Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales High Court (Patents Court) Decisions
You are here: BAILII >> Databases >> England and Wales High Court (Patents Court) Decisions >> Saint-Gobain Adfors SAS v 3m Innovative Properties Co [2022] EWHC 1018 (Pat) (09 May 2022)
URL: http://www.bailii.org/ew/cases/EWHC/Patents/2022/1018.html
Cite as: [2022] EWHC 1018 (Pat)
[New search] [Printable PDF version] [Help]
Neutral Citation Number: [2022] EWHC 1018 (Pat)
Claim No: HP-2020-000024
IN THE HIGH COURT OF JUSTICE
BUSINESS AND PROPERTY COURTS OF ENGLAND AND WALES
INTELLECTUAL PROPERTY LIST (ChD)
PATENTS COURT
Rolls Building
Fetter Lane
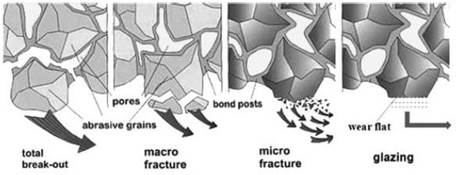
London, EC4A 1NL
9 May 2022
Before :
MICHAEL TAPPIN QC
(sitting as a Deputy Judge of the High Court)
- - - - - - - - - - - - - - - - - - - - -
Between :
|
|
SAINT-GOBAIN ADFORS S.A.S. (a company existing under the laws of France) |
Claimant |
|
|
- and -
|
|
|
|
3M INNOVATIVE PROPERTIES COMPANY (a company existing under the laws of Delaware, United States) |
Defendant |
- - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - -
James Abrahams QC & Michael Conway (instructed by Powell Gilbert LLP) for the Claimant
Michael Hicks & Nicholas Zweck (instructed by Wiggin LLP) for the Defendant
Hearing dates: 30 March - 1 April & 5 April 2022
- - - - - - - - - - - - - - - - - - - - -
Judgment Approved
This judgment was handed down by the judge remotely by circulation to the parties’ representatives by e-mail and release to The National Archives. The date and time for hand-down is deemed to be 10.30 am on 9 May 2022.
the Skilled Person and the common general knowledge.. 4
Abrasive products and abrasive particles. 5
Interpretation of the claims. 25
Invalidity as a result of practising Rowenhorst. 32
Obvious modifications of Rowenhorst. 34
Lack of technical contribution over Rowenhorst. 37
Uncertainty-type insufficiency.. 41
Undue burden to produce anything within the claims. 46
Undue burden to produce particles across the scope of the claims. 50
The Deputy Judge:
1. This is a claim for revocation of European Patent (UK) 2 373 755 (“the Patent”) which is registered in the name of the Defendant (“3M”). The Patent is entitled “Dish-shaped abrasive particles with a recessed surface” and claims a priority date (which is not challenged) of 17 December 2008 (“the priority date”).
2. The Claimant (“SG”) contends that the Patent is invalid on the following grounds:
i) lack of novelty over a 3M patent, US patent 5,366,523, published on 22 November 1994 and entitled “Abrasive article containing shaped abrasive particles” (“Rowenhorst”);
ii) lack of inventive step over Rowenhorst, including an allegation that the Patent does not disclose any plausible technical benefit associated with the products claimed compared to those disclosed in Rowenhorst;
iii) insufficiency, of both the “uncertainty” and “undue burden” types.
The Witnesses
3. SG’s expert was Professor Alan Atkinson. Prof Atkinson currently holds the position of Emeritus Professor of Materials at Imperial College London. He graduated in Natural Sciences from Cambridge University in 1967 and completed a PhD in Physics at Leeds University in 1971, where he then held a research fellowship. Between 1975 and 1995 Prof Atkinson worked at the Harwell Laboratory, becoming Head of Materials Chemistry in 1990, at which point his research included ceramic processing including sol-gel methods. He was appointed Chair of Materials Chemistry at Imperial College in 1995, where he continued research on ceramic processing including sol-gel methods, and subsequently held various positions at Imperial College.
4. Prof Atkinson has published a number of papers on sol-gel chemistry, focussing on the basic science. He also has experience of using sol-gel methods in a variety of industrial applications. That included using boehmite sol-gels to produce coatings for catalyst supports for automotive exhaust systems. However, he did not have any direct experience of manufacturing abrasive particles, whether by the sol-gel process or otherwise. That meant that his evidence as to the issues which might face a skilled person seeking to implement the teaching of the Patent or of Rowenhorst was given without the benefit of such direct experience. I have taken that into account in assessing his evidence. No criticism was made of the way in which Prof Atkinson gave his evidence, and rightly so; he was straightforward and clear in his answers to the questions put to him and at all times sought to assist the court to the best of his ability.
5. SG also led evidence from Benjamin Rowlatt, a senior associate at Powell Gilbert. His statement explained how the Cubitron II particles referred to in Prof Atkinson’s reports were obtained. He was also asked some questions about SG’s experiments on the Cubitron II particles and the Rowenhorst particles (of which, more below) and about the models which had been produced based on those experiments. He was, as one would expect, entirely straightforward in his answers.
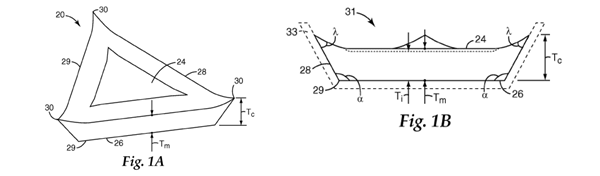
6. 3M’s expert was Dr Mark Schwabel. Dr Schwabel holds a BSc in Ceramic Engineering from the New York State College of Ceramics at Alfred University and a PhD in Ceramics from the same institution. In 1977 he began work as a Senior Research Chemist in the Abrasive Systems Division (“ASD”) of 3M Company (the parent company of the Claimant). In about 1980 he started working on 3M Company’s sol-gel abrasive grains project (which focussed on crushed grain, rather than shaped abrasive grain) and continued working on that project until after his promotion to Technical Manager within ASD in 1990. He held that position until 1997, during which time his focus shifted from the development of the grain itself to developing applications for the grain, including in cut off wheels and other abrasive articles. In 1997 he moved away from working on abrasive grain technology, apart from a period of about a year in 2005-2006 when he once more acted as Technical Manager. However, he continued to work on cut off wheels which used the abrasive grain. He retired from 3M Company in 2016.
7. Until mid-1993, Mr Rowenhorst had worked for the Automotive Aftermarket Division (“AAD”) of 3M Company (during which period the Rowenhorst patent was filed). Following the merger of AAD into ASD, Dr Schwabel supervised Mr Rowenhorst as he worked to evaluate the commercial value of the triangular shaped abrasive grain described in Rowenhorst. However, for practical and economic reasons related to the scale up of the production process, neither the grains themselves nor any products using the grains were commercialised, and the project was abandoned in 1995. Dr Schwabel was not involved in the research conducted by 3M Company in the 2000s into shaped sol-gel abrasive grain which led to the Patent and the other 3M patents discussed below.
8. Dr Schwabel had more experience of manufacturing abrasive particles from boehmite using the sol-gel process than did Prof Atkinson. He also had some indirect knowledge of the issues faced by Mr Rowenhorst when seeking to make triangular shaped abrasive particles. However, I did not understand him to have been closely involved with that work, nor to have any direct experience of seeking to manufacture shaped abrasive particles using the sol-gel process. Again, I have taken that into account in assessing his evidence.
9. Dr Schwabel, as a former long-serving employee of 3M Company, holding shares and stock options and receiving a pension from its pension fund, ran the risk of being accused of failing properly to fulfil the role of an independent expert. No such accusation was made, and again rightly so; Dr Schwabel gave his evidence in an entirely fair and balanced manner and without any hint of partiality. At points he acknowledged that he knew certain facts about what Mr Rowenhorst had done when working on his triangular shaped abrasive grains project, but I am satisfied that he was able to put that out of his mind when answering questions about how the skilled person would have proceeded given Rowenhorst.
10. I am grateful to both experts for their evidence and the assistance they gave me, and to the legal teams on both sides for the way in which they prepared and presented this case.
the Skilled Person and the common general knowledge
11. There was no real dispute as to the nature of the skilled person. The skilled person (who may in reality have been a team of people) would have had knowledge and experience of abrasives, including the production and testing of abrasive articles, and of the fabrication of ceramics, in particular of abrasive particles from boehmite using the sol-gel process, and the characterisation of such particles.
12. The parties produced a document which set out the matters agreed by the parties to be within the common general knowledge (“CGK”) of the skilled person at the priority date. That document also indicated certain matters which one of the parties contended were part of the CGK, but which were not agreed to be CGK by the other party. In fact, by the end of the trial there was no dispute about any material aspect of the CGK. What follows is an edited version of the document produced by the parties, including those aspects which are material to the dispute or necessary to understand this judgment.
Abrasive products and abrasive particles
13. Abrasive products made from abrasive particles are used to abrade, cut, grind, finish or polish a wide variety of materials and surfaces and have applications in a wide range of industries.
14. In order to grind, the abrasive particles used in the abrasive product have to (i) penetrate the workpiece (i.e. there needs to be a sufficient initial force per abrasive particle contact to push the particles into the workpiece) and (ii) wear in such a way during use that removal of material from the workpiece is sustained (i.e. the force per area has to remain sufficiently high in use).
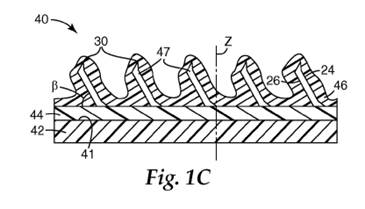
15. During use (i.e. when force is applied), the abrasive particles can wear down by attritious wear (the particles interact with the workpiece to form dull, flat areas on the surfaces of the particles known as “wear flats”) and/or by fracture wear (the particles splinter or fracture where high tensile stresses concentrate to form cracks that may propagate through the particles) or may break out completely. The different types of wear that may occur during grinding are illustrated below.
16. At high forces per area there is a greater chance of the particles breaking out (such that they are removed from grinding completely without doing any useful work) or wearing back very quickly by macro-fracturing (without the full amount of useful work being obtained). At lower forces per area, persistent wear flats form, which increase the friction between the particles and the workpiece, thereby increasing the grinding forces and causing heating in the workpiece. If the wear flats get too large without fracture (micro or macro) or break-out occurring as the wear flat grows, this causes the force per area of contact of the particles with the workpiece to fall to a level where fracture of the abrasive particles ceases. At this point, material removal falls, grinding efficiency is lost and the wear flats continue to grow.
17. Optimum grinding occurs when the particles fracture in such a way that only microparticles break off from each particle in a limited and controlled manner (i.e. micro-fracture) thereby shedding the wear flat which has developed on the surface of the particles contacting the workpiece and exposing a new grinding surface with fresh, uncontaminated and unworn edges to cut the substrate (a process known as self-sharpening). The ideal state of wear is limited attritious wear and controlled micro-fracture.
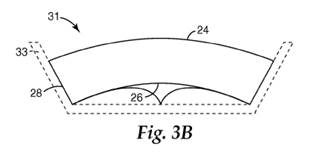
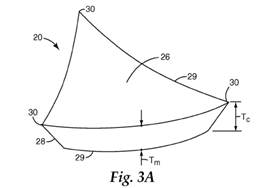
18. The type of fracture that occurs depends on the material from which the abrasive particles are made. Sol-gel abrasive particles show advantageous performance in high force per area (high load) grinding situations compared to fused alumina grains, because their toughness and fracture behaviour (they micro-fracture) means that higher loads can be applied before they fracture and, when they do fracture, the area that fractures off is relatively small. This results in the particles being able to remove material from the workpiece at a faster rate and wearing back more slowly.
19. Abrasive products that could be made using abrasive particles included products in the following categories:
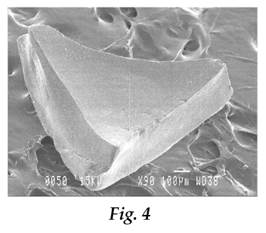
i) Coated abrasives – such articles (which may be in the form of a disc, belt or sheet) comprise a backing covered by a layer of abrasive particles, attached to the backing by means of a resin binder (often known as a make coat), and a second coating of binder covering the abrasive particles (often known as the size coat) which further attaches or adheres the particles to the backing and reinforces the abrasive particles.
ii) Bonded abrasives – these products (which include cut off wheels and grinding discs and wheels) comprise a plurality of abrasive particles bonded together by means of a binder to form a three-dimensional shaped mass.
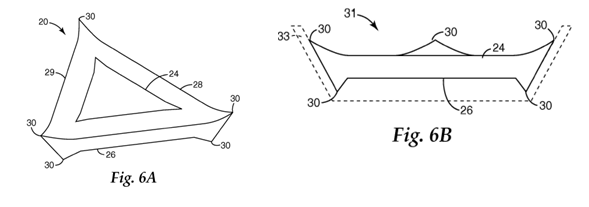
20. Electrostatic coating is typically used to apply the layer of abrasive particles to the make coat in a coated abrasive product. The particles tend to orient themselves in accordance with their geometry in the electrostatic field, i.e. the particles have a greater tendency to be consistently aligned with the longest axis perpendicular to the backing rather than being applied in a completely random manner. This is done so that the abrasive grains fracture (micro-fracture in the context of sol-gel grains) and wear over the largest possible distance perpendicular to the backing. Since the cut rate reduces as the single layer of particles in coated abrasives wear down, if more elongated grains are used (preferably with a uniform cross-sectional area) they maintain a more uniform contact area and cut rate as the abrasive particles wear in use and the distance between the backing and the workpiece decreases. Although a degree of randomness remains with electrostatic coating, the tendency of the particles to be consistently aligned with the longest axis perpendicular to the backing increases the chances that the particles cut in the most efficient manner and at the highest cut rate possible for as long as possible.
21. In bonded abrasive products, the nature of the grinding face is approximately uniform with a certain depth of abrasive particles (rather than a single layer of abrasive particles as in coated abrasives) and the abrasive particles are to a great extent randomly oriented on the surface (not aligned in any way as in coated abrasives).
22. Types of abrasive particles available at the priority date included so-called conventional abrasive grains, which had wide application in both bonded and coated abrasive products. They may be further subdivided into categories including fused alumina abrasives (grains made from fused aluminium oxide) and sol-gel abrasives (aluminium oxide based, ceramic abrasive grains made from sol-gel processing).
23. Important properties of abrasive grains, such as the levels of toughness and hardness, which have an impact on the wear resistance and cutting/grinding performance of an abrasive product in which the abrasive grains are used, are determined by the micro-crystalline structure of the abrasive grain material. Alumina abrasive grains prepared by means of the sol-gel process tend to have a finer micro-crystalline structure than fused alumina grains.
24. The following types of sol-gel abrasive particles were commercially produced at the priority date:
i)
 |
Crushed grains such as 3M’s Cubitron crushed grain (shown below) and certain of SG’s Cerpass grains:
These sol-gel abrasive grains were made by crushing the abrasive material after it had been dried, and then screening the particles by size. The form (and size) of crushed particles cannot be precisely controlled so the shapes of these types of grains were not precisely defined. However, the particles could be encouraged to have a particular type of form, e.g. more angular and elongated (in one or two dimensions) or more “blocky”.
ii) Explosive comminution grains such as SG’s exploded Cerpass DGE grains (referred to by SG as “Extra Sharp”):
 |
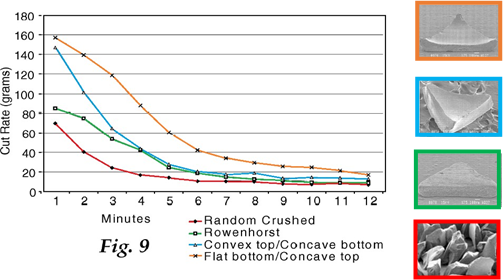
The comminution process involves the rapid heating of lumps or extruded rods of partially dried gel in a calcining machine, resulting in the gels explosively fracturing during calcination because of the high gas pressure in the particles.
iii) Rod-shaped grains such as SG’s sol-gel alumina “Targa” grains produced using extrusion techniques:
 |
25. At the priority date, no commercially available abrasive sol-gel particles were known to be made using a mould or tooling comprising individual cavities to produce particles of a precise, defined shape and size.
The sol-gel process
26. The sol-gel process has been known for many decades and has been used in a wide range of applications, including making optical components and coatings for articles. Although there were industrial applications of the sol-gel process, these were limited by the complexity of manufacturing economically on an industrial scale (in contrast it was not particularly complex to undertake on a laboratory scale).
27. The first stage in the sol-gel process is the preparation of a sol. Very small solid particles are dispersed into a liquid, commonly an acidic aqueous solution. This mixture can be referred to as a “dispersion”. The low pH of the solution causes particles to become electrically charged. The like charge between the particles creates a slight repulsive effect and helps maintain the particles in suspension (rather than the particles agglomerating and settling out of solution by gravity). This process of lowering the pH and creating the suspension is called peptizing, and accordingly chemicals such as strong acids were referred to as peptizing agents. The resulting colloidal suspension is often called a “sol”.
28. The next step in the process involves bringing the suspended particles in the sol together in a controlled way to form a “gel”. A polymeric gel forms where the particles covalently bond to form molecular polymers. A particulate (or colloidal) gel forms where the particles remain molecularly distinct but form three-dimensional networks by inter-particulate forces. A particulate gel can be visualised as the suspended particles coming together to form “strings” of particles, in a process called gelation.
