substitutions covered by the claims can cause loss of activity is much more directly applicable and cogent.
- I do not think it undermines my conclusion that it is not possible to say how many compounds would be expected to lack activity (other than that there would be many). The test is not a quantitative one and anyway lack of ability and information in the Patents to predict for any compound or group of compounds with confidence whether they would have activity must surely be a point against plausibility, not in favour of it.
- Since I find that the skilled team would positively think that a substantial number of compounds in the claims would lack activity, the Patents are invalid on the ab initio plausibility test and on the ab initio implausibility test.
- I have particularly reviewed Prof Seley-Radtke’s written and oral evidence as relied on by NuCana in coming to these conclusions. While she expressed more optimism in relation to the changes that were possible at each position than I think was justified by the CGK, and likewise placed more faith in (bio)isosterism, my overall impression was that the furthest she was really prepared to go was that the majority of the compounds would be cytotoxic (see e.g. T5/761, relied on by NuCana in its written closing submissions), and she could not really dispute that there would be an expectation that others would not.
- The written evidence of Prof Micklefield and Dr Galmarini, from their different perspectives, was that the Patents contained data for only a few gemcitabine analogues while permitting a wide range of changes at the various positions specified by Formula I. They said that it was not possible to make predictions about activity across the scope of the claims. In their first reports this was expressed in very general terms. In his second report, Prof Micklefield commented on Prof Seley-Radtke’s “SAR” analysis, the parts of which relevant to the 2’ and 5 positions I have commented on above. He said that it was not really an SAR analysis at all because it did not include systematic modifications tested by reference to a particular target, but was a disparate collection of individual analyses. I agree with this (which applies to the other positions in Formula I).
- Prof Seley-Radtke’s conclusion in her first report was that “The Skilled Team would therefore have understood that a range of different modifications could be made at the 5 position of a nucleoside while preserving its potential for cytotoxic activity”. She said the same, mutatis mutandis, about the 2’ position. If all she meant was that it could be predicted that there probably would be some modifications, identity unknown, that would work, I agree. If she meant that predictions could be made for any particular substitutions then that is clearly to be rejected, and if she meant that a prediction could be made as to the substituents called for at those positions by the claims of the Patent then I reject that too. But I do not read her evidence as making either of the latter two statements in any event. And as I say, the gist of her oral evidence was at its highest that the majority of compounds would have activity and others would not.
- The above is the main basis for my conclusions. I also reject NuCana’s case on the additional bases that:
- NuCana also sought to make a case under this heading.
- “Research tool” is a somewhat ambiguous phrase. It can mean something that is an actual physical tool which can be used to accomplish a task, as with a nucleotide probe which would be used to detect or amplify a complementary strand. That is not NuCana’s case. It can also mean something which generates information that can be used in research, and that is what NuCana relies on.
- NuCana relies on paragraphs 57-61 of Prof Seley-Radtke’s second report that I have set out above. It also relies on paragraph 27 of her second report:
- NuCana also relies on the following passage from her oral evidence, T5/756-757, to similar effect:
- NuCana submitted that Dr Galmarini agreed with paragraph 27 of Prof Seley-Radtke’s second report at T3/34815-24, but what he said has to be seen in context. The questions were part of (at the end of) a longer section in which he was asked about a theoretical situation of a resistant cell line for which the skilled person had compounds which were cytotoxic and compounds which were not and could thereby investigate the mechanism of resistance. That situation is not applicable to the question of plausibility/technical effect of the Patents because they are not pleaded or argued to provide compounds which overcome resistance. In any event Dr Galmarini agreed that having compounds like that would allow “basic research”. I think his answers at the end of T3/348 have to be understood in the same sense, and what he agreed with was simply that because there is such a stupendous number of nucleoside analogue structures that are possible, having some that provide cytotoxic activity would allow further research.
- A further shortcoming of the passage of evidence was that it was not at all clear what level of cytotoxicity was meant by the questions. I think in context Dr Galmarini probably understood it in the sense of meaningful cytotoxicity (and indeed NuCana also relied on a passage of Prof Smyth’s evidence which was about meaningful cytotoxicity). I certainly do not think, given all his other evidence, that Dr Galmarini understood it in the sense of any measurable value. Finally, I do not think he understood “technical achievement” in the sense that patent law means in relation to “technical contribution”.
- In any event, the passage from Prof Seley-Radtke’s cross-examination at T5/756-757 is about using nucleoside analogues to investigate mechanisms of action and the Patents do not provide any information about the mechanism of action of the compounds of Formula I.
- Furthermore, this argument has to be seen in the context of the very limited information in the Patents about the compounds of Formula I: just the tests in three cell lines of three compounds. In that context, Prof Smyth more or less accepted that there was no utility as a research tool (T6/998-999).
- This whole argument assumes the provision of information (resistance, mechanisms of action) that is not provided by the Patents or even pleaded by NuCana as being part of its contribution. What it boils down to, even assuming the provision of that information, is that the Patents provide compounds which could be used to generate information, both as to their success and failure in terms of cytotoxicity, to feed into a research project to explore mechanisms and SARs and thus, much later, to find out which ones work and why. As I mentioned when dealing with the law, I reject NuCana’s argument that the decision of the Supreme Court in HGS supports the contention that the mere generation of information for taking research forward is enough for industrial application (or sufficiency).
- Given my analysis of the law and the facts I do not think that the attack of lack of industrial application requires separate consideration. It was basically deployed, in the end, by Gilead so as to be able to argue that if some degree of cytotoxicity was rendered plausible but had no practical utility, then the objection of lack of industrial application could succeed if lack of plausibility was not good enough. I think such considerations can be raised under the heading of lack of plausibility for reasons I explained in Sandoz.
- The parties used different expressions to refer to the question of whether/which compounds within the scope of the claim do in fact have whatever level of cytotoxicity is called for. NuCana called it “sufficiency” and Gilead called it “existence in fact”; I prefer the latter but nothing substantive turns on it. It is a question of what the position actually is based on evidence available at trial and not a question of what the skilled person would have said was plausible based on the Patents and the CGK at the priority date.
- Although existence in fact is a different question from plausibility, the parties made submissions about whether the facts as they appear now are what would have been expected at the priority date and I will address that briefly.
- There is really no material dispute about the facts as to what assay results have been achieved, the question is one of interpretation. As a result, the parties were able to agree a comprehensive spreadsheet of the results, which is Annex B to this judgment.
- Annex B is split into sections:
- These overlap a little bit because e.g. some of the results for sofosbuvir come from Zhou and some from Mengshetti, and there was another paper called Le Cher et al ‘Discovery of a 2’-Fluoro, 2’-Bromouridine Phosphoramidate Prodrug Exhibiting Anti-Yellow Fever Virus Activity in Culture and in Mice’ (2022) which covered compounds that overlap with other sections.
- For each compound the substituents at the positions stipulated by the claims of the Patent are given, then there are four columns in which either a green block or a red block indicates that the compound is or is not within the claims in issue for EP190 and EP365. In the remaining columns to the right, there is a space for a result for each compound in respect of each of a large number of cell lines; a number indicates that the compound was tested in that cell line, a blank indicates that it was not tested in that cell line. In general there are about 4 to 6 cell lines per compound.
- I have explained at the start of this judgment what sofosbuvir is: it has X as Me.
- Within the results in Annex B:
- There are no compounds in Annex B where X = F. NuCana sought to portray this as a retreat or at least concession by Gilead but I do not see it that way. It is in fact quite consistent with Prof Seley-Radtke’s “sugar pucker” evidence that the difluoro substitution at the 2’ position is an important aspect of gemcitabine and that changing it would be likely to have a significant effect.
- In Annex B, an asterisk is used to mark compounds which achieved a measurable cytotoxicity. Absence of an asterisk denotes no measurable toxicity at the highest concentration used.
- The overall position is:
- As to sofosbuvir:
- Gilead does not advance a case in relation to existence in fact if the standard is any measurable toxicity, since to do so would be inconsistent with its position that anything has a measurable activity if the concentration is made high enough and the assay conditions lenient enough. So I have to assess matters in relation to NuCana’s 100µM case. In all the circumstances I also think I am able to and will comment on the position as to meaningful cytotoxicity.
- Clearly, there are many compounds for which no measurement was achieved at 100µM. They are not a majority but they are a significant minority, and they span a variety of possible substitution patterns. The problem is particularly evident for the X = Me compounds from Gilead’s disclosure but also applies to e.g. Mengshetti in most instances, and Zhou in two. It is fair to say that Quintiliani is not nearly as bad.
- NuCana’s answers to this were:
- As to the number of cell lines used, I accept that it was less than the full NCI panel (although NuCana can hardly credibly deploy that as the “gold standard” given that sofosbuvir produced no result in any NCI cell line). My task is to look at the totality of the evidence that I have, however, and I think there is no basis in the evidence or in common sense for accepting what NuCana submits. I think it is inherently very unlikely against the broad spread of failure in the materials I do have that there would be at least one “golden” cell line for each compound or even for most.
- In addition:
- As to the assertion that the “vast majority” of the compounds in the peer reviewed articles showed cytotoxicity below 100µM that is not a fair summary. Each article painted a different picture.
- As to the point about not knowing the assay conditions in the Gilead work, I accept that as an uncertainty and take it into account but I do not think it is realistic to argue that it transforms the picture.
- So my conclusion is that Gilead has shown on the balance of probabilities that for a significant number of compounds within each of the claims in issue, there is no cytotoxicity at or below 100µM.
- I would also say that even for those compounds where there are one or a sprinkling of results below 100µM, it is more likely than not that they have no “meaningful” cytotoxicity in the sense that Prof Smyth explained and which I have discussed above. There are a non-trivial number of compounds where the lowest measured value was about 50µM up to about 80µM. When considering plausibility I have noted that there are a small number of cases in this field, to which special considerations apply, where those sorts of values can translate into useful potential anticancer applications, but in general those values are indicative of no potential utility. It is so wildly implausible as to be dismissed that all the ones at that sort of level in Annex B do in fact have potential as anti-cancer agents. Most of them are probably of no more meaningful use than the ones where no cytotoxicity was measured at all.
- Finally, I said I would comment on consistency between what would be predicted in relation to plausibility and the actual results. In my view the actual results correspond closely to what Gilead argued on plausibility. A spread of substitutions at a number of positions would each have been seen as having the potential to reduce or extinguish the activity seen with gemcitabine or the three compounds in Table I in the Patents. Changing X would look especially likely to be a problem. The position would be expected to be unpredictable in respect of each position and all the more so when multiple substitutions were made. That all matches the picture of widespread loss of activity seen in Annex B, to which there is no pattern, as explained above.
- As I have said above, improved intracellular delivery (“ICD”) is now only run by NuCana as bolstering cytotoxicity, but in any event Gilead seeks to meet it on the facts.
- There are two aspects to this. The first is that Gilead says that the data in the Patents do not support the conclusion that ProTide analogues of gemcitabine have improved cytotoxicity compared with the unmodified nucleoside, so that that effect is not made plausible. I have considered this above and concluded that Gilead is right. If there is not improved cytotoxicity then it cannot be inferred that there is improved ICD.
- The second is that Gilead argued that SAR work done by Prof McGuigan’s group had shown that for some amino acid changes within the scope of the Patents’ claims, activity got worse, not better. This was significant because the Patents do not contain any specific teaching of their own to render improved ICD plausible; NuCana has to seek to get plausibility from the CGK. Gilead’s argument was developed in the cross-examination of Prof Seley-Radtke by reference to Cahard (‘Aryloxy Phosphoramidate Triesters as Pro-Tides’ (2004)); that paper showed graphically data which was also contained in the McGuigan papers identified in [0007] - [0008] of the Patents.
- I agree with Gilead on this point. Counsel for NuCana’s response was that the point did not make any difference because it did not undermine the general correlation to be expected between improved ICD and cytotoxicity; so that all it meant was that if one obtained a lower ICD one would also have worse cytotoxicity. I do not see that that is any answer at all, though. Gilead’s point based on Cahard (which NuCana did not contradict on the facts) was that the claims cover NAs which are worse than the bare nucleoside. So if there is an improvement in ICD it does not apply across the scope of the claim and cannot be relied on by the patentee.
- Gilead maintains the argument that the Patents are obvious over Shepard. Shepard is about the successful application of the McGuigan ProTide phosphoramidate strategy in the context of BVdU.
- Gilead’s experts did not give any written evidence that the Patents were classically obvious over Shepard and Gilead’s written opening submissions were directed purely to arguing for obviousness over Shepard based on the lack of any technical contribution. I could not see how this in practice added anything to the plausibility/insufficiency arguments and asked Counsel for Gilead about it during oral opening submissions. He said that Gilead was not saying there was a conventional obviousness case but that on the basis of how Prof Seley-Radtke saw matters there might be. So it was a squeeze between conventional obviousness and plausibility.
- Dr Galmarini was asked very briefly about this by Counsel for NuCana during his cross-examination about Shepard/NB-1011, which of course was part of the picture in relation to plausibility. He said that if the skilled team were going to progress Shepard they would do it by further work on NB-1011. So in respect of Dr Galmarini there remained no evidence in support of classical obviousness.
- Prof Seley-Radtke was then cross-examined by Counsel for Gilead. It was suggested to her that gemcitabine was known to have problems entering the cell (which she agreed with) and that applying the ProTide strategy to it would therefore be attractive. So far this was just a case over the CGK alone, which was not pleaded.
