Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales Court of Appeal (Civil Division) Decisions
You are here: BAILII >> Databases >> England and Wales Court of Appeal (Civil Division) Decisions >> Actavis Group PTC EHF v ICOS Corp [2017] EWCA Civ 1671 (01 November 2017)
URL: http://www.bailii.org/ew/cases/EWCA/Civ/2017/1671.html
Cite as: [2017] EWCA Civ 1671, [2018] RPC 7, (2018) 159 BMLR 108, 159 BMLR 108
[New search] [Printable PDF version] [Help]
ON APPEAL FROM THE HIGH COURT OF JUSTICE
CHANCERY DIVISION (PATENTS COURT)
THE HON MR JUSTICE BIRSS
Strand, London, WC2A 2LL |
||
B e f o r e :
LORD JUSTICE KITCHIN
and
LORD JUSTICE FLOYD
____________________
| (1) Actavis Group PTC EHF (a company incorporated under the laws of Iceland) (2) Actavis UK Limited (Appellants in Appeal A3/2016/4110) (Respondents in Appeal A3/2016/4103) (1) TEVA UK Limited (2) TEVA Pharmaceutical Industries Limited (a company incorporated under the laws of Israel) (Appellants in Appeal A3/2016/4094) (Respondents in Appeal A3/2016/4103) Generics (UK) Limited (t/a Mylan) (Appellant in Appeal A3/2016/4104) (Respondents in Appeal A3/2016/4103) |
||
-and- |
||
| (1) ICOS Corporation (a company incorporated under the laws of the State of Washington, USA) |
||
| (2) Eli Lilly & Company (a company incorporated under the laws of the State of Indiana, USA) (Respondents in Appeals A3/2016/4110, 4094, 4104) (Appellants in Appeal A3/2016/4103) |
____________________
(instructed by Bird & Bird LLP) appeared for Actavis UK Limited
(instructed by Taylor Wessing LLP) appeared for Generics (UK) Limited (t/a Mylan)
(instructed by Pinsent Masons LLP) appeared for Actavis Group PTC EHF, TEVA UK Limited and TEVA Pharmaceuticals Industries Limited
Andrew Waugh QC and Katherine Moggridge (instructed by Allen & Overy LLP)
appeared for ICOS Corporation and Eli Lilly and Company
Hearing dates: 12/13 July 2017
____________________
Crown Copyright ©
- These appeals are concerned with the validity and infringement of a patent concerning tadalafil. Tadalafil is the generic name of a drug which is sold under the brand name CIALIS for the treatment of male erectile dysfunction ("ED") and benign prostatic hyperplasia, and under the brand name ADCIRCA for the treatment of pulmonary arterial hypertension. CIALIS has enjoyed considerable commercial success. In 2014 worldwide sales amounted to about $2.29 billion and UK sales amounted to about $99 million. In that same year UK sales of ADCIRCA amounted to about $1 million.
- The patent, EP (UK) 1,173,181 (the "181 patent" or "the patent"), is owned by ICOS and exclusively licensed to Lilly. It relates to the use of tadalafil in a dosage form and is entitled "Compositions comprising phosphodiesterase inhibitors for the treatment of sexual dysfunction". It was filed on 26 April 2000 and claims priority from US application 132036P filed on 30 April 1999. It was granted on 15 October 2003. The form of the patent in issue is a B3 specification following centralised amendments made in the EPO on 25 March 2015. Tadalafil is also protected by an SPC (SPC/GB03/007) that expires in November of this year.
- The claimants, Actavis, TEVA and Mylan, began these proceedings to revoke the 181 patent and so clear the way for the marketing of their own products. ICOS and Lilly, collectively "Lilly", defended the claim and counterclaimed that the claimants were threatening to infringe the patent.
- The claimants sought the revocation of the 181 patent on various grounds. They contended first, that the matter disclosed in the specification extended beyond that disclosed in the application for the patent as filed; secondly, that the patent lacked novelty over three publications, WO 01/08688 ("Anderson"), WO 00/53148 ("Stoner") and WO 01/08686 ("Oren"); thirdly, that the patent was obvious in the light of another publication, WO 97/03675 ("Daugan"); and finally, that the specification of the patent did not disclose the invention clearly enough and completely enough for it to be performed by a person skilled in the art.
- The position in relation to the novelty citations was a little complicated but may be summarised as follows. Anderson, Stoner and Oren were all published after the filing date for the 181 patent but were available in support of an allegation of lack of novelty under s.2(3) of the Patents Act 1977 ("the 1977 Act") (corresponding to Article 54(3) of the EPC) if and in so far as they contained relevant matter which was entitled to a priority date earlier than that of the invention of the 181 patent. Anderson and Oren both had a priority date of 3 August 1999. Accordingly, if the 181 patent was not entitled to its claimed priority date, they were each relevant prior art for novelty purposes. Stoner was filed on 3 March 2000 and so, if the 181 patent lost priority, it too was relevant prior art for novelty purposes. However, Stoner claimed priority from a US filing on 8 March 1999 which is earlier than the claimed priority date for the 181 patent. It followed that, in so far as Stoner was entitled to this earlier priority date, it was citable for novelty purposes even if the 181 patent was entitled to its claimed priority date. Lilly did not suggest that the matter relied upon in Stoner might not be entitled to priority on substantive grounds but contended that the claimants had failed to establish that its owner was entitled to make the priority claim.
- The action came on for trial before Birss J in June and early July 2016 and he handed down judgment on 10 August 2016 ([2016] EWHC 1955 (Pat)). He found that all of the claims of the 181 patent, save claims 2 and 12, were entitled to their claimed priority date; claims 2 and 12 lacked novelty in light of the disclosures of Anderson and Oren; Stoner was entitled to its claimed priority date but none of the other claims lacked novelty in light of its disclosure; the allegation of obviousness in light of the disclosure of Daugan failed; and that the allegation of insufficiency fell away in light of the findings concerning the validity of claims 2 and 12. As for the counterclaim, the judge held that the claimants would infringe the 181 patent were they to launch their intended products.
- I should also mention that there was before the judge a claim for the revocation of another Lilly patent, EP (UK) 1,200,092. The judge found that all of the claims of this patent were invalid. The appeal by Lilly against this finding has not been pursued and so I need say no more about it.
- Upon these appeals, brought with the permission of the judge, the claimants contend as follows:
- I will address each of these contentions in turn but must first summarise aspects of the technical background and consider, albeit briefly, the disclosure of the 181 patent.
- The technical background is set out in a technical primer, the contents of which were largely agreed by the parties before the trial. The following summary is sufficient to understand the issues arising on this appeal.
- ED is an extremely common medical condition affecting a significant percentage of men between forty and seventy years of age. It may be caused by a considerable number of disorders, both physiological and psychological.
- The penis contains smooth muscle. In the normal resting state, this smooth muscle contracts and so restricts the arteries supplying blood to the penis. The penis is, as a result, detumescent. When an erection is triggered, the smooth muscle relaxes and no longer restricts the supply of arterial blood. The penis then fills with blood and becomes tumescent.
- Smooth muscle relaxation leading to an erection is the result of a cascade of complex biochemical reactions within the body. Sexual stimulation causes the release of the neurotransmitter nitric oxide ("NO") which enters the smooth muscle cells where it leads to an increase in the production of a second messenger, cyclic guanosine-3', 5'-monophosphate ("cGMP"). cGMP in turn binds to and activates an enzyme which regulates the activity of other intracellular proteins and leads to the relaxation of the smooth muscle. An increase in the intracellular level of cGMP, through NO production, therefore promotes smooth muscle relaxation, while a decrease in the intracellular level of cGMP tends to cause the smooth muscle to return to its ordinary contracted state.
- The intracellular concentrations of cGMP and another second messenger, cyclic adenosine-3', 5'-monophosphate ("cAMP"), are regulated by a class of enzymes known as cyclic nucleotide phosphodiesterases ("PDEs"). By 1999 at least six PDE families had been identified, PDE1 to PDE6, but it was known that the most prevalent in the penis is PDE5. This binds cGMP and hydrolyses it to its non-cyclic form GMP so leading to a reduction in smooth muscle relaxation and the prevention of penile tumescence.
- The first orally administered PDE5 inhibitor to be marketed for the treatment of ED was sildenafil which is sold under the brand name VIAGRA. By inhibiting PDE5, sildenafil prevents it from hydrolysing cGMP to the inactive GMP. As a result, cGMP levels remain elevated which promotes smooth muscle relaxation. This leads to greater arterial blood flow into the penis and results in penile tumescence. Tadalafil is another PDE5 inhibitor and it operates in essentially the same way as sildenafil.
- As for the specificity of the PDE families known in 1999, PDEs 1 and 2 were known to act on both cGMP and cAMP; PDE3 and PDE4 were known to act specifically on cAMP; and PDE5 and PDE6 were known to act specifically on cGMP.
- Potency is the amount of the drug required to produce a defined biological effect of given intensity. It can be measured as the concentration (EC50) or dose (ED50) of a drug required to produce 50% of the drug's maximal effect as depicted by a dose-response curve. The graph below, figure 4 in the technical primer, shows concentration response curves for a series of agonists which show the same maximal effect, but different potencies.
- If the drug inhibits the action of another substance, potency can be expressed as the concentration (IC50) of a drug required to inhibit a given biological process by half, that is to say the in vitro concentration of that drug which is required for 50% inhibition. The graph below, figure 5 in the technical primer, shows concentration response curves for a series of inhibitors which show the same maximal inhibition but different potencies:
- The selectivity of the drug to its target is determined by measuring the ability of the drug to bind not only to the target but also to other closely related receptors or binding sites and may be determined by comparing the IC50 values for other binding sites to the IC50 for the target. It is desirable for a drug to be highly selective because off-target and non-specific binding may give rise to unwanted side-effects.
- There was no dispute between the parties as to the composition of the skilled team of persons to whom the 181 patent is addressed. The team would include a clinical pharmacologist with experience in pharmacokinetics and a clinician specialising in urology. The judge found that both would be important. Quantification of doses and dose response would be matters primarily for the clinical pharmacologist, working with the clinician; and the clinician would take the lead when assessing the clinical significance of an effect, whether a desired effect or a side effect. The clinical pharmacologist would be primarily responsible for the selection of the doses to be tried in the dose ranging study or studies which would follow a successful Phase IIa study, albeit with input from the clinician.
- The judge had the benefit of hearing expert evidence from both sides as to the knowledge and perceptions of the skilled team. So far as relevant to this appeal, the claimants called Mr Muirhead, a clinical pharmacologist. Lilly called Dr Saoud, a clinical pharmacologist, and Dr Brock, a clinical urologist.
- The judge found that the common general knowledge of the skilled team would include all of the technical background that I have summarised. It would also include various other matters, aspects of which I must now describe.
- Clinical research into a new medicine follows a standard pathway consisting of a series of phases. The judge helpfully described these in his judgment from [76] to [81]. The following summary is drawn from that exposition. A new drug, identified through appropriate in vitro testing and pre-clinical animal studies, is taken forward into human tests. The first such tests are known as Phase I and they are carried out in healthy volunteers and test safety rather than efficacy. The tests provide pharmacokinetic information and allow an assessment of bio-availability. If these tests are positive, the next step is to move the drug into Phase II.
- Phase II studies are generally carried out in two stages, Phase IIa and Phase IIb. Phase IIa, which consists of what are sometimes known as 'go, no-go' studies, provides proof of concept. The studies are generally carried out at one dose, selected to be high enough to give the drug the best chance of showing a positive effect on the disease, albeit not too high to risk serious side effects.
- Phase IIb, the stage upon which the claimants particularly focus for the purpose of this appeal, involves testing a range of doses to show the effect of dose. In the judge's words, the idea is that the highest dose will show a larger clinical effect than the smallest dose.
- If the decision after Phase II is positive, the next phase is Phase III. This is a large scale clinical trial designed to generate data to support an application for regulatory approval. Phase IV studies take place after regulatory approval and have no bearing upon the issues arising in this appeal.
- The minimum effective dose is the smallest dose of a drug which will cause a clinically relevant effect. In other words and having regard to the dose response curves depicted at [17] and [18] above, it is the smallest dose in the dose response part of the curve at which a clinically significant effect can be seen.
- The concept of the minimum effective dose would be known to the skilled team and they would be aware that regulators could ask for it to be identified. But they would also know that it is not always required.
- The judge found at [85] that it had not been established that the skilled team would always seek to pin down what the minimum effective dose for a given drug actually was. Knowing that a minimum effective dose was somewhere in a range might be sufficient, and was sufficient in the case of sildenafil. The skilled team would also know that its identification depends upon a value judgment. Furthermore, the primary task of the skilled team was and remains to make safe, tolerable and effective medicines. In the context of ED, there was no agreed definition of a minimum clinically relevant effect and this had a bearing on the judge's reasoning in relation to obviousness, as I shall explain.
- By the claimed priority date of the 181 patent, sildenafil was a blockbuster drug, and it was well known that the market for oral ED medication was large and attractive.