29. The process of gelation can occur simply with time. The gelation process can also be actively encouraged in a number of ways. For example, by removing some of the solvent which forces the suspended particles closer together, at some point the repulsive force between the suspended particles (tending to keep them apart) will be exceeded by the compression caused by the reduced volume. In any event, the result is a “wet gel” consisting of a solid-like three-dimensional particle network within a liquid.
30. A dispersion is not simply in a sol state or a gel state, rather there is a continuum of properties the dispersion can have. In particular, the extent of gelation has a major impact on the properties of the dispersion. “Rheology” is a term used to describe the deformation and flow characteristics of a dispersion. The rheological properties of a gel can be described by reference to a number of quantifiable parameters - viscosity, elastic modulus (stiffness at stresses below the yield point) and yield stress/point (below the yield point the gel is elastic and will return to its original shape when the stress is removed, above the yield stress the dispersion behaves as a fluid) - although measuring such parameters was not always straightforward. The property of time-dependent reversible shear thinning is referred to as thixotropy. In simple terms such a dispersion may be relatively viscous and solid-like at rest but will become less viscous and fluid-like when subjected to stresses greater than the yield point (e.g. through stirring). If allowed to stand undisturbed the dispersion will over time return to the solid gel state.
31. A common starting material to form sol-gel alumina ceramics at the priority date was boehmite, which comprises aluminium oxide hydroxide. Boehmite was in the form of a powder tens of microns in size consisting of agglomerated sub-micron crystals, such as DISPERAL, manufactured by Sasol. Boehmite powder would typically be dispersed into an aqueous acid solution. Boehmite particles are sub-micron, although larger agglomerates would also persist in the dispersion. Boehmite particles are platelet-shaped (similar but not identical to clays). The fact that they are platelet-shaped will have an impact on the detailed properties of a dispersion. Sols made using boehmite precursors form particulate gels rather than polymeric gels. Sols made from boehmite and their resulting gels are thixotropic.
32. Boehmite sols and their resulting gels are very sensitive to a number of variables (many of which are interlinked), which are liable to have a significant effect on the behaviour and properties exhibited by the material:
i) Solids content: the solids content has one of the biggest influences on how a sol or gel behaves. As solids content increases, the frequency of collisions between particles in a sol to form weak van der Waals bonds increases dramatically. As a result, the viscosity of sols produced at higher solids increases much faster and the time to gelation decreases. Gels produced from higher solids sols have higher yield strength for a given ageing time. When gels made from higher solids sols are sheared to form flowable sols, these sols will gel again much faster than lower solids sols. Drying (i.e. removal of some of the solvent) is one way to achieve higher solids content but it must be remembered that drying also involves adding ageing time so, during drying, effects from ageing also come into play in addition to the effect of a higher solids content.
ii) Ageing time: greater ageing time allows more time for the bonds (i.e. weak van der Waals forces) between particles of the sol to increase in number. It results in “stronger gels”, i.e. gels with a higher yield point where higher shear forces are needed to make the gel revert to the flowable sol state.
iii) Temperature: increased temperature accelerates bond formation between particles therefore accelerating ageing and gelation.
iv) Acid content: in electrostatically stabilised aqueous boehmite sols, hydrated hydrogen ions from the peptizing agent (e.g. nitric acid) are attracted to the surface of the boehmite particles. This gives the boehmite particles the positive charge that causes the particles to repel one another and form the sol. As the acid content is increased, the surface of the boehmite is covered with a hydrated hydrogen ion layer. Addition of more acid drives the pH of the solution lower and boehmite is dissolved. This helps during sol dispersion with the separation of boehmite particles (that were agglomerated and bonded together during drying in the production of commercial boehmite), but it also increases the ionic strength of the solution in the sol. The added acid and the resulting aluminium species in the solution between the sol particles in suspension causes the sol to gel more quickly. A gel with higher acid content that is subjected to shear forces to revert to a flowable sol state will gel again more quickly than a sol with a lower acid content.
33. Dispersions can simply be dried in a simple container like a tray, which may be the case if the intention is to crush the final product. Alternatively, the dispersion can be shaped using a variety of different methods, including using a mould. There are a number of different factors which can alter the shape of the final object, and therefore the shape of the mould alone cannot guarantee that the final product will be the desired shape – the process needs to be carefully controlled to achieve this, as detailed when considering drying below. In general, in the conversion from the dispersion to the final ceramic there is considerable shrinkage in the dimensions of the object.
34. A mould release agent may be added to the surface of the mould before the dispersion is added in order to aid its removal after the drying stage - it is important that the dried gel is easy to remove, otherwise the dried gel may break during the removal process. A wide range of mould release agents were available, including silicone-based agents, synthetic polymers, natural oils or greases (e.g. vegetable) or man-made (e.g. mineral oils). Mould release agents could be applied by hand (e.g. with a cloth or brush), with an aerosol spray, or “baked” onto the mould (e.g. a PTFE-coated mould). The level of adhesion of the dispersion to the mould will depend upon the dispersion (e.g. its chemical properties), and the mould material (e.g. adhesion to metal and plastics is often different). Depending on these factors, a mould release agent may or may not be needed. Mould release agents were common laboratory practice, although on an industrial scale the added process step and environmental, safety and contamination concerns favoured avoiding their use.
35. Sol-gel abrasives were typically made either by crushing lumps of dried gel, explosive comminution during calcining or by being shaped through extrusion (e.g. to produce rods) - see paragraph 24 above. As mentioned above, at the priority date commercial sol-gel abrasives were not known to be made using moulds with cavities to produce particles of a defined shape and size.
36. The next stage of the sol-gel process is to dry the wet gel to remove the free solvent that is not bound to the particles. The wet gel is extremely porous, and the pores are impregnated with the solvent. The wet gel is dried, for example in a drying chamber or oven, to remove the solvent through evaporation. The rate of evaporation depends upon a number of factors, including the temperature, humidity (which can be controlled for example with air flow) and the ability of the solvent to move through the gel structure.
37. The initial stage of drying is known as the “constant rate period”, during which the rate of evaporation of the solvent from the gel is constant. At this stage, the solvent is evaporating from the surface of the gel, and the solvent is being drawn from inside the pores in the gel by capillary action. As the solvent evaporates, the gel begins to shrink and, during the constant rate period, the volumetric shrinkage of the gel is equal to the volume of liquid evaporated. The level of shrinkage can be extreme, with the gel halving in volume or more.
38. As the gel shrinks, the particles in the network move closer together and the gel becomes more rigid. Shrinkage stops when a “critical point” is reached (also known as the “leatherhard point”), that is when the gel network has become so stiff that it is able to resist the stresses imposed by the capillary forces. The capillary pressure is inversely related to the radius of curvature of the liquid meniscus at the surface of the gel. It reaches a maximum when the radius of curvature of the meniscus becomes equal to the radius of the pores between the particles. After the critical point, drying then enters the “falling rate period” where the drying front moves within the gel and solvent is flowing through the partially empty pores.
39. The above is an idealised description of drying, however, so where drying occurs unevenly, or the gel is prevented from shrinking freely (which is often the case), a gel can be distorted or even crack.
40. If a gel plate dries from only one side, the capillary forces drawing the solvent from the pores inside the gel to the surface create a pressure gradient perpendicular to the drying surface, which acts to transport the solvent towards the drying surface. This results in a greater compressive force on the gel near the drying surface and hence more shrinkage. In a colloidal, spherical particle (e.g. a non-boehmite) gel plate this causes the gel to warp, becoming concave towards the drying side.
41. As the gel continues to dry from the top surface and reaches the critical point, the drying front then moves inside the gel plate, and the maximum compressive force moves similarly. Eventually the lower part of the gel, which is still saturated in solvent, is subject to the maximum compressive force and shrinkage that causes the bowing to be reduced or even reversed. While boehmite gels would not warp on drying in the way described in the previous paragraph, in late stages of drying they could warp to form a concavity away from the drying side.
42. The shape will also be affected by adhesion of the gel to the sides or base of the mould or substrate in / on to which it is placed. Additionally, the differential drying stresses are linked to the drying rate (influenced by the external environment such as temperature and humidity). This is the result of two effects. First, a greater rate of evaporation causes a greater pressure gradient within the liquid in order to supply solvent to the drying surface at a rate to satisfy the rate of evaporation, whereas low rates of evaporation afford the opportunity for the internal pressures to equilibrate. Secondly, the particles in the gel cannot redistribute instantaneously and time is required for them to form the denser drying structure. In addition, the permeability of the gel to the solvent was also understood to be a factor which would influence the drying stresses. Furthermore, it was understood that the strength and stiffness of the gel would help enable the drying object to resist and accommodate the drying stresses.
43. The occurrence of distortions, such as warping, is not the only effect of drying stresses. If such stresses become too great, then cracking can occur, particularly when free shrinkage is constrained. Steps can be taken to avoid cracking (and in some cases complete fracture of the gel), such as adding surfactants to the solvent to decrease the capillary forces and therefore the pressure gradient. A greater degree of gelation before drying can strengthen the gel and therefore also reduce the risk of fracture.
44. Overall, drying conditions (e.g. temperature and humidity) need to be considered during the drying stage, but depend greatly on the object being dried (e.g. its size, permeability, strength), and so the occurrence of issues such as distortion, warping and cracking are difficult to predict, but are generally more likely the higher the drying rate and/or the larger the object is.
45. Once the free solvent has been removed through evaporation, the dried gel is formally known as a “xerogel”. More colloquially it is known as the “green body”. ![]() The green body is resistant to deformation, but is very friable (i.e. quite easy to break) and therefore needs to undergo further heat treatment in order to convert the dried gel into the final product by removal of the pores between the particles.
The green body is resistant to deformation, but is very friable (i.e. quite easy to break) and therefore needs to undergo further heat treatment in order to convert the dried gel into the final product by removal of the pores between the particles.
46. The green body is substantially dry, although there may be some residual solvent remaining. In addition, solvents can become bound to the solid particles by physical or chemical forces, and the particles themselves can contain unwanted chemical constituents. Calcination involves heating to moderately high temperatures (typically several hundred degrees) to remove any residual free and bound solvent. Other components which are no longer required (e.g. the acid) are also removed by calcination. In simplified terms, calcination “burns off” unwanted impurities. During calcination of objects made from boehmite (which would typically take place at around 500 to 800oC), the boehmite goes through a series of phase changes, and the aluminium oxide is in the gamma or other “transition” alumina phases following calcination.
47. Sintering, in the context of the sol-gel process, involves heating the calcined green body to high temperatures (higher than the calcination temperature but below the bulk melting point of the green body), and sintering temperatures are typically around 0.5-0.75 of the absolute melting temperature. In the case of objects made from boehmite, sintering would typically take place at around 1200 to 1500oC. Sintering achieves two main purposes. The first purpose is to transform the green body into the desired crystal phase. The second purpose is effectively to convert what is a collection of loosely agglomerated particles into a more singular mass and substantially remove the porosity between the particles (densification). The interface at which crystal particles join during sintering is called the grain boundary. Pore removal continues by diffusion of material along the grain boundaries into the pores.
48. As during the sintering process the size of the pores between particles decreases, the density of the green body increases. This densification process causes further shrinkage, although the amount of shrinkage is typically much less drastic than seen when drying the wet gel. The shrinkage may be uniform, although if the density of the green body is not reasonably uniform it can be uneven. In abrasive particles made from boehmite, the phase transformation from transition alumina to alpha alumina is highly exothermic and the alpha phase has a significantly higher density than the transition alumina, so there is considerable shrinkage just from the phase transformation in addition to shrinkage from eliminating pores.
Rowenhorst
49. Prof Atkinson described the primary teaching of Rowenhorst (accurately in my judgment) as being that intentionally shaped alumina ceramic particles, in particular in the form of triangles, made by the sol-gel process are useful as abrasive particles.
50. Most attention at trial focussed on the general description of the process for preparing the abrasive particles, and on the examples. Rowenhorst identifies seven steps in the process, in brief: (1) preparing a dispersion comprising a volatile component, (2) providing a mould, (3) filling the mould with the dispersion, (4) removing volatile liquid from the dispersion to increase its viscosity, (5) removing the precursor abrasive particles from the mould, (6) calcining the precursor abrasive particles and (7) sintering to produce the abrasive particles.
51. The description of the first, sixth and seventh steps of the process is essentially the same as that of the corresponding steps in the Patent, discussed below. Significant aspects of the description of the second to fifth steps are as follows:
i) The mould cavities can either “extend for the entire thickness” of the mould or “can extend only for a portion of the thickness” of the mould. The preferred mould shape is triangular, but other shapes can be used, including (SG emphasised) frusto-pyramidal.
ii) 3M emphasised that, when introducing the dispersion into the mould cavities:
“…it is preferred that no exposed surfaces of the dispersion extend substantially beyond the planes formed by the planar surfaces of the mould to ensure uniformity in thickness of the abrasive particles. It is also preferred that the planar surface of the mould surrounding the cavities be substantially free of dispersion.”
iii) It is preferred to apply a release coating to the surface of the mould cavities prior to introduction of the dispersion, to allow the particles to be removed easily. Release coatings may typically be made of silicone or PTFE.
iv) It is preferred to remove the volatile liquid by evaporation, which may be at elevated temperatures:
“The elevated temperatures can range from about 40oC to about 300oC. However, at higher temperatures, high drying rates are obtained that produce undesirable cracks in the resulting abrasive particle. It is preferred to heat the mould containing the dispersion at a temperature of from about 50oC to about 80oC for from about 10 to about 30 minutes in a forced air oven.
v) It is explained (in a passage emphasised by 3M) that:
“The removed precursors of the abrasive particles have approximately the same shape as the cavities of the mold from which they were formed. Exact replication is unlikely for three reasons. First, the dispersion will shrink, so the precursors of the abrasive particles will be smaller. Second, when the precursors of the abrasive particles are removed from the mold cavities, some of their edges may break off or become rounded. Third, when the dispersion is introduced in the cavities, the dispersion may not completely fill the cavities. It should be noted that care should be taken throughout the process to minimize the foregoing factors.”
52. Following the description of the seven steps of the process, reference is made to a continuous process which can be used to make the abrasive particles, using the apparatus shown in Fig. 8. That employs a belt as a mould (with cavities that pass through the belt) which, after being filled with dispersion, is passed between wiper blades and then doctor blades before entering an oven preferably set at about 75oC (or higher, or lower, depending on the speed of the belt and the solids content of the dispersion). 3M emphasised this teaching:
“It is preferred that the exposed surface or surfaces of the dispersion in the cavities not extend substantially beyond the plane of the belt in order to guarantee that the abrasive particles prepared from the process be substantially uniform. Any excess dispersion surrounding the openings of the cavities and remaining on the non-recessed portion of the belt 62 is removed, preferably by leading-edge wiper blades 68 positioned down the belt 62 from the die body 66. The top and bottom surfaces of the belt 62 can be wiped by the leading-edge wiper blades 68. These blades 68 are mounted between leveling doctor blades 70 and the die body 66. The leveling doctor blades 70 further ensure that abrasive precursor particles will have a uniform thickness.”
53. There is then a “procedure for making shaped abrasive particles”:
“A dispersion (44% solids) was made by the following procedure: alpha aluminum oxide monohydrate powder (1,235 parts) having the trade designation "DISPERAL” and alpha iron oxide (206 parts, 10% FeOOH) were dispersed by continuous mixing in a solution containing water (3,026 parts) and 70% aqueous nitric acid (71 parts). The sol that resulted was mixed with magnesium nitrite (429 parts) to form a gel which was then dried at a temperature of approximately 125°C in a continuous dryer to produce the 44% solids dispersion. The dispersion was introduced into the cavities of the desired shape in a mold by means of a rubber squeegee. The cavities were coated with a release coating, either a silicone material or polytetrafluorethylene. The filled mold was placed in a forced air oven maintained at a temperature of 71° C for 20 minutes….”
54. That procedure was then used to make abrasive particles for use in examples 1-10 (though the magnesium nitrite is omitted in example 9). Example 1 involves triangular-shaped particles, while examples 2 and 3 involve disc-shaped and square-shaped particles respectively. Those particles are compared with Cubitron grain in a specified grinding test. The results show a significant improvement for the triangular-shaped particles compared with the other particles and the Cubitron grain. Further tests are then conducted on the triangular-shaped particles in examples 4-10, which as a whole show a significant improvement compared to Cubitron grain.
55. Example 11 involves making precursors of abrasive particles using the apparatus of Fig. 8 and a dispersion prepared as in the “procedure for making shaped abrasive particles”. The example shows the benefit of wiping the mould during the process of production.
56. A question arose as to the disclosure of Rowenhorst regarding the moulds used in examples 1-10. Counsel for SG submitted that the skilled person would understand that they were of what he called the “muffin tray” type, i.e. with a base rather than with cavities extending through the mould. While Rowenhorst plainly envisages both types, and uses punched-through moulds in the Fig. 8 continuous system employed in example 11, there is only slight basis in the text for the submission that examples 1-10 used muffin tray type moulds. SG relied simply on the fact that the moulds are described as being “placed” in a forced air oven for a specified length of time. Prof. Atkinson’s reports did not suggest that the disclosure of Rowenhorst was that examples 1-10 were carried out using muffin tray type moulds, though in oral evidence he explained convincingly why it would be attractive to use such moulds in a laboratory scale process. Dr Schwabel accepted that “it looks as though examples 1 to 10 are with a muffin tray of cavities with a bottom”, but he was not asked whether it was clear that they were. In case it matters, in my judgment there is no clear disclosure in Rowenhorst of the use of muffin tray type moulds in examples 1 to 10, though it would plainly be an obvious implementation to use such moulds.