- It was then put to Prof Seley-Radtke in the context of Shepard that the skilled person, having seen that the ProTide approach worked for BVdU, would expect it to work for gemcitabine. She first said that they were different nucleosides with different mechanisms of action, but later gave an answer (at T6/905) which appeared to accept that there would be an expectation of success, based not only on Shepard but all of the literature.
- Although the last answer to which I have referred looks impressive in isolation, I found this whole attack sketchy and unconvincing. The fact that it was explained to me in oral openings that there would be a squeeze between classical obviousness and plausibility and that that passed without objection from NuCana leads me to conclude that it is formally open to Gilead to run the point, indeed NuCana having seen the point was no doubt the reason for the (brief) cross-examination of Dr Galmarini to which I have referred above. But the fact that Dr Galmarini gave no written evidence in support and that Gilead’s written opening did not run classical obviousness, even as a squeeze, are symptoms of the attack being put together without the proper groundwork.
- As to the cross-examination of Prof Seley-Radtke, although at key points the questions were phrased to explicitly exclude consideration of the Patents (partly at my request), there were significant discussions of the Patents in the same passages which I am not satisfied were put out of mind by Prof Seley-Radtke at the critical junctures. There was also a strong sense of the very abstract kind of case over CGK alone which the case law deprecates (although Shepard was plugged in at a late stage of the logic, so it was not pure CGK), and the passage at T6/905 followed immediately after the putting of a 2004 document whose contents were not, I find, established to be CGK.
- So I reject the classical obviousness attack over Shepard. I still do not see how the lack of technical contribution attack adds anything to Gilead’s plausibility case.
- As pleaded, Gilead advanced its case on undue burden in two ways:
- The second of these did not form part of Gilead’s case at trial, however.
- It can be a little hard to understand the different ways in which the key compounds at issue in this part of the case are referred to. It was well explained in NuCana’s opening skeleton, on which the following paragraphs are closely based.
- As mentioned above, at [0073] EP190 discloses that compounds of the invention may be prepared by reacting a nucleoside derivative (Formula III) with a phosphochloridate (Formula IV). There is no dispute that this particular reaction is enabled. However, Gilead pleaded that compounds where the nucleoside is configured with methyl (up) and fluorine (down) at the 2' position of the ribose ring (2MU2FD) could not be made without undue burden. An example is as follows:
- This is the nucleoside component of sofosbuvir (i.e. the base is uracil) and is referred to by Prof Micklefield as compound 1e (whereas Prof Davies refers to it as compound 1b in his first report). Prof Micklefield’s nomenclature was mostly used in the oral evidence at trial and in the parties’ written submissions.
- It was not alleged that the di-fluoro nucleoside (as in gemcitabine), or various other options at the 2’ position were not enabled. The focus of Gilead‘s case was on compound 1e.
- In claim 1 of both Patents (as granted and as proposed to be amended), the nucleobase is either uracil (as shown above) or cytosine. It was common ground that conversion of compound 1e to the corresponding cytidine nucleoside, which Prof. Micklefield refers to as compound 1f (and vice versa), would be straightforward. I mention this because there were references in the evidence at trial to compound 1f from time to time.
- NuCana referred to the Court of Appeal in FibroGen v Akebia (supra) in relation to the law of undue burden as it applies to Markush groups. It made three points:
- The first two of these points really went to the second way in which Gilead had pleaded its case and therefore were no longer relevant. The third point has the consequence that in order to be sufficient the Patents must allow the skilled person to make the 2MU2FD intermediates without undue burden, otherwise the claim would not be enabled across its scope. This was not really in dispute (Gilead cited Regeneron v Kymab [2020] UKSC 27 for the same point); NuCana did not, for example, say that the 2MU2FD intermediates related only to a de minimis or irrelevant part or aspect of the claims.
- Therefore the principle of law which actually mattered was the relevant standard to be applied in assessing undue burden.
- On this, Gilead cited Mentor v Hollister [1991] FSR 557 at 562 (Aldous J, as he then was):
- And Gilead also relied on Aldous J’s reference to and agreement with the EPO decision in T226/85 Unilever/stable bleaches:
- I agree that these two statements capture the basic test for “classical”, undue burden insufficiency. NuCana did not dispute them in its closing submissions.
- Finally, Gilead referred to the decision of Laddie J in Evans Medical’s Patent [1998] RPC 517 at 536-527:
- Although this has some resonance for the present case at the level that some real teams who tried to make 2MU2FD compounds failed and some succeeded, the statement is really about how to assess enablement from an ambiguous document (in that case in the context of priority), which is not the point I am considering.
- So there was in the end no material dispute that the question I am asking is whether making the 2MU2FD compounds would require undue burden, and that the standard I should apply is the one identified by Aldous J in his own words and by reference to the EPO case law in Unilever/Stable bleaches.
- I think it worth articulating two other matters which arise from this:
- Where the issue is one of obviousness the courts in this jurisdiction are very used to separating primary evidence (that of the experts) from secondary evidence, such as the experience of real world workers who did or did not make the invention, and applying appropriate caution to the latter, based for example on whether they represented the ordinary skilled person, whether they had the cited art, and so on.
- In my view, although I was not addressed on it in any detail, similar considerations must apply to assessing undue burden. The question for decision is whether the ordinary skilled person, a notional figure, could make the products concerned, in this case using CGK alone, without undue burden. The primary evidence on this will be that of the expert witnesses, who have the task of putting themselves in the position of the skilled person and then explaining to the court what would be involved in making the products, and also what is a normal degree of effort in the field concerned.
- On the other hand, where evidence is advanced about real workers who either succeeded or failed in the task (in the present case, both), that is secondary evidence. It may well be of lower assistance and lower value to the judge deciding the case.
- I mention this because the sheer forensic effort that was necessary for the parties to get on top of the secondary evidence, analyse it and present it to me led, in my view, to sight rather being lost of the primary evidence of the experts. I acknowledge the potential usefulness of the secondary evidence in this case in giving me the possibility of a real-world cross-check on what the experts said was normal in this field, but it remains secondary evidence.
- I also identify at this stage that the usefulness of secondary evidence must depend in significant part on how complete and how testable it is. In the present case I did not hear oral evidence from any of the real world workers relied on, and the documentary record is patchy, including because the documentation created at the time was poor (in the case of Mr Clark). This makes it especially hard to assess why the workers in question succeeded or failed, as the case may be. Secondary evidence on obviousness is often discounted by a trial judge on the basis that it is simply unknown why (for example) the invention was not made before and in my view the same should apply to undue burden, as part of the overall exercise of assessing the secondary evidence.
- It will assist if I give an overview of the synthetic routes to compound 1e discussed at trial.
- The routes fall into two broad categories:
- In the glyceraldehyde routes the fluorine is there from the outset. At a general level, the basis for this approach is the synthesis of gemcitabine (hence references in the evidence to “analogous gemcitabine”).
- In the fluorination routes, as the name suggests, the fluorine is added later on. Prof Micklefield split these into nucleoside routes and sugar routes based on whether the starting material had a base or not.
- As to the glyceraldehyde routes:
- Reformatsky and Aldol both generate the same products; the desired diastereoisomer is isolated, cyclised and modified further, and then the base is added.
- Parenthetically, Prof Davies suggested the use of SuperQuat, which is a variant of the Aldol route using techniques developed in his laboratory. NuCana did not rely on it, no doubt because it was not CGK.
- Among the fluorination routes:
- So Route 2 can only be done with a sugar whereas all the other routes in this family can be done with a sugar or nucleoside.
- Route 2 is not relied on by NuCana on the basis that it would be prioritised below other possibilities, a point on which Prof Davies and Prof Micklefield essentially agreed. That does not mean that the work involved in thinking about it, looking at the literature and so on can be discounted and I do not think either expert entirely discounted the need to resort to it if other approaches failed.
- There was much argument, whose merits I assess below, about the fluorinating agent for use in Route 3.1. Two possibilities were DAST and Deoxofluor. An alternative was the variant referred to in the evidence as Route 3.1A, in which the tertiary hydroxyl on the ribose/nucleoside is transformed into a sulphonate derivative (in particular, a triflate).
- When working to fluorinate a tertiary alcohol, Concept tried what were called the Protected Uridine and Unprotected Uridine routes (two nucleoside variants), first according to Route 3.1A (this was also referred to as the Sugar Grignard route). When they ran into difficulties they changed to Route 3.1 using DAST as the fluorinating agent.
- Route 3.2 is not relied on by NuCana since it accepts that it cannot be shown to work at all. Furthermore it was the first route tried by Idenix so NuCana did not argue that it can be discounted. But NuCana says that if there was a failure with Route 3.2 the skilled chemist would switch to Route 3.1 and that that would be facilitated by the fact (which was common ground) that they share some early steps.
- Route 3.3, like Route 2, was not relied on by NuCana on the basis that it would be low priority. Again, however, that does not mean that the research effort into getting to it as a proposal can be ignored.
- I was provided with excellent agreed depictions of the routes which I have referred to in preparing this judgment but do not reproduce here. They were important to my understanding but the issues I have to resolve are at a higher level than that of the detail the depictions contain.
- An important topic for the assessment of the undue burden arguments generally and the secondary evidence in particular is the fluorination conditions used. In the very complex situations that have to be considered it is hard to be confident about what factors caused success or failure, but it appears reasonably clear that the choice of fluorination conditions was important for Mr Clark, for Idenix and for Concept.
- It is common ground that the fluorination step would have been regarded by the skilled chemist as a risky and unfamiliar one. It is also common ground that the information they would need was not part of the CGK as such and would need to be looked for in the literature.
- Prof Micklefield undertook an analysis of this topic in his first report. Possibilities that were referred to extensively at trial included DAST and Deoxofluor and a publication that Prof Micklefield said would be identified was Singh.
- Prof Micklefield pointed out that although there are numerous examples in Singh, only one relates to the fluorination of tertiary alcohols; that sole example is to be found in Wachtmeister.
- Based on his analysis of these and other materials, Prof Micklefield’s opinion in his first report was that the skilled chemist pursuing route 3.1 would initially attempt fluorination using DAST under standard conditions, which would mean in anhydrous dichloromethane (“DCM”), with and without pyridine and at room temperature. He said that if that did not work, a troubleshooting exercise would be needed.
- Prof Davies’ first report devoted very little attention to this issue, by comparison. In his early interactions with NuCana’s representatives he also dealt with the topic at a very high level. He dealt with it in more detail in his second report in the context of the secondary evidence.
- I think two points have to be borne in mind in considering this issue:
- This was one of the areas where I found Prof Micklefield more thorough and convincing than Prof Davies. This was not just the case in relation to the fluorination conditions in general, but in relation to important points of detail. By way of example and in particular, Mr Clark tried a variety of fluorination conditions at Pharmasset. Some worked and others did not. Prof Davies reviewed these in his third report and concluded that Mr Clark was usually if not always successful. But this was not correct and Prof Davies had to make significant corrections in his fourth report, which corrections had themselves to be revised later.
- There are two specific points of detail from the secondary evidence that I should address on this topic.
- This is a document dated 22 January 2004 prepared by Mr Clark.
- It refers in the text of the first, main paragraph to “typical DAST conditions”. It was accepted by NuCana that for Mr Clark, such “typical” conditions involved toluene at low temperature. But NuCana also argued that the first three sentences of the paragraph taken with the reaction sequence at the top of the page indicated the use of DCM at room temperature, with and without pyridine, reporting yields of 25% and 20% respectively.
- Considerable time was given at trial to seeking, through the witnesses and Counsel’s submissions, to work out what precisely Mr Clark did. It was accepted by Prof Davies that there is not actually a laboratory notebook page showing those conditions, without pyridine, giving a yield of 20%, while there is a notebook of 28 April 2002 entry indicating (by the word “mess!”) a failure with such conditions.
- I do not think it is possible to resolve, from the materials I have, exactly what the Fluorination Schemes document is talking about or whether Mr Clark did use DCM at room temperature without pyridine successfully at some point. I agree with NuCana that the document on one reading suggests as much, but it is very unclear. I note that Prof Davies’ view in his third report having looked at all the notebooks was that Mr Clark did not succeed with DCM. In any event, given that Mr Clark clearly failed with DCM at room temperature on one occasion I think this all goes to show just how chancy the choice of fluorination conditions was, and the route which Mr Clark found in general to be successful was plainly the low temperature toluene one.
- In May 2019 Concept ran into trouble with the fluorination step. They regarded this step as key.
- After about two weeks of failure with the fluorination it was decided to ask Prof Davies for help and this was done by email from Dr Atkinson. Prof Davies provided two suggestions which did not include different fluorination conditions and although Concept took them on board it is not suggested that either succeeded.
- Meanwhile and without the involvement of Prof Davies, a call was held between HGF and Concept. Fluorination conditions were discussed, and it can be seen from Concept’s report of 4 June 2019 that a variety were in the course of being tried. DAST with DCM at low temperature appeared to have succeeded but Ishikawa’s reagent failed. Then in the report of 11 June 2019 Concept reported that it had succeeded with DAST in Toluene at -20oC (Mr Clark’s conditions) and with Deoxofluor in DCM at -78oC, but had been unsuccessful with 1,1,2,2,-tetrafluoroethyl-N,N-dimethylamine.
- The manner in which the key conditions were arrived at by Concept is essentially unexplained, but the inference is strong that they came from HGF and are very likely to have been influenced by knowledge of what Mr Clark had done. I reject any submission by NuCana that this aspect of Concept’s work can be relied on as indicating what the ordinary skilled chemist would, or could, do. It is true that the actual experiments to show success did not take long once the conditions were identified but that is not the point. Concept were stuck until they got help.