- Sildenafil is administered on demand. Its onset of action is about one hour after administration but can be delayed if it is taken with food. It has a half-life of about four hours which means that, if taken once daily, it does not accumulate significantly in the body. The lack of spontaneity associated with the need to take sildenafil on demand and about one hour before it has a clinical effect was a known drawback.
- As for sildenafil's mode of action, it was known that it acts as a PDE5 inhibitor but it was also known that its potency for PDE6 is only ten times less than for PDE5. Sildenafil was also known to have various side effects, some, such as its effects on the eye, were thought to be associated with its mode of action on PDE6 but others, such as flushing, headache and dyspepsia were thought to be related to its mode of action on PDE5 because PDE5 was known to exist in a number of peripheral sites in the body such as visceral and vascular smooth muscle. It was also contra-indicated for concomitant administration with nitrates. Nitroglycerin is a cardiac medicine which produces a rapid release of NO which in turn leads to an increase in cGMP levels and vasodilation. Exposure to high levels of NO in conjunction with PDE5 inhibition can cause hypotension.
- As for dosing, it was known that sildenafil was marketed in doses of 25mg, 50mg and 100mg and that broadly efficacy increased with dose, as did side effects. The judge found that those three doses were the doses upon which a skilled team would focus although it was also known that a 10mg dose of sildenafil had been investigated in trials and shown to be efficacious.
- Finally, I should say a word about the concept of second in class. Sildenafil was a first in class drug which validated the rationale for trying to treat ED using an oral PDE5 inhibitor. Any other PDE5 inhibitor for ED would be known as a second in class drug. A clinical pharmacologist would have an enhanced expectation that a second in class drug would also be efficacious. The idea of investigating chronic dosing of a drug for the treatment of ED was not part of the common general knowledge, however.
- Paragraph [0002] of the specification explains that the invention relates to a highly selective and potent PDE inhibitor and its use in a pharmaceutical unit dosage form. In particular, it is said, the invention relates to a potent inhibitor of PDE5 which, when incorporated into a pharmaceutical product, is useful for the treatment of sexual dysfunction. It continues that the unit dosage form described is characterised by selective PDE5 inhibition and accordingly provides a benefit in therapeutic areas where inhibition of PDE5 is desired with minimisation or elimination of adverse side effects resulting from the inhibition of other PDE enzymes.
- There follows, from [0003] to [0010], a description of the background of the invention. It discusses the sildenafil formulation sold as VIAGRA and, while acknowledging sildenafil's considerable commercial success, explains that it has only a 10-fold selectivity for PDE5 as against PDE6 and that this is thought to be the basis for the abnormalities it causes relating to colour vision. It continues that sildenafil has fallen short due to its significant other adverse side effects, including facial flushing, and says that these side effects have limited its use in patients suffering from vision abnormalities or hypertension or who are using organic nitrates.
- After acknowledging Daugan, the specification says that the present invention discloses an effective product having a reduced tendency to cause flushing. It continues that the invention discloses an effective therapy for sexual dysfunction in individuals who previously were untreatable or suffered from unacceptable side effects, including individuals having cardiovascular disease and requiring nitrate therapy or suffering from congestive heart failure or vision abnormalities.
- After a summary of the invention and an explanation that it comprises, inter alia, the use of a unit dose of 1 to 5mg of tadalafil for the manufacture of a medicament for administration up to a maximum total dose of 5mg of the compound per day for the treatment of sexual dysfunction, there appears a detailed description.
- Paragraphs [0019], [0024] and [0025] explain that the claimed dosage form is preferably packaged as an article of manufacture for human pharmaceutical use comprising a package insert, container and a dosage form comprising about 1 to about 5mg of the claimed compounds. This insert is said to provide a description of how to administer any such compound, together with safety and efficacy data required to allow the physician, pharmacist and patient to make an informed decision regarding its use.
- Paragraphs [0024] and [0025] are of some importance and read as follows:
- Paragraph [0031] says that the invention is based upon detailed experiments and clinical trials and the unexpected observations that side effects previously believed to be indicative of PDE5 inhibition can be reduced to clinically insignificant levels by the selection of a compound and unit dose. It continues that this unexpected observation has enabled the development of a unit dosage incorporating about 1 to 5mg of Compound (I) which, when orally administered, minimises undesirable side effects previously believed unavoidable. It says that these side effects include facial flushing, vision abnormalities and a significant decrease in blood pressure. It concludes that the minimal effect of Compound (I), administered in about 1 to about 5mg unit dosage forms, on PDE6 allows the administration of a selective PDE5 inhibitor to patients suffering from retinal disease.
- The specification then describes data and various examples, the details of which are set out in the judgment from [108] to [113]. The judge was satisfied on the basis of the expert evidence given by Dr Brock that side effects such as headache, backache, myalgia and flushing have a significant impact on patient tolerability in ED, and that the lower incidence of these effects seen at doses of 2mg and 5mg would be seen as a real advantage. The judge also found at [116] that, at the claimed doses, tadalafil is not only an effective treatment for ED but has a reduced tendency to side effects associated with its mode of action as a PDE inhibitor as compared to sildenafil.
- As I have mentioned, three claims are in issue, namely claims 1, 7 and 10. They, together with the claims upon which they depend, read as follows:
- There was no issue between the parties as to the correct approach to the construction of the claims in issue. The question is what the person skilled in the art would have understood the patentee to be using the language of the claims to mean. Nothing in the recent decision of the Supreme Court in Actavis UK Ltd & Ors v Eli Lilly & Co [2017] UKSC 48 affects the application of this approach in the context of this case. The parties are not agreed, however, as to the meaning of claims 7 and 10 when interpreted in this way.
- I must start with claim 1. This is a claim to a dosage form comprising 1 to 5mg of tadalafil, such form being suitable for oral administration up to a maximum total dose of 5mg per day. The expression "suitable for" makes it clear that the claim is not limited to the use of this dosage form for any particular purpose, as the judge correctly held at [119]. It follows that the words "up to a maximum total dose of 5mg per day" have no effect upon the scope of this claim.
- Claim 7 as dependent upon claim 6 (and claim 1) introduces the purpose limitation that the dosage form must be for use in treating a condition where inhibition of PDE5 is desirable and where the condition is a sexual dysfunction. Mr Adrian Speck QC, who has appeared on this appeal with Mr Mark Chacksfield and Mr Thomas Jones on behalf of the claimants, submits that the words "up to a maximum total dose of 5mg per day" in claim 1 form no part of this purpose limitation and that the judge fell into error in finding that they do. I agree with Mr Speck that, read literally, the maximum dose per day is no more a limitation of this claim than it is of claim 1. But, as I have said, the claims are to be read not literally but purposively. I do not think that the skilled person would understand this claim when read in the context of the specification to have the meaning for which Mr Speck contends. I agree with the judge that the words "up to a maximum total dose of 5mg per day" now make sense, and I believe they do so because they can attach to and qualify the use to which the dosage form is to be put. In my judgment the skilled person would understand that the patentee intended the maximum dose per day to constitute part of the purpose limitation of the claim and I would reject the contention of Mr Speck to the contrary.
- Claim 10 is a conventional purpose limited Swiss form claim. The purpose limitation of this claim is plainly directed to both the indication, sexual dysfunction, and the dosing regimen, up to a maximum total dose of 5mg per day.
- That brings me to the second issue between the parties, namely the meaning of the words "up to a maximum total dose of 5mg per day" in claims 7 and 10. It is important, not least because the allegation of infringement of these claims depends on it. The claimants argued at trial that these words apply to the population as a whole and so the claims exclude the case in which a doctor might prescribe tadalafil for some patients at a daily dose of 5mg and others at, say, 20mg. Here, so the argument went, the maximum daily dose would be 20mg per day.
- The judge rejected this submission. He recognised that the invention presented in the specification is the discovery that tadalafil can be administered at low doses of up to 5mg per day in a manner which is clinically effective but also has low adverse side effects. This is said to be surprising in that the side effects of a PDE5 inhibitor were thought to be concomitant with efficacy. But he also recognised that the specification does not suggest that higher doses of tadalafil are not safe and effective. So, the specification says in relation to example 6 at paragraph [0074] that a 10mg dose of tadalafil was fully efficacious and demonstrated only minimal side effects. Similarly, the specification teaches that doses of 25mg and above are efficacious but that their side effects must be considered. Against this background the heart of the judge's reasoning appears at [135] to [136]:
- In short, so the judge found, claims 7 and 10 are directed to the treatment of sexual dysfunction by the administration of a dose of no more than 5mg tadalafil per day. Treatment of sexual dysfunction by the administration of higher doses of tadalafil per day falls outside their scope.
- Upon this appeal Mr Speck contends, as he did at trial, that the judge has here fallen into error and that the maximum dosage referred to in these claims is indeed the maximum for the patient population as a whole. He points out that the Summary of Product Characteristics (the "SmPC") for each of the claimants' formulations provides prescribing and dosing information for 2.5, 5, 10 and 20mg tablets. He continues that the SmPCs go on to address recommended doses and make reference to 10 and 20mg for on demand use and 5 and 2.5mg for daily use. He submits that since these SmPCs give prescribing information not just for 2.5mg and 5mg tablets but also for 10 and 20mg tablets, and for doses both within and substantially in excess of the claimed limits, all of this use falls outside the scope of the claims.
- I believe the judge was right to reject these submissions. As I have said, the invention is the application of the discovery that sexual dysfunction may be treated by administering a dose of no more than 5mg tadalafil per day. This posology combines efficacy with minimal side effects. The fact that tadalafil may be prescribed safely at higher doses is neither here nor there. As Mr Andrew Waugh QC, who has appeared on this appeal on behalf of Lilly with Katherine Moggridge, submits and I agree, the correct question is whether the clinician is authorised to prescribe a maximum dose of 5mg per day for the treatment of sexual dysfunction and whether this is the dose for which the tablets in issue are made and packaged. If the answer is 'yes' then these activities will take advantage of the invention.
- The claimants have marketing authorisations to make and sell tablets containing 2.5mg and 5mg tadalafil for administration up to a maximum total dose of 5mg of tadalafil per day for the treatment of sexual dysfunction. If the claimants were to place such tadalafil tablets on the market based upon those marketing authorisations and with the appropriate SmPCs and product information leaflets, their activities would constitute an infringement of claims 7 and 10 of the patent.
- The legal principles concerning entitlement to priority were explained by the Court of Appeal in Unilin Beheer NV v Berry Floor NV [2005] FSR 6 and Medimmune Ltd v Novartis Pharmaceuticals Ltd [2012] EWCA Civ 1234, [2013] RPC 27 at [151] to [154]. In summary, the question is whether, as a matter of substance not form, the skilled person can derive the subject matter of the claim in issue directly and unambiguously, using common general knowledge, from the disclosure of the priority document.
- There is a second requirement. The matter disclosed in the priority document must be enabling, that is to say it must disclose the invention in a way which will enable it to be performed by a person skilled in the art without undue effort: Asahi Kasei Kogyo KK's Application [1991] RPC 485.
- There was at trial a hard fought dispute between the parties as to whether claims 7 and 10 were entitled to priority. But before turning to the judge's findings and the submissions made by the parties on this appeal, I must summarise the important aspects of the priority document's disclosure.
- It begins with a description of the field of the invention. It says that the invention relates to highly selective PDE enzyme inhibitors and to their use in pharmaceuticals. It continues that the invention relates to potent inhibitors of PDE5 that, when incorporated into a pharmaceutical product, are useful for the treatment of sexual dysfunction. These inhibitors are selective and so minimise adverse side effects resulting from the inhibition of other PDE enzymes.
- The background section of the document contains a discussion about sildenafil. It says that sildenafil, marketed under the brand name VIAGRA, is a potent PDE5 inhibitor which is sold in the form of 25, 50 and 100mg tablets and is described in the package insert as being 4,000 fold selective for PDE5 as against PDE3 and but only 10 fold selective for PDE5 as against PDE6. It also says that sildenafil has achieved considerable commercial success but that side effects have limited its use in patients suffering from visual abnormalities or hypertension or who use organic nitrates.
- The present invention, by contrast, is said to provide an article of manufacture for pharmaceutical use comprising a package insert, a container and an oral dosage form comprising a selective PDE5 inhibitor at dosages "between 1 and 20mg/dosage form". This is said to have become available through the applicants' discovery that a selective PDE5 inhibitor meeting the criteria below allows for the effective administration of a 1 to 20mg/dosage form without the contra-indications or warnings generally required for PDE5 inhibitor products, and with reduced flushing and clinically insignificant reactions with nitrates. The criteria are:
- On page 4 there appears a summary of the invention. It is said, in substantially the same terms as before, that the invention provides an article of manufacture for human pharmaceutical use, but continues that the invention also provides a method of treating conditions where inhibition of PDE5 is desired and that this method comprises administering to a patient an oral dosage form having 1 to 20mg of a selective PDE5 inhibitor as needed up to a total dose of 20mg/day.
- There follows a detailed description of the invention. It is reiterated that the invention comprises an article of manufacture for human pharmaceutical use comprising a package insert, a container and a dosage form comprising 1 to 20mg of a selective PDE5 inhibitor per dosage form.