The Patent
The description
57. The Patent starts by acknowledging certain US patents, including Rowenhorst, as disclosing triangular shaped abrasive particles and abrasive articles using such particles, and states that such particles are useful in manufacturing abrasive articles having enhanced cut rates.
58. The summary of the invention starts at [0003], which states:
“Shaped abrasive particles, in general, can have superior performance over randomly crushed abrasive particles. By controlling the shape of the abrasive particle it is possible to control the resulting performance of the abrasive article. The inventors have discovered that by making the abrasive particle dish-shaped with either a recessed or concave surface unexpected grinding benefits occur.”
59. [0004] then explains that:
“Without wishing to be bound by theory, it is believed that the recessed or concave face improves the amount of material removed by the dish-shaped abrasive particle. In particular, an ice cream scoop or a spoon has a concave shaped end that effectively digs into materials and removes a significant quantity of the material. A scoop is much more effective than a knife or a flat thin body when digging into and removing large quantities of material. Similarly, a hollow ground chisel having a concave surface produces a sharper edge. In a similar manner, placing a recessed or concave face onto the shaped abrasive particle thereby forming a dish-shaped abrasive particle can increase the grinding performance of the dish-shaped abrasive particle over a similarly shaped abrasive particle having a planar first face and a planar second surface.”
60. [0005] then explains a further benefit arising from the dish-shaped particles being additionally provided with a sloping sidewall, i.e. having what the Patent calls a draft angle of over 90o, so that they can be arranged so as to present a rake angle of less than 90o:
“Secondly, by additionally forming the dishshaped abrasive particles with a sloping sidewall, the dish-shaped abrasive particles with the sloping sidewall tend to rest on the make coat of a coated abrasive article at an angle corresponding to the draft angle of the sidewall. It is believed that a draft angle other than 90 degrees results in the dish-shaped abrasive particles leaning instead of having a 90 degree orientation to the backing in a coated abrasive article since the sidewall, which the dish-shaped abrasive particle in the coated abrasive rests on, is sloped due to the draft angle. Because the dish-shaped abrasive particles are mostly tipped or leaning to one side due to the angled sidewall they rest on, they can have a rake angle less than 90 degrees relative to the workpiece thereby enhancing cut rates. It is believed that this rake angle enhances the cut rate of the dish-shaped abrasive particles.”
61. [0009]-[0012] contain some definitions. Of significance, “comprise” is said to have an open-ended meaning equivalent to “include” (as is normal in patent specifications) and it is made clear that “precursor dish-shaped abrasive particle” refers (as in Rowenhorst) to the particle after forming but before sintering, to distinguish it from the final, sintered dish-shaped abrasive particle.
62.
 |
63. As will be seen, the first face 24 is recessed such that the thickness Tc at the corners 30 is greater than the thickness Ti between the lowest point of the first face and the second face 26. Also, in this instance, the particle has sloping sidewalls 28 with a draft angle a. The combination of the recessed first face and the draft angle a leads to sharp points at the corners, with angle l.
64.
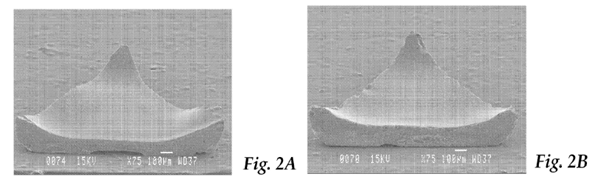 |
It is explained in [0017] that it is believed that the recessed face is formed by the sol-gel in the mould cavity forming a “meniscus”. [0017] also explains that the recessed first face may have a substantially flat centre portion, as in the particle shown in Fig. 2A, or a substantially concave centre portion, as in the particle shown in Fig. 2B.
65. While the term concave is not used in the Patent in relation to the first face of all particles of this embodiment, the term planar/concave was used at trial to describe them, and it is convenient to retain that terminology (they were also referred to as Fig. 2 type particles). In relation to these particles, [0018] restates the inventors’ belief that the recessed first face improves the amount of material removed by the dish-shaped abrasive particle, repeating the theory advanced in [0004] (which was referred to at trial as the “ice cream scoop theory”). [0019] adds a further theory (which was referred to at trial as the “thinness theory”):
“Additionally, it is believed that having a thinner interior portion of the shaped abrasive particle may help grinding performance of the dish-shaped abrasive particle once the sharp upturned point or corner is worn away. When the interior portion is thinner, two factors may come into play that improves grinding performance. First, a corresponding wear flat generated during use of the dishshaped abrasive particle will have less area as compared to a shaped abrasive particle having a thicker interior section. If one particle is half as thick as the next particle then the resulting wear flat will be half the size due to the change in the thickness. Secondly, the thinner interior portion may result in increased fracturing of the dishshaped abrasive particles during use thereby enhancing the particle’s ability to re-sharpen itself through fracture mechanics. A thicker particle is less likely to fracture than a thinner particle.”
66. [0021]-[0022] address the thickness ratio Tc/Ti:
“The thickness ratio of Tc/Ti is between 1.25 to 5.00, or between 1.30 to 4.00, or between 1.30 to 3.00. To calculate the thickness ratio, fifteen randomly selected dish-shaped abrasive particles are screened. The height of each corner of each particle is measured and then all of the heights are averaged to determine an average Tc. For example, a triangle would have three Tc measurements per shaped abrasive particle and 45 measurements total for use in determining the average for Tc.
Next, the smallest thickness, Ti, for the interior of the first face 24 of each shaped abrasive particle is measured. Often the translucency of the shaped abrasive particle can be used to find the minimum interior thickness and the 15 results are averaged to determine an average Ti. The thickness ratio is determined by dividing the average Tc by the average Ti. A light microscope equipped with an X-Y stage and a vertical location measurement stage can be used to measure the thickness of various portions of the dish-shaped abrasive particles.”
67. As will be seen, the method given by the Patent for determining the thickness ratio Tc/Ti involves measuring the heights of the corners of 15 randomly selected dish-shaped particles, averaging those heights, and then dividing that average by the average of the thicknesses of the 15 particles at their narrowest points. Where I refer in this judgment to an “average Tc/Ti ratio” I mean a Tc/Ti ratio obtained using this methodology, thereby distinguishing it from the Tc/Ti ratio for an individual particle.
68. [0022] continues (in this and other quotes I have corrected obvious typographical errors):
“Triangular dish-shaped abrasive particles produced by the invention have been measured to have thickness ratios between 1.55 to 2.32 in some embodiments. Triangular shaped particles produced by the prior art method disclosed in U.S. patent number 5,366,523 entitled Abrasive Article Containing Shaped Abrasive Particles to Rowenhorst et al. have been measured to have thickness ratios between 0.94 to 1.15 meaning they are essentially flat and are just as likely to be slightly thicker in the middle as they are to be slightly thinner in the middle. Dish-shaped abrasive particles having a thickness ratio greater than 1.20 are statistically different from the Rowenhorst particles at the 95% confidence interval.”
69. In my judgment the skilled person would understand the thickness ratios stated in this passage to have been obtained using the method of averaging over 15 randomly selected particles, rather than being ratios of Tc/Ti for individual particles. That is consistent with the fact that this passage immediately follows the explanation of the method for determining the thickness ratio Tc/Ti, and the fact that a statistical comparison is made between particles of the invention and those of Rowenhorst on the basis of their respective Tc/Ti ratios. Of course, in the case of the Rowenhorst particles referred to, the thickness ratios cannot have been obtained by randomly selecting 15 dish-shaped particles. On the contrary, as the Patent explains, the Rowenhorst particles are “essentially flat” (and an illustrative example of such particles is shown in Fig. 5, referred to in [0020]) and the skilled person would not think that a thickness ratio of 0.94 could have been obtained by applying the methodology to 15 dish-shaped particles.
70. [0023] refers to the draft angle a shown in Fig. 1B, which can be varied. It then says:
“As discussed in copending patent application U.S. patent application serial number 12/337,075 entitled "Shaped Abrasive Particle With A Sloping Sidewall", filed on December 17, 2008, and having attorney docket number 64869US002, having a draft angle a greater than 90 degrees is believed to improve the grinding performance of shaped abrasive particles. Furthermore, a slight increase in the draft angle from 90 degrees to 98 degrees has been found to double the cutting performance of triangular shaped abrasive particles and the increased performance is present until the draft angle becomes greater than about 130 degrees.”
71. When the case was opened, 3M was contending that the skilled person reading the Patent could refer to the cited US patent application (“US 075”). However, by the time of closing it was common ground that such a cross-reference could not be made, because the conditions for such a cross-reference identified by Pumfrey J in Halliburton Energy Services Inc v Smith International (North Sea) Ltd [2005] EWHC 1623 (Pat) at [294] were not met. Specifically, it was common ground that US 075 had not been published until after the date on which the PCT application which led to the Patent was published, and so the test applied by the European Patent Office (“EPO”) for permissible cross-references was not satisfied.
72. That meant that it was not necessary to decide on the correctness of the view expressed (obiter) by Pumfrey J that the decision of the House of Lords in Biogen Inc v Medeva plc [1997] RPC 1 required a stricter test, namely that the cited document must have been published before the date of application for the patent in question. Such a test would introduce an unfortunate disparity between the UK and the EPO. Pumfrey J appears to have taken the view that because Biogen v Medeva established that sufficiency is to be judged as of the application date (in fact in cases where, unlike Biogen v Medeva, a patent is entitled to an earlier priority date, it is judged as of that earlier priority date), that meant that reference could only be made to a document available to the public at that date. However, the application for a patent itself is not available to the public at the date of application, so I find it hard to see why a cross-reference should be excluded for that reason. But, as I say, it was not in the end necessary to decide that point.
73.
 |
The Patent continues to explain the effect of the draft angle on planar/concave particles by reference to Fig. 1C, which shows the particles attached to a backing 42 by a make coat 44 and covered by a size coat 46.
74. As stated in [0026], Fig. 1C shows that the sloping sidewalls lead to the dish-shaped particles having an orientation or rake angle b of less than 90o relative to the backing. After saying that is unexpected, given the electrostatic coating method which tends to orient particles at 90o to the backing, [0026] continues:
“As seen, once the dish-shaped abrasive particles with a sloping sidewall are applied and allowed to lean, the highest corners 30 are at a favorable rake angle for abrading a workpiece. In particular, the first face 24 by being recessed results in an acute angle l between the sidewall 28 and the first face 24 resulting in a very sharp point or corner instead of the rounded corner of the prior art. This gives the dish-shaped abrasive particle a saw tooth point 47 that engages and removes more material; especially, when the draft angle a is greater than 90 degrees.”
75. [0029] adds this:
“Without wishing to be bound by theory, it is believed that an orientation angle b less than 90 degrees results in enhanced cutting performance of the dishshaped abrasive particles with a sloping sidewall. Surprisingly, this result tends to occur regardless of the particles’ rotational orientation about the Z axis within the coated abrasive article. While FIG. 1C is idealized to show all the dish-shaped abrasive particles aligned in the same direction, an actual coated abrasive disc would have the dish-shaped abrasive particles randomly distributed and rotated at various orientations relative to the Z axis.”
76.
 | |||
 | |||
The second principal embodiment is described from [0031]-[0037] and illustrated by Figs 3A and 3B:
77. [0033] explains that:
“In this embodiment, the first face 24 is convex and the second face 26 is concave (concavo-convex) such that the dish-shaped abrasive particle substantially comprises a triangular section of a spherical shell. As will be discussed in more detail, it is believed that the convex face is formed by the sol-gel in the mold cavity 31 releasing from the bottom surface of the mold due to the presence of a mold release agent such as peanut oil during evaporative drying of the sol-gel. The rheology of the sol-gel then results in the convex/concave formation of the first and second face while the perimeter 29 is formed into a triangular shape during evaporative drying.”
78. An example of such a particle is shown in Fig. 4 and such particles were referred to at trial as concave/convex, or as Fig. 4 type particles.
 |
79. In relation to these particles, the Patent advances the ice cream scoop theory (at [0034]), but not the [0019] theory. That is because such particles are not thinner in the middle than at the corners - rather they are substantially “a triangular section of a spherical shell”. However, they are consistently referred to as dish-shaped.
80. [0038] assumed some significance at trial. In that paragraph, the Patent introduces further embodiments of dish-shaped particles in which both faces are recessed:
“Referring now to FIGS. 6A and 6B, in other embodiments of the invention, the first face 24 and the second face 26 of the dish-shaped abrasive particles 20 can both be recessed. In some embodiments, the dishshaped abrasive particles can be biconcave having a concave first face 24 and a concave second face 26. Such shaped abrasive particles can be made by making the bottom surface of the mold cavity 31 convex such that a concave second face 26 is formed on the shaped abrasive particle. Alternatively, other recessed structural geometries can be formed on the second face 26 by appropriately designing the contour of the bottom surface of the mold cavity. For example, in FIG 6B, the bottom surface of the mold can have a substantially planar center portion and recessed corners that form a plurality of upturned points or a plurality of raised corners 30 on the second face 26. In such embodiments, the degree of curvature or flatness of the first face 24 can be controlled to some extent by how the dish-shaped abrasive particles are dried thereby resulting in a recessed or curved first face or a substantially planar first face.”
81. As can be seen, these embodiments are illustrated by Figs 6A and 6B; there is no further discussion of these embodiments in the Patent.
 |
82. [0049]-[0050] make it clear that dish-shaped abrasive particles of the invention can be mixed with other abrasive or non-abrasive particles and envisage that the proportion of dish-shaped abrasive particles of the invention can be as low as 5%.
83. [0056]-[0074] set out a generalised method for producing dish-shaped abrasive particles. As in Rowenhorst, this is arranged under seven process steps, in brief: (1) providing a dispersion, (2) providing a mould, (3) filling the mould with the dispersion, (4) controlling the rheology of the sol-gel in the mould to make different types of dish-shaped particles, (5) removing the precursor dish-shaped abrasive particles from the mould, (6) calcining and (7) sintering.
84. The teaching of the Patent in this section is broad and general. The following are notable:
i) [0056] explains that the dispersion should comprise “a sufficient amount of liquid for the viscosity of the abrasive dispersion to be sufficiently low to enable filling the mold cavities and replicating the mold surfaces, but not so much liquid as to cause subsequent removal of the liquid from the mold cavity to be prohibitively expensive.” It is then said that the dispersion can contain from 2 to 90% of boehmite (in fact the evidence was that dispersions of over about 50% boehmite would be challenging to make); narrower ranges such as 40 to 50% are then provided. [0057] then adds that examples of commercially available boehmite “include products having the trademarks “DISPERAL” and “DISPAL”, both available from Sasol”.
ii) [0062]-[0066] discuss moulds. They explain that the mould can either be of a type with a bottom surface and a plurality of cavities (i.e. of the muffin tray type) or can take the form of a belt or the like with cavities punched through the entire thickness. The mould is made from polymeric material, or from other materials with a polymeric coating. [0065] makes reference to the fact that the moulds can have sloping sidewalls and explains that this is “believed to enable easier removal of the precursor abrasive particles from the mould”.
iii) [0067]-[0068] discuss filling the cavities in the mould with the dispersion by conventional techniques. The internal surfaces of the mould can be coated with a mould release agent.
iv) [0069] starts by saying that “The fourth process step involves controlling the rheology of the sol-gel in the mold to make different types of dish-shaped abrasive particles.” However, as SG observed, it says nothing about how to control the rheology of the sol-gel so as to achieve such particles. Instead, it focusses entirely on the impact of mould release agents, saying that the use of no, or a small amount of, mould release agent tends to lead to the formation of a “meniscus” in the first face of the particle (i.e. to a planar/concave particle), whereas when more, or an excess of, mould release agent is used, the particles tend to release from the bottom surface of the mould during drying, leading to concave/convex particles. As SG observed, although this stage of the process involves drying, the conditions of which were known to have an important impact, the Patent says nothing about how to control or vary the drying conditions so as to achieve any particular form of dish-shaped particle.
v) [0070]-[0073] discuss removal of the particles from the mould, an optional additional drying step, calcining and sintering. There is nothing in these paragraphs which adds to the teaching of Rowenhorst about these steps of the process.
85. [0074] adds that:
“More information concerning methods to make shaped abrasive particles is disclosed in copending U.S. patent application serial number 12/337,001 entitled "Method Of Making Abrasive Shards, Shaped Abrasive Particles With An Opening, Or Dish-Shaped Abrasive Particles", having attorney docket number 63512US002, and filed on December 17, 2008.”
86. In this instance it was common ground that the requirements for cross-referencing identified in [294] of Halliburton v Smith were met. SG contended that nevertheless the cross-reference was not a legitimate one, referring to [58] and [61]-[62] of Halliburton v Smith. I shall deal with the cross-reference to the US application cited in [0074] (“US 001”) when I come to consider insufficiency.