- It was effectively common ground that the skilled chemist would be used to choosing between different possible routes if designing and implementing a new synthesis, and prioritising which route to attempt first. It was also common ground that the skilled chemist might run different routes, or at least stages in such routes, in parallel. And finally, it was common ground that the skilled chemist would have in mind aspects in which different routes had some steps in common; in the particular context of this case, that was of potential relevance because routes 3.1 and 3.2 shared some steps so hitting a problem in one would leave open the option of reusing those steps in the other.
- Prof Micklefield gave detailed and, I find, balanced evidence about the choice in the present case. He said that his own preference based on his personal background would have been routes 3.1 and 3.2 because they were the shortest. The downside was that the fluorination step required was unknown. He said that the sugar routes would remain available as a fallback. He said that routes 1.1 and 1.2 were the best precedented but involved more unknown steps than routes 3.1 and 3.2, and would pose issues with the stereochemistry.
- Prof Davies said the skilled chemist’s preference would instead be driven by the fact that the Patent flagged up gemcitabine and the skilled chemist would want to work by analogy with it.
- In my view this is an area where it is particularly difficult to put aside the position of real people and think about the notional skilled chemist. Prof Micklefield laid out the relevant factors but made clear that he was also expressing what would be his own personal choice. Prof Davies did not quite put it that way but his personal perspective was also apparent from putting forward the chiral auxiliary route. Similarly, the secondary evidence shows the influence of personal choice, for example Dr Griffon’s past experience with fluorination was important in setting out his strategy.
- Also, the fact that the skilled chemist would at a general level have the ability to make these strategic choices in the planning stage of a project does not mean that the need to do so in the present case is irrelevant to the issue of undue burden. My overall conclusion is that the choice in the present case would be a difficult, relatively complex and finely balanced one. The notional skilled chemist would, I find, have chosen either nucleophilic fluorination or a glyceraldehyde route as a starting point but not both. Which was chosen would depend essentially on personal taste and experience, so close is the decision, and either would be in keeping with the CGK. The difficulty of the choice in the present case is due to the complexity of the situation and the fact that each option would be perceived at the outset to have serious and unpredictable potential problems. This is a factor pointing towards the burden of the task being undue.
- Whichever way the skilled chemist went in the first instance they might fail and have to switch to the other.
- In relation to the sub-choice between Reformatsky and Aldol I again do not think that it matters much if at all to my overall conclusion but on balance I prefer Gilead’s position that the Reformatsky route would be the first attempted, and this matches the secondary evidence of the Clark and Griffon teams. There was evidence both ways on this, with Prof Micklefield accepting, for example, that the Aldol route was better in the condensation step.
- Having identified the context, I will first summarise and assess the primary evidence.
- Prof Micklefield’s primary evidence on this issue was contained in his first report, section 8, pages 39 to 75. He then covered the secondary evidence concerning Pharmasset and Idenix at pages 76 to 97 before returning to his overall views in the light of it. He expressed his views on the primary evidence before he was shown the materials concerning Pharmasset or Idenix.
- Prof Micklefield’s evidence started with a discussion of making compounds of Formula III, including specifically 2MU2FD compounds by reference to compound 1e (he said that compound 1d would be seen as impossible and I will not say anything more about it).
- He then set out three general routes for consideration (nucleoside, sugar, glyceraldehyde). He said that the skilled chemist would not know how to apply them to make the particular compounds he was considering and that reference to the literature would be needed. He said that the skilled chemist would start with general fluorination methods in a textbook by March (see the SACGK paragraph 22) and look at selected literature references from it. His conclusion at that stage was that some general fluorination information would be obtained but not sufficiently useful for the Formula III compounds.
- He therefore said that a literature search would be needed and gave extensive details of what it would entail. He said that the task of doing the search would itself be a major one.
- Against that background, Prof Micklefield returned to the question of whether the skilled chemist could make the compounds of Formula III. He explained that with the literature he had identified the skilled chemist would not have adequate examples and would not know which of the routes identified would work. The overall assessment he gave at this stage of his report was that the skilled chemist would need six months per compound if they were really fortunate but would need to plan for up to three years.
- Prof Micklefield then considered the 2MU2FD compounds specifically, by reference to compound 1e and compound 1f (1e having the uracil base and 1f, cytosine). To do so he drew on what he had already said about literature searching and the like. He set out five “overarching” routes that would be considered and then went through them in detail with literature references. He identified areas where the skilled chemist would expect difficulty, such as control of stereochemistry and tertiary fluorination.
- Having been through these routes, he expressed his views on prioritising them at paragraphs 247 and 248:
- I deal with the secondary evidence in more detail below; suffice it to say for now that Prof Micklefield thought the Idenix work supported his views and that the Pharmasset work showed that results had been obtained more quickly, but consistently with the sorts of difficulties he had predicted prior to seeing what they had done (and with failures on the way). He noted that the journal papers in which Pharmasset reported their success highlighted difficulties experienced.
- The primary evidence of Prof Davies was contained in his first report. He dealt with matters much more briefly than Prof Micklefield.
- He first said that he had been asked to explain how the skilled chemist would synthesise the relevant compounds without regard to information learned through the EPO proceedings, but that he would also comment on whether the latter affected his views.
- Prof Davies’ evidence went directly from the task set (synthesising the compounds) to two potential routes without including any stage of a preliminary literature review or the like.
- In section B.1 he covered the “Analagous Gemcitabine Route” with some supporting citations, covering the Concept work on that route in section B.1.1.
- In section B.2 he covered the “Chiral Auxiliary Route” which employed his laboratory’s SuperQuat technique. This is not relied on by NuCana in these proceedings, as I have already indicated and was done by another CRO called FOB.
- In section B.3 he covered the “Compound 22 Route”, which corresponds to Prof Micklefield’s route 3.1 (nucleophilic fluorination of tertiary alcohols), and covered the Concept and FOB work on that.
- I found Prof Micklefield’s primary evidence much more thorough and convincing than that of Prof Davies. It was more systematic and detailed and was much more clearly directed to how difficult the syntheses would have been for the ordinary skilled chemist. I was also impressed by his elaboration of how and why the synthetic routes would be difficult and the perceived “pinch points”.
- By contrast, although Prof Davies’ primary evidence was in places expressed to be in relation to the task that would face the ordinary skilled chemist, effectively Prof Davies went straight to putting down synthetic schemes which he regarded as possible ways to the compounds which could work. There was little to no recognition of the fact that there could be difficulties, or why, or where in the schemes they might lie.
- Although at this stage I am assessing the primary evidence and not the secondary evidence, I think it is appropriate to have regard to the fact that whatever ultimate conclusion one might reach about detailed aspects of the work done by real chemists that were covered at trial, it is plain that they experienced very real problems and setbacks at multiple stages. Synthesis of these compounds plainly has real difficulties. Prof Davies’ extremely high expectation of an easy and quick result was simply badly misplaced if proper regard is had to the ordinary skilled chemist. It reflected instead his own exceptional skill, experience and confidence.
- I have next to consider the secondary evidence of real teams who tried to make 2MU2FD compounds. I use “real” to distinguish them from the CROs who were undertaking work on the instructions of legal advisers for litigation.
- The work at Idenix was well summarised over 15 pages by Prof Micklefield in his first report. There is no dispute about the various routes that Idenix tried or the chronology. The routes were referred to as “Strategy 1” through to “Strategy 11”.
- The work started in February 2002 when Dr Griffon was put in charge. He began by doing a literature search which took until June (at least by the time he attempted Strategy 6 in early 2003 he conducted further research and had found Wachtmeister, which he came to via Singh).
- Work began and focused on a number of different fluorination approaches. At the time these were all considered to have failed. It is possible that in fact Dr Griffon did in February 2003 synthesise compound 1e as a minor product mixed in with others, but certainly at the time he did not think so. I regard that as a failure and not a success; identifying the result of an experiment is a necessary part of making it work.
- Eight fluorination strategies were tried between the commencement of work and November 2003, when the project was suspended. It was reinstated in February 2004 when Dr Griffon tried Strategy 1 again without success. Following internal discussion Dr Griffon proposed three more fluorination strategies but by April 2004 they had also failed.
- Lastly, Strategy 11, a glyceraldehyde approach, was tried in May 2004 but also failed.
- The overall picture is therefore one of failure. NuCana meets it in a number of ways. The main points are:
- NuCana points out that Dr Griffon was relatively junior and the project was only one of his responsibilities.
- Both these submissions are correct and I will bear them in mind. However, I think a much more significant factor is that the team as a whole was quite a large one. Dr Griffon reported to Dr Richard Storer and Prof Gilles Gosselin, both of whom were very experienced in nucleoside chemistry.
- In addition, Dr Griffon had input from external experts, Prof George Fleet from Oxford who was an expert in carbohydrate chemistry and Dr Paul Coe who was an expert in fluorination chemistry.
- On the evidence I am therefore satisfied that there was a good, indeed excellent, degree of scientific input, certainly in relation to fluorination routes.
- NuCana also relies on the fact that the glyceraldehyde work was undertaken mainly by Elodie Pecheux, who was an internship student. I agree that this is also a factor, and in addition that work took place in the last part of the timespan of the project when focus and optimism may (although this is speculative) have been flagging. Nonetheless Ms Pecheux was acting under the supervision of Dr Griffon and I have no positive reason to think she was doing other than making the best effort that she could.
- In his EPO evidence Prof Davies expressed in very strong terms the opinion that Dr Griffon lacked skill as a “wet” chemist. He maintained that position in his evidence in this case, although his views were not set out until his third report. A problem with this approach was that Prof Davies had already reached that conclusion for the EPO proceedings but could not say on the basis of what materials, except that they had included Arnold J’s judgment in Idenix v Gilead, Gilead’s Grounds of Opposition, and some laboratory notebook extracts.
- By contrast, Prof Micklefield expressed the view that the team was a good one (I have covered this), that the bench work was subject to supervision, that the strategies and choices matched well what Prof Micklefield had said was likely before seeing it (i.e. his primary evidence) and that all the decisions taken in prioritising and executing the routes were reasonable. Prof Micklefield’s views were based on a review of a “Briefing Bundle” which seems to me to have been very comprehensive.
- A problem with Prof Davies’ having focused on this issue only in his later evidence was that Prof Micklefield did not have a chance to respond to it in writing. And apart from some questions on the reasons for failure of the glyceraldehyde work, he was not really asked about the general competence of the Idenix team in his oral evidence, either. In a case such as the present where there was so much to get through during the trial it is understandable that some issues receive less attention in cross-examination than others, but on this point I think it is right for me to put significant weight on the fact that Prof Micklefield’s careful evidence about the competence of Dr Griffon is essentially unchallenged. I also think the close match between what Prof Micklefield had said about the notional skilled team’s approach and what actually happened at Idenix is supportive of my accepting his evidence.
- By contrast, Prof Davies’ opinions that Dr Griffon was not competent were intemperately expressed and excessive and the lack of transparency about the materials on which he had formed his views on this point further undermine their cogency.
- I also think it is inherently unlikely that Dr Griffon was given the responsibility for, and then for over two years left in charge of, a project such as this at a significant organisation when under very experienced supervision, if he was so lacking in competence.
- Finally, one reason that Prof Davies gave for questioning Dr Griffon’s competence was the failure to make compound 1e despite it being (Prof Davies said) easy to do so. This is obviously circular and Prof Davies was not open to the other interpretation, which was that Dr Griffon failed because the task is in fact very difficult.
- I therefore reject NuCana’s argument on this point.
- Prof Davies said that Dr Griffon had an undue focus on getting high yield and that this shaped his decisions in a way which did not represent the approach of the ordinary skilled person.
- This point is different in its nature and support from the general attack on competence. There are many references in Dr Griffon’s witness statements to the fact that he wanted a high yield, and that that focus both affected his choice of fluorination conditions and led to his not concentrating on minor products (which lends some support to NuCana’s point about whether Dr Griffon did in fact make compound 1e in February 2003, but not enough for me to change my mind about whether that was a “success”).
- NuCana submits that the test for sufficiency must be whether the ordinary skilled person could make a small quantity, enough to characterise, and does not require the ability to make production quantities. I agree with this. Therefore this is a factor that I should take into account. But it is only one factor and secondary evidence never perfectly matches the situation of the notional skilled person, so I think it is far from being enough to lead me to reject reliance on the Idenix work.
- Dr Griffon already had experience of DAST from his PhD and it was therefore usually his first choice of fluorinating agent. However, he did not opt for it in the Idenix work because of his concern about yield, which I have just mentioned, and because he was unaware, at least at the outset of the project, of literature supporting its use for tertiary alcohols.
- Accordingly Dr Griffon only used Deoxofluor, and NuCana through Prof Davies criticised the fact that he did not try DAST as well, especially in the work going on in February 2003. NuCana also submits that Prof Micklefield and Prof Davies agreed that the skilled chemist would be open to trying more than one option.
- However, there is more to it than NuCana submits. What happened was that Dr Griffon had read Wachtmeister, which he had identified via Singh. Wachtmeister does describe the use of DAST and Deoxofluor, but it describes the latter as an improvement over the former, and also gives more detailed conditions for its use. So in my view Dr Griffon’s approach, while influenced by his concern with yield, was a reasonable one.
- Even had Dr Griffon tried DAST as well, there would still be the question of the temperature, solvent and so on to consider. For reasons explained in relation to Mr Clark’s work, those details matter, and it appears that the use of standard DAST conditions (room temperature, DCM) does not lead to success. So I do not think this point assists NuCana.
- Idenix tried both the Reformatsky and Aldol approaches (in that order) and failed with both.