- Then, bridging pages 6 and 7, this important passage appears:
- The priority document continues that preferred conditions to be treated include sexual dysfunction, including ED. On page 8 it is said that the invention is based upon the applicants' detailed experiments and clinical trials and their unexpected observation that the side effects believed to be indicative of PDE5 inhibition can be reduced to clinically insignificant levels by the selection of a selective PDE5 inhibitor having the specific characteristics to which I have referred. Then, on page 9, lines 16 to 24, it says:
- It was agreed before the judge that this chemical name contains an error which the skilled person would identify and correct. There was, however, a dispute between the parties as to whether the skilled person would identify the compound, so corrected, as tadalafil. The judge resolved that issue in favour of Lilly. He found that it would be identified as tadalafil and the claimants do not have permission to appeal against that finding.
- It is to be noted that this passage states that this compound, tadalafil, is a selective PDE5 inhibitor, as defined, and that clinical studies have shown it to have a minimal impact upon systolic blood pressure when administered in conjunction with nitrates. In this regard it is a significant improvement upon sildenafil.
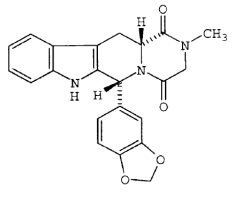
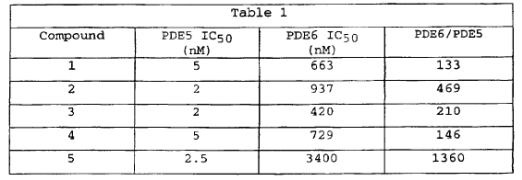
- The priority document proceeds with a description of a generalised structure of the preferred compounds, the IC50 values of five of which are said to have been tested against PDE5 and PDE6, yielding the results set out in table 1 on page 11:
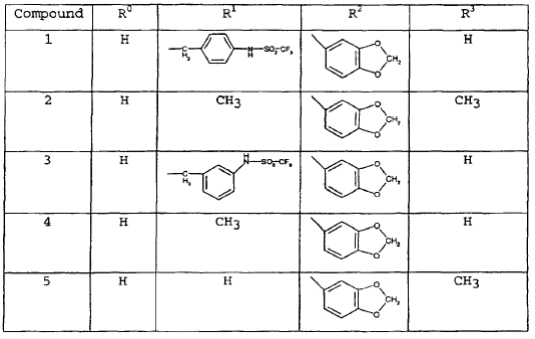
- The structures of these five compounds are then given in this further table on page 12:
- There follows this further description:
- This especially preferred compound is, as all parties accept, tadalafil but, confusingly, it corresponds to compound 4 in the table set out in [67] above. This gave rise to another dispute between the parties as to whether the data for compounds 4 and 5 have been erroneously inverted. After careful analysis of the expert evidence, the judge found that the priority document does not disclose that compound 5 is tadalafil. The reader would understand that it might be but would be left in real doubt.
- The priority document then describes a series of examples. Examples 1 to 3 concern formulations comprising a selective PDE5 inhibitor in unit dosage forms of 1mg, 5mg and 10mg. Example 4 is a clinical pharmacology interaction study that evaluated the effects of a single nitroglycerin exposure upon a group of healthy male volunteers who had received either placebo or study drug (agreed by the parties to be tadalafil) at 10mg doses for the six or seven preceding days. The study shows minimal impact on systolic blood pressure. Example 5 describes two randomised double blinded placebo studies in which a selective PDE5 inhibitor was administered on demand and by daily dosing. The inhibitor is identified as compound 5. In the second study it was found that daily dosing of 10mg was fully effective and demonstrated minimal side effects.
- That brought the judge to the critical question, namely whether, as a matter of substance, the subject matter of each of claims 7 and 10 was derivable directly and unambiguously, using common general knowledge, from the priority document. The judge was of the view that the priority document does clearly disclose tadalafil and that, for dosing, the key passage is the one bridging pages 6 and 7 and set out at [62] above. He considered that the preferences referred to in the second sentence are alternatives to the "total dose of 20mg per day" referred to in the first sentence. In other words, the skilled person would understand the document to be teaching that the dosage forms can be administered up to a total dose of 20mg per day or preferably 5 to 20mg per day, or more preferably 5 to 15mg per day; and that the most preferable approach is to administer a dose of 10mg per day by using a 10mg dosage form. Thus, the judge concluded, a total daily dose of 5mg of tadalafil is disclosed, as is a total daily dose of 20mg. He found that this, coupled with the disclosure of dosage forms of between 1 and 5mg of the active drug does amount to a clear and unambiguous disclosure of the claimed invention. The judge expressed his conclusion in these terms at [229]:
- Finally, the judge dealt with the issue of enablement. He asked himself whether the skilled person would consider the disclosure of the priority document to be plausible. I think he was right to do so because, at the priority date, the skilled person would not have had any difficulty making the compositions the subject of claims 7 and 10. The critical question is whether the skilled person would regard the disclosure of the priority document as credible as opposed to speculative.
- The idea that tadalafil would produce efficacy at the claimed doses with minimal side effects was, the judge thought, plausible for the reasons he summarised at [234]:
- Mr Speck has attacked these findings. He submits that the invention disclosed in the priority document is a way of identifying a wide class of PDE5 inhibitors by their properties rather than by their chemical structure. It is, he says, striking that the document mentions very many compounds of widely varying structures, only one of which is tadalafil. Further, the document contains no disclosure of any test in relation to tadalafil save for that described in example 4, and that is a test designed to assess patients' tolerance to a dose of 10mg tadalafil daily for seven days. More specifically, Mr Speck submits that the priority document does not disclose:
- I am not persuaded by Mr Speck's general point as to the nature of the invention described in the priority document. It discloses, among other things, an article of manufacture for human pharmaceutical use comprising a package insert, a container and a dosage form containing 1 to 20mg of a selective PDE5 inhibitor. A selective PDE5 inhibitor is defined as an inhibitor which satisfies the three criteria set out at [59] above. The priority document also teaches that these articles of manufacture, when administered, do not cause the undesired side effects of flushing, vision abnormalities or significant decreases in blood pressure, whether the articles are administered alone or in combination with an organic nitrate. On page 9 of the priority document, tadalafil is specifically identified as being one such inhibitor and on page 12, tadalafil is identified as being especially preferred.
- Turning now to the first of the specific matters relied upon by Mr Speck, the judge found that a dosing regimen of up to a maximum total dose of 5mg per day is disclosed in the passage of the priority document bridging pages 5 and 6 which bears repetition:
- Mr Speck submits that, read fairly in the context of the priority document as a whole, this passage discloses the administration of different preferred doses but in all cases with an overall limit upon the total dose administered per day of 20mg.
- I reject this submission. In my judgment this passage teaches, in its first sentence, the administration of different possible dosage forms as needed up to a maximum dose of 20mg per day. The second sentence then describes alternatives to that maximum total dose. Thus, preferably the maximum dose administered per day is between 5 and 20mg; more preferably between 5 and 15mg; and most preferably 10mg administered in a 10mg dosage form. I am therefore satisfied that the judge understood this passage correctly and that he was right to find that it discloses not just the administration of a total dose per day of 20mg but also a total dose per day of 5mg. This aspect of claim 1 of the patent is therefore disclosed in the priority document.
- I can take the second and third points together. It is said that there is no disclosure of the efficacy of tadalafil when administered at a maximum dose of 5mg per day, and still less is there any disclosure of the efficacy of tadalafil when administered at any dose at or below 5mg per day.
- In my judgment there is a disclosure in the priority document of the efficacy of tadalafil when administered in 1 to 5mg dosage forms as needed up to a maximum total dose of 5mg per day. As I have explained, the document teaches that the administration of a selective PDE5 inhibitor, as defined, is effective for the treatment of ED while minimising or eliminating undesirable side effects. Tadalafil is expressly disclosed as one such inhibitor and is described as being especially preferred. The passage in the priority document bridging pages 6 and 7 also discloses that selective PDE5 inhibitors, necessarily including tadalafil, are preferably administered at up to a total dose per day of between 5 and 20mg or, more preferably, between 5 and 15mg. The judge was therefore right to conclude as he did at [229] that there is a disclosure of tadalafil in dosage forms of between 1 and 5mg up to a maximum total dose of 5mg per day.
- Finally, submits Mr Speck, the judge ought to have found that the priority document does not make it plausible that tadalafil will be efficacious in treating ED when administered at a maximum total dose of 5mg per day.
- Mr Speck submits that the judge fell into error at [234] because the priority document does not contain any plausible teaching that tadalafil will be efficacious when administered in the form of a daily dose of 5mg. He points out, correctly, that only example 4 concerns tadalafil and that this is a test of its safety when administered at a dose of 10mg. However, says Mr Speck, it says nothing about efficacy. While it is true to say that example 5 is directed to efficacy at doses of 5mg per day and above, there is no disclosure that the compound tested was tadalafil.
- I am not persuaded by these submissions. It is quite clear that a claimed invention will not be implausible if the inventor provides a reasonably credible theory as to why it will work: see, for example, Warner-Lambert v Generics (UK) Ltd [2016] EWCA Civ 1006 at [46]. There is ample material in the priority document to satisfy this criterion. Tadalafil is specifically identified and is said to be especially preferred. The data in table 1 show that it selectively inhibits PDE5 and, as the judge observed, the skilled person would know that sildenafil works in this way. Example 4 shows that a 10mg dose of tadalafil is well tolerated and example 5 shows that a selective PDE5 inhibitor, as defined, is efficacious for the treatment of ED at doses of 5mg per day. In my judgment the requirement of plausibility is satisfied.
- I can deal with this ground of appeal quite shortly. The claimants contend that the 181 patent must be revoked because the matter disclosed in the specification extends beyond that disclosed in the application as filed, and the judge should have so held.
- The matters I have addressed in relation to priority also underpin this ground of objection to the patent's validity, although it of course requires a comparison of the patent with the application as filed. Mr Speck submits that the application as filed does not disclose dosage forms of 1 to 5mg of tadalafil or the administration of tadalafil for the treatment of ED up to a maximum total dose of 5mg per day.
- The problem facing the claimants, however, is that, as the judge correctly held at [236], the passage bridging pages 6 and 7 of the priority document also appears on page 8, lines 12-18 of the application as filed. It is true that the application contains a good deal more information than the priority document but in my judgment none of this further information qualifies in any way the disclosure of the subject matter of the claims in the form they now take. For like reasons to those I have given in considering priority, I am satisfied that the specification (including, in particular, claims 7 and 10) does not disclose matter extending beyond that disclosed in the application as filed.
- If, as I would hold, claims 7 and 10 are entitled to their claimed priority date, the only possible relevant prior art for novelty purposes is Stoner. As I have explained, this is taken to be part of the state of the art for novelty purposes by operation of s.2(3) of the 1977 Act if it is itself entitled to its own claimed priority date.
- The allegation that the patent lacks novelty in light of Stoner gave rise to two issues before the judge: first, whether the relevant matter in Stoner is entitled to its claimed priority date, namely the date of filing of US application 60/123,244; and secondly, if it is entitled to that priority date, whether its disclosure anticipates claim 7 (or claim 10 which here stands or falls with claim 7). The judge resolved the first of these issues in favour of the claimants but the second in favour of Lilly.
- As for Stoner's priority date, it was not suggested that its disclosure is not supported by matter disclosed in US application 60/123,244, the inventors of which are identified as Ms Stoner and Ms Waldstreicher. The live issue between the parties was and remains whether Merck & Co. Inc. ("Merck") and Ms Waldstreicher, the applicants for the relevant designations of Stoner, were legally entitled to claim that Stoner's priority date was the filing date of US application 60/123,244.
- The difficulty facing the judge was that he had nothing before him by way of evidence on this issue save for Stoner and US application 60/123,244. Neither side adduced any other evidence. Prior to the trial, the claimants served a notice asking Lilly to admit that Merck and Ms Waldstreicher had the relevant title but Lilly declined to make that admission.
- The claimants contended at trial that, while the legal burden of proof rested with them to establish that Merck and Ms Waldstreicher were entitled to claim that Stoner's priority date was the filing date of US application 60/123,244, the available evidence raised a prima facie case that they were so entitled with the consequence that the evidential burden to rebut that inference shifted onto Lilly. Lilly responded that the legal and evidential burden of establishing that Stoner's priority is that of the earlier US application fell and remained upon the claimants and they had failed to discharge it.
- The judge resolved this issue in favour of the claimants. He considered that the contents of Stoner supported an inference that its priority date was indeed the filing date of US application 60/123,244. Since Ms Waldstreicher was named as one of the inventors of that application, prima facie her claim to title was strong. Further, Merck's entitlement to make the priority claim was likely to be on the basis that it had derived title from Ms Stoner. He considered that Merck, a major international pharmaceutical company, must have had a professional patent department, and part of the function of that department must have been to ensure that formalities of this kind were complied with. Moreover, the fact that Merck's patent department had gone to the trouble of distinguishing between Ms Stoner and Ms Waldstreicher on the face of Stoner supported the inference that someone must have taken care over these formalities. The judge found that in these circumstances the evidential burden had shifted to Lilly to call evidence to rebut the inference of an entitlement to priority. It had not done so and accordingly Stoner was entitled to its claimed priority date.