87. The Patent then presents three examples. They are not numbered, but were referred to at trial as examples 1-3. Example 1 is said to produce planar/concave particles, example 2 to produce concave/convex particles, and example 3 to produce prior art shaped abrasive particles (it is very similar to example 9 in Rowenhorst). The significant aspects of example 1 read as follows:
“A sample of boehmite sol-gel was made using the following recipe: aluminum oxide monohydrate powder (7333 parts) having the trade designation "DISPERAL" was dispersed by high shear mixing a solution containing water (11000 parts) and 70% aqueous nitric acid (293 parts) for 10 minutes. The resulting sol-gel was aged for 1 hour before coating. The sol-gel was forced into production tooling having triangular shaped mold cavities of 28 mils depth and 110 mils on each side. The draft angle a between the sidewall and bottom of the mold was 98 degrees. … The sol-gel was forced into the cavities with a vacuum slot die coating station so that all the openings of the production tooling were completely filled. The sol-gel coated production tooling was passed through a 27 foot convection air oven at 10 feet per minute set to 300 degrees Fahrenheit at 40% air velocity in the 13.5 foot zone 1 section and 325 degrees Fahrenheit at 40% air velocity in the 13.5 foot zone 2 section. …”
88. Example 2 differs in the following respects:
i) The proportions of components of the dispersion are different (4824 parts DISPERAL, 7087 parts water, 212 parts 70% nitric acid). This produces a similar solids content to example 1 (about 40%) but the nitric acid level is slightly higher (about 0.33M compared to 0.30M).
ii) The high shear mixing lasts for 13 minutes rather than 10 minutes.
iii) A mould release agent, 2% peanut oil in water, was used to coat the mould at about 1 mg/in2.
iv) The drying temperatures are lower (280oF in the zone 1 section and 250oF in the zone 2 section).
89. Example 3 differs from example 1 in the following respects:
i) The proportions of components of the dispersion are different (1235 parts DISPERAL, 3026 parts water, 71 parts 70% nitric acid) and mixing is said to be “continuous” rather than high shear mixing for a defined time. There is no reference to ageing; instead the resulting sol was dried at about 125oC in a continuous drier to produce a 44% solids dispersion.
ii) The drying conditions are different. Once the mould had been filled with the dispersion, it was placed in a forced air oven at a temperature of 71oC for 20 minutes. Once the particles were removed from the mould they were dried at 121oC for three hours.
90. After calcining and sintering, the particles from each example (and 3M’s Cubitron grain) were each graded, mixed with graded calcium carbonate particles and coated onto fibre disc backings at a level of 18 g per disc, using not only a make coating and a size coating but also a KBF4 supersize coating. The grinding performance of each disc was evaluated using a specified grinding test on a specified stainless steel workpiece.
91. The results are shown in Fig. 9. Prof Atkinson provided a version of Fig. 9 in which the plotted lines are helpfully colour coded and the types of particles tested are illustrated:
92. The Patent comments on these results in [0083]:
“Referring to FIG. 9, the dish-shaped abrasive particles 20 performed significantly better than the prior art triangular shaped abrasive particles disclosed in U.S. patent number 5,366,523 to Rowenhorst et al. having two parallel planar surfaces (FIG. 5), or the random crushed grain. In particular, the dish-shaped abrasive particles had almost twice the initial cut rate of the prior art shaped abrasive particles, which is a tremendous improvement for an abrasive disc. Furthermore, the dishshaped abrasive particles maintained a higher cut rate throughout the test as compared to the prior art shaped abrasive particles.”
The claims in issue
93. Claim 1 is in the following terms, broken down into an agreed set of integers:
(1) Abrasive particles comprising:
(2) dish-shaped abrasive particles (20) each having a sidewall (28),
(3) each of the dish-shaped abrasive particles (20) comprising alpha alumina
(4) and having a first face (24) and a second face (26) separated by varying thickness (T);
(5) wherein the first face (24) is recessed
(6) and a thickness ratio of Tc/Ti for the dish-shaped abrasive particles (20) is between 1.25 and 5.00, wherein Tc is the thickness at a corner (30) of the sidewall (28) and Ti is the smallest thickness of the interior of the first face (24),
(7) wherein the sidewall (28) forms a perimeter (29) of the first face (24) and a perimeter (29) of the second face (26)
(8) and the geometric shape of the perimeter (29) is triangular, rectangular, star-shaped or that of other regular or irregular polygons,
(9) wherein, in order to calculate the thickness ratio
(a) fifteen randomly selected dish-shaped abrasive particles (20) are screened,
(b) the height of each corner (30) of each particle (20) is measured
(c) and then all of the heights are averaged to determine an average Tc,
(d) Ti of each particle (20) is measured
(e) and then the results are averaged to determine an average Ti,
(f) and the thickness ratio is determined by dividing the average Tc by the average Ti.
94. 3M also contended that claims 2, 4, 5, 6, 7 and 8 were independently valid:
2. The abrasive particles of claim 1 wherein the second face (26) is substantially planar.
4. The abrasive particles of claim 2 wherein the first face (24) is concave.
5. The abrasive particles of claim 1, 2, 3 or 4 comprising a draft angle (a) between the second face and the sidewall (28), and wherein the draft angle (a) is between about 95 degrees to about 130 degrees.
6. The abrasive particles of claim 1, 2, 3 or 4 comprising a draft angle (a) between the second face and the sidewall (28), and wherein the draft angle (a) is between about 95 degrees to about 110 degrees.
7. The abrasive particles of claim 1, 2, 3 or 4 wherein the perimeter (29) comprises a triangular shape.
8. The abrasive particles of claim 7 wherein the triangular shape comprises an equilateral triangle.
95. In its closing submissions 3M abandoned reliance on claims 7 and 8. It was impossible to understand how they could ever have been independently valid and they should have been abandoned much earlier. Counsel for 3M did not, however, abandon reliance on any of claims 2, 4, 5 and 6. He said that he would identify their relevance when addressing each of the invalidity attacks. However, he did not do that for either claim 2 or claim 4 and in my judgment neither of those claims could be independently valid over any of the attacks advanced. Nor did he explain how claim 6 could be valid if claim 5 was not. In my judgment, the issues between the parties can be decided by reference to claims 1 and 5.
Interpretation of the claims
96. The law on claim interpretation is well-known. The claims must be given a purposive construction; the question being what a skilled person would have understood the patentee to be using the words of the claim to mean - see Icescape Ltd v Ice-World International BV [2018] EWCA Civ 2219 at [60].
97. The issues between the parties on interpretation of claim 1 concerned the meaning of the term “dish-shaped abrasive particles” and the thickness ratio in integer (6) and the method for its calculation in integer (9).
98. In opening, 3M submitted that:
i) “dish-shaped” meant that a particle had an individual Tc/Ti ratio of between 1.25 and 5.00;
ii) the method for calculation of the thickness ratio in integer (9) involved the random selection of 15 particles, whether or not they were dish-shaped.
99. I believe that these submissions were abandoned in 3M’s closing, and in any event I have no hesitation in rejecting them. As to the first point, the Patent uses “dish-shaped” to refer not only to planar/concave particles, but also to concave/convex particles which are no thicker at the corners than in the middle. There is no basis for limiting “dish-shaped” in the claims to the first category of particles. As to the second point, integer (9) is clear - the random selection is of 15 dish-shaped particles. I cannot see how the term “dish-shaped” can be read out of integer (9).
100. However, I agree with the submission made by 3M (and I do not think that SG really disputed this) that the skilled person would understand that the purposes of the claim were or included: (1) to claim particles which would be expected to have the beneficial effects identified in the Patent because of their dishing in the centre and (2) to distinguish the claimed particles from the essentially flat Rowenhorst particles.
101. SG’s primary position was that the claim was unconstruable as a whole, but nonetheless it made submissions on the interpretation of various aspects of the claim.
102. The starting point for SG’s submissions was the fact that the claim is to “abrasive particles comprising dish-shaped abrasive particles”. It made the point, supported by [0009] and [0049]-[0050], that the claim contemplated that the majority of the abrasive particles present in a sample could be non-dish-shaped (as mentioned above, [0049] contemplates as little as 5% dish-shaped abrasive particles in a sample). I do not think 3M disputed this, and in any event I accept it. However, as will be seen, I do not think it has the significance or consequences which SG sought to attach to it.
103. Another key aspect of SG’s submissions concerned the interaction of integer (9) with the remainder of the claim, and in particular integer (6). SG submitted that integers (2)-(8) defined what it referred to as “Patent Particles”, one characteristic of which was that they, individually, had a Tc/Ti ratio of between 1.25 and 5.00. Then SG said that it was possible to envisage collections of particles, none of which had that individual Tc/Ti ratio, which would satisfy the integer (9) test, and also collections of particles, most of which did have that individual Tc/Ti ratio, which would fail the integer (9) test. It therefore submitted that the test provided by integer (9) was not a reliable test for whether a batch of particles was within integers (2)-(8). However, SG’s submission about “Patent Particles” is wrong. Integer (9) provides the test for determining the thickness ratio of integer (6). There is no separate assessment of individual particle Tc/Ti ratios for the purpose of integer (6), and the two integers are not in tension in the way suggested by SG.
104. Then SG submitted that, if “dish-shaped” included Fig. 4 type particles, then a blend of 33% Fig. 2 type particles (each assumed to have an individual Tc/Ti ratio of 2) and 67% Fig. 4 type particles (each assumed to have an individual Tc/Ti ratio of 1) would fall outside the claim, because the method in integer (9) would produce a thickness ratio of 1.2. That, SG said, would be inconsistent with what is said in [0009] and [0049]-[0050]. I do not agree. [0009] and [0049]-[0050] do not require that any collection of particles which comprises dish-shaped particles should fall within the claims. It is plain that the claims limit the claimed invention to collections of particles which comprise dish-shaped abrasive particles with the thickness ratio specified in integer (6) determined by the method in integer (9). Further, as will be seen from Fig. 9, improved results were obtained with planar/concave particles (which the Patent indicates had thickness ratios in the range 1.55 to 2.32) than with concave/convex particles (which will have thickness ratios of about 1). The skilled person would not be surprised that the claims were limited to collections of abrasive particles in which the average thickness ratio was above 1.25. (I should add that a person who made the blend postulated by SG by using a batch of Fig. 2 type particles each with an individual Tc/Ti ratio of 2, would infringe the claim by using that batch to make the blend.)
105. In my judgment the interpretation of the claim is clear. Integer (9) requires the random selection of 15 dish-shaped particles. They are then measured, and the thickness ratio calculated, as specified in integer (9). The thickness ratio so produced is that which is relevant for integer (6).
106. However, I do not agree with SG that this means that any sample which contains 15 dish-shaped particles which, when subjected to the integer (9) method, produce a thickness ratio of between 1.25 and 5.00, falls within the claim. The skilled person would understand that the selection of the 15 dish-shaped particles was required to be random because the patentee’s purpose was to provide a test which produced an average that was representative of a bulk of dish-shaped particles (whether it has succeeded in providing a test which is sufficiently certain is one which I shall consider below). The skilled person would appreciate that, in a sample which contained only a few dish-shaped particles, it was not possible to make a truly random selection for the purpose of the integer (9) test. The sample must contain enough dish-shaped particles to allow a truly random selection so as to achieve the patentee’s purpose. Further, I agree with what Prof Atkinson said:
“if a batch of abrasive particles contains only a small number of particles with a particular feature it may not obtain any benefit from that feature. Therefore, in my opinion a technically sound understanding of the claim may be that a batch of abrasive particles must comprise a sufficient proportion of dish-shaped particles to confer the alleged benefit of the invention.”
107. SG also raised the question of how the skilled person would determine whether a particle was dish-shaped. It is, of course, possible to envisage a continuum of particle shapes, from completely flat to undoubtedly dish-shaped. Prof Atkinson sought to illustrate such a continuum with his exhibit AA-5, which showed 42 randomly selected particles from a sample of Cubitron II, arranged roughly in order from least to most dish-shaped. He said that he did not think that the skilled person would be able to identify an objective basis to determine at what point along such a continuum the criterion of being dish-shaped was fulfilled.
108. Whether a particle is dish-shaped or not is of course a question of degree. When such questions arise they are often met with objections of the type identified by Holmes J, quoted by Jacob J in Milliken Denmark A/S v Walk Off Mats Ltd [1996] FSR 292 at 302:
“When he has discovered that a difference is a difference of degree, that distinguished extremes have between them a penumbra in which one gradually shades into the other, a tyro thinks to puzzle you by asking where you are going to draw the line, and an advocate of more experience will show the arbitrariness of the line proposed by putting cases very near it on one side or the other.”
109. In this case, in my judgment the skilled person would determine whether a particle is dish-shaped or not by reference to the teaching of the Patent, which (i) identifies exemplary dish-shaped particles; (ii) distinguishes them from the “essentially flat” particles of Rowenhorst, which can none the less have (on average) centres that are slightly thinner than their corners - see [0022]; and (iii) explains that dish-shaped particles are believed to have grinding benefits compared with “essentially flat” particles because of their dished shape (the ice cream scoop theory). In my judgment, a skilled person would not regard as being dish-shaped a particle that did not have an appearance which would be thought likely, on the basis of the ice cream scoop theory, to produce benefits compared with an “essentially flat” particle.
110. For example, SG put a model of a particle (the second Rowenhorst particle - see below) to Dr Schwabel and asked him whether it would be expected to have an ice cream scoop effect. He said it would not. In my judgment such a particle would not (contrary to the submission made by Counsel for 3M in closing) be regarded by the skilled person as being “dish-shaped” for the purposes of the Patent. The same applies to Rowenhorst particle 24 (see below).
111. SG submitted that any construction of “dish-shaped” has to be able to provide a sensible answer to the question of whether each of the following is dish-shaped:
i) The particle shown in Fig. 4 of the Patent. That is plainly dish-shaped - the Patent makes that clear.
ii) A particle which looks like those in Figs 1A/1B/2A but which has a thickness ratio of 1.2. In my judgment such a particle is dish-shaped if it resembles the particles in Figs 1A/1B/2A, and that is not affected by the fact that, when selected and measured, it has an individual Tc/Ti ratio of 1.2.
iii) A particle which looks like those in Figs 1A/1B/2A but which has a thickness ratio of 5.1. The same answer applies as in ii) above.
iv) A particle with a thickness ratio of >1.25 but which looks nothing like Figs 1A/1B/2A/2B (i.e. nothing like a dish), e.g. the second Rowenhorst particle or Rowenhorst particle 24. In my judgment, for the reasons given above, such a particle would not be regarded as dish-shaped by the skilled person.
112. In my judgment this interpretation of claim 1 (which is similar to, but not exactly the same as, that I understood to be advanced by 3M in closing) achieves the purposes identified in paragraph 100 above.
113. When the case was opened, there appeared to be a dispute about interpretation of some of the subsidiary claims, and in particular about the draft angle in claim 5, but I was told by Counsel for SG in closing that it was no longer necessary to decide any such points (unless Counsel for 3M raised a particular issue, which he did not).
the factual material
114. Before addressing the issues that arise on the validity of the claims over Rowenhorst, and on the insufficiency of the Patent, it is necessary to set out the factual material that is available which is relevant to those issues. In my judgment it is particularly important to have that material in mind given the nature of the case that was advanced by SG.
115. First, the case of anticipation run by SG was one of inevitable result in implementation of Rowenhorst. Such a case has to be assessed against the available material regarding implementations of Rowenhorst.
116. Secondly, SG did not run a case that it would be obvious, given Rowenhorst, to make dish-shaped particles. Rather, SG contended that it would have been obvious to explore the multi-dimensional space of the disclosure of Rowenhorst and that, in doing so, the skilled person would (or could) end up making dish-shaped particles within the claims. However, there are only two sets of conditions which are known to produce planar/concave particles - a set of conditions within example 1 of the Patent and a set of conditions within example 1 in another 3M patent discussed in paragraph 121 below (as will be seen below, these examples contain uncertainties about some of the conditions). So, for this case of lack of inventive step it was necessary for SG to contend that the skilled person would (or could) end up using the conditions actually used in those examples.
117. Thirdly, the case of insufficiency run by SG included (though importantly was not limited to) an allegation that it would require undue burden for the skilled person to put example 1 of the Patent into effect to obtain planar/concave particles within the claims. That case was that, because example 1 is insufficiently specific, the skilled person might initially fail to obtain such particles, and the Patent does not contain sufficient teaching to enable them to fix that problem. However, there was no evidence as to what would be obtained by carrying out example 1 in any particular manner (other than the evidence in the Patent that planar/concave particles were obtained by some implementation of example 1).
118. As 3M observed, SG did not carry out any experiments to show what would be produced by any particular implementation of the teaching of Rowenhorst, nor by any particular implementation of example 1 of the Patent. There was no reason to think that it would have been difficult to carry out such experiments, given SG’s capabilities. In any event, the result is that the amount of factual information before the court as to the result of the use of different recipes and processes is limited.
119. The information that is available is of two types. First, there are examples in various 3M patents. In the Patent itself, example 1 is said to produce planar/concave particles, example 2 to produce concave/convex particles, and example 3 to produce essentially flat particles. In Rowenhorst, examples 1 and 9 are said to produce essentially flat particles.
