Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales Court of Appeal (Civil Division) Decisions
You are here: BAILII >> Databases >> England and Wales Court of Appeal (Civil Division) Decisions >> FibroGen Inc v Akebia Therapeutics Inc [2021] EWCA Civ 1279 (24 August 2021)
URL: http://www.bailii.org/ew/cases/EWCA/Civ/2021/1279.html
Cite as: [2021] EWCA Civ 1279
[New search] [Printable PDF version] [Help]
Neutral Citation Number: [2021] EWCA Civ 1279
Case No: A3/2020/0895 and A3/2020/0893
IN THE COURT OF APPEAL (CIVIL DIVISION)
ON APPEAL FROM THE HIGH COURT OF JUSTICE
BUSINESS AND PROPERTY COURTS OF ENGLAND AND WALES
INTELLECTUAL PROPERTY LIST
PATENTS COURT
Lord Justice Arnold
HP-2018-000036 and IL 2019-000031
Royal Courts of Justice
Strand, London, WC2A 2LL
Date: 24/08/2021
Before :
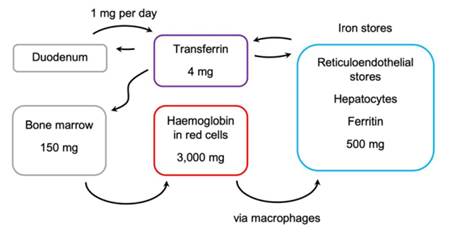
LORD JUSTICE PHILLIPS
LORD JUSTICE BIRSS
and
SIR CHRISTOPHER FLOYD
- - - - - - - - - - - - - - - - - - - - -
Between :
|
|
FibroGen Inc. |
Appellant in A3/2020/0895 |
|
|
- and - |
|
|
|
(1) Akebia Therapeutics Inc. (2) Otsuka Pharmaceutical Company Ltd |
Respondents in A3/2020/0895 |
|
|
|
|
|
|
|
|
|
|
Astellas Pharma Inc |
Appellant in A3/2020/0893 |
|
|
- and - |
|
|
|
(1) Akebia Therapeutics Inc. (2) Otsuka Pharmaceutical Company Ltd (3) FibroGen Inc. |
Respondents in A3/2020/0893 |
- - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - -
Tom Mitcheson QC and Joe Delaney (instructed by Carpmaels & Ransford LLP) for FibroGen
Justin Turner QC, Kathryn Pickard and Michael Conway (instructed by Potter Clarkson LLP) for Astellas
Iain Purvis QC, Piers Acland QC and Anna Edwards-Stuart (instructed by Hogan Lovells) for Akebia and Otsuka
Hearing dates: 8th, 9th and 10th June 2021
By Teams
- - - - - - - - - - - - - - - - - - - - -
Judgment Approved
Covid-19 Protocol: This judgment was handed down remotely by circulation to the parties’ representatives by email, release to BAILII and publication on the Courts and Tribunals Judiciary website. The date and time of the hand-down was deemed as 10:30am on
24th August 2021.
Lord Justice Birss:
1. This appeal relates to six patents belonging to FibroGen and exclusively licensed to Astellas. The patents relate to the use of medicines known as HIF-PH inhibitors for the treatment of anaemia. Astellas obtained the first marketing authorisation for a HIF-PH inhibitor for this disease in Japan in 2019. The authorisation was based on FibroGen’s and Astellas’ roxadustat product, which it hopes will turn out to be a blockbuster ($1 billion sales) by 2023.
2. Akebia and Otsuka together have a rival HIF-PH inhibitor which is in clinical trials for anaemia. It is called vadadustat. The proceedings in this jurisdiction began with a claim for revocation of the patents brought by Akebia and Otsuka together against FibroGen as patentee. Astellas as exclusive licensee then brought a claim for quia timet infringement against Akebia and Otsuka, contending that the launch of vadadustat would infringe. As patentee, FibroGen was joined as a defendant to that claim. Before this court FibroGen and Astellas make common cause and the patentee’s/licensee’s side of the case can simply be referred to as FibroGen. Also Akebia and Otsuka make common cause and so it is convenient to refer to the side contending for invalidity and non-infringement as Akebia.
3. The patents form two families. Family A, based on application WO 03/053997 (“WO 997”), consists of EP (UK) 1,463,823, EP (UK) 2,289,531 and EP (UK) 2,298,301. Family B, based on application WO 2004/108121 (“WO 121”), consists of EP 1,633,333, EP (UK) 2,322,153 and EP (UK) 2,322,155. The earliest claimed priority date for Family A is 6 December 2001. WO 997 was published on 3 July 2003. It is common ground that the validity of the Family B patents should be assessed as at its second claimed priority date of 29 April 2004. Thus WO 997 is full prior art with respect to the Family B patents.
4. There are two kinds of anaemia - CKD anaemia and ACD. CKD anaemia is a chronic condition associated with kidney disease, the abbreviation standing for Chronic Kidney Disease. ACD is different and the abbreviation stands for Anaemia of Chronic Disease. Broadly speaking the claims of the Family A patents all relate to a class of compounds defined in structural and functional terms for use in the treatment of CKD, while the claims of the Family B patents relate to the same compounds, but for the treatment of ACD. Nevertheless, although the claims of Family A relate to CKD, the content of the Family A application WO 997 includes some material relating to ACD as well, which gives rise to difficulties for the later Family B patents. Moreover, to be precise not all claims in the Family A include a limitation to chronic kidney disease, e.g. claim 17A of EP 531 does not do so, but nothing turns on that for present purposes.
5. In addition to the claims based on classes of compounds, there are two claims which relate to single compounds. In Family A it is claim 17A of EP 531, which relates to a compound called Compound C. In Family B it is claim 36A of EP 333 which relates to the same compound.
6. Akebia alleged the Family A patents were invalid for lack of inventive step and insufficiency. The inventive step challenge was that the use of Compound C was obvious over prior art called Epstein. If that case succeeded then, subject to a conditional application to amend the broad claims, all the claims in the unconditionally amended form before the court would be invalid. There was also an Agrevo obviousness case which corresponded to the insufficiency arguments but there was no wider obviousness case based on prior art advanced by Akebia against Family A. On the other hand the insufficiency objection was advanced across the board, contending that all the claims (save for the claim based on Compound C alone) were invalid on the grounds of excessive claim breadth on the basis of a lack of plausibility and of undue burden. There was also a specific point based on uncertainty taken against claims which included the requirement that a compound had to be a “structural mimetic of 2-oxoglutarate”.
7. On infringement of Family A there were two questions: whether vadadustat was a compound within the wider claims on a normal construction, and whether for the claim limited to Compound C (claim 17A of EP 531) vadadustat infringed that claim by equivalence. (The same equivalence was alleged relating to claim 36A of EP 333 in Family B.)
8. In relation to Family B the inventive step challenge was based on WO 997 and advanced against all claims. The insufficiency case was the same as the one against Family A, in other words it applied to all claims save the one which was limited to the single compound alone (claim 36A of EP 333).
9. FibroGen brought a quia timet infringement case related to Family B. The point was that the current clinical trials for vadadustat are aimed at CKD and the current draft marketing authorisation, if granted, would authorise the use of vadadustat for the treatment of CKD. It would not authorise use for treating ACD. However FibroGen contended that for various reasons it was possible to conclude that Akebia were today threatening and intending to market vadadustat in the future in a manner which would infringe the ACD based Family B patents under s60(2) of the Patents Act 1977.
10. At the trial there was extensive expert evidence relating to nephrology, medicinal chemistry and clinical practice. The judgment below had to grapple with all the issues identified above, putting them into context with the technical background and the common general knowledge. As one would expect from a judge of Arnold LJ’s experience, the judgment is well structured and closely reasoned. The conclusions on the matters in issue are summarised at the end (paragraph 640). The conclusions are that the Family A patents are not obvious over Epstein but all the relevant claims of Family A both lack plausibility and cannot be performed across their scope without undue burden, and therefore are invalid for insufficiency (and would be Agrevo obvious for the same reason). The uncertainty insufficiency attack also succeeds. On infringement vadadustat is found to be within the relevant chemical definition (Formula I) and so the wide Family A claims would be infringed by vadadustat on a normal construction if they were valid. The equivalence infringement case based on claim 17A of EP 531 is rejected. On Family B, the obviousness attack based on WO 997 succeeds. In addition the relevant claims of Family B are insufficient for the same reasons as Family A. Finally on quia timet infringement, there was no current threat to infringe the Family B patents even if they were valid.
11. On appeal FibroGen challenges the conclusions on insufficiency (and Agrevo obviousness). This aspect of the appeal applies to both patent families (save for the claims in EP 531 and EP 333 which relate only to a single compound). FibroGen also appeals the conclusion that Family B is obvious over WO 997, arguing that the conclusion is inconsistent with the findings made in the course of rejecting the quia timet infringement case. By a Respondent’s Notice Akebia challenges the conclusion that vadadustat is within Formula I. All these appeals are with the permission of the court below.
12. Thus before this court the conclusion that the Family A patents are not obvious over Epstein is unchallenged, as is the rejection of the equivalence case based on claims 17A or 36A and the finding of no threat to infringe the Family B patents. There is also no challenge to any of the conclusions on the skilled team, the extensive findings on common general knowledge at either priority date or the summary of the disclosure of the patents. It will be necessary to return to some of those issues below but for present purposes the best place to start is with the claims of Family A.
The claims of Family A
13. Although a variety of different claims were before the court, it is only necessary at this stage to set out two claims from Family A. I will start with what can be called claim 8A of EP 823, as dependent on claim 1 as unconditionally amended (judgment paragraph 155) and then move to claim 19A of EP 823 as dependent on claim 8A and unconditionally amended claim 2 (judgment paragraph 157). I will refer to these claims by shorthand as claim 8A and claim 19A even though a proper definition ought to retain the references to the claims on which they depend.
14. Claim 8A is set out below. I have added labels to the various parts for ease of reference:
A Use of a heterocyclic carboxamide compound selected from the group consisting of
B pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and beta-carboline carboxamides
C that inhibits hypoxia inducible factor (HIF) prolyl hydroxylase enzyme activity
D in the manufacture of a medicament for
E increasing endogenous erythropoietin
F in the prevention, pretreatment, or treatment of anemia associated with kidney disease,
G wherein the anemia is associated with chronic kidney disease.
15. Claim 19A, broken down in a similar way, is:
A A heterocyclic carboxamide compound selected from the group consisting of
B pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and betacarboline carboxamides
C that inhibits hypoxia inducible factor (HIF) prolyl hydroxylase enzyme activity
D for use in
E increasing endogenous erythropoietin
F in the prevention, pretreatment, or treatment of anemia associated with kidney disease,
G wherein the anemia is associated with chronic kidney disease,
H wherein the compound is a compound of Formula (I) wherein [chemical Markush formula]”
16. Claim 8A is a Swiss form use claim whereas claim 19A is an EPC 2000 product for use claim, but nothing turns on that difference. The labels A to H illustrate the many common features between the claims and the differences, such as they are.
17. Starting with claim 8A, the claim begins with structural features. The compounds must be heterocyclic carboxamides (feature A). The judge explained the relevant chemistry at [116] - [120]. Briefly, a heterocyclic compound is one with a ring system which includes at least one atom different from carbon in the ring structure, and a heterocyclic carboxamide is one in which the carboxamide group is attached to the heterocyclic ring. Then in feature B that definition is qualified in that the compound has to be selected from one of a number of narrower sub-classes: pyridine carboxamides, quinoline carboxamides, and so on. The detail of what these are does not matter. What is true is that these are essentially infinitely large classes because, as defined, they would include any compound which had the right kind of heterocyclic carboxamide in it somewhere and could have any other sort of chemical structure attached to it.
18. I will refer to compounds satisfying features A and B as “heterocyclic carboxamides of the claimed structure”. In the judgment the term “Carboxamides” is defined in the same way.
19. Then at C there is a functional limitation. The compound must be one which inhibits hypoxia inducible factor (HIF) prolyl hydroxylase enzyme activity. This bears some explanation.
20. The judgment fully explains the general physiology behind all this at [48] to [73] and explains the detailed biochemistry at [169] to [203]. Briefly, HIF-PH stands for hypoxia inducible factor - prolyl hydroxylase. HIF-PH is an enzyme, although there is in fact more than one. HIF-PH belongs to a family of enzymes called the 2-oxoglutarate dioxygenase family. The rationale on which the treatment of CKD anaemia by using roxadustat and vadadustat is based, can be summarised as follows. Anaemia is the result of a lack of red blood cells (erythrocytes). The natural process of making red blood cells is called erythropoiesis. There is a natural protein called erythropoietin (epo) which stimulates erythropoiesis. In the past “exogenous” epo has been manufactured using genetic engineering technology. Administering that exogenous epo as a medicine was and is a standard treatment for anaemia. By contrast with exogenous epo, the term “endogenous” epo refers to epo which the body makes naturally. Turning to the present case, there is a factor called hypoxia inducible factor (HIF) which plays a part in the gene regulation of the production of endogenous epo. More HIF causes more epo to be made, which in turn stimulates erythropoiesis and so on. The enzyme HIF-PH plays a role in this regulation because the enzyme initiates a process which breaks down HIF. So the action of the HIF-PH enzyme reduces the amount of HIF present, which leads to less epo, and so on. Thus if you could inhibit the action of HIF-PH, you could increase the amount of HIF, leading to an increase in epo, which promotes erythropoiesis, making more red blood cells and thus treating anaemia. The compounds of the invention inhibit the action of HIF-PH and so should have the desired effect. There is a lot more detail, and some of it is important for some issues, but for present purposes this simplistic explanation is sufficient to understand the issues, also bearing in mind that not all of this was known or known for sure before the priority date.
21. Returning to the claim, feature C requires that the compound must inhibit HIF-PH. There is an important issue of claim construction which arises here. The question can be posed by asking - what compounds are within the claim? Is the patent (and the claim) directed to each and every heterocyclic carboxamide of the claimed structure and then, by feature C, asserting that they will be inhibitors of HIF-PH? Or is the patent here only claiming those heterocyclic carboxamides of the claimed structure which are themselves inhibitors of HIF-PH? Looking ahead, if the right construction of the patent is the latter, then a compound which is a heterocyclic carboxamide of the claimed structure but is not an inhibitor of HIF-PH is not an example of a claimed compound, nor is its existence evidence that part of what is claimed does not work.
22. Turning to feature D, this is part of the conventional framework language for a Swiss style claim to make it a claim to the use of a compound for making a medicament for treating a disease. Feature D in claim 19A performs the corresponding task in that claim, which is an EPC 2000 “product for use” claim. In this case the differences between Swiss style claims and EPC 2000 claims do not matter.
23. Then at feature E there is a reference to another function - increasing endogenous erythropoietin. A similar question as for feature C arises on this claim feature.
24. Feature F is the place in the claim where the disease (or “indication”) to be treated is defined. The first few words are a form of the conventional framework language in Swiss (and in claim 19A, EPC 2000) claims. At feature F the disease is defined as anaemia associated with kidney disease and then at feature G this definition is further narrowed down to require the disease to be chronic. Together these features make the claim directed to compounds for preventing, pre-treating or treating CKD.
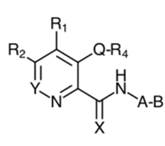
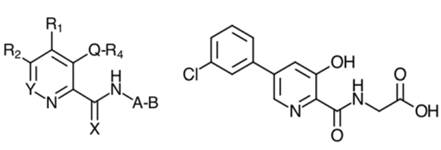
25. Turning to claim 19A, in terms of features A to G, the only differences between it and claim 8A arise from their different conventional formats. The important difference between claim 19A and claim 8A is the extra final feature H. This feature applies a further structural limitation to the claim by means of a chemical formula called Formula I. In patent jargon this kind of chemical formula is called a Markush formula, I believe after an inventor in the 1920s who first used such a formula in a patent. It is a way of writing a generalised chemical formula. Formula I was itself very long and the judgment sets it out in an appendix. The question whether vadadustat infringed turned on an issue of construction of Formula I.
26. The only other claim from Family A worth mentioning is claim 24A of EP 823. As dependent on claim 19A, 8A and claim 1 as unconditionally amended it is:
“A heterocyclic carboxamide compound selected from the group consisting of pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and betacarboline carboxamides that inhibits HIF prolyl hydroxylase enzyme activity for use in increasing endogenous erythropoietin in the prevention, pretreatment, or treatment of anemia associated with kidney disease, wherein the anemia is associated with chronic kidney disease, wherein the compound is a compound of Formula (I) …,
wherein the compound is a structural mimetic of 2-oxoglutarate”
[emphasis added]
27. It is not necessary to divide up the first part of the claim (which is the same as claim 19A above). What claim 24A adds to claim 19A is a further requirement that the compound is a “structural mimetic of 2-oxoglutarate”. This is the language which was held to lead to uncertainty insufficiency.
The Family A patents
28. With this understanding of the claims, it is only necessary to mention a few points arising from the disclosure of the patents. The judgment deals with this fully at paragraphs [107] to [153]. It was not criticised on appeal and I adopt it in full. The judgment uses the paragraph numbers in WO 997 for Family A however before us the parties’ arguments were advanced using paragraph numbers in EP 823. I will use the paragraph numbering in EP 823 for that reason.
29. There are three aspects of the disclosure which are worth highlighting on appeal.
30. First, in the section entitled “Invention” from paragraph [0058], there is a discussion of the mechanism by which the compounds might work. The patent explains that what is “disclosed herein” are methods of increasing endogenous erythropoietin and explains they can be used to treat “the EPO-associated condition anemia”. The compounds are to be used to increase the synthesis of endogenous epo by inhibiting the hydroxylation of the alpha subunit of HIF (HIFa). At [0067] the patent proposes that the enzyme responsible for hydroxylation of HIFa is a member of the 2-oxoglutarate dioxygenase family, and then sets out a number of members of that family, including prolyl-4-hydroxylase. It points out at paragraph [0068] that several small molecule inhibitors of prolyl-4-hydroxylase have been identified and includes a reference to a prior art paper called Majamaa (1985) which I will return to. Then paragraph [0069] refers to the idea of using structural mimetics of 2-oxoxglutarate. A structural “mimetic” is something which mimics the relevant structure. This whole section in the patent is the same as the section quoted in the judgment at paragraph [135].
31. Second, an aspect of the specification relevant to the issues is at [0072]–[0077]. The judgment deals with this at paragraph [139]. Here the patent cross-refers to eleven other patents in which, the patent in suit asserts, there are “exemplary compounds”. The paragraphs also assert that “all compounds listed” for each of those cross-referred to patents “may be used”, and in addition the paragraphs go to the trouble of listing out approximately 100 actual compounds from these sources. Amongst them are compounds which are labelled in the patent in suit as compounds C to K. The judgment recognised all this in paragraph [139]. Then at EP 823 paragraph [0078] (WO 997 para [0084], judgment para [140]) certain papers are cross-referred to in a similar manner to the previous cross-references to other patents.
32. Third, is the examples. Here the patent sets out test results for eleven compounds A to K and also describes some other tests, without giving results. As judgment paragraph [144] notes, while compounds C to K are within Formula (I), compounds A and B are not.
