Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales High Court (Patents Court) Decisions
You are here: BAILII >> Databases >> England and Wales High Court (Patents Court) Decisions >> Eli Lilly And Company & Ors v Genentech, Inc [2019] EWHC 387 (Pat) (01 March 2019)
URL: http://www.bailii.org/ew/cases/EWHC/Patents/2019/387.html
Cite as: [2019] EWHC 387 (Pat)
[New search] [Printable PDF version] [Help]
Neutral Citation Number: [2019] EWHC 387 (Pat)
Case No: HP-2017-000041
IN THE HIGH COURT OF JUSTICE
BUSINESS AND PROPERTY COURTS
INTELLECTUAL PROPERTY LIST (CHANCERY DIVISION)
PATENTS COURT
Rolls Building
Fetter Lane, London, EC4A 1NL
Date: 1 March 2019
Before :
MR JUSTICE ARNOLD
- - - - - - - - - - - - - - - - - - - - -
Between :
|
|
(1) ELI LILLY AND COMPANY (2) LILLY FRANCE SAS (3) LILLY DEUTSCHLAND GMBH (4) ELI LILLY ITALIA SPA (5) ELI LILLY AND CO (IRELAND) LIMITED (6) ELI LILLY KINSALE LIMITED (7) LILLY SA (8) ELI LILLY AND COMPANY LIMITED |
Claimants |
|
|
- and - |
|
|
|
GENENTECH, INC |
Defendant |
- - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - -
Andrew Waugh QC , Thomas Mitcheson QC and Stuart Baran (instructed by Allen & Overy LLP ) for the Claimants
Michael Tappin QC , Justin Turner QC, Mark Chacksfield and William Duncan (instructed by Marks & Clerk Solicitors LLP ) for the Defendant
Hearing dates: 16-19, 21-25, 30-31 January, 1 February 2019
- - - - - - - - - - - - - - - - - - - - -
Approved Judgment
I direct that pursuant to CPR PD 39A para 6.1 no official shorthand note shall be taken of this Judgment and that copies of this version as handed down may be treated as authentic.
.............................
MR JUSTICE ARNOLD
MR JUSTICE ARNOLD:
Contents
Topic Paragraphs
Introduction 1
The witnesses 4-38
Fact witnesses 4-6
Expert witnesses 7-38
The dermatologists 8-18
The rheumatologists 19-25
The antibody experts 26-38
General technical background 39-133
Nucleic acids 40-41
Proteins 42-52
Recombinant expression of proteins 53-55
Innate vs adaptive immunity 56-64
Antigen-presenting cells 65-67
B cells 68-70
Antibody expression 68-69
B cell differentiation 70
T cells 71-77
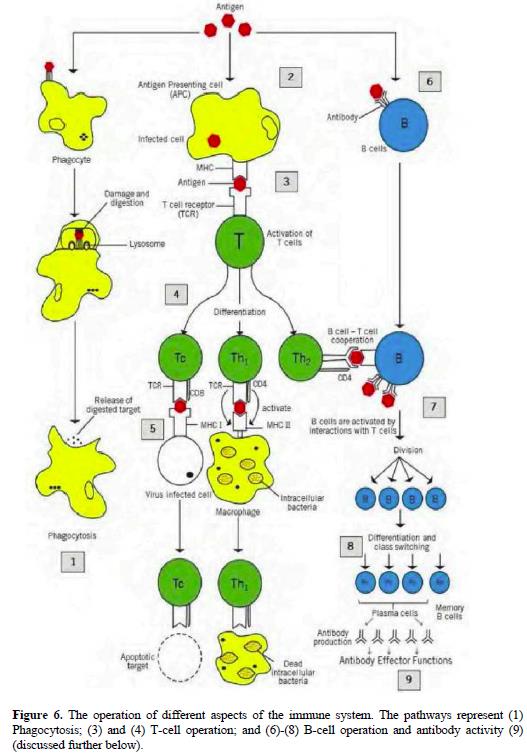
Interaction with APCs 71
CD4 + /CD8 + Cells 72-73
T H 1/T H 2 cells 74-76
Memory T cells 77
Inflammation 78-79
Cytokines 80-83
Tumour necrosis factor alpha 84-85
Interferon gamma 86
Interleukin-6 87-88
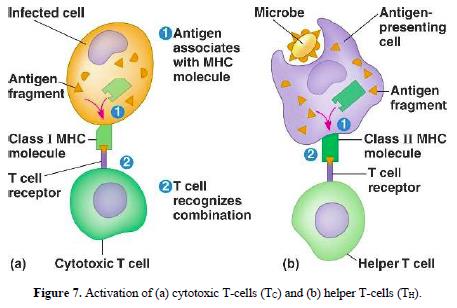
Interleukin-8 88
The interleukin-17 family 89-92
Antibody structure 93-98
Antibody classes 99-102
Antigen binding 103-110
Epitopes 103-104
Binding affinity 105-107
Measuring antibody binding affinity 108-110
X-ray crystallography 111-112
Generating antibodies by immunising animals 113-117
Generating antibodies using phage display 118
ELISA 119-124
Immunocytochemistry/immunohistochemistry 125-126
Neutralisation assay 127-129
Fc fusion proteins 130
Therapeutic antibodies 131-133
The Patent 134-178
Field of the invention 135
Background of the invention 136-143
Summary of the invention 144-148
A. Embodiments 144-147
B. Additional embodiments 148
Brief description of the drawings 149
Detailed description of the preferred embodiments 150-160
I. Definitions 150-152
II. Compositions and methods of the invention 153-159
Examples 160-178
Example 1 161-167
Example 2 168-170
Examples 3 to 11 171-178
The claims 179-181
The skilled team 182-187
Common general knowledge 188-292
The CGK of the rheumatologist 189-208
RA and its immuno-pathophysiology 190-191
Animal models 192
IL-6 and IL-8 193
IL-17A 194-196
IL-17 family 197-199
IL-17A and RA 200-206
Use of IL-17A for treating RA 207-208
The CGK of the antibody engineer 209
The CGK of the dermatologist 210-292
Psoriasis 211
Psoriatic arthritis 212
PASI 213
PGA 214
The immunological basis for psoriasis 215-216
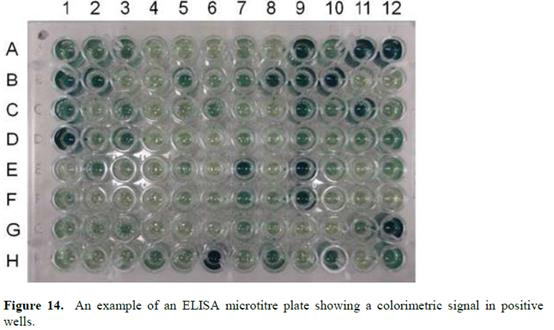
Models of psoriasis 217-218
Treatment options for psoriasis 219-220
Relationship with RA 222-224
Cytokines involved in psoriasis 225-229
TNFα 230-231
IFNγ 232
IL-12 and IL-23 233-234
IL-6 235-253
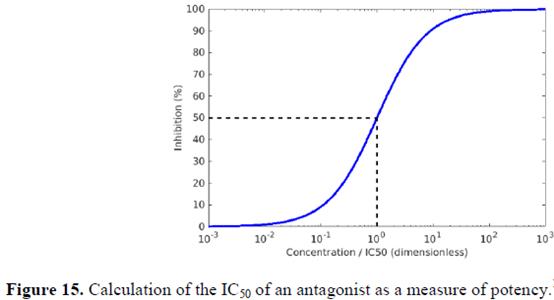
IL-8: general 254-255
IL-8: Abgenix’s antibody 256-257
IL-8: Prof Krueger’s evidence 258-260
IL-8: Prof Prens’ evidence 261-276
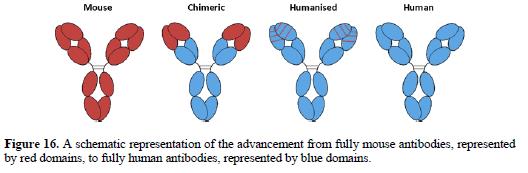
IL-8: PPP 277-279
IL-8: Other later evidence 280-281
IL-8: conclusion 282-284
IL-17 285
IL-1 286-287
NF-kB 288
ICAM-1 289
GM-CSF 290
MIP/CCL20 291
Overall 292
Construction 293-314
The law 294
Which specifically binds to 295-312
Inhibits the activity of …IL-17A/F…to induce the production 313
of IL-8 and L-6
Use of an antagonist anti-IL A/F antibody … for 314
Genentech’s amendment applications 315
Clarity 316-318
Extension of protection 319-321
Added matter 322-351
The law 324-328
The absence of specific evidence 329-330
The “complex” point 331-334
The “IL-8 and IL-6” point 335-349
The “combination” point 340-344
The “Kd” point 345-347
The “conditions” point 348-351
Conclusion in relation to Genentech’s amendment applications 352
The prior art 353-396
The IL-17A/F prior art: US344 354-358
The IL-17A prior art: W0717, US711, JP046 and Lubberts 2001 359-396
W0717 360-369
US711 370-384
JP046 385-396
Obviousness over US344 397-410
The disclosure of US344 398-399
Obviousness of claims 1,2,13,14 and 15 400-403
Claim 1 400
Claims 2 and 15 401
Claims 13 and 14 402
Obviousness of claims 12, 20 and 20 in so far as directed to RA 403-410
Novelty over the IL-17A/A prior art 411-412
Obviousness over the IL-17A/A prior art 413-521
An outline of the issues 416-418
The use of mAbs 5, 16 and 25 as a starting point 419-430
Taking mAbs 5, 16 and 25 forward 431-435
The humanisation work 436
Use of IMGT definitions of CDRs 437-440
Use of germline sequences 441-444
Choice of residues to back mutate 445-472
Adair 453-457
Carter 458-460
Queen 461-471
Conclusion 472
The affinities of the humanised antibodies 473-481
Inevitability of binding to and inhibition of IL-17A/F 482-485
as well as IL-17A/A
Structures of IL-17A, IL-17 F/F IL-17A/F and 486-497
binding to receptors
Antibodies that bind to and inhibit IL-17 A/A 498-503
Immunodominance 504-506
Immune self-tolerance 507-508
Epitope clustering 509
The known antibodies 510-517
Conclusion on obviousness 518-521
Insufficiency: plausibility of the psoriasis claims 522-578
The law 523-531
Assessment 532-577
Fossiez 533
Chabaud 534-535
Jovanovic 536
Teunissen 537-542
Albanesi 543-548
Homey 549-550
Aggarwal 2002 551
Aggarwal 2003 552
The skilled person’s perception of the potency and 553
range of effects of IL-17A
Examples 1 and 2 of the Patent 554-556
Prof Krueger’s evidence 557-571
Prof Prens’ evidence 572-574
Prof Kamradt’s evidence 575
Conclusion 576-578
Insufficiency: other grounds 579-581
Ambiguity 579
Undue burden 580-581
The development of ixekizumab 582-593
Infringement 594-617
Ixekizumab 594
Which specifically binds to 595-599
Use … for: contribution of inhibition of IL-17A/F 600-606
Infringement of claims 1 and 2 607
Infringement of claims 13, 14, 15 and 22 608-613
Infringement of claims 12 and 20 614-615
Infringement of claims 13, 14 and 20 if conditionally 617
amended
Summary of principal conclusions 618
Introduction
2. Lilly seek revocation of the Patent, alleging that all of the claims are invalid on grounds of lack of novelty, obviousness and insufficiency, and a declaration that dealings in ixekizumab do not infringe the Patent in any event. There is no challenge to the earliest claimed priority date of 8 July 2003. Genentech counterclaims for infringement. Genentech has also applied to amend the Patent both unconditionally and conditionally. Lilly opposes the amendments on grounds of added matter, extension of protection and lack of clarity as well as contending that they do not cure the invalidity of the claims. Although both the application for the Patent and the Patent as granted contained claims directed to the treatment of any immune-related disorder, Genentech only maintains claims directed to rheumatoid arthritis (“RA”) and psoriasis. Claims directed to inflammatory diseases generally and asthma specifically were abandoned as recently as 4 January 2019.
3. It is pertinent to observe at the outset that this is one of the most complex patent cases I have ever tried (and I have considerable experience of trying complex patent cases). There are a large number of issues, and a formidable body of material addressing them. Some indication of this is provided by the following metrics. Lilly’s written closing submissions run to 607 paragraphs and Genentech’s to 423 paragraphs, and both documents incorporate by reference additional sections from the parties’ respective opening skeleton arguments. There are 24 reports from nine expert witnesses running to 676 pages (including annexes, but excluding exhibits). The experts were efficiently cross-examined over seven and a half days. There are over 300 scientific papers (including a few abstracts) in the trial bundles (although I estimate that only about half were referred to), plus extracts from two books. I have done my best to take all this material into account; but I cannot possibly refer to all of it in this judgment. As will appear, I have been able to deal quite briefly with some of the issues. Even so, it cannot avoid being a lengthy judgment.
The witnesses
5. Dr Jean-Jacques Pin is a founder and President of Dendritics SAS (“Dendritics”), a position he has occupied since 2005. While working for Schering-Plough in 1993-1994, he was the scientist primarily responsible for the preparation of the mAb5, mAb16 and mAb25 monoclonal antibodies that Lilly relies on as representative of prior art murine antibodies raised to IL-17A/A. Genentech did not require Dr Pin to attend for cross-examination.
6. Dr Ian Wilkinson has been the Chief Scientific Officer of Absolute Antibody Ltd (“Absolute”) since 2012. Absolute carried out the humanisation of the mAb5, mAb16 and mAb25 antibodies as part of Lilly’s experiments. Genentech did not require Dr Wilkinson to attend for cross-examination.
7. Lilly called five experts and Genentech called four. Both parties called a dermatologist, a rheumatologist and two or more witnesses to address topics relating to antibody engineering.
9. The main focus of Prof Krueger’s research since the early 1990s has been on skin inflammation, and in particular psoriasis. In addition to psoriasis, he has carried out research in relation to skin cancers and he has collaborated with investigators of other inflammatory skin diseases such as atopic dermatitis and psoriatic arthritis. He has participated in over 50 clinical trials, many involving psoriasis treatments, including ones that selectively deplete activated T cells, block early T cell activation signals, block T cell mitogenic receptors, alter T cell differentiation toward regulatory cells, and antagonise specific inflammatory cytokines. Prof Krueger has published over 300 peer-reviewed publications, primarily in relation to psoriasis biology and treatment. Since 1995 he has been a member of advisory boards for a number of pharmaceutical companies and he has been consulted by a number of companies that have being developing treatments for psoriasis, including both Lilly (in relation to ixekizumab) and Genentech (in relation to efalizumab, an anti-CD11 antibody which received approval from the US Food and Drug Administration in October 2003, but was withdrawn from the market in 2009 due to adverse reactions). He has received a number of awards and honours, including the Distinguished Achievement Award and the Psoriasis Research Achievement Award from the American Skin Association in 2001.
10. Counsel for Genentech accepted that Prof Krueger was an eminent psoriasis expert, but submitted that his expertise significantly exceeded that of the relevant skilled person. I accept that, but this is a common attribute of expert witnesses in patent litigation in this country. Counsel also submitted that Prof Krueger had occasionally found difficulty in answering questions from the perspective of the person skilled in the art rather than from his own personal perspective. I also accept this, but again it is a common problem. I found Prof Krueger to be an impressive witness, and in general I have no hesitation in preferring his evidence to that of Prof Prens where they conflict. As always, however, it remains necessary to consider the evidence on each issue as a whole.
11. Prof Krueger was well placed to speak to the common general knowledge of the skilled person in July 2003 since he had written a review for the continuing medical education of dermatologists which was published in January 2002 (Krueger, “The immunologic basis for the treatment of psoriasis with new biologic agents”, J Am Acad Dermatol , 46, 1-23, “Krueger 2002”) and had co-authored two reviews published in 2004 (Lowes et al , “Current concepts in the immunopathogenesis of psoriasis”, Dermatol Clin , 349-369, “Lowes” and Lew et al , “Psoriasis vulgaris: cutaneous lymphoid tissue supports T-cell activation and ‘Type 1’ inflammatory gene expression”, Trends in Immunol , 25, 295-305, “Lew”) which were probably written in around July 2003. He also gave a presentation entitled “IL-17 Family Cytokines and Psoriasis” at a Psoriasis: Gene to Clinic meeting in December 2017 in which he reviewed the history of discoveries relating to the IL-17 family and its role in psoriasis. Counsel for Genentech criticised Prof Krueger for not re-reading Kreuger 2002 when writing his reports even though he had referred to it, but Prof Krueger explained that he remembered it very well. In any event, as Prof Krueger also explained, the field had moved on by July 2003. Thus I do not accept counsel for Genentech’s submission that Prof Krueger’s evidence was inconsistent with Krueger 2002.
12. Counsel for Genentech submitted that some of Prof Krueger’s evidence was wrong, but the principal example he relied upon was what Prof Krueger had said about allergic contact dermatitis in his first report. As discussed below, Prof Krueger modified his position in cross-examination, but maintained the thrust of the point he was making. Counsel for Genentech also submitted that Prof Krueger had adopted an unduly negative attitude to some of the prior publications in the field, but I consider that Prof Krueger was simply giving his opinion as a scientist.
13. Finally, counsel for Genentech submitted that Prof Krueger’s evidence was coloured by the fact he personally had not considered IL-17A to be a target for psoriasis in 2003, but had considered IFN-γ to be an important target. I accept that the view of the skilled person in July 2003 would not necessarily have coincided with that of Prof Krueger, and that it is necessary to take this into account when considering the evidence as a whole.
14. Genentech’s dermatologist is Professor Errol Prens, who is a Professor of Experimental Dermatology at the Erasmus University Medical Centre in Rotterdam (“Erasmus MC”), where he also practices clinically as a dermatologist. He obtained a medical degree from the University of Groningen in 1981. Following training at the Erasmus University, he became a certified dermatologist in 1985. He joined the Department of Immunology at Erasmus MC in 1986 where he completed a PhD on the immunopathophysiology of psoriasis in 1992. In 1993 he became a Researcher and group leader. In 2005 he was appointed to his current position.
15. Prof Prens’ research interests have centred on psoriasis and other inflammatory skin diseases such as atopic dermatitis and more recently hidradenitis suppurativa (also known as acne inversa). His focus has been on the immunological cells, cytokines and inflammatory signalling pathways involved in the initiation and maintenance of psoriasis. He has published over 170 articles, the majority of which relate to psoriasis and immunology, about 100 abstracts and about 10 book chapters. He has participated in a number of clinical trials of treatments for psoriasis and other inflammatory skin diseases. He is a member of advisory boards for a number of pharmaceutical companies.
16. Counsel for Lilly submitted that Prof Prens’ evidence was unsatisfactory, but accepted that, in some respects, this appeared to be due to the way in which he had been instructed, and his reports prepared, by Genentech’s legal team. Counsel gave three examples of this. The first was the exhibition of a selective extract from Freedberg et al (eds), Fitzpatrick’s Dermatology in General Medicine (6th ed, 2003). Prof Prens explained that the particular pages had been selected by the legal team, and he had not seen the selection at the time he signed his report. I regard this as unfortunate, but of no further significance.
17. The second example was the reliance upon a paper on cyclosporine Prof Prens had published in 1995 (Prens et al , “Effects of cyclosporine on cytokines and cytokine receptors in psoriasis”, J Am Acad Dermatol , 33, 947-953) rather than a review he had published in the same year (Prens et al , “T lymphocytes in psoriasis”, Clinics in Dermatol , 13, 115-129). This in itself does not strike me as significant, particularly given the age of the review. What is more significant is, as counsel for Lilly submitted, the brevity and superficiality of Prof Prens’ exposition of the common general knowledge of the skilled person in his first report compared to that of Prof Krueger, something which I noted when I first read the reports. For example, there was little discussion of the complexity of the cytokine network, and no mention at all of the phenomenon of redundancy.
18. The third example was Prof Prens’ evidence concerning Abcream. I shall deal with this topic in context below. As this stage, it is sufficient to record that I do not accept that the cross-examination of Prof Prens on this matter was, as counsel for Genentech submitted, “bizarre”, “inappropriate”, “aggressive” or “bullying”, although it is fair to say that some of the questions were mis-directed. I found Prof Prens’ evidence on the topic deeply unsatisfactory for the reasons I shall explain. While it may be said to be an isolated and somewhat peripheral topic which does not necessarily affect Prof Prens’ evidence on the other issues in the case, I am bound to say that it did reduce my confidence in Prof Prens’ evidence and thus the weight which I am able to give it.
19. The rheumatologists . Lilly’s expert on RA was Dr Erik Lubberts, who is Head of the Research Laboratory of Immune Mediated Inflammatory Diseases and an Associate Professor in the Department of Rheumatology at Erasmus MC. He obtained a Master’s in Biology and Medical Biotechnology from the University of Groningen in 1994 and a PhD on the role of interleukin (IL)-4 and IL-10 in the regulation of experimental arthritis from the University of Nijmegen in 1999. From 1999 to 2002 he was a post-doctoral fellow in Prof van den Berg’s group in the Department of Rheumatology working on two projects on the role of IL-17 in arthritis. From November 2002 to August 2003 he carried out research as a visiting scientist in three US laboratories, including work on IL-17. From September 2003 to March 2005 he was a post-doctoral researcher in the Department of Rheumatology at the University of Nijmegen. He then moved to the Department of Rheumatology at Erasmus MC as an Assistant Professor and became an Associate Professor in 2009.
21. Much of Dr Lubberts’ evidence was unchallenged in cross-examination. Consistently with that, counsel for Genentech accepted that Dr Lubberts was well qualified to give the evidence he had given and made no criticism of that evidence.
22. Genentech’s expert on RA was Professor Thomas Kamradt, who is Professor of Immunology at the Institute of Immunology at Jena University Hospital in Germany. He undertook his medical training at the Universities of Cologne, Vienna and Berlin, obtaining his medical licence in 1984. From 1984 to 1989 he was a resident in internal medicine at the Medical School of the University of Bonn. During that period, he obtained a Dr. med degree at the Free University of Berlin in 1987. From 1989 to 1991 he was a post-doctoral associate in the Department of Biology at the Massachusetts Institute of Technology, where he researched T-cell immunology. From 1991 to 1994 he was an Assistant Professor of Medicine in the Department of Rheumatology/Clinical Immunology at Tufts Medical School in Boston. During this period, his research focus was Lyme disease, in particular Lyme arthritis. From 1994 to 2004 he was the Group Leader of T-cell Immunology at the Deutsches RheumaForschungszentrum (German Rheumatism Research Centre, “DRFZ”) in Berlin. In 1998, his group became interested in IL-17-producing Th cells through their work on Lyme disease. In parallel with his work at the DRFZ, from 1995 to 2003 he was a practising clinician at the rheumatology outpatients clinic at Charité University Hospital in Berlin. He has been head of the Institute of Immunology at Jena University Hospital since 2004. He has collaborated with pharmaceutical companies on a number of occasions.
23. In the area of autoimmunity, Prof Kamradt’s research group primarily investigates the induction, chronification and modulation of pathological immune responses in (models of) arthritis and autoimmune encephalitis. In the area of immunoregulation, they primarily investigate the induction, function and stability of Th17 cells, and how cytokine receptors (in particular IL-33R) interact with other cellular receptors. He has published over 130 articles on immunology and rheumatic diseases, including RA. In addition, he has written chapters in about 10 textbooks, including the Oxford Textbook of Rheumatology .
25. Counsel for Lilly also criticised Prof Kamradt’s oral evidence with respect to US344. As counsel himself submitted, however, Prof Kamradt’s final position was essentially the same as that set out in his first report.
26. The antibody experts . Lilly’s principal expert on antibody engineering was Dr John Tite. Dr Tite is a director of Pannier Consulting Ltd, a biotechnology consultancy company, part-time Scientific Advisor at Touchlight Genetics Ltd, a biopharmaceutical company developing synthetic DNA manufacturing technology, and a non-executive director of Iquar Ltd, a biopharmaceutical company developing a vaccine platform. He obtained a degree in Zoology from University College London in 1974 and a PhD in Immunology from the Department of Immunology of the Middlesex Hospital Medical School, University of London in 1977. From 1977 to 1980 he was a post-doctoral researcher in the Medical Research Council (MRC) Immunobiology Unit, Department of Pathology at the University of Bristol Medical School. From 1980 to 1985 he held a position in the Division of Immunobiology, Department of Pathology at Yale University School of Medicine. In 1986 he joined the Wellcome Foundation as a postdoctoral Research Associate. Prior to the merger of Wellcome plc with Glaxo plc, he managed the Wellcome Therapeutic Antibody programme. After the formation of GlaxoWellcome in 1995 he held the positions of Unit Head of the Immunology Research Unit overseeing the early Discovery Portfolio (1995-1999), Head of the Immunology and Virology Department (1999-2001) and Acting Director of Biological Sciences Division (2000-2001). After the merger of GlaxoWellcome and SmithKline Beecham to form GlaxoSmithKline plc, he was Vice-President, Gene and Protein Therapeutics, Discovery Research (2001-2003) and Vice-President for Discovery Biology within the Biopharm Centre of Excellence for Drug Discovery (2003-2008). During the latter period he was also Chair of the Board of Trustees of the Edward Jenner Institute for Vaccine Research. From 2009 to 2012 he was the founding Chief Executive Officer of Bicycle Therapeutics Ltd, which had a proprietary technology for the development of highly stable bicyclic peptides as novel biopharmaceutical agents. He established Pannier Consulting Ltd in 2012.
27. Counsel for Genentech made no criticism of Dr Tite as a witness. Counsel submitted that it was regrettable that certain points made by Dr Tite in his oral evidence had not been included in Dr Tite’s written reports, but accepted that this may have been the fault of Lilly’s legal team. More importantly, counsel pointed out that Dr Tite was an immunologist, not an expert in structural biology, and that he had had no experience of working with IL-17 cytokines or antibodies to them. Accordingly, Dr Tite accepted that he was not in a position to question Prof Carr’s structural analysis of what antibodies would be expected to be raised against IL17A and how they would be expected to interact with IL-17A/F. As counsel for Lilly pointed out, however, Dr Tite emphasised that he was in a position to consider the question from the perspective of an immunologist.
28. Dr Lutz Riechmann was Lilly’s expert on antibody humanisation. He is now a consultant in antibody engineering. He obtained a degree in biology from the University of Bremen in 1984 and a PhD in biology from the same institution in 1986. From 1986 to 1988 he was a post-doctoral researcher in the laboratory of Professor Sir Gregory Winter CBE FRS (as he now is). During this period, Dr Riechmann produced the first ever fully humanised antibody, CAMPATH-1H. Following work at the Scripps Institute in La Jolla in 1988 to 1989, he was employed by MRC Laboratory of Molecular Biology as a Group Leader (1989-1997) and then Senior Scientific Officer (1997-20008 and 2009-2011). He was Director of Antibody Display Technology at F-star Cambridge from 2008 to 2009. He has been a consultant since 2011. He has published over 40 articles and patents.
29. Counsel for Genentech advanced no real criticism of Dr Riechmann as a witness, as opposed to the substance of certain points that he made.
30. Finally, Lilly called Professor Arthur Lesk as an expert in computational biology. He is a Professor in the Department of Biochemistry and Molecular Biology at the Pennsylvania State University, where he also holds an honorary appointment in the Department of Computer Science and Engineering. He obtained an AB in Biochemical Sciences from Harvard University in 1961 and a PhD in Physics and Physical Chemistry from Princeton University in 1966. Following this, he held positions including Professor of Chemistry at Fairleigh Dickinson University (1971-1987); a Visiting Scientist at the MRC Laboratory of Molecular Biology (1977-1979 and 1981-1990); Group Leader of the Biocomputing Programme at the European Molecular Biology Laboratory in Heidelberg (1987-1990); and Senior Research Associate in the Department of Haematology at the University of Cambridge (1990-2003). He has held his current position since 2003.
31. Prof Lesk’s research focuses on genomics; protein structure, function and evolution; and the structures and functions of biological networks. His work in relation to the canonical-structure model and its application to the analysis of antibody germ line genes, which he carried out in collaboration with Cyrus Chothia at the MRC Laboratory of Molecular Biology in the late 1980s, supported the humanisation of antibodies for therapy. His publications are cited in the Adair, Carter and Queen patents referred to below. He has published over 230 articles and several textbooks, including Introduction to Bioinformatics (4th ed, Oxford University Press, 2013; 5th ed in press), Introduction to Protein Science (3rd ed, Oxford University Press, 2016) and Introduction to Genomics (3rd ed, Oxford University Press, 2017).
32. As counsel for Genentech pointed out, Prof Lesk had one main task in this case (although he also produced some 3D images for Dr Tite). His main task was to produce models that reflected those which a skilled person in 2003 would have produced if humanising mAbs 5, 16 and 25 so that Dr Riechmann could use the inter-atomic distances to apply his 3.5 Å distance criterion. In that task he singularly failed, for the reasons explained below. Counsel for Genentech submitted that the sequence of events discussed there did not reflect well on Prof Lesk. I have to say that I agree with this.
33. Genentech’s principal expert on antibody engineering was Professor Andrew Martin, who is Professor of Bioinformatics and Computational Biology in the Department of Structural and Molecular Biology, Division of Biosciences at University College London (“UCL”). He obtained a degree in Biochemistry from the University of Oxford in 1986 and a DPhil in the molecular modelling of antibody combining sites from the same institution from 1986 to 1990. From 1990 to 1994, he was self-employed doing contract work for Oxford Molecular Ltd. and The National Grid Company as well as independently developing scientific software. In 1994 he joined UCL as a post-doctoral Research Fellow and in 1998-1999 he was seconded four days a week to Inpharmatica Ltd, a spin-off from UCL, where he held the position of Technical Director. From 1999 to 2003, he was Lecturer in Bioinformatics at the University of Reading. In 2004 he took the same position at UCL, becoming Senior Lecturer in Bioinformatics in 2005 and Reader in Bioinformatics and Computational Biology in 2014. He was appointed to his current position in 2018.
34. Prof Martin has major research interests in (a) the sequence, structure and function of antibodies, creating databases and tools for studying these proteins, performing analyses and making predictions; and (b) the effects of mutations on protein structure and how these are related to disease. A major element of his work has concerned humanisation aspects of antibody development. He has published a considerable number of articles and seven book chapters. He has collaborated extensively with UCB Biopharma, and he has considerable experience as an expert witness.
35. Counsel for Lilly made no real criticism of Prof Martin as a witness, but pointed out that Prof Martin’s expertise was in computational biology rather than immunology and that he had done no “wet” laboratory work since part-way through his undergraduate degree. Counsel for Lilly submitted that Prof Martin had strayed into a field in he did not have expertise, namely SPR. As Prof Martin explained, however, although he did not have experience of performing SPR, he did have experience in interpreting SPR results. More generally, he had experience of working as part of teams dealing with antibody engineering.
36. In addition, Genentech called Professor Mark Carr , an expert in structural biology. He is Professor of Biochemistry in the Department of Molecular and Cell Biology at the University of Leicester, and has a leadership role in the Leicester Institute of Structural and Chemical Biology. He obtained a degree in Biochemistry from the University of Birmingham in 1983 and a D.Phil. in Biochemistry from the University of Oxford in 1987. From 1987 to 1989 he held Post-doctoral Fellowships at the Max Planck for Medical Research and the Max Planck Society in Heidelberg. From 1989 to 2003 he held a post-doctoral research position in the Laboratory of Molecular Structure at the National Institute for Medical Research. From 1993 to 1997 he was a Group Leader in the Laboratory of Molecular Structure at the National Institute for Biological Standards and Control. From 1997 to 2001 he was Lecturer in Structural Biology in the Department of Biosciences at the University of Kent. From 2001 to 2009 he was Reader in Biological NMR Spectroscopy in the Department of Biochemistry at the University of Leicester. He was appointed to his present position in 2009. From 2011 to 2015, he was also Director of Enterprise for the College of Life Sciences at the University of Leicester. In addition to his roles at the University of Leicester, he has acted as a senior scientific advisor to UCB since 2002.
37. Prof Carr’s research focuses on determining the structures, functions, interactions and mechanisms of action of proteins and protein complexes involved in key biological processes of significant medical importance, including the characterisation of interactions with potential new therapeutics. He has published over 60 articles covering structural and functional studies of a diverse range of proteins and protein complexes. Importantly, he was very familiar with the IL-17 cytokines, having worked with them as part of a two-to-three year project carried out by his group in collaboration with UCB. He was thus well qualified to opine on what would be expected in terms of antibodies binding to these molecules from a structural perspective. He also had experience of working with collaborators in therapeutic antibody projects. As counsel for Lilly pointed out, however, Prof Carr was not an immunologist and he accepted that he was not as well qualified as Dr Tite to speak about such matters as B-cell recognition, maturation and somatic hypermutation.
38. Counsel for Lilly pointed out that it had emerged from Prof Carr’s oral evidence that he had received some help in the preparation of his first report from his research assistant Dr Lorna Waters which was not fully or properly acknowledged. I agree that this should have been fully disclosed in the report, but Prof Carr was candid about the assistance he had received in cross-examination and was clear that the evidence he gave was his own.
General technical background
39. The following account of the general technical background is based on the technical primer which the parties helpfully agreed, save that I have slightly expanded the description of cytokines. For convenience this account is mainly expressed in the present tense, but it refers to what was known in July 2003.
Nucleic acids
40. Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) consist of chains of nucleotides. Nucleotides are phosphate esters of a pentose sugar (containing five carbon atoms) covalently linked to a nitrogenous base. There are four types of nucleotide in each type of nucleic acid, determined by the nature of nitrogenous base: adenine (A), thymine (T) (in DNA) / uracil (U) (in RNA), guanine (G) and cytosine (C). Nucleotides are joined together by a ligation reaction. The ligation covalently links the phosphate group on the second nucleotide and the hydroxyl group on the first nucleotide to form a sugar-phosphate backbone from which the bases are projected (see Figure 1). Repetition of this reaction creates a long chain of nucleotides (a polynucleotide chain).
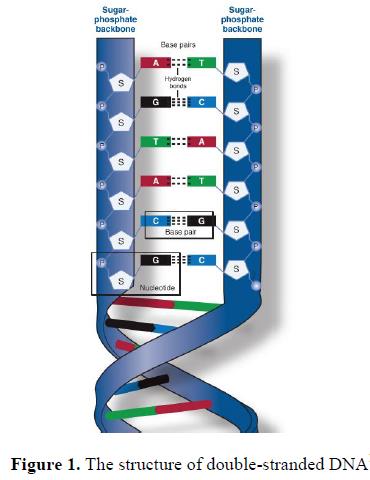
41. A gene is a code created by the collection of nucleotides into a specific order. This code is stored as DNA which forms a stable double-stranded helical structure through the hydrogen bonding of complementary base pairs, adenine to thymine and guanine to cytosine, in two polynucleotide chains aligned in opposite directions (polynucleotides are read from the 5’-end to the 3’-end). A gene encodes a transmissible trait, generally through the encoding of a protein or RNA that is functional.
Proteins
42. A protein is the functional product of the blueprint encoded by DNA. In order for the product of the blueprint to be made, the DNA must be decoded through two sequential processes termed transcription and translation. During transcription the double-stranded DNA is unzipped and the relevant single strand is used as a template to create a complementary RNA polynucleotide chain (in which uracil takes the place of thymine). This RNA undergoes post-transcriptional modification to enable it to be processed correctly by the machinery within the cell. Such modifications include the removal of the complementary RNA which does not encode part of the gene product (introns), also referred to as splicing, and the addition of a poly-adenosine tail to the 3’-end of the RNA polynucleotide. After modification the single strand is referred to as messenger RNA (mRNA).
43. The DNA code, once transcribed into mRNA, is then translated in order to generate a sequence of amino acids, the building blocks for proteins.
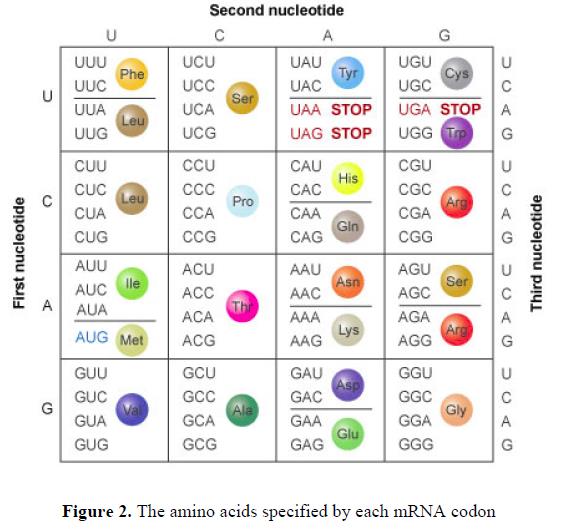
44. Each group of three nucleotides within the mRNA is referred to as a codon. The combination of bases within each codon translates to a particular amino acid (or a signal to stop the translation process). Multiple codons can specify the same amino acid (see Figure 2).
45. Amino acids share a common structure with a primary amino group (-NH 2 ), a carboxylic acid group (-COOH), a single hydrogen and a variant (R) side-chain group branching from a central carbon atom (the α-carbon) (Figure 3).

46. Each amino acid differs in the composition of its R-group and there are twenty common types. This R-group determines the characteristics of the amino acid.
47. These twenty amino acids may be classified on the basis of the properties of their R-group into one of four groups: (I) non-polar; (II) polar, uncharged; (III) acidic; and (IV) basic. Other systems of classification based on the structure or chemical characteristics of the amino acid side chains may also be used.
48. During the translation process the amino acids are joined together into linear chains through peptide bonds between the carboxyl group of one amino acid and the amino group of another in which a water molecule is lost. A protein is made from a long chain of amino acids (also referred to as a polypeptide) and each of the amino acids is referred to as a residue. Each protein will have an N-terminal residue (an exposed amino group) and a C-terminal residue (an exposed carboxyl group).
49. There are four levels of structural organisation in a protein (see Figure 4). The primary structure is simply the sequence of the amino acids in the polypeptide.
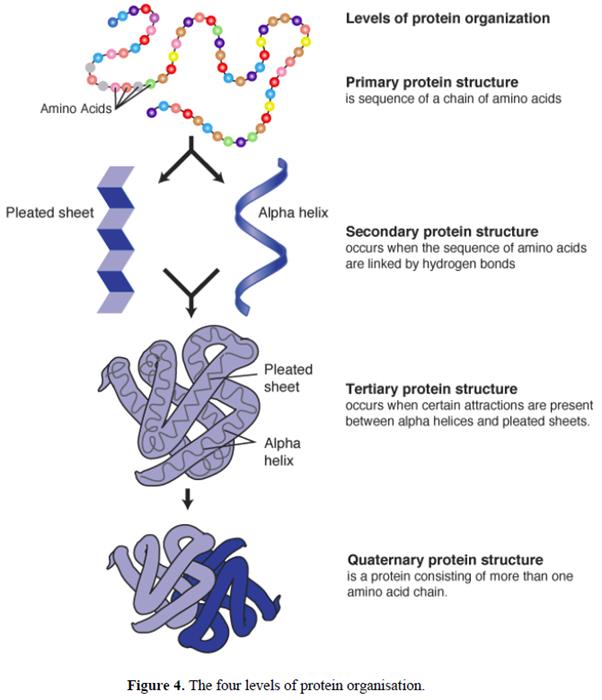
50. The secondary structure is defined by the conformation of the polypeptide backbone, which generally forms a regular arrangement of amino acids such as an α-helix or β-strand. An α-helix is a spiral structure within a protein in which hydrogen bonding links one amino acid to the amino acid four residues along in the chain. This binding forms a backbone core that is tightly packed, with the amino acid side chains extending away from this central axis. In contrast, a β-strand consists of an extended amino acid chain that must interact via backbone hydrogen bonds with another β-strand to form a stable structure known as a β-sheet. Such strands may be arranged in a parallel fashion (where the strands run in the same direction) or an anti-parallel fashion (where the strands run in opposite directions). In some cases two anti-parallel strands can be formed with a tight turn between them; in other cases extensive loops or other regions of secondary structure may occur between adjacent β-strands.
51. The tertiary structure is formed by the folding of the secondary structural elements of a protein and is determined by the properties of the side chains of the amino acids that make up the primary structure. The tertiary structure is formed largely due to the hydrophobic (non-polar) side chains being buried in the core (where water is largely excluded) and the hydrophilic (polar) side chains being exposed largely on the surface. Some hydrophilic groups are found internally and act to stabilise the structure through electrostatic interactions or hydrogen bonding.
