Contents
Topic Paragraphs
Introduction 1-6
The witnesses 7-47
Technical background 48-106
The Family A Patents 107-153
The claims of the Family A Patents: unconditionally amended 154-163
The claims of the Family A Patents: conditional amendments 164
The skilled team 165-167
Common general knowledge as at the Family A Priority Date 168-203
The documents cited in the Family A Patents 204-258
Construction of Family A claims 259-303
Obviousness of Family A over Epstein 304-346
Insufficiency of EP 823 and EP 301 348-405
AgrEvo obviousness of EP 823 and EP 301 406
Infringement of Family A by vadadustat 407-462
Amendment of EP 531 463
The Family B Patents 464-501
The claims of the Family B Patents 502-518
The skilled team 519
Common general knowledge as at the Family A Priority Date 520-543
The documents cited in the Family B Patents 544-548
Construction of Family B claims 549-553
Obviousness of Family B over WO 997 554-574
Insufficiency and AgrEvo obviousness of Family B 575-578
Infringement of Family B by vadadustat 579-639
Summary of principal conclusions 640
Introduction
1. These proceedings were originally brought by Akebia Therapeutics Inc (“Akebia”) and Otsuka Pharmaceutical Co Ltd (“Otsuka”) seeking to revoke six patents (“the Patents”) belonging to FibroGen, Inc (“FibroGen”) in order to clear the way for their product vadadustat. Subsequently the exclusive licensee under the Patents, Astellas Pharma Inc (“Astellas”), brought a cross-claim for threatened infringement. For convenience, I shall refer to Akebia and Otsuka collectively as “the Defendants” and to FibroGen and Astellas collectively as “the Claimants”. There were also parallel proceedings involving two GlaxoSmithKline companies (“GSK”) and their product daprodustat which were to be tried together with these proceedings, but those proceedings were settled on the working day before trial.
2. The Patents concern the use of inhibitors (referred to as HIF-PHIs) of an enzyme called hypoxia inducible factor-prolyl hydroxylase (HIF-PH) for treating various types of anaemia and related conditions. Astellas obtained a marketing authorisation for the first oral HIF-PHI product, roxadustat, in Japan in September 2019, and intends to launch the product more widely, including in the UK. It hopes that the product will achieve blockbuster status by 2023. Vadadustat and daprodustat are HIF-PHI products which are both undergoing Phase III clinical trials at present.
3. The Patents have been grouped into two families of three patents, each deriving from a common international application, designated “Family A” and “Family B”:
Family A Family B
WO 03/053997 (“WO 997”) WO 2004/108121 (“WO 121”)
EP (UK) No 1,463,823 (“EP 823”) EP (UK) No 1,633,333 (“EP 333”)
EP (UK) No 2,289,531 (“EP 531”) EP (UK) No 2,322,153 (“EP 153”)
EP (UK) No 2,298,301 (“EP 301”) EP (UK) No 2,322,155 (“EP 155”)
4. There is no challenge to the earliest claimed priority date of the Family A Patents, which is 6 December 2001. It is common ground that the validity of the Family B Patents should be assessed as at the second claimed priority date, which is 29 April 2004. I shall refer to these dates as “the Priority Dates”.
5. It will be convenient to describe the disclosure of the Patents by reference to the two international applications listed above (“the Applications”), in particular because WO 997, which was published on 3 July 2003, is relied upon by the Defendants as prior art against the Family B Patents. Nevertheless, caution is required, because there are some small, but nevertheless potentially significant, textual differences between the Applications and the respective Patents. I shall return to this point below.
6. The Defendants contend that the Family A Patents are obvious over A.C.R. Epstein et al , “C. elegans EGL-9 and Mammalian Homologs Define a Family of Dioxygenases that Regulate HIF by Prolyl Hydroxylation”, Cell , 107, 43–54 (5 October 2001) (“Epstein”), that the Family B Patents are obvious over WO 997 and that all the Patents are insufficient. The Defendants also dispute that they threaten to infringe any of the Patents. Furthermore, FibroGen has applied to amend the Patents both unconditionally and conditionally. Most of the amendment applications are unopposed save on the ground that they do not cure the alleged invalidity of the Patents, but one is. The result is a case of considerable complexity, as indicated by the fact that the parties’ written closing submissions run to 434 paragraphs (Claimants) and 537 paragraphs (Defendants), and cross-refer to further material in their respective opening skeleton arguments.
The witnesses
7. Each side called two principal expert witnesses, a nephrologist and a medicinal chemist. Helpfully, each pair of experts was called back-to-back. Less helpfully, the medicinal chemists were called before the nephrologists. The logical order would have been the other way around. This is a problem which I have encountered before. I appreciate that the availability of experts can make scheduling their testimony in the logical order difficult, but I would urge legal teams to do their utmost to try to ensure that this is done.
8. The Defendants also called a second medicinal chemist, Prof Fishwick. Originally, Prof Fishwick’s evidence was directed solely to the number of compounds covered by Formula (I) in what was then claim 19 of EP 823 as proposed to be amended (now claim 19A). It was justifiable for the Defendants to wish to call a second expert for that discrete and limited purpose, particularly given that claim 19 of EP 823 was asserted by the Claimants against the Defendants, but not against GSK. As part of that exercise, however, Prof Fishwick interpreted Formula (I). The Defendants’ main medicinal chemist, Prof Ward also interpreted Formula (I), and at that stage appeared to reach the same conclusion. No objection to this was raised by the Claimants prior to the trial, even though the Claimants did (successfully) raise an objection to a different instance of duplication of expert evidence by the Defendants (and GSK) at the pre-trial review. Shortly before the trial, both Prof Ward and Prof Fishwick served supplementary reports acknowledging errors in documents prepared by the Defendants’ solicitors which they had exhibited illustrating their interpretation; but at that stage it became clear that they were interpreting Formula (I) differently. Although the Claimants did object to this after the trial had commenced, given that no objection had been raised previously, I permitted the Defendants to call Prof Fishwick not merely to give evidence as to his calculations, but also as to the interpretation of the claim. (The admissibility of the latter evidence is a separate point, to which I will return below.) Sensibly, counsel kept their cross-examination of all three medicinal chemistry experts on this issue brief.
9. In addition to the experts referred to above, each side called an additional nephrologist to address questions of current and future clinical practice in the United Kingdom which are relevant to the issue of infringement. I will refer to these witnesses as “the clinical practice experts”. Again, it was justifiable for the parties to call additional experts to address these questions, because (for differing reasons) their principal nephrology experts were unable to do so. Without objection from the Defendants, counsel for the Claimants also cross-examined the Defendants’ clinical practice expert on some questions of common general knowledge at the Priority Dates.
Expert evidence in patent cases
10. Before turning to consider the experts individually, it is once again necessary for me to address some general questions concerning expert evidence in patent cases. The Patents Court depends on the assistance it receives from expert witnesses, many of whom are scientists of considerable distinction in their own fields. Particularly in complex cases such as this, preparing expert reports and giving oral evidence can be an arduous task in terms of the time, effort and concentration involved. It is vital that the task of the experts is not made more difficult by the lawyers than it needs to be.
11. I considered the preparation of expert reports in a passage in MedImmune Ltd v Novartis Pharmaceuticals UK Ltd [2011] EWHC 1669 (Pat) at [99]-[114] which is frequently cited, not least in experts’ reports. The key point I made in that passage is that “the lawyers who instruct expert witnesses bear a heavy responsibility for ensuring that an expert witness is not put in a position where he can be made to appear to have failed in his duty to the court even though he conscientiously believes that he has complied with that duty”.
12. I considered the cross-examination of experts in a passage in Merck Sharp and Dome Ltd v Shionogi & Co Ltd [ 2016] EWHC 2989 (Pat) at [87]-[93] which is perhaps less well known. The key point I made in that passage is that “too much time is spent by cross-examiners in patent cases on ad hominem attacks that are unfair to the witness, unhelpful to the court and waste expensive time”.
13. The present case has demonstrated that the warnings I gave in MedImmune v Novartis and MSD v Shionogi are still not being sufficiently heeded. As I shall explain, both Prof Winearls and Prof Haase were let down by those instructing them with respect to the preparation of their expert reports, and Prof Haase was in one respect cross-examined unfairly. It should not be necessary for me to say that this is unacceptable. These are matters of professional responsibility. If practitioners continue not to observe the standards required of them, the Patents Court will have to take steps to enforce those standards.
The nephrologists
14. Prof Winearls . The Claimants’ expert was Professor Christopher Winearls. On 31 August 2019 he retired from practice as an NHS consultant nephrologist in the Oxford Kidney Unit at the Churchill Hospital, part of the Oxford University Hospital NHS Foundation Trust. Prof Winearls obtained an MBChB from the University of Cape Town in 1973 and was awarded a DPhil in transplant immunology by the University of Oxford in 1979. He undertook his training in nephrology in Oxford and then at Hammersmith Hospital. He was appointed a consultant nephrologist at the Churchill Hospital in 1988 and remained in full-time practice until 2016, after which he was part-time. He was the Clinical Director of the Oxford Kidney Unit from 1995 to 2009. He was a Lecturer, and then Senior Lecturer, at the Royal Postgraduate Medical School between 1985 and 1988, after which he became an Associate Professor of Medicine at the University of Oxford. He was an editor of Erythropoietin - Molecular, Cellular and Clinical Biology (Johns Hopkins University Press, 1991) and of the Oxford Textbook of Clinical Nephrology (now in its 4th edition), and an author of a considerable number of published papers. In addition to other professional memberships, he was formerly Secretary and later Clinical Vice President of the Renal Association (of the United Kingdom). He was on the Editorial Board of the American Journal of Kidney Diseases until 2016, and he contributed to the Standards Document produced by the Royal College of Physicians and the Renal Association and the Kidney Disease Improving Global Outcomes (“KDIGO”) Guidance on Chronic Kidney Disease (2012). He described his role in 2001 as having been “a clinician, a clinical researcher and an educator and trainer of students and doctors”. His research interests included renal anaemia. He was the nephrologist on the team which first investigated the effect of recombinant human erythropoietin in uraemic man (P.M. Cotes et al , “Characterization of the anaemia of chronic renal failure and the mode of its correction by a preparation of human erythropoietin (r-HuEpo): an investigation of the pharmacokinetics of intravenous erythropoietin and its effects on erythrokinetics”, Q J Med , 70(262), 113-37 (1989)) and he was a Principal Investigator on the PIVOTAL trial describing the effects of two dose regimens of intravenous iron in haemodialysis patients also receiving erythropoiesis-stimulating agents (ESAs) (I.C. Macdougall et al , “Intravenous iron in patients undergoing maintenance hemodialysis”, N Engl J Med , 380(5), 447-458 (2019)).
15. As Prof Winearls explained in his first report, he was instructed to read EP 823 as exemplifying the Family A Patents. Despite that, he was asked to consider the obviousness of the claims of EP 531. In paragraph 134 of his first report he identified the inventive concept of the claims of EP 531 as being “the use of HIF-PHIs to increase endogenous Epo production in prevention, pre-treatment or treatment of anaemia associated with kidney disease, CRF or CKD”. As Prof Winearls naturally accepted in cross-examination, that statement of the inventive concept is applicable to EP 823, but not to EP 531. Counsel for the Defendants rightly did not suggest that this was the fault of the witness: it is evident that, at some point in the drafting of the report, a section dealing with the obviousness of EP 823 was re-drafted to address EP 531 without all the necessary changes being made. An expert in Prof Winearls’ position cannot possibly be expected to spot points like this, and must rely on those instructing them. Fortunately, this error did not matter.
16. Counsel for the Defendants accepted that Prof Winearls had given his oral evidence fairly, but submitted that his first report had contained a number of significant errors with regard to the common general knowledge concerning hypoxia inducible factor (HIF). I will consider the substance of this issue later. At this stage it suffices to say that I make no criticism of Prof Winearls. It became clear from his oral evidence that, as a careful reading of his reports had suggested might well be the case, he had some difficulty in distinguishing between what would have been known by an ordinary clinical nephrologist and what would have been known by a nephrologist with a research interest in renal anaemia. (The significance of this distinction will become apparent later.)
17. This would be understandable in any event, but there is a specific reason why Prof Winearls is entirely to be forgiven for this. As he explained, he was a colleague for more than 30 years of Professor Sir Peter Ratcliffe, who features in the case as an author of some of the key papers and who (together with Professor Gregg Semenza and Professor William Kaelin) won the Nobel Prize in Physiology or Medicine in 2019 for their work on oxygen sensing, and whose work on the control of erythropoietin (Epo) Prof Winearls had followed with great interest. As Prof Winearls vividly put in his oral evidence, he was “next door to it”. Prof Winearls made it clear that he had (rightly) attempted to put that special knowledge out of his mind. But I think that made it particularly difficult for him to identify the common general knowledge of a nephrologist with a research interest in renal anaemia who did not have that special knowledge, and it is not surprising that he over-compensated.
18. As counsel for the Defendants pointed out, Prof Winearls accepted that two sentences in his first report were badly drafted. First, in paragraph 81 Prof Winearls said that “[i]t is now known that HIF … is responsible for promoting Epo production [emphasis added]”. As he accepted, in fact this was known in 2001 (and indeed, had been known for some time before that). I regard this mis-statement as regrettable, but it appears to me that it flowed from the difficulty discussed above.
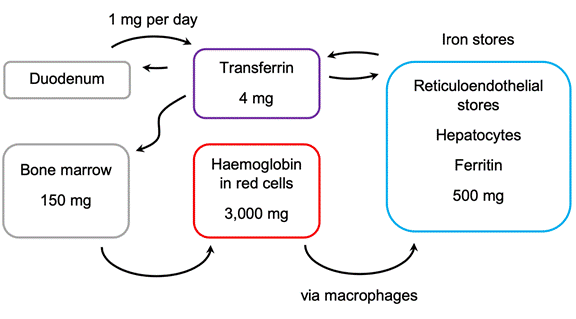
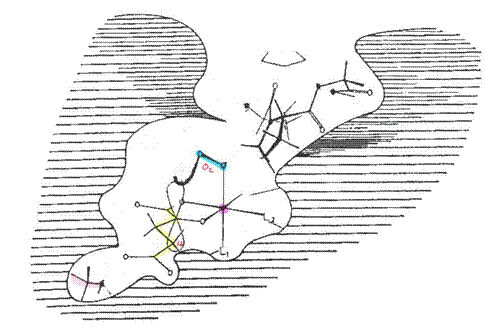
19. The second instance actually concerns a different topic to HIF. In paragraph 83 of his first report Prof Winearls referred to “the damaged kidney” being “incapable of producing Epo in renal anaemia patients [emphasis added]”. Prof Winearls accepted that that sentence was over-stated. As counsel for the Claimants pointed out, however, elsewhere in his first report, Prof Winearls stated the position in a more nuanced manner. Thus in paragraph 136 he said that in patients with kidney disease “the ability of the kidneys to produce Epo was thought to be reduced because of damage to or phenotypic change in the interstitial fibroblasts [emphasis added]”, and he used very similar language at paragraph 48. It is clear that the latter statement represents the opinion that Prof Winearls was attempting to convey. Accordingly, I do not regard the poor drafting of the former statement as significant.
20. Prof Haase . The Defendants’ expert was Professor Volker Haase. He obtained an MD degree from the Johann Wolfgang Goethe University School of Medicine in 1987 and a higher research doctorate in 1990 for work on tumour immunology. His career since then has been primarily in the United States, where he held a variety of research and clinical positions in the 1990s. From 1990 to 1993 he undertook a research fellowship at the Massachusetts General Hospital and the MGH Cancer Center. From 1993 to 1996 he was an Intern and then a Resident in Internal Medicine at Emory University in Atlanta, Georgia. From 1996 to 1999 he was a Clinical and Research Fellow in the Renal Division at Beth Israel Deaconess Medical Center and Harvard Medical School, and he obtained board certification in nephrology in 1999. From 1997 to 2001 he was a Research Fellow at the Whitehead Institute for Biomedical Research at Massachusetts Institute of Technology. From 1999 to 2001 he was an Instructor in Medicine at Harvard Medical School, and from 2001 to 2008 was Assistant Professor of Medicine at the University of Pennsylvania School of Medicine. From 2008 he held various Assistant and Associate Professor roles, and in 2015 he was appointed as full Professor of Medicine, full Professor of Molecular Physiology and Biophysics and full member of the programme in Cancer Biology at Vanderbilt University School of Medicine in Nashville, Tennessee. Since 1997 his research activities have focused on the regulation of the HIF and von Hippel-Lindau (VHL) pathways and their involvement in renal and other diseases, and he started his own laboratory group in 2001 to undertake research focused on the role of HIF signalling. He is the author of over 95 scientific publications, and he is on the editorial boards of a number of journals. Alongside his research activities, he has practised as a clinical nephrologist for over 20 years. He was an Attending Physician at the Beth Israel Deaconess Medical Center and Harvard Medical School from 1999 to 2001, and at the Hospital of the University of Pennsylvania from 2001 to 2008. Since 2010 he has served as an Attending Physician at the VA Medical Center in Nashville, and since 2011 he has served in the same capacity at the Vanderbilt University Medical Center in Nashville.
21. Counsel for the Claimants pointed out that, as at December 2001, Prof Winearls had considerably more clinical experience than Prof Haase: at that time Prof Haase was spending about 6-8 weeks a year working as a clinician. Counsel for the Claimants submitted that this meant that more weight should be given to Prof Winearls’ views. I do not accept this. Prof Haase had sufficient clinical experience to give the evidence that he did, and the parts of the case his evidence addressed do not turn on questions of clinical practice. What is more important in my judgment is that Prof Haase was working on HIF in December 2001, and therefore it is possible that he had more knowledge about HIF than the skilled nephrologist discussed below.
22. As I have indicated, Prof Haase’s evidence raises both of my concerns about expert evidence. The first concern is over the preparation of his reports. One of the points I made in MedImmune (at [113]) was the need for the lawyers instructing an expert to make sure that the expert discloses their own previous relevant publications and, where appropriate, explains them in their report. Despite this, Prof Haase failed to disclose or discuss two relevant papers of his.
23. First, Prof Haase did not mention C. Peyssonnaux et al , “Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs)”, J Clin Invest , 117, 1926–1932 (2007) (“Peyssonnaux”), of which he was a co-author, in his first report. The significance of this is that this was - apart from the Family B Patents - the first report of a link between HIF and hepcidin (as to which, see below). Prof Haase explained in his fourth report, served in reply to Prof Winearls’ third report, which drew attention to Peyssonnaux, that there had subsequently been conflicting reports in the literature on this point. I consider that Prof Haase should have been instructed to address this in his first report, but I do not see any reason for thinking that Prof Haase is to be blamed for this failure as counsel for the Claimants submitted.
24. Secondly, Prof Haase was asked to consider and comment in his fourth report on a number of papers concerning HIF-PHIs which Prof Winearls had discussed in his second report. What Prof Haase did not mention in his report was that he and a co-author had recently published a review (N.S. Sanghani and V.H. Haase, “Hypoxia-Inducible Factor Activators in Renal Anemia: Current Clinical Experience”, Adv Chronic Kidney Di s, 26(4), 253-266 (2019), “Sanghani”) in which they had discussed the same papers. It was put to Prof Haase that the conclusions he drew from some of the papers in his fourth report were inconsistent with those drawn in Sanghani. Before turning to the substance of the criticism, I again consider that Prof Haase should have been instructed to reference Sanghani in his report and, if and to the extent that he was now drawing different conclusions, to explain why. Turning to the substance of the criticism, in the case of the principal alleged inconsistency, the real point is, as explained below, not that Prof Haase was inconsistent; but that, for a reason he missed both times, one of the papers could be said to support a different conclusion. In any event, as counsel for the Defendants pointed out, Prof Haase’s task in writing his report was different to his task in writing Sanghani because he had been instructed specifically to consider the papers in question and to see what conclusions could be drawn from them that were relevant to the issues in this case.
25. I would add that I find it strange that Prof Haase was not instructed to exhibit his CV to his report, as the other experts were and as is conventional. Nothing turns on this, however.
26. I turn to the cross-examination of Prof Haase. For the most part, this was exemplary: it was well-constructed, appropriately thorough and courteously conducted. It was marred, however, by one passage which should not have taken place. In paragraph 43 of his first report Prof Haase expressed the opinion that cobalt salts were well known (i) to have been used to treat anaemia from the 1930s to the 1970s, (ii) to stimulate the expression of Epo by inducing HIF-α and (iii) to mimic hypoxia by stabilising HIF and inducing Epo. In support of his opinion on the first point, Prof Haase cited H.H. Corner, “Cobalt and nutritional anaemia”, Br Med J , 2, 169-170 (1939), L. Berk et al , “Erythropoietic effect of cobalt in patients with or without anemia”, N Engl J Med , 240, 754-61 (1949) and J.P. Kriss et al , “Hypothyroidism and thyroid hyperplasia in patients treated with cobalt”, J Am Med Assoc , 157, 117-21 (1955) (“Corner”, “Berk” and “Kriss”). In paragraphs 37-40 of his fourth report Prof Haase returned to this topic, explaining that he was not suggesting that Corner, Berk and Kriss were themselves common general knowledge and citing an additional publication.
27. Counsel for the Claimants asked Prof Haase whether he had found Corner, Berk and Kriss or whether the Defendants’ solicitors had provided them to him. That was a legitimate question to ask, because it went to the cogency of the witness’ opinion that (some of) the information contained in them was common general knowledge. Prof Haase’s answer was that he had cited papers on cobalt in previous review articles he had written, for example one he and a co-author had published in 2015 (M.J. Koury and V.H. Haase, “Anaemia in kidney disease: harnessing hypoxia responses for therapy”, Nat Rev Nephrol , 11, 394-410, “Koury”), and therefore he had been aware of the papers prior to his involvement in this case. He added that he had been aware of cobalt in 2001, having published an article referring to it (which he was able specifically to identify) in that year, but he was not sure that he knew about Corner, Berk or Kriss at that time.
28. Despite these clear answers, counsel returned to the subject the following day. Having referred to Prof Haase’s answer about Koury, counsel asked the witness “did you find Corner, Berk and Kriss for the purposes of this case, or were they supplied to you by the solicitors?”. That was not a proper question, because it was an attempt to get the witness to choose between two possibilities, neither of which reflected the evidence he had previously given.
29. Worse, counsel then put it to the witness that Koury did not refer to Corner, Berk and Kriss. It did, however, discuss cobalt and it did cite a number of earlier papers including at least one review (“Ebert”). I still do not know whether any of the articles cited in Koury cite Corner, Berk or Kriss. What I do know is that counsel made no attempt to demonstrate that none of them did. From memory, Prof Haase thought that Ebert did, although he was not 100% sure. Counsel for the Claimants asserted in a footnote in their written closing submissions that Ebert did not cite Corner or Berk. I will assume that that is correct; but that implies that Ebert did cite Kriss, and Kriss cites Berk (but not Corner). Even if none of the articles had cited Corner, Berk or Kriss, that would still not have disproved the witness’ evidence, because it would remain possible that he had read the papers for the purposes of writing Koury, but had decided not to cite them, or had read them for a previous review. Moreover, that would still leave his previous awareness of cobalt.
30. Worse still, having again asked whether Prof Haase had chosen Corner, Berk and Kriss for the purposes of his report or whether they were provided by the solicitors, and received the clear answer
“I brought this [cobalt] up. This was not something that was brought to me by the solicitors. … Then I picked papers for this expert witness report”,
counsel first mischaracterised the witness’ evidence by putting it to the witness that the papers were provided to him and then badgered the witness for a page of transcript with further questions apparently designed to try to undermine the answer the witness had given without having any material to contradict it. This is not an acceptable way in which to treat an expert witness. The cogency of Prof Haase’s opinion as to the use of cobalt salts to treat anaemia being part of the common general knowledge is a separate question to which I will return below. As I will explain, it is not even the point which matters most when it comes to the subject of cobalt.
31. In addition to his failure to mention the two papers discussed above, counsel for the Claimants advanced a number of other criticisms of Prof Haase. First, counsel pointed out that Prof Haase strayed into giving long and discursive answers on occasion. That is undoubtedly correct, and it unnecessarily prolonged the cross-examination, but I do not consider that it detracts from the cogency of his opinions.
32. Secondly, counsel for the Claimants submitted that at times it appeared that Prof Haase had spent too long with the lawyers in this case, which had perhaps unwittingly led him to act as an advocate for the Defendants. In addition to the alleged discrepancies between what he had said in his reports and what he had said in the Peyssonnaux and Sanghani articles, which I have already dealt with, counsel submitted that this was demonstrated by Prof Haase’s evidence that it had been proposed that hepcidin might be regulated by Epo, when the Claimants contend that this is not supported by the contemporaneous literature. I do not accept this demonstrates partiality on the part of Prof Haase. On the contrary, it appeared to me that it represented Prof Haase’s genuine opinion. Again, whether that opinion accurately reflected the common general knowledge at the Family B Priority Date is a different question which I will consider below.
33. Counsel also suggested that this tendency may have been “exacerbated” by Prof Haase’s relationship with Akebia: he has served on its Scientific Advisory Board since 2009 and was involved in the Phase I and II trials for vadadustat. Prof Haase was open about his relationship with Akebia in his first report, however. He was also open about his relationship with FibroGen: he has attended several conferences and sponsored meetings organised by FibroGen and its licensee for the USA and China, AstraZeneca, to discuss their HIF-PHI pipeline and he recently served as an advisor to FibroGen and AstraZeneca with regard to their Phase III programme for roxadustat. I do not accept this begins to demonstrate partiality towards Akebia on the part of Prof Haase.
34. Thirdly, counsel for the Claimants submitted that Prof Haase had been wrongly instructed as to, or had misunderstood, the concept of common general knowledge. I see no evidence that he was wrongly instructed. He may not have fully understood the concept, but this is a common problem for expert witnesses in patent cases. As noted above, Prof Winearls also had some difficulty with the concept.
35. Finally, counsel for the Claimants pointed out that Prof Haase had explained that the first document he was shown in the case was WO 997 (which discloses HIF biology), and he was asked, based on this, what he thought the skilled team would look like. Counsel submitted that, as a result, his entire analysis was tainted by the knowledge that the invention was HIF-related, when that would not have been apparent at the Family A Priority Date.
36. This submission illustrates why it can be advantageous to try to instruct expert witnesses in sequence, first asking them about the common general knowledge, then showing them the prior art and asking them questions such as what steps would be obvious in the light of it and only then showing them the patent in suit. This is a procedure known as “sequential unmasking” in the psychological literature (see generally on this subject C.T. Robertson and A.S. Kesselheim (eds), Blinding as a Solution to Bias , Academic Press, 2016). The point of it is to try to avoid, or at least reduce, hindsight. In my opinion, it is desirable to try to minimise hindsight on the part of expert witnesses where possible. There is no rule or principle that experts must be instructed sequentially, however. Moreover, there are often real practical problems in doing so. To take just one obvious example, any discussion about the common general knowledge must start by identifying the skilled person or team. How is this to be done if the expert cannot be shown the patent? One way is to ask the expert to make an assumption, which they can check later when they see the patent; but that is not necessarily a perfect solution. Other problems can be caused by the pre-existing knowledge of the expert and by amendments to the parties’ cases (such as the introduction of new prior art after the expert has read the patent). Still further, instructing experts in this way can make their task even more burdensome, particularly when it comes to cross-examination, because they may find it difficult to recall what they knew when unless it is clearly documented. (It should be borne in mind, however, that some cross-examination as to the way in which the expert has been instructed is often justified in any event.)
37. In the present case, Prof Haase explained in his first report that he had discussed the common general knowledge with those instructing him before he had seen the Patents, so to that extent he was instructed sequentially. Nevertheless, it appears that Prof Haase was asked to read WO 997 before commenting on Epstein because at that time the Defendants were not advancing a case of obviousness of the Family A Patents, but were relying upon WO 997 as prior art against the Family B Patents. In any event, given Prof Haase’s prior knowledge of HIF-PHIs, which he acknowledged in his first report, it would not have been possible to instruct him in a manner which was free from hindsight. Accordingly, I do not criticise the manner in which he was instructed. In evaluating his evidence, however, I accept that it is necessary to take into account that he read WO 997 before forming his opinion concerning obviousness over Epstein.
The medicinal chemists
38. The Claimants’ expert was Dr Gurdip Bhalay, who has been Team Leader, Medicinal Chemistry at the Institute of Cancer Research since 2018. He obtained a degree in chemistry and a PhD from the University of Nottingham in 1989 and 1992 respectively. From 1993 to 1995 he was a post-doctoral scientist at the University of Oxford. From 1995 to 1997 he was employed by a start-up company on drug discovery projects for Pfizer Central Research. From 1998 to 2014 he was employed by Novartis, initially as a Research Investigator (until 2008) and then as Senior Research Investigator. There his role involved co-leading drug discovery projects as the medicinal chemistry lead, as well as evaluating emerging scientific methodology. From 2014 to 2018 he was Group Leader, Medicinal Chemistry at Charles River Early Discovery. He is an author of 34 scientific publications and a named inventor on 21 patents.
39. Counsel for the Defendants made no criticism of Dr Bhalay’s evidence. As counsel pointed out, cross-examination showed that there was relatively little between Dr Bhalay and Prof Ward.
40. The Defendants’ main expert was Professor Simon Ward, who has been the Sêr Cymru Professor in Translational Drug Discovery at Cardiff University and a Director at the Medicines Discovery Institute since 2017. He received an MA (natural sciences) in 1993 and a PhD (synthetic organic chemistry) in 1997 from the University of Cambridge. He then held various roles in the pharmaceutical industry as a medicinal chemist, joining GSK in 2001 as an associate/assistant director of medicinal chemistry, where he led medicinal chemistry and multi-disciplinary teams working on CNS drug discovery projects, including on enzyme inhibitors. Prof Ward left GSK in 2010 to become Professor of Medicinal Chemistry and Director of the Sussex Drug Discovery Centre at the University of Sussex. Among other things, he is Joint Editor-in-Chief of Comprehensive Medicinal Chemistry Vol III (3rd ed, Elsevier, 2017). He is an author of two books, 49 scientific publications and a named inventor on 44 published patent applications.
41. Counsel for the Claimants criticised Prof Ward for using what counsel characterised as “invective” when he described the number of compounds covered by Formula (I) in the Patents as “staggeringly” large. This criticism is wholly unjustified: on any objective view the number is staggeringly large, as I will explain. That is not altered by the fact that Prof Ward is a named inventor on a patent in respect of which the same observation might be made.
42. In addition, as mentioned above, the Defendants called Professor Colin Fishwick, who is Professor of Medicinal Chemistry and Head of the School of Chemistry at the University of Leeds. He received his undergraduate degree and PhD in chemistry from the University of Liverpool in 1982 and 1985 respectively, and joined the staff at the University of Leeds in 1985, and was appointed Professor in 2009. He was Head of Organic Chemistry from 2013 to 2018. He is an author of more than 145 publications.
43. Counsel for the Claimants appeared to suggest that both Prof Ward and Prof Fishwick were in some way to be criticised for the fact that, as mentioned above, both their respective first reports exhibited documents prepared by the Defendants’ solicitors which were intended to illustrate their interpretations of Formula (I), but which turned out to contain errors. In my view no criticism of either expert for failing to spot these errors is merited. As will become clear when I come to the issue of construction, interpretation of Formula (I) is far from easy. Moreover, it is entirely understandable that those charged with preparing illustrative documents made what amount to formatting errors given the length and complexity of Formula (I).
The clinical practice experts
44. The Claimants’ expert was Dr Mark Devonald, who is a consultant nephrologist at Nottingham University Hospitals NHS Trust (“NUH”). He qualified in medicine at the University of Edinburgh in 1993. He obtained specialist accreditation in 2004, and has worked as a consultant nephrologist within the NHS since then. He obtained a PhD from the University of Cambridge in 2005. He has been at NUH since 2007. He estimates that he is responsible for over 100 patients a year who receive treatment for anaemia associated with CKD. He was a member of the guideline development group for the National Institute for Health and Care Excellence (NICE) Clinical Guidance NG8 Management of Anaemia in Chronic Kidney Disease . He was a member of NUH’s Drugs and Therapeutics Committee from 2007 to 2014.
45. Counsel for the Defendants made two criticisms of Dr Devonald’s evidence, both of which I consider well founded. First, Dr Devonald was strangely unwilling to accept that his first report was based on Prof Winearls’ evaluation of the literature, despite the fact that Dr Devonald had expressly stated in his report that he was asked to assume that Prof Winearls’ evaluation was reasonable. Dr Devonald attempted to suggest that he had undertaken his own analysis based on a review by E.K. Batchelor et al , “Iron Deficiency in Chronic Kidney Disease: Updates in Pathophysiology, Diagnosis and Treatment”, J Am Soc Nephrol , 10 February 2020 (e-publication), but he had not seen that review at the date of his first report. Secondly, Dr Devonald was reluctant directly to answer a question he was asked as to whether a change in clinical practice was likely with HIF-PHIs. (Counsel for the Defendants submitted that Dr Devonald twice failed to answer the question, whereas counsel for the Claimants submitted that he had answered it the third time it was asked. I think counsel for the Claimants is correct, but that does not detract from the point made by counsel for the Defendants.)
46. The Defendants’ expert was Dr Neil Ashman, who is a consultant nephrologist at the Royal London Hospital, a partner hospital of Barts Health NHS Trust (“Barts”), and also the Chair of the Medicine Board at Barts. He obtained an MBChB from the University of Cape Town in 1991 and his certificate in nephrology in 2004. He obtained a PhD from Queen Mary University of London in 2008. He has managed patients with anaemia of chronic kidney disease in the UK since at least 2004 when he became a consultant nephrologist. Barts has one of the largest renal units in the UK.
47. Counsel for the Claimants made no criticism of Dr Ashman. He was considerably more impressive than Dr Devonald as a witness, and to the extent that they conflict I have no hesitation in preferring the evidence of Dr Ashman.
Technical background
48. Regrettably, no technical primer was prepared in this case. I was told that, at the time of the case management conference, the parties were disagreed as to the need for a primer, and the judge decided not to order one. With the benefit of hindsight, it is clear to me that that was a mistake. This case involves two moderately complex areas of science, and the preparation of a primer at an early stage would have saved considerable time and effort at later stages. This is particularly so because, as is often the case, there was no dispute as to much of the technical background. In future, the preparation of a technical primer should be regarded as mandatory in Category 4 and 5 cases unless there are good reasons to the contrary. The following account is largely based on the helpful summaries of the experts’ evidence contained in the parties’ skeleton arguments, supplemented with some additional material from their written closing submissions and the expert evidence. I shall mainly express myself in the present tense, but I am referring to what was known at the Priority Dates.
Erythropoiesis, iron metabolism, anaemia and hypoxia
49. Erythropoiesis . The interstitial fibroblasts located in the kidneys are the main source of endogenous Epo, although some is produced by hepatocytes and other cells in the liver. Epo stimulates erythropoiesis, the process of red blood cell formation. Epo causes the erythroid bone marrow to generate erythroblasts which develop into reticulocytes. In turn, reticulocytes mature into erythrocytes (red blood cells).
50. Iron metabolism . Iron is required for the synthesis of haemoglobin, and hence for the production of healthy red blood cells. Inadequate iron supply results in red blood cells that are small (microcytic) and pale (hypochromic).
51. Iron is absorbed mainly by the duodenum, typically amounting to about 1 mg a day. Once the iron has passed from the enterocyte cells of the gut into the bloodstream via the ferroportin transporter, a protein called transferrin delivers the iron to the tissues, such as the erythroblasts in the bone marrow, where it binds to transferrin receptors (“TfR”) located on the erythroblast membrane. TfR internalise the iron to the erythroblast via receptor-mediated endocytosis for use in haem synthesis.
52. Free (i.e. unbound) iron is toxic in vivo , and so iron is normally stored in the body bound to ferritin, a protein that keeps the iron stores in a non-toxic and accessible form. Ferritin complexes are located within macrophages of the reticuloendothelial system and in hepatocytes in the liver. Ordinarily, there are only limited levels of ferritin detectable in the blood. Serum ferritin is often used as a proxy measure for the overall levels of iron stored in the body.
53. Serum iron is a measure of circulating ferric (Fe3+ ) ions bound mainly to transferrin. Transferrin saturation (TSAT) is calculated as a percentage of the serum iron compared to the total iron binding capacity (i.e. the number of iron-binding sites on transferrin).
54. The main iron pathways are shown diagrammatically in a figure from Prof Winearls’ first report which I reproduce below.
55. On the left is shown absorption of dietary iron in the duodenum, and the use of iron in bone marrow for erythropoiesis. The central rectangles (in purple and red) show iron in the bloodstream, where it is either bound to transferrin or in the form of haemoglobin in red blood cells. On the right are the iron stores, from where iron is released into (or to which iron is removed from) the circulating, transferrin-bound pool. When red blood cells reach the end of their 120-day life, their iron is also recycled into these stores via macrophages.
56. Iron deficiency . It is important to distinguish between absolute iron deficiency and functional iron deficiency.
57. Absolute iron deficiency occurs when a patient does not have enough iron in stores to supply the body’s needs. Absolute iron deficiency may be caused by a low-iron diet, reduced iron absorption and/or bleeding. Absolute iron deficiency is characterised by a low TSAT and a low level of serum ferritin, namely, a TSAT < 20% (or < 16% in more extreme cases) and serum ferritin < 50-100 ng/ml.
58. Functional iron deficiency occurs where there is sufficient iron in stores, but where there is inadequate delivery of that iron from stores to the bone marrow. Inflammation can result in iron being sequestered into iron stores and the reticuloendothelial blockade, which prevents release of the stored iron, being activated. Functional iron deficiency is characterised by a low TSAT and normal or high serum ferritin.
59. Patients who are “iron replete” are usually defined as those with a TSAT of at least 20% and a serum ferritin level of at least 100 ng/ml; but TSAT measurements show considerable diurnal and day-to-day variation for a given patient.
60. Anaemia . Anaemia is a class of conditions characterised by an inability to produce sufficient quantities of healthy red blood cells to meet the oxygen requirements of the body. Insufficient red blood cell production can cause fatigue, lethargy, pale skin, and dizziness.