- Prof Davies said in his third report that two possible reasons for these failures were the omission to “crack” the glyceraldehyde by distillation immediately prior to use, in case it had formed a trimer, and the danger that the lithium reagents had “gone off”.
- The facts on “cracking” the glyceraldehyde were not very clear. Prof Davies agreed that on one occasion (3 May 2004) Ms Pecheux did it the day before the Reformatsky step was attempted and said that that may have been because she was aware of this issue. Had she been aware of it, it would then be odd that she did not follow the same approach before the second Reformatsky attempt on 14 May, although it is not recorded in her notebooks, and Prof Davies said her record keeping was generally good.
- This point was put to Prof Micklefield, but only to elicit that he was not familiar enough with the specific context to know how long it was all right to leave the glyceraldehyde for after distillation.
- NuCana’s case on these points is very speculative. Prof Davies went no further in his written evidence than to say they “may” be the explanation for failure. In all the circumstances I do not think any real weight can be placed on them.
- Mr Clark at Pharmasset, it is common ground, did manage to make the 2MU2FD nucleoside in May 2003. The ultimately successful route involved fluorination by a sugar route. He used DAST as a fluorinating agent, in dichloromethane at room temperature.
- As I have said above, it is not Gilead’s case that the task is an impossible one, but rather that it involves undue effort. So Mr Clark’s success is not the end of the inquiry and I have to consider the effort involved and how his work corresponded to that of the ordinary skilled chemist.
- NuCana submitted that there are more materials available to me than there were for Arnold J in his analysis of the Clark work in Idenix v Gilead. Since Gilead gave quite a lot of disclosure in the present case I expect that is true but as I have said above I do not think a comparative analysis is appropriate.
- I know relatively little about Mr Clark himself; his deposition is not very easy to follow. I do not feel well placed to assess the extent to which he corresponded to the notional ordinary skilled addressee.
- It is clear that he had advice and input from Prof Krzysztof (“Kris”) Pankiewicz, not from the outset of the project but probably from very early in 2003.
- Gilead said that Prof Pankiewicz was an expert in the field of synthesising nucleoside analogues. Prof Davies rather disobligingly said that he did not accept Prof Pankiewicz was an expert, despite being the author of a review article on which Prof Davies had positively relied in his first report. Further, Prof Seley-Radtke confirmed that Prof Pankiewicz was an expert in the field, and she was more involved in that than Prof Davies. I hold that Prof Pankiewicz was an expert, that his skill and knowledge would have significantly exceeded that of the ordinary skilled person, and that his input to Mr Clark’s work was of real significance.
- Prof Pankiewicz gave discouraging advice about the DAST fluorination route.
- Gilead submitted that although it was clear when Mr Clark achieved a successful result, it was not clear when he started. I agree that it is not completely clear but that does not matter; he started work in either November 2002 or December 2002 and the difference is not material.
- It is also clear that Mr Clark carried out an extensive and time-consuming literature search prior to starting work. It is not possible to be precise about its duration but Mr Clark said in his deposition for US proceedings that it was of the order of months. This is broadly consistent with his having had the idea to attempt the synthesis in October 2002 or possibly before.
- The overall timing is inconsistent with Prof Davies’ assessment of the time that the skilled chemist ought to take, although I agree with NuCana that the position is less than completely clear because the project was only part time for Mr Clark for some of the time. It is roughly consistent with the time that Prof Micklefield estimated for success if things went well (six months), but things did not go smoothly for Mr Clark so there is a bit of a mismatch. However, the overall timing and the need for a literature search and its duration match Prof Micklefield’s evidence much better that Prof Davies’.
- Mr Clark tried the Reformatsky approach but did not succeed and abandoned it. So far as one can tell from his deposition, the abandonment was before he did the successful DAST work but he may well have been doing them in parallel for some of the time and so far as it matters I agree with NuCana that he probably was. However, I disagree with Prof Davies that Mr Clark may have stopped work on the Reformatsky approach because the DAST work was going well. The documents are much more consistent with his giving up because of the problems he was having.
- Doing the best one can, it seems that he spent up to about one month on the Reformatsky work and certainly not longer than that; he did not put a specific duration to it in his deposition and said, understandably, that it seemed like a long time because he was failing.
- The problems that prevented progress were volatility of the reagents and difficulties with the stereochemistry.
- Mr Clark did not try the aldol route.
- Mr Clark’s work on glyceraldehyde routes is considerably more consistent with Gilead’s case than NuCana’s. His failure is consistent with Prof Micklefield’s evidence that the route would have difficulties (in particular in relation to stereochemistry), and corroborates to some extent the fact that Idenix also failed, although details are rather sparse in both cases. In addition, although it is a minor point it is to be noted that Mr Clark tried the Reformatsky approach rather than starting with the Aldol route as Prof Davies suggested would be the case.
- The key issue with this route was the fluorination conditions which I have addressed separately above. Mr Clark succeeded with non-standard conditions and having gone ahead in the teeth of discouragement from Prof Pankiewicz. The exact reasons for Mr Clark’s success are just not entirely clear. Counsel for Gilead said that luck was involved; it is possible that that is so but I am not able to tell.
- What I can say is that Mr Clark’s decisions and the conditions he chose were not conventional and that his success cannot be taken to be representative of what the experience and journey of the ordinary skilled chemist would be. His success came after quite a struggle, as well.
- So I do not think that the ultimately successful fluorination route assists NuCana much.
- Concept tried a number of routes.
- The key issue with Concept’s success on the Unprotected Uridine route relates to the fluorination conditions. I have considered this separately. My conclusions mean that NuCana is unable to rely on Concept’s success as representative of what the ordinary skilled chemist would experience or achieve.
- It also has to be borne in mind that Concept failed altogether on the Sugar Grignard fluorination route. NuCana does not rely on that route but that does not mean that I should ignore what happened. Concept did not even get as far as attempting the fluorination step, and this despite the route being one that Prof Davies put forward at the outset of his involvement in the EPO work. So this is another example where real world experience did not match Prof Davies’ optimistic assessments.
- Concept also succeeded with the Aldol glyceraldehyde route. It took from April to June 2019. Gilead took two related points about this.
- The first was that Concept had the advantage of:
- As to these, NuCana responded that:
- In general I agree with NuCana on the individual points. Concept will have saved some time as a result of these matters, and how much is hard to say, but they did not make the difference between success and failure.
- Gilead’s second point was that Concept had difficulty with the mesylation that was necessary to couple the lactone to the base. This took about two or three weeks to resolve. Here, the availability of the commercial lactone was probably more significant and Prof Micklefield pointed out that Concept never took starting material all the way through this route.
- Idenix also had trouble with the coupling aspect of this kind of route and I must consider that together with Concept’s relative success. In addition, Synthonix had an issue at that stage (in a Reformatsky context, and without the involvement of Prof Davies); NuCana does not rely on Synthonix but that does not prevent Gilead referring to it and this is a convenient point to address it, so I will briefly digress.
- Synthonix reported to Ms Knowles by email on 4 September 2020 that the mesylation was “not working”. Dr Allred of Synthonix said that he was not sure there was anything that could be done. But then in another email less than an hour later he provided “clarification” that the problem was during purification of the mesylate and that if the material was taken on “crude” to the next step he was confident that it would work.
- Dr Allred’s sudden change of heart is unexplained by NuCana. Gilead said that in the intervening time between the emails Ms Knowles must have provided further input. I reject this allegation which amounts to saying that Ms Knowles provided that input but then she and Dr Allred connived to create a misleading paper trail. There is no basis for such an inherently implausible explanation and the fact that Ms Knowles on other occasions encouraged Prof Davies not to commit things to paper is of a completely different nature.
- In closing oral submissions Counsel for NuCana said that he was not relying on the idea of taking the material straight through without separating but on isolating and then coupling. The fact remains that Synthonix failed at the latter.
- Having regard to the position with Idenix and Synthonix alongside what Concept did with the availability of commercial sugar lactone, I think the secondary evidence suggests that the mesylation/coupling stage in the glyceraldehyde route was tricky and uncertain.
- NuCana did not rely before me on FOB’s results and I heard little about it at trial. It was given the same five routes as Concept. In the EPO it was asserted that it succeeded with protected uridine and chiral auxiliary. Gilead invited the inference that it did not succeed with any of the other three. NuCana did not respond to this. I cannot see any reason why NuCana would not provide positive results if they existed, so I agree with the inference in broad terms but if it is assumed that the other routes did not succeed there could be many reasons. This is all too vague to give weight to it.
- I take as my starting point Prof Micklefield’s careful evidence that making the 2MU2FD compounds was intrinsically a difficult task, in absolute terms and in comparison to various otherwise similar nucleoside analogues. I also accept his assessment that the time involved would be considerable and very dependent on chance.
- I have already stated my reasons for accepting Prof Micklefield’s primary evidence over Prof Davies’, but I reiterate that he was to my mind really addressing whether he himself could succeed, not whether the task was a routine one for the skilled chemist.
- That being my starting point, I must still weigh the evidence against the legal standard with care. In particular, just as the fact that some succeeded does not mean that the standard is met, the fact that others failed (completely or initially) does not mean the effort required was undue; the law permits initial failures and recognises that in some fields they are just a fact of life.
- In that light, I think the following factors are important:
- My overall assessment of the work by those who actually attempted the work is that:
- Taking this as a whole, I conclude that no person genuinely representative of the ordinary skilled person and unassisted by super-experts and/or hindsight has been shown to have succeeded. I recognise that this cannot be conclusive and is not a substitute for the overall assessment that I have to make but it is important. I think the complete failure by Idenix and the patchy successes and failures of the other workers paints a picture of the task being well beyond the routine and of uncertain result.
- I think that the secondary evidence is, broadly and taken as a whole, consistent with Prof Micklefield’s primary evidence. The work of Idenix and Pharmasset stretched over the sort of time periods that he said were likely, and met with difficulty as he said they would. Even with the advantages they had, the CRO workers met the sort of problems he predicted, too.
- My conclusions are:
- I will hear Counsel as to the form of Order if it cannot be agreed. I direct that time for seeking permission to appeal shall not run until after the hearing on the form of Order (or the making of such Order if it is agreed). I draw attention to paragraph 19.1 of the Patents Court Guide, which says that a hearing on in the form of Order should take place within 28 days of hand down. In the present case, 28 days from hand down will be 18 April 2023.
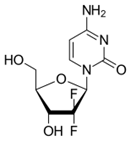
Gemcitabine
Sofosbuvir
i) Added matter: Gilead says that the Markush group definitions of the claims of the Patents are not clearly and unambiguously disclosed in the relevant original application.
ii) Lack of plausibility: Gilead says that the Patents do not plausibly disclose any technical contribution common to substantially all the claimed compounds. NuCana says that there is a plausible technical contribution in relation to the compounds having cytotoxic activity or improved intracellular delivery; it says that the cytotoxicity means that the compounds have the potential to be used in the treatment of cancer. Lack of plausibility is run under the legal heads of:
a) Obviousness over US 2003/0109697 (“Shepard”) on the basis that given the lack of plausibility there is no technical contribution;
b) Insufficiency.
iii) Lack of industrial applicability. This is very closely related to the lack of plausibility attack.
iv) Lack of technical effect in fact: Gilead alleges that in a significant number of cases the compounds of the Patents do not in fact have cytotoxic activity or improved intracellular delivery.
v) Undue burden insufficiency: Gilead says that it would be an undue burden for the skilled team to make a certain category of “2’ Me(up), 2’ F(down)” (“2MU2FD”) compounds, which are precursors for making some of the compounds of the claims, including sofosbuvir.
vi) That the proposed amendments to EP190 are not allowable. The main point is added matter to which I have referred already, but there is also a clarity point. Since amendment is sought unconditionally, refusal of the amendments would lead to revocation.
i) Litigation experiments (originally done for the EPO) by which NuCana engaged a number of contract research organisations (“CROs”) to synthesise relevant compounds. The evidence about the CROs focused on a company called Concept.
ii) Evidence of two real teams (Pharmasset, where the key player was a Mr Clark, and Idenix, led by a Dr Griffon) who sought to make 2MU2FD compounds at around the priority date.
EPO timing
Relevance of Idenix v Gilead
The witnesses
Professor Micklefield - Gilead’s medicinal chemistry expert
Doctor Galmarini - Gilead’s oncology expert
Prof Matthias Götte
Professor Seley-Radtke - NuCana’s medicinal chemistry expert (non-synthesis)
Professor Smyth - NuCana’s oncology expert
i) A mistake about the IC50s reported in papers called Zhou and Mengshetti. I agree that Prof Smyth was fairly obviously in error on this.
ii) Inconsistency about whether the HT115 colon cell line in the Patents had developed resistance. I agree that his oral evidence did not match his written evidence.
iii) Inconsistency about whether Figure 4 of the Patents allowed conclusions to be drawn about whether CPF31 had improved efficacy compared to gemcitabine. Again, there was some inconsistency.
iv) Overstatement in a declaration in the German proceedings about whether the NCI-60 panel results provided evidence of anti-cancer activity for sofosbuvir. I agree that there was an overstatement.
v) Not making clear in his reports that there was a difference between there being any measurable cytotoxicity and “meaningful” cytotoxicity. I agree that this distinction only came out when put to him during his oral evidence.
