Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales High Court (King's Bench Division) Decisions
You are here: BAILII >> Databases >> England and Wales High Court (King's Bench Division) Decisions >> Harcombe & Anor v Associated Newspapers Ltd & Anor [2024] EWHC 1523 (KB) (25 June 2024)
URL: http://www.bailii.org/ew/cases/EWHC/KB/2024/1523.html
Cite as: [2025] WLR 405, [2024] WLR(D) 295, [2024] EWHC 1523 (KB), [2025] 1 WLR 405
[New search] [Printable PDF version] [View ICLR summary: [2024] WLR(D) 295] [Buy ICLR report: [2025] 1 WLR 405] [Help]
Neutral Citation Number: [2024] EWHC 1523 (KB)
Case No: QB-2020-000799, QB-2020-000801
IN THE HIGH COURT OF JUSTICE
KING'S BENCH DIVISION
MEDIA & COMMUNICATIONS LIST
Royal Courts of Justice
Strand, London, WC2A 2LL
Date: 25 June 2024
Before :
THE HONOURABLE MR JUSTICE NICKLIN
- - - - - - - - - - - - - - - - - - - - -
Between :
|
|
(1) Zoë Harcombe PhD (2) Dr Malcolm Kendrick |
Claimants |
|
|
- and –
| |
|
|
(1) Associated Newspapers Limited (2) Barney Calman |
Defendants |
- - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - -
Adrienne Page KC and Godwin Busuttil (instructed by Carter-Ruck)
for the Claimants
Catrin Evans KC and Sarah Palin (instructed by Reynolds Porter Chamberlain LLP)
for the Defendants
Hearing dates: 3-11 July 2023
- - - - - - - - - - - - - - - - - - - - -
Approved Judgment
This judgment was handed down remotely at midday on 25 June 2024 by circulation to the parties or their representatives by e-mail and by release to the National Archives.
The Honourable Mr Justice Nicklin :
- This judgment is divided into the following sections:
- The First Claimant is a professional researcher, writer, and public speaker on diet health and nutritional science. She is a graduate of the University of Cambridge, in economics and mathematics and has a PhD from the University of West Scotland in public health nutrition. In her PhD, her thesis title was "An examination of the randomised controlled trial and epidemiological evidence for the introduction of dietary fat recommendations in 1977 and 1983: A systematic review and meta-analysis".
- The Second Claimant is a general practitioner, writer and lecturer. As a GP, he works in general practice, intermediate care and out of hours for two NHS Trusts in Cheshire. As a writer and lecturer, he has a specialist interest in the epidemiology of cardiovascular disease. He has contributed to papers that have been published in the British Medical Journal and other journals. He has written several books, including "The Great Cholesterol Con" (2008), "Doctoring Data" (2015) and "A Statin Nation: Damaging Millions in a Brave New Post-Health World" (2018). He was an early member of the Centre for Evidence Based Medicine at the University of Oxford and of The International Network of Cholesterol Sceptics, the latter comprising scientists, doctors and researchers who, the Second Claimant states, share the belief that cholesterol does not cause cardiovascular disease. The Second Claimant has also worked for the European Society of Cardiology and the National Institute for Clinical Excellence.
- The First Defendant is the publisher of the national newspapers, The Daily Mail and The Mail on Sunday, and of the global website MailOnline. Articles published by the Defendant, whether in the print editions of its newspapers or online, will be read by millions of people within this jurisdiction and beyond.
- The Second Defendant is the Health Editor of the Mail on Sunday and editor of the health pages in the "Health, Wealth and Holidays" pull-out section of the Mail on Sunday ("Mr Calman").
- The Claimants have brought a claim for libel arising from the publication of a series of articles that were published by the Defendants in both the print and online editions of the Mail on Sunday of 3 March 2019. In total, the following three separate articles were published:
- Mr Calman was credited as being the author of all three articles. The Articles, as they appeared in the print edition are reproduced in Annex 1 to this judgment. I have included these because, although some of the text is illegible, they demonstrate the layout of the articles, which can be a factor in deciding their overall impact and meaning. The text of the News Article, the Main Article (including the Text Box) and the Editorial is set out in Annex 2 to this judgment.