61. Though the anaemic conditions share common symptoms, their pathophysiologies are distinct. The causes include inadequate red cell production, defective iron acquisition or availability, defective haem synthesis, red cell destruction and blood loss. Some of these causes involve Epo.
62. Anaemia of CKD . Chronic kidney disease (CKD) describes a diminution in renal function through irreversible damage to the kidneys to an extent that has negative consequences for the patient, including an impairment of Epo production, and hence anaemia.
63. In kidneys affected by CKD, it was thought that the interstitial fibroblasts had been partially destroyed or had undergone a phenotypical change such that the kidneys had a reduced capacity to produce physiological amounts of Epo. Although this basic point is not in dispute, there is an important issue concerning it which I will address below.
64. In patients with CKD, iron deficiency is often seen, of both the absolute and functional kind.
65. Anaemia of chronic disease (ACD) . ACD is characterised by normal or high ferritin levels but low transferrin and serum iron, indicative of functional iron deficiency. ACD was thought to be caused by an underlying chronic disorder involving inflammation, such as rheumatoid arthritis or cancer, that activated the reticuloendothelial blockade (trapping iron in macrophages and thereby causing functional iron deficiency) and suppressed Epo production and bone marrow activity through the effect of inflammatory cytokines.
66. Treatment of anaemia . At the Priority Dates, anaemias were treated in the manner described below.
67. Iron deficiency anaemia (without another underlying cause) was treated by increasing iron in the diet, giving iron supplements, or in extreme cases by blood transfusion.
68. Anaemia of CKD was first treated with oral iron, to see if this alone achieved the desired haemoglobin response, and if not, intravenous (IV) iron. Patients with more severe CKD, receiving haemodialysis, were typically put straight on IV iron because this could be done easily during dialysis and because of their increased iron needs.
69. If iron supplementation did not raise haemoglobin to the target range, patients were given an ESA. ESAs include recombinant human Epo (r-HuEpo) (such as epoetin alfa, epoetin beta) and various analogues (including darbepoetin alfa), all of which stimulate erythropoiesis in the presence of adequate iron. Anaemia of CKD was considered a treatable condition, as ESAs circumvented the damaged kidneys’ reduced Epo production by providing an exogenous source. ESAs did have certain disadvantages, however, in that they were expensive and had to be administered either intravenously or subcutaneously.
70. ESAs tend to cause (or exacerbate) iron deficiency, because they stimulate the demand for iron, and so patients are often given supplementary iron with ESAs. The norm was to aim for a TSAT of 30% or more before starting treatment with ESAs.
71. A small number of patients are “refractory” or “resistant” (the terms appear to be interchangeable) to ESAs. I shall consider the definition of “refractory” below. In rare cases, patients do not respond to ESAs at all.
72. Treatment of ACD . The primary goal of treating ACD focussed on resolving the underlying inflammation. It is common ground that ESAs were sometimes administered to patients with ACD, but there is a dispute about the effectiveness of such treatment which I will address below.
73. Hypoxia . Hypoxia is lack of oxygen, while normoxia refers to adequate levels of oxygen. HIF was known to regulate physiological responses to hypoxia, including the expression of Epo, but there is a dispute as to the extent of such knowledge which I will address below.
Medicinal chemistry
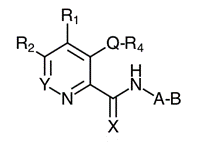
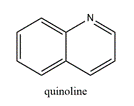
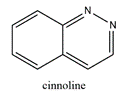
74. Drug development . Having identified a therapeutic target, the first stage of the drug development process is to find one or more “hit” compounds that will form the starting point for further investigation. A “hit” compound is generally identified by demonstrating that it has some biological activity in a relevant assay. This will often be an in vitro assay, but it may be an in vivo animal model. If no better approach is available, “libraries” of compounds can be screened for activity. Where possible, it is preferable, however, to start with known modulators of the therapeutic target or a related therapeutic target.
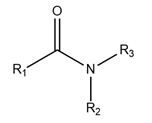
75. Another approach is to use the natural ligand or substrate of the target protein as the starting point for the synthesis of analogues which bind to the same site of action, but which modulate the physiological effect of the natural ligand. The theory that underpins this approach is that a compound that differs structurally from the substrate enough to be chemically unreactive (or react very slowly compared to the substrate), but structurally resembles the substrate enough to the extent that it is able to bind to the substrate binding site on the target, might have some pharmacological activity against the target. The more closely the compound resembles the substrate, the more likely it may bind to the substrate binding site in competition with the natural substrate. Conversely, the less the compound resembles the substrate, the less likely it may bind to the substrate binding site in competition with the natural substrate.
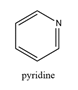
76. Structure-activity relationships . Having identified a biologically active compound, the next stage of the process typically involves a structure-activity relationship (SAR) analysis. The aim of SAR is to discover which parts of the compound are important to its biological activity and which are not. By making a series of structural modifications in which a particular functional group is removed or altered or added, and then measuring the effect on biological activity in each case, it is possible to identify which functional groups are essential for the desired activity and should be retained, and which regions of the molecule are tolerant towards modification while retaining the desired biological effect.
77. To be robust, an SAR investigation should:
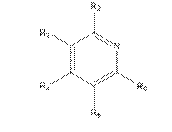
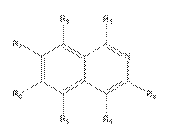
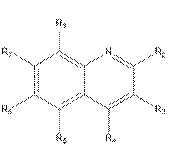
i) consider a sufficient number of molecules;
ii) generally involve making one modification at a time;
iii) involve a diverse set of modifications (conservative modifications often do not reveal much about the relationship between structure and function);
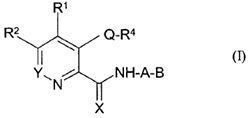
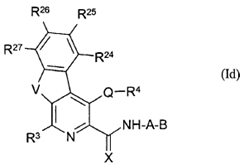
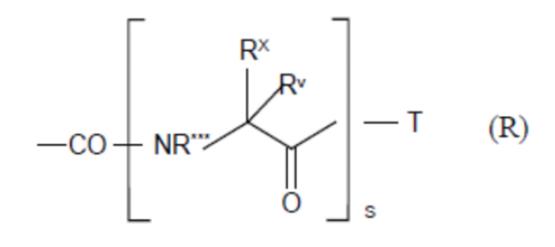
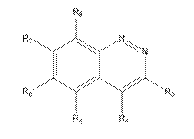
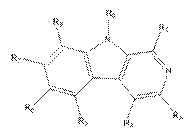
iv) involve sufficient changes to enable the medicinal chemist broadly to understand the environment around the compound (assuming it is bound into the active site of the enzyme);
v) include molecules that are both active and inactive; and
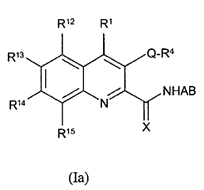
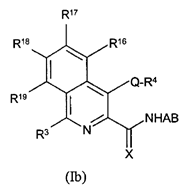
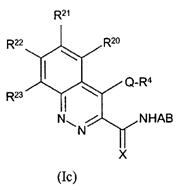
vi) be based on data that is repeated (i.e. not generated in single experiments).
78. Even in the case of something as simple as a hydroxyl (-OH) group, it may be necessary to undertake a number of modifications (such as conversion to hydrogen, methyl ether or ester) in the course of an SAR analysis to determine how important a particular functional group is to biological activity.
79. SARs are preferably conducted using inhibition studies on isolated enzymes. If cell-based assays or in vivo models are used, the results tend to be much less clear cut because changes in activity could be due to the inability of the compound to reach the target enzyme (as a consequence of poor metabolic stability, solubility or permeability to cell membranes).
80. SAR studies will often result in the identification of one or more “lead” compounds that have superior activity to the original “hit” compound. Ideally, the SAR will enable the medicinal chemist to define a “pharmacophore”, that is to say, the functional groups which are required for activity and their relative positions in space with respect to each other. In so doing the medicinal chemist may learn something about the three-dimensional shape and chemistry of that part of the target protein with which the compound is interacting.
81. An SAR is useful in trying to understand the activity of the molecules used to generate it. However, the impact of a modification beyond the set of modifications examined in the SAR is frequently unpredictable and must be tested empirically. Furthermore, SARs are focussed on the interaction with the proposed active site. SARs do not address pharmacokinetic or safety issues.
82. By its very nature, SAR analysis is an exercise of genuine research in which the medicinal chemist is trying to discover new information. Moreover, it involves matters of choice and judgement.
83. Lead optimisation . Having identified one or more lead compounds through SAR, the next stage of the process is often termed “lead optimisation”. This aims to modify the lead compound such that it interacts more effectively and selectively with its molecular target in the body. Stronger drug-target interactions should increase the activity of the drug, while an increase in target selectivity will lower side-effects. Lead optimisation involves learning more about the shape and chemistry of the part of the target protein with which the compound is interacting than has been revealed by SAR.
84. Strategies used in lead optimisation include variation of substituents, extension of the structure, chain extensions/contractions, ring expansions/contractions, ring fusions, the use of isosteres (molecules with a similar shape and often similar electronic properties), simplification of the structure and rigidification of the structure.
85. Lead optimisation is assisted by x-ray crystallography of the lead compound bound to the target protein, because this will typically reveal more detailed information about the three-dimensional arrangement of the interacting functional groups on the lead compound and on the protein.
86. Pharmacokinetics . Pharmacokinetics is a branch of pharmacology that describes how the body affects a compound after it is administered. In order for a compound to cause a therapeutic response, it must be maintained at an adequate concentration at the target site long enough for the compound to have a therapeutic effect. The therapeutic response is dependent upon the processes of absorption, distribution, metabolism and excretion (ADME).
87. A compound’s pharmacokinetic profile largely depends both on the presence of specific functional groups vulnerable to metabolism and the bulk physicochemical properties of the compounds (the balance between the polarity/charge and lipophilicity of the compound). That balance depends in turn on the functional groups present. There are no hard and fast rules, but there are certain rules of thumb (such as Lipinski’s Rules, also known as the rule of five) that are used to try to predict when, for example, poor absorption or cell membrane permeation is more likely.
88. Strategies may be deployed in an effort to enhance the pharmacokinetic profile of a compound. For example, a drug may be manufactured as a pro-drug. Pro-drugs are compounds that are themselves inactive, but are converted into the active drug inside the body. For example, a carboxylic acid functional group is ionised inside the body. It may have a role to play in the binding of a drug via ionic or hydrogen bonding, but the fact that it is ionised is likely to restrict it from crossing a lipid cell membrane. Commonly, the carboxylic acid is protected as an ester. The less polar ester is able to cross cell membranes into the blood stream where it is hydrolysed to the ionised carboxylic acid by esterases in the blood.
89. Enzymes . Enzymes are a class of proteins with which drugs can interact. They speed up, or catalyse, biological reactions without themselves being consumed. Substrates bind to and react at a specific part of the enzyme called the active site (also referred to as the binding site or binding pocket). The active site usually consists of an indentation on the surface of the enzyme that has a unique three-dimensional structure and functional group distribution. Only molecules with the right shape and functional group distribution can bind to the active site and form the enzyme-substrate complex required for catalysis.
90. One model for enzyme-substrate binding is the “lock and key” model (in which both enzyme and substrate are seen as rigid, with the substrate fitting like a key into a lock). Another model is the “induced fit” model. This model assumes that the active site of an enzyme has a degree of flexibility. It proposes that the substrate is not quite the perfect shape for the active site but when it enters the active site the latter changes shape slightly to maximise bonding interactions.
91. Enzymes may require co-factors (or helper molecules) to carry out the catalysis. Co-factors include
i) metal ions (such as Fe2+ or Zn2+ ) which may assist in holding the substrate(s) in an optimal configuration and/or be involved in electron transfer reactions; and
ii) small organic molecules called co-enzymes which are transiently and loosely bound in the active site during an enzymatic reaction, and which undergo a chemical transformation as part of the enzymatic catalytic cycle. A co-enzyme may itself be considered a substrate of the enzyme (and is sometimes referred to as a co-substrate).
92. Related enzymes . Phylogenetically-related enzymes that are structurally and mechanistically similar are grouped into so-called “super families”. Enzymes within the same super family may catalyse different reactions, but do so by the same or very similar reaction mechanisms and have one or more of their substrates in common. Enzymes that have a substrate in common will all have a space in their active site that is complementary to the three-dimensional shape and chemistry of the functional groups of the relevant substrate into which the relevant substrate can bind. There are certain amino acids in the active site that are conserved between enzymes of the same super family.
93. Beyond such conserved amino acids, the sequences may well diverge between related enzymes. For this reason, it is common to be able to develop specific inhibitors against different members of a family of enzymes.
94. Enzyme inhibition . Compounds may inhibit the normal activity of an enzyme in a number of different ways.
95. A competitive inhibitor is a compound which competes with the natural substrate for the enzyme’s active site. As discussed above, competitive inhibitor usually bears some features of the substrate to the extent that it specifically binds to the active site, but differs from the substrate enough to be chemically unreactive (or react very slowly). The effect of a competitive inhibitor is reversed by increasing the concentration of substrate because the frequency of successful collisions between inhibitor and active site is reduced. A competitive inhibitor acts by reducing the concentration of free enzyme available for substrate binding.
96. A non-competitive irreversible inhibitor acts by reducing the concentration of free enzyme available for substrate binding. Such a compound typically shows some sort of similarity to the natural substrate, but they often contain a functional group which reacts with an amino acid in the active site, forming a covalent bond. This blocks the active site. The formation of a covalent bond means the inhibitor molecule cannot be displaced by the natural substrate. The effect of this type of inhibitor is not reversed by increasing the concentration of natural substrate.
97. Non-competitive allosteric inhibitors bind into an allosteric site (i.e. a binding site distal from the active site of the enzyme) which changes the affinity of the enzyme for its substrate by triggering a change in the 3D shape of the active site. This inhibits the enzyme’s activity because the natural substrate can no longer bind to the active site. The inhibitor may bind reversibly, in which case the active site of the enzyme will return to the correct 3D shape for catalysis. Many enzymes are regulated naturally by allostery.
98. Inhibitors of iron-dependent enzymes . Metal ions can exist in different forms within cells. Metal ions can be present “free” in the intracellular solution. Free metal ions form coordinate (covalent dative) bonds to water molecules. For example, free Fe2+ may exist inside the cell coordinated by six water molecule ligands. Metal ions can also be present inside the binding sites of proteins (such as the active sites of enzymes). These metal ions are held in place by coordinate bonds between them and ligands that are also present inside the active site. These ligands can be functional groups on amino acids and/or substrates.
99. A molecule which is capable of forming two or more separate coordinate bonds to a metal ion is known as a chelator. A chelate complex (also called a coordination complex) is the complex that forms when a metal ion is coordinated by a chelator. The formation of such complexes is known as chelation (or coordination). During chelation, a chelator forms multiple coordinate bonds with the metal ion (two bonds are formed if the chelator is bidentate, more than two bonds are formed if the chelator is polydentate), displacing its pre-existing ligands. Metal chelators that are able to access the inside of a cell, fit inside the active site of an enzyme and chelate the active site-bound metal ion by displacing its natural ligands may inhibit that enzyme’s reaction.
100. Free metal ions can also be chelated in a bidentate or polydentate fashion by chelators that are able to access the inside of a cell. For example, a chelator may be able to displace some of the water molecules coordinating free Fe2+ , forming a Fe2+ chelate complex. As the concentration of chelate complexes inside a cell rises, and the concentration of free metal ion falls, Le Chatelier’s principle predicts that protein-bound metal ions will disassociate from their ligands to redress the balance (including, for example, active-site bound metal ions). Thus, the chelation of free metal ions by chelators can also result in the indirect inhibition of metal ion-dependent, protein-driven processes (like metal ion-dependent enzyme catalysis).
101. Metal-ligand coordinate bonds are a type of covalent bond where one of the atoms in the ligand provides both electrons in the bond. Groups which are capable of forming a coordinate bond to a metal possess a lone pair of electrons. The availability of a lone pair of electrons to form a coordinate bond can be influenced by the surrounding chemical context, including, for example, the inductive effect of an electron-withdrawing group, the inductive effect of an electron-donating group and steric factors. There are an essentially limitless range of modifications that may, or may not, reduce or eliminate the availability of a lone pair of electrons to form a coordinate bond with a metal ion. The impact of these modifications is unpredictable.
102. Enzyme kinetics . Enzyme kinetics is the study of the rate of enzymatic reactions. The rate of catalysis depends on the concentration of enzyme, substrate and inhibitor present, as well as factors such as pH and temperature.
103. Enzyme kinetic studies can be used to determine whether an inhibitor is competitive or non-competitive. The reaction rate of the enzyme is measured with respect to varying substrate concentration in the presence of different concentrations of inhibitor. In the case of competitive inhibition, the maximum rate of reaction is unaffected because increasing substrate concentration will overcome inhibition (at high substrate concentrations, the tables turn and the substrate outcompetes the inhibitor). In the case of a non-competitive inhibitor, the maximum rate of reaction is reduced because the presence of the non-competitive inhibitor affects the enzyme such that no amount of substrate can restore the maximum rate of catalysis.
104. The Ki (inhibition constant) of a compound is the concentration of inhibitor required to produce half maximum inhibition and is an indication of how potent an inhibitor is. The lower the Ki , the higher the affinity of the enzyme for the inhibitor. Ki values can be converted into IC50 values. IC50 is the concentration of inhibitor required to inhibit 50% of the enzyme's maximal activity.
105. The Km (Michaelis-Menten constant) of an enzyme is the substrate concentration at which the reaction rate of the enzyme is half the maximal rate. It is determined by measuring the reaction rate of the enzyme with respect to varying concentrations of substrate. Km is a measure of the affinity of the substrate for the enzyme’s active site. The lower the Km value of an enzyme, the higher the affinity of the enzyme for its substrate.
106. Km , Ki and IC50 values determined under different experimental conditions are generally not comparable.
The Family A Patents
107. As noted above, I shall set out the disclosure of the Family A Patents by reference to WO 997. I shall do so using the headings in the specification and I shall note some, but not all, of the respects in which the texts of the Patents differ. Before I do so, I must note two important and related points the significance of which will become apparent below.
108. The first point is that both WO 997 and the Family A Patents which derive from it refer to a considerable number of scientific papers and books and earlier patents and patent applications. The numbers of such documents referred to in each of the four are not precisely identical, but for example EP 823 refers to 47 papers or books and 26 patents and applications (including four prior art citations referred to in EP 823 at [0007] which are not mentioned in WO 997). I shall refer to a number of instances of such references below.
109. The second point is that, in some places, WO 997 states that the earlier publications are, or information contained in such publications is, incorporated by reference in its entirety; but that language is not present in the Family A Patents (this is because the European Patent Office’s practice is to require such language to be deleted). I shall refer to some examples of this below.
Field of the invention
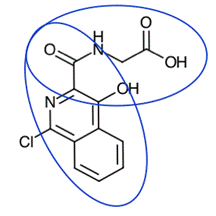
110. The specification begins by explaining at [0002] that the invention “relates to methods for increasing endogenous erythropoietin, ex vivo and in vivo , and to compounds that can be used in the methods”. The language in the corresponding paragraph ([0001]) of the Family A Patents is as follows:
i) EP 823: “relates to medicaments [sic ] for increasing endogenous erythropoietin in the prevention, pretreatment or treatment of anemia, and to compounds that can be used in the methods”;
ii) EP 531: “relates to compounds for use in the treatment or prevention of anemia”;
iii) EP 301: “relates to compounds for use in the treatment or prevention of anemia associated with kidney disease”.
Background of the invention
111. At [0003]-[0005] the specification explains the role of Epo in stimulating the production of red blood cells, that anaemia is typically associated with a condition in which the blood is deficient in red blood cells or haemoglobin and that anaemia may be caused by (among other things) iron deficiency, inflammatory disorders or renal dysfunction.
112. Epo is discussed further at [0006]. The paragraph ends by citing, in brackets, five papers. The specification does not in terms explain why these papers are referred to. The natural inference is that they are being cited as providing scientific support for the statements made in the paragraph. There is no apparent reason why papers are cited in [0006], but not in the rest of this section of the specification.
113. In [0007] reference is made to the introduction of genetically engineered Epo for the treatment of anaemia in chronic renal failure patients and the limitations of such treatment, including cost and the need for intravenous administration. At [0008] it is explained that there remains a need for methods and compounds effective in the treatment of Epo-associated conditions such as anaemia, including anaemia associated with kidney failure, cancer and infection, and specifically a need for methods and compounds that increase endogenous Epo.
Summary of the invention
114. This section begins by explaining that the invention relates generally to methods for increasing endogenous Epo. Four methods are identified at [0009]:
i) stabilising the alpha subunit of HIF (HIFa );
ii) inhibiting the hydroxylation of HIFa ;
iii) inhibiting 2-oxoglutarate (“2-OG”) dioxygenase enzyme activity; and
iv) inhibiting HIF-PH enzyme activity.
115. At [0013] the specification states that, in methods relating to inhibition of 2-OG dioxygenase activity, embodiments are provided in which the 2-OG dioxygenase enzyme is selected from “the group consisting of EGLN1, EGLN2, EGLN3, procollagen prolyl 4-hydroxylase, procollagen prolyl 3-hydroxylase, procollagen lysyl hydroxylase, PHD4, FIH-1, and any subunit or fragment thereof”. For methods of increasing endogenous Epo by inhibiting HIF-PH enzyme activity, the enzyme is selected from “the group consisting of EGLN1, EGLN2, EGLN3, and any subunit or fragment thereof”.
116. At [0015] the specification explains that, in a particular embodiment, the compound is “a heterocyclic carboxamide selected from the group consisting of pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and beta-carboline carboxamides”. I shall refer to this group of compounds as “the Carboxamides”.
117. A carboxamide is a functional group with the general structure R1 -CO-NR2 R3 where R1 , R2 and R3 can be organic substituents or hydrogen as shown below.
118. Pyridine is a heterocycle with the formula C5 H5 N as shown below. The parent compound can be substituted at up to five different positions around the ring (excluding substitution of the nitrogen atom) with substituents R2 , R3 , etc as shown on the right.
119. The term “pyridine carboxamides” comprises all organic compounds containing: at least one pyridine functional group, substituted at any of positions R2 -R6 by at least one carboxamide functional group, and at the other R positions by: H; a further carboxamide; or any other substituent (except for a substituent in which adjacent R-groups are joined to form a ring, as this would then make a different class of heterocycle), and on the other end of the carboxamide by any group at all.
120. The other types of Carboxamide referred to in [0015] are depicted below. Again, substitutions can be made at any of positions R1 , R2 , R3, etc of the parent compounds as shown on the right.
121. At [0017] the specification states that the invention specifically relates to methods for treating, preventing or pre-treating anaemia in a subject. One embodiment is said to be a method comprising increasing endogenous Epo including, in various embodiments, stabilising HIFa , inhibiting 2-OG dioxygenase enzyme activity, inhibiting HIF-PH enzyme activity, etc.
122. At [0018] the specification specifies that the anaemia is associated with a condition selected from a group which includes cancer, kidney disease, infection and inflammation. It is also stated that it is “contemplated in specific embodiments that the anemia can be associated with defects in iron transport, processing, or utilization”.
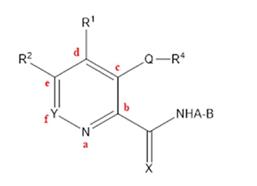
123. At [0026] the specification states that in certain embodiments compounds used in this invention are selected from a compound of Formula (I):
124. The specification then sets out over 10 pages a very long and complicated set of lists of possible substituents at positions A, B, Q, R1 , R2 , R4 , X and Y in Formula (I) and then adds “including the physiologically active salts and prodrugs derived therefrom”. I shall consider these lists when I come to the claims.
125. The compounds embraced by Formula (I) include four sub-formulae of Formula (I): Formula (Ia) (an optionally substituted quinoline); Formula (Ib) (an optionally substituted isoquinoline); Formula (Ic) (an optionally substituted cinnoline); and Formula (Id) (an optionally substituted beta-carboline):
126. I shall refer to the compounds embraced by Formula (I) as “the Formula I Compounds”. A long list of specific Formula I compounds is set out in [0027].
127. At [0028] the specification describes how the compounds of the invention increase endogenous Epo plasma levels by increasing the synthesis of Epo in, amongst other things, renal tissues.
Brief description of the drawings
128. This section introduces the Figures which show the results of the experiments set out in the Examples described later in the specification.
Description of the invention
129. This section contains some general statements about the way in which the invention is described in the specification, including the following at [0041]:
“Unless defined otherwise, all technical and scientific terms used herein have the same meanings as commonly understood by one of ordinary skill in the art to which this invention belongs. Although any methods and materials similar or equivalent to those described herein can be used in the practice or testing of the present invention, the preferred methods, devices, and materials are now described. All publications cited herein are incorporated herein by reference in their entirety for the purpose of describing and disclosing the methodologies, reagents, and tools reported in the publications which might be used in connection with the invention. …”
In the corresponding paragraph of the Family A Patents ([0037]) in EP 823) the words “incorporated herein by reference in their entirety” are replaced by the word “mentioned”.
Definitions
130. From [0043] to [0063] the specification sets out a series of definitions, including of “anemia” (at [0043]-[0046]) (which is defined to include anaemia due to infections, inflammation and cancer and specifically rheumatoid arthritis and sideroblastic anaemia), “HIFa ” (at [0050]), “related proteins” (at [0053]) (which is defined to include procollagen lysyl hydroxylase and procollagen prolyl 4-hydroxylase) and “HIF-PH” (at [0054]) (which is defined to include EGLN1, EGLN2 and EGLN3). A number of these paragraphs include citations of papers, including Epstein at [0054].
Invention
131. At [0064] the specification repeats that the invention provides methods of increasing endogenous Epo and further provides methods of increasing endogenous Epo levels to prevent, pre-treat or treat Epo-associated conditions including conditions associated with anaemia. Various examples of such conditions are listed, including cancer and inflammation. Various patient groups who might benefit from an increase in endogenous Epo are discussed at [0065]-[0066].
132. At [0070] it is stated that the methods of the invention increase the haematocrit and blood haemoglobin levels in animals treated in vivo . Haematocrit is the volume percentage of red blood cells in blood.
133. At [0072] the specification states:
“The invention also contemplates increasing iron transport, processing, and utilization using the methods of the invention. (See, e.g., commonly owned, copending U.S. Patent Application No. ____, entitled ‘Stabilization of Hypoxia Inducible Factor (HIF) Alpha,’ filed of even date, and incorporated herein by reference in its entirety.) Specifically, the methods of the invention may increase enzymes and proteins involved in iron uptake, transport, and processing. Such enzymes and proteins include, but are not limited to, transferrin and transferrin receptor, which together facilitate iron transport to and uptake by, e.g., erythroid tissue, and ceruloplasmin, a ferroxidase required to oxidize ferrous iron to ferric iron. As transferrin can only bind and transport ferric iron, ceruloplasmin is important for supply of iron to tissues. The ability of the methods of the invention to increase both endogenous erythropoietin and transport and utilization of iron in a single course of treatment provides benefits not addressed by current anemia therapeutics, such as administration of recombinant erythropoietin, in the treatment of anemic disorders including, but not limited to, rheumatoid arthritis, sideroblastic anemia, etc.”
134. At [0073] it is stated that, although the invention is not limited by the method in which endogenous Epo is induced, one specifically contemplated mechanism by which the compounds increase the synthesis of endogenous Epo is by inhibiting hydroxylation of HIFa .
135. The specification continues:
“[0074] As HIFa is modified by hydroxylation, a reaction requiring oxygen and Fe2+ , the present invention contemplates in one aspect that the enzyme responsible for HIFa hydroxylation is a member of the 2-oxoglutarate dioxygenase family. Such enzymes include, but are not limited to, procollagen lysyl hydroxylase, procollagen prolyl 3-hydroxylase, procollagen prolyl 4-hydroxylase a (I) and a (II), thymine 7-hydroxlase, aspartyl (asparaginyl) β-hydroxylase, ε-N-trimethyllysine hydroxylase, γ-butyrobetaine hydroxylase, etc. These enzymes require Fe2+ , 2-oxoglutarate, and ascorbic acid for their hydroxylase activity. (See, e.g., Majamaa et al. (1985) Biochem J 229:127-133 [‘Majamaa 1985’]; Myllyharju and Kivirikko (1997) EMBO J 16:1173-1180 [‘Myllyharju and Kivirikko 1997’] ; Thornburg et al. (1993) 32:14023-14033; and Jia et al. (1994) Proc Natl Acad Sci USA 91: 7227-7231.)
[0075] Several small molecule inhibitors of prolyl 4-hydroxylase have been identified. (See, e.g., Majamaa et al., supra ; Kivirikko and Myllyharju (1998) Matrix Biol 16:357-368 [‘Myllyharju and Kivirikko 1998’] ; Bickel et al. (1998) Hepatology 28:404-411; Friedman et al. (2000) Proc Natl Acad Sci USA 97:4736-4741; and Franklin et al. (2001) Biochem J 353:333-338; all incorporated herein in their entirety.) The present invention contemplates the use of these compounds in the methods provided herein.
[0076] Compounds that can be used in the methods of the invention include, e.g., structural mimetics of 2-oxoglutarate. Such compounds may inhibit the target 2-oxoglutarate dioxygenase family member competitively with respect to 2-oxoglutarate and noncompetitively with respect to iron. (Majamaa et al. (1984) Eur J Biochem 138:239-45 [‘Majamaa 1984’]; and Majamaa et al., supra .).”
136. The words “all incorporated herein in their entirety” in [0075] do not appear in the corresponding paragraphs of the Family A Patents. In addition, the last sentence of [0075] differs in the Family A Patents as follows:
i) EP 823 [0068]: “The present invention contemplates the use of these compounds that are selected from the group consisting of pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and beta-carboline carboxamides.”
ii) EP 531 [0064]: “ The present invention contemplates the use of these compounds that are of Formula (I) as defined herein.”
iii) EP 301 [0064]: “ The present invention contemplates the use of these compounds that are of Formula (I) as defined herein.”
137. There is no further explanation of the term “structural mimetics of 2-oxoglutarate” than appears in [0076].
138. At [0077] the specification repeats that in some embodiments compounds used in the methods of the invention are selected from a compound of Formula (I).
139. At [0078]-[0083] the specification states that “exemplary” or “additional” “compounds according to Formula (I) [or (Ia) or (Ib)]” are described in two European and nine US patents, including US Patent No. 5,620,995 (“US 995”). WO 997 states that all compounds listed in these patents are incorporated into the application by reference, but these statements do not appear in the Family A Patents. A considerable number of specific examples of these compounds are also identified in these paragraphs, including certain compounds which are labelled as Compounds D, E, F, G, J, C, H, I and K. Compounds D, E, F and G are said at [0082] to be exemplary compounds according to Formula (Ia) which are described in US Patent No. 5,719,164 and US Patent No. 5,726,305 (“US 305”). Compounds J, C, H, I and K are said at [0083] to be exemplary compounds according to Formula (Ib) which are described in US Patent No. 6,093,730 (“US 730”).
140. At [0084] the specification states that, additionally, “compounds for use in the methods of the invention are compounds described by” five of the papers cited in [0075] and [0076], all of which are said to be incorporated by reference in their entirety (this text is not included in the Family A Patents).
141. At [0085] and [0087] the specification states that, in other embodiments, compounds for use in the invention are selected from two further formulae, Formula (II) and Formula (III).
It should be noted, however, that Formula (III) does not feature in EP 823 and that neither Formula (II) nor Formula (III) feature in EP 531 and EP 301.
142. At [0086] and [0088] the specification states that “exemplary” compounds of Formula (II) and Formula (III) are described in two US patents and two international patent applications. Various compounds are identified in these paragraphs, including two compounds which are labelled as Compounds A and B. Compound A is said at [0086] to be an exemplary compound of Formula II which is described in US Patent No. 5,916,898 (“US 898”), US Patent No. 6,200,974 (“US 974”) and international application WO 99/21860 (“WO 860”). Compound B is said at [0088] to be an exemplary compound of Formula III which is described in international application WO 00/50390.
143. At [0090]-[0096] the specification describes methods of using the compounds of the invention. At [0097]-[0117] it describes pharmaceutical formulations and routes of administration. At [0118]-[0120] it describes methods of compound screening and identification. At [0121]-[0123] it describes methods for producing endogenous Epo in vitro .
Examples
144. WO 997 discloses test results for the eleven compounds referred to as compounds A-K, two of which (A and B) are not covered by Formula (I) even in its widest form. The names and structures of these compounds were conveniently set out by Prof Ward in his report:
145. In Example 1 ([0125]-[0126]) cells from the Hep3B hepatocarcinoma cell line were incubated in tissue culture medium overnight with one of compounds A to I (or DMSO as a negative control). Following incubation, the tissue culture media was collected and analysed for Epo levels. This experiment represents a preliminary screen for in vitro activity in respect of the ability of the compounds to increase the level of endogenous Epo. The data presented in Figure 1 appear to show that each of compounds A to I induced an increase in the Epo level of the tissue culture media compared to the control, although it appears from the absence of error bars that a single experiment was performed for each compound without replicates. The range of the observed increase in Epo was from about 2-fold (Compounds E and F) to about 32-fold (Compounds A and I). Compounds C, D and H were the next best performing compounds after Compounds A and I.
146. Example 2 describes two experiments. In Experiment I ([0127]-[0131]) mice were treated with Compound C (in carboxymethyl cellulose, CMC) or CMC alone (as a negative control) twice a day for 2½ days. After the final dose blood samples were obtained (to measure Epo and haematocrit) before the mice were sacrificed to isolate their liver and kidneys. The level of Epo gene expression in the liver and kidney tissue was measured with reverse-transcription quantitative PCR (RT-qPCR). The data presented in Figure 2A shows that Epo gene expression was increased relative to the negative control in both the liver (32-fold) and kidney (580-fold) of the mice treated with Compound C. Those mice also showed a significant increase in Epo plasma levels (Figure 2B) and haematocrit (Figure 2C) relative to the negative controls.
147. In Experiment II ([0132]-[0133]) mice were treated with Compounds E, K or CMC (negative control) and blood samples were analysed for Epo and haematocrit levels. Other mice were treated with Compounds F, J or CMC (negative control) and blood samples analysed for haematocrit levels. The data in Figure 3A shows that Epo levels were increased in mice treated with Compounds E and K relative to the negative control. The data in Figure 3B shows that haematocrit levels were higher in mice treated with Compounds E, K, F and J relative to negative controls.
148. In Example 3 ([0134]-[0135]) three groups of rats were treated with Compound C in a series of different dose regimens conducted over 19 days. Blood samples were taken at various points throughout the 19 days and analysed for Epo, reticulocytes (immature red blood cells), haemoglobin and haematocrit. Figures 4A and 4B show that Compound C caused an increased level of Epo within 24 hours which in turn led to increased reticulocyte levels. Figures 4C and 4D show that Compound C increased haemoglobin and haematocrit levels, and those increases were maintained over an extended period of time.
149. In Example 4 ([0136]-[0138]) the effect of Compound C in rats with cisplatin-induced acute renal failure was investigated. As shown in Figure 5A, prior to treatment with Compound C, exposure to cisplatin resulted in a reduction of haematocrit; following treatment with Compound C, haematocrit levels increased. As shown in Figure 5B, the increase in haematocrit was associated with an increase in reticulocyte levels.
150. Example 5 ([0139]-[00140]) discusses an animal model for haemolytic anaemia. Example 6 ([00141]-[00142]) discusses an animal model of anaemia associated renal failure involving nephrectomy (surgical removal of the kidney). No data are provided for either Example.
151. In Example 7 ([0143]-[0149]) mice were treated with Compound C, CMC (negative control) or blood collected daily to induce anaemia. Blood samples were collected and various tissues (kidney, liver, brain, lung, skeletal muscle) were harvested. Epo gene expression was measured using RT-qPCR. Figures 6A, 6B and 6C show an increased Epo expression in brain, kidney and liver tissues and that the expression level was equivalent to or exceeded the expression levels observed in the bleed-induced anaemia in the same tissues (although Epo expression in the bleed mice did not appear to be significantly different from the negative control).
152. In Example 8 ([0150]-[0152]) the ability of Compound C to induce endogenous Epo production in rats in the absence of functioning kidneys (both kidneys having been surgically removed) was investigated. Figure 7 shows that serum Epo levels were significantly increased in rats treated with Compound C relative to “sham-operated animals” in which the kidneys were not removed and serum Epo levels were also increased in the nephrectomised animals compared with control animals not treated with Compound C.
153. Example 9 ([0153]) describes a screening assay by which it is said that further compounds that increase endogenous Epo levels by inhibiting HIF-PH activity and stabilising HIFa can be identified. No data from the assay are presented. Indeed, no HIF-PH data are disclosed in WO 997 at all.
The claims of the Family A Patents: unconditionally amended
154. The claims of the Family A Patents as proposed unconditionally to be amended which were relied upon by the Claimants at trial are as follows. The numbering is a result of the fact that FibroGen made a sequence of applications to amend. As will appear, one of the claims is a Swiss-form claim while the others are EPC 2000 claims. In most cases, where dependent claims are dependent on both types of claim, I have only included the versions which are dependent on the EPC 2000 claims.
EP 823
155. Claim 8A (formerly 9) as dependent on (unconditionally amended) claim 1:
“Use of a heterocyclic carboxamide compound selected from the group consisting of pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and beta-carboline carboxamides that inhibits hypoxia inducible factor (HIF) prolyl hydroxylase enzyme activity in the manufacture of a medicament for increasing endogenous erythropoietin in the prevention, pretreatment, or treatment of anemia associated with kidney disease ,
wherein the anemia is associated with chronic kidney disease.”
156. Claim 8A (formerly 9) as dependent on (unconditionally amended) claim 2:
“A heterocyclic carboxamide compound selected from the group consisting of pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and beta-carboline carboxamides that inhibits hypoxia inducible factor (HIF) prolyl hydroxylase enzyme activity for use in increasing endogenous erythropoietin in the prevention, pretreatment, or treatment of anemia associated with kidney disease ,
wherein the anemia is associated with chronic kidney disease.”