Professor Davies - NuCana’s synthetic chemistry expert
Prof Davies’ instructions for the EPO
The skilled team
CGK
Approach to CGK - the law
Agreed CGK
Nucleotides and nucleosides
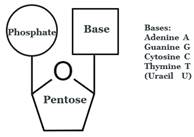
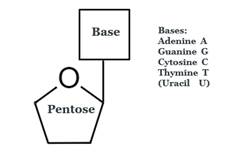
The structure of a nucleoside
Nucleoside analogues
Gemcitabine
|
|
|
|
Gemcitabine (dFdC) |
Deoxycytidine (dCyd) |
Gemcitabine (dFdC) alongside its natural nucleoside counterpart deoxycytidine (dCyd)
Mechanisms of action
Metabolic pathways
Overcoming resistance/intracellular drug delivery issues with nucleoside analogues
Measuring activity
i) IC50 - this is the concentration of a test compound at which cell proliferation is inhibited by 50% compared to the control. Researchers sometimes used CC50 and GI50 as synonyms for IC50. IC50 stands for Inhibitory Concentration (50%).
ii) EC50 - this is the concentration of a test compound where 50% of its maximal effect is obtained. This presents the issue that the maximal effect may be large or small, so one has to think about EC50s with care.
Disputed CGK –issues other than synthetic chemistry
Mechanisms of action and metabolism of NAs in cancer
AraU
dFdUMP
Positional changes
2’ position of the sugar, 5 position of the pyrimidine base
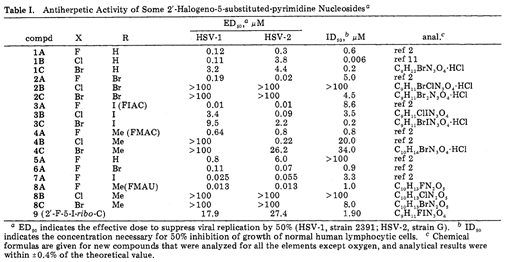
i) She pointed to the cytotoxic activity of floxuridine, capecitabine and 5-chloro-2’-deoxyuridine. Gilead accepted that the cytotoxicity of the first two was CGK, but said that the third was not, and I agree. In any event all three were, Prof Seley-Radtke accepted, TS inhibitors and so could only found predictions in relation to that mechanism of action, which is not relevant to gemcitabine analogues. These were isolated data points and I cannot see how they could found a prediction across the scope of the claims of the Patents in relation to the 2’ and 5 positions.
ii) Prof Seley-Radtke also relied on a publication by Prober and others in 1987 (‘A System for Rapid DNA Sequencing with Fluorescent Chain-Terminating Dideoxynucleotides’ (1987)), which concerned NAs used in DNA sequencing. I find that neither the publication nor the field of modifying nucleosides for such application was CGK and anyway the chemistry was significantly different because of the use of a spacer.
i) She referred to three AraC derivatives but I find that they were not CGK.
ii) She relied on a paper by Harris and others (Harris et al, ‘2-Deoxy-2’-halonucleotides as Alternate Substrates and Mechanism-Based Inactivators of Lactobacilllus leichmannii Ribonucleotide Reductase’ (1987)). Its relevance was not clear and NuCana did not appear to rely on it.
Q position of the sugar
i) In De Clercq itself the change of base (a point I consider in more detail next) from cytosine in Ce-Cyd to uracil in Ce-Urd abolished activity.
ii) In Balzarini, 1996 (‘Herpes Simplex Virus Thymidine Kinase Gene-Transfected Tumor Cells: Sensitivity to Antiherpetic Drugs’ (1996)), in three instances, using BVdU and analogues, making the compounds carbocyclic at position Q led to a loss of cytotoxicity, as Prof Seley-Radtke accepted. It may be noted that Prof Micklefield had been challenged in his cross-examination to give an example where replacing the furanose oxygen with CH2 led to a loss of activity and he said he had not looked for it, but Balzarini clearly provides such an example.
Changing the base
(Bio)isosterism
i) “The goal of analog design is twofold: (1) to modify the chemical structure of the lead compound to retain or to reinforce the desirable pharmacologic effect while minimizing unwanted pharmacological (e.g., toxicity, side effects, or undesired routes of and/or unacceptable rates of metabolism) and physical and chemical properties (e.g., poor solubility and solvent partition characteristics or chemical instability), which may result in a superior therapeutic agent; and (2) to use target analogs as pharmacological probes (i.e. tools used for the study of fundamental pharmacological and physiological phenomena) to gain better insight into the pharmacology of the lead molecule and perhaps to reveal new knowledge of basic biology. Studies of analog structure-activity relationships may increase the medicinal chemist’s ability to predict optimum chemical structural parameters for a given pharmacological action.”
ii) “The ideal program of analog design should involve a single structural change in the lead molecule with each new compound designed and synthesized…On a practical basis, it is frequently chemically impossible to effect only one discrete change in the lead molecule”
iii) “Although the strategy of bioisosteric replacement may be a powerful and highly productive tool in analog design, Thornber has emphasized that fundamental chemical and physical chemical changes can be expected to result from these molecular modifications, which may in themselves profoundly affect the pharmacological action of the resulting molecules. Contributing factors include change in the size of the atom or group introduced, which may affect the overall shape and size of the molecule; changes in bond angles; change in partition coefficient; change in the pKa of the molecule; alteration of the chemical reactivity and chemical stability of the molecule, with accompanying qualitative and quantitative alteration of in vivo metabolism of the molecule; and change in hydrogen-bonding capacity. The chemical and biological results and pharmacological significance of many of these factors are unpredictable and must be determined experimentally.”
i) “Isosteres are atoms or groups of atoms which have the same valency (or number of outer shell electrons). … Isosteres can be used to determine whether a particular group is an important binding group or not by altering the character of the molecule in as controlled a way as possible. Replacing O with CH2, for example, will make little difference to the size of the analog, but will have a marked effect on its polarity, electronic distribution and bonding. … Isosteric groups could be used to determine whether a particular group is involved in hydrogen bonding. For example, replacing OH with CH3 would completely destroy hydrogen bonding, whereas replacing OH with NH2 would not.”
ii) “Isosteres…have often been used in drug design to vary the character of the molecule with respect to such features as size, polarity, electronic distribution and bonding in such a way that any alteration in drug character can be rationalized. Therefore, some isosteres would be used to determine the importance of size towards activity, whereas a different isostere would be used to determine the importance of electronic factors.”
i) “Bioisosteres are substituents or groups that have chemical or physical similarities, and which produce broadly similar biological properties. Bioisosterism is a lead modification approach that has been shown to be useful to attenuate toxicity or to modify the activity of a lead, and it may have a significant role in the alteration of metabolism of a lead.”
ii) “It is, actually, quite surprising that bioisosterism should be such a successful approach to lead modification.”
i) Isosterism provided an analytical tool to facilitate SAR-type work by allowing the rational design of changes to a known compound or classes of compounds. It provided a fairly simple way to identify candidate, relatively small changes with some understanding of the means by which the change might have an effect. Thus, as the example in Patrick says, replacing O with CH2 would not be expected to change size but might well change other qualities (polarity etc).
ii) Nonetheless, it was understood that such changes were indeed changes and might well have an effect on activity, including a major effect. Numerous examples were given at trial. It was, in short, CGK that small changes could have a big effect.
iii) Isosterism was not a predictive tool, at least in the sense that where a change of the kind just mentioned was made to a compound, the skilled person would know that its effect had to be tested.
iv) On the other hand, changes made on the basis of this kind of analysis were supported with a degree of understanding of what the reason for a change would be if it were observed.
v) Changes of this kind ought to be made one at a time. The reason for this was to be able to understand the cause of a change in activity if one were observed.
vi) Overall the approach was structured and relatively conservative.
vii) When Silverman said that bioisosterism was a successful approach to lead modification that meant that leads had frequently been successfully improved by its use. That does not mean that it was a predictive technique and is perfectly consistent with its being a well thought out empirical approach.
Prof McGuigan’s ProTide work
Potential utility as anti-cancer agents
i) The “any measurable value” standard.
ii) An IC50 or EC50 of 100µM or less in an assay such as the MTT assay.
i) There was no single numerical cut off;
ii) Potential as an anti-cancer agent had to be assessed in a highly context-dependent way;
iii) Applying an appropriately context-dependent approach it would be possible to say in broad terms whether a compound was a strong candidate as an anti-cancer agent, a hopeless candidate, or a borderline candidate.
Disputed CGK - synthetic chemistry
Literature search
Experience of nucleosides
Fluorination conditions
Analytical techniques
i) Whether it was CGK that a TLC spot could be scraped off an analytical TLC plate. Prof Micklefield said not and Prof Davies said yes. Prof Micklefield was not really challenged about this and I accept his evidence. Prof Davies’s evidence only persuaded me that people in his laboratory were able to do it, and probably he was talking about after the priority date. In any event this is a very small point, being a minor facet of the argument about whether Dr Griffon was competent.
ii) Whether it was CGK to carry out an NMR analysis on every reaction product, including crude products. Prof Micklefield said not, particularly in the case of “messy” products. Prof Davies said that with messy products a complete NMR analysis might not be done but that it would be possible to characterise the main products in the circumstances of this case. I agree with Prof Davies that that would be seen as a reasonable way to go in some circumstances but I reject any contention that not doing it would be contrary to accepted practice so as to be an indication of a lack of competence (this was another point touching on Dr Griffon).
The Patents
Teaching of the specification
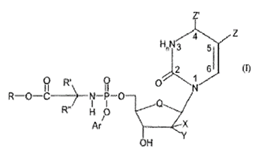
Quality of the experiments in the Patents
In vitro data
In vivo data
Assessment
Differences in EP365
i) Much of the difference is accounted for by the removal of the synthetic routes for the BVdU derivatives. This does not have any impact on the issues. The tests on the BVdU derivatives in Table I are unchanged.
ii) The discussion that appears at paragraphs [0050]-[0058] of EP190 is removed. But nothing appeared to turn on this given the arguments at trial, since there are still assertions of utility in the treatment of cancer in earlier paragraphs.
iii) [0020] contains a statement that in a preferred embodiment X is independently selected from F and methyl. This had a potential impact on the added matter points, but given my conclusions below there is added matter without it, and it just makes the position worse. So it does not require any further comment.
Claims in issue
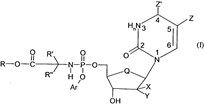
|
PCT |
EP’190 |
EP’365 | ||||
|
Formula (I) |
Claim 1 (uncond.) |
Claim 1 (cond.) |
Claim 1 |
Claim 2 | ||
|
R |
alkyl, aryl or alkylaryl |
methyl (-CH3), ethyl (-C2H5), n- or i-propyl (-C3H7), n- or i-butyl (-C4H9), or benzyl (-CH2C6H5) 720-21 | ||||
|
R’ |
H, alkyl or alkylaryl, or together form an alkylene chain |
one is H and one is methyl (i.e. D- or L-alanine) 816-17 | ||||
|
R’’ | ||||||
|
Q |
O (i.e. furanose) or CH2 (i.e. cyclopentyl) |
O (i.e. furanose) 914 | ||||
|
X |
H, F, Cl, Br, I, OH or methyl (-CH3) |
F, Cl, Br, or methyl (-CH3) 322-23 |
F, Br or methyl (-CH3) 322-23 |
F or methyl (-CH3) 322-23 | ||
|
Y |
F 322-23 | |||||
|
Ar |
a monocyclic aromatic ring moiety or a fused bicyclic aromatic ring moiety, either of which said ring moieties is carbocyclic or heterocyclic and is optionally substituted; |
-C6H5, pCF3C6H4-, pFC6H4-, pNO2C6H4-, pClC6H4- or oClC6H4-; 111-3 | ||||
|
Z |
H, alkyl or halogen |
H, alkyl or halogen |
H, acyclic C1-6 alkyl or halogen 115-7 | |||
|
Z’ |
The base can either be cytosine (Z’=NH2) or uracil (Z’=O) | |||||
Claim Scope and infringement
Validity
Added matter
i) To avoid covering compounds disclosed in the prior art, in particular Shepard.
ii) To avoid covering compounds which cannot be made.
iii) To avoid covering compounds which are not active.
The central argument
The law
“Added matter - the law
46. The objection is founded upon Article 123(2) EPC :
‘A European patent application or a European patent may not be amended in such a way that it contains subject matter which extends beyond the content of the application as filed.’
47. The test for added matter was stated by Aldous J in Bonzel v Intervention (No 3) [1991] RPC 553 at 574 in these terms:
‘The decision as to whether there was an extension of disclosure must be made on a comparison of the two documents read through the eyes of a skilled addressee. The task of the Court is threefold:
(1) To ascertain through the eyes of the skilled addressee what is disclosed, both explicitly and implicitly in the application.
(2) To do the same in respect of the patent,
(3) To compare the two disclosures and decide whether any subject matter relevant to the invention has been added whether by deletion or addition. The comparison is strict in the sense that subject matter will be added unless such matter is clearly and unambiguously disclosed in the application either explicitly or implicitly.’
48. In Case G 2/10, 30 August 2011, the Enlarged Board of the EPO explained in similar terms that an amendment can only be made “within the limits of what the skilled person would derive directly and unambiguously, using common general knowledge, and seen objectively and relative to the date of filing, from the whole of the application as filed”.
49. In Vector Corp v Glatt Air Techniques Ltd [2007] EWCA Civ 805, [2008] RPC 10 , Jacob LJ elaborated aspects of the test to be applied and drew together various statements of principle from earlier cases at [4]-[9]:
‘4. In Richardson-Vicks' Patent [1995] RPC 568 at 576 I summarised the rule in a single sentence:
‘I think the test of added matter is whether a skilled man would, upon looking at the amended specification, learn anything about the invention which he could not learn from the unamended specification.’
I went on to quote Aldous J in Bonzel. His formulation is helpful and has stood the test of time.