52. The quaternary structure refers to the number and spatial arrangement of multiple folded protein subunits which bind together to form larger protein molecules (see Figure 4). The structure of a dimer is shown in Figure 4, which consists of two subunits that may be identical (a homodimer) or different (a heterodimer). The quaternary structure is often maintained by non-covalent bonds between the protein subunits, and in some cases stable disulphide linkages that are covalent bonds.
Recombinant expression of proteins
53. Recombinant expression of proteins is a process which involves inserting DNA, which contains the code for a specific protein, into a host cell in such a way that the host cell treats it as its own. The host cell then uses its internal machinery to produce (or “express”) the protein of choice.
54. This process involves the following steps:
i) Identification and DNA sequencing of the gene encoding the particular protein or fragment which is to be produced.
ii) Generating DNA encoding the protein/fragment of interest – usually by amplification of the DNA sequence encoding the protein of interest.
iii) Insertion of the DNA into a “vector” that is able to carry the DNA sequence into the host cell and cause the protein(s) encoded by the DNA sequence to be expressed by the host cell.
iv) Introducing the vector into the host cell by transfection, transduction, or transformation.
v) Expression of the protein by the host cell, followed by collection and purification of the resultant protein or fragment.
55. The proteins that result from the above process are called “recombinant proteins”. This form of genetic engineering can be applied to the generation of recombinant proteins, including antibodies, in sufficient quantities for laboratory studies or industrial application.
Innate vs adaptive immunity
56. The cells and molecules responsible for immunity constitute the immune system, and their collective and coordinated response to the introduction of foreign substances is called the immune response. When an immune response is produced in response to proteins or molecules expressed by the host (a self-antigen), this is known as auto-immunity and may result in so-called auto-immune disease.
57. The body’s immune system has two types of defence against pathogens (micro-organisms that can cause disease when they infect the host) and any toxic molecules they produce: (i) innate immunity, non-adaptive mechanisms that rapidly provide protection against pathogens, and (ii) adaptive immunity against specific pathogens.
58. Innate immunity provides the first line of defence against infection. Some components of innate immunity, e.g. production of anti-microbial proteins by epithelial cells, are constitutive, but can also be induced or increased rapidly to augment the constitutive levels. Other components are cellular or cytokine pathways that can be rapidly activated to provide protective responses. Innate mechanisms can be divided into a number of categories: anatomic (e.g. epithelial cell surfaces), physiologic (e.g. body temperature), engulfment (e.g. phagocytes) and the complement system (plasma proteins that induce a series of inflammatory responses). Generally, microbes or microbial products directly trigger cellular pathways which produce an “active” innate immune response, e.g. lipopolysaccharide from Gram negative bacteria activate macrophages to release pre-synthesised tumour necrosis factor (TNF) which then induces a “chain reaction” of subsequent cytokines (IL-1, IL-6, IL-8, amongst others) which rapidly (within hours) bring neutrophils to a site of infection. Neutrophils release anti-microbial products that kill bacteria, and bacteria are also engulfed by macrophages, neutrophils, and other phagocytes that kill them in the cytoplasm. Natural killer (NK) cells are a type of lymphocyte of the innate immune system that when activated also release inflammatory cytokines and can kill cellular targets using products stored in the cytoplasm.
59. The general nature of this type of immunity means that its components are pre-existing or rapidly synthesised upon microbial contact. Constitutive elements and triggered reactions do not require any previous contact with a microbe to be fully mobilised.
60. In contrast, adaptive immunity develops in response to a primary exposure to the antigenic stimulus and functions by expansion and differentiation of immune cells into “effector cells” that target specific antigens and “memory cells”. These effector cells are known as lymphocytes and can be divided into B-lymphocytes (B cells) and T-lymphocytes (T cells) (see Figure 5). T cells are further sub-divided into helper T-cells (CD4 + ) and cytotoxic T cells (CD8 + ). In 2003 it was also known that a subset of CD4 + T-cells could suppress T cell activation responses and these cells were termed regulatory T cells (T R ).
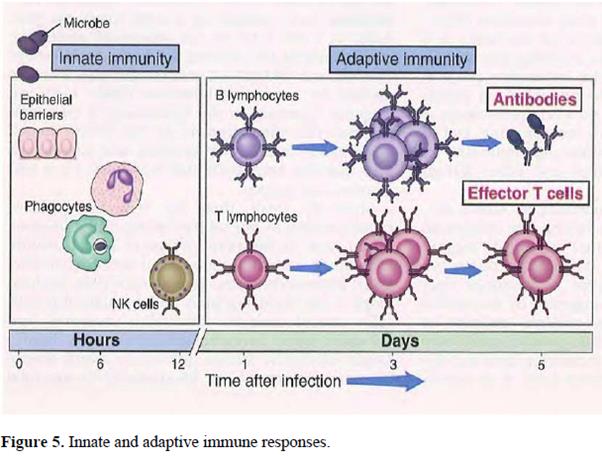
61. The primary role of B cells is to generate antibodies and this pathway is referred to as “humoral immunity”. Helper T cells (T H or Th cells) have a wide range of functions, one of which is to interact with B cells to facilitate their activation and differentiation into plasma cells to produce highly potent antibodies. Cytotoxic T cells on the other hand destroy host cells that have become infected with viruses or other intracellular pathogens. The T cell immune response is referred to as cell-mediated immunity.
62. Figure 6 illustrates the operation of different aspects of the immune system, including innate immunity and adaptive immunity split between B cell (humoral) and T cell (cell-mediated) pathways.
Phagocytes
63. Phagocytes include macrophages, neutrophils, monocytes and dendritic cells. In phagocytosis the pathogen is surrounded by the phagocyte membrane and is then internalised in a membrane-bound vesicle called a phagosome, which becomes acidified. The phagosome then fuses with a lysosome creating a phagolysosome into which the proteolytic enzymes contained in the lysosome are released to destroy the pathogen via proteolysis. This process forms part of both innate and adaptive immunity, as shown in Figure 6.
64. In adaptive immunity, macrophages can be activated by a subset of T H cells called T H 1 cells. Activated macrophages have increased phagocytic activity and fuse their lysosomes more efficiently to form phagosomes and also make a variety of other toxic products that assist with the destruction of pathogens, including oxygen radicals and nitric oxide (both of which have antimicrobial activity), as well as synthesising antimicrobial peptides and proteases that can be released to attack extracellular microbes.
Antigen-presenting cells
65. A major function of the innate immune system is to present antigens to the cells of the adaptive arm of the immune system and thereby activate the adaptive immune response. An antigen is any substance that can be recognised by the adaptive arm of the immune system. This substance may derive from a micro-organism, an allergen (such as grass pollen or house dust mite) or may be an alloantigen, a neo-antigen, or a component of a vaccine. In the case of auto-immunity, the antigen may be a self-protein derived from the host.
66. Certain cells of the innate immune system, such as monocytes, macrophages and dendritic cells are known as antigen-presenting cells (APCs). These cells can take up an antigen, for example, by engulfing a micro-organism, an allergen or a vaccine component by phagocytosis, using a variety of ubiquitous recognition systems. Alternatively, cells that are infected by a pathogen can also act as APCs. B cells can also serve as APCs in some circumstances.
67. Once inside the APC, the antigen is degraded, generally resulting in the formation of short peptide fragments. The peptides are then externalised and “presented” at the surface of the APC. An antigen is presented on the surface of APCs complexed with a molecule known as major histocompatibility complex (MHC) class I or class II. This is shown in section 3 of Figure 6.
B cells
68. Antibody expression . Prior to their activation through interaction with either T H 1 or T H 2 cells, B cells are referred to as “naïve”. Naïve B cells express proteins known as antibodies. Initially the antibodies are bound to the cell surface membrane and act as a receptor for an antigen (known also as the B cell receptor or BCR). An antigen is any substance capable of inducing an adaptive immune response. Each B cell expresses antibodies that have the same unique antigen binding site. Naïve B cells produce an immunoglobulin type called IgM after they become activated.
69. When an antigen is recognised by a B cell receptor (BCR), as shown in section 6 of Figure 6, the antigen is internalised. Once inside the B cell, the antigen is processed and presented at the B cell surface (similar to the processing and presentation of antigen by APCs) by the MHC class II molecules. The antigen-MHC complex on the B-cell surface can then interact with T cell receptors on the surface of activated T H cells. T cells which become activated by this process produce cytokines that influence the type of immunoglobulin (IgG vs. IgE) that are made by memory B cells through a process called class switching. Thus a B cell/T cell interaction regulates activation, proliferation and differentiation of B cells. The activated B cells then expand in number (“clonal expansion”), as shown in section 7 of Figure 6. B cells can also be activated to produce immunoglobulins without T cell “help,” but if this happens the antibody class is IgM, as explained further in below.
70. B cell differentiation . As the response to the antigen matures, further interactions occur between cells of the innate immune system and lymphocytes. These take place within specialist parts of lymphoid organs called germinal centres and result in the differentiation of the B cells. B cells differentiate into either plasma cells or memory B cells, as shown in section 8 of Figure 6. The differentiation process generates antibodies with increasing affinity for the antigen (affinity maturation and somatic hypermutation). At the same time, the “class” of the antibody may change, in a process known as class switching (antibody classes are explained below).
T cells
71. Interaction with APCs . The antigen-MHC complex on the surface of APCs is recognised by T cells. This represents the direct interaction between cells of the innate immune system (APCs) and the adaptive immune system (T cells). The T cells bind to the APCs via specific receptors on the surface of the T cells known as T cell receptors (TCRs) (see Figure 7). Each individual T cell expresses a particular TCR, which demonstrates specificity for a single antigen. However, the T cell population will consist of millions of cells with different TCRs and antigen specificities. Binding of the antigen on the surface of the APC to the antigen binding site on the TCR, in conjunction with co-stimulation by accessory molecules, activates the T cell.
72. CD4 + /CD8 + Cells . In addition to cell-surface expression of TCRs, T cells express a variety of other membrane molecules, called co-receptors, which augment and stabilise the initial antigen-MHC/TCR interaction. Differential expression of CD4 and CD8 co-receptors allows T-cells to be subdivided into helper T cells (T H ), which express CD4, and cytotoxic T cells (T C ), which express CD8 (shown in section 4 of Figure 6). On the basis of this subdivision, T H and T C cells are referred to as being CD4-positive (CD4 + ) and CD8-positive (CD8 + ), respectively.
73. Typically, CD4 + T H cells recognise antigen bound to MHC class II molecules on the APC’s surface with the CD4 molecule binding to the MHC class II molecule. CD8 + T C cells recognise antigen bound to MHC class I molecules and the CD8 molecule binds to the MHC class I molecule.
74. T H 1/T H 2 cells . Following activation, CD4 + T cells undergo programmed differentiation into two major subsets, T H 1 and T H 2 (or Th1 and Th2) cells. These subsets can be distinguished on the basis of their function and pattern of cytokine production (cytokines are discussed in more detail below).
75. T H 1 cells preferentially activate macrophages, although they can also activate cytotoxic T cells and B cells. Their predominant role is in defending against intracellular pathogens. Another important action of T H 1 T cells is that they direct B cells to “class switch” to produce IgG antibodies which are important for helping to control bacterial, fungal and viral infections.
76. By contrast, T H 2 cells are predominantly concerned with defending against extracellular pathogens and their responses are mediated by cytokines such as interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13). The predominant action of T H 2 cells is to activate (“class switch”) B cells to produce IgE antibodies which help to control helmith (worm) infections. However, IgE antibodies can also be produced to environmental antigens (pollens) or food, leading to hayfever or other seasonal allergies and food allergies. After forming an antigen-antibody complex, IgE antibodies trigger mast-cells and basophils, types of innate immune cells, to release histamine and other “allergic” mediators, as well as products that are toxic to helmiths.
77. Memory T cells . Following activation, some T cells differentiate to form a population of long-lived memory T cells, which respond with greater reactivity on a subsequent exposure to the same antigen. This creates a secondary immune response, which provides more rapid protection against re-challenge with the same pathogen.
Inflammation
78. Inflammation is the response of tissue to injury or infection. It is a process that increases the local concentration of immunomodulatory molecules and cells at the site of damage or infection, resulting from an increase in vascular permeability and increased migration of cells of the adaptive and innate immune systems from the blood to inflamed tissue.
79. The inflammatory response occurs in different phases and the initial events typically amplify the immune response. The second phase usually involves the resolution of the immune response and the repair of tissue damage. In the context of auto-immunity, such inflammatory responses are associated with a failure to resolve the immune response properly (and can become chronic) and this is relevant to a number of auto-immune inflammatory diseases, including rheumatoid arthritis, psoriasis, inflammatory bowel disease (including ulcerative colitis and Crohn’s disease), multiple sclerosis, etc.
Cytokines
80. Cytokines are proteins released by cells of the innate and adaptive immune systems. They can function as immune-modulating agents, for example inducing cells of the immune system to proliferate or differentiate, or to generate an inflammatory response.
81. Cytokines are typically given names based on their cellular source or key property. Cytokines which are produced by leukocytes (white blood cells) such as macrophages or T cells, and modulate the activity of other leukocytes, are called interleukins (IL).
82. Cytokines are recognised by specialised receptors which are usually expressed on the surface of cells. Upon the binding of a cytokine to its receptor, the functions of the cell that bears the receptor are altered. For some cytokines, natural inhibitors (or antagonists) exist that tightly control the cytokine’s actions.
83. To block the actions of a cytokine, several avenues are possible, including the administration of: (i) an antibody that binds to the cytokine in such a manner as to inhibit its activity; (ii) a soluble receptor that binds to the cytokine in circulation and thus prevents it from binding to the patient’s cell-bound cytokine receptors; (iii) an antibody that binds to the cytokine receptor in a manner which blocks the activation of that receptor; or (iv) a recombinantly-produced naturally-occurring receptor antagonist.
Tumour necrosis factor alpha
84. Tumour necrosis factor alpha (TNFα) is primarily secreted by macrophages, but antigen stimulated T cells, mast cells and natural killer cells also produce the protein.
85. The principal physiological role of TNFα in inflammation is to stimulate the recruitment of neutrophils and monocytes to sites of infection and to activate these cells to eradicate microbes. The action of TNFα on endothelial cells and macrophages induces secretion of chemokines (cytokines with chemoattractant properties), which in turn promotes the recruitment and infiltration of leukocytes from the blood (chemotaxis). TNFα also stimulates mononuclear phagocytes to produce interleukin-1 (IL-1). The binding of TNFα to its receptor leads to the activation of a transcription factor called nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which responds by upregulating a number of genes linked to inflammation including those relating to T-cell proliferation and survival.
Interferon gamma
86. Interferon gamma (IFNγ) is produced by natural killer cells during the innate immune response and T H 1 and T C 1 cells once adaptive immunity develops. Its principal actions are to activate macrophages in both innate and adaptive cell-mediated immune responses leading to an increase in the efficiency of hydrolytic cell destruction of phagocytosed pathogens. IFNγ also promotes a positive feedback loop by stimulating undifferentiated CD4 + T cells (T H 0) to differentiate into T H 1 cells, which in turn will produce more IFNγ.
Interleukin-6
87. Interleukin-6 (IL-6) is a pro-inflammatory cytokine produced by many cell types, including macrophages, bronchial epithelial cells (the cells lining the airways), fibroblasts (cells found in connective tissue) and synoviocytes (fibroblast-like cells found within joints). IL-6 stimulates adaptive immunity by promoting the growth of differentiated B cells which secrete antibodies i.e. plasma cells.
Interleukin-8
88. Interleukin-8 (IL-8), also referred to as CXCL8, is produced by a range of cells including macrophages, neutrophils, synoviocytes and fibroblasts. IL-8 is a chemokine which promotes chemotaxis of leukocytes, in particular the infiltration, and subsequent degranulation, of neutrophils into sites of inflammation.
The interleukin-17 family
89. The first member of the interleukin-17 (IL-17) family was originally called IL-17, but since the discovery of other members of the same family it is now referred to as IL-17A or IL-17A/A (I shall use all these terms interchangeably.) IL-17A is a secreted disulphide-linked homodimer (hence IL-17A/A) with a molecular weight of 30-35kD. IL-17A was first identified from a murine T-cell cDNA library; the human IL-17A gene was subsequently identified and the protein sequence was published. From rodents to humans, IL-17A is synthesised mainly by activated T cells.
90. Stimulation of various cell types with IL-17A promotes the production of other pro-inflammatory cytokines such as IL-6 as well as the chemokine IL-8. The NF-κB transcription factor is activated by IL-17A leading to upregulation of pro-inflammatory gene expression which, for example, contributes to T cell proliferation.
91. Following the identification of IL-17A, five further related cytokines were identified in human cells and designated IL-17B to IL-17F. Each of the members of this “family” is also a homodimer (thus IL-17F may also be referred to as IL-17F/F).
92. By 2003 one receptor for IL-17A had been identified, designated IL-17R. (It is convenient to note, however, that subsequently a second receptor has been identified and the two are now referred to as IL-17RA and IL-17RC.)
Antibody structure
93. An antibody (or immunoglobulin (Ig)) is a large (approximately 150 kDa) protein produced by B cells which recognises an antigen (as described above). Antibodies consist of four polypeptide chains, two identical light chains (each around 220 amino acids in length) and two identical heavy chains (each around 440 amino acids in length). The four chains are bound together by a combination of non-covalent interactions and disulphide bridges and form a Y-shaped structure with each of the “arms” containing a heavy and a light chain joined together (see Figure 8).

94. Both light and heavy immunoglobulin chains can be separated into two regions based on the variability of the amino acid sequence between individual antibodies. Towards the N-terminus of each heavy and light immunoglobulin chain is a variable region identified as V H or V L , respectively. The V H and V L regions are made up of around 110 amino acids.
95. Conversely, the remaining region of the heavy and light immunoglobulin chains towards the C-terminus is a region which does not change significantly between individual antibodies; this is termed the constant region, C H or C L , respectively. Each C L region is made up of around 110 amino acids whereas the C H regions are much larger. The C H regions are subdivided into three units (Ig domains) each of around 110 amino acids, C H 1 to C H 3 in IgG, IgD, and IgA antibodies (see Figure 8).
96. Within both variable regions (V H and V L ) there are three segments of particular variability, designated the “hypervariable” regions, which form loop structures which are commonly referred to as the complementarity-determining regions (CDRs). There are six CDR loops in each arm of the Y-shaped antibody, three in the V H region and three in the V L region (see Figure 9). These CDRs are primarily responsible for determining antigen specificity by forming a binding site that is complementary to the binding site on the antigen. The CDRs are supported by the remaining stretches of the variable region, which are called framework regions, and which may contribute to antigen binding by ensuring that the CDRs adopt the correct conformation.

97. Antibody molecules can be cleaved in vitro into various fragments by enzymes, known as proteases, which cleave proteins at specific sites. The protease papain cleaves an antibody of the IgG class into two Fab fragments (short for “Fragment antigen-binding”) and an Fc fragment (short for “Fragment crystallisable”) (see Figure 10, left hand panel). The Fab fragment is composed of the V H and V L regions with the first constant Ig domain (C H 1 and C L ) held together by a disulphide bond. The Fc fragment comprises the remaining two constant domains of the Ig heavy chain (C H 2 and C H 3) also held together by a disulphide bond in the “hinge” region. In an intact antibody, the part of the antibody that corresponds with the Fc fragment is known as the Fc region.
98. A different protease, pepsin, cleaves an antibody of the IgG class into a F(ab’)2 fragment and degrades the constant region into several smaller fragments. A F(ab’)2 fragment comprises of two Fabs held together by disulphide bonds in the hinge region (Figure 10, right hand panel).

Antibody classes
99. Antibodies are divided into different classes and subclasses depending on their heavy chain. There are five distinct classes (also referred to as isotypes) of antibody called IgA, IgD, IgE, IgG and IgM, the heavy chains of which are known as α, δ, ε, γ and μ respectively. The classes differ in a number of aspects, most importantly in size and amino acid sequence. In the blood of humans and mice, the most common class of antibody is IgG, which accounts for about 75–80% of the total antibody pool.
100. IgG and IgA are further divided into subclasses. Human IgG is divided into four subclasses which differ only slightly in their amino acid sequences: IgG1, IgG2, IgG3 and IgG4. Human IgA is divided into IgA1 and IgA2.
101. Mouse IgG is also divided into subclasses, namely, IgG1, IgG2a, IgG2b and IgG3. Despite the similarity in nomenclature to human IgG subclasses, the human and mouse IgG sub-classes are not equivalent either in terms of sequence or function.
102. The Fc region of an antibody mediates the effector functions of the antibody (e.g. promoting clearance of pathogens which express the antigen) via binding to Fc receptors on the cells of the immune system. Different Fc receptors exist which show specificity for different classes and subclasses of antibody. The receptors that recognise IgG are known as Fcγ receptors (FcγRs). The Fc region of some antibody classes can also bind an activated complement complex which leads to direct lysis of cells that bind the antibody/complement complex.
Antigen binding
103. Epitopes . Areas that interact between an antibody and an antigen are commonly referred to as the paratope (on the antibody) and the epitope (on the antigen). The paratope is primarily generated by the CDRs on the antibody as have been described above (see Figure 9).
104. In general terms, an epitope comprises a region on the antigen which interacts with the paratope. An epitope may be classified in the following ways:
i) A continuous epitope (also referred to as consecutive or linear), in which the epitope is formed by a stretch of neighbouring amino acid residues along the primary sequence of the antigen.
ii) A discontinuous epitope (also referred to as non-consecutive or conformational), in which the amino acid residues forming the epitope are discontinuously arranged along separate parts of the primary sequence of the antigen, but are brought into close proximity through the native folding of the polypeptide chains and/or arrangement of the polypeptide chains that form the protein (see Figure 11).

105. Binding affinity . The binding of an antigen and an antibody is driven by non-covalent reversible interactions such as hydrophobic interactions, hydrogen bonds, ionic bonds and Van der Waals interactions.
106. The binding affinity of an antibody is a measure of the combined strength of the non-covalent interactions between the antibody and antigen. In essence, antibodies with low affinity associate with antigens weakly and tend to dissociate quickly, whereas antibodies with high affinity associate with antigens more quickly and tend to dissociate less readily.
107. The affinity of a binding interaction between an antigen and an antibody is often represented by the equilibrium dissociation constant (K D or Kd). The K D is defined as the ratio between (i) the product of the free antibody concentration (Ab) and the free antigen concentration (Ag); and (ii) the concentration of antibody:antigen complexes (AbAg): K D = [Ab][Ag]/[AbAg].
108. Measuring antibody binding affinity . A common method employed to measure the binding affinity of an antibody to its antigen is surface plasmon resonance (SPR). SPR is based on the measurement of the refractive index near a sensor surface. The phenomenon occurs when a surface plasmon, which is a charge density wave that occurs at the interface between a metal (often gold) and a dielectricum, is excited by light. When the light is directed at a particular angle, the photon energy is transferred to the charge density wave, which is observed as a sharp dip in the refracted light intensity. If an antigen is adsorbed onto the metal and an antibody is passed over the surface, binding of the proteins causes a shift in the SPR angle (see Figure 12).

109. As an example, Biacore is one of several commercial immunosensing systems based on SPR. The equipment measures the binding between molecules (the ligand) bound to a gold-coated sensor chip and molecules (the analyte) that are passed over the surface.
110. To assess antibody binding affinity, the experiment starts, after calibration, by binding the antigen of interest to the surface of a chip. The Biacore instrument then passes the antibody of interest over the chip. The machine detects the rate of association (k on ) and dissociation (k off ) and calculates the dissociation constant (K D ).
X-ray crystallography
111. X-ray crystallography involves the analysis of the diffraction pattern produced by the scattering of a collimated X-ray beam as it passes through a crystal.
112. In the context of epitope mapping, X-ray crystallography involves analysis of a crystal of the antibody bound to the antigen. X-ray crystallography provides the coordinates of all the non-hydrogen atoms of the molecules that were crystallised (hydrogen atoms can only be seen in very high resolution structures) and thus, can be used to calculate the contact distances between residues in the antibody:antigen complex. Such contact distances may be used, along with other parameters, as a basis to identify the epitope region on an antigen recognised by the paratope of an antibody.
Generating antibodies by immunising animals
113. Antibodies against an antigen of interest may be generated by immunising an animal. The antigen of interest may act as an immunogen (a foreign protein eliciting an adaptive immune response).
114. B-cells are isolated and fused with cells which are “immortal” i.e. able to multiply in culture indefinitely.
115. The cell created after fusion of a B cell with the immortal cell is called a hybridoma. These cells are able to be maintained in culture and will continually secrete the particular antibody which the B cell expresses. Antibodies to an antigen of interest may be monoclonal, meaning they derive from a single B-cell or hybridoma clone and all recognise the same epitope, or polyclonal, meaning they derive from different B cells or hybridomas and recognise different epitopes.
116. After fusion, the hybridomas are initially plated into wells of a cell culture plate. Wells containing hybridoma cells producing antibodies which recognise the antigen of interest are identified through the use of screening with immunoassays (discussed below). The hybridomas from a well which tests positive in these assays are redistributed into new wells to isolate a single hybridoma cell in each well. This technique, referred to as sub-cloning, generates a population of cells in each well which stem from a single parent cell and produce monoclonal antibodies.
117. The wells are screened again using immunoassays to identify the monoclonal antibodies which recognise the antigen of interest.
Generating antibodies using phage display
118. Phage display is a technique, of which there are a number of variants, for the identification of antibodies which bind an antigen of interest in vitro . A bacterial virus, a bacteriophage (or phage for short), is engineered to express and display on its surface an antibody Fab or single-chain variable fragment (scFv). A “library” of phage expressing different antibody Fabs or scFvs is prepared and screened for binding to the antigen of interest. To do so, the antigen of interest may be coated to a surface and exposed to the phage in order that the phage that express a Fab/scFv which binds the antigen of interest are captured while the phage that do not bind are washed away. Bacteria may be infected with the captured phage to amplify the relevant phage. The DNA from captured phage may be isolated and used to recombinantly produce the Fab/scFv which bind the antigen of interest for further testing. The Fabs/scFvs can be converted into full antibodies through genetic engineering.
ELISA
119. An enzyme-linked immunosorbent assay (ELISA) is a high throughput solid phase immunoassay, meaning that one of the components (i.e. antigen or antibody) is fixed to a solid surface. There are three types of ELISA: direct, indirect and sandwich.

120. In a direct ELISA, an antigen (Ag, shown as a green circle in Figure 13) is immobilised on the surface of a well in a microtitre plate and then incubated with the antibody of interest which has been linked (conjugated) to an enzyme (the primary antibody conjugate in panel A of Figure 13 below) after which the plate is washed to remove any antibody that has not bound to the antigen. The enzyme is able to produce a detectable response by catalysing a reaction in a substrate. Common enzymes used for this purpose include horseradish peroxidase (HRP) or alkaline phosphatase (AP).
121. The response is measured to assess the amount of antibody bound to the antigen on the plate. A darker colour will indicate a higher level of bound antibody (see Figure 13). A quantitative measure of the signal in each well can be generated using a microtitre plate reader which measures the absorbance at a specific wavelength of light depending on the colour of the signal, this is termed optical density (OD).
122. An indirect ELISA involves coating the antigen of interest onto a microtitre plate but the detectable response is provided by a secondary antibody which recognises any portion of the primary antibody bound to the antigen (see panel B of Figure 13 above). The secondary antibody is applied after washing away any unbound antibody and is conjugated to an enzyme. The enzyme attached to the secondary antibody catalyses a reaction leading to a detectable change in the substrate and the amount of binding can be measured, for example, as a change in colour (see Figure 14).
123. A sandwich ELISA employs two antibodies, or three for an indirect assay (see panel C of Figure 13 above). The first antibody for the antigen is attached to the bottom of the wells of a microtitre plate; this is referred to as the capture antibody. The test solution containing the antigen of interest is then introduced. The antigen is captured by the fixed antibody and any unbound proteins are washed away. An antibody which recognises the antigen via a different epitope is then added and the binding of this second antibody, referred to as a detection antibody, will be used to detect that the antigen has been captured. Figure 14 shows this being performed using an indirect method, but a direct method using an enzyme conjugated to the detection antibody is possible. The signal is developed and the detectable response is measured as outlined above.
124. A competitive ELISA can be performed, amongst other ways, by coating the antigen of interest on to the microtitre plate and incubating with a primary antibody conjugated to an enzyme. The level of binding of the primary antibody is then measured. A second antibody which is not conjugated is then introduced step-wise in increasing concentrations. The amount of primary antibody bound is detected at each concentration of the unlabelled antibody. If the antibodies bind to the same or an overlapping epitope the signal from the primary antibody will be reduced.
125. Immunocytochemistry/immunohistochemistry . Immunocytochemistry and immunohistochemistry are techniques used to identify an antigen within, or on the surface of, a cell. The cells of interest are fixed to immobilize the antigen while maintaining the relevant structural features. The antigen may be contained within the interior of a cell and therefore the cells are also permeabilised to allow the antibody to access the intracellular space and bind to the antigen.
126. The presence of the antigen is observed by detecting the presence of bound antibody; either directly where the antibody is fluorescently labelled or indirectly where a fluorescently labelled secondary antibody binds to the detecting antibody (see panels A and B of Figure 13, respectively). The fluorescence can then be observed in distinct locations under a microscope.
Neutralisation assay
127. A neutralisation assay can be used to measure the ability of an antibody to inhibit the activation of a downstream signal which is elicited when a ligand binds to a receptor. The ligand is applied to cells or a tissue in which it is known to elicit a response, such as the release of a cytokine or other messenger. The reduction in the defined response in the presence of increasing concentrations of the test antibody is measured.
128. The IC 50 is the concentration of antibody which is able to inhibit the defined response by 50% (Figure 15).
129. The physical blockade of a ligand-receptor interaction by an antibody can be assessed using SPR (described above).
Fc fusion proteins
130. The Fc region of an antibody can be linked to a peptide or protein of interest to create an Fc fusion protein (typically identified as X:Fc, where X is the protein of interest).
Therapeutic antibodies
131. Murine monoclonal antibodies can lead to an immune response in humans due the recognition of the murine antibody as “foreign”, leading to the generation of antibodies against the mouse antibody. This was termed the human anti-mouse antibody (HAMA) response.
132. Chimeric antibodies may be generated by expressing the murine variable regions together with human constant regions, with the aim of reducing the immunogenicity of the antibody.
133. Humanised antibodies may be generated by inserting (“grafting”) the CDRs from a murine antibody into a human antibody framework (see Figure 16). Commonly it is necessary to also change framework residues in the variable domains, typically to the residues found in the murine antibody. A number of variations on the basic CDR-grafting technique have been developed. Techniques for humanising antibodies other than by CDR-grafting have also been developed.
The Patent
134. The Patent is of considerable length and complexity, the specification running to no less than 461 paragraphs and 127 pages. I shall summarise the disclosure as briefly as I can, using the headings and sub-headings of the specification.
Field of the invention
135. The specification states at [0001] that the invention “relates generally to the identification and isolation of a novel human cytokine designated as interleukin-17A/F (IL-17A/F)”.
Background of the invention
136. The specification sets out in [0002]-[0006] some basic information about extracellular, secreted and membrane-bound proteins and receptors, explaining that they have various industrial applications, including as pharmaceuticals and diagnostics.
137. At [0007] the specification states that the invention “relates to identifying novel secreted polypeptides of the interleukin-17 (IL-17) family which have been shown to be related to immune-mediated and inflammatory disease”. At [0008] it is noted that, although the genesis of these diseases often involves multi-step pathways, intervention at critical points, either by antagonism of a detrimental pathway or stimulation of a beneficial one, can have a therapeutic effect.
138. At [0009] the specification states (emphasis added):
“Many immune related diseases are known and have been extensively studied. Such diseases include immune-mediated inflammatory diseases (such as rheumatoid arthritis , immune mediated renal disease, hepatobiliary diseases, inflammatory bowel disease (IBD), psoriasis , and asthma), non-immune-mediated inflammatory diseases, infectious diseases, immunodeficiency diseases, neoplasia, etc.”
139. The specification continues with a brief discussion of the nature of the mammalian immune response system, focussing on the role of T cells, their maturation and proliferation. At [0014] it points out that immune suppressants such as neutralising antibodies can be used in the treatment of immune-related diseases.
140. From [0015] to [0019] the specification provides a detailed overview of IL-17A and the other members of the IL-17 family (i.e. IL-17B-F). Since this passage is central to Genentech’s case on plausibility, it is necessary to quote it in full. It can be divided into three parts. I shall highlight references to certain articles which featured prominently in the evidence and which are referred to below. I shall also highlight references to RA and psoriasis.
141. The first part discusses IL-17 i.e. IL-17A:
“[0015] lnterleukin-17 (IL-17) is a T-cell derived pro-inflammatory molecule that stimulates epithelial, endothelial and fibroblastic cells to produce other inflammatory cytokines and chemokines including IL-6, IL-8, G-CSF, and MCP-1 [ see , Yao, Z. et al., J. Immunol., 122(12):5483-5486 (1995); Yao, Z. et al., Immunity, 3(6):811-821 (1995); Fossiez, F., et al., 30 J. Exp. Med., 183(6): 2593-2603 (1996) ; Kennedy, J., et al., J. Interferon Cytokine Res., 16(8):611-7 (1996); Cai, X. Y., et al., Immunol. Lett, 62(1):51-8 (1998); Jovanovic, D.V., et al., J. Immunol. 160(7):3513-21 (1998) ; Laan, M., et al., J. Immunol., 162(4):2347-52 (1999); Linden, A., et al., Eur Respir J, 15(5):973-7 (2000); and Aggarwal, S. and Gurney, A.L., J Leukoc Biol, 71(1):1-8 (2002) ]. IL-17 also synergizes with other cytokines including TNF-a and IL-1β to further induce chemokine expression ( Chabaud, M., et al., J. Immunol. 161 (1):409-14 (1998) ). Interleukin 17 (IL-17) exhibits plei[o]tropic biological activities on various types of cells. IL-17 also has the ability to induce ICAM-1 surface expression, proliferation of T cells, and growth and differentiation of CD34 + human progenitors into neutrophils. IL-17 has also been implicated in bone metabolism, and has been suggested to play an important role in pathological conditions characterized by the presence of activated T cells and TNF-α production such as rheumatoid arthritis and loosening of bone implants (Van Bezooijen et al., J. Bone Miner. Res., 14: 1513-1521 [1999]). Activated T cells of synovial tissue derived from rheumatoid arthritis patients were found to secrete higher amounts of IL-17 than those derived from normal individuals or osteoarthritis patients (Chabaud et al., Arthritis Rheum., 42: 963-970 [1999]). It was suggested that this proinflammatory cytokine actively contributes to synovial inflammation in rheumatoid arthritis . Apart from its proinflammatory role, IL-17 seems to contribute to the pathology of rheumatoid arthritis by yet another mechanism. For example, IL-17 has been shown to induce the expression of osteoclast differentiation factor (ODF) mRNA in osteoblasts (Kotake et al., J. Clin. Invest., 103: 1345-1352 [1999]). ODF stimulates differentiation of progenitor cells into osteoclasts, the cells involved in bone resorption. Since the level of IL-17 is significantly increased in synovial fluid of rheumatoid arthritis patients, it appears that IL-17 induced osteoclast formation plays a crucial role in bone resorption in rheumatoid arthritis . IL-17 is also believed to play a key role in certain other autoimmune disorders such as multiple sclerosis (Matusevicius et al., Mult. Scler., 5: 101-104 (1999); Kurasawa, K., et al., Arthritis Rheu 43(11):2455-63 (2000)) and psoriasis ( Teunissen, M.B., et al., J Invest Dermatol 111 (4):645-9 (1998) ; Albanesi, C., et al., J Invest Dermatol 115(1):81-7 (2000) ; and Homey, B., et al., J. Immunol. 164(12:6621-32 (2000) ).
[0016] IL-17 has further been shown, by intracellular signalling, to stimulate Ca 2+ influx and a reduction in [cAMP] in human macrophages ( Jovanovic et al., J. Immunol., 160:3513 [1998] ). Fibroblasts treated with IL-17 induce the activation of NF-κB, [Yao et al., Immunity, 3:811 (1995), Jovanovic et al., supra], while macrophages treated with it activate NF-κB and mitogen-activated protein kinases (Shalom-Barek et al., J. Biol. Chem., 273:27 467 [1998]). Additionally, IL-17 also shares sequence similarity with mammalian cytokine-like factor 7 that is involved in bone and cartilage growth. Other proteins with which IL-17 polypeptides share sequence similarity are human embryo-derived interleukin-related factor (EDIRF) and interleukin-20.
[0017] Consistent with IL-17's wide-range of effects, the cell surface receptor for IL-17 has been found to be widely expressed in many tissues and cell types (Yao et al., Cytokine, 9:794 [1997]). While the amino acid sequence of the human IL-17 receptor (IL-R) (866 amino acids) predicts a protein with a single transmembrane domain and a long, 525 amino acid intracellular domain, the receptor sequence is unique and is not similar to that of any of the receptors from the cytokine/growth factor receptor family. This coupled with the lack of similarity of IL-17 itself to other known proteins indicates that IL-17 and its receptor may be part of a novel family of signaling proteins and receptors. It has been demonstrated that IL-17 activity is mediated through binding to its unique cell surface receptor (designated herein as human IL-17R), wherein previous studies have shown that contacting T cells with a soluble form of the IL-17 receptor polypeptide inhibited T cell proliferation and IL-2 production induced by PHA, concanavalin A and anti-TCR monoclonal antibody (Yao et al., J. Immunol., 155:5483-5486 [1995]). As such, there is significant interest in identifying and characterizing novel polypeptides having homology to the known cytokine receptors, specifically IL-17 receptors.”
142. The second part discusses IL-17B to F, and in particular IL-17F:
“[0018] Interleukin 17 is now recognized as the prototype member of an emerging family of cytokines. The large scale sequencing of the human and other vertebrate genomes has revealed the presence of additional genes encoding proteins clearly related to IL-17, thus defining a new family of cytokines. There are at least 6 members of the IL-17 family in humans and mice including IL-17B, IL-17C, IL-17D, IL-17E and IL-17F as well as novel receptors IL-17RH1, IL-17RH2, IL-17RH3 and IL-17RH4 (see W001/46420 published June 28, 2001). One such IL-17 member (designated as IL-17F) has been demonstrated to bind to the human IL-17 receptor (IL-17R) (Yao et al., Cytokine, 9(11):794-800 (1997)). Initial characterization suggests that, like IL-17, several of these newly identified molecules have the ability to modulate immune function. The potent inflammatory actions that have been identified for several of these factors and the emerging associations with major human diseases suggest that these proteins may have significant roles in inflammatory processes and may offer opportunities for therapeutic intervention.
[0019] The gene encoding human IL-17F is located adjacent to IL-17 (Hymowitz, S.G., et al., Embo J, 20(19):5332-41 (2001)). IL-17 and IL-17F share 44% amino acid identity whereas the other members of the IL-17 family share a more limited 15-27% amino acid identity suggesting that IL-17 and IL-17F form a distinct subgroup within the IL-17 family (Starnes, T., et al., J Immunol, 167(8):4137-40 (2001); Aggarwal, S. and Gurney, A.L., J. Leukoc Biol, 71 (1):1-8 (2002) ). IL-17F appears to have similar biological actions as IL-17, and is able to promote the production of IL-6, IL-8, and GCSF from a wide variety of cells. Similar to IL-17, it is able to induce cartilage matrix release and inhibit new cartilage matrix synthesis (see US-2002-0177188-A1 published November 28, 2002). Thus, like IL-17, IL-17F may potentially contribute to the pathology of inflammatory disorders. Recently, these authors have observed that both IL-17 and IL-17F are induced in T cells by the action of interleukin 23 (IL-23) ( Aggarwal, S., et al., J. Biol. Chem., 278(3):1910-4 (2003) ).”