157. Claim 19A (formerly 20) as dependent on claims 8A and (unconditionally amended) claim 2:
“A heterocyclic carboxamide compound selected from the group consisting of pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and beta-carboline carboxamides that inhibits hypoxia inducible factor (HIF) prolyl hydroxylase enzyme activity for use in increasing endogenous erythropoietin in the prevention, pretreatment, or treatment of anemia associated with kidney disease ,
wherein the anemia is associated with chronic kidney disease,
wherein the compound is a compound of Formula (I) wherein ….”
158. For reasons that will appear, I have reproduced the list of substituents in Formula (I) which follows the word “wherein” in the precise form in which it appears in granted claim 20 of EP 823 at page 34 line 13 to page 37 line 7 of the specification in the Annex to this judgment.
159. (New) claim 24A as dependent on (unconditionally amended) claim 2:
“A heterocyclic carboxamide compound selected from the group consisting of pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and beta-carboline carboxamides that inhibits hypoxia inducible factor (HIF) prolyl hydroxylase enzyme activity for use in increasing endogenous erythropoietin in the prevention, pretreatment, or treatment of anemia associated with kidney disease ,
wherein the compound is a structural mimetic of 2-oxoglutarate .”
160. (New) claim 24A as dependent on claim 19A, claim 8A and (unconditionally amended) claim 2:
“A heterocyclic carboxamide compound selected from the group consisting of pyridine carboxamides, quinoline carboxamides, isoquinoline carboxamides, cinnoline carboxamides, and beta-carboline carboxamides that inhibits hypoxia inducible factor (HIF) prolyl hydroxylase enzyme activity for use in increasing endogenous erythropoietin in the prevention, pretreatment, or treatment of anemia associated with kidney disease ,
wherein the anemia is associated with chronic kidney disease,
wherein the compound is a compound of Formula (I) wherein …,
wherein the compound is a structural mimetic of 2-oxoglutarate .”
EP 531
161. (Unconditionally amended) claim 17A (formerly 1):
“A compound for use in the treatment or prevention of anemia associated with kidney disease , wherein the compound is a compound of formula (I) … [Compound C]. ”
EP 301
162. (New) claim 2 as dependent on claim 1:
“A compound for use in preventing or treating anemia associated with kidney disease in a subject, wherein the compound is a compound of [Formula (I) wherein A is (C1 -C4 )-alkylene],
wherein the compound inhibits HIF prolyl hydroxylase .”
163. (New) claim 4 as dependent on (new) claim 2 and claim 1:
“A compound for use in preventing or treating anemia associated with kidney disease in a subject, wherein the compound is a compound of [Formula (I) where A is (C1 -C4 )-alkylene but otherwise as defined previously],
wherein the compound inhibits HIF prolyl hydroxylase ,
wherein the compound is a structural mimetic of 2-oxoglutarate .”
The claims of the Family A Patents: conditional amendments
164. In addition, if necessary, FibroGen seeks conditionally to amend EP 823 and EP 301. The purpose of these amendments is to exclude Compound C from the scope of the claims if it is held that claim 17A of EP 531, which is limited to Compound C, is obvious over Epstein. It is not necessary to set out the precise details of these amendments.
The skilled team
165. It is common ground that the Family A Patents are addressed to a multi-disciplinary team investigating potential therapies for anaemia caused by kidney disease. The skilled team would include at minimum:
i) a pre-clinical researcher involved in the investigation of anaemia associated with renal disease;
ii) a clinical nephrologist involved in the treatment of patients with renal disease; and
iii) a medicinal chemist involved in the development of new pharmaceuticals.
166. It is also common ground that the pre-clinical researcher and the clinical nephrologist might be the same person, which is why both sides were able to call a single expert witness to address both fields of expertise. As Prof Winearls put it in his first report, he gave evidence “from the perspective of a research scientist with knowledge of the pathophysiology of anaemia caused by kidney disease and also as a clinician with first-hand clinical experience in the treatment of such conditions”. I shall refer to this person (or combination of persons) as “the skilled nephrologist” save where it is necessary to differentiate between the pre-clinical researcher and the clinical nephrologist.
167. There is a dispute between the parties as to the extent of the skilled nephrologist’s knowledge about HIF prior to reading the Family A Patents. Contrary to the suggestion made at points by the Claimants, this is, as counsel for both sides agreed in oral closing submissions, not a question of the composition of the skilled team. Rather, it is a question of the skilled nephrologist’s common general knowledge, and I shall therefore address it under that head.
Common general knowledge as at the Family A Priority Date
168. There is no dispute that everything I have set out under the heading “technical background” above was common general knowledge. There are three main areas of dispute as to the common general knowledge of the skilled nephrologist as at December 2001. Before turning to consider those, I should note that there is no dispute that, in order to be common general knowledge, the information must be common general knowledge to the skilled person in the UK. There is also no dispute that, in December 2001, there were certain differences between the practices of clinical nephrologists in the UK and those in the USA: in particular, higher doses of epoetin alpha were prescribed in the USA than the UK (epoetin beta was not available in the USA at that date). In my view nothing turns on this.
Endogenous production of Epo by diseased kidneys
169. As noted above, there is no dispute that it was common general knowledge that in patients with CKD the ability of the kidneys to produce Epo is reduced. This is because of a reduction in the number of Epo-producing cells. Nor is it in dispute that it was known that the amount of Epo produced in such circumstances is, although actually slightly higher than in patients with normal kidneys, insufficient to prevent anaemia. There is a dispute, however, as to whether the skilled nephrologist would have thought that damaged kidneys could be stimulated to produce more Epo, in particular by hypoxia.
170. In his first report Prof Winearls appeared to be saying that the skilled nephrologist would not have thought that this was possible. He referred in this context to an “influential” paper from the Ratcliffe group (P.H. Maxwell et al , “The interstitial response to renal injury: Fibroblast-like cells show phenotypic changes and have reduced potential for erythropoietin gene expression”, Kidney Int , 52, 715-724 (1997), “Maxwell”) which he noted had been presented before publication to “a wide audience of nephrologists” at a meeting of the Renal Association in 1997, and thus was common general knowledge.
171. Prof Haase’s evidence in his third report was that the skilled nephrologist would have appreciated from Maxwell that, even in severely damaged kidneys, it is possible to stimulate the production of significant quantities of Epo. Prof Haase added that this was consistent with observations that, in patients with anaemia of CKD, Epo production was induced as a result of blood loss and acute hypoxia. In support of the latter point, he cited A. Kato et al , “Erythropoietin production in patients with chronic renal failure”, Ren Fail , 16(5), 645-51 (1994) (“Kato”) and R. P. Ross et al , “Erythropoietin response to blood loss in hemodialysis patients is blunted but preserved”, ASAIO J , 40(3), M880-5 (1994) (“Ross”).
172. Maxwell examined Epo production in three types of renal injury: ureteric obstruction, global ischemia and focal needlestick injury. It states in the first paragraph in the discussion section at 723 (divided into sub-paragraphs for ease of comprehension):
“The principal finding of this study is that following renal injury a reduced number of interstitial cells expressed the Epo-TAg gene in response to anemia or hypoxia. This reduction in the number of positively staining cells was observed irrespective of the nature of the injury, and was apparent in both anemically and hypoxically stimulated animals. Several features of the response to injury were defined.
First, the reduction in the number of cells expressing Epo-TAg was regionally correlated with severity of injury. In needlestick injured kidneys the reduction in positive cells was focal. In post-ischemic kidneys, where the most severe injury was observed in the outer medulla, the most striking reduction in interstitial cells expressing the Epo-TAg gene was also in this region.
Second, even in severely injured regions occasional positive cells were observed, and there was no apparent difference in the intensity of staining in these cells when compared with those in normal kidneys.
Third, in an individual injured area there was no apparent difference between the fibroblast-like cells that did or did not express the Epo-TAg gene. In particular, the intensity of desmin staining was similar.
Fourth, although there were clear reductions in the number of positive cells in injured regions at all levels of stimulation, more intense anemic or hypoxic stimulation (as judged by the number of positive cells in the control kidney) resulted in a greater recruitment of cells in the injured kidney, so that the greatest disproportion between the injured and uninjured kidney was seen under conditions of mild stimulation.”
173. As Prof Haase explained in cross-examination, what the last sub-paragraph is saying is that the injured kidney retains a capacity to respond to more intense hypoxic stimulus to produce more Epo. As he agreed, the same point is made in the following paragraph:
“Our finding that a reduced proportion of fibroblast-like cells was induced to express Epo-TAg in injured kidneys, and that more of these cells could be recruited by more severe stimulation is consistent with previous observations that rodents and humans with renal failure produce significant amounts of erythropoietin from the kidney and increase erythropoietin production in response to severe anemia or hypoxia [19—23]. It indicates that the cells are neither destroyed nor rendered completely refractory to stimulation. Rather, they have an apparently altered threshold for gene expression. …”
(It should perhaps be noted that references 19-23 do not include Kato or Ross).
174. Kato showed that Epo production could be stimulated either by hypoxia or by acute bleeding in patients with end-stage renal disease (ESRD). Systemic hypoxia was shown to increase Epo in the blood to up to 24.6 times the normal level. As it is put in the abstract:
“These data suggest that the ability of the Epo production is well preserved in ESRD, indicating that acute hypoxic stimuli provoke a significant increase in serum Epo.”
Kato suggests that one explanation of this effect may due to enhanced production in the liver, but as Prof Haase explained, Maxwell provides direct evidence that it is due to the activity of kidney cells.
175. The message of Ross is aptly, and sufficiently for present purposes, summarised in the title of the paper.
176. In cross-examination, Prof Winearls agreed that, if anyone had educated themselves in the question of the production of Epo in patients with damaged kidneys, they would have been aware, from Maxwell and Kato, that the damaged kidneys could be stimulated to produce more Epo. Thus the evidence of both experts is consistent that this would have been common general knowledge.
177. A point was put to Prof Haase in cross-examination that had not been foreshadowed in any of Prof Winearls’ evidence (written or oral), namely that this effect was transient. Nothing in Maxwell or Ross was relied upon as supporting this, but it was pointed out that Kato reports that, out of a group of eight patients with hypoxemia (due to pulmonary edema or pneumonia), serum Epo returned to about the normal level within 1-2 days “despite continued hypoxemia in 3 patients whose serial blood samples were obtained (data not shown)” (page 649). Moreover, it states in the discussion section at page 650 that this is consistent with a prior report and refers to “the transient response to hypoxic stimuli”. This is not a point picked up in the abstract, however, which simply says that the serum Epo level in all eight patients “declined to or near the normal level after recovery from acute hypoxic stress”.
178. More importantly, perhaps, it was pointed out to Prof Haase that Schrier and Gottschalk, Diseases of the Kidney (vol III, 6th ed, Little Brown, 1997), an extract from which had been included by the Defendants in a bundle of documents for Prof Winearls’ cross-examination, states at 2583:
“Diseased kidneys are incapable of augmenting erythropoietin production chronically in response to an appropriate anemic hypoxic stimulus, yet an acute blood loss can transiently increase erythropoietin levels [47, 55]. The reason for the inability of diseased kidneys to sustain augmented production in response to chronic anemic ‘hypoxia’ is unknown. Although little data are available on sequential erythropoietin levels with progressive renal failure, erythropoietin production in such anemic patients is probably also inadequate.”
(Reference 47 is Ross.)
179. Prof Haase accepted that this reflected the common general knowledge, but he pointed out that a similar transient response was observed in normal persons at high altitude. He made the same point when asked about the increase in Epo levels in the three hypoxic patients in the Kato paper: “It is temporary, but the Epo response is temporary in the normal patient as well.”
180. In those circumstances I conclude that the increase in Epo production in response to hypoxia was thought to be a transient one despite the absence of supporting evidence from Prof Winearls. The role that this information would play in the skilled nephrologist’s thinking is a separate question which I will consider below.
HIF
181. The Claimants contend that the skilled nephrologist would not have any significant knowledge of HIF, whereas the Defendants contend that the skilled nephrologist would have a very good knowledge of the biochemistry of the HIF pathway.
182. In resolving this issue, the starting point is that I do not understand it to be in dispute that the clinical nephrologist would have little knowledge of HIF. That is evidenced by the fact that it is not mentioned in textbooks such as R.J. Johnson and J. Freehally, Comprehensive Clinical Nephrology (2nd edition, Mosby, 2003). That is beside the point, however. What matters is what the pre-clinical researcher would have known - the “research scientist with knowledge of the pathophysiology of anaemia caused by kidney disease” in Prof Winearls’ words.
183. Prof Winearls opined in paragraph 82 of his first report that:
“Although a few nephrologists may have been working on oxygen sensing or related projects in a research setting (as was the case for example in Peter Ratcliffe’s research group), the vast majority of practising nephrologists would likely have been unaware of the HIF system and it would not have formed part of the CGK of the Skilled Clinical Nephrologist [emphasis added].”
184. In paragraph 8 of his third report, replying to Prof Haase’s first report, Prof Winearls said that:
“I remain of the opinion set out in Winearls 1. For the reasons I set out there, details concerning the molecular pathways involved in renal anaemia were not issues that occupied the mind of the notional Skilled Clinical Nephrologist at the Priority Dates and the sort of research being carried out by the Semenza, Ratcliffe and Kaelin groups would have been seen as ‘fundamental science’ without immediate clinical application [emphasis added]”
185. It can be seen that in these passages Prof Winearls appears to have been focussing his mind on the knowledge of the clinical nephrologist as opposed to that of the pre-clinical researcher. Consistently with that, the sources of common general knowledge identified by Prof Winearls in his first report were clinical textbooks, four journals of interest to clinicians and clinical treatment guidelines. This reading of his evidence was confirmed by what he said in cross-examination.
186. Prof Winearls accepted that, by 2001, a lot of work had been done on HIF and its connection with Epo. He also accepted that, as Prof Haase had explained in his third report, a whole edition of Kidney International (a journal which was among those listed by Prof Winearls as being read regularly by the skilled nephrologist) in February 1997 had been devoted to hypoxia, and a number of the articles discussed HIF and the fact that HIF was the mechanism by which hypoxia induced Epo. When asked why, in those circumstances, he had said that the vast majority of practising nephrologists would likely have been unaware of the HIF system, Prof Winearls explained (emphases added):
“Clinical nephrologists concentrate on a very large number of subjects, and this was a mechanistic concept and research, which was very basic, and mostly published in scientific journals, though in that particular issue in 1997 Peter Ratcliffe described Epo as a model of oxygen-sensing. However, I think because it was in Kidney International in a single issue entitled ‘Hypoxia’ does not mean the general nephrologist would have read that. They tend to read things that are of immediate interest to them and their practice. If you were researching anaemia, you might well have read that , and, of course, because Peter Ratcliffe was a UK nephrologist originally, we had kept up with this particular story, but I do not think the ordinary nephrologists were as interested as you are implying, in the nuts and bolts of how the story had eventually been put together.”
187. Prof Winearls went on to accept that “a skilled clinical nephrologist with an interest in anaemia research would certainly have known about HIF”. Furthermore, he explained that his view of the way in which the skilled team would work was that the clinical nephrologist “would be educated by the very skilled [person] who he is working alongside” i.e. the pre-clinical researcher.
188. Consistently with Prof Winearls’ approach, the cross-examination of Prof Haase was essentially directed to establishing that HIF biology was not part of the common general knowledge of a clinical nephrologist. No real attempt was made to challenge Prof Haase’s evidence that it would be known to the pre-clinical researcher. In particular, no challenge was made to Prof Haase’s evidence that real teams in the field - not only those led by Ratcliffe, Kaelin and Semenza, but also others he identified - had significant knowledge of pre-clinical research. Nor was there any challenge to Prof Haase’s evidence that Epo and HIF had been inextricably linked since HIF had first been identified (by Semenza and G.L. Wang in 1992) in the context of its regulation of the Epo gene.
189. Accordingly, I conclude that the pre-clinical researcher, and hence the skilled nephrologist, would have known the information set out by Prof Haase. This may be summarised as follows.
190. HIF plays an essential role in oxygen homeostasis. It activates the transcription of multiple genes that mediate adaptive cellular responses to hypoxia. As noted above, HIF stands for Hypoxia Inducible Factor. In fact, the response to hypoxia is not “induction” in the sense of making new HIF protein, but rather the stabilisation of one subunit of the HIF complex, HIF-α (also referred to as HIFα, two iso-forms of which were known in 2001, HIF-1α and HIF-1β). Under normoxic conditions, HIF-α is rapidly degraded, whereas hypoxia blocks this degradation, allowing it to translocate to the nucleus, associate with its partner HIF-β (also known as ARNT) and carry out transcriptional regulation. The way that HIF-α is marked for degradation is by covalent modification. One of its proline residues becomes hydroxylated in an oxygen-dependent reaction by HIF-PHs, causing it to be recognised by the VHL protein complex, resulting in its degradation by the proteasome.
191. The two pathways (degradation in normoxia and functioning as a transcription factor in hypoxia) are conveniently shown in the following figure taken from the Nobel Prize website (which is of course recent, but reflects what was known at the time, and which refers to the HIF-1α isoform).
192. When oxygen levels are low (hypoxia), HIF-α is protected from degradation and accumulates in the nucleus, where it associates with ARNT and binds to specific DNA sequences (HRE) in hypoxia-regulated genes (1). This activates the transcription of those genes, including the Epo gene. At normal oxygen levels, HIF-α is rapidly degraded by the proteasome (2). Oxygen regulates the degradation process by the addition of hydroxyl groups (OH) to HIF-α (3). The VHL protein can then recognise and form a complex with HIF-α leading to its degradation in an oxygen-dependent manner.
193. In addition to oxygen, HIF-PHs utilise 2-OG for the hydroxylation of HIF-1α in an iron-dependent manner. By December 2001, three HIF-PHs had been identified, known as PHD1, PHD2 and PHD3.
194. Th e role of HIF prolyl hydroxylation in the regulation of the HIF-α subunit was independently discovered by Kaelin and Ratcliffe, and published in two seminal, back-to-back papers in the 20 April 2001 edition of Science : M. Ivan et al , “HIFα Targeted for VHL-Mediated Destruction by Proline Hydroxylation: Implications for O2 Sensing”, 292(5516), 464-468 (“Ivan”) and P. Jaakkola et al . “Targeting of HIF-α to the von Hippel-Lindau Ubiquitylation Complex by O2 -Regulated Prolyl Hydroxylation” 292(5516), 468-72 (“Jaakkola”). These were accompanied by a commentary by H. Zhu and H. F. Bunn, “How do cells sense oxygen?”, 292(5516), 449-451 drawing attention to the significance of this work. It is convenient to note here that these publications were followed six months later by a further paper from the Ratcliffe group, namely Epstein.
195. Jaakkola also reported the testing of a series of 2-OG analogues that had previously been shown to act as competitive inhibitors of collagen prolyl-4-hydroxylase, which plays a central role in collagen biosynthesis by catalysing the formation of 4-hydroxyproline in collagens (“P4H”). Complete inhibition of HIF prolyl hydroxylation was observed with N-oxalylglycine (NOG), which could be competed with by 2-OG. To assess the potential for inhibition of HIF-PH activity to regulate the HIF system in vivo , a cell-penetrating (i.e. pro-drug) ester of NOG, dimethyl-oxalylglycine (DMOG), was used. Exposure of cells to DMOG was found to result in rapid induction of HIF-α.
196. In addition to regulating the expression of Epo, the HIF transcription factor was known to regulate the expression of a number of other genes involved in physiological responses to hypoxia, as shown in the following figure which Prof Haase reproduced from a short review by Semenza (“HIF-1, O2 and the 3 PHDs: How Animal Cells Signal Hypoxia to the Nucleus”) which was published in the same issue of Cell as Epstein.
197. As can be seen from this figure, the genes whose expression was known to be regulated by HIF included a number of genes involved in iron homeostasis, namely those for transferrin (the iron-binding protein of the blood), transferrin receptor (to which transferrin binds) and ceruloplasmin (which converts Fe2+ to Fe3+ , allowing serum iron to bind to transferrin) in addition to Epo.
Cobalt salts
198. The third area of dispute concerns cobalt salts. As I have already indicated, the Claimants expended quite a lot of energy in attacking part (but only part) of Prof Haase’s evidence on this topic. In addressing this issue, it is important to distinguish between five different questions.
199. The first concerns the therapeutic use of cobalt chloride to treat anaemia of CKD. It is clear that cobalt chloride was used for this purpose at least to some extent from the 1940s to the 1970s, when it was discontinued due to concerns over toxicity. Prof Haase thought that this would have been common general knowledge. In support of this, he relied upon Corner, Berk, Kriss and (rather more persuasively) an editorial in The Lancet published on 3 July 1976 (which cites Kriss and a fair number of other papers, but not Corner or Berk). Prof Winearls’ opinion was that this would not have been common general knowledge to the clinical nephrologist, and he gave cogent reasons for this: many clinical nephrologists had trained after the 1970s; it was not mentioned in clinical sources; he personally had never heard of cobalt being prescribed for anaemia (although he had been aware of the historical usage in 2001); and there was no real need for it once r-HuEpo became available. As he made clear in paragraph 37 of his fourth report, Prof Haase did not dispute this. He maintained, however, that in his opinion it was likely to be something that the pre-clinical researcher would have come across. I am not persuaded that it was common general knowledge even to the pre-clinical researcher, however. The materials relied on are simply too old and obscure.
200. The second question concerns the fact that Epo production by the kidneys can be stimulated by (among other things) the administration of cobalt salts. Prof Haase and Prof Winearls were agreed that this was common general knowledge. Prof Winearls explained that “everybody” knew that cobalt was an Epo stimulant because that was how the kidneys were identified as the main source of Epo (referring to Dr Eugene Goldwasser’s ground-breaking work in 1957 showing that the only thing that stopped Epo being stimulated by cobalt administration to rats was the removal of the kidneys). As Prof Haase explained, there were other reasons why this was well known as well.
201. The third question concerns the use of cobalt salts as a research tool. Prof Haase’s opinion was that the use of cobalt to stimulate the production of Epo in pre-clinical research was widespread. He gave a number of examples of this, including papers by M.A. Goldberg et al in 1987 and 1988 and by Semenza and Wang in 1993 and 1995 (three of which were published in Science and Proc Natl Acad Sci USA ). By contrast with Corner, Berk and Kriss, these are not old or obscure papers. On the contrary, Prof Haase gave unchallenged evidence that the 1988 Goldberg paper had been cited 610 times by the end of 2001. He also pointed out that cobalt was used in both Ivan and Jaakkola. Prof Winearls gave no contrary evidence, and I am satisfied that the use of cobalt salts as a research tool was common general knowledge.
202. The fourth question concerns the fact that cobalt chloride stimulates the expression of Epo by the induction of HIF-α. This was demonstrated by Wang and Semenza, “Purification and characterization of hypoxia-inducible factor 1”, J. Biol. Chem ., 270(3), 1230-7 (1995). Prof Winearls’ comment on this paper was that there was no “clinical focus” and nothing to suggest that “cobalt was a suitable treatment for anaemia”. Again, therefore, he was looking at it more from the perspective of the clinical nephrologist than that of the pre-clinical researcher. Prof Haase did not take issue with Prof Winearls’ comment, but maintained that the fact that cobalt chloride stimulates the expression of Epo by the induction of HIF-α would have been well known to the pre-clinical researcher. That evidence was not challenged, and I accept it. (This is also consistent with my conclusion as to the pre-clinical researcher’s knowledge of HIF.)
203. The fifth question is whether it was generally accepted in December 2001 that cobalt stimulated Epo by the same mechanism as hypoxia. Prof Haase accepted in cross-examination that this was debated. Accordingly, it was not common general knowledge that it did, as opposed to might do.
The documents cited in the Family A Patents.
204. Before turning to consider the interpretation of the claims of the Family A Patents, it is convenient first to consider a more general question which bears upon a number of issues in the case. This is whether, and if so to what extent, the skilled team, and in particular the skilled medicinal chemist, would read the documents cited in the Family A Patents. The Claimants’ case on this question evolved over time.
205. Dr Bhalay stated in his first report that FibroGen’s solicitors had asked him to review the six papers cited in [0068]-[0069] of EP 823 (corresponding to [0075]-[0076] of WO 997) and to review US 305, US 995 and US 730 (which are among the patents cited in [0074] and [0076]-[0077] of EP 823 corresponding to [0080] and [0082]-[0083] of WO 997). He did not suggest that he would otherwise have done so, nor did he suggest that a skilled medicinal chemist reading EP 823 in December 2001 would have done so. Still less did he suggest that the skilled person would have read any of the other documents cited in the Family A Patents.
206. Prof Ward annexed to his first report two annexes commenting on the 15 patents and applications cited in WO 997 at [0078]-[0088] and on the six papers cited in WO 997 at [0075]-[0076]. In Annex I he explained that he had been asked to assume that the compounds of these patents and applications were incorporated by reference into WO 997 as appropriate and to address what, if anything, the skilled medicinal chemist would have learnt if the medicinal chemist had turned them up. In Annex III he explained that he had been asked to address what, if anything, the medicinal chemist would have learnt if the medicinal chemist had turned up the six papers. He did not suggest that a skilled person reading the Family A Patents in December 2001 would in fact have turned up any of these documents.
207. Dr Bhalay commented in his third report on Prof Ward’s Annex III. He also said, in the context of a discussion about Compounds C-K, that the Family A Patents “cross-refer” to the six papers in question. He did not comment on Prof Ward’s Annex I. Nor did he say anything about whether the skilled person would follow up any of the references. In his fourth report Dr Bhalay commented further on Majamaa 1984 and Bickel, but again did not address the question of whether the skilled person would turn up these papers.
208. Prof Ward commented in his third report on what Dr Bhalay had said in his first report about the six papers and about US 305, US 995 and US 730. In the former context Prof Ward referred to what the medicinal chemist would have noted “if he had read” one of the papers. In the latter context Prof Ward noted that WO 997 provided “very little guidance or direction” as to why the 15 patents cited at [0078]-[0088] “might be important”.
209. In their opening skeleton argument the Claimants discussed the disclosure of the Family A Patents by reference to WO 997. In the context of insufficiency, the Claimants said that the specification “refers the Skilled Person to a series of publications describing known small-molecule inhibitors of collagen prolyl-4-hydroxylase”, which must be a reference to [0075], and that it “also refers the Skilled Person to several US patent applications which provide further examples of heterocyclic carboxamides that are small molecular inhibitors of prolyl-4-hydroxylase”, which seems to be a reference generally to [0078]-[0088]. The Claimants also mentioned that in [0076] “reference is made to” Majamaa 1984 and Majamaa 1985. The Claimants did not address the question of which documents cited in the Family A Patents, if any, the skilled team would read or why.
210. The Defendants stated in their skeleton argument that they did not accept that the skilled person would review the documents cited in the Family A Patents considered by Dr Bhalay. They also made the point that, if the skilled person had gone beyond the lengthy specification to consider the numerous cited prior publications and patents, there was no basis for the selective treatment of the cited prior art undertaken by Dr Bhalay.
211. During the course of counsel for the Claimants’ oral opening submissions I was taken briefly through WO 997 as being representative of the disclosure of the Family A Patents. When he came to [0075], counsel noted the references to the five papers cited there and said “obviously we will be … exploring these in cross-examination”. I queried whether the statement “all incorporated by reference herein in their entirety” was reproduced in the granted Patents. Counsel said he would check. He did not address the question of which documents cited in the Family A Patents, if any, the skilled team would read or why.
212. In cross-examination Dr Bhalay confirmed that the only reason why he had read the six papers cited in [0075]-[0076] of WO 997 was because he had been asked to. Furthermore, Dr Bhalay accepted that the skilled medicinal chemist would have a choice as to whether to turn up the documents referred to in [0068]-[0069] of EP 823 (corresponding to [0075]-[0076] of WO 997) and that it would be reasonable not to do so. He added, however, that “if you were doing a due diligence, I think you would be compelled to actually follow up these papers”. Similarly, Dr Bhalay confirmed that the only reason why he had read US 305, US 995 and US 730 was because he had been asked to. He also accepted that the skilled person would have a choice as to whether to turn up any of the cited patents. When pressed as to whether it would be reasonable not to do so, Dr Bhalay said that he would do “a thorough job” and spread the work among other members of the team with (unspecified) instructions as to what to look for. He was unable to think of any reason why the skilled person would single out US 305, US 995 and US 730 for review.
213. Prof Ward’s evidence in cross-examination was that he thought that, although it depended on the time available and the information coming from the skilled nephrologist, the skilled medicinal chemist would be more likely to follow up references which were cited as disclosing particular compounds for use in the invention. Furthermore, he agreed that it would be reasonable for the medicinal chemist to read the references in [0068]-[0069] of EP 823, but focussing on the chemistry, that is to say, the compounds and their activities. No reason was put to him as to why the skilled person would single out US 305, US 995 and US 730 for review from the other cited patents and applications.
214. In their written closing submissions the Claimants submitted that:
“.. the skilled person is deemed to read with interest the papers and patents [to which reference is made in the specification] which are expressly advanced as being relevant to the sufficiency of the patent. In the alternative we contend that the skilled person would in the context of this case read those papers and patents.”
The Claimants did not, however, identify which papers and patents would be read on this basis, beyond making it clear that they would include the papers cited in [0075]-[0076] of WO 997.
215. The Defendants submitted in their written closing submissions that the skilled medicinal chemist would not necessarily review either the papers cited in [0075]-[0076] of WO 997 or US 305, US 995 and US 730, relying upon the evidence of Dr Bhalay.
216. During counsel for the Claimants’ oral closing submissions I asked whether it was the Claimants’ case that the skilled team would read all 47 papers and books and 26 patents and patent applications cited in EP 823. After a great deal of equivocation during which he said at least twice that the answer was no, counsel’s final submission was that that the skilled team would read all the cited publications “for the purpose for which they are identified”, alternatively would read the publications cited in [0068]-[0069] and [0078] of EP 823 since those paragraphs refer to compounds for use in the invention.
217. Astonishingly, neither side referred during the course of the trial to the statement made in [0041] of WO 997, and the corresponding paragraphs of the granted Patents, about the cited publications. I only noticed it when writing this judgment.
218. In my judgment there are two problems with the submissions advanced in the Claimants’ written closing submissions. First, there is no principle of law that the skilled team are deemed to read all documents cited in a patent. It is a context- and fact-dependent question, and thus it depends firstly upon the wording of the specification and secondly on the evidence. Secondly, none of the documents cited in the specifications of the Family A Patents is “expressly advanced as relevant to the sufficiency of the patents”.
219. Turning to the submissions made in oral closing submissions, the first submission amounts to saying that the skilled team would read all the cited publications. But as I have said there is no principle of law that leads to that conclusion. Nor is there any evidence to support it. In the absence of any evidence (or even submissions) addressing it, I consider that the statement in [0041] of WO 997 would be interpreted by the skilled reader simply as instructing them that the cited publications contain information which might be useful in practising the invention.
220. As for the second submission, this appears to proceed on the assumption that the skilled team, or at least the medicinal chemist, would follow up those publications that are cited as disclosing compounds for use in the invention. But that approach does not lead one to the publications cited in [0068]-[0069] and [0078] of EP 823 (corresponding to [0075]-[0076] and [0084] of WO 997), since EP 823 also cites 15 patents and patent applications as disclosing compounds for use in the invention. In my view the skilled reader would regard these references as simply providing sources of compounds which could be used if desired. In any event, no reason was identified as to why, if looking for compounds for use in the invention, the medicinal chemist would go to the cited papers and not the cited patents and publications.
221. My conclusion based on the texts of the granted Patents and the evidence of the experts is that the skilled team would not necessarily follow up any of the cited publications. They would appreciate that there were a large number of them, and that the specification gives little, if any, direction as to which would be worth following up. They would also appreciate that the papers appear to be cited as providing scientific support for statements made in the specification, and that the cited papers would inevitably refer to a considerable number of other papers, leading to the question of how far they should go in reviewing such material. (Indeed, counsel for the Claimants put it to Prof Ward, and submitted in closing, that the skilled medicinal chemist would follow up a reference in Friedman even though no such suggestion was ever advanced or assumed by Dr Bhalay.) Whether they would review the cited publications would therefore depend in part on the time and resources available, which would vary between skilled teams. In my view they would be most likely to read Majamaa 1984 and Majamaa 1985, since these papers are cited in a manner that suggests that they may shed light on what is meant by “structural mimetics of 2-OG” and, as discussed below, the skilled reader would find that expression unclear. Even if the medicinal chemist did follow up all the six papers cited in [0075]-[0076] and [0084] of WO 997, what they would be looking for would be information about the chemistry and activities of specific compounds discussed in those papers, rather than for explanations of such activity. There is no basis whatsoever for thinking that the skilled team would single out US 305, US 995 and US 730 from the other cited patents and applications for review.
222. Assuming that, contrary to the conclusion I have reached, the skilled medicinal chemist would review all six papers cited in [0075]-[0076] and [0084] of WO 997, what would they learn? I will consider them in chronological order. I will also consider two other papers which, for reasons that will appear, the skilled person would also be likely to turn up if they got that far.
Majamaa 1984
223. As is explained in the second paragraph of the introduction, the work reported in this paper is premised on the stereochemical mechanism suggested by two of the authors, M. Hanauske-Abel and V. Günzler, in a prior paper in which the binding of 2-OG to P4H was suggested to occur via a positively-charged amino acid residue (subsite I) and an enzyme-bound ferrous ion (subsite II). Hanauske-Abel and Günzler also suggested a sequence of subsequent events in which molecular oxygen binds, leading to the decarboxylation of 2-OG (giving rise to succinate), the formation of a ferryl (Fe4+ ) ion and hydroxylation of the proline residue in the polypeptide substrate. This hypothesis indicated that the binding of the C5 carboxyl group of 2-OG to subsite I was a pre-requisite for the subsequent reactions.
224. Figure 1 of Majamaa 1984 shows the proposed binding slightly modified in the light of the data reported in Majamaa 1984. I reproduce this below as marked up by the Claimants for ease of comprehension: the 2-OG backbone is in yellow, molecular oxygen in blue and the ferrous ion shown as a pink sphere.
225. As Majamaa 1984 explains in the third paragraph, the purpose of the work was to study the inhibitory characteristics of substances likely to compete with 2-OG at the postulated subsites I and II. To that end, two series of “structural analogs of 2-oxoglutarate” were prepared, 11 aliphatic and 13 aromatic, and tested as inhibitors of P4H, including whether inhibition was competitive with respect to 2-OG and Fe2+ .
226. Majamaa 1984 reports at pages 241-242 that, with the exception of compound 15 (pyridine 2,6-dicarboxylate), all the analogues inhibited competitively with respect to 2-OG and non-competitively with respect to iron. This includes compounds which the skilled medicinal chemist would see as structurally related to 2-OG, although lacking the capacity for bidentate iron chelation (for example compounds 5, 6, 7 and 8).
227. Majamaa 1984 provides the inhibition constant for each compound in Table 1. Pyridine 2,5-dicarboxylate (compound 14) is more potent than pyridine 2,4-dicarboxylate (compound 13) by a factor of 2.5 (Ki values of 0.008 mM and 0.002 mM respectively). The remaining compounds all inhibit P4H, albeit with higher Ki constants.
228. In the discussion section starting at page 243 the authors conclude that P4H has three distinct subsites for binding 2-OG: the two previously proposed and an additional hydrophobic binding subsite III.
229. The requirement for domain I (the C5 carboxyl group in 2-OG) was confirmed, but the authors were surprised to find that the optimal location for this domain in the aromatic compounds was found at ring position 5 rather than position 4 (i.e. pyridine 2,4-dicarboxylate was less potent than pyridine 2,5-dicarboxylate), since the latter would seem to correlate more closely with 2-OG in its most favourable conformation.
230. The requirement for an intact domain II (that thought to chelate the enzyme-bound iron) was also confirmed. The authors conclude that it is plausible that the enzyme-bound ferrous iron becomes chelated by the two oxygen atoms in C1 and C2 moiety of 2-OG.
231. Comparison of the Ki value for benzene 1,4-dicarbonate (compound 23) with that for adipinate (compound 6) suggested the existence of subsite III which would preferentially interact with aromatic structures in the C3-C4 region of 2-OG and might contribute to the very low Ki values of pyridine 2,4-dicarboxylate and pyridine 2,5-dicarboxylate.
232. The authors draw attention at page 242 to the fact that pyridine 2,6-dicarboxylate (compound 15) differed from the other compounds “as its inhibition was competitive with respect to Fe2+ … and noncompetitive with respect to 2-oxoglutarate at low concentrations and nonlinear at high concentrations”. They then discuss the iron chelating properties of pyridine 2,6-dicarboxylate. They return to this subject in the discussion section at page 244:
“Pyridine 2,6-dicarboxylate differed from all the other compounds in its inhibition pattern. This compound acts as a terdentate ligand [17] and thus cannot chelate the enzyme bound iron which provides two cis-positioned sites for co-substrate binding only (Fig. 1). Nevertheless, pyridine 2,6-dicarboxylate can interact with free Fe2+ , thus competing with the enzyme for the metal.”
233. It is common ground that Majamaa 1984 amounts to an SAR analysis of the components of 2-OG and of constrained analogues of 2-OG that are important for binding. The question is what, if anything, more the skilled medicinal chemist would get out of it.
234. Prof Ward and Dr Bhalay agreed that the structural diversity of the compounds tested in Majamaa 1984 is very small and very closely clustered around the natural co-factor (2-OG).
235. Accordingly, Prof Ward’s evidence was that the predictive capacity of the SAR described in Majamaa 1984 for other classes of molecules was very limited. Dr Bhalay did not really disagree. He characterised the work as a “very, very early, very rudimentary SAR”, and when asked whether Majamaa 1984 enabled the medicinal chemist to make predictions about the three-dimensional shape of the P4H active site, he said that additional work would be required.