120. Three other 3M patents were relied on: EP 2 445 982 (“982”), EP 2 373 747 (“747”) and EP 2 373 458 (“458”). 982 has a later priority date than the Patent (22 July 2009). 747 and 458 have the same priority date as the Patent and are the European patents which derive from US 075 and US 001 respectively (it was agreed that each contained the same relevant disclosure as the corresponding US filing). It was common ground that the contents of these documents could be relied on as evidence of fact, even if they were not available as cross-references to the skilled person reading the Patent.
121. These three patents contained examples of making various types of shaped abrasive particles, but the most relevant was example 1 of 458 (set out in its [0085]-[0086]). That example was in two parts, differing only in the addition of a mould release agent. Under the conditions of that experiment, when no mould release agent was used, shards were produced, whereas when the mould release agent was used, dish-shaped particles (of the planar/concave type) were produced. 3M also relied (for purposes which will be explained later in this judgment) on example 3 of 458, which produced particles with an opening.
122. The parties produced a document setting out the recipes and processes (to the extent they are described) used in all the examples in the various 3M patents. However, the examples on which most attention focussed were examples 1 and 2 of the Patent, example 9 of Rowenhorst, the two parts of example 1 of 458 and example 3 of 458. Below is a cut-down version of the annex, focussing on those examples.
|
|
Rowenhorst example 9 - essentially flat |
Patent example 1 - planar / concave |
Patent example 2 – concave / convex |
458 example 1 [0085] - shards |
458 example 1 [0086] - planar / concave |
458 example 3 - with openings |
|
Dispersion composition (parts) |
DISPERAL 1235 Water 3026 70% nitric acid 71 |
DISPERAL 7333 Water11000 70% nitric acid 293 |
DISPERAL 4824 Water 7087 70% nitric acid 212 |
DISPERAL 1235 Water 3026 70% nitric acid 71 |
DISPERAL 1235 Water 3026 70% nitric acid 71 |
DISPERAL 4824 Water 7087 70% nitric acid 212 |
|
Mixing method |
Continuous mixing |
High shear (10 mins) |
High shear (13 mins) |
Continuous mixing |
Continuous mixing |
High shear (13 mins) |
|
Pre-mould drying |
125oC in a continuous dryer |
|
|
125oC in a continuous dryer |
125oC in a continuous dryer |
- |
|
Solids content |
~44% |
39.4% |
39.8% |
~44% |
~44% |
39.8% |
|
Ageing |
Not specified |
1 hour |
1 hour |
Not specified |
Not specified |
1 hour |
|
Mould material |
Metal coated with silicone or PTFE |
Polymeric (unknown) |
Polymeric (unknown) |
Polypropylene |
Polypropylene |
Polypropylene |
|
Cavity walls |
Vertical |
98o |
98o |
98o |
98o |
98o |
|
Release agent |
None |
None |
Peanut oil (2% in water) 1 mg/in2 |
None |
Peanut oil (0.1% in methyl alcohol) sprayed on (amount unknown) |
Residual from example 2 which uses peanut oil (2% in water) 1 mg/in2 |
|
Drying conditions |
71oC, ~20 mins, airflow not indicated |
149 oC & 163 oC, ~2.7 mins, airflow unclear |
137 oC & 121 oC, ~2.5 mins, airflow unclear |
110 oC, ~40 mins, airflow not indicated |
110 oC, ~40 mins, airflow not indicated |
135 oC & 121 oC, ~2.7 mins, airflow unclear |
123. In addition, SG relied on certain images and measurements of particles contained in two samples of particles disclosed by 3M and in a sample of Cubitron II particles extracted from 3M discs purchased in 2019. The first sample disclosed by 3M (“the first Rowenhorst sample”) was taken from a container labelled 87872-110 (a designation which matches a page from a lab notebook of Mr Rowenhorst which was also disclosed). It appears that this sample was produced in August 1991, and 3M has stated that it was made in a manner consistent with the teaching of Rowenhorst; however, the specific recipe and process used to make the sample is not known and the associated notebook page does not provide much clear useful information. The second sample disclosed by 3M (“the second Rowenhorst sample”) is believed to have been made in 1993 and to have been the particles referred to in example 3 of the Patent (which is very similar to example 9 of Rowenhorst); however again the associated notebook page does not provide much clear useful information about the specific recipe and process used. Dr Schwabel explained that in fact Mr Rowenhorst had made particles using a continuous belt system (as in Fig. 8 of Rowenhorst), with punched-through mould cavities in the belt but with a sheet underneath the cavities when they were being filled.
124. The first Rowenhorst sample was provided to a company called OR3D, which produced scans of 30 particles, said to have been randomly selected - those scans formed exhibit AA-10. The whole sample was then visually inspected to identify particles which appeared to have some degree of curvature. Particles which were not substantially full triangles were discarded, leaving about 5% of the total sample (as the sample contained about 1200-1800 particles, 5% amounted to about 60-90 particles). The remaining particles were imaged and two particles were selected for measurement. It was not suggested that the two particles were representative of the other particles in the remaining 5% of the sample. Images of the two measured particles are below:
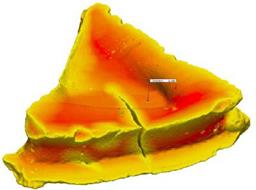

125. The measurements taken by OR3D of these particles indicate that the left hand particle (“the first Rowenhorst particle”) had a Tc/Ti ratio of 2.16 (using the “top to bottom” manner for measuring Ti which corresponds to the approach shown in Fig. 1B of the Patent), while the right hand particle (“the second Rowenhorst particle”) had a Tc/Ti ratio of 1.61 on the same basis.
126. OR3D also produced scans of 30 particles said to have been randomly selected from the second Rowenhorst sample - those scans formed exhibit AA-11. Even though Prof Atkinson expressed the view that these 30 particles showed more variation than the 30 imaged particles in the first Rowenhorst sample, no measurements were taken of any particles in the second Rowenhorst sample.
127. Finally, OR3D produced scans of 42 particles said to have been randomly selected from the Cubitron II sample - those scans formed exhibit AA-5. One particle (particle 32 in exhibit AA-5 - “the Cubitron II particle”) was measured and found to have a Tc/Ti ratio (on the top to bottom basis) of 1.32.
128. SG produced, for use at trial, 3D printed models of the first Rowenhorst particle, the second Rowenhorst particle and the Cubitron II particle. It appears that 3M also produced a 3D printed model of a particle from the first Rowenhorst sample (particle 24 in exhibit AA-10 - “Rowenhorst particle 24”). That led to SG asking OR3D to measure Rowenhorst particle 24, and introducing those measurements in opening (without objection by 3M), which showed a Tc/Ti ratio (on the top to bottom basis) of 1.20.
Validity over Rowenhorst
129. SG’s case of invalidity over Rowenhorst had three limbs. First, it contended that (a) it was inevitable that practising either example 1 or example 9 of Rowenhorst would produce abrasive particles within the claims because such batches would inevitably comprise 15 particles with individual Tc/Ti ratios of 1.25 to 5.00; alternatively (b) even if that was not inevitable, it would happen frequently, such that it was obvious to produce such particles by practising example 1 or example 9 of Rowenhorst. Secondly, it contended that obvious modifications to the teaching of Rowenhorst would lead to the production of abrasive particles within the claims. Thirdly, it contended that the abrasive particles of the claims made no technical contribution to the art compared to the abrasive particles of Rowenhorst. I shall deal with each limb of the case in turn.
Invalidity as a result of practising Rowenhorst
130. As indicated above, SG did not contend that Rowenhorst described the claimed invention of the Patent; rather it contended that Rowenhorst anticipated the claimed invention because practising either example 1 or example 9 would inevitably lead to something within the claims - see Synthon BV v SmithKline Beecham plc [2005] UKHL 59 at [22]-[23]. SG’s fall-back was to say that even if that was not inevitable (for example because it was possible to avoid producing something within the claims by taking great care) then it would happen in numerous instances of practising example 1 or example 9 and so the claimed invention was obvious. These arguments were advanced on the basis of what Counsel for SG referred to as the [0009] or “comprising” construction, i.e. that it was enough that a batch produced by following example 1 or example 9 contained 15 particles with individual Tc/Ti ratios of 1.25 to 5.00.
131. The basis for these arguments was the two Rowenhorst samples disclosed by 3M, and in particular the measurements conducted on the first Rowenhorst particle (Tc/Ti ratio 2.16) and the second Rowenhorst particle (Tc/Ti ratio 1.61) from the first Rowenhorst sample. SG suggested that any attempt to put example 1 or example 9 of Rowenhorst into practice would result in a similar level of variation to that seen in the two Rowenhorst samples, and so any production run would contain a certain number of particles (SG suggested well over 15, and put it at the level of a single-figure percentage) which have individual Tc/Ti ratios of 1.25 or greater.
132. I do not accept these arguments. First, in my judgment the evidence comes nowhere near to establishing that carrying out example 1 or example 9 of Rowenhorst would inevitably (or even frequently) produce batches containing particles with individual Tc/Ti ratios of 1.25 or greater at the levels contended for by SG. The Rowenhorst examples allow for variation in numerous aspects of their implementation and, as explained above, there is little specific information about the recipe and process used to make the Rowenhorst samples, though it appears that they were made using a continuous process using a punched-through mould (with a sheet under the cavities while they were being filled) whereas it is common ground that the Rowenhorst examples at least allow the use of muffin tray type moulds. The Rowenhorst samples therefore do not allow general conclusions to be drawn about implementation of the Rowenhorst examples. Nor, I should add, does the Cubitron II sample (there was no evidence of the recipe or process used to make that sample).
133. Further, the only Tc/Ti measurements provided and relied on by SG were of the first and second Rowenhorst particles. As explained above, these particles were selected for measurement and were not suggested to have been representative of the 5% of the sample (about 60-90 particles) which had been identified as having “some degree of curvature”. This provides no basis for SG’s submission that any batch (or of many batches) of particles produced according to one of the Rowenhorst examples will contain a single-figure percentage of particles with individual Tc/Ti ratios of 1.25 or greater. I cannot see how this is improved by reference to the Cubitron II particle (with a Tc/Ti ratio of 1.32, selected from 42 imaged particles).
134. I have not been able to identify any expert evidence which established that a single-figure percentage of particles would have individual Tc/Ti ratios of 1.25 or greater, or even that at least 15 particles in a batch would have such Tc/Ti ratios. It was put to Dr Schwabel, and he agreed, that a skilled person making their first attempt to implement Rowenhorst would inevitably make “some particles” that looked like those shown on a page which depicted the Cubitron II particle and the first and second Rowenhorst particles. That does not establish the number or percentage of such particles that would be produced. In closing submissions, SG referred to some evidence of Prof Atkinson that “it is inevitable that there will be some variation in the shape of abrasive particles produced by any particular method”, but again that is far too non-specific for SG’s purposes.
135. SG also referred to some oral evidence of Prof Atkinson (day 2, 213/11 - 214/14) relating to the aspect of [0022] of the Patent which I have quoted in paragraph 68 above. SG submitted that Prof Atkinson’s evidence was to the effect that this passage indicated that the skilled person could make a batch in which 95% of the particles had individual Tc/Ti ratios of between 0.94 and 1.15, with 2.5% having individual ratios above 1.15, and 2.5% having individual ratios of below 0.94. Even if [0022] did teach what SG says Prof Atkinson suggested (which I do not accept), that would not help SG, because it would not indicate the proportion of particles with individual Tc/Ti ratios above 1.25.
136. Secondly, for reasons that I have explained above, I do not accept what Counsel for SG called the [0009] or “comprising” construction - it is not enough for a batch to fall within claim 1 that it comprises 15 particles with individual Tc/Ti ratios of 1.25 or greater. Nor do I accept that it is sufficient only to focus on whether particles have individual Tc/Ti ratios of 1.25 of greater. In order to be selected for analysis in the integer (9) test, the particles must also be dish-shaped and, as I have stated, in my judgment a skilled person would not regard the second Rowenhorst particle as being dish-shaped.
Obvious modifications of Rowenhorst
137. As I have mentioned, SG did not advance a case that it would have been obvious for the skilled person, given Rowenhorst, to seek to make dish-shaped abrasive particles. Rather, its case was that it would have been obvious for the skilled person, given Rowenhorst, to make “workshop modifications” to the teaching of Rowenhorst which in fact would (or could) lead to the production of dish-shaped abrasive particles, even though the skilled person was trying to make the essentially flat abrasive particles of Rowenhorst. Given the limited evidential material, that case focussed on the skilled person ending up using a recipe and process in accordance with either example 1 of the Patent or example 1, [0086] of 458.
138. In approaching this limb of SG’s obviousness case, I do so on the basis that the Patent does indeed plausibly disclose a technical benefit of the claimed dish-shaped particles over the essentially flat particles of Rowenhorst (I shall consider whether it does in fact do so in the following section).
139. In relation to this aspect of the case, both parties referred me to the summary of the law by Lord Hodge in Actavis Group PTC EHF v ICOS Corp [2019] UKSC 15 at [52]-[73].
140. 3M highlighted what Lord Hodge said in [70] about the relevance of the motive of the skilled person, who is not assumed to undertake technical trials for the sake of doing so but only because they have some end in mind. It also highlighted what he said in [72] about the dangers of a step-by-step analysis informed by hindsight.
141. In that paragraph, Lord Hodge cited a statement by Floyd J (as he then was) in Gedeon Richter plc v Bayer Schering Pharma AG [2011] EWHC 583 (Pat). 3M drew my attention to the fact that in his judgment in the Court of Appeal in Actavis v ICOS [2017] EWCA Civ 1671 at [158]-[160], Floyd LJ had adhered to that statement, and also to a similar statement he had made as a first instance judge in Teva UK Ltd v Merck & Co Inc [2009] EWHC 2952 (Pat) at [98]:
“I think that Mr Birss is right that one must proceed with caution when faced with an obviousness attack based on a suggestion that the skilled person would embark on a research program in the course of which he would discover that a product or compound was effective. That is particularly so where the technical effect is one which is newly discovered, or impossible or very hard to predict. That is because the expectation of success may be zero, or inadequate to drive the research forward. In the end it will all depend on weighing the various factors as they appear from the evidence in the case.”
142. To that one could add what Jacob LJ said in Leo Pharma A/S v Sandoz Ltd [2009] EWCA Civ 1188 at [7]-[10]:
So the case is unusual. The ordinary obviousness attack consists of a contention that the skilled person would, using his technical knowledge, discern the invention from the prior art. The case here is that the skilled person would have come upon the invention (the hydrate and its benefit) without any expectation of successfully finding a better product.
That sort of obviousness attack should be scrutinised with great care. I do not say it could not succeed, but one must be very confident that the steps said to lead to the discovery of a new and beneficial product "by accident" as it were, were at the least, really likely, almost mandated. If you need to do research to find an invention then, for a finding of obviousness, that research must be of a kind which a skilled man would do, not which he might do.”
143. When considering SG’s case, logically the first question is whether the skilled person would have any motivation to conduct any workshop modifications of Rowenhorst. Rowenhorst was 14 years old at the priority date, and it was common ground that in the intervening period no commercial implementation of it had emerged. Dr Schwabel pointed out that there were no commercially available sol-gel abrasive particles made by casting into shaped moulds, and the focus was on abrasive grains with high aspect ratios such as SG’s Extra Sharp and Targa grains. Dr Schwabel suggested that in those circumstances the skilled person might disregard Rowenhorst completely. I do not agree. Rowenhorst discloses something which would be new to the skilled person at the priority date (the idea of making abrasive particles using the sol-gel process by shaping them using moulds). It also contains data showing that triangular shaped abrasive particles had significant advantages over Cubitron grain. Further, the skilled person would not know why 3M had decided not to commercialise the Rowenhorst particles - there could have been a variety of reasons and it could not be concluded that there was nothing to be gained from investigating Rowenhorst particles.
144. Prof Atkinson’s evidence was that the skilled person would want to “explore the landscape that is set out in Rowenhorst as being interesting” and “explore the boundaries of its operational envelope”, in order to explore how sensitive the process was to changes in its numerous variables, and with a view to trying to increase the speed of production. More specifically, his evidence was that the skilled person would consider varying the solids contents of the dispersion, altering the material used for the mould and/or a mould release agent, and increasing the drying temperature. He pointed out that example 1 of the Patent lay within the scope of the variations he had proposed and that accordingly the skilled person could arrive at a process which leads to the production of planar/concave particles.
145. More specifically still, in annexes D and E to his second report, Prof Atkinson identified the differences between the general teaching of Rowenhorst and example 1 of the Patent (annex D) and between example 9 of Rowenhorst and example 1 [0086] of 458 (annex E) and explained why in his view the changes were ones which the skilled person could have made without invention. (As I have indicated above, one of the problems for SG was that only two sets of conditions were known, as a matter of fact, to produce planar/concave particles, and even those sets of conditions contained various uncertainties.)
146. In closing, SG made it clear that its case based on annex E was the stronger of the two. The differences of significance between example 9 of Rowenhorst and example 1 [0086] of 458 lay, as can be seen from the table in paragraph 122 above, in the mould material, the mould release agent and the drying conditions. However, while Prof Atkinson explained that the skilled person would want to increase the drying rate to improve process efficiency, he did not explain why that would result in the drying conditions used in example 1 [0086] of 458, nor why it would have been obvious simultaneously also to make the changes to mould material and mould release agent between example 9 of Rowenhorst and example 1 [0086] of 458.