- Turning to the second issue, this gave rise to two sub-issues: first, whether Stoner contains clear and unmistakable directions to do what Lilly claims to have invented, namely to make dosage forms containing 1 to 5mg of tadalafil for administration to a patient at doses of 1 to 5mg per day in a method of treating sexual dysfunction; and secondly, whether the disclosure in Stoner is enabling.
- The judge decided the first of these two sub-issues in favour of the claimants. He found that Stoner does contain clear and unmistakeable directions to do what Lilly claim to have invented. He resolved the second sub-issue in favour of Lilly, however. He found that although Stoner asserts efficacy for the various doses it describes, Dr Brock gave evidence which established that this assertion is speculative. The judge therefore rejected the allegation that Stoner anticipated the claims.
- Upon this appeal, Lilly contend that the judge fell into error on the first sub-issue and the claimants contend that he fell into error on the second. Of course the claimants have to succeed on both issues in order to secure the revocation of claims 7 and 10 for lack of novelty.
- Mr Waugh, for Lilly, submits that the judge fell into error in finding that the evidential burden had shifted on to Lilly to show that Stoner does not have the earlier priority date. He contends that, in accordance with principle, the burden of establishing an issue of fact must fall upon the person who asserts it and here, the claimants having pleaded that Stoner has a priority date earlier than its own date of filing, it was up to them to prove it.
- I accept that the legal burden of establishing that Stoner is entitled to a priority date earlier than its own date of filing lay on the claimants. But it does not follow that the evidential burden also lay on them throughout. The applicants for Stoner were Merck and Ms Waldstreicher and it carries on its face a reference to US application 60/123,244. The applicants for that US application are identified as Ms Stoner and Ms Waldstreicher, who are both said to share Merck's address in Rahway, New Jersey. Furthermore, the relevant subject matter in Stoner is disclosed in that US application. It is not suggested that Ms Stoner, Ms Waldstreicher or Merck had any relevant connection with the claimants or, indeed, with Lilly. In these circumstances and while it is of course possible that Merck was not the successor in title to Ms Stoner, it seems to me to be very unlikely. As a large pharmaceutical company with skilled legal advisers, it must have been well used to ensuring that the necessary formalities were complied with. In my judgment all of these matters were such as to raise a presumption or prima facie case that the claim by Ms Waldstreicher and Merck that Stoner was entitled to a priority date earlier than its own date of filing was properly and regularly made, in Merck's case as the successor in title to Ms Stoner. I am also satisfied the judge was both entitled and right to find that, although the legal burden of establishing that Stoner is entitled to the earlier priority date lay upon the claimants throughout, the circumstances were such as to shift the evidential burden on to Lilly to produce some evidence from which a lack of entitlement could be inferred. No such evidence was ever produced. Subject to the question of enablement, I therefore agree with the judge that Stoner is entitled to priority from the filing date of US application 60/123,244.
- I turn now to the disclosure of Stoner. It describes a combination therapy for sexual dysfunction involving a cGMP PDE specific inhibitor together with an alpha-adrenergic receptor antagonist such as Melanotan-II. These alpha-adrenergic receptor antagonists were known to be centrally acting drugs which initiated erections in men with psychogenic ED. Among the cGMP PDE inhibitors described are PDE5 inhibitors such as sildenafil and tadalafil (identified as IC-351). A wide range of dosage forms are disclosed including compositions containing from 0.01 to 500mg of each such ingredient, although it is right to acknowledge that there is also a specific disclosure of tablets containing 1, 2.5 and 5mg of each ingredient. Dosage levels are said to be between about 0.001 to 50mg/kg of body weight daily, preferably about 0.005 to about 25mg/kg per day, and more preferably about 0.01 to 10mg/kg per day. Even the narrowest of these ranges is very much broader than that of the patent. This impression of the breadth of the dosing level ranges is confirmed by the uncontroverted evidence of Dr Brock that the skilled clinician would consider such a wide range, in the absence of efficacy data, to be speculative and so broad as effectively to provide no meaningful guidance. Moreover the fact that it is directed to a combination therapy would also affect how the skilled person would understand its disclosure. The judge summarised the evidence given by Dr Brock under cross-examination in these terms at [254]:
- As I have mentioned, the judge found that Stoner does disclose the invention claimed in the 181 patent. More specifically, he found that Stoner discloses the dosage forms and daily doses claimed in the patent. Further, to the judge's mind, the fact that Stoner is directed to a combination therapy does not affect the position because the claims of the patent are broad enough to encompass such therapies.
- I have some doubt as to whether the judge was correct on this issue. As Dr Brock said in evidence and the judge accepted, a combination such as that described in Stoner muddles the situation because the skilled person would not know whether or not the centrally acting agent, the adrenergic receptor antagonist, is going to modify totally the response of the patient to the PDE5 inhibitor. In the words of Dr Brock, "all bets are off". Furthermore, the range claimed in the patent is narrow, far removed from even the narrowest range described in Stoner and is certainly not arbitrary. In these circumstances it seems to me to be well arguable that Stoner does not make available to the skilled person as a technical teaching the subject matter of the claims of the patent; and further, that read through the eyes of the skilled person, Stoner does not disclose that it is possible to use the whole of the dosing range to which it refers. However, it is not necessary to express a final view upon this in light of the judge's finding on the final question, with which I entirely agree.
- That final question is whether Stoner amounts to an enabling disclosure. The judge considered that it does not, because although it asserts that various combinations are efficacious, Dr Brock's evidence showed that there was no basis for concluding that the particular combination of tadalafil with a centrally acting alpha-adrenergic receptor antagonist would be efficacious. There was nothing which made it credible or plausible that such a combination would work.
- I believe that the judge was entitled to make this finding in light of the evidence of Dr Brock to which I have referred. Once again it must be borne in mind that Stoner only discloses a combination therapy and, having regard to Dr Brock's evidence, the skilled person would not regard it as plausible that, in combination with a centrally acting alpha-adrenergic receptor antagonist, administration of tadalafil in the claimed dosing range would be efficacious for the treatment of ED.
- There can be no doubt that the allegation that the claimed invention was obvious was, in the circumstances of this case, a powerful one. It was founded upon Daugan, an application which was published on 6 February 1997, that is to say before the earliest possible priority date of the 181 patent. Daugan teaches the use of PDE5 inhibitors for the treatment of ED. Tadalafil (compound A) is specifically disclosed, its IC50 against PDE5 is given and examples of a tablet containing a 50mg dose are described. It explains that doses of tadalafil will generally be in the range of from 0.5 to 800mg daily for the average adult patient.
- Adoption of the structured approach to the assessment of obviousness described by the Court of Appeal in Pozzoli v BDMO [2007] EWCA Civ 588, [2007] FSR 37 requires the identification of the differences between the disclosure of the prior art and the claims in issue. The judge observed that in this regard all the claims stood or fell together. I agree that claims 7 and 10 stand or fall together. But claim 1 is different. It is a claim to a product which comprises 1 to 5mg of tadalafil and which is suitable for oral administration up to a maximum total dose of 5mg per day. But it is not limited to a tablet intended for use or in fact used in that way.
- The differences between the disclosure of Daugan and the subject matter of claims 7 and 10 are that Daugan does not specifically disclose a 5mg daily dose of tadalafil or that such a dose is an effective treatment for sexual dysfunction. The difference between Daugan and the subject matter of claim 1 is rather more limited. It is simply that Daugan does not specifically disclose a tablet containing 5mg of tadalafil.
- The claimants' case at trial ran as follows. At the priority date it would have been perfectly obvious for the skilled team, given Daugan, to take tadalafil forward into a routine pre-clinical and clinical trial programme to assess its use as an oral treatment for sexual dysfunction. In the course of that programme, a 5mg daily dose of tadalafil would be used in patients and it would reveal the invention, that is to say that a 5mg daily dose is a safe, tolerable and effective treatment.
- Lilly responded that the claimants' case was really one of "obvious to try" and that this could only lead to a finding of invalidity if the skilled team would consider that the programme had a fair prospect of success. That was certainly not the case here because, at the start of the programme and given Daugan, the skilled team would have had no idea whether or not a 5mg daily dose of tadalafil would be a safe, tolerable and effective treatment of sexual dysfunction, still less that it would be both efficacious and have minimal PDE5 related side effects.
- The judge resolved this difference in favour of Lilly. He found that claim 7 (and necessarily, therefore, claim 10 too) involved an inventive step. After evaluating the arguments and the evidence, he found that a 5mg daily dose of tadalafil as a treatment for sexual dysfunction was not obvious.
- Mr Speck has subjected this conclusion to serious criticism. Mr Waugh counters that the judge carried out a proper and careful evaluation and it is one with which we should not interfere. Before addressing their respective submissions, I must explain the findings made by the judge on the evidence and the essential steps in his reasoning.
- The judge found that it would be entirely obvious for a skilled team, starting with Daugan, to take tadalafil forward into a routine pre-clinical and clinical trial programme as an oral treatment for male ED. The statements in Daugan were sufficient to make this obvious and the success of sildenafil made it very obvious. Tadalafil would be an attractive second in class medicine to develop.
- Standard pre-clinical studies would be undertaken with the firm expectation that they would produce useful data and a reasonable expectation that tadalafil would turn out to be a viable drug. In light of the information which is now known about tadalafil, these studies would produce favourable results.
- The drug would therefore be carried forward into routine Phase I safety studies in healthy volunteers. Here a wide range of doses would be tested and the maximum tolerated dose established. Repeated daily doses would also be tested. All of this work would be routine and carried out in the reasonable expectation that tadalafil would prove to be a safe drug.
- The judge made the following findings (at [289]) about the knowledge, understanding and activities of the skilled team at this point in the programme:
- At this point the skilled team would decide whether to investigate use on demand or by chronic daily dosing or both. The judge was in no doubt that the team would investigate use on demand, but later in his judgment (at [340]) he held that they would also investigate chronic daily dosing. I shall return to this finding and its consequences after following the course of the on demand investigations.
- Given the positive results of the preclinical and Phase I results and the great success of sildenafil, the skilled team would embark on the next stage of the programme, a "go, no-go" Phase IIa study of the efficacy of a single dose of tadalafil in a relatively small group of patients. The team would be likely to select a dose of 50mg and would embark upon the study with a reasonable expectation that, at this dose, the drug would be safe, tolerable and effective. Given what is now known, this Phase IIa study would produce favourable results. The decision of the team would therefore be to "go".
- The next step would be routine Phase IIb dose ranging studies in larger groups of patients. For this the skilled team would have to select a range of doses. The judge found that the team would select 25, 50 and 100mg for the first study and would hope that the results would show a dose response relationship although they would not necessarily expect the effects of the 25mg dose to be clinically relevant. As for side effects, they would think that any reduction in these effects would be accompanied by a reduction in efficacy, their perception being that efficacy and side effects have the same root causes.
- The skilled team would find the results of the Phase IIb study rather surprising. The results would show that the 25, 50 and 100mg doses were all equally efficacious, demonstrating an apparent therapeutic plateau or, in terms of the graphs shown at [17] and [18] above, that all of these results were on the upper part of the characteristic sigmoidal dose response curve. Based upon the results of a real study of tadalafil, termed the LVBG study, the skilled team would find that the common side effects of headache, back pain and myalgia did show a dose response, however. In this LVBG study, doses of 25mg were well tolerated and doses up to 100mg were "generally" well tolerated.
- There was a substantial dispute between the parties as to what the skilled team would do next. The claimants argued that it would be obvious to conduct further dose ranging studies to investigate lower doses to ascertain the dose response relationship and find the minimum effective dose. Lilly, on the other hand, argued that the skilled team would not investigate lower doses at this stage. They also contended and the judge accepted (at [323]) that characterisation of the minimum effective dose would require a value judgment and different teams might arrive at different conclusions. It was also relevant that Pfizer did not ascertain with any degree of precision the minimum effective dose for sildenafil. It was found to lie somewhere between 5 and 25mg.
- The judge weighed up the evidence and the submissions of the parties upon this aspect of the case at [327]. He found that it was not inevitable but very likely that the skilled team would investigate lower doses. As he put it:
- The judge considered next what the skilled team's expectations would be were they to investigate lower doses and for this purpose he assumed a dose ranging study including both 5 and 10mg doses. He reiterated that by this stage the team would have ascertained that a dose of 25mg was a marketable dose and was safe, tolerable and effective. He also found that they would have defined a minimum clinically relevant effect for their own purposes. Returning to expectations, the judge found that the team would not have any expectation that the minimum effective dose was substantially lower than 25mg and they would have no reasonable expectation that 10mg would produce a clinically relevant effect as they had defined it. An entirely feasible outcome would be that the minimum effective dose would be found to lie somewhere between 10 and 25mg.
- The judge also considered (at [332]) the possibility of the skilled team conducting the testing in two further stages, that is to say, testing doses of 10, 25, 50 and 100mg first, finding that the dose of 10mg was also on the therapeutic plateau, and then testing a range of doses down to 5mg. He found that the expectations of the team at this stage would be no different from those they had earlier. Moreover, in the judge's words, "an argument which requires three rounds of dose ranging to arrive at the invention is beginning to look like hindsight".