236. Consistently with this, Dr Bhalay agreed with Prof Ward’s evidence that Figure 1 of Majamaa was purely schematic and would not be understood to represent the actual available space or chemical characteristics of the active site of P4H. Dr Bhalay agreed that this applied as much to those parts of Figure 1 that are drawn in and around the iron-chelating motif as to the substrate recognition site.
Majamaa 1985
237. This paper compares the inhibitory activity of the same analogues of 2-OG as those described in Majamaa 1984 against P4H, prolyl 3-hydroxylase (“P3H”) and lysyl hydroxylase (“LH”). Although inhibitors that were active against P4H were generally active against the other two enzymes, there were differences. Thus Ki values reported in Table 1 on page 132 show that the relative potencies of pyridine 2,5-dicarboxylate and pyridine 2,4-dicarboxylate differ significantly between P4H (where the former is 2.5 times more potent than the latter) and P3H (where the latter is five times more potent than the former).
Myllyharju and Kivirikko 1997
238. This is not one of the six papers cited in [0075]-[0076] of WO 997, but it is cited in [0074]. Dr Bhalay agreed that, if the skilled medicinal chemist reviewed the papers cited in [0075]-[0076], they would also review those cited in [0074]. Moreover, the skilled person would have a particular reason to review this one because it is by the same authors as one of the six cited papers.
239. In this paper, the authors use site-directed mutagenesis to identify three amino acid residues in P4H which are likely to provide the ligands for binding Fe2+ , the residue required for binding the C5 carboxyl of 2-OG and an additional critical residue in the active site.
240. Dr Bhalay agreed that this additional information (the identity of specific amino acid residues) provided the medicinal chemist with nothing in terms of the three-dimensional shape of the binding pocket for 2-OG or the collagen substrate recognition site. He would have asked a computational chemist to try to create a 3D image of the catalytic site. But there is no evidence as to the basis upon which such an image could have been created or how much work that would have entailed or what the exercise would have revealed.
Myllyharju and Kivirikko 1998
241. This is a mini-review of P4H enzymes, and it includes a schematic representation of the hydroxylation reaction by reference to the amino acid residues identified in Myllyharju and Kivirikko 1997.
242. Under the heading “Nonpeptide inhibitors” at page 362, the authors say:
“It has been pointed out ... that pyridine 2,5-dicarboxylate and 2-oxoglutarate may bind to P4H in different ways, and thus the data concerning modifications of this compound must be interpreted with caution (Cunliffe et al , 1992).”
243. Dr Bhalay agreed that, in the light of that cautionary note, the skilled medicinal chemist would have good reason to turn up the cited paper, C.J. Cunliffe et al , “Novel Inhibitors of Prolyl 4-Hydroxylase 3; Inhibition by the Structural Analogue N-oxaloglycine and Its Derivatives”, J Med Chem , 35, 2652-2658 (1992) (“Cunliffe”).
Cunliffe
244. Cunliffe reports on the inhibition of P4H by NOG and a number of its derivatives. The authors observe at page 2655-2656 that increasing the distance between the terminal carboxyl groups (referred to as “homologation”) of NOG and 2-OG has a detrimental effect on inhibitory activity whereas the analogous modification to pyridine 2,5-dicarboxylate is not attended by any significant change in activity. At the end of the results and discussion section on page 2656, the authors conclude:
“… It seems very likely that oxaloglycine (3) and 2-oxoglutarate (1) bind to prolyl hydroxylase in a closely analogous way. Thus, the discovery that the structure-activity relationships in oxaloglycine series differ so completely from those in the pyridine-2,5-dicarboxylic acid series calls into question the published assumptions22 about the nature of the pyridine-2,5-dicarboxylic acid (2) binding site on prolyl hydroxylase and suggests caution in interpreting the physical reality underlying the observation of strictly competitive inhibition.”
245. Reference 22 is Majamaa 1984. As Dr Bhalay agreed, this illustrates the well-known point that small changes to compounds can have unpredictable effects on biological activity and the need to carry out further work to understand better the relationships between structure and function. ( Dr Bhalay also accepted that the same point was illustrated by some of the other papers.)
Bickel
246. There is no dispute that, if the skilled medicinal chemist did review the papers cited in EP 823, they would find that Bickel is the only one that discloses a compound which falls within Formula (I), namely a compound identified as S 4682.
247. The authors state at page 408:
“The novel P4H inhibitor, S 4682, directly inhibited purified P4H, being substantially more potent than the structurally related 2,4-PDCA [pyridine 2,4-dicarboxylate] and 2-PCA [pyridine 2-carboxylic acid] at the enzyme level. Based on the IC50 values, S 4682 was 42 times more potent than 2,4-PDCA and 580 times more potent than 2-PCA (Table 1)”.
Dr Bhalay agreed that the skilled medicinal chemist would see this as very significant.
248. Bickel illustrates the structural similarities between 2-OG, pyridine 2,4-dicarboxylate and S 4682 in Figure 1, which I reproduce below.
249. Dr Bhalay agreed that, based on the structure of S 4682, the medicinal chemist would assume that the pyridyl nitrogen and adjacent carbonyl were effecting bidentate chelation of iron at the active site. They would also note the presence of a carboxyl group equivalent to that on the C5 of 2-OG, positioned the same distance away from the chelating motif.
250. The authors state at page 407:
“S 4682 differs from other structural analogs of 2-oxoglutarate, such as pyridine 2-carboxylic acid (2,4-PDCA) and pyridine 2-carboxylic acid (2-PCA) by inhibiting P4H in a noncompetitive fashion with respect to the cosubstrate, 2-oxoglutarate (Fig 3A).”
251. In his first report Dr Bhalay specifically noted this statement and did not suggest that the skilled medicinal chemist would question it (although he did mention that no explanation was provided by the authors). Nor did he suggest this when discussing Bickel in his fourth report. This was consistent with the statement that Dr Bhalay made early on in his cross examination that the medicinal chemist would take in good faith the conclusions made by the biochemists within the wider project team and that it would be extremely rare for the medicinal chemist to challenge those conclusions. When questioned about Bickel, however, he was reluctant to accept the statement quoted above. He did not offer any reason to doubt the methodology, the results or the presentation of the data. Rather, the gist of his oral evidence was that the medicinal chemist would be intrigued by the results and would ask for input from an enzymology specialist. In my judgment the skilled medicinal chemist would proceed in the manner suggested by Dr Bhalay’s written evidence and would take the statement at face value.
Friedman
252. This paper investigates the activity of two known P4H inhibitors in Caenorhabditis elegans (the nematode worm). As the skilled reader would appreciate, the compounds in question are ester-based pro-drugs. Neither side suggested that the skilled medicinal chemist would get much that was useful out of Friedman.
253. As noted above, however, despite the fact that it was nowhere referred to by Dr Bhalay, the Claimants contend that the medicinal chemist would follow up reference 42 of the 50 in Friedman, namely V. Günzler and K. Weidmann, “Prolyl 4-Hydroxylase Inhibitors” in N. A. Guzman (ed), Prolyl Hydroxylase, Protein Disulfide Isomerase, and Other Structurally Related Proteins (Marcel Dekker, 1998) at pages 65-95. Indeed, in the Claimants’ written closing submissions, Günzler and Weidmann was identified as one of three “key publications” (together with Majamaa 1984 and Majamaa 1985) that the medicinal chemist would read. Counsel for the Claimants submitted that Prof Ward had accepted that the medicinal chemist would read Günzler and Weidmann, but I disagree. In the first place, the witness did not accept that the medicinal chemist would go beyond the abstract of Friedman, because it was a detailed biological paper in an organism of no interest to them. What the witness then accepted was that, if he had to read Friedman, and if he got to the last paragraph of Friedman, he would “look at what the reference was” cited in that paragraph. I am not satisfied that the medicinal chemist would read Günzler and Weidmann even if they got as far as Friedman.
Franklin
254. The authors describe an SAR investigation of a series of phenanthrolinone compounds as inhibitors of P4H in three assays. Two of the compounds (1 and 5) are said at page 335 to be competitive inhibitors of 2-OG. Dr Bhalay agreed that the skilled medicinal chemist would be uncertain whether, and if so how, these compounds would chelate iron.
US 730
255. For completeness, even if the skilled medicinal chemist did read US 305, US 995 and US 730, I do not understand it to be in dispute that they would find that the only one which contained any useful information about the activities of the compounds disclosed was US 730 (see further below on this point).
256. Table 1 of US 730 sets out the activities of 15 compounds. The text states at column 9 lines 44-43 that the results are for “antifibrotic activity” as measured in an animal model described at column 9 line 62 - column 10 line 8. It is common ground, however, that the skilled reader would deduce that this must be a mistake and that the results are actually for inhibition of P4H as measured in an assay referred to at column 9 lines 36-39.
257. Some of these compounds correspond to the exemplified isoquinolines in EP 823:
US 730 (IC50 μM)
EP 823
Example 1 (0.12)
Compound K
Example 3 (0.79)
Compound J
Example 9 (2.30)
Compound C
Example 13 (9.30)
Compound I
Example 15 (0.66)
Compound H
258. Example 15 (Compound H) is shown to be more potent than Example 13 (Compound I) by a factor of around 14. By contrast, the relative potencies of Compounds I and H are reversed in Example 1 of EP 823. Figure 1 shows that Compound I induces more Epo than Compound H by a factor of about 2. This is another illustration of the unreliability of predictions about inhibitory activity of individual compounds, even between related enzymes.
Construction of Family A claims
259. There are two issues of construction of the Family A claims. The first concerns the definition of Formula (I) in claim 19A of EP 823 and claim 2 of EP 301. The second concerns the expression “structural mimetic of 2-OG” in claim 24A of EP 823 and claim 4 of EP 301.
260. Before turning to consider those issues, it is important to note a point that is common ground, which is that the claims require the therapeutic effects mentioned to be achieved. It is also common ground that the criteria for efficacy for this purpose are that indicated by the Examples in the specification, namely that the compound in question must inhibit HIF-PH in an appropriate biochemical (in vitro ) assay and induce the production of endogenous Epo in vivo in a suitable animal model (such as a mouse or rat). (I would have thought that it must also increase haematocrit in a suitable animal model, but this point was not explored in closing submissions and so I shall say no more about it.) The claims obviously cover using the compounds for the treatment of human patients, but neither side suggests that they require efficacy in humans to be demonstrated even to the level of a Phase II clinical trial.
Formula (I)
261. The issue concerns the definition of R2 in Formula (I). It is common ground that R2 may be “(C6 -C12 )-aryl”: see page 36 line 40 of EP 823 (as mentioned above, the lists of substituents in the definition of Formula (I) in granted claim 20 of EP 823 are reproduced in the Annex). The Claimants contend that the words “where an aryl radical may be substituted by 1 to 5 substituents selected from … halogen …” at page 37 lines 50-51 apply to (among other things) the option “(C6 -C12 )-aryl” at page 36 line 40, and hence halogen-substituted (C6 -C12 )-aryl is a permitted option for R2 . The Defendants dispute this.
262. It bears pointing out before proceeding further that the reason why this can be in dispute is because of the length and complexity of the lists of possible substituents in Formula (I), which makes it difficult to work out precisely which substituents are encompassed by the formula and which are not. Counsel for the Claimants reminded me that it is not appropriate to interpret a claim by a process of meticulous verbal analysis; but this claim forces the skilled person to wrap a cold towel round their head when trying to understand it.
263. Since this is a question of the interpretation of a chemical formula, the relevant skilled person through whose eyes it must be considered is the medicinal chemist. It was addressed by all three medicinal chemists in their evidence. As will appear, however, the issue of interpretation depends not on medicinal chemistry, but on the structure of the lists of substituents, and in particular the manner in which the relevant list is punctuated and laid out, and on the way in which certain substitutions are described, and in particular the fact that some groups are said to be “optionally substituted” without more. All three medicinal chemists agreed in cross-examination that there was no chemical reason why the substitution in question should or should not be permitted. It follows that their opinions as to the correct interpretation of Formula (I) are all inadmissible.
264. As I have just indicated, the interpretation of Formula (I) involves two main aspects. The first concerns the structure of the lists of the substituents. The second concerns the language in which possible substitutions are described.
265. The structure of the lists of the substituents . At the highest level, the demarcation between different parts of the definition of Formula (I) presents no difficulty. The various options for the substituents are identified as follows:
A page 34 lines 13-26:
B page 34 line 27 - page 35 line 40:
X page 35 line 41;
Q-R4 page 35 line 42 - page 36 line 34;
Y page 36 line 35;
R1 , R2 and R3 page 36 line 35 - page 39 line 3 (note that Y may be N or CR3 ).
266. In the case of at least A, B, R1 , R2 and R3 , the permitted options are separated by semi-colons. By way of example, there are four semi-colons embedded in the list of options for A at page 34:
line 13: semi-colon before “or (C1 -C4 ) alkylene”;
line 15: semi-colon before “(C1 -C6 )-alkylmercapto”;
line 19: semi-colon before “or by a substituted (C6 -C12 )-aryloxy”;
line 24: semi-colon before “or wherein A is -CR5 R6 ”.
267. It is common ground that these semi-colons in the definitions of A and B would be understood to delineate between different classes of substituent.
268. I do not understand it to be in dispute that the semi-colons in the definition R1 , R2 and R3 would be assumed to function in a similar way. In this case, however, there are three complicating factors. The first is that some of the semi-colons are followed by line breaks. The semi-colons and line breaks are as follows:
i) page 37 line 5: semi-colon before “CON(CH2 )h ''” - no line break;
ii) page 37 line 7: semi-colon before “a carbamoyl radical” - no line break;
iii) page 37 line 26: semi-colon before “or R* and R**”– no line break;
iv) page 37 line 29: semi-colon before “carbamoyloxy” - no line break;
v) page 37 line 50: semi-colon before “where an aryl” - no line break;
vi) page 38 line 20: semi-colon before “carbamoyloxy” - no line break;
vii) page 38 line 35: semi-colon after “(C7 -C16 )-aralkylsulfonyl” - line break;
viii) page 38 line 41: semi-colon after “ring” - line break;
ix) page 38 line 42: semi-colon after “ring” - line break;
x) page 38 line 41: semi-colon after “cinnoline” - line break;
xi) page 39 line 2: semi-colon after “above” - line break after “and”;
xii) page 39 line 3: semi-colon after “R3 ” - line break;
xiii) page 39 line 4: semi-colon after “8” - line break;
xiv) page 39 line 5: semi-colon after “(2f+1)” - line break;
xv) page 39 line 6: semi-colon after “3” - line break after “and”;
xvi) page 39 line 7: semi-colon after “7” - line break.
269. The second complicating factor is the definition of R1 , R2 and R3 includes two sub-formulae, (R) and (Id). I have already set out Formula (Id) in paragraph 125 above. Formula (R) is as follows:
270. The third complicating factor is that the definition of Formula (R) includes a definition of T. As will appear, part of the difficulty in resolving the issue of construction lies in determining where the definitions of R and of T end.
271. If the definition of R1 , R2 and R3 is considered as a whole, taking into account the semi-colons followed by line breaks, it appears that it is divided into six sections as follows:
i) “R1 , R2 and R3 are identical or different” at page 36 line 36 to page 38 line 35;
ii) “or wherein R1 and R2 , or R2 and R3 form a chain” at page 38 lines 36-39;
iii) “or wherein the radicals R1 and R2 , or R2 and R3 ” at page 38 lines 40-41;
iv) “or wherein R1 and R2 , or R2 and R3 form a carbocylic” at page 38 line 42;
v) “or where R1 and R2 , or R2 and R3 , together with” at page 38 lines 43-45; and
vi) “or wherein the radical R1 and R2 , together with the pyridine carrying them, form a compound of Formula Id” at page 38 line 46 to page 39 line 3.
272. Although at first blush it might be thought that, as both sides suggested, the definitions of f, g, x and h at page 39 lines 4-7 are part of the sixth section of the definition of R1 , R2 and R3 , this cannot be correct for two reasons. First, Formula (Id) does not itself include f, g, x or h. Secondly, and perhaps more importantly, this is the only place where one can find definitions of f, g and x even though f, g and x are used to define A and B (see e.g. page 34 line 21 and page 34 line 39). (By contrast, the definition of h appears to be largely, if not entirely, duplicative.)
273. The dispute concerns the first section of the definition of R1 , R2 and R3 , which includes the definitions of Formula (R) and of T. As noted above, it is not immediately clear how this section is structured, and in particular where the definitions of R and T end.
274. Taking into account the semi-colons, however, it appears that it is structured as follows. First, there is a block which extends from “are identical or different” at page 36 line 36 to the semi-colon after “-carbamoyl” at page 37 line 5. As the Claimants point out, this block begins with “hydrogen, halogen, cyano, trifluoromethyl, nitro, carboxyl” at page 36 line 36. Next there is a set of substituents that may be broadly described as hydrocarbons (although some include oxy- groups) at page 36 lines 37-46. Then there are three fluorine-containing compounds at page 36 line 46. Then there is a series of carbonyl substituents at page 36 lines 46-55. Then there is a series of carbamoyl substituents at page 36 line 55 - page 37 line 5.
275. Secondly, there is CON(CH2 )h ˈˈ defined at page 37 lines 5-7.
276. Thirdly, there is “a carbamoyl radical of the formula R” the definition of which begins at page 37 line 7. The definition of Formula (R) includes the definition of substituent T which begins at page 37 line 23. Where do these definitions end? Given the absence of line breaks, one could be forgiven for thinking that either or both extended to page 38 line 35.
277. The Claimants suggest, however, that the both definitions end with the semi-colon after “h is from 3 to 7” at page 37 line 29. This reading is supported by the following points. First, T is defined as OH or NR*R**, and then there are two lists of possibilities for R* and R** (in the first case, with R***), the second being where R* and R** together are –[CH2 ]h . It therefore does not appear that the text beginning with “carbamoyloxy” at page 37 line 29 can be part of the definition of T. Similarly, R is defined as a carbamoyl radical with the structure depicted. It therefore does not appear that the text beginning with “carbamoyloxy” at page 37 line 29 can be part of the definition of R.
278. Thus it appears that the text beginning with “carbamoyloxy” at page 37 line 29 forms the fourth block of options. This block comprises a set of carbamoyloxy substituents (down to page 37 line 34) followed by a series of amino substituents (page 37 lines 34-42) followed by a series of sulphur-containing substituents (page 37 lines 42-50).
279. This takes me to the key passage of text starting “where an aryl radical may be substituted by 1 to 5 substituents” at page 37 lines 50-51. It is not obvious how this fits into the structure of the definition of R1 , R2 and R3 . As the Claimants point out, however, what can be seen is that the pattern of substituents that follows repeats the pattern discussed above: “hydrogen, halogen, cyano, trifluoromethyl, nitro, carboxyl” (page 37 lines 51-52) followed by hydrocarbon (page 37 line 52 - page 38 line 2) followed by fluorine-containing (page 38 line 2) followed by carbonyl (page 38 lines 2-10) followed by carbamoyl (page 38 lines 10-18) followed by CON(CH2 )h ˈˈ (page 38 lines 18-20) followed by carbamoyloxy (page 38 lines 20-26) followed by amino (page 38 lines 26-33) followed by sulphur-containing substituents (page 38 lines 33-35). Although the pattern is the same, the options are not always the same: for example, the second list of sulphur-containing substituents is rather shorter than the first.
280. The language in which possible substitutions are described . Formula (I) includes aryl and aryl-containing groups at various positions. In some cases, there appears to be no suggestion that the relevant group may be substituted: see, for example, the 1,2-, 1,3- and 1,4-arylidenes at the beginning of A (page 34 line 14).
281. In other cases, it is clear that substitution of aryl and aryl-containing groups is permitted. Such substitutions fall into one of two categories:
i) Type 1: instances in which the aryl group is specifically described as having a certain number and/or type of possible substitutions. See, for example, “or by a 6 -C12 )-aryloxy, (C7 -C11 )-aralkyloxy, (C6 -C12 )-aryl, (C7 -C11 )-aralkyl radical, which carries in the aryl moiety one to five identical or different substituents selected from halogen …” in A (page 34 lines 19-24) and “wherein radicals which are aryl or contain an aryl moiety, may be substituted on the aryl by one to five identical or different hydroxyl, halogen …” in B (page 35 lines 10-40).
ii) Type 2: instances where the aryl group is merely said to be “optionally substituted” without any indication as to the nature of the permitted substitution: see “optionally substituted (C7 -C16 )-aralkylcarbonyl, optionally substituted (C6 -C12 )-arylcarbonyl” in R4 (page 36 lines 32-33), “optionally substituted (C7 -C16 )-aralkanoyl, optionally substituted (C6 -C12 )-aroyl” in T (page 37 lines 25-26) and “optionally substituted (C7 -C16 )-aralkanoyl, or optionally substituted (C6-C12)-aroyl” in the second section of the definition of R1 , R2 and R3 (page 38 line 39).
282. The Defendants contend that there must be some limit to the possible substitutions encompassed by Type 2, whereas the Claimants contend that there is no limit. In my view the skilled person would assume that some limit was intended, since otherwise the formula would embrace compounds that would be practically impossible to make and/or insoluble and a formula that covers a staggeringly large number of compounds anyway (as explained below) would cover a limitless number of compounds.
283. What is the purpose of the passage starting at page 37 line 50? Against this background, the key question is what the skilled person would think that the purpose of the passage starting at page 37 line 50 was. The Defendants contend that the skilled person would conclude that its purpose was to provide a “pick list” of substituents which could be used for Type 2 substitutions. As counsel for the Defendants submitted, this interpretation avoids “optionally substituted” being limitless. On the other hand, as counsel for the Defendants accepted, it has the consequence that the “pick list” wording would not only apply to the “optionally substituted” language in the first section of R1 , R2 and R3 , but also to that language in Q-R4 and in the second section of R1 , R2 and R3 . That does not fit with the way in which the passage in question is positioned within the overall structure of the definition of Formula (I).
284. In the alternative, the Defendants contend that the passage starting at page 37 line 50 would be understood to apply to the aryl or aryl-containing groups within the preceding block of text i.e. page 37 lines 29-50. That reading lacks the merit of providing a limit for “optionally substituted”, but fits somewhat better within the structure of the definition.
285. The Claimants contend that the passage starting at page 37 line 50 would be understood to apply generally to the aryl or aryl-containing groups within the first section of the definition of R1 , R2 and R3 , including “(C6 -C12 )-aryl”. The Claimants submit that this not only best fits the structure of the definition, but also makes sense because there is no apparent reason why it should only apply to some aryl or aryl-containing groups in the first section of R1 , R2 and R3 , but not others. On the other hand, it does not provide a limit for “optionally substituted”.
286. The conclusion I have reached is that the Claimants’ interpretation is the better one. Although the skilled reader would assume that some limit to “optionally substituted” was intended, there is nothing to indicate that the passage in question was intended to supply that limit. The skilled reader would consider that the most important factor was the structure of the definitions, and in particular the structure of the definition of R1 , R2 and R3 . While the skilled reader would consider the possibility that the passage starting at page 37 line 50 was only intended to qualify the preceding passage (the Defendants’ alternative interpretation), they would conclude that there was nothing in the structure to limit its application to that passage. Moreover, they would conclude that there was no reason why such substitutions should be available for aryl carbamoyloxy, amino and sulphur-containing groups and not for aryl hydrocarbon, carbonyl and carbamoyl groups. Thus they would conclude that it applies to all the aryl groups in the first section of the definition of R1 , R2 and R3 .
287. Before leaving this topic, however, I should mention for completeness a point which the Claimants relied upon as supporting their interpretation which I do not find persuasive. As noted above, and as discussed more fully below, EP 823 refers to 26 other patents and applications. One such reference is in [0076]:
“Exemplary compounds according to Formula (Ia) are described in U.S. Patent Nos, 5,719,164 and 5,726,305. All compounds listed in the foregoing patents, in particular, those listed in the compound claims and the final products of the working examples, may be used. Exemplary compounds according to Formula (Ia) …”
288. It is common ground that Example 16 of the first patent (“US 164”) is a compound which falls inside Formula (I) on the Claimants’ interpretation, but outside it on the Defendants’ interpretations. The Claimants contend that this shows that the Defendants’ interpretations cannot be right. I do not accept this. Example 16 is just one of a very large number of compounds identified in patents cited in EP 823. Even if, contrary to the conclusion I have reached above, the skilled medicinal chemist would read at least the 15 patents cited in [0078]-[0088] of WO 997, no reason has been given as to why the skilled person seeking to interpret Formula (I) would alight on Example 16 of US 164 as a means of checking their interpretation. US 164 is not even one of the patents singled out for review by Dr Bhalay. Nor has any reason been given as to why the skilled person would comb systematically through all the compounds listed in cited patents and applications to see which ones fell inside or outside on different interpretations, an exercise which would be a very laborious and time-consuming task indeed.
Structural mimetic of 2-OG
289. It is common ground that the second sentence of [0076] of WO 997 is all that is provided by way of a definition of “structural mimetics of 2-oxoglutarate”. For convenience, I will repeat it here:
“Such compounds may inhibit the target 2-oxoglutarate dioxygenase family member competitively with respect to 2-oxoglutarate and noncompetitively with respect to iron.”
290. The Claimants contend that the skilled medicinal chemist would understand the expression “structural mimetic of 2-oxoglutarate” to be synonymous with the term “structural analog of 2-oxoglutarate” used in Majamaa 1984, and that they would understand both expressions to mean a compound that has sufficient structural similarity to 2-OG to locate in the active site of the target enzyme (HIF-PH or P4H, respectively) and to inhibit the hydroxylation reaction competitively with respect to with 2-OG (the natural substrate) and non-competitively with respect to iron.
291. The Defendants contend that the expression is uncertain, with the consequence that the relevant claims are invalid for insufficiency. Although Prof Ward was not comfortable with the use of the term “structural mimetic”, as opposed to “structural analogue”, the Defendants do not contend that this in itself means that the expression “structural mimetic of 2-oxoglutarate” is uncertain. Rather, the Defendants say that it would not be clear to the skilled medicinal chemist from the specification (or from the cited papers) what does and what does not qualify as a “structural mimetic of 2-oxoglutarate”.
292. As already indicated, the only light that the specification throws on this is the statement quoted in paragraph 289 above, which is followed by references to Majamaa 1984 and Majamaa 1985. The Defendants point out that this statement says that such compounds “may inhibit [emphasis added]”, not “inhibit”. The Claimants contend that, in context, “may” means “have the capacity to”. But even that reading would leave the skilled reader unclear as to whether inhibition competitively with respect to 2-OG and non-competitively with respect to iron is intended to be the criterion for whether compounds qualify as a “structural mimetic of 2-oxoglutarate” or not.
293. It is for this reason that the skilled reader would be more likely to turn to Majamaa 1984 and Majamaa 1985 to see if they assist than the skilled reader would be to follow up any of the other cited publications. It is sufficient for this purpose to concentrate on Majamaa 1984, since neither side suggests that Majamaa 1985 adds anything.
294. As the Claimants point out, Majamaa 1984 uses the term “structural analogs of 2-oxoglutarate”. The authors do not define what they mean by this expression, however. They simply use it descriptively to refer to all the 11 aliphatic and 13 aromatic compounds which were studied. As noted above, the authors report that all 11 aliphatic and 12 of the aromatic “structural analogs” inhibited P4H competitively with respect to 2-OG and non-competitively with respect to iron, but they draw attention to the fact that the pyridine 2,6 dicarboxylate “structural analog” inhibited non-competitively with respect to 2-OG (at least at low concentrations) and competitively with respect to iron. The latter observation is not an incidental one: on the contrary, it forms a significant part of the teaching of the paper.
295. It follows that reading Majamaa 1984 would not assist the skilled medicinal chemist to ascertain how to distinguish between compounds which qualify as a “structural mimetic of 2-oxoglutarate” and those which do not. On the contrary, it would lead the skilled reader to conclude that, when the inventors said “may” at [0076], they meant “may” (i.e. “may or may not”).
296. The uncertainty does not end, there, however. Dr Bhalay’s evidence in his first report was as follows:
“165. Claim 24 states that the compound of claim 1, 2, 19 or 20 is a ‘structural mimetic of 2-oxoglutarate’. Based on the discussion in the description of EP 823 and the prior art cited in EP 823 …, the Skilled Medicinal Chemist would understand this to be an additional mechanism-based feature by which I mean that, for a compound to fall within the claim, it needs to be one that is replicating or mimicking the key interactions of 2-oxoglutarate in the enzyme binding pocket (particularly bidentate coordination to Fe2+ via dative bonding from the lone electron pairs possessed by the aromatic nitrogen and the carbonyl oxygen of the carboxamide moiety). If a compound replicates or mimics the other two interactions of 2-oxoglutarate in the enzyme binding pocket …, this would also be expected to contribute to that compound acting as a structural mimetic of 2-oxoglutarate. ….
166. To assess whether a given compound is a ‘structural mimetic of 2-oxoglutarate’, the Skilled Medicinal Chemist would be able to make a visual assessment of its structure. In particular, the Skilled Medicinal Chemist would determine whether it is capable of replicating or mimicking the key interactions of 2-oxoglutarate with the active site of the enzyme, primarily whether it has at least two groups capable of forming dative bonds with Fe2+ , for bidentate coordination in the active site. This could then be confirmed experimentally by assessing whether the compound in question competitively inhibits binding of 2-oxoglutarate.”
297. In cross-examination Dr Bhalay confirmed that, in his opinion, the skilled medicinal chemist would see the capacity for bidentate chelation of enzyme-bound iron as a necessary feature of a “structural mimetic of 2-oxoglutarate”. As indicated above, however, Majamaa 1984 discloses a number of compounds that lack the capacity for bidentate chelation, but nevertheless inhibit P4H competitively with respect to 2-OG.
298. Furthermore, if the skilled reader read Bickel, they would see that it discloses a compound, S 4682, which (i) is structurally related to 2-OG, and (ii) that the medicinal chemist would assume to chelate iron in the active site, but (iii) is reported to be non-competitive with respect to 2-OG. (As Dr Bhalay explained in his fourth report, there are a number of possible ways in which S 4682 might inhibit non-competitively despite being structurally related to 2-OG, namely: (i) as an allosteric inhibitor; (ii) by chelating free iron; (iii) by binding irreversibly to the active site; or (iv) as a “pseudo irreversible” inhibitor.)
299. In the light of the foregoing, the skilled medicinal chemist would not conclude that the capacity for bidentate chelation was the, or even a, key feature. Moreover, Dr Bhalay accepted that the medicinal chemist would be confident that the inventors did not regard the ability to inhibit the target enzyme competitively with respect to 2-OG to be a defining characteristic of a “structural mimetic of 2-oxoglutarate”.
300. When it was put to Dr Bhalay that the medicinal chemist would be left wholly uncertain as to what that expression would be understood to mean, he had no coherent explanation. Instead his approach seemed to require the medicinal chemist to conduct enzyme kinetic experiments using isolated HIF-PH to discover whether Compounds C-K compete with respect to 2-OG. If they turned out to be competitive, then he would use similar kinetic analyses to determine whether or not other compounds were structural mimetics. This is not a criterion that is suggested anywhere in the Family A Patents.
301. In any event, the uncertainty is further compounded by the issue of potency. Let it be assumed that competition with respect to 2-OG is understood to be the acid test for whether a compound is or is not a structural mimetic of 2-OG. On this basis, the compound must necessarily inhibit the hydroxylation reaction. There is no guidance in the specification (or the common general knowledge), however, as to what constitutes the relevant threshold in terms of Ki . Dr Bhalay accepted that different medicinal chemists could arrive at different thresholds, depending on a variety of considerations, including resources, individual predilections and knowledge about the potential to inhibit other members of the same enzyme family.
302. Prof Ward’s written evidence was that the skilled medicinal chemist would be uncertain as to the meaning of “structural mimetic of 2-oxoglutarate”. In cross-examination, at least two interpretations of this term were put to him, namely “structural analogs and functional mimetics of 2-OG” and a compound which has “structural similarity with 2-OG and which binds to the HIF-PH competitively with 2-OG and inhibits enzymatic activity”. Prof Ward identified a number of other reasonable definitions, however, such as compounds which: (i) simply mimic the structure of 2-OG regardless of function; (ii) mimic the binding interactions of 2-OG in the active site; (iii) bind irreversibly to the active site; or (iv) mimic the downstream function of 2-OG.
303. I therefore conclude that the skilled medicinal chemist would be uncertain as to the meaning of the expression “structural mimetic of 2-oxoglutarate”. More specifically, they would be unable to determine either from the specification or from the cited papers what criterion to apply to distinguish between a compound which is a “structural mimetic of 2-oxoglutarate” and a compound which is not. I will consider the consequences of this when I come to the issue of insufficiency.
Obviousness of Family A over Epstein
304. The Defendants contend that claim 17A of EP 531, i.e. Compound C for use in the treatment of anaemia associated with CKD, is obvious in the light of Epstein. The Defendants further contend that, if Compound C is obvious over Epstein, then so too are claims 8A, 19A and 24 of EP 823 and claims 2 and 4 of EP 301 (being claims covering Compound C, in the case of claim 24A of EP 823 and claim 4 of EP 301 on the assumption that Compound C is a “structural mimetic of 2-oxoglutarate”).
The disclosure of Epstein
305. As noted above, Epstein is a paper from the Ratcliffe group. Its authors include five with a background as nephrologists.
306. Epstein identifies the enzyme that performs the prolyl hydroxylation of HIF-α, first in the nematode worm, C. elegans , and then as a series of homologues in mammalian cells. It investigates the biochemistry of recombinantly expressed proteins, revealing the HIF-PH enzyme to be the oxygen sensor in the hypoxia pathway. Prof Haase’s explanation of the disclosure of Epstein in his first report was agreed by Prof Winearls in his third report. I would summarise this as follows.
307. The introduction first recaps what is known about HIF as a key regulator of oxygen homeostasis in mammalian cells, playing a central role in both local and systemic responses to hypoxia. It notes that the HIF system is activated by hypoxia, iron chelators and cobaltous ions. Oxygen- and 2-OG-dependent hydroxylation of a proline residue in HIF-α had suggested involvement of a member of the superfamily of 2-OG-dependent oxygenases.
308. Epstein then describes the work which the authors carried out. In summary:
i) A candidate worm version of HIF-α, termed HIF-1, was identified by database searches of the genome, and was found (like mammalian HIF-α) to encode a protein regulated by hypoxia and iron chelation.
ii) The worm version of VHL (i.e. the protein that ubiquitylates HIF-α, resulting in its destruction) was then also identified. Mutants in the gene encoding worm VHL had abnormally high levels of HIF-1 in the presence of oxygen, indicating that HIF-1 destruction was defective.
iii) The physical association between recombinantly-expressed HIF-1 and VHL was found to depend upon an activity found in worm extract, and this activity was identified (like in the mammalian system) as causing hydroxylation of a particular proline residue in a peptide found in HIF-1. This proline hydroxylation was dependent on 2-OG: it could be inhibited by an analogue of 2-OG, and the inhibition was competed with by excess 2-OG. A cell-penetrating analogue of 2-OG, DMOG, strongly induced HIF-1 in normoxic worms.
iv) Further database searches identified worm homologues of the 2-OG-dependent oxygenase superfamily. Mutants of one of these, EGL9, had high levels of HIF-1 in normoxia and showed upregulation of hypoxia-inducible transcripts (even in the absence of hypoxia).
v) The biochemical function of recombinant EGL-9 as a HIF-PH was demonstrated. It was found to depend on 2-OG, iron and oxygen, and was directly inhibited by cobalt ions.
vi) Human homologues were then identified in the sequence databases, which the authors term PHD-1, PHD-2 and PHD-3. Recombinantly-expressed versions of these proteins resulted in prolyl hydroxylation of human HIF-α, and this activity was again strongly inhibited by iron chelation, cobalt ions, and by the 2-OG analogue NOG, and to depend upon oxygen concentration.
309. Prof Winearls very fairly described this work in his oral evidence as “a beautiful demonstration of the final piece in the jigsaw puzzle of oxygen-sensing”.
310. In its discussion section Epstein states (at page 51-52) that:
“… the classical features of HIF induction by hypoxia, cobaltous ions, and iron chelators can be explained, at least qualitatively, by the properties of recombinant HIF-PH enzymes.”
311. It goes on to say (at 52):
“In mammals, the HIF system regulates not only cellular responses to oxygen, but also a range of systemic functions such as regulation of angiogenesis, erythropoiesis, and vasomotor control. …”
312. The paper concludes (at 52):
“Finally, the identification of the HIF-PHIs also raises therapeutic possibilities. Inhibitors of HIF-PHs might be used to activate HIF and enhance angiogenesis in ischemic/hypoxic disease. Application of the 2-oxoglutarate analog dimethyloxalylglycine to tissue culture cells strongly induces HIF target genes (D.R.M., unpublished observations). Though this compound is not specific for the HIF-PHs and inhibits other 2-oxoglutarate dioxygenases, structural and mechanistic studies of the defined enzymes may now permit design of more specific inhibitors for therapeutic development.”
313. DMOG was the compound that the group’s earlier paper, Jaakkola, had shown to result in rapid induction of HIF-α in cultured cells, and Epstein reports that this indeed resulted in induction of HIF target genes. As the skilled team would be aware from their common general knowledge, the archetypal HIF target gene was Epo, and Epstein specifically refers to this role of HIF. Thus, as Prof Winearls accepted, the skilled nephrologist would appreciate that Epstein provides a biochemical explanation of the increase in gene targets, and in particular Epo, as a result of hypoxia.
314. Both NOG and DMOG are described by Epstein (at page 47 and in the concluding paragraph on page 52 quoted above) as “2-oxoglutarate analog[s]”. The skilled medicinal chemist would appreciate that, as mentioned above, DMOG is an ester pro-drug of NOG. As Dr Bhalay accepted, it would be apparent to the medicinal chemist that NOG inhibits the hydroxylation of HIF-α by PHD-1 and that 2-OG competes with NOG. Thus NOG and DMOG are “structural mimetics of 2-OG” on the Claimants’ interpretation of that expression.