- The Articles were also published online substantially in the form in which they appeared in the print edition of the newspaper, with some changes in context including to headlines and furniture (see further [16(4)] below). On one page of Mail Online, the three Articles were published together - Main Article (minus the Text Box), News Article and Editorial ("Online Publication 1"). On another page of the website, the News Article was published separately but with a hyperlink to the Main Article ("Online Publication 2"). Finally, the Editorial was published on a further website page ("Online Publication 3").
- The Claimants issued separate Claim Forms, on 26 February 2020. Particulars of Claim were not immediately provided. Instead, the Claimants sought the trial of various preliminary issues, including the natural and ordinary meaning that the Articles bore. That application was originally granted, but ultimately it became apparent that the Defendants' reliance upon certain qualified privilege defences meant that a conventional trial of preliminary issues was not appropriate (see my decision of 22 February 2022 [2022] EWHC 543 (QB)).
- Joint Particulars of Claim were filed on 8 April 2021. The Claimants complained that they had been defamed in the online and print versions of the Articles that had been (and continued to be) published by the Defendant.
- The natural and ordinary meaning that the Claimants contended the Articles bore was:
- In terms of the online publication, the Claimants complained of the publication of the three Online Publications (see [8] above).
- The Claimants contend that the Online Publication 1 (the three print Articles, minus the Text Box) and Online Publication 2 (the News Article (read with the Main Article)) bear the same natural and ordinary meaning as attributed to the print publication. The same meaning is also attributed to the online publication of the News Article, when read with the Main Article, advanced as an innuendo meaning as "a substantial but necessarily unquantifiable number of readers of the online version of the News Article will have clicked on the link provided and read the Main Article".
- Online Publication 3 (the Editorial), is alleged by the Claimants to bear the following natural and ordinary meaning:
- Each Claimant contends that the publication of the Articles has caused serious harm to her/his reputation, and both seek damages (including aggravated damages) - and other remedies - for libel.
- The Defendants filed their Defence on 2 July 2021. With some important express reservations, the Defendants admitted publication of the Articles. The Defendants' case on publication is:
- The Defendants made no admissions as to the Claimants case of serious harm to their reputation alleged to have been caused by publication of the Articles.
- The Defence advanced substantive defences of:
- The Defence was a 75-page document, but it contained the following summary:
- The Defendants contend that, in the following meanings, the Articles (save for Online Publication 3), are defensible as honest opinion:
- The Defendants contend that paragraph (1) consists of a non-defamatory statement of fact, with paragraphs (2) to (4) representing expression of defamatory opinions.
- As required by CPR PD 53B §4.4, the Defence contains particulars of the matters upon which the Defendants rely to support the honest opinion defence.
- The Defendants contend that, in the following meanings, the Articles are substantially true:
- As required by CPR PD 53B §4.3, the Defence contains particulars of the matters upon which the Defendants rely to support the truth defence.
- The Defendants contend that publication of certain parts of the Articles is protected by privilege:
- As required by CPR PD 53B §4.4, the Defence contains particulars of the matters upon which the Defendants rely to support the s.15 reporting privilege.
- The Defendants contend that publication of further parts of the Articles, highlighted in green in Annex 1 (and shown with dotted-underlining in Annex 2) [3], are protected by qualified privilege as an extract and/or summary of a peer-reviewed statement in a scientific or academic journal pursuant to s.6(5) Defamation Act 2013. (The relevant section is set out in [326] below).
- The Defendants relied upon the following particulars to support this qualified privilege defence:
- Finally, the Defendants contend that publication of the Articles was on a matter of public interest, that they reasonably believed (and continue to believe) that publishing the Articles was in the public interest, and, in consequence, they have a defence under s.4 Defamation Act 2013 for both the original and continuing online publication of the Articles (the section is set out in [270] below).
- As required by CPR PD 53B §4.5, the Defence contains particulars of the matters upon which the Defendants rely to support the public interest defence.
- The Claimants' Reply was filed on 29 November 2021 (and was subsequently Amended on 5 December 2022). In this substantial document, running to over 200 pages, the Claimants gave the following summary of their case:
- In the balance of the Reply, the Claimants (a) set out their response to the substantive defences advanced in the Defence; and (b) advanced a case of malice against the Defendants. The plea of malice alleged the following:
- Common to both pleas of malice were two essential elements. On the assumption that the relevant qualified privilege defence was established:
- It is no part of the Claimants' plea of malice to allege that Mr Calman had a dominant intention to injure the Claimants, or that his dominant motive was spite towards the Claimants.