143. The third part discusses IL-17A/F. As such, the skilled reader would appreciate that it is actually concerned with the invention, rather than what was known before:
“The observation that IL-17 and IL-17F share similar chromosomal localization and significant sequence similarity [as] well as the observation that IL-17 and IL-17F appear to be induced with the same cell population in response to a specific stimuli has lead to the identification of a new human cytokine that is comprised of a covalent heterodimer of IL- 17 and IL-17F (herein designated IL-17A/F). Human IL-17A/F is a distinctly new cytokine, distinguishable from human IL-17 and 1L-17F in both protein structure and in cell-based activity assays. Through the use of purified recombinant human IL-17 A/F as a standard, a human IL-17AF-specific ELISA has been developed. Through the use of this specific ELISA, the induced expression of human IL-17A/F was detected, confirming that IL-17A/F is naturally produced from activated human T cells in culture. Hence, IL-17A/F is a distinctly new cytokine, detectable as a natural product of isolated activated human T cells, whose recombinant form has been characterized, in both protein structure and cell-based assays, as to be different and distinguishable from related cytokines. Thus, these studies provide and identify a novel immune stimulant (i.e. IL-17 A/F) that can boost the immune system to respond to a particular antigen that may not have been immunologically active previously. As such, the newly identified immune stimulant has important clinical applications. This novel IL-17A/F cytokine or agonists thereof, would therefore find practical utility as an immune stimulant, whereas molecules which inhibit IL-17A/F activity (antagonists) would be expected to find practical utility when an inhibition of the immune response is desired, such as in autoimmune diseases. Specifically, antibodies to this new cytokine which either mimic (agonist antibodies) or inhibit (antagonist antibodies) the immunological activities of IL-17 A/F would possess therapeutic qualities. Small molecules which act to inhibit the activity of this novel cytokine would also have potential therapeutic uses.”
It can be seen that no specific clinical applications or therapeutic uses are identified in this passage. The nearest one gets is the reference to auto-immune diseases.
Summary of the invention
144. A. Embodiments . From [0020] to [0048] the specification identifies some 29 embodiments of the invention, many of which encompass multiple possibilities. Of note are [0020], which concerns “compositions and methods useful for the diagnosis and treatment of immune related diseases in mammals, including humans”, stating that “the IL17A/F polypeptides of the present invention and antagonists thereof as defined in the claims are also useful to prepare medicines and medicaments for the treatment of immune-related and inflammatory diseases”; and [0021], which concerns “methods of identifying agonists and antagonists of an IL-17A/F polypeptide”.
145. At [0023] the specification states (emphases added):
“In another embodiment, the invention relates to a method of treating an immune related disorder in a mammal in need thereof, comprising administering to the mammal a therapeutically effective amount of an IL-17A/F polypeptide, an agonist thereof, or an antagonist thereto. In a preferred aspect, the immune related disorder is selected form the group consisting of: systemic lupus erythematosis, rheumatoid arthritis , osteoarthritis, juvenile chronic arthritis, spondyloarthropathies, systemic sclerosis, idiopathic inflammatory myopathies, Sjögren's syndrome, systemic vasculitis, sarcoidosis, autoimmune hemolytic anemia, autoimmune thrombocytopenia, thyroiditis, diabetes mellitus, immune-mediated renal disease, demyelinating diseases of the central and peripheral nervous systems such as multiple sclerosis, idiopathic demyelinating polyneuropathy or Guillain-Barré syndrome, and chronic inflammatory demyelinating polyneuropathy, hepatobiliary diseases such as infectious, autoimmune chronic active hepatitis, primary biliary cirrhosis, granulomatous hepatitis, and sclerosing cholangitis, inflammatory bowel disease, gluten-sensitive enteropathy, and Whipple's disease, autoimmune or immune-mediated skin diseases including bullous skin diseases, erythema multiforme and contact dermatitis, psoriasis , allergic diseases such as asthma, allergic rhinitis, atopic dermatitis, food hypersensitivity and urticaria, immunologic diseases of the lung such as eosinophilic pneumonia, idiopathic pulmonary fibrosis and hypersensitivity pneumonitis, transplantation associated diseases including graft rejection and graft-versus-host-disease.”
146. At [0024] the specification states that, in another embodiment, the invention “provides an isolated antibody which specifically binds to any of the above or below described IL-17A/F polypeptides”.
147. The specification goes on to describe a series of diagnostic embodiments:
“[0028] Described herein is a method of diagnosing an immune related disease in a mammal, comprising detecting the level of expression of a gene encoding an IL-17A/F polypeptide …
[0029] In another embodiment, the present invention concerns a method of diagnosing an immune disease in a mammal, comprising (a) contacting an anti-IL-17A/F antibody with a test sample of tissue cells obtained from the mammal, 15 and (b) detecting the formation of a complex between the antibody and an IL-17A/F polypeptide, in the test sample; wherein the formation of said complex is indicative of the presence or absence of said disease. …
[0030] In another embodiment, the invention provides a method for determining the presence of an IL-17A/F polypeptide in a sample comprising exposing a test sample of cells suspected of containing the IL-17A/F polypeptide to an anti-IL-17A/F antibody and determining the binding of said antibody to said cell sample …
[0031] In another embodiment, the present invention may concern an immune-related disease diagnostic kit, comprising an anti-IL-17A/F antibody and a carrier in suitable packaging. The kit preferably contains instructions for using the antibody to detect the presence of the IL-17 A/F polypeptide. …
[0032] In another embodiment, the present invention may concern[] a diagnostic kit, containing an anti-IL-17A/F antibody in suitable packaging. The kit preferably contains instructions for using the antibody to detect the IL-17A/F polypeptide.”
148. B. Additional embodiments . From [0049] to [0066] the specification describes 18 additional embodiments, many of which encompass multiple possibilities.
Brief description of the drawings
149. At [0067] the specification identifies and explains 12 Figures, all of which relate to Examples 1 and 2.
Detailed description of the preferred embodiments:
150. I. Definitions . From [0068] to [0140] the specification sets out a long series of definitions. It is necessary to quote three of these definitions which are relevant to issues considered later. In the case of the second definition, I have broken the paragraph down into four sections which I have labelled A, B, C and D for ease of identification in the discussion below:
“[0115] A ‘species-dependent antibody,’ e.g., a mammalian anti-human lgE antibody, is an antibody which has a stronger binding affinity for an antigen from a first mammalian species than it has for a homologue of that antigen from a second mammalian species. Normally, the species-dependent antibody ‘bind[s] specifically’ to a human antigen (i.e., has a binding affinity (Kd) value of no more than about 1 x 10 -7 M, preferably no more than about 1 x 10 -8 and most preferably no more than about 1 x 10 -9 M) but has a binding affinity for a homologue of the antigen from a second non-human mammalian species which is at least about 50 fold, or at least about 500 fold, or at least about 1000 fold, weaker than its binding affinity for the human antigen. The species-dependent antibody can be of any of the various types of antibodies as defined above, but preferably is a humanized or human antibody.
[0118] [A] An antibody, oligopeptide or other organic molecule ‘which binds’ an antigen of interest, e.g. a tumor-associated polypeptide antigen target, is one that binds the antigen with sufficient affinity such that the antibody, oligopeptide or other organic molecule is useful as a diagnostic and/or therapeutic agent in targeting a cell or tissue expressing the antigen, and does not significantly cross-react with other proteins. In such embodiments, the extent of binding of the antibody, oligopeptide or other organic molecule to a ‘non-target’ protein will be less than about 10% of the binding of the antibody, oligopeptide or other organic molecule to its particular target protein as determined by fluorescence activated cell sorting (FACS) analysis or radioimmunoprecipitation (RIA).
[B] With regard to the binding of an antibody, oligopeptide or other organic molecule to a target molecule, the term ‘specific binding’ or ‘specifically binds to’ or is ‘specific for’ a particular polypeptide or an epitope on a particular polypeptide target means binding that is measurably different from a non-specific interaction. Specific binding can be measured, for example, by determining binding of a molecule compared to binding of a control molecule, which generally is a molecule of similar structure that does not have binding activity. For example, specific binding can be determined by competition with a control molecule that is similar to the target, for example, an excess of non-labeled target. In this case, specific binding is indicated if the blinding of the labeled target to a probe is competitively inhibited by excess unlabeled target.
[C] The term ‘specific binding’ or ‘specifically binds to’ or is ‘specific for’ a particular polypeptide or an epitope on a particular polypeptide target as used herein can be exhibited, for example, by a molecule having a Kd for the target of at least about 10 [-]4 M, alternatively at least about 10 -5 M, alternatively at least about 10 -6 M, alternatively at least about 10 -7 M, alternatively at least about 10 -8 M, alternatively at least about 10 -9 M, alternatively at least about 10 -10 M, alternatively at least about 10 -11 M, alternatively at least about 10 -12 M, or greater.
[D] In one embodiment, the term ‘specific binding’ refers to binding where a molecule binds to a particular polypeptide or epitope on a particular polypeptide without substantially binding to any other polypeptide or polypeptide epitope.
[0131] ‘Active’ or ‘activity’ for the purposes herein refers to form(s) of an IL-17A/F polypeptide which retain a biological and/or an immunological activity of native or naturally-occurring IL-17A/F polypeptides, wherein ‘biological’ activity refers to a biological function (either inhibitory or stimulatory) caused by a native or naturally-occurring IL-17A/F polypeptide other than the ability to induce the production of an antibody against an antigenic epitope possessed by a native or naturally-occurring IL-17A/F polypeptide and an ‘immunological’ activity refers to the ability to induce the production of an antibody against an antigenic epitope possessed by a native or naturally-occurring IL-17A/F polypeptide. One preferred biological activity includes inducing activation of NF-[κB] and stimulation of the production of the pro inflammatory chemokines IL-8 and IL-6. Another preferred biological activity includes stimulation of peripheral blood mononuclear cells or CD4 + cells. Another preferred biological activity includes stimulation of the proliferation of T-lymphocytes. Another preferred biological activity includes, for example, the release of TNF-α from THP1 cells. Another activity includes an enhancement of matrix synthesis in articular cartilage. Alternatively, another activity includes promoting breakdown of articular cartilage matrix as well as inhibiting matrix synthesis. Another preferred biological activity includes modulating the level of the interleukin-17 signalling pathway during mild to severe stages of inflammatory bowel disease or during stroke.”
151. Although it is not strictly a definition, the specification states at [0136] (emphases added):
“ Examples of immune-related and inflammatory disease, some of which are immune or T cell mediated, which can be treated according to the invention, include systemic lupus erythematosis, rheumatoid arthritis , juvenile chronic arthritis, osteoarthritis, spondyloarthropathies, systemic sclerosis (scleroderma), idiopathic inflammatory myopathies (dermatomyositis, polymyositis), Sjögren's syndrome, systemic vasculitis, sarcoidosis, autoimmune hemolytic anemia (immune pancytopenia, paroxysmal nocturnal hemoglobinuria), autoimmune thrombocytopenia (idiopathic thrombocytopenic purpura, immune-mediated thrombocytopenia), thyroiditis (Grave's disease, Hashimoto's thyroiditis, juvenile lymphocytic thyroiditis, atrophic thyroiditis), diabetes mellitus, immune-mediated renal disease (glomerulonephritis, tubulointerstitial nephritis), demyelinating diseases of the central and peripheral nervous systems such as multiple sclerosis, idiopathic demyelinating polyneuropathy or Guillain-Barré syndrome, and chronic inflammatory demyelinating polyneuropathy, hepatobiliary diseases such as infectious hepatitis (hepatitis A, B, C, D, E and other non-hepatotropic viruses), autoimmune chronic active hepatitis, primary biliary cirrhosis, granulomatous hepatitis, and sclerosing cholangitis, inflammatory bowel disease (ulcerative colitis: Crohn's disease), gluten-sensitive enteropathy, and Whipple's disease, autoimmune or immune-mediated skin diseases including bullous skin diseases, erythema multiforme and contact dermatitis, psoriasis , allergic diseases such as asthma, allergic rhinitis, atopic dermatitis, food hypersensitivity and urticaria, immunologic diseases of the lung such as eosinophilic pneumonia, idiopathic pulmonary fibrosis and hypersensitivity pneumonitis, transplantation associated diseases including graft rejection and graft versus-host-disease.”
152. Although at first sight this list of conditions appears to be the same as that in [0023], in fact it is a longer list: in particular, it specifies “Grave’s disease, Hashimoto’s thyroiditis, juvenile lymphocytic thyroiditis, atrophic thyroiditis” as varieties of thyroiditis and “hepatitis A, B, C, D, E and other non-hepatotropic viruses” as instances of hepatobiliary diseases. (In the latter case it appears that these words were accidentally omitted from [0023].) Curiously, neither the list in [0023] nor the list in [0136] includes stroke, which is mentioned at the end of [0131].
153. II. Compositions and methods of the invention . From [0141] to [0382] the specification sets out at great length illustrative compositions and methods of the invention. Little of the detail of this matters for present purposes, but it is important to appreciate the extremely broad scope of this section. This is sufficiently indicated by the following table of contents:
A. Full-length IL-17A/F polypeptides: [0141];
B. IL-17A/F polypeptide variants: [0142]-[0150];
C. Modifications of IL-17A/F: [0151]-[0161];
D. Preparation of IL-17A/F: [0162]-[0188];
E. Uses for IL-17A/F: [0189]-[0232];
F. Tissue distribution: [0233]-[0235];
G. Antibody binding studies: [0236]-[0239];
H. Cell-based assays: [0240]-[0250];
I. Animal models: [0251]-[0264];
J. Immunoadjuvant therapy: [0265];
K. Screening assays for drug candidates: [0266]-[0269];
L. Compositions and methods for the treatment of immune related diseases: [0270]-[0274];
M. Anti-IL-A/F antibodies: [0275]-[0332];
N. IL-17A/F binding oligopeptides: [0333]-[0337];
O. IL-17A/F binding organic molecules: [0338];
P. Screening for anti-IL-17A/F antibodies, oligopeptides and organic molecules having the desired properties: [0339]-[0342];
Q. Pharmaceutical compositions: [0343]-[0349];
R. Methods of treatment: [0350]-[0378];
S. Articles of manufacture: [0379];
T. Diagnosis and prognosis of immune related disease: [0380]-[0383].
154. In the sub-section concerning tissue distribution (F), the specification states at [0235]:
“Gene expression in various tissues, alternatively, may be measured by immunological methods, such as immunohistochemical staining of tissue sections and assay of cell culture or body fluids, to quantitate directly the expression of gene product. Antibodies useful for immunohistochemical staining and/or assay of sample fluids may be either monoclonal or polyclonal, and may be prepared in any mammal. Conveniently, the antibodies may be prepared against a native sequence of an IL-17 A/F polypeptide or against a synthetic peptide based on the DNA sequences encoding the IL-17A/F polypeptide or against an exogenous sequence fused to a DNA encoding an IL-17 A/F polypeptide and encoding a specific antibody epitope. General techniques for generating antibodies, and special protocols for Northern blotting and in situ hybridization are provided below”
155. In the sub-section concerning animal models (I), the specification refers to a considerable number of animal models for various diseases. It states at [0259]:
“Additionally, the compounds of the invention can be tested on animal models for psoriasis like diseases. Evidence suggests a T cell pathogenesis for psoriasis. The compounds of the invention can be tested in the scid/scid mouse model described by Schon, M. P. et al., Nat. Med., 3:183 (1997), in which the mice demonstrate histopathologic skin lesions resembling psoriasis. Another suitable model is the human skin/scid mouse chimera prepared as described by Nickoloff, B. J. et al., Am. J. Path., 146:580 (1995).”
156. In the sub-section concerning anti IL-17A/F antibodies (M), the specification states at [0289] that “The anti-IL-17A/F antibodies of the invention may further comprise humanized antibodies or human antibodies”.
157. This sub-section goes on to discuss humanisation techniques. In this context the specification states at [0292]:
“It is further important that antibodies be humanized with retention of high binding affinity for the antigen and other favourable biological properties. To achieve this goal, according to a preferred method, humanized antibodies are prepared by a process of analysis of the parental sequences and various conceptual humanized products using three-dimensional models of the parental and humanized sequences. Three-dimensional immunoglobulin models are commonly available and are familiar to those skilled in the art. Computer programs are available which illustrate and display probable three-dimensional conformational structures of selected candidate immunoglobulin sequences. Inspection of these displays permits analysis of the likely role of the residues in the functioning of the candidate immunoglobulin sequence, i.e., the analysis of residues that influence the ability of the candidate immunoglobulin to bind its antigen. In this way, FR residues can be selected and combined from the recipient and import sequences so that the desired antibody characteristic, such as increased affinity/or the target antigen(s), is achieved.”
158. In the sub-section concerning methods of treatment (R), the specification states at [0351] that “Exemplary conditions or disorders to be treated with the polypeptides, antibodies and other compounds of the invention, include, but are not limited to” the same list of conditions as was set out in [0136]. These conditions are described at [0352]-[0371]. Whereas the description of rheumatoid arthritis in [0353] runs to 16 lines, all that is said about psoriasis at [0369] is:
“Psoriasis is a T lymphocyte-mediated inflammatory disease. Lesions contain infiltrates of T lymphocytes, macrophages and antigen processing cells, and some neutrophils”
“[0372] Other diseases in which intervention of the immune and/or inflammatory response have benefit are infectious disease including but not limited to viral infection (including but not limited to AIDS, hepatitis A, B, C, D, E and herpes) bacterial infection, fungal infections, and protozoal and parasitic infections (molecules (or derivatives/agonists) which stimulate the MLR can be utilized therapeutically to enhance the immune response to infectious agents), diseases of 55 immunodeficiency (molecules/derivatives/agonists) which stimulate the MLR can be utilized therapeutically to enhance the immune response for conditions of inherited, acquired, infectious induced (as in HIV infection), or iatrogenic (i.e., as from chemotherapy) immunodeficiency, and neoplasia.
[0373] It has been demonstrated that some human cancer patients develop an antibody and/or T lymphocyte response to antigens on neoplastic cells. It has also been shown in animal models of neoplasia that enhancement of the immune response can result in rejection or regression of that particular neoplasm. Molecules that enhance the T lymphocyte response in the MLR have utility in vivo in enhancing the immune response against neoplasia. Molecules which enhance the T lymphocyte proliferative response in the MLR (or small molecule agonists or antibodies that affected the same receptor in an agonistic fashion) can be used therapeutically to treat cancer. Molecules that inhibit the lymphocyte response in the MLR also function in vivo during neoplasia to suppress the immune response to a neoplasm; such molecules can either be expressed by the neoplastic cells themselves or their expression can be induced by the neoplasm in other cells. Antagonism of such inhibitory molecules (either with antibody, small molecule antagonists or other means) enhances immune-mediated tumor rejection.
[0374] Additionally, inhibition of molecules with proinflammatory properties may have therapeutic benefit in reperfusion injury; stroke; myocardial infarction; atherosclerosis; acute lung injury; hemorrhagic shock; burn; sepsis/septic shock; acute tubular necrosis; endometriosis; degenerative joint disease and pancreatitis. …”
Examples
160. The specification describes 11 examples from [0384] to [0461]. The specification states at [0383] that these are “not intended to limit the scope of the present invention in any way”.
161. Example 1 ([0385]-[0399]). Example 1, headed “Recombinant expression of a novel IL-17 cytokine identified as IL-17A/F”, describes the expression of recombinant IL-17A/F by (in summary) transfecting human 293 kidney cells with plasmids encoding IL-17A, IL-17C and IL-17F. More specifically, cells were made to express: (i) IL-17A in isolation, (ii) IL-17F in isolation, (iii) IL-17A in combination with IL-17F, (iv) IL-17C in isolation, and (v) IL-17A in combination with IL-17C. The resulting media was fractioned, and a Western blot analysis utilising IL-17A and IL-17F antibodies was then conducted. The extracted samples were immunoprecipitated with either the IL-17A antibodies (lanes 1-5, Figures 1A and 1B) or the IL-17F antibodies (lanes 6-10, Figures 1A and 1B). The immunoprecipitated proteins were then blotted with either the IL-17A antibodies (Figure 1A) or the IL-17F antibodies (Figure 1B) and resolved.
162. The skilled reader would appreciate that the theory behind the inventors’ detection method was that, if an IL-17A/F heterodimer existed, it might be capable of interacting with certain antibodies that can bind to IL-17A (but not IL-17F) and certain antibodies that can bind to IL-17F (but not IL-17A), and would therefore be detected by the Western blot analysis. Conversely, the IL-17A/A and IL-17F/F homodimers would not be capable of interacting with both of these categories of antibodies, and so would not be detected by the Western blot analysis.
163. Column chromatography was subsequently used to purify and isolate the novel protein (Figures 2B-1 and 2B-2). The inventors then used a variety of techniques to verify the identity of the protein, including: (i) SDS-PAGE analysis to determine the molecular mass of the protein (Figures 2A and 3A), (ii) N-terminal peptide sequence analysis to compare the sequence of the protein to the known sequences of the N-termini of IL-17A and IL-17F (Figures 3B and 3C), and (iii) mass spectrometry to characterise the existence and location of disulphide bonds linking the IL-17A and IL-17F chains of the protein (Figure 4). The specification states in [0396]:
“Western Blot analysis indicated that this novel protein species is also able to interact both with an antibody that is able to bind to IL-17 and with an antibody that is able to bind to IL-17F. Each of these observations and the distinct molecular mass of the novel isolated protein species suggest that the isolated protein IL-17A/F is a novel protein species comprised of a covalent association of IL-17 and IL-17F.”
164. Example 1 goes on to describe a method for identifying antibodies that bind to IL-17A/F using a synthetic Fab phage display library. 34 independent clones encoding distinct Fab antibody sequences were identified which were able to mediate binding to IL-17A/F, as shown in Figure 6 and Table 7. The specification states at the end of [0398]:
“Thus, specific antibodies which bind selectively to the novel heterodimeric complex of IL-17A/F have been identified which may serve to modulate the activity of this novel cytokine”.
165. The final part of Example 1 describes how the authors used the TK-10 human kidney carcinoma cell line to analyse the ability of purified IL-17A/F to induce the production of IL-6 and IL-8 using ELISAs. Dose response curves comparing IL-6 and IL-8 induction by IL-17A/F, IL-17A/A, and IL-17F/F are set out in Figures 5A and 5B. These show that IL-17A/F, IL-17A/A and IL-17F/F all induce the production of IL-6 and IL-8 in TK-10 cells in a dose-dependent fashion.

166. The specification comments on these results in [0399] as follows:
“Interestingly, IL-17 A/F was observed to have a unique potency that differs from that of either IL-17 or IL-17F. The difference in activity differs from IL-17 and IL-17F by roughly an order of magnitude in each case. The substantially greater activity of IL-17 A/F than IL-17F in this assay suggests that IL-17A/F may comprise a critical component of the cytokine activity resulting from the IL-17F gene product. This unique potency may enable the molecule to possess distinct range of actions in vivo. IL-17A/F also induced production of IL-6 from this cell line (Figure 5B). Additionally, it is likely that IL-17A/F may possess additional characteristics not present in either IL-17 or IL-17F as a result of its novel heterodimeric composition that may alter the kinetics and utilization of receptor subunits in vivo, resulting in unique biological consequences.”
167. The skilled reader would understand from this paragraph and from Figures 5A and 5B that the activity of IL-17A/A in inducing IL-6 and IL-8 was about 10 times that of IL-17A/F which in turn was about 10 times that of IL-17F/F, and that this was all that was meant by the statement that IL-17A/F has a “unique” potency. Given that IL-17A/F is a heterodimer of A and F, the skilled reader would not be surprised to find that its potency is intermediate between that of IL-17A/A and IL-17F/F.
168. Example 2 ([0400]-[0407]). Example 2, headed “Identification of a novel IL-17 cytokine produced in activated human T cells”, demonstrates that IL-17A/F is naturally produced in activated human T-cells. Human blood was first extracted from a healthy donor. Human T-lymphocytes were then isolated from the blood and activated using anti-CD3 and anti-CD28 antibodies. Samples of media were collected at various time points following plating and assayed for IL-17A/F by a sandwich ELISA. An anti-human IL-17A antibody was coated onto a microtitre plate, and after the test sample had been added a biotinylated anti-human IL-17F antibody as a detection antibody diluted in assay buffer. A positive signal would only be detected in response to the IL-17A/F heterodimer – the anti-human IL-17A coat antibody first binds to the IL-17A chain and immobilises the molecule, and the anti-human IL-17F antibody binds to the IL-17F chain to visualise it. IL-17A and IL-17F were used as positive controls to demonstrate that the assay was specific for IL-17A/F.
169. The results, which are set out in Figures 11 and 12, show that IL-17A/F was produced by the activated human T-cells, but that no production of IL-17A/F was detected from the non-activated T-cells (as expected from the negative control). This confirms that the ELISA was able to selectively and reproducibly detect IL-17A/F (but not IL-17A/A or IL-17F/F).
170. The specification comments on these results as follows:
“[0404] … Hence, IL-17A/F is a distinctly new cytokine, detectable as a natural product of isolated activated human T cells, whose recombinant form has been characterized, in both protein structure and cell-based assays, as to be different and distinguishable from related cytokines.
[0405] This new cytokine can act to modulate the activity of IL-17 in vivo , acting as a competitive inhibitor to binding sites for IL-17 or other related cytokines. IL-17A/F can also modulate the activity of other related cytokines by down regulation of binding sites for itself and/or binding sites for other related cytokines. IL-17A/F can exhibit activity through intracellular adapters or signaling molecules which act to affect its own signaling activity or that of other related cytokines. IL-17A/F has the ability to affect the pairing of receptors and co-receptors found at the surface of cells or within the intracellular compartment.
[0406] Thus, these studies provide and identify a novel immune stimulant (i.e. IL-17A/F) that can boost the immune system to respond to a particular antigen that may not have been immunologically active previously. As such, the newly identified immune stimulant has important clinical applications. …
[0407] Thus, antibodies to this new cytokine which either mimic (agonist antibodies) or inhibit (antagonist antibodies) the immunological activities of IL-17A/F would possess therapeutic qualities. …”
171. Examples 3 to 11 . Examples 3 to 11 all appear to be “armchair” or “prophetic” examples, that is to say, they describe experiments which are proposed, rather than experiments which the inventors have actually carried out, since no data or results are reported for any of these examples.
172. Example 3 ([0408]-[0411]) concerns the use of a nucleotide sequence encoding IL-17A/F as a hybridisation probe to screen cDNA and genomic libraries.
173. Examples 4-7 ([0412]-[0433]) describe the recombinant expression of IL-17A/F in a variety of eukaryotic and prokaryotic systems.
174. Example 8 ([0444]-[0449]) is said at [0444] to illustrate “preparation of monoclonal antibodies which can specifically bind IL-17A/F”. It describes a method for producing murine antibodies to IL-17A/F, and then screening for them by ELISA. No such antibodies are disclosed by the Patent. The example does not propose to demonstrate that any such antibodies inhibit the production of IL-6 or IL-8.
175. Example 9 ([0450]-[0453]) describes the purification of IL-17A/F using specific antibodies by a column chromatography process.
176. Example 10 ([0454]-[0457]) describes the use of IL-17A/F in drug screening techniques.
177. Example 11 ([0458]-[0461]) describes the production of structural analogues of IL-17A/F and small molecules with which it interacts.
178. None of the examples concerns the use of IL17A/F for the treatment of any disease. There is no reference to psoriasis, RA or any other condition in the examples. There is no example proposing to test IL-17A/F, or an antibody thereto, in any in vitro or animal model of disease, let alone any results from such a test.
The claims
179. Genentech does not seek to defend the validity of the claims as granted. It has applied to amend the claims both unconditionally and conditionally. The unconditional amendments include the deletion of granted claims 1-26, namely claims relating to isolated nucleic acids (1-11), vectors (12-13), host cells (14-15), a process for producing IL17A/F (16) and isolated polypeptides and/or complexes (17-26).
180. The proposed amended claims which Genentech contends to be independently valid as are follows. The conditional amendments are shown in bold in square brackets
“ 27 1 . An isolated antibody which specifically binds to the an isolated IL-17A/F heterodimeric complex according to claim 23 or claim 24 and which inhibits the activity of the IL-17A/F heterodimeric complex to induce production of IL-8 and IL-6, wherein the isolated IL-17A/F heterodimeric complex comprises [consists of] SEQ ID NO:3 and SEQ ID NO:4, without their associated signal peptides, and further comprises two interchain disulfide linkages between SEQ ID NO:3 and SEQ ID NO:4; and wherein the antibody is either human or humanized .
2. The isolated antibody of Claim 1, wherein said antibody has a Kd for the IL-17A/F heterodimeric complex of at least about 10 -8 , 10 -9 , 10 -10 , 10 -11 or 10 -12 M .
40 12 . Use of an antagonist anti-IL-17A/F antibody as defined in Claim 27 1 or 2 in the preparation of a medicament for
(i) the treatment of an immune related disorder;
(ii) inhibiting the proliferation of T-lymphocytes; or
(iii) decreasing the infiltration of inflammatory cells into a tissue in a mammal in need thereof.
41. The use according to Claim 39 or Claim 40 wherein the immune related disorder is systemic lupus erythematosis, rheumatoid arthritis, osteoarthritis, juvenile chronic arthritis, a spondyloarthropathy, systemic sclerosis, an idiopathicinflammatory myopathy, Sjogren's syndrome, systemic vasculitis, sarcoidosis, autoimmune haemolytic anemia, autoimmune thrombocytopenia, thyroiditis, diabetes mellitus, immune-mediated renal disease, a demyelinating disease of the central or peripheral nervous system, idiopathic demyelinating polyneuropathy, Guillain-Barre syndrome, a chronic inflammatory demyelinating polyneuropathy, a hepatobiliary disease, infectious or autoimmune chronic active hepatitis, primary biliary cirrhosis, granulomatous hepatitis, sclerosing cholangitis, inflammatory bowel disease, gluten-sensitive enteropathy, Whipple's disease, an autoimmune or immune-mediated skin disease, a bullous skin disease, erythema multiforme, contact dermatitis, psoriasis , an allergic disease, asthma, allergic rhinitis, atopic dermatitis, food hypersensitivity, urticaria, an immunologic disease of the lung, eosinophilic pneumonia, idiopathic pulmonary fibrosis, hypersensitivity pneumonitis, a transplantation associated disease, graft rejection or graft-versus-host-disease .
13. The isolated antibody of Claim 1 or 2 for use [as an antagonist of the IL-17A/F heterodimeric complex in] a method of medical treatment.
14. An isolated antibody which specifically binds to an isolated IL-17A/F heterodimeric complex and which inhibits the activity of the IL-17A/F heterodimeric complex to induce production of IL-8 and IL-6, wherein the isolated IL-17A/F heterodimeric complex comprises [consists of] SEQ ID NO:3 and SEQ ID NO:4, without their associated signal peptides, and further comprises two interchain disulfide linkages between SEQ ID NO:3 and SEQ ID NO:4; and wherein the antibody is for use [as an antagonist of the IL-17A/F heterodimeric complex] in a method of medical treatment.
15. The isolated antibody for use of Claim 14, wherein said antibody has a Kd for the IL-17A/F heterodimeric complex of at least about 10 -8 , 10 -9 , 10 -10 , 10 -11 or 10 -12 M.
20. Use of an antagonist anti-IL-17A/F antibody as defined in Claim 14, 15 or 16 in the preparation of a medicament for [antagonizing the IL-17A/F heterodimeric complex in] the treatment of rheumatoid arthritis or psoriasis.
22. The isolated antibody for use of Claim 14, 15 or 16 wherein the method of medical treatment is a treatment of rheumatoid arthritis or psoriasis. ”
181. The polypeptide sequences SEQ ID 3 and SEQ ID 4 referred to in claims 1 and 14 are the sequences of the prior art IL-17A and IL-17F polypeptide monomers . In relation to claims 2 and 15, Genentech asserts independent validity for each Kd value. In relation to claims 12, 20 and 22, Genentech asserts independent validity for each of RA and psoriasis.
The skilled team
182. It is common ground that, as proposed to be amended, the Patent is addressed to two different, but overlapping, teams of persons skilled in the art, namely a psoriasis team and an RA team. The psoriasis team consists of (i) a dermatologist with both clinical experience of, and a research interest in, the treatment of psoriasis and (ii) one or more persons with expertise in antibody engineering. The RA team consists of (i) an immunologist with both clinical experience of, and a research interest in, the treatment of RA and (ii) one or more persons with expertise in antibody engineering.
183. The dermatologist would have experience in: (i) immunology and the role of the immune system in psoriasis; (ii) an understanding of human skin biology and skin structure; (iii) an understanding of skin pathology and how this impacts upon development of therapeutics (including the limitations of animal models); (iv) treatment of psoriasis; and (v) the PASI and PGA clinical scores for psoriasis (as to which, see below).
184. The rheumatologist would have experience in: (i) persistent joint inflammation, cartilage destruction and bone erosion; (ii) research into the immune-pathophysiology of RA and the cytokine networks involved in RA disease processes; and (iii) work with human cells from RA patients in vitro , and/or with animal models for arthritis.
185. The expert(s) in antibody engineering would be someone with a biological sciences degree and a doctorate in molecular biology or immunology. They would have two-three years’ post-doctoral experience of biopharmaceutical research, focussing on (a) antibody generation and characterisation and (b) humanisation of antibodies. If the antibody engineer did not have enough experience in humanisation, then a specialist with more experience would be engaged. For convenience, however, I shall refer to a singular antibody engineer. The antibody engineer would be familiar with: (i) sequencing murine antibodies, then selecting homologous human framework sequences for CDR grafting; (ii) engineering the recombinant humanised antibody; and (iii) reviewing predicted or known antibody structures to determine distances between atoms of interest and making appropriate choices to carry out so-called “back mutations” where the murine and human framework residues differ.
186. It is common ground that the antibody engineer would, if they did not have sufficient expertise themselves, have access to advice from an expert in the use of computer models of antibodies.
187. For some purposes it does not matter which of the two skilled teams is to be considered, whereas for other purposes it does. Where it matters, I should be taken to be referring to the relevant skilled team even if I do not explicitly say so. In some contexts, only one member of the skilled team is relevant, and I shall therefore refer to the skilled person.
Common general knowledge
188. It is common ground that everything I have set out in the technical background section above was part of the common general knowledge of the relevant skilled team. There was little, if any, dispute as to the respective common general knowledge of either the rheumatologist or the antibody engineer, but considerable dispute as the common general knowledge of the dermatologist. There was no dispute as to the principles to be applied when determining what is, and what is not, common general knowledge. Nor was it disputed that what matters is whether something is common general knowledge to a person skilled in the art in the UK.
The CGK of the rheumatologist
189. Dr Lubberts was well placed to speak to the common general knowledge of the rheumatologist in July 2003, having written a review which was published in May 2003 (Lubberts, “The role of IL-17 and family members in the pathogenesis of arthritis”, Curr Op in Invest Drugs , 4, 572-577). Dr Lubberts set out in paragraph 80 of his first report a helpful summary of the common general knowledge he had discussed in the preceding paragraphs of his report. In his second report Dr Lubberts responded to Prof Kamradt’s account of the common general knowledge in the latter’s first report, agreeing with much, but not all, of what Prof Kamradt had said. Dr Lubberts’ evidence on this topic was not challenged in cross-examination. I reproduce Dr Lubberts’ summary below with minor additions from the preceding paragraphs of his first report and from his second report.
190. RA and its immuno-pathophysiology. RA is a chronic inflammatory autoimmune disease that mainly affect the small joints. RA affects about 1% of the population worldwide. It causes disability and is associated with increased morbidity and mortality. Persistent joint inflammation, cartilage destruction and bone erosion are features of RA. Memory T cells which infiltrate the synovium (a thin cellular lining in joints) play a central role in the pathogenesis of RA.
191. TNFα and IL-1 were considered to be key cytokines in RA and had been shown to stimulate joint inflammation and damage. TNFα (etanercept sold as Enbrel and infliximab sold as Remicade) and IL-1 (anakinra sold as Kineret) inhibitors had been approved for the treatment of RA by July 2003 and were on the market. Whilst Remicade and Enbrel were known to be very effective, there was still a significant proportion of patients who did not respond. Kineret was less effective.
192. Animal models . Collagen-induced arthritis (CIA) is a well-accepted experimental animal model for RA in which mice are injected with collagen. CIA was used to test the efficacy of therapeutic agents for RA prior to clinical trials in humans. Another animal model that was used to evaluate treatments for RA was adjuvant-induced arthritis (AIA), in which rats are injected with an adjuvant containing mycobacterium.
193. IL-6 and IL-8 . IL-6 is a pro-inflammatory cytokine considered to be important in bone destruction and joint inflammation and to be a target for the treatment of RA. IL-8 is a chemokine involved in neutrophil recruitment which had been shown to be upregulated in RA.
194. IL-17A . IL-17A is secreted as a homodimer by activated memory T helper cells (CD4 + ). IL-17A binds to its receptor, IL-17R. IL-17R is ubiquitously expressed on almost all cell types .
195. IL-17A binds to IL-17R with low affinity. As a relatively low concentration of IL-17A was required to produce a biological response, however, it was suggested that IL-17A may interact with an unidentified additional receptor component present on IL-17A-responsive cells . Lack of IL-17R in mice results in increased susceptibility to lung bacterial infection.
197. IL-17 family. IL-17A belongs to a family of cytokines (IL-17A to IL-17F). Of the other members of the IL-17 family, IL-17F has the highest amino acid homology with IL-17A (approximately 50% homology).
198. IL-17F has similar effects to IL-17A, but is less potent than IL-17A in its effects on cartilage turnover. Other IL-17 family members (IL-17B to IL-17E) have different expression patterns compared with IL-17A. IL-17B, IL-17C and IL-17E had been reported not to bind to IL-17R.
199. IL-17F did not bind to purified IL-17R, but had been suggested to be able to bind to IL-17R in combination with an additional unidentified receptor component.
200. IL-17A and RA. IL-17A is found in synovial fluid and tissue of patients with RA, and IL-17A-producing T cells are present in RA synovium.
202. IL-17A is less potent than TNFα and IL-1 based on in vitro studies. IL-17A has additive or synergistic effects with TNFα and IL-1 based on in vitro studies .
204. Neutralisation of IL-17A suppresses arthritis and reduces joint damage, whereas overexpression of IL-17A worsens synovial inflammation and joint damage in in vivo animal models of RA.
205. IL-17A has additive effects with IL-1 on cartilage destruction in vitro and induces inflammation and joint damage independently of IL-1 in CIA.
206. IL-17A induces receptor activator of NF-κB (RANKL, a key cytokine in osteoclast formation and activation) in in vitro studies. The addition of IL-17A leads to bone erosion in in vitro studies and in in vivo animal models of RA.
208. Not only was Dr Lubberts’ evidence that the skilled person would have expected inhibiting IL-17A to be useful in treating RA not challenged, but also Prof Kamradt agreed that there was (as he variously put it) “an a priori expectation”, “a fair expectation” and “a good expectation” on the part of the skilled person that blocking IL-17A by administration of either an antibody or a soluble receptor would be effective to treat RA.
The CGK of the antibody engineer
209. The antibody engineer’s common general knowledge included:
i) the use of different cell systems for generating recombinant proteins;
ii) the need to use humanised or fully human antibodies in a therapeutic agent to avoid inducing an immune response to the antibody itself;
iii) methods for generation of murine antibodies;
iv) antibody characterisation methods including an understanding of ELISA and SPR techniques;
v) the requirements for antagonistic activity, namely:
a) recognition of an epitope on the protein of interest so as to cause a steric (spatial blocking) effect, or a conformational change in that protein, so as to prevent the protein interacting with its receptor; and
b) a greater binding affinity for the protein than the protein-receptor binding affinity;
vi) bioassay methods to screen for antagonistic antibodies;
vii) criteria to select antibodies for humanisation, including based on affinity for their targets; and
viii) (with assistance from a humanisation expert as required) methods for humanising antibodies.
The CGK of the dermatologist
210. Although there is a fair amount of common ground as to the common general knowledge of the dermatologist, there are considerable disputes. Rather than distinguishing between what is common ground and what is disputed, and setting out the rival contentions as to the latter, I shall simply set out my findings. In general, these findings are based on my overall assessment of the evidence of Prof Krueger and Prof Prens on each topic. I have also taken into account the various review articles in evidence, although for obvious reasons I have given more weight to those which are close to the relevant date (such as those by Prof Krueger and Prinz, “The role of T cells in psoriasis”, J Eur Acad Dermatol Venereol , 17, 257-70 (2003, “Prinz”)) than older ones (such as Bonifati and Ameglio, “Cytokines in psoriasis”, Int J Dermatol , 38, 241-251 (1999, “Bonifati”)). I have also given more weight to reviews which were mentioned by at least one of the experts in at least one of their respective reports, as opposed to reviews (such as Bonifati) which were not mentioned by either expert in either of their reports. In relation to some of the more heavily disputed points, I shall set out my reasoning in further detail.