33. Example 1 is an in vitro cell culture test to see if compounds can increase expression of epo in liver cells. On the face of it there is an effect, and compounds C, D and H were the best at achieving it. Example 2 describes two in vivo tests in mice. The results show that compounds C, E, K, F and J increased epo levels and hematocrit (a measure of red blood cells) relative to controls.
34. Examples 3, 4, 7 and 8 then report tests of compound C in rats and mice. The results are positive.
35. Examples 5 and 6 do not provide results but discuss animal models. Example 9 also does not provide results, but discusses an in vitro screening assay for testing compounds for their ability to inhibit a HIF-PH enzyme.
36. With this introduction I can turn to the live issues on appeal.
37. The judgment on insufficiency starts at [347] and deals with the law relating to excessive breadth, noting that the Supreme Court were due to give judgment in Regeneron v Kymab. They have now done so at [2020] UKSC 27. At paragraph [353] a two stage enquiry is proposed, the first stage based on plausibility and the second based on undue burden.
38. After dealing with plausibility by reference to the Supreme Court in Warner Lambert v Generics [2018] UKSC 56, at paragraph [361] the judgment addresses the law on undue burden, citing the relevant cases (such as Regeneron v Genentech). At [365] the question arises of how the law handles claims which combine functional and structural features. There is no reference in the judgment to the decision of the German Supreme Court (Bundesgerichtshof or BGH) Dipeptidyl-Peptidase-Inhibitoren X ZB 8/12 (11th Sept 2013) on this point, although it was cited by the appellant below. The judgment turns to the EPO case law and summarises the conclusion reached as follows:
“[366] As counsel for the Defendants accepted, this does not mean that the skilled person or team must be able to identify all compounds covered by the claim without undue burden. Rather, what is required is that the skilled person or team must be able to identify substantially all compounds covered by the claim without undue burden.”
39. Having dealt with the law the judgment goes on to apply the two stage approach referred to, starting with plausibility at [368] and reaching a conclusion at [381] that all the claims in issue are insufficient for want of plausibility. The patent (implicitly) promises that substantially all compounds which satisfy the structural definitions in the claims will have the therapeutic efficacy. However that is not plausible. The skilled person would have no real reason to think that substantially all the compounds in Formula I would be effective in inhibiting HIF-PH, or increasing epo, or otherwise treating anaemia. Nor would they be able to make a reasonable prediction that substantially all those compounds would be effective. Thus the claims are insufficient. FibroGen’s submission that this approach is wrong in principle based on the true construction of the claims and on the law is rejected as being contrary to Idenix v Gilead [2016] EWCA Civ 1089. Also, taking into account the technical contribution actually made, based on the findings made in the course of rejecting the obviousness attack on claim 17A, that contribution does not support claims of the width they are. A large number of heterocyclic carboxamides of the claimed structure are not likely to work.
40. In case the conclusion on plausibility is wrong, the judgment then addresses whether the invention can be performed across the scope of the claim without undue burden, starting at [381]/[382]. The approach applies the test identified in [366] (set out above) that the skilled team must be able to identify substantially all compounds covered by the claim without undue burden.
41. The judgment first addresses what can be derived from the patent, and finds that the skilled person would not be taken very far forward beyond being presented with a small number of compounds which worked. FibroGen’s case that the papers and patents cited in the specification can be taken into account is rejected but in the alternative, at [387], even if they would be, they do not help. The evidence of the two experts, Dr Bhalay and Prof Ward is addressed in detail. The evidence was that the medicinal chemist would embark on a Structure Activity Relationship (SAR) analysis starting from the compounds in the patent. That such an analysis would be a great deal of work was not in dispute, but Dr Bhalay called by FibroGen expressed the view that it was routine in nature for a medicinal chemist. What could be derived from certain disclosure documents was then addressed, including figures showing what percentages of various compounds tested passed various tests. A point of detail arises on that.
42. The overall conclusion is that the claims are insufficient on the undue burden basis too, as follows:
“[399] Taking all of the evidence into account, the conclusion I reach is that the invention cannot be performed across the scope of the claims in issue without undue burden. It would require a substantial research project to identify any compounds other than those specifically identified in the specification which met the criteria for efficacy, and success would not be guaranteed. While it is probable that, if sufficient resources were thrown at the project, the skilled medicinal chemist would be able to identify some compounds falling within Formula (I) (and more which constituted Carboxamides) which were effective, they would not be able even in many lifetimes of sustained effort to make and test more than a tiny fraction of such compounds, and a substantial proportion either could not be made or would not work. This is not only setting the skilled team a research project and claiming the results, it is a never-ending one. Accordingly, on this ground also I conclude that the claims in issue are insufficient”
43. Akebia submitted that a finding of fact was made by the court which it calls the “not a single compound finding”. That is a reference to the second sentence in paragraph [399] above. A substantial research project would be required to identify any useful compounds (my emphasis). The “not a single compound finding” would be a conclusion that the skilled team were not able to find even a single compound which worked, aside from compounds C-K. If that is the finding of fact then, assuming it was open to the court to do so, it would be fatal to the patentee’s case on undue burden. Also I will say now that while FibroGen did seek to argue such a conclusion was plainly wrong in fact, I was not convinced. In my judgment such a finding was open to the court on the evidence before it.
44. However I do not believe it is fair to look at that sentence in isolation. The sentences before it and following it need to be taken into account too. The sentence before makes clear that the focus is performance across the whole scope of the claim, which the judgment earlier characterises as staggeringly large in scope. The sentence after states that it is probable that if enough resources were thrown at the project some compounds would be identified which worked. I also bear in mind the preceding analysis in this section of the judgment, and in particular the last sentence of the previous paragraph ([398]) which acknowledges unchallenged evidence of Dr Bhalay based on the timing of how Akebia found vadadustat, concluding that “all this shows” is that it is possible to identify another compound which works without difficulty.
45. I cannot read the judgment as a whole as reaching the conclusion contended for by Akebia. If the conclusion was simply that not any further useful compound at all could be found without an undue burden then there would be no need to go any further. The judge’s conclusion is more nuanced that that. To perform the invention across the scope of the claims (my emphasis) would require a substantial research project and success would not be guaranteed that any useful compounds would be found if that was the task. However given enough resources, it was probable (i.e. more likely than not) that a team would identify some other compounds which were useful over and above the specific compounds C-K identified in the patent. It would involve a great deal of work. However the fact that some useful compounds would be found can only ever scratch the surface of what is required by law given the scope of the claims and therefore does not save the patent from insufficiency.
46. Thus, contrary to Akebia’s submission, the judgment does contain a finding, plainly open on the evidence, that although it would involve a great deal of work, the skilled team would be able to identify some other useful compounds.
The arguments on appeal
47. FibroGen contends that the judgment’s conclusions are flawed for the following main reasons:
i) The judgment misconstrues the claim. The correct construction of the claims (taking claim 8A) is that functional features C and E limit the class of claimed compounds within whatever structural limitations are also in the claim.
ii) The judgment is based on an error of law in that plausibility has nothing to do with functional features like features C and E. The correct approach to functional features of this kind is that explained by the BGH in Dipeptidyl-Peptidase-Inhibitoren. Provided they do not introduce an undue burden, there is no breadth of claim problem caused by such limitations. As far as EPO decisions on this issue are concerned, the most that can be said by the respondent is that there are decisions going both ways on the issue.
iii) Putting claim construction and the law together, contrary to the finding at paragraph [376], the patent does not promise that substantially all compounds which satisfy the structural definitions in the claims will have the claimed therapeutic activity. The classes were not said to be new compounds. They had previously been described in earlier patents and scientific literature. They were known as inhibitors of other members of the enzyme family to which HIF-PH belongs. Contrary to [376] the technical contribution of the Family A patents resides not in the identification of a novel class of molecules per se, but in the teaching that certain classes are HIF-PH inhibitors and may be used in the treatment of anaemia associated with CKD, by increasing the endogenous production of epo (appeal skeleton para [15]).
iv) Plausibility is relevant to the claimed therapeutic efficacy in a Swiss or EPC 2000 claim (features F and G) but the answer on the facts is simple because (as was never in dispute) given what is shown in the patent it is indeed entirely plausible that a compound with the relevant structural features which satisfied the functional features C and E, i.e. was an inhibitor of HIF-PH and showed epo induction in cell and in vivo assays, would have the relevant therapeutic effect.
v) Also in the plausibility section, the conclusion about the technical contribution by reference to aspects of the findings on obviousness (paragraph [379]) was wrong because it falls into the error identified in Conor v Angiotech [2008] UKHL 49. Validity is to be judged by reference to the claims themselves. In writing Fibrogen had cited Generics v Lundbeck [2009] UKHL 12 on this topic but the point was put orally by reference to Conor v Angiotech. The distinction does not matter.
vi) The finding on undue burden was also wrong in principle, this time because the judge was distracted by the number of compounds within Formula I. The approach taken was to posit a skilled team undertaking a single programme whose objective was to try to identify substantially all the compounds within the claim (paragraphs [366], [363] on the law and see e.g. the reference to many lifetimes in conclusion paragraph [399]). That is not the right question.
vii) The distraction relating to the number of compounds within Formula I, which makes the task effectively endless so that the quantity of work required is inevitably an undue burden, meant that the judgment never seeks to resolve the issue of whether the quality of the significant work which a SAR analysis does involve is nevertheless routine in nature. On the evidence it is the routine bread and butter work of medicinal chemists.
viii) On three points of detail, first the distraction about numbers means that the judgment did not give proper credit for the fact that the skilled person will make sensible choices as part of their work, which answers the issue about ADME (absorption, distribution, metabolism and excretion) and similar problems. Second the judgment gives no credit for the fact that as a matter of construction the patent expressly describes about 100 other “exemplary” compounds aside from the compounds C-K which are said to be exemplary compounds which may be used. Given the disclosure, if Akebia had wanted to suggest to the contrary that they did not work for some reason, Akebia could and should have done so but did not. Third, the dismissal of the cross-references to the other patents was wrong in principle.
ix) Thus the two conclusions, of lack of plausibility and undue burden are wrong and should be reversed on appeal.
48. In response Akebia supported the judgment in relation to claim construction, technical contribution, and on the law on plausibility and undue burden. Akebia submits that having got those things right, there is no error with which this court could interfere in the findings on the facts and evidence about plausibility and undue burden.
The law - insufficiency
49. To grapple with this, I start with the legislation. The 1977 Act provides that to be valid the specification must disclose the invention “clearly enough and completely enough for it to be performed by a person skilled in the art”. This corresponds to Art 83 EPC although the Act uses the word “performed” instead of the Convention’s phrase “carried out”, but there is no difference. Everything else is judge-made law, working out how this principle applies in different sets of circumstances. As the judgment does in paragraph [347] it is useful to see that this single ground can be classified into three types of objection - classical insufficiency, Biogen insufficiency aka excessive claim breadth, and uncertainty. Nevertheless one does need to take care not to read too much into brief summaries of what those categories amount to and not to treat them like statutes.
50. Just as the kinds of insufficiency can be put into categories, so too can the kinds of case to which they apply. The issue in this case is about alleged excessive claim breadth as it applies to inventions which are concerned with compounds and classes of compounds whose utility is in some kind of medical therapy.
51. The most up to date general statement of the relevant law of insufficiency, particularly as it relates to claim breadth in this context, is that made by Kitchin LJ in Regeneron v Genentech in the Court of Appeal at paragraphs [95] to [103]. The whole passage repays careful reading. It is not necessary to set it all out. The fourth principle of the six which Kitchin LJ identifies relates to inventions defined in general terms and the requirement of a reasonable prediction:
“98 Fourth, it is permissible to define an invention using general terms provided the patent discloses a principle of general application in the sense that it can reasonably be expected the invention will work with anything falling within the scope of these terms. As Lord Hoffmann said in Biogen Inc. v Medeva plc [1977] R.P.C. 1 at pp.48–49:
‘If the invention discloses a principle capable of general application, the claims may be in correspondingly general terms. The patentee need not show that he has proved its application in every individual instance. On the other hand, if the claims include a number of discrete methods or products, the patentee must enable the invention to be performed in respect of each of them.
Thus if the patent has hit upon a new product which has a beneficial effect but cannot demonstrate that there is a common principle by which that effect will be shared by other products of the same class, he will be entitled to a patent for that product but not for the class, even though some may subsequently turn out to have the same beneficial effect: see May & Baker Ltd v Boots Pure Drug Co. Ltd. (1950) 67 R.P.C. 23, 50. On the other hand, if he has disclosed a beneficial property which is common to the class, he will be entitled to a patent for all products of that class (assuming them to be new) even though he has not himself made more than one or two of them.’
99 In Kirin-Amgen Inc v Hoechst Marion Roussel Ltd [2004] UKHL 46, [2005] RPC 9 Lord Hoffmann further explained the concept of a principle of general application in this way:
“112. In my opinion there is nothing difficult or mysterious about [a principle of general application]. It simply means an element of the claim which is stated in general terms. Such a claim is sufficiently enabled if one can reasonably expect the invention to work with anything which falls within the general term. For example, in Genentech I/Polypeptide expression (T 292/85) [1989] O.J. EPO 275, the patentee claimed in general terms a plasmid suitable for transforming a bacterial host which included an expression control sequence to enable the expression of exogenous DNA as a recoverable polypeptide. The patentee had obviously not tried the invention on every plasmid, every bacterial host or every sequence of exogenous DNA. But the Technical Board of Appeal found that the invention was fully enabled because it could reasonably be expected to work with any of them.
113. This is an example of an invention of striking breadth and originality. But the notion of a ‘principle of general application’ applies to any element of the claim, however humble, which is stated in general terms. A reference to a requirement of ‘connecting means’ is enabled if the invention can reasonably be expected to work with any means of connection. The patentee does not have to have experimented with all of them.”
100. It must therefore be possible to make a reasonable prediction the invention will work with substantially everything falling within the scope of the claim or, put another way, the assertion that the invention will work across the scope of the claim must be plausible or credible. The products and methods within the claim are then tied together by a unifying characteristic or a common principle. If it is possible to make such a prediction then it cannot be said the claim is insufficient simply because the patentee has not demonstrated the invention works in every case.
101. On the other hand, if it is not possible to make such a prediction or if it is shown the prediction is wrong and the invention does not work with substantially all the products or methods falling within the scope of the claim then the scope of the monopoly will exceed the technical contribution the patentee has made to the art and the claim will be insufficient. It may also be invalid for obviousness, there being no invention in simply providing a class of products or methods which have no technically useful properties or purpose.
52. It may be a matter of taste only but I prefer to refer to this fourth principle as reasonable prediction rather than simply plausibility, however whatever it is called, it is the same principle.
53. To apply the reasonable prediction principle one has to take three steps. First one must identify what it is which falls within the scope of the claimed class. Second one must determine what it means to say that the invention works. In other words what is it for? Once you know those two things, the third step can be taken: to answer the question whether it is possible to make a reasonable prediction the invention will work with substantially everything falling within the scope of the claim.
54. In a paradigm case of a Swiss style claim to the use of a class of compounds defined in a Markush formula to treat a disease, the first two steps are simple and the question will be whether it is possible to make a reasonable prediction that substantially all the molecules within the Markush class will work to treat the disease. In terms of functional and structural limitations in claims, in this simple case the structural limitation defines the class and is considered at the first step and the functional limitation defines the therapeutic effect and is addressed at the second step. The significance of the existence of inactive compounds within the Markush formula will be a matter of fact and degree but the fact they exist does not matter if it does not falsify the reasonableness of the prediction. Also and similarly the fact that active compounds within the formula turn out to be unsuitable as clinically approved agents for reasons unrelated to efficacy itself, such as side effects profiles, bioavailability and the like, is also unlikely to falsify the reasonableness of the prediction, depending again on this being a matter of degree. These issues will also play a role in analysis of any undue burden.
55. However in other cases the first step also involves a separate functional limitation too, in addition to the use to treat a disease. Claims with such double functional features are not so unusual. Twenty years ago the crucial claim in Lilly ICOS v Pfizer [2000] EWHC Pat 49) was to the use of a cGMP PDE enzyme inhibitor for the treatment of male erectile dysfunction. There was no structural limitation in that claim at all. The claim in Regeneron v Genentech is another example. Although there was a debate before us about how to characterise that claim, essentially it was a claim to the use of a product defined at least partially in functional terms for use in treating certain non-cancerous diseases characterised by excessive blood vessel growth. The functional definition of the products claimed was that they had to be antagonists to human vascular endothelial growth factor (VEGF). Amongst other things the court below in that case had held that it was possible to make a reasonable prediction that VEGF antagonism could be used to treat all the relevant diseases, and on appeal the Court of Appeal rejected the insufficiency attack holding at [134] that “The judge had ample evidence before him upon which to conclude that it was plausible that VEGF antagonism could be used to treat any non-neoplastic neovascular disease.
56. Thus Regeneron is an example of the three step test I have referred to applied to a claim with double functional features. To distinguish between these two kinds of functional feature I will refer to “step one functional features” (such as VEGF antagonism) and “step two functional features” (such as treating the relevant diseases). It will be a matter of construction to work out what sort of functional features one is dealing with.
57. In some cases the second step is the aspect which is a bit more involved. So in Idenix v Gilead, claim 1 was to a Markush class of molecules (see Kitchin LJ para [61]). The claim language did not include any reference to what they were for and so one could not answer the question at the second step by looking at the words of the claim. This is also not unusual. If the compounds are new, then a claim to those compounds will be novel without including a claim feature which refers to what they are actually for. However that does not prevent the reasonable prediction principle being applied. In fact the answer in Idenix was clear from the patent specification. That showed that the point of the invention was to treat infections caused by viruses in the Flaviviridae family. So one can assess the validity of the claim on the basis that it is a claim to compounds with anti-Flaviviridae activity, which is what Kitchin LJ said at paragraphs [113] and [124]. So, in the language coined above, anti-Flaviviridae activity was a step two functional feature. The issue in Idenix arose in the context of inventive step but the same approach applies to reasonable prediction/plausibility. Note that this does not mean that claims to compounds per se are actually limited to using the compounds for treating Flaviviridae infections, but for the purposes of assessing questions like inventive step and reasonable prediction/plausibility, one needs to know what the compounds are supposed to be useful for. In fact in Idenix the outcome of the third step was against the patentee. The court held that it was not plausible that substantially all the claimed molecules would be effective against Flaviviridae infections, and hence it was Agrevo obvious and also insufficient for lack of plausibility for the same reason (see paragraphs [129] and [140]).
58. Before leaving this it is worth expanding briefly on Agrevo. If one was performing a Pozzoli analysis of inventive step in such a case, the inventive concept would be the compounds for treating Flaviviridae infections. In the EPO, one would ask what the problem to be solved is, and the answer would be the same - to treat Flaviviridae infections. Just as in Agrevo itself, so in Idenix, the claim was to a Markush class of compounds with no limitation to the use they were for, but that did not prevent the tribunal from determining what they were for by reading the patent specification. In Agrevo itself the use was as herbicides. So the EPO’s problem/solution approach would ask the question whether the claimed molecules were or were not obvious to use as herbicides. They may well not have been. However Agrevo is authority for the proposition that there is a prior question. Before one can investigate inventive step that way, the tribunal must be satisfied that the alleged problem to be solved is indeed solved by the claimed subject matter. The Agrevo question is whether it is credible or plausible that the claimed compounds have the alleged beneficial property. If they do then that useful property can be employed to formulate the problem to be solved. If they do not then the claim lacks inventive step because drawing up a list of compounds with no plausible utility is not an act of invention. As Regeneron v Genentech makes clear in the passage cited above, the Agrevo question is the same as the question whether it is possible to make a reasonable prediction that the invention will work with substantially everything falling within the scope of the claim.