147. The nature of Prof Atkinson’s evidence became clear in a passage of evidence (day 2, 164/18 - 166/8) by reference to a diagram which he drew which became XX/12 (the table being referred to is one at XX/5 setting out the various examples, similar to that eventually agreed by the parties and referred to in paragraph 122 above):
“I think we have discussed now that there are lots of different parameters that affect the shape of the particle as it is drying in a mould that are listed in this particular table. They are listed there as process parameters but perhaps they could be reclassified in some way as four major parameters; for example, there is the solids content, the rheology when it goes into the mould, the stickiness of the mould, which is related to the mould material, and there is the drying rate and the amount of water that is lost. So there is a multi-dimensional space here and we could represent that by some kind of two-dimensional representation with one of the examples of Rowenhorst sitting there, and the skilled person is looking out in different directions interested in where this boundary is. Somewhere within this space is Example 1 of the patent, and also within this space, as you can see from this table, is another set of conditions that gives a dished particle. So let us say that is here - these are not representing anything in particular - and I think that appears as the third from the last, no, not the third from the last column, it is EP '458, dish-shaped particles Figure 7, yes, Figure 2, Example 1 in paragraph 86 of EP '458. So there are two that are known, and presumably there is some spread around these. They may even overlap. All I am saying is that by moving around this space, by changing the variables that Rowenhorst has indicated could be changed within certain ranges, then there is a chance that you would hit these conditions. So obtaining these Figure 2-type dish-shaped particles would be a deviation from the Rowenhorst central teaching, if you like, to give you an example, but it is still within the scope of the overall parameters and it produces a particle that is interesting. If we look at this table more generally, then you can see set out there that there is a whole range of different outcomes that are possible that are scattered around the boundaries of this general procedure and may be included within them as well, is probably included within them as well. So there are lots of different outcomes that you can get just by playing around - in my opinion, without invention - with the parameters that are suggested in the Rowenhorst patent.”
148. To my mind, this evidence demonstrated, in a vivid fashion, why this case of obviousness fails. The case relies on the skilled person chancing on a combination of conditions within a multi-dimensional space. While there may have been reasons why the skilled person would consider making changes to certain variables, Prof Atkinson did not identify why the particular combination of variables would be arrived at by the skilled person. Once one knows the combinations of variables which produce planar/concave dish-shaped particles (from example 1 of the Patent and example 1 [0086] of 458, though even then not all the precise conditions are known) it is possible to explain how the skilled person could have arrived at those combinations of variables, and to produce reasons why each change of variable could have been made. But in my judgment this case is a classic step-by-step analysis infected by hindsight knowledge of the answer.
149. I should add that I do not accept that any assistance can be gained from the work done by 3M which led to the Patent and the other 3M patents, which SG suggested was merely “routine tinkering”. The fact that 3M did that work and applied for patents for it gives rise to a question about whether the claimed invention was obvious; it is entirely circular to suggest that it also answers that question.
150. There is another, independent, reason why in my judgment this case fails. Rowenhorst emphasises the importance of taking steps to ensure that the abrasive particles produced are flat (see the passages quoted in paragraphs 51(ii)&(v) and 52 above). Further, SG’s case involves accepting that the skilled person would be trying to make abrasive particles which were flat. Its contention is that the skilled person would, by mistake, end up at the end of the fourth stage of the Rowenhorst process (which involves drying in the mould) with particles which are not flat but planar/concave. But these are not abrasive particles, rather they are precursors of abrasive particles. In order to produce abrasive particles, it is necessary to subject the precursors to the sixth and seventh steps of the process (calcining and sintering). In my judgment, a skilled person who was trying to produce flat abrasive particles would not carry on and subject planar/concave precursor particles to calcining and sintering. Rather, they would remove the precursor particles from the moulds, discard them, and alter the conditions of the process to try to avoid repeating the mistake. Prof Atkinson thought that planar/concave precursor particles would be regarded as “interesting” and would not be “disregarded”, but in my judgment that would not be the reaction of a non-inventive skilled person trying to make flat triangular particles following the teaching of Rowenhorst.
151. In case I am wrong about the above, I should say that the evidence clearly established that there was no invention in introducing a draft angle of about 95o to about 130o (or about 110o). Rowenhorst suggests making particles in the shape of a frusto-pyramid, and the experts were agreed that doing so would make it easier to fill the moulds, and to get the dried particles out of the moulds. The range of angles claimed include obvious draft angles to choose if making a frusto-pyramid for either of those purposes. It follows that, if claim 1 were invalid for obviousness over Rowenhorst, so would be claim 5 (and claim 6).
Lack of technical contribution over Rowenhorst
152. I was referred to numerous authorities on what is often called AgrEvo obviousness. It is sufficient to cite what Floyd LJ said in Generics (UK) Ltd v Yeda Research & Development Co Ltd [2013] EWCA Civ 925 at [49]:
“I would summarise the position thus far in the following way:
i) Article 56 of the EPC is in part based on the underlying principle that the scope of the patent monopoly must be justified by the patentee’s contribution to the art;
ii) If the alleged contribution is a technical effect which is not common to substantially everything covered by a claim, it cannot be used to formulate the question for the purposes of judging obviousness;
iii) In such circumstances the claim must either be restricted to the subject matter which makes good the technical contribution, or a different technical solution common to the whole claim must be found;
iv) A selection from the prior art which is purely arbitrary and cannot be justified by some useful technical property is likely to be held to be obvious because it does not make a real technical advance;
v) A technical effect which is not rendered plausible by the patent specification may not be taken into account in assessing inventive step;
vi) Later evidence may be adduced to support a technical effect made plausible by the specification;
vii) Provided the technical effect is made plausible, no further proof of the existence of the effect is to be demanded of the specification before judging obviousness by reference to the technical effect propounded.”
153. Floyd LJ went on to hold that later technical evidence could also be used to disprove the existence of a technical effect made plausible by the specification, but that does not arise in the present case because neither party relied on such evidence.
154. Floyd LJ also went to observe (at [65]) that the fact that the primary technical contribution advanced by a patent turns out to be implausible or untrue does not necessarily lead to the conclusion that a patent is obvious. The patentee may still be able to advance an alternative less ambitious technical contribution, and the party attacking the patent will still have to convince the court that the invention was obvious on that basis. For example, it may be possible to contend that a patent provides alternative products with the same properties as those of the prior art. In my judgment such an argument cannot succeed where the claimed invention is a selection from the prior art, as such a selection would be arbitrary. However, it is not necessary to get into that debate, or consider whether the invention in this case is a selection from the prior art (because the conditions which lead to the claimed invention are within the scope of those disclosed in Rowenhorst) because Counsel for 3M accepted that if the technical effect advanced in the Patent was not plausible, then the Patent was invalid. He did not advance an alternative technical effect.
155. As to the standard of plausibility, I was referred to the well-known passage in the judgment of Lord Sumption in Warner-Lambert Co LLC v Generics (UK) Ltd [2018] UKSC 56 at [37]. In summary, the specification must disclose some reason for supposing that the assertion as to the technical effect is true, i.e. something that would cause the skilled person to think that there was a reasonable prospect that the assertion would prove to be true. That may be experimental data, or it may consist of a priori reasoning.
156. In the present case, the technical effect asserted by the Patent is that dish-shaped particles produce grinding benefits compared to the essentially flat particles of Rowenhorst. In support of that, the Patent advances two theories - the ice cream scoop theory, which is advanced in relation to both planar/concave and concave/convex particles, and the thinness theory, which is advanced in relation to planar/concave particles. It also provides the experimental data in Fig. 9 (annotated, coloured version set out in paragraph 91 above).
157. I shall start by considering the theories (the a priori reasoning). 3M placed most emphasis on the thinness theory, perhaps because it was advanced only in relation to planar/concave particles. The thinness theory can be seen, from [0019], to have two aspects which are said to arise from having “a thinner interior portion of the shaped abrasive particle”. The first is that a wear flat “will have less area as compared to a shaped abrasive particle having a thicker interior section”. The second is that “a thicker particle is less likely to fracture than a thinner particle”, so thinner particles are more likely to be able to re-sharpen through fracture.
158. However, as is apparent from what is stated in [0019], and as was convincingly demonstrated in cross-examination of Dr Schwabel, the key factor for this theory depends on the absolute thickness of the interior portion of the particle rather than on its shape or thickness ratio. Assuming the theory to be true, whether a particle will demonstrate improved grinding benefits compared to a Rowenhorst particle depends solely on how thick the interiors of each particle are. I therefore agree with SG that the thinness theory is not capable of supporting the claimed invention across its breadth.
159. Prof Atkinson did not suggest, in his reports, that the ice cream scoop theory was implausible, and in cross-examination he expressed the view that it was plausible. His concern was that the Fig. 9 data did not support it (a point which I shall consider below). He did make the point that, because it would not be possible to orient all the particles so that they presented a scoop to the workpiece (as shown in Fig. 1C) some of the particles would present the workpiece with the back of the scoop. He said that would be worse than a flat Rowenhorst particle, but he did not suggest that it would cancel out any effect from those particles which did present a scoop.
160. It was put to Dr Schwabel, and he agreed, that one would not want to scoop out balls of material from a workpiece in the way that one would scoop out ice cream from a tub, because such balls of material would fill the spaces between the grains and create friction, so interfering with the abrading process. However, it was not suggested to him that the result of using particles of the type claimed would be to remove balls of material, or that the Patent said that it would be, nor was it put to him that the ice cream scoop theory was implausible as a basis for claiming that the claimed particles had grinding benefits.
161. As mentioned above, Prof Atkinson’s concern was about the data in the Patent. In closing, SG submitted that any otherwise plausible theory in the Patent was disproven by the data which it contained and was therefore not plausible in the light of that data.
162. Fig. 9 shows the results of a grinding test using four different grains: planar/concave particles from example 1, concave/convex particles from example 2, flat particles from example 3 and random crushed Cubitron grain. As can be seen, the performance in that test of the planar/concave particles (the orange line in the diagram in paragraph 91 above) is initially double that of the flat particles (the green line) and remains superior to all other particles at least before 12 minutes. The performance of the concave/convex particles (the blue line) initially is close to that of the planar/concave particles, but declines to become comparable to that of the flat particles by 4 minutes.
163. Prof Atkinson made the point that there were three significant differences between the two sets of dish-shaped particles tested and the prior art particles tested: (1) dish-shaped rather than flat main faces, (2) use of a 98o draft angle for the side walls, rather than a 90o side wall, and (3) sharp, rather than rounded, edges and corners. He said that the teaching of the Patent was that difference (2) alone should cause a doubling in performance, and that it would be expected that difference (3) should also improve performance. Therefore, he said, the difference in performance between the planar/concave particles (the orange line) and the flat particles (the green line) could be explained by difference (2) and at least by differences (2) and (3) in combination. He therefore concluded that there was no credible evidence of any benefit from difference (1).
164. I do not agree that the data in the Patent disprove the ice cream scoop theory so as to render it implausible, or establish that there is no technical benefit associated with the claimed particles (or make it implausible that there is).
165. In my judgment, Prof Atkinson’s analysis seeks to draw conclusions from the data which cannot be justified. First, as he observed in another context (a comparison between the processes used in examples 1 and 2 of the Patent), it is an important scientific principle that in order to obtain comparable data it is important to change only one variable at a time. As a result, I do not believe one can seek to analyse the reasons for the difference between the performance of the planar/concave particles and the flat particles in the way that he did.
166. Perhaps more importantly, his analysis relies on the statement in [0023] that “a slight increase in the draft angle from 90 degrees to 98 degrees has been found to double the cutting performance of triangular shaped abrasive particles” as being a statement of fact which is entirely general, applying to all triangular abrasive particles regardless of their other characteristics, and regardless of the test in which their performance is being analysed. In my judgment a skilled person would not understand it that way, but as reporting a result which had been obtained in US 075, which has been referred to in the immediately preceding sentence (but which the skilled person cannot consult). Further, as Dr Schwabel said (and as Counsel for SG pointed out in closing, he was an expert in abrasives whereas Prof Atkinson was not) the skilled person would know that one cannot generalise from performance in one type of abrasives test to performance in all other types of abrasives test. Certainly, I do not believe that the statement in [0023] can be treated as factually correct, such that the doubling of performance from use of a 98o draft angle can be used in combination with the Fig. 9 data to disprove the alleged technical effect.
167. Indeed, as Prof Atkinson himself observed, comparison between the performance of the concave/convex particles with a 98o draft angle (the blue line) and the flat particles with 90o side walls (the green line) showed that a doubling of performance was not achieved. In my judgment the skilled person would reconcile that by recognising that the statement in [0023] was not of entirely general applicability, rather than postulating that the comparison between the blue and green lines is to be explained by the dish-shaped faces of the concave/convex particles having a negative effect.
168. For these reasons, in my judgment SG has not established that the Patent does not disclose a plausible technical effect associated with the claimed invention.
169. SG suggested that an inference could be drawn from the fact that 3M’s commercial product, Cubitron II, comprises essentially flat triangular particles. It suggested that the inference was that 3M had found that dish-shaped particles provided no technical benefit. I do not agree that such an inference can be drawn. It is entirely likely that, as Dr Schwabel explained, 3M decided that Cubitron II particles were the ones which could routinely provide the best performance at reasonable cost.
170. I should add that SG made no attempt to suggest that there was no plausible technical effect associated with the invention of claim 5 - indeed it was a fundamental plank of its case on lack of plausible technical effect of claim 1 that there was a technical effect associated with the draft angles of claim 5. However, had claim 1 been invalid for lack of any plausible technical effect, claim 5 would also have been invalid for the reasons explained in paragraph 151 above.
Insufficiency
Uncertainty-type insufficiency
171. There is, of course, only one statutory ground of invalidity due to insufficiency, namely that provided by s.72(1)(c) Patents Act 1977 (which is intended to have the same effect as Art. 83 EPC):
“the specification of the patent does not disclose the invention clearly enough and completely enough for it to be performed by a person skilled in the art.”
172. However, it has been recognised that this ground of invalidity can arise in a number of ways, one of which is now, following the judgment of the Court of Appeal in Anan Kasei Co Ltd v Neo Chemicals & Oxides Ltd [2019] EWCA Civ 1646, referred to as uncertainty.
173. In Kirin-Amgen Inc v Hoechst Marion Roussel Ltd [2004] UKHL 46, the claim in question was to recombinant erythropoietin (rEPO) distinguished by having a higher molecular weight by SDS-PAGE than EPO from urinary sources (uEPO). But the patent did not identify the uEPO to be used as a comparator and the evidence established that not all uEPOs had the same molecular weight by SDS-PAGE, such that comparing a sample of rEPO with different samples of uEPO could give different results. The House of Lords held that in those circumstances the lack of clarity as to which uEPO to use meant that it was not possible for a skilled person to perform the invention.
174. In Sandvik Intellectual Property AB v Kennametal UK Ltd [2011] EWHC 3311 (Pat), the claim required a parameter to be within certain numerical limits (1.3 to 1.5). The patent required a comparison between measured intensity and a standard intensity, but did not identify the standard to be used (i.e. which PDF card to use) or whether to apply a particular approach (Ka2 stripping). The evidence was that the choice of PDF card, and whether or not Ka2 stripping was applied, each made a difference to the result (about 0.1 in the former case and about 5-10% in the latter case). Arnold J held that the patent was insufficient because it was uncertain what the correct test was to determine whether a product was within the claim.
175. In both those cases, the court distinguished the case before it from one which merely “throws up the possibility of doubtful cases”, i.e. one in which the claim has a “fuzzy boundary”. As Floyd LJ put it in Anan Kasei at [23], having referred to Kirin-Amgen:
“The House of Lords did not throw any doubt on the principle that a claim is not rendered insufficient because there is some room for doubt, or fuzziness, at the edge of the claim. The claim in Kirin-Amgen was insufficient because it was conceptually uncertain.”
176. Floyd LJ went on at [25] to explain that such cases arise where the process of interpretation could not resolve the question of what the patentee had in mind for the necessary test. Lewison LJ put it similarly at [101]:
“If the court cannot ascertain the boundary, having used all the interpretative tools at its disposal, it must conclude that the specification does not disclose the invention clearly enough and completely enough for it to be performed by a person skilled in the art.”
177. Lewison LJ went on, at [103]-[104], to approve the following statements from Generics UK Ltd v Yeda Research & Development Co Ltd (the first being by Arnold J [2012] EWHC 1848 (Pat) at [193], with the substitution of “uncertain” for “ambiguous”, and the second being by Floyd LJ [2013] EWCA Civ 925 at [78]):
“It is also common for claims to have a fuzzy boundary, because an integer of the claim involves some question of degree or an imprecise functional limitation. It is well established that is not itself objectionable. If a claim is truly [uncertain], so that it is not clear what is the correct test to determine whether or not a product or process infringes, however, then the claim is insufficient.”
“It is sometimes difficult to determine where the precise boundary of a claim lies. In such cases what matters is whether the skilled person knows what the test is he has to apply to determine infringement.”
178. In Anan Kasei, the allegation of uncertainty-type insufficiency failed for the reasons explained by Floyd LJ at [28]-[32]. The process of interpretation identified the question to be asked (whether the added ingredient had a material effect on the essential characteristics of the product). The evidence did not establish that there was any more than a fuzzy boundary to the claim as so interpreted. Further, it was wrong to ask whether a purchaser of a product could test for infringement; the question was whether the patent disclosed the invention clearly enough and completely enough for it to be performed by a person skilled in the art.