5. The reason for the rule was explained by the Enlarged Board of Appeal of the EPO in G1/93 ADVANCED SEMICONDUCTOR PRODUCTS/Limiting feature [1995] EPOR 97 at [Reasons 9]:
“With regard to Article 123(2) EPC , the underlying idea is clearly that an applicant shall not be allowed to improve his position by adding subject-matter not disclosed in the application as filed, which would give him an unwarranted advantage and could be damaging to the legal security of third parties relying upon the content of the original application.”
6. Mr Richard Arnold Q.C. provided a clear articulation as to how the legal security of third parties would be affected if this were not the rule:
“The applicant or patentee could gain an unwarranted advantage in two ways if subject-matter could be added: first, he could circumvent the “first-to-file” rule, namely that the first person to apply to patent an invention is entitled to the resulting patent; and secondly, he could gain a different monopoly to that which the originally filed subject-matter justified.”
7. Kitchin J has recently helpfully elaborated upon the Bonzel formulation in European Central Bank v Document Security Systems [2007] EWHC 600 (Pat), 26th March 2007 :
“[97] A number of points emerge from this formulation which have a particular bearing on the present case and merit a little elaboration. First, it requires the court to construe both the original application and specification to determine what they disclose. For this purpose the claims form part of the disclosure (s. 130(3) of the Act), though clearly not everything which falls within the scope of the claims is necessarily disclosed.
[98] Second, it is the court which must carry out the exercise and it must do so through the eyes of the skilled addressee. Such a person will approach the documents with the benefit of the common general knowledge.
[99] Third, the two disclosures must be compared to see whether any subject matter relevant to the invention has been added. This comparison is a strict one. Subject matter will be added unless it is clearly and unambiguously disclosed in the application as filed.
[100] Fourth, it is appropriate to consider what has been disclosed both expressly and implicitly. Thus the addition of a reference to that which the skilled person would take for granted does not matter: DSM NV's Patent [2001] RPC 25 at [195]-[202]. On the other hand, it is to be emphasised that this is not an obviousness test. A patentee is not permitted to add matter by amendment which would have been obvious to the skilled person from the application.
[101] Fifth, the issue is whether subject matter relevant to the invention has been added. In case G1/93, Advanced Semiconductor Products, the Enlarged Board of Appeal of the EPO stated (at paragraph [9] of its reasons) that the idea underlying Art. 123(2) is that that an applicant should not be allowed to improve his position by adding subject matter not disclosed in the application as filed, which would give him an unwarranted advantage and could be damaging to the legal security of third parties relying on the content of the original application. At paragraph [16] it explained that whether an added feature which limits the scope of protection is contrary to Art. 123(2) must be determined from all the circumstances. If it provides a technical contribution to the subject matter of the claimed invention then it would give an unwarranted advantage to the patentee. If, on the other hand, the feature merely excludes protection for part of the subject matter of the claimed invention as covered by the application as filed, the adding of such a feature cannot reasonably be considered to give any unwarranted advantage to the applicant. Nor does it adversely affect the interests of third parties.
[102] Sixth, it is important to avoid hindsight. Care must be taken to consider the disclosure of the application through the eyes of a skilled person who has not seen the amended specification and consequently does not know what he is looking for. This is particularly important where the subject matter is said to be implicitly disclosed in the original specification.”
8. When amendment of a granted patent is being considered, the comparison to be made is between the application for the patent, as opposed to the granted patent, and the proposed amendment (see the definition of ‘additional matter’ in s.76(l)(b)). It follows that by and large the form of the granted patent itself does not come into the comparison. This case was to some extent overcomplicated by looking at the granted patent, particularly the granted claim 1.
9. A particular, and sometimes subtle, form of extended subject matter (what our Act calls ‘additional matter’) is what goes by the jargon term ‘intermediate generalisation’. Pumfrey J described this in Palmaz's European Patents [1999] RPC 47 , 71 as follows:
“If the specification discloses distinct sub-classes of the overall inventive concept, then it should be possible to amend down to one or other of those sub-classes, whether or not they are presented as inventively distinct in the specification before amendment. The difficulty comes when it is sought to take features which are only disclosed in a particular context and which are not disclosed as having any inventive significance and introduce them into the claim deprived of that context. This is a process sometimes called “intermediate generalisation”.”
Domestic law about selection from lists
The EPO case law
“1.6.3 Deletion of elements from lists - shrinking the lists without singling out a combination of features
According to the boards' consistent case law, the guiding principle is that deleting meanings of residues in a generic chemical formula must not lead to the selection, in the respective lists, of a particular combination of single, specific but originally undisclosed meanings of residues (see T 615/95 and T 859/94).
In T 615/95 there were three independent lists of sizeable length specifying distinct meanings for three residues in a generic chemical formula in a claim. One originally disclosed meaning was deleted from each of the three independent lists. The board stated that the present deletions did not result in singling out a particular combination of specific meanings, i.e. any hitherto not specifically mentioned individual compound or group of compounds, but maintained the remaining subject-matter as a generic group of compounds differing from the original group only by its smaller size. Such a shrinking of the generic group of chemical compounds was not objectionable under Art. 123(2) EPC 1973, since these deletions did not lead to a particular combination of specific meanings of the respective residues which was not disclosed originally or, in other words, did not generate another invention. (See also T 948/02, which refers in detail to this case law and which did not allow the amendment of a generic chemical formula. For another decision distinguishing its case from T 615/95, see T 1150/15; see also T 894/05, T 888/08).
In T 50/97 the board explained that in the case at issue the shrinking of the lists of alternative definitions disclosed in the application as filed was not objectionable as that limitation did not result in singling out a particular combination of specific definitions, i.e. a hitherto not specifically mentioned sub-class of compounds, but maintained the remaining subject-matter of claim 1 as generic lists of alternative definitions differing from the original lists only by their smaller size (with reference to T 615/95 and T 859/94).
In T 942/98 the board held that, through the deletion of all other meanings, residues X1, X2 and R5 had been narrowed down to a single meaning, leading to a combination of specific meanings of residues not disclosed in the application as filed. Consequently, claim 1 as filed did not in itself provide adequate support for claim 1 as amended (cited by T 2013/08 in connection with the established case law concerning "singling out").
In T 1506/13 the board, referring to T 948/02, summarised that a deletion of genes from a list of specific genes was allowable if it fulfils two conditions: First, the deletion must not result in singling out any hitherto not specifically mentioned individual compound or group of compounds, but maintains the remaining subject-matter as a generic group of compounds differing from the original group only by its smaller size. Second, the deletion does not lead to a particular combination of a specific meaning which was not disclosed originally, i.e. it does not generate another invention, or in other words it merely restricts the required protection but does not provide any technical contribution to the originally disclosed subject-matter.
In T 98/09, which concerned the "singling out" of combinations of active ingredients not originally disclosed from lists, the board held that, contrary to the appellant's view, a deletion from a list could also constitute an inadmissible extension if the singling out of one individual ingredient led to a selection of combinations which, even if conceivably covered by the application as filed, had not been specifically disclosed. It was the boards' settled case law that such a selection is to be regarded as an inadmissible extension and so as an infringement of Art. 123(2) EPC (see e.g. T 727/00 and T 686/99). The case at issue concerned two lists (six elements and 47 elements). The applicant sought to individualise one of the lists to one element. The board held that this selection was contrary to Art. 123(2) EPC. For a similar case, in which the board found that the deletion of elements of two lists led to an unallowable selection, see T 1808/08.
In T 10/97 not all the compounds listed in the original claim were included in amended claim 1. However, since the claimed group of compounds was obtained not by restricting an originally disclosed generic definition of a substituent in a generic formula to a specific one selected from worked examples, but by deleting some members from a list of individualised equally useful compounds in order to improve the chances of patentability over the available prior art, the board found that such deletions must be considered admissible in accordance with the case law of the boards of appeal (see T 393/91). For the remaining compounds, no particular technical effect was either disclosed or alleged.
In T 783/09 the board referred to T 10/97. All forty-four combinations resulting from the combination of the elements of the two lists (one list with two elements, the other list with 22 elements) were directly and unambiguously disclosed. However, a further issue was whether or not the claiming of only three of the forty-four combinations disclosed extended the content of the application as filed in an unallowable way. The forty-four combinations were referred to as "very preferred embodiments"; by this statement the skilled person was taught that each of the forty-four combinations had the same quality, i.e. they were all very preferred combinations in the context of the invention. Nothing else was derivable from the remainder of the application, i.e. a particular quality, for example a particular technical effect, was attributed neither to the three combinations of claim 1 nor to the remaining forty-one. Hence, the group of claim 1 was to be considered as the result of the deletion of forty-one elements from a list of forty-four qualitatively equal elements (see T 10/97). In summary, the subject-matter of claim 1 complied with the requirements of Art. 123(2) EPC.
In T 1075/12 the patent proprietor restricted the definitions of groups to lists of specific substituents. The board held that the more precise definitions of the groups did not result in a particular combination of specific meanings of the respective groups being singled out, namely no particular structural feature of the compounds concerned was now claimed which was not disclosed originally. The board distinguished its case from T 859/04 and T 801/02, in which more than one variable in the respective chemical formulae had been individualised.”
i) Do the deletions single out a particular combination of specific meanings, i.e. a hitherto not specifically mentioned individual compound or group of compounds?
ii) Or, do the deletions merely maintain the subject matter as a generic group of compounds differing only from the original group by its smaller size?
iii) It is relevant to consider whether the deletions “generate another invention”. Another invention will be generated if the smaller group provides a technical contribution.
T615/95
i) A limitation does not necessarily result in novel subject matter (at 4.3);
ii) It may instead merely exclude subject matter without giving unwarranted advantage and with no impact on legal security (for third parties), citing G1/93 (ibid.);
iii) Deletions which do not result in “singling out” a compound or group of compounds” and which maintain the remaining subject matter as a generic group differing only in size did not “generate another invention”.
T50/97
T948/02 Great Lakes
T1150/15 Shionogi
G2/10
“4.3 The basic principle underlying Article 123(2) EPC, in the jurisprudence of the Enlarged Board
The importance and the applicability, without exception, of Article 123(2) EPC was underlined in the jurisprudence of the Enlarged Board of Appeal as early as in its opinion G 3/89 and decision G 11/91 (OJ EPO 1993, 117 and 125, relating to amendments by way of correction). From these rulings it follows that any amendment to the parts of a European patent application or of a European patent relating to the disclosure (the description, claims and drawings) is subject to the mandatory prohibition on extension laid down in Article 123(2) EPC and can therefore, irrespective of the context of the amendment made, only be made within the limits of what a skilled person would derive directly and unambiguously, using common general knowledge, and seen objectively and relative to the date of filing, from the whole of these documents as filed, points 1., 1.3 and 3 of the Reasons.”
Relevance of motive
The preliminary opinion of the TBA on EP190
“6.3 The Board observes that in accordance with established jurisprudence regarding the deletion of meanings from multiple lists defining variables in a generic formula (see Case Law of the Boards of Appeal, 10th Edition, II.E.1.6.3) it is not sufficient that the remaining subject-matter still relates to a generically defined group of compounds. In order to comply with Article 123(2) the deletion must not result in a particular combination of specific meanings which was not originally disclosed and which thereby generates another invention. In other words the amendment may not lead to a combination that is suitable to provide a technical contribution to the originally disclosed subject-matter as opposed to a mere restriction of the required protection.
In the patent as granted the definition of the compounds of formula I had already been limited with respect to the originally disclosed group of compounds by restriction of Y to a single meaning. Whilst such limitation is not objectionable as sole amendment, the combination of this limitation of Y with the further deletion of H in the meaning of X seems to single out those compounds in which Y represents F and in which X does not represent H. The original disclosure does not seem to provide any pointer to such combined limitation. The combined limitation effectively results in a group of compounds which is distinguished from the originally defined group of compounds by not comprising those compounds which are also covered by the teaching of document D36 [Shepard] and seems thereby suitable to provide a technical contribution to the originally disclosed subject-matter.”
The facts
Relevant disclosure of the PCT
“According to a first aspect of the invention there is provided a compound of formula I:
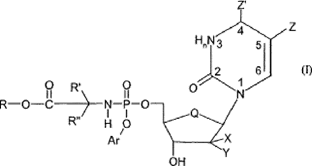
wherein:
R is selected from the group comprising alkyl, aryl and alkylaryl;
R’ and R” are, independently, selected from the group comprising H, alkyl and alkylaryl, or R’ and R” together form an alkylene chain so as to provide, together with the C atom to which they are attached, a cyclic system;
Q is selected from the group comprising -O- and -CH2-;
X and Y are independently selected from the group comprising H, F, Cl, Br, I, OH and methyl (-CH3);
Ar is a monocyclic aromatic ring moiety or a fused bicyclic aromatic ring moiety, either of which ring moieties is carbocyclic or heterocyclic and is optionally substituted;
Z is selected from the group comprising H, alkyl and halogen; and
n is 0 or 1,
wherein when n is 0, Z’ is -NH2 and a double bond exists between position 3 and position 4, and
when n is 1, Z’ is =O;
or a pharmaceutically acceptable derivative or metabolite of a compound of formula I;
with the proviso that when n is 1, X and Y are both H, R is methyl (-CH3), one of R’ and R” is H and one of R’ and R” is methyl (-CH3), then Ar is not phenyl.”
“Reference in the present specification to an alkyl group means a branched or unbranched, cyclic or acyclic, saturated or unsaturated (e.g. alkenyl or alkynyl) hydrocarbyl radical.”
“Preferably, X and Y are, independently, selected from the group comprising F, H and OH.