59. I turn to the third step in reasonable prediction. The solidity of the basis for a given prediction, or putting it another way, the degree of plausibility required, was something addressed by Lord Sumption in the Supreme Court in Warner Lambert v Generics. As far as I know nothing turns on that aspect of this issue in the present case.
60. That takes one to the fifth principle articulated by Kitchin LJ in Regeneron v Genentech which involves undue burden:
102. Fifth, patentees not infrequently seek to avoid the possibility that a claim covers products or methods which do not work by inserting a functional limitation. Such a claim may be allowed by the EPO if the invention can only be defined in such terms or cannot otherwise be defined more precisely without unduly restricting its scope. But, it must still be possible to perform the invention across the scope of the claim without undue effort. As I said in Novartis v Johnson & Johnson at [244]:
“. . . In the case of a claim limited by function, it must still be possible to perform the invention across the scope of the scope of the claim without undue effort. That will involve a question of degree and depend upon all the circumstances including the nature of the invention and the art in which it is made. Such circumstances may include a consideration of whether the claims embrace products other than those specifically described for achieving the claimed purpose and, if they do, what those other products may be and how easily they may be found or made; whether it is possible to make a reasonable prediction as to whether any particular product satisfies the requirements of the claims; and the nature and extent of any testing which must be carried out to confirm any such prediction.”
61. The idea of an undue effort or burden comes up in various aspects of insufficiency. For example when examining whether a skilled person can make the invention work at all, that may involve the skilled person doing some trials and experimentation. One way of characterising the difference between testing which does not give rise to insufficiency, and testing which does, is the idea of reasonable trial and error (see Mentor v Hollister [1991] FSR 577 and [1992] RPC 1). Another is to say that the patent sets a research project for the skilled person, which is insufficient, e.g. Idenix v Gilead (CA) at [197]. Another way of putting the same idea is undue burden - see Idenix again, this time in the section dealing with an insufficiency challenge to the Markush claim on the basis that an entire sub-class of compounds within that formula (called the 2'-methyl-up-2'-fluoro-down molecules) could not be made at all without an undue burden (see the conclusion at [192]).
62. So undue burden is not always concerned with functional limitations, but it can be. In Regeneron v Genentech itself the undue burden issue was the suggestion that too much work was required to satisfy the step two functional feature of therapeutic efficacy. The submission was that to get the invention to work by turning a given VEGF antagonist into an approved treatment for a particular disease within the claimed class such as rheumatoid arthritis was too difficult. This was rejected because it set too high a standard. The law does not require absolute proof that the compound is clinically approved before it can be claimed as such (see [156] - [158]).
63. However there is a different sense in which functional limitations can create an undue burden. Some step one functional features themselves can give rise to an undue burden on their own. Relevant issues are whether the test itself is one which is already established in the art, whether is too difficult to carry out or how easy it is to interpret the results. Other issues can be whether the skilled person already knows of compounds likely to satisfy the test or whether instead they have been left to fish in an infinitely large ocean with each catch giving them no pointers to the next one.
64. As the judgment below observes the present case involves a claim with a mixture of structural and functional features (paragraph [365]). The holding is that the way undue burden is to be applied in such a case was that stated in paragraph [366], i.e. that the skilled person must be able to “identify substantially all compounds covered by the claim without undue burden”. This principle was derived from EPO cases and I will come to them below. The correctness of this principle is the major legal issue in this case.
65. None of the earlier authorities on insufficiency which were cited below go this far. AHP v Novartis [2001] RPC 8 at [41]-[47] and Halliburton v Smith [2006] EWCA Civ 1715 at [18] are relevant for a different point, that even routine work can amount to insufficiency if too much of it is required (a “gigantic project” in Halliburton). However it is also worth bearing in mind, as Idenix at paragraph [197] makes clear, that a claim is not insufficient just because it covers a very large number of molecules.
66. The paragraph [366] principle, applied to Regeneron v Genentech, would mean that for sufficiency it would have been necessary for the skilled person to identify substantially all VEGF antagonists within the claim, without undue burden. That would clearly have been an impossible task. However the point was not argued in that way in that case. Which takes me to a case in which the issue did arise, the German Supreme Court decision in Dipeptidyl-Peptidase-Inhibitoren. The case was concerned with a claim in the following form (with numbered features added for clarity):
1. The use of inhibitors of the enzyme activity of dipeptidyl peptidase IV (DP IV)
2. for lowering the blood glucose level below the glucose concentration characteristic of hyperglycaemia in the serum of mammals with diabetes mellitus
67. This is another double functional feature claim without any structural limitation at all. The first step functional feature is to be an inhibitor of the relevant enzyme (DP IV). Manifestly it is not plausible that substantially every chemical compound of whatever sort satisfies that functional definition. The point of the invention in that case was the discovery that a compound which was an inhibitor of the relevant enzyme would be useful to reduce blood glucose levels below the level of diabetic hyperglycaemia. So the second step functional feature was to lower blood glucose level etc.
68. The German Federal Patent Court had found the patent invalid on the ground of insufficiency. Paragraph 10 of the English translation of the BGH’s judgment (para B II in the original) summarises the lower court’s reasons. They included the following points: The claim was directed to the use of inhibitors which were only characterised by functional features regardless of any structure (the translation uses the term “substance feature”). The specification only identified four compounds and the skilled person “is given no clues as to which further compounds they might consider for solving the problem.” “The generalising wording, which goes beyond the solution disclosed to the skilled person in the documents in their entirety, generalises the claimed subject matter to such an extent that the patent protection sought goes beyond the contribution the invention makes to the prior art.”
69. On the question of generalisation, the BGH held that:
“15 b) Generally, the applicant is free to choose not to limit the claimed protection to embodiments expressly described in the documents as filed, but to make certain generalisations. If a patent claim contains generalising wording, the effect can be that it also comprises embodiments which are not specifically addressed in the description. Yet it does not necessarily follow from this that the invention as a whole or parts of it are no longer disclosed in manner that allows the skilled person to carry it out. What is decisive instead are the circumstances of the individual case.
16. If protection is claimed for a product, the applicant is generally required to describe the object by means of physical features. If protection is sought for a chemical substance, this can be identified e.g. by its scientific name or its structural formula. Yet it can also be identified in a different way, if it is not possible or not practicable to capture the disclosed teaching in any other way.”
[In the original these paragraphs are the first two paragraphs at III. 1. b)]
70. I agree with the BGH.
71. Then after addressing certain claim categories, the BGH continued:
“18 A generalising wording in a patent claim violates the requirement of a clear and complete disclosure if it generalises what is protected by the patent beyond the solution according to the invention which is disclosed to the skilled person in the description ([citations, including the EPO’s T 435/91 Unilever Detergents) It is also inadmissible to characterise an object or a method to which the invention relates with parameters that only describe the object underlying the invention ([citations])).
19 Subject to this, it may be admissible to recite a group of substances in a generalised form, even if not all substances that belong to this group are suitable for the purpose of the invention, provided the skilled person is easily able to determine the suitability of the individual substances by experiments ([citations]). That a claim worded in this manner also covers substances that do not yet exist or that have not been discovered yet, is no cause for concern. As long as using them makes use of the invention, it is not problematic that substances are also covered that cannot be found without an inventive effort.”
[The next two paragraphs of III. 1. b) in the original]
72. I expressed my agreement with this reasoning in Illumina v Latvia MGI [2021] EWHC 57 (Pat) - at paragraph [269]-[275]. I will take the opportunity here to agree with it again.
73. It is worth dwelling briefly on what is said at the end of paragraph 18 of Dipeptidyl-Peptidase-Inhibitoren. There the BGH is reiterating the important point that it is not acceptable simply to claim “everything which works” without telling the skilled person how to achieve success. However the BGH clearly did not think that that was what was happening in the case before them, and it is easy to see why not. A claim simply to the use of any compound for achieving the therapeutic efficacy feature would fall foul of that principle. That would be a claim to anything which satisfied the step two functional feature, in other words a claim to everything which works. However a claim to the use of any compound which has the step one functional feature (of being a DP IV inhibitor) for that step two therapeutic purpose does not simply claim everything which works. The skilled person must be able to identify such compounds with the step one functional feature without undue burden, but that is a different issue. Moreover the fact the claim would cover compounds which may not have been invented yet is not a problem either.
74. In the next paragraph of Dipeptidyl-Peptidase-Inhibitoren (paragraph 20 of the translation, III. 1. c) of the original) the BGH explains that its statement of the law is in line with major well known decisions of the EPO as well as the UK’s Biogen v Medeva and Kitchin LJ’s judgment in the Court of Appeal in Regeneron v Genentech.
75. Turning to the case before it, the BGH held that the claim was not insufficient. The whole reasoning is instructive and I set it out:
23. The patent claim according to the main request contains a functional feature. What is claimed is not only the use of a specific substance or a plurality of substances identified in concrete terms for lowering the blood glucose level in the case of diabetes mellitus, but the use of all substances that act as inhibitors of dipeptidyl peptidase IV (DP IV). Contrary to the opinion of the Patent Court and [a parallel EPO case], this wording of the claim cannot already be criticised due to an insufficient disclosure.
24. It may be true that this claim wording does not only cover the dipeptidyl derivatives identified in the description, but all inhibitors of dipeptidyl peptidase. But, as we have explained, this alone is not sufficient to establish an insufficient disclosure. The facts on which the decision on the appeal on points of law must be based, do not justify the assumption that the wording of the patent claim according to the main request goes beyond what the skilled person derives from the patent document as the most general form of the technical teaching described.
25. a) According to the patent document, the invention concerns a simple method of lowering the blood glucose level. The technical problem is described as providing a simple, cost-efficient method for lowering the blood glucose level. This problem is supposed to be solved by administering inhibitors of dipeptidyl peptidase. According to the description, this is based on the following: [the mechanistic rationale for achieving the result is set out there and in the following paragraph].”
76. Thus the biochemical rationale showed why it was credible that compounds with the step one functional feature (DP IV inhibition) would be likely useful for achieving the step two efficacy feature (lower blood glucose). Provided there is no undue burden in finding compounds which satisfied the functional assay feature, then such a claim does not exceed the technical contribution. The fact that the specification “only” discloses four actual compounds does not falsify that result given the existence of a credible rationale and the ability, without undue burden, to find other compounds.
77. In conclusion, the BGH’s decision is a clear and persuasive authority that the legal principle in [366] is wrong. The functional language at step one means that the claim can cover compounds which may not have been invented yet. Given that, it cannot be the law that the functional language requires the skilled person to be able to identify substantially all compounds covered by the claim.
78. The judgment at [365] goes to the EPO cases to grapple with the application of the law of insufficiency to claims with broad structural and functional features, and so I now turn to the EPO decisions. The well-established principle is that functional language can be used provided the whole subject matter of the claim must be capable of being carried out without undue burden. Authority for that goes back to Unilever/Detergents T435/91.
79. At paragraph [365] the judgment cites an extract from the second paragraph of 6.6.9 of the 9th (2019) Edition of the EPO’s text book on the Case Law of the Boards of Appeal. The extract cited in the judgment is:
“In T 544/12 the board confirmed that a definition of a group of compounds in a claim by both structural and functional features is generally acceptable under Article 83 EPC as long as the skilled person is able to identify, without undue burden, those compounds out of the host of compounds defined by the structural feature(s) in the claim which also fulfil the claimed functional requirements (following T 435/91 and T 1063/06).
[emphasis in para [365] of the judgment]
80. This passage could be read in two ways. I would agree with a narrow reading of the cited extract, as understood simply to mean that the undue burden refers to the ability of the skilled person to identify compounds within the structural class as having the functional feature. In other words if one has a claim with a first step functional feature, it must be possible without undue burden, to find compounds which satisfy the test and to find out whether a given compound satisfies the test.
81. However I recognise that this extract can be read as the judgment below takes it, with a broader meaning, as stated in the conclusion at paragraph [366] of the judgment (quoted above). Absent authority, in my judgment the conclusion would be odd. No functional language could ever satisfy such a test because by definition a functional feature is capable of covering something which has not been invented yet. As long as the whole subject matter anywhere within the claim can be carried out without undue burden, I do not see there is any reason in principle why the skilled person should have to embark on trying to positively identify substantially every compound within the claim.
82. If, as a matter of fact, identifying all conceivable compounds which passed the test was a necessary step to identifying any useful compounds at all, then that is another matter, but the principle as stated in [366] is not confined in that way. There is no reason why, absent those facts, a skilled person would want to identify all or substantially all compounds within a claim.
83. There is more support for a broad approach to the principle in the remainder of the same paragraph 6.6.9 of the EPO Case Law text book from which the judgment cited the extract above. The remainder of the paragraph is:
“In T 544/12 it was up to the skilled person to identify within the almost infinite host of alternatives covered by the structural definition of claim 1 those compounds that were phosphorescent. Claim 1 extended to classes (of iridium complexes) that were entirely different from the concept as argued by the proprietor (non-compliance with Art. 83 EPC). The board did not share the view taken by the German Federal Court of Justice (Bundesgerichtshof) in its decision of 11 September 2013 (X ZB 8/12).”
[X ZB 8/12 is Dipeptidyl-Peptidase-Inhibitoren]
84. So in fact the authors of the EPO’s Case Law textbook recognised that what was being proposed was contrary to Dipeptidyl-Peptidase-Inhibitoren.
85. Turning to the text book, the main authority cited is therefore T 544/12 Princeton University/OLEDs (22 November 2013), which itself cites T435/91 Unilever/Detergents and T 1063/06 Bayer/Reach through claim. The judgment at [365] also cited further EPO decisions: T 555/12 Cytec Technology/Flexible Polymer Element (30 July 2015) at [5.1] and T 323/13 Princeton University/L2MX Complexes (5 March 2015) at [7.1.1].
86. I considered Princeton University/OLEDs in my judgment in Illumina. I recognised and agreed with paragraphs 4.2 and 4.5 of that EPO decision as statements of the basic legal principles. However I went on at [274] - [275] to look in more detail at Princeton University/OLEDs and the reasoning which led to the disagreement with Dipeptidyl-Peptidase-Inhibitoren. I explained there that Princeton University/OLEDs cannot be taken too far, and I maintain that view. The point can be seen in paragraph [275] of Illumina as follows:
“[275] […] The Board [in Princeton/OLEDs] appears to take the view that a functional definition will be necessarily insufficient simply because, as the BGH noted in the [Dipeptidyl-Peptidase-Inhibitoren] case, such language covers things which have not been invented yet. Stated in such a general way I respectfully disagree with the Board and I note that Lord Briggs [in Kymab] did not take that view either. This absolutist approach would strike down all functional language and represent a radical change for no discernible benefit to the public. A functional definition cannot help cover things which are not yet invented. That may not necessarily matter at all. What matters is that the skilled person must be able to put the invention into practice without undue burden. They need to be able to come up with components which will work and, if that involves testing things, that testing must not introduce an undue burden.”
87. On the other hand if Princeton/OLEDs is understood only as another way of saying that the whole subject matter of the claim must be capable of being carried out without undue burden, then it is unobjectionable. However if that is all it amounts to then it does not support paragraph [366] of the judgment below.
88. T 1063/06 Bayer/Reach through claim is concerned with particular facts. Briefly the invention was said to be a new kind of research tool which could identify compounds which operated by a new mechanism of action. The claim in that case had double functional features and no structural limitation. The claim was to the use of compounds which were capable of stimulating the enzyme guanylate cyclase in a certain way (independently of the heme group in the enzyme) for use in the treatment of cardiovascular disorders such as angina. The first step functional feature was the new mechanism of action. Since the functional feature itself was new, at the date of the application there were no compounds known to have the relevant property. The board held that this was an attempt to claim the fruits of research and was insufficient.
89. Within the reasoning, paragraph 5.2 does include a reference to the skilled person having to test every conceivable compound. However that is not put on the basis that there is a general legal principle applicable to claims of this type that the skilled person must be able to identify all suitable compounds. The point arose in the detail. The difficulty was that in the particular circumstances, if the skilled person did find one suitable compound which passed the test, that would take them no further forward in being able to find any others. So the only way to find more useful compounds would be to test every conceivable compound. Pulling one fish out of the infinite ocean would not help find another fish. This factual issue will come up again below on the judgment because Akebia’s case is that these are the facts irrespective of whether there is a general legal principle.
90. In T 555/12 Cytec Technology/Flexible Polymer Element, as is common in the EPO, the decision simply repeats the words of the para 6.6.9 extract. On its facts the case is not concerned with the difference between narrow and broad interpretations of the passage.
91. T 323/13 Princeton University/L2MX Complexes at 7.1.1 also repeats the words of the para 6.6.9 extract, however the way the principle is applied on its facts is consistent with the narrow interpretation. The Board rejects the insufficiency allegation because the skilled person would have been able to select without undue burden appropriate structures to make the invention work (7.1 - 7.8).
92. The most recent EPO decision cited to us on the topic was T 2015/20 Inhalation Composition/Almirall (23rd February 2021). The statement of the law on sufficiency is fuller than some of the previous cases. It is at 2.5, as follows:
“2.5 The Boards of Appeal have indeed recognized that in the context of the requirement of sufficiency functional features require particular attention, as such features are defined by means of an effect that has to be achievable (see G 1/03, reasons 2.5.2 ).
Occasional failure to achieve a defined effect does not necessarily imply insufficiency and reasonable experimentation by trial and error may be permissible, if the skilled person has adequate information, from the specification or on the basis of the common general knowledge, to achieve success in spite of initial failure (see Case Law of the Boards of Appeal, supra, section II.C.6.6.1, see also T 14/83, OJ 1984, 105, Headnote, and T 2220/14, reasons 63).
Similarly, the definition of a group of compounds by both structural and functional features may be acceptable under Article 83 EPC, if the skilled person is able to identify without undue burden the compounds which fulfil the claimed functional requirements within the structurally defined group of compounds (see Case Law of the Boards of Appeal, supra, section II.C.6.6.9 with reference to T 544/12).
However, claims may not represent an invitation to perform a research programme without effective guidance towards success (see T 435/91, OJ 1995, 188, Headnote and Reasons 2.2.1).
A crucial consideration in the assessment of the requirement of sufficiency in relation to functional definitions is the need for fair protection commensurate with the disclosed actual technical contribution (see T 1063/06, OJ 2009, 516, Headnote, T 694/92, OJ 1997, 408, Headnote and T 409/91, OJ 1994, 653, Headnote).”
93. I agree with all this. The way this board refers to paragraph 6.6.9 in the Case Law text book and to T 544/12 Princeton/OLED is equally consistent with the narrow interpretation of the extract.