The differences between Epstein and claim 17A of EP 531
315. The differences between Epstein and claim 17A of EP 531 are as follows:
i) Epstein makes no reference to the treatment or prevention of anaemia associated with kidney disease, or even to enhancing endogenous Epo. Epstein mentions possible therapeutic uses of HIF-PHIs to activate HIF and enhance angiogenesis in ischaemic/hypoxic disease, but does not refer to erythropoiesis in this context.
ii) Although Epstein discloses the use of inhibitors of HIF-PH to stabilise HIF, it discloses only one inhibitor, namely DMOG, and provides no results beyond stating that it “strongly induces HIF target genes” in tissue culture cells. It is not disclosed that DMOG induces the production of endogenous Epo in an animal model. Thus Epstein does not show that DMOG satisfies the second of the two criteria for therapeutic efficacy discussed in paragraph 260 above.
iii) Epstein does not disclose Compound C.
Is claim 17A of EP 531 obvious?
316. In the light of the differences between Epstein and claim 17A, the issue of obviousness involves four questions, the first two of which lie in the province of the skilled nephrologist and the fourth in the province of the skilled medicinal chemist while the third concerns both of them:
i) Would it be obvious to consider using a HIF-PHI to treat or prevent anaemia associated with CKD?
ii) If so, would the skilled person have a reasonable expectation of success, in the sense of satisfying the criteria for therapeutic efficacy discussed above?
iii) If so, would it be obvious to search for other compounds in addition to DMOG to test?
iv) If so, would a routine search lead to Compound C?
317. I should note before proceeding further that neither side really distinguished either in their cross-examination of the experts or in their submissions between the first and second questions. I do not criticise them for that, because the questions are inter-related; but for the purposes of analysis I have found it easier to address them separately.
318. So far as the first question is concerned, the starting point is, of course, that the skilled team is interested in new treatments for anaemia. I have found that the skilled nephrologist (or at least the pre-clinical researcher) would have been aware from their common general knowledge of the role of HIF in regulating the expression of Epo. In any event, Epstein specifically mentions this.
319. Counsel for the Claimants submitted that the skilled team, having read Epstein, would regard it as a purely academic study which was of no clinical application. That submission is contrary to the express teaching of Epstein, which is that the identification of HIF-PHIs “raises therapeutic possibilities”, albeit that the only therapeutic application which is mentioned is ischaemic/hypoxic disease.
320. I understood Prof Winearls to accept that, reading Epstein through the eyes of the skilled nephrologist, for whom Epo would have been the key target gene, it would be obvious to consider the possibility of using a HIF-PHI to treat or prevent anaemia associated with CKD. As he put it, he would have said, “How very interesting. Very elegant, but it is going to be clinically applicable?”. He went on to agree that he would have seen the potential therapeutic benefit, but he would have had some question marks. As I see it, Prof Winearls’ question marks go to the second question identified above. I shall therefore consider them in that context.
321. Prof Haase maintained that it was obvious to consider using a HIF-PHI to treat or prevent anaemia associated with CKD. No reason was put to Prof Haase as to why this possibility would not even occur to the skilled person. (Rather, as discussed below, reasons were put as to why the skilled person would not have thought that there was a reasonable prospect of success.) Even so, the Claimants contend that this is only obvious with hindsight. Three main points are relied on this regard.
322. First, Epstein itself does not suggest this application. Counsel for the Claimants submitted that, given the background of five of the authors, if it had been obvious, they would have been bound to mention it. Prof Haase did not accept this, and nor do I. As Prof Haase explained, the authors may have chosen to single out ischaemic/hypoxic disease in the concluding paragraph because it is one of the two main causes of mortality and morbidity in Western societies, and thus would emphasise the significance of their research. This is particularly so given that Epstein was published in Cell , a general journal rather than one aimed specifically at nephrologists.
323. Secondly, counsel for the Claimants put to Prof Haase a series of 10 papers about HIF, including Ivan and Jaakkola, published in the period from 2000 to 2003 which do not mention the treatment of anaemia as a possible application of HIF-PHIs, whereas there are a number of mentions of ischaemia and a couple of mentions of stroke and cancer. Again, counsel for the Claimants submitted that, if the treatment of anaemia had been obvious, at least some of these papers would have been bound to mention it. Again, Prof Haase did not accept this, and nor do I. The evidence relied upon is a dog that did not bark in the night; but it is only probative if there is some reason why the dog should have barked. I am not persuaded that there is. One of the papers (Jaakkola) does not discuss clinical applications at all. More importantly, it was not established, or even put to Prof Haase, that the authors of all the papers included one or more persons corresponding to the skilled nephrologist: although some did, it appears that some did not (such as a review written by at least two oncologists). Even in the case of papers which do satisfy that criterion, the point remains that a perfectly good explanation why cancer, heart disease and stroke are mentioned is to draw attention to the significance of HIF, particularly in journals not aimed at nephrologists. Furthermore, an alternative explanation for the failure to mention the potential application of HIF-PHIs to induction of Epo is that it was considered by many to be unremarkable in the light of Epstein. Yet further, as Prof Haase pointed out in his third report, two review articles, one by Ratcliffe and one by Patrick Maxwell, were published during the same period (in September 2002 and 2003 respectively) which cited Epstein and which did expressly mention “erythropoietin deficiency” and “promot[ing] erythropoiesis” as potential therapeutic applications for HIF-PHIs.
324. Thirdly, the Claimants pointed out that on 21 March 2002 Isis Innovation Ltd (“Isis”) filed a priority patent application in the UK (0206711.4) naming a number of inventors, including Ratcliffe, for an invention relating to HIF-PHIs which mentioned a number of potential therapeutic applications but not anaemia, although it did mention the “critical” role of HIF in (among other things) erythropoiesis. When Isis came to file an international application (WO 03/080566) claiming priority from the UK filing on 21 March 2003, additional applications were included, including “the treatment of anaemia”. Counsel for the Claimants suggested that this showed this application had not occurred to the Ratcliffe group by March 2002, but only later. This does not necessarily follow. They might, for example, have thought that it went without saying. They did not need to mention this specific application, because the claims in the priority document did not include any claims directed to specific applications, but did include a general claim directed to “treatment of a condition associated with increased or decreased HIF levels or activity”.
325. Counsel for the Claimants also suggested to Prof Haase that Prof Ratcliffe had got the idea from FibroGen at a meeting in May 2002. That was a wholly improper suggestion for two reasons. First, Prof Haase had no way of knowing whether there was any such meeting, or if so, what was said; and the Claimants adduced not a jot of evidence to show that there even was such a meeting when they would have been well able to do so if it had happened. Secondly, it amounts to an accusation of impropriety on the part of Professor Ratcliffe in circumstances where he was unable to defend himself. As counsel for the Defendants submitted, the making of this suggestion smacks of desperation on the part of the Claimants.
326. As noted above, Prof Ratcliffe did write a review article that mentioned this application which was published in September 2002. It is entirely possible that that article was written prior to May 2002 or even 21 March 2002. Accordingly, I see no reason to conclude that Prof Ratcliffe had not appreciated the possibility of using HIF-PHIs to treat anaemia at the time of publishing Epstein.
327. My conclusion on the first question is as follows. Epstein specifically draws attention to HIF’s role in regulating erythropoiesis in its penultimate paragraph. Erythropoesis is the second target mentioned after angiogenesis. The authors go on in the next paragraph explicitly to say that HIF-PHIs might be used to enhance angiogenesis. It is implicit, particularly to the skilled nephrologist, that they might also be used to enhance erythropoiesis. That is objectively obvious. It is not hindsight.
328. I turn, therefore, to consider the second question. Prof Winearls raised two concerns that the skilled nephrologist would have had as to the clinical applicability of HIF-PHIs in the treatment of anaemia of CKD, which I will consider in turn.
329. Prof Winearls’ first, and principal, concern was that, for the reasons discussed above in relation to Maxwell, damaged kidneys could not produce sufficient Epo. As he put in cross-examination:
“… we know that the damaged kidneys can produce extra Epo. What we would have been concerned about is, if the original stimulus to the HIF system was not working adequately in these patients, why would an alternative work?”
330. After it was put to Prof Winearls that the answer to this question was that a HIF-PHI would artificially stimulate the system rather than relying upon the natural hypoxia, he accepted that this would obviously be well worth testing to see if it worked, but not that it was obvious that it would do so. As he put it, “… I would have said, ‘It could work, but I am not sure that it will’.” He went on to accept that it would be obvious in particular to test compounds in rats with cisplatin (as in Example 4 in the Family A Patents).
331. Although Prof Winearls was clear and consistent throughout his evidence that, in his opinion, the skilled nephrologist would be sceptical as to the ability of damaged kidneys to produce sufficient Epo to alleviate the patients’ anaemia, not once did he suggest that the skilled nephrologist would think that HIF-PHIs were unlikely to work because the enhanced production of Epo in response to hypoxia was thought to be transient . Despite this, the case that was put to Prof Haase in cross-examination was that the skilled nephrologist would think that the effect on damaged kidneys would be both insufficient and transient . Given that it was never mentioned by Prof Winearls, I do not accept that transience would have been a factor in the skilled nephrologist’s thinking. In any event, Prof Haase did not agree that either factor meant that it was not obvious to try using HIF-PHIs to treat anaemia.
332. Turning to Prof Winearls’ second concern, this was that there could be a problem due to the fact that HIF turns on other genes as well as Epo. This is not a concern he had raised in his written evidence, however. Moreover, as he accepted, it is not something that is addressed in the Family A Patents. Nor was it put to Prof Haase as being something that would deter the skilled nephrologist from testing HIF-PHIs for anaemia.
333. The final point that requires consideration before reaching a conclusion on the second question concerns cobalt. As counsel for the Claimants pointed out, part of Prof Haase’s reasoning in relation to this question in his first report was that “it was well established … that the induction of HIF by cobalt salts was clinically effective in stimulating erythropoiesis …”. I have concluded that it was not common general knowledge that cobalt chloride had been used to treat anaemia. To that extent, Prof Haase’s opinion was misplaced. On the other hand, I have concluded that it was common general knowledge that cobalt stimulated the production of Epo, and that it did so by the induction of HIF-α, although there was still debate as to whether this was the same mechanism as hypoxia. This would have given the skilled reader of Epstein, which refers to the role of cobalt in HIF induction, reason to believe that the authors were correct in implying that inhibition of HIF might affect erythropoiesis.
334. In any event, this was an additional reason which was introduced by Prof Haase after he had already concluded that the skilled nephrologist reading Epstein would readily appreciate that inhibition of HIF-PHs by 2-OG analogues such as DMOG, resulting in the stabilisation of HIF-α, would result in an increase in the expression of (among other genes) Epo. That in itself was a sufficient foundation for his opinion that the skilled team would take DMOG forward into pre-clinical testing for the treatment of anaemia.
335. The conclusion I reach on the evidence as a whole is that the skilled nephrologist would be uncertain as to whether HIF-PHIs would be effective in stimulating endogenous Epo, and in particular would be uncertain as to whether they would stimulate the production of sufficient Epo to have a therapeutic effect against anaemia, but they would consider that the prospects of success were sufficient to warrant carrying out first in vitro , and then (for successful candidates) in vivo , tests of suitable compounds. In other words, it was obvious to try.
336. Turning to the third question, as discussed above, the only HIF-PHI disclosed in Epstein is DMOG. I do not understand it to be in dispute that, if the skilled team decided that it was worth carrying out pre-clinical testing of DMOG, it would obvious to search for other compounds to test as well.
337. That brings me to the fourth question. Prof Haase gave unchallenged evidence that the skilled nephrologist would have asked the skilled medicinal chemist to identify known inhibitors of prolyl hydroxylases, such as collagen prolyl hydroxylases (which, as Epstein explains, were the best characterised hydroxylases at that time), for testing.
338. Prof Ward gave evidence in his first report that, given such a request, the medicinal chemist would have carried out a literature search using one of the standard tools which existed in December 2001. He carried out a search using one of the most commonly used tools, SciFinder, limited by date which produced two lists of citations set out in his exhibits SW2 and SW3. Dr Bhalay agreed in his third report that the search carried out by Prof Ward was the sort of search that would be carried out. He did not suggest that the results would have been affected by Prof Ward’s use of a contemporary version of the software to carry out the search.
339. Exhibit SW2 contained the results for the exact string “prolyl hydroxylase inhibitor”, running to 60 citations; while exhibit SW3 contained the results for the concept “prolyl hydroxylase inhibitor”, running to 320 citations.
340. Prof Haase’s evidence in his first report was that the skilled nephrologist would have been interested in screening the compounds mentioned in these publications, for Epo induction activity in a cell line such as Hep3B, prior to testing in an animal model for an increase in both Epo expression and haematocrit. In cross-examination, he clarified that he was not suggesting that all the compounds would be screened, and said that he would defer to Prof Ward as to which to screen.
341. Prof Ward agreed that the medicinal chemist would sift the results by relevance, first by a quick review of the abstract and then by a small study of the remaining papers, before studying a subset in detail. This might lead to only 10 papers out of the 60 in SW2 being the subject of full consideration.
342. Dr Bhalay noted in his third report that SW3 included Majamaa 1985, Bickel, Franklin, and the two European patents cited in WO 997. It also includes US 974. More importantly, it includes German patent application No. 197 46 287 (“DE 287”). It can be seen from US 730 (which, as noted above, is WO 997’s source for Compound C) that this was the priority document for US 730. As is common ground, US 730 contains both in vitro and in vivo results demonstrating the efficacy of Compound C as a P4H inhibitor. The same data are contained in DE 287; but the skilled team would have to obtain a translation of DE 287 to find that out.
343. The Defendants contend that the compounds that would be identified by this route would all be obvious compounds to test first in vitro and then in vivo , including Compound C. In essence, the argument is that this would all be routine work once the skilled team had decided that the proposition that HIF-PHIs would stimulate Epo, and hence treat anaemia, was worth testing.
344. As counsel for the Claimants submitted, however, the evidence simply does not establish that Compound C would be found by this route. Given that SW3 contains 320 citations, it is likely that the skilled medicinal chemist would focus on SW2. Moreover, as discussed above, the medicinal chemist would be likely to concentrate on a subset of the 60 citations in SW2. Although the question of how many compounds would be screened initially was not discussed with any of the experts, it is inherently probable that the skilled team would proceed iteratively, screening a certain number of compounds at a time in the search for a hit compound. If no hit compound was identified, another batch would be screened. If a hit compound was identified, then the medicinal chemist would embark upon a drug development programme beginning with an SAR investigation.
345. I therefore conclude that claim 17A of EP 531 is not obvious over Epstein.
Other claims
346. Given that claim 17A is not obvious over Epstein, and the way in which the Defendants put their case, it follows that nor are the other claims of the Family A Patents in issue. If claim 17A was obvious, the Claimants did not identify any reason why the other claims would be independently valid. The validity of the other claims would be saved, however, by the conditional amendments proposed by FibroGen.
Insufficiency of EP 823 and EP 301
347. A patent is invalid if “the specification does not disclose the invention clearly and completely enough for it to be performed by a person skilled in the art” (section 72(1)(c) of the Patents Act 1977 giving effect to Article 138(1)(b) EPC). As I have observed in a number of previous judgments, although insufficiency is a single ground of invalidity, it embraces three distinct types of objection: where the invention cannot be performed at all without undue burden (sometimes called “classical insufficiency”); where the invention cannot be performed across the breadth of the claim without undue burden (sometimes called “Biogen insufficiency” and also referred to as “excessive claim breadth”); and where the claim does not enable the skilled person to know whether they are within the claim or outside (previously called “ambiguity” and recently re-named “uncertainty”). In the present case the Defendants advance both of the latter types of objection against EP 823 and EP 301, but not the first.
Excessive claim breadth
348. The law . One might have thought that, given that it has been reviewed in a number of recent Court of Appeal decisions, the law on this subject was fairly well settled subject to whatever the Supreme Court may say in its forthcoming decision in Regeneron Pharmaceuticals Inc v Kymab Ltd . Despite this, there was a vigorous debate before me as to the law, and a considerable number of authorities was cited. I have taken into account all of the submissions made and all the authorities cited, but I do not consider that it is necessary to discuss them all in this judgment.
349. In Eli Lilly & Co v Human Genome Sciences Inc [2012] EWCA Civ 1185 , [2013] RPC 22 at [11] Sir Robin Jacob, and in Idenix Pharmaceuticals Inc v Gilead Sciences Inc [2016] EWCA Civ 1089 at [133] Kitchin LJ, cited with approval the following summary of the basic principles given by Kitchin J (as he then was) at first instance in the former case [2008] EWHC 1903 (Pat) , [2008] RPC 29 at [239]:
“The specification must disclose the invention clearly and completely enough for it to be performed by a person skilled in the art. The key elements of this requirement which bear on the present case are these:
(i) the first step is to identify the invention and that is to be done by reading and construing the claims;
(ii) in the case of a product claim that means making or otherwise obtaining the product;
(iii) in the case of a process claim, it means working the process;
(iv) sufficiency of the disclosure must be assessed on the basis of the specification as a whole including the description and the claims;
(v) the disclosure is aimed at the skilled person who may use his common general knowledge to supplement the information contained in the specification;
(vi) the specification must be sufficient to allow the invention to be performed over the whole scope of the claim;
(vii) the specification must be sufficient to allow the invention to be so performed without undue burden.”
350. As Kitchin LJ added in Idenix v Gilead at [135]:
“The extent of the disclosure necessary to make the patent sufficient depends on the nature of the invention, the scope of the claims and the art in which the invention is made …”
351. The objection of excessive claim breadth concerns the requirement that the invention must be capable of being performed over the whole scope of the claim without undue burden (points (vi) and (vii) in Kitchin J’s summary quoted above). As will appear, this requirement must not be taken too far.
352. It is well established that it is permissible for a claim to describe an invention in general terms provided it is plausible in the light of the disclosure and the common general knowledge that the invention will work with anything falling within the scope of those terms. As Kitchin LJ explained in Regeneron Pharmaceuticals Inc v Genentech Inc [2013] EWCA Civ 93 , [2013] RPC 28 :
“98. … it is permissible to define an invention using general terms provided the patent discloses a principle of general application in the sense that it can reasonably be expected the invention will work with anything falling within the claim. As Lord Hoffmann said in Biogen Inc. v Medeva plc [1977] R.P.C. 1 at pp.48–49:
‘If the invention discloses a principle capable of general application, the claims may be in correspondingly general terms. The patentee need not show that he has proved its application in every individual instance. On the other hand, if the claims include a number of discrete methods or products, the patentee must enable the invention to be performed in respect of each of them.
Thus if the patent has hit upon a new product which has a beneficial effect but cannot demonstrate that there is a common principle by which that effect will be shared by other products of the same class, he will be entitled to a patent for that product but not for the class, even though some may subsequently turn out to have the same beneficial effect: see May & Baker Ltd v Boots Pure Drug Co. Ltd. (1950) 67 R.P.C. 23, 50. On the other hand, if he has disclosed a beneficial property which is common to the class, he will be entitled to a patent for all products of that class (assuming them to be new) even though he has not himself made more than one or two of them.’
99. In Kirin-Amgen Inc v Hoechst Marion Roussel Ltd [2004] UKHL 46 , [2005] RPC 9 Lord Hoffmann further explained the concept of a principle of general application in this way:
‘112. In my opinion there is nothing difficult or mysterious about [a principle of general application]. It simply means an element of the claim which is stated in general terms. Such a claim is sufficiently enabled if one can reasonably expect the invention to work with anything which falls within the general term. For example, in Genentech I/Polypeptide expression (T 292/85) [1989] O.J. EPO 275, the patentee claimed in general terms a plasmid suitable for transforming a bacterial host which included an expression control sequence to enable the expression of exogenous DNA as a recoverable polypeptide. The patentee had obviously not tried the invention on every plasmid, every bacterial host or every sequence of exogenous DNA. But the Technical Board of Appeal found that the invention was fully enabled because it could reasonably be expected to work with any of them.
113. This is an example of an invention of striking breadth and originality. But the notion of a “principle of general application”' applies to any element of the claim, however humble, which is stated in general terms. A reference to a requirement of “connecting means” is enabled if the invention can reasonably be expected to work with any means of connection. The patentee does not have to have experimented with all of them.’
100. It must therefore be possible to make a reasonable prediction the invention will work with substantially everything falling within the scope of the claim or, put another way, the assertion that the invention will work across the scope of the claim must be plausible or credible. The products and methods within the claim are then tied together by a unifying characteristic or a common principle. If it is possible to make such a prediction then it cannot be said the claim is insufficient simply because the patentee has not demonstrated the invention works in every case.
101. On the other hand, if it is not possible to make such a prediction or if it is shown the prediction is wrong and the invention does not work with substantially all the products or methods falling within the scope of the claim then the scope of the monopoly will exceed the technical contribution the patentee has made to the art and the claim will be insufficient. It may also be invalid for obviousness, there being no invention in simply providing a class of products or methods which have no technically useful properties or purpose.”
353. Accordingly, the authorities establish that the court must undertake a two-stage enquiry. The first stage is to determine whether the disclosure of the patent, read in the light of the common general knowledge of the skilled team, makes it plausible that the invention will work across the scope of the claim. At this stage, it is not permissible for either the patentee or the party attacking the patent to rely upon evidence which post-dates the patent. If the disclosure does make it plausible, the second stage is to consider whether the evidence establishes that in fact the invention cannot be performed across the scope of the claim without undue burden. At this stage, evidence which post-dates the patent is admissible.
354. The criterion of plausibility has received the most detailed consideration by the courts in the context of claims involving medical applications. The authoritative statement of the law is that of the majority of the Supreme Court in Warner-Lambert Co LLC v Generics (UK) Ltd [2018] UKSC 56 , [2019] Bus LR 360 , which was given by Lord Sumption. That case was concerned with a second medical use claim in Swiss form of a known pharmaceutical. This part of the present case is concerned with a first medical use of (largely) known compounds, even though there are claims framed as second medical use claims both in Swiss form and in EPC 2000 form. There is no dispute that the guidance given by Lord Sumption is applicable, although the Claimants contend that, for the reasons explained below, it is not the whole story. (To avoid returning to this subject later, I would add that the claims in the Family B Patents are true second medical use claims, and thus Lord Sumption’s guidance is directly applicable.)
355. Lord Sumption began at [17] with the fundamental principle that, as it was put by the Board of Appeal of the European Patent Office in T 409/91 Exxon/Fuel oils [1994] OJ EPO 63 at [3.3] and [3.4], “the extent of the patent monopoly, as defined by the claims, should correspond to the technical contribution to the art”, that is to say, “the patent monopoly should be justified by the actual technical contribution to the art”. As he observed, the requirements of novelty, inventive step, industrial applicability and sufficiency are all, in one way or another, directed to ensuring that this principle is satisfied.
356. At [19]-[20] Lord Sumption noted that the problem with interpreting the requirement of sufficiency in the context of a second medical use claim as merely requiring the disclosure of the new purpose was that “it would enable a patent to be obtained on a wholly speculative basis”. Importantly for the present context, he said at [22]:
“The Court of Appeal's reference to ‘armchair inventors’ suggests that what they meant by speculative claiming was claiming by persons who had done nothing new or inventive at all but had simply sought to patent abstract possibilities. That may well be a particular risk in the case of patents for new uses of known compounds, especially when they are commercially successful in their existing use. In reality, however, speculative claiming of this kind is simply one of a number of ways in which a patentee may attempt to claim a monopoly more extensive than anything which is justified by his contribution to the art. Other ways in which this can happen include claiming a monopoly wider than the disclosure in the patent can support. An over-broad claim will not necessarily be speculative. The inventor may really have invented something corresponding to the full breadth of the claim. Research may subsequently demonstrate this. But the claim will still exceed his contribution to the art if that contribution is not sufficiently disclosed in the patent.”
357. From [23]-[35] Lord Sumption reviewed the case law of the Boards of Appeal of the EPO, where, as he explained, the concept of plausibility had originated “as a response to over-broad claims”.
358. At [36] Lord Sumption disagreed with the Court of Appeal’s statement of the effect of the plausibility test, saying:
“The principle is that the specification must disclose some reason for supposing that the implied assertion of efficacy in the claim is true. Plausibility is not a distinct condition of validity with a life of its own, but a standard against which that must be demonstrated. Its adoption is a mitigation of the principle in favour of patentability. It reflects the practical difficulty of demonstrating therapeutic efficacy to any higher standard at the stage when the patent application must in practice be made. The test is relatively undemanding.
359. Lord Sumption went on at [37] (emphases and line breaks added):
“Plausibility is not a term of art, and its content is inevitably influenced by the legal context. In the present context, the following points should be made.
First , the proposition that a product is efficacious for the treatment of a given condition must be plausible.
Second , it is not made plausible by a bare assertion to that effect, and the disclosure of a mere possibility that it will work is no better than a bare assertion. ….
But, third , the claimed therapeutic effect may well be rendered plausible by a specification showing that something was worth trying for a reason, ie not just because there was an abstract possibility that it would work but because reasonable scientific grounds were disclosed for expecting that it might well work. The disclosure of those grounds marks the difference between a speculation and a contribution to the art. This is in substance what the Technical Board of Appeal has held in the context of article 56, when addressing the sufficiency of disclosure made in support of claims extending beyond the teaching of the patent. In my opinion, there is no reason to apply a lower standard of plausibility when the sufficiency of disclosure arises in the context of EPC articles 83 and 84 and their analogues in section 14 of the Patents Act. In both contexts, the test has the same purpose.
Fourth , although the disclosure need not definitively prove the assertion that the product works for the designated purpose, there must be something that would cause the skilled person to think that there was a reasonable prospect that the assertion would prove to be true.
Fifth , that reasonable prospect must be based on what the TBA in SALK (para 9) called ‘a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se.’
Sixth , in SALK , this point was made in the context of experimental data. But the effect on the disease process need not necessarily be demonstrated by experimental data. It can be demonstrated by a priori reasoning. For example, and it is no more than an example, the specification may point to some property of the product which would lead the skilled person to expect that it might well produce the claimed therapeutic effect; or to some unifying principle that relates the product or the proposed use to something else which would suggest as much to the skilled person.
Seventh , sufficiency is a characteristic of the disclosure, and these matters must appear from the patent. The disclosure may be supplemented or explained by the common general knowledge of the skilled person. But it is not enough that the patentee can prove that the product can reasonably be expected to work in the designated use, if the skilled person would not derive this from the teaching of the patent.”
360. At [40] Lord Sumption added:
“The question is not whether [the medicament] works but whether the contribution to the art consisting in the discovery that it can be expected to work has been sufficiently disclosed in the patent. The inherent difficulty of demonstrating this before clinical trials is taken into account in the modest standard (ie plausibility) which is applied to test it. … This does not mean that subsequent data is never admissible in a dispute about sufficiency, but the purpose for which it is admitted is strictly limited.
361. So far as the question of undue burden is concerned, in Regeneron v Genentech Novartis v Johnson & Johnson at [236]:
“Whether the specification discloses an invention clearly and completely enough for it to be performed by a person skilled in the art involves a question of degree. It is impossible to lay down any precise rule because the degree of clarity and completeness required will vary depending on the nature of the invention and of the art in which it is made. On the one hand, the specification need not set out every detail necessary for performance. The skilled person must be prepared to display a reasonable degree of skill and use the common general knowledge of the art in making routine trials and to correct obvious errors in the specification, if a means of correcting them can readily be found. Further, he may need to carry out ordinary methods of trial and error, which involve no inventive step and generally are necessary in applying the particular discovery to produce a practical result. On the other hand, he should not be required to carry out any prolonged research, enquiry or experiment: Mentor Corporation v Hollister Inc. [1993] R.P.C. 7.”
362. Kitchin LJ went on to consider the requirement that the specification should enable the skilled person to perform the invention without undue burden in the context of a claim to the use of a product to make a medicine for a particular therapeutic purpose:
“102. … patentees not infrequently seek to avoid the possibility that a claim covers products or methods which do not work by inserting a functional limitation. Such a claim may be allowed by the EPO if the invention can only be defined in such terms or cannot otherwise be defined more precisely without unduly restricting its scope. But, it must still be possible to perform the invention across the scope of the claim without undue effort. As I said in Novartis v Johnson & Johnson at [244]:
‘… In the case of a claim limited by function, it must still be possible to perform the invention across the scope of the scope of the claim without undue effort. That will involve a question of degree and depend upon all the circumstances including the nature of the invention and the art in which it is made. Such circumstances may include a consideration of whether the claims embrace products other than those specifically described for achieving the claimed purpose and, if they do, what those other products may be and how easily they may be found or made; whether it is possible to make a reasonable prediction as to whether any particular product satisfies the requirements of the claims; and the nature and extent of any testing which must be carried out to confirm any such prediction.’
103. … the Boards of Appeal of the EPO have recognised that in the case of a claim to the use of a product to make a medicine for a particular therapeutic purpose it would impose too great a burden on the patentee to require him to provide absolute proof that the compound has approval as a medicine. Further, it is not always necessary to report the results of clinical trials or even animal testing. Nevertheless, he must show, for example by appropriate experiments, that the product has an effect on a disease process so as to make the claimed therapeutic effect plausible. It was put this way in T609/02 Salk at [9]:
‘… It is a well-known fact that proving the suitability of a given compound as an active ingredient in a pharmaceutical composition might require years and very high developmental costs which will only be borne by the industry if it has some form of protective rights. Nonetheless, variously formulated claims to pharmaceutical products have been granted under the EPC, all through the years. The patent system takes account of the intrinsic difficulties for a compound to be officially certified as a drug by not requiring an absolute proof that the compound is approved as a drug before it may be claimed as such. The boards of appeal have accepted that for a sufficient disclosure of a therapeutic application, it is not always necessary that results of applying the claimed composition in clinical trials, or at least to animals are reported. Yet, this does not mean that a simple verbal statement in a patent specification that compound X may be used to treat disease Y is enough to ensure sufficiency of disclosure in relation to a claim to a pharmaceutical. It is required that the patent provides some information in the form of, for example, experimental tests, to the avail that the claimed compound has a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se. Showing a pharmaceutical effect in vitro may be sufficient if for the skilled person this observed effect directly and unambiguously reflects such a therapeutic application (T 241/95, OJ EPO 2001, 103, point 4.1.2 of the reasons, see also T 158/96 of 28 October 1998, point 3.5.2 of the reasons) or, as decision T 158/96 also put it, if there is a “clear and accepted established relationship” between the shown physiological activities and the disease (loc. cit.). Once this evidence is available from the patent application, then post-published (so-called) expert evidence (if any) may be taken into account, but only to back-up the findings in the patent application in relation to the use of the ingredient as a pharmaceutical, and not to establish sufficiency of disclosure on their own.’”
363. Consistently with this statement of the law, it has been held in a number of cases that a patent will be insufficient if the specification requires the skilled person to undertake a substantial research project in order to perform the invention (either at all or across the breadth of the claim) and claims the results: see e.g. American Home Products Corp v Novartis Pharmaceuticals UK Ltd [2001] RPC 8 at [41]-[47] (Aldous LJ), Halliburton Energy Services Inc v Smith International (North Sea) Ltd [2006] EWCA Civ 1715 at [18] (Jacob LJ), Novartis AG v Johnson & Johnson Medical Ltd [2010] EWCA Civ 1039 , [2011] ECC 10 at [50]-[92] (Jacob LJ) and Idenix v Gilead at [197] (Kitchin LJ).
364. Kitchin LJ returned to the question of the extent to which an invention must be enabled across the whole scope of the claim in Regeneron Pharmaceuticals Inc v Kymab Ltd [2018] EWCA Civ 671 , [2018] RPC 14 . Having reviewed a number of decisions of Boards of Appeal of the EPO, he concluded:
“231. First, it is not the law that a specification must necessarily enable the skilled person to make or perform all of the embodiments of a claimed invention. Were it otherwise, claims would be insufficient if they covered inventive improvements. But, as the decision in GENENTECH I/Polypeptide expression makes clear, in appropriate cases, a claim may embrace variants which may be provided or invented in the future and which achieve the same effect in a manner which could not have been envisaged without the invention.
232. Secondly, the assessment of insufficiency must be sensitive to the nature of the invention and the facts of the particular case. If the character of the invention is one of general methodology or is such that the invention is of general application then it may be permissible to claim it in general terms, even though the specification does not enable every way of arriving at its subject matter. Otherwise, as the Board explained in Modifying plant cells/MYCOGEN , no dominant patent could ever exist and each developer of a new method of arriving at that subject matter would be free of earlier patents. In many cases in the field of biotechnology, patent protection would then become illusory.
233. Thirdly, it is a general principle that the protection afforded by the claims must correspond to the technical contribution to the art made by the disclosure of the invention. The patentee is entitled to fair protection having regard to the nature and character of the invention he has described.
…
248. Th[e] exposition [of the law in Regeneron v Genentech at [173]] is, we believe, entirely consistent with the principles we have identified. A claim is not insufficient simply because it encompasses inventive embodiments provided they embody the technical contribution the disclosure of invention has made to the art.”
365. A particular issue which arises in this case concerns the sufficiency of claims which combine both broad structural and functional features. Counsel for the Claimants submitted that such a claim was valid if the skilled person or team could identify, without undue burden, some compounds having the claimed structural features which also fulfilled the claimed functional requirements. In my judgment this is not the law. Rather, the law is correctly stated in Case Law of the Boards of Appeal of the European Patent Office (9th edition, 2019) at page 368 (emphasis added):
“In T 544/12 the board confirmed that a definition of a group of compounds in a claim by both structural and functional features is generally acceptable under Article 83 EPC as long as the skilled person is able to identify, without undue burden, those compounds out of the host of compounds defined by the structural feature(s) in the claim which also fulfil the claimed functional requirements (following T 435/91 and T 1063/06).”
The statement of principle in T 544/12 Princeton University/Very High Efficiency Organic LEDs (22 November 2013) at [4.2] has subsequently been followed in other Board of Appeal decisions which counsel for the Claimants cited and relied on, such as T 555/12 Cytec Technology/Flexible Polymer Element (30 July 2015) at [5.1] and T 323/13 Princeton University/L2MX Complexes (5 March 2015) at [7.1.1].
366. As counsel for the Defendants accepted, this does not mean that the skilled person or team must be able to identify all compounds covered by the claim without undue burden. Rather, what is required is that the skilled person or team must be able to identify substantially all compounds covered by the claim without undue burden.
367. Assessment. It is convenient to consider the sufficiency of the claims by reference to those which feature Formula (I) in its widest form, namely claims 19A and 24A as dependent on 19A of EP 823. Although claims 2 and 4 of EP 301 are slightly narrower in that substituent A in Formula (I) is limited to (C1 -C4 )-alkylene and claim 8A is wider since it embraces any Carboxamide, neither side suggests that these differences affect the assessment.
368. As noted above, the number of compounds covered by Formula (I) is on any view staggeringly large. There is no dispute as to the accuracy of any of Prof Fishwick’s (conservative) estimates as to the number of options at the following positions:
369. Plausibility . For the reasons explained above, the first question to consider is whether the disclosure of the Family A Patents, read in the light of the skilled team’s common general knowledge, makes it plausible that the invention will work across the scope of the claims in issue. As the Claimants emphasise, the Defendants do not contend that claim 17A of EP 531 is insufficient. Accordingly, there is no dispute that the specification makes it plausible that Compound C is effective in the treatment or prevention of anaemia associated with CKD. Indeed, it fully demonstrates that Compound C satisfies the criteria for efficacy discussed in paragraph 260 above. It is convenient to note here that it is common ground that the specification also adequately demonstrates efficacy for Compounds E, F, J and K.
370. In the case of the claims in issue, however, the Defendants rely upon the unchallenged evidence of Prof Ward that the skilled medicinal chemist would have no real reason for supposing that substantially all the Formula I Compounds would be effective in inhibiting HIF-PH or increasing Epo or otherwise treating anaemia, nor would they be able to make a reasonable prediction that substantially all the compounds would be effective.
371. Furthermore, Prof Ward’s unchallenged evidence was there are good reasons for believing that a significant number of the Formula I Compounds would not be effective. First, there are compounds which would not be expected to cross the cell membrane and/or to have suitable ADME profiles. Second, many of the compounds would be predicted to be ineffective drugs because of the presence of groups known to have the potential to cause toxic side effects. Third, many of the compounds are likely to be challenging to synthesise either because of steric effects due to the close proximity of large substituents or due to a lack of literature precedent. Prof Ward said that the number of compounds that would be expected not to work for these reasons would be large.
372. The Claimants contend that this evidence is predicated upon either an incorrect construction of the claims in issue or an incorrect understanding of the law. The Claimants emphasise that the claims are not merely to compounds having the defined structural features, but to ones which satisfy the defined functional limitations, and in particular therapeutic efficacy according to the criteria discussed above. In short, the Claimants argue, the claims are limited to compounds which work, and therefore it necessarily follows that it is plausible that the invention will work across the scope of those claims.
373. I do not accept this argument. In my judgment it is precluded by the decision of the Court of Appeal in Idenix v Gilead , which is binding on this Court. In that case claim 1 of the patent was, on its face, a pure compound claim based on a Markush formula which embraced a very large number of compounds. The parties agreed at trial, however, that the validity of claim 1 should be assessed on the basis that it was to be construed as a claim to compounds which had anti-Flaviviridae activity (see [2014] EWHC 3916 (Pat) at [306]). I concluded that claim 1 was invalid for lack of inventive step on AgrEvo grounds because it covered compounds which the skilled team would not have considered plausible had anti-Flaviviridae activity and which therefore did not plausibly solve the technical problem of providing compounds which did have such activity, and thus the claim covered compounds which made no technical contribution to the art (see [449]-[450)]). For the same reasons, I concluded that the disclosure of the patent, read in the light of the common general knowledge of the skilled team, did not make it plausible that the invention would work across the scope of the claim, and therefore the claim was insufficient (see [469]).
374. The Court of Appeal upheld the conclusion that the claim lacked an inventive step for want of plausibility (see [2016] EWCA Civ 1089 at [129]), and held that it inevitably followed that it was also insufficient for the same reasons (see [140]). Importantly, when considering the question of plausibility for the purposes of inventive step, Kitchin LJ referred in his judgment not once but twice to the fact that it was agreed that the claim should be construed as a claim to compounds which had anti-Flaviviridae activity (see [116] and [124]).