When n is 1, preferably each of X and Y is H.
When n is 0, preferably each of X and Y is F, or X is OH and Y is H, or X is H and Y is OH.”
“When Z is F, Q is O, n is 1 and X and Y are each H, the base moiety of the compound of formula I corresponds to that of fluorodeoxyuridine i.e. compound (1) above.
When Z is H, Q is O, n is 0 and X is OH and Y is H, the base moiety of the compound of formula I corresponds to that of cytarabine i.e. compound (2) above.
When Z is H, Q is O, n is 0 and X and Y are each F, the base moiety of the compound of formula I corresponds to that of gemcitabine i.e. compound (3) above.
When Z is H, Q is O, n is 0 and X is H and Y is OH, the base moiety of the compound of formula I corresponds to that of cytidine.”
“Compounds of formula I wherein n is 0 and X and Y are F are preferred. Particularly preferred are compounds of formula I wherein n is 0, X and Y are F, Q is O and Z is H, corresponding to phosphoramidated gemcitabine.
Also preferred are compounds of formula I wherein n is 0 and X is OH and Y is H. Particularly preferred are compounds of formula I wherein n is 0, X is OH, Y is H, Q is O and Z is H, corresponding to phosphoramidated cytarabine.
Also preferred are compounds of formula I wherein n is 0 and X is H and Y is OH. Particularly preferred are compounds of formula I wherein n is 0, X is H, Y is OH, Q is O and Z is H, corresponding to phosphoramidated cytidine.”
“Preferably Z is selected from the group comprising H, F, optionally substituted C1-6 alkyl particularly Me (-CH3), optionally substituted C1-6 alkenyl and optionally substituted C1-6 alkynyl, the optional substituents being as recited immediately above.
When n is 1, Z’ is O, Q is O and X and Y are each H, preferably Z is a substituted C2 alkenyl (i.e. ethenyl or vinyl) moiety (-CH=CH-); more preferably, Z is bromovinyl (-CH=CHBr) or methylpropenoate (-CH=CHCO2Me); and most preferably, Z is -CH=CHBr.”
“16. A compound according to any one of the preceding claims wherein n is 1, each of X and Y is H.
17. A compound according to any one of claims 1 to 15 wherein n is 0, each of X and Y is F.
18. A compound according to any one of claims 1 to 15 wherein n is 0, X is OH and Y is H.
19. A compound according to any one of claims 1 to 15 wherein n is 0, X is H and Y is OH.”
Relevant disclosure of EP190 as proposed to be amended
Comparison
Is there added matter?
i) It said that the PCT disclosed at page 6 line 4 that the substituents could include halogen atoms, meaning F, Cl, Br and I. I fail to see how this could help since neither X nor Y is defined in those terms in the amended form of EP190 and additionally X includes Me.
ii) It said that the overall invention had not in fact changed because the amended claims were to a class of ProTided gemcitabine analogues and such was also disclosed in the PCT, in particular in the form of CPF31, 40 and 41. In my view this is an exercise in describing two very different disclosures in such a general way that the same description (which did not appear in the PCT anyway) applies to both. The two classes are extremely different in size and nature. Comparisons for the purposes of added matter are to be carried out with much more rigour. There would have been basis for claiming those three compounds but then of course there would have been no trial because Gilead would not have infringed.
Alkyl
Conclusion on EP190 added matter
UKIPO comments
EP365
i) R is selected from methyl, ethyl, n- and i-propyl, n- and i-butyl and benzyl (rather than alkyl, aryl and alkylaryl, with the PCT’s definition of alkyl);
ii) one of R’ and R” is H and the other is Me (rather than each being any of H, alkyl and alkylaryl or together with an alkylene chain forming a cyclic system);
iii) Q is -O- (rather than -O- or -CH2-);
iv) Ar is selected from phenyl, para-F/CF3/NO2-phenyl and para/ortho-Cl-phenyl (rather than an optionally substituted carbocyclic or heterocyclic monocyclic or fused bicyclic aromatic ring moiety);
v) Z is selected from H, halogen and acyclic C1-6 alkyl (rather than H, halogen and alkyl, with the PCT’s definition thereof).
Industrial applicability and plausibility
Law on capable of industrial application
“The general principles are:
(i) The patent must disclose “a practical application” and “some profitable use” for the claimed substance, so that the ensuing monopoly “can be expected [to lead to] some ... commercial benefit” (T 0870/04, para 4, T 0898/05, paras 2 and 4);
(ii) A “concrete benefit”, namely the invention’s “use ... in industrial practice” must be “derivable directly from the description”, coupled with common general knowledge (T 0898/05, para 6, T 0604/04, para 15);
(iii) A merely “speculative” use will not suffice, so “a vague and speculative indication of possible objectives that might or might not be achievable” will not do (T 0870/04, para 21 and T 0898/05, paras 6 and 21);
(iv) The patent and common general knowledge must enable the skilled person “to reproduce” or “exploit” the claimed invention without “undue burden”, or having to carry out “a research programme” (T 0604/04, para 22, T 0898/05, para 6)”
Law on plausibility
i) First, what falls within the scope of the claimed class?
ii) Second, what does it mean to say that the invention works?
iii) Third, is it possible to make a reasonable prediction the invention will work with substantially everything falling within the scope of the claim?
i) Plausibility must be shown by the specification of the patent in issue, along with the CGK. I agree with this and Gilead relied on it. But I return to this in a little more detail below.
ii) A patentee is not necessarily limited to relying on the most demanding teaching of utility in the specification. I accept this too. I dealt with it in Sandoz at [63] - [69], primarily by reference to what Floyd LJ said in Generics v Yeda [2013] EWCA Civ 925 at [64]-[65].
iii) A technical contribution must be of some, even if low, real significance. Disclosing a uselessly low degree of activity as a comparator or “what not to do” is not good enough. This is one of the things that I said in Sandoz, at [74] - [76].
Ab initio plausibility or implausibility
Technical contribution - NuCana’s case
i) The first technical contribution advanced by NuCana was a new class of phosphoramidate nucleosides of the chemical structure of the various claims of the Patents.
ii) The second technical contribution advanced was a new class of phosphoramidate nucleosides with improved intracellular delivery.
iii) The third technical contribution alleged was a new class of phosphoramidate nucleosides with cytotoxic activity.
“[NuCana’s] contention is that all or substantially all [the claimed] compounds are characterised by improved intracellular delivery (compared to that of the corresponding bare nucleoside analogues) and/or cytotoxic activity. As a consequence of said improved intracellular delivery, the Patents disclose that the phosphoramidate pro-drug approach devised by Professor Christopher McGuigan can be successfully applied to gemcitabine and all or substantially all the gemcitabine-like nucleosides defined in the claims. Further, as a consequence of said improved intracellular delivery and/or cytotoxic activity, all or substantially all the claimed compounds (i) have the potential to be used as therapeutic agents and/or (ii) have utility in non-therapeutic applications, including as research tools in scientific research.”
“57. At paragraph 298, Dr Galmarini states that “in my view, the Patent is directed to a drug discovery team that was interested in developing a NA drug for cancer”. While that can be the end goal of a drug discovery process (Professor Micklefield refers to it as the “ultimate” goal in paragraph 64 of his report), the medicinal chemist reading the Patents would see the compounds in question as promising therapeutic agents with the potential to be used in the treatment of cancer or other diseases that were known to involve abnormal cellular proliferation such as immune-related disorders and inflammatory diseases (for example rheumatoid arthritis).
58. The data in the Patents are limited to showing that the compounds have cytotoxic activity - although some in vivo mouse experiments are reported, no clinical data are presented. In order to confirm whether any particular compound was clinically effective, it would be necessary to carry out a significant amount of further work, including clinical studies, to demonstrate efficacy in patients and the absence of toxicity (off-target effects). Therefore, the medicinal chemist would see the Patents as providing a class of novel compounds that have cytotoxic activity and (because of the general properties of ProTides) improved intracellular delivery with consequent potential for development for clinical use. As I explained at paragraph 27 above, the identification of such compounds would have been seen as a valuable technical achievement in the drug development process.
59. Furthermore, as acknowledged by Dr Galmarini in paragraph 32, at the Priority Date cytotoxic nucleoside analogues were the subject of study for the purpose of elucidating the precise molecular mechanism by which they were causing cytotoxicity. This included studying the effect of cytotoxic agents on cell lines of interest in order both to explore the molecular biology of those cells and mechanisms related to drug resistance. For example, gemcitabine ProTides can be used to explore mechanisms of gemcitabine resistance as they do not depend upon the activity of nucleoside transporter proteins or a kinase for the first phosphorylation event, which were both known to be involved in resistance mechanisms.
60. Cytotoxic agents, such as nucleoside analogues, were also used to study the biological processes involved in the development of diseases characterized by abnormal cellular proliferation. As indicated above, in addition to cancer, such diseases include a number of immune-related disorders and inflammatory diseases.
61. For these reasons, the compounds that are the subject of the Patents would have been recognized to be useful as research tools in addition to representing a valuable technical achievement in the drug development process.”
“NuCana’s primary case is that cytotoxic activity is demonstrated by a measurable IC50/EC50 etc value in an in vitro cytotoxicity/antiproliferative assay such as the MTT assay.
NuCana’s secondary case is that cytotoxicity is demonstrated by a value of 100 μM or less in such an assay.”
Disclosure of the contributions
Cytotoxicity - plausibility facts and assessment
i) There are large numbers of compounds in the claims.
ii) The Patents do not contain information about their mechanisms of action.
iii) Small changes in structure can make a big difference in activity (this was accepted by NuCana).
iv) The claims of the Patents allow not just one change from gemcitabine or the compounds tested in Table I and in the mouse model, but several. Yet Prof Seley-Radtke’s analysis only considered one change at one position.
v) It was CGK that an important structural feature of gemcitabine and the compounds tested in the patent is the presence of two fluorines at the 2’ position of the sugar (the “sugar pucker”).
vi) It was CGK that changes at the 2’ position of the sugar or the 5 position of the base could remove activity.
vii) Likewise it was CGK that changing the furanose oxygen could remove activity.
viii) It was CGK that changing the base between cytosine and uracil could remove activity.
Two aspects of Dr Galmarini’s evidence
“Cytotoxic nucleoside analogues are clinically important anticancer drugs. The newer member of this family, gemcitabine, has shown great activity in solid tumors and thus enlarged the spectra of malignancies treated by this family of molecules. However, the clinical use of nucleoside analogues is limited by important side-effects and primary or acquired drug resistance, and there is an unmet medical need for the development of new molecules and technologies allowing a suitable treatment of cancer patients with these agents.”
And
“Recently, Macguigan [sic.] published a patent in which he describes the development of phosphoramidate derivatives of nucleoside analogues [53]. In contrast to the phosphotriester derivatives described above, phosphoramidates contain a P-N link, and therefore require another biological system for intracellular delivery of the monophosphorylated nucleoside analogue. One interesting derivative described in this patent is the CPF31 ((17), Fig. 10). In cell culture cytotoxicity assays, this compound is 100-fold more active than gemcitabine on colon cancer HCT-115 cells. In mice bearing human colon cancer cells or murine prostate cancer PC-3 cells, CPF31 was more efficient in reducing tumor growth, and less toxic than gemcitabine. This patent does not include any data on nucleoside analogue resistant cell lines, but, in theory, these compounds should be efficient against transporter- or kinase-deficient cells.”
13 MR. MITCHESON: Just stepping back from this, doctor, I think you
14 would accept that although there is data suggesting that some
15 compounds falling within some of the claims of the patents do
16 not give a number below 100 micromolar in relation to some of
17 the cell lines against which they have been tested; yes?
18 THE WITNESS: Mmm-hmm.
19 Q. You accept they have only been tested against a very limited
20 number of cell lines; yes?
21 A. Yes.
22 Q. Compared to, say, the 60 standard lines in the NCI test; yes?
23 A. Yes.
24 Q. So you just cannot say whether any of these molecules would,
25 in fact, provide a result under 100 micromolar in one or more
2 of the standard cell lines to be tested?
3 A. The molecules that are expressed in the table?
4 Q. I am stepping back and talking about all the numbers you have
5 commented on in the papers; yes?
6 A. Yes. And the question was, sorry?
7 Q. You just cannot say whether any of these molecules would, in
8 fact, provide a result under 100 micromolar in one or more of
9 the standard cell lines to be tested?
10 A. No, because that was not done.
…
22 Q. So what I am suggesting to you, doctor, is that given the
23 spread of results under 100 micromolar from the molecules in
24 these peer-reviewed papers, you would not be surprised to find
25 one or more cell lines which have not been tested yet in which
2 each of these remaining molecules gave you a figure of less
3 than 100 micromolar?
4 A. Yes, that is speculation, but we can assume that there are
5 going to be cell lines that are going to be over that value
6 and cell lines that are going to be below that value. You
7 have to perform the test.
Assessment
i) The fact that the claims cover large numbers of compounds in which multiple changes known to be prone to remove activity are made makes it extremely unlikely that substantially all would have activity at the levels discussed above, so much so that the skilled team would positively think that many would not.
ii) The fact that the claims cover combinations at the 2’ position which could be seen in the CGK such as Watanabe, and inferred from the “sugar pucker” thinking, to be really quite fundamental differences from gemcitabine and liable to remove activity.
i) “Any measurable activity” is a meaningless standard and while it could (as Gilead itself contends) be predicted to be possessed by any compound in some cell line under some conditions and at some concentration it does not reflect a technical contribution.
ii) The 100µM IC50 standard does not translate to any real utility in most cases. There are rare instances of compounds with IC50s approaching that level having utility but most do not. I refer to my findings in relation to the CGK.