211. Psoriasis . Psoriasis is a chronic inflammatory human disease of the skin involving hyperproliferation of skin cells (also called keratinocytes). Psoriasis is characterised by scaly red patches on the skin which can be itchy as well as unsightly. Depending on its severity, psoriasis can have a great impact on a patient’s quality of life. The incidence of psoriasis varies from country to country. In 1999 it was estimated to affect 2.6% of the US population. There are five main types with plaque psoriasis (also called psoriasis vulgaris) being the most common.
212. Psoriatic arthritis . Psoriatic arthritis is a related disorder. Psoriasis can lead to psoriatic arthritis, but does not necessarily do so. It is also possible for a patient to suffer from psoriatic arthritis without displaying overt symptoms of psoriasis.
213. PASI . The Psoriasis Area and Severity Index (or PASI) is a widely used standardised scale for the measurement of severity of psoriasis in clinical trials. PASI combines the assessment of the severity of lesions and the area affected into a single score. The PASI score is calculated by grading the intensity of the redness, thickness and scaling of psoriasis plaques in four body regions (head, trunk, arms and legs) on a five-point scale of 0-4 and multiplied by the extent of psoriasis plaques found in the region and by the proportion of that region with respect to the entire body. The total PASI score ranges from 0 to 72. In July 2003 it was common to specify a primary efficacy endpoint of a 75% or greater reduction in PASI from the start of treatment, referred to as PASI75, in clinical trials.
214. PGA. Another measure of the severity of psoriasis is the Physician Global Assessment (or PGA). This is a score of 0-5 based on degree of redness, thickness and scaliness averaged over the entire body. An alternative endpoint used for assessing the efficacy of systemic therapies was the achievement of PGA 0 or 1 (“clear” or “almost clear”).
215. The immunological basis for psoriasis . A significant contribution to the understanding that psoriasis had an immunological basis had stemmed from observations that some immunosuppressants could be effective against psoriasis. This led to research into the role of the immune system in psoriasis. By 2003, it was known there was a great deal of complexity regarding the immune system pathways involved in the pathogenesis of psoriasis and they were the subject of continuing active debate and study.
216. Evidence supported a role for both aspects of the immune system, innate immunity and adaptive immunity, in the pathogenesis of psoriasis. Their relative contribution was unclear, with some key opinion leaders in the field supporting a more important role for innate immunity and others supporting a more important aspect for adaptive immunity. According to Prof Prens, the majority view was that the adaptive system was dominant, but I find that the skilled person would be aware some distinguished scientists held the contrary view.
217. Models of psoriasis . In 2003 there were certain in vitro models for psoriasis involving keratinocyte growth on a surface or in a cell, but there was no reliable animal model. Although several mouse models had been proposed (such as the first model referred to in the Patent at [0259]), a fundamental problem with mouse models was that murine skin is quite different to human skin. One approach was to graft healthy and lesional human skin into subacute combined immunodeficient (SCID) mice (this is the second model referred to in [0259]), but that approach suffered from the defect that many critical elements of immune cells normally found in psoriasis lesions were absent. Accordingly, the pre-clinical testing of potential therapeutic treatments was typically carried out in models of T cell mediated diseases of other organs such as CIA or experimental autoimmune encephalomyelitis (EAE, a model for multiple sclerosis). This enabled potential immune-modifying drugs to be identified, but did not enable efficacy against psoriasis to be predicted.
218. In order for a molecule to be considered a potential therapeutic target for psoriasis, it would need to be shown that it was highly expressed (upregulated) in psoriasis lesions. The skilled person would also need to have a reason to believe that the immune pathway in question was pathogenic in psoriasis.
219. Treatment options for psoriasis . There was in July 2003, and still is, no cure for psoriasis. The aim of any treatment is to manage the symptoms of the disease rather than to cure it. Treatment options for psoriasis can be divided into three categories: topical treatment, phototherapy and systemic treatment.
220. Topical treatments included creams and ointments which are applied directly to the skin. The creams and ointments included corticosteroids, modified vitamin D3 ointments (calcipotriol), coal tar and ditranol. Phototherapy involves exposing the skin to certain types of ultraviolet light. Whereas both topical treatment and phototherapy seek to target the diseased skin directly, systemic treatment involves administering drugs that seek to prevent the underlying mechanism causing the symptoms. The drug options available in July 2003 included general immunosuppressants such as methotrexate and cyclosporine.
221. It was known that various biologic drugs were under development for the treatment of psoriasis. These included molecules with proven efficacy in the treatment of RA, such as infliximab and etanercept, which were in Phase III clinical trials for psoriasis.
222. Relationship with RA . RA was considered to be different in nature to psoriasis for a number of reasons, including:
i) RA was considered to be a mixed T cell and B cell disease, whereas psoriasis was not considered to have a B cell component;
ii) RA involves tissue destruction, whereas psoriasis involved an increase in the number of keratinocytes resembling a healing response; and
iii) RA affects discrete and relatively small amounts of joint tissue, whereas psoriasis is a disease affecting the skin, the largest organ in the body.
223. Some drugs for treating RA were also effective in psoriasis, whereas some others were ineffective in psoriasis or their use in psoriasis was discouraged.
224. More was known about the pathology of RA than about that of psoriasis in July 2003, which is why the development of targeted therapies for psoriasis lagged behind the development of such therapies for RA.
225. Cytokines involved in psoriasis . By 2003 a large number of cytokines had been shown to be upregulated (or in a few cases downregulated) in psoriatic skin compared to normal skin. As Prof Prens vividly expressed it in his first report, “Researchers referred to this as the ‘cytokine soup’ in psoriasis skin lesions”. Prof Krueger reproduced in his first report the following table from Lowes listing the cytokines which had been implicated as having a possible role in psoriasis.
226. The skilled dermatologist would be aware, however, that upregulation (or downregulation) of a cytokine in psoriasis lesions could be either a cause or a consequence of the disease.
227. Cytokines can be pleiotropic, meaning that that they have multiple targets. Some cytokines are more pleiotropic than others. Equally, cytokines can often be redundant, meaning that more than one cytokine has a similar effect. Thus inhibiting one cytokine may make little difference. But on the other hand, redundancy may involve the cytokines binding to the same receptor or to a different receptor. If the receptor is different, then inhibition of one cytokine may have a different effect to inhibition of the other. Moreover, cytokines can be additive, synergistic or antagonistic in their effects.
228. Whether a cytokine was considered therapeutically relevant for psoriasis would depend on a number of factors, including its range of biological effects, the relevance of those effects in the pathogenesis of psoriasis, its potency and the redundancy of its effects with other cytokines that are also expressed in psoriasis lesions.
229. The two cytokines which were generally thought to be the most important in psoriasis pathogenesis in July 2003 were TNFα and IFN g . For this reason, TNFα and IFN g were considered to be the frontrunners as targets for psoriasis therapy.
230. TNFα . TNFα was known to be produced by many innate immune cells as well as type 1 T cells. It was thought to mediate inflammatory effects in psoriasis and to be a key driver in its pathogenesis. Elevated expression of TNFα had been detected in psoriasis lesions in 1994. By chance, an anti-TNFα antibody, infliximab, had been found to clear psoriasis in a patient being treated for inflammatory bowel disease in 2000. A clinical trial of a TNF-receptor-Ig fusion protein, etanercept, which as noted above is also a TNFα inhibitor, in psoriatic arthritis patients reported in 2000 showed efficacy in some patients, but the relevance of that finding to psoriasis was uncertain. Subsequently a small clinical trial of infliximab in psoriasis patients was reported in 2001 showing impressive efficacy and good tolerability. That led to Phase III trials which were ongoing in July 2003 (and subsequently led to infliximab being approved for psoriasis).
231. Thus TNFα antagonism had been shown to be highly effective in treating psoriasis with much greater responses than T cell specific therapies. TNFα antagonism was the only anti-cytokine therapeutic approach that had been shown to be successful in psoriasis as at July 2003.
232. IFN g . IFN g was considered to be the archetypal cytokine produced by type 1 T cells of the adaptive immune system. It was known to have wide-ranging biological effects. Many of the genes that were upregulated in psoriasis lesions could be traced to activation of STAT-1 (a transcription factor) which it was thought was likely to be mediated by IFNγ. For reasons explained in more detail below, for those who considered that the adaptive immune system was dominant in psoriasis, much of the pathogenic inflammation was thought likely to be due to the release of IFN g from activated T cells in skin lesions. As such, IFN g was a therapeutic target of considerable interest. This interest led to clinical trials of a humanised IgG1 antibody against IFN g in psoriasis patients which were ongoing in July 2003 (subsequently it was found that the antibody was minimally effective, however).
233. IL-12 and IL-23 . IL-12 had been reported to be strongly upregulated in psoriasis lesions. IL-12, which is a heterodimer composed of p35 and p40 subunits, was thought to be essential for T cell dependent inflammatory responses on the basis of studies using p40 knockout mice and neutralising antibodies to p40. Accordingly, an antibody against IL-12 was being developed for the treatment of psoriasis.
234. In February 2003 an important paper was published in Nature (Cua et al , “Interleukin-23 rather than inteleukin-12 is the critical cytokine for autoimmune inflammation of the brain”, 421, 744-748) reporting that IL-23, rather than IL-12, was the key cytokine mediating inflammation in EAE. IL-23 is a heterodimer composed of p19 and p40 subunits. It was therefore appreciated that the previous studies had knocked out or inhibited both IL-12 and IL-23.
235. IL-6 . IL-6 was known to be expressed at a considerably higher level in psoriasis lesions than in normal skin and to simulate keratinocyte proliferation. A number of other factors such as transforming growth factor alpha (TGFα) and IL-1 were also known to be present in psoriasis lesions and to induce keratinocyte proliferation, however. Thus there was a potential problem of redundancy. Although the stimulus for IL-6 overproduction in psoriasis was not known, studies had shown that both TNFα and IFNγ induced IL-6 production by keratinoctyes. In summary, it was uncertain as to whether IL-6 had a therapeutically relevant role in psoriasis.
236. Counsel for Genentech put to Prof Krueger in cross-examination, and relied in closing submissions on, a review of IL-6 from 2011 (Jones et al , “Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling”, J Clin Invest , 121, 3375-3383). The passage relied on discusses how IL-6 was recognised as a major growth factor in multiple myeloma in the early 1990s. This led to clinical trials with neutralising anti-IL-6 antibodies, but it was found that this led to massive systemic elevation of IL-6. To overcome this problem, blockade of IL-6’s receptor, IL-6R, was targeted. This led to the development of tocilizumab, which inhibits the binding of IL-6 to IL-6R. This was the subject of clinical trials in a variety of conditions after 2003, and tocilizumab has been approved for the treatment of RA since 2010. There is no reference to psoriasis in this passage (or anywhere else in the review so far as I can see), and Prof Krueger said that he was not familiar with this work. Moreover, Prof Prens confirmed that information relating to tocilizumab was not publicly available before July 2003. As Prof Krueger had pointed out in his first report, it has recently been found that tocilizumab can lead to the onset of psoriasis in patients with no prior history of it.
237. As Prof Krueger acknowledged in his first report, an anti-IL-6 antibody, clazakizumab, has more recently been the subject of a clinical trial for psoriatic arthritis. When it was put to him that this showed that IL-6 continued to be considered to be a relevant target for psoriasis even after 2003, Prof Krueger disagreed, explaining that the two diseases were different. Although there was some overlap in the cytokine networks, the position was far less clear for psoriatic arthritis and there were far less satisfactory treatments of it available. In any event, this work was published in 2016.
238. Counsel for Genentech asked Prof Krueger if he could point to any publications which said that IL-6 was not a target of interest for treating psoriasis in 2003. Unsurprisingly, Prof Krueger was unable to do so, but he said that one should consider the totality of the reviews and that at best the role of IL-6 was uncertain. As will appear, what the reviews do not say is more significant than what they do say. For reasons that will appear, it is relevant also to consider what they say about redundancy.
239. By contrast, Prof Prens expressed the view in his reports that IL-6 was considered to play an important role in the pathogenesis of psoriasis. The only publications he cited in support of this opinion, however, were papers dating from 1989 to 1996 reporting such matters as its promotion of the proliferation of keratinocytes and other cells. He did not refer to any reviews addressing this point.
240. Turning to the reviews, Prof Prens’ own review in 1995 discusses the role of IL-6 together with IL-1, IL-8 and various other factors. The passage concludes (at page 121) that:
“In summary, the data presented here clearly illustrate that T cell-keratinocyte interactions in psoriasis are responsible for enhanced bidirectional cellular activation. Minute quantities of cytokines released during these interactions may trigger a cascade of intercellular cytokine signals which induce or boost cutaneous inflammation, or both. Therefore, the identification of the primary signal and its cellular source in psoriasis remains extremely difficult.”
When cross-examined on this passage, Prof Prens accepted that redundancy could be an issue in targeting a cytokine, but said that one did not know until an agent was tested.
241. Bonafati lists IL-6 as one of 22 cytokines (including IL-8) whose production in psoriatic skin is upregulated (or in one case downregulated) and have “possible” effects on keratinocyte proliferation and inflammatory changes in psoriasis. In their discussion of IL-6, the authors note that the possible importance of IL-6 in the “psoriasis cytokine network” has mainly been suggested by its ability to promote keratinocyte proliferation in vitro and T lymphocyte activation and the fact that psoriatic keratinocytes have been reported to be more sensitive to the growth-promoting effect of IL-6 than normal ones. They go on to note that IL-6 production is stimulated by “most” of the cytokines involved in psoriasis and that its level in psoriatic lesions correlates with the PASI score. In their conclusions the authors state (at page 247) that “cytokines are characterized by pleiotropic effects and redundancy, and therefore their individual role(s) in vivo cannot be precisely defined”.
242. Turning to Krueger 2002, this discusses a variety of therapeutic strategies, including the use of cytokines and anti-cytokine therapeutics. Although this section discusses a number of interleukins and other cytokines, IL-6 is not one of them. In paragraph 255 below I quote what Prof Krueger says about IL-8. Although the comments about redundancy refer to chemokines, I consider that the skilled person would regard them as equally applicable to inflammatory cytokines such as IL-6.
243. Aggarwal and Gurney, “IL-17: Prototype member of an emerging cytokine family”, J Leukocyte Bio , 71, 1-9, which was also published in January 2002 (“Aggarwal 2002”), reviews what is known about IL-17 generally. It explains that IL-17 is a potent pro-inflammatory cytokine produced by activated memory T cells and that at least six members of the family are known. It notes (at page 1, references omitted) that IL-17:
“ … increases the local production of chemokines such as IL-8, monocyte chemoattractant protein-1 (MCP-1) and Gro-α, thereby promoting the recruitment of monocytes and neutrophils, and stimulates production of G-CSF and GM-CSF. … Other actions such as the stimulation of IL-6 and PGE2 production enhance the local inflammatory environment.”
244. Later, it reviews the association of IL-17 with a range of diseases. In relation to RA, it notes that IL-17 is found in RA synovial fluid and states (at page 4) that “IL-17 together with … IL-1β and TNF stimulate osteoblasts to secrete cytokines such as GM-CSF and IL-6, which in turn regulate osteoclast and chondrocyte-mediated resorption and hence bone and cartilage destruction”. IL-17 induced release of IL-6 is also mentioned in connection with obstructive airway diseases and cervical cancer. All that Aggarwal 2002 says about psoriasis, however, is that it is one of a number of diseases with which IL-17 has been associated. I shall return to this point below.
245. Cather and Menter, “Novel Therapies for Psoriasis”, Am J Clin Dermatol , 3, 159-173, which appears to have been published in March 2002 (“Cather”), discusses a number of cytokine therapies for psoriasis, including IL-10, IL-11, TNFα and anti-IL-8 antibodies. There is no mention of IL-6.
246. Kirby and Griffiths, “Novel immune-based therapies for psoriasis”, Br J Dermatol, 146, 546-551 (2002, “Kirby”) discusses cytokine-targeted therapies for psoriasis, concentrating on IL-10, anti-IL-8 and anti-TNFα. There is no mention of IL-6.
247. Asadullah et al , “Cytokine therapy in dermatology”, Exp Dermatol , 11, 97-106 (2002, “Asadullah”) discusses cytokine therapy for chronic inflammatory skin diseases, beginning with psoriasis. The authors discuss IL-4, IL-10, IL-11, anti-IL-8 antibodies and anti-TNFα. There is no mention of IL-6. When I asked Prof Prens why, in his opinion, it had not been mentioned, he said that he thought it was because IL-6 had not been tried and so there was nothing to report.
248. Prinz 2003 includes IL-6 in a table of eight principal cytokines involved in pathogenic steps leading to psoriasis. In his discussion of therapeutic mechanisms for targeting T cells in psoriasis, however, there is no mention of IL-6. (By contrast, IL-8 is mentioned, as discussed below.)
249. Victor et al , “Changing paradigms in dermatology: tumor necrosis factor alpha (TNFα) blockade in psoriasis and psoriatic arthritis”, Clinics in Dermatol , 23, 392-39 (2003) states (at page 392, references omitted):
“It has been shown that TNFα is capable of increasing production of interleukin (IL)-1, IL-6, IL-8 and nuclear transcription factor κB (NFκB). These proinflammatory cytokines can be synthesized by stimulated T lymphocytes and keratinocytes, exerting specific effects in the pathogenesis of psoriasis”.
It goes on to say that IL-6 is involved in proliferation of keratinocytes and that NFκB stimulates transcription of cytokines including TNFα, IL-6, IL-8 and various adhesion molecules. There is no discussion of targeting IL-6 for therapy (as the title suggests, the focus of the review is on TNFα).
250. Kanitakis et al , “Novel biological immunotherapies for psoriasis”, Expert Opin Investig Drugs , 12, 1111-1121, which appears to have been published in July 2003 (“Kanitakis”), discusses a series of cytokine-targeting treatments: anti-TNFα (infliximab, etanercept and also adalimumab and onercept), anti-IL-8, IL-4, IL-10, IL-10 and IL-11. There is no mention of IL-6.
251. As can be seen from paragraph 225 above, Lowes lists IL-6 as one of a large number of cytokines which had been found to be upregulated or downregulated in psoriatic lesions. IL-6 is not discussed in the text, however, although IL-8 is mentioned. By contrast, the authors state in their conclusion:
“The authors’ belief of the importance of IFN-γ as a pivotal cytokine in the initiation or maintenance of psoriatic lesions has been supported with evidence throughout this article, while acknowledging that TNFα plays an important and probably synergistic role.”
Similarly, IL-6 is not mentioned in Lew (while IL-8 is mentioned in passing).
252. Finally, there is Fitzpatrick’s . Table 42-6 on page 421 lists IL-6 and IL-8 as two of 12 cytokines which are or may be upregulated (or in one case downregulated) in lesional skin. (The exhibit to Prof Prens’ report included page 420, which specifically mentions IL-6 and IL-8 as well as IL-1α and IL-1β in the text, but not page 421, even though the text refers to Table 42-6).
253. The conclusion I draw from the evidence as a whole is that the skilled person would be aware of IL-6 as one of a large number of cytokines potentially implicated in the pathogenesis of psoriasis, but whose role was uncertain. In the case of IL-6, the skilled person would be aware that redundancy was a potential problem, but would not rule out targeting it solely on that account. Thus the skilled person would regard IL-6 as a possible therapeutic target, but a low priority one. As a result, there was little interest in targeting it in July 2003. Thus it does not appear from the evidence that any anti-IL-6 antibody was under development at that time.
254. IL-8: general . One of the most fiercely contested issues at trial concerned the common general knowledge of the skilled dermatologist with respect to IL-8. Accordingly, it is necessary for me to deal with this topic in some detail. As always with such disputes, it is necessary first to have careful regard to the relevant date and secondly to distinguish between what was known to some and what was common general knowledge.
255. It is common ground that IL-8 is a pro-inflammatory cytokine, more specifically a chemokine, which was known to be expressed in psoriasis lesions and was thought to recruit neutrophils into the skin. As can be seen, it is referred to in most of the reviews discussed in paragraphs 240-252 above. There is no dispute that, prior to May 2002, it was regarded as a plausible therapeutic target. This is despite the fact that it was recognised that there was a potential problem with redundancy, particular with respect to growth-regulated oncogene-alpha (Gro-α, also known as CXCL1). As Prof Krueger put it in Krueger 2002 (at page 18):
“According to the pathogenic model drawn in Fig 6, IL-8 is a chemokine that amplifies T-cell–‘driven’ inflammation by recruiting neutrophils into psoriasis lesions. Although this chemokine might also affect T-cell recruitment into lesions, there is no evidence for selectivity of its receptor (CCR1) in regulating type 1 T-cell responses. ABX–IL-8 (Abgenix, Inc, Fremont, Calif), [is] a fully human anti–IL-8 antibody that neutralizes this chemokine. Moderate clinical improvements observed in most patients with psoriasis treated with anti–IL-8 135 support the role of this chemokine as a part of an inflammatory cascade, but not as a sole mediator. There is also a difficult anatomic problem with respect to IL-8 neutralization in that upper spinous keratinocytes synthesize large amounts of this chemokine, whereas penetration of large proteins (anti-IL-8 antibodies) into the epidermis is likely to be quite limited. In addition, a general problem with antagonizing single chemokines is that considerable redundancy exists. For example, both IL-8 and Gro-α are neutrophil chemoattractants that bind to surface receptors CXCR1 or CXCR2. Gro-α is highly expressed in psoriatic lesions 85 and could still stimulate neutrophil trafficking even though IL-8 is fully neutralized by an antibody. The situation in T lymphocytes is similar in that multiple chemokines control T-cell migration responses. However, there is additional redundancy in that some receptors bind 2 or more chemokine ligands (Fig 5). Despite these problems, chemokines and chemokine receptors are attractive therapeutic targets because highly specific immune blockade can be obtained with[out] producing generalized immune suppression.”
256. IL-8: Abgenix’s antibody . As mentioned in the passage from Krueger 2002 quoted in the preceding paragraph, Abgenix had developed an anti-IL-8 antibody which had been tested in clinical trials. There is no dispute that, in fact, Abgenix stopped development of its anti-IL-8 antibody in May 2002 due to its failure in one of those trials. There is a vigorous dispute, however, as to whether this fact was common general knowledge in July 2003, and if so, the impact that would have had on the skilled person’s thinking.
257. The facts concerning the clinical trials of Abgenix’s anti-IL-8 antibody are as follows:
i) There was a Phase I trial in 26 patients in which 25% of patients achieved PASI75, but efficacy was only shown in a subgroup. The results were reported in an abstract, Lohner et al , “Clinical trials of a fully human anti-IL-8 antibody for the treatment of psoriasis, Br J Dermatol , 141, 989 (1999, “Lohner”). In addition to being mentioned in Krueger 2002 in the passage quoted above (reference 135 is Lohner), this trial is referred to in Cather (which does not mention the efficacy results), in Kirby and in Kanitakis. Both Krueger 2002 and Kanitakis describe the results as showing “moderate clinical improvements”.
ii) There was a Phase I/II trial in 45 patients in which 21% of patients achieved at least PASI50. Abgenix reported the results in a press release dated 30 November 1999 which stated that they were to be presented at the Psoriasis: From Gene to Clinic meeting in London on 2 December 1999. Asadullah states, however, that preliminary results presented at the American Academy of Dermatology meeting in 2001 suggested that the antibody had no significant effect on the PASI score of psoriasis patients.
iii) There was a Phase IIa trial in 94 patients in which 24% of patients achieved at least PASI75. The results were reported at the 63rd Annual Meeting of the Society of Investigative Dermatology in Los Angeles in May 2002 and published in an abstract, Horowitz et al , “ABX-IL8 in the treatment of psoriasis: clinical results”, J Invest Dermatol , 119, 239 (2002). They are mentioned in Kanitakis and in Prinz (although Prinz does not give the figures, simply saying “Reduction in PASI has been observed”, and the reference he gives is erroneous).
iv) There was a Phase IIb trial in 276 patients which failed. Abgenix announced this in a press release on 14 May 2002. The press release stated:
“ … treatment with ABX-IL8 did not result in a significant improvement in PASI scores, the primary efficacy end point of the trial. Based on these findings, Abgenix is discontinuing clinical development of ABX-IL8 in psoriasis. In addition, the company will not proceed with a previously planned clinical study of ABX-IL8 in melanoma and will wind down its ongoing Phase 2a study in chronic obstructive pulmonary disease (COPD). Abgenix currently has no plans to conduct further clinical studies involving ABX-IL8.”
The results were also presented at the 63 rd Annual Meeting. Although Kanitakis does not mention the Phase IIb trial or its failure, it does state that the results of the Phase IIa trial were “modest” and therefore “the sponsor decided to discontinue clinical trials with this product”.
258. IL-8: Prof Krueger’s evidence . Prof Krueger explained in his first report that interest in targeting IL-8 had led to the development of an anti-IL-8 antibody by Abgenix, but that Abgenix had discontinued development after it failed to meet its efficacy endpoint in a Phase II trial. He said that, as a result of this failure, IL-8 had been ruled out as having any therapeutically relevant role in the pathogenesis of psoriasis before July 2003. He said that this was “an example of how redundancy in chemokine/cytokine could mean that blocking one factor would not be sufficient to have a therapeutic”. As he acknowledged, he had had personal knowledge about Abgenix’s anti-IL-8 antibody, because Abgenix had consulted him about whether to test it in psoriasis. He had advised against this, but Abgenix went ahead anyway.
259. In his second report Prof Krueger said that the failure of Abgenix’s antibody had had a significant impact on the psoriasis community. He considered that it established that IL-8 was not a pathogenic cytokine in psoriasis.
260. Prof Krueger maintained these views in cross-examination. As he graphically put it, his experience with his colleagues and with pharmaceutical companies was that IL-8 was “dropped like a hot potato” after the Abgenix announcement. As he explained, at that time it was customary not to publish a full report of a negative trial.
261. IL-8: Prof Prens’ evidence . In his first report Prof Prens said that IL-8 was well-known to be highly upregulated in psoriasis and to attract neutrophils, citing a paper published in 1991. He said that in 2003, and still today, IL-8 was regarded as having an important role in the pathophysiology of psoriasis and thus had been targeted for potential therapies. He made no reference to the Abgenix anti-IL-8 antibody.
262. In his second report (dated 11 December 2018) Prof Prens disagreed with Prof Krueger’s evidence in his first report that IL-8 had been ruled out as a therapeutic target for psoriasis before July 2003 as a result of the failure of Abgenix’s anti-IL-8 antibody. He gave two reasons for his disagreement. First, he did not think that the skilled person would have been aware of the failure of Abgenix’s antibody in July 2003 because it was not the subject of any articles and he (Prof Prens) did not recall finding out about it until much later. In cross-examination Prof Prens accepted that it was likely that word would have got around in the USA by July 2003, but maintained that it had not in Europe.
263. Secondly, Prof Prens expressed the opinion that, even if the skilled person had known about the failure of Abgenix’s anti-IL-8 antibody, the skilled person would have remained of the view that IL-8 had an important role in the pathogenesis of psoriasis. This was because the anti-IL-8 antibody could have been discontinued for a number of reasons; because endpoints in trial reflected commercial considerations; and because positive results had previously been reported by Abgenix in a Phase IIa trial (although the document he exhibited was in fact Abgenix’s press release reporting the Phase I/II trial). I am unimpressed by this reasoning. The fact remains that Abgenix discontinued its antibody because it failed to demonstrate statistically significant efficacy against psoriasis in the Phase IIb trial.
264. By way of support for his second reason, Prof Prens stated in paragraph 25:
“I note that further research into anti-IL-8 therapy continued after the failed Abgenix trial. In particular, Anogen (a Canadian biopharmaceutical company) conducted studies on a topical anti-IL-8 therapy called Abcream. A phase 2/3, double blind, placebo-controlled trial involving 412 psoriasis patients demonstrated that efficacy was higher in the active treatment group than the control group (49% versus 14.9%). Subsequently, a phase 4 clinical trial of 1452 psoriasis patients showed that following Abcream treatment for 6 weeks, 62% of patients achieved PASI60, and 18% of patients achieved PASI90. Abcream has been approved in China for psoriasis treatment. 10 ”
265. Footnote 10 is to a review by Tsai and Tsai, “Anti-interleukin and interleukin therapies for psoriasis: current evidence and clinical usefulness”, Ther Adv Musculoskel Dis , 9, 277-294 (2017). The relevant part of the review states (at 284):
“ Abcream (Enboke) . A phase II/III, double-blind, placebo-controlled trial enrolled 412 psoriasis patients to test the efficacy of Abcream, a topical IL-8 inhibitor. After 6 weeks, the efficacy was higher in the active treatment group than in the control group (49% versus 14.9%). The adverse reactions including irritation, pain, itch and edema were 5.9% in the Abcream group and 6.6% in the control group. The therapeutic effect of IL-8 monoclonal antibody is thought to be related to the decrease of neutrophil recruitment and angiogenesis. 85 Then, a phase IV clinical trial (n = 1452) showed that after Abcream treatment for 8 weeks, 62% and 18% of patients achieved PASI 60 and PASI 90, respectively. 86 Abcream has been approved by China for psoriasis treatment.
…
Although anti-IL-8 is approved as a topical treatment for psoriasis in China, it has not been accepted as an effective treatment for psoriasis elsewhere. The use of anti-IL-8 biologics is still under development for oncologic indication, but no further development was in progress for psoriasis.”
266. In addition, table 3 of Tsai and Tsai (page 283) states that Abcream is “on market”, but no reference is given to support this statement. There is no reference in Tsai and Tsai to Anogen or to it being a Canadian company.
267. Reference 85 in Tsai and Tsai is Huang, “En bo ke zhi liao yin xie bing de xin ji yuan”, China Prescription Drug , 5, 54-56 (2002). Reference 86 is Liu et al , “En bo ke ru gao zhi liao xun chang xing yin xie bing 172 li lin chuang guan cha”, Chin J Dermatol Int Trad Western Med , 4, 252–253 (2003). It can be seen from their titles that these papers are in a Chinese language (presumably Mandarin).
268. When cross-examined about this, Prof Prens said that he had not heard of Abcream before this litigation and had found Tsai and Tsai by a search on the well-known PubMed platform. Unsurprisingly, he said that he had not read either reference 85 or reference 86, but he had Googled the latter. He said that, although it was in Chinese, “there was an abstract in English, and they also showed the pictures of the patients treated”. When he was asked where he had got the information that Anogen was a Canadian company from, he said that it was “also on the internet”. He went on to say that he had not seen any list of ingredients in Abcream. When challenged as to why he was prepared to rely upon the information in Tsai and Tsai when he had not read the underlying papers, he said:
“I have checked the clinical paper. I have seen the results in the patients being treated before and after pictures. They are there. [Reference] 86”.
When I asked him if he read Chinese, he said no, but he could see the pictures and he had read the abstract which gave the patient numbers.
269. After he had finished giving evidence, Prof Prens was asked for a copy of the English abstract of reference 86 he had mentioned. As result of this, he realised that he had been mistaken, and that the document to which he had been referring was not an abstract of reference 86, but a different document which he had downloaded from Anogen’s website (“the Anogen document”). He therefore made a third report apologising for his mistake and exhibiting a copy of the Anogen document and a print-out of the relevant webpage. By agreement between the parties, Prof Prens’ third report was admitted into evidence on the basis that he would be recalled for further cross-examination upon this topic. This was done by videolink in order to avoid the need for Prof Prens to return to the UK.
270. When he was recalled, counsel for Lilly showed Prof Prens a copy of reference 86 and a machine translation of it. The authors match those credited by Tsai and Tsai. Just as the title to the reference as reproduced in Tsai and Tsai contains the Arabic numeral 172, so does the title of the actual paper. The same Arabic numeral appears repeatedly in the text. The number 1452 does not appear. The translation of the title is “Clinical observations on 172 cases of psoriasis vulgaris treated with Enboke cream”. The paper states that 172 patients were treated with the cream during the period from November 2001 to April 2002 and reference is made to “approval number S20010003”. After eight weeks the “cure rate” was 13.4% and the “total effective rate” was 48.3%. There was no placebo control. Accordingly, reference 86 does not support the statement made in Tsai and Tsai about a Phase IV clinical trial. I would add that the paper cites four references (three of which are given in English in the original and the fourth of which is cited using Arabic numerals for the date, volume and page numbers) which were all published in the period 1996 to 1998.
271. Turning to the Anogen document, this is entitled “Abcream: A topical treatment of psoriasis with monoclonal antibodies that neutralize interleukin 8 (IL-8)”. No authors of the Anogen document are credited. Section 1 of the document (on page 1) states that “Anogen-Yes Biotech Laboratories Ltd was the first company in the world to carry out the research and development of the revolutionary anti-IL-8 treatment of psoriasis in 1993”. Section 2 of the document (page 1-13) describes certain in vitro studies. No dates for these are given. Section 3 (pages 13-27) describes a Phase II/Phase III trial which is attributed to six people (not including Huang) from various different institutions in China. The document states that the trial was carried out from October 1999 to June 2000. It describes the trial as a randomised, double-blinded, placebo-controlled trial, but with the inclusion of an open-label treatment group. It states at pages 14-15 that 208 patients (treatment group), 234 patients (control group) and 231 patients (open-label group) were enrolled, of whom 202, 221 and 210 respectively completed the trial. Later on page 15, however, it gives the numbers of the first two groups as 197 and 215 respectively, giving a total of 412. The total figure of 412 is reported at the beginning of paragraph 3.4 on page 17, although the end of the same paragraph gives the numbers of the treatment and control groups as 202 and 228. After six weeks, it is reported the efficacy in the treatment group was 49% against 14.9% in the control group, a statistically significant result. It appears that this was based on a system of scoring symptoms on a scale of 0 to 4 (i.e. not PASI or PGA). The adverse reaction rates reported are 5.9% and 6.6%. The document concludes with a set of “before and after” photographs. There is no list of ingredients of Abcream in the document. Nor is there any mention of Abcream having been approved for marketing. Nor is there any mention in it of a Phase IV trial, let alone one with 1452 patients.
272. When it was put to Prof Prens that none of these documents showed that “further research into anti-IL-8 therapy continued after the failed Agenix trial”, he maintained that Tsai and Tsai, with its statement that Abcream was on the market, did support that statement. Only when pressed did he concede that he had no evidence that Anogen had conducted any trials after May 2002. Moreover, despite having made that concession, he almost immediately withdrew it, saying that testing was required to get a product approved in any country in the world. Moreover, he declined to retract or qualify paragraph 25 of his second report.
273. When I asked Prof Prens when Abcream was approved in China, he said that he had seen from the Wikipedia entry for Anogen, which he had looked at when tracing the company, that it was in 2003. When I asked him why he had not mentioned this in either his second or his third report, he said that it was “a detail”. (What Prof Prens did not say is that the same entry stated that the Phase IV trial was completed in 2002. The numbers reported in the Wikipedia entry match those reported in Tsai and Tsai. The source cited by Wikipedia, however, is reference 86 in Tsai and Tsai.)
274. To err is human, and I attach no great significance to the fact that Prof Pens mis-remembered the nature of the English document he had seen when he first gave evidence (although I do find it surprising that he can have thought that a 26 page document was an abstract). Nevertheless, as stated above, I regard Prof Prens’ evidence on this topic as deeply unsatisfactory. Given the dates of references 85 and 86 in Tsai and Tsai (2002 and 2003), Prof Prens should have been cautious about relying upon Tsai and Tsai as evidence of “further research” after May 2002. Given that he looked at the Wikipedia entry and saw the date of 2003 given for approval of Abcream, he should have been even more cautious. When he downloaded and read the Anogen document, he should have appreciated that it was not safe to rely upon this material at all because (a) the dates mentioned in it pre-date May 2002, (b) the description of the Phase II/III trial is at best unclear, (c) there is no mention of Abcream having been approved and (d) there is no mention of the supposed Phase IV trial. Even if he considered that it was appropriate to rely upon the material, he ought to have put the material properly before the court in his second report, which would have involved him explaining the searches he had done and exhibiting the Anogen document. Finally, I was particularly concerned by Prof Prens’ reluctance to accept, even after being shown the translation of reference 86, that the material does not demonstrate there was “further research” after May 2002.
275. In my judgment the evidence relating to Abcream does not demonstrate that there was any further research into anti-IL-8 therapy after May 2002. It may be the case that Abcream was approved by the Chinese authorities sometime in 2003; but if so, the evidence indicates that that approval was based on studies conducted prior to May 2002.
276. It is convenient to note that at this point that, although one of Genentech’s counsel (Dr Turner QC) submitted that Prof Prens had not been giving an opinion on the efficacy of Abcream, even though some of the cross-examination was directed to that question, and that the efficacy of Abcream was not in issue, another (Mr Chacksfield) submitted that Abcream showed that IL-8 inhibition worked in psoriasis. In my judgment Dr Turner was correct on this point, and therefore Mr Chacksfield was wrong. In any event, I do not consider that there is any reliable evidence that Abcream, and therefore IL-8 inhibition, is efficacious against psoriasis. Mr Chacksfield relied upon the results of the Phase II/Phase III trial reported in the Anogen document, but that document has not been published in the scientific literature and there is no evidence that it has ever been peer-reviewed. Given the document is internally inconsistent as to the numbers of patients in the relevant groups, I do not consider it a reliable report of whatever trial was carried out. Moreover, the trial included an open-label arm, which, as Prof Prens agreed, is irregular. Finally, as noted above, there is no list of ingredients, and thus one cannot be sure that the cream did not include e.g. a corticosteroid. Reliance was also placed on the supposed fact that Abcream is on the market in China, but the only evidence of that is the statement in Tsai and Tsai which I do not regard as reliable. In any event, that would not demonstrate that it is effective.
277. IL-8: PPP . Counsel for Genentech relied on a paper by Skov et al , “IL-8 as antibody therapeutic target in inflammatory diseases: reduction of clinical activity in palmoplanar pustulosis”, J Immunol , 181, 669-679 (2008) which reports a trial concerning a human anti-IL-8 antibody developed by Genmab and Medarex for the treatment of palmoplanar pustulosis (PPP, an inflammatory condition affecting the palms and soles). In this paper, which was submitted for publication on 14 December 2007, the authors state (at page 677):
“Previously, the efficacy of murine IL-8 Ab in acute inflammatory diseases has been demonstrated in a number of animal models, suggesting Ab-mediated neutralization of IL-8 can potentially be used for various human inflammatory disorders (21, 23, 24, 34– 38). Few studies have been performed in chronic inflammatory diseases. The anti-IL-8 activity of another human IL-8 mAb, ABX-IL-8 (IgG2/κ), was demonstrated in vitro and in animal models in vivo (30). The result of a placebo-controlled phase IIb clinical trial for treatment of moderate-to-severe psoriasis with this Ab, however, was disappointing (39). This failure might well have resulted from the low and infrequent dosing used in these studies, leading to insufficient Ab concentrations in situ. Additionally, heterogeneity in clearance rates of the human IgG2 Ab resulting from a polymorphism for FcγRIIa that is known to affect IgG2 serum concentrations (40) may have played a role.”
278. Their conclusion (at page 678) is:
“In conclusion, we show IL-8 to play a central role in PPP. Using an IL-8-neutralizing Ab, we demonstrate that targeting a single critical factor in this disease characterized by high IL-8 overexpression leads to clinically relevant reductions in disease activity. This observation bears promise for the treatment of other diseases characterized by IL-8 overexpression.”
279. When this paper was put to Prof Krueger, he pointed out that PPP is a different disease to psoriasis. In PPP the primary pathology in the skin is accumulation of neutrophils, and it is not a hyperplasia scaling phenotype of the kind that happens in psoriasis. Thus the pathogenesis is different, and it is widely debated even today whether there was any similarity between the two. (Prof Krueger also pointed out the data appeared to show that efficacy was flat across the dose ranges tested and that there was no placebo. Accordingly, he questioned whether the study had any validity. That is a separate issue, however.) In my judgment this paper does not show that IL-8 was considered a therapeutically relevant target for psoriasis in July 2003.