375. It is fair to say that counsel for the appellant do not appear to have submitted that, because the claim was limited to compounds which had anti-Flaviviridae activity, it necessarily followed that it was plausible that all compounds covered by the claim would work. Nor, consequently, did Kitchin LJ reject any such submission. It is not hard to see, however, why the very experienced team of specialist counsel who represented the appellant did not make that submission, and why neither of the two very experienced specialist judges (the other being Floyd LJ) who sat on the appeal thought that it was an answer to the objection of lack of plausibility. The reason is that it was implicit in the agreed construction of the claim that the patent was promising that substantially all compounds having the defined structure did have anti-Flaviviridae activity. Otherwise the patentee would have been saying, in effect, “I claim those compounds which are among the billions covered by the structural definition which happen to have anti-Flaviviridae activity, but I make no promise that any of them do, and you, dear reader, can go and find out which if any do have such activity”. That would not have involved an inventive step, because it would not have solved the technical problem of providing compounds which did have anti-Flaviviridae activity. Equally, it would have meant that the specification did not sufficiently disclose the invention, because it was leaving the task of finding compounds which had anti-Flaviviridae activity to the reader. As Lord Sumption pointed out in Warner-Lambert , the underlying consideration in both contexts is the same: what actual technical contribution has the patentee made to the art which justifies the scope of the monopoly claimed?
376. Turning to the present case, the patent is implicitly promising that substantially all compounds which satisfy the structural definitions in the claims in issue will have the claimed therapeutic efficacy. Otherwise, the skilled team would be faced with a situation where the structural definition covers around 10183 compounds (or a little less or even more), but the specification only demonstrates that five compounds, namely Compounds C, E, F, J and K, satisfy the criteria for therapeutic efficacy. That would amount to no more than an invitation to the skilled team to find the other compounds covered by the claim which work. It would not involve an inventive step, because it would not solve the technical problem of identifying compounds which have the desired activity, and it would not sufficiently disclose the invention, because it would leave most of the work to the reader.
377. The Claimants do not contend that it is plausible that substantially all the compounds covered by the structural definition in the claims in issue do have the claimed therapeutic efficacy. This is no doubt for the very good reason that there is nothing in the specification which could support such a claim. Indeed, the specification makes no attempt whatsoever to explain, let alone justify, the choices which have been made in Formula (I) or to explain why substantially all the Carboxamides should be expected to work. (Nor does the specification attempt to explain why Compounds A and B, which are shown to induce Epo in Example 1 (and indeed, in the case of Compound A, to give one of the two best results), are not claimed (not being Carboxamides), although the skilled team might deduce that it is because A and B act as chelators of free iron.) In any event, it is clear from the evidence that it is not plausible that substantially all the compounds covered by the structural definition in the claims in issue do have the claimed therapeutic efficacy.
378. Instead, the Claimants argue that the technical contribution of the Family A Patents is the teaching that “heterocyclic carboxamides, being prolyl hydroxylase inhibitors, can be used to treat anaemia associated with kidney disease by inhibiting HIF-PH”, and that they are entitled to claims commensurate with that technical contribution. As I understand it, what the Claimants mean by this statement is that some heterocyclic carboxamides (i.e. Carboxamides as I have defined that term) can be used to treat anaemia. They do not mean that all, or even substantially all, heterocyclic carboxamides can be used for that purpose.
379. I do not accept this argument. In the first place, it does not reflect my conclusions with regard to Epstein. In the light of those conclusions, the actual technical contribution to the art is no more than the identification of Compounds C, E, F, J and K as being ones that have therapeutic efficacy for anaemia associated with CKD. That might well justify a claim to a wider group of compounds that could plausibly be predicted to have similar efficacy for given structure-activity reasons. It does not begin to justify the claims in issue.
380. Secondly, even if I am wrong to conclude that Epstein makes it obvious to test suitable compounds for efficacy in increasing endogenous Epo and hence treating anaemia, I still do not accept that the postulated technical contribution justifies the breadth of the claims in issue. A finding that some (specifically, five) heterocyclic carboxamides can be used to treat anaemia does not justify a claim to “whichever ones out of 10183 compounds in addition to those five that you the reader are able to find that work through your own researches”. To put the same point another way, that finding is not a principle of general application across the breadth of the claims. It is not a principle of general application because the evidence shows that a large number of heterocyclic carboxamides are not likely to work.
381. I therefore conclude that all the claims in issue are insufficient for want of plausibility. (I should make it clear that this includes claims limited to “structural mimetics of 2-oxoglutarate” if, contrary to the conclusion reached above, it is possible to identify compounds which satisfied that criterion since it would still embrace a very large number of compounds.) In case I am wrong in reaching that conclusion, however, I shall nevertheless go on to consider whether the invention can be performed across the scope of the claims in issue without undue burden.
382. Undue burden . The starting point here is to consider in a little more detail what the skilled team would learn from the specification.
383. It is common ground that, despite the absence of any experiment involving isolated HIF-PH, the skilled nephrologist would assume that the results reported in the Examples for Compounds A-K were attributable to the inhibition of this enzyme. Nowhere in the specification, however, do the inventors identify the mechanism of such inhibition, for example, whether the compounds do so as competitive, non-competitive or allosteric inhibitors.
384. As Dr Bhalay accepted, it was common general knowledge that one of the properties of phenanthrolines was their capacity to chelate free iron, and so the skilled medicinal chemist might attribute the effect of Compound A on Epo levels reported in Figure 1 to the chelation of free iron. Accordingly, in the case of Compounds C-I, the medicinal chemist might reasonably attribute their effects on Epo reported in Figure 1 to the chelation of free iron as well.
385. Nor do the inventors attribute the biological activity of any of the exemplified compounds to any particular functional group or groups. As Prof Ward explained, no meaningful information can be derived about the relationship between the structure of the compounds and their function from the experimental data for three reasons, none of which was challenged. First, none of the Examples interrogates the inhibitory activity of the Compounds against HIF-PH in an isolated enzyme assay, which makes it impossible to tell the extent to which the differences between them are attributable to differences in cellular permeability, stability or solubility. Secondly, none of Compounds A-K is reported to be inactive, which means it impossible to determine which functional groups are key for activity. Thirdly, the structural differences between Compounds C-K are both very limited and non-systematic, while Compounds A and B are too structurally distinct to be compared to the others yet are shown to be active in inducing Epo (indeed, as noted above, Compound A is one of the two most active compounds in this respect). Thus the medicinal chemist cannot build even a rudimentary SAR on the basis of the information disclosed.
386. Nor does the specification give the skilled medicinal chemist any assistance at all with regard to such matters as finding compounds within Formula (I) which have suitable ADME profiles. The medicinal chemist could, of course, apply their common general knowledge rules of thumb such as Lipinski’s rules, but predictions made using such rules would not always be correct.
387. The next question is what the skilled medicinal chemist would learn if, contrary to my conclusion above, they read the six papers cited in [0075]-[0076] of WO 997 and (as Dr Bhalay was instructed to do) US 995, US 305 and US 730. I have set out what they would learn as a result of that exercise above. The upshot is that, as Dr Bhalay accepted, the medicinal chemist would conclude that Compounds C-K could be inhibiting HIF-PH competitively with respect to 2-OG, but there are other plausible mechanisms by which they could be having that effect. Moreover, the medicinal chemist would be no better informed as to the relationship between structure and function in Compounds C-K, still less as to the relationship between structure and function in the vast array of compounds covered by Formula (I). Nor would the medicinal chemist have much idea of the three-dimensional shape and chemistry of the active site of HIF-PH.
388. Dr Bhalay’s evidence in paragraph 168 of his first report was that, having read the six papers and the three US patents, it would be straightforward and routine for the skilled medicinal chemist “to identify compounds other than those tested in the specific examples which exhibit similar activity and have potential use in therapy”. It is clear from what he said both in this paragraph and elsewhere in his first report that what he meant by this was some compounds. The same goes for his third report.
389. It is clear from Dr Bhalay’s evidence that this would require a substantial amount of work. The medicinal chemist would start by making Compounds C-K in order to verify the teaching of the Family A Patents and for benchmarking purposes. The medicinal chemist would then synthesise and test other compounds using the same or similar assays to those disclosed in EP 823. The medicinal chemist would make sensible choices (e.g. avoiding excessively and unnecessarily bulky substitutions). Relatively large numbers of compounds could be tested in vitro and those that which showed activity could be tested further in vivo .
390. Just making the compounds is not simple. The Family A Patents do not teach how to make any of the compounds that they describe. There is a dispute as to how useful the information contained in the cited patents would be in synthesising new compounds, and the extent to which combinatorial synthesis could assist. Prof Ward thought that a single skilled medicinal chemist could make approximately 150 compounds a year, while Dr Bhalay said that using combinatorial or parallel synthesis it would have been feasible to make “many thousands of compounds in a year”. It does not matter who is right about this, and I will assume Dr Bhalay is, at least if “many” is interpreted as being low single digits.
391. Once synthesised, the compounds would have to be assayed and then the results used for further development. In vitro assays would be quicker to carry out than in vivo ones, but even so some time would be required to test large numbers of compounds.
392. Dr Bhalay clarified the nature of this exercise in cross-examination:
i) the medicinal chemist would start with an SAR analysis, involving tens, hundreds or even thousands of compounds to see what kind of changes are tolerated, testing for activity in an enzyme assay;
ii) depending on the strategy, there might be “spot checks” to see whether the compounds were competitive with respect to 2-OG;
iii) compounds which looked promising would be progressed to cell-based Epo induction assays;
iv) after that, promising compounds would go on to in vivo Epo induction assays, but not before completion of some initial pharmacokinetic studies.
393. The number of compounds involved in this initial SAR analysis (even if ran to thousands) would obviously not scratch the surface in terms of the number of permutations envisaged by Formula (I). Dr Bhalay envisaged that the medicinal chemist would then undertake further SAR “streams”, each involving a different chemotype, by which he meant pyridine, isoquinoline, quinoline etc. In each case, the medicinal chemist would adopt the lead optimisation strategies described in paragraphs 83-85 above.
394. In summary, the search for active compounds beyond Compounds C-K would be a very substantial undertaking, even for a well-resourced company. By its very nature, it would be a research programme, as Dr Bhalay accepted. Moreover, the difficulty of making any reliable prediction of what would work is vividly illustrated by Dr Bhalay’s comment that, even having read the six papers and three patents, it would be “a leap of faith” to conclude that (as the Claimants contend) useful guidance could be obtained from the information they provided about collagen prolyl hydroxylases given the absence of information that the three-dimensional shape of the active site of HIF-PH was the same.
395. Prof Ward’s evidence was to the same effect. Indeed, he was cross-examined on the basis that the search for active compounds constituted a “development programme”. It was not put to Prof Ward that the medicinal chemist would be able to predict reliably that a given molecule (even one that was closely related to any of Compounds C-K) would be active. He maintained that the exercise was one of iterative research. Moreover, consistently with the approach taken by Dr Bhalay, it was not suggested to Prof Ward that the skilled medicinal chemist could possibly synthesise and test even an infinitesimal proportion of the compounds covered by Formula (I) no matter how long they spent on the exercise. The case that was put was merely that the medicinal chemist could find some compounds.
396. In addition to the opinion evidence of the experts summarised above, there is a certain amount of evidence that post-dates the Family A Patents from the parties’ disclosure. This consists of results from various assays in respect of compounds which qualify as Carboxamides even if they do not fall within Formula (I). Leaving aside the fact that there is no witness evidence concerning this work, as the Defendants point out, some caution is required because it is evident that some of it was carried out after the publication of a crystal structure of HIF-PH and Compound C in 2006 (see N.C. Warshakoon et al , “Design and synthesis of substituted pyridine derivatives as HIF-1α prolyl hydroxylase inhibitors”, Bioorg Med Chem Lett , 16, 5616-5620 (2006) (“Warshakoon”) at 5616 and references 9 and 20). That is information which obviously was not available at the Family A Priority Date, and as explained above would have made it easier to design active compounds.
397. The more informative evidence comes from FibroGen’s disclosure, which includes information about 2,884 compounds, of which there is assay data for 1,151. The assays comprise enzyme assays against PHD1, PHD2 and PHD3, cell-based Epo induction assays and in vivo Epo induction assays, but not every compound has data from all these assays. The data show that success in the HIF-PH assay (applying Dr Bhalay’s criteria) is not predictive of success (again applying Dr Bhalay’s criteria) in cell-based/and or in vivo Epo-induction. Moreover, of the compounds for which there is assay data, only 182 (16%) are shown to meet Dr Bhalay’s criterion for Epo-induction in vivo (which is not to say that 84% fail - in fact, Dr Bhalay’s evidence was that the pass rate amongst those tested was 86%). The majority of these compounds are isoquinolines. In the class of pyridines, there are only two compounds which were tested for in vivo Epo-induction. Only one of them passed, namely vadadustat. The other failed.
398. Finally, reliance was placed by the Claimants on Dr Bhalay’s unchallenged evidence, based on his reading of the Defendants’ disclosure documents, that, starting from Compound C, it took Akebia only just over two months, proceeding by standard modifications, to arrive at vadadustat, which the Claimants contend exemplifies the claimed inventions. All this shows, however, is that it is possible to identify another compound which works without difficulty.
399. Taking all of the evidence into account, the conclusion I reach is that the invention cannot be performed across the scope of the claims in issue without undue burden. It would require a substantial research project to identify any compounds other than those specifically identified in the specification which met the criteria for efficacy, and success would not be guaranteed. While it is probable that, if sufficient resources were thrown at the project, the skilled medicinal chemist would be able to identify some compounds falling within Formula (I) (and more which constituted Carboxamides) which were effective, they would not be able even in many lifetimes of sustained effort to make and test more than a tiny fraction of such compounds, and a substantial proportion either could not be made or would not work. This is not only setting the skilled team a research project and claiming the results, it is a never-ending one. Accordingly, on this ground also I conclude that the claims in issue are insufficient.
Uncertainty
400. The law . In Generics (UK) Ltd v Yeda Research & Developments Co Ltd [2012] EWHC 1848 (Pat) I said at [193]:
“… it is necessary to distinguish between claims that are difficult to construe or that have a ‘fuzzy boundary’ (in the words of Lord Hoffmann in Kirin-Amgen Inc v Hoechst Marion Roussel Ltd [2004] UKHL 46 [2005] RPC 9 at [126]) on the one hand from claims that are truly ambiguous on the other. It is regrettably common for claims to be difficult to construe, but the court will nevertheless strive to give such claims a sensible meaning having regard to the inventor's purpose. It is also common for claims to have a fuzzy boundary, because an integer of the claim involves some question of degree or an imprecise functional limitation. It is well established that is not itself objectionable. If a claim is truly ambiguous, so that it is unclear what is the correct test to determine whether or not a product or process infringes, however, then the claim is insufficient…”
That statement of the law was approved by the Court of Appeal in the same case: [2013] EWCA Civ 925 , [2014] RPC 4 at [78] (Floyd LJ).
401. In Anan Kasei Co. Ltd v Neo Chemicals and Oxides Ltd [2019] EWCA Civ 1646 , [2020] FSR 8 at [24]-[25] (Floyd LJ) and [101]-[104] (Lewison LJ) the Court of Appeal held that this type of insufficiency is better described as “uncertainty” rather than ambiguity. At [26]-[27] Floyd LJ rejected in the following terms a submission that such insufficiency was only available where it was impossible to tell in any case whether a product infringed:
“I think that Lord Hoffmann's emphasis [in Kirin-Amgen ] was simply intended to draw attention to the distance between the judge’s finding and a case which presented doubtful cases at the edge of a claim. For my part, I do not agree that the objection of uncertainty is answered simply because there is something within the claim which is clear, if there is a large territory (more than a fuzzy boundary) where the claim is uncertain. ”
402. Assessment . The Defendants contend that the claims of EP 823 and EP 301 which include the expression “structural mimetic of 2-oxoglutarate”, namely claim 24A of EP 823 and claim 4 of EP 301, are insufficient on the ground of uncertainty. I accept that contention. For the reasons given above, the skilled team would not know what constituted a “structural mimetic of 2-oxoglutarate”, and in particular what test to apply to distinguish between a compound which is, and a compound which is not, a “structural mimetic of 2-oxoglutarate”. The claims are therefore uncertain, and hence invalid for insufficiency.
403. In addition to their arguments on the construction of this expression, the Claimants assert that Prof Ward accepted that Compound C is a “structural mimetic of 2-oxoglutarate”, and rely upon that evidence as supporting their contention that the term is not uncertain. I do not accept this for two reasons.
404. First, Prof Ward did not accept the proposition asserted. What he accepted were two different points. The first was that, having read Majamaa 1984 and Bickel, the skilled person would consider that it was a reasonable hypothesis that Compound C was a competitive inhibitor of 2-OG because of its similarity of structure to 2-OG, but they would have to test it. The second was that Warshakoon, with the benefit of the crystal structure, was fair to state at 5616 that Compound C (isoquinoline 3 in Warshakoon) “may inhibit EGLN enzymes by acting as a 2-OG mimetic”.
405. Secondly, even if it is assumed that the skilled team would conclude that the inventors regarded Compound C as a “structural mimetic of 2-oxoglutarate”, that would not solve the problem of determining what the test was for identifying other “structural mimetics of 2-oxoglutarate”. As Floyd LJ held in Anan v Neo , it is not an answer to the objection of uncertainty that it is clear that some things do fall within the claim.
AgrEvo obviousness of EP 823 and EP 301
406. The Defendants contend that the claims of EP 823 and EP 301 are obvious on AgrEvo grounds. In support of this contention, the Defendants rely upon the same arguments as they rely upon in support of their case on insufficiency through lack of plausibility. Given my conclusion on insufficiency, it follows that the claims in issue are also obvious through lack of an inventive step applying the principles laid down by the Court of Appeal in cases such as Idenix v Gilead . As noted above, the underlying consideration is the same. This point would only matter if an appellate court were in future to decide that there was some relevant difference between AgrEvo obviousness and insufficiency.
Infringement of Family A by vadadustat
407. There are a number of issues as to whether vadadustat infringes the claims of the Family A Patents. There is no dispute, however, that vadadustat is a Carboxamide which is suitable for use in the treatment of anaemia associated with CKD in that it meets the criteria for efficacy discussed in paragraph 260 above. Nor is there any issue concerning the intention requirements in claims 1 and 2 of EP 823. Accordingly, if it was valid, vadadustat would infringe claim 8A of EP 823.
Infringement on a normal interpretation of the claims
408. Claims involving Formula (I) . There is a dispute as to whether vadadustat infringes claims in which the class of compounds is defined by reference to Formula (I). Vadadustat is shown beside Formula (I) below.
409. It is common ground that vadadustat satisfies the requirements for A (which includes CH2 ), B (which includes COOH), Q-R4 (which includes OH), Y (which includes CH) and R1 (which includes H). As for R2 , there is no dispute that one of the permitted groups at this position is (C6 -C12 )-aryl, which would include phenyl. The issue is whether Formula (I) allows such an aryl group to be substituted with halogen. This is the question of construction considered above. Given my conclusion on that question, it follows that vadadustat does fall within Formula (I).
410. There being no dispute as to any of the other requirements of the claims, it follows that vadadustat would infringe claim 19A of EP 823 and claim 2 of EP 301 if they were valid.
411. Claims requiring “a structural mimetic of 2-oxoglutarate” . The only issue here is as to the validity of these claims. It follows that vadadustat would infringe claim 24A of EP 823 and claim 4 of EP 301 if they were valid.
Infringement by equivalence
412. Claim 17A of EP 531 is limited to Compound C. It is common ground that this claim is not infringed on a normal interpretation of the claim, because vadadustat is different from Compound C, as can be seen from the side-by-side comparison below.
413. The Claimants contend that vadadustat nevertheless infringes claim 17A by virtue of the doctrine of equivalents.
414. The law . In Actavis UK Ltd v Eli Lilly and Co [2017] UKSC 48 , [2017] Bus LR 1731 the Supreme Court held that a patent may be infringed by virtue of this doctrine even if the product or process does not fall within the relevant claim(s) as a matter of interpretation. In order to determine this, Lord Neuberger said at [66] that the court should generally ask itself the following three questions:
“(i) Notwithstanding that it is not within the literal meaning of the relevant claim(s) of the patent, does the variant achieve substantially the same result in substantially the same way as the invention, i.e. the inventive concept revealed by the patent?
(ii) Would it be obvious to the person skilled in the art, reading the patent at the priority date, but knowing that the variant achieves substantially the same result as the invention, that it does so in substantially the same way as the invention?
(iii) Would such a reader of the patent have concluded that the patentee nonetheless intended that strict compliance with the literal meaning of the relevant claim(s) of the patent was an essential requirement of the invention?
In order to establish infringement in a case where there is no literal infringement, a patentee would have to establish that the answer to the first two questions was ‘yes’ and that the answer to the third question was ‘no’.”
415. At the same time, however, Lord Neuberger said that these questions are guidelines, not strict rules, and that the language of some or all of the questions may sometimes have to be adapted to apply more aptly to the specific facts of a particular case.
416. Although the questions are framed to begin from the “literal” meaning of claim(s), it has since been clarified by the Court of Appeal that this means the “normal” or purposive meaning: Icescape v Ice-World International [2018] EWCA Civ 2219 , [2019] FSR 5 at [60] (Lord Kitchin).
417. Counsel for the Defendants advanced a series of criticisms of the decision in Actavis v Lilly . It is pointless for me to consider these criticisms, however, given that the decision is binding upon me.
418. Counsel for the Defendants also submitted that, if infringement by equivalents is found, then the same scope of claim should be adopted when considering any issue of validity. Having regard to my other conclusions, it is not necessary for me to consider this submission.
419. Assessment: question (i) . The Claimants put their case on question (i) in three alternative ways with increasing levels of particularity. I will consider these in turn. Before doing so, it is pertinent to observe that question (i) is partly a question of interpretation of the specification (what is the inventive concept revealed by (the relevant claim(s) of) the patent?) and partly a question of fact (does the variant achieve substantially the same result in substantially the same way?). In so far as it is a question of fact, it is clear that the burden of proof must lie on the patentee.
420. First, the Claimants say that the inventive concept embodied by claim 17A of EP 531 is the use of Compound C for treating anaemia associated with kidney disease. The skilled team would understand from EP 531 that Compound C solves the problem of treating renal anaemia by inhibiting HIF-PH. Vadadustat treats renal anaemia the same way, by inhibiting HIF-PH. Thus it achieves the same result in the same way.
421. I accept the premise of this argument, but not the conclusion. The inventive concept of claim 17A is indeed the use of Compound C (a specific molecule) for treating anaemia associated with kidney disease. The inventive concept is not the use of any compound that inhibits HIF-PH for treating anaemia associated with kidney disease.
422. The skilled team would appreciate from reading the specification of EP 531 (as proposed to be amended) that Compound C was different even to Compounds D-K both structurally and in terms of the experimental results obtained. As noted above, Compound C is not the best of those tested for Epo expression in Example 1; but it is the only compound tested in a number of the Examples, notably Example 4 showing the increase in haematocrit (which is what matters for treating anaemia). Even in the case of Compound C, HIF-PH inhibition is not actually demonstrated, although this may be inferred.
423. Vadadustat has a quite different structure to Compound C. Vadadustat does not have the bicyclic aromatic system of Compound C. It is therefore not an isoquinoline, as Compound C is. Instead, it has a monocyclic pyridyl ring, which is chlorophenyl-substituted. There is no teaching in EP 531, or anything in the common general knowledge, that would suggest to the skilled team, and in particular the medicinal chemist, that the bicyclic ring was not essential to the function of Compound C, or that it could be replaced by a chlorophenyl group with no material effect on binding or specificity.
424. Vadadustat also differs from the commonality between Compounds C-K in other ways. It has:
i) a hydroxyl group at the Q-R4 position of Formula (I), which Compound D does not have;
ii) a hydrogen at R3 , which is found only in Compounds H and I; and
iii) a hydrogen at R1 , which is only found in Compounds D, E, F and G.
425. Counsel for the Claimants asserted in closing submissions that it is now known from crystal structures that Compound C and vadadustat bind to HIF-PH in materially the same way, relying on T.-L. Yeh et al , “Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials”, Chem Sci , 8, 7651-7688 (2017). Although a passage from this paper was put to Prof Ward in cross-examination, however, that proposition was not to put to him, and it does not appear to me that the paper goes quite that far. What the authors say at 7653-7655 is that vadadustat shows a “similar binding mode” to FG-2216 (Compound C) involving metal coordination via glycinamide oxygen and pyridine/isoquinoline nitrogen and electrostatic interactions of glycinamide carboxylate with Tyr-239 and Arg-383 in PHD2. They go on, however, to discuss the rotational freedom of the chlorophenyl group around the C-C axis that connects the two aromatic rings in vadadustat, something that has no counterpart in Compound C.
426. Although there is no direct comparison in evidence, it is likely, given the differences between them, that vadadustat has different activity to Compound C in terms of its binding and specificity to HIF-PH. Indeed, it seems likely that vadadustat has superior binding and specificity than Compound C given that vadadustat has progressed to a Phase III trial, whereas Compound C has not.
427. Furthermore, there are no data available about vadadustat’s ability to inhibit collagen PH. It is not, therefore, known whether vadadustat is a specific inhibitor of HIF-PH or whether (like Compounds C to K) it inhibits both collagen PH and HIF-PH, although for the reason just given it is likely that it is more specific.
428. In those circumstances, I do not consider that it has been shown that vadadustat achieves the same result in the same way (or even substantially the same way) as Compound C.
429. Secondly, the Claimants say that Compound C solves the problem of treating anaemia by inhibiting HIF-PH through being a 2-OG structural mimetic and that vadadustat also inhibits HIF-PH competitively with respect to 2-OG.
430. I do not accept this either. The mechanism by which Compound C inhibits HIF-PH is nowhere explained in EP 531, and the skilled team does not know whether it acts as a competitive, non-competitive or allosteric inhibitor. As noted above, Prof Ward accepted that, having read Majamaa 1984 and Bickel, the skilled medicinal chemist would consider that it was a reasonable hypothesis that Compound C was a competitive inhibitor to 2-OG because of its similarity of structure to 2-OG, but he said they would have to test it. He also accepted that Warshakoon was fair to state that Compound C acts as a 2-OG mimetic. The latter piece of information would not, however, be available to the skilled team reading EP 531 in December 2001. Moreover, it is still not known even now whether Compound C is a competitive inhibitor to 2-OG. On the other hand, there is evidence (in a conference abstract from a number of Akebia scientists published in November 2019) that vadadustat shows 2-OG competitive inhibition against human HIF-PHDs.
431. Thirdly, the Claimants say that vadadustat and Compounds C–K all have a “common structural motif” consisting of a carbonyl glycine group attached to a heterocyclic ring, where the heterocyclic ring features at least one coordinating group adjacent to the carbonyl glycine moiety. This is shown by the blue rings below.
432. Again, I do not accept this. First, there is no “common structural motif” in the invention of claim 17A of EP 531. That claim is to Compound C alone, and the inventive concept is no broader than the use of Compound C itself. The claim is not to Compounds C-K. Accordingly, it cannot be right to search for an inventive concept that is common to those Compounds.
433. Secondly, there is no description, or even hint, in EP 531 of the existence of a “common structural motif”, and no such motif would have been apparent to the skilled medicinal chemist.
434. Thirdly, the alleged common structural motif is in any event not a proper characterisation of the features shared by Compounds C-K. T he Claimants have identified only common features said to be found in the alleged infringements (i.e. including daprodustat), and omitted features not found in them, in order to suit the infringement case. Prof Ward’s evidence was that the skilled medicinal chemist would not have cherry-picked these features, even if tasked with extracting some sort of commonality from Compounds C-K.
435. Nor did Dr Bhalay’s evidence support this “common structural motif”. The representation of the “common structural motif” (i.e. the blue rings) assumes that the oxygen atom appended to the pyridine ring has some significance in terms of biological activity. The most that Dr Bhalay could say was that the biological data in EP 531 suggested that an oxygen atom in this position (whether as an ether or part of a hydroxyl group) was tolerated. But in order to ascertain the significance or otherwise of this oxygen atom, he said that the skilled medicinal chemist would make and test equivalent compounds from which the hydroxyl group had been deleted and “build around the pharmacophore”, possibly with the assistance of computational modelling software.
436. Next, compounds C-K are all aromatic bicyclic systems. Dr Bhalay accepted that (at least in terms of an initial assessment) the bicycle should be included as a common feature. His only caveat was that the medicinal chemist could do some further work using the literature cited in the specification and SciFinder to see if there was any activity when jumping from an isoquinoline or quinoline to a pyridine. Yet at the same time, Dr Bhalay agreed that such a modification could result in changes in potency and other behavioural characteristics. Equally, he accepted that, as a matter of common general knowledge, there is a big difference between a bicyclic core and a monocycle, in that the former is capable of making additional Van der Waals and/or hydrophobic interactions with the active site.
437. Next, Dr Bhalay agreed that the medicinal chemist would see the benzyl ether on the pyridine ring as a key difference between Compound D and the other Compounds (all of which bear a hydroxyl group) and which could change the behaviour of the molecule.
438. In the light of the foregoing, the “common structural motif” would look more like the combination of the two blue circles superimposed on Compound C below.
439. As Dr Bhalay agreed, this motif is plainly not satisfied by vadadustat.
440. Fourthly, Dr Bhalay’s evidence that the presence of a carbonyl glycine moiety adjacent to the nitrogen of the heterocyclic ring in vadadustat are key contributors to its potency as an HIF-PH inhibitor was inconsistent with a large number of compounds that had been made and tested by FibroGen. Similarly, his evidence that the medicinal chemist would be confident that a compound having the capacity for bidentate iron-chelation coupled with a carboxyl group positioned equivalent to the C5 of 2-OG would be a HIF-PH inhibitor was inconsistent with other compounds made and tested by FibroGen.
441. Fifthly, if the claim language is ignored entirely and the skilled medicinal chemist is told to extract some core structural features from the description as a whole that the patentee has indicated as being of importance, I agree with the Defendants that they would look to Formula (I). There at least the patentee has identified structural features of the compounds considered to be important for activity. Formula (I) is very broad in some respects, but narrow in others. This can be demonstrated by reference to the following version of Formula (I) in which the ring positions have been labelled a-f.
442. In Formula (I):
i) the heterocyclic ring has a mandatory nitrogen at position (a), so this is not just a feature of Compounds C-K but of all the huge number of compounds covered by Formula (I);
ii) the mandatory nitrogen at position (a) of the heterocyclic ring cannot be substituted;
iii) Y cannot be a substituted nitrogen;
iv) R2 cannot be oxygen (so as to form a carbonyl group);
v) the ring atom at position (d) must be carbon; and
vi) the heterocyclic ring must be aromatic.
443. Despite this, none of these attributes appears in the “common structural motif” relied upon by the Claimants.
444. Finally, I should deal with a point which I understood counsel for the Claimants to rely upon in support of all three ways in which the Claimants put their case. This is that, as noted above, Akebia developed vadadustat starting from Compound C. I cannot see that this has any bearing on the matters considered above.
445. Accordingly, I conclude that the answer to question (i) is “no”. It follows that the remaining questions do not arise. I will nevertheless go on to consider them on the assumption, contrary to the conclusion I have reached, that the answer to question (i) is “yes”,
446. Question (ii) . Given the way in which question (ii) has been formulated by the Supreme Court, there will rarely be scope for a negative answer if the answer to question (i) is “yes”, and I do not consider that there is in the present case.
447. Question (iii) . As Lord Neuberger pointed out in Actavis v Lilly at [71], the answer to question (iii) cannot be dictated by the fact that the variant does not fall within the wording of the claim on its normal interpretation, because otherwise there would be no point in answering the question. As he went on to explain at [74], what matters in this context is the reason why the addressee would think that the claim was limited in the relevant respect (in that case, to the disodium salt).
448. In the present case I consider that it is clear that the skilled team would conclude that the patentee intended that strict compliance with the normal meaning of “Compound C” was an essential requirement of the invention of claim 17A for a number of reasons.
449. First, claim 17A is limited by structure to Compound C. On its face, it is not intended to cover anything that works or anything that does so by competing with 2-OG (which would be purely functional definitions) or even anything which shares the “common structural motif” (which would be an open-ended structural feature).
450. Secondly, read in the context of the specification, the claim is clearly intended to be a narrow one, and much narrower than either Formula (I) or Formulae (Ia) to (Id). The skilled team would understand that it was the function of Formulae (Ia) to (Id), and not claim 17A of EP 531, to define protection narrower than Formula (I), but broader than an individual compound.
451. Thirdly, Compound C would be understood to be the most promising and best-explored of the exemplified compounds in EP 531. The skilled team would realise that the patentee had limited the claims accordingly, and was not claiming different or untested compounds. In other words, a technical choice had been made.
452. Fourthly, it is common ground that the skilled team is to be taken to be aware that granted claim 1 has been amended down to claim 17A and that the other granted claims have been deleted. I do not understand it to be disputed that they are also to be taken to be aware that the reason for the amendment was that the broader claims were invalid (or at least that there was a substantial risk that they would be found to be invalid).
453. As counsel for the Defendants submitted, it is contradictory for the Claimants on the one hand to be amending the claim down to just Compound C, particularly in order to save its validity, and yet at the same time to be asserting that the scope of protection of the amended claim extends well beyond Compound C to a structurally rather different compound, and by implication to a large number of other compounds as well. By amending down to Compound C, the Claimants are disclaiming the other ways of achieving the same effect disclosed in the specification, and in particular everything covered by the broader granted claims.
454. This is an extreme instance of a principle which is well established in the jurisprudence of the Bundesgerichtshof (German Federal Court of Justice). As the BGH held in Case X ZR 16/09 - Okklusionsvorrichtung (Occlusion Device):
“If the description discloses a plurality of possibilities for achieving a specific technical effect, but only one of those possibilities is catered for in the patent claim, the utilisation of any of the other possibilities properly does not constitute infringement of the patent with equivalent means.”
455. Fifthly, the skilled team would recognise that vadadustat is less structurally similar to Compound C than Compounds D to K of EP 531. Accordingly, the skilled team would conclude that, having disclaimed Compounds D to K of EP 531, it was not the patentee’s intention that a claim to Compound C of EP 531 would extend to a product such as vadadustat.
456. Again, this is an instance of a principle recognised by the BGH that, where the specification discloses several ways in which a particular technical effect can be achieved but only one way is claimed, the conclusion that use of the other (disclosed but not claimed) ways to achieve the technical effect cannot amount to infringement as an equivalent extends to further undisclosed ways in which the technical effect can be achieved where the further ways operate in a manner more similar to the disclaimed than the claimed methods: see Case X ZR 69/10 - Diglyzidverbindung (Diglycid compound) at [45]-[46].
457. Sixthly, at least on the Claimants’ broader approaches to question (i), the scope of claim 17A by equivalence would extend to the compounds which are shown to be HIF-PHIs in Epstein. Since the case of obviousness over Epstein has only failed because it did not lead to Compound C, the result would be an invalid claim. This is a good reason to conclude that the scope of claim 17A is not intended to extend beyond Compound C.
458. Seventhly, the scope of claim 17A by equivalence would be a claim that was so broad that it would suffer the same problems with insufficiency (both plausibility and undue burden) as the claims of EP 823 and EP 301. Again, that is a good reason to conclude that the scope of claim 17A is not intended to extend beyond Compound C.
459. Eighthly, there is the prosecution history. FibroGen chose to limit the scope of claim 1 of EP 531 to compounds of Formula (I) in order to overcome an objection from the examiner that the previous claims, which were directed to “a heterocyclic carboxamide compound”, lacked novelty over prior art referred to as D11. FibroGen thereby represented that it was not seeking to contend that the patent, if granted, would have a scope that extended to heterocyclic carboxamides beyond the confines of Formula (I). Yet extending the scope of claim 17A in this way is precisely what the Claimants are now seeking to do. In those circumstances, this is one of those cases referred to by Lord Neuberger in Actavis v Lilly at [88] where it would be contrary to the public interest for the contents of the prosecution file to be ignored.
460. Contrary to the Claimants’ submission, I do not accept that it is an answer to this objection that, in its response, FibroGen stated that “Any deleted subject matter is not abandoned” and that “Although the applicant maintains that the previous claims were patentable, the applicant has amended the claims to simplify the outstanding issues in the hope of progressing the applicant to grant”. FibroGen cannot have it both ways. If it wanted a broader claim, it should have maintained its position in the face of the examiner’s objection and, if necessary, appealed to the Board of Appeal (as was its right). It did not do so, but accepted a narrower claim. It is inconsistent with that acceptance for it now to say that the claim extends beyond that. (The fact that the Claimants have subsequently further limited the claim to Compound C does not detract from this, but simply makes it even worse.)
461. If, finally, one cross-checks the foregoing conclusion with the Protocol on the Interpretation of Article 69 of the European Patent Convention, I consider that it is manifest that extending the scope of protection of claim 17A in the manner contended for by the Claimants would go well beyond fair protection for the patentee and would not afford a reasonable degree of legal certainty for third parties.
462. Accordingly, I conclude that claim 17A of EP 531 is not infringed by vadadustat.
Amendment of EP 531
463. The only issue as to the allowability of the amendments to the Family A claims which remained live at the end of the trial concerns the amendments to granted claim 1 of EP 531 to produce amended claim 17A. On its face, these amendments amount to a drastic narrowing of the claim. Nevertheless, the Defendants contend that, if claim 17A is infringed by virtue of the doctrine of equivalents, then the amendments are not permissible because they extend the scope of protection of granted claim 1. If I am correct that vadadustat does not infringe claim 17A, this issue does not arise. If I am wrong in that conclusion, I do not consider the amendments are impermissible. Vadadustat would, on my construction of Formula (I), have fallen within the granted claim as well as the amended claim, and thus infringement would not demonstrate any extension of protection. The fact that the granted claim would, having regard to my conclusions on insufficiency, be invalid, whereas claim 17A is valid, does not mean that the scope of protection of the claim has been extended.