94. Two further EPO decisions were cited to us in this connection: T1300/05 RET screening assay/Progenics (11th July 2006) and T21/05 CTL responses to HCV/Scripps (13th July 2016). Scripps does not support the narrow interpretation. In Progenics the Examining Division had held that an antibody claim with functional limitations was insufficient because it would be an undue burden to make all the antibodies falling within the claim. The Board of Appeal overturned that decision, holding that the claim was not insufficient on the facts. The testing would involve a lot of work and testing a large number of clones, but the testing method was known and that there was no doubt as to the outcome of the experiment (para 9). The Board concluded as follows:
“17. The examining division denied sufficiency of disclosure for the reason that it would be undue burden to obtain all antibodies falling within the scope of the claims. As explained above, it is the board's opinion that all necessary information for doing so is contained within the application. Thus, assuming for the sake of discussion that the skilled person would ever want to isolate all of the antibodies falling within the scope of the claims, the possibly undue amount of work involved would not stem from deficiencies in the way the invention was described but rather from the task which he/she chose to accomplish.”
95. Having run through the EPO decisions, I conclude as follows. There is clear support for a test based on the narrow reading of the extract from paragraph 6.6.9. The principle based on the narrow reading would not be contentious. Also, if the facts are like those in the Bayer/Reach through case then a question along the lines of paragraph [366] may arise. However the only decision which supports the principle of law as it is stated in the judgment at [366] is T 544/12 Princeton/OLED itself. That is not a sufficient basis to reach such a radical conclusion. In my judgment paragraph [366] is wrong. The right test is as follows. If one has a claim with a functional feature which defines the claimed compounds, or a mix of such structural and functional features, it must be possible, without undue burden, both to identify compounds which satisfy the relevant test, and to find out whether any given compound satisfies the test. However it is not necessary as a matter of law, for sufficiency (or for Agrevo), simply because a claim contains functional features (or a mix of functional and structural features) to establish that the skilled person can identify all or substantially all the compounds which satisfy the test.
96. Finally, if the law does not require the identification of substantially all such compounds, the question remains, how many is enough? Take the facts of the present case. The claims like claim 8A with structural and functional language at step one clearly claim a wider class than the particular compounds C, E, F, J and K identified in the patent as likely to have therapeutic efficacy. Even if one adds on the 100 or so compounds identified in the patent at paragraphs [0072]–[0077], the claim is plainly intended to be much wider than that too. In terms of a promise, the wider claim is a promise or assertion that there are more useful compounds within the class than the ones identified by name in the patent. Bearing in mind the ultimate issue is all about breadth of claim, in such a case the question is how many is enough?
97. I believe the answer is in two parts. For claims of this type, it must be possible for the skilled person, without undue burden, to identify some compounds beyond those named in the patent, which are within the claimed class and therefore are likely to have therapeutic efficacy. Otherwise the contribution is no more than the named compounds and the wider claim is too wide and unsupported by the disclosure. Second and separately, it must also be possible for the skilled person to work substantially anywhere within the whole claim (Kymab is one example, in which inventive step was needed to be able to work in a part of the claim which was not otherwise available to the skilled team from the specification, and another is the non-functional 2'-methyl-up-2'-fluoro-down sub-class of the Markush formula in Idenix). So it must be possible for the skilled person, given any sensible compound within the structural class (or substantially any), to apply the tests without undue burden and work out if it is a claimed compound.
The judgment on reasonable prediction/plausibility
98. The question is whether it is possible to make a reasonable prediction the invention will work with substantially everything falling within the scope of the claim, and as I have described, there are three steps. The judgment does not approach the question this way, and instead asks a rolled up question whether it is plausible that compounds within the structural class will have the claimed therapeutic efficacy e.g. paragraph [376], [378] and [380], concluding that it is not. However whether that is the right question depends on taking the three step approach I have outlined.
99. Considering claim 8A as the best example, it is manifest that the answer at the first step, to identify what is it which falls within the scope of the claimed class, is as follows. What falls within the scope of the claim are compounds satisfying both the structural features A and B (and in claim 19A feature H) and also functional features C and E - HIF-PH inhibition and increasing endogenous epo. Those are the claimed compounds. If someone took a compound within Formula I which was not a HIF-PH inhibitor and did not increase epo, but used it successfully to treat CKD anaemia, they would not infringe the claim.
100. It is also clear (see judgment paragraph [260]) that functional feature C (HIF-PH inhibition) can be tested for in an appropriate biochemical in vitro assay and functional feature E (increase in endogenous epo) can be tested in vivo in a suitable animal model (mouse or rat). Whether these tests are difficult to perform is a matter for undue burden, below.
101. Thus the conclusion at step one ought to have been that the claimed compounds are compounds within the relevant structures which satisfy the in vitro tests for feature C and the in vivo animal model tests for feature E. The judgment contains no finding to this effect. That is an error of principle and approach.
102. The answer to the second step, determine what it means to say that the invention works, is also clear. Working, in this case, means treating the CKD form of anaemia. In terms of the problem to be solved, it is to treat CKD anaemia (or to find compounds which will treat anaemia, the difference does not matter). In other words it is the achievement of the therapeutic effect, features F and G. The fact that it is not necessary to demonstrate success in a clinical trial (paragraph [260]), does not mean that the claims do not require the therapeutic effect to be achieved.
103. Accordingly I agree with FibroGen that on the facts of this case it is not relevant or necessary to ask whether it is plausible that compounds which have the structural features which are written in the claim will satisfy the functional aspects C and E, in other words whether they would be HIF-PH inhibitors or would increase endogenous epo. However the reason it is an error is not because plausibility is irrelevant to this claim (as some of FibroGen’s wider submissions appeared to suggest). On the contrary, as FibroGen did recognise on other occasions, the requirement for plausibility does indeed apply in this case, as I have explained above.
104. Having now taken the first two steps above, the right question can be asked at the third step. The question is whether it is possible to make a reasonable prediction that compounds which satisfy the structural features and which also satisfy the functional features C and E, will be useful to treat CKD anaemia. Or, asking the same question a different way, is it plausible that compounds which satisfy structural features A and B, and function features C and E, will be useful to treat CKD.
105. The answer is clear that this is indeed plausible. Moreover this approach is not a sleight of hand based on functional language, it is the essence of the invention as it is disclosed in the patent specification.
106. Putting it another way, for the reasons already explained, the fact that the skilled person would have no reason to suppose that substantially all compounds within Formula I satisfy the step one functional features (that is paragraph [370]), is irrelevant to reasonable prediction. And it is also irrelevant whether the skilled person could make a reasonable prediction that all compounds within Formula I would work (i.e. would be effective to treat CKD) (that is paragraph [371]). The relevant question is the one stated above.
107. It was not disputed before the court below that if the third step question is posed as explained above, then the answer is in the patentee’s favour. The judgment holds that this is not the right way of asking the question for three reasons. The first reason, at [372] to [375], is that it is precluded implicitly by Idenix v Gilead. However as I have endeavoured to show above, that is not correct. This approach to asking the question arises from an orthodox application of the law. Apart from anything else Idenix was not concerned with a case in which a claim had double functional features or had a mix of structural and functional features at step one.
108. The judgment at [373] to [375] contains a slightly involved reading of Idenix which it is not necessary to examine. The point is a simpler one. The achievement of the claimed therapeutic effect is not a get out of jail free answer to plausibility in this or any other case on the footing that the claim only actually claims those compounds which achieve the therapeutic effect. If it was then the various cases on plausibility would all be wrong. Idenix is indeed an example of a case why FibroGen’s extreme submission as the judge characterised it below, is wrong but in that respect it does not go further than the other UK cases such as Regeneron v Genentech and Warner Lambert.
109. Before leaving paragraph [372] itself, it is fair to note that the way FibroGen characterised its own case was that the claims “are limited to compounds which work”. I am not surprised that this was rejected because it elides the difference between the step one functional features, which limit the scope of the compounds to which the patent’s promise applies, and step two, the therapeutic effect, which certainly is subject to a requirement for reasonable prediction /plausibility.
110. The second reason why the judgment does not approach the third step in the right way is because, at [376], it is held that the patent implicitly promises that substantially all the compounds which satisfy the structural definitions will have the claimed therapeutic efficacy. That is not correct and is an error of construction of the patent specification. The patent does not promise that explicitly or implicitly. Its promise, for good or ill, is that it is compounds which satisfy both the structural definitions and the step one functional features that are the ones which will have the claimed therapeutic activity.
111. The justification given in [376] why the patent should in effect be taken to make the implicit promise referred to is not one based on construction, rather it is said to be because the structural definition covers around 10183 compounds and that the specification only demonstrates that five compounds, namely Compounds C, E, F, J and K, satisfy the criteria for likely therapeutic efficacy. However that is not a complete summary of what the patent discloses. Crucially the patent also discloses that the compounds which ought to be used to achieve the therapeutic effect are the ones within the structural class which also satisfy the step one functional features in the claim. That is why it is not a fair statement in the context of reasonable prediction/plausibility to say that it is “no more than” an invitation to the skilled team to find other compounds covered by the claim which work. Again undue burden is a different issue and needs to be addressed separately.
112. The last sentence of paragraph [376] states that it would not involve an inventive step because it would not solve the technical problem of identifying other compounds beyond C, E, F, J and K which have the desired activity. This wrongly elides reasonable prediction with undue burden for the reasons already explained.
113. The third reason why the judgment rejects what I have called the right way of asking the reasonable prediction question is because of the technical contribution which is identified in the course of rejecting the obviousness case ([378] - [379]). To grapple with this it is necessary to turn to the reasoning on inventive step. The findings on inventive step can be stated briefly:
i) The concept of inhibiting prolyl 4-hydroxylase enzymes and HIF-PH in particular was known. This was not in dispute. After all that is why the patent can cite a number of previous patents as disclosing prolyl 4-hydroxylase inhibitor molecules.
ii) The prior art Epstein paper specifically draws attention to HIF’s role in regulating erythropoiesis (amongst other things) and the judge concluded at paragraph [327] that it was obvious to the skilled nephrologist that HIF-PH inhibitors might be used to enhance erythropoiesis.
iii) The skilled nephrologist would be uncertain whether HIF-PH inhibitors would be effective in stimulating endogenous epo either at all or sufficiently to have a therapeutic effect against anaemia but they would consider the prospects of success sufficient to warrant first in vitro and then, for successful candidates, in vivo tests of suitable compounds. They were obvious to try (paragraph [335]).
iv) However Epstein only discloses one compound which is a HIF-PH inhibitor. It is called DMOG. It is not a heterocyclic carboxamide of the claimed structure. The judgment concludes that it was obvious to search for other compounds beyond DMOG to test (paragraph [335]).
v) The skilled nephrologist would ask the medicinal chemist to identify known HIF-PH inhibitors, such as collagen prolyl hydroxylases. The question on obviousness was whether Compound C would be found by this route. In the end the evidence did not establish that Compound C would be found this way (paragraph [344]).
114. In paragraph [378] the judgment holds that it is relevant to insufficiency that these findings with regard to Epstein mean that the “actual contribution to the art is no more than the identification of Compounds C, E, F, J and K as being ones that have therapeutic efficacy for anaemia associated with CKD”. This is an example of the Conor v Angiotech error. The validity of the claim has to be judged by the terms of the claim itself. If that claim was obvious then it would be invalid for lack of inventive step. Moreover if it was not possible to reasonably predict that the therapeutic effect could be achieved using the claimed compounds then it would be invalid for insufficiency. But it is not the right approach to cut down the technical contribution by reference to partial steps in a failed obviousness attack.
115. Furthermore the fact that it is obvious to try, with the appropriate level of success, to test whether HIF-PH inhibitors actually do stimulate endogenous epo either at all or enough to be sufficiently therapeutically relevant to treat anaemia does not mean that the state of the art (let alone the common general knowledge) included knowledge of the outcome of such a test. It simply did not. Therefore something which the patent unquestionably does contribute on the findings in the judgment is the idea that a certain (wide) structural class of molecules which are also HIF-PH inhibitors contains members which do indeed stimulate endogenous epo enough to be likely to be therapeutically relevant to treat CKD anaemia. On the judgment, that was not known.
116. Nor, on any view, is it appropriate on these grounds to limit the technical contribution to “no more than” the identification of five compounds with therapeutic efficacy. For one thing identifying any compound at all with therapeutic efficacy based on a biochemical rationale which the skilled person did not previously know would work, can be a valuable technical contribution in its own right. More importantly in this case however, the patent ties the claim and the disclosure to a particular mechanism and claims accordingly. A claim of that kind must also satisfy the law relating to undue burden but, as I have repeated, that is a different issue.
117. The final paragraph before the conclusion, [380], starts by reference to a premise which does not apply since there is no challenge before this court to the conclusion that Epstein makes testing some compounds obvious to try. However the paragraph does contain a conclusion that a finding that five heterocyclic carboxamides can be used treat anaemia is not a principle of general application across the breadth of the claims because the evidence shows that a large number of heterocyclic carboxamides are not likely to work. This is the same flaw as has been addressed at length above. Based on the claim in this case the question is not whether heterocyclic carboxamides of the claimed structure “work” in the sense of achieving the therapeutic effect.
118. Before leaving reasonable prediction, I will mention paragraphs [370] and [371], which have not been addressed so far. Paragraph [370] summarises Prof Ward’s evidence that the skilled person would have no real reason to think that substantially all the compounds in Formula I would be effective in inhibiting HIF-PH, or increasing epo, or otherwise treating anaemia, and paragraph [371] summarises the technical factors why a significant number of Formula I compounds would not be effective. These arise from three factors: (i) the ability of a compound to cross the cell membrane and ADME; (ii) potential toxic side effects; and (iii) difficulties in synthesis for various reasons including steric effects. The short answer to these points is that they are only relevant on the erroneous approach to the three steps required to address reasonable prediction. For example if a compound does not cross the cell membrane then it is unlikely to pass the in vivo cell tests and therefore will not satisfy claim feature E. If it does not satisfy feature E then it is not a claimed compound and the fact it does not have the therapeutic effect (feature F/G) is not an instance of the invention not working. Similarly for many ADME issues and also for the inability to make a compound. If it cannot be made then it cannot be shown to pass the functional tests. This illustrates why, used appropriately, functional limitations are legitimate. On the other hand one can see that a cumulation of failures of that kind could well play a role in undue burden.
119. Side effects are a different issue. First, the relevant prediction is that substantially all the claimed compounds will be efficacious, not that they will all necessarily be free of side effects. If the skilled person would predict that all the claimed compounds would have toxic side effects then that might be a different matter but it was not Prof Ward’s evidence. Evidence about side effects of the kind given by Prof Ward does not falsify the reasonable prediction.
120. I therefore disagree with the conclusion on reasonable prediction/plausibility. The same result follows in relation to Agrevo obviousness.
The judgment on undue burden
121. As explained above the law of undue burden in this context does not require the skilled person to identify substantially all compounds covered by the claim, rather it applies by asking whether it is possible to perform the invention across the scope of the claim without undue effort. For claims of this type, this has two elements. It must be possible for the skilled person, without undue burden, to identify some other compounds, beyond those individually named in the patent, which are within the claimed class and therefore are likely to have therapeutic efficacy. Second and separately, it must also be possible for the skilled person to work substantially anywhere within the whole claim. That will involve the skilled person, given any sensible compound within the structural class (or substantially any), being able to apply the tests without undue burden and work out if it is a claimed compound.
(i) possible to identify some further useful compounds without undue burden
122. The court below found that although it would be a great deal of work, the skilled team would be able to find some compounds which were effective. The nature of the work required to get there is a SAR analysis involving a process of iteration, with information being built up as compounds are tested, and with optimisation of the leading compounds which are identified. It is addressed in paragraphs [392] - [393] addressing Dr Bhalay’s cross-examination:
“392. Dr Bhalay clarified the nature of this exercise in cross-examination:
i) the medicinal chemist would start with an SAR analysis, involving tens, hundreds or even thousands of compounds to see what kind of changes are tolerated, testing for activity in an enzyme assay;
ii) depending on the strategy, there might be “spot checks” to see whether the compounds were competitive with respect to 2-OG;
iii) compounds which looked promising would be progressed to cell-based Epo induction assays;
iv) after that, promising compounds would go on to in vivo Epo induction assays, but not before completion of some initial pharmacokinetic studies.
393. The number of compounds involved in this initial SAR analysis (even if ran to thousands) would obviously not scratch the surface in terms of the number of permutations envisaged by Formula (I). Dr Bhalay envisaged that the medicinal chemist would then undertake further SAR “streams”, each involving a different chemotype, by which he meant pyridine, isoquinoline, quinoline etc. In each case, the medicinal chemist would adopt the lead optimisation strategies described in paragraphs 83-85 above.”
123. Dr Bhalay’s evidence was that the exercise which produced some useful compounds involved work which is routine for the medicinal chemist. What the judgment was based on was that this would never scratch the surface since the task was to identify substantially all useful compounds within the formula. In other words the undue burden found lay in the quantity of the work to be done, not its quality.
124. There are a number of issues to address briefly. First is the effort involved in performing the tests themselves. Second is predictability and third is the characterisation of the work as a “research programme” or “research project”.
125. On the first issue, it is clear that actually performing the in vitro or in vivo tests on a given candidate compound itself was not unduly difficult. There is no finding in the judgment to that effect. It is not the source of an undue burden. The fact that large numbers of compounds would be made and tested is a different issue.
126. On the second issue, the evidence was that based on the information in the patent the skilled person would not be able to predict whether a given compound would be a HIF-PH inhibitor before doing a test, even if it was closely related to Compounds C to K. One problem was the lack of evidence in the patent of the three-dimensional shape of the active site of HIF-PH. FibroGen had sought to suggest that useful information could be found in the three-dimensional shape of the active site of a different prolyl-hydroxylase (collagen PH) which is in the papers cited in the patent. Using that to draw an inference about HIF-PH was what Dr Bhalay called a leap of faith, referred to (judgment [394]). The difficulty of making a reliable prediction whether a compound would be a HIF-PH inhibitor was something Prof Ward agreed with [395].
127. However the significance of these findings about the difficulty in making any reliable prediction of what would be likely to pass that assay screen has to be kept in context. Of course if the skilled team was able to predict a priori from the patent which compounds would pass the HIF-PH inhibitor test and which would not that would make the task easier, but it is not a legal requirement that the outcome of these tests necessarily needs to be predictable from the start.
128.
A point on prediction also arises relating to the documents produced in disclosure. Paragraph [396] notes that there is no witness evidence about the work and some of it post-dates publication of the crystal structure of HIF-PH and Compound C which would make it easier to design active compounds. At [397] however the results of assays on 1,151 compounds are summarised. These include in vitro HIF-PH enzyme inhibition assays, in vitro cell culture based epo induction assays, and in vivo epo induction assays. The judgment states:
“[397] The data show that success in the HIF-PH assay (applying Dr Bhalay’s criteria) is not predictive of success (again applying Dr Bhalay’s criteria) in cell-based/and or in vivo Epo-induction. Moreover, of the compounds for which there is assay data, only 182 (16%) are shown to meet Dr Bhalay’s criterion for Epo-induction in vivo (which is not to say that 84% fail - in fact, Dr Bhalay’s evidence was that the pass rate amongst those tested was 86%). The majority of these compounds are isoquinolines. In the class of pyridines, there are only two compounds which were tested for in vivo Epo- induction. Only one of them passed, namely vadadustat. The other failed.”
129.