- What is more, the judge continued, the team would expect efficacy and PDE5 related side effects to go hand in hand and, although side effects had been shown to be dose responsive at doses of 25, 50 and 100mg while efficacy had plateaued, they would not believe that there might be a dose below 25mg at which a clinically relevant effect might be found but with reduced side effects.
- Summarising the position in relation to the skilled team's expectations, the judge found that they would not have a reasonable expectation that a dose of 5mg per day (on demand) would provide a useful treatment for ED, nor any expectation at all that this would produce a clinically relevant effect but with minimal side effects. However, they would discover that this dose is both effective and has reduced side effects and this would be a surprise. The judge explained the position in these terms at [335] to [336]:
- The judge turned next to daily dosing. The claimants, supported by the evidence of their expert, Mr Muirhead, contended that the relatively long half-life of tadalafil as compared to sildenafil would suggest daily dosing; and further, that this half-life would have emerged from the pre-clinical and Phase I studies. Lilly responded, on the basis of the evidence of their expert, Dr Brock, that the skilled team would think that chronic dosing would exacerbate or prolong tadalafil's side effects.
- The judge accepted Mr Muirhead's evidence that the skilled team would investigate chronic daily dosing but held they would not have a strong expectation that it would lead to a useful drug. They would make this decision after Phase I and would carry out Phase IIa studies with a dose of 50mg/day. They would find, just as they would find for on demand dosing, that the results were favourable.
- The skilled team would then proceed to Phase IIb studies and would include a 10mg dose as an additional arm having regard to what the judge described as the accumulation factor. As he held at [341]:
- The judge then stepped back and considered the issue of obviousness by reference to the programme as a whole. He explained that a clinical programme such as this has many routine and obvious steps. He continued that, although Daugan only describes a 50mg tablet, he was sure that a 25mg/day dose of tadalafil for the treatment of ED was obvious and involved no inventive step. He continued that the 5mg/day dose was not obvious, however. Basing himself in part upon the various factors referred to by me in Generics (UK) Ltd v H Lundbeck [2007] EWHC 1040 (Pat), [2007] RPC 32 at 72, he explained the basis for that conclusion:
- Mr Speck began his attack on the judge's reasoning by taking us to the decision of the Court of Appeal in Actavis UK Ltd v Merck & Co Inc [2008] EWCA Civ 444, [2008] RPC 26. In giving the judgment of the court, Jacob LJ said at [32]:
- Mr Speck continues that there is nothing unusual about this case and that the invention would be found by starting with Daugan, adopting standard practice and following the well-trodden path through the phases of the clinical trials' process. He argues that the purpose of the Phase IIb studies is to provide an understanding of the dose response relationship of a drug and the fact that it is not possible to predict in advance whether the drug will be safe and efficacious at any particular dose is neither here nor there. He also submits that it is striking that, despite finding that taking tadalafil forward into a clinical testing programme was "very obvious", and despite finding that the skilled team would test a dose of 5mg of tadalafil and find it safe and efficacious for the treatment of ED, the judge held that the claimed dosing regime amounted to an invention. This, says Mr Speck, was irrational and wrong.
- Mr Waugh responds that the judge directed himself entirely properly as to the law and that he was right to have regard to the skilled team's expectation of success in embarking on the programme of research that was necessarily involved in arriving at the invention. Indeed, says Mr Waugh, it is now clearly established that expectation of success is a critical consideration in all 'obvious to try' cases. Here the evidence established and the judge correctly found that the skilled team would have to make a series of value judgments in order to arrive at the invention, that they would have had no expectation that a dose of 5mg of tadalafil per day would be efficacious, and that they would have been surprised to find that a dose of 5mg was both efficacious and had reduced side effects.
- It is readily understandable why Mr Speck places so much reliance upon the passage in Actavis v Merck to which I have referred. But it is important not to take it too far. It does not establish that investigations into appropriate dosage regimens cannot yield patentable inventions. Indeed, Jacob LJ made it clear at [29] of his judgment that research into new and better dosage regimens is clearly desirable and that there is no policy reason why the discovery of a novel and non-obvious dosing regimen should not be rewarded by a patent.
- The correct approach to the assessment of obviousness in a case such as this was explained by the Court of Appeal in MedImmune v Novartis [2012] EWCA Civ 1234, [2013] RPC 27 at [90] to [93] and [178] to [182]. In short, the court must have regard to all of the relevant circumstances in order to answer the single and relatively simple question: was it obvious to the skilled but unimaginative addressee in light of the prior art and the common general knowledge to make a product or carry out a process falling within the claim? The court has to weigh up all of the relevant evidence and decide whether the invention was obvious. That is the statutory task.
- In that same year the Court of Appeal gave guidance as to the relevance of whether a particular route was obvious to try and skilled team's expectation of success in Novartis AG v Generics (UK) Ltd (trading as Mylan) [2012] EWCA Civ 1623. In a judgment with which Lewison and Munby LJJ agreed, I said this at [55]:
- I have no doubt the judge had all of these principles well in mind in carrying out his assessment. Furthermore, where no question of principle is involved, an appellate court will be very cautious in differing from a judge's evaluation. However, says Mr Speck, in this case the judge did make errors of principle, failed to take into account relevant matters, asked himself the wrong questions and failed to distinguish relevant from irrelevant factors.
- Mr Speck elaborated these submissions and Mr Waugh responded to them primarily by reference to claims 7 and 10. It is also necessary to address claim 1, however. As I have explained, this is directed to a dosage composition comprising 1 to 5mg of tadalafil which is suitable for oral administration up to a maximum total dose of 5mg per day. But it has no purpose limitation and encompasses a unit dosage composition comprising 1 to 5mg of tadalafil which is suitable for administration up to a maximum total dose of 5mg per day but which is intended for use and is in fact used for administration up to a total dose of, for example, 25 or 50mg per day. On the judge's findings, it was entirely obvious to develop such a composition in light of Daugan. On any basis, claim 1 is therefore invalid for obviousness and the judge should so have held.
- I come then to claims 7 and 10, the real battleground between the parties. The heart of the judge's reasoning and his key findings are drawn together at [343]. Neither side has quarrelled with the finding at [343(i)]. The skilled team would be highly motivated by Daugan and the success of sildenafil to investigate tadalafil for the treatment of ED. But, as Mr Waugh fairly points out, this is a necessary but not sufficient requirement for the obviousness case to succeed.
- The judge considered possible avenues of research at [343(ii)]. He explained that the claimed dose of 5mg per day is significantly lower than the 50mg dose described in Daugan which would be chosen for the first test of efficacy in Phase IIa. Thus far, Mr Speck has no criticism of his reasoning. Nor does Mr Speck criticise his finding that 5mg per day would not be chosen for the first dose ranging study. But Mr Speck says that thereafter the judge has fallen into error in so far as he has suggested that subsequent dose ranging studies or the investigation of daily dosing constitute different avenues of research or are other than routine.
- Mr Waugh responds that the judge correctly found that, at various stages of testing, the skilled team would have to make value judgments as to what to do and which doses to take forward, and that it was not inevitable that they would investigate lower doses given the results of the Phase IIb study. He submits that the first Phase IIb study would involve the testing of 25, 50 and 100mg doses of tadalafil on demand, and that if the team decided to look further into the dose response relationship, they would test 10mg. In making the choice to pursue the dose response relationship, particularly in light of the finding that a dose of 25mg on demand was safe and efficacious, the team would no longer be pursuing 'one avenue'. Further, if they were to decide to test 5mg, this would be a further step away from 'one avenue'. He also points to the judge's observation at [332] that an argument which requires three rounds of dose ranging in order to arrive at the invention is beginning to look like hindsight. Moreover, says Mr Waugh, the decision whether to pursue on demand and daily dosing bifurcates the 'one avenue' in another way.
- I have no doubt the judge was right to consider whether the skilled team, starting with Daugan, would be faced with various possible avenues of research. Once an invention has been made it is often all too easy to see how it might have been arrived at by taking a particular combination of apparently routine steps. But the notional skilled team seeking an improved product or process is equipped only with the common general knowledge and the prior art and it may be far from obvious to them how to proceed for they may have little or no idea which of the possible avenues of research will prove fruitful. What is more, if they do choose one avenue over the others, they may be faced with choices as to how to pursue it. In these circumstances it may require ingenuity to arrive at the invention and this is a characteristic that the notional skilled team does not have.
- I also recognise that, in the present case, the notional skilled team embarking on Phase II studies would be faced with a number of choices and, in particular, how to proceed with the dose ranging studies and whether to pursue on demand or daily dosing. But in assessing the relevance of these choices to the issue of obviousness it is important to have a number of matters well in mind. First, the decision about daily and on demand dosing would be taken after Phase I and with the knowledge of the various matters I have set out at [113] above, including half-life. The judge found that at this point the skilled team would decide to pursue both of them (see, in particular, the judge's findings at [340]). Secondly, although the judge found that the skilled team would not test the efficacy of a dose of 5mg tadalafil in the first or possibly even, in the case of use on demand, the second dose ranging study, they would very likely investigate it thereafter (see, in particular, the judge's findings at [327] and [343(iv)]).
- These findings are not at all surprising. The purpose of the dose ranging studies relating to efficacy is to ascertain the dose response relationship of the drug under test. If the studies yield results for efficacy which are all on the upper plateau of the sigmoidal dose response curve then the dose response relationship has not been determined. Furthermore, the judge's finding that, following the discovery of a plateau starting at 25mg or 10mg, there would very likely be a subsequent dose ranging study which included 5mg is also entirely consistent with the evidence of both Mr Muirhead and Dr Saoud, the experts in clinical pharmacology. Their evidence on this issue is particularly important in light of the judge's finding that, while the clinician's view is important, dose selection is an issue on which the clinical pharmacologist member of the team would take the lead. Mr Muirhead's evidence was clear and unshaken in cross-examination. It was his opinion that the skilled team would test lower doses. Dr Saoud was asked in cross-examination about the LVBG study and the results of testing doses of 10, 25, 50 and 100mg of tadalafil per day. He agreed that the results showed that all of these doses were equally efficacious and that the skilled person would recognise that they were all on the upper plateau of the dose response curve. He was then asked whether the skilled team would investigate lower doses. This led to the following interchange (on day 5, page 662):
- A little later, Dr Saoud also gave this illuminating evidence concerning the invention of the patent (on day 5, page 685):
- Accordingly, this is not a case in which the skilled team would be faced with a series of parallel avenues of study and would not have any expectation that any one of those avenues would prove fruitful or that would be more likely to prove fruitful than any other. Nor is it a case where most or even some of the avenues of investigation would not lead to the invention. It is instead a case where the two possibilities of on demand and daily dosing would both be addressed in light of the earlier routine work which would reveal tadalafil's half-life, and where each would be very likely to lead the skilled team to the invention. Rather than focussing on these important points, as I believe he should have done, the judge has instead attached weight to the fact that a dose of 5mg is considerably less than the 50mg dose which would be used in Phase IIa efficacy test, and to the fact that a dose of 5mg would not be chosen for the routine first dose ranging study in Phase IIb. It is of course true that a dose of 5mg is considerably less than a dose of 50mg but it must also be borne in mind that the two parts of Phase II have different purposes. Phase IIa is designed to provide proof of concept and is generally carried out at one dose selected to be high enough to provide the best chance of showing a positive effect while not causing serious side effects. Phase IIb is carried out at different doses chosen to provide an understanding about the dose response relationship. It is also true that a dose of 5mg would not be chosen for the first study in Phase IIb. However, as the judge himself found, the skilled but uninventive team would very likely investigate it thereafter.
- At [343(iii)], the judge considered the resources required for the programme and the attitude of the skilled team as it progresses. Mr Speck does not challenge the judge's finding that the programme would involve very substantial resources in terms of time, money and researchers. However, he submits that it is necessary to consider with care the further finding that, by the time the idea of investigating lower doses would present itself, the team would have established safe, tolerable and efficacious doses of tadalafil at 25mg on demand and 10mg for daily dosing and that, at that stage, the impetus to investigate lower doses would be reduced but not eliminated. He continues that the impetus would remain because the team would know that the clinical response to all of the tested doses had been the same.
- The point made by Mr Speck is, in my judgment, entirely fair. The impetus would indeed remain for, while the results are all on the top plateau of the dose response curve, the purpose of the Phase IIb study, namely to understand the dose response relationship of the drug, has not been fulfilled. This is reflected too in the judge's own finding that the skilled team would very likely proceed to test the efficacy of lower doses, including the dose of 5mg.
- The judge addressed the skilled team's expectation of success at various points throughout his judgment on obviousness, including [307] to [308], [312], [331] to [336], [340] to [341] and [343(iv)] and he did so overall and in relation to particular studies. He found that the team would embark on the project with a reasonable prospect of success in establishing tadalafil as a safe, tolerable and effective treatment for ED. However, he continued, it had not been shown that the efficacy of tadalafil at a dose of 5mg was predictable or worth considering by the skilled team based on the properties of tadalafil as against sildenafil. He also found that, assuming the skilled team decided at any point to test a 5mg per day dose, they would not have had a reasonable expectation that this dose would produce a clinically useful response with reduced side effects.