The Family B Patents
464. As noted above, I shall set out the disclosure of the Family B Patents by reference to WO 121. Again, I shall do so using the headings in the specification. The same caveats apply as in the case of the Family A Patents. Quite a lot of the specification of WO 121 is repeated from WO 997.
Field of the invention
465. This is identified at [0002] in the following terms:
“The present invention relates to methods and compounds for regulating or enhancing erthropoiesis [sic ] and iron metabolism, and for treating or preventing iron deficiency and anemia of chronic disease.”
This statement is repeated in the corresponding paragraph ([0001]) of the Family B Patents.
Background of the invention
466. At [0003]-[0004] the specification refers to anaemia associated with chronic disease of inflammation (ACD), and points out that ACD is often associated with absolute or functional iron deficiency. At [0005] it notes that numerous physiological deficiencies are observed in patients with ACD, including reduced Epo production and impaired iron metabolism, which contributes to impaired erythropoiesis.
467. At [0006] the specification notes that ACD is associated with increased production of inflammatory cytokines. It goes on to discuss evidence that such cytokines mediate Epo production. This paragraph cites, at various points, ten papers. The specification does not in terms explain why these papers are referred to. The natural inference is that they are being cited as providing scientific support for the statements made in the paragraph. There is no apparent reason why papers are cited in [0006], but not in the rest of this section of the specification.
468. At [0007] the specification identifies a need for methods of treating or preventing ACD, and in particular in terms of overcoming Epo issues and iron issues.
469. At [0008]-[0013] the specification discusses iron deficiency, describing absolute and functional iron deficiency (the latter being frequently associated with ACD). Since iron deficiency can lead to impaired erythropoiesis, a need is identified for methods of treating or preventing disorders associated with iron metabolism and of enhancing iron metabolism.
Summary of the invention
470. The specification states at [0015]:
“The present invention relates to methods and compounds for inducing enhanced or complete erythropoiesis in a subject. In particular, the methods comprise inducing enhanced or complete erythropoiesis by stabilizing HIFα in a subject. Methods of inducing enhanced erythropoiesis by inhibiting HIF prolyl hydroxylase are specifically contemplated. In specific embodiments, the methods comprise administering to a subject a compound of the invention. In various embodiments, the subject can be a cell, tissue, organ, organ system, or whole organism.”
471. The summary of the invention then continues at some length from [0016] to [0083]. At [0034] the specification states:
“The invention provides various methods of regulating/enhancing iron processing and iron metabolism. In one aspect, the invention provides methods for increasing iron transport, uptake, utilization, and absorption in a subject, each of the methods comprising administering to the subject an effective amount of a compound that stabilizes the alpha subunit of hypoxia inducible factor (HIF). In particular embodiments, the invention provides methods for increasing transferrin expression, transferrin receptor expression, IRP-2 expression, ferritin expression, ceruloplasmin expression, NRAMP2 expression, sproutin expression, and ALAS-2 expression in a subject, each method comprising administering to the subject an effective amount of a compound that stabilizes the alpha subunit of hypoxia inducible factor (HIF). In other embodiments, the invention provides methods for decreasing hepcidin expression, the method comprising administering to the subject an effective amount of a compound that stabilizes the alpha subunit of hypoxia inducible factor (HIF). Methods for increasing heme synthesis in a subject by administering to the subject an effective amount of a compound that stabilizes the alpha subunit of hypoxia inducible factor (HIF) are also provided.”
472. At [0038] the specification states that the invention encompasses a compound for use in a method for treating or preventing iron deficiency in a subject, the method comprising administering to the subject an effective amount of a compound that stabilises HIFα. The iron deficiency may be functional iron deficiency. [0044] and [0057] refer to compounds which stabilise HIFα, thereby treating or preventing functional iron deficiency. [0056] refers to a method of decreasing hepcidin expression by the administration of a compound that stabilises HIFα.
473. At [0083] the specification states that exemplary compounds of the invention include four specific compounds labelled A-D. Compound A in WO 121 is the same as Compound C in WO 997 and Compound D in WO 121 is the same as Compound B in WO 997. Compounds A-C are isoquinoline carboxamides, while Compound D is a hydroxamic acid. The names and structures of these compounds were conveniently set out by Prof Ward in his report:
Brief description of the drawings
474. This section introduces the Figures which show the results of the experiments set out in the Examples described later in the specification.
Description of the invention
475. This section contains some general statements about the way in which the invention is described in the specification, including a statement at [0113] which corresponds to the statement in WO 997 at [0041] quoted in paragraph 129 above.
Definitions
476. From [0115] to [0121] the specification set out a series of definitions. Reference is made in some of the definitions to a number of scientific papers.
477. At [0122] the specification states:
“In particular embodiments, the present invention provides for use of structural mimetics of 2-oxoglutarate. Such compounds may inhibit the target 2-oxoglutarate dioxygenase family member competitively with respect to 2-oxoglutarate and noncompetitively with respect to iron. (Majamaa et al. (1984) Eur J Biochem 138:239-45 [‘Majamaa 1984’]; and Majamaa et al. (1985) Biochem J 229:127-133 [‘Majamaa 1985’].) …”
No further explanation is provided, however.
Invention
478. At [0123] the specification repeats the statement of the invention made in [0015].
479. At [0124] the specification describes ACD specifically by reference to inflammatory disorders, cancer, rheumatoid arthritis etc.
480. At [0125] the specification introduces the invention as providing methods for “inducing enhanced or complete erythropoiesis” in subjects with ACD and a TSAT of less than 20%. It goes on:
“… Reduced or ineffective erythropoiesis is a common pathology in patients with anemia of chronic disease. Reduced or ineffective erythropoiesis can result from various metabolic abnormalities in the erythropoietic pathway including, for example, …abnormal iron processing including for example abnormal or ineffective iron uptake, mobilization, storage, and absorption.”
481. At [0127] the specification states:
“The present invention provides advantages over existing therapies for anemia of chronic disease, such as, for example, recombinant EPO administration. Reduced EPO production is only one aspect of decreased erythropoiesis and it is recognized that administration of recombinant EPO does not address other deficiencies associated with reduced erythropoiesis that exist in patients with anemia of chronic disease. These deficiencies include, for example, reduced EPO responsiveness of the bone marrow, as well as numerous aspects of iron metabolism that contribute to complete or total erythropoiesis including iron absorption from the gut, transenterocyte transport, oxidation of the iron to the ferric state by hephaestin or ceruloplasmin, binding and uptake of iron by transferrin and transferrin receptor and iron transport to the marrow where iron utilisation occurs, including heme synthesis. Many patients are refractory to administration of recombinant EPO for the reasons described above, in which responses to recombinant EPO administration are reduced or absent, even at high doses of recombinant EPO.”
482. At [0129] the specification states:
“Anemia of chronic disease is associated with increased levels of ferritin. Despite high levels of ferritin, subjects with anemia of chronic disease are not able to utilize iron effectively. High levels of ferritin are indicative of reduced iron recycling to the marrow and enhanced iron storage, a functional iron deficiency often associated with anemia of chronic disease and a pseudo-inflammatory state often existing in uremic chronic kidney disease patients. By decreasing ferritin levels, methods and compounds of the present invention decrease stored iron and enhance iron recycling through transferrin and transferrin receptor. Reduced serum ferritin levels would be indicative of enhanced iron utilization and enhanced iron recycling to the marrow, thus increasing iron availability for heme production and erythropoiesis.”
483. At [0135] the specification provides a further explanation of functional iron deficiency. It goes on:
“Iron is not available at a rate sufficient to allow normal hemoglobinization of erythrocytes, leading to reduced reticulocyte and erythrocyte cellular hemoglobin content. Functional iron deficiency is often seen in healthy individuals with apparently normal or even increased iron stores but with impaired iron availability, as measured, e.g., by low levels of percent transferrin saturation. This type of iron deficiency is frequently associated with acute or with chronic inflammation.”
484. At [0140]-[0141] the specification states:
“[0140] Numerous proteins mediate iron metabolism, including proteins such as … transferrin, transferrin receptor, iron transporters …, ceruloplasmin etc. Increases in transferrin and transferrin receptor expression stimulate iron uptake by erythroid progenitors and transport to marrow by macrophage ... Ceruloplasmin increases the oxidation of ferrous iron to ferric so that binding to transferrin occurs … In certain aspects, methods of the present invention increase iron metabolism by increasing expression or activity of proteins involved in iron metabolism including… transferrin, transferrin receptor… In other aspects, methods disclosed increase iron metabolism by decreasing expression or activity hepcidin and by modulating expression of ferritin.
[0141] In one embodiment, the disclosure provides methods and compounds for increasing expression of genes whose products are involved in iron metabolism and processing including iron uptake, storage, transport, absorption etc. Such genes include but are not limited to transferrin receptor, ceruloplasmin … Therapeutic upregulation of genes involved in iron metabolism and processing will effectively increase iron availability and, thereby produce a beneficial effect in patients with anemia of chronic disease, anemia of iron deficiency, functional iron deficiency etc. In another embodiment, the disclosure provides methods and compounds for decreasing expression of hepcidin, a protein associated with iron regulation.”
485. At [0148] the specification explains that decreased hepcidin expression is associated with increased iron release from reticuloendothelial cells and increased intestinal iron absorption.
486. A subsection of the specification headed “Compounds” at [0156]-[0177] discloses compounds for use in the invention in similar, but not identical, terms to WO 997. This subsection begins by stating that exemplary compounds that stabilise HIFα are disclosed in WO 03/049686 (“WO 686”) and WO 997 (in WO 121 these are said to be incorporated by reference in their entirety, but that wording is missing from the granted Family B Patents).
487. [0160]-[0162] are in almost identical terms to [0074]-[0076] of WO 997.
488. [0163]-[0164] identify the same Formula (I) and sub-formulae Formula (Ia), (Ib), (Ic) and (Id) as WO 997. There is no equivalent of the Carboxamides or Formula II in WO 121, but [0171] refers to embodiments of the invention selected from Formula (III) and (IIIa).
489. At [0165]-[0169] and [0172] the specification identifies “exemplary” or “additional” compounds within Formula (I), Formula (Ia), Formula (Ib) or Formula (III) by reference to 12 patents and patent applications. The same patents and applications are cited as in WO 997 at [0078]-[0088] save for US 898, US 974 and WO 860 which relate to Formula II. Each paragraph identifies a number of specific compounds. [0169] identifies Compound A as an example of Formula (Ib). [0170] identifies additional compounds for use in the invention without referring to any prior art, including Compounds B and C. [0172] identifies Compound D as an example of Formula (III).
490. Pharmaceutical Formulations and Routes of Administration are dealt with at [0178] onwards.
Examples
491. Examples 1-10 ([0198]-[0222]) are experiments in which the effects of Compounds A-C are examined in cells treated with various inflammatory cytokines. These experiments indicate that Compounds A-C are able to stimulate the production of endogenous Epo in the presence of these cytokines.
492. Example 11 ([0223]-[0224]) tests the effect of Compounds A and B on transferrin receptor expression. The specification concludes at [0224]:
“Therefore, compounds of the present invention are useful for increasing transferrin receptor expression in various cell types. In addition, increased transferrin receptor expression would result in increased transferrin receptor-mediated endocytosis of ferric transferrin, thereby increasing iron transport, utilization, storage, and metabolism Therefore, compounds of the present invention are useful for enhancing erythropoiesis by increasing iron transport, utilization, storage, and metabolism.”
493. Examples 12-14 ([0225]-[0229] are examples containing methods for testing for the effect of compounds of the invention on transferrin receptor expression, iron-regulatory protein-2 and iron utilisation. No results are reported.
494. Example 15 ([0230]-[0236]) demonstrates that Compound B increases the expression of genes encoding erythropoietic proteins in Hep3B cells.
495. Example 16 ([0237]-[0239]) is a method for testing the effect of compounds of the invention on measures such as serum iron and haematocrit in rats. No results are reported.
496. Example 17 ([0240]-[0254] shows the effect of expression of genes encoding iron-processing proteins in mice in vivo when treated with Compound A. Ceruloplasmin expression was increased in mouse kidneys over a period up to 72 hours, as shown in Table 3. Table 4 shows down-regulation of hepcidin expression over a period up to 16 hours. The figures reported do not appear to show a clear trend, however, and there is no statistical information. As Prof Haase explained, and Prof Winearls accepted, there is nothing to demonstrate that this effect is independent of erythropoiesis, although both witnesses considered that this was a possibility. Data are also provided showing upregulation of expression of the transferrin receptor (Figure 6A), the gut duodenal transporter NRAMP2 (Figure 6A; Figure 6B) and the first enzyme in the haem synthetic pathway ALAS-2 (Figure 6C).
497. Example 18 ([0255]-[0256]) is a method for testing the effect of compounds of the invention on erythropoiesis in vivo in mice. No results are reported.
498. In Example 19 ([0257]-[0258]) serum iron levels in rats in vivo were measured and found to be increased with administration of Compound A as shown in Table 5.
499. In Example 20 ([0259]-[0278]) an animal model of ACD was used in two series of experiments in which various measurements taken to ascertain the efficacy of Compound A, namely reticulocyte count, haematocrit, haemoglobin, red blood cell count, mean corpuscular volume and mean corpuscular haemoglobin. The same model was also used to measure serum iron and transferrin saturation, which are both reduced in patients with ACD. As shown in Figures 18A and 18B, administration of 40 mg/kg of Compound A resulted in a significant increase in both serum iron and TSAT in non-anaemic control animals. This increase was not observed to the same extent in anaemic animals, however, although administration of 40 mg/kg of Compound A produced lower levels of serum iron and TSAT than 20 mg/kg. Compound A was also found to increase expression of NRAMP2 and sproutin in the intestine (Figure 19), suggesting a beneficial effect on iron absorption.
500. In Example 21 ([0279]-[0284]) human subjects were given compound A and found to have increased reticulocyte count, increased haematocrit, increased red blood cell count, increased soluble transferrin receptor and decreased serum ferritin levels, consistent with increased iron utilisation.
501. It should be noted, for reasons that will appear, that the Family B Patents do not contain any data comparing the effects of HIF-PHIs to those of ESAs, and in particular no data showing that any of Compounds A-D have superior effects on iron mobilisation to ESAs.
The claims of the Family B Patents
502. The claims of the Family B Patents as proposed unconditionally to be amended which were relied upon by the Claimants at trial are as follows. All of these claims are EPC 2000 claims.
EP 333
503. Claim 1:
“A compound of formula (I) that stabilizes HIFα for use in treating anemia of chronic disease in a subject … wherein the subject has a percent transferrin saturation of less than 20%.”
504. (New) claim 2:
“The compound of claim 1 for the use of that claim, wherein the subject has a percent transferrin saturation of less than 16% in adults. ”
505. Claim 9 (formerly 6):
“A compound of formula (I) that stabilizes HIFα for use in treating anemia that is refractory to treatment with exogenously administered erythropoietin (EPO) in a subject wherein A, B, Q, R1 , R2 , R4 , Y and X are as defined in claim 1.”
506. (Unconditionally amended) claim 22A (formerly 15):
“A compound of formula (I) that stabilizes HIFα for use in treating functional iron deficiency in a subject, and wherein the functional iron deficiency is associated with anemia wherein A, B, Q, R1 , R2 , R4 , Y and X are as defined in claim 1.”
507. (Unconditionally amended) Claim 31A (formerly 24):
“The compound for use according to any of the preceding claims 1, 2, 7, 8, 9, 15, 16, 17, 20, 21, 22, 27 and 28 wherein the compound is for decreasing hepcidin expression in a subject.”
508. (New) claim 34A:
“The compound for use according to any of claims 102, 7-9, 15-17, 20-23, 27, 28, 31 and 32, wherein the compound is a structural mimetic of 2-oxoglutarate. ”
509. Claim 36A (formerly 27):
“The compound for use according to any of the preceding claims wherein the compound is [Compound A].”
EP 153
510. (Unconditionally amended) claim 1A:
“A compound that inhibits hypoxia inducible factor (HIF) prolyl hydroxylase activity for use in treating or preventing functional iron deficiency associated with anemia in a subject, wherein the compound is a structural mimetic of 2-oxoglutarate.”
511. (New) claim 2:
“The compound according to claim 1, where the compound is of Formula (I) … ”
512. (New) claim 11A:
“The compounds of claim 1, 2 or 3 for the use of that claim, wherein the compound is for decreasing hepcidin expression. ”
EP 155
513. Claim 1:
“A structural mimetic of 2-oxoglutarate that inhibits hypoxia inducible factor (HIF) prolyl hydroxylase activity for use in treating anemia in a subject having a percent transferring saturation of less than 20%.”
514. (New) claim 2:
“The mimetic of claim 1 for the use of that claim, wherein the subject has a percent transferrin saturation of less than 16% in adults. ”
515. (Unconditionally amended) claim 3B (formerly 2):
“The mimetic of claim 1 3A for the use of that claim, wherein the anemia is anemia of chronic disease is associated with a condition selected from the group consisting of an inflammation, an infection, an immunodeficiency disorder, and a neoplastic disorder .”
516. (Unconditionally amended) claim 6A (formerly 5) as dependent on (unconditionally amended) claim 5A (formerly 4):
“The mimetic of claim 1 or 2 for the use of that claim, wherein the anemia is associated with iron deficiency,
wherein the iron deficiency is functional iron deficiency”
517. (Unconditionally amended) claim 8A (formerly 7:
“The mimetic of any one of claims 1, to 4, 5 or 6 for the use of that claim, wherein the mimetic is a compound of Formula I …”
518. (New) claim 15A:
“The mimetic of any preceding claim for the use of that claim, wherein the mimetic is for decreasing hepcidin expression. ”
The skilled team
519. It is common ground that the Family B Patents are addressed to the same skilled team as the Family A Patents.
Common general knowledge as at the Family B Priority Date
520. There is no dispute that everything which was common general knowledge at the Family A Priority Date remained common general knowledge at the Family B Priority Date. There are three areas of dispute as to the common general knowledge of the skilled nephrologist at the latter date. In the case of the first two, I understand it to be common ground that there was little, if any, relevant difference between the state of the common general knowledge as at December 2001 and as at April 2004, but the issues are relevant to Family B rather than Family A.
Treatment of ACD with ESAs
521. As noted above, there is no dispute that patients with ACD were sometimes treated with ESAs, but there is a minor dispute as to the effectiveness of such treatment. ACD was not an approved indication for ESAs, and so such treatment was “off-label” (i.e. prescribed by the responsible clinician on the basis of their own clinical judgment of the potential of the treatment to meet the needs of a specific patient). Prof Winearls agreed that this use of ESAs was conventional (although he later contradicted himself by saying that it was not conventional, but was done on an ad hoc basis). He said that in his view it was “somewhat misguided”, but nevertheless volunteered that it was an obvious thing to try because it was hoped to compensate for reduced Epo production by the kidneys and reduced responsiveness to Epo by the bone marrow. His opinion was that the treatment was generally ineffective, but he accepted that it worked in some patients.
522. Prof Haase’s opinion was that ESAs were effective in the treatment of ACD in some patients, particularly when used at higher doses. As he pointed out, that view is supported by several contemporaneous textbooks, such as E. Beutler et al (eds), Williams Hematology (6th ed, McGraw-Hill, 2001) at pages 484-485. Prof Haase agreed that it was thought that ESAs achieved such therapeutic effect as they did by compensating for reduced Epo production and responsiveness to Epo, and that ESAs were not thought to act by unblocking iron. Thus there was little difference between the experts.
Anaemia that is resistant to exogenous Epo
523. The main cause of resistance to exogenously administered Epo was thought to be iron deficiency and the second most common cause was the presence of infection or inflammation.
524. The 2002 UK Renal Association Guidelines defined “resistance” to Epo as failure to reach target haemoglobin or the need for doses of Epo above 300 IU/kg/week. Dr Ashman agreed this was a recognised definition of Epo resistance in 2001/2, and that this group of patients was a recognised patient cohort.
525. The US guidelines suggested that there was no point in giving Epo to patients who had stopped responding to it. Similarly, UpToDate , a multi-volume treatise published in the USA in CD-ROM form in 2001 states in volume 9 number 2 (edited by Schrier) that “It is not worthwhile to continue Epo in patients who do not have a clinically meaningful response by 12 weeks”. Prof Haase accepted that this reflected the common general knowledge, although his personal view was that completely stopping Epo would be a mistake.
Hepcidin
526. The protein hepcidin was only discovered in around 2000-2001. Hepcidin binds to ferroportin, the iron export channel found in intestinal cells, reticuloendothelial cells and hepatocytes, in an inhibitory fashion. This inhibition prevents the export of iron to the transferrin in the blood plasma and instead leads to iron sequestration in cells. Thus, increased hepcidin levels reduce iron absorption from the duodenum and also reduce the release of iron from macrophage stores. Conversely, decreased levels of hepcidin increase iron absorption and increase its release from stores.
527. Prof Winearls’ evidence in his first report was that the role of hepcidin in iron homeostasis was not known to the skilled nephrologist in April 2004.
528. Prof Haase’s evidence in paragraphs 74-76 of his first report was that the common general knowledge was as follows. It was known that the body systematically balances iron levels through the absorption of dietary iron and the release of iron from macrophages, regulated via hepcidin. Hepcidin is synthesised in the liver and its production is regulated in response to circulating iron levels. Hepatocytes sense the increased levels of transferrin-bound iron and hepcidin production is induced, resulting in inhibition of iron uptake in the duodenum and the prevention of iron release from macrophages. It was therefore thought that ACD, which is associated with functional iron deficiency, may be due to elevated plasma levels of hepcidin. Hepcidin transcription is suppressed in response to anaemia and hypoxia, resulting in the increased mobilisation of iron for use in erythropoiesis. It had therefore been proposed that Epo might be involved in the transcriptional downregulation of hepcidin. Hepcidin transcription was also thought to be upregulated by inflammatory cytokines. In support of this account, Prof Haase cited a number of papers.
529. Prof Winearls stated in paragraph 47 of his third report that, having reviewed the papers cited by Prof Haase, he “did not take issue with the substance” of what Prof Haase had said in his paragraphs 74-76, but “this knowledge had not reached a significant majority of nephrologists, let alone entered the therapeutic arena or become CGK for the Skilled Clinical Nephrologist”. In my judgment Prof Winearls was again focussing here on the clinical nephrologist as opposed to the pre-clinical researcher. Prof Haase’s unchallenged evidence in paragraph 40 of his third report was that the papers would have been known to the pre-clinical researcher.
530. Furthermore, Prof Winearls accepted in cross-examination that it was known by 2003 that Epo was involved in transcriptional down-regulation of hepcidin, adding “if you give erythropoietin to a dialysis patient the hepcidin comes down”.
531. Despite this, counsel for the Claimants submitted that the papers Prof Haase had mentioned in his reports did not show that it was known that Epo was involved in transcriptional down-regulation of hepcidin.
532. The paper which Prof Haase had relied upon his first report in this connection was G. Nicolas et al , “The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation”, J Clin Inv , 110, 1037-1044 (October 2002) (“Nicolas”). This states (at page 1042):
“A speculative scheme in the regulation of iron balance by hepcidin is proposed in Figure 7. When anemia/hypoxia occurs, e.g., following severe bleeding or after PHZ [phenylhydrazine] treatment, erythropoietin expression increases, leading to a stimulation of the erythropoietic activity. In parallel, hepcidin gene expression is decreased, inducing a rapid mobilization of iron from reticuloendothelial cells to supply sufficient amounts of iron for the erythropoietic activity. Indeed, we demonstrated previously that a deficiency in hepcidin gene expression results in a dramatic decrease in iron stores in reticuloendothelial cells (6). At the moment, we cannot specify whether erythropoietin is involved in hepcidin downregulation or whether hepcidin and erythropoietin responses to hypoxia are independent.”
533. Counsel for the Claimants drew attention to the uncertainty expressed in the last sentence (and reflected in a question mark in Figure 7), which Prof Haase accepted reflected the thinking at that time (i.e. 2002).
534. Nicolas goes on, however, to say (at 1043):
“These results [namely those reported in two prior papers] reinforce our hypothesis that hepcidin per se is a key component of the erythropoietic regulator of intestinal iron absorption.”
Counsel for the Claimants himself put it to Prof Haase that this was the understanding that a reader of the paper would take away from it, and Prof Haase agreed.
535. Prof Haase had referred in paragraph 74 of his first report to a review by T. Ganz, “Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation”, Blood , 102, 783-788 (August 2003) (“Ganz”).
536. Prof Haase was cross-examined on some passages towards the end of Ganz, in particular in the final two sections (at page 787):
“Therapeutic implications of hepcidin
…
Elucidation of the receptor and its transduction pathways should lead to the development of hepcidin antagonists, some of which could be useful in treatment of anemia of inflammation, a condition often resistant to erythropoietin therapy45 .
….
Conclusions
Hepcidin may be the principal iron-regulatory hormone, the key mediator of anemia of inflammation, and a bridge between innate immunity and iron metabolism (Figure 2). Studies of the molecular mechanisms of hepcidin activity could transform our understanding of the regulation of iron transport and should lead to new therapies for hemochromatosis and anemia of inflammation.”
537. Prof Haase agreed that these passages reflected the state of knowledge at the time. It was then put to him that there was no evidence that hepcidin was regulated by Epo or induction of erythropoiesis. He was not referred, however to the sections of Ganz which discuss Nicolas (reference 23). These sections state (at 785-786):
“Induction of hepcidin by infection and inflammation
…
[reference to Nicolas]
…
In the aggregate, the increase of hepcidin production by inflammation and the ability of transgenic or tumor-derived hepcidin to suppress erythropoiesis by iron starvation strongly suggest that hepcidin is the key mediator of anemia of inflammation. However, it still remains to be shown that hepcidin peptide administration to mice or humans will cause iron sequestration and iron-limited erythropoiesis.
Suppression of hepcidin by anemia or hypoxia
In addition to iron stores and inflammation, anemia and hypoxia also affect iron metabolism. These stimuli would be expected to decrease hepcidin production and remove the inhibitory effect on iron absorption and iron release from macrophages so that more iron is available for compensatory erythropoiesis. Weinstein et al21 and Nicolas et al23 confirmed that these effects indeed take place.”
538. Prof Haase referred in his third report to two papers, the first being R.N. Greenwood et al , “Erythropoietin dose variation in different facilities in different countries and its relationship to drug resistance”, Kidney International , 64, S78-S86 (2003). This states in the concluding two sentences (at page S85):
“Evidence from transgenic mice expressing mRNA for hepcidin suggests that it reduces iron absorption and iron release from macrophages. In inflammation, hepcidin production may increase substantially, so that it is possible that this substance plays an important role in EPO resistance.”
539. The second was an editorial by R. Deicher and W.H. Hörl, “Hepcidin: a molecular link between inflammation and anaemia”, Nephrol Dial Transplant , 19, 521–524 (March 2004). Prof Haase was cross-examined on certain passages, and in particular a passage (at pages 522-523) which states:
“To date, the relevance of hepcidin for the effectiveness of erythropoietin in chronic kidney disease patients is unclear.”
540. The next sentence states, however:
“Absolute iron deficiency rapidly develops during therapy with exogenous erythropoietin, and persistently high levels of hepatic hepcidin expression might explain why duodenum iron absorption remains inadequately low.”
Moreover, these statements follow immediately on from the preceding section of the review, headed “Hepcidin: a target for erythropoietin?”.
541. I would add that the point which Prof Haase made in his third report was that the review explained that hepcidin formed a molecular link between inflammation and anaemia. That evidence was not challenged.
542. Considering the evidence as whole, the conclusion I draw is that the common general knowledge was accurately stated in paragraph 76 of Prof Haase’s first report, namely that it had been “proposed that EPO might be involved in the transcriptional downregulation of hepcidin”.
543. What is not in dispute is that, at the Family B Priority Date, it had not been suggested that HIF suppresses hepcidin, and that that would have been pure speculation. The link between HIF and hepcidin was not published in the scientific literature until after the Family B Priority Date, in Peyssonnaux.
The documents cited in the Family B Patents
544. Like the Family A Patents, the Family B Patents refer to a considerable number of papers, books, patents and patent applications. EP 333, for example, refers to 64 papers and books and 20 patents and patent applications. Again, the question arises as to which, if any, of these documents the skilled team, and in particular the medicinal chemist, would read. There was less evidence on this topic than in relation to the Family A Patents.
545. In his first report Dr Bhalay reviewed WO 686. Although he did not say so in terms, it is clear from what he said that this was because he was asked to do so. He also noted that EP 333 cited the same (six) papers that he had been asked to read in relation to EP 823 and cited at [0138]-[0142] the same (11) patent documents as sources of Formula (I) compounds as EP 823 at [0072]-[0077]. Prof Ward in his first report simply referred to his previous comments on the cited documents.
546. In cross-examination Dr Bhalay said that the skilled medicinal chemist would read all six cited papers and all 11 cited patent documents when reading EP 333, but gave no reason for taking a different approach than in relation to EP 823.
547. In my judgment the skilled team is most likely to read Majamaa 1984 and Majamaa 1985 for the same reason as in the case of the Family A Patents. Given that the Family B Patents refer to even more papers and patent documents than the Family A Patents, they would be even less likely to read the other documents.
548. No reason was given by Dr Bhalay, or put to Prof Ward, as to why WO 686 would be singled out for review as it was by Dr Bhalay in his first report. WO 686 presents experimental data in respect of 17 compounds (A-Q), one of which (B) is exemplified in EP 333. Six (A, C, D-F, N and Q) are not within Formula (I). The remainder are isoquinoline (J-M), quinoline (H, I and O) or pyridine (G and P) carboxamides. Prof Ward’s unchallenged evidence was that very little meaningful information (if any) could be derived about the relationship between the structures of the heterocyclic carboxamides and their biological activity or mechanism of action from the experimental data presented in WO 686.
Construction of Family B claims
549. A number of points of interpretation of the Family B claims arise, although not all of these are in dispute.
Formula (I) and structural mimetic of 2-oxoglutarate
550. The same issues arise in relation to these features as in the context of the Family A Patents, and it is common ground that they should be resolved in the same way.
For use in treating anaemia of chronic disease
551. It is common ground that treatment of ACD involves treatment of anaemia associated with an underlying chronic inflammatory condition.
For use in treating anaemia that is refractory to treatment with exogenously administered erythropoietin
552. It is common ground that the relevant claims are to be interpreted as covering use of a HIF-PHI for the purpose of treating anaemia in circumstances where the patient’s anaemia has already been established to be refractory to treatment with exogenous Epo. It is also common ground that “refractory” covers both a complete absence of response and hyporesponsiveness, i.e. a lowered response, to Epo. Although there was at earlier stages a dispute as to what criterion the skilled nephrologist would apply for this purpose, by closing submissions it was not disputed that they would apply the criterion for resistance set out in paragraph 524 above.
For use in treating functional iron deficiency associated with anaemia
553. Although the Defendants contended in their opening skeleton argument that this required an absolute increase in serum iron, typically characterised by an increased TSAT, that contention was disputed by the Claimants and was not pursued by the Defendants in closing submissions. Thus it is not in dispute that the relevant claims do not require there to be any measurable increase in iron parameters. A different point was raised by the Claimants which I will address in context below.
Obviousness of Family B over WO 997
554. The Defendants contend that all the Family B claims in issue are obvious over WO 997. It is common ground that this is essentially a question of whether it would be obvious to use the compounds disclosed in WO 997 for the purposes claimed in the Family B Patents. Although claim 36A of EP 333 is limited to Compound A, corresponding to Compound C of WO 997, the Claimants do not suggest that there would be any invention in choosing that compound given that it is the one that is the subject of the most experimental data in WO 997.
WO 997
555. I have set out the disclosure of WO 997 above. Before turning to the issues on obviousness, it is convenient first to consider in general terms what the skilled team, and in particular the nephrologist, would make of [0072] (quoted in paragraph 133 above). As is common ground, there are no data in WO 997 to support the suggestion that the methods of the invention increase iron transport, processing and utilisation. Prof Winearls described this as “a very bold claim” that was “totally unsubstantiated”, but he accepted that it would have been an interesting one. Prof Haase agreed that, as a scientist, he would want to see data before accepting that the effect was a real one. Prof Winearls agreed that the pre-clinical researcher in the skilled team, who would have known that HIF regulated transferrin, transferrin receptor and ceruloplasmin, would have thought that the statements about HIF-PHIs increasing the amount of transferrin, transferrin receptor and ceruloplasmin were plausible. On the other hand, Prof Haase agreed that none of transferrin, transferrin receptor and ceruloplasmin had been implicated in 2004 as a cause of iron deficiency or ACD or anaemia in general.
The differences between WO 997 and the claims in issue
556. The differences between WO 997 and the claims in issue depend on which claim one is considering, but in essence the difference in each case is that WO 997 does not expressly disclose the therapeutic use claimed. There are four such uses: (i) use in treating ACD in subjects with TSAT less than 20%/16% in adults, (ii) use for treating anaemia that is refractory to exogenous Epo, (iii) use in treating functional iron deficiency associated with anaemia and (iv) use for decreasing hepcidin expression. I will consider the obviousness of these in turn.
557. Before doing so, however, I should address the Claimants’ over-arching point that the obvious way forward for the skilled team reading WO 997 in April 2004 would be to investigate the use of HIF-PHIs for the treatment of anaemia of CKD, and that in order properly to test that they would want to exclude co-morbidities and ensure that patients were iron replete. Unsurprisingly, Prof Haase agreed that that would be an obvious course to adopt. It simply does not follow, however, that other possibilities were not obvious.
For use in treating ACD in subjects with TSAT less than 20%/16% in adults
558. WO 997 expressly teaches use of the compounds for the treatment of anaemia associated with inflammation (see [0004], [0018], [0044] and [0064]) and inflammatory conditions such as cancer and infection ([0008], [0018], [0044] and [0064]) and rheumatoid arthritis and sideroblastic anaemia ([0044], [0046] and [0072]). It is common ground that these are ACD conditions. Indeed, they are some of the ACD conditions specifically referred to by WO 121 in [0124].
559. In any event, it was accepted by Prof Winearls that it would have been obvious to use the compounds disclosed by WO 997 for purposes including the treatment of ACD.
560. It is common ground that, in the case of patients with ACD, the standard of care in 2004 was (in addition to treating the underlying disease) to administer supplemental iron either orally or intravenously. Prof Haase’s evidence was that there were a small number of patients for whom this treatment was not effective. The Claimants suggest that this means that there was no need to treat patients with a TSAT of less than 20% or 16% with anything else, but that would mean that there was no technical problem to solve. In any event, the evidence is clear that it is precisely because supplemental iron was not always effective that ESAs were used to treat ACD, as discussed above. (The Claimants also rely upon Dr Ashman’s evidence that, in CKD patients being treated with ESAs, IV iron would almost always correct any absolute iron deficiency; but this is irrelevant to the present issue.)
561. Prof Winearls explained in his first report that TSAT < 16% was the cut-off for iron deficiency in the normal population, while TSAT < 20% was the cut-off for iron deficiency in the CKD population. He also explained that (based on the disclosure of the Family B Patents) one would expect administration of HIF-PHIs to increase TSAT and to treat iron deficiency anaemia, including anaemia associated with ACD, in both populations.
562. The Defendants contend that, given that it was obvious from WO 997 to use the disclosed HIF-PHIs to treat ACD, then it follows that the skilled team would inevitably be treating at least some subjects with a TSAT at the claimed levels. I accept this. In any event, Prof Haase’s evidence was that patients with a TSAT of less than 20% or 16% were treated with ESAs, including some patients whose TSAT dipped below those levels due to diurnal or periodic variation. Given that HIF-PHIs are disclosed by WO 997 as an alternative to ESAs, they would be administered to patients with the relevant TSAT levels.
563. As the Defendants point out, the Family B Patents do not show, or even attempt to show, that HIF-PHIs confer any benefit over ESAs in terms of iron delivery. If and in so far as there is such a benefit, however, the Defendants contend that this would have been discovered by the skilled team by taking obvious steps. As Prof Winearls accepted, the skilled team would have been motivated by WO 997 to do some relatively straightforward tests. These would have included the transferrin and transferrin receptor tests in animal cells in WO 121, which would (if WO 121 is correct in its assertions) have shown an “enhancement” of erythropoiesis through increasing iron transport etc. This would have led to the other tests done in WO 121 to measure iron uptake, including measuring haemoglobin levels, and then ultimately comparative tests on iron uptake over ESAs . The Defendants submit, and I agree, that, taken as a whole, Prof Haase’s evidence was also consistent with this.
564. Accordingly, I conclude that claims 1 and 2 of EP 333 and claims 1, 2 and 3B of EP 155 are obvious over WO 997.
For use in treating anaemia that is refractory to treatment with exogenous Epo
565. The Defendants contend that, given the disclosure of WO 997 of HIF-PHIs for the treatment of anaemia explicitly as alternatives to ESA treatment with potential benefits, it would be entirely obvious to administer a HIF-PHI as a replacement therapy for a patient who had proved refractory to ESAs. Indeed, there would be a huge motivation to do so, and no reason not to do so. As the Defendants submit, this is supported by the evidence of Dr Devonald, which although given in relation to a latter point in time is equally applicable to April 2004.
566. The Claimants rely upon evidence that, if a patient was found to be refractory to Epo, treatment with Epo should cease, such as the statement in UpToDate that “it is not worthwhile to continue EPO in patients who do not have a clinical meaningful response by 12 weeks”. Counsel for the Claimants submitted that this showed that Epo should not be given to such patients at all, but it shows no such thing. What it shows is that Epo was discontinued where the patient’s anaemia was found to be refractory precisely for that reason. This supports, rather than undermines, the Defendants’ case.
567. Accordingly, I conclude that claims 9 and 36A of EP 333 are obvious over WO 997.
For use in treating functional iron deficiency associated with anaemia
568. [0018] of WO 997 expressly teaches use of the compounds of the invention for the treatment of anaemia “associated with defects in iron transport, processing or utilisation” i.e. functional iron deficiency. The same message appears from [0072]. Yet further, as discussed above, ACD involves functional iron deficiency. The Claimants do not suggest that the mode, format or dosage involved in treating functional iron deficiency in accordance with the Family B Patents is any different to those involved in treating ACD. Nor, as discussed above, is it a requirement of the relevant claims that there should be any increase in iron parameters. It follows that WO 997 also makes it obvious to use the compounds for the treatment of functional iron deficiency associated with anaemia.