280. IL-8: Other later evidence . More relevantly, to my mind, counsel for Genentech also relied upon two other papers published after July 2003 concerning psoriasis as casting light backwards. The first is Arican et al , “Serum levels of TNFα, INF-γ, IL-6, IL-8, IL-12, IL-17 and IL-18 in patients with active psoriasis and correlation with disease severity”, Mediators Inflamm , 5, 273-279 (2005). This states (at page 276):
“Elevated amounts of IL-8 have been detected in psoriatic lesional skin [30]. Many studies indicate that IL-8 may be involved in the pathomechanism of psoriasis. In fact, data currently available suggest that this cytokine exerts a critical role as a potent chemoattractant for neutrophils and T lymphocytes, as well as a factor prompting keratinocyte proliferation [10].”
Reference 10 is Bonifati.
281. The second paper is Numerof and Asadullah, “Cytokine and anticytokine therapies for psoriasis and atopic dermatitis”, Biodrugs , 20, 93-103 (2006). This states (at page 96):
“A fully human anti IL-8 antibody (ABX-IL-8) has been developed and tested in a dose-escalation study for psoriasis.[21] At the highest dose (3 mg/kg intravenously), 30% of the patients had a >50% reduction in PASI scores, but this was on the edge of clinical significance and lower doses were ineffective. Drug-specific issues cannot be excluded as the reason for the failure of the anti-IL-8 therapy; for example, whether the antibody achieved sufficient levels in the skin has not been conclusively determined. However, a consensus is emerging that IL-8 is not a suitable target for the treatment of psoriasis.”
Reference 21 is Lohner.
282. IL - 8: conclusion . I accept Prof Krueger’s evidence that, amongst the circles in which he moved, it was well known that Abgenix’s anti-IL-8 antibody had failed and that IL-8 had ceased to be regarded as a therapeutic target by July 2003. It does not necessarily follow that this was part of the common general knowledge of the skilled person in Europe, and in particular the UK. Despite my misgivings about Prof Prens’ evidence on this topic, there is no reason to doubt his unchallenged evidence that he had not heard of it by that date. Nor is there any evidence to show that he ought to have heard of it. There was no scientific article reporting the failed trial, nor was it picked up in contemporaneous reviews. Moreover, the later evidence supports the view that there were at least some in the field who had not heard of the failure and had not ruled out IL-8 by July 2003.
283. Accordingly, I conclude that the state of the common general knowledge concerning IL-8 was that it was regarded as a plausible therapeutic target, and it was known that an anti-IL-8 antibody was under development, but that the antibody had only yielded moderate clinical improvements in early trials and further results were awaited.
284. Although it does not go to the skilled person’s common general knowledge, it is convenient to address a related point here. Prof Prens accepted in cross-examination that a skilled person who was thinking about taking the Patent further for psoriasis would do a literature search, would find Kanitakis and would see that clinical trials of Abgenix’s anti-IL-8 antibody had been discontinued due to insufficient efficacy. (I would add that, if the skilled person wanted to find out more, they would search for and find the Abgenix press release.) In those circumstances, I consider that the skilled person would question IL-8 as having a pathogenic role in psoriasis.
285. IL-17 . It is common ground that, while the skilled dermatologist would probably be aware of its existence, IL-17 was not considered to be therapeutically relevant to psoriasis in July 2003. There was disagreement between Prof Krueger and Prof Prens as to how much the skilled person would have known about IL-17, with Prof Krueger opining that the skilled person would know more about it than Prof Prens considered they would. As counsel for Genentech submitted, however, it is not necessary to resolve this dispute because, as explained below, it is common ground that the skilled person would obtain and read the relevant papers when considering the question of plausibility. Accordingly, what matters is what the perception of the skilled person would be after having read those papers. I will address that question below.
286. IL - 1 . IL-1 has two isoforms, IL-1α and IL-1β. IL-1 was important historically as it was one of the first cytokines to be measured in psoriasis lesions. It was known to be implicated in keratinocyte hyperplasia. It was also known to have direct mitogenic effects on keratinocytes. Prof Prens’ evidence in his first report was that IL-1 was considered to be one of the most important cytokines in the pathology of psoriasis. Prof Krueger disagreed with this in his second report, referring to several papers which reached differing conclusions as to the upregulation and bioactivity of IL-1α and IL-1β in psoriasis lesions. In cross-examination Prof Krueger convincingly explained that the picture was a complicated and confusing one. Counsel for Genentech submitted that Bonifati undermined Prof Krueger’s evidence. In my view it does the opposite. Thus Bonafati states (at page 242):
“Although the assumption of a key role of IL-1 in the psoriatic cytokine network seemed very promising, many conflicting results have been reported by different authors on the relative amounts of IL-1alpha and IL-1beta in psoriatic skin and their roles in the pathomechanisms of the dermatosis. 10-18 ”
287. There is nothing to show that the confusion had been cleared up by July 2003. Accordingly, I conclude that the state of the common general knowledge was that IL-1 might have a role in the pathogenesis of psoriasis, but it was unclear.
288. NF-κB . NF-κB is a protein complex that controls transcription of DNA and cytokine production, playing an important role in regulating the immune response to infection. This activation pathway was known to be active in psoriasis and would therefore have been of interest to the skilled dermatologist as an indicator of inflammation. It plays an important role in inflammatory pathology in skin disease, and in psoriasis in particular. The activation of NF-κB results in the secretion of TNFα, IL-1, IL-6 and IL-8.
289. ICAM-1. Intracellular Adhesion Molecule 1 (ICAM-1) is an adhesion molecule expressed on keratinocytes and APCs which interacts with l ymphocyte function-associated antigen 1 (LFA-1, an integrin found on lymphocytes and other leukocytes) expressed on T cells. This interaction also enables adhesion between APCs and T cells to enable T cell activation. It was therefore understood to be involved in the migration of T cells from the endothelium to the skin and their adhesion to keratinocytes to produce a psoriatic plaque.
290. GM-CSF . G ranulocyte-macrophage colony stimulating factor (GM-CSF) is a cytokine which regulates the production of granulocytes and myelocytes, activates neutrophils, induces keratinocyte proliferation and the expression of TNFα and IL-8. It was thought that GM-CSF may be involved in the pathogenesis of psoriasis and also contribute to the immune activation or leukocyte accumulation in psoriasis.
291. MIP/CCL20 . Macrophage inflammatory protein (MIP), also known as CCL20, is a protein which stimulates the migration of T cells, dendritic cells and macrophages into sites of inflammation. CCL20 was known to be associated with psoriatic lesions and is highly expressed in lesional psoriatic skin. Its receptor is CCR6.
292. Overall . As can be seen from the foregoing discussion, overall, the common general knowledge as to psoriasis pathogenesis in July 2003 was that there was considerable uncertainty and debate. The many competing factors at play meant the skilled person faced great difficulty in attempting to predict the potential efficacy of targeting a particular immune pathway. The front runners were thought to be TNFα and IFN g .
Construction
293. There are three issues as to the construction of the claims.
The law
294. There is no dispute as the legal principles to be applied. The claim must be given a “normal” interpretation: Actavis UK Ltd v Eli Lilly & Co [2017] UKSC 48, [2017] RPC 21 at [54], [58] (Lord Neuberger) This means a “purposive” interpretation, that is to say, an interpretation which takes into account the purpose of the Patent, which is to describe and claim an invention to a person skilled in the art: Icescape Ltd v Ice-World International BV [2018] EWCA Civ 2219 at [60] (Kitchin LJ, as he then was) and [96] (Floyd LJ). As HHJ Hacon sitting as a High Court Judge pointed out in Regen Lab SA v Estar Medical Ltd [2019] EWHC 63 (Pat) at [202]-[207], it is no longer necessary to take equivalents into account in such an interpretation, because it is now possible for a patentee to contend that a patent has been infringed by virtue of the doctrine of equivalents even if it is not infringed when the claims are given a normal interpretation.
Which specifically binds to
296. Lilly’s written closing submissions on this issue run to 36 paragraphs and Genentech’s to 28 paragraphs. I shall not set out all the rival submissions, although I have taken them all into consideration. Instead, I shall simply set out the reasons which have led me to the conclusion stated below.
297. The starting point is that, although he had appeared to be suggesting otherwise in his reports, Dr Tite immediately and unreservedly accepted in cross-examination that the meaning of the expression “specifically binds to”, and cognate expressions, in the field of antibodies was dependent on context (as Prof Martin had stated in his second report). Dr Tite identified two meanings as being used in the art. The first was that the antibody bound authentically as an antibody, rather than non-specifically due to proteins adhering to each other without binding. The second was that the antibody bound to a single antigen. Even in the second case, he acknowledged that it was possible for someone to speak of an antibody being specific for one antigen, but cross-reactive to other antigen to which it bound with lower affinity.
298. Given that the meaning of the expression “which specifically binds to” depends on context, the crucial question is how the skilled team would understand the patentee to be using it in the context of the Patent. As is common ground, the interpretation of the wording of the claim is a matter for the court, but expert evidence as to the technical content of the specification which bears upon the question is both admissible and of assistance. Given the issue is one of antibody binding, this would fall within the province of the antibody engineer. Hence it was primarily addressed by Dr Tite for Lilly and by Prof Martin for Genentech. Some of the other experts also gave relevant evidence on the point, however.
299. Given that the specification contains a series of definitions, I consider that the skilled person would turn first to the definition in [0118]. Lilly’s case is that this paragraph either supports Lilly’s construction or is incoherent. Dr Tite’s evidence was that it is somewhat confusing. It was clear from his oral evidence, however, that the principal source of his confusion was the first sentence of section A, and in particular the statement that an antibody “which binds” is “one that binds the antigen with sufficient affinity …, and does not significantly cross-react with other proteins”. Dr Tite’s view was that the effect of this was to conflate the meaning of “binds” and “specifically binds”.
300. The first sentence of section [B] gives a definition of “specifically binds” which, as Dr Tite accepted, accords with the first meaning he identified. Thus this definition supports Genentech’s case. Section [B] goes on to describe two example approaches. As the experts agreed, the first involves a control molecule which is similar to the molecule that is doing the binding (e.g. a control IgG antibody), whereas the second involves a control molecule which is similar to the target. Dr Tite accepted that this passage is consistent with the first sentence.
301. Section [C] explains that “specific binding” and related terms “can be exhibited” by molecules having a Kd of the values stated. As the experts agreed, this is not a definition of the terms in question.
302. Section [D] states that “in one embodiment” the term “specific binding” refers to binding to a particular polypeptide or epitope without substantially binding to any other polypeptide or epitope (i.e. in accordance with Lilly’s interpretation of the claim). Again, as the experts agreed, this is plainly not a general statement or a definition. Moreover, as I will discuss, there is indeed at least one embodiment in which specific binding in this more restricted sense is required.
303. Thus the skilled reader is faced with a choice between two readings of [0118]. The first is to take the definition of “which binds” in section [A] literally, and therefore to read the definition of “specifically binds to” in section [C] as meaning something different to its literal meaning. The second is to take the definition of “specifically binds to” in section [C] as being the key definition, and to seek to reconcile the definition of “which binds” in section [A] with it. In my judgment the skilled reader would do the latter. The skilled reader is deemed to read the whole of the definition (in its context of the specification as a whole) and with a mind willing to understand it. It is section [C] which defines the words used in the claim, and the skilled reader would give that most weight. Moreover, the skilled reader would consider that this is supported by the statement in section [D] that “specific binding” has a more restricted meaning in one embodiment. Although the skilled reader might on a first reading be confused by the definition of “which binds” in section [A], on reflection after reading [0118] as a whole, the skilled reader would conclude that, as Prof Martin explained, it was possible to reconcile section [A] with the remainder of the paragraph by interpreting the words “does not significantly cross-react with other proteins” as meaning that the antibody does not react non-specifically with other proteins i.e. by adherence.
304. Although the definition of “species-dependent antibody” in [0115] uses the defined term “bind[s] specifically”, neither side suggested that the skilled person would consider that this shed any light on the question presently under consideration.
305. Next, the skilled person would consider the teaching of the specification to see whether this confirmed or contradicted their reading of [0118]. Lilly rely upon the fact that the focus of the invention is on what the specification repeatedly refers to as a “novel” or “new” cytokine, IL-17A/F: see [0001], [0019], [0396], [0399] and [0404]-[0407] and the headings to both Example 1 and 2. Moreover, the specification refers to this novel cytokine as having “unique potency” and “unique biological consequences”: see [0399]. None of this requires the skilled reader to interpret the words “specifically binds to” in a different manner to the way in which they are defined in section [B] of [0118], however.
306. The skilled reader would appreciate that the central core of the teaching of the specification is to be found in Examples 1 and 2. In Example 1, the Patent describes using phage display to prepare antibodies which bind to IL-17A/F. Although counsel for Lilly relied upon the statement at [0396] that IL-17A/F is able to interact both with an antibody that binds to IL-17 and an antibody that binds to IL-17F, this is nothing in this statement to indicate that the claimed antibodies to IL-17A/F must not bind to IL-17A/A or IL-17F/F.
307. More importantly, it is common ground that the process by which the antibodies were produced is not described as having included any negative screening step, i.e. the antibodies were not screened for their ability to bind IL-17A/A or IL-17F/F and selected only if they did not do so. They were only screened for their ability to bind IL-17A/F. The specification describes these antibodies in [0398] as “specific antibodies which bind selectively to the novel heterodimeric complex of IL-17A/F”. In his oral evidence (unlike in his report), Dr Tite appeared at one point to suggest that the word “selectively” implied that a test must have been carried out for binding to other proteins, but he accepted that there was nothing in the use of the word “selectively” which led to a different conclusion than the word “specific”. In other words, the skilled reader would conclude that the word “selectively” was being used to mean the same thing as “specifically” (and perhaps was being used to avoid repeating “specific”). Overall, the skilled reader would understand from Example 1 that antibodies “specific” to IL-17A/F were not required not to bind to other members of the Il-17 family.
308. Example 8 reinforces the conclusion to be drawn from Example 1. Example 8 is said to illustrate preparation of “antibodies which can specifically bind IL-17A/F” (see [0444]). Again, no step of selecting out antibodies that bind IL-17A/A (or any other related cytokine) is described. Again, the skilled person would understand that such a step was not needed to produce “antibodies which can specifically bind IL-17A/F”.
309. It is common ground that the specification describes embodiments in which the skilled person would understand that an antibody that bound to IL-17A/F but not to IL-17A/A or IL-17F/F was required, that is to say, embodiments which accord with the more restricted meaning of “specific binding” in section [D] of [0118]. Example 9 provides a specific instance of this. Lilly rely on the fact that Example 9 does not describe a negative selection step either, and ask the rhetorical question why the skilled reader would understand that it was to be performed in Example 9 but had not been performed in Example 1. In my judgment the answer to this is supplied by the point which Lilly make that the skilled reader would appreciate that Example 9 is a prophetic example. Thus Example 9 merely sets out an outline of a proposed experiment. By contrast, Example 1 describes an actual experiment carried out by the inventors, reports the results obtained and analyses those results, which as I have already observed form part of the central core of the teaching of the Patent. In those circumstances, the skilled person would expect that, if an important step like negative selection had been carried out in Example 1, the inventors would have described it, but would consider that Example 9 fell into a different category. The same applies to the diagnostic applications referred to in [0028]-[0032] and [0235]. I should add that it is common ground that the skilled reader would know how to carry out a negative selection step from their common general knowledge.
310. As the skilled person would appreciate, there are other applications in which antibodies that bind to IL-17A/F, but not IL-17A/A or IL-17F/F, are not required. A specific instance of this is the drug screening process of Example 10.
311. Dr Tite accepted that there is nothing in the Patent to show that one must use an antibody that only binds IL-17A/F for a therapeutic application. In particular, there is nothing which requires the skilled person to identify actions of IL-17A/F which are distinct from those of IL-17A/A or IL-17F/F when using an antibody. This evidence is consistent with that of Prof Kamradt looking at the matter from the perspective of the rheumatologist. Prof Krueger’s opinion looking at the matter from the perspective of the dermatologist was different, but I do not find his reasoning persuasive so far as the interpretation of the expression “specifically binds” is concerned (its impact on other aspects of the case is another matter). As counsel for Genentech submitted, given the significance of therapeutic applications, there is all the more reason not to limit the claims to antibodies that are required for applications such as that in Example 9.
312. For the reasons given above, I conclude that the expression “specifically binds” is to be interpreted in the manner contended for by Genentech.
Inhibits the activity of … IL-17A/F … to induce production of IL-8 and IL-6
313. Claims 1 and 14 both require the claimed antibody “inhibits the activity of … IL-17A/F … to induce production of IL-8 and IL-6”. Lilly contend that the skilled team would understand that claims 12 and 20, which are dependent on claims 1 and 14 respectively, require the therapeutic effect to be mediated through the inhibition of IL-6 and IL-8. In support of this contention, Lilly point to Genentech’s reliance upon IL-6 and IL-8 inhibition as part of its case on plausibility. Genentech disputes this interpretation, and contends that the skilled team would understand that inhibition of IL-6 and IL-8 is part of the test used in claims 1 and 14 to identify the claimed antibodies, and is not a functional requirement of the therapeutic uses in claims 12 and 20. In my judgment Genentech is correct about this. Plausibility is a separate question which I will consider below.
Use of an antagonist anti-IL-17A/F antibody … for
314. Claims 12 and 20 require “use” of the claimed antibody “for” the treatment of RA or psoriasis. It is common ground that the skilled team would understand this to mean that the antibody must have a discernible therapeutic effect in some patients. Lilly contend that the skilled team would understand inhibition of IL-17A/F must contribute to the therapeutic efficacy to a significant extent. Genentech accepts that inhibition of IL-17A/F has to make a contribution to the therapeutic effect of the antibody. In my judgment the skilled team would not regard an insignificant contribution as material for this purpose. The same goes for claim 22, which requires the antibody to be “for use in” the treatment of RA or psoriasis.
Genentech’s amendment applications
315. As noted above, Genentech seeks to amend the claims of the Patent and Lilly oppose the amendments on grounds of added matter, extension of protection and clarity. It is convenient to address these objections in reverse order. I shall consider the validity of the claims as proposed to be amended later.
Clarity
316. Lilly contend that, if the words “which specifically binds to” in claims 1 and 14 are not construed in the manner contended for by Lilly, then they are unclear. I disagree. On Genentech’s interpretation, which I have accepted, the words have a clear meaning. In any event, as Genentech points out, these words were contained in the granted claims. Accordingly, no objection arises on the application to amend: see G3/14 Clarity [2015] EPOR 29.
317. Lilly also contend that the word “comprises” in claims 1 and 14 is unclear. I disagree. As I see it, Lilly’s real objection is one of extension of protection.
318. Finally, Lilly contend that the words “induce production of IL-6 and IL-8” are unclear. Counsel for Genentech pointed out that Lilly had not pleaded this objection, but sensibly did not object to Lilly advancing it on that ground. As he also pointed out, however, there is no evidence that this requirement is unclear. In any event, the relevant words were again contained in the granted claims and therefore no objection can be taken on the application to amend.
Extension of protection
319. Lilly contend that the substitution of the word “comprises” in new claims 1 and 14 for the words “consists of” in granted claim 17 results in an extension of protection. Claim 17 was in the following terms:
“An isolated polypeptide or complex having at least 80% amino acid sequence identity to an IL-17A/F heterodimeric complex consisting of SEQ ID NO:3 …and SEQ ID NO:4 …with or without their associated signal peptides”
320. New claims 1 and 14 permit the complex to comprise not merely the sequences in SEQ ID NO:3 and SEQ ID NO:4, but also other material. Counsel for Genentech relied upon the requirement of 80% amino acid sequence identity in granted claim 17, but this makes Lilly’s point, since no such limit appears in new claims 1 and 14.
321. As is common ground, however, this objection is easily fixed by Genentech’s conditional application to re-instate the words “consists of”. I shall consider claims 1 and 14 on that basis.
Added matter
322. Lilly advance five different added matter objections to the amendments. Although these objections require a comparison to be made between the application for the Patent as filed (“the Application”) and the Patent as proposed to be amended, in the present case the only material differences between the Application and the Patent lie in the claims: the substantive content of the documents is otherwise the same. For convenience, I shall therefore refer to the passages in the Patent which correspond to the passages in the Application which are relied upon by Genentech as providing support for the amended claims.
323. Although objections of added matter are sometimes lacking in substance, Lilly’s objections must be taken seriously for two reasons. First, in opposition proceedings concerning the Patent in the European Patent Office, the Opposition Division held in a decision dated 24 November 2016 that Genentech’s main and auxiliary requests were all unallowable on this ground. That decision is presently under appeal, and I am informed that a decision of the Technical Board of Appeal is not expected before January 2020 at the earliest. (I am not aware that any request has been made for acceleration due to these proceedings. Certainly, the parties did not request this Court to support such a request, as they could have done. This is particularly unfortunate given that the Opposition Division did not determine any of the other grounds of opposition, and thus if Genentech’s appeal is allowed the case will need to be remitted to the Opposition Division with the potential for a further appeal to the Board of Appeal thereafter.) Secondly, the Comptroller-General of Patents has filed comments on Genentech’s amendment application in a letter dated 3 January 2019 stating that, in the Comptroller’s opinion, the proposed amendments are unallowable on this ground.
The law
324. Article 123(2) of the European Patent Convention provides:
“The European patent application or European patent may not be amended in such a way that it contains subject-matter which extends beyond the content of the application as filed.”
This provision is transposed into UK law by section 76(2) and (3)(a) of the Patents Act 1977.
325. There is no dispute as to the applicable principles, which have been laid down in a series of decisions of the Enlarged Board of Appeal of the EPO and of the Court of Appeal of England and Wales. As it is put in Case Law of the Boards of Appeal of the European Patent Office (8 th ed) at 401:
“The ‘ gold standard ’ … for assessing compliance with Art. 123(2) EPC is the following: any amendment to the parts of a European patent application or European patent relating to the disclosure (the description, claims and drawings) is subject to the mandatory prohibition on extension laid down by Art. 123(2) EPC and can therefore, irrespective of the context of the amendment made, only be made within the limits of what a skilled person would derive directly and unambiguously, using common general knowledge, and seen objectively and relative to the date of filing, from the whole of these documents as filed …”
326. As it was put by Jacob LJ in Vector Corp v Glatt Air Techniques Ltd [2007] EWCA Civ 805, [2008] RPC 10 at [4] approving his own earlier statement as Jacob J in Richardson-Vicks Inc’s Patent [1995] RPC 568 at 576:
“I think the test of added matter is whether a skilled [person] would, upon looking at the amended specification, learn anything about the invention which he could not learn from the unamended specification.”
327. Two points must be noted about the way in which the English courts apply these principles. First, the English courts consider it important to distinguish between what a claim covers and what it discloses . As Birss J explained in IPCom GmbH & Co KG v. HTC Europe Co Ltd [2015] EWHC 1034 (Pat) at [125]:
“… as the line of cases leading from AC Edwards to AP Racing … explains, English patent law draws a distinction between coverage and disclosure. To amount to added matter, the intermediate generalisation must be a generalisation in terms of disclosure, not coverage. In other words, to characterise a claim as an intermediate generalisation is not sufficient to establish the presence of added matter. Proving that a claim is an intermediate generalisation in terms of coverage does not establish added matter.”
328. Secondly, there is a line of cases in the Boards of Appeal of the EPO concerning the addition of matter by selections from multiple lists. The English courts recognise that matter may be added in this way, just as a selection from multiple lists may be novel over the disclosure of those lists. As Henry Carr J said in GlaxoSmithKline UK Ltd v Wyeth Holdings LLC [2016] EWHC 1045 (Ch) at [119], however:
The absence of specific evidence
330. On the other hand, I should say that I do not accept counsel for Lilly’s riposte, namely that the absence of expert evidence was a point against Genentech since Genentech bore the burden of proof. While it is true to say that Genentech, as the party seeking to amend the Patent, bears the legal burden of showing that the proposed amendments satisfy the applicable statutory requirements, the question of added matter is not one which falls to be resolved by reference to the burden of proof since it involves an objective comparison by the court of the two documents. In any event, as will appear, counsel for Genentech did elicit evidence from Dr Tite as to the disclosure of the Patent which he relied upon in support of Genentech’s answers to Lilly’s objections.
331. Lilly’s first objection is that added matter arises from the fact that new claims 1 and 14 describe the antibody as specifically binding to an “IL-17A/F heterodimeric complex” rather than to an IL-17A/F polypeptide.
333. More generally, Dr Tite agreed that various terms are used in the Patent (and there is no difference between the Patent and the Application in this respect) to describe the same thing: IL-17A/F, IL-17A/F polypeptide, heterodimeric polypeptide, dimeric complex, covalent heterodimer and heterodimeric complex, and that all these different terms would be understood by the skilled person to refer to the same thing. Thus the use of the expression “IL-17A/F heterodimeric complex” in new claims 1 and 14 is a permissible generalisation from Example 1.
334. Accordingly, I conclude that the reference to an “IL-17A/F heterodimeric complex” in new claims 1 and 14 does not amount to added matter. I therefore respectfully disagree with the Opposition Division, which held at [3.6] that there was added matter in this respect.
336. Genentech relies upon the fact that Example 1 states in the passage corresponding to [0398] that “specific antibodies which bind selectively to the novel heterodimeric complex of IL-17A/F have been identified which may serve to modulate the activity of this novel cytokine [emphasis added]”. It then immediately proceeds to refer to the pro-inflammatory activity of IL-17A/F of inducing production of IL-6 and IL-8, with reference to Figure 5. As Dr Tite agreed, the skilled person would understand that the reference to the antibodies modulating the activity of the cytokine was a reference to inhibiting the activity of inducing IL-6 and IL-8 production.
337. Thus the Application does indeed single out the activity of inducing IL-6 and IL-8 production and associates inhibition of that activity with specific antibodies to IL-17A/F. Moreover, the Application provides context for this by noting (in the passages corresponding to the first sentence of [0015] and the third sentence of [0019] of the Patent) that both IL-17A and IL-17F had been found to promote the production of IL-6 and IL-8.
338. Accordingly, I conclude that the requirement that the antibody “ inhibits the activity of the IL-17A/F heterodimeric complex to induce the production of IL-8 and IL-6” in new claims 1 and 14 does not amount to added matter. I therefore respectfully disagree with the Opposition Division, which held at [3.7] that there was added matter in this respect, and with the Comptroller.
339. Lilly have a subsidiary point, based on the fact that the passage corresponding to [0131] states that “one preferred biological activity includes inducing activation of NF-[κB] and stimulation of the production of proinflammatory chemokines IL-8 and IL-6”. The suggestion is that referring to inducing the production of IL-8 and IL-6 in the claims, but not referring to activation of NF-κB, adds matter. I disagree with this for the reasons already explained. Furthermore, Prof Krueger agreed that the skilled person would know that production of IL-6 and IL-8 requires activation of NF-κB, so the reference in the claims to IL-6 and IL-8 production carries with it activation of NF-κB.
341. Genentech contends that, if the question is approached through the eyes of the skilled team, there is no new technical teaching. I agree with this.
342. I have already explained why I consider that there is no added matter in identifying the antibody as specifically binding to the IL-17A/F heterodimeric complex or as inhibiting its production of IL-6 and IL-8.
343. Nor is there any added matter in including the feature that the antibody is human or humanised. As Dr Tite accepted, the skilled person would be well aware that, for therapeutic applications, human or humanised antibodies would be used. In the passage corresponding to [0289] of the Patent, the Application states in general terms that the anti-IL-17A/F antibodies of the invention “may … comprise humanized antibodies or human antibodies”. Furthermore, the antibodies of Example 1 are human.
344. As for the argument that combining these features amounts to a prohibited selection from multiple lists, I do not consider that new claim 1 teaches the skilled team anything new about the invention. It simply narrows the claim to part of the disclosure of the Application. Again, therefore, I respectfully disagree with decision of the Opposition Division at [3.5].
346. In my judgment this does not mean that the claims add matter. The Application discloses that “specific binding” can be exhibited by a molecule having any of the Kds in the list. Moreover, Dr Tite accepted that the skilled person would understand that, the lower the Kd, the better the antibody would be as an inhibitor, and that the skilled person would want antibodies with Kds in the range of 10 -8 or better.
347. Lilly argue that the Kds span a range from about 10 -4 to about 10 -12 and that the claim is to an undisclosed subset of that range. This is incorrect. What the Application discloses is not a range of affinities. Rather, each Kd is disclosed individually in a series of narrowing embodiments; they are independent disclosures. Accordingly, it does not add matter to identify only some of the disclosed Kds in the claim.
349. The question is whether these claims teach the skilled team something about the invention which they were not taught in the Application. In my judgment the answer is that the skilled team would learn nothing new from these claims.
350. The skilled team is told by the Application that RA and psoriasis are conditions that can be treated according to the invention. The Application makes it clear (though the skilled team would know anyway) that RA and psoriasis are auto-immune disorders and that, for conditions where an inhibition of the immune response is desirable, antagonist antibodies to IL-17A/F should be used. Although the greatest focus of the Application is upon RA, psoriasis is one of only two other conditions specifically mentioned in the passage corresponding to [0015] of the Patent. The fact that the Application also identifies a wide range of other conditions that it asserts can be treated does not matter for this purpose, given the teaching in relation to RA and psoriasis.
351. Lilly rely upon the decision of the Enlarged Board of Appeal in G1/93 Advanced Semiconductor Products/Limiting feature [1995] EPOR 97 that matter may be added by the addition of a limiting feature where that creates an inventive selection not disclosed in the application as filed or derivable therefrom. That statement of principle is not in doubt, but in my view it does not apply to the present case because the narrowing of these claims to RA and psoriasis is not an inventive selection. I should make it clear, however, that the impact of this on the question of plausibility is a separate question. I shall consider that below.
Conclusion in relation to Genentech’s amendment applications
352. For the reasons given above, I conclude that the amendments are allowable save for the word “comprises” in new claims 1 and 14, but the conditional amendment to “consists of” in place of “comprises” is allowable.
The prior art
353. When opening the case, Lilly relied upon six prior art citations. These fell into three groups, two of which are considered in this section. I will refer to the sixth citation later.
The IL-17A/F prior art: US344
354. United States Patent No 6,043,344 (“US344”) entitled “Human CTLA-8 and uses of CTLA-8-related proteins” assigned to Genetics Institute Inc was filed on 4 March 1998 and published on 28 March 2000. The abstract states:
“Polynucleotides encoding human CTLA-8 and related proteins are disclosed. Human CTLA-8 proteins and methods for their production are also disclosed. Methods of treatment using human CTLA-8 proteins, rat CTLA-8 proteins and herpesvirus herpes CTLA-8 proteins are also provided.”
355. US344 states at column 4, lines 15-26:
“Golstein et al. … reported a species they initially identified as ‘human CTLA-8’. However, examination of the sequence of the Golstein et al. species and the human CTLA-8 (B18) sequence of the present invention readily reveals that they are two different proteins, although they are homologous with each other and with the rat CTLA-8 and herpes CTLA-8 identified herein. The Golstein et al. species has now been renamed interleukin-17 (IL-17). Because of the homology between applicants’ human CTLA-8 (B[1]8) and IL-17, these proteins are expected to share some activities.
It has also been preliminarily determined that human CTLA-8 (B18) forms homodimers when expressed. As a result, human CTLA-8 proteins may possess activity in either monomeric or dimeric forms. Human CTLA-8 proteins can also be produced as heterodimers with rat and herpes CTLA-8 proteins and with human IL-17. These heterodimers are also expected to have activities of the proteins of which they are comprised.”
356. It is common ground that the skilled reader would ascertain from the sequence information provided in US344 that what US344 refers to as “human CTLA-8” and “B18” is IL-17F.
357. At column 8 lines 19-31 US344 states (emphasis added):
“Autoimmune disorders which may be treated using a protein of the present invention include, for example, multiple sclerosis, systemic lupus erythematosus, rheumatoid arthritis , autoimmune pulmonary inflammation, Guillain-Barre syndrome, autoimmune thyroiditis, insulin dependent diabetes mellitis, myasthenia gravis, graft-versus-host disease and autoimmune inflammatory eye disease. Such a protein of the present invention may also to be useful in the treatment of allergic reactions and conditions, such as asthma or other respiratory problems. Other conditions, in which immune suppression is desired (including, for example, asthma and related respiratory conditions), may also be treatable using a protein of the present invention.”
358. In Example 6 of US 344, human CTLA-8 is tested and demonstrated to induce production of IL-6 and IL-8.
The IL-17A/A prior art: WO717, US711, JP046 and Lubberts 2001
359. In opening, Lilly relied upon no less than four items of prior art as disclosing IL-17A/A as a target for RA therapy, one of which was Lubberts et al , “IL-1-Independent Role of IL-17 in Synovial Inflammation and Joint Destruction During Collagen-Induced Arthritis”, J Immunol , 167, 1004-1013 (2001, “Lubberts 2001”), a paper co-authored by Dr Lubberts. Both in Lilly’s skeleton argument and in counsel for Lilly’s oral opening submissions, the disclosure of each of these four items of prior art was summarised in some detail. In Genentech’s skeleton argument it was accepted that the key findings of Lubberts 2001 were part of the skilled rheumatologist’s common general knowledge. Although Lilly’s written closing submissions referred back to their skeleton argument, no arguments were advanced specifically by reference to any of these items of prior art. I asked counsel for Lilly if Lilly were still relying on Lubberts 2001, and he replied that they were not, having regard to the common ground as to the common general knowledge concerning IL-17A/A. With the benefit of hindsight, my question was misdirected. In reality, given the evidence as to the common general knowledge, Lilly do not need specifically to rely upon any of these items of prior art. I will nevertheless summarise the disclosure of the three citations that, formally at least, are still pursued.
360. WO717 . International Patent Application No WO 02/0587717 entitled “Methods for treating rheumatoid arthritis using IL-17 antagonists” in the name of Immunex Corp (“WO717”) was filed on 18 October 2001 and published on 1 August 2002.
361. The heading “Field of the invention” WO717 states at page 1 lines 14-18 that “the present invention involves treating rheumatoid arthritis by administering an IL-17 inhibitor or IL-17 antagonist, in particular IL-17 receptor, to an individual afflicted with such rheumatoid arthritis”.
362. Under the heading “Description of related art”, WO 717 describes at page 1 lines 26-35 the discovery of IL-17A as a cytokine produced by activated T cells that stimulates the secretion of various proinflammatory molecules including TNFα, IL-1β and PGE2 and explains that TNFα and IL-1 were believed to play a role in the inflammation and bone destruction in RA. It also notes that elevated levels of IL-17A had been found in RA patients’ synovial fluid and may play a role in the bone destruction characteristic of RA.
363. Under the heading “Summary of the invention”, WO717 states at page 2 lines 7-12:
“The present invention relates to a method of treating a mammal afflicted with a condition that relates to an inflammatory response, in particular, rheumatoid arthritis, by administering an IL-17 antagonist that inhibits IL-17 mediated signalling to a cell via membrane-bound IL-17 receptor. Suitable IL-17 antagonists include soluble IL-17 receptor, antagonistic antibodies that specifically bind IL-17 and antagonistic antibodies to the IL-17 receptor and combinations thereof.”
364. Under the heading “Detailed description of the invention”. WO717 states at page 2 lines 28-32:
“The subject methods involve administering to the patient an IL-17 antagonist or IL-17 inhibitor that is capable of reducing the effective amount of endogenous biologically active IL-17, by preventing the binding of IL‑17 to its receptor. Such antagonists include … antibodies directed against IL-17 (antibodies that bind IL-17 and inhibit binding thereof to IL-17 receptor) ….”.
365. At page 4 lines 27-34 WO717 states:
“… antibodies that specifically recognize a component of the IL-17 receptor and that prevent signalling through the receptor by IL-17 can be used to inhibit IL-17 activity. IL-17 antagonists that are antibodies include but are not limited to polyclonal antibodies, monoclonal antibodies (mAbs), humanized or chimeric antibodies… Thus, such antibodies can … be utilized as part of inflammatory disorder treatment methods.”
366. At page 5 lines 31-33 WO 717 states that “Preferably, for use in humans, the antibodies are human or humanised; techniques for creating such human or humanised antibodies were well known and commercially available ”.
368. Example 1 (page 10 lines 20-34) describes how an IL-17R/Fc fusion protein – a soluble form of IL-17R fused to the Fc region of human IgG1 – can be expressed. It is purified in Example 2 (page 10 line 35 – page 3 line 8).
369. In Example 3 (page 11 line 10 – page 12 line 19), a fusion protein is tested in CIA. No statistical analysis is provided for the results, but the results in Table 1 show a lower average final score in arthritis symptoms in the mice treated with IL-17R/Fc and TNF receptor/Fc as compared to control group, and the mice in the combination group are described as having a “markedly” lower average final score than the mice treated with monotherapy. A second similar set of experiments is then described, and WO717 concludes that these results “indicate that IL-17 receptor ameliorates the symptoms of arthritis in an animal model of rheumatoid arthritis”.
370. US711 . US Patent No 6,274,711 entitled “Purified mammalian CTLA-8 antigens and related reagents” assigned to INSERM and Schering Corp (“US711”) was filed on 2 May 1995 and published on 14 August 2001.
371. The summary of the invention describes a new “cytokine-like” protein which is designated as CTLA-8 (i.e. IL‑17A). The invention is said to include (column 2 lines 13-17 and 34-40):
“… isolated genes encoding proteins of the invention, variants of the encoded protein, e.g., mutations (muteins) of the natural sequence, species and allelic variants, fusion proteins, chemical mimetics, antibodies and other structural and functional analogs. …
an antibody which specifically binds to a primate CTLA-8 protein or peptide thereof; the antibody is raised against a protein sequence of SEQ ID NO: 2 [mouse IL-17A], 4 [viral IL-17A], 6 [human IL-17A fragment], 8 [human IL-17A] or 10 [mouse IL-17A fragment]; the antibody is a monoclonal antibody; the antibody blocks the CTLA-8 induced secretion of an inflammatory mediator, e.g., IL-6, IL-8 and/or PGE2; or the antibody is labelled.”
372. Methods of modulating the physiology of a cell by regulating CTLA-8-induced secretion of an inflammatory mediator are described including by using an antibody which specifically binds mammalian IL-17A (column 2 lines 47-54). The cell can be a synovial, epithelial, endothelial, fibroblast or carcinoma cell (column 2 lines 55-57).
“Purified CTLA-8, when cultured with synoviocytes, is able to induce the secretion of IL-6 from these cells. This induction is reversed upon the addition of a neutralizing antibody raised against human CTLA-8-8. Endothelial, epithelial, fibroblast and carcinoma cells also exhibit responses to treatment with CTLA-8. This data suggests that CTLA-8 may be implicated in inflammatory fibrosis, e.g., psoriasis, sclerodermia, lung fibrosis, or cirrhosis. CTLA-8 may also cause proliferation of carcinomas or other cancer 25 cells inasmuch as IL-6 often acts as a growth factor for such cells.”
374. Under the sub-heading “II Nucleic acids”, the nucleotide and amino acid sequences for murine and human CTLA-8 (i.e. IL-17A) are set out in Tables 1 and 3.
375. Under the sub-heading “III Purified CTLA-8 Protein”, US711 explains that “[t]he peptide sequences allow the preparation of peptides to generate antibodies to recognize segments” (column 15 lines 61-63).
376. Under the subheading “IV Making CTLA-8 Protein; Mimetics”, US711 refers to the necessary steps to transfect the CTLA-8 gene in a host cell using an expression vector with a view to producing fragments or full length CTLA-8 protein for use as an antigen in the generation of antibodies (column 17, lines 29 ff ). A range of host cell types is described which may be used for expression of CTLA-8. Specific examples of some useful cell lines are provided, followed by a list of suitable expression vectors.
377. Under the sub-heading “VII Antibodies”, US711 describes the production and screening of antibodies to CTLA-8. It is said that (column 26 lines 28- 32):
“Monoclonal antibodies are prepared from cells secreting the desired antibody. These antibodies can be screened for binding to normal or defective CTLA-8 proteins, or screened for agonistic or antagonistic activity, e.g., mediated through a binding partner. These monoclonal antibodies will usually bind with at least a K D of about 1mM, more usually at least 300µM, typically at least about 10µM, more typically at least about 30µM, preferably about 10µM, and more preferably at least about 3µM or better.”