- As I have explained, Mr Waugh submits that these are findings which the judge was entitled to make and which are highly relevant to and supportive of his overall conclusion of non-obviousness. In my judgment there are a number of problems with this reasoning, however. The fact that the skilled team could not make any prediction at the outset that a dose of 5mg of tadalafil per day would be safe and efficacious is of little weight because at least one of the purposes of carrying out the Phase IIb studies is better to understand the dose response relationship of the drug and so identify the appropriate dose range for the target population. Much the same point applies to the judge's assessment of the expectation of success were the team to assess a dose of 5mg per day. By this time the skilled team would know that the doses of 10, 25, 50 and 100mg of tadalafil per day (or doses of 25, 50 and 100mg on demand) all yielded results for efficacy which lay along the top plateau of the dose response curve. The team would also know that, as doses are reduced, there will come a point at which the efficacy of the drug will start to fall, as depicted in the sigmoidal dose response curves shown at [17] and [18] above. The team would not know in advance at which dose that point would be reached or how steep the curve would be but ascertaining that relationship is a purpose of the routine Phase IIb study. More fundamentally, however, the judge has found that the notional non-inventive team would be very likely to include a dose of 5mg per day as part of a dose ranging study. It is very hard to see why a notional skilled but non-inventive skilled team would do so unless they had a reasonable expectation that it would assist them better to understand the dose response relationship. Moreover, were they to test the dose of 5mg per day they would find it is both clinically effective and has reduced side effects.
- The judge addressed unexpected or surprising results at [336], [341] and [343(v)]. He found that the path to the invention includes unexpected or surprising results: first, the plateau in the dose response at doses from 10 to 100mg; and secondly, that a 5mg per day dose of tadalafil is efficacious and has reduced side effects. It is of course true that the discovery of a surprising or unexpected technical effect may be suggestive of invention, and this is a point upon which Mr Waugh heavily relies. On the other hand, if it is already obvious for the skilled person to arrive at something falling within the claim then it does not cease to be obvious because some unexpected or bonus effect is found. In this case I do not consider that either of the points identified by the judge takes Lilly very far. The skilled but uninventive team would find tadalafil an attractive potential second in class medicine to develop. Daugan made it obvious to do so and the success of sildenafil made it very obvious. So it was entirely obvious for the skilled team to take tadalafil forward into a routine pre-clinical and clinical trial programme. In the course of that programme and on the judge's own findings, the skilled team would very likely carry out a dose ranging study including a 5mg dose. If they did so, they would find it was efficacious and had reduced side effects and so they would take it forward into Phase III. They would have arrived at the invention.
- Finally, the judge had regard, at [343(vi)], to the value judgments that the skilled team would have to make in a programme which reached the claimed invention. He identified three: first, the identification of the level of clinical effect to be regarded as relevant; secondly, whether to embark on an investigation of daily dosing; and thirdly, what to do when an unexpected plateau has been identified at the same time as a marketable dose. In my judgment these provide no effective support for the judge's conclusion.
- It is convenient to take the first and third of these value judgments together for they are interconnected. The judge found that the skilled team would know that characterisation of the minimum effective dose for the treatment of ED would require a value judgment; and further, that different groups would have different views as to what constituted the minimum clinically relevant effect. The judge also found that the skilled team would find that the first dose ranging study of 25, 50 and 100mg doses of tadalafil on demand yielded results which were efficacious, well tolerated and safe; and that the skilled team would have had both of these matters in mind in deciding whether to investigate lower doses. But, as I have explained, the judge found that it was very likely they would proceed and test the 5mg dose, and that, so it seems to me, is the critical finding.
- The judge's second point has no independent life of its own for, as I have also explained, the judge found the skilled team would consider both on demand and daily dosing. Moreover, on the judge's findings, the only difference between them is that, in the case of daily dosing, the skilled team would include a dose of 10mg in the first dose ranging study. In each case, the skilled team would be very likely to investigate a dose of 5mg thereafter.
- Drawing the threads together, I am satisfied that Mr Speck has made good his criticisms of the judge's reasoning. The judge has lost sight of the fact that, on his own findings, the claimed invention lies at the end of the familiar path through the routine pre-clinical and clinical trials' process. The skilled but non-inventive team would embark on that process with a reasonable expectation of success and in the course of it they would carry out Phase IIb dose ranging studies with the aim of finding out, among other things, the dose response relationship. It is very likely that in so doing they would test a dose of 5mg tadalafil per day and, if they did so, they would find that it is safe and efficacious. At that point they would have arrived at the claimed invention. In my judgment claims 7 and 10 are therefore invalid.
- I would allow the appeal. Claims 1, 7 and 10 of the 181 patent are invalid for lack of inventive step and the judge ought so to have held.
- I agree with Kitchin LJ's conclusions on each of the issues of construction, infringement, priority, added matter and novelty over Stoner and have nothing to add to his reasons for upholding the judge on these issues. I also agree with his conclusion and reasoning on obviousness, but as we are differing on this one issue from a very experienced patents judge, I wish to add a few words of my own.
- There is no difficulty about identifying the question which the court has to answer on the issue of obviousness of claims 7 and 10. It is whether it was obvious to the skilled team, in the light of Daugan, and without knowledge of the invention, that a 5 mg daily dose of tadalafil would be a safe and effective treatment, with minimal side effects, for sexual dysfunction. If one notionally asks that question of the skilled team before it embarked on the investigations which Birss J chronicles in his judgment, the answer would be, of course, that it was not obvious. The skilled team would respond that it could not know without conducting appropriate tests what if any dose of tadalafil would achieve that goal.
- The law, as it has developed at least in this jurisdiction, does not halt its enquiry at this point, however. If it did, this would have been a very short issue to decide. It is recognised that a patent will not be granted for an invention which, though not obvious in this a priori sense, is nevertheless an invention which would be arrived at by a line of routine and uninventive enquiry which would be carried out by a skilled team.
- It is important not to let this approach to obviousness extend beyond its proper bounds. There will hardly ever be an invention for which it is not possible to "show how it might be arrived at by starting from something known, and taking a series of apparently easy steps": see per Moulton LJ in British Westinghouse v Braulik (1910) 27 RPC 209 at 230. Nearly 100 years later, Moulton LJ's view that this approach was "not countenanced by English law" was said by Jacob LJ (with whom Mummery and Pill LJJ both agreed) in Technip France's SA's Patent [2004] RPC 46 at [112] to be "as true today as when it was first said".
- I said something about the dangers of too relentless an approach to the "obvious line of enquiry" principle in Gedeon Richter v Bayer Schering [2011] EWHC 583 (Pat), in the context of experimentation. Having reviewed the cases on obvious to try I said:
- To similar effect is Teva v Merck [2009] EWHC 2952 (Pat) at [98], where I said:
- I adhere, not surprisingly it might be said, to the views I expressed in both of those judgments. However, there are some steps which can be characterised as so routine that the skilled person would carry them out simply because they are routine, and irrespective of any prospect of success. An example is routine dose ranging studies in the clinical testing of a known drug: see the passage from Actavis v Merck [2008] EWCA Civ 444 cited by Kitchin LJ at [128] above.
- We must also keep in mind, as we were asked to more than once by Mr Waugh QC, that this court should be extremely slow to interfere with the finding of a trial judge on the issue of obviousness. Lord Hoffmann put it in this way in Biogen v Medeva:
- The "evaluation" to which Lord Hoffmann referred is the value judgment as to whether, overall, what is required of the skilled person in order to arrive at the invention merits the description "obvious". That is the statutory question at the outset, and however the question is divided up for the purpose of the decision-making process, that is what it remains. Of course, if the judge's findings of fact compel a finding of obviousness, in the sense that no other conclusion was open to him on those findings, then this court will be bound to conclude that the invention was indeed obvious.
- To my mind the important findings of the judge were as follows:
- For the purpose of his analysis the judge then assumed that there would be either one (involving 10 mg and 5 mg) or two (including 10 and then 5 mg) further studies. He went on to consider what the skilled team's thinking would be in advance of such notional studies. He concluded that the skilled person would regard a 25 mg dose of tadalafil as a marketable dose. It would be safe, tolerable and effective. The skilled team will have defined a minimum clinically relevant effect for their own purposes. They would have no expectation that the minimum effective dose was substantially lower than 25 mg. The most that could be said was that the team would expect that somewhere below 25 mg there would be a dose of tadalafil which did not work. They would hope to see a dose response but even if they expected to see any statistically significant effect at 10 mg, they would have no reasonable expectation that 10 mg tadalafil would produce a clinically relevant effect as they had defined it. In terms of expectations, an entirely feasible outcome would be that the minimum effective dose would to be found to be between 10 and 25mg. As for side effects, the skilled team would expect efficacy and PDE5 related side effects to go together. The fact that side effects showed a dose response in the first dose ranging study whereas efficacy was on the plateau would not lead the team to expect that there might be a dose below 25mg at which a clinically relevant effect was found with reduced side effects.
- Thus, the judge found that the skilled team when carrying out testing of a 5mg per day dose of tadalafil (on demand) would not have a reasonable expectation that the drug at this dose would be a useful treatment for erectile dysfunction nor any expectation at all that the drug would produce a clinically relevant effect but with minimal side effects.
- The judge went on to hold that a skilled team which carried out a dose ranging study including a 5mg dose would discover that this dose was efficacious and had reduced side effects. They would be surprised by this. The team would go ahead to do Phase III studies at 5mg/day and seek clinical approval for a 5mg/ dose of tadalafil.
- The judge then stood back and evaluated the investigatory programme which he had outlined as a whole. He rightly gave the claimants credit for the fact that the skilled team would be highly motivated by Daugan and the success of sildenafil to investigate tadalafil as a treatment for erectile dysfunction. Nevertheless he recognised that 5 mg/day is a significantly lower dose than the 50 mg dose exemplified in the Daugan prior art and the marketed doses of sildenafil, and significantly lower than the 50 mg dose which would be chosen for the first test of efficacy at Phase IIa. It would not be chosen in the routine first dose ranging study. Further, the overall programme (which would be pursued) would involve very substantial resources of time, money and people. By the time the idea of investigating lower doses presented itself, the team would have established safe, tolerable and effective doses of tadalafil at 25mg on demand and 10 mg for daily dosing. At that stage the impetus to investigate lower doses would be reduced, albeit not eliminated.
- Overall, the judge considered that the team would embark on the project with a reasonable expectation of success in establishing tadalafil as a safe, tolerable and effective treatment for tadalafil. However, the claimants had failed to prove that efficacy at 5mg tadalafil was predictable or worth considering by the skilled team based on the properties of tadalafil as compared to sildenafil. The team would know that in principle there would be a minimum effective dose for tadalafil but would also know that its definition depends on a value judgment made by the team. In relation to the dose ranging studies, the team would conduct them hoping for a dose response. Following discovery of a plateau starting at 25 mg or 10mg, there would "very likely" be a subsequent dose ranging study which included 5 mg. The team would include a 5 mg dose in this study hoping to see a dose response but that does not mean they would have a reasonable expectation that 5mg would produce a clinically relevant effect at all nor one with minimal side effects. Assuming a 5 mg /day dose of tadalafil was tested, it would not be tested with a reasonable expectation of success. The result for the 5 mg/day dose was a surprising one: the existence of a useful effect with reduced side effects. A number of value judgments would be required of a skilled team in a programme which reaches the claimed invention. One was to define the level of clinical effect to be regarded as relevant. Another was to embark on investigating daily dosing. An important value judgment was what to do when an unexpected plateau in the dose response has been identified at the same time as a marketable dose.
- I think that it was in these final steps in his reasoning that the judge fell into error. The whole purpose of embarking on the routine Phase IIb dose ranging study was to identify a dose response. The discovery of a plateau indicated that the routine study would have to be repeated at a lower dose, because it was not complete. Completion of the study would inevitably lead the skilled team to test 5 mg/day, whether that dose was still on the plateau, or in a region of the curve where a dose effect is observed. Which it is does not matter, because the result is that the skilled person would at this stage have arrived at a dosing regimen within the claim.
- Whilst the existence of value judgments on the road to the invention are of course highly material, the judgments identified by the judge in this passage of his reasoning were collateral ones which had no impact on the decision to complete the Phase IIb study, or were not true value judgments at all. Thus the judge relied on the need to define the level of clinical effect to be regarded as relevant, i.e. the minimum effective dose. The identification of a dose response and the identification of a minimum effective dose are, however, different things. The need to complete the Phase IIb study and identify a dose response does not involve exercising a judgment about minimum effective dose. Likewise, if by referring to the decision to embark on investigating daily dosing the judge was intending to refer to chronic daily dosing, that is also not an obstacle to completing the Phase IIb trial with the 5 mg maximum daily dose and arriving within the claim by that route. Finally, the judge relied on the decision as to what to do when faced with the plateau. There was ample evidence to suggest that, far from being a value judgment, investigating lower doses was something the skilled team would do without further thought.