- On 10 February 2022, I made the order directing the preliminary issues that have been tried in this case. The judgment given on that occasion explains the difficult issues with which the Court had to grapple: [2022] EWHC 543 (QB) ("the PIT Judgment"):
- After a full day of argument, I directed the trial of the following preliminary issues:
- I note, here, so that the parameters of the exercise are clear, that, at the trial, the Claimants accepted that the references to the Kearney Statement in the Articles are privileged under s.15 Defamation Act 1996 Act. Issue 1.3 therefore falls away. Other concessions made by the Claimants have slightly narrowed the scope of the issues that require resolution, and those are explained in Section H below.
- The preliminary issues directed to be tried were more extensive than would ordinarily be the case in conventional defamation where the trial of preliminary issues has become commonplace. The resolution of the preliminary issues in this case required a substantial trial. The reasons for making the order, in this unusual case, are explained in the PIT Judgment, at the end of which I decided that the benefits of a split trial narrowly outweighed the downsides: [44]-[45]. A key factor was the fact that resolving the issues of privilege and public interest would - if they did not dispose of the claim entirely - allow determination of the meaning of the Articles and thereafter set the parameters for the defences of truth and honest opinion: [38]. Having all issues relating to the state of mind of the Defendants (including any issues of malice and s.3(5) Defamation Act 2013), resolved in Trial 1, would mean that there would not be a risk of overlapping issues between Trial 1 and any Trial 2, if a trial of remaining issues was required: [29]-[32].
- As determination of these preliminary issues required resolution of disputed issues of fact, directions were subsequently given for Trial 1, on 6 April 2022, for costs budgeting and the case management phases of disclosure and witness statements.
- Finally, before turning to the issues to be resolved following this Preliminary Issue Trial, it is important that I set out clearly what is, and what is not, being resolved by the Court in this trial and this judgment.
- As part of consideration of the public interest defence, I shall be referring to various claims that have been made against several people, including the Claimants, but extending also to other third parties. It is necessary for me to refer to these claims - because they are relevant to the information that Mr Calman had prior to publication of the Articles - but I am not able (because I do not have the relevant evidence) and it is not necessary (because it is outside the scope of the exercise as I have explained it) to resolve whether the claims are true or not. The relevance to the public interest defence is not whether the claims were true or not, but the fact that they had been made and what impact that had (or could or should have had) on the terms in which the Articles were published. At some points in this judgment, where I have judged it important to do so, I have re-emphasised this point, as an appropriate reminder as to the parameters of the exercise and in fairness to third parties who have not had an opportunity to give evidence during the trial (and who have not otherwise had a chance to comment) upon various claims that have been published. Those reading, and reporting upon, this judgment must have these parameters clearly in mind.
- The Defendants have relied upon evidence from the following witnesses at the trial of the preliminary issues:
- The Claimants did not file any witness statements for the trial of preliminary issues. That is not surprising. As I have noted, the issues that arise for determination are not ones on which the Claimants themselves had any relevant evidence.
- Substantial documentation in the trial bundles has been relied on by the parties.
- Mr Calman, Mr Wellington and Mr Verity were cross-examined at the trial. Mr Adams' evidence, given in his witness statement, was not challenged by the Claimants.
- In the next section of the judgment, I will set out the relevant facts. The vast majority of these facts are uncontroversial, being either established by contemporaneous documents or unchallenged evidence. In respect of the few areas of material factual dispute, I state my conclusions reached on the totality of the evidence, including the evidence of the key witness for the Defendants, Mr Calman.
- My assessment of the witnesses who gave evidence at the trial is that they were all obviously honest witnesses who were seeking conscientiously to assist the Court with their recollection of events and to answer, in a straightforward way, the questions that they were asked. Mr Calman, particularly, was an impressive, intelligent, careful, and thoughtful witness. He clearly felt (and feels still) passionately about the issue of statins. Save in the limited respects identified below, I have generally accepted his evidence.