378. This section goes on (column 27 lines 32-34):
“The antibodies … of this invention can have significant diagnostic or therapeutic value. They can be potent antagonists that bind to a binding partner and inhibit antigen binding or inhibit the ability of an antigen to elicit a biological response.” (column 26, lines 33-37)
“The polypeptides and antibodies of the present invention may be used with or without modification, including chimeric or humanized antibodies”
379. Under the sub-heading “VIII Uses”, US711 states (column 28 lines 5-17 and lines 25-26):
“The CTLA-8 protein (naturally occurring or recombinant), fragments thereof, and antibodies thereto, along with compounds identified as having binding affinity to CTLA-8 protein, should be useful in the treatment of conditions associated with abnormal physiology or development, including abnormal proliferation, e.g. cancerous conditions or degenerative conditions. Abnormal proliferation, regeneration, degeneration, and atrophy may be modulated by appropriate therapeutic treatment using the compositions provided herein. For example, a disease or disorder associated with abnormal expression or abnormal signal[l]ing by a CTLA-8 antigen should be a likely target for an agonist or antagonist of the protein.”
“Recombinant antibodies which bind to CTLA‑8 can be purified and then administered to a patient.”
380. US711 contains a number of examples. The isolation of human CTLA-8 and its biochemical characterisation using SDS-PAGE are described in Example III (columns 34-35). A human genomic library was obtained and screened with murine CTLA-8. Three human cDNA clones covering the full sequence of human CTLA-8 were identified. The open reading frame identified encoded a 155 amino acid polypeptide with a predicted molecular weight of 17kDa.
381. In Example IV (column 35), the inventors describe the biochemical characterisation of recombinant and naturally occurring CTLA-8 proteins. First, they describe the production of rhuCTLA-8 in 2 different cell lines. The results are that CTLA-8 is secreted as a glycosylated homodimer. They confirmed the production of CTLA-8 as a glycosylated homodimer in stimulated (PMA/ionomycin) human CD4 + T-cells. The inventors go on to describe how antibodies for human CTLA-8 were created using hybridoma technology (columns 36-37).
382. Two antibodies, Ab25 and Ab16, referred to as being antibodies specific for CTLA-8, were selected for use in a sandwich ELISA (Ab25 was used as the coat antibody) used to determine levels of human CTLA-8 in various cells and patient samples. No further details about those antibodies are provided (in particular, no amino acid sequences are disclosed). The inventors claim that the lowest concentration of human CTLA-8 detected was 0.015ng per ml, but no underlying data is included.
383. In Example V, synoviocytes were taken from controls and RA patients and incubated with increasing concentrations of human CTLA-8-8 (columns 37-38). The reference to “CTLA-8-8” is not properly explained, although there is a reference to it in column 36, line 34 which appears to indicate that it is used as a way of describing the purified human CTLA-8 protein. Concentrations of IL-6 secretions are measured using ELISA. No data are shown, but the inventors report that IL-6 was secreted in a dose-dependent manner.
384. Further bioassays measuring IL-6 secretions are described using two different kidney epithelial carcinoma cell lines (TUMT and CHA) and human lung fibroblasts (MRC-5) which were incubated with increasing human CTLA-8-8 concentration and are reported to show dose-dependent increases of IL-6. Again, none of the data is shown. Similar results are reported to have been obtained in human dermal fibroblasts, human brain epithelial cells and human bronchus epithelial cells.
385. JP046 . Japanese Patent Application N JP2000-186046A entitled “Chronic rheumatoid arthritis treatment drug and diagnostic method” in the name of Snow Brand Milk Products Co Ltd and Sankyo Co Ltd (“JP046”) was filed on 14 October 1999 and published on 4 July 2000.
386. Under the heading “Prior art” JP046 explains:
“[0003] Various cytokines, such as IL-1, IL-6, and TNF-a play an important role in the pathological development of RA. A particularly representative inflammatory cytokine is IL-6, and RA treatment by blocking IL-6 signal transmission has been attempted. …
[0004] In recent years, interleukin-17 (hereinafter, ‘IL-17’) was discovered and its function was investigated (Clinical Immunology, 29, 678-682 (1997)). IL-17 is known not only to cause the production of inflammatory cytokines such as IL-6, IL-8, and GCSF, but also to induce the differentiation of mature neutrophils. Recently, IL-17 was surmised to possibly be involved in inflammatory diseases. However, IL-17 is a new cytokine derived from T-cells, so its role in the pathological development of RA is still unknown. …
[0008] The cells that control bone metabolism are osteoblasts and osteoclasts, and these cells intimately interact with one another in a phenomenon called coupling. Osteoblast/osteoblast-like stromal cells are known to adhere to osteoclast precursor cells and mature osteoclasts, respectively inhibiting osteoclast differentiation/maturation and the bone resorption activity of mature osteoclasts. The theory has been proposed that osteoblast/osteoblast-like stromal cells produce osteoclastogenesis differentiation factors (ODFs) on the cell membrane by receiving signals via 3 different signal transmission systems for the various bone resorption factors: specifically, intranuclear D3 receptors in the case of activated vitamin D3, protein kinase in the case of interleukin-1 (IL-1), parathyroid hormone (PTH), prostaglandin fa (PGfa), etc., and gp130 in the case of the inflammatory cytokine IL-6 …”
387. Under the heading “Problems to be solved by the invention”, JP046 states at [0010]:
“Although IL-17 is a new cytokine derived from T-cells, its role in osteoclast formation is almost completely unknown. The present inventors discovered that IL-17 has an important relationship with the pathology of RA….”
388. Under the heading “Means for solving the problem”, JP046 states at [0011]:
“The present invention relates to a chronic rheumatoid arthritis treatment drug whose active ingredient is a substance that suppresses or neutralizes IL-17 activity in the body and/or a substance that inhibits the transmission of IL-17 signals that induce osteoclastogenesis. More specifically, examples of the substance that neutralizes interleukin-17 activity may be interleukin-17 neutralizing antibodies …”
389. Under the heading “Mode for carrying out the invention”, JP046 states:
“[0012] As shown in the embodiments below, the research of the present inventors showed that IL-17 levels in synovial fluid are significantly higher in RA patients than in patients with osteoarthritis (p<;0.001). Also, it was discovered that IL-17-positive cells exist among CD4+ and CD45RO+ T-cells in synovial fluid and tissue. In this way, IL-17 is suggested to be deeply involved in the pathological development of RA. … As described above, the present inventors discovered for the first time that IL-17 was markedly increased in RA patients, that increased IL-17 promoted the formation of osteoclasts and bone destruction, and that this sort of bone destruction was selectively inhibited by osteoclastogenesis inhibitory factors (OCIFs).
[0013] As described above, IL-17 levels were shown to be significantly higher in RA patients compared with osteoarthritis patients (p<;0.001), and since IL-17 produced a bone resorption effect by markedly inducing osteoclastogenesis, it was discovered to be a cytokine that was deeply involved in RA lesions such as joint and bone resorption. In addition, these results revealed that suppressing or neutralizing IL-17 activity in the body and/or inhibiting osteoclastogenesis inducing signal transmission could be a treatment for RA. Therefore, a substance with properties that suppress or neutralize IL-17 activity and/or a substance that inhibits IL-17 osteoclastogenesis inducing signal transmission could be used as an active ingredient in a drug to treat RA … use of an IL-17 neutralizing antibody or OCIF is particularly preferable.”
390. At [0014] JP046 states that “Preferably, anti-human IL-17 monoclonal humanized antibodies are used”. It goes on:
“[0015] … Also, for a humanized antibody, a monoclonal antibody that neutralizes human IL-17 activity and that also has high affinity for human IL-17(lowest dissociation constant possible, for example, 10 -10 M or below) is selected from among the mouse anti-monoclonal antibodies obtained by the above-described methods.
[0016] … By using a protein A column to purify the culture solution of the antibody-producing hybridoma, a complete human antihuman IL-17 monoclonal antibody can be obtained. The humanized anti-human IL-17 monoclonal antibody that is the objective of the present invention can be obtained by selecting a human monoclonal antibody that neutralizes human IL-17 activity and exhibits high affinity for human IL-17.”
391. Embodiment 1 ([0023]) describes use of polyclonal anti-IL-17A antibodies to neutralise IL-17A activity thereby supressing osteoclastogenesis in a co-culture of osteoblasts and bone marrow cells. The inventors comment that the “results clearly show that polyclonal neutralizing antibodies to IL-17A supress joint and bone resorption caused by increased IL-17A levels in the synovial fluid in RA and that these antibodies have utility as a drug for treating RA.”
392. Embodiment 2 ([0024]-[0025]) is similar, but relates to osteoclastogenesis suppression using OCIF. An in vitr o assay was carried out using osteoblasts and bone marrow cells and IL-17A as stimulus to induce osteoclast differentiation, to which OCIF was added in increasing amounts. The results show that OCIF inhibits IL-17A induced osteoclastogenesis in a dose-dependent manner by binding to what the inventors call the “OCIF binding molecule (OBM)”, which, they say, “the IL-17 causes to be expressed on the surface of osteoblasts and blocking the osteoclastogenesis signals of OBM”. The inventors conclude that OCIF “therefore has much promise as an extremely safe and effective RA treatment”.
393. Embodiment 4 ([0030]) describes the measurement of IL-17 in the synovial fluid of 43 patients with RA and nine patients with OA, four patients with trauma and seven patients with gout. It is shown that the RA patients’ IL-17 levels are significantly higher.
394. Embodiment 5 ([0031]) describes an experiment in which IL-17 is shown to induce osteoclastogenesis in an in vitro co-culture system (using mice osteoblasts and bone marrow cells). Osteoclast formation is dose-dependently induced in response to IL-17A in mouse bone marrow cells and osteoblasts.
395. Embodiment 6 ([0032]) describes osteoclast formation in response to IL-17 in an in vitro co-culture system (using bone marrow cells and osteoblasts). The results show the complete inhibition of IL-17A induced osteoclast formation in the presence of a COX-2 inhibitor (labelled NS398) and, separately, indomethacin (an NSAID).
396. Under the heading “Effect of the invention”, JP046 states at [0033]
“The present invention provides a RA treatment drug whose active ingredient is a substance that suppresses or neutralizes IL-17 activity in the body and/or a substance that inhibits osteoclastogenesis inducing signal transmission due to IL-17 …”
Obviousness over US344
397. Lilly contend that claims 1, 2, 13, 14 and 15 are obvious over US344, and so too are claims 12, 20 and 22 in so far as those claims are directed to RA. There is no dispute as to the relevant principles of law, which are very familiar, and so there is no need for me to set them out.
The disclosure of US344
398. Counsel for Genentech did not dispute in his closing submissions that, as the evidence of Prof Krueger and Prof Kamradt confirmed, column 4 lines 30-32 of US344 is an enabling disclosure of the recombinant production of a heterodimer between “human CTLA-8” (i.e. IL-17F) and “human IL-17” (i.e . IL-17A), that is to say, IL-17A/F. Although it is not explicitly stated that the heterodimer is to be produced recombinantly, this is implied by the reference to forming heterodimers between human CTLA-8 and rat and herpes CTLA-8 proteins. Although actual production of the heterodimer is not described in US344, there is no dispute that the skilled team would know how to do this using their common general knowledge. Although counsel for Lilly at times appeared to suggest that US344 disclosed naturally occurring IL-17A/F, it plainly does not.
399. US344 states that the heterodimers (including IL-17A/F) are expected to have activities of the proteins of which they are comprised; but it does not disclose any evidence of this.
Obviousness of claims 1, 2, 13, 14 and 15
400. Claim 1. Lilly contend that, given the disclosure of IL-17A/F in US344, it was obvious to make and isolate antibodies to IL-17A/F since it was a routine procedure in July 2003 and there is nothing unexpected about the properties of the anti-IL-17A/F antibodies disclosed and claimed in the Patent. Lilly contend that such antibodies will inherently inhibit production of IL-6 and IL-8, but in any event rely upon the evidence of Prof Kamradt that it was common practice by July 2003 to test IL-17 family members for inducing IL-6 and IL-8 production as showing that it was obvious to raise antibodies which inhibited this. Lilly further rely upon the evidence of Prof Martin that the effect on IL-6 and IL-8 was what the skilled person would be looking for as confirming this. In any event, Example 6 of US344 itself would make it obvious to do this. Finally, Lilly rely upon the evidence of Prof Martin that humanised antibodies were desired not only for use in therapy, but also for use in diagnostics, as showing that it was obvious to make humanised antibodies. I accept all of these points. Accordingly, I conclude that claim 1 is obvious over US344.
401. Claims 2 and 15. It is clear from the evidence that the skilled team would aim to make antibodies with as high an affinity (as low a Kd) as possible, and would aim for a Kd of lower than 10 -8 M. It follows that claim 2 is also obvious and that claim 15 is obvious if claims 13 and 14 are.
402. Claims 13 and 14 . Claims 13 and 14 add the purpose limitation “for use in a method of medical treatment”. Given that these claims extend to absolutely any form of medical treatment, I consider that these claims cannot be independently valid. Either they require no threshold of efficacy, in which case it would be obvious to try humanised anti-IL-17A/F antibodies for treating various inflammatory diseases to see what happened; or they do require a threshold of efficacy, in which case it was and remains utterly implausible that IL-17A/F antibodies are efficacious for all forms of medical treatment.
Obviousness of claims 12, 20 and 22 in so far as directed to RA
403. Lilly contend that it was obvious in the light of US344 to try humanised anti-IL-17A/F antibodies for the treatment of RA. As noted above, it was common ground between Dr Lubberts and Prof Kamradt that the skilled rheumatologist would have a good expectation that inhibiting IL-17A/A would be effective to treat RA. Genentech agrees that, given the disclosure of the Patent, the RA skilled team would have considered it plausible that humanised anti-IL-17A/F antibodies would be efficacious in the treatment of RA. Genentech contends, however, that, absent the disclosure of the Patent, the RA skilled team would not have had any expectation that humanised anti-IL-17A/F antibodies would be efficacious. This is because it was the Patent which showed for the first time that IL-17A/F existed in humans, being produced in activated T cells and having the effect of inducing the production of IL-6 and IL-8. It was this disclosure, Genentech contends, which made IL-17A/F a therapeutic target for RA.
404. Lilly rely in support of this aspect of their case on the evidence of Dr Lubberts. As counsel for Genentech pointed out, however, Dr Lubberts was not instructed by Lilly’s legal team to address it in his first expert report, even though he had been given US344 to read before reading the Patent. (This was because Lilly’s legal team had asked Prof Krueger to address it in his first report, and were trying to avoid duplication. As counsel for Lilly acknowledged, this was a mistake on the part of Lilly’s legal team.) It was only in his second report that Dr Lubberts was asked to consider it, by which time he was familiar with the Patent. Counsel for Genentech submitted that, in those circumstances, it was likely that Dr Lubberts’ evidence on this point was tainted by hindsight. I agree that this method of proceeding created a real risk of hindsight. It remains necessary, however, to consider the cogency of Dr Lubberts’ reasoning.
405. Dr Lubberts’ evidence in his second report was that, given that IL-17A and IL-17F were known to be homodimers produced by the same type of cells and to have a high degree of homology, and given the statement in US344 that a heterodimer of IL-17A and IL-17F, i.e. IL-17A/F, can be produced (albeit without any supporting evidence), the skilled rheumatologist would consider it “possible” that IL-17A/F existed in nature. Moreover, given that IL-17A and (to a lesser extent) IL-17F were both considered to be targets for treating RA, the skilled rheumatologist would consider that there was “a good likelihood” that IL-17A/F would bind to and activate the same receptor as IL-17A and be involved in RA pathogenesis. Dr Lubberts concluded:
“The skilled person would expect IL-17A/F to have similar effects to IL-17A and would conduct studies in relation to the function of IL-17A/F including to compare the effects and potency of IL-17A/F against IL-17A and IL-17F in inducing the production of proinflammatory cytokines by synovial fibroblasts. If, as expected, those studies were positive, IL-17A/F would be a therapeutic target as with IL-17A for RA.”
406. Dr Lubberts’ oral evidence on this point was consistent with his written evidence. Indeed, if anything, he was more positive as to the skilled person’s expectation that IL-17A/F existed in nature. Moreover, he rejected the suggestion that his reasoning was based on hindsight.
407. As noted above, Prof Kamradt accepted that the skilled person had a good expectation that blocking IL-17A would be efficacious for RA. He also agreed that it was known that IL-17F had similar properties to IL-17A. As he pointed out, however, less was known about IL-17F in July 2003 than about IL-17A. He accepted that, in the light of US344, the skilled person would think that IL-17F represented a promising target for the treatment of RA and that they would have reason to believe that blocking IL-17F could be beneficial in treating RA; but his view was that the skilled person would want to conduct further studies to feel “confident” that blocking IL-17F would have a therapeutic effect.
408. When it was put to Prof Kamradt that, given the known similarities between IL-17A and IL-17F and given the disclosure of IL-17A/F as a heterodimer of A and F which is “expected to have activities of the proteins of which they are comprised” in US344, the skilled person would regard IL-17A/F as at least as promising as IL-17F, he disagreed. His main reason for this disagreement was that IL-17A/F had not been shown to exist in nature, although he accepted that “speculation” that it did “may be reasonable”. As he put it, “Once it was known, you would include it on your list”. He also said that the skilled person would want to know more about IL-17A/F.
409. The conclusion I draw from the evidence is that the skilled person would consider it reasonably likely that IL-17A/F existed in nature and would consider it a promising target for RA therapy. Given that less was known about IL-17F than IL-17A, the skilled person would not have a positive expectation that targeting IL-17A/F would be efficacious, but would consider that success was sufficiently likely to warrant studies of the kind described by Dr Lubberts, leading to tests in animal models and then to trials in humans. I do not understand it to be in dispute that such tests and trials would be likely to be successful. Furthermore, there is nothing in the Patent that would give the skilled person any greater reason to believe that an anti-IL-17A/F antibody would be efficacious against RA. (This means that, if the skilled person’s expectation of success based on the prior art was not sufficient for obviousness to try, the claims would fail for lack of plausibility applying the principles discussed below; which is not to imply that the legal tests are the same.)
410. Accordingly, I conclude that claims 12, 20 and 22 are obvious over 344 in so far as those claims are directed to RA.
Novelty over the IL-17A/A prior art
411. Lilly contend that, if the claims are construed in the manner contended for by Genentech, as I have accepted, then claims 1, 2, 13, 14 and 15 are lacking in novelty over all the IL-17A/A prior art, and claims 12, 20 and 22 in so far as those claims are directed to RA are lacking in novelty over WO717 and JP046. Lilly put their case in three different ways. First, Lilly contend that it is an inevitable result of working the IL-17A/A prior art that antibodies which also bind to IL-17A/F are produced. Secondly, Lilly rely upon case law of the Boards of Appeal to the effect that a claimed class of compounds lacks novelty if it overlaps with a class of compounds disclosed in the prior art. Thirdly, Lilly rely upon the principle that novelty cannot be established by just providing more information about the same invention.
412. Without intending any disrespect to the lengthy submissions I received on these issues, I do not propose to discuss them in any detail. For reasons that will become apparent when I consider obviousness, I have concluded that it is not inevitable (or at least has not been proved to be inevitable) that anti-IL-17A/A antibodies produced in accordance with the prior art will bind to IL-17A/F as well. In those circumstances, I do not consider that it can be said that the Patent merely provides more information about the prior inventions. Nor do I consider that this is a case of overlapping classes of compounds: compare Dr Reddy’s Laboratories (UK) Ltd v Eli Lilly and Co Ltd [2009] EWCA Civ 1362, [2010] RPC 9.
Obviousness over the IL-17A/A prior art
414. This part of the case has led to experiments being conducted by Lilly which have been heavily criticised by Genentech. This has led to extensive expert evidence from Dr Tite, Dr Reichmann and Prof Lesk on the one hand and Prof Martin and Prof Carr on the other hand. A number of issues of considerable complexity have been raised. It is Lilly’s contention, however, that, despite the number and complexity of the issues that have been raised, in substance Genentech’s criticisms of the experiments consist of points which do not make any practical difference. Moreover, Lilly contend that, for reasons that will appear, many of the criticisms of the experiments, even if well-founded, do not prevent Lilly from succeeding in their obviousness case.
415. I would add that it seems to me that at least some of these issues arose because the case was presented by Lilly primarily as a novelty attack and only secondarily as an obviousness attack.
An outline of the issues
416. Lilly’s experiments involved characterising three murine antibodies referred to as mAb 5, mAb 16 and mAb 25, producing certain humanised versions of those murine antibodies, and characterising those humanised versions. The experiments can be divided into the following groups:
i) Experiments carried out by the laboratory of Professor Anton van de Merwe at the University of Oxford on mAbs 5, 16 and 25 to assess their characteristics:
a) indirect ELISA experiments to assess binding activity against IL-17A/A, IL-17F/F and IL-17A/F (producing EC 50 values);
b) SPR experiments to assess binding affinity to IL-17A/A and IL-17A/F (producing Kd values as well as on- and off-rates);
c) bioassays to assess the inhibition of IL-17A/A-induced production of IL-6 and IL-8 (producing an IC 50 value with respect to each of IL-6 and IL-8); and
d) bioassays to assess the inhibition of IL-17A/F-induced production of IL-6 and IL-8 production (producing an IC 50 value with respect to each of IL-6 and IL-8).
ii) Humanisation of mAbs 5, 16 and 25 to produce 12 humanised versions of each antibody. As noted above, this work was carried out by Absolute.
iii) Experiments conducted on a subset of 11 of the 36 humanised mAbs, namely similar indirect ELISA, SPR and IL-6 and IL-8 bioassays to those set out in (i) above.
418. In summary, Genentech contends that the experiments suffer from the following defects:
i) mAbs 5, 16 and 25 are not representative of murine antibodies that would be produced following the teaching of the prior art.
ii) The humanisation techniques used involved the use of post-July 2003 or other unjustified techniques, including:
a) the use of IMGT definitions of the CDRs;
b) the use of germline sequences for the antibody frameworks;
c) the use of a post-July 2003 modelling program called Phyre 2 ;
d) the use of post-priority structures for homology modelling; and
e) the choices for the combinations of residues to back-mutate.
The use of mAbs 5, 16 and 25 as a starting point
419. Dr Tite was asked by Lilly’s solicitors, Allen & Overy, to produce protocols for the production of antagonistic human or humanised antibodies to IL-17A/A following the teaching of WO717 and US711. He did so on 26 October 2017. He indicated that the skilled person would proceed by using purified biologically active recombinant human IL-17A/A either for the immunisation of animals and the generation of hybridomas producing IL-17A/A-specific monoclonal antibodies or to select scFv antibodies which bound to IL-17A/A from a phage display library. In the former case the skilled person would then test the panel of resultant antibodies for binding to and neutralising activity against IL-17A/A and select antibodies for humanisation using certain criteria. In the latter case the skilled person would convert the scFv antibodies into full human antibodies. Genentech makes no criticism of Dr Tite’s protocols. It points out, however, that Lilly did not follow the immunisation part of the first two protocols. Nor did Lilly follow the phage display protocols.
420. Dr Tite explained that Allen & Overy subsequently informed him that, due to time and ethical constraints, it was not practical to perform the immunisation sections of his first two protocols for the purposes of this litigation. Those stages would have taken at least four months to carry out assuming that all the capabilities were aligned and there were no experimental delays, which was typically not the case. Furthermore, the appropriate ethical permissions would have been required for the use of the animals. In his experience, getting a Home Office licence could be tricky and time-consuming. What Dr Tite did not say (presumably because he did not know), but counsel for Lilly informed me without contradiction from counsel for Genentech, is that experiments on animals are not permitted for the purposes of patent litigation under section 5C of the Animals (Scientific Procedures) Act 1986.
421. Counsel for Genentech submitted that Lilly could have followed Dr Tite’s phage display protocols instead, which would have avoided the ethical constraint. It would still have taken time, however. It is not clear to me from the evidence whether or not there was sufficient time available in which to do this. Moreover, I think there is something in the point made by counsel for Lilly that, since ixekizumab is a humanised (rather than human) antibody, it made sense for Lilly to focus on methods of producing humanised (rather than human) antibodies. But in any event, Genentech’s principal point is a different one.
422. Dr Tite explained that Allen & Overy asked him to assume that the steps of his protocols that relate to generating murine antibodies had been completed and they presented him with three particular antibodies (mAbs 5, 16 and 25) for characterisation, rather than a panel of antibodies as would have been produced by his protocols. It was not until after the characterisation experiments on these antibodies had been completed that Dr Tite was shown the witness statement of Dr Pin describing the generation of these antibodies by Schering-Plough in 1994 and their subsequent acquisition by Dendritics. (Allen & Overy informed Dr Tite that Dr Pin had worked with the group that filed US711, although he is not a named inventor and Dr Pin does not himself say this.)
423. As Dr Tite explained, the form of antigen used by Dr Pin in 1994 was not the same as that prescribed in his protocols. Dr Pin used cell lysate for the initial immunisations rather than purified biologically active recombinant protein because the latter was not available. For later immunisations, however, purified protein was used. The first round of immunisations using cell lysate contained complete Freund’s adjuvant and the second round contained incomplete Freund’s adjuvant (i.e. without mycobacteria). The last round of immunisations with purified protein used isotonic saline solution.
424. It is not in dispute that, by July 2003, purified recombinant biologically active IL-17A/A was commercially available. (It could also have been made, but that would have taken more time and effort.) Dr Tite accepted that, in those circumstances, the skilled person would have used that rather than cell lysate.
425. The question is whether this made a material difference to the nature of the antibodies. After all, as Prof Carr accepted, people had used cell lysate for immunising mice in such procedures before they used recombinant proteins. Dr Tite expressed the view in his reports that it was a reasonable approach, although he acknowledged that there were good reasons why purified protein was generally preferred if it was available.
426. Prof Carr suggested that it was likely that IL-17A monomer would be present in the cell lysate which would bias the population of antibodies generated towards those that recognised epitopes on a single IL-17A chain (thus making them more likely to bind to IL-17A/F). He acknowledged that there were no studies that show that the secreted form of IL-17 is found as a monomer, but said that the monomer would be present in the secretory pathway in the cell and hence in cell lysate. He agreed, however, that there would also be dimer present in the cell lysate.
427. Moreover, Prof Carr accepted that the use of purified IL-17A/A for the booster immunisations would bias the antibodies towards the dimeric form. He also acknowledged that it would be standard to confirm the bioactivity of the purified immunogen prior to its use, and he had no reason to suspect that was not exactly what Dr Pin had in fact done.
428. Most importantly, Prof Carr accepted that anyone injecting IL-17A/A with complete Freund’s adjuvant would also be likely to bias the antibodies because of the denaturing effect of the adjuvant on the protein. Furthermore, he accepted that he had no reason to think that mAbs 5, 16 and 25 were any different in character to those made in 2003 using IL-17A injected with Freund’s. The reason this is important is because both US711 (at column 36 lines 36-42) and the Patent (in Example 8 at [0446]) specify the use of Freund’s, which was common in 2003.
429. For his part, Dr Tite said he did not think that the use of lysate as the immunogen would be expected to bias the antibodies towards IL-17A monomers as Prof Carr had suggested. His first main reason was that he did not think that isolated monomer would be an effective immunogen once it had been put in complete Freund’s adjuvant. His second main reason was that it did not appear that the lysate was a very effective immunogen at all. Rather, it appeared that it had mainly served to prime antigen-specific B cells to IL-17A, leading to a greater increase in the numbers of antibodies in the last round of immunisations with the purified protein. In addition, his view was that, owing to the functional criteria that were applied to the antibodies that were taken forward, the particular immunogen that was used to generate them in the first place was less significant.
430. The conclusion I reach on the evidence as a whole is that it is unlikely that the use of cell lysate rather than purified recombinant IL-17A in the initial immunisations made a material difference. Accordingly, mAbs 5, 16 and 25 are representative of those that would have been made following the prior art in July 2003. In any event, given that the use of cell lysate was a well-known, (if older) procedure, mAbs 5, 16 and 25 represent the results of an obvious way of implementing the prior art in July 2003.
Taking mAbs 5, 16 and 25 forward
i) the murine antibody’s level of binding to other known IL-17 family members and a primate orthologue of human IL-17A/A;
ii) the antibody’s potency in inhibiting the stimulation of cells by IL-17A/A to produce IL-8 and IL-6; and
iii) the antibody’s affinity of binding to IL-17A/A as measured by SPR.
432. His conclusion was that the skilled person would take forward mAb5 and mAb16 to humanisation, and perhaps mAb25, depending on resource availability. Although all three had affinities for IL-17A/A of around 2 nM, whereas the skilled person would have been aiming for 10-100 pM, overall they were good enough to progress.
433. Prof Martin accepted that the selection criteria for humanisation candidates set out by Dr Tite were reasonable. He also agreed that mAbs 5, 16 and 25 showed sufficient affinity and potency that they would have been taken forward in 2003, at least in the absence of anything better.
434. As Prof Martin agreed, the skilled team in 2003 would expect in the vast majority of cases to be able to humanise antibodies using routine techniques so as to achieve comparable affinity for the target and equivalent biological activity to those of the murine antibodies. The skilled person would expect the humanised version of an antibody to bind the same epitope, and certainly that would be their aim. Prof Carr agreed that, if an antibody binds IL-17A/A so as to inhibit its activity, then if it bound to IL-17A/F it should also inhibit it.
435. Lilly contend that it follows from this evidence that if (as I have concluded) mAbs 5, 16 and 25 are representative of the murine antibodies that would have been produced in accordance with the IL-17A/A prior art, then claims 1 and 2 are obvious on Genentech’s construction, because mAbs 5, 16 and 25 bind to and inhibit IL-17A/F as well as well IL-17A/A, it would be obvious to humanise them using routine techniques and success would be expected, meaning that the humanised antibodies would also be expected to bind to and inhibit both IL-17A/A and IL-17A/F. To that extent, it does not matter whether the humanisation work carried by Absolute as part of Lilly’s experiments was representative of what the skilled person would have done in 2003. I accept this contention. Nevertheless, I must go on to consider the humanisation work.
The humanisation work
436. So far as relevant to the present dispute, the process of humanisation involves two main steps:
i) First, the grafting of the CDRs from the murine antibody onto a suitable human antibody framework. This is designed to make the antibody appear more human and less immunogenic to the patient (via the human framework regions), without losing its specificity to the desired epitope (which comes from the murine CDRs). The CDRs need to be identified in order to be grafted. Further, a suitable framework has to be identified from the available sequences for human antibodies.
ii) Secondly, since the grafting process tends to reduce the affinity of the antibodies for their target, often below acceptable levels, residues in the framework regions are “back mutated” to those residues from the original murine antibody to try to reinstate some or all of the affinity of the humanised antibodies. Accordingly, the skilled person needs to identify the framework residues for back mutation.
Use of IMGT definitions of CDRs
437. In July 2003 at least three CDR definitions were known, referred to as Kabat, Chothia and IMGT. The IMGT definitions were introduced as part of the international ImMunoGeneTics (IMGT) database of antibody sequences established in 1989. They are shorter in some instances and longer in others than either the Kabat or the Chothia definitions. Absolute used the IMGT definitions of CDRs in its humanisation work for Lilly’s experiments. Genentech contends that this approach would not have been used by the skilled person.
438. Counsel for Genentech submitted that Dr Riechmann had accepted this. This is an issue where the evidence needs to be analysed with care. In paragraph 13 of his second report Dr Riechmann said that the IMGT CDR definitions were known prior to 2003, but accepted that they were “not common” because, like Prof Martin who had addressed this question in his first report, he knew of no examples of them being used for humanisation prior to 2003. In cross-examination it was suggested to Dr Riechmann, without showing him the actual passage, that he had agreed in his second report that the skilled person would not have used the IMGT CDRs definitions, and Dr Riechmann agreed. It was then put to him that, in that regard, what Absolute had done was not representative of what the skilled person in 2003 would have done, and Dr Riechmann again agreed. In my assessment, all that Dr Riechmann can be taken to have accepted was that it was far from inevitable that the skilled person would have used the IMGT CDRs for humanisation. No reason was put to him as to why the skilled person could not or would not have used the IMGT CDRs if the skilled person wished to do so. All that was being relied upon was Dr Riechmann’s acceptance that there was no known example of it actually having been done by July 2003.
439. For his part, Prof Martin accepted that using the IMGT database to define the CDRs was common general knowledge in 2003, although he maintained that he knew of no example of this approach being used for humanisation purposes prior to July 2003 and thought it was not common even after that. He did not identify any reason why the skilled person could not or would not have used the IMGT definitions for this purpose if the skilled person wished to do so.
440. Accordingly, I conclude that the use of the IMGT CDR definitions for humanisation was an obvious possibility in July 2003. In any event, the question is whether it makes a material difference in terms of the residues that Absolute back mutated. I will consider that question below.
Use of germline sequences
441. This a similar kind of issue to the preceding one. Absolute used the closest human germline sequences, rather than sequences of mature human antibodies, as the human framework for grafting the CDRs onto. As Dr Riechmann accepted, we do not know what frameworks would have been chosen if Absolute had used mature antibody sequences, but they are most likely to have been different. That means there would have been a different starting point for the back mutation exercise and one cannot know what humanised antibodies would have been produced (although Dr Riechmann thought one could guess).
442. Dr Riechmann accepted that using the closest human germline sequences for the framework was something that had only been rarely done by July 2003, since there were only four publications using the approach by then. His evidence, however, was that by that date it was obviously an attractive alternative to the approach of using mature human antibodies which had previously been used because it retained as much human sequence as possible which the skilled person would expect to increase its tolerability.
443. Prof Martin agreed that the use of human germline sequences for the human acceptor scaffold was known in 2003 and was one of the options which the skilled person could use although it was not common practice. He pointed out that it had not been established by then that germline sequences did in fact reduce immunogenicity, but he accepted that that was the theory and that it was a reasonable theory (although not one he particularly agreed with).
444. Again, therefore, my conclusion is that the use of germline sequences was an obvious option in July 2003, albeit that it was not something that the skilled person would inevitably have done.
Choice of residues to back mutate
445. It is common ground that the choice of back mutations would be made by the skilled person based upon the methods taught in the prior art, in particular patents and applications from CellTech (US Patent No 5,829,205 published on 12 June 1999 (“Adair”)), Genentech (US Patent No 6,407,213 published on 18 June 2002 (“Carter”)) and Protein Design Labs (International Patent Application No WO 90/07861 published on 26 July 1990 (“Queen))”. In each case, there are also relevant papers: Adair et al , “Humanization of the murine anti-human CD3 monoclonal antibody OKT3”, Hum Antibody Hybridomas , 5, 41-47 (1994); Carter et al , “Humanization of an anti-p185HER2 antibody for human cancer therapy”, Proc Natl Acad Sci USA , 89, 4285-4289 (1992); and Queen et al , “A humanized antibody that binds to the interleukin-2 receptor”, Proc Natl Acad Sci USA , 86, 10029-10033 (1989).
446. A small point which is convenient to address here is that counsel for Genentech suggested that Dr Riechmann had only relied on the Adair and Carter patents when preparing his reports, and not the corresponding papers. This is incorrect: Dr Riechmann listed the papers in paragraph 7 of his first report as being ones he had read for the purposes of preparing that report; he referred to the skilled person relying upon the “publications and patents” at paragraphs 30 and 32 of that report; in paragraph 41 he stated that he had “reviewed … the selection of academic literature and patents that would have been well known to the skilled person in 2003 (listed in paragraph 7 … above)” in order to assess Absolute’s choice of back mutations; and in paragraph 42 he said that “the back mutations … are supported overall by the literature”. Moreover, in paragraph 29 of his second report he referred to both the Adair and Carter papers. It is fair to say that in his annexes justifying each back mutation he only made specific reference to the Adair and Carter patents, but he was clearly relying upon the Adair and Carter papers as well.
447. In brief summary, the methods outlined in the patents and used in the corresponding papers are as follows:
i) Adair involves a “hierarchy of positions” for identifying important locations within the framework, identifying lists of residues which should be donor.
ii) Carter uses a consensus sequence for the human acceptor. Carter also explains the use of models to identify residues of interest which have the effect of (i) non-covalent binding of the antigen, (ii) interacting with a CDR or (ii) participating in the V H /V L interface, and thus should be back mutated. The patent further provides a list of residues one or more of which can be selected for substitution.
iii) Queen uses a distance criterion approach to identifying residues for back mutation as explained in more detail below.
448. As Dr Riechmann explained in paragraph 40 of his first report, and Prof Martin accepted:
“As there is a degree of flexibility and personal judgement, on which residues to back mutate, different labs starting from the same murine antibodies would in all likelihood produce different humanised variants. However, all of these would be designed applying well established and routine principles. And while the exact framework sequence of the final humanised antibodies produced in the different labs may vary, each successfully humanised antibody will have an antigen affinity comparable to its parental murine antibody.”
449. As Dr Riechmann and Prof Martin also agreed, the strategy in humanisation would be to minimise the amount of non-human structure in order to minimise potential immunogenicity, whilst retaining suitable binding. The number of necessary mutations would vary from case to case, but the guiding principle which the skilled person would follow would be that the fewer mutations made, the better. Prof Martin accepted that a single-figure number of mutations would be typical in 2003.
450. Lilly did not adduce any evidence from Dr Wilkinson explaining why Absolute had chosen to back mutate the residues they did. It is common ground, however, that Absolute used a modelling program called Phyre 2 which was not available in July 2003 and used structures which were not available in July 2003.
451. Instead, Lilly adduced evidence from Dr Riechmann seeking to justify Absolute’s choices by reference to each of the Adair, Carter and Queen approaches and information which would have been available in July 2003. As I will explain, in the case of the Queen approach, Dr Riechmann relied upon the models produced by Prof Lesk.
452. The analysis in Dr Riechmann’s first report assumed that the skilled person would have used the IMGT CDR definitions. The analysis in his second report instead assumed that the skilled person would have used the combined Kabat and Chothia CDR definitions. Counsel for Genentech relied upon Dr Riechmann’s acceptance that, because the skilled person would not have used the IMGT CDRs, his first analysis could be forgotten about. For the reasons explained above, however, I do not accept this.
453. Adair . As mentioned above, in the annexes to his reports, Dr Riechmann cited passages in the Adair patent, which sets out a protocol for humanisation. Prof Martin advanced two criticisms of Dr Riechmann’s analysis which are maintained by Genentech.
454. The first involved residues H71, H73 and H78. Prof Martin pointed out that the Adair patent suggests should either all three should be mutated, or none. On this basis, since Absolute back-mutated H71, they should have back mutated H73 and H78 as well, alternatively they should not have back mutated H71.
455. This criticism assumes, however, that the skilled person would follow the protocol in the Adair patent rigidly and without modification. Dr Riechmann pointed out, however, that in the Adair paper the authors had mutated H78 and not H71 and H73. Prof Martin’s interpretation of the paper was that what the authors had done was first to mutate all three and then try only mutating H78. Even if that is the correct reading of the paper, however, the upshot is the same, which is that the skilled reader would appreciate that it may not be necessary to back mutate all three residues, consistently with the general principle that one should do the minimum. The same thinking would justify back-mutating only H71 (rather than only H78). Accordingly, I conclude that an obvious possibility would be for the skilled person to be guided by the message of the Adair paper, rather than blindly following the protocol in the Adair patent, and hence to choose only to back mutate H71.
456. The second criticism is similar. This is that the protocol in the Adair patent suggests back mutating residues H23, H24 and H49, but Absolute did not mutate H49. In my view the answer is the same, namely that, if it was possible to achieve satisfactory results without mutating a particular residue, then the skilled person would not do so. They would not blindly follow the protocol regardless of whether all the suggested mutations were necessary.
457. Furthermore, as counsel for Lilly pointed out, these two criticisms do not apply to two of the humanised mAb5 antibodies and two of the humanised mAb25 antibodies (in the case of the first point) and to the mAb16 antibodies (in the case of the second point). That leaves two humanised mAb5 and two humanised mAb25 antibodies to which neither point applies.
458. Carter . Again, Prof Martin advanced two criticisms of Dr Riechmann’s analysis. The first was a rather complicated point the essence of which was that Absolute had not mutated various residues suggested in the Carter patent. As I understand it, this analysis depends on the point about which definitions of the CDRs are used. In any event, however, Prof Martin accepted that the Carter patent teaches the skilled person to make “at least one” of certain mutations. Furthermore, I understood him to accept Carter itself did not make some of the mutations which he criticised Absolute for making. Again, I conclude that, applying the conservative approach of doing the minimum, the skilled person would appreciate that it was not necessary to make these mutations if they could get satisfactory results without doing so.