179. In my judgment this final point has some significance in the present case. It places the focus on whether a skilled person could perform the invention and, in the case of a product claim, performing the invention means making or otherwise obtaining the product (see Generics UK Ltd v H Lundbeck A/S [2008] EWCA Civ 311 at [30]).
180. The allegations of uncertainty-type insufficiency were twofold. First, SG contended that the claim was uncertain because it did not specify how to identify whether a particle was dish-shaped and/or eligible for inclusion in the integer (9) test. I have dealt with SG’s points above when considering interpretation of the claims. I have identified how the skilled person would determine whether a particle was dish-shaped for the purpose of the Patent, and while that is a question of degree which will give rise to a fuzzy boundary, in my judgment that does not give rise to insufficiency.
181. Secondly, SG contended that the results of the integer (9) analysis could differ depending on the selection of dish-shaped particles made. The point was pleaded in this way:
“Whether a plurality of particles will fall within the claim cannot be determined with certainty since it will depend on the thickness ratio of the individual particles identified by the random selection process. Precisely the same plurality of particles may fall within the claim if one set of 15 was selected from within that plurality, but may not fall within the claim if another set of 15 was selected.”
182. I accept that in principle this could give rise to an uncertainty-type insufficiency. At one level, the test which the skilled person has to conduct is clear - namely, make a random selection of 15 dish-shaped particles, measure their Tcs and Tis, and perform the averaging. But the same could be said of the test in Kirin-Amgen - select a uEPO and compare its molecular weight with the rEPO using SDS-PAGE. The problem in Kirin-Amgen was that the particular uEPO to select was not identified and the skilled person could select ones with materially different molecular weights by SDS-PAGE. So too here - the skilled person could in theory make different random selections of 15 dish-shaped particles which would give materially different average Tc/Ti ratios.
183. I was not convinced by 3M’s attempts to draw parallels with the application of the de minimis principle in cases of patent infringement (see Napp Pharmaceutical Holdings Ltd v Dr Reddy’s Laboratories (UK) Ltd [2016] EWHC 1517 (Pat) at [136]-[149]). That principle applies when the proportion of a product which falls within a claim is so trifling that the law ought not to concern itself with it. The problem here is that the test provided by integer (9) is intended to determine whether a batch as a whole falls within the claim or not.
184. However, I do not accept that it is enough for a possibility that different random selections will give materially different average Tc/Ti ratios to exist in theory. I do not believe that if, in Kirin-Amgen, the differences in the molecular weights of different uEPOs by SDS-PAGE were so small as to be undetectable, or “lost in the noise” of the test given the other sources of error or uncertainty in such an experiment, the claim would have been held invalid for insufficiency. In my judgment it is necessary for a party advancing a case of insufficiency of this type to show that the uncertainty in the test is one which can give rise to a difference in the result obtained in practice. Only then can it be said that it causes a problem for the skilled person seeking to perform the invention. In my judgment as part of such an assessment it will be appropriate to consider whether there are other sources of experimental error which will swamp any variation in result which may be attributable to adopting different versions of the test.
185. Prof Atkinson recognised that “if the variation in the shape of a batch of abrasive particles is narrow then the answer to whether that batch falls within the claim should be quite consistent over any number of random samples of 15 abrasive particles”. He said that this would not be the case if the variation was not so narrow. However, the evidence did not address, in the level of detail which in my judgment would be required, the range of variation in the shape of dish-shaped particles, and the distribution of those different shapes, which could realistically be expected from a production run or, importantly, the difference in average Tc/Ti ratios which would arise from different random selections of 15 particles from such a distribution. I suspect that, in order to determine the distribution of average Tc/Ti ratios that would arise from different random selections of 15 particles from within a distribution of different particle shapes, evidence from a statistician would have been needed, as well as evidence about the nature of the distribution from which the selections were being made.
186. As Prof Atkinson pointed out, there was little information about the variability that might be observed in the average Tc/Ti ratio. For example, no attempt was made by SG to measure the Tcs and Tis for a large number of particles in the first or second Rowenhorst samples (or the Cubitron II) sample and show what variation in average Tc/Ti ratios could be obtained by different selections of particles. Prof Atkinson noted that the Patent reports values of 1.55 to 2.32 for particles of the invention and 0.94 to 1.15 for particles produced according to Rowenhorst. However, in my judgment these would not be understood as resulting from different random selections on the same batch, but as resulting from random selections on different batches (if it were otherwise, the patentee would scarcely have advanced the integer (9) test as the means for determining infringement); certainly, it is not clear that they are an indication of variation of the average Tc/Ti ratios that arise from different random selections from a given distribution.
187. Prof Atkinson advanced some potential scenarios, including a batch of particles with individual Tc/Ti ratios distributed evenly over a range from 1.20 to 1.30, and a batch of particles with individual Tc/Ti ratios forming a bell curve distribution between 1.20 and 1.30. He made the point that in either case, the chances of making a random selection of 15 particles giving an average Tc/Ti ratio of above 1.25 would be 50% (and 50% of giving an average Tc/Ti ratio below 1.25).
188. SG seized upon 3M’s concession that it was possible to come up with such a “thought experiment” as being an admission of defeat. I do not agree. First, there was no evidence to demonstrate that either distribution was one which was likely to arise from a real production run (and I believe that Prof Atkinson recognised that his first scenario was unrealistic). Further, there was no evidence of the distribution of average Tc/Ti ratios that would result from random selections of 15 particles in such distributions. For example, in Prof Atkinson’s second scenario, it is intuitively obvious that the distribution of average Tc/Ti ratios will be narrower than the distribution of Tc/Ti ratios for individual particles, but no attempt was made to identify what it would be. Of course, it can be said that 50% of the average Tc/Ti values will be above 1.25 (and 50% below), but it may be that the differences in average Tc/Ti values caused by making different random selections are insignificant when compared with the errors and uncertainties introduced by measuring Tcs and Tis for individual particles. If so, the level of uncertainty introduced by the fact that the test allows for different random selections of 15 particles would not be a material one.
189. In closing submissions, Counsel for SG advanced a different scenario. He postulated a case in which a production run had been carried out which produced particles with a Tc/Ti ratio of 1.2, and another production run had produced particles with a Tc/Ti ratio of 1.3, and those two batches had been mixed together. He then said that one had to be able to answer the question of whether that mixed batch infringed. I do not agree that this kind of scenario shows that there is an uncertainty-type insufficiency. Ultimately, the question is whether the patent is sufficient to enable the skilled person to perform the invention. In Counsel for SG’s scenario, the skilled person is plainly performing the invention by making the batch of particles with a Tc/Ti ratio of 1.3 and using that to produce a mixed batch. Following the approach of Floyd LJ in Anan Kasei, it does not matter for these purposes that a purchaser of a mixed batch who does not know how the mixed batch has been produced might have difficulties determining whether or not it fell within the claims.
190. For the reasons I have explained above, in my judgment SG has not succeeded in demonstrating that the fact that the test in integer (9) allows for different random selections of particles gives rise to a practical problem in performing the invention, as opposed to a theoretical one.
Undue burden
191. Both parties referred me to the summary of the law by Kitchin J in Eli Lilly & Co v Human Genome Sciences Inc [2008] EWHC 1903 (Pat) at [239]:
i) the first step is to identify the invention and that is to be done by reading and construing the claims;
ii) in the case of a product claim that means making or otherwise obtaining the product;
iii) in the case of a process claim, it means working the process;
iv) sufficiency of the disclosure must be assessed on the basis of the specification as a whole including the description and the claims;
v) the disclosure is aimed at the skilled person who may use his common general knowledge to supplement the information contained in the specification;
vi) the specification must be sufficient to allow the invention to be performed over the whole scope of the claim;
vii) the specification must be sufficient to allow the invention to be so performed without undue burden.”
192. As to what amounts to undue burden, I was referred to what Aldous J said in Mentor Corp v Hollister Inc [1991] FSR 557 at 562:
“[The skilled person] must seek success. He may need to carry out the ordinary methods of trial and error, which involve no inventive step and generally are necessary in applying the particular discovery to produce a practical result. In each case, it is a question of fact, depending on the nature of the invention, as to whether the steps needed to perform the invention are ordinary steps of trial and error which a skilled man would realise would be necessary and normal to produce a practical result.”
193. Aldous J went on to say that he regarded his view as being consistent with what the Technical Board of Appeal of the EPO had said in T 226/85 Unilever / stable bleaches at [8]:
“Even though a reasonable amount of trial and error is permissible when it comes to the sufficiency of disclosure in an unexplored field or - as it is in this case - where there are many technical difficulties, there must then be available adequate instructions in the specification or on the basis of common general knowledge which would lead the skilled person necessarily and directly towards success through the evaluation of initial failures or through an acceptable statistical expectation rate in case of random experiments.”
194. SG pressed me with the decision of the Technical Board of Appeal in T 1743/06 Ineos / amorphous silicas. I do not believe that this establishes any different principle of law; indeed the Board’s statement of the law at [1.9] is essentially identical to the passage from T 226/85 quoted above.
195. On the need for the specification to be sufficient to allow the invention to be performed without undue burden across the whole scope of the claim, I was referred to the judgment of Lord Briggs in Regeneron Pharmaceuticals Inc v Kymab Ltd [2020] UKSC 27 and to Birss LJ’s analysis in Illumina Cambridge Ltd v Latvia MGI Tech SIA [2021] EWHC 57 (Pat) at [248]-[279].
196. In its written closing submissions (but not its oral closing) 3M suggested that it was not necessary for the Patent to disclose how to make (without undue burden) particles at each end of the claimed range (i.e. particles with an average Tc/Ti of 5.00 as well as particles with an average Tc/Ti of 1.25). I do not agree. In my view it is clear that the range of 1.25 to 5.00 in the claim is what Birss LJ referred to in Illumina as a range relevant in the Regeneron sense. It is plain that the teaching of the Patent is that the average Tc/Ti ratio is a variable which significantly affects the value or utility of the product in achieving its relevant purpose, because it is shown to affect the grinding benefits conferred. Indeed, the fact that the particles are dish-shaped with an average Tc/Ti ratio in the range 1.25 to 5.00 is the essence or core of the claimed invention.
197. It follows that, to avoid insufficiency, it is necessary for the Patent to enable the skilled person to make, without undue burden, abrasive particles with average Tc/Ti ratios at the upper end of the claimed range as well as those with an average Tc/Ti ratio at the lower end of that range. I accept 3M’s submission that it is not necessary for the skilled person to be able to “produce at will particles which have any one of the infinite number of Tc/Ti ratios which fall within the range specified in claim 1”, but that does not mean that the Patent is sufficient if it only enables the skilled person to produce particles with an average Tc/Ti up to, say, 2.5.
198. SG’s case on undue burden had two aspects to it. First, it contended that the skilled person was faced with an undue burden to produce anything within the claims. Secondly, it contended that the skilled person could not, without undue burden, produce particles with an average Tc/Ti ratio at the upper end of the claimed range. I shall consider each aspect in turn.
Undue burden to produce anything within the claims
199. SG’s case on undue burden starts with the evidence of Dr Schwabel relating to Rowenhorst (paragraphs 220-221 of his first report):
“Furthermore, not only would the skilled person be unlikely, in my view, to think about varying the shape of the planar triangular Rowenhorst particles to create dish-shaped triangular particles, I do not think it would be obvious to the skilled person how to make particles with this shape. As discussed further in section J below, forming sol-gel alumina abrasive particles of a specified shape using a mould requires knowledge of the phenomena occurring during the drying of sol-gels, including shrinkage and compressive stresses, as well as an understanding of the complex interplay of factors that influence the shape and level of curvature/warping in the resulting particle, such as the level of adhesion of the gel to the mould (affected by the presence and/or amount of release agent used), the drying rate, the rheology of the sol-gel and the nature of the particles (in this case boehmite) in the sol-gel.
Whilst I consider that the skilled person following the set of instructions provided in the Patent on how to make planar/concave particles would be able to make them, it is quite a different matter whether a method to make such particles would be obvious in light of Rowenhorst. First of all, as discussed above, the skilled person would first need a reason to want to make such shapes, and there is nothing in Rowenhorst that would suggest such a reason. Secondly, in my opinion, if the skilled person started from the set of instructions in Rowenhorst on how to make planar shaped abrasive particles, it would not be obvious to the skilled person how to change the sol-gel composition or the processing parameters such as to form the claimed particles. The composition of the dispersion to be used, the aging parameters, the mould, the extent to which a release agent is needed, and the drying conditions would all have to be determined experimentally and would typically be an iterative process where each of the parameters would be changed in turn until a combination of the relevant conditions that worked together to produce particles with the features claimed in the Patent was achieved.”
200. As SG submitted, in these paragraphs Dr Schwabel recognised that there was a complex interplay of factors that influenced the shape of the particle, including the nature and rheology of the dispersion, the level of adhesion to the mould (influenced by the mould material and any release agent) and the drying conditions.
201. Therefore, when he came to consider how the skilled person would go about seeking to implement the teaching of the Patent to produce dish-shaped particles, Dr Schwabel focussed on performing examples 1 and 2, supplemented as necessary with information from the general description and the CGK. He summarised his views in paragraph 303 of his first report:
“In my opinion, the majority of the information required to make the precursor abrasive particles in Examples 1 and 2 is expressly specified in the Examples and in respect of the few variables where the Examples do not specify the exact parameters, the appropriate conditions can be established by reading the Patent as a whole and using CGK and routine trial and error methods. Given the empirical nature of the work in the field of sol-gel abrasive grains, the skilled person would expect to perform test runs and would understand that it may take a number of attempts to make the particles described. In my opinion, the Patent specification provides sufficient information that the skilled person would not be required to carry out prolonged research or experimentation in order to make the dish-shaped abrasive particles claimed.”
202. Prof Atkinson’s response to that, in paragraphs 134-135 of his second report, was to draw together what he said were the uncertainties in example 1 (which is said to produce planar/concave particles) and explain why they mattered:
“As I have noted above, at various points Dr Schwabel suggests that the skilled person can simply follow Example 1 in the Patent, and that this compensates for the lack of detail in the description of the Patent. However, as I have explained above, in Example 1 the skilled person is left with the following uncertainties:
(a) The nature of the dispersion - Example 1 does not specify the type of DISPERAL used which, as the datasheet states, will impact its properties.
(b) The rheology of the dispersion - Example 1 does not provide any indication of the properties of the dispersion (particularly rheology) which the skilled person should aim for.
(c) The level of adhesion - although no mould release agent was used, the adhesion of the dispersion to the mould will be impacted by the polymer chosen which is not identified in Example 1.
(d) The drying rate - although temperature and duration can be determined, the airflow cannot be deduced from Example 1.
If just one of these uncertainties existed, it could be resolved by simple testing. For example, if the desired rheology had been indicated then the imprecision in the dispersion components could be addressed without great difficulty. However, with all of these factors at play it is a much more challenging task. I reiterate that I do not believe that the skilled person would doubt that figure 2-type (planar/concave) particles could be produced, however, if the skilled person tries to implement Example 1 and fails to achieve dish-shaped particles (which seems rather likely) they will not know if it is because the dispersion is too viscous or too fluid; or because the polymer used is too ‘sticky’ or not sticky enough; or whether the airflow should be increased (to increase the drying rate) or decreased; or a combination of all of these factors. The skilled person would have to experiment with all of these factors with little idea in respect of any given choice of parameter or parameters whether they would achieve the desired result, and the Patent would offer them no assistance in this task.”
203. I can dispose quickly of Prof Atkinson’s point (a). Example 1 of the Patent refers to the use of aluminium oxide monohydrate powder “having the trade designation DISPERAL” (in contrast to the general teaching in [0057] which refers to “products having the trademarks DISPERAL and DISPAL”). The datasheet in question refers to a number of products, including one identified simply as DISPERAL and others where there is an additional suffix, such as DISPERAL 20 and DISPERAL HP 10. In my judgment Dr Schwabel was right to say that the skilled person reading example 1 would understand that the material used was the product designated DISPERAL, with no suffix. That is not an uncertainty in example 1.
204. The other points have much more substance. The experts were agreed that the rheology of the dispersion had an important influence on the shape of the particles produced. However, the Patent does not define any rheological properties for the dispersion, and the evidence showed that the rheology can be affected by variations in particle size in the DISPERAL product, by the mixer (and settings) used for the high shear mixing, by the ageing temperature and by the equipment and process used to introduce the aged gel into the mould. However, the evidence did not establish what level of variation in rheology would arise from these various factors, nor whether the level of variation would be sufficient to lead to failure to produce planar/concave particles with the level of dishing said to result from example 1.
205. The experts were also agreed that the level of adhesion of the gel to the mould was an important factor in determining the shape of the particles produced, and that the level of adhesion was affected by the material from which the mould was made. However, example 1 does not specify the mould material, beyond stating that it was polymeric. Dr Schwabel pointed out that the polymeric materials which were identified in [0062] as suitable had surface energies of between 30 and 46 dynes/cm. Prof Atkinson agreed that the skilled person would know that if they wanted more or less stickiness, they could choose a material with a higher or lower surface energy; his point was that the skilled person would not know how much stickiness they wanted. However, again it was not established that choosing one polymeric material would lead to failure in production of planar/concave particles whereas choosing another would lead to success.