- Before turning to the facts and the evidence, I should say something about the limits of the contemporaneous documents. The availability of emails, some notes of Mr Calman, and drafts of the Articles provide a reliable record of certain parts of the pre-publication process (which is of particular relevance to the public interest defence). However, there are significant gaps in this evidence. Two areas of significance should be identified at the outset. First, there is a complete absence of any contemporaneous record of how, and on what basis, Mr Calman concluded that publication of the Articles was in the public interest. Second, and of relevance to the first, there is no reliable record of what public statements, or claims, of the Claimants Mr Calman had read and considered prior to publication (this is the subject of Section F(4) and H below). On these critical issues, save for the very few documents that shed light on them, the Court substantially has only the witness evidence of Mr Calman. For example, in his witness statement, Mr Calman says this about his research for the Articles:
- As there is no contemporaneous record, save for deductions that can be made from the available documents, it is very difficult to identify what Mr Calman had read, whether in relation to the identification of the Claimants' claims, or the medical research that he relied upon as contradicting these claims. As I observed in Lachaux -v- Independent Print Ltd [2022] EMLR 2, the absence of contemporaneous records is likely to impair a defendant's ability to prove subsequently that his/her belief, at the time, that publication was in the public interest, was reasonable (see [277] below). It is not open to a defendant to prove that his/her belief would have been reasonable based on material that was never considered at the time.
- Mr Calman has worked at The Mail on Sunday since 2008, first as Deputy Health Editor, and then, from 2011, as the Health Editor. At the time of publication, Mr Calman's deputy was Eve Simmons ("Ms Simmons").
- Mr Calman had previously worked together with the First Claimant on articles that he had published. In his evidence, based on their prior dealings, Mr Calman described the First Claimant as having a particular strength in analysing medical studies and identifying weaknesses in them, or disputing their conclusions, and offering a different view. Mr Calman regarded the First Claimant as qualified to comment on nutrition and described her as a "valuable contributor" on stories in this area. But Mr Calman added, in his evidence, that "[her] take on nutrition can... be controversial". Mr Calman was a subscriber to the First Claimant's weekly newsletter and, from that, described himself as being "well aware of her strong views on cholesterol and heart health, on statins, and on the conflicts of interest she believes in".
- As to the First Claimant's views on statins, Mr Calman stated that he was "generally aware of her views", but it was not until she had intervened in an issue relating to Dr Malhotra that he said he began to "examine her position... and the things she had said". The incident to which Mr Calman was referring was an email that the First Claimant had sent to him, on 26 November 2018, following an article on "fake news" published by the Second Defendant. The First Claimant had objected to the misattribution of a quotation to Dr Malhotra from an article in The Daily Express, which had subsequently been corrected, and stated: "Aseem's position on statins is simply that he wishes the patient to be able to make an informed choice when presented with the benefit and risks (thennt.com data)". Beyond that, Mr Calman had no specific knowledge about the First Claimant's stance on statins. He said:
- As for the Second Claimant, Mr Calman said that he did not know him personally but had long been aware of him. He was, Mr Calman said, best known for his argument that high cholesterol does not lead to heart disease and that lowering cholesterol is disadvantageous.
- The Second Claimant had published several books. Mr Calman had read "The Great Cholesterol Con", published in 2007, which he described as a "seminal work in that debate" and said was frequently cited by "statin sceptics". Mr Calman had also read parts of "A Statin Nation", published in 2018, as research for articles in the Health Section of The Mail on Sunday. In his witness statement, Mr Calman said:
- The Mail on Sunday launched its 'Fight Fake Health News' campaign in late 2018 ("the Campaign"). Mr Calman stated that health misinformation and disinformation was, and is, "very much a topic of our time". He suggested vaccine disinformation was perhaps the best example and most widely recognised, but commonly there were also misleading claims about cancer treatments and diets. As part of the Campaign, the newspaper (and Mr Calman) intended to examine what they believed were the most commonly circulated misinformation, "and offer the facts, relayed by trusted healthcare professionals, scientists and others, explaining for example whether they [were] entire fabrications with zero evidence or whether they [were] more nuanced where there [were] shades of truth and distortion".
- Mr Calman stated that he had always known that statins would feature as part of the Campaign. It was, he believed, one of the "big areas of concern", particularly since the publication of "two infamous articles" - a study and opinion piece, published by the British Medical Journal ("BMJ") in October 2013 ("the 2013 BMJ Articles") (as to which see description of the Articles in [206] below). Mr Calman said that the 2013 BMJ Articles had "ignited debate", and that:
- Mr Calman said that he was aware of what he called the 'anti-statin lobby'. Dr Malhotra had approached the newspaper, prior to publication of the 2013 BMJ Articles, and an article had been published about the potential side effects of statins.