459. Secondly, Prof Martin pointed out that Dr Riechmann’s analysis did not justify the mutations made by Absolute to residues H1, H61 and L1, although Prof Martin acknowledged that H61 could have been justified. This point appears to be a sound one. As counsel for Lilly pointed out, however, it only affects the humanised mAb5 antibodies (in relation to H1) and two of the humanised mAb25 antibodies (H61) tested in the repeat experiments. Thus it does not apply to two of the humanised mAb25 antibodies or any of the humanised mAb16 ones.
460. Finally, I should note that it was common ground between Dr Riechmann and Prof Martin that under the Carter approach the use of modelling was optional.
461. Queen . The Queen method involves considering the proximity of the framework residues (FRs), which are different between the donor and acceptor sequences, to the CDRs measured in Ångstrom units. The proximity is determined by measuring the distance between all atoms of a FR residue of interest and all atoms of the CDR residues. If a pair of FR and CDR residues is within a certain distance, it is inferred that they interact with one another and that the FR residue provides structural support to the CDR. Alternatively, FR residues in close proximity to the CDRs may make direct contact with the antigen and contribute. Queen suggests applying a distance criterion of “about” 3Å, and Dr Riechmann chose 3.5Å.
462. As the structure of the murine antibody to be humanised is typically not known, this proximity analysis relies upon known structures of other antibodies deposited in the Protein Bank Database (PDB) established in 1971. A common method is that of homology modelling, in which a computer program is used to predict the position of each of the residues in the murine (donor) antibody by comparing the sequence of the donor antibody to other antibodies in the PDB and identifying those that are most homologous. Alternatively, the PDB can be searched for antibodies of known structure which are homologous to the murine donor and preferably have equivalent CDR lengths to the murine donor. Distances between the atoms in the models of the predicted or known structure are then calculated.
463. In order to apply the distance criterion suggested in Queen, Dr Riechmann relied upon models created by Prof Lesk. Prof Lesk noted in his first report that Phyre 2 was not available in 2003, but he said he was not aware of a pre-2003 version of a homology modelling program that was still available for use today. Accordingly, he also used Phyre 2 . In cross-examination, however, he accepted the statement put on instructions from Prof Martin that there were pre-2003 versions of three programs that could have been used (although it appears from a subsequent answer of Prof Martin’s that only one, Modeller, may have been available).
464. Prof Lesk generated two homology models for each of the three antibodies (mAbs 5, 16 and 25). His “High Rank” models used the pre-priority structures ranked highest by Phyre 2 . Given that the ranking algorithm in Phyre 2 uses post-priority methods, he also created “Low Rank” models based on the structure ranked in the top five by Phyre 2 which he assessed as being most different from the highest ranked structure. As Prof Martin pointed out, however, neither was something that the skilled person would have produced in 2003. Prof Lesk accepted that the result was that his models were contaminated by post-2003 software and data.
465. Furthermore, as Prof Martin also pointed out in his second report (served on 11 December 2018), Prof Lesk’s models generated numerous distances that were less than the van der Waals radii of the two atoms in question. That meant that the atoms were effectively superimposed in the models, which was not realistic and meant that the models could not reliably be used in a distance criterion approach. Prof Lesk said that some of the apparent clashes would properly be regarded as short hydrogen bonds, rather than true clashes, but did not suggest that this applied to all of the clashes identified by Prof Martin.
466. Although Prof Lesk admitted in paragraph 9 of his fourth report (served on 20 January 2019) that he was aware of this problem when he created his models, and he was also aware that Dr Riechmann was relying on the distances they generated, it appears that he did not mention the problem to anyone on Lilly’s side at that time.
467. In paragraph 13 of his fourth report he accepted that his models were “unrealistic” and “incorrect” (and in cross-examination he volunteered a further adjective, “terrible”).
468. Even then Prof Lesk sought to underplay the defects, saying (wrongly) that they were limited to inter-domain clashes and clashes between side-chains, and that the differences between his incorrect models and correct ones were minor, when many clashes in his models were greater than 1 Å and several greater than 2 Å.
469. He also said that it would have been possible for the skilled person in 2003 to have removed sidechain clashes by using software. But, as counsel for Genentech submitted, that just goes to highlight the difference between the models he had created and those which the skilled person in 2003 would have created.
470. Prof Lesk attempted to defend his models on the basis that they provided a qualitative (rather than quantitative) measure of the distances. As Prof Martin explained, however, that is simply not how the models were used by Dr Riechmann. As noted above, Dr Riechmann used them to apply a distance criterion, which meant that errors in the models would move residues in or out of consideration for back mutation. In the context of a cut-off of 3.5 Å, the errors in the models, many of which are over 1 Å and several of which are over 2 Å, are very large.
471. Counsel for Genentech submitted that Prof Lesk’s models could not be relied on for the purposes of applying a distance criterion as it would have been applied in July 2003. I accept that submission.
472. Conclusion . Overall, I conclude that, for the majority of the humanised antibodies produced by Absolute, the choices of residues to back mutate can be justified by reference to the Adair and/or Carter methods, albeit not by reference to the Queen method. It follows that the majority of the back mutations are ones that that the skilled team could have chosen to make applying two obvious methods. In the case of the remainder, the evidence does not establish that the skilled team could have chosen to make them, but nor does it establish that the back mutations are ones that the skilled team could not have chosen to make.
The affinities of the humanised antibodies
473. As well as criticising the methods used in Lilly’s experiments, Genentech relies upon the end results, in terms of the affinities of the humanised antibodies, as indicating that there was something unusual about them.
474. The humanised versions have affinities for IL-17A/A as measured by SPR which are 3-10 fold (for mAb 5), 3-7 fold (for mAb 25) and 4-12 fold (for mAb 16) greater than the affinities of their murine parents. Prof Martin said that in his experience it was highly unusual to see such a consistent and high level of improvement in affinity. He accepted that an increase of three-fold would not be surprising, but said that the increases found in this case would not be expected. He acknowledged that it might happen very occasionally, however. Dr Riechmann’s experience was any increase was highly unusual, although he noted that the Carter paper claimed a three-fold increase.
475. As Dr Tite explained, however, the SPR data for the murine and humanised antibodies are not strictly comparable because of the use of a different capture antibody for SPR as between the murine and humanised species. He accepted that the results indicated that there was an increase, but said that the use of different reagents meant that it was not possible to make a direct quantitative comparison.
476. Counsel for Lilly asked Prof Carr about the possible effect of using a polyclonal murine capture antibody of the kind sold by GE Healthcare Life Sciences as a standard reagent versus a monoclonal human capture antibody. Prof Carr did not know exactly how the polyclonal capture antibody would bind, but said that the reagent would not be fit for purpose if it affected the binding of the captured antibody. In his opinion this did not explain the differences in the SPR data. As he acknowledged, however, in order definitively to answer the question, further experiments would be required.
477. As Dr Tite pointed out, there is another set of experimental data which sheds light on this question, namely chimera ELISA data generated by Absolute which was annexed to Lilly’s Notice of Experiments as part of the work-up material they were required to disclose. The chimera has all of its variable region from the mouse and all of its constant region from a human source, so can be compared to humanised antibodies because the same anti-human detection antibody is used (unlike when comparing to the original murine antibody, which must use an anti-murine detection antibody). The data indicates that the humanised antibodies bound slightly less well than the chimera.
478. Counsel for Genentech submitted that Lilly were not entitled to rely upon the results of the chimera ELISA experiments because they were not the subject of any request for admissions in the Notice of Experiments. In my judgment Lilly should be permitted to rely upon these results for the following reasons. First, the party seeking to make a quantitative comparison between the SPR results is Genentech, not Lilly, but Genentech did no experiment in reply on this point. Secondly, although Genentech served a response to the Notice of Experiments, it did not notify Lilly that it intended to take a point based on a quantitative comparison between the SPR results until it served its expert evidence in reply. Had Genentech raised the point sooner, Lilly could have included the chimera as a benchmark in the witnessed SPR repeats. Thirdly, the chimera ELISA experiments were disclosed with the Notice of Experiments and thus will not have taken Genentech by surprise when Dr Tite referred to them.
479. Counsel for Genentech also pointed out that the chimera ELISA data had not been put to Prof Carr or Prof Martin. Neither of them were immunologists, however, unlike Dr Tite. In any event, this does not detract from the point that the chimera could have been used as a benchmark. Furthermore, the results of the chimera ELISA experiments are consistent with the ELISA and bioassay data included in Lilly’s Notice of Experiments, which Prof Martin was asked about. As he accepted, the latter are more consistent with what one would expect. Prof Martin made the point that SPR is the gold standard, and that the SPR results suggested that something different was happening. His conclusion was that it was “just puzzling”.
480. Consistently with that conclusion, Prof Martin did not say, nor was it put to either Dr Tite or Dr Riechmann, that the higher SPR affinities recorded for the humanised antibodies than for the murine equivalents demonstrated that something had gone wrong with the humanisation process. The fact that they appear to be unusually good in this respect is not enough. It is possible that that is an artefact of the SPR experiments. This is particularly so given the evidence to which I have already referred that the skilled team would expect to be able to produce humanised antibodies with acceptable binding to and inhibition of IL-17A/F.
481. Thus I conclude that the majority of the humanised antibodies produced in Lilly’s experiments are representative of what the skilled team would have produced implementing the IL-17A/A prior art using obvious methods. In the case of the remainder it is uncertain whether they are representative or not.
Inevitability of binding to and inhibition of IL-17A/F as well as IL-17A/A
482. As I have explained, it is Lilly’s case that it is inevitable that antibodies to IL-17A/A produced in accordance with the prior art will also bind to and inhibit IL-17A/F. As well as attacking Lilly’s experiments, Genentech relies upon the evidence of Prof Carr as showing that this is not inevitable, but on the contrary it is probable that some IL-17A/A antibodies will not bind to IL-17A/F at all and some may not inhibit IL-17A/F even if they bind. If I am right in my conclusions so far, it is not strictly necessarily for me to address this issue. For reasons that will appear, however, I consider that the evidence on it reinforces the conclusions that the claims in dispute are not lacking in novelty, but are obvious.
483. It may be helpful if I make two observations at the outset. The first is that the parties’ cases on this issue were to some extent like the proverbial ships sailing in the night. As explained above, Prof Carr approached the matter as a structural biologist and not as an immunologist. By contrast, Dr Tite approached it as an immunologist and not as a structural biologist. Consistently with that approach to the evidence, Genentech contends that it is the structural analysis that matters, while Lilly contends that it is the immunological considerations which matter.
484. The second is that Lilly rely heavily upon the fact that a considerable number of antibodies have been reported which bind to and inhibit IL-17A/F as well as IL-17A/A, but none that only bind IL-17A/A. In particular, there is no suggestion that Genentech has found one, despite working in this field for many years. Genentech contends that this does not prove that such antibodies cannot exist.
485. These observations lead me to a threshold submission advanced by counsel for Lilly with respect to this part of the case. He pointed out that, as I shall explain in more detail below, Prof Carr had not been instructed to consider what the skilled person would in fact have achieved by working the prior art. Rather, he had been instructed to consider the matter from a theoretical perspective based on current knowledge. Counsel for Lilly submitted that this was the wrong question, of purely academic interest and of no assistance to the Court. I disagree. Prof Carr’s evidence was directed to the issue of inevitability. For that purpose, it was appropriate for Genentech to instruct him in the manner it did.
486. Structures of IL-17A/A, IL-17F/F and IL-17F/F and binding to receptors . Prof Carr was instructed by Genentech to explain what was known today about the structures of IL-17A/A, IL-17F/F and IL-17A/F and their interactions with their receptors based on public available information. He set this out in paragraphs 37-96 of his first report. Prof Lesk agreed with this part of Prof Carr’s report, and thus there is no dispute as to its accuracy. Prof Carr’s evidence may be summarised as follows.
487. There are five key papers which report on the structures of IL-17A/A, IL-17F/F and IL-17A/F, either alone or bound either to IL-17RA or to an antibody fragment, as determined by X-ray crystallography, although certain residues are missing in these structures, either because of disorder in the crystal at those locations or because those parts of the structure are so flexible that crystals could only be obtained by using a protein missing the flexible residues :
i) Hymowitz et al , “IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding”, EMBO J , 19, 5332-5341 (2001) disclosed the structure of Il-17F/F;
ii) Ely et al , “Structural basis of receptor sharing by interleukin 17 cytokines”, Nature Immunol , 10, 1245-1252 (2009) published a structure of IL-17F/F bound to IL-17RA;
iii) Gerhardt et al , “Structure of IL-17A in complex with a potent, fully human neutralizing antibody”, J Mol Biol , 321, 851-862 (2009) published a structure of IL-17A/A homodimer bound by an inhibitory Fab fragment;
iv) Liu et al , “Crystal structures of interleukin 17A and its complex with IL-17 receptor A”, Nature Comm , 4, 1-9 (2013) published a structure of free IL-17A/A and a structure of IL-17A/A bound to IL-17RA; and
v) Goepfert et al , “The human IL-17A/F heterodimer: a two-faced cytokine with unique receptor recognition properties”, Nature Sci Rep , 7, 1013 (2017) published the IL-17A/F structure and a structure of IL-17A/F bound to IL-17RA.
488. As noted above, IL-17A/A and IL-17F/F have about 50% amino acid identity. IL-17A/A, IL-17F/F and IL-17A/F share a similar overall structure – a dimer adopting a so-called “cystine knot” fold with two pairs of antiparallel β strands and disulphide bonds connecting the strands together. The dimers are tightly associated with large buried contact surfaces between the monomers – these buried residues are more highly conserved between IL-17A and IL-17F. The structures have been described as resembling a “garment”, with “sleeves” formed of strands 1 and 2 of each chain, “a collar” formed by the cystine knot disulphide region, a “body” formed of part of strands 3 and 4 and part of the N-terminal extension and a “skirt” region formed of the three-stranded sheets (involving strand 0 and part of strands 3 and 4). These areas are shown below on the structure of the A/F heterodimer (taken from Goepfert):
489. This diagram also shows regions of the A/F heterodimer that show conformational differences from the respective subunits in the homodimers (in red for the A subunit and in orange for the F subunit). These differences can be seen in more detail in Figure 7 from Prof Carr’s first report (reproduced below: note that the cytokine is shown the other way up, with the C-terminal “collar” region at the bottom, and that the images show the same structure rotated in the manner shown by the arrows). This shows the conformational differences between IL-17A/F (A subunit in red, F subunit in blue) and IL-17A/A (grey and pink), as well as the fact that the distance between the subunits is reduced in IL-17A/F.
490. In addition to these structural differences, there are also differences between IL-17A/F and IL-17A/A / IL-17F/F arising from the differences in sequences between the A subunit and the F subunit. These are shown by Prof Carr in Figure 14 of his first report (this is not a part that Prof Lesk agreed with, but I do not understand there to be any dispute about the accuracy of these images, although Dr Tite made the point that the central image shows the F “face” whereas the A face is more heavily conserved as between IL-17A/A and IL-17A/F):

491. This shows IL-17A/F, with residues that are identical in the sequences of IL-17A and IL-17F shown in white (A) or grey (F), residues that are similar in IL-17A and IL-17F shown in pink (A) or cyan (F) and residues that are dissimilar in IL-17A and IL-17F shown in red (A) or blue (F). The insets show the A subunit in yellow and the F subunit in green to assist in identification.
492. As mentioned above, structures of each of IL-17F/F, IL-17A/A and IL-17A/F bound to IL-17RA have been published. In each case three sites on each face of the cytokine at which it can interact with IL-17RA have been identified. On each face, site 1 is formed from the N-terminal skirt region and is primarily made up of residues from the first subunit, site 2 is made up of residues at the major interface between the two subunits and consists of a mixture of residues from each subunit, and site 3 is primarily formed at the cysteine knot region at the collar and is made up principally of residues from the second subunit. So all sites involve some residues from both subunits.
493. Of the various representations of the sites in the papers, perhaps the clearest is that of the sites in the IL-17F/F – IL-17RA complex (below, taken from Ely). Broadly the same locations of sites are found in the A/A and A/F – IL-17RA complexes, but there are differences between the sites in the three cytokines because of the different residues in the subunits and, in some cases, because of conformational differences.
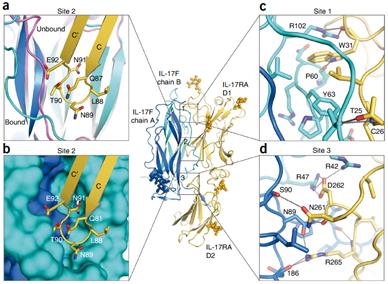
494. As mentioned above, each cytokine has two sets of interaction sites (one on each face) and can bind IL-17RA with either face. Once one IL-17RA is bound to the cytokine, the other face of the cytokine has far weaker binding affinity for another IL-17RA receptor.
495. Liu suggested that the reduced affinity for a second IL-17RA may be the result of an allosteric mechanism associated with the conformational changes that take place on binding to IL-17RA. These changes are illustrated in the figure below (from Liu) in which green and cyan represent the bound IL-17A/A subunits compared to the unbound subunits in grey. As can be seen there are substantial changes, particularly in the N-terminal half of the green subunit but also in the loops of the blue subunit.

496. Conformational changes between IL-17A/F in the free form and when bound to IL-17RA were also observed by Goepfert, as shown in the figure below in which bound IL-17A/F is shown in blue and free IL-17A/F in red (A subunit) and orange (F subunit). Goepfert concluded that an allosteric mechanism must be responsible for the binding of only a single IL-17RA to IL-17A/F, though the mechanism may be more subtle than that suggested by Liu for IL-17A/A.
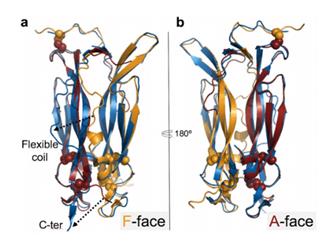
497. It is also known that a second receptor (IL-17RC) also needs to be bound to induce signalling, but no structure of any of the dimers bound to IL-17RC has yet been published. Accordingly, the precise manner in which IL-17 complexes binds to IL-17RC is unknown and cannot reliably be predicted.
498. Antibodies that bind to and inhibit IL-17A/A . It is common ground that there are two possible ways in which antibodies which bind to and inhibit IL-17A/A may operate. They may physically obstruct the interaction between the cytokine and either receptor (IL-17RA or IL-17RC) – so-called orthosteric inhibition. Or they may induce a conformational change in the cytokine which prevents binding of either receptor, or prevent a conformational change in the cytokine which is needed for binding either receptor – so-called allosteric inhibition.
499. Prof Carr expressed the opinion in his reports that, because of the nature of the IL-17A/A - IL-17RA binding process, it was likely that some antibodies will inhibit IL-17A/A by an allosteric mechanism and that antibodies which did so may well not inhibit IL-17A/F. Furthermore, he expressed the opinion that even antibodies which inhibit IL-17A/A by an orthosteric mechanism may not bind to and/or inhibit IL-17A/F. In his view some antibodies which bind IL-17A/A across the extensive dimer interface and therefore recognise parts of each subunit would be expected not to bind IL-17A/F. This is because of the different residues in, and/or the conformational differences between, the two cytokines. In general, a single residue difference in an epitope can destroy (or at least significantly reduce) antibody binding. Similarly, antibodies which bind to part of a single IL-17A or IL-17F subunit which adopts a different conformation in IL-17A/F may not be able to bind the heterodimer due to those conformational differences. Furthermore, antibodies which can bind only to the A face of IL-17A/F may not be sufficient to inhibit binding to IL-17RA via the F face and inducing signalling (whereas such antibodies would bind and block both faces of IL-17A/A).
500. Dr Tite expressed the opinion in his reports that antibodies inhibited IL-17A/A orthosterically and would bind to and inhibit IL-17A/F. He suggested that an inhibitory antibody to IL-17A/A would have to bind to epitopes in or close to the regions important for interaction with the receptor and that these regions are either completely conserved in, or highly homologous to, the corresponding regions in IL-17A/F. He also suggested that any difference in the epitopes will be tolerated because the affinity of the IL-17RA receptor for IL-17A/A is over 10 times its affinity for IL-17/F.
501. This dispute led to extensive cross-examination of the experts, and the resulting evidence was analysed at some length in the parties’ closing submissions. It is not necessary for me to consider the various complicated points that were discussed, however, because the bottom line is that Dr Tite accepted that, based on the available evidence about the structures of the IL-17s and their binding to the receptors, it was theoretically possible for there to be antibodies which bound to and inhibited IL-17A/A but not IL-17A/F. As he pointed out, however, this begs the question why no such IL-17A/A-only antibody has yet been reported. In his view, there were immunological reasons why such antibodies might not actually be produced.
502. For his part, Prof Carr acknowledged that there were at least two areas of uncertainty as to how the antibodies would bind. First, he accepted that the missing information about the A face from the available structure of free IL-17A meant that it was difficult to make predictions about whether antibodies would bind to the homodimer or the heterodimer around the loop region. Secondly, Prof Carr’s response to Dr Tite’s point about the 10-fold higher affinity of the IL-17RA receptor was to point out that it might be the case that the antibody initially bound to the IL-17RC receptor, in which case the affinity would be the same. He also pointed out that it might depend on the concentration of antibody present. It follows in my view that Prof Carr’s theoretical analysis cannot be taken to be conclusive as to what actually happens in practice.
503. Furthermore, when Prof Carr was asked what proportion of antibodies he estimated would bind to IL-17A/A, but not IL-17A/F, he said that it was speculation, but he estimated “may be between 1 in 5, 1 in 25, something like that”. As he accepted, that estimate was based on the top third of the molecule where the most diverse surfaces were, and the ratio of dual binders to sole binders would go up once the whole molecule was considered. It is not clear to me from the transcript whether he also agreed that the ratio would be increased by the functional issues raised by Dr Tite, and I will assume he did not. Even on that basis, it is clear that the great majority of antibodies would bind to both IL-17A/A and IL-17A/F.
504. Immunodominance . Immunodominance is the result of affinity maturation and somatic hypermutation. It accounts for the clustering of epitopes found in the real world: although epitopes could in theory be found anywhere on the surface of a molecule, in fact the immune system will favour antibodies binding at particular sites. So among all the various epitopes which are theoretically possible, in practice the immunisation may be driven to a subset.
505. Moreover, the inefficient fusion step in the hybridoma technique means that “rare” antibodies to the more obscure parts of the molecule may not result from a standard monoclonal antibody generation approach in 2003.
506. Dr Tite accepted that he could not point to any evidence that immunodominance was actually occurring in the present situation, but maintained that it was a potential factor which might explain the behaviour of the real world antibodies that had been reported. For his part Prof Carr accepted that this was beyond his expertise.
507. Immune self-tolerance . A related manifestation is the phenomenon of self-tolerance, whereby antibodies are unlikely to be raised to parts of a human molecule which look similar to the mouse’s own native protein. Dr Tite thought there was some evidence of self-tolerance in the present context in the epitope clustering evidence discussed below, but his main point was that there was no reason to think that IL-17 was exempt from this phenomenon.
508. Prof Carr agreed that one would expect clustering of epitopes if there were regions of the molecule which were identical between human and mouse and that there are areas of the respective human and mouse IL-17A proteins which are the same as one another. He accepted that this suggested that one was likely to get epitope clustering in this case, but said that he expected the antibodies to go round the whole molecule.
509. Epitope clustering . Dr Tite attempted to analyse the information which is available concerning IL-17A antibodies so as to map their epitopes in exhibit JT44 to his second report. Taken at face value, this appears to show that the epitopes all overlap with one or more of the three binding sites for the IL-17RA receptor. Although Dr Tite accepted that there were some flaws in the exercise, he maintained that it appeared to show that the epitopes were clustered in what he called “hotspots” of diversity between the murine and human, whereas there were other areas that were relatively conserved between the two species. Prof Carr accepted that the evidence from the epitope mapping exercise was consistent with epitope clustering, but considered that it did not provide any positive evidence of it, particularly once one took into account the small size of the sample given that some of the antibodies had been generated by phage display rather than mouse immunisation.
510. The known antibodies . The upshot regarding immunodominance, immune self-tolerance and epitope clustering is that there is little positive evidence that these are playing a role, but they are potential explanations as to why antibodies that are theoretically possible from a structural point of view may not be produced in practice.
511. Dr Tite searched for antibodies that bind to IL-17A/A, but not to IL-17A/F, but found none. His searches turned up the following murine, humanised and human antibodies which bind to and inhibit IL-17A/A and which also bind to IL-17A/F (many of which also inhibit IL-17A/A, although some have not been tested):
i) mAbs 5, 16 and 16 and their humanised variants discussed above;
ii) ixekizumab;
iii) secukinumab, an antibody marketed by Novartis under the trade mark Cosentyx for the treatment of psoriasis and other conditions;
iv) A2027F/c631 and A2031F/c632 disclosed in International Patent Application No WO 2009/082624 in the name of Zymogenetics Inc;
v) OREG 203, 207 and 210 disclosed in International Patent Application No WO 2014/001356 in the name of Orega Biotech (although OREG 203 and 207 have the same CDRs and so are not independent examples);
vi) mAb6785/Fab6468 disclosed in US Patent No 8,519,107 in the name of Janssen Biotech Inc;
vii) XAB1 disclosed in International Patent Application No WO 2014/122613 in the name of Novartis AG;
viii) CA 028_496 disclosed in US Patent No 8,679,494 in the name of UCB Pharma SA; and
ix) 14 antibodies disclosed in US Patent No 8,779,101 in the name of AbbVie Inc.
512. In addition, there is confidential evidence concerning the following:
i) two murine antibodies produced by eBioscience, one of which binds to IL-17A/F and one which may or may not bind; and
ii) five murine antibodies produced by Genentech, all of which bind to IL-17A/F but whose inhibitory effect has not been determined.
513. Thus there are 33 known antibodies that bind to both IL-17A/A and IL-17A/F, and no known antibodies that bind to IL-17A/A only.
514. Genentech points out that Lilly do not contend that ixekizumab is representative of antibodies that would be produced by the skilled person following the prior art in 2003. That is true, but irrelevant for present purposes just as it is irrelevant that Prof Carr relied upon post-July 2003 information for his analysis. It remains the case that ixekizumab is an IL-17A/A antibody which binds to IL-17A/F (which is why it is alleged to infringe).
515. Genentech contends that some of these antibodies may have been selected for their ability to bind IL-17A/F as well as IL-17A/A. Except in the case of the AbbVie antibodies, there is no positive evidence that this was the case, however. In any event, the fact remains that they bind to both.
516. Genentech points out that mAb6785/Fab6468 was raised against a mutant IL-17A/A and not native IL-17A/A. Genentech says that an antibody raised to native IL-17A/A might not have bound to IL-17A/F, but there is no evidence of any such antibody.
517. Conclusion on inevitability . As stated above, the overall conclusion I draw on this part of the case is that Lilly have not established that it is inevitable that anti-IL-17A/A antibodies also bind to IL-17A/F. In my judgment the evidence shows, however, that it is highly probable that such antibodies will also bind to and inhibit IL-17A/F.
Conclusion on obviousness
518. Accordingly, it is highly probable that a skilled team implementing the IL-17A/A prior art by obvious methods in July 2003 would produce an antibody that also bound to and inhibited IL-17A/F. Thus claim 1 is obvious.
519. Genentech contends that, even if claim 1 is obvious, claim 2 is not obvious, in particular in respect of the higher affinities specified. Genentech argues that the skilled team would aim for an antibody with an affinity to IL-17A/A in the range of 10-100 pM. Even if they succeeded in making an antibody which bound to IL-17A/A with an affinity of 10 pM which also bound to IL-17A/F, it would bind to IL-17A/F with lower affinity, and hence with an affinity lower (i.e. worse) than 10 -10 M. In my judgment this is again a point which is good against lack of novelty, but not against obviousness. It is clear from the evidence of Dr Tite and Prof Martin that would be obvious to aim for the highest possible affinity for IL-17A/A. Although the evidence shows that the affinity to IL-17A/F would tend to be lower, it would remain obvious to obtain antibodies with the affinities specified. There is no evidence that this could not be achieved or would require special, non-obvious techniques. (If there was, claim 2 would be insufficient because the Patent does not disclose any such techniques.)
520. In the draft of this judgment which I circulated to the parties before it was handed down, I stated that I did not understand there to be any dispute that, if claims 1 and 2 were obvious over the IL-17A/A prior art, then so too were claims 13, 14 and 15, and claims 12, 20 and 22 in so far as they are directed to RA (or at least, in the case of claims 12, 20 and 22, obvious over WO717 and JP046). Counsel for Genentech informed me that this was incorrect, and that Genentech relied upon the conditional amendments to claims 13, 14 and 20 to incorporate the language “[for use] as an antagonist of the IL-17A/F heterodimeric complex” or “[for] antagonizing the IL-17A/F heterodimeric complex” as an answer to Lilly’s case that those claims were obvious. As counsel for Lilly pointed out in response, however, Genentech’s submissions at trial on this point were only directed to Lilly’s lack of novelty case. Be that as it may, I do not consider that the new integer added by the conditional amendments does provide an answer to the obviousness case because the antibodies which I have found it would be obvious to make would satisfy this requirement.
521. Accordingly, I conclude that claims 1, 2, 13, 14 and 15, and claims 12, 20 and 22 in so far as they are directed to RA, are obvious over the IL-17A/A prior art.
Insufficiency: plausibility of the psoriasis claims
522. As is common ground, in order for claims 12, 20 and 22 to be valid in so far as they are directed to psoriasis, it must have been plausible to the skilled dermatologist reading the Patent in July 2003 in the light of the common general knowledge that an anti-IL-17A/F antibody would have some therapeutic efficacy for treating psoriasis. If not, the claims will be insufficient. (Lilly contend that they would be obvious as well, but I consider that in the present case the objection is more conveniently analysed as one of insufficiency.)
The law
523. The law has recently been considered by the Supreme Court in Warner-Lambert Co LLC v Generics (UK) Ltd [2018] UKSC 56. The Court divided 3:2 on this issue. The judgment of the majority was given by Lord Sumption. That case was concerned with a second medical use claim in Swiss form of a known pharmaceutical. The present case is concerned with a first medical use, given that the claimed antibodies were not known, although there are claims framed as second medical use claims both in Swiss form (purpose-limited process claims, namely claim 12 and 20) and in EPC2000 form (a purpose-limited product claim, namely claim 22). There is no dispute that the guidance given by Lord Sumption is applicable, although Genentech contends that it is necessary when applying it to bear in mind the different context. I accept that.
525. At [19]-[20] Lord Sumption noted that the problem with interpreting the requirement of sufficiency in the context of a second medical use claim as merely requiring the disclosure of the new purpose was that “it would enable a patent to be obtained on a wholly speculative basis”. Importantly for the present context, he said at [22]:
“The Court of Appeal's reference to ‘armchair inventors’ suggests that what they meant by speculative claiming was claiming by persons who had done nothing new or inventive at all but had simply sought to patent abstract possibilities. That may well be a particular risk in the case of patents for new uses of known compounds, especially when they are commercially successful in their existing use. In reality, however, speculative claiming of this kind is simply one of a number of ways in which a patentee may attempt to claim a monopoly more extensive than anything which is justified by his contribution to the art. Other ways in which this can happen include claiming a monopoly wider than the disclosure in the patent can support. An over-broad claim will not necessarily be speculative. The inventor may really have invented something corresponding to the full breadth of the claim. Research may subsequently demonstrate this. But the claim will still exceed his contribution to the art if that contribution is not sufficiently disclosed in the patent”
526. From [23]-[35] Lord Sumption reviewed the case law of the Boards of Appeal, where, as he explained, the concept of plausibility had originated “as a response to over-broad claims”.
527. At [36] Lord Sumption disagreed with the Court of Appeal’s statement of the effect of the plausibility test, saying:
“The principle is that the specification must disclose some reason for supposing that the implied assertion of efficacy in the claim is true. Plausibility is not a distinct condition of validity with a life of its own, but a standard against which that must be demonstrated. Its adoption is a mitigation of the principle in favour of patentability. It reflects the practical difficulty of demonstrating therapeutic efficacy to any higher standard at the stage when the patent application must in practice be made. The test is relatively undemanding. But it cannot be deprived of all meaning or reduced … to little more than a test of good faith.”
528. Lord Sumption went on at [37] (emphases and line breaks added):
“Plausibility is not a term of art, and its content is inevitably influenced by the legal context. In the present context, the following points should be made.
First , the proposition that a product is efficacious for the treatment of a given condition must be plausible.
Second , it is not made plausible by a bare assertion to that effect, and the disclosure of a mere possibility that it will work is no better than a bare assertion. ….
But, third , the claimed therapeutic effect may well be rendered plausible by a specification showing that something was worth trying for a reason, ie not just because there was an abstract possibility that it would work but because reasonable scientific grounds were disclosed for expecting that it might well work. The disclosure of those grounds marks the difference between a speculation and a contribution to the art. This is in substance what the Technical Board of Appeal has held in the context of article 56, when addressing the sufficiency of disclosure made in support of claims extending beyond the teaching of the patent. In my opinion, there is no reason to apply a lower standard of plausibility when the sufficiency of disclosure arises in the context of EPC articles 83 and 84 and their analogues in section 14 of the Patents Act. In both contexts, the test has the same purpose.
Fourth , although the disclosure need not definitively prove the assertion that the product works for the designated purpose, there must be something that would cause the skilled person to think that there was a reasonable prospect that the assertion would prove to be true.
Fifth , that reasonable prospect must be based on what the TBA in SALK (para 9) called ‘a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se.’
Sixth , in SALK , this point was made in the context of experimental data. But the effect on the disease process need not necessarily be demonstrated by experimental data. It can be demonstrated by a priori reasoning. For example, and it is no more than an example, the specification may point to some property of the product which would lead the skilled person to expect that it might well produce the claimed therapeutic effect; or to some unifying principle that relates the product or the proposed use to something else which would suggest as much to the skilled person.
Seventh , sufficiency is a characteristic of the disclosure, and these matters must appear from the patent. The disclosure may be supplemented or explained by the common general knowledge of the skilled person. But it is not enough that the patentee can prove that the product can reasonably be expected to work in the designated use, if the skilled person would not derive this from the teaching of the patent.”
529. At [40] Lord Sumption added:
“The question is not whether [the medicament] works but whether the contribution to the art consisting in the discovery that it can be expected to work has been sufficiently disclosed in the patent. The inherent difficulty of demonstrating this before clinical trials is taken into account in the modest standard (ie plausibility) which is applied to test it. … This does not mean that subsequent data is never admissible in a dispute about sufficiency, but the purpose for which it is admitted is strictly limited. Where the asserted therapeutic effect is plausible in the light of the disclosure in the patent, subsequent data may sometimes be admissible either to confirm that or else to refute a challenger's contention that it does not actually work… But it cannot be a substitute for sufficient disclosure in the specification.”
530. As counsel for Genentech pointed out, there is no reference in any of the judgments of the Supreme Court to the previous decision of the Supreme Court on plausibility in Human Genome Sciences Inc v Eli Lilly and Co [2011] UKSC 51, [2012] RPC 6 given just seven years previously, even though it was applied by the lower courts and even though it was cited in argument. The legal context of HGS was different in that the issue was that of industrial applicability. As Lord Sumption said, however, the fundamental principle is the same. Counsel for Genentech submitted that the test laid down in Warner-Lambert was the same as that in HGS , and that HGS was of assistance in applying that because, like the present case, it was concerned with a new member of a known family.
Assessment
532. It is important to be clear as to two points at the outset. First, Genentech does not rely upon any common general knowledge of the skilled dermatologist regarding IL-17. By contrast, it does rely upon their common general knowledge concerning IL-6 and IL-8. For their part, Lilly rely upon the common general knowledge of the skilled person concerning the other cytokines implicated in psoriasis, and in particular their knowledge regarding TNFα and IFN g . Secondly, it is common ground that, in considering the plausibility of the claims, the dermatologist would obtain and read the key papers cited in the Patent at [0015]-[0019], in particular the three papers cited at the end of [0019] as concerning the role of IL-17 in psoriasis, but also certain other papers. Accordingly, it is necessary to begin by considering what the skilled person would learn from those papers if he or she was not already aware of them.
533. Fossiez . Fossiez et al , “T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines”, J Exp Med , 183, 2593-2603 (1996) reported that IL-17 had no major effect on the proliferation, cytokine secretion (IFN-β, IL-4, IL-6, IL-10), phenotype (CD3, CD4, CD8), or cytotoxicity of CD4 + and CD8 + T cells, regardless of whether these cells had been activated with PHA, tetanus toxoid, or IL-2. By contrast, IL-17 induced the production of IL-6, IL-8, prostaglandin E2 (PGE2) and G-CSF by synovial fibroblasts and IL-6 by endothelial cells and epithelial cells. In addition, it was found that IL-17 and TNFα in combination induced synovial fibroblasts to produce GM-CSF, whereas neither did so on their own. The authors concluded:
“In conclusion, the induction of secretion by stromal cells of IL-6, IL-8, and PGE 2 but not of IL-1 or TNF, and the lack of detectable activity on monocytes suggest a limited proinflammatory role of IL-17 in T cell-driven inflammatory pathological processes such as psoriasis …”
534. Chabaud . Chaubaud et al , “Enhancing effect of IL-17 on IL-1-induced IL-6 and leukaemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation by Th2 cytokines”, J Immunol , 161, 409-414 (1998) reported synergy between IL-1β and IL-17 in the production of IL-6 by RA synoviocytes.
535. Prof Krueger pointed out that Chabaud is mis-cited in the Patent at [0015]. The Patent cites Chabaud in support of the statement that IL-17 “synergizes with other cytokines including TNFα and IL-1β to further induce chemokine expression”. Chabaud does not itself contain any data about synergism between IL-17 and TNFα, although it does report the finding in Fossiez concerning GM-CSF. GM-CSF is a haemopoetic cytokine which was not considered to play an important role in psoriasis pathogenesis. Moreover, Chabaud is concerned with IL-6, which is not a chemokine either. These are minor points, however.
536. Jovanovic . Jovanovic et al , “IL-17 stimulates the production and expression of proinflammatory cytokines, IL-β and TNF-α, by human macrophages”, J Immunol , 160, 3513 (1998) reported that IL-17 induced the production of IL-1β, TNFα, IL-6, IL-10, IL-12 and PGE2 from activated macrophages, contrary to the earlier findings of Fossiez. The skilled reader would be aware that macrophages are found in psoriasis lesions.
537. Teunissen . Teunissen et al , “Interleukin-17 and Interferon-γ synergize in the enhancement of proinflammatory cytokine production by human keratinocytes”, J Invest Dermatol , 111, 645-649 (1998) reports a study comparing IL-17 (i.e. IL-17A) and IFN g with regard to modulating cytokine production and cell-surface molecule expression of keratinocytes.
538. In the introduction the authors note that:
i) CD4 + T cells can be detected in early and fully developed psoriatic lesions;
ii) keratinocytes in these lesions exhibit unusual expression of MHC class II and ICAM-1, which are induced on keratinocytes by IFN g ; and
iii) IL-17 has been demonstrated to stimulate production of IL-6, IL-8 GM-CSF and PGE2 in epithelial, endothelial and fibroblastic cells and to induce ICAM-1 expression on fibroblasts.
539. The authors demonstrate that:
i) the dosing of normal human keratinocytes with IL-17 increased the expression of the pro-inflammatory cytokines IL-6 and IL-8, but not IL-1α or IL-15, and was synergistic in the presence of IFN g , leading to a 10-fold increase in IL-6 and IL-8 after co-stimulation;
ii) IL-17 weakly induced expression of ICAM-1 and human leukocyte antigen DR (HLA-DR), and had a slight additive effect with IFN g on the expression of ICAM-1;
iii) of 12 CD4 + and five CD8 + T cell clones derived from psoriatic skin, nine CD4 + and three CD8 + clones produced IL-17 mRNA when stimulated; and
iv) IL-17A mRNA was detected in skin biopsies from lesional skin using normal skin as a control.
540. The authors observe in the discussion that IL-17 appears to be working by a different intracellular signalling pathway to IFN g . Although IFNγ triggers a broader spectrum of activities in keratinocytes, IL-17 enhances the production of IL-6 and IL-8, which are thought to be important mediators of inflammation. Thus IL-17 can also be considered as a pro-inflammatory cytokine. The authors suggest that the synergy between IL-17 and IFN g “may probably” occur in vivo leading to vigorous production of IL-8 and IL-6 by the keratinocytes in lesional skin. The authors conclude that the results of the study suggest that skin-infiltrating T cells are able to produce IL-17 and that IL-17 might amplify the development or sustain chronic inflammatory responses in the skin through stimulation of normal human keratinocytes to increase the secretion of proinflammatory cytokines IL-6 and IL-8.
541. The authors report the activity of the IFN g used in their study by reference to the manufacturer’s units, not the international standard IU. Prof Krueger had personal knowledge about the conversion which enabled him to conclude that 10-fold higher concentrations of IL-17 than IFN g were used to produce the same response. As I think he accepted, the skilled reader without that knowledge would not be able to compare the concentrations used. Counsel for Genentech submitted that the skilled person would therefore take the results at face value. In my judgment the skilled person would appreciate that there was some uncertainty as to the true significance of the results so far as the comparison between IL-17 and IFN g was concerned, and hence as to the relative potencies of IL-17 and IFN g . Moreover, the skilled person would appreciate that it was not known if the same concentrations of IL-17 were present in vivo .
542. Prof Krueger accepted, however, that the skilled person would understand from Teunissen that there was evidence that IL-17 was produced in psoriasis lesions by T cells.
543. Albanesi . Albanesi et al , “Interleukin-17 is produced by both Th1 and Th2 lymphocytes and modulates interferon-γ- and interleukin-4-induced activation of human keratinocytes”, J Invest Dermatol , 115, 81-87 (2000) performed IL-17 ELISAs on CD4 + T-cells obtained from patients with allergic contact dermatitis (ACD) to nickel.
544. In the introduction the authors note that:
i) IL-17 is a pleiotropic cytokine active on a wide variety of cell types, in particular it stimulates macrophages, fibroblasts and endothelial and epithelial cells to release cytokines and prostaglandins and to express ICAM-1;
ii) the effects of IL-17 are potentiated by TNF a , IFN g and IL-1 b ;
iii) IL-17A has been detected in skin affected by ACD and psoriasis and is expressed by a portion of nickel-specific CD4 + cells;
iv) IL-17 exerts important regulatory effects on human keratinocytes by enhancing IFN g -induced ICAM-1 expression and regulating IL-8 and RANTES (another chemokine); and
v) T cell-driven keratinocyte activation plays a relevant role in the pathogenesis of chronic inflammatory skin disorders including ACD and psoriasis involving the infiltration of T lymphocytes and the production of cytokines.
i) about 50% of nickel-specific Th0, Th1 and Th2 clones released IL-17 on activation;
ii) IL-17 on its own and in combination with IL-4, and IL-4 and IFN g , decreased the ratio of IL-1R to IL-1 a , which is proinflammatory;
iii) IL-17 caused production of cytokines from keratinocytes including GM-CSF, IL-6, Gro- a and was synergistic with IFN g and IL-4; and
iv) IL-17 strongly increased IFN g -induced expression of ICAM-1 on keratinocytes.
546. The authors conclude that IL-17 appears to be deeply implicated in the amplification of ACD reactions.
547. Prof Krueger said in his first report that Albanesi taught the skilled person that IL-17 could be a cytokine relevant to skin inflammatory diseases through its effects on keratinocytes. He expressed the view, however, that the findings would be considered less relevant to psoriasis than to ACD because, to the extent that psoriasis was considered to be a T cell mediated disease, it was thought to be type 1 T cell rather than type 2 T cell mediated, whereas ACD was thought to be type 2 T cell mediated. Prof Prens replied that ACD was thought to be a type 1 T cell mediated disease, a point he was not challenged on. In cross-examination Prof Krueger modified his position and said that ACD involved a mixed response which depended on the genetic background of the animal in question and on whether one was focussing on delayed hypersensitivity or the allergic reaction and that both type 1 and type 2 T cells could be involved. He maintained that Albanesi’s findings would be considered less relevant to psoriasis, but re-iterated his acceptance that it supported the idea that IL-17 induced a limited range of cytokines in keratinocytes and that its effect may be augmented by its reactions with other cytokines. For his part, Prof Prens accepted that ACD differed from psoriasis in that it can often be cured by removing the source of the allergy whereas psoriasis cannot (although, as Prof Prens pointed out, psoriasis can be triggered by an allergic reaction).
548. In my judgment the skilled person would not dismiss Albanesi as irrelevant to psoriasis, and would conclude that it provided some support for the role of IL-17 in inflammatory skin diseases, but would not treat the detailed findings as directly applicable to psoriasis.
549. Homey . Homey et al , “Up-regulation of macrophage inflammatory protein-3a/CCL20 and CC chemokine receptor 6 in psoriasis”, J Immunol , 164, 6621-6632 (2000) reports that the chemokine CCL20 and its receptor CCR6 are significantly upregulated in psoriasis. From table 2 it can be seen that IL-17 increased CCL20 and was synergistic in this regard in the presence of TNFα.
550. Prof Krueger’s evidence was that the skilled person would understand from this paper that IL-17 could potentially contribute to T cell infiltration into psoriasis lesions by inducing CCL20, but that IL-17 was one of a number of inflammatory cytokines that had this effect.
551. Aggarwal 2002 . I have summarised this review in paragraph 243 above. Genentech relies upon it for the statement about IL-17 inducing IL-8 and Groα. In relation to the association between IL-17 and psoriasis, however, Aggarwal 2002 simply cites Teunissen, Albanesi and Homey. Thus it would add nothing to the skilled person’s understanding of IL-17’s role in psoriasis after reading those papers. It is convenient to note here that Lilly pleaded Aggarwal 2002 as prior art, to show that everything in the Patent about the role of IL-17 in psoriasis was in the prior art and thus to support a case that the Patent made no technical contribution with regard to psoriasis. Counsel for Lilly did not rely upon Aggarwal 2002 as founding a case of obviousness in closing submissions, however.
552. Aggarwal 2003 . Aggarwal, “Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17”, J Bio Chem , 278, 1910-1914 (2003) reports that IL-23 induced the production of IL-17 in a distinct T cell activation state. The authors suggest that IL-17 does not fit into the Th1/Th2 paradigm. The authors observe that IL-17 is known to promote recruitment of monocytes and neutrophils through induced chemokine production and the induction of ICAM thereby providing important co-stimulation of further T cell responses.
553. The skilled person’s perception of the potency and range of effects of IL - 17A . Prof Krueger’s evidence was that IL-17 (i.e. IL-17A) was considered to be, as he put it, a “wimpy” cytokine, meaning one of low potency, compared to TNFα and IFN g . There is no dispute that that was Prof Krueger’s perception, at least after July 2003: he stated as much in one of his slides in his December 2017 presentation. Moreover, he was not alone. Thus Mylle et al , “Targeting the IL-23/IL-17 pathway in psoriasis: the search for the good, the bad and the ugly”, J Clin Dermatol , 19, 625-637 (2018, “Mylle”) states (at page 628) that “the potency of IL-17 was long underestimated because its weak inflammatory effect in vitro, masking its true pro-inflammatory character in vivo”. Prof Prens did not agree that this was the perception of the skilled person in July 2003. For the reasons explained previously, it does not matter whether or not it was common general knowledge in July 2003. What matters is what the perception of the skilled person would have been after reading the papers discussed above. In my judgment, considering the evidence as a whole, the skilled person would have concluded that (a) there was some uncertainty as to IL-17’s potency in vitro and (b) it was not known what potency it had in vivo . I do not understand it to be in dispute that the skilled person would have considered IL-17 to have a narrower range of effects than either TNFα or IFNγ, and in any event that is my finding.
554. Examples 1 and 2 of the Patent. It is common ground that the skilled person would understand from Example 1 that IL-17A/F binds to the same receptor as IL-17A/A (i.e. IL-17R) and has the same function, but binds with lower affinity. Thus the skilled person would agree with the statement in the Patent at [0405] that IL-17A/F may compete with IL-17A/A for binding to IL-17R (although the Patent does not provide any evidence as to whether it does or not).
555. Prof Krueger made some minor criticisms of the experiments in Example 2 in his reports. Counsel for Genentech submitted that these criticisms had been undermined in cross-examination. It is not necessary for me to consider whether this is correct because counsel for Lilly did not rely upon these points in closing submissions. Nor were they of significance to Prof Krueger’s analysis of plausibility.
556. It is common ground that the Patent contains no experimental evidence demonstrating the presence of IL-17A/F in psoriasis lesions or otherwise indicating that IL-17A/F has a pathogenic role in psoriasis.
557. Prof Krueger’s evidence . Prof Krueger’s opinion was that the skilled reader of the Patent in July 2003 would not consider it plausible that an antibody to IL-17A/F would be efficacious for the treatment of psoriasis. Although he gave quite a lot of evidence on this topic, I think the main points can be summarised as follows.
558. First, the skilled person would note the very broad lists of conditions which the Patent claims can be treated with (inter alia) anti IL-17A/F antibodies. The diseases in the Patent’s laundry list include ones characterised by: (i) auto-antibodies produced by the abnormal activation of B cells; (ii) platelet destruction by auto-antibodies; (iii) cytotoxic T cell mediation; (iv) auto-antibody diseases that may be produced by structural defects in keratins or adhesion proteins in the skin; and (v) IgE antibody mediation. There is no unifying characteristic between these conditions beyond the fact that they involve the immune system in some way, and no unifying rationale or theory for regarding IL-17A/F as of pathogenic relevance to them. The skilled person would regard it as highly unlikely that targeting IL-17A/F would be beneficial in some of them and at best a guess for many. There was no challenge to this aspect of Prof Krueger’s evidence.
559. Secondly, the skilled person would note the teaching of the papers cited in the Patent at [0015]-[0019] and discussed above. In brief summary, the skilled person would note that there was evidence in the literature that IL-17A/A was produced in psoriasis lesions, that it induced the production of IL-6 and IL-8 and was synergistic with IFNγ in vitro , that there was some evidence that IL-6 played a role in inflammatory skin diseases, and that there was evidence of redundancy with respect to IL-17.
560. Thirdly, the skilled person would note the limited nature of the experimental evidence presented in Examples 1 and 2, and that there was no experimental evidence directed to the role or effect of IL-17A/F in psoriasis. In the absence of any evidence in the Patent, the skilled person would not assume that IL-17A/F was produced in psoriasis lesions. Even if the skilled person did make that assumption, they would have no idea what the relative concentrations of IL-17A/A and IL-17A/F were, and thus where the system was on the dose-response curves in Figure 5. (Indeed, this work has still not been done even now.)
561. Fourthly, the skilled person would not regard IL-17A/A as a candidate to take forward to a clinical trial, and would consider IL-17A/F as even less promising given that the data in the Patent showed that it was an order of magnitude less potent than IL-17A/A.
562. Prof Krueger summarised his opinion in paragraph 74 of his second report by reference to the considerations mentioned in Warner-Lambert as follows:
“…. I consider that the disclosure of the Patent amounts to no more than the disclosure of a mere possibility that an antibody which binds to and inhibits IL-17A/F (whether only or in addition to IL-17A/A and/or IL-17F/F) will work to treat psoriasis. The Patent discloses no reasonable scientific grounds for expecting that such an antibody might well work to treat psoriasis. There is nothing in the Patent that would cause the skilled person to think that there was a reasonable prospect that the assertion that such an antibody would treat psoriasis would prove to be true. The skilled person could not point to a direct effect of such an antibody on a metabolic mechanism specifically involved in psoriasis, this mechanism being either known from the prior art or demonstrated in the Patent itself. The Patent does not point to a property of such an antibody which would lead the skilled person to expect that it might well produce the claimed therapeutic effect of treating psoriasis nor any unifying principle that relates to such an antibody which would suggest such an effect to the skilled person. I do not consider that the skilled person can derive from the Patent that such an antibody can reasonably be expected to work to treat psoriasis.”
Prof Krueger maintained these views in cross-examination, describing the claims in the Patent at one point as “tenuous”.
563. Counsel for Genentech submitted that the teaching in the Patent at [0015]-[0019] was retrospectively vindicated by Prof Krueger’s December 2017 presentation. I do not accept this. It is true that Prof Krueger referred to the findings of Fossiez and Teunissen; but he identified the turning point as being the publication by Cua of two critical papers in 2003 and 2005 (the first of these is the paper discussed in paragraph 234 above) which led to the discovery of Th17 T cells as a distinct subset under IL-23 regulation mediated by IL-17A. It is this “IL-23/Th17 axis” or “pathway” (as Prof Krueger described it) that is targeted by ixekizumab.
564. Similarly, counsel for Genentech submitted that the teaching in the Patent was also retrospectively vindicated by Mylle, but Mylle’s analysis (at page 628) is entirely consistent with that of Prof Krueger in his presentation:
“Now, the IL-23/Th17 axis has been suggested as the main attributer for psoriatic disease. For instance, intradermal injection of IL-23 in mice induced erythema and induration, histopathologically resembling psoriasis [51]. IL-17A promotes the production of IL-6, IL-8, intercellular adhesion molecule (ICAM)-1 and granulocyte–macrophage colony-stimulating factor (GM-CSF) in keratinocytes, which strongly resembles the psoriatic phenotype found in humans. Finally, inhibition of IL-17A in humans consecutively resulted in reductions in hyperplasia and infiltration of the dermis and epidermis [52].”
References 51 and 52 were published in 2006 and 2012 (the latter is a paper co-authored by Prof Krueger).
565. Counsel for Genentech also sought to contrast Prof Krueger’s evidence concerning IL-17A/F with his evidence concerning two other therapeutic targets for psoriasis, namely CTLA4Ig and IFNγ.
566. It is convenient to take these in reverse order, since I have already briefly reviewed the skilled dermatologist’s common general knowledge concerning IFNγ in paragraph 232 above. As is reflected in the conclusion of Lowes quoted in paragraph 251 above, there was quite a lot of evidence for the role of IFNγ in psoriasis in July 2003 which is discussed in that review. For present purposes it is sufficient to quote one passage from the review (at pages 351-352):
“The current authors believe that IFN-γ is a pivotal cytokine in the development and maintenance of psoriatic lesions. Fig. 3 outlines a sequential pathway of type 1 T-cell activation, release of T-cell–derived cytokines, and production of several inflammatory mediators that the authors term the type 1 pathogenic pathway. IFN-γ is produced by effector memory CD8 + T cells, epidermal Tc1, CD4+ T cells, and NK and NK-T cells. Psoriatic CD8 + Tc1 cell lines and clones have been shown to produce heterogeneous levels of IFN-γ [15]. There is also evidence of the effects of IFN-γ at the tissue level in psoriatic lesions: keratinocytes show increased levels of HLA-DR, intercellular adhesion molecule-1 (ICAM-1) [27], and CD40 [28]; increased CXCR3 expression on lymphocytes [8]; and greater levels of keratinocyte-derived MIG and IP-10 [8]. Furthermore, this cytokine may also increase expression of costimulatory molecules on DCs [29]. IFN-γ potently activates macrophages and may also induce TNF-α release from monocytes and macrophages, which acts synergistically with IFN-γ in an inflammatory response [30]. Endothelial cells are also responsive to IFN-γ, up-regulating several adhesion molecules, such as ICAM-1 and vascular cell adhesion molecule-1 (VCAM-1), which facilitates the complex process of leukocyte trafficking into tissues. The sum of cytokines and chemokines made in response to IFN-γ and TNF-α (see Fig. 3) can explain many features of the pathogenic process: angiogenesis and vascular ectasia, T-cell and neutrophil emigration into lesions, and some components of the psoriatic epidermal response.”
References 8, 15, 27, 28 and 30 were published prior to 2003, while reference 29 appears to have been published in June 2003.
567. Prof Krueger accepted that the problem of redundancy did not put him off from considering IFNγ to be a therapeutic target. This is consistent with my finding as to the role of redundancy in the skilled person’s thinking set out above, namely that it was a factor to be taken into account but not determinative on its own.
568. Counsel for Genentech pointed out that there was no proof that IFNγ was going to be a clinically effective target in psoriasis, but that did not prevent it from being a plausible target. That I accept, but I do not consider that this detracts from Prof Krueger’s evidence regarding the lack of plausibility of targeting IL-17, and in particular IL-17A/F. There was simply more evidence that IFNγ had a pathogenic role in psoriasis at that stage.
569. Turning to CTLA4Ig, there is much less evidence about this in the case. Prof Krueger mentioned his work on CTLA4Ig in passing in his first report as an example of his collaborations with pharmaceutical companies (in that instance, Bristol-Meyers Squibb), and cited a paper he and others had published in 1999 (Abrams et al , “CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris”, J Clin Invest , 103, 1243-1252, “Abrams”)). Prof Krueger was asked a few questions about the work reported in this paper in cross-examination, but my attention was not drawn to any other evidence about CTLA4Ig.
570. As discussed above, there was already evidence at the time that suggested that activated T cells played an important role in triggering and perpetuating psoriasis. As Abrams explains, the B7 family of molecules on APCs regulate T-cell activation by delivering antigen-independent stimulatory signals through CD28 and inhibitory signals through CD152 (also known as CTLA-4). CTLA4Ig is a soluble chimeric protein consisting of the extracellular domain of human CTLA-4 and a fragment of the Fc portion of human IgG1. CTLA4Ig had been found to bind to B7-1 (CD80) and B7-2 (CD86) molecules on APCs and thereby block the CD28-mediated costimulatory signal for T cell activation. Biological activity of CTLA4Ig had been demonstrated in a variety of animal models of auto-immunity. In some animal models of auto-immunity, CTLA4Ig not only prevented the induction of an auto-immune process, but also suppressed disease activity late in the course of an established auto-immune response. In vitro experiments had shown that CTLA4Ig inhibited, in a dose-dependent fashion, the capacity of B7 molecules present on epidermal Langerhans’ cells and dermal dendritic cells to serve as costimulatory molecules for the proliferation of T cells in a primary immune response. Based on this previous work, Prof Krueger and his co-works carried out a Phase I open-label dose-escalation trial of CTLA4Ig in psoriasis patients.
571. Prof Krueger agreed that, prior to the trial, no work had been done with CTLA4Ig on psoriasis lesions or cells taken from psoriasis lesions. As he explained, however, the particular strategy that was being tested was one that was believed to be antigen-independent, in the sense that it did not really matter what the antigen was that was driving the T cell reaction. It was not put to Prof Krueger that this work was inconsistent with his evidence about IL-17. In any event, in my view it again does not detract from that evidence given what was known about CTLA4Ig and the rationale for the trial.
572. Prof Prens’ evidence . Prof Prens’ opinion was that the skilled person would regard it as plausible that IL-17A/F had a role in psoriasis and that an anti-IL-17A/F antibody would be beneficial in the treatment of psoriasis, although this would remain a hypothesis that needed further study. The basis for this opinion was what the skilled person would learn from reading the key papers cited in the Patent at [0015]-[0019], and in particular Teunissen, Albanesi, Homey and Aggarwal 2003, combined with the key teachings of the Patent that IL-17A/F stimulates the production of IL-6 and IL-8 with a potency intermediate between IL-17A and IL-17F and that IL-17A/F is produced by activated T cells.
573. A central aspect of Prof Prens’ reasoning on this question was his conception of the skilled person’s common general knowledge concerning IL-6 and IL-8 as being that they were thought to be key agents with a significant role in the pathogenesis of psoriasis. I have found, however, that this was not the case. IL-6 was simply thought to be one of a large number of cytokines potentially implicated in the pathogenesis of psoriasis whose role was uncertain and which might suffer from redundancy, and so there was little interest in targeting it. As a matter of common general knowledge, there was more interest in IL-8 as a potential therapeutic target; but the skilled person considering plausibility would do a literature search that would lead them to discover that Abgenix had discontinued trials of its anti-IL-8 antibody and to question IL-8 as a therapeutic target.
574. In addition, Prof Prens’ analysis also depended heavily on what the skilled person would extract from Teunissen, Albanesi, Homey and Aggarwal 2003. I have considered this question above.
575. Prof Kamradt’s evidence . Although Prof Kamradt was not in a position to speak to the plausibility of the claims from the perspective of a dermatologist, counsel for Lilly relied on his evidence by way of contrast to that of Prof Prens. Prof Kamradt said that “the two pillars of checking if something is a valid therapeutic target are … finding it overexpressed in the diseased tissue versus controls and finding a role in an animal model”. Neither of Prof Kamradt’s “pillars” is present in the Patent with respect to psoriasis.
576. Conclusion . In considering the question of plausibility, it is important to focus on the right question. As discussed above, claims 12, 20 and 22 are to be interpreted as requiring a discernible therapeutic effect on psoriasis. Accordingly, the question which must be considered is whether the skilled reader would consider it plausible that an IL-17A/F antibody would have such an effect. It would not be enough for the skilled person to conclude that IL-17A/F was a potential target for psoriasis therapy which was worthy of further research to find out whether an anti-IL-17A/F antibody was likely to be efficacious.
577. In my judgment the skilled person would not regard it as plausible that an anti-IL-17A/F antibody would have a discernible therapeutic effect on psoriasis for the reasons given by Prof Krueger. I would emphasise five points. First, the absence from the Patent of any experimental data concerning the role or effect of IL-17A/F, let alone an anti-IL-17A/F antibody, in psoriasis. Secondly, the absence of any discussion of the role or effect of IL-17A/F in psoriasis. Thirdly, the limited support for IL-17A/A (let alone IL-17A/F) having a pathogenic role in psoriasis provided by the papers cited in the Patent, particularly given the common general knowledge as to all the other cytokines which were implicated in psoriasis. Fourthly, the fact that the Patent shows that IL-17A/F is an order of magnitude less potent than IL-17A/A. Fifthly, the fact that the specification claims efficacy against a broad list of conditions which it is wholly implausible that an anti-IL-17A/F antibody (or any form of IL-17A/F therapy) would be effective against. Moreover, there is no emphasis on psoriasis in the specification. Such emphasis as there is concerns RA, which the skilled dermatologist would appreciate raised different considerations to psoriasis. In short, the claim of efficacy against psoriasis is speculative.
578. Given that I have concluded that plausibility has not been established on the basis of the Patent and the common general knowledge, later evidence is not admissible to demonstrate plausibility. Genentech did not in terms rely upon later evidence as supporting its case on plausibility, but did rely upon it in relation to infringement. Accordingly, I shall discuss the evidence relied on in that context. It is convenient to note here, however, that, so far as the evidence goes, no-one has made an antibody which binds to IL-17A/F, but not IL-17A/A, and tested its effect on psoriasis. As the Patent notes, it is theoretically possible that, by binding to IL-17A/F, such an antibody could remove a competitive antagonist to IL-17A/A, thus exacerbating IL-17A/A’s pro-inflammatory effects. Nor is there any evidence that blockade of IL-6 or IL-8 is therapeutically beneficial in psoriasis. Indeed, current evidence suggests otherwise. More generally, as Prof Krueger explained, there is later evidence that inhibition of IL-17A/F has failed as a treatment for three of the indications listed in the Patent, namely (i) chronic obstructive pulmonary disease, (ii) asthma and (iii) inflammatory bowel disease.
Insufficiency: other grounds
Ambiguity
579. Lilly contend that the claims are insufficient because the requirement “which specifically binds to” is ambiguous if it is not construed in the manner contended for by Lilly. The short answer to this is that is not ambiguous on Genentech’s construction, which I have accepted, either.
Undue burden
580. Lilly also contend that the claims are insufficient because it would be an undue burden on the skilled team to identify human or humanised antibodies that bind and inhibit IL-17A/F, not IL-17A/A (or IL-17F/F). Genentech contends that this insufficiency does not arise on its construction of the claims, since they are satisfied by antibodies that bind to IL-17A/F and IL-17A/A and there is no dispute that the skilled person can make such antibodies without undue burden. I do not accept this. It is well established that the specification must be sufficient to allow the invention to performed without undue burden across the full scope of the claims: see e.g. Regeneron Pharmaceuticals Inc v Genentech Inc [2013] EWCA 93, [2013] RPC 8 at [95] (Kitchin LJ, as he was). Here the claims plainly encompass anti-IL-17A/F only antibodies. If the skilled team cannot make such antibodies, or cannot do so without undue burden, then the claims are insufficient.
The development of ixekizumab
582. Lilly rely as part of their case on the evidence of Dr Kikly concerning the development of ixekizumab. Having regard to my other conclusions, this evidence is of little relevance. I should nevertheless make the appropriate findings of fact in case they become relevant to any appeal.
583. In July 2002 Dr Ling Liu (Dr Kikly’s predecessor as Lead Biologist on the project) presented a proposal to develop an antibody to target IL-17A for RA and asthma together with a research plan to a Cell and Molecular Biology meeting. The analysis and justification for the studies proposed was all focussed on IL-17A.
584. In December 2002 the Lilly team sent a third-party contractor a quantity of human IL-17A and requested it to generate antibodies specific to IL-17A. The contractor used a proprietary phage display technique to generate a pool of murine Fabs (antigen-binding fragments) specific to IL-17A. Lilly put these through initial tests, and selected an appropriately genetically diverse group of 10 individual Fabs to evaluate further in about July 2003. After the contractor had produced and purified further quantities of these, further tests were carried out, which showed (among other things) that none of the 10 Fabs cross-reacted with any of the other IL-17 family members then known.
585. In November 2003 the four most promising Fabs were selected for conversion to Mabs (monoclonal antibodies) based on various criteria, including high affinity for IL-17A. The four Mabs were then subjected to further testing to determine which should be humanised.
586. The result of this work was the identification and presentation of a lead candidate (Mab 2321 also known as LA426-2321) to Lilly’s Program Sanction committee in May 2004. Mab 2321 was chosen on the basis of various criteria, including binding with high affinity to both human and cynomolgus IL-17A and specificity to IL-17A in that it did not cross-react with other IL-17 family members.
587. After having obtained Program Sanction, the Lilly team proceeded to humanise Mab2321. This work was subsequently described in Liu et al , “Generation and characterization of ixekizumab. A humanized monoclonal antibody that neutralizes interleukin-17A”, J Infl Res , 9, 39-50 (2016). In summary, six humanised versions were created. Four of these Mabs were subjected to various tests. In May 2005 the humanised antibody LY2439821 (later named ixekizumab) was selected as a candidate for human clinical trials for RA and multiple sclerosis.
588. Between May 2005 and July 2006 the Lilly team carried non-clinical pharmacology studies on LY2439821. A Phase I safety, tolerability and efficacy study in patients with RA receiving background disease-modifying drugs (DMARDs, a diverse group of drugs such as methotrexate which slow the progression of RA) was carried out between November 2006 and February 2008. The results were positive. A Phase II dose-ranging study in patients with concomitant DMARD therapy was commenced in August 2009.
589. Psoriasis was first suggested as a possible indication in July 2006 due to several findings concerning IL-17A which emerged from the literature in 2005 and 2006. Psoriasis was included as an indication in a revision to the Investigator’s Brochure in October 2007. A Phase I safety and tolerability study in patients with psoriasis vulgaris was carried out between July 2008 and April 2010. This was followed by further clinical trials, including a Phase II dose-ranging study, six Phase III studies and a Phase IIIB study.
590. Ultimately, Lilly applied for an EU marketing authorisation for psoriasis and psoriatic arthritis on 23 April 2015. The marketing authorisation was granted almost exactly a year later.
591. Dr Kikly explained that IL-17A/F played no role at all in the development of ixekizumab. She first became aware of the heterodimer sometime in late 2008 or early 2009 through four publications (three of which were published in 2007 and one of which was published in 2008) thrown up by literature searches carried out by her team. By this point, ixekizumab had already completed the Phase I study in RA patients and had commenced the Phase I study in psoriasis patients.
592. Once they knew of IL-17A/F, Lilly took it upon themselves to undertake various studies to establish its relevance or otherwise to ixekizumab. Initial studies showed that ixekizumab did not interfere with the binding of IL-17A/F in an ELISA obtained from a commercial source. At that stage Lilly could not carry out experiments with IL-17A/F itself as they did not have a readily available source of the heterodimer. Once it became commercially available, Lilly carried out further tests between February 2010 and April 2011. The key information Lilly ascertained as a result of these tests is included in paragraph 595 sub-paragraphs (i) and (iv) below.
593. Ixekizumab is the subject of a family of patents obtained by Lilly.
Infringement
Ixekizumab
594. Ixekizumab is a recombinant humanised IgG4 monoclonal antibody. Its sequence and various other properties are set out in Lilly’s Amended Confidential Product Description. For present purposes the only points that matter are as follows:
i) ixekizumab binds to purified recombinant human IL-17A/A homodimer and to IL-17A/F heterodimer with the same measured affinity (Kd < 3 pM);
ii) ixekizumab neutralises IL-17A/A- and IL-17A/F-induced GROα secretion from the human colorectal adenocarcinoma epithelial cell line HT-29;
iii) ixekizumab can neutralise human IL-17A/A-induced secretion of IL-8 from the human foreskin fibroblast cell line Hs27;
iv) ixekizumab can block human IL-17A/A binding to the human IL-17RA subunit; and
v) the expression of genes in psoriasis lesions including the IL-8 gene was reduced in patients after two weeks of treatment with 150 mg of ixekizumab.
Which specifically binds to
595. There is no dispute that, as I have construed this requirement of the claims, it is satisfied by ixekizumab. (Nor is there is any dispute that ixekizumab inhibits the activity of IL-17A/F to induce the production of IL-6 and IL-8, a matter which Genentech established by experiment.) Accordingly, dealings in ixekizumab would infringe claims 1, 2, 14 and 15 if valid.
596. Genentech contends that, even if the claims are construed as limited to antibodies which bind only to IL-17A/F, dealings in ixekizumab infringe by virtue of the doctrine of equivalents established by the decision of the Supreme Court in Actavis v Lilly (cited above).
597. Given my conclusion on the normal interpretation claim, this issue does not arise. In case I am wrong on that point, I will briefly consider it.
598. In my judgment the answers to the three questions identified by Lord Neuberger in Actavis v Lilly at [64] are as follows:
i) Does the variant achieve substantially the same result in substantially the same way as the invention? The variant on this hypothesis would be an antibody which inhibited IL-17A/A as well as IL-17A/F, rather than just IL-17A/F. On current evidence, both of these are pro-inflammatory molecules involved in the pathogenesis of psoriasis, and which act upon the same receptor and through the same inflammatory pathways. It is theoretically possible that inhibiting just IL-17A/F may increase inflammation, but there is no evidence that this is actually the case (see further paragraph 605 below). It is more probable that an antibody which binds and inhibits IL-17A/A as well as IL-17A/F has an extra effect, but that in my view does not detract from the proposition that the variant does achieve substantially the same result in substantially the same way.
ii) Would it be obvious to the skilled person reading the Patent at the priority date, but knowing that the variant achieves substantially the same result as the invention, that it does so in substantially the same way as the invention? There are likely to be few cases in which this question will be answered in the negative. In the present case the answer must be yes.
iii) Would the reader conclude that the patentee nevertheless intended that strict compliance with the literal meaning of the claims was an essential requirement of the invention? The skilled addressee would see that there is nothing in the Patent to indicate that it is essential to the invention that the antibodies should bind to IL-17A/F only when used for therapeutic purposes. Even were the scope of the Patent’s claims to be limited due to considerations concerning other applications as Lilly contends, the skilled addressee would not see those as applicable or relevant when considering therapy. On the contrary, binding IL-17A/A in addition to IL-17A/F would be seen as likely to be beneficial.
599. Accordingly, if necessary, I would hold that this integer of the claims is infringed applying the doctrine of equivalents.
Use … for: contribution of inhibition of IL-17A/F to the therapeutic effect
600. Although it is common ground that ixekizumab has been shown to be efficacious in the treatment of psoriasis, ixekizumab binds to both IL-17A/A and IL-17A/F. As Prof Prens acknowledged, these is no direct evidence of IL-17A/F having been found in psoriasis lesions or of it being upregulated compared to normal skin. Genentech nevertheless contends that there is evidence that IL-17A/F has a pathogenic role in psoriasis, such that inhibition of IL-17A/F would be expected to make a contribution to the therapeutic effect of ixekizumab. Genentech relies upon four items of evidence.
601. First, Genentech relies on the European Medicines Agency’s assessment report relating to Taltz dated 25 February 2016 which states (at paragraph 2.1) that the “biologically active form of IL-17A consists of either IL-17A homodimers or IL-17A-IL-17/F heterodimers”. Dr Kikly accepted that the document was based on information by Lilly and therefore this statement represented Lilly’s understanding at that date. She was not asked about the meaning of this statement, however. In her witness statement, she had referred to tests Lilly carried out to see “whether IL-17A/F was biologically active (i.e. could activate human cell receptors)”, which were in vitro tests. The results corroborate Figure 5 of the Patent, but no more.
602. Secondly, Genentech relies upon a passage in Papp et al , “Brodalumab, an anit-interluekin-17-receptor antibody for psoriasis”, New Eng J Med , 366, 1181-1189 (2012), of which Prof Krueger was a co-author (at page 1187):
“Increased understanding of the immunopathogenesis of psoriasis has led to the development of multiple biologic drugs targeting specific molecules that are essential for the development of psoriatic plaques. Overproduction of interleukins 17A, 17F, and 17A/F induces the expression of proinflammatory cytokines with pathologic consequences, including the proliferation of keratinocytes and inflammation of epithelial cells in psoriasis. Therapies targeting this pathway, including interleukin-17 and interleukin-17R, are currently under investigation for the treatment of inflammatory conditions, such as psoriasis and rheumatoid arthritis. 19,28-30 Brodalumab, which targets interleukin-17RA, blocks signalling of interleukins 17A and 17F and the interleukin-17A/F heterodimer, all of which play a role in the inflammation of psoriasis.”
603. Prof Krueger was embarrassed by this passage when it was put to him in cross-examination, since his evidence was that there was no evidence to demonstrate that IL-17A/F had a pathogenic role in psoriasis, and the paper does not provide any. The most he was prepared to accept was that this was a possibility.
604. Thirdly, there is evidence in the post-July 2003 literature and in Lilly’s own Investigators’ Brochure that both IL-17A and IL-17/F are upregulated in psoriasis lesions. Prof Prens’ opinion was it was therefore it was inherently likely that T cells in psoriasis lesions also expressed IL-17A/F. Prof Krueger’s opinion was that this did not necessarily follow, since there was evidence that some T cell clones expressed IL-17A and others IL-17F.
605. Fourthly, and most importantly, Genentech relies upon Liang et al , “An IL-17A/F heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment”, J Immunol , 179, 7791-1199 (2007). This shows that differentiated T cells expressed IL-17A/F in significantly higher amounts than either IL-17A or IL-17F. Although this was an in vitro test, it was common ground between the experts that differentiated T cells are an approximation to how T cells present in vivo in peripheral organs (such as skin) respond after secondary stimulation. Prof Prens considered that this supported the hypothesis that T cells in psoriatic lesions expressed IL-17A/F. Prof Krueger maintained that one did not know what the position was in vivo until one had done the test, a point that Prof Prens accepted.
606. For the purposes of infringement, the question to be answered is whether inhibition of IL-17A/F makes a more than insignificant contribution to the therapeutic effect of ixekizumab. Genentech bears the burden of proof on this question, and it has not carried out any experiment to establish that there is such a contribution (but nor has Lilly established by experiment that there is not). Counsel for Genentech accepted that there was no evidence which clearly established the position one way or the other. He nevertheless submitted that, on the available evidence, it was more likely than not that it did. Having regard in particular to the Liang paper, I accept that submission.
Infringement of claims 1 and 2
607. Claim 1 and 2 are straightforward product claims. Genentech alleges infringement of these by Lilly pursuant to section 60(1)(a) of the Patents Act 1977. Lilly admits that Eli Lilly & Co Ltd has kept, disposed of and offered for disposal Taltz, the active ingredient of which is ixekizumab, and that Eli Lilly & Co is jointly liable for those acts. Accordingly, it follows from my previous conclusions, that if claims 1 and 2 are valid, they have been infringed by Lilly.
Infringement of claims 13, 14, 15 and 22
608. Claims 13, 14, 15 and 22 are all EPC2000 purpose-limited product claims. Genentech alleges infringement of these by Lilly pursuant to section 60(1)(a) alternatively section 60(2). It is sufficient to deal with Genentech’s case under section 60(2).
609. Genentech contends that Taltz is means relating to an essential element of the invention of these claims and that Eli Lilly & Co and Eli Lilly & Co Ltd know, or it is obvious, that the means are suitable and intended for putting the invention into effect in the UK.
610. The law on the mental ingredient of infringement under section 60(2) was set out in Grimme Landmaschinenfabrik GmbH & Co KG v Scott [2010] EWCA Civ 1110, [2011] FSR 7 at [105] and KCI Licensing Inc v Smith & Nephew plc [2010] EWCA Civ 1260, [2011] FSR 8 at [53] . In summary, it is enough if (at the time of supply or offer to supply) the supplier knows (or it is obvious to a reasonable person in the circumstances) that some ultimate users will intend to put the invention into effect in the UK using the “means essential”.
612. Further, I find that Eli Lilly & Co and Eli Lilly & Co Ltd know (or at least it is obvious) that some ultimate users will intend to put the invention into effect. As mentioned above, Taltz is authorised for the treatment of psoriasis. Further, Eli Lilly & Co and Eli Lilly & Co Ltd know (or it is obvious) that ixekizumab acts as an IL-17A/F antagonist. The only question is whether they know (or it is obvious) that inhibition of IL-17A/F makes a more than insignificant contribution to the therapeutic effect of ixekizumab. In my judgment Lilly acquired this knowledge, or it became obvious to a reasonable person, as a result of the evidence given at trial, although I am not satisfied that this was the case before that. I will fix the relevant date as the last day of the trial (1 February 2019).
613. Accordingly, it follows from my previous conclusions that, if valid, Eli Lilly & Co and Eli Lilly & Co Ltd have infringed these claims by acts committed since 1 February 2019.
Infringement of claims 12 and 20
614. Claims 12 and 20 are Swiss form claims directed to the treatment of, so far as relevant to infringement, psoriasis. Genentech alleges infringement of these by Lilly pursuant to section 60(1)(c). In Warner-Lambert (cited above) the Supreme Court divided 2:2:1 on question of whether infringement of Swiss form claims pursuant to section 60(1)(c) involved a mental element, and if so what it was. Moreover, all of the judgments on this question were obiter.
615. In the present case, however, I do not think it matters which test is to be applied for the purposes of claims 12 and 20. This is because (i) the parties alleged to infringe and the manufacturers of Taltz are Lilly companies, (ii) Lilly intend that Taltz is to be used for the treatment of psoriasis and (iii) the outward presentation of Taltz makes it clear that it is for use for the treatment of psoriasis. Counsel for Lilly submitted that there was no evidence of (ii) and (iii), but I disagree: both facts are plain from the marketing authorisation for Taltz.
616. Accordingly, it follows from my previous conclusions that, if valid, Eli Lilly & Co and Eli Lilly & Co Ltd have infringed these claims.
Infringement of claims 13, 14 and 20 if conditionally amended
617. Genentech’s conditional amendments to claims 13, 14 and 20 incorporate the language “[for use] as an antagonist of the IL-17A/F heterodimeric complex” or “[for] antagonizing the IL-17A/F heterodimeric complex”. This potentially gives rise to a difficult question on infringement. Although Genentech made written submissions on it, Lilly barely addressed it. Given my other conclusions, the issue does not arise. I should, however, make a finding of fact in case the issue becomes live in another court. That concerns the date on which Lilly acquired knowledge of the antagonistic effect of Taltz on IL-17A/F, if at all. Genentech contends that Lilly had such knowledge at least from the date of its application for a marketing authorisation. I accept that contention.
Summary of principal conclusions
618. For the reasons given above, I conclude that:
i) Genentech’s unconditional amendments to the claims are allowable, with the minor exception of “comprises” in new claims 1 and 14, but the conditional amendment to “consists of” is allowable.
ii) Claims 1, 2, 13, 14 and 15 are obvious over US344, as are claims 12, 20 and 22 in so far as those claims are directed to RA.
iii) Claims 1, 2, 13, 14 and 15 are novel but obvious over the IL-17A/A prior art, as are claims 12, 20 and 22 in so far as those claims are directed to RA.
iv) Claims 12, 20 and 22 are insufficient for lack of plausibility in so far as they are directed to psoriasis. Lilly’s other insufficiency objections are rejected.
v) If (contrary to my conclusions) the claims are valid, they have been infringed by Eli Lilly & Co Ltd and Eli Lilly & Co.