Research tools
“These first two phases [identifying compounds for testing and in vitro testing] represented a significant challenge. The chemical space (i.e. the number of possible compounds that could be made and tested) is vast. The medicinal chemist seeking to develop new nucleoside analogues is faced with an almost infinite number of choices in terms of the structure of potentially useful compounds, even if factors such as the ease of synthesis and druggability of the compounds are taken into account. The identification of a defined class of new compounds with cytotoxic activity would therefore have represented a valuable technical achievement at the Priority Date, as it still does today.”
19 ...the skilled person looks at the field, looks at what has
20 been done, and there was a huge interest in modified
21 nucleosides for research tools. Many, many groups were
22 looking at this, including ours, to explore enzyme mechanisms,
23 to explore -- well, frankly, how far you can push mother
24 nature in terms of making modifications and changes. But
25 I think very useful to have a variety of different structures
2 available to you to sort of interrogate enzyme mechanisms, you
3 know what are the requirements for that particular mechanism
4 of action for a particular set of compounds? So they are very
5 useful, in addition to the McGuigans, being able to overcome
6 two major problems with nucleoside analogues.
Lack of Industrial Application
“Sufficiency”/“existence in fact”
i) The results in the Patents;
ii) Results for sofosbuvir;
iii) Results from three scientific papers:
a) Zhou ('2’-Chloro-fluoro Ribonucleoside Prodrugs with Potent Pan-genotypic Activity against Hepatitis C Virus Replication in Culture’ (2017));
b) Mengshetti (‘Discovery of a Series of 2’-alpha-Fluoro, 2’-beta-bromo-ribonucleosides and Their Phosphoramidate Prodrugs as Potent Pan-Genotypic Inhibitors of Hepatitis C Virus’ (2019));
c) Quintiliani “(‘Design, synthesis and biological evaluation of 2’-deoxy-2’,2’-difluoro-5-halouridine phosphoramidate ProTides’ (2011));
iv) Results from Gilead’s disclosure;
v) Two results from “other disclosure”.
i) The compounds in Zhou have X = Cl.
ii) The compounds in Mengshetti have X = Br.
iii) The compounds in Quintiliani have Z = halogen.
iv) The compounds in Gilead’s disclosure have X = Me.
i) As to the compounds in the Patents, as set out in the section of this judgment dealing with the data from the specification.
ii) There are many compounds in Annex B that do not achieve a measurable cytotoxicity even at the highest concentration tested.
iii) There are other compounds that have a mixture of results with some cell lines giving no result at the highest concentration and other cell lines giving a result.
iv) Results, where achieved, vary quite widely, from (excluding the Patent compounds) mostly around 20µM up to 200µM, with a couple from Gilead’s disclosure being less than 1µM.
v) Quite a lot of the results achieved are over 100µM or only slightly under.
vi) Although there are of course fewer results for the narrower claims, they do not present a materially different overall picture from the broader claims (because the narrowing excludes compounds with and compounds without measurable activity) and NuCana did not argue that they did.
vii) Neither side said there was a pattern to the results based on different substitutions.
i) No cytotoxicity was found up to 100µM in any of the 60 NCI cell lines (these are not all shown in Annex B, just covered by the title of the right hand most column).
ii) Nor was any cytotoxicity found up to 100µM in Zhou or Mengshetti.
iii) There have been measurable results in Huh7, HepG2 and SKBR3 cell lines, ranging from just under 100µM down to about 60µM in one instance.
Assessment
i) Fewer cell lines were tested than e.g. in the NCI screen.
ii) The “vast majority” of the compounds in the peer reviewed articles show cytotoxicity below 100µM in at least one cell line.
iii) Gilead’s disclosure showed more “failures” (NuCana said 23 out of 53, the exact numbers do not matter) but less is known about the assay conditions and it cannot be known what would happen if more cell lines e.g. the full NCI panel were used. It is likely that measurable cytotoxicity, including at the 100µM level, would have been found in one or more cell lines had that been done.
i) My conclusions on plausibility are that the substituent changes compared to gemcitabine are such that loss of activity was likely to result from them.
ii) Prof Seley-Radtke made clear that her written evidence was given on the basis of any measurable activity, not 100µM.
iii) I have rejected NuCana’s reliance on the particular part of Dr Galmarini’s cross-examination at T3/492-495.
Improved intracellular delivery - plausibility facts and assessment
Shepard
Undue burden insufficiency
i) Undue burden to synthesise 2MU2FD intermediates.
ii) Undue burden to identify any use for the class of compounds claimed other than treating cancer or viral infections.
Terminology
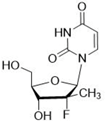
The Law
i) The size of the structural class is not relevant; the test for undue burden is not the cumulative burden of identifying and testing for efficacy all the compounds covered by the structural formula.
ii) Instead, the skilled person must be able, without undue burden, to identify some compounds beyond those named in the patent.
iii) It must also be possible for the skilled person to work substantially anywhere within the whole claim.
The standard for undue burden
“[The skilled person] must seek success. He may need to carry out the ordinary methods of trial and error, which involve no inventive step and generally are necessary in applying the particular discovery to produce a practical result. In each case, it is a question of fact, depending on the nature of the invention, as to whether the steps needed to perform the invention are ordinary steps of trial and error which a skilled man would realise would be necessary and normal to produce a practical result.”
“Even though a reasonable amount of trial and error is permissible when it comes to the sufficiency of disclosure in an unexplored field or - as it is in this case - where there are many technical difficulties, there must then be available adequate instructions in the specification or on the basis of common general knowledge which would lead the skilled person necessarily and directly towards success through the evaluation of initial failures or through an acceptable statistical expectation rate in case of random experiments.”
“It is not enough if the instructions are such that a number of equally qualified notional addressees can arrive at completely different end points, some within the scope of the claimed invention and some not. If reasonable addressees can come to different conclusions there is a conundrum as to which is right. That is not enablement. This view appears to be consistent with the approach of the Technical Board of Appeal of the EPO in Unilever/Stable bleaches (Decision T226/855) [1988] OJEPO 336, which was referred to with approval by Aldous J and the Court of Appeal in Mentor Corp v. Hollister Inc.”
i) The test is not whether it is impossible to make the compounds. Of course that would be an insufficiency as well, but it is not Gilead’s case; we know that compound 1e and thence sofosbuvir can be made. The attack is one of undue burden.
ii) In assessing undue burden one assumes that the skilled team has the benefit of the teaching of the patent concerned, and the CGK. In the present case there is no relevant teaching in the Patents to help with the synthesis. That means that NuCana has to rely on the CGK alone (and NuCana did not dispute this). Gilead repeatedly relied on this as if there was something inherently wrong with it, but there is not. A patent can perfectly well be sufficient based on the CGK alone, although of course the patentee will typically be much better off if the patent contains specific instructions.
The role of primary and secondary evidence
Overview of the routes
Two broad categories
i) Glyceraldehyde;
ii) Fluorination.
Glyceraldehyde routes
i) Route 1.1 was referred to as Reformatsky, based on a citation called Hertel.
ii) Route 1.2 was referred to as Aldol, based on Weigel.
Fluorination routes
i) Route 2 is performed on a lactone and is carried out only with a sugar. This is an electrophilic fluorination approach in contrast to 3.1 to 3.3 which are all nucleophilic.
ii) Route 3.1 uses a tertiary alcohol and may be carried out with a sugar or a nucleoside.
iii) Route 3.2 uses an alkene and may be carried out with a sugar or a nucleoside.
iv) Route 3.3 uses an epoxide and may be carried out with a sugar or a nucleoside
Fluorination conditions
i) This would be unfamiliar territory for the skilled chemist.
ii) There are many parameters to be adjusted, including the choice between DAST and Deoxofluor, temperature, solvent and use of pyridine.
Fluorination Schemes Document
Choice of successful fluorination conditions used by Concept
Prioritisation
Primary evidence
Prof Micklefield
“247. If I had to personally prioritise routes, based upon my background and experience I would say that routes 3.1-3.2 (nucleophilic fluorination) would be my first preference because they potentially offer the shortest route to the target compound from starting materials which are described in the literature; however, the fluorination step required by these routes is unknown. An attractive feature of these routes is that there is the option of doing each with the base on (i.e. a nucleoside route), or the base off (i.e. a sugar route). Trying the ‘base on’ reactions first would be slightly more attractive to me, because if the fluorination works ‘base on’ would mean a smaller number of steps to make the final molecule for the biologist to test. If the base has to be added, then there are potential problems with the stereocontrol for the addition of the base (discussed in paragraph 203). The sugar routes would remain a fall-back option if there were problems with the nucleoside routes, that would have been my initial preference.
248. In contrast, Routes 1.1 and 1.2 are probably the best precedented of all the routes have discussed above because the plan would be to essentially follow the Hertel synthesis if the initial step could be achieved. Routes 1.1-1.2 also avoid the need for difficult and unknown fluorination reactions on complex molecules. The downsides are that routes 1.1-1.2 involve a larger number of unknown steps than Routes 3.1-3.2.”
Prof Davies
Comparison
Secondary evidence: real teams
Idenix
i) Personnel on the team at Idenix.
ii) Competence of Dr Griffon and those working for him.
iii) Focus on yield.
iv) Fluorination conditions.
v) Reasons for the failure of the glyceraldehyde work.
Personnel
Competence of Dr Griffon
Focus on yield
Fluorination conditions
Failure of the glyceraldehyde work
Pharmasset
Mr Clark and Prof Pankiewicz
Overall timing
Glyceraldehyde routes
Fluorination route
Secondary evidence: CRO experiments
Concept
i) Better chromatographic techniques than were available in 2003;
ii) Access to published NMR data, not available in 2003 to characterise the sugar lactone;
iii) Access to commercial sugar lactone in the base-coupling reactions.
i) The evidence does not show that the improvement in chromatographic techniques made a difference to Concept’s success;
ii) Concept also confirmed the stereochemistry with NOE, a 2003 CGK technique and anyway the NMR peaks to be looked for were easy to spot so this was no more than a comfort blanket;
iii) The time saved was small and in 2003 the alternative of just repeating the steps to make the sugar lactone was available.
FOB
Analysis
i) Quite a number of routes were put forward by the experts and tried by real workers.
ii) This was not because they were spoilt for choice but because of the nature and difficulty of the task and because it was not a well precedented one. A number of routes were needed as candidates because of the very real probability that each would fail. But it was not predictable which would fail or why.
iii) So this was not a situation where the right general approach was clear but tweaks or fine tuning would be expected if there were initial failures. It would have been a quite a different matter if it was plain, for example, that a particular fluorination approach ought to work and it was just a question of empirically identifying the reaction time and temperature, expecting both that the first try would not work but that a subsequent one in due course would.
iv) The experts could not and did not agree about which routes were preferred, and did not put forward the same possibilities. Again, this was not just a matter of taste or embarras de richesses, but because of the complexity of the task.
v) Each route had its “pinch points” or areas of greater expected difficulty. Prof Davies did not really recognise these, but Prof Micklefield did.
vi) The pattern of success and failure for the different real teams with different routes was different. There was not a clear winning strategy.
vii) There was a big role for sheer luck.
viii) Whatever one might conclude about the detailed reasons for success or failure with each route by each team, none of the real workers went into the exercise with the expectation that it would be straightforward.
ix) Of the real world workers who tried the task (excluding the CROs) and succeeded, both Mr Clark’s team and Prof Seley-Radtke’s team had their work reported in peer-reviewed journals, and Prof Seley-Radtke’s group reported that the work was “nontrivial” and had produced only low yields (I recognise that the paper also reported much other work). This is a rather prosaic point, perhaps, and it might be said that it is somewhat indirect, but I think it is significant. One would not expect a merely routine synthesis to generate publications like this.
i) Idenix failed because of the difficulty of the task. I discount to some degree the attempts somewhat late in the project with the glyceraldehyde approach because I accept that it is likely that the effort and resources deployed were limited and the experience of those involved was modest. But I reject the suggestion by NuCana that the main thrust of the work under Dr Griffon was not competently done. In my view there was a very significant effort which failed even despite highly expert input/advice, well above the level of the ordinary skilled person.
ii) Mr Clark succeeded, particularly in relation to the fluorination approach. But he had his struggles, and also had expert outside advice rising above the ordinary, for which reason he cannot be taken to represent the ordinary skilled chemist. The evidence of how he succeeded and why is not clear or complete. Looking at the work overall, including failures as well as successes, despite the relatively short overall time from start to success (at the lower end of what Prof Micklefield thought might be expected), I do not think it represented a routine effort, and in relation to the key point, the fluorination conditions, NuCana has not shown that he was following a routine path.
iii) Concept had both success and failure in the routes tried. However, I do not think the workers there can be taken to be representative of the ordinary skilled chemist. Their initial instructions may have been based at a high level on Prof Davies’ suggestions, but much of the detail appears to have come from NuCana’s legal team and from whichever source it came, the inputs to their work were influenced, directly or indirectly, by knowledge of the successful efforts by Mr Clark, and by hindsight generally. In addition they were able to call on Prof Davies when they got stuck, and did so.
Conclusions
i) EP190 and EP365 are both invalid for lack of plausibility and for added matter. They are also insufficient because they cover compounds which do not in fact have the relevant characteristics, and because of the undue burden involved in making the 2MU2FD compounds.
ii) The proposed amendments to EP190 also fail for added matter.
iii) The attack over the Shepard prior art fails.
iv) Had the Patents been valid they would have been infringed.