- Publication of the Articles was prompted, Mr Calman said, by a joint article, published in the European Heart Journal in late January 2019, by the editors of all the major heart‑health medical journals warning of the risk of lives due to what they saw as the spread of medical misinformation on statins ("the Joint Editorial").
- Mr Calman said that he was generally aware that (1) the use of statins was supported by the established medical community, but that there existed a "small but vocal group of doctors" who shared the belief that the prevailing consensus, generally agreed upon by cardiovascular specialists, and relied upon in the clinical guidelines, on the causal link between cholesterol and cardiovascular disease, was wrong; (2) that this "anti‑statin lobby" claimed, therefore, that statins, as cholesterol-lowering drugs, have no, or only negligible, effects in the prevention and treatment of cardiovascular disease; and (3) that this lobby also claimed that the side effects of statins were being downplayed.
- Mr Calman said that it was clear to him, as soon as he began researching the Articles, that they would identify individuals. The two Claimants (and Dr Malhotra) were, he said, high profile commentators who had appeared on television and radio, had written books and newsletters "with controversial titles" and had sway with journalists across the media and politicians; "that is why we named them". The Claimants were two of the three UK-based members of the International Network of Cholesterol Skeptics which Mr Calman regarded as doing "a lot of work to try to debunk mainstream evidence on statins and the link between cholesterol and cardiovascular disease".
- In his evidence at trial, Mr Verity said that, prior to publication, he and Mr Calman had had a "long chat" in his office "about the whole issue of fake news" in which Mr Calman had identified statins as a target area. Mr Verity continued:
- Another "key document", identified by Mr Calman, which substantially informed his research and understanding, was a review published in The Lancet on 8 September 2016: "Interpretation of the evidence for the efficacy and safety of statin therapy" (Professor Rory Collins and others) ("The Lancet Review"). Mr Calman described The Lancet Review as "the most authoritative evidence" on statins. The study included the following summary:
- In his witness statement, Mr Calman identified what he regarded as the principal conclusions from The Lancet Review:
- On 2 February 2019, The Lancet published a further study by the Cholesterol Treatment Trialists' Collaboration titled: "Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials" ("The Lancet 2019 Study"). The Lancet 2019 Study included the following summary:
- The Lancet 2019 Study included the following tables:
- Although the possibility of an investigation into statins had been under active consideration since late 2018, the first real indication that it was being actively pursued for publication appear in Mr Calman's emails on 4 February 2019.
- During his investigations (and as set out in more detail below), Mr Calman spoke to several experts in the field:
- As set out in more detail below, Mr Calman spoke personally to Professor Baigent on 6 February 2019; to Professor Samani on 13 February 2019; to Professor Collins on 14 February 2019; to Professor Sever on 15 February 2019; and Professor Smeeth on 22 February 2019. He also communicated with the experts by email prior to publication of the Articles.
- As noted, Mr Calman's work on the Articles really began in earnest on 4 February 2019. At 10.33, that day, Mr Calman received an email update alerting him to a new article on the First Claimant's blog, titled "Statins in the over 75s". Mr Calman was a subscriber to emails from the First Claimant. In his witness statement, he described this article as one of the "prompts" for his Articles.
- The text of the First Claimant's article is set out in Annex 3(A) to the judgment, but in summary the First Claimant challenged the claim that statins prescribed to the over 75s could save thousands of lives. She argued that such a claim was not supported by statistical data from The Lancet 2019 Study. In particular, relying upon the data from the study - particularly that presented in Figure 4 (see [65(1)] above) - she said that "there was no statistically significant difference between the statin group and the control group in people over 75 (or in the 70-75 age group)" and therefore "benefit in those who have 'no history of cardiovascular problems' cannot be claimed."
- At 10.41, that same day, Mr Calman sent the following by email to Professor Baigent:
- At 11.16, Mr Calman sent an email to X (a consultant cardiologist, whose identity as a source the Defendants have not disclosed), which included the following:
- Another important document that Mr Calman had seen and read (because it was quoted in the Main Article), prior to publication of the Articles, was an article on the Second Claimant's blog titled "Response to the Lancet paper", published on 3 February 2019. As the title suggested, it was the Second Claimant's response to The Lancet 2019 Study (see [64] above). The text of this article is set out in Annex 3(B).
- On 5 February 2019, arrangements were made for Professor