- I think the judge also allowed himself to be side-tracked by his undoubtedly correct conclusion that the skilled team would not have been able to predict that the 5 mg/day dose would be effective. If the only thing which was driving the skilled team to test the 5 mg dose was its level of expectation that a 5 mg dose might be effective, the judge's conclusion about expectation of success as to efficacy would be highly material. The skilled team does not generally undertake steps with a given objective in mind without a reasonable expectation of success in obtaining that objective. Phase IIb tests are, however, conducted with a separate objective, namely to identify a dose response. The judge rightly held that it is very likely that these routine tests would be conducted for precisely that reason. Indeed that is the only basis on which his conclusion that the skilled person was very likely to do such tests is understandable. The absence of an expectation of success as to efficacy is, in these particular circumstances, not relevant.
- It is true that the judge made a finding that the skilled team would be surprised by the result, namely efficacy at 5 mg/day. However it is a result which on his findings would be arrived at by the standard, routine enquiries into dose response which are required by Phase IIb clinical trials. The surprising result, once uncovered, does not make these routine enquiries inventive.
- On this one issue, therefore, I agree that the appeal must be allowed.
- I agree with the judgments of Kitchin and Floyd LJJ. I, too, wish to add a few words of my own on the question of obviousness.
- Mr Waugh's main argument on behalf of Lilly was that an expectation of success is an integral component of an "obvious to try" case. In my judgment that is not the law. At one point in his judgment the judge appears to have rejected that argument. In Gedeon Richter v Bayer Schering at [114] Floyd LJ said:
- The judge referred to this at [275] and correctly held at [276]
- It is true that whether or not the skilled person or team has an expectation of success can be relevant to the question whether he or they would undertake a particular test or experiment. But it is not a necessary condition in all cases, as Kitchin LJ explained in Novartis AG v Generics (UK) Ltd (which he has quoted at [133] above).
- The judge made a number of highly relevant findings. At [287] he held that it was "entirely obvious" to take tadalafil forward into a "routine pre-clinical and clinical trial programme." At [293] he found that following Phase IIa the next step would be to conduct "routine dose ranging studies… in order to establish the dose response".
- The judge went on to hold at [327], having considered all the evidence, that the skilled team would be "very likely" to have investigated lower doses than 25mg. The expectation of success, if relevant at all, should come in before making the decision on what the skilled team would do. If the skilled team had no expectation of success, that might be a reason why they would not undertake the step in question. But having come to the conclusion that they would, it seems to me that the question has been answered and that there is no further need to examine an expectation of success. That is the way in which Jacob LJ dealt with the question in Actavis UK Ltd v Merck & Co Inc (to which Kitchin LJ has referred). At [116] to [117] he summarised the point by posing the question whether the expectation of success had fallen so low that you would never start.
- This court has been at pains to warn against the over-elaboration of the "obvious to try" line of cases. While there are a number of factors which, depending on the circumstances, may bear on the question it is not always necessary for all of them to be ticked off as if on a checklist. As Kitchin and Floyd LJJ have demonstrated, in a case which involves routine pre-clinical and clinical trials, what would be undertaken as part of that routine is unlikely to be inventive.
- I, too, would allow the appeal.
Lord Justice Kitchin:
Introduction
i) the judge failed properly to construe the claims of the 181 patent which are said on these appeals to be independently valid, namely claims 1, 7 and 10;
ii) the judge ought to have found that none of these claims is entitled to its claimed priority date;
iii) the amendments made to the claims mean their disclosure extends beyond the disclosure of the application as filed and the judge should have so held;
iv) the judge fell into error in failing to find that Stoner deprived the claims of novelty; and
v) the judge fell into error in approaching the issue of obviousness and ought to have found there was nothing inventive in any of these claims.
The technical background
Potency and selectivity
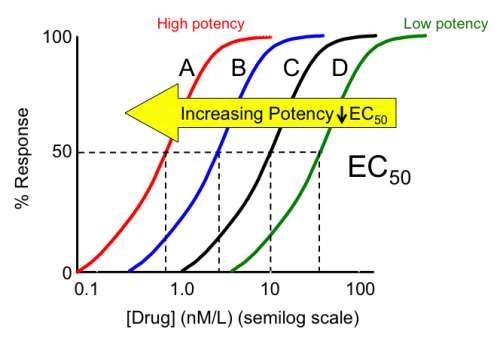
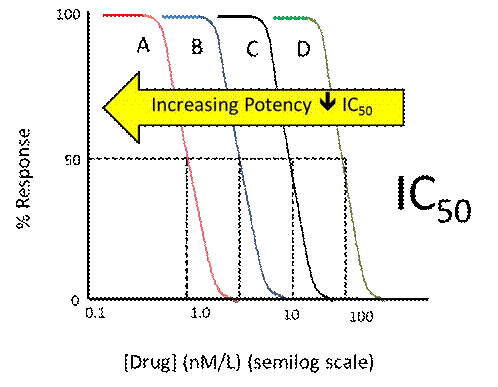
The skilled team
The common general knowledge
The phases of clinical research
Minimum effective dose
Sildenafil
Second in class
The 181 patent
"[0024] The presently claimed dosage form preferably is packaged as an article of manufacture for human pharmaceutical use, comprising a package insert, a container, and a dosage form comprising about 1 to about 5 mg of Compound (I) [tadalafil].
[0025] The package insert provides a description of how to administer a pharmaceutical product, along with the safety and efficacy data required to allow the physician, pharmacist, and patient to make an informed decision regarding the use of the product. The package insert generally is regarded as the label of the pharmaceutical product. The package insert incorporated into the article of manufacture indicates that Compound (I) is useful in the treatment of conditions wherein inhibition of PDE 5 is desired. The package insert also provides instructions to administer one or more about 1 to about 5 mg unit dosage forms as needed, up to a maximum total dose of 5 mg per day."
1. A pharmaceutical unit dosage composition comprising 1 to 5mg of a compound having the structural formula:
said unit dosage form suitable for oral administration up to a maximum total dose of 5 mg per day.
2. The dosage form of claim 1 comprising 2.5mg of the compound in unit dosage form.
3. The dosage form of claim 1 comprising 5mg of the compound in unit dosage form.
4. The dosage form of any one of claims 1 through 3 wherein the unit dose is in a form selected from a liquid, a tablet, a capsule, or a gelcap.
5. The dosage form of any one of claims 1 through 3 wherein the unit dose is in the form of a tablet.
6. The dosage form of any of claims 1 through 3 for use in treating a condition where inhibition of PDE5 is desirable.
7. The dosage form of claim 6 wherein the condition is a sexual dysfunction.
10. Use of a unit dose containing 1 to 5mg of a compound having the structure [of tadalafil] for the manufacture of a medicament for administration up to a maximum total dose of 5mg of said compound per day in a method of treating sexual dysfunction in a patient in need thereof."
Construction and infringement
"135. I will approach the matter of construction focussing on claim 7 or 10 since they include the clinical indication. For this purpose the focus is not on the part of the claim concerned with how much drug is in the tablet, it is on dosing. Subject to the word "maximum", a skilled reader would understand that what the inventor was using the words of those claims to mean is that the invention is concerned with treating sexual dysfunction by administering a dose of no more than 5mg tadalafil per day to a patient. Doing this provides the efficacy with minimal side effects provided for in the patent. Again subject to the word "maximum", the skilled reader would not think the inventors intended to exclude the idea that higher doses of tadalafil could be administered to patients if the doctor (and clinical regulators) regarded the balance of efficacy and side effects to be acceptable. Those higher doses would just not be taking advantage of the invention. So marketing authorisation documents from the regulators which approved doses of 2.5 mg per day or 5mg per day as well as 10 mg per day would not mean that a use in accordance with the invention had not been approved by the regulator. On the contrary. It would have been. Both a dose of 2.5 mg per day and a dose of 5 mg per day would take advantage of the discovery presented by the patent.
136. What then of the word "maximum"? The phrase in both claims is "administration up to a maximum total dose of 5mg […] per day". The language is being used to mean that the claim does not purport to cover administration of higher daily doses. The maximum referred to is the total dose of 5 mg bearing in mind that a doctor may administer "up to" a total dose of 5mg. So if the regulators only approved 20 mg daily tadalafil then (assuming there is no off-label use) the claim would never be infringed. But if the regulator approved both 20mg daily tadalafil and also 5mg daily tadalafil then the latter use would infringe and the existence of the additional higher approved dose would make no difference. The claim refers to a population in that sense."
Priority
i) at least a 100 fold differential in the IC50 for the inhibition of PDE5 versus PDE6;
ii) at least a 1,000 fold differential in the IC50 for the inhibition of PDE5 versus PDE1c; and
iii) an IC50 less than 10nM.
They form the substance of the definition of the term "selective PDE5 inhibitor" on page 5 of the priority document.
"The package insert is generally regarded as the label of the pharmaceutical product. The package insert incorporated into the present article of manufacture indicates that the PDE5 inhibitor is useful to treat conditions wherein the inhibition of PDE5 is desired. The package insert also provides instructions to administer one or more 1 to 20mg dosage forms as needed up to a total dose of 20 mg per day. Preferably, the dose administered is 5 to 20mg/day; more preferably 5 to 15mg; and most preferably a 10mg dose form administered once per day, as needed."
"One such inhibitor, (6R-trans) -6- (1, 3-benzodioxol-5-yl) - 2, 3, 4, 7, 12, 12a - hexahydro-2-methyl-pyrazino [1',2':1,6]-pyrido [3,4-b] indole-1,4-dione, was demonstrated in human clinical studies to have minimal impact on systolic blood pressure when administered in conjunction with nitrates. By contrast, sildenafil demonstrates a 4 fold greater decrease in systolic blood pressure over placebo, which leads to the contraindications and warnings in certain patients."
Immediately thereafter, it is said that compound 5 also demonstrated an IC50 against PDE1c of 10,000 and a ratio of PDE1c/PDE5 of 4,000.
The data in Table 1 indicate that a compound of Formula (I), wherein R1 is hydrogen or C1-6 alkyl, R2 is
and R3 is hydrogen; is especially preferred. Preferably A is
.
"229. The combination of tadalafil as one of the drugs to which the teaching relates, the content of the dosage forms (1-5mg drug) and the maximum total dose of 5mg per day are disclosed in the priority document. Based on the general teaching, the reader would understand that what is disclosed is that at all the doses described (including 5mg per day and also up to 20mg per day) the drug will be an efficacious treatment for sexual dysfunction, will have minimal side effects (including vision and flushing) and can be administered without significant interactions with nitrates. The fact that the document is focussed on a wider idea of finding selective PDE5 inhibitors defined by the three criteria does not mean that the more particular disclosures relating to tadalafil are absent."
"234. Claims 7 and 10 (and the other use claims) are specific to using tadalafil to treat sexual dysfunction. The priority document makes what is claimed plausible because the document provides data showing that tadalafil (Compound 4) is a PDE5 inhibitor and since, as the skilled reader would know but the document explains anyway, sildenafil works to treat this disease by being a PDE5 inhibitor, the basic effect is plausible. The idea that tadalafil would produce efficacy at the doses claimed with minimal side effects is also plausible because the document expressly discloses the doses, sets out a rationale that this effect is due to being a "selective PDE5 inhibitor" based on the three criteria, and explains that tadalafil is a selective PDE5 inhibitor. The claimants did not suggest that the rationale was not credible. The rationale is also supported by Examples 4 and 5. Although Example 5 is not clearly tadalafil, it nevertheless supports the teaching that a selective PDE5 inhibitor based on the three criteria would have the desired effect. If one compound with the desired property does this then it is credible that another will, albeit no doubt tests will be needed. Example 4 supports the general rationale albeit since it is for 10mg tadalafil rather than a 5 mg dose it cannot directly support the claims. Overall in my judgment the use claims are supported by what is disclosed in the priority document and so all the claims (bar claims 2 and 12) maintain priority."
i) a dosing regimen of up to a maximum total dose of 5mg per day for any drug; or
ii) that such a regimen using tadalafil is efficacious in the treatment of ED; or
iii) that any dose within such a regimen using tadalafil is efficacious in the treatment of ED.
"The package insert also provides instructions to administer one or more 1 to 20mg dosage forms as needed up to a total dose of 20mg per day. Preferably, the dose administered is 5 to 20mg/day; more preferably 5 to 15mg; and most preferably 10mg dosage form administered once per day, as needed."
Added matter
Novelty
"254. However Stoner is not concerned with monotherapy, it teaches the use of a combination of the two kinds of drug. Dr Brock's evidence was that the combination with the centrally acting agents drug made a significant difference. He thought the synergistic therapy discussed in Stoner was problematic and said in cross-examination that "all bets are off". Dr Brock also said that the skilled person reading Stoner would not believe that it disclosed increased efficacy from the combination, that the skilled person would understand that centrally acting agents have a very intolerable side-effect profile and that the combination may actually negate any positive benefit of the peripherally acting PDE5. On the question of dose Dr Brock said that if you look at combination therapy it muddles the situation, because you do not know if that centrally acting agent is going to modify totally the response you may see from a dose."