FibroGen contends that there is an error in the passage above because in fact the data showed that 80-90% of the compounds which had success in the HIF-PH assay succeeded in the epo induction testing. There is no dispute about what the data shows or about the criteria. Therefore either the first sentence in [397] means that 80-90% is not predictive of success or there is a mistake. In my judgment if 80-90% of the compounds shown to be HIF-PH inhibitors demonstrated epo induction, that indicates something which is reasonably predictive of success.
130.
Moreover the 16% figure is not useful other than to indicate the relatively small number of compounds to which this part of the evidence applies. In terms of success rate the figure which matters is that 86% of the compounds tested showed in vivo epo induction.
131.
The analysis of the disclosure concludes at [398] with the reference to Dr Bhalay’s unchallenged evidence relating to the short time it took Akebia to develop vadadustat starting from Compound C. As already mentioned much earlier in this judgment, the paragraph concludes that “All this shows, however, is that it is possible to identify another compound which works without difficulty”. 132. An important aspect, which Akebia’s expert Prof Ward’s evidence supported, is that the SAR work is an iterative process. This provides the answer to whether this case is like T 1063/06 Bayer/reach through claims, in which the team has no alternative if they want to find some compounds but to test every conceivable compound. The fact the skilled team can perform the SAR analysis described by the experts which produces some useful compounds shows that the results of the tests on individual compounds build on one another. In other words the skilled team’s work is cumulative. Thus Bayer/reach through claims does not apply. 133. Third is the “research project” point. Dr Bhalay agreed the SAR exercise was a research programme [394]. Prof Ward’s evidence was summarised as follows: “Prof Ward’s evidence was to the same effect. Indeed, he was cross-examined on the basis that the search for active compounds constituted a “development programme”. It was not put to Prof Ward that the medicinal chemist would be able to predict reliably that a given molecule (even one that was closely related to any of Compounds C-K) would be active. He maintained that the exercise was one of iterative research. Moreover, consistently with the approach taken by Dr Bhalay, it was not suggested to Prof Ward that the skilled medicinal chemist could possibly synthesise and test even an infinitesimal proportion of the compounds covered by Formula (I) no matter how long they spent on the exercise. The case that was put was merely that the medicinal chemist could find some compounds.” 134. And of course the characterisation of the work as a research project is in the concluding paragraph [399]. 135. On appeal Akebia submitted that the finding below was that the work was a “research project”, and therefore necessarily unduly burdensome. In addition to the passages above, Akebia also referred to the section of the judgment on common general knowledge which explains SAR analysis and lead optimisation, stating at [82] that: “By its very nature, SAR analysis is an exercise of genuine research in which the medicinal chemist is trying to discover new information. Moreover, it involves matters of choice and judgement.” 136. I do not accept Akebia’s submission for two reasons. 137. First it is clear that the finding below that the work required was a “research project” was closely connected to the difference between only being able to identify some useful compounds and having to identify substantially all of them. That is reflected in paragraph [394] above relating to Prof Ward’s evidence which draws attention to the point that what was put could only ever deal with an “infinitesimal portion” of the claimed compounds. Another example is earlier at [388] in which the judgment deals with part of Dr Bhalay’s evidence that in certain circumstances (subject to an issue about reading papers cited in the patent) it would be straightforward and routine for the skilled medicinal chemist to identify compounds across the scope of Formula (I) which exhibit “similar activity” (i.e. HIF-PH inhibition) and would have potential use in therapy. The concern raised in the next paragraph, at [389], was focussed on the amount of work that would require and not the quality of the work itself. Furthermore at [390] there was a dispute on the evidence about how many compounds a skilled team could make in a year. Here the judgment finds that this does not matter because neither figure comes close to the size of the structural class. The judgment also repeatedly characterises the structural class as “staggeringly large” (at [41], [282] and [368]). The fact Prof Ward said it was staggeringly large is not the point. The point is that the size of the structural class is not relevant to the application of the test of undue burden. Finally the conclusion expressed at [399] with the reference to many lifetimes and a never ending task, also shows that that is the approach taken. 138. Thus the “research project” identified is the work to identify substantially all compounds within the claim, which is not the right legal question. 139. Second, there is a relationship between inventive step and insufficiency. As the Supreme Court has now clarified in Actavis v ICOS [2019] UKSC 15, the fact that in a programme of work experiments may be undertaken to discover new information and involving choice and judgment does not necessarily preclude a finding that what is found in such a programme lacks inventive step and was obvious. Although obviousness and insufficiency are not mere mirror images of one another for all kinds of reasons, nevertheless there is scope for parity of reasoning. The same thinking applies to sufficiency. The fact that a programme of work involves experiments undertaken to discover new information, and involves choice and judgment, does not necessarily preclude a finding that the result is sufficiency rather than insufficiency. It is all a matter of degree. 140. The issue is not the correctness of the statement in the common general knowledge section of the judgment that work on a SAR involves genuine research because the medicinal chemist is trying to discover new information using choice and judgment. That is plainly right, and in any case was a finding plainly open to the court. However it cannot be taken as a finding that all such SAR work necessarily involves an undue burden for the skilled team. Notably the judgment does not say that. 141. Nor does the judgment state there, or anywhere else, that merely because a SAR analysis would be performed of whatever kind, it necessarily follows that the claim must be insufficient. Much less would have been needed to be said if the conclusion was as simple as that. Moreover given that SAR analysis plays a part in many pharmaceutical patent cases, it would be a remarkable result if merely because a SAR of any sort was undertaken, the claim was insufficient. 142. Standing back, the finding was that although it would be a great deal of work, the skilled team would be able to find some compounds which were effective. The judgment does not expressly state that this result would be reached without undue burden but I believe that is the only answer. It would take a great deal of work but it would be routine for the medicinal chemist and iterative in nature. What made the whole process an undue burden below was the number of compounds in the claim and the fact the exercise would not scratch its surface. Other points 143. One of the points taken by FibroGen was that the judgment gives no credit for the 100 or so exemplary compounds identified in the specification at [0072]–[0077]. I would hold that a proper assessment of undue burden needs to take the whole teaching of the specification into account and in this case that would include the further approximately 100 exemplary compounds. They are disclosed as being compounds which may be used in the invention and if Akebia had wanted to show that they did not work, the evidential onus was on Akebia. 144. However as the issues have developed on appeal, I do not believe this issue is relevant. That is because no matter how many compounds within a claimed class are positively identified in a patent specification and disclosed as useful, if there is a claim to an even larger class, then for that wider claim to be sufficient, some other compounds within it must be available to the skilled person without undue burden. 145. Another issue is the correctness of the conclusion (mentioned above in relation to Dr Bhalay) that the skilled person would not follow up any of the cited publications in the patent specification. The reasoning in this point concludes at [221] (starting at [204]) that based on the texts of the granted patents and the evidence of the experts, the skilled team would not necessarily do that, holding at [218] that: “ there is no principle of law that the skilled team are deemed to read all documents cited in a patent. It is a context- and fact- dependent question, and thus it depends firstly upon the wording of the specification and secondly on the evidence. ” 146. FibroGen contended this was wrong in principle because: “(a) The skilled person is a legal construct and is not put off by having to read large amounts of material (citing Rockwater v Technip [2004] RPC 46 at paragraphs 6-7). (b) If a patent advances a document as being relevant to putting the invention into effect the skilled person is deemed to read that document with interest - unless there are special reasons why he or she would not do so. (c) The notional skilled team is deemed to have sufficient resources to read the literature. The evidence was that to review all the papers and the patent applications in these passages would take one person 4 days, which is plainly proportionate.” 147. Akebia did not agree, contending that there was no such principle of law and that the finding, which was a matter of fact and depended on the evidence, was open to the court. 148. There is something to be said for the argument on both sides of this debate. I must say I find it difficult to see how it can be right as a matter of construction of the specification (bearing in mind construction is ultimately a matter for the court) for the skilled person (bearing in mind they are a construct) not to take into account the particular patents and papers referred to expressly in paragraphs [0072] to [0078] in an assessment of sufficiency of disclosure, given that the patent expressly characterises them as places in which exemplary compounds which may be used in the invention can be found. However as with the 100 identified compounds I do not believe it is necessary to resolve this issue to decide this appeal and I prefer to leave the point for a case in which it actually matters. (ii) work substantially anywhere 149. The second and separate requirement for sufficiency is that it must be possible for the skilled person to work substantially anywhere within the whole claim. This is conceptually different from the previous issue. It is not concerned with what the skilled team would actually do given the patent document - as to which the evidence was unsurprisingly that they would start from the specific compounds shown to be useful. This arises from the scope of the claim and is concerned with whether there is a specific region of the claimed scope for which testing the functional features would be an undue burden. 150. There was evidence that a significant number of compounds within the structural class as a whole would not work for ADME and other reasons - side effects and difficulties in being able to make them. The judgment refers to this at [371] in the context of reasonable prediction/ plausibility, and ADME comes up again in the context of undue burden at [386], which is as follows: “386. Nor does the specification give the skilled medicinal chemist any assistance at all with regard to such matters as finding compounds within Formula (I) which have suitable ADME profiles. The medicinal chemist could, of course, apply their common general knowledge rules of thumb such as Lipinski’s rules, but predictions made using such rules would not always be correct. ” 151. FibroGen’s answer to that point is that the skilled person is deemed to make sensible choices. So for example on ADME in particular the judgment expressly recognises that the medicinal chemist can apply their common general knowledge rules of thumb such as Lipinski’s rules and avoid trying out chemical structures likely to have unfavourable ADME profiles. The fact the predictions are not always correct does not amount to insufficiency. That is why the in vitro and in vivo tests are undertaken. 152. I accept that the skilled person needs to be considered as someone making sensible choices, but the fact remains that the conclusion in the judgment is that significant numbers of compounds would be ineffective. The real answer to this is that evidence about ADME and the other factors was generic to the claim as a whole and was not made about a specific region of the scope (cf again the 2'-methyl-up-2'-fluoro-down sub-class in Idenix). It did not undermine the overall conclusion that some useful compounds would be found, nor does it indicate that there are particular regions of the claim scope which cannot be tested. Accordingly on the facts found below, no issue of the relevant sort arises. Insufficiency - uncertainty 153. The modern law on uncertainty insufficiency is the judgment of the Court of Appeal in Anan Kasei v Neo Chemicals [2019] EWCA Civ 1646, demonstrating that it is now well established as a free standing kind of insufficiency, and therefore a ground on which a patent claim can be held invalid. However as so often the law is relatively easy to state but not always easy to apply. An important part of the background is that in the legislation the requirement for a claim to be “clear and concise” (Art 84 EPC and s14 of the 1977 Act) is a ground on which grant can be refused by the relevant patent office but is not a ground for post grant revocation for invalidity. I reviewed the history of all this in Unwired Planet v Huawei [2016] EWHC 576 (Pat). At that time it was called “ambiguity”. In reaffirming the principle as a ground of invalidity Anan Kasei re-named it uncertainty or conceptual uncertainty. That is a better name for it, for the reasons explained by Floyd LJ at paragraphs 21-25 and Lewison LJ at paragraphs 101-104. 154. The conclusion I reached in Unwired Planet was at paragraph [163] and, apart from the fact it uses the term “truly ambiguous” instead of “conceptually uncertain”, I believe it remains pertinent. With that change in language the conclusion is: … I agree that claims can often be difficult to construe. Sometimes those difficulties are due to avoidable obscurity for which the patentee should get no sympathy, but it can be because trying fairly to describe an invention in words is not always an easy task. I also agree with Arnold J that the existence of a fuzzy boundary in a claim is not objectionable. The contrast is between that and a claim which is conceptually uncertain. The factual circumstances in which such a conceptually uncertain claim has been identified so far in the modern law (Kirin-Amgen and Sandvik) are ones which depend on carrying out a technical test to find out if a product or process is within the claim or not. If the skilled person cannot know whether they are carrying out the right test, then the claim is conceptually uncertain and therefore insufficient. That makes sense. However, while the principle cannot be limited just to technical tests, after all SmithKline Beecham was not that sort of case, nevertheless it does not apply simply because one can imagine difficult cases to judge at the edge of a claim. When a defendant has been found to infringe, demonstrating that the claim’s scope is at least clear enough to work that out, an argument that the claim should be regarded as conceptually uncertain is likely to be met with scepticism.” 155. Now in Anan v Neo Floyd LJ said the following at paragraph [27]: “[…] For my part, I do not agree that the objection of uncertainty is answered simply because there is something within the claim which is clear, if there is a large territory (more than a fuzzy boundary) where the claim is uncertain.” 156. On its face the last sentence of Unwired Planet does not sit easily with this, but I will take this opportunity to say that I believe both statements are correct. Each case has to be decided on its own facts. A finding of infringement does not preclude a finding of conceptual uncertainty, but I do maintain it ought to cause the court to look closely at what the alleged uncertainty really amounts to. 157. The issue is whether claims which included the term “a structural mimetic of 2-oxoglutarate” were conceptually uncertain and invalid. The technical background to this is as follows. Recall that HIF-PH, the enzyme which the claimed compounds are supposed to inhibit, operates by initiating the process which breaks down HIF (which in turn regulates epo). In order to function some enzymes require further factors which can be called co-substrates. HIF-PH requires iron, oxygen and 2-oxoglutarate (2-OG). So perhaps if one could find a molecule which mimics the natural substrate 2-OG, it might be able to inhibit the operation of the enzyme. For example, put crudely, the mimic molecule would slot into the pocket in the enzyme designed to accommodate 2-OG but jam it up so that it does not work anymore. There can be competitive inhibitors which compete with the natural substrates in a reversible way, non-competitive irreversible inhibitors which block the active site in an irreversible manner and non-competitive “allosteric” inhibitors which actually bind to a different active site on the target enzyme altogether which changes the way the enzyme operates. The judgment explains this at [95]-[97]. The science of enzyme reaction kinetics can allow one to distinguish between competitive and non-competitive inhibition. Enzyme kinetics is explained in more depth at [102] - [106]. 158. Although broadly the term “a structural mimetic of 2-oxoglutarate” would be understood to mean a molecule which mimicked 2-OG, that alone does not make the term conceptually certain. The judgment approaches the issue in the following way. Paragraphs [289] - [303] address the meaning starting with reference to the only paragraph in the patent which is provided by way of a definition of the term. The relevant part of the paragraph ([0069] of EP 823 and [0076] of WO 997) is: “[0069] […] Such compounds may inhibit the target 2-oxoglutarate dioxygenase family member competitively with respect to 2-oxoglutarate and noncompetitively with respect to iron. (Majamaa et al. (1984) Eur J Biochem 138:239-45; and Majamaa et al., supra.)” 159. After considering the Majamaa 1984 paper referred to the judgment then decides that the skilled person would construe the reference in the patent to “may … inhibit competitively” as “may or may not inhibit competitively” (paragraph [295]). Then [296] to [299] considers and rejects evidence from Dr Bhalay that an ability to carry out so called “bidentate chelation of iron” was a necessary feature of being a structural mimetic of 2-OG. There is no appeal on that. Paragraph [300] then refers to enzyme experiments which Dr Bhalay said would be done to see if the compounds were competitive with 2-OG and then paragraph [301] refers to a different point, about potency, concluding that even if competition with 2-OG was the acid test, the skilled person would not know what constitutes the relevant threshold of potency in that enzymatic reaction (measured using the enzyme kinetics parameter Ki). Different skilled people would give different answers. Finally paragraph [302] notes a number of different definitions which were put to Prof Ward, and other reasonable definitions which he suggested in his evidence. 160. The conclusion is at [303]: “303. I therefore conclude that the skilled medicinal chemist would be uncertain as to the meaning of the expression “structural mimetic of 2-oxoglutarate”. More specifically, they would be unable to determine either from the specification or from the cited papers what criterion to apply to distinguish between a compound which is a “structural mimetic of 2-oxoglutarate” and a compound which is not. I will consider the consequences of this when I come to the issue of insufficiency.” 161. Then, turning to insufficiency itself, the judgment deals with this at paragraphs [400] - [405]. Paragraph [402] concludes that the skilled person would not know what constituted a “structural mimetic of 2-oxoglutarate” and also that in particular they would not know what test to apply to distinguish between a compound which is and a compound which is not a “structural mimetic of 2-oxoglutarate” and so the claims are uncertain and invalid. 162. Paragraphs [403] - [405] address an additional point and reject it. Below FibroGen had contended that Prof Ward accepted that Compound C was a structural mimetic of 2-oxoglutarate and argued that this supported its case that there was no uncertainty. The paragraphs hold that that was not Prof Ward’s evidence ([404]) and go on to hold that if the skilled person would conclude that the inventors regarded Compound C as a structural mimetic of 2-oxoglutarate it is no answer to the objection, referring to what Floyd LJ said in Anan v Neo at paragraph 27. The point was that to know that the patent was asserting that Compound C was a structural mimetic of 2-oxoglutarate did not tell you what test to use to determine whether another compound is one. 163. FibroGen’s case on appeal is simple enough: i) In law (Anan Kasei) there is a distinction to be drawn between a claim that is difficult to construe, or has some room for doubt or fuzziness at the edge, and one that is conceptually uncertain. ii) Despite the judgment, in fact the concept of a structural mimetic was readily understood. It describes a compound which is competitive with 2-OG at its binding site. The concept of mimicry, of a compound sitting in the binding pocket which would otherwise be occupied by 2-OG, was known in the art. iii) The target HIF-PH enzyme requires various co-substrates to function, including iron and 2-OG. Structural mimetics of 2-OG are to be distinguished from compounds which achieve HIF-PH inhibition activity by competing with iron - compounds which sit outside the binding site and mop up the iron required for enzymatic activity. The specification of EP 823 makes this distinction clear at [0069]. iv) The construction of “may” in [0069] of EP 823 as meaning “may or may not” is not correct. In this context “may” would be understood by the skilled person to mean has the capacity to inhibit in that manner (competitively). Actual inhibition will depend on concentration. There is no problem in identifying competitive inhibition and this is the appropriate way to determine whether a compound is a structural mimetic. v) In fact the judgment made a clear finding at paragraph [411] that Akebia’s compound vadadustat, about which infringement was in issue, was a structural mimetic of 2-oxoglutarate. Akebia has not appealed that finding. 164. Akebia’s case is to support the judgment. In addition Akebia contended that even if there is an error in relation to competitive inhibition, the point on potency at [301] would be a free standing basis on which to conclude there was uncertainty. 165. I agree with FibroGen’s submissions, as I shall explain. Starting with the patent, in my judgment the right construction of paragraph [0069] of EP 823 is that it refers to a capacity which the compounds have. They have the capacity to inhibit the target 2-oxoglutarate dioxygenase family member competitively with respect to 2-oxoglutarate and noncompetitively with respect to iron. That is what the skilled person would understand the patentee is trying to say. In context to read this as if it means “may or may not” deprives it of any meaning. 166. Nor do I agree that reading the Majamaa paper demands such a conclusion. The paper describes 24 compounds as structural “analogs” of 2-OG. For present purposes there is no difference between an analog and a mimetic. 