206. The experts were also agreed that the drying conditions were an important factor in determining the shape of the particles. Example 1 specifies the temperature and drying time, but not the airflow level. Dr Schwabel agreed that the airflow level will affect humidity, which affects the drying rate and hence the shape of the particles. However, once again it was not established that the skilled person could sensibly choose an airflow level which would lead to failure to produce planar/concave particles with the level of dishing said to result from example 1.
207. Ultimately, Dr Schwabel accepted that if the skilled person were to fail in their first attempt to produce planar/concave particles by implementing example 1, they would not know in which of these areas the problem lay. However, I do not believe that he was challenged directly on his evidence that it would be possible to identify conditions that led to success by routine trial and error.
208. When asked about the skilled person trying to implement example 1 to obtain particles with a level of dishing shown in Fig. 2 by varying the parameters, Prof Atkinson said this (day 2, 198/6 - 199/7):
“A. Yes, but I think what we are now looking at is a design of experiments approach to optimising this process and finding out where the parameters, the conditions are that bring success. There is a well-defined approach to this so-called design of experiments where you change one parameter, you measure something in the product that has changed as a result, and you get a sensitivity for that and you change another one and then so on. So, in that space you move around in a controlled way to enable you to home in on the conditions that give you the result that you want. That is not simple trial and error, that is a project.
Q. Professor Atkinson, surely changing the mould and its release agent and changing the drying speed are exactly the same sort of changes which when you were considering the move from Rowenhorst to Example 1 of the patent you described as routine and obvious changes.
A. Yes, I accept that. But my understanding is that what we are talking about now with implementing Example 1 of the patent is one of undue burden, not whether it is interesting from the point of the view of the skilled person to move around in this space to see what happens and explore the parameters. That is done in a more relaxed environment, if you like, with more time available. Now we are talking about can this be done without making this a substantial body of effort to actually find out where the sweet spot lies in forming these dish particles.”
209. In my judgment SG has failed to establish that it would require undue burden to make any particles within the claims. The fundamental problem for its case is that the range of values for the relevant parameters within the bounds of example 1 (rheological properties, “stickiness” of the mould material, airflow) which will give rise to planar/concave particles with the degree of dishing said to be produced by example 1 is unknown. It is not known how big what Prof Atkinson called “the sweet spot” is. As he said in the passage of evidence I have quoted in paragraph 147 above, there is presumably some spread around the points at which success was achieved; but there is no information about how big that spread is. The consequence is that it is impossible to know what the chances are of a skilled person succeeding with their first attempt at implementing example 1, nor how hard it would be to find a successful set of conditions by routine trial and error. If the landscape within the boundaries of example 1 was such that successful combinations of conditions were rare, like scattered islands in a sea of failure, then I would agree with Prof Atkinson that finding those islands without navigational aids would amount to an undue burden. But if the landscape is one of sunlit uplands with scattered sinkholes, then the position is otherwise. The problem for SG is that the evidence did not establish which was the case in fact.
210. I should add that SG also suggested that there was undue burden in carrying out the test in integer (9). There is nothing in this point. The test in integer (9) requires the skilled person to make a random selection of 15 dish-shaped particles, measure their Tcs and Tis and average them. There was no evidence to suggest that this was onerous or placed an undue burden on the skilled person.
Undue burden to produce particles across the scope of the claims
211. In my judgment the position is different when it comes to consider whether the skilled person could make particles across the breadth of the claims without undue burden. The Patent indicates in [0022] that “triangular dish-shaped particles produced by the invention have been measured to have thickness ratios between 1.55 to 2.32 in some embodiments.” While it is clear from the Patent that it is possible to make dish-shaped particles with such average Tc/Ti ratios using example 1, by contrast there is no indication that it has been possible to make dish-shaped particles with average Tc/Ti ratios above 2.32, and in particular with ratios towards the upper end of the range claimed (i.e. approaching 5.00). However, for the reasons I have explained, in order for the claims to be valid, the Patent needs to enable the skilled person to make such dish-shaped particles.
212. In his first report, Prof Atkinson expressed the view that the Patent did not provide sufficient information to allow the skilled person to control the average Tc/Ti ratio from 1.25 to 5.00. In his first report (the expert reports were served sequentially) Dr Schwabel recorded that he had been instructed that the specification must be sufficient to allow the invention to be performed over the whole scope of the claims, but he did not address the point about whether an average Tc/Ti ratio of up to 5.00 could be achieved without undue burden (as noted above, he focussed on performing examples 1 and 2). In his second report Prof Atkinson noted Dr Schwabel’s views about the skilled person’s ability to produce dish-shaped particles following example 1. He then said that, even if that was so, it would lead to particles with a Tc/Ti ratio of about 2, that the Patent provided no indication of how the relevant process parameters should be adjusted to vary the Tc/Ti ratio across the range of the claim, and that the skilled person would not know how to go about that.
213. That led to a brief response from Dr Schwabel in paragraph 30 of his second report, under the heading “Varying the Tc/Ti ratio”:
“I explained in paragraphs 272, 273 and 279-280 of my first report that the faster the drying rate the more pronounced the warping. Therefore, to reduce the degree of warping/dishing a skilled person would consider reducing the drying rate (lower temperature) and to increase warping/dishing the skilled person would consider increasing the drying rate.”
214. In response to that, in paragraphs 16-21 of his third report, Prof Atkinson made the following points:
i) The Patent says virtually nothing about drying conditions in the mould and makes no suggestion that the Tc/Ti ratio can be controlled by adjusting the drying temperature.
ii) The skilled person would not expect there to be a simple relationship between the drying rate and the degree of curvature in the final product - on the contrary there was a complex interaction of parameters.
iii) Example 1 already has a high drying temperature (up to 163oC). Increasing the temperature could increase the potential for fracture of the particles and could lead to degradation of the polymeric mould.
iv) In any event, there was nothing to suggest that increasing the drying temperature could produce particles with an average Tc/Ti ratio of up to 5.00.
215. That prompted paragraph 3 in Dr Schwabel’s third report:
“In paragraphs 16 to 21 of Atkinson III, Professor Atkinson discusses changing the Tc/Ti ratio by controlling the amount of shrinkage in the context of the planar/concave particles made in a mould with no or a small amount of release agent present. This is not the only way the Tc/Ti ratio can be varied. Paragraph [0038] of the Patent explains that a concave lower face of the particles can be formed by moulding with the degree of curvature or flatness of the upper face being controlled to some extent by how the particles are dried. Increasing the solids content of the gel placed into the mould and drying slowly would reduce drying shrinkage and encourage accurate transfer of the mould shape and a substantially planar upper face to the dish-shaped particles.”
216. Prof Atkinson responded to that in a fourth report in which he addressed what he understood Dr Schwabel to be suggesting in his third report, illustrated with some diagrams. In case he had misunderstood, he confirmed in his oral evidence in chief that his view also applied if what Dr Schwabel was suggesting was better represented in some other diagrams (XX/9). The points made by Prof Atkinson included:
i) The idea of creating a particle with a flat face and a concave face by using a mould with a convex base and faithfully mirroring that shape in a concave face of the particle, while keeping the upper face of the particle flat, was precisely the opposite of the approach of example 1 of the Patent.
ii) The approach suggested by Dr Schwabel was also contrary to the teaching of example 1 of the Patent in that it would involve increasing the solids content of the dispersion, drying slowly, and using a mould release agent so that the particles would not stick to the mould.
iii) Achieving such a particle with a draft angle of greater than 90o would involve producing a mould with an undercut, which would be difficult to create and to fill.
iv) Drying slowly would not reduce shrinkage, only the rate of shrinkage. It would be very difficult to control the drying conditions to achieve the desired result.
v) The skilled person would be faced with the need to achieve a suitable combination of dispersion rheology, drying conditions and mould adhesion with no guidance from the Patent.
vi) The problems are exacerbated as the desired Tc/Ti ratio increases.
217. When Dr Schwabel was cross-examined about what was referred to as his “drying theory” (paragraph 30 of his second report) I understood him to accept what Prof Atkinson had said in paragraphs 16-21 of his third report. In particular Dr Schwabel accepted that an increase in drying temperature would not cause a particle to shrink more in the middle, and that the complexity of the system made it very difficult to predict the effect of increasing temperature. He also acknowledged that increasing the temperature would increase the risk of fracture of the particles and of degradation of the polymeric mould. There was no significant challenge to Prof Atkinson’s comments on the drying theory in his cross-examination. In my judgment, having regard to the evidence as a whole, the skilled person would not have come up with the drying theory as a way of trying to increase the Tc/Ti ratio, nor would they have been able to achieve average Tc/Ti ratios at the upper end of the claim by using such an approach.
218. When asked about what was referred to as his “inverted moulding theory” (paragraph 3 of his third report) Dr Schwabel explained that 3M’s lawyers had pointed him to [0038] of the Patent, and that led him to start to think about other ways in which dish-shaped particles could be made. He agreed that it would involve using a different recipe and process to example 1, and that the Patent would not provide any guidance as to the dispersion or drying conditions to use or the amount of release agent to use. He also accepted that it would be very difficult to predict what the ultimate shape of the particle would be, because the bottom corners of the particle would be likely to become deformed during drying, so reducing the Tc/Ti ratio. I do not believe that the inverted moulding theory, as explained in paragraph 3 of Dr Schwabel’s third report, was actually put to Prof Atkinson. Again, in my judgment, the skilled person would not have come up with that theory as a way of trying to increase the Tc/Ti ratio, nor would they have been able to achieve average Tc/Ti ratios at the upper end of the claim by such an approach.
219. A different approach was put to Prof Atkinson in cross-examination (day 2, 205/19 - 208/23). It involved using the same recipe and process as in example 1, but using a mould with a convex lower face (apparently with a view to making particles similar to those shown in Figs 6A and 6B). Prof Atkinson explained that would not work, because to make a particle with a concave upper face it would be necessary to have a moderately sticky interface between the mould and the dispersion, whereas to achieve a concave lower face it would be necessary for the particle to be able to slide relative to the mould. The remaining cross-examination was somewhat confused, and in my judgment it did not establish that this approach would have succeeded in producing particles with an average Tc/Ti ratio at the upper end of the claim. In any event, given that it was not an approach that had occurred to either expert, in my judgment it would not have occurred to the skilled person.
220. Finally, 3M sought to rely on the cross-reference to US 001 in [0074] of the Patent. In order to see why it did so, it is necessary to explain a little more about what that document discloses. For these purposes, it was common ground that 458 could be used instead, as its relevant disclosure was the same.
221. The document is entitled “Method of making abrasive shards, shaped abrasive particles with an opening, or dish-shaped abrasive particles”. In [0005] it explains that:
“The inventors have determined that by controlling the process parameters and by using a polymeric production tooling having a plurality of mold cavities, different types of the shaped abrasive particles can be produced from the exact same mold. In particular, the inventors have determined a method to fracture the shaped abrasive particles while still in the mold to produce abrasive shards instead of solid, intact shaped abrasive particles. The inventors have also determined a method of controlling the formation of the shaped abrasive particles while residing in the mold in order to form an opening through the shaped abrasive particle. Lastly, the inventors have also determined a method of controlling the formation of the shaped abrasive particle while residing in the mold to form a concave surface on the shaped abrasive particle to make a dish-shaped abrasive particle. Thus, depending on the process parameters, the same identical production tooling can produce solid, intact shaped abrasive particles, abrasive shards, shaped abrasive particles with an opening, or dish-shaped abrasive particles.”
222. The detailed description contains a section headed “abrasive shards” ([0016]-[0025]), one headed “shaped abrasive particles with an opening” ([0026]-[0034]), and one headed “dish-shaped abrasive particles” ([0035]-[0047]) which is very similar to [0013]-[0019], [0021]-[0024], [0026](final part)-[0028], and [0030](initial part) of the Patent. In [0041] it states that “triangular dish-shaped particles produced by the invention have been measured to have thickness ratios between 1.55 to 2.32 in some embodiments.” Counsel for 3M suggested that this was a reference to particles made according to example 1 in the Patent, rather than to dish-shaped particles made in accordance with the examples of 458, but I cannot see why the skilled person would read it that way.
223. There follows a section headed “method of making different types of shaped abrasive particles” which spans [0048]-[0083] and includes sub-sections on “abrasive shards” ([0065]-[0071]), “shaped abrasive particles with an opening” ([0066]-[0076]) and “dish-shaped abrasive particles” ([0077]-[0083]).
224. Finally, there are three examples. Example 1 is headed “preparation of abrasive shards” but only [0085] relates to that - as already explained above, [0086] is a variation in which a mould release agent is used and leads to the production of dish-shaped abrasive particles. Example 2 is headed “preparation of REO-doped dish-shaped abrasive particles” but no real attention was paid to this at trial. Example 3 is headed “preparation of REO-doped shaped abrasive particles with openings”. The key features of examples 1 and 3 are set out in the table in paragraph 122 above.
225. In closing, Counsel for 3M relied in particular on the statement in [0063] of 458 that:
“In general, by increasing the drying rate when there is mold release agent on the surface of the polymeric mold will increase the size of the meniscus in contact with air in a dish-shaped abrasive particle. The forming of an even larger meniscus eventually produces an opening in the shaped abrasive particle.”
226. He also relied on Table 1, which sets out typical process parameters to control the type of abrasive particle obtained, and on [0073] which indicated that rapid removal of volatile liquid from the dispersion leads to rapid solidification, thereby forming a large meniscus that leads to formation of an opening.
227. Counsel for 3M submitted that these passages (a) established as a matter of fact that dish-shaped particles with an average Tc/Ti ratio of 5.00 can be made, because a large “meniscus” can be formed, to the point of producing an opening in the particle, and (b) provided guidance to the skilled person as to how such particles could be made.
228. The fundamental problem for 3M is that none of this was addressed by Dr Schwabel in any of his reports, nor put to Prof Atkinson in cross-examination. The only point that was put to Prof Atkinson (day 2, 201/12 - 203/9) was that Table 1 of 458 would indicate to the skilled person that increasing the drying rate was likely to increase the rate of dishing. I do not accept that I can conclude, on the basis of the statements in 458 without the assistance of expert evidence, that it would be possible for the skilled person, without undue burden, to produce particles with an average Tc/Ti ratio towards the upper end of the claim by increasing the drying rate while using a mould release agent.
229. Further, there is the question of whether the skilled person would, on the basis of the cross-reference in [0074] of the Patent, consult US 001 and alight on the passages relied on by 3M, and in particular that in [0063] of 458. SG relied on what Pumfrey J said in Halliburton v Smith at [62], as part of his discussion in [60]-[62] of a cross-reference to a paper, namely that “if the disclosure is essential to the patent that fact should be made abundantly clear”. I do not think Pumfrey J was saying that, for a skilled person to consult a cross-referenced document, it must be stated that the document contains material essential for implementation of the patent. Rather, my understanding of his judgment is that in order for a skilled person to consult a document for a particular purpose, it must be made clear that the skilled person should consult a document for that purpose. Further, in my judgment, the question of what a skilled person would do, when faced with a cross-reference and the document cross-referred to, is one on which expert evidence is admissible, and indeed required if a party wishes to establish that the skilled person would alight on particular text and find it of assistance in a particular way.
230. In the present case, [0074] says that “more information concerning methods to make shaped abrasive particles is disclosed” in US 001, the title of which is then given. There is no indication that US 001 contains information that will assist the skilled person to make dish-shaped particles with average Tc/Ti ratios towards the upper end of the claim of the Patent. Further, even if the skilled person were to turn to US 001, he would focus on the sections concerning dish-shaped particles and their manufacture. I would have required evidence that the skilled person would have alighted on the passages relied on by Counsel for 3M and have appreciated that they would provide a solution to the problem of making dish-shaped abrasive particles with an average Tc/Ti ratio towards the upper end of the claim.
231. However, no such evidence was given by Dr Schwabel, who did not mention the cross-reference in [0074] in any of his reports. Nor, when he considered 458 in response to it being raised by Prof Atkinson, did he point to the passages relied on by Counsel for 3M and suggest them as providing a solution to the problem. Further, no such suggestion was made by Counsel for 3M in cross-examination of Prof Atkinson.
232. For these reasons I reject the attempt by 3M to rely on the cross-reference to US 001 to supplement the disclosure of the Patent in relation to making dish-shaped abrasive particles with an average Tc/Ti ratio towards the upper end of the claim.
233. In summary, in my judgment the evidence establishes that the skilled person could not, without undue burden, make dish-shaped abrasive particles with an average Tc/Ti ratio towards the upper end of the claim. Accordingly, the Patent is insufficient in that regard and claim 1 is invalid. None of the subsidiary claims relied on contains a limitation which avoids that finding of insufficiency and accordingly the Patent should be revoked.
Conclusion
234. In conclusion, my findings are as follows:
i) The Patent is not invalid for lack of novelty or lack of inventive step over Rowenhorst.
ii) SG’s case of invalidity for uncertainty-type insufficiency fails, as does its case that it would require an undue burden to produce something within the claims.
iii) However, the Patent is invalid because it would require an undue burden to produce particles across the scope of the claims.
iv) Accordingly, SG’s claim succeeds and the Patent should be revoked.
235. This judgment will be handed down remotely, and I will adjourn consideration of the form of the order which should be made to a hearing on a date to be fixed. I direct that the time for lodging any Appellant’s Notice shall not begin to run until the date of that further hearing.