Obviousness
i) The team would find that the maximum tolerated doses for tadalafil are 500mg in a single dose and 100mg when taken daily.
ii) The team would also find that tadalafil has a higher IC50 against PDE6 than sildenafil. This would be an indication to them that tadalafil might have fewer visual disturbance side effects than sildenafil.
iii) Although the IC50 for tadalafil against PDE5 is reported in Daugan as 2nM, the team would measure it themselves, yielding a value of between about 2 to 2.5nM.
iv) The routine testing would include a study of tadalafil's pharmacokinetics and this would reveal that it has a half-life of 17.5 hours, which is much longer than the sildenafil half-life of four hours.
v) The team would identify tadalafil's mean accumulation ratio.
vi) The team would ascertain tadalafil's molecular weight.
"327. Weighing all this up, the results of the Phase IIb study would require the team to make value judgments. It is not inevitable that a skilled team would investigate lower doses given the plateau in the results of the Phase IIb study, because by identifying a dose (at least 25 mg) which is safe, tolerable and effective they have secured the prime objective of the programme, but it is very likely. Such an investigation would not be quite as routine for the skilled team as the work which has gone before but that cannot be taken too far. Multiple dose ranging studies in general terms are something the skilled team would be familiar with as necessary."
"335. In other words the skilled team testing a 5mg per day dose of tadalafil (on demand) when carrying out these investigations would not have a reasonable expectation that the drug at this dose would be a useful treatment for erectile dysfunction nor any expectation at all that the drug would produce a clinically relevant effect but with minimal side effects.
336. A skilled team which carried out a dose ranging study including a 5mg dose would discover that this dose was efficacious and had reduced side effects. They would be surprised by this. The team would go ahead to do Phase III studies at 5mg/day and seek clinical approval for a 5mg/day dose of tadalafil."
"341. The initial Phase IIb dose ranging study which was for daily dosing would probably include a 10mg dose as an additional arm owing to the accumulation factor. It would not include a dose lower than 10mg/day. The team would expect to see a dose response but would see the same plateau in efficacy down to 10 mg and dose response for side effects as discussed already. All the reasoning I have considered above would be the same. Assuming the team decided to conduct an investigation into lower doses in a second, daily dosed, dose ranging study including a 5mg/day dose of tadalafil, they would not have a reasonable expectation that the drug at this dose would be a useful treatment for erectile dysfunction nor any expectation that the drug would produce a clinically relevant effect but with minimal side effects. They would find that it did. They would be surprised."
"343. However in my judgment a 5mg daily dose of tadalafil as a treatment for erectile dysfunction is not obvious over Daugan. That is for the following reasons articulated having regard to the factors I identified above based in part on Lundbeck:
i) In terms of motives to find a solution to the problem the patent addresses, the skilled team would be highly motivated by Daugan and the success of sildenafil to investigate tadalafil as a treatment for erectile dysfunction.
ii) As for possible avenues of research, overall tadalafil would be obvious to investigate. In terms of doses however, 5 mg/day is a significantly lower dose than the 50 mg dose exemplified in the Daugan prior art and the marketed doses of sildenafil. It is also significantly lower than the 50 mg dose which would be chosen for the first test of efficacy at Phase IIa. It would not be chosen in the routine first dose ranging study. The team would not have anticipated daily dosing as something to be studied from the outset but once the half-life was discovered it is likely that daily dosing would be included.
iii) In terms of effort, overall the programme would involve very substantial resources of time, money and people but it would be pursued. However, by the time the idea of investigating lower doses presents itself, the team would have established safe, tolerable and effective doses of tadalafil at 25mg on demand and 10 mg for daily dosing. At that stage the impetus to investigate lower doses would be reduced but not eliminated.
iv) Expectations of success can be considered overall and in relation to particular studies. Overall the team would embark on the project with a reasonable expectation of success in establishing tadalafil as a safe, tolerable and effective treatment for tadalafil. However, the claimants failed to prove that efficacy at 5mg tadalafil was predictable or worth considering by the skilled team based on the properties of tadalafil as compared to sildenafil. The team would know that in principle there would be a minimum effective dose for tadalafil but would also know that its definition depends on a value judgment made by the team. In relation to the dose ranging studies, the team would conduct them hoping for a dose response. Following discovery of a plateau starting at 25 mg or 10mg, there would very likely be a subsequent dose ranging study which included 5 mg. The team would include a 5 mg dose in this study hoping to see a dose response but that does not mean they would have a reasonable expectation that 5mg would produce a clinically relevant effect at all nor one with minimal side effects. Assuming a 5 mg /day dose of tadalafil was tested, it would not be tested with a reasonable expectation of success.
v) Considering unexpected or surprising results, the position is as follows. The path to a 5 mg dose requires the discovery of new information such as the half life and the IC50 vs PDE6. That information would inevitably be found in any clinical programme. The path includes an important result which is unexpected even if it is not actually surprising, i.e. the plateau in the dose response from 10 to 100 mg. There is also a surprising result: the existence of a useful effect with reduced side effects. The claimed 5mg /day dose has that property.
vi) A number of value judgments would be required of a skilled team in a programme which reaches the claimed invention. One is to define the level of clinical effect to be regarded as relevant. Another is to embark on investigating daily dosing. An important value judgment is what to do when an unexpected plateau in the dose response has been identified at the same time as a marketable dose."
"32. So holding is far from saying that in general just specifying a new dosage regime in a Swiss form claim can give rise to a valid patent. On the contrary, nearly always such dosage regimes will be obvious – it is standard practice to investigate appropriate dosage regimes. Only in an unusual case such as the present (where, see below, treatment for the condition with the substance had ceased to be worth investigating with any dosage regime) could specifying a dosage regime as part of the therapeutic use confer validity on an otherwise invalid claim."
"55. …. In deciding whether the invention was obvious to the skilled but unimaginative addressee at the priority date the court will have regard to all the circumstances of the case including, where appropriate, whether it was obvious to try a particular route with a reasonable or fair expectation of success. What is a reasonable or fair expectation of success will again depend upon all the circumstances and will vary from case to case. Sometimes, as in Saint Gobain, it may be appropriate to consider whether it is more or less self-evident that what is being tested ought to work. So, as this court explained in that case, simply including something in a research project in the hope that something might turn up is unlikely to be enough. But I reject the submission that the court can only make a finding of obviousness where it is manifest that a test ought to work. That would be to impose a straightjacket upon the assessment of obviousness which is not warranted by the statutory test and would, for example, preclude a finding of obviousness in a case where the results of an entirely routine test are unpredictable."
"Q: We know we are on the top plateau for efficacy and we know that going lower will reduce the side effects. It is really a no brainer, is it not doctor?
A: To go to a lower dose?
Q: Yes.
A: Yes. In this patient population, yes.
Q: Indeed, what we see … in the discussion and overall conclusions: "This study confirms that [tadalafil] is safe, well tolerated, and effective when administered as once-daily therapy for the treatment of mild to moderate MED at doses of 10 to 100mg. The results support further investigation evaluating the safety and efficacy of doses < 10mg, which may identify a broader dose range providing a high degree of efficacy with a minimum of undesirable side effects". That is the conclusion that an ordinary skilled man would come to as well if he had done this study; agreed?
A: Yes, agreed"
"Q: … As I understand your evidence, you think Dr Pullman [one of the two inventors] did something inventive. Yes?
A: Yes.
Q: Exactly what was that?
A: Thinking that a lower dose will need to be tested before the results came out --- before the results of the 10-100 came out.
Q: Oh, so you mean thinking to test lower before you saw that LVBG study?
A: Yes.
Q: Right, because I think you accept that once they came out you would have gone lower anyway. Yes?
A: Yes, based on the report, yes."
Conclusion
Lord Justice Floyd:
"113. Where, therefore, the evidence reveals that to arrive at the invention, the skilled person has to embark on an experiment or series of experiments where there was no fair expectation of success, the conclusion will generally be that the invention was not obvious. …
114. I think that the guiding principle must be that one has to look at each putative step which the skilled person is required to take and decide whether it was obvious. Even then one has to step back and ask an overall question as to whether the step by step analysis, performed after the event, may not in fact prove to be unrealistic or driven by hindsight. …
115. How one would proceed after purely routine steps have been performed may involve more in the way of a value judgment. The mere fact that further steps can be characterised as being performed in order to make an informed decision cannot prevent those steps from contributing to a finding of inventiveness."
"I think that Mr Birss is right that one must proceed with caution when faced with an obviousness attack based on a suggestion that the skilled person would embark on a research program in the course of which he would discover that a product or compound was effective. This is particularly so where the technical effect is one which is newly discovered, or impossible or very hard to predict. That is because the expectation of success may be zero, or inadequate to drive the research forward. In the end it will all depend on weighing the various factors as they appear from the evidence in the case."
"The question of whether an invention was obvious has been called "a kind of jury question" (see Jenkins L.J. in Allmanna Svenska Elektriska A/B v. The Burntisland Shipbuilding Co. Ltd (1952) 69 R.P.C. 63, 70) and should be treated with appropriate respect by an appellate court. It is true that in Benmax v. Austin Motor Co. Ltd [1955] A.C. 370 this House decided that, while the judge's findings of primary fact, particularly if founded upon an assessment of the credibility of witnesses, were virtually unassailable, an appellate court would be more ready to differ from the judge's evaluation of those facts by reference to some legal standard such as negligence or obviousness. In drawing this distinction, however, Viscount Simonds went on to observe, at p. 374, that it was "subject only to the weight which should, as a matter of course, be given to the opinion of the learned judge." The need for appellate caution in reversing the judge's evaluation of the facts is based upon much more solid grounds than professional courtesy. It is because specific findings of fact, even by the most meticulous judge, are inherently an incomplete statement of the impression which was made upon him by the primary evidence. His expressed findings are always surrounded by a penumbra of imprecision as to emphasis, relative weight, minor qualification and nuance (as Renan said, la vérité est dans une nuance), of which time and language do not permit exact expression, but which may play an important part in the judge's overall evaluation. It would in my view be wrong to treat Benmax as authorising or requiring an appellate court to undertake a de novo evaluation of the facts in all cases in which no question of the credibility of witnesses is involved. Where the application of a legal standard such as negligence or obviousness involves no question of principle but is simply a matter of degree, an appellate court should be very cautious in differing from the judge's evaluation."
i) it would be entirely obvious for a skilled team given Daugan to set out on taking tadalafil forward into a routine pre-clinical and clinical trial programme as an oral treatment for male erectile dysfunction at the priority date;
ii) The statements in Daugan are quite sufficient to make it obvious to do this but the huge success of sildenafil, an oral PDE5 inhibitor, make it very obvious.
iii) Routine pre-clinical studies would be done first. They would be undertaken with the firm expectation that they would produce useful data and a reasonable expectation that tadalafil would turn out to be a viable drug.
iv) We know those tests would produce favourable results given what we know now about tadalafil. The skilled team would then conduct routine Phase I safety studies in healthy volunteers. A wide range of doses would be tested and the maximum tolerated dose would be established. Repeated daily doses would be tested, albeit from the point of view of safety. This is all routine. Given the positive pre-clinical data, the Phase I studies would be undertaken with a reasonable expectation that tadalafil would prove to be a safe drug. Overall those tests would produce favourable results given what we know now about tadalafil.
v) After the phase I tests and based on their results the skilled team would design and undertake a "go no-go" phase IIa study of a single dose of tadalafil in a relatively small group of patients.
vi) The dose which would be selected by the team would be 50mg.
vii) Given the positive pre-clinical and Phase I results and success of sildenafil as an oral PDE5 treatment for erectile dysfunction, the skilled team would embark on this Phase IIa study with a reasonable expectation that the drug would be safe, tolerable and effective at this dose.
viii) From what we now know, such a Phase IIa study would produce favourable results for efficacy and therefore result in a decision to "go".
ix) The next step would be to conduct routine Phase IIb dose ranging studies in larger groups of patients. In order to do that a range of doses has to be selected in order to establish the dose response. It was common ground that doses in a first dose ranging study would include 25 mg, 50 mg and 100mg.
x) The results of this study would show that there was no difference in efficacy end point between the doses, demonstrating an apparent therapeutic plateau. The most common side effects would show a dose response and all doses were well tolerated.
xi) Weighing all this up, the results of the Phase IIb study would require the team to make value judgments. It is not inevitable that a skilled team would investigate lower doses given the plateau in the results of the Phase IIb study, because by identifying a dose (at least 25 mg) which is safe, tolerable and effective they have secured the prime objective of the programme, but it is very likely. Such an investigation would not be quite as routine for the skilled team as the work which has gone before but that cannot be taken too far. Multiple dose ranging studies in general terms are something the skilled team would be familiar with as necessary.
Lord Justice Lewison:
"Such tests would be performed in ignorance of the results of the testing and in ignorance of whether any particular formulation strategy would have a fair expectation of success. But they would nevertheless be an obvious thing to do. They are obvious because the evidence shows that the skilled person would do them anyway, as part of his routine work."
"This passage explains that some experiments undertaken without a particular expectation as to the result are obvious."