23 of the compounds inhibit the prolyl 4-hydroxylase enzyme competitively with respect to 2-OG and non-competitively with respect to iron. It is true, as the judgment notes, that the paper observes that one compound, pyridine-2,6-dicarboxylate, was competitive with respect to iron and not competitive with respect to 2-OG (at least at low concentrations) and it is also true, as held, that this forms a significant part of the teaching of the paper. Majamaa shows that the difference between competitive and non-competitive inhibition can be seen in the experimental results (figure 5). However none of that would falsify the skilled person’s understanding of what the patent means in [0069] of EP 823 nor would it lead them to conclude that paragraph [0069] is meaningless because “may” means “may or may not”. Thus they would understand the teaching of the patent to be that what is meant by a structural mimetic of 2-oxoglutarate was one which had the capacity to inhibit the target competitively with respect to 2-OG. 167. Since the skilled person can distinguish between competitive and non-competitive inhibition based on enzyme kinetics, the conclusion on construction disposes of all the issues on uncertainty save for potency. However a lack of clarity about the relevant threshold for potency on its own cannot amount to conceptual uncertainty, not least in the light of the conclusion that vadadustat is a structural mimetic of 2-oxoglutarate. Bear in mind that unlike the argument over Compound C, vadadustat is not a molecule identified individually in the patent, and yet the conclusion of infringement was arrived at. It is fair to note that the judgment does not contain any reasoning for that conclusion but neither has Akebia appealed the result. No doubt on potency, the position is that vadadustat is manifestly a sufficiently potent inhibitor of HIF-PH to work, given how far it has come through clinical trials. 168. The potency point is a classic example of the difference between conceptual uncertainty and a fuzzy boundary. The Majamaa paper itself shows that skilled people are able to characterise compounds as ones which did inhibit prolyl 4-hydroxylase competitively. The fact that reasonable experts may disagree about exactly where the boundary will lie between something which is and something which is not an inhibitor means there is a “fuzzy” boundary to the claim. The remedy for that would be that the patentee could not satisfy the court of infringement. But to find the claim invalid in those circumstances would be a disproportionate response, which in my judgment the law does not require. Obviousness of Family B over WO 997 169. The issues relevant to the Family B patents relate to levels of iron in the body. Iron matters because it forms part of the molecule haemoglobin which is the molecule in red blood cells which carries oxygen. So the body needs iron to make red blood cells. 170. The level of iron in a patient is expressed as the TSAT (Transferrin Saturation). Transferrin is a protein to which iron which is in the serum is mainly bound. The judgment explains this in paragraph [54]-[55]: “54. The main iron pathways are shown diagrammatically in a figure from Prof Winearls’ first report which I reproduce below. 55. On the left is shown absorption of dietary iron in the duodenum, and the use of iron in bone marrow for erythropoiesis. The central rectangles (in purple and red) show iron in the bloodstream, where it is either bound to transferrin or in the form of haemoglobin in red blood cells. On the right are the iron stores, from where iron is released into (or to which iron is removed from) the circulating, transferrin-bound pool. When red blood cells reach the end of their 120-day life, their iron is also recycled into these stores via macrophages. ” 171. There are two kinds of iron deficiency and it is necessary to distinguish between them. They are absolute iron deficiency and functional iron deficiency. The judgment explains this at paragraph [57]-[59]: “57. Absolute iron deficiency occurs when a patient does not have enough iron in stores to supply the body’s needs. Absolute iron deficiency may be caused by a low-iron diet, reduced iron absorption and/or bleeding. Absolute iron deficiency is characterised by a low TSAT and a low level of serum ferritin, namely, a TSAT < 20% (or < 16% in more extreme cases) and serum ferritin < 50-100 ng/ml. 58. Functional iron deficiency occurs where there is sufficient iron in stores, but where there is inadequate delivery of that iron from stores to the bone marrow. Inflammation can result in iron being sequestered into iron stores and the reticuloendothelial blockade, which prevents release of the stored iron, being activated. Functional iron deficiency is characterised by a low TSAT and normal or high serum ferritin. 59. Patients who are “iron replete” are usually defined as those with a TSAT of at least 20% and a serum ferritin level of at least 100 ng/ml; but TSAT measurements show considerable diurnal and day-to-day variation for a given patient. ” 172. The final aspect of relevant background which needs to be explained is the relationship, similarities and differences, between CKD and ACD. The following is based on judgment paragraphs [62]-[72]. 173. CKD describes a diminution in renal function through irreversible damage to the kidneys to an extent that has negative consequences for the patient, including an impairment of epo production, and hence anaemia. In patients with CKD, iron deficiency is often seen, of both the absolute and functional kind. 174. By contrast ACD is characterised by normal or high ferritin levels but low transferrin and serum iron, indicative of functional iron deficiency. ACD is thought to be caused by an underlying chronic disorder involving inflammation, such as rheumatoid arthritis or cancer, that amongst other things suppresses epo production and bone marrow activity through the effect of inflammatory cytokines. 175. At both priority dates anaemias were treated in the following ways. Iron deficiency anaemia (without another underlying cause) was treated by increasing iron in the diet, giving iron supplements, or in extreme cases by blood transfusion. 176. Anaemia of CKD was first treated with oral iron, to see if this alone achieved the desired haemoglobin response, and if not, intravenous (IV) iron was given. If iron supplementation did not raise haemoglobin to the target range, patients were given an erythropoiesis stimulating agent (ESA). ESAs include recombinant human epo and various analogues, all of which stimulate erythropoiesis in the presence of adequate iron. CKD was considered a treatable condition, as ESAs circumvented the damaged kidneys’ reduced epo production by providing an exogenous source. ESAs did have certain disadvantages, however, in that they were expensive and had to be administered by injection. 177. ESAs tend to cause (or exacerbate) iron deficiency, because they stimulate the demand for iron, and so patients are often given supplementary iron with ESAs. The norm was to aim for a TSAT of 30% or more before starting treatment with ESAs. A small number of patients are “refractory” or resistant to ESAs. 178. In terms of treating ACD, the primary goal focussed on resolving the underlying inflammation. It was common ground that ESAs were sometimes administered to patients with ACD, but there was a dispute about the effectiveness of such treatment. The judgment 179. With that background I can turn to the judgment relating to the Family B patents. It starts at paragraph [464] with a review of the patent specification by reference to the published application WO 121 which is common to all three patents. Neither party criticises the summary of the specification in the judgment. For the purposes of this appeal only four points about the patent specification matter. 180. First, in general terms the disclosure of the Family B patents relates to the use of essentially the same compounds as in Family A, but for treating ACD rather than CKD. 181. Second, four specific compounds are focussed upon, labelled A-D. Compound A in Family B is Compound C in Family A. In this judgment I will refer to the Family B usage as A', B' etc. to distinguish that terminology from the usage in Family A. So from now on Compound A' means the compound referred to as A in Family B, which is in fact the same molecule as the compound referred to as C in Family A. 182. Third, in Examples 1 to 21 the specification contains the results of a number of experiments. It is not necessary to examine the experiments in any detail. Examples 1 to 10 indicate that Compounds A' to C' are able to stimulate the production of endogenous epo in the presence of various inflammatory cytokines. Other tests relate to effects on proteins called transferrin and ceruloplasmin (such as Examples 11 and 17). Example 19 shows an increase in iron levels. Example 21 sets out results of administering Compound A' to human subjects. They include increased red blood cell count and other results, all consistent with increased iron utilisation. 183. The experimental results also include evidence of down-regulation of a protein called hepcidin by the administration of a HIF-PH inhibitor compound (Compound A'). Hepcidin was discovered in 2000/2001 ([526]). It has a role in iron homeostasis (although that was not known at the Family B priority date in 2004 [527]). Homeostasis is the maintenance by the body of things at a given level, e.g. body temperature. FibroGen emphasised that the judge held (at [543]) that it had not been suggested in the art before the publication of the Family B patents that there was any link between HIF and hepcidin. Therefore, submitted FibroGen, the hepcidin result published in the Family B patents was unexpected. 184. The significance of this to FibroGen’s case is as follows. ESAs were the standard treatment for CKD based on the idea that endogenous epo needed to be supplemented. One way of expressing the rationale on which the claims of Family A are based is that HIF-PH inhibitors are a substitute for ESAs because they stimulate endogenous epo and so there is no need to supplement it. Now when ESAs were administered one would expect the patient’s iron levels to go down because the increased rate of red blood cell production stimulated by the ESA would take up iron in the body. By contrast, FibroGen submitted, what the Family B patents are based on is the surprising discovery that the compounds work in a different way such that when they are administered, the iron levels go up. 185. Briefly the possible rationale for this effect can be put simplistically as follows. The HIF-PH inhibitor inhibits the PH enzyme which flags HIF for destruction, so by administering the compound the HIF level goes up and since (we now know) HIF suppresses (or “down-regulates”) hepcidin, such that more HIF means less hepcidin, it follows that administering the inhibitor compound down-regulates hepcidin. And since (we now know) hepcidin plays a role in iron homeostasis, perhaps the compound has a direct effect on iron levels other than by its being just a substitute for an ESA. 186. Fourth, as the judgment notes in paragraph [501] the Family B patents do not contain any data comparing the effects of HIF-PH inhibitors to those of ESAs and no data that any of compounds A' to D' have superior effects on iron mobilisation as compared to ESAs. The significance of the absence of such a comparison can be seen below. 187. The judgment then deals with the sixteen different relevant claims of the three Family B patents at paragraphs [503] to [518]. There is no need to set them all out because their variety derives from being different combinations of what is in fact a relatively small number of features. They are all EPC 2000 compounds for use claims. The compounds claimed are defined by reference to Formula (I) and/or by the expression “a structural mimetic of 2-oxoglutarate”. Sometimes they are also defined by reference to being an inhibitor of HIF-PH or to being a compound that “stabilizes HIF-α”. For the purposes of the obviousness case, nothing turns on the differences between these expressions. The claims are all, one way or another, limited to a use which involves treating anaemia. Sometimes this is expressly limited to ACD but other times the word used is simply “anemia” and the fact the claim is concerned with (in effect) ACD arises for other reasons. However again, for the purposes of the obviousness case, nothing turns on the difference between those expressions. 188. Where the claims do differ is that they all also contain one of four different kinds of further feature which qualifies the use. The first kind is a limitation on the patients treated by reference to their TSAT level. This can be either less than 20% or less than 16%. The second kind is a requirement to treat anaemia which is refractory to treatment with exogenously administered epo. The third kind is a requirement to treat functional iron deficiency, and the fourth kind is to use the compound for decreasing hepcidin expression. 189. The judgment then turns to the common general knowledge at [520]. It was common ground that everything which was common general knowledge at the December 2001 priority date of Family A was also common general knowledge at the April 2004 priority date of Family B. 190. Paragraphs [521] - [522] address the issue, foreshadowed above, about how effective it was thought using ESAs to treat ACD was. The conclusion is that ESAs were thought to work in some ACD patients, and achieved such therapeutic effect that they had by compensating for reduced epo production and reduced responsiveness to epo by the bone marrow. ESAs were not thought to act by unblocking iron. 191. Another aspect of the common general knowledge was the recognition that there were patients with anaemia which were resistant to exogenous epo. The main cause of that was thought to be iron deficiency and the second most common cause was infection or inflammation [523]. 192. At paragraphs [526] - [543] the judgment addresses hepcidin. The conclusion at [543], that the link between HIF and hepcidin was first revealed in the Family B patents, has already been mentioned. 193. None of the points on claim construction which were alive below now matter and at [554] the judgment turns to deal with obviousness over WO 997. Without any need to point it out, the judgment follows the structured Pozzoli approach. The skilled team is the same as for the Family A patents ([paragraph 519]) and the common general knowledge has already been identified. The judgment then addresses the disclosure of WO 997 at paragraph [555], identifies the differences at [556] and then deals with whether the inventions characterised by various different kinds of claim are obvious at [557] to [574]. 194. To understand the issues on appeal it is necessary to examine the prior art WO 997 in a bit more detail. 195. Of course WO 997 discloses the idea of using the HIF PH inhibitor compound to increase epo production and treat CKD. That is the basis for the claims of Family A. However it is not all that is disclosed in WO 997. As the judgment holds in paragraph [558] the document expressly teaches the use of the compounds to treat various disease states, such as anaemia associated with inflammation, with rheumatoid arthritis and with sideroblastic anaemia, which are ACD conditions and are some of the ACD conditions referred to in the Family B application WO 121 itself. 196. In addition paragraph [0072] of WO 997, which is one of the places in which ACD conditions are mentioned, also specifically proposes that the compounds may also have an effect on iron transport, processing and utilization. The paragraph is as follows: “The invention also contemplates increasing iron transport, processing, and utilization using the methods of the invention. (See, e.g., commonly owned, copending U.S. Patent Application No. ____, entitled ‘Stabilization of Hypoxia Inducible Factor (HIF) Alpha,’ filed of even date, and incorporated herein by reference in its entirety.) Specifically, the methods of the invention may increase enzymes and proteins involved in iron uptake, transport, and processing. Such enzymes and proteins include, but are not limited to, transferrin and transferrin receptor, which together facilitate iron transport to and uptake by, e.g., erythroid tissue, and ceruloplasmin, a ferroxidase required to oxidize ferrous iron to ferric iron. As transferrin can only bind and transport ferric iron, ceruloplasmin is important for supply of iron to tissues. The ability of the methods of the invention to increase both endogenous erythropoietin and transport and utilization of iron in a single course of treatment provides benefits not addressed by current anemia therapeutics, such as administration of recombinant erythropoietin, in the treatment of anemic disorders including, but not limited to, rheumatoid arthritis, sideroblastic anemia, etc.” 197. Bearing in mind the experimental results in the Family B patents, it is notable that transferrin, transferrin receptor and ceruloplasmin are referred to in this passage of the prior art. Also relevant is that the passage refers to the idea of using the compounds to increase both (my emphasis) endogenous epo, and transport and utilization of iron, in a single course of treatment. This is said to provide benefits for anaemia not addressed by current anaemia therapeutics. 198. There was a dispute about what the skilled person would make of this paragraph. The judgment summarises the evidence of the two experts Prof Winearls and Prof Haase: “[555] I have set out the disclosure of WO 997 above. Before turning to the issues on obviousness, it is convenient first to consider in general terms what the skilled team, and in particular the nephrologist, would make of [0072] (quoted in paragraph 133 above). As is common ground, there are no data in WO 997 to support the suggestion that the methods of the invention increase iron transport, processing and utilisation. Prof Winearls described this as “a very bold claim” that was “totally unsubstantiated”, but he accepted that it would have been an interesting one. Prof Haase agreed that, as a scientist, he would want to see data before accepting that the effect was a real one. Prof Winearls agreed that the pre-clinical researcher in the skilled team, who would have known that HIF regulated transferrin, transferrin receptor and ceruloplasmin, would have thought that the statements about HIF-PHIs increasing the amount of transferrin, transferrin receptor and ceruloplasmin were plausible. On the other hand, Prof Haase agreed that none of transferrin, transferrin receptor and ceruloplasmin had been implicated in 2004 as a cause of iron deficiency or ACD or anaemia in general. ” 199. FibroGen emphasised Prof Winearls’ evidence that the suggestion in WO 997 that the compounds would increase iron transport (etc.) was a “very bold claim” etc. but it can be seen that the judge had this well in mind, and noted that the witness had also accepted that it was interesting and that a pre-clinical researcher in the skilled team would have thought the statements were plausible. FibroGen make the point that plausibility is not the test for obviousness, which is true of course, but there is nothing wrong in the judgment characterising that evidence at this stage in that way. 200. Turning to the differences between the prior art and the claims, these were accurately summarised in the judgment, as follows: “556. The differences between WO 997 and the claims in issue depend on which claim one is considering, but in essence the difference in each case is that WO 997 does not expressly disclose the therapeutic use claimed. There are four such uses: (i) use in treating ACD in subjects with TSAT less than 20%/16% in adults, (ii) use for treating anaemia that is refractory to exogenous Epo, (iii) use in treating functional iron deficiency associated with anaemia and (iv) use for decreasing hepcidin expression. I will consider the obviousness of these in turn. 201. Next at [557] the judgment holds as follows: 557. Before doing so, however, I should address the Claimants’ over-arching point that the obvious way forward for the skilled team reading WO 997 in April 2004 would be to investigate the use of HIF-PHIs for the treatment of anaemia of CKD, and that in order properly to test that they would want to exclude co-morbidities and ensure that patients were iron replete. Unsurprisingly, Prof Haase agreed that that would be an obvious course to adopt. It simply does not follow, however, that other possibilities were not obvious.” 202. This is an application of the well-established principle found in Brugger v Medicaid [1996] RPC 635 and now approved by the Supreme Court in Actavis v ICOS that just because one thing is an obvious way forward does not necessarily mean that other ways forward are not obvious too. The important word is necessarily. In some cases one dominant approach may crowd out other possibilities, but in other cases it may not. It all depends on the particular facts and circumstances. There is no error here. 203. Then at paragraph [559] there is a finding that it was obvious to use the compounds disclosed by WO 997 for the treatment of ACD. Given that WO 997 expressly proposes using the compounds for disease conditions which the skilled person would understand as conditions of ACD, and given paragraph [557] on the Brugger v Medicaid point, this conclusion is not surprising and was manifestly open to the court. It cannot be gainsaid on appeal. On its own it does not mean the claims are invalid - that is why the four further features such as <20%/<16% TSAT matter - but it provides important context. 204. The judgment then turns to address the claims based on the first use: treating ACD in subjects with TSAT less than 20%/16% in adults. The reasoning has a number of steps. First (paragraph [559]) that it was obvious to use the compounds in WO 997 for the purposes of treating ACD. Second (paragraphs [560] - [562]) that this would inevitably involve treating at least some ACD subjects with a TSAT at the claimed levels. As part of this finding the judgment also concludes that since some patients with TSATs below 16% were treated with ESAs and since the compounds are disclosed in WO 997 as an alternative to ESAs, that is another reason why they would be administered to such patients. 205. Then [563] addresses FibroGen’s case that the Family B patents disclose an unexpected beneficial property of the compounds concerning iron delivery over ESAs. The paragraph points out that the patents do not show such a benefit but then accepts Akebia’s case that even if such a benefit does exist, it would be discovered as a result of taking obvious steps during the treatment of ACD with the compounds. 206. Therefore the TSAT level claims are obvious, and the judgment moves on to consider the second use, for treating anaemia refractory to exogenous epo. The conclusion is that that too is obvious because WO 997 explicitly discloses the idea of using the compounds as alternatives to ESAs (paragraphs [565] - [566]). Then at [568] - [570] the judgment concludes that the functional iron deficiency claims are obvious as well because the disclosure in WO 997 to use the compounds to treat anaemia associated with defects in “iron transport, processing or utilisation” is a teaching to use it to treat functional iron deficiency. That is what those conditions are. A point on the mode of action is rejected at [569] because it is not a claim feature. The appeal on obviousness 207. FibroGen’s grounds of appeal take five points, The first one is that the conclusions on obviousness are inconsistent with conclusions reached on the quia timet infringement side of the case (grounds paragraphs 13-18). The second is a submission that the judgment misconstrues the functional iron deficiency claim because that claim in fact does include the mode of action and so paragraph [569] is wrong. The third is that the finding on inherency amounted to an unpleaded novelty case which had been disclaimed and was not open to Akebia. Moreover the inherency finding was contrary to the law as laid down in Mobil/Friction Reducing Additive G2/88. The fourth was that judgment is in error because it does not ask whether the skilled person would have had any relevant expectation of success that the HIF PH inhibitor compounds could successfully be used to treat the patient groups identified in the Family B Patents. Instead the reasoning adopts a stepwise approach contrary to authority. The fifth ground was that judgment mischaracterises the contribution of the Family B patents, wrongly finds (at [563]) that the patents do not show that the HIF PH inhibitors confer a benefit over ESAs in terms of iron delivery, and wrongly finds (at [569]) that the patents do not provide evidence of the mode of action referred to in the context of the functional iron deficiency claims. 