569. The Claimants submitted that the relevant claims require the HIF-PHIs to act “by overcoming the reticuloendothelial block which prevents the release of iron from stores”. This is not a feature of the claims, however. Nor is there is any evidence in the Family B Patents that HIF-PHIs achieve this. In any event, even if it did happen, it would be an inherent effect of administering the compounds of WO 997 to an ACD patient.
570. Accordingly, I conclude that claim 22A of EP 333, claims 1A and 2 of EP 153 and claim 6A of EP 155 are obvious over WO 997.
For decreasing hepcidin expression
571. The Defendants contend that this requirement adds nothing to the claims in issue. The Claimants contend that it is a further limitation on the relevant method of treatment, namely, the particular means by which the condition is treated, and a means which was not known in April 2004.
572. In my judgment the Defendants are correct on this point. As Prof Winearls accepted, any effect of HIF-PHIs on hepcidin is an inevitable and inherent part of administering the drug. Moreover, there is no evidence that decreasing hepcidin expression was in April 2004, or even now, a therapeutic objective in its own right.
573. Although there was some debate during the evidence as to whether there was an independent relationship between HIF and hepcidin (that is to say, a relationship not mediated through erythropoiesis), the Claimants did not rely upon any such phenomenon as part of their non-obviousness case in their closing submissions. It is therefore not necessary for me to consider the evidence in detail. It suffices to say that Prof Winearls and Prof Haase were agreed that, even now, this was entirely speculative.
574. Accordingly, I conclude that claim 31A of EP 333, claim 11A of EP 531 and claim 15A of EP 155 are obvious over WO 997.
Insufficiency and AgrEvo obviousness of Family B
575. The issues on insufficiency in relation to the Family B Patents are the same as in relation to the Family A Patents. Accordingly, I can deal with them briefly.
576. Plausibility . There is no dispute that the specification makes it plausible that Compound A (Compound C in Family A) achieves the effects claimed for it. It is not plausible, however, that substantially all the compounds embraced by Formula (I) do. Nor is it plausible that substantially all “structural mimetics of 2-oxoglutarate” would do so if it was possible to identify compounds which satisfied that criterion. Accordingly, all the claims in issue except for claim 36A of EP 333 are invalid on the grounds of insufficiency (and AgrEvo obviousness).
577. Undue burden . Save for Compound A, the skilled team would be unable to identify substantially all the compounds embraced by the structural features of the claims which satisfied the functional limitations without undue burden. Accordingly, all the claims in issue except for claim 36A of EP 333 are invalid on the grounds of insufficiency on this basis as well.
578. Uncertainty . All the claims of EP 153 and EP 155 are insufficient on this ground, as is claim 34A of EP 333.
Infringement of Family B by vadadustat
579. For the purposes of the Family B Patents, it is necessary to explain the basis of the Claimants’ infringement case more fully than I did in relation to the Family A Patents. As noted at the outset of this judgment, vadadustat is presently undergoing Phase III trials. It follows that it has not yet received a marketing authorisation. It is common ground, however, that, if and when vadadustat is authorised, the Defendants intend to market it in the UK. Moreover, the Defendants have given evidence (by way of a Product and Process Description) as to the scope of the marketing authorisation that they presently intend to seek. The therapeutic indications for which authorisation will be sought are “treatment of anaemia associated with chronic kidney disease (CKD) in adults who are non-dialysis-dependent (NDD) and those who are dialysis-dependent (DD)”.
580. The Claimants contend that the Defendants thereby threaten to infringe the Family B Patents despite the fact that the proposed marketing authorisation does not on its face include any of the uses claimed in the Family B Patents, but only the use taught by WO 997 and claimed in the Family A Patents, and that the proposed Summary of Product Characteristics (“SmPC”) states that “[TSAT] and serum ferritin should be evaluated per standard of care”, alternatively “prior to and during treatment with vadadustat”, and “Administration of supplementary iron therapy is recommended as needed”.
The law
581. The Claimants’ infringement case requires consideration of three areas of law, namely (i) the law concerning infringement of medical use claims, (ii) the law concerning indirect infringement and (iii) the law concerning quia timet claims for infringement.
582. Infringement of medical use claims . As noted above, most of the claims in issue are in EPC 2000 form, whereas claim 1 of EP 823 is in Swiss form. The difference between the two is that a Swiss-form claim is a purpose-limited process claim (see Generics (UK) Ltd v Warner-Lambert Co LLC [2018] UKSC 56 , [2019] Bus LR 360 at [2], [63]), whereas an EPC 2000-form claim is a purpose-limited product claim. As such, they have a different scope of protection: see T 1373/11 GENZYME/Treatment of Pompe’s Disease [2016] EPOR 33 at [21] cited in Case Law of the Boards of Appeal of the European Patent Office at page 522. In particular, direct infringement of EPC 2000 claims falls to be determined under section 60(1)(a) of the Patents Act 1977, whereas direct infringement of Swiss-form claims falls to be determined under section 60(1)(c). Furthermore, whereas claims for indirect infringement of Swiss-form claims by downstream dealings in the product of the manufacturing step are unsustainable for the reasons explained by Lord Sumption (with whom all the other members of the Supreme Court agreed on in this issue) in Generics v Warner-Lambert at [87]-[88], no such problem arises in the case of EPC 2000 claims.
583. In Generics v Warner-Lambert the Supreme Court were divided three ways as to the correct approach to the mental element required for direct infringement of a Swiss-form claim. Fortunately, it is not necessary for me to decide what the correct test is, or whether direct infringement of an EPC 2000 claim requires the same or a different mental element. This is because counsel for the Claimants sensibly put the Claimants’ case on the basis of indirect infringement, and accepted that, if that failed, the Claimants could not succeed on the basis of direct infringement.
584. Indirect infringement . Under section 60(2) of the 1977 Act a person infringes if:
“while the patent is in force and without the consent of the proprietor, he supplies or offers to supply in the United Kingdom a person other than a licensee or other person entitled to work the invention with any of the means, relating to an essential element of the invention, for putting the invention into effect when he knows, or it is obvious to a reasonable person in the circumstances, that those means are suitable for putting and are intended to put, the invention into effect in the United Kingdom.”
585. The background to Article 26 CPC, and hence section 60(2) of the 1977 Act, was explained by Jacob and Etherton LJJ, with whom Sir David Keene agreed, in Grimme Landmaschinenfabrik GmbH v Scott [2010] EWCA Civ 1110 , [2011] FSR 7 at [82]-[98]. They went on at [105]-[131] to consider the requirements of knowledge and intention in section 60(2). They found helpful guidance in relation to these questions in a number of decisions of the Bundesgerichtshof (Federal Court of Justice) on the corresponding German provision, which also derives from Art 26 CPC. In KCI Licensing Inc v Smith & Nephew plc [2010] EWCA Civ 1260 , [2011] FSR 8 at [53] Jacob LJ delivering the judgment of the Court of Appeal summarised the key parts of Grimme v Scott with regard to the requirements of knowledge and intention as follows:
“i) The required intention is to put the invention into effect. The question is what the supplier knows or ought to know about the intention of the person who is in a position to put the invention into effect - the person at the end of the supply chain, [109].
ii) It is enough if the supplier knows (or it is obvious to a reasonable person in the circumstances) that some ultimate users will intend to use or adapt the ‘means’ so as to infringe, [107(i)] and [114].
iii) There is no requirement that the intention of the individual ultimate user must be known to the defendant at the moment of the alleged infringement, [124].
iv) Whilst it is the intention of the ultimate user which matters, a future intention of a future ultimate user is enough if that is what one would expect in all the circumstances, [125].
v) The knowledge and intention requirements are satisfied if, at the time of supply or offer to supply, the supplier knows, or it is obvious to a reasonable person in the circumstances, that ultimate users will intend to put the invention into effect. This has to be proved on the usual standard of the balance of probabilities. It is not enough merely that the means are suitable for putting the invention into effect (for that is a separate requirement), but it is likely to be the case where the supplier proposes or recommends or even indicates the possibility of such use in his promotional material, [131]”
586. It is clear from these decisions that it is sufficient that a proportion of users will intend to use the means so as to infringe. Even if the majority of users will not intend to use the means to infringe, that is only relevant to remedies, and in particular financial remedies (see Grimme v Scott at [134]-[137]). On the other hand, one should disregard “speculative, maverick or unlikely use” of the means (see Grimme v Scott at [116], [124], [127] and [129]-[130] and KCI v Smith & Nephew at [47])”.
587. I considered what is meant by the term “means relating to an essential element of the invention” in Nestec SA v Dualit Ltd [2013] EWHC 923 (Pat) , [2013] RPC 32 at [168]-[175], and held that it must be “something that … contribute[s] to the technical teaching of the invention”.
588. Quia timet claims for infringement . Having reviewed the relevant authorities, Birss J summarised the relevant principles in Merck Sharp Dohme Corp v Teva Pharma BV [2013] EWHC 1958 (Pat) , [2014] FSR 13 as follows:
“56. The principle I derive from these authorities is that the question the court is asking in every case is whether, viewed in all the relevant circumstances, there was a sufficiently strong probability that an injunction would be required to prevent the harm to the claimant to justify bringing the proceedings. In adding the word sufficiently to the word strong I do not mean to put a gloss on the words of Chadwick LJ, rather I am seeking to encapsulate the idea that the degree of probability required will vary from case to case depending on all the circumstances but that mere possibilities are never enough. To justify coming to court requires there to be a concrete, strong and tangible risk that an injunction is required in order to do justice in all the circumstances”
57. If a defendant really does, at the date of the proceedings, have no intention to do the act then in the majority of cases that will be conclusive of the question whether there was a sufficiently strong probability to justify proceedings. (e.g. London Borough of Islington ). However it seems to me that the question is not confined to the defendant's subjective intentions. A defendant's overt acts must be capable of being relevant. To take an extreme case, if a man began taking actual preparatory steps to commit some unlawful act seriously damaging to the claimant and in infringement of the claimant's rights and did so in full view of the claimant and well aware that the claimant could see them, he could hardly complain if the claimant started proceedings and the court decided to grant a final injunction to prevent it. A statement at trial that he had never intended to go through with it would get short shrift.
58. I bear in mind that intentions are not necessarily simple. A state of mind need not merely be either one thing or another. Also in this case the defendants are corporate entities to whom an intention can only be imputed.
59. The way the matter is put in the Particulars of Claim contains the allegation that the defendant ‘threatens and intends’ to infringe. I think this is a useful expression in that it encompasses both the defendant's intentions and also the idea that the court should look from the outside at what the defendant is threatening to do. Both are relevant.”
Assessment
589. If vadadustat falls within Formula (I) (as I have concluded) and/or it is a “structural mimetic of 2-oxoglutarate” (if claims containing that expression are valid, contrary to my conclusion), then it fulfils the structural requirements of the Family B claims (except for claim 36A of EP 333, which is alleged to be infringed on the basis of equivalence on the same grounds as claim 17A of EP 531, but on the basis of my conclusion in relation to claim 17A does not infringe). I do not understand it to be in dispute that, on that basis, vadadustat will constitute “means, relating to an essential element of the invention” if the other requirements of section 60(2) are satisfied.
590. Accordingly, the question is whether the Defendants are threatening to market vadadustat in circumstances where they will know, or it would be obvious to a reasonable person in the circumstances, that vadadustat is suitable for putting and intended to put the claimed inventions into effect in the United Kingdom.
591. It is worth breaking this down a little before proceeding further. As I have already explained, vadadustat is not yet on the market, nor is it likely to be marketed for some time. The claim therefore requires the Court to consider both the Defendants’ state of mind (at least in terms of what would be obvious to a reasonable person in those circumstances) and the state of mind of clinicians prescribing vadadustat (in terms of their intentions) at some indeterminate future point in time.
592. This situation has come about because the Claimants have chosen to bring an infringement claim now, rather than wait and see what happens if and when vadadustat is marketed. Counsel for the Claimants recognised, however, that the Court might conclude that it is not possible to form a view as to infringement at this stage because there are too many uncertainties. Counsel submitted that, in that event, the appropriate course would be for the Court to stay the cross-claim and give the parties permission to apply in the event of a change of circumstances. In my judgment, that would not be appropriate. If the evidence before the Court does not establish that the Defendants are presently threatening to infringe the Family B Patents, then the cross-claim should be dismissed. Given that there is no claim by the Defendants for a declaration of non-infringement, there would be nothing to prevent the Claimants from bringing a further claim for infringement if the circumstances change in the future.
593. As to whether vadadustat is suitable and intended for the uses claimed in the Family B Patents, as both sides recognised, for practical purposes the question which matters is whether vadadustat has advantages compared to ESAs with respect to such uses, since it is only likely to be prescribed by clinicians if it does have such advantages.
594. As is common ground, there has not yet been any Phase III trial comparing vadadustat to ESAs with respect to its effect on ACD in patients with TSAT < 16% or 20%, or its effect on patients with anaemia that is refractory to treatment with ESAs or its effect in patients with functional iron deficiency; nor is there any evidence that such a trial is presently planned. (Indeed, although there are quite a lot of Phase III trials underway in which HIF-PHIs are being compared with ESAs, there is no evidence that iron parameters are a primary end point in any of them.) The Claimants do not dispute that it follows that at present there is no foreseeable prospect of vadadustat receiving a marketing authorisation for such uses. The Claimants nevertheless contend that it is foreseeable that clinicians will prescribe vadadustat for such uses off-label because there is a growing body of clinical evidence that HIF-PHIs have advantages over ESAs in terms of their effects on increasing iron mobilisation. This contention requires me to consider two questions. First, what is the current state of the clinical evidence? Second, is it foreseeable that clinicians will prescribe vadadustat off-label for such uses?
595. The current clinical evidence . As Prof Winearls explained in his second report, in seeking further evidence of the claimed therapeutic effects of HIF- PHIs on iron parameters as promised by the Family B Patents, the most relevant experiments would be those in which:
i) HIF-PHIs and ESAs were compared so as to investigate whether the effects of HIF-PHIs are additional to those provided by ESAs; and
ii) the treatment of patients was conducted irrespective of their iron status and without pre-treatment with iron, such that the response of low iron patients could be compared to that of iron replete patients.
596. Reports of a large number of Phase II clinical trials of various HIF-PHIs appear in the evidence. As Prof Winearls explained in his first report, however, many of these trials do not provide reliable evidence for present purposes:
“The primary endpoint of a clinical trial is the endpoint for which the subjects are randomised and for which the trial is powered. While iron metabolism parameters such as serum iron, TSAT and hepcidin may have been secondary endpoints, it is more difficult to draw any conclusions about the effect of the compounds on these parameters without an appropriately powered trial designed to investigate them. Interpretation of the results is further complicated by the fact that between different studies (and in some cases within the same study) some patients were provided with iron supplementation and some were not.”
597. For the reasons given by Prof Winearls, the debate centres on a small subset of these studies that came closest to meeting one or other of the two criteria mentioned in paragraph 595 above, namely three trials of roxadustat sponsored by FibroGen: R. Provenzano et al , “Oral Hypoxia–Inducible Factor Prolyl Hydroxylase Inhibitor Roxadustat (FG-4592) for the Treatment of Anemia in Patients with CKD”, Clin J Am Soc Nephrol , 11, 982–991 (2016) (“Provenzano 1”); R. Provenzano et al , “Roxadustat (FG-54920 Versus Epoeitin Alfa for Anemia in Patients Receiving Maintenance Hemodialysis: A Phase 2, Randomised, 6- to 19-week, Open-Label, Active Comparator, Dose-Ranging, Safety and Exploratory Efficacy Study”, Am J Kidney Dis , 67(6), 912-924 (2016) (“Provenzano 2”); and N. Chen et al , “Roxadustat Treatment for Anemia in Patients Undergoing Long-Term Dialysis”, N Engl J Med , 381, 1011-1022 (2019) (“Chen”).
598. Provenzano 1 was not a study comparing HIF PHIs with ESAs. In his second report, Prof Winearls relied on the authors’ observation (at page 989) that “Hb response in patients who were not iron replete and not on oral iron at baseline was as good as those who were iron replete and on oral iron”. As Prof Haase pointed out in his fourth report, however, supplemental iron was made available to the patients and the paper does not identify those patients who received the supplemental iron or whether or not those patients who received supplemental iron were iron replete at the outset or not. Accordingly, as Prof Winearls accepted, it is not possible to conclude whether the reported effects of increased iron availability for erythropoiesis was the result of exogenous iron or the administration of the HIF-PHI (roxadustat). Furthermore, as a matter of common sense, it is likely that those who were not iron replete at the start of the trial were the most likely patients to receive oral iron.
599. Ultimately, Prof Winearls accepted that in the absence of a comparative study between roxadustat and ESAs, Provenzano 1 could not provide any real clinical evidence that roxadustat had an effect on iron metabolism over and above that of ESAs. He described it as a “weak paper” in which the authors had “mined the data” in a manner that amounted to “speculation”. Even though patients with TSATs of <20% were included in the study, it was well known that such patients may also respond to ESAs.
600. Provenzano 2 did seek to compare roxadustat with an ESA, epoetin alpha. As Prof Winearls accepted, however, because all the patients were iron replete at baseline, the paper provides no clinical evidence about whether patients could be safely treated with roxadustat at lower TSAT levels. Furthermore, whilst patient cohorts were administered different doses of roxadustat, only one dose of Epo was used. The study did not therefore seek to compare patient cohorts who were experiencing the same degree of erythropoiesis (from roxadustat and Epo), and so the effects of roxadustat and Epo other than stimulating erythropoiesis could not be ascertained.
601. Chen was another trial (described by the authors as a Phase III trial, although it was in some ways closer in design to a Phase II trial) in which roxadustat and epoetin alpha patients were compared, the primary endpoint being haemoglobin level. Although patients were included in both groups with TSATs at baseline above and below 20%, the proportion of patients with low TSAT levels was less in the roxadustat group and the results were not stratified according to TSAT at baseline. As such, Prof Winearls accepted that it was not possible to conclude that patients with a TSAT below 20% would benefit more from roxadustat than epoetin alpha.
602. Prof Haase considered all three of these papers (together with others) in his second report and in more detail in his fourth report. His conclusion in his fourth report was as follows:
“Regarding patients with low baseline iron, it is hard to draw any firm conclusions from the studies given that the patients in the relevant studies were permitted to take oral iron during the treatment period notwithstanding low baseline parameters. Furthermore, none of the studies provide support for HIF prolyl hydroxylase inhibitors being suitable for the treatment of iron deficiency or any other condition for which ESAs are not also suitable.”
603. As counsel for the Defendants submitted, this conclusion is entirely consistent with Prof Winearls’ oral evidence as summarised above.
604. As noted above, it was put to Prof Haase in cross-examination that his evidence was inconsistent with what he had said in Sanghani. Counsel for the Claimants particularly relied upon the apparent inconsistency between Prof Haase’s statement in paragraph 24 of his fourth report that “there is no evidence in [Provenzano 2] to show that the effect of roxadustat on hepcidin is different to that of epoetin alfa” and the statement in Sanghani (at page 255) that “The decrease in hepcidin was statistically significant in the 2 mg/kg roxadustat cohort (part 1 of study) compared to epoetin alfa”.
605. The sentence in Sanghani refers to the authors’ finding in part 1 of the study that, as shown in Figure 4B, there was a statistically significant difference in the patients given 2 mg/kg roxadustat (but not 1 mg/kg or 1.5 mg/kg) compared to epoetin alpha at 2 weeks and at 5 weeks 4 days. This is a thoroughly dubious result, however, since Figure 4B shows that there was no statistically significant difference even in the 2 mg/kg group at 6 weeks. It is therefore understandable that Prof Haase was unimpressed by this when writing his report.
606. As Counsel for the Claimants pointed out, however, it can be seen from Table 4 of Provenzano 2 that it does in fact report a statistically significant (p=0.04) difference at 19 weeks in part 2 of the study. This is a finding that appears to have escaped Prof Haase’s attention both when writing the text of Sanghani (although it is recorded in the summary in Table 3) and when writing his report.
607. Thus the real point is not one of inconsistency on the part of Prof Haase, but that, upon analysis, the data in Provenzano 2 support a different conclusion to that which he drew in his report with respect to hepcidin. The primary endpoint in Provenzano 2 for both parts of the study, however, was the patients’ haemoglobin level. The hepcidin level was merely an “exploratory analysis” (meaning that, as explained by Prof Winearls in the passage quoted above, the study was not designed to produce statistically meaningful results in that respect). Moreover, it was, as the title indicates, an open-label (i.e. unblinded) study. In those circumstances the finding with respect to hepcidin is indicative rather than properly substantiated.
608. More generally, Prof Haase did not dispute that there was evidence in the various studies that had been carried out of HIF-PHIs having an effect in reducing hepcidin as well as raising haemoglobin. Given the emphasis placed on part of it by counsel for the Claimants, I will quote more fully what Prof Haase said in Sanghani in this respect (at page 260) (references omitted):
“Another major advantage of HIF-PHI therapy would be the suppression of hepatic hepcidin production and its negative effects on iron mobilisation. Hepcidin plays a central role in the pathogenesis of functional iron deficiency as it inhibits gastrointestinal iron uptake and iron release from internal stores by down-regulating the surface expression of ferroportin, the only known cellular iron exporter. Clinical data from phase II studies have consistently shown ‘positive’ effects on iron metabolism as manifested by a reduction in plasma ferritin and hepcidin and simultaneous increase in plasma transferrin and TIBC … These results are consistent with experimental data from animal and cell culture studies, which demonstrated that the PHD/HIF axis coordinates iron metabolism with erythropoiesis via transcriptional regulation of genes involved in iron uptake, iron release and transport. … The observed effects on plasma hepcidin levels in CKD patients receiving HIF-PHIs are most likely indirect, as hepcidin is not a direct transcriptional target of HIF. Transcriptional suppression of hepcidin in the context of HIF activation requires erythropoietic activity and is mediated by bone-marrow derived factors such as erythroferrone. It is unclear, however, whether the effects of oral HIF-PHI therapy on iron metabolism are primarily mediated via the hepcidin-ferroportin axis (increase in erythropoietic activity with subsequent suppression of hepcidin and increased ferroportin-mediated iron release) or through direct transcriptional regulation of iron metabolism gene expression. Although several iron metabolism genes … are bona fide HIF-regulated genes and can be upregulated by oral prolyl hydroxylase inhibition, it has not been examined whether HIF-PHI doses currently used in clinical trials are sufficient to induce the expression of these genes in CKD patients. Nevertheless, the added HIF-PHI effect on iron mobilisation has the potential to reduce the need for IV iron supplementation in patients with renal anemia as suggested by Besarab and colleagues.”
609. The qualifications and uncertainties expressed in this passage, and in particular the cautious reference to “positive” results in inverted commas, confirm that these are matters that are not yet established and require further investigation. (Another recent review relied upon by the Claimants, namely Batchelor, is to similar effect, although less analytical and slightly more upbeat in tone.)
610. The matter does not end there, however, because the real question is not whether HIF-PHIs have an effect on hepcidin, but whether any such effect is a clinical useful one with respect to iron mobilisation, in particular compared to ESAs. It can be seen that in the last sentence of the passage from Sanghani quoted in paragraph 608 above, Prof Haase referred to a paper by A. Besesarab et al , namely “Roxadustat (FG-4592): Correction of Anemia in Incident Dialysis Patients”, J Am Soc Nephrol , 27, 1225–1233 (2016) (“Besarab”).
611. Prof Haase discussed Besarab in paragraphs 74-77 of his second report. As he explained, this is the only study to stratify subjects by reference to exogenous iron supplementation. It was a Phase IIb study of roxadustat in correcting anaemia in newly initiated dialysis patients naïve to ESAs, randomly assigned to different iron supplementation regimens (oral iron, IV iron and no iron). One group of 24 patients received no iron, and that group showed a statistically significant decrease in levels of both serum iron and TSAT. The authors noted (at page 1228) that “Neither transferrin saturation (TSAT) levels nor reticulocyte Hb content changed significantly in the groups receiving oral or IV iron, but both decreased in those not receiving iron”. The iron parameters were not primary endpoints in this study, however, and so, consistently with his other evidence, Prof Haase said that further studies were needed to form a definitive view.
612. Prof Haase returned to the subject of Besarab in paragraphs 86-91 of his third report, where he pointed out that Besarab represents the best clinical available evidence as to the effect of roxadustat (or indeed any of the HIF-PHIs) on iron, and that it suggests that, far from increasing iron mobilisation, it has the opposite effect.
613. Prof Winearls stated in paragraph 11 of his fourth report that he did not disagree with what Prof Haase had said in his second report about Besarab. Consistently with that, Prof Haase’s evidence about Besarab was not challenged in cross-examination. (I should take this opportunity to apologise to Prof Haase for the fact that, having forgotten Prof Winearls’ concession and having lost patience due to some long answers Prof Haase had given, I cut him off when he raised Besarab during the course of his cross-examination, saying “We will come to that in due course”. Contrary to my expectation at that moment, counsel for the Claimants did not come to Besarab. The witness did return to the topic in a later answer, however.)
614. Finally on the subject of Besarab, despite not challenging any of Prof Haase’s evidence about it, counsel for the Claimants relied in closing submissions on the suggestion made by Besarab which was picked up in the last sentence in the passage from Sanghani quoted above. As can be from page 1229 of Besarab, however, the suggestion is that it may be possible to administer oral iron instead of IV iron with roxadustat.
615. In any case, Prof Haase did not resile from the conclusion in his fourth report quoted in paragraph 602 above. While he expressed the hope that HIF-PHIs would be beneficial, and accepted that it could turn out to be the case, he maintained that the data were not there yet.
616. Is it foreseeable that vadadustat will be prescribed off-label? The first point to note here is that, as Prof Winearls accepted, the interpretation of clinical trials, and in particular the statistical analysis of clinical trials, is a science in itself. Prof Winearls volunteered that it was one in which he was an amateur. Counsel for the Claimants submitted that this was immaterial because nephrologists were capable of reading and drawing conclusions from papers like Provenzano 1, Provenzano 2 and Chen. The relevance of this evidence, however, is that, in the absence of a proper analysis of the clinical evidence by persons who are qualified to undertake it of the kind that is available to, for example, NICE when formulating its Guidelines, it is less likely that clinicians will be inclined to take the risk of prescribing vadadustat off-label.
617. Furthermore, as Dr Devonald explained, since there is less clinical experience of new drugs, prescribing clinicians are likely to act more cautiously, notwithstanding claims made by manufacturers of the drugs in their labels and promotional literature. It follows that clinicians will be even more cautious about prescribing a new drug off-label, particularly in the absence of any change in the Guidelines and in the absence of any proper statistical analysis of the clinical trials.
618. In considering the likelihood of clinicians prescribing vadadustat off-label, it is logical, as counsel for the Defendants submitted, to begin with CKD, given that vadadustat is intended to be marketed for the treatment of CKD.
619. Current clinical practice in the treatment of CKD . There was substantial agreement between Dr Ashman and Dr Devonald as to the current practice. It was common ground between the experts that where a patient presents with CKD, nephrologists will follow the relevant guidelines (i.e. the Renal Association’s Clinical Practice Guideline Anaemia of Chronic Kidney Disease and NICE Guideline NG8), which provide as follows.
620. The patient will be assessed. That assessment will include a consideration of any underlying cause of the anaemia and an assessment of the patient’s iron status, typically by measuring a combination of serum ferritin and TSAT. If iron deficiency is detected, it is corrected with oral or intravenous iron supplementation. Iron repletion alone may result in adequate improvement in haemoglobin levels and symptoms.
621. Where iron repletion is insufficient to improve haemoglobin levels and symptoms, the clinician will then consider whether exogenous Epo (i.e. an ESA) is appropriate. ESAs are not prescribed before iron status is replete (recognised, for example, by evidence of both ferritin greater than 200 m g/L and TSAT > 20%). Haemoglobin, ferritin and TSAT are monitored regularly in any patients receiving ongoing treatment with iron and/or an ESA. These results are used to adjust doses of iron and/or the ESA, in general with the aim of keeping haemoglobin between 100-120 g/L (for patients receiving ESAs), ferritin at 200-800 m g/L and TSAT > 20%.
622. Guideline 2.1 of the Renal Association Guidelines provides as follows:
“Guideline 2.1 - Treatment of Anaemia with Iron therapy - Iron repletion
We recommend that patients should be iron replete to achieve and maintain target Hb whether receiving ESAs or not. (1B)
Iron repletion is usually defined as:
• %HRC <6% / CHr >29 pg / ferritin and TSAT (>100 microgram/L and >20%)
• For children, aim for a target ferritin level greater than 100 microgram/L for CKD patients on dialysis as well as CKD patients not on ESA therapy. (ungraded)”
623. It is clear from this guideline that CKD patients should be iron replete to achieve and maintain target haemoglobin whether receiving ESAs or not. Unless there is a change in the Guidelines, there is no reason to believe that nephrologists would depart from their usual practice of assessing a patient’s iron status and correcting iron deficiency if it exists.
624. Unsurprisingly, it was common ground between the experts that, in accordance with the RA Guidelines, patients with anaemia of CKD will first be administered iron. As Dr Devonald put it:
“[ESAs] would not be offered until the patient’s iron status is considered replete. In our unit we would consider this to be when serum ferritin is greater than 200 micrograms per litre and TSAT greater than 20% … For patients with anaemia related to CKD who are not iron replete according to these criteria, a clinical nephrologist would offer oral or intravenous iron until their iron status was replete.”
As Dr Devonald explained, iron repletion alone may be effective.
625. The current tendency in clinical practice in respect of iron is to administer higher levels of iron than formerly (albeit still within the RA Guidelines range). This tendency is due to the influential PIVOTAL study published in 2019. This was one of the largest renal clinical trials ever undertaken exclusively in the UK, and was designed to investigate the optimum amount of intravenous iron that can be given to patients on dialysis to treat anaemia effectively and safely. Previously it was thought that giving high doses of IV iron might lead to a greater risk of infection, but the PIVOTAL trial showed that a high dose regime is safe and more effective than the (more commonly used) low dose regime, and leads to a reduction in the dose of ESAs required, which is both clinically and financially advantageous. The PIVOTAL study therefore provides a basis for nephrologists to use iron supplementation more liberally in haemodialysis patients in order to achieve better patient outcomes, and Dr Ashman explained that Barts (one of the leading renal units in the UK) has already changed to a high-iron regime.
626. Dr Ashman summarised the position as follows:
“For nearly a century the management of iron deficiency anaemia has included the use of iron supplementation; there is a level of comfort with the use of iron supplementation. Furthermore, iron is cheap and, as explained above, the results from the PIVOTAL study provide a basis for nephrologists to use iron supplementation more liberally in haemodialysis patients in order to achieve better patient outcomes. I therefore cannot see any motivation for nephrologists to move away from the use of iron therapy to treat iron deficiency anaemia.”
627. Dr Devonald agreed that the direction of travel was to administer higher levels of iron, although his unit had generally run high ferritin and TSAT levels well before PIVOTAL so there was no need to change post-PIVOTAL. Nevertheless, he acknowledged that it might yet change to administering the iron proactively rather than reactively.
628. Expected clinical practice with respect to vadadustat for CKD . The Claimants contend that it is foreseeable that at least some clinicians will at some point approach iron supplementation differently with vadadustat compared to what they do currently with ESAs. The suggestion is that HIF-PHIs may start to be used without iron repletion. This relies on the theory that the hepcidin effects of HIF-PHIs may have a clinically beneficial effect on iron mobilisation, such that patients may be given HIF-PHIs without first being made iron replete, i.e. when they have a TSAT <16% or even <20%.
629. On the evidence presently available, it is pure speculation as to whether any such change will occur at all. As noted above, the best evidence (Besarab) does not support such a move. Indeed, at least in the short term, clinicians are likely to move towards using higher levels of iron than presently due to the results of the PIVOTAL trial, and this will apply as much to HIF-PHIs as to ESAs.
630. The highest that Dr Devonald put it was that, if the effects referred to in the clinical trial papers (for which he relied on Prof Winearls’ assessment of the literature in his second report) continued to be observed in Phase III clinical trials and subsequent studies, then there was a reasonable expectation that clinical guidelines would consider it acceptable to administer these drugs to patients with ferritin and TSAT levels below current guideline levels for epoetin ESA prescription. In terms of prescribing to patients with TSAT <20%, he suggested that the label “would be unlikely to deter nephrologists from taking this approach if they obtained adequate reassurance, from relevant academic publications, that this approach is safe and effective”. But on the evidence, it is clear there is no such reassurance to be found in the existing clinical trials.
631. If matters were to change, and a substantial body of evidence were to emerge to support administering HIF-PHIs to patients with TSAT <20%, it is likely that it would take several years before this were to trickle down into clinical practice. Dr Ashman gave the example of the PIVOTAL results, where it took 12 years from the generation of the hypothesis in 2007 to the publication of definitive patient outcome results in 2019, which only now is resulting in changes in clinical practice. As he pointed out, even where robust clinical data is available, clinicians may not change their practice, particularly in circumstances where to do so would be against guidelines or contrary to regulatory authorisation.
632. Furthermore, it would appear to be against the goal of achieving clinical benefits for patients and against the mechanism of action of HIF-PHIs to treat non-iron-replete patients with vadadustat. As the mechanism of action of HIF-PHIs involves stimulating erythropoiesis (which uses iron), it is only logical to achieve iron repletion first to ensure sufficient iron levels to be used in making red blood cells to allow the HIF-PHIs to achieve their best therapeutic effects for the patients. There is no apparent clinical justification for a doctor to avoid iron repletion of the patient before the administration of HIF-PHIs, particularly given that iron is cheap and easy to administer.
633. Expected clinical practice with respect to vadadustat for ACD/anaemia refractory to ESAs/functional iron deficiency . The wording of the SmPC for vadadustat is not within the Defendants’ control and has not been finalised. With one exception, all of the options proposed by the Defendants include the following text in section 4.2, Posology and method of administration :
“All other causes of anaemia (e.g. iron deficiency, vitamin deficiency, metabolic or chronic inflammatory conditions, bleeding, etc.) should be evaluated and treated prior to initiating therapy with [vadadustat]. The management of anaemia due to chronic renal failure should be individualized.”
634. This wording clearly says that anaemia caused by iron deficiency, and anaemia caused by chronic inflammatory conditions (i.e. ACD) should be treated prior to starting treatment with vadadustat. Despite this, the Claimants allege that vadadustat will be used to treat ACD and functional iron deficiency. As the Defendants submit, there is no evidence to show that such off-label use is likely.
635. Treatment of ACD. Whilst there was some suggestion that HIF-PHIs might be of benefit to patients with (for example) raised inflammation (as in ACD) and that HIF-PHIs might, in the future, prove to be suitable for the treatment of patients with conditions such as ACD, there is no evidence at all that vadadustat might actually be prescribed to treat patients with indications (such as ACD) for which it is not licensed. The evidence of Dr Ashman was to the contrary.
636. Treatment of anaemia that is refractory to exogenous EPO . Dr Ashman acknowledged that there were small groups of patients who are difficult to treat and for whom the existing treatment options are limited (e.g. those who are hyporesponsive and those who are blocked within the reticuloendothelial system). Dr Ashman also acknowledged that HIF-PHIs may be an attractive drug for these patients, but only if the clinical benefits could be established and it was a cost-effective agent. Since the clinical benefits have not yet been established and the cost of vadadustat is unknown, however, there is no current indication that vadadustat will be used for treating such patients.
637. The high water mark of the Claimants’ case appears to be Dr Devonald’s evidence that, whilst a wholesale change in clinical practice replacing with ESAs with HIF-PHIs was unlikely to happen (whatever the outcome of the Phase III clinical trials), there was a possibility that it could happen in some cases. As the Defendants submit, this evidence does not establish that it is foreseeable now.
638. Treatment of functional iron deficiency . A nephrologist treats a patient’s anaemia, not their functional iron deficiency. As explained above, at the outset of such treatment the patient’s iron status is assessed and any iron deficiency corrected with supplemental iron. If the patient is still showing symptoms of anaemia once iron replete, additional lines of treatment will be considered. In these circumstances, the patient’s iron deficiency having been corrected, I agree with the Defendants that the administration of HIF-PHIs to treat the continuing anaemia could not sensibly be said to be for “treating” the functional iron deficiency, particularly where appropriate TSAT levels will be maintained through the course of the treatment.
639. Conclusion . On the evidence available to this Court, the Defendants are not threatening to market vadadustat in circumstances where they will know, or it would be obvious to a reasonable person in the circumstances, that vadadustat is suitable for putting and intended to put the claimed inventions into effect in the United Kingdom. Accordingly, even if the claims in issue are valid, there is no threat by the Defendants to infringe the Family B Patents.
Summary of principal conclusions
640. For the reasons given above, I conclude that:
i) the Family A Patents are not obvious over Epstein, and since this is the only attack on the validity of claim 17A of EP 531 that claim is valid (subject to the allowability of the amendment);
ii) all the claims in issue of EP 823 and EP 301 both lack plausibility and cannot be performed across their scope without undue burden, and therefore are invalid for insufficiency;
iii) claim 24A of EP 823 and claim 4 of EP 301 are uncertain, and therefore invalid for insufficiency;
iv) all the Family A claims in issue other than claim 17A of EP 531 would be infringed by vadadustat if they were valid;
v) claim 17A of EP 531 is not infringed by vadadustat on the basis of equivalence, and the same goes for claim 36A of EP 333;
vi) the amendments to produce claim 17A of EP 531 are permissible;
vii) all the claims in issue of the Family B Patents are obvious over WO 997;
viii) all the claims in issue of the Family B Patents except for claim 36A of EP 333 are invalid on the grounds of insufficiency for the same reasons as the Family A Patents; and
ix) even if the claims in issue are valid, there is no threat by the Defendants to infringe the Family B Patents.
Annex