Mr Justice Birss:
|
Topic
|
Paragraphs
|
|
Introduction
|
1
|
|
The witnesses
|
13
|
|
The skilled team and the common general knowledge
|
27
|
|
The patent and claim construction
|
56
|
|
Amendments
|
107
|
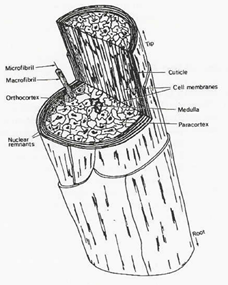
|
Infringement
|
111
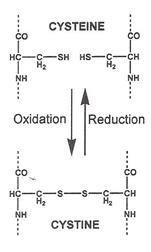
|
|
Priority
|
118
|
|
The prior art
|
119
|
|
The prior use
|
120
|
|
Catzy
|
157
|
|
WO 768
|
187
|
|
Kim
|
228
|
|
The second claimant’s status
|
254
|
|
Conclusion
|
264
|
Introduction
1.
This is a patent case about hair care products. The patent is UK patent
GB 2 525 793 entitled “Keratin treatment formulations and methods”. The patent
was granted on 2nd November 2016 following an application made on 15th
May 2015 claiming priority from US filing 61/994,709 dated 16th May
2014. The patent belongs to the first claimant. The second claimant is the
exclusive licensee. The claimants can be referred to together as Olaplex.
2.
Olaplex makes and sells hair products including a product called Olaplex
No. 1 Bond Multiplier. The key ingredient in Bond Multiplier is a diamine salt
of maleic acid. Sales of Bond Multiplier have grown dramatically since the
product was launched in June 2014. It sold $100 million worth of sales in its
first year. Olaplex Bond Multiplier has had extensive coverage in the press
and has been used by many female celebrities. The defendants (L’Oréal) sell a
product called Smartbond Step 1. It contains maleic acid too, albeit not in
the same form as in Bond Multiplier. Olaplex says that L’Oréal first attempted
to buy the Olaplex business but has now chosen to adopt the patented
ingredient. Whether that is so has no bearing on the issues I have to decide.
3.
Olaplex contends that L’Oréal’s Smartbond infringes the patent. L’Oréal
denies infringement and contends the patent is invalid. L’Oréal also seeks a
declaration of non-infringement relating to an alternative formulation of
Smartbond. In response to the validity attack Olaplex has applied to amend the
patent in various ways.
4.
Claims 1 and 11 of the patent as granted are in this form:
Claim 1
A method for providing bleached hair comprising:
(a) applying to the hair a first formulation comprising a
bleaching agent; and
(b) applying to the hair a second formulation comprising an
active agent, wherein the active agent is
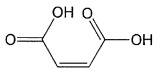
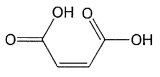
or a simple salt thereof;
and wherein step (a) occurs simultaneously with step (b).
Claim 11
The use of an active agent which is

or a simple salt thereof
simultaneously with a bleaching agent
to reduce or prevent hair damage due to a treatment to
provide bleached hair.
5.
The chemical formula shown in these claims is maleic acid. There are
issues of claim construction. The main two are the scope of the term
“providing bleached hair” and a point about the chemical formula along with the
reference to simple salt. The bleached hair point is whether the term refers
only to the process of hair lightening which changes the colour of hair by
oxidation but does not involve hair dye or whether it also includes a process
of hair dyeing using oxidation dyes, which does involve some use of bleaching
agents but also involves dye. The real importance of this issue is about prior
art. L’Oréal relies on a prior Korean patent application known as Kim
(publication number PAT 2003-0003970). Kim discloses using maleic acid and
derivatives of it in a process using oxidation dyes. If the Olaplex claims
cover using oxidation dyes then they have a problem of lack of novelty
(although there may be dependent claims which are novel). If the claims are
limited to bleaching without dyeing then the issue is one of obviousness.
6.
The issue about the formula and simple salt engages issues of
construction, priority, amendment and infringement. In its form as granted the
claim refers to maleic acid (by the formula) or a simple salt thereof. L’Oréal
contends that claims are not entitled to priority because “simple” salt is not
in the priority document. If the claims lose priority then they are all
invalid because Olaplex’s Bond Multiplier product was made available to the
public between the priority date and the filing date of the patent in suit.
Olaplex does not accept the granted claims are not entitled to priority but
offers an unconditional amendment to remove the reference to a simple salt.
Accordingly in its opening skeleton Olaplex did not get into the detail of what
simple salt meant. Olaplex contended that the unconditionally amended claims,
with the reference to simple salt struck through, relate to any relevant
chemical system containing the species which maleic acid produces in solution. It
is not limited to the undissociated form of maleic acid depicted in the formula
essentially because the skilled person would be well aware that when maleic
acid was put into aqueous solution, it would dissociate into ions such as maleate
ion and hydrogen maleate. The equilibrium would be determined by the pH.
7.
Now Olaplex’s submission about the scope of the claim may be right or
wrong but starting at the amended claim risks leading to trouble. That is
because one of the objections raised by L’Oréal to the amendment is a question
of extension of scope. If one starts by focussing on the granted claim, the
reference to the maleic acid formula or a simple salt thereof could be
understood by the skilled person as an attempt by the patentee specifically to
limit the claim only to maleic acid itself or only simple salts and not, for
example, forms of maleic acid derivatives which are neither maleic acid nor
simple salts or to maleate ions however formed. And if that is right then the
construction of the amended claim advanced by Olaplex could be seen as making
it wider in scope than the granted claim. But that is forbidden by s76 of the
Patents Act, implementing Art 123(3) of the European Patent Convention.
Tangled up with all this are questions raised by an alternative formulation of
the L’Oréal Smartbond product the subject of a claim for a declaration of
non-infringement. Whereas the Smartbond formulation alleged to infringe
includes maleic acid and ethanolamine as distinct species, in the alternative
formulation the species is a salt – ethanolamine maleate. The debate is also
illustrated by a question of whether Olaplex’s own Bond Multiplier formula is
within the claim. The diamine salt in the formulation may not be a simple
salt. A further dimension to this general issue is about amendments to the
specification. Usually when claims are amended, there may need to be
corresponding amendments to the specification. They are normally not
controversial but they can be when an issue of construction like this arises.
8.
The way to resolve these issues will be to start in the right place (the
granted patent including the claims as granted) and work from there.
9.
Aside from these issues there are three more validity attacks. L’Oréal
relies on the description of a hair lightening product called Catzy. This was
published before the priority date. It included maleic acid as an excipient.
Known uses of maleic acid as an excipient were as a buffering or chelating
agent. The amount of maleic acid in the Catzy formulation is not stated in the
published material (although it is now known). There is a question of claim
construction whether the claims would cover such a formulation in any event.
Olaplex also relies as a fall back on dependent claims which involve ranges
specifying the amount of maleic acid in the formulation.
10.
L’Oréal relies on a s2(3) citation WO 2015/017768 A1. This is a
published international patent application under the PCT made by Liqwd Inc. I
will refer to it as WO 768. It was published on 5th February 2015
and designates the United Kingdom. The WO 768 application was filed on 1st
August 2014 claiming priority from a series of US filings, most of which were
before the claimed priority date of the patent in suit. Therefore although WO
768 itself was filed after the claimed priority date of the patent in suit,
matter disclosed within it may be entitled to a priority earlier than the
patent in suit. L’Oréal refers to example 8 of the published application. If
the matter disclosed by that example is entitled to a priority date before 16th
May 2014 then it is prior art against all claims. As a s2(3) citation WO 768
is only relevant for novelty, not inventive step.
11.
The other major issue in the case is prior use. It is not in dispute
that before the 15th May 2014 priority date, Olaplex distributed its
Bond Multiplier product to some hair care professionals in California and
elsewhere in the USA. Most of the hair care professionals are referred to as
colorists. It is convenient to use that term and to use the US spelling since
that is how they refer to one another.
12.
If the distribution to the colorists made the contents of the product
available to the public then the patent is invalid. L’Oréal contends that it
did and refers to extensive social media posts by the hair professionals
promoting the product, promoting its use on celebrities, and saying how
wonderful it was. Initially Olaplex took two points. The first was and is
that this distribution exercise was part of testing the product and that the
recipients were not told what the secret formula was and were not free to
analyse it or to give it away. So information about the contents was not made
available to the public. The fact that this also created a social media buzz
(which was intended too) does not make any difference. The second argument was
that L’Oréal’s attempt to show that analysing the product would inform the
skilled person of the identity of the crucial ingredient failed to reach the
relevant legal standard. That second point was abandoned at trial after
further evidence about the analyses was served.
The witnesses
13.
The expert witness called by Olaplex is Professor David Haddleton. He
is Professor of Chemistry at Warwick University and Adjunct Professor at Monash
University (Pharmacy and Materials Engineering). The current focus of his work
at the University of Warwick is on polymers for healthcare and therapeutics,
and controlled free radical polymerisation. He has also worked extensively
with industry on various consumer products including hair care products. He
has done a lot of work with Unilever and also worked with L’Oréal.
14.
L’Oréal criticised Prof Haddleton, submitting that his evidence should
be treated with caution. L’Oréal is correct that Prof Haddleton had much less
practical hands on experience of oxidative dyeing, hair bleaching and the
formulation of such products than L’Oréal’s expert Dr Hefford. I will take
that into account. L’Oréal submitted that his first report had not been
prepared with diligence and showed his lack of expertise. I will refer to two
matters. Prof Haddleton’s first report contained a fundamental error about the
way oxidative dyeing works which the professor had picked up from a passage in
the patent. He did correct it in a later report but the fact the error was
made at all is indicative of the limits of the Prof Haddleton’s expertise in
this field. Someone with substantial actual experience in this field would
have been immediately puzzled by the passage the professor relied on.
15.
The second matter arose from a passage in the professor’s first report
in which he said “The solution described in the patent is not one that
occurred to me when I considered what would be obvious for the skilled person
starting from the prior art”. In his report as written this statement was
made in a context in which he had been given the prior art but not the patent.
It therefore makes sense. It is not really expert evidence at all. It is
really evidence of fact, apparently recording that Prof Haddleton considered
the prior art before he ever saw the patent, thought about what might occur to
him over that prior art in circumstances necessarily devoid of hindsight –
since he had not seen the patent – and did not think of the solution described
in the patent. In other judgments I have questioned the utility of this sort
of evidence but in any event its clear purpose was to support Olaplex’s case that
the patent is not obvious from the prior art.
16.
However it turned out that the professor had made an error in relation
to the circumstances in which he had been given documents by Olaplex’s
lawyers. In fact at the time this sentence is referring to he had already seen
the patent as well. If that is true then the sentence makes no sense. In
cross-examination Prof Haddleton said this was just a general statement about
obviousness over the prior art, but that is not correct. When Prof Haddleton
was being cross-examined about this he did not seem to me to see why this point
appeared to be so significant to L’Oréal. I sympathise with him to some extent
because my impression of Prof Haddleton was that he was entirely honest and
trying his best to assist the court. He was a bit argumentative but that did
not indicate anything other than a witness trying to help the court as best he could.
17.
I will take both these points into account when considering the weight
to be attached to Prof Haddleton’s views. I reject the submission that I
should treat all his evidence with caution.
18.
L’Oréal’s main expert was Dr Robert Hefford. Dr Hefford is a chemist and
worked in industry for a number of years. He was at Unilever from 1977-1989,
where he worked on the research and development of hair products and skin
products. Dr Hefford then moved to the UK Clairol Division of Bristol-Myers
until 2002, where his role moved from concentrating on formulations to covering
all aspects of product development. Since 2002 Dr Hefford has been a
consultant to the cosmetics and haircare industries.
19.
Olaplex submitted that the evidence Dr Hefford had given in parallel US
proceedings about the same invention and the Kim prior art meant that his
ability to put himself in the position of the unimaginative skilled person
simply reading Kim with interest was massively prejudiced. I reject that.
Experts in patent cases know, and Dr Hefford certainly did, that even though
the case is conducted ex post facto, the ultimate task is to decide without
hindsight what the unimaginative skilled person would do (if anything) given
the prior art. That is why the law has developed methods for assessing
obviousness which aim to identify and remove hindsight. The task is not easy
but it is not made any harder just because the expert has considered the
invention and the prior art in detail in evidence for the American court.
20.
L’Oréal also called Professor Robert Law. He is Professor of Biological
Materials at Imperial College London. He collaborates with the Department of
Materials, Chemical Engineering and Cell and Molecular Biology, and has
successfully established an interdisciplinary research centre for solid state NMR
at Imperial College London. His evidence was about what a skilled analytical
chemist would do if a formulator of hair care products asked him or her to
identify what a product contained, assuming it came with no ingredient list.
21.
Olaplex also called fact evidence from a number of individuals. The
fact witnesses who gave oral evidence and were cross examined were:
i)
Dean Christal is the owner, CEO and Manager of Olaplex LLC. His evidence
is about the prior use of Bond Multiplier. His evidence was that he made sure
the people he gave Bond Multiplier to knew they were being given it to test and
that it was a secret formula. They must keep it confidential and not give it
to others.
ii)
Dr Eric Pressly is one of the inventors named on the patent and is a
co-owner of Olaplex. His witness statement verified the product description of
Bond Multiplier. He was cross-examined about issues relating to the prior use.
There was a suggestion he was uncomfortable in the witness box. I did not
think so.
iii)
Jordan Alexander is Director of Special Projects at Olaplex LLC. Before
that he worked at the Méche salon in Los Angeles. He was one of the assistants
of a famous colorist at Méche called Tracey Cunningham. His evidence is about
the prior use.
iv)
Sarah Lim (sometimes known as Slim) also worked at the Méche salon from
around late 2012 to 2014 as one of Tracey Cunningham’s assistants. By the time
that she left Méche in 2014, Ms Lim was the head assistant. Her evidence is
about the prior use.
v)
Vicki Laris is a colorist based in Chicago. She worked with Tracey
Cunningham at the Méche salon in March 2014. Her evidence is about the prior
use.
vi)
Sylvie Vaught is a colorist and stylist. In 2014 she worked at the
Estilo Salon in Los Angeles and give evidence about the prior use.
22.
Mr Alexander, Ms Lim, Ms Laris and Ms Vaught gave evidence by video link
to the USA.
23.
Olaplex also relied on fact evidence from a number of individuals under
Civil Evidence Act hearsay notices. Alan Gold and Gina Monaci are colorists who
gave witness statements about the prior use. They were unable to attend trial.
Tracey Cunningham gave a short witness statement stating that she did not wish
to give evidence in this case. She has relationships with both Olaplex and
L’Oréal.
24.
L’Oréal also called evidence from Rachel Boakes, an associate at
L’Oréal’s solicitors Baker McKenzie. Her evidence arose from efforts by both
herself and another solicitor at the firm to speak with potential witnesses about
the prior use of Olaplex’s Bond Multiplier. She was cross-examined briefly on
that evidence.
25.
L’Oréal called Frederic Legrand to verify the Product and Process
Description of Smartbond. He was cross-examined about a table in the
Alternative PPD. Olaplex submitted he gave inconsistent evidence about responsibility
for it and submitted that he was apparently ignorant of “basic chemistry”. I
do not agree. The explanation for the point on the table was that M. Legrand
was not inconsistent. At worst there was a simple misunderstanding, probably caused
in part by the existence of a PPD which he had signed and an Alternative PPD
which he had always made clear he had not signed. The point on salt was that
the questioner put to him a question which was far from basic chemistry. The
question was not a general one about salts per se, it was a particular one
about whether, at the particular pH of the formulation which includes
ethanolamine maleate, the salt was solely in the form of dissociated ions. M.
Legard’s answer meant he was simply saying that he did not know whether the
material entirely dissociated. The point was not explored further with M.
Legrand. However there was an issue in this case, as no doubt M. Legrand was
aware, about the true extent to which molecules fully dissociate at different
pHs. There was also evidence that maleic acid itself (rather than ethanolamine
maleate) is not entirely dissociated at pH 3. And there was an issue on one
view of claim construction about whether that mattered for infringement. In my
judgment M. Legrand was a good witness seeking to help the court. He was a
fluent English speaker but it is not his mother tongue.
26.
Finally, L’Oréal relied on evidence from two scientists who had
performed analyses on a sample of Olaplex material in order to show what
information about the prior used formulation might be made available to the
public. They were Dr Shen Luk and Dr Huw Williams. Dr Luk is Chief
Scientific Officer at Juniper Pharma Services (“Juniper”). His expertise is in
analysis for the pharmaceutical and chemical industries. Dr Williams is a
nuclear magnetic resonance Facility Manager at the School of Chemistry at the
University of Nottingham. He performed NMR analysis to assist Dr Luk. Neither
Dr Luk nor Dr Williams were cross-examined.
The skilled team
and the common general knowledge
27.
In this case the person skilled in the art is a team.
28.
The patent is addressed to a team responsible for producing and
developing hair-care products. The principle member of the team would be a
chemist/formulator with experience developing hair treatments. This person
would have an undergraduate degree in chemistry or a related field and either a
relevant PhD and a few years’ experience developing, formulating and testing
hair care products or no PhD and more years’ experience. When considering the
issues of construction I will refer to the skilled team as the skilled reader.
29.
If relevant the team would also include an analytical chemist to
determine the composition of a sample of an unknown product.
30.
The relevant common general knowledge relates to hair, hair treatments
and chemistry.
Hair and hair structure
31.
Hair is mostly made of keratin, a naturally occurring polymer of amino
acid monomers. A strand of hair has three components known as the cuticle, the
cortex and the medulla. A representation of a hair strand is:
32.
The cuticle forms an outer protective sheath which can act as a barrier
to protect the cortex, and minimise friction between hair fibres. It controls
the movement of molecules/chemicals between the fibre’s central regions and the
outside environment, including moisture and vice versa. The cuticle has lipids
bound to its surface by thioester bonds, which give the hair natural shine and
a soft feel.
33.
The cortex provides hair with its strength. It contains nested
longitudinal bundles of keratin fibres and melanin granules. Melanin is
responsible for the hair’s natural colour. The cortex does not have a great
deal of lateral strength; part of the function of the cuticle is to hold the
fibres of the cortex together.
34.
The medulla is a central region normally found in thick hair. It is not
always present in hair. Naturally blonde and fine hair generally does not have
a medulla.
35.
Keratin proteins are the major contributor to hair strength at a
molecular level. Keratin has a high level of cysteine residues that result in
disulphide crosslinking throughout the hair. These crosslinks are formed by
the two cysteine side chains which have thiol (-SH) groups reacting to form
cystine (also known as a cysteine bridge), which has a disulphide (-S-S-) bond
between the two chains. Going from the thiols to the disulphide is an
oxidation reaction while going from the disulphide to the thiols is a reduction
reaction:
36.
Hair damage is a complex process. Hair damage can arise from both
chemical and mechanical processes. Recognised sources of damage are from
reducing agents (for example in perms), from oxidising agents (such as in
bleaching), from the deposition of dye in the cuticle of the hair fibre, from
mechanical processes such as grooming (brushing, combing, drying), and from
heat treatment.
Hair treatments
37.
One of the most common ways to bleach hair is by the destructive
oxidation of the chromophores in melanin, by applying a bleaching mixture. The
chromophores are the groups of atoms in the melanin molecules responsible for
giving the colour.
38.
As mentioned already, one of the issues in the case involves considering
two methods of changing the colour of hair. I will call one well known method
“hair lightening” because it changes the colour of hair by oxidation but does
not involve hair dye. The other well known method is a process of dyeing hair
using oxidation dyes. There are other methods of changing hair colour
involving dyes which are not oxidation dyes.
Hair lightening
39.
The mixture used for hair lightening principally comprises an oxidising
agent such as hydrogen peroxide and a further material such as a persulfate.
The further material can be called variously a bleach booster or accelerator.
The mixture is applied at an alkaline pH. This is a very common way of
changing the colour of hair. It involves no dye at all. The colour change
comes entirely from the process of bleaching or oxidation. If all the colour
is removed the result is a silver white colour – hence the term peroxide
blonde.
40.
The cuticle of the hair was known to open and swell in treatment with a
high pH, causing the permeability of the cuticle to increase. This facilitates
the entry and diffusion of chemicals deeper into the hair, and in particular to
the cortex to allow penetration of, for example, hair dyes. The side effect of
this is that the hair can become less hydrophobic, especially when natural oils
are removed by chemical processes. There is also an increase in the amount of
swelling due to ingress of moisture when the hair is wet.
41.
While the exact chemical steps involved in the peroxide
and persulfate interaction were and are still not well understood, it was known
that both were (and are) needed for a bleaching process to work in a
practically useful time frame. It was also understood that they need to be
kept apart until the point of application to avoid a premature chemical
reaction.
42.
The aggressive chemistry used in bleaching causes damage. One source of
damage is due to the oxidising agents decreasing the hydrophobicity of the hair
fibres. This affects, for example, the binding of the natural oils to the
hair, causing the oil to be removed and the hair becoming dry and losing its
shine.
43.
In terms of chemical mechanisms, an aspect of damage by oxidising
agents was believed to be due to the conversion of the disulphide bond (S-S) to
cysteic acid groups (SO3).
Oxidation dyes
44.
Oxidation dyes are used in the majority of hair dye treatments in the US
and Europe. The process uses intermediate colouring agents which require the
intervention of an oxidation agent (usually hydrogen peroxide) to react with
them in order to produce permanent coloured compounds through oxidative
condensation. The chemical processes involved are complex.
45.
The hydrogen peroxide in the formulations could act to oxidise the hair
(and therefore cause essentially the same damage as described above for hair
lightening). However the extent to which this occurred is in dispute. I find
that the skilled person knew, as a matter of common general knowledge, that
oxidative damage was something which could occur in oxidation dye systems,
especially with repeated dyeing. It was less severe than the damage caused in
hair lightening owing to the less aggressively oxidising formulations used in
dyeing as compared to those used in hair lightening. So it was known that
oxidation could be a cause of damage but looking at the matter the other way
round, it was not the case that the skilled person necessarily would assume
that any damage seen must have been caused by oxidation rather than having some
other cause.
46.
Another related issue is the degree to which the formulations contain an
excess of the oxidation agent over and above that needed to oxidise the dye
precursor molecules to form the dyes. I find that the common general knowledge
was that many formulations did have a substantial excess of hydrogen peroxide
but not all such formulations.
47.
It was understood that another thing which happened with the use of
oxidative dyes was the deposition of the oxidised dye precursor and product
molecules on the cuticle of the hair fibre. This negatively affects the look
and feel of the hair and is distinct from any damage that might arise from the
oxidative processes.
Trying to treat
or prevent hair damage
48.
The damage to hair caused by oxidation was known and those in the art
had to deal with it. Professionals tried not to bleach hair too often but that
was not always possible. For example actresses in film and television might
have to undergo treatments which involved oxidation of hair very frequently.
49.
Conditioners were applied to the hair afterwards. These improved the
combability of hair. If one tried to draw a comb through hair which had been
oxidised but to which no conditioner had been applied there was a risk of
breaking the hair. The conditioner is not acting to actually reduce or prevent
the damage to the disulphide bonds themselves but rather to treat the symptoms
of that damage. Conditioners were very effective for the period they were on
the hair but that was a temporary effect because they wash out when hair is
washed and have to be reapplied again and again.
Testing for hair
damage
50.
If a hair treatment was being developed the skilled team would test it.
A common approach would be to test the prototype product on swatches of hair in
a laboratory. One common test is the tensile strength of the hair. Machines
from the company Diastron were well known. There is more than one Diastron
machine and even in the same machine a tensile test could be set up in
different ways. For example a test could be done on wet hair or dry hair.
Part of the common general knowledge was that bleaching could weaken the
tensile strength of hair when measured with wet hair.
51.
Another kind of testing is consumer testing. There is no doubt that
this sort of sensory testing will form part of the testing process. Tensile
strength is related to the cortex whereas the condition of the cuticle does not
correlate to the tensile strength of the hair. The state of the cuticle is
important for consumer perception. An issue is the relative significance of
consumer testing results over laboratory tests like tensile strength. I find
that the skilled person would find positive results of either kind sufficiently
interesting to take forward even if they were reported without the other kind.
So good tensile results alone would be worth investigating.
Chemistry
52.
When an acid reacts with a base the result is a salt plus water. In
solid form salts are crystalline ionic compounds made up at least one cation
(positively charged ion) and at least one anion (negatively charged ion). When
the crystals are dissolved in water to make an aqueous solution the crystal
lattice is lost and the solution is a mixture of separate cations and anions.
53.
Maleic acid was part of the common general knowledge of the skilled
team. It is a diprotic acid, i.e. it has two protons which could dissociate. When
one comes off the result is a proton and a hydrogen maleate ion in solution.
When the second proton comes off the hydrogen maleate the result is two protons
and a maleate ion in solution. Maleic acid has a pKa1 of 1.94 and
pKa2 of 6.22. Therefore at low pH (e.g. pH 3 or 3.5) the majority
ionic species is hydrogen maleate and there will be some undissociated maleic
acid; whereas at high pH (e.g. pH 8 or above) both protons will dissociate and
the predominant species is maleate ion. In the context of hair care, before
the priority date the skilled team would only have been aware of maleic acid’s
potential use as a chelating agent or pH buffer/modifier.
54.
Peracids are acids with an [-OOH] group and so are related to hydrogen
peroxide [HOOH]. These are strong oxidising agents. The use of peracids as
bleaching agents was well known at the priority date.
Sources of
common general knowledge
55.
The field has a number of textbooks which those skilled in this art
refer to. The ones referred to in evidence were Chemical and Physical Behavior
of Human Hair, 5th Ed. by C.R. Robbins; The Science of Hair Care, 2nd Ed.
edited by Bouillon & Wilkinson; Hair and Hair Care, edited by Dale H
Johnson and Fundamentals of Human Hair Science Issue 1, by J Alan Swift. Not
every line of every textbook represents common general knowledge (nor for that
matter is every single statement accurate either) but they provide an important
resource.
The patent and
claim construction
56.
There was no dispute about the law applicable to the interpretation of
patent claims. However given the recent decision of the Supreme Court in Actavis
UK Ltd v Eli Lilly and Co [2017] UKSC 48, and subsequent decisions
of judges sitting in the Patents Court about it, I will make some brief
observations of my own.
57.
Prior to Actavis the approach based on Kirin-Amgen
Inc v Hoechst Marion Roussel Ltd [2004] UKHL 46 was that whatever
account was required in law to be taken of equivalents when applying the
Protocol to the Interpretation of Art 69 EPC and its equivalent provisions in
national law, that was achieved by the process of purposive construction. The
Supreme Court in Actavis has decided that that is not the
right approach. The scope of protection is now determined by a two stage
process. The first stage is a process of construction and then the second stage
is the application of a doctrine of equivalents.
58.
An outstanding question has been whether the first stage, also referred
to as a process of normal interpretation, is the same as what was previously
called purposive construction. Part of the reason this question arises is because
in Actavis the questions formulated to be answered in
applying the equivalents analysis refer to variants from the “literal meaning”
of the claim language. Prior to Actavis a “purposive” approach
to construction was intended not to be a purely literal one.
59.
So far the judges of the Patents Court who have had the opportunity to
express themselves on the point have unanimously held that the normal
interpretation stage required by Actavis is the same as
purposive construction (Arnold J in Generics v Yeda [2017] EWHC 2629 (Pat), Richard Meade QC in Fisher & Paykel v Resmed
[2017] EWHC 2748 and Henry Carr J in Illumina v Premaitha
[2017] EWHC 2930 (Pat)). I agree for the reasons given by those judges. As
Henry Carr J put it in paragraph 202 of Illumina, normal
interpretation means purposive construction.
60.
I will add two further observations. They are points which at least on
one view of the issues in this case might have mattered but in the end did
not. The first is about taking equivalents into account in the process of
construction. One consequence of Kirin-Amgen was that
account was taken of equivalents in the process of determining what the true
purposive construction of the claim was. I will say only that I can see scope
for debate about whether, following Actavis, that sort of
approach might or might not produce the same result at the normal
interpretation stage as would have been arrived at following Kirin
Amgen. In other words, construing a patent purposively to identify
the normal interpretation in the manner described in those first instance decisions
which I do agree with, may not be precisely the same as every nuance of the
process of the determination of claim scope which was mandated by Kirin-Amgen
prior to Actavis.
61.
The second point is about validity and claim scope. One of the issues
involves whether an amendment might extend the scope of protection and
therefore be impermissible (or if it had been made already, invalid). This has
caused me to think about the relationship between validity and the Actavis
approach to claim scope (including the scope determined by the second stage of Actavis
as well as the scope produced by the process of normal interpretation). I will
say only that I can see room for arguing that for validity purposes some
account ought to be taken of the wider scope.
62.
I turn to consider the patent in this case.
63.
The patent starts by explaining that the field of the invention relates
to formulations and methods for providing bleached hair (p1 ln3). What that
term means is in issue. I will come back to that.
64.
Next in the background section the first line (p1 ln8) refers to hair
bleaching as a globally accepted fashion phenomenon. Next (p1 ln9) is a
reference to the use of reducing agents to break disulphide bonds allowing for
the deeper penetration of the bleaching agents into the hair. This is not
correct and the skilled reader would not think it was correct. Reducing agents
are used in perming. From this passage onwards there is a muddled section which
does mention oxidation but it really focussed on perming. Reactions with the
thiols in keratin are mentioned, again in the context of perming.
65.
After this muddled section there are statements setting out what is
needed in the art. At p3 ln6 it is stated that “there is a need for hair
formulations and treatments that repair and/or strengthen keratin in hair
damaged from bleaching treatments” and at p3 ln10 it is stated that “there is
also a need for formulations and treatments that can repair damage to keratin
present in hair”. The skilled reader would accept and agree with these
statements. In between is a reference to thiols but that does not matter.
66.
At p3 ln13 the patent states that it is an object of the invention to
provide improved formulations and methods for repairing and/or strengthening
damaged hair. Given their common general knowledge the skilled team would be
very interested. The text section goes on to state at line 15 that:
“The present invention provides a method for providing
bleached hair which comprises simultaneously applying to the hair a first
formulation comprising a bleaching agent and a formulation comprising an active
agent as described herein.”
67.
Next is a section called “summary of the invention”. The first
paragraph here (p4 ln9-13) provides:
“Formulations, kits and methods for restoring hair that has
been broken during a bleaching treatment are disclosed. The formulations have
similar benefits when used with different color chemical processes, such as
bleaching, highlights, lowlights, semi-permanent, demi-permanent, and permanent
color.”
68.
The skilled reader would not think the first sentence there meant that
the patent was suggesting that hair which has actually been broken could be put
back together again. The reference to “broken” would be understood most likely
either as meaning damaged or as a reference to breaking disulphide bonds.
69.
Each side relied on the second sentence in support of their case on the
meaning of “providing bleached hair” in the claims. L’Oréal submitted that the
skilled reader would regard permanent colour as a synonym for the use of
oxidation dyes. I think the skilled reader would regard oxidation dyes as the
paradigm case of permanent colour, and I agree that the skilled reader would understand
what is being said here as including a statement that the benefits would be
found if the formulation was used with oxidation dyes.
70.
Olaplex submitted the list in the second sentence would indicate to the
reader that the inventors distinguished between bleaching and dyeing; and in
particular between bleaching and the use of oxidation dyes. L’Oréal submitted
that the sentence supported its case that the term “bleached hair” included
hair bleached in an oxidation dye process since the invention is said to work
for colouring chemical processes in general and in particular including
permanent colour (i.e. oxidation dyeing).
71.
As I have said L’Oréal is correct that the passage would be understood
to assert that the invention will work in an oxidation dyeing process. That
would mean that the reader would not be surprised if a claim was made to a
method which included oxidation dyeing. However the reader would also see that
this passage shows what the inventors are using words to mean. Here a
distinction is being drawn between bleaching and (for example) permanent
colour; in other words between bleaching and oxidation dyeing. They are both
kinds of colour chemical process – which is correct because they both change
the colour of hair using chemicals – but they are distinct processes. The
reader would not think that the reference to bleaching in that sentence was
used to include the bleaching which will at least to some extent take place in
an oxidation dyeing process.
72.
The next passage in the patent states:
“The
methods disclosed herein use active agents to repair the hair; these active
agents are washed from an individual’s
hair on the same day that they are applied to the hair. Under the same
conditions, such as temperature and moisture, hair treated with the
formulations disclosed herein takes a longer time to revert to its prior state
as compared to the same hair that is treated with hydrogen peroxide.
The formulation is applied at the same time as the hair
bleaching treatment.”
73.
The reference to the hair which has been treated reverting to its prior
state is puzzling but nothing turns on it.
74.
Next the detailed description starts at p5 with an uncontroversial
series of definitions (section I). The organisation of the next section in the
document from p10 on to p30 is confusing. At page 30 is the first example –
numbered Example 2. There is no example 1 even though an example 1 is referred
to in some of the later examples.
75.
At p11 is a statement that the formulations and methods in the patent
are to treat keratin in hair and may reduce to prevent hair damage due to hair
bleaching processes. At p11 ln7 is a statement that the formulations contain
maleic acid or a simple salt thereof. The sentence then defines “active
agents” as those. A similar statement is made on p12 albeit the structural
formula for maleic acid is used instead of the name in words.
76.
Wide pH ranges (about pH 3 to about pH 12, preferably pH 5 to pH 8) are
given. Wide ranges are stated for the weight % (wt%) active agent. The widest
wt% is from 0.01 wt% to about 50 wt%. (More figures for wt% are given at p22.)
Excipients are listed from p13 and include wide ranges for the wt% of the
excipients. Forms of the formulation are described such as sprays,
conditioners, shampoos, creams and liquid active agent formulations.
77.
Methods of use are discussed at p22. The method involves applying a
colouring formulation to the hair which may be a highlighting formulation made
from mixing bleach powder and developer. The application of the active agent
is described at p23 onwards. At the end of this section at p25 ln6 is the
following:
“The formulation described herein improves hair quality, such
as appearance (e.g., sheen) and feel, and decreases hair breakage when the hair
is subjected to treatments, such as coloring or permanent waving.
In some embodiments, hair breakage decreases by 5, 10, 15,
20, 25, 30, 35, 40, or 50% or higher after treatment with the active agent
compared to untreated hair from the same individual. Hair breakage is a
significant problem encountered during coloring and other treatments.”
78.
Next, on p25 at line 10, is a section about perming and reducing
agents. Again it is a bit confusing but it is entitled “B Reference – Chemical
treatment of hair with a reducing agent”. The reader would understand the word
“Reference” indicates that this text is not purporting to describe something
within the claims. Another “Reference” section starts at p27 line 18 about
applying active agents to skin or nails. Then a “kit for treating hair” is
described from p28-29. One possibility is that the active agent is provided as
a dry powder (p22 ln10-11).
79.
The examples start at p30 with example 2. Examples 2, 4, 5, 6, 7 and 8
are all labelled Reference.
80.
The only example of what is claimed is example 3. It describes taking two
swatches of hair from the same head. The hair was medium brown. Both swatches
were lightened using a developer and powder bleach. With Swatch 1 the active agent
formulation was added, with Swatch 2 it was not. The active agent formulation
contained maleic acid at a concentration of 2.0g in 10g water. Confusingly the
reference to the active agent formulation refers to example 1 and to
concentrations (plural) but nothing turns on that.
81.
The products were applied with a brush as the hair lay on aluminium
foil. This is like a highlighting process.
82.
The patent explains that after the process “a noticeable difference in
hair quality … was observed”. The hair treated with the active ingredient was
“softer, less frizzy, appeared hydrated with more shine”. Both swatches were
washed and treated 5 more times “with the same noticeable benefits” of the
treated sample as opposed to the control.
83.
L’Oréal points out that the analysis in this example is subjective and
has no statistics. That is true. Nevertheless the skilled reader would take
this result at face value and would be interested in it. It is indicative that
something beneficial is taking place.
84.
Although not relevant to construction, it is convenient at this stage to
note that using after acquired knowledge we now know today that the invention
does work. To the extent it matters, it is legitimate to take that knowledge
into account since (I find) the disclosure in the patent renders the invention
plausible despite the thin nature of Example 3. Dr Hefford noted that the
invention does “do something”.
The points on
construction in the claims
85.
Claim 1 calls for a method which is for providing bleached hair. That
means it is a method suitable for achieving that result. Two formulations are
defined, one with a bleaching agent and the other with the active agent. The
bleaching agent could be hydrogen peroxide but need not be.
86.
There is a point on the terms “active” or “active agent” but that is
best addressed in context (Catzy).
87.
The claim does require two formulations to be produced but they are then
applied to the hair simultaneously. I suppose that means they could be applied
separately but at the same time. In any case it clearly also includes mixing
them together in advance and then applying the mixture to the hair.
88.
Claim 11 is a claim to the use of an active agent defined in the same
way as claim 1. The active agent is used simultaneously with a bleaching
agent. The active agent is used “to reduce or prevent hair damage due to a
treatment to provide bleached hair”. The reference to providing bleached hair
would be understood in the same way as in claim 1.
89.
The achievement of the result of reducing or preventing damage (etc.) is
a functional technical feature of claim 11. That may well involve examining
the state of mind of the person formulating a maleic acid treatment and putting
it in a bottle to sell, the state of mind of the person selling the product
and/or the state of mind of the colorist or consumer using the product.
However nothing turns on that in this case.
Provide bleached
hair
90.
The first issue is about providing bleached hair. Read in context and
with the common general knowledge I find this means a process of lightening
hair. It is true that the patent uses the term colouring to refer to processes
which involve bleaching alone and also dyeing but “colouring” is not the word
used in the claim. The reader would understand the inventors to have used the
words “a method for providing bleached hair” as a reference to a hair
lightening process. That is at least a (if not the) natural meaning of those
words. The reader would also see that if the inventors had wanted to cover
both hair lightening and colouring using dye they could simply have used the
word colouring (or “a method for providing coloured hair”). It is noticeable
that the inventors did not do that. In context “bleached hair” would be
understood to refer to hair that has been the subject of a hair lightening
process. It would not be understood to refer to hair dyed using oxidation dyes
even though the skilled reader understands perfectly well that strictly
speaking the hair produced at the end of an oxidation dye process has also been
subject to bleaching at least to some extent.
91.
It is also true that the patent teaches that the invention would work in
an oxidation dyeing process and the skilled reader would see that since the
invention seems to work by ameliorating chemical damage caused by oxidation, it
is likely to work to some extent also when oxidation dyes are used, since those
methods do involve some oxidation of the hair. However this does not justify
reading the words of claim 1 as if they include an oxidation dyeing process.
92.
I do not believe anything turns on the fact that the claim does not
refer to an accelerator or booster.
93.
The same conclusion follows for claim 11.
Maleic acid or a
simple salt thereof
94.
In terms of construction there is no relevant difference between the
words “maleic acid” and the formula shown in unamended claim 1.
95.
The skilled reader would understand that strictly speaking maleic acid –
and the formula – refer to a chemical compound in which the two hydrogens
(protons) are bound to the oxygens in the carboxylic acid groups. The skilled
reader would also understand that as soon as maleic acid was dissolved in
water, the molecule would dissociate and the species present would depend on pH.
The skilled reader would also expect that the thing which actually mattered as
far as achieving a relevant effect on the hair is concerned was one or both of
the ions forms, hydrogen maleate and maleate. If they had to distinguish
between the two ions (I do not believe it matters) they would think the maleate
was the relevant ion since that will predominate at high pH and high pH
represents the conditions when the bleaching takes place.
96.
So there are two feasible constructions of the term maleic acid. One is
limited strictly to the un-ionised molecule and the other includes that
molecule and the ions it forms in aqueous solution. I will refer to these two
as Meanings A and B respectively. It is clear that out of context the skilled
person could interpret that term either way but it is also clear that in
general, in the context of aqueous systems, the skilled person would favour
Meaning B.
97.
Turning to “simple salt”, that term also has multiple possible
meanings. Focussing first on the word salt, rather like maleic acid, that term
could refer to the undissociated form only (in effect the ionic crystalline
solid) or it could include a solution in which the anion and cation which were
together in the solid form of the salt are in solution. Either is tenable.
Just thinking about table salt, the term can refer to the white powder but one
can also refer to a salt solution.
98.
Focussing on the term “simple” or “simple salt”, neither is commonly
used in hair care. Dr Hefford suggested the following. The opposite of a
simple salt could be a double salt such as NaKCl2 which as a solid
would have a different lattice from either NaCl or KCl. Or the opposite could
be a complex salt in the sense of a salt in which one of the ions is a complex
such as the hexaminecobalt ion made up of a cobalt atom and six amine elements
in hexaminecobalt (III) chloride. Another possibility raised by Dr Hefford was
that a complex salt meant that the ion made more than one atom but I reject
that one.
99.
Yet another approach is to interpret the reference to simple salts of
maleic acid as an attempt to draw a distinction between salts of maleic acid in
which the counter ion has some structural feature or functional group which the
skilled person would expect to perform a significant function or affect the way
the invention works as compared to maleic acid itself. So for example if the
counter ion acted as a linker molecule which affected the way the maleic acid
worked, maybe the salt of the maleic acid plus that counter ion was not a
simple salt. A simple salt would be one in which the counter ion had no such
effect. I will call this construction the non-functional counter ion
construction. This construction of simple salt was advanced by Olaplex in its
statements of case (see paragraph 3 of the Claimants’ Amended Reply Statements
of Case on Validity (KR790, WO768 and CATZY)), cross-referred to in paragraph 4
of the equivalent statement of case relating to the prior use. This latter
statement of case advanced Olaplex’s case that the prior used maleic acid
diamine salt was not a simple salt.
100.
However at trial Olaplex submitted that the correct construction of
maleic acid was Meaning B and the correct construction of salt was as a
reference to the undissolved solid. The consequence of Meaning B would be that
any salt of maleic acid, once it was in solution and produced the relevant
ions, would be encompassed within the claim regardless of the scope of “simple
salt”. That would have the result that the prior used formulation of Olaplex would
fall within the claim because whether or not the counter ion in the diamine
salt was functional or not would be irrelevant to the question whether the
formulation was encompassed by “maleic acid”. As Olaplex submitted in its
Closing at paragraph 93, on this basis “it does not matter from the point of
view of infringement what other ions may be in solution along with the
free-floating maleate or hydrogen maleate ions.” Therefore at trial Olaplex
was abandoning the non-functional counter ion construction of simple salt
advanced in its Statements of Case. It submitted that its approach to
construction of maleic acid was not inconsistent with the Statement of Case
because the document did not say the that the prior used Olaplex formulation was
not maleic acid.
101.
The potential tangles caused by this construction are tolerably clear
although complicated to explain. If simple salt bears the non-functional
counter ion construction then the reference to maleic acid in the composite
expression “maleic acid or a simple salt thereof” makes more sense bearing
Meaning A than it does bearing Meaning B. That is because the non-functional
counter ion construction of simple salt is inconsistent with the wider Meaning
B of maleic acid. So on that basis, the composite expression “maleic acid or a
simple salt thereof” in which Meaning A applies would therefore encompass the
undissociated maleic acid molecule and also maleate or hydrogen maleate ions
but only when those ions do not have a functional counter ion. And on that
basis, for example, the prior used diamine salt would fall outside claim 1
altogether because it was neither undissociated maleic acid nor did it have a
non-functional counter ion. So far so good but there is a problem. Amending
to delete “simple salt” from claim 1 leaves the claim just with the reference
to maleic acid. Without the term “simple salt”, the skilled person would
favour Meaning B for maleic acid. So claim 1 as amended would encompass the
prior used diamine salt. But that kind of extension of scope by amendment is
forbidden by Art 123(3) EPC and s76 of the 1977 Act. And it could get even
more complicated if one considers equivalents. If after amendment the term
maleic acid remains construed as Meaning A, perhaps nevertheless the diamine
salt, while not within the normal construction of Meaning A, would still satisfy
the second stage of the Actavis analysis as an
equivalent? Whereas it would not have satisfied that second stage based on the
un-amended claim because it was expressly excluded by the non-functional
counter ion construction of simple salt.
102.
Despite this potential complexity, in the end I believe the issues are
relatively straightforward to resolve if one comes back to basic principles and
reads the claim through the eyes of the skilled reader and in the context of
the patent as a whole. The skilled reader would see that the formulations used
in the invention are largely aqueous (although they do not have to be). They
would favour Meaning B for the term maleic acid. In other words they would
think the inventors by using that term intended to mean both the undissociated
molecule and the maleate and hydrogen maleate ions. The term simple salt has
no well defined meaning for the skilled reader. The idea that it has something
to do with functional counter ions would never occur to them. Given the width
of Meaning B, they would think salt referred to the solid form of the material
and this is consistent with the idea that the active agent could be in powder
form (p22 ln 10 of the specification). Therefore the composite phrase “maleic
acid or a simple salt thereof” would make sense as including a formulation in
solution in which there could be undissociated maleic acid species and/or
maleate and hydrogen maleate ions and also covering simple salts in solid
form. The term simple would be understood in one of the ways referred to by Dr
Hefford – most probably as a reference to the ion complex.
103.
Accordingly the prior used formulation of the diamine maleate salt would
fall within claim 1 in its granted form and amending to delete the reference to
simple salt has the effect of narrowing the scope of the claim. It does not
have the effect of extending the scope of protection.
104.
The same conclusion follows for claim 11.
Independently
valid claims
105.
By closing Olaplex relied on claims 1, 3, 4 and 11 as being
independently valid.
106.
Claim 3 adds a limitation that “the first formulation and the second
formulation are mixed at the time of use and prior to application”. Claim 4 limits
the amount of active agent in the mixture applied to the hair to the range 0.1
– 50 wt%.
Amendments
107.
Four sets of amendments had been proposed. The first set were advanced
unconditionally to delete “or a simple salt thereof” from claim 1 and 11. On
the construction I have reached of claim 1 this deletion does not extend the
scope of protection. Olaplex proposed that the right way to bring the
specification into conformity with that amendment was to delete the same words
from page 11 (line 7) and page 12 (line 11). I agree. I will therefore allow
that amendment in that form.
108.
The second, third and fourth sets of amendments were conditional,
intended to cure various kinds of invalidity if contrary to Olaplex’s case, the
relevant attack succeeds. By closing only the second one, Fall Back 2, was
pressed. I will refer to it as Fall Back 2 to maintain consistency with the
terms used in the trial bundles.
109.
Fall back amendment 2 changes claims 1 and 11 as follows:
Fall back 2 Claim 1
A method for providing bleached hair comprising:
(a) applying to the hair a first formulation comprising a
bleaching agent comprised of bleach powder and developer; and
(b) applying to the hair a second formulation having a pH
range from 3 to 8 and comprising an active agent, wherein the active agent
is
or a simple salt thereof;
and wherein step (a) occurs simultaneously with step (b).
Fall back 2 Claim 11
The use of an active agent which is

or a simple salt thereof
simultaneously with a bleaching agent
to reduce or prevent hair damage due to a treatment to
provide bleached hair the use comprising:
(a) applying to the hair a first formulation comprising a
bleaching agent comprised of bleach powder and developer; and
(b) applying to the hair a second formulation having a pH
range from 3 to 8 and comprising the active agent.
110.
There is no formal objection to this amendment (over and above the
arguments about the deletion of simple salt). The point of the amendment
really applies if providing bleached hair were to be construed as including a
dyeing process. By requiring both a bleaching agent and a developer the claim
does not cover conventional dyeing formulations irrespective of the scope of
providing bleached hair because although those dyeing formulations include
hydrogen peroxide they do not include persulfate developer. Therefore even if
unamended claim 1 lacked novelty over Kim on the construction of providing
bleached hair which included dyeing, this claim is novel over Kim.
Infringement
111.
The relevant ingredients of L’Oréal’s Smartbond product are maleic acid
and ethanolamine. The product description gives the pH of the formulation as 3
±0.2. At that pH the maleic acid in the formulation will mostly be in the form
of hydrogen maleate, with some undissociated maleic acid. Therefore Smartbond
is a means relating to an essential element of the invention claimed in claims
1 and 11 (unamended and unconditionally amended). That conclusion also applies
to claim 3 (the product falls within the wide wt% range) and claim 4 (no point
arose on the way the product would be used or the directions for use).
112.
At this stage I can also deal with the alternative formulation the
subject of L’Oréal’s application for a declaration of non-infringement. In the
alternative formulation the two ingredients maleic acid and ethanolamine are
put together to form a salt before being dissolved to make the formulation.
This might have been relevant on different constructions of unamended claim 1
but as I have construed the claim the issue does not arise. This alternative
approach would infringe.
113.
Claims 1 and 11 of the Fall-Back 2 amendments introduce two features.
No issue arises relating to the fact the bleaching agent comprises bleach
powder and developer. The other feature places a lower limit on the pH of the
“second formulation”, i.e. the one containing maleic acid. L’Oréal contended
that since the formulation was described in the product description as having a
pH 3 ±0.2 it followed that not every batch of the material would infringe.
Olaplex did not agree.
114.
It was said to be common ground that the lower limit of the pH range in
this claim was “exactly 3”, by which I understood the parties to mean 3 with as
many zeros after the decimal point as you like. Therefore it was common ground
that a material with a pH which is 2.9 and not 3.0 would not infringe. So
L’Oréal submitted that since the product description allowed for material with
a pH 3 ±0.2, it encompassed material with a pH 2.9 (for example) and that would
not infringe. Therefore L’Oréal submitted while the court could find there was
probably infringement sometimes (since material with pH 3.0 would occur as well
and one might assume that pH 3.1 is as likely as pH 2.9 assuming a normal
distribution), the court should leave the question of the extent of
infringement to any inquiry as to damages. There might also be a point on any
injunction if the process produces material which dips in and out of
infringement.
115.
I am not satisfied that this case is one in which the material alleged
to infringe varies as it is made such that sometimes it has a pH outside the
claimed range and sometimes it falls within the claimed range. The level of
precision of the ingredients in the Smartbond formulation stated in the product
description varies. It is not giving anything secret away to set out the
figures after the decimal points for the wt% for the six ingredients:
Maleic acid X.701
Ethanolamine X.4
Ingredient X.0001
Ingredient X.000011
Ingredient X.00008
Ingredient X.898809
116.
The pH of a mixture is just the result of the aggregate effect of the various
ingredients in the concentrations they have. The precision stated for the ingredients
indicates that the formulation is made to quite a high precision. Mr Legrand
confirmed in his cross-examination if you make up the formulation of Smartbond,
it is always going have a pH of 3. The reason for the stated range of ±0.2 is
not due to a variation in the pH of the underlying material, it is to deal with
possible variations in the calibration of the measuring equipment. I find that
the pH of the Smartbond material which is made by L’Oréal has a pH of 3. The
material infringes. There is no issue of variation which might need to be
addressed at an inquiry.
117.
Accordingly L’Oréal infringes all relevant claims.
Priority
118.
The only live issue of priority was one which would only arise if I
refused to allow the unconditional amendment. I will address it in case the
matter goes further. The question is whether “simple salt” is entitled to
priority. The priority document discloses a much wider range of active agents
than are claimed in the granted claims. Included within the disclosure of the
priority document (e.g. at p12 ln14-15) is a reference to especially preferred
compounds being maleic acid and salts thereof. See also claims 7, 21 and 27.
Nowhere is the word salt qualified by the word simple. If it was necessary to
do so I would reject the claim to priority. I accept part of Olaplex’s case
that it is not necessarily the case that the word simple has to be found expressly
in the priority document. And I would find that the common general knowledge
of the skilled person would include the idea that salts can be classified into
different types. Having held that simple salt distinguishes from other salts in
one of the ways described by Dr Hefford, it seems to me that for simple salt to
be able to claim priority the priority document would have to contain at least
some teaching somewhere that it might be relevant to think about that
distinction (whichever one it was) between types of salt. That is absent. So
there is no basis for a distinction between kinds of salt and no priority.
The prior art
119.
Normally I would address validity by legal category – novelty then obviousness.
In this case it is more convenient to approach the issues by taking each item
of cited prior art and working through the case arising from it.
The prior use
120.
There is no doubt that substantial quantities of Olaplex Bond Multiplier
were distributed to colorists in the USA before the 16th May 2014
priority date. Nor is there any dispute that if a person had such a sample and
analysed it they would find it contained an appreciable amount of the diamine maleate
salt. As a matter of fact the information they would acquire would, if the
person was free to obtain and use it, make the invention available to that
person. All the claims including all proposed amendments and all allegedly
independently valid claims stand or fall together over this alleged prior use.
The issue is to decide on the terms on which the samples were distributed.
121.
The law is clear and not in dispute. As Lord Hoffmann explained in the House
of Lords in Merrell Dow v Norton [1996] RPC 76, the use of
a product makes the invention part of the state of the art only so far as that
use makes available the necessary information. Pall Corp v Commercial
Hydraulics [1990] FSR 329 demonstrates that whether giving someone
a sample of product makes its contents available to the public or not depends
on the terms on which the sample was provided. In Pall Corp
experimental samples were given to potential customers for testing. Two
instances of use were relied on. The first instance was supply of six samples
of the microporous 6.6 nylon membrane to Motorola which were then used in
public tests in the presence of trade rivals. Falconer J held that the samples
to Motorola were experimental and secret and that the testing did not reveal
any information about the nature of the product. Accordingly although the
tests were in public, they did not make available to the public the information
necessary to reveal the invention. The second instance involved the supply of
more samples to other customers. Again they were experimental and secret and
the recipients knew they were confidential and so again there was no making
available to the public of the relevant information.
122.
Olaplex submitted that in the case of a product whose chemical
composition is not identified and could only have been identified by a process
of chemical analysis it would have to be shown that the recipient of the
product was free as a matter of law and equity both to send the composition
away for analysis and then subsequently to make use of the information for his
or her own purposes including the public disclosure of that information. I
accept that submission.
123.
Olaplex also submitted, correctly, that English law recognises an equitable
obligation of confidence in appropriate circumstances, citing Coco v
Clark [1969] RPC 41 and the Spycatcher case (A-G v
Guardian Newspapers (No 2) [1990] 1 AC 109, 281 (per Lord Goff):
“I start with the broad general principle (which I do not
intend in any way to be definitive) that a duty of confidence arises when
confidential information comes to the knowledge of a person… in circumstances
where he has notice, or is held to have agreed, that the information is
confidential, with the effect that it would be just in all the circumstances
that he should be precluded from disclosing the information to others.”
124.
The main witness for Olaplex was Dean Christal. He has experience in
the hair care industry. He explained that the product was developed by the
inventors Dr Hawker and Dr Pressly. They did some work in a garage in Santa
Barbara. Olaplex was a tiny company. In early 2013 Mr Christal was given some
product to test and he did so by perming some swatches of hair. He liked the
results. Mr Christal identified a person called Joe Santy. Joe Santy was a
well known hair stylist referred to as the King of Perms. Neither Mr Christal
nor the inventors knew him personally. They asked him to sign a non-disclosure
agreement (NDA) which he did on 17th July 2013. After that for a
few months Mr Santy tried out the prototype which had been developed. At that
time Mr Santy was not told the identity of the ingredients in the liquid being
tested. Mr Christal did not know either. At some stage (late 2013 or early
2014) Mr Christal wanted to work with colorists and others in the industry to
test and further develop the product.
125.
The first patent application was filed on 1st August 2013. A
number of further patent applications followed. Mr Christal’s evidence was
that his only involvement with patent applications was in paying for them.
126.
Mr Christal met the first colorist who was going to test the product in
February 2014. Her name was Tracey Cunningham. Ms Cunningham is and was a
co-owner of the Mèche salon in Los Angeles. She was and remains a famous
colorist with many celebrity clients. When Mr Christal was introduced to Ms
Cunningham she was coloring the hair of a famous actress. Mr Christal says in
his witness statement that he gave samples to Ms Cunningham to test, telling
her that they contained a new secret formula that was for her use only and that
she had to keep the product in her possession at all times, was not to leave it
lying around and was not to take it out of the salon unless she was doing
celebrity house calls. For the next month or two Mr Christal hand delivered
samples to Ms Cunningham. He was a frequent visitor to the salon. Part of the
testing programme involved considering and adjusting the instructions for use.
He sought and obtained feedback from the testers.
127.
Since then a number of other colorists became testers too. Mr
Christal’s evidence is that this was on the same essential terms, i.e. that the
samples they were given were experimental samples for testing only and not to
be distributed further. Mr Christal said that he did not want to give
colorists NDAs to sign because in his experience that was not how they worked.
Mr Christal said that his approach was to explain the confidential terms to the
colorists personally. That way they could clear up anything they were not sure
about. If they were not prepared to agree to his terms he would not have given
them the product.
128.
It is clear that the testing was on a reasonably large scale. Somewhere
in the region of 20 colorists received samples before the priority date. Ms
Cunningham clearly used a lot of material herself. There was a significant
social media buzz about the Olaplex product, encouraged by Mr Christal. There
are numerous references on Instagram before the priority date to famous women
who had had Olaplex used on their hair. So this is a case, like Pall Corp,
in which the public did know that a product of some kind was being tested.
129.
The bottles of the product used did not carry a list of ingredients nor
did they carry any words to indicate that they contained a secret formula or
that they must not be distributed.
130.
Both Mr Christal and the other witnesses concerned with the prior use,
and also Dr Pressly, were cross-examined about all this. As L’Oréal points
out, there is no documentary evidence to support Olaplex’s case. No NDAs were
sought (after Joe Santy) and there is no email in which Mr Christal puts the
terms on which he was providing the samples to the testers in writing or in
which a tester asks any question about those terms. So the evidence in favour
of Olaplex’s case is primarily the testimony of Mr Christal, corroborated, to
the extent it is, by the other witnesses.
131.
In closing L’Oréal confined its case to three disclosures: (i) to Tracey
Cunningham herself, (ii) to Guy Tang, another colorist unconnected with Ms Cunningham,
and (iii) to Esther Vasquez and Sylvie Vaught, who worked at a salon called Estilo.
This was a realistic approach because if L’Oréal’s case does not succeed on
those instances, it is not going to succeed on the others. Since the issues
overlap I will summarise the three of the instances relied on and the points
they raise before deciding the issues.
132.
The involvement of Ms Cunningham has been described already. L’Oréal’s
case is that the restrictions said to have been put on Tracey Cunningham’s use
of Olaplex are demonstrably false - she considered herself free to take Olaplex
to Dubai, she provided samples to other hair colourists in New York at the Met
Ball and invited a colorist called Vicki Laris to use product in the Mèche
Salon. She used 100s of bottles of Olaplex and posted widely on social media.
L’Oréal contends that for reasons not properly explained she has not given
evidence to the court notwithstanding she owns 4% of Olaplex. L’Oréal submits
there is no evidence to support the suggestion that she was unable to do as she
pleased with the samples and so, overall, the disclosure to Ms Cunningham
should be held to be free of restriction and invalidating.
133.
Guy Tang is a colorist who approached Mr Christal directly asking to use
Olaplex. He was unknown to Mr Christal. Mr Tang had heard about Olaplex from
social media posts by Ms Cunningham. Mr Tang had an internet celebrity client
and he wanted to use the product on her. The first contact between Mr Tang and
Mr Christal was by email. Mr Tang was clearly enthusiastic. His emails
emphasise his social media following. Mr Christal’s testimony was that he
spoke to Mr Tang on the telephone and then met him in Los Angeles. Mr Christal
gave the samples to him at the meeting and at that meeting Mr Christal
explained what the parameters were about being a tester, that it was a secret
formula and that he had to keep it confidential and could not share it.
134.
L’Oréal contended that Mr Tang was unknown to Dean Christal. Mr
Christal was particularly interested in using Mr Tang because he was treating
the particular internet celebrity and Mr Christal made particular efforts to
get Olaplex to him before her treatment. Mr Christal was plainly interested in
Mr Tang because of his enthusiasm and his huge following on social media. In
his written evidence he said he was “initially reluctant” to use Mr Tang
but L’Oréal submit this is not apparent in the email chain. L’Oréal submitted
that the witness statement implied that an explanation as to confidentiality
took place at Mr Tang’s salon (Salon Republic) but that in cross-examination when
it was pointed out that Mr Christal’s email said he would only need 10 minutes with
Mr Tang at the salon, Mr Christal suggested that he had a long conversation on
the telephone. L’Oréal submitted that the court should hold that on the balance
of probabilities the disclosure to Mr Tang was not under conditions of
confidence and Mr Tang was not prevented from using the sample he had been
provided with as he pleased.
135.
Finally L’Oréal relies on Esther Vasquez and Sylvie Vaught. Ms Vasquez
had a telephone conversation with L’Oréal’s solicitor Rachel Boakes. She said to
Ms Boakes that she did not remember Mr Christal talking about confidentiality
or any restrictions on use. Ms Vasquez then filed a statement stating that “Dean
made it clear that the product was for our use only and it could not be shared
with other or given to third parties”. She did not make herself available for
cross-examination. L’Oréal contends that it is notable that Mz Vasquez does
not mention the phone conversation with Rachel Boakes and seek to explain the
change of recollection; nor does she say that Ms Boakes misunderstood her or
that on reflection she realised she was wrong.
136.
Sylvie Vaught worked at the Estilo salon. Ms Vasquez was her assistant
in 2014. Ms Vaught knew Tracey Cunningham and respected her as a colorist. She
would be interested in trying out anything Ms Cunningham was using. Ms Boakes’s
notes of the conversation with Ms Vasquez were put to Ms Vaught in cross-examination.
She said she did not remember it in the way described in the note. L’Oréal
submitted that Ms Vaught said she could not clearly remember a conversation
about confidentiality. She did indeed say that but she also said that it
struck her that the confidentiality was a given, because she was testing a
product. She said she absolutely knew that there were aspects of the product that
were confidential, because she and Ms Vasquez were testing a product.
137.
I turn to decide the issues. It is clear that Tracey Cunningham did
take the Olaplex samples to Dubai and used them there. L’Oréal say this shows
that Mr Christal’s evidence about the restrictions on Ms Cunningham was
demonstrably false because one of the restrictions was that she was not
supposed to use the samples outside her salon save for celebrity house calls. In
cross-examination Mr Christal sought to equate the activity in Dubai with a
form of celebrity house call. That was unconvincing. I find that Ms Cunningham
did use the samples of the Olaplex material in at least one salon in Dubai.
She clearly felt free to use it in that salon. I very much doubt it was used
solely on celebrities as some kind of attempt to fit this activity into a
“celebrity house call” exception.
138.
This indicates that Mr Christal’s evidence is not entirely accurate.
However he maintained, in the face of sustained questioning, that he did make
it clear to Tracey Cunningham (and all the other testers to whom he gave
product) that the product was a secret formula for testing and that they must
only use the sample for testing and not distribute it to anyone else. I will
refer to these core restrictions as the “general restrictions”. The question
is whether Mr Christal’s demonstrably inaccurate testimony about the exact
scope of the restrictions applicable to Ms Cunningham shows that the main
thrust of his evidence, which is about the general restrictions, should not be
accepted.
139.
The other bases on which L’Oréal submit that Mr Christal’s evidence was
demonstrably false are less clear cut. Tracey Cunningham clearly took samples
of Olaplex to New York on the occasion of a glamourous event called the Met
Ball. Ms Lim, one of Ms Cunningham’s assistants also gave evidence about
this. At least some of the samples Ms Cunningham was taking were because she
was supposed to be delivering product to another stylist called Harry Josh, who
had agreed to be a tester and with whom Mr Christal says he had discussed the
general restrictions. She seems not to have given Mr Josh the product at the
Met Ball itself because the proceedings were very busy with celebrities
apparently needing to get their hair done at the event itself. But giving
samples to Harry Josh does not falsify Mr Christal’s testimony. The question
is whether Ms Cunningham gave samples to anyone else.
140.
This is a puzzling episode and I was doubtful about the attempts to
minimise the significance of the samples made to be taken to New York. As best
one can tell the preparation of the samples with instruction sheets inside and
the likely quantity (I am not convinced the relevant emails were just talking
about two bags) looks more consistent with samples being prepared to be handed
out to a variety of people rather than just making a lot of bags to give to
Harry Josh. But in the end there is just no reliable evidence that that is
what actually happened. I find that the only person to whom Tracey Cunningham gave
samples on that trip was Harry Josh. That means Mr Christal’s evidence on this
was not wrong.
141.
The final issue relating to acts carried out by Tracey Cunningham relied
on to show that Mr Christal’s testimony about her was wrong, is L’Oréal’s submission
that she invited Vicki Laris to use product at the Mèche Salon. The point is
that, based on Mr Christal’s evidence, Ms Cunningham was not supposed to be
handing out samples for others to use (even if she did make clear they were
confidential).
142.
Vicki Laris is a professional hair colourist in Chicago. She gave
evidence at the trial. In cross-examination she explained that she could
remember talking to Mr Christal on the telephone but could not remember when
that call took place. She could remember that he asked her not to give Olaplex
product to anyone else, and that she was the only one to use it. She also
remembered that Mr Christal was going to be sending her Olaplex product samples
so that she could test the product, and that he wanted her feedback sent back
to him.
143.
However the point against the claimants is that even assuming this is
true, it must have happened after Vicki Laris had already encountered Olaplex
at the Méche salon. It is clear that Ms Laris did
use some Olaplex material whilst she was working at the Méche salon. Mr
Christal’s suggestion about this in cross-examination (which Ms Laris did not
hear) was that she could very well have been assisting Ms Cunningham, and that
he would assume and know that Ms Cunningham would have explained the
confidentiality to Ms Laris as part of that exercise.
144.
Vicki Laris’s evidence in cross-examination was that she went out and
worked with Tracey Cunningham at the Méche salon quite often. She could not
remember why she was there in March 2014; she may have been working. When at
Méche she assisted Ms Cunningham and also dealt with clients at the salon. Ms
Laris said that Ms Cunningham gave her some Olaplex and told her that it was
being tested for the prevention of breaking the hair during the chemical
process. She wanted Ms Laris to try it; Ms Laris ended up trying it on herself.
145.
So in her cross-examination Vicki Laris did say that when Tracey
Cunningham gave her some Olaplex to try she was told it was for testing but Ms
Laris did not say that Ms Cunningham said anything to her about
confidentiality.
146.
Whether Tracey Cunningham did tell Vicki Laris that the material was
confidential (or words to that effect) or not, this episode does show that Mr
Christal’s evidence about the restrictions placed on Ms Cunningham is either
inaccurate or else, even if such restrictions were put to Ms Cunningham in the
precise terms Mr Christal described in his written evidence, they were not in
fact adhered to (and if that is true I am sure Mr Christal knew that since he
spoke to Vicki Laris on the phone afterwards). So at best this demonstrates again
that the evidence given in Mr Christal’s written statements is inaccurate.
Either that or positively misleading.
147.
Given the manner in which L’Oréal put their case, the relevance of the
issues relating to Vicki Laris is about impugning Mr Christal’s evidence. I do
not believe it is strictly necessary to go further and decide the basis on
which Tracey Cunningham gave the product to Vicki Laris. However in case it is
relevant, I will say this. Having heard Vicki Laris give evidence, in my
judgment that question stands or falls with the question of the terms on which
the samples were given to Ms Cunningham in the first place. If Ms Cunningham
was given the samples subject to the general restrictions (defined above) then
I would not find that Ms Cunningham failed to comply with those restrictions
when she gave product to Vicki Laris. If Ms Cunningham had been told the
product was subject to the general restrictions then it is more likely than not
that she told the same thing to Vicki Laris when she gave the product to Ms Laris
to test.
148.
I did not get much assistance from the evidence of Jordan Alexander.
L’Oréal submitted it showed that Tracey Cunningham was distributing samples free
of any restrictions to colorists at Mèche before
the priority date for them to use. Mr Alexander had been confused about dates
but he was wrong to say that the product was only used at Mèche by Tracey
Cunningham or her assistants. Others clearly did use it. But I cannot infer
from this that those others were not given the product subject to the general
restrictions.
149.
The next major issue about Tracey Cunningham is her reasons for not
attending trial. Ms Cunningham gave a very short witness statement from which,
Olaplex submits, the obvious inference is that the reason she does not wish to
give evidence is because she has significant commercial relationships with both
parties. She is a 4% shareholder in Olaplex and a Redken product ambassador
(Redken is a major L’Oréal product). L’Oréal submits that this inference is
not what the witness statement actually says and the inference does not
follow. L’Oréal contends that the true reason Tracey Cunningham has not given
evidence is because her truthful evidence would be very bad for Olaplex’s case and
she has a valuable shareholding. I am not prepared to draw that inference. If
L’Oréal thought her evidence could assist them they could have approached her
or even used the evidence gathering techniques available in the USA to obtain
evidence for use in foreign proceedings. I do not draw any inference about the
evidence Ms Cunningham would have given. I do infer that she did not wish to
give evidence because she has a relationship she values with both sides.
150.
Accordingly in conclusion on Tracey Cunningham, the key evidence that
she was given samples subject to general restrictions and therefore was not
free in law and equity to distribute them or analyse them herself is the
testimony of Mr Christal. I will address that at the end.
151.
The next issue is Guy Tang. There is a point of contrast between Mr
Tang and Joe Santy – since both were unknown to Mr Christal or the inventors
but only Mr Santy was required to fill in an NDA. But Mr Christal’s answer is
that he wished to and did speak to Mr Tang personally. There is also a point
that time was short and a point on the tone of the email exchange. But in the
end the issue is the same as for Tracey Cunningham. Like Ms Cunningham, Mr
Tang was provided with samples. The evidence from which the claimants invite
the court to find that Mr Tang was subject to general restrictions is the
testimony of Mr Christal. That depends on his credibility.
152.
Finally the Estilo salon. That is a different matter. Based on Ms
Vaught’s evidence in cross-examination I reject this aspect of L’Oréal’s case.
I find that Ms Vaught and Ms Vasquez were only provided with samples of Olaplex
product under the conditions I have called the general restrictions. I do not
need to consider this point further.
153.
So of the three episodes, for the two I have not yet decided (Cunningham
and Tang) looking at the evidence as a whole, the issue turns on the
reliability of Mr Christal’s evidence.
154.
The other points taken about Mr Christal’s evidence were the following.
It was said that he failed to explain why there were no confidentiality
agreements in writing. He did not fail to explain that. He explained that he
wished to work face to face. That is not inherently improbable. L’Oréal also
suggested that his evidence that he thought colorists would not sign NDAs was
falsified by the fact that colorists sign NDAs to work with celebrities. I
reject that point. As Mr Christal explained the relative power in the
relationship is different and the fact colorists sign NDAs so that they can
work with celebrities does not undermine Mr Christal’s evidence that he thought
they would not be prepared to sign an NDA with a start up company they had
never heard of. There was a disagreement between Dr Pressly and Mr Christal
about Mr Christal’s planned approach of not requiring NDAs from the colorist
testers. Mr Christal was not frank about that. Also L’Oréal contends that Mr
Christal received emails from people he did not know who appeared to have used
Olaplex which must have appeared to him at the time as breaches of the general
restrictions. He did not react to them in what L’Oréal says is the way you
would have if that is what you thought. Mr Christal explained this however.
He said he wished to maintain good relations with people and speak to them. I
have dealt with the other matters concerning Mr Christal’s evidence in context.
155.
Standing back, this issue turns not simply on Mr Christal’s general
credibility but on his truthfulness. I put it that way because given what Mr
Christal said, the idea that he could have given the extensive testimony he
gave about what happened but had somehow misremembered what occurred is
fanciful. So also is it fanciful to suppose that Mr Christal could have
mistakenly convinced himself that all that took place happened in the manner he
said when it did not. This is not a case in which the witness could be right
about some occasions but wrong about others. I reject the idea that, for
example, Mr Christal could have gone to the trouble of imposing a duty of
confidence by speaking to Ms Cunningham but misremembered doing so for other
testers. Given his testimony that makes no sense. Mr Christal’s evidence, if
true, is sufficient to make the claimant’s case. Either Mr Christal is lying
and the discussions he described are pure invention, or he is telling the
truth.
156.
In my judgment Mr Christal was not lying. I will not go back over all
the detail. The points raised against Mr Christal’s evidence, particularly
concerning Ms Cunningham, do show that his written evidence is inaccurate but I
do not believe they show he gave untruthful evidence. Those are the errors
made by someone seeking to tell the truth as they saw it and making mistakes of
recollection coupled with a view about what “must have” happened. Standing
back, I find that Mr Christal has told the truth. He did impose the general
restrictions on Tracey Cunningham and on Guy Tang (and a number of others).
Therefore the Olaplex samples, although they were tested in the presence of
members of the public, were always subject to material restrictions on their
use. They did not make any relevant information available to the public. I
reject the validity attack based on prior use.
Catzy
157.
An entry for the Catzy product was published in the Mintel Global New
Products database in about July 2007. Although at one stage L’Oréal also
relied on the availability of the product itself, by the trial only the
publication was relied on. The published information shows a photograph of the
packaging with the title “Catzy Blonde” and indicates that it is a product of
the company Midelfart & Co (which apparently is or was a Norwegian hair
products company). The product description is:
“Catzy Hair Colourant is especially developed for
Scandinavian women. The range is comprised of 14 shades and incorporates
UV-filtration and provitamin B5.”
158.
The product is a hair lightener including three formulations: a
bleaching power which includes persulfate, a mixing cream which includes
hydrogen peroxide; and an after treatment. There is no dye or dye precursor.
159.
The ingredients are stated as follows:
“Bleaching powder: Sodium Silicate, Potassium
Persulfate, CI 77713, Ammonium Persulfate, Paraffinum Liquidum (Liquid),
Silica, Cellulose Gum, Urea, Carbomer, EDTA, Sodium Lauryl Sulfate, CI 77007
Mixing cream: Aqua, Hydrogen Peroxide, Cetearyl
Alcohol, Olea Europaea Fruit, Lanolin, parfum, Benzyl Salicylate, Hexyl
Cinnamal, Hydroxycitronellal, L-limonene, Alpha-isomethyl Ionone, Glyceryl
Stearate, Sodium Stannate, Tartaric Acid, Maleic Acid, Sodium Lauryl Sulfate,
Sodium Cetearyl Sulfate, Sodium Hydroxide
After treatment: Aqua, Cetyl Alcohol, Stearyl Alcohol,
Distearoylethyl Hydroxyethylmonium Methosulfate, Caprylic/Capric Triglyceride,
Helianthus Annuus Extract, Panthenol, Wheat Amino Acids, Triticum Vulgare Germ
Oil, Sorbitol, Cetyl Palmitate, Cetearyl Alcohol, Cetrimonium Chloride,
Isopropyl Alcohol, Ethylhexyl Methoxycinnamate, parfum, Butylene Glycol,
Phenoxyethanol, Propylparaben, Methylparaben, Potassium Sorbate, Disodium EDTA,
Sodium Chloride, BHT, Benzyl Salicylate, Hexyl Cinnamal, L-limonene,
Alpha-isomethyl Ionone (Alpha)”
160.
The point is that one of the ingredients in the mixing cream along with
hydrogen peroxide is maleic acid (fourth from the end).
161.
The amount of maleic acid in Catzy is not stated. To address this
Olaplex produced a letter from a Swedish company called Hardford AB under a
Civil Evidence Act notice. The letter is undated but was obviously produced
for the purpose of this action. The letter explains that Hardford manufactured
the product for Midelfart & Co A/S although they did not develop it. In
the mixing cream the maleic acid content is 0.094 wt%. The evidence is that on
that basis the wt% in the mixture applied to the hair would therefore be 0.071%.
162.
Catzy is only relevant to the method claims of the Olaplex patent.
L’Oréal rightly does not press an allegation of invalidity against use claims
11 or 12. They are novel over Catzy on conventional grounds. There is no
suggestion that the use of maleic acid in order to reduce or prevent hair
damage is obvious over Catzy.
163.
However Catzy is pressed as rendering the method claims lacking novelty
or lacking inventive step. Turning to claim 1, it makes no difference whether
one is considering the granted form of the claim or the unconditional amended
form in which simple salt is deleted, because Catzy discloses maleic acid. Nor
does the “bleached hair” issue matter because Catzy is a hair lightening
product. The Mintel publication discloses a method for providing bleached hair
by using Catzy. The Catzy bleaching powder is a first formulation within claim
1 since it comprises persulfates, well known bleaching agents. Claim 1 has no express
lower limit on the amount of maleic acid in the formulation. Nor is there a
functional limitation other than the need to provide bleached hair. While that
might mean that there has to be enough bleach to work, it does not place a
lower limit on the amount of maleic acid. For the purposes of novelty, what
matters is what is disclosed by the prior art expressly or implicitly (or by
inevitable result). The wt% figures from Hardford are not part of that
disclosure.
164.
Olaplex contended that since it is manifest the maleic acid in Catzy is
an excipient, the mixing cream is not a second formulation within claim 1 because
the maleic acid in Catzy is not an “active agent”. Olaplex pointed out that
the patent specification expressly distinguishes between active agents and
excipients. I agree that the specification does list excipients separately
from the active agents but I do not agree this justifies excluding from a claim
a formulation containing what the patent calls an active agent (maleic acid)
just because the skilled person would assume the original formulator added it
as an excipient, no doubt being unaware of its properties as a molecule which
the patent has disclosed.
165.
Without knowledge of the patent the skilled person looking at Catzy
would say that the maleic acid in the formulation was an excipient. That does
not mean they would think it did not perform any function at all. As a matter
of common general knowledge excipients are by no means necessarily just inert
substances. For example the first class of excipients described by the patent
after the heading excipients on page 13 is surfactants at p14. That reflects
the common general knowledge. The skilled person would identify maleic acid in
the Catzy formulation as being present to perform a function – either pH adjuster/modifier
or as a chelating agent. It would not be seen as inactive.
166.
To construe “active agent” in a manner which excludes maleic acid in a
hair lightening formulation involves either turning claim 1 into some kind of
subjective, purpose limited claim or reading in a functional limitation along
the lines of “in a sufficient amount to do the job”. The former construction is
just not what the words say and since the patent includes use claims anyway
there is no reason the reader would contemplate that such an ordinary method
claim should be understood that way. The latter construction is also
unwarranted. There is nothing in the patent to justify that interpretation. In
argument Olaplex characterised the amount of maleic acid in Catzy as “tiny”.
However the ranges actually stated expressly in the patent include “typical”
amounts with a lower limit of 0.01wt% page 11.
167.
Accordingly I find that the publication describing Catzy, which was made
available to the public before the priority date, deprives claim 1 of novelty.
That applies to the granted form of claim 1 and the unconditionally amended
form.
168.
Claim 3 was said to be independently valid although I did not have my
attention drawn to any circumstance in which it could survive if claim 1 was
anticipated. It might have bare novelty over the published information but
mixing the bleaching powder and mixing cream at the time of use is plainly
obvious.
169.
Claim 4 is relied on as being independently valid on the basis that it
has a lower limit of 0.1 wt% for the maleic acid content. That limit relates
to the wt% in the mixture of the two formulations and so the Catzy product as
described in the Hardford letter is outside claim 4 because the maleic acid in
the mixture would be 0.071%. In any event, irrespective of the Hardford letter
the question is one of obviousness over the Mintel published information rather
than novelty since the particular amount of maleic acid in Catzy is not made
available to the public by the publication in the database.
170.
Based on Dr Hefford’s evidence, I find it would be entirely obvious for
a skilled person, given the information on the Mintel database, to set about
making a product based on it. Copying such a product to make a “me too”
formulation for the same market was not inventive. This is not a reverse
engineering exercise since the skilled person has an ingredient list which does
not specify amounts and does not have the product itself to analyse. Dr
Hefford described the exercise which would be carried out. The formulator
would use their skill to come up with a list of levels for the various
ingredients to try first. It would be educated guesswork.
171.
The skilled person would be able to identify the likely functions of the
various ingredients. Maleic acid was a known ingredient and the skilled person
would identify its function as pH control and/or chelating metal ions.
172.
Pausing at this stage, in my judgment no inventive step would be involved
for the skilled person given the published information about Catzy to produce a
hair lightening formulation based on the ingredients list which included some
maleic acid. They would make up a product using their skill to specify the
amounts for the various components. It would then be tested and the amounts
adjusted as necessary. I find that after adjustment the product would work
satisfactorily and would include a maleic acid component. All of this would be
routine work for the formulator member of the skilled team.
173.
The issue turns on the level of the maleic acid component in the product
produced this way. The skilled person would assume that the ingredients in the
list were listed in order of amount down to 1%, while the ingredients below 1%
could be in any order. The skilled person would know that neither assumption
was necessarily correct but it would be the natural and obvious way to look at
the information. In the mixing cream ingredient list the skilled person would take
it that “parfum” was not above 1%. Therefore (and in any event) the skilled
person would expect that the maleic acid component was in an amount less than
1%.
174.
Dr Hefford’s view expressed before he saw the Hardford letter was that when
the skilled person formulated a product they would end up with about 0.3% to
0.5% maleic acid. He also said he would be extremely surprised if the amount
of maleic acid did not lie between 0.1% and 1% in Catzy.
175.
Olaplex submitted that this approach by Dr Hefford was a “pretty bizarre
approach” and that it was unclear why Dr Hefford considered that this process
(which Olaplex called speculative) would end up with the 0.3% to 0.5% range
since he had never actually tried to do the process with the formulation of
Catzy. Olaplex submitted that a more sensible approach was to start from the
proposition that maleic acid was there for a particular purpose and that you
would simply add enough to do the job. Dr Hefford agreed that you would need
just enough to do the job. The fallacy in Olaplex’s submission here is the
idea that adding enough to do the job is different from Dr Hefford’s view that
the end result would be likely to be 0.3% to 0.5%. Dr Hefford’s position was
that the skilled person would indeed aim to add enough to do the job in the circumstances
they found themselves. The job of maleic acid would be to control pH or as a
chelating agent. His view, given his experience in formulation, was that the
result was likely to be a concentration in that range. I reject the submission
that this was pretty bizarre or speculative. It was solidly based in his
experience.
176.
But Dr Hefford’s opinion based on his experience is not the only
evidence of what the amount of maleic acid might end up being. There is also
the Hardford letter. It was put to Dr Hefford that this represented the best
evidence of what is needed for maleic acid to perform its function. The
formulators will have added enough to do the job and we know how much they
used. Dr Hefford did not agree that this was the best evidence. Olaplex
submitted it was not clear why he did not agree but in fact Dr Hefford
explained his reasons clearly in cross-examination. He explained that he would
look at the sodium stannate, the tartaric acid and the maleic acid as a stabilising
system to prevent the hydrogen peroxide product “going off”. The stability of
hydrogen peroxide relates to pH and to metal ions. The
amount of stabiliser depends on the grade of hydrogen peroxide the manufacturer
has actually used in the formulation. For example cheap hydrogen peroxide
might require a lot of stabiliser. The amount of stabiliser also depends on
the manufacturing tank, filling line and packaging because if those produce a
high level of metal ions, then a high level of metal chelating agent is needed
to take the metal ions out. The skilled person would pass the product through their
manufacturing plant and see how stable it was. If at 0.04% maleic acid, it was
stable then the skilled person would be happy with that. Dr Hefford maintained
that he was surprised the level of chelating agent (by which he meant maleic
acid) was as low as is stated in the Hardford letter but noted that they were
obviously happy with it. However his view was that using Hardford’s level for
that particular ingredient was not relevant to things made in other
manufacturing facilities.
177.
Dr Hefford’s point was not that he did not believe the number in the
Hardford letter (in his second report he had described it as “on the low side”).
His point was, as explained above, that it did not demonstrate what level the
skilled person would produce in practice.
178.
It was also suggested that Dr Hefford was embarrassed by the Hardford
letter given his opinion expressed in his first report. I do not believe Dr
Hefford was embarrassed at all. His evidence explaining how the skilled person
would proceed given the published information was a convincing and credible
explanation of routine work by the skilled person. I accept it.
179.
Olaplex submitted that the obvious thing to do given the Mintel database
was ask the actual manufacturers, and that if that was done the information in
the Hardford letter would be produced. I reject this for the following
reasons. First, although Prof Haddleton expressed the view that there was no
motivation to change the amount of maleic acid in the Catzy product given that
it was on the market, in fact as Dr Hefford’s explanation shows, knowing precisely
how much maleic acid is in the Catzy product does not tell the skilled person
how much they will need in a product made in their own factory. So it is not a
critical item of information.
180.
Second I am not satisfied that even if they asked, the skilled person would
have received the information in any event. Clearly sometimes manufacturers
will divulge this sort of information, but sometimes they will not. Prof
Haddleton had experience of asking for it and being given it. But both sides
in this case jealously guarded the quantities of the ingredients in their
formulations and the trial was conducted on that basis. The evidence does not
establish how the Hardford letter came to be produced. The correspondence,
presumably starting with an approach to Midelfart, has not been produced.
Therefore the simple fact that Hardford was prepared to divulge the level of
maleic acid today, without knowing how they were approached or by whom, is not
a sound basis from which to infer that they would have done so then when
approached by a person skilled in the art.
181.
It is certainly not inevitable that a skilled person given the published
information about Catzy would end up with a product within claim 4, or strictly
a product whereby its use as a hair lightener would be a method within claim
4. So claim 4 is novel. However in my judgment claim 4 does not involve an
inventive step. It would be obvious to produce a product in which there was more
than 0.1 wt% maleic acid in the mixture. The product produced without any
inventive step would have a level of 0.3 wt% to 0.5 wt%.
182.
Putting it another way, a formulation producing a mixture with a level
of between 0.3 wt% to 0.5 wt% maleic acid is not an inventive solution to any
technical problem given Catzy. It arises from the uninventive application by
the skilled person of their common general knowledge of maleic acid as a pH
adjuster and metal chelating agent in order to help stabilise hydrogen
peroxide.
183.
Therefore Claim 4 is invalid.
184.
No other granted or unconditionally amended method claims are alleged to
be independently valid. That leaves so called Fall-Back 2 which proposes
amendments to claim 1 to include bleach power and developer and also a pH
range. The real purpose of that amendment was as a fall back over Kim in case
the construction of “providing bleached hair” would include the oxidation dye
process, since developers are (normally) used in oxidation dye formulations.
Fall-Back 2 in effect makes sure the claimed method is a hair lightening method
rather than an oxidation dye method. I think strictly speaking the condition
advanced by Olaplex in which Fall-Back 2 was advanced is not triggered by having
decided all the method claims are invalid over Catzy but I will consider it
briefly anyway. This sort of difficulty is an illustration of the problems
caused by multiple conditional amendments. They need to be ranked in a clear
way.
185.
I find claim 1 of Fall-Back 2 is invalid over Catzy. Since Catzy is a
hair lightening formulation with both peroxide and persulfate the only issue is
pH. Prof Haddleton accepted (2nd report paragraph 120) that it
would have been obvious over Catzy to formulate the component containing the
maleic acid in Catzy to a pH in the general range claimed in Fall-Back 2 claim
1 (pH 3 to pH 8).
186.
Accordingly all the method claims 1-10 however they are amended are
invalid over Catzy.
WO 768
187.
The WO 768 application describes its “Field of Invention” as methods and
compositions for treating hair or skin, particularly for repairing disulphide
bonds in hair or on the skin (p1 ln18). A wide range of “binding agents” are
described. They include salts of maleic acid.
188.
The important dates are set out below.
1st
August 2013 filing date of earliest priority filing from which WO 768
claims priority
12th
November 2013 filing date of fourth priority filing
from which WO 768 claims priority. This is US 61/903,239 (“US 239”)
21st
April 2014 filing date of fifth priority filing from which WO768
claims priority. This is US 14/257,089 (“US 089”)
15th
May 2014 filing date of US 61/994709. This is the priority filing
from which the patent in suit claims priority
1st
August 2014 international filing date of WO 768
5th
February 2015 publication date of WO 768
15th
May 2015 filing date of patent in suit
189.
Thus for any claim of the Olaplex patent in suit which is not entitled
to claim priority from the US filing on 15th May 2014, WO 768 is
full prior art. That is because the publication date of WO 768 is before the
filing date of the patent in suit. But unconditionally amended claims 1 and
11 and the allegedly independently valid claims dependent on them are all
entitled to claim priority from 15th May 2014. Accordingly the only
issue is novelty under s2(3) of the Act. Furthermore the only matter disclosed
in WO 768 which is relevant to that novelty attack is matter which is itself
entitled to a priority date earlier than 15th May 2014. That is
because the filing date of WO 768 itself is after the filing date of the patent
in suit.
190.
L’Oréal focussed on Example 8 of WO 786 (at p48) and contended it was
entitled to priority from the fourth and fifth priority filings. If it is then
all the claims of the patent in suit are invalid.
191.
Example 8 describes a comparison test between two hair highlighting
treatments. In one treatment a single highlighting formulation is applied to
the hair. That formulation includes a developer and a bleach. It comprises a
bleaching agent on any view and therefore is a first formulation within claim 1
of the Olaplex patent. In the other treatment the same highlighting
formulation is applied together with another formulation called a binding
formulation. The binding formulation comprises a “bismaleate binding agent” at
a concentration of 2400mg in 10g water. The example refers back to Example 1
for the nature of the bismaleate binding agent. In Example 1 on p43 it is
described as 2,2’-(ethane-1.2-diylbis(oxy))bis(ethan-1-amine) di-maleate.
This is the same diamine salt which was in the sample formulation given by
Olaplex to colorists before the priority date. It has the following structure:
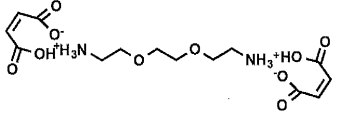
192.
Dr Hefford’s evidence, which I accept, is that in the concentration
described this will make up 15 wt% maleate ions and 9 wt% of the diamine and
will likely be mostly present as hydrogen maleate and the diamine in its fully
ionised form. When mixed with the highlighting formulation, the mixture will
contain about 2 wt% maleate and 1.2 wt% diamine. In that formulation the
maleic acid will be in the form of maleate ions in the presence of diamine and
other cations and anions.
193.
Accordingly the binding formulation in Example 8 is a second formulation
within claim 1. The method of Example 8 is within claim 1 as granted and as
unconditionally amended. The method is within claim 4. Example 8 also
discloses the use claimed in claim 11 as granted and as unconditionally
amended. Neither side addressed the point but in any event I find that the
claims of Fall-Back 2 would lack novelty over Example 8 read in the context of
the pH ranges discussed for the formulations in WO 768 and in the relevant
priority documents.
194.
Therefore if the matter disclosed in Example 8 is entitled to a priority
earlier than 16th May 2014 then the Olaplex patent in suit is
entirely invalid.
195.
For the priority argument L’Oréal focussed on the fifth priority
document US 089 in opening. However in closing L’Oréal focussed on the fourth
priority document US 239 which was filed on 12 November 2013. The
circumstances which led to this change are addressed below. Example 4 of US
239 discloses the use of a “bismaleimide crosslinking agent” in a hair highlighting
treatment. Example 4 of US 239 is in the same form as Example 8 of WO 768. It
is a comparison between two hair highlighting treatments. There is a highlighting
formulation which includes a developer and a bleach. On one treatment this
formulation alone is applied to the hair. In the other treatment the
highlighting formulation is mixed with a second formulation containing a maleic
acid derivative in water. However unlike Example 8 of WO 768, in Example 4 of
US 239, the maleic acid derivative is stated to be a maleimide. The structure
of that maleimide is:
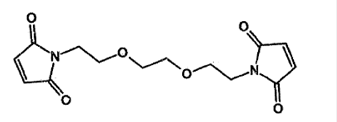
196.
Although this is a maleic acid derivative (the two maleic acid moieties
can be seen at either end of the molecule) it is common ground that this covalent
molecule is not the same thing as the diamine salt referred to in Example 8. The
molecule above is an imide rather than an amine (as in the salt above) or for
that matter an amide. Essentially the difference between an amide and an imide
is that an amide has a single carbonyl group bound to the nitrogen whereas in
an imide there is a pair of carbonyl groups bound to the nitrogen. The
molecule above has two imide groups.
197.
The synthesis of the bismaleimide agent is set out in Example 1 of US
239. The synthesis is depicted in the document in this way:
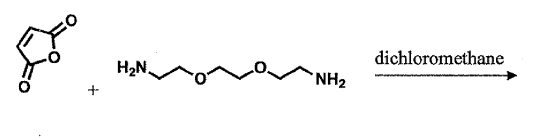
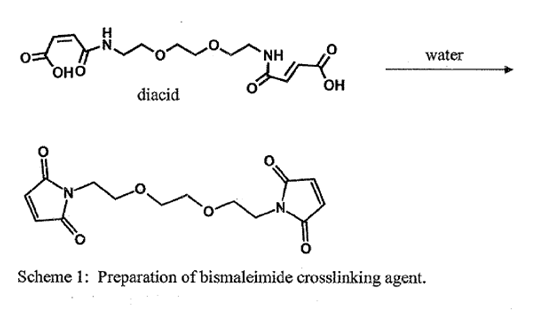
198.
The scheme starts with maleic anhydride and the diamine shown. That
diamine is the same diamine as is in the salt described in Example 8 of WO 768
and in the prior used Olaplex samples. The reaction is carried out in dichloromethane
and produces the molecule shown above known as the diacid. It is a diacid
because at each end there is a carboxylic acid group. The diacid is then
dispersed in water and heated to reflux for 2 hrs and then the water removed in
vacuo to produce the bismaleimide as a white solid.
199.
One might think that the fact that Example 4 of US 239 described a
completely different chemical entity from Example 8 of WO 768 was fatal to any
claim to priority. However in Dr Hefford’s expert report he expressed the view
that the bismaleimide agent was sensitive to water and would undergo a
hydrolysis reaction in aqueous solution to form the diacid. The diacid has two
secondary amide linkages and Dr Hefford then expressed the view that although
amide linkages cannot easily be hydrolysed in physiological conditions, by
thinking about what happens to amide bonds in hair, his opinion was that it was
feasible that the diacid would undergo at least some hydrolysis over time on
storage or when mixed with an alkaline bleaching/colouring formulation, to form
the maleate and the diamine species.
200.
What Dr Hefford was talking about was a possible reaction along the
lines set out below:
↓ in water
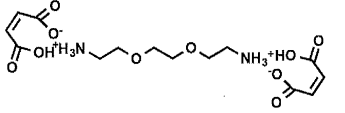
201.
However, as Dr Hefford concluded, it was very hard to predict the rate
or extent of that reaction.
202.
Prof Haddleton agreed that the first step (maleimide to diacid) was
possible if the maleimide was stored for a time in water but he was not sure
about the rate and noted there was no teaching in the priority document to
store it that way. In fact he looked at US 089 rather than US 239 but nothing
turns on that. As for the second hydrolysis which is supposed to turn the
diacid into the diamine salt, his view was that such a reaction would be
expected to require heat. That is because it would need to break the amide
linkages in the diacid. There was also a point on a description of the diacid
in a scientific manual (Thermo) but following the cross-examination I am not
satisfied that reference is relevant to this issue. In any event however in the
cross-examination Prof Haddleton maintained that the amides (which are in the
diacid) would require extreme conditions.
203.
This was the argument advanced in L’Oréal’s opening skeleton. It was a
thin basis on which to found an argument that Example 8 was entitled to
priority from Example 4 of US 239 and it was not advanced in closing. If and
to the extent it is still pressed, I reject it. I am not satisfied the diacid
hydrolyses to the diamine salt to any relevant extent in relevant
circumstances. That basis for the claim to priority is rejected.
204.
However during the cross-examination of Prof Haddleton L’Oréal put a
different point to the witness. This was based on a proton nuclear magnetic
resonance (NMR) spectrum of the product made by Example 1 of US 239. The NMR
spectrum was taken in dimethylsulfoxide (DMSO) as the solvent. Figure 1 of US
239 is the NMR spectrum. Prof Haddleton was cross-examined about it first by
Dr Turner QC and then by Mr Chacksfield. Normally the court will not permit
two counsel for the same party to cross-examine the same witness but as
sometimes happens in patent cases a particular detailed scientific issue is
handled by experienced junior counsel like Mr Chacksfield instead of leading
counsel. In this case Mr Chacksfield was handling the Notice of Experiments
which analyse the sample of the Olaplex prior use samples. So even though the
main cross-examination of Prof Haddleton was undertaken by Dr Turner, he asked
and I agreed that Mr Chacksfield could follow and cross-examine the professor
on that topic.
205.
Another part of the context is this. On 25th April (i.e. the
second day of trial and the day on which Prof Haddleton went into the witness
box) L’Oréal had served a second expert’s report of Professor Law which
included further analysis he had carried out on the NMR spectra produced by
Juniper. Prof Law explained two things. One was that two additional
components in the Olaplex product could be identified - benzoic acid and
phenoxy ethanol. That accounted for what had previously been seen as an
unidentified 5% of the material. The other was a point explaining how the
peaks in the NMR correspond to the maleic acid diamine salt. One peak in
particular was a broad peak at 7.4ppm which he said was caused by the labile
proton from the amine groups on the diamine. Olaplex had indicated there was
no objection to this evidence and was not seeking to cross-examine Prof Law.
206.
Prof Haddleton was first cross-examined about the NMR spectrum at Figure
1 of US 239 by Dr Turner QC. He was asked about a broad peak which appears in
it at about 7.9 ppm and it was suggested it shows a labile proton. He thought
it might be from water but was not sure. He thought the spectrum was consistent
with the maleimide and not with the diacid. He was not asked directly about
the diamine salt. Then Mr Chacksfield cross-examined the professor about the
Juniper report of the analyses of the Olaplex product and the benzoic acid and
phenoxy ethanol components as making up the unidentified 5%.
207.
After that Mr Chacksfield put to Prof Haddleton the Juniper NMR spectrum
of the Olaplex product side by side with the NMR spectrum in figure 1 of US
239. Counsel drew the professor’s attention to the broad peak representing the
labile protons in the amine at 7.4ppm in the Juniper NMR spectrum and put to
him that the explanation for the broad peak at 7.9ppm in the spectrum at figure
1 of US 239 was the presence of such labile protons. In other words the point
being put was that the spectrum in figure 1 was in fact a spectrum of the
maleate diamine salt, the same material as in the Olaplex product, and was not
a spectrum for the maleimide, even though US 239 says it was. The shift
between 7.4 and 7.9 was said to be likely due to the difference in solvents.
208.
Prof Haddleton made clear that he would have preferred to have had a
chance to think about this and use the software tool he uses for NMR analysis
but it was clear that ascribing peaks to NMR spectra is very much part of the
professor’s work and experience. Prof Haddleton agreed that the broad peak was
not from the maleimide. L’Oréal contends that in the end Prof Haddleton
accepted that it was probably right that the spectrum was the maleic acid
diamine salt.
209.
Prof Haddleton was not re-examined on these points and the evidence
closed.
210.
In closing L’Oréal submitted that the court should be satisfied on the
balance of probabilities that despite what the priority document says, in fact the
salt was the true product of the reaction. L’Oréal also contended that if one
tracks through the later priority filings it seems that the inventors appear to
have realised that in fact the substance was the salt and not the imide. Hence
it was submitted that priority for the salt in Example 8 of WO 768 was
established because the product of the synthesis in Example 1 of US 239 is in
fact the very same salt even though it is called the maleimide.
211.
In closing Olaplex made two submissions. The first was that this was an
entirely new case, a deliberate ambush and should not be entertained. The
court should exercise the power in CPR Part 3 rule 3.1(2)(k) and exclude the
issue from consideration. The second submission was that in any event the
argument fails on the facts.
212.
On the first submission Olaplex submitted that the new case was
potentially of fundamental significance to the issue of priority and therefore
of validity. It was a self-contained technical issue. Olaplex had not been
told when the point was thought of but it must have been in advance of the
cross-examination. It was a point on which Olaplex could have considered
obtaining and filing a range of evidence: such as investigating how the
original synthesis and NMR were recorded, whether there was a point about a
water (or strictly OH) peak, where the alleged labile proton peak would appear
in the particular solvent being used or what other groups might have given rise
to a peak at the point in issue. If the point had been raised by Prof Law in
his second Report served on 25th April then it would have given rise
to serious objections, and could not have been run without an adjournment,
which Olaplex contended was unlikely to have been allowed. The point must have
been considered in detail by the Defendants in the course of preparing their
cross-examination. This must have been before Prof Haddleton went into the
witness box and before Prof Law provided his final Report which did not mention
the point at all.
213.
L’Oréal accepted that matters had developed during the trial which were
not ventilated in the expert’s reports. Dr Turner explained that it was not a
deliberate ambush because the significance of the NMR in Figure 1 only became
apparent to the team during the course of the first week of trial. He
submitted that priority from US 239 was a pleaded issue and that no objection
was taken while the evidence was open. Had objection been taken before the
evidence closed, the court would have been faced with various options, and
would have heard argument. It cannot be assumed that such an objection, if it
had been raised at that stage, would have been successful. The options for the
court would have included hearing further evidence and/or further
cross-examination. Also L’Oréal pointed out (i) that one of the inventors was
in court for the whole trial (Mr Pressly) and so instructions could have been
taken about the NMR; and (ii) that Olaplex had not hitherto addressed the
fourth priority document and on such an application would have had to explain
why not.
Assessment of the first submission
214.
No authorities were cited on the extent of the court’s powers under CPR
Part 3 rule 3.1(2)(k). Although no doubt the power in rule 3.1(2)(k) would normally
be exercised before trial, there is nothing stated in the rule which limits the
occasions on which it could be exercised and in my judgment it could be
exercised at any time prior to judgment being given. The fact that no
objection had been taken to the line of questioning in cross-examination or
that the point was only raised after the evidence had closed are relevant
matters to take into account but they do not preclude the exercise of the power
in circumstances which would otherwise be a furtherance of the overriding
objective.
215.
Olaplex made submissions about the principles by which the Patents Court
operates. I agree with them. Some are elementary but are worth restating anyway.
I set them out here with minor modifications:
(a)
The
critical points which are sought to be proven on each of the issues in the case
need to be laid out in advance so that they can be properly addressed in
evidence. Either in a Statement of Case, or (if the Statement of Case is
broadly pleaded) in an expert’s report served well in advance of trial.
(b)
It
is not acceptable to keep a new critical point going to a central issue in the
case for ambush in cross-examination. Such points are commonly thought of late
in the day, but they should be disclosed as soon as the decision is taken to
run them so the judge can decide how to deal with them having heard the
submissions of the other side.
(c)
Where
a new point of substance requiring investigation and technical analysis is
thought of and intended to be run at trial, it is incumbent on the party who
wishes to run it to give proper notice to the other party and not to seek to
ambush an expert witness with the point at trial.
(d)
If
a new point of this nature requires expert evidence to prove it (as this one),
it is incumbent on the party running it to serve his own expert evidence in
advance setting out what the point is and the technical reasons why it is
considered to be correct, to give the other side an opportunity to consider it
and file their own counter-evidence. It may even be incumbent to file a new
Statement of Case.
(e)
A
fortiori where (as here) the point may well have required research, experiment
and historical evidence to deal with.
216.
A further principle submitted by Olaplex was that the Patents Court procedure
encourages counsel not to interrupt cross-examination to make objections as to
lines of questioning. If there is a proper objection to a question then it
ought to be taken, but I entirely agree that cross-examination should not be
interrupted unless it is strictly necessary. That has the great advantage of avoiding
a disruptive style of trial process. It is particularly advantageous when the
questions involve highly technical subject matter whose significance can be
quite unclear at the time. However a consequence of encouraging counsel not to
interrupt cross-examination unless they really have to, is that the court must
be prepared to exercise its power, in a proper case, to exclude an issue from
consideration even after some testimony going to that issue has been given.
Otherwise it would be too late once the witness has spoken. That is not a
sensible way of proceeding.
217.
I accept what I have been told on behalf of L’Oréal that the point only
became apparent during the first week of trial. Nevertheless it obviously was
not thought of during the time Prof Haddleton was under oath and therefore it
could, and so should, have been raised with the other side before the professor
was called as a witness. He was clearly the only relevant witness then being
called by Olaplex to whom the point could be put. The fact it was not raised
in advance is a relevant factor in the exercise of the power under r3.1(2)(k).
218.
The point is a highly technical piece of science about the attribution
of peaks in a proton NMR spectrum. That itself is another a reason why it
ought to have been foreshadowed either in an expert’s report, a Statement of
Case or at least in a solicitor’s letter. Moreover Olaplex is correct that
there is a range of evidence which could be investigated to address the issue,
not only about NMR itself but about the surrounding circumstances. To take one
example: for all anyone knows Figure 1 is indeed a spectrum for the salt not
the imide but the explanation is simply the spectra were muddled up at the time
and the wrong one was filed and thus the figure does not tell you anything
about what the experimental method of example 1 actually produces.
219.
L’Oréal’s stance includes in effect a hint that Olaplex must have always
known this was a problem. That is the implication of the point L’Oréal makes
that if the matter had been raised Olaplex would have to have explained why it had
not called evidence about US 239. But until the cross-examination of Prof
Haddleton this new point had not been mentioned at all. There is no basis for
an inference that before 26th April Olaplex was aware of this
problem (if that is what it is).
220.
L’Oréal’s stance also in effect suggests that Olaplex must have worked
with Mr Pressly between Thursday 26th April and Monday 30th
April when the written closings were filed and found that L’Oréal is correct
and there is nothing Olaplex can put forward to gainsay the submission. Again
I draw no such inference. Given what would have to be investigated the time available
is far too short.
221.
The forensic purpose of these two aspects of L’Oréal’s stance is to
support a criticism of Olaplex that the objection ought to have been taken on
Thursday and it is too late to take it on paper on the Monday. I reject that
criticism. Olaplex has not had a proper opportunity to investigate this
matter.
222.
This action is an important case between substantial commercial
organisations in a high value market. Both sides have sophisticated legal
teams well able to handle issues of this kind if they are raised at short
notice. Even with that in mind however and even though the objection was taken
in closing rather than during the cross-examination, in my judgment the issue
of what is to be inferred from the NMR spectrum which forms figure 1 of US 239
should be excluded from consideration. To decide it now would not be to deal
with this case justly and at proportionate cost.
Conclusion on WO 768
223.
Example 8 in WO 768 is not supported by US 239 and does not have
priority from it. The Olaplex patent does not lack novelty over WO 768.
The second
submission – the argument on the facts
224.
Since I had the benefit of hearing Prof Haddleton, in case the matter
goes further, I will address the issue as best I can on the materials which are
available in any event.
225.
Prof Haddleton was put in some difficulty by the questioning about the
peaks but it was a subject he was very familiar with and he was able to look
after himself. Prof Haddleton did not think he had definitive proof and wanted
more experimental work to be done. However listening carefully to him, the concession
that he made that the broad peak in figure 1 was probably from labile protons
in the maleate salt was not lightly made. If he could have thought of any
other possible explanation he would have said so. Definitive proof is not the
standard required by the law. If I had to decide the point on the material I
have, I would find on the balance of probabilities that the sample analysed to
produce Figure 1 of US 239 contained appreciable amounts of the maleate diamine
salt.
226.
Would that mean Example 8 of WO 768 is entitled to a priority derived
from US 239? Not in the least. This sort of priority argument is a variant of
the “rose by any other name” type of case (cf Synthon v SmithKline
Beecham [2005] UKHL 59). To found priority it must be inevitable
that the skilled person given US 239 would make the maleate salt even though the
disclosure is telling the skilled person in terms to make the maleimide. The
text identifies one molecule and the spectrum identifies another one. There is
no obvious way to resolve the issue without repeating the experiment and seeing
what happens. That has not been done. Moreover the fact that (if true) a
maleate salt was produced on one occasion does not prove it is inevitable, all
the more so when the skilled person will be aiming to make an imide not a
salt. Maybe the synthesis actually undertaken by the inventors was not done
strictly in accordance with what is written in Example 1, we do not know. Perhaps
there was a muddle with the spectra and the wrong one was used as figure 1.
Perhaps the further experiments required by Prof Haddleton for definitive proof
would turn out to show that the broad peak is not from the salt at all but from
something else.
227.
All this goes to show that the argument to support a claim to priority
for Example 8 of WO 768 from US 239 supported by conclusions drawn from the NMR
spectrum at figure 1 is US 239 is too speculative. If I had to decide it I
would reject it.
Kim
228.
Kim is a Korean patent application entitled “Hair dye composition”. It
was published in January 2003 and filed in July 2001. At trial an agreed
English translation was used.
229.
Kim is concerned with formulations of a hair dye including additives
which are maleic acid derivatives. There is no description in Kim of a hair
lightening formulation, by which I mean a formulation which bleaches the hair
and changes the hair colour without use of a dye.
230.
The abstract of Kim states:
“This invention relates to a novel hair dye composition, more
specifically, to a hair dye composition which contains an oxidation dye
precursor and further a maleic acid derivative, thus having a hair protective
effect that is superior to conventional dyes.”
231.
In a passage from p2 ln13-21 of the translation Kim refers to
conventional hair dye compositions and refers to the fact that they include a
first agent which contains dye precursors which typically form a dye on being
oxidised and a second agent which includes hydrogen peroxide. The purpose of the
compositions is to dye the hair as completely as possible, whilst achieving a
long-lasting hair dyeing effect. Kim goes on to explain that the currently
available hair dyes have the drawback that the hair is damaged and becomes
rough or loses its lustre because in order to increase the dyeing effect on the
hair the first agent has high alkalinity and the second agent uses hydrogen
peroxide.
232.
Kim then poses the “Problem the Invention is intended to Solve” and
explains a discovery that the inventors have made through thorough research of
oxidation hair dye compositions that can reduce hair damage after dyeing. The
discovery is that hair damage after dyeing can be further reduced by using a
maleic acid derivative in combination with a hair dye composition containing an
oxidation hair dye precursor. A summary of the invention is then set out as being
a:
“hair dye composition wherein the hair dye composition
contains one or more oxidation dye precursors and further maleic acid, maleic
acid anhydride, maleamic acid, maleimide, and metal salts thereof.”
233.
The detailed description follows. It refers to commonly used oxidation
dye precursors and also refers (at p3 ln22-24) to direct dyes. Olaplex place a
lot of emphasis on this reference to direct dyes. The point is that such dyes
do not need to be oxidised. They would not be formulated with hydrogen
peroxide.
234.
The next important section is a discussion of the mechanism by which the
maleic acid derivatives protect the hair after dyeing. It is as follows:
“The thiol groups that are present in the hair bring about
damage to the hair accompanied by a reduced tensile strength or breaking
strength compared to the original hair through thiol-disulphide bond
interchange reactions during a dyeing process. The thiol groups in the hair
can undergo an addition reaction with a substance that has an a,b-unsaturated
carbonyl group, such as maleic acid. Thus, the addition reaction of the thiol
groups with maleic acid can lower the possibility of a reaction among the
thiols within the hair, resulting in reduction of hair damage.”
235.
Although this mechanism involves the disulphide bridges
in the hair, an important point in the context of this case is that this
concept of thiol-disulphide interchange causing damage to the hair involves a
process of reduction rather than oxidation. When reduction breaks a disulphide
bridge the result is two thiols, in effect [-S-S-] is turned into [-SH HS-].
By contrast when oxidation breaks a disulphide bridge the result is two group
known as cysteic acid groups or sulphoxides, in effect [-S-S-] is turned into
[-SO3- -O3S-].
236.
There was a debate about what the skilled person would make of the
mechanism in Kim. I will come back to that.
237.
After some more detail, which suggests the inventors of Kim may have
carried out a range of experiments on wt % of maleic acid derivatives the
results of which are not set out, Kim then provides four examples of a hair dye
composition in Table 1. Example 1 contains maleic acid, example 2 sodium
maleate, example 3 maleamic acid, and example 4 maleimide.
238.
Kim then reports the results of experiments using those example
formulations to dye hair and measure the effect on the tensile strength of the
hair. The hair is dyed five times. The results are set out in Table 2. The
way the results work is that a control (with no maleic acid derivative) is run
as Comparative Example 1. That has no maleic acid derivative. The effect of
the dyeing treatment is to reduce the tensile strength of the hair by 19.4%.
The results for the four examples can be compared with that. They all either
produce a lesser reduction in tensile strength or in one case a small increase.
The idea is that the greater the degree of reduction in breaking strength, the
more severe the hair damage. The results are:
239.
The figures are based on an average of the strength of 50 hairs.
Nothing of substance turns on it but one of Prof Haddleton’s less convincing
passages of testimony was his suggestion that this was done by taking one
measurement of a clump of 50 hairs and dividing the result by 50. That is
plainly not what Kim means by the statement that the values in the table are
the average of those of 50 hairs. Measuring a single value for a 50 hair clump
does not involve taking the average of the values (plural, my emphasis) of 50
hairs.
240.
Kim explains that the lowered tensile strength indicates severe hair
damage due to changes in the protein structure within the hair and concludes
from the results in the table that the maleic acid derivatives give a markedly
lower reduction in tensile strength than the control.
Novelty over Kim
241.
On the construction of “providing bleached hair” I have accepted, the
claims are all novel over Kim. That is because Kim is a dyeing process.
Obviousness over
Kim
242.
The skilled person and the common general knowledge have been identified
already. In terms of inventive concept whether I am considering claim 1 or
claim 11 whether as granted or unconditionally amended, the inventive concept
is the same. Focussing on claim 11 it is the use of maleic acid (which
includes maleate and hydrogen maleate ions) simultaneously with a bleaching
agent to reduce or prevent hair damage due to a treatment to provide bleached
hair.
243.
Comparing Kim to claim 11, the document does disclose the use of maleic
acid (and maleate ions) to reduce or prevent hair damage due to a hair
treatment. Identifying the differences between Kim and claim 11, the only
difference is that Kim is concerned with a dyeing treatment whereas the claim
is concerned with hair lightening by bleaching and without dye.
244.
Another aspect to have in mind is that only three of the four maleic
acid derivatives in Kim are within the claims of the Olaplex patent. Kim
Example 4 is a maleimide. It is not the same maleimide as the one in WO 768
but in any case it is not within the claims.
245.
The question in the end is whether it would be obvious to the skilled
person to apply the idea disclosed in Kim of using an additive such as maleic
acid to reduce or prevent hair damage due to a hair lightening treatment
involving bleaching without dyeing.
246.
Despite the elaboration given to this issue at trial, in my judgment
this question turns on a short and simple point. The skilled person knows as
part of their common general knowledge that a hair lightening treatment
involving bleaching without dyeing is a highly oxidative environment. The
mixtures used generally include hydrogen peroxide and persulfate. The damage
caused by that sort of treatment was known to be damage caused by that
oxidative environment. For the skilled person to think it was worth using any
of the additives disclosed in Kim, they have to believe that those additives
might have an effect in a system in which the damage the additive is there to deal
with is caused by oxidation.
247.
In my judgment it would not be obvious to the skilled person that the
maleic acid derivatives in Kim might (let alone would) have a protective effect
against damage caused by oxidation. That is for the following reasons. First
and foremost the thiol-disulphide mechanism actually proposed
by Kim is concerned with reduction not oxidation. The skilled person would
regard it as scientifically credible. Dr Hefford agreed that the mechanism
would be viewed as credible although in cross-examination (but not in his
report) he suggested that while it was credible as a phenomenon, it was not as
an explanation for a reduction in tensile strength. I was not convinced by
that qualification. As a matter of common general knowledge, both oxidation
and reduction were known to damage disulphide bonds and were known to reduce
the tensile strength of hair. Since reduction was known to cause damage, the
idea that an additive might act to reduce that damage by interacting with thiol
groups to prevent it is credible. There was then a point on the availability
of thiol groups but I am not satisfied the skilled person’s thinking would go
so far as to delve into the likely number and availability of thiol groups so as
to lead to doubts about Kim’s mechanism. That degree of insight and thought is
a hallmark of inventiveness (or hindsight).
248.
Second the Kim document goes out of its way to propose a mechanism. It
does not simply present data and leave the reader to infer how it is working.
On the face of it the inventors of Kim have done the tests they say they have
done and perhaps done more tests too.
249.
Third, although oxidative dye compositions do use oxidising agents, they
are known to have a much less aggressively oxidising effect than the hair
lightening treatments which involved bleaching without dyeing. The latter had
two aggressive oxidisers – hydrogen peroxide and persulfate. The former had
hydrogen peroxide alone. Olaplex overstate the case sometimes when seeking to
downplay the significance of the hydrogen peroxide in an oxidative dyeing
composition. While its function was in part to oxidise the dye precursors, it
is clear that the skilled person would, as a matter of common general
knowledge, understand that the hydrogen peroxide would often act by bleaching
the hair as well. That would not always happen to an appreciable extent but it
often would. That effect of the hydrogen peroxide was understood to be the
cause of hair damage with repeated use of oxidative dyeing treatments. However
Kim does not say anything which purports to link the hydrogen peroxide in the
oxidative dye formulations described with the damage mitigated by the maleic
acid derivatives. That would be contrary to the mechanism Kim proposes.
250.
Fourth, although Kim does make clear that the proposal
relates to oxidative dyes and a system with oxidative dye precursors, it does
also expressly contemplate a system with direct dyes and therefore no hydrogen
peroxide at all. Albeit that no results are presented for direct dyes, that
suggestion is inconsistent with the effect being one associated with oxidative
damage.
251.
For a skilled person to think that maleic acid would
work to prevent damage in a pure bleaching system with no dye would involve
that person thinking they knew better than Kim. It is not the law that the
skilled person is bound to follow whatever mechanism is proposed in a prior
teaching nor is it the law that it is necessarily inventive to go against or
beyond such a teaching. It always depends on the facts of the particular
case. I accept this is an empirical art and that the skilled person would be
interested in the data in Kim (“the data is the data” as Prof Haddleton said). The skilled person would not reject the data, for example,
just because the particular Diastron instrument on which the tensile tests were
measured was not identified.
252.
The problem for L’Oréal is that
the skilled person is aware that chemical reduction can cause damage to hair
and so there is no reason for an uninventive skilled person to disbelieve Kim.
For a skilled person to go ahead and test maleic acid in a hair lightening
formulation involving hydrogen peroxide, persulfate and no dye would be an act
of invention. I reject the obviousness case over Kim.
253.
I have not taken into account the unpleaded commercial
success argument advanced by Olaplex, including the cross-examination materials
referring to the Holy Grail and some parts of Mr Christal’s testimony. The
evidence does suggest that maleic acid or derivatives of it do have some genuine
effect to reduce hair damage cause by oxidation, but that just goes to show
that the invention works and so there is no insufficiency. Whether it is all
worth the social media hype is quite another matter and so too is whether any
of that properly establishes a long felt want. This was another transparent
attempt to avoid the clear provisions in the rules (CPR Part 63 PD63
paragraphs 4.6 and 6.3) similar to the one I encountered in Blue
Gentian LLP v. Tristar Products (UK) Ltd [2013] EWHC 4098 at
paragraph 13.
The second
claimant’s status
254.
A point which emerged in closing was about the second claimant’s
status. The second claimant Olaplex, LLC claims to be an exclusive licensee
under the patent and therefore claims to have a cause of action to sue for
patent infringement by s67 of the 1977 Patents Act. L’Oréal did not admit that
the company was an exclusive licensee within the meaning of the Act and put the
claimants to proof. No evidence about this was led by either side and it was
not mentioned in opening. In the written closing submissions L’Oréal submitted
that the claimants had failed to prove that the second claimant was an
exclusive licensee and so the action as brought by that company should be
dismissed. In response the claimants submitted that following a debate about
this at the CMC disclosure of the relevant licences had been given, the
documents were in a disclosure list and there was no issue about their
authenticity. Mr Purvis QC for Olaplex handed up the documents and submitted
they showed that the second claimant was an exclusive licensee. L’Oréal’s case
in response was that the materials did not establish that the second claimant
was an exclusive licensee. Mr Chacksfield for L’Oréal handed up a clip of
extracts from some further documents to explain the point.
255.
Taken as a whole the documents show the following:
i)
By a licence agreement dated 20th May 2014 Liqwd Inc the
first claimant granted to Olaplex, LLC the second claimant a non-exclusive
licence relating to products and certain patents. The products include the
relevant products and the patents include the relevant patent.
ii)
By a distributorship agreement dated 22nd August 2014
Olaplex, LLC appointed Anglo International Management Limited (“Anglo”) a
Gibraltar registered company as exclusive distributor of the relevant product
in territory which includes the UK.
iii)
By an amendment agreement dated 31st October 2016 Liqwd Inc and
Olaplex, LLC amended their 20th May 2014 licence agreement so that
Olaplex, LLC became an exclusive licensee under the patent in suit to practise
the patent. The relevant clause expressly permits Olaplex, LLC to grant
sublicences including exclusive sublicences.
iv)
On 31st October 2016 an amendment to the 22nd
August 2014 distributorship agreement with Anglo was agreed between Olaplex,
LLC and Anglo. As a result new clauses were inserted into the 22nd
August 2014 agreement. They inserted a definition of “Patents” by reference to
a new schedule in which the patents were listed and inserted a new clause 2.6 whereby
Olaplex, LLC granted Anglo an exclusive sublicence under the Patents in the
relevant territory. The Patents includes the patent in suit. I have not seen
all the terms of the original distributorship agreement but I infer it did not
include an express patent licence before this amendment.
v)
On 2nd November 2016 the claim form was issued in this
action. In addition to the first and second claimant the claim form also named
Anglo as third claimant and a UK company called Star Qualities Ltd (“Star”) as
fourth claimant. In the original Particulars of Claim Star was said to have an
exclusive sublicence from Anglo, which in turn had an exclusive sublicence from
Olaplex, LLC and so on. I have not been shown the licence between Anglo and
Star.
vi)
On 30th November 2016 Tiffany Walden, General Counsel of
Olaplex, LLC wrote to Leslie Spears, director and chairman of Anglo, giving
notice terminating the exclusive licence in clause 2.6. The termination was
said to take effect 14 days after the date of the email.
vii)
The CMC took place on 16th March 2017. Upon certain
undertakings given by Anglo and Star to protect the defendants’ position and undertakings
that they would give disclosure if need be, both Anglo and Star were removed
from the action and permission was given to the first and second claimants to
amend the Particulars of Claim generally and to reflect the fact that Anglo and
Star were no longer exclusive licensees of the patent in suit and therefore not
parties to the litigation. That amendment to the Particulars of Claim was
made.
256.
The relevant provisions of the Act are s67 itself and the definition of
exclusive licence in s130(1). Mr Chacksfield’s referred to Dendron v
University of California [2004] EWHC 1163 (Ch), [2004] FSR 43. He
submitted that this case was authority for the proposition that the holder of
what purports to be an exclusive licence, who grants a licence to another
party, is not then an exclusive licensee within the meaning of the Act and has
no right to bring proceedings. In my judgment that submission is wrong. Dendron
is authority for the proposition that an exclusive licensee A, who grants an
exclusive licence to another party B, is no longer an exclusive licensee. The
exclusive licensee is now B. B would have the right to sue but not A. In this
I am assuming a simple case in which there is only one patent and all the acts
exclusively licensed to A are then exclusively sub-licensed to B.
257.
However Dendron is concerned with exclusivity.
What Dendron is not authority for is the wider submission
made by counsel, that if A is an exclusive licensee and had granted a
non-exclusive licence to B, somehow A’s status as an exclusive licensee is
undermined. That is simply not what the judge was addressing in Dendron.
Indeed Pumfrey J made the point that the Act contemplates that an exclusive
licence within section 67 can include the power to grant sublicences. In my
judgment the holder of an exclusive licence can grant non-exclusive licences
without undermining their status as an exclusive licensee under the act. The
exclusive licensee in that situation still has the right to exclude all other
persons, including the patent proprietor, from working the invention. The
words of s130 show that “other persons” means other than himself and persons
authorised by him.
258.
So in order for L’Oréal’s point to succeed the facts must be that
despite terminating the exclusive licence by the 30th November 2016
email, Anglo’s position remains that of an exclusive licensee under the patent.
The fact that Anglo may retain some form of patent licence is not enough.
Anglo has to still have an exclusive licence for this point to succeed.
259.
It was not clear to me whether L’Oréal actually submitted that Anglo was
still an exclusive licensee despite the termination of the exclusive licence by
the 30th November 2016 email but I will consider the matter in any
event. Mr Chacksfield’s clip of papers included a second witness statement of
Stephen Bennett of Hogan Lovells, the claimants solicitors, dated 14th
March 2017. It was served for the CMC. Mr Chacksfield referred to paragraph 9
in which Mr Bennett addresses a point made by Mr Sheraton (L’Oréal’s solicitor
at Baker McKenzie) in Mr Sheraton’s witness statement. Mr Sheraton was
responding to something said in Mr Bennett’s first witness statement. I have
not been shown either of the earlier statements. All I have been shown is Mr
Bennett’s 14th March 2017 statement.
260.
Paragraph 9 states that Mr Sheraton may have misunderstood something Mr
Bennett had said. Mr Bennett then refers to the termination email and states
that “The termination of the patent licence does not impact the business
relationship between the claimants. Anglo and its subsidiary Star continue to
distribute the Olaplex product on behalf of Olaplex in certain territories on
the same terms as before the amendment agreements were made.”
261.
Reading paragraph 9 in context, I do not accept the submission, if made,
that Anglo remained an exclusive licensee under the patent after the
termination. I appreciate that the original distributorship agreement appoints
Anglo as exclusive distributor of the product and so one might be tempted to
argue that if that amounted to an implicit exclusive licence under the patent
(despite the patent only being added into the agreement by amendment later)
then the reference by Mr Bennett to the relationship continuing under the same
terms as before could be said to imply that that implicit exclusive patent
licence continues. But this is far fetched. The email clearly and expressly
terminated the exclusive patent licence. No doubt the business relationship
continued entirely as before but the legal relationship after that did not
involve Anglo having an exclusive patent licence.
262.
Therefore I find that from 14th December 2016 Olaplex, LLC
was the exclusive licensee under the patent and remains so. Prior to 31st
October 2016 Olaplex, LLC only had a non-exclusive licence under the patent and
so had no right to sue under s67. In the six week period in between, although
Olaplex, LLC had what purported to be an exclusive licence, on the authority of
Dendron, it was not an exclusive licensee under the Act
and had no right to sue because Olaplex, LLC had granted an exclusive licence
to Anglo which remained in force until 14th December 2014.
263.
Since the second claimant’s right to sue has been perfected during the
proceedings, the fact it had no right to sue on the day the claim form was
issued will have an effect on damages but does not have an effect on any
forward looking relief such as an injunction.
Conclusion
264.
Claims 1 to 10 of the patent are invalid. So are claims 1 to 10 of the
amendments in Fall Back 2. Claim 11 as unconditionally amended is valid and
infringed.