208. There is no appeal from the conclusions relating to the claims characterised by the fourth use - for decreasing hepcidin expression. The grounds of appeal are directed to the claims limited by the other three uses. 209. Appeals on obviousness often fail because obviousness is a multifactorial assessment balancing all the evidence (Biogen v Medeva [1977] R.P.C. 1). Moreover by the end of a trial in the Patents Court, and despite the rhetoric of the parties, a conclusion either way on obviousness is frequently open to the court. In such a case, absent an error of principle, there is little scope for a successful appeal. 210. The first ground of appeal, that the conclusions are inconsistent with the findings on quia timet infringement, is capable of being such an error of principle. The point has a superficial attraction. One can characterise the issue this way. The obviousness finding is that it was obvious for a skilled team involving clinicians concerned with anaemia to use the HIF PH inhibitor compounds disclosed in Family A to treat CKD, as drugs to treat ACD. Whereas the rejection of the quia timet infringement case was on the basis that given Phase III clinical trials showing the utility of HIF PH inhibitor compounds in Family A for the treatment of CKD, it would not be “obvious to a reasonable person in the circumstances” (part of the test for indirect infringement in s60(2) of the 1977 Act) that that compound would be prescribed by doctors to treat ACD. 211. However the submission breaks down as soon as the detail is examined. The obviousness question is concerned with what the notional skilled team, that is a team working in clinical research and development in 2004, would do given an item of prior art which does not just focus on CKD but also expressly makes reference to conditions which relate to ACD. The infringement question starts from a different place altogether. As a quia timet claim, it is concerned with whether it can be shown today that Akebia or its licensees are threatening to market vadadustat at some future date in circumstances in which they will know or it will be obvious to a reasonable person in the circumstances that the drug is suitable for putting and intended to put the claimed inventions into effect in the United Kingdom (judgment paragraph [590]). There is not yet a marketing authorisation for vadadustat but the issue proceeded on the assumption that when it was granted it would be expressly limited to CKD and, pointedly, would not authorise the use of the drug for ACD [594]. The states of mind of two different persons are in issue [591]. Neither of them is a notional clinical R&D team. The question starts with what either the real pharmaceutical sales team at Akebia or its licensees (or a reasonable team) would think. The question about what is obvious to them is not the invention, it is what real clinicians are likely to do with a drug which is actually on the market for CKD and whether it is foreseeable that those doctors will prescribe it off-label for ACD. 212. Thus the two sets of findings are simply not comparable. I would dismiss the first ground of appeal. 213. From here it is convenient to focus on the grounds in the following order: first deal with the third ground (unpleaded lack of novelty), then the fifth ground (technical contribution), then the fourth ground (expectation of success) and finally the second ground (functional iron deficiency claims, and at the same time address the refractory to epo claims). 214. To recap, the starting point of the reasoning on what is obvious is the unassailable finding at [559] that it was obvious to use the compounds disclosed by WO 997 for the treatment of ACD. 215. The next step is the finding that in doing this, ACD patients with TSATs within the relevant claims would in fact be administered the compound. That is the finding in [560] to [562] based on how ACD patients were treated at the time (up to 2004). The concluding paragraph [562] is as follows: “562. The Defendants contend that, given that it was obvious from WO 997 to use the disclosed HIF-PHIs to treat ACD, then it follows that the skilled team would inevitably be treating at least some subjects with a TSAT at the claimed levels. I accept this. In any event, Prof Haase’s evidence was that patients with a TSAT of less than 20% or 16% were treated with ESAs, including some patients whose TSAT dipped below those levels due to diurnal or periodic variation. Given that HIF-PHIs are disclosed by WO 997 as an alternative to ESAs, they would be administered to patients with the relevant TSAT levels.” 216. FibroGen’s main challenge to this on appeal was the inconsistency with the infringement argument which I have rejected. The conclusions at judgment [562] contain no error. They include that the skilled team would inevitably be treating ACD patients with TSAT levels below 20% and below 16% and also that, since WO 997 discloses the compounds as alternatives to ESAs (and given that ESAs were in fact used to treat ACD as had been held previously) the compounds would be administered to ACD patients with the relevant TSAT level. 217. It is true that there was evidence the other way, and on appeal FibroGen took us to evidence (such as from Dr Ashman) that he had expressed the view that giving epo to iron deficient patients was a waste of time and money. FibroGen says it was clearly established even at 2004 that you would look to raise TSAT levels about 20% before giving a patient an ESA. However the finding to the contrary in paragraph [562] was plainly open to the court for the reasons that paragraph explains. 218. The second attack on paragraph [562] is the third ground of appeal. It is said to be vitiated because it amounts to a finding of lack of novelty on an unpleaded basis which was not open to Akebia and which was in error anyway because it failed to take account of law arising from the Mobil line of cases. 219. It is wrong. The simple answer is that there is no finding of lack of novelty at all and therefore no finding not open to Akebia to propose or the court to reach. The reason why not is because the whole of [562] follows from the decision at [559] that it was obvious to use the compounds for purposes including the treatment of ACD. Everything else follows from that. The sort of novelty argument FibroGen is referring to works as follows. Assume the prior art WO 997 actually discloses the use of the compounds for actually treating ACD. Consider the finding in [562] that patients treated would inevitably include some with TSATs within the claim. Therefore, goes the alleged novelty argument, this is a conclusion that the claim lacks novelty based on the principle of inevitable result (General Tire v Firestone [1972] RPC 457). However it is quite apparent that the judgment does no such thing. And in case this is misunderstood, in setting out the argument to explain what is going on here, I am not endorsing it. 220. Furthermore, if such an argument had been made, and if the judgment had approached it that way, then I agree with FibroGen that reasoning of that sort might engage the Mobil line of cases, raising questions which have troubled the courts all over the world for years. The submission would be that if (as FibroGen contends) the Family B patents disclose a new technical benefit such as an effect on iron metabolism useful for treating disorders of iron metabolism, and if the court was dealing with novelty, then the claims containing an appropriate limitation to that effect might still be valid even if the beneficial effect was in fact being achieved when what was disclosed in WO 997 was practised by the skilled team, albeit unbeknownst to them. So in Mobil a claim to the use of an engine oil additive for reducing friction was not anticipated by a disclosure to use the additive in the same way in engine oil, but for the allegedly different purpose of inhibiting rust, in circumstances in which the lubrication effect would inevitably have occurred albeit supposedly unbeknownst to the skilled person. 221. However, it is not desirable to get into this unless necessary, and it is not necessary because the judgment does not proceed that way either expressly or by any sort of inherent implicit logic. The judgment in fact addresses the beneficial effect in the very next paragraph [563] and that is the subject of the fifth ground of appeal. 222. There is one more issue arising on the third ground of appeal, relating to patient sub-populations. FibroGen’s submission on this ground specifically makes the point that the fact that a patent discloses a different patient subgroup would satisfy the novelty test anyway. All I will say is that I agree that there can, in a proper case, be novelty in the context of second medical use inventions in the identification of a specific patient sub-population. However since the judgment does not make a finding of lack of novelty on this basis, whatever the legal principle is, it is irrelevant. 223. I can now turn to the fifth ground of appeal. The argument relates to the next step in the reasoning at [563]. FibroGen submits that the judge mischaracterised the technical contribution. If correct, this would be a point capable of undermining a conclusion of obviousness. The argument is focussed on the first sentence of judgment paragraph [563], as follows: “As the Defendants point out, the Family B Patents do not show, or even attempt to show, that HIF-PHIs confer any benefit over ESAs in terms of iron delivery.” 224. This is submitted to be “just plain wrong” on the basis of the arguments summarised below, because in fact the compounds do exhibit a different effect on iron levels from those produced by ESAs. 225. The point is put in different ways. It is submitted that the judge failed to understand the contribution the patents make by failing to take into account Prof Winearls’ unchallenged evidence that there was an additional effect of giving the compounds to patients with iron deficiencies that is neither predicted nor disclosed in the prior art. We were taken to paragraph [0223] of one of the Family B patents EP 333. This discusses hepcidin expression data reported as part of Example 17. It states: “As shown above in Table 4, administration of compound A', resulted in reduced expression of hepcidin mRNA in mouse liver. Decreased hepcidin expression is associated with increased iron release from reticuloendothelial cells and increased intestinal iron absorption. Therefore, compounds of the present invention are useful for decreasing hepcidin expression and increasing intestinal iron absorption.” [A' added] 226. This is the disclosure which Prof Haase agreed was plausible. 227. We were also taken to Example 19, in which serum iron levels are being increased by Compound A'. Counsel contended this was clear evidence of the unexpected effect of the compound having the opposite effect on iron levels to the one produced by ESAs. This also is said to explain why the compounds are useful to treat patients with ACD functional iron deficiency and patients who were refractory to epo. All in all it is said to go to support FibroGen’s case that the technical contribution should have been recognised as follows: “that HIF prolyl-hydroxylase inhibitors of Formula (I) have a beneficial and unexpected effect on iron metabolism, such that they can be used to treat disorders of iron metabolism, and, in particular, the three particular disorders which you have seen in the claims.” 228. The problem with all this, as Akebia’s counsel submitted, is that the judge clearly did understand what FibroGen contended was the technical contribution but reached conclusions which rejected it as a matter of fact and on a basis which was open to the court. To see how and why requires one to pull together a number of different findings and passages. 229. First in relation to the common general knowledge of hepcidin. The judgment contains a thorough review of the evidence on hepcidin and common general knowledge at [526] to [543]. One of the conclusions, as FibroGen emphasise, is that the link between HIF and hepcidin was not known, but the other, based on Prof Haase, was that it had been proposed that epo might be involved in the transcriptional downregulation of hepcidin (paragraph [542]). That conclusion was open to the court and cannot be undermined on appeal. Its significance, looking ahead, is that it might show that if downregulation of hepcidin is observed, it could be an effect of an ESA. 230. Next, in relation to Example 17 and what the Family B patents actually disclose. The judge held that Example 17 does show the down-regulation of hepcidin in mice when treated with Compound A' however, crucially given the way the argument is put, the judge also held that: “[496] … The figures reported do not appear to show a clear trend, however, and there is no statistical information. As Prof Haase explained, and Prof Winearls accepted, there is nothing to demonstrate that this effect is independent of erythropoiesis, although both witnesses considered that this was a possibility.” 231. The second sentence is particularly important because it bears on the issue of whether it has been shown that the effect of the HIF-PH inhibitor compounds on iron delivery is indeed something independent of their utility as ESAs. 232. Then paragraph [501] concludes in relation to the Family B patents that they do not contain any data comparing the effects of HIF-PH inhibitors to those of ESAs and no data showing that any of Compounds A' to D' have superior effects on iron metabolization to ESAs. These findings were open to the court. 233. Thus, what matters is that paragraph [563] is not rejecting an absolute question about whether the compounds do or do not have an effect on iron delivery, it is rejecting a relative question about the compounds’ properties as compared to ESAs. What is rejected is whether any such effect confers a benefit over ESAs, and that matters because it was the alleged difference between the behaviour of the compounds and ESAs which was the basis for the alleged unexpectedness of the effect. In this way the finding said to be “just plain wrong” in the first sentence of judgment paragraph [563] is a conclusion the court was entitled to reach, accepting Akebia’s submission in doing so. The judgment does not mischaracterise the technical contribution. It rejects the patentee’s case on what the technical contribution is, in a manner soundly based in the evidence. This is the core point of the fifth ground of appeal. I reject it. 234. The next ground of appeal is the fourth ground. It relates to the remainder of judgment paragraph [563] which addresses what tests the skilled team would do when they were embarking on a programme of treating ACD using HIF-PH inhibitors. Paragraph [563] is as follows: “563. As the Defendants point out, the Family B Patents do not show, or even attempt to show, that HIF-PHIs confer any benefit over ESAs in terms of iron delivery. If and in so far as there is such a benefit, however, the Defendants contend that this would have been discovered by the skilled team by taking obvious steps. As Prof Winearls accepted, the skilled team would have been motivated by WO 997 to do some relatively straightforward tests. These would have included the transferrin and transferrin receptor tests in animal cells in WO 121, which would (if WO 121 is correct in its assertions) have shown an “enhancement” of erythropoiesis through increasing iron transport etc. This would have led to the other tests done in WO 121 to measure iron uptake, including measuring haemoglobin levels, and then ultimately comparative tests on iron uptake over ESAs. The Defendants submit, and I agree, that, taken as a whole, Prof Haase’s evidence was also consistent with this.” [emphasis added] 235. The relevance of this reasoning to the obviousness case is that the conclusion reached is that even if there really is an unexpected beneficial effect of HIF-PH inhibitors over ESAs, that beneficial effect would be discovered by the skilled team without any inventive step because the team would do tests the same as the tests reported the Family B patents, which would lead to more tests. Overall this would lead to the skilled team finding out the existence of the alleged beneficial effect (if that is what it is). That is an example of the well-established “bonus effect” problem characterised clearly in Hallen v Brabantia [1991] RPC 195 and confirmed in Actavis v ICOS (Lord Hodge paragraph 73). Unexpected beneficial effects are the sort of thing which usually lead to inventions and valid patents but, no matter how unexpected a beneficial effect is, if in fact it would present itself to the skilled person who took obvious steps over the prior art for a different purpose, then the existence of such a beneficial effect will not provide a basis for a valid patent. 236. The fifth ground of appeal is that this conclusion about tests which the skilled team would perform, particularly the latter set which are comparative tests with ESAs, is flawed because it does not ask or answer the question whether the skilled team would undertake these tests with a reasonable prospect of success. While the question of prospects of success was addressed in the judgment in relation to the Family A patents, FibroGen submit it was ignored here and so the judgment never asks the right, statutory, question. Moreover it is said to be a classic stepwise analysis deprecated in Technograph v Mills & Rockley [1972] RPC 346 (HL). 237. The law relevant both to the stepwise analysis and on expectation of success is clearly stated in Actavis v ICOS by Lord Hodge. Stepwise analysis was addressed at paragraph [72]. There is no need to set it out. Lord Hodge explained that the Technograph warning against stepwise analysis has no bearing in a case in which the steps which the notional skilled person would take can readily be ascertained without the taint of hindsight. 238. Then, on expectation of success, the relevant passages of Lord Hodge’s judgment are [63] to [69]. Questions like whether something is obvious to try and what the expectations of success of a given avenue are, are relevant. However the law cannot be summarised as simply as saying that for any test which the skilled team might undertake, the outcome only matters if the test was obvious to try with a reasonable prospect of success. Lord Hodge explained this at paragraph [65]: “[65] First, it is relevant to consider whether at the priority date something was ‘obvious to try’, in other words whether it was obvious to undertake a specific piece of research which had a reasonable or fair prospect of success: Conor v Angiotech (above) para [42] per Lord Hoffmann; MedImmune Ltd v Novartis Pharmaceuticals UK Ltd [2012] EWCA Civ 1234, [2013] IP & T 536, [2013] RPC 659 (paras [90] and [91]) per Kitchin LJ. In many cases the consideration that there is a likelihood of success which is sufficient to warrant an actual trial is an important pointer to obviousness. But as Kitchin LJ said in Generics (UK) Ltd (t/a Mylan) v Novartis AG [2012] EWCA Civ 1623, [2012] All ER (D) 126 (Dec) (para [55]), there is no requirement that it is manifest that a test ought to work; that would impose a straightjacket which would preclude a finding of obviousness in a case where the results of an entirely routine test are unpredictable. As Birss J observed in this case (para [276]), some experiments which are undertaken without any particular expectation as to result are obvious. The relevance of the ‘obvious to try’ consideration and its weight when balanced against other relevant considerations depend on the particular facts of the case.” 239. Before leaving Actavis v ICOS, it is also worth highlighting that the outcome of the case itself was an example of a finding of obviousness as a consequence of the results of routine tests undertaken as part of a mammoth but obvious drug development programme which followed from the prior art without hindsight. 240. With these principles in mind, I turn to the fifth ground starting with the submission that the judgment involves an impermissible stepwise approach. The reasoning as a whole, and the particular part of it in paragraph [563] contains no such error. The finding was that the steps involving the test were obvious given the motivation in WO 997 itself. That was clearly open to the court on the evidence. I reject FibroGen’s submission. 241. On expectation of success, it is true that if one focusses on the partial sentence in paragraph [563] which relates to what were ultimately the comparative tests on iron uptake over ESAs, the words “expectation of success” do not appear. But there is no error here either. The whole finding about tests begins with the statement that the skilled team would be motivated by the prior art WO 997 to do some relatively straightforward tests. Thus contrary to FibroGen’s case there is a finding about motivation. Moreover the tests concerned are relatively straightforward. 242. Now there is a distinction to be drawn between the tests which are the same as those in the Family B patents which are not comparative with ESAs (hence why the patent does not show that there is a beneficial effect compared to ESAs) and further tests which do compare the activity of the compounds to ESAs. Recall that in paragraph [563] the tests in Family B are referred to as the tests in WO 121. They are the tests on transferrin etc. 243. However this difference is clearly reflected in paragraph [563] and explains why the ultimately comparative tests with ESAs appear in the paragraph in the location they do. Counsel for Akebia took us to the passage in cross-examination of Prof Winearls in which he accepted that once one they had done the same tests as are in the Family B patents, if the skilled team had thought they had a drug which would have an improved iron uptake over ESAs, it would have been obvious to carry out those comparative tests. 244. Now it is true to observe that the premise put to Prof Winearls was that the comparative tests would be obvious if the skilled team had thought they had a drug which would have an improved iron uptake over ESAs. However this too is reflected in the reasoning in [563] because the conclusion is premised on the idea that what happens when the Family B transferrin etc. tests are undertaken is that an enhancement of erythropoiesis through increased iron transport would be seen if Family B is correct in its assertions. 245. In other words, as judgment reflects, the patentee is firmly on the horns of a dilemma. FibroGen asserts that the results in the patent support a technical contribution based on the idea that the compounds have an effect which confers a benefit as compared to ESAs. But there are no comparative tests in it. If the patent is wrong in its assertions then there is no useful technical effect and it lacks inventive step for that reason. However if it is right in its assertions then, since the skilled team would get as far as what is in the patent, based on what was taught in WO 997, without invention the skilled team would also do the comparative tests and discover the comparative effect. 246. The reasoning in paragraph [563] is compressed but none the worse for that. All the conclusions were open to the court on the evidence and I reject the fifth ground of appeal. 247. That leaves the second ground of appeal. For this it is convenient to address the claims in the same order as the judge, starting with claims to the use for treating anaemia which is refractory to epo, and then addressing the functional iron deficiency claims. 248. The basis for the claims to treating anaemia refractory to exogenous epo is closely related to FibroGen’s case on technical contribution that these compounds have a beneficial effect distinct from any action as a substitute for an ESA. So if giving a patient an ESA such as exogenous epo did not work, how, one asks rhetorically, could it be obvious to administer an agent which worked in the same way? The answer in the judgment [565] is simply that WO 997 itself, and paragraph [0072] in particular, explicitly proposes that the HIF-PH inhibitors are alternatives to treatment with current anaemia therapeutics such as recombinant epo. As the judgment also holds at [566] the fact that clinical practice was that if a patient was found to be refractory to epo then epo treatment should cease does not undermine the obviousness case when the content of the prior art WO 997 is taken into account. 249. Turning to functional iron deficiency, the main problem for FibroGen is the familiar one, as put by the judge in [568] (my emphasis): “[0018] of WO 997 expressly teaches use of the compounds of the invention for the treatment of anaemia “associated with defects in iron transport, processing or utilisation” i.e. functional iron deficiency. The same message appears from [0072]. Yet further, as discussed above, ACD involves functional iron deficiency. The Claimants do not suggest that the mode, format or dosage involved in treating functional iron deficiency in accordance with the Family B Patents is any different to those involved in treating ACD. Nor, as discussed above, is it a requirement of the relevant claims that there should be any increase in iron parameters. It follows that WO 997 also makes it obvious to use the compounds for the treatment of functional iron deficiency associated with anaemia.” [emphasis added] 250. That would be enough to dispose of the claims limited to the use to treat functional iron deficiency subject to a point on claim construction, the submission that the claim requires a particular mode of action. This is dealt with in [569]: “The Claimants submitted that the relevant claims require the HIF-PHIs to act “by overcoming the reticuloendothelial block which prevents the release of iron from stores”. This is not a feature of the claims, however. Nor is there is any evidence in the Family B Patents that HIF-PHIs achieve this. In any event, even if it did happen, it would be an inherent effect of administering the compounds of WO 997 to an ACD patient. ” 251. On appeal FibroGen repeats the point of construction and also contends that the answer at the end of the paragraph about inherent effects, is contrary to Mobil. 252. The issue is about claim 22A of EP 333 and it is worth setting that out, as follows: “A compound of formula (I) that stabilizes HIFα for use in treating functional iron deficiency in a subject, and wherein the functional iron deficiency is associated with anemia wherein A, B, Q, R1, R2, R4, Y and X are as defined in claim 1.” 253. Obviously the mode of action (overcoming reticuloendothelial block etc.) is not spelled out in the claim. However the argument is that it was thought that functional iron deficiency was caused by a reticuloendothelial block which prevented the release of iron from stores. It is not necessary to examine that in detail. It is explained in paragraph [58] of the judgment which is quoted above at the start of the section on Family B. But none of this means that, as a matter of claim construction, it necessarily follows that the skilled person would understand the inventors to have used the words “functional iron deficiency” in claim 22A to include a mandatory requirement that the treatment of that condition had to take effect by reference to a particular physiological mechanism. I agree that, as a matter of construction, the mechanism is not a feature of the claim. 254. As Akebia pointed out in argument, imagine if later on it was found that contrary to current assumptions HIF-PH inhibitors or some of them actually improved the delivery of iron by a mechanism which did not overcome reticuloendothelial blockade but (say) worked by enhancing iron uptake in bone marrow. In that case the drugs when used for the treatment of functional iron deficiency would still infringe the claim, for the very reason that as it stands there is no reference to the mechanism. This illustrates why it makes sense for the patentee not to have written the mechanism into the claim. 255. Thus paragraph [569] is right on the construction of the claim and I would dismiss this ground of appeal on that basis. 256. In the last sentence of [569] the judgment goes on to find that if overcoming the blockade was an inherent and necessary feature of the claim then the claim would be invalid anyway for inherency. I see the point, but to address it properly may (or may not) engage the Mobil principle and I prefer not to get into that as it is not necessary to do so. 257. Finally, I will stand back. Addressing the grounds of appeal involves examining individual parts of the overall reasoning on the obviousness of the Family B patents with some care. Reviewing the conclusions as a whole I can see no error of principle at all nor any finding of fact not open to the court on the evidence as it was. I would reject this appeal on obviousness. Does vadadustat fall within Formula I? 258. The issue is about claim construction. The legal principles are not in dispute. As paragraph [263] of the judgment correctly explained: “263. Since this is a question of the interpretation of a chemical formula, the relevant skilled person through whose eyes it must be considered is the medicinal chemist. It was addressed by all three medicinal chemists in their evidence. As will appear, however, the issue of interpretation depends not on medicinal chemistry, but on the structure of the lists of substituents, and in particular the manner in which the relevant list is punctuated and laid out, and on the way in which certain substitutions are described, and in particular the fact that some groups are said to be “optionally substituted” without more. All three medicinal chemists agreed in cross-examination that there was no chemical reason why the substitution in question should or should not be permitted. It follows that their opinions as to the correct interpretation of Formula (I) are all inadmissible.” 259. The conclusion was that vadadustat falls within Formula I on a normal construction. Akebia challenges that conclusion. Formula I is shown below: Formula I [In EP 823 and the judgment the numerical indices are superscripts rather than subscripts but nothing turns on that. In this judgment I will use superscripts.] 260. This formula specifies a certain chemical structure which is mandatory. It includes the various lines without letters which are part of normal chemical notation. The unlabelled junctions between the lines represent carbon atoms and lines represent chemical bonds. The normal notation also includes the letters N and H, which refer to nitrogen and hydrogen. The letters A, B, R1, R2, Q-R4, X and Y work in a different way. They do not have a fixed conventional meaning but are labels which are then defined in the remainder of the formula, not shown above. The definitions, which are what paragraph [263] is referring to as the lists of substituents, are exceptionally wordy and lengthy. The judgment sets the whole thing out in an appendix. There is no need to set it all out on appeal. 261. The heterocycle is the hexagonal structure on the left. The carboxamide substitution is on the right coming off the ring. X is defined as being oxygen or sulphur. The two lines above the X indicate a double bond to the carbon atom. Carbons have four bonds as can be seen for that carbon with the double bond and the two other lines coming out on either side. Bonded to the carbon on the right is a nitrogen (N). Nitrogen has three bonds. In this case the top one is to a hydrogen H and the third one is down to the right to A. When X is oxygen (O) this collection of atoms is a carboxamide. The fact that X could be sulphur (S) does not matter because S is chemically related to O. 262. Thus it can be seen that whatever atoms and groups are specified by the letters A, B, R1, R2, Q-R4, X and Y, the molecules will be heterocyclic carboxamides. The debate turns on the definitions of those letters and the structure of that definition, as the judgment explains starting at [265]. The whole definition runs from p34 ln12 to p39 ln3. The judgment carefully analyses the structure of that definition as a whole by identifying it as being divided into a hierarchical arrangement of parts, sections and blocks. The blocks make up a section, the sections make up a part and the parts make up the whole definition. This arrangement of parts, sections and blocks is a convenient way of looking at the definition. The structure the judgment identifies is rightly not challenged on appeal. 263. There as six parts which, in order, define A, B, X, Q-R4, Y and finally R1, R2, R3 together. Note that R3 derives from Y. The part which defines of R1, R2, R3 is from p36 ln35 to p39 ln3. The case is about that part. It is clear that the part defining R1, R2, R3 is divided into six sections (judgment [271]). The first section runs from p36 ln36 to p38 ln35. The case is about that first section. The first section is divided into a series of blocks (judgment [273] - 279]). 264. At this point it is useful to see the relationship between Formula I and vadadustat: Formula I vadadustat 265. What is important for infringement is what is on the left of the formulae. There is no doubt that the various atoms and groups on the right in vadadustat satisfy Formula I. It is also clear that R2 in Formula I allows for a second hexagonal ring (an aryl group) as on the left in vadadustat. This is the result of a passage which is in the first block of the first section of the part which defines R1, R2, R3. It starts at p36 ln 36 and the key passage is at line 40, as follows: “R1, R2 and R3 are identical or different and are …. (C6-C12)-aryl …”. 266. The issue is the chlorine atom substituted in the left hand aryl group in vadadustat. The judgment concludes that that is covered by Formula I, Akebia contends it is not. 267. The question is about a further passage which makes up the last block in the same first section of the part which defines R1, R2, R3. This passage states “where an aryl radical may be substituted by 1 to 5 substituents selected from … halogen …”. It is not in dispute that this language provides that, for whatever aryl groups it is referring to, those groups may be substituted with a halogen. Chlorine is a halogen and so, if this language applies to the left hand aryl group in vadadustat, then vadadustat falls within the claim. The debate is about which aryl groups the language applies to. Based on the way Akebia put it in argument I will refer to this language as “the Permitted Aryl Language”. 268. The expression “aryl” appears frequently in the definition as a whole. Sometimes when that language is used, there is no suggestion, at the point at which it is used, that the relevant group may be substituted. The example given in the judgment [280] is at the very start of the definition in the part dealing with label A. Another example would be reference to aryl in the first block which is said to cover vadadustat. On other occasions the definition expressly refers to the possibility that the aryl group could be substituted. These occasions can be sub-divided into two types as the judgment explains in paragraph [281]. With Type 1 the language expressly defines the possible substituents by number or type (such as “wherein radicals which are aryl or contain an aryl moiety, may be substituted on the aryl by one to five identical or different hydroxyl, halogen …” which appears in the part defining label B (p35 ln 10-40). With Type 2 the aryl group is merely said to be “optionally substituted” but without an indication as the nature of the substitution. Judgment paragraph [281] notes three instances of this language in the definition as a whole. One is at p37 ln25-26 and therefore within the third block of the same relevant first section of the part which defines R1, R2, R3. One is within the part defining Q-R4 (at p36 ln32-33) and the other is in the second section of the part which defines R1, R2, R3. 269. Akebia’s case here and below is that the Permitted Aryl Language relates and only relates to those three Type 2 instances. Akebia’s main point is that this makes sense because it would provide a limit on what would otherwise be an unbounded definition in Type 2. 270. The judgment deals with the rival submissions and reaches the conclusion in favour of FibroGen. The reasoning starts by recognising Akebia’s submission about the logic of having a limit on Type 2: “282. The Defendants contend that there must be some limit to the possible substitutions encompassed by Type 2, whereas the Claimants contend that there is no limit. In my view the skilled person would assume that some limit was intended, since otherwise the formula would embrace compounds that would be practically impossible to make and/or insoluble and a formula that covers a staggeringly large number of compounds anyway (as explained below) would cover a limitless number of compounds.” 271. However the judgment then turns to address what the skilled person would think the purpose of the Permitted Aryl Language at p37 ln 50 was: 283. What is the purpose of the passage starting at page 37 line 50? Against this background, the key question is what the skilled person would think that the purpose of the passage starting at page 37 line 50 was. The Defendants contend that the skilled person would conclude that its purpose was to provide a “pick list” of substituents which could be used for Type 2 substitutions. As counsel for the Defendants submitted, this interpretation avoids “optionally substituted” being limitless. On the other hand, as counsel for the Defendants accepted, it has the consequence that the “pick list” wording would not only apply to the “optionally substituted” language in the first section of R1, R2 and R3, but also to that language in Q-R4 and in the second section of R1, R2 and R3. That does not fit with the way in which the passage in question is positioned within the overall structure of the definition of Formula (I). [paragraph [284] rejects a now abandoned alternative case] 285. The Claimants contend that the passage starting at page 37 line 50 would be understood to apply generally to the aryl or aryl-containing groups within the first section of the definition of R1, R2 and R3, including “(C6-C12)-aryl”. The Claimants submit that this not only best fits the structure of the definition, but also makes sense because there is no apparent reason why it should only apply to some aryl or aryl-containing groups in the first section of R1, R2 and R3, but not others. On the other hand, it does not provide a limit for “optionally substituted”. 272. The conclusion (at [286]) is that the skilled person would conclude that the Permitted Aryl Language applies to all the aryl groups in the first section of the definition of R1, R2 and R3. Leaving aside the now abandoned alternative case, the reasons are: “286. The conclusion I have reached is that the Claimants’ interpretation is the better one. Although the skilled reader would assume that some limit to “optionally substituted” was intended, there is nothing to indicate that the passage in question was intended to supply that limit. The skilled reader would consider that the most important factor was the structure of the definitions, and in particular the structure of the definition of R1, R2 and R3. […]” 273. This reasoning cannot be faulted. In my judgment, although the definition as a whole looks daunting, as the judgment explains it does have a structure and when that structure is understood, as the skilled person would, the question only has one sensible answer. The Permitted Aryl Language is located at the end of the first section, and makes sense as referring generally to the aryl or aryl-containing groups within that the first section. It does not make sense at all as a qualification to the instance of Type 2 language which appears in a completely different part of the definition nor does it make sense as referring to the Type 2 passage in a later section within the same part dealing with R1, R2 and R3. 274. The fact that it leaves aspects of the definition as a whole unconstrained is true but does not trump the clear structure of the language. 275. Akebia take a point that the Formula I definition is clearly intended to be more limited than the wide words in claim 1 which relate to heterocyclic carboxamides in general, with any substituents. I agree with that submission but it does not assist Akebia. Formula I is a very wide definition but it is still narrower than simply “heterocyclic carboxamides” or what I have defined as heterocyclic carboxamides of the claimed structure, and that remains true whether Formula I is unbounded in certain respects or not. There will always be compounds excluded by Formula I, irrespective of this debate, which would be included by the general language of claim 1. Just because two definitions are unbounded does not mean they have the same scope. Far from it. 276. The fact, as Akebia also pointed out, that this approach also renders redundant the words “optionally substituted” in the Type 2 passage within the first section, is also true but does not trump the clear structure of the language. 277. On appeal a further point, not taken below, was advanced by FibroGen to support its construction. The point was about a passage defining permitted substitutions in aryl groups which appears to perform the same function for the part dealing with label B, as the Permitted Aryl Language does for the first section of the part dealing with R1, R2 and R3. The two lists of defined substitutions in aryl groups are similar but not identical. There is no Type 2 passage in the part defining label B and therefore little doubt this passage would be understood to apply generally to the aryl or aryl-containing groups within the relevant part but not otherwise. In oral argument Akebia accepted that that was the right construction of this language in part B. 278. In my judgment this passage does support FibroGen’s case for two reasons. First because it is another instance of a similar passage working in a similar way to the Permitted Aryl Language, and second because, if Akebia’s case was right, then the same logic should apply to this part B language too. Akebia’s case involves the Permitted Aryl Language applying to Type 2 language in a different part (Q-R4) and also to Type 2 language which appears below it in the definition in a later section. If either of these were appropriate, then it is hard to see why the same approach ought not also to apply to the earlier set of permitted aryl substitutions in part B, but that would be rejected by the skilled person. 279. Finally a further point emerged in appeal that in fact there is another place in the definition at which, even on Akebia’s case, there is an unbounded reference to substituents in the ring systems - that is p38 ln 43-45. I would prefer to place no weight on this since it involves very different language from the lists of substituents above. If weight was to be given to this factor it would be a point against Akebia’s case in its own terms since the logic of the submission was to reach a result with no unbounded lists of substituents. However as I say I prefer to place no weight on it. 280. Therefore I would dismiss Akebia’s respondent’s notice. Conclusion 282. I would dismiss the appeal on obviousness of the Family B patents. Thus even though the conclusion on sufficiency applies to those patents as well, they remain invalid for obviousness. 283. I would dismiss the respondent’s notice seeking to overturn the finding below that vadadustat is a compound within Formula I. Sir Christopher Floyd: 284. I agree with Birss LJ as to the disposition of this appeal on validity and the respondent’s notice on infringement. I add a few words of my own because, on the issues of sufficiency, we are differing from a patent judge of enormous experience and distinction, and the issues addressed in this case are of importance to the patenting of inventions in this important area of technology. 285. The judge said at [376]: “Turning to the present case, the patent is implicitly promising that substantially all compounds which satisfy the structural definitions in the claims in issue will have the claimed therapeutic efficacy. Otherwise, the skilled team would be faced with a situation where the structural definition covers around 10183 compounds (or a little less or even more), but the specification only demonstrates that five compounds, namely Compounds C, E, F, J and K, satisfy the criteria for therapeutic efficacy. That would amount to no more than an invitation to the skilled team to find the other compounds covered by the claim which work. It would not involve an inventive step, because it would not solve the technical problem of identifying compounds which have the desired activity, and it would not sufficiently disclose the invention, because it would leave most of the work to the reader.” 286. Earlier the judge had said at [366]: “As counsel for the Defendants accepted, this does not mean that the skilled person or team must be able to identify all compounds covered by the claim without undue burden. Rather, what is required is that the skilled person or team must be able to identify substantially all compounds covered by the claim without undue burden.” 287. I do not agree with the judge’s construction of the claim or with the proposition that, with claims of the kind we are dealing with here, it is necessary for the skilled person, as a cumulative task, to be able to identify substantially all the compounds covered by the claim without undue burden. By “a cumulative task” I mean working one’s way through all the compounds claimed. 288. As to construction, and taking claim 8A as the example, it is wrong to say that the structural features are all that is required to achieve the claimed therapeutic efficacy. The claim is to use of compounds of a particular class which satisfy particular functional requirements which can be tested by appropriate assays or animal models. It is only those compounds which meet these functional requirements which the skilled person would understand to be predicted to have therapeutic utility. The skilled person would understand that mere membership of the class was not enough to predict therapeutic efficacy, not least because he or she would immediately recognise that there were compounds which would not be sensible choices, and the functional requirements would be otiose if compliance with the structural formula was all that was necessary for success. 289. The judge’s reason for rejecting this construction is a comparison between the number of compounds shown by the patent to satisfy the criteria for therapeutic efficacy and the number of compounds covered by the structural formula of the claim. I do not accept that this is a relevant comparison, when, as I have said, the claim is further limited by functional requirements which can be tested by assays and models. The question of whether this imposes an undue burden on the skilled reader falls to be decided at a later stage, and does not justify, far less does it compel, the broad construction adopted by the judge. 290. The judge’s finding on construction made his conclusion on plausibility inevitable, because the patentee did not contend that it was plausible that all the compounds covered by the claim had the claimed therapeutic efficacy. On the correct construction of the claims, however, as Birss LJ has explained, the question of plausibility answered itself. 291. As to the judge’s paragraph [366] there is a danger inherent in posing the question in this way. The Board of Appeals put their finger on in it in T1300/05 RET screening assay/Progenics (11 July 2006). The test for undue burden is not the cumulative burden of identifying and testing for efficacy all the compounds covered by the structural formula, or even by the functional requirements of the claim. That is an objective which is almost bound to produce the response that the burden is excessive. As the Board pointed out, however, in the passage cited by Birss LJ at [94] above, the fact that a skilled reader might choose to accomplish this rather unrealistic task is not the correct question, and cannot found an objection of insufficiency if the skilled person is given all the necessary information in the patent for obtaining those compounds which work. There are clear indications in the judgment that the judge approached the question of undue burden on a cumulative basis, for example his reference in [393] to the fact that an “initial SAR analysis (even if it ran to thousands) would obviously not scratch the surface in terms of the number of permutations envisaged by Formula (1)”. For this, and all the other reasons identified by Birss LJ, if the judge had approached the question of undue burden correctly he would have found the patents met the test for sufficiency. Lord Justice Phillips: 292. I agree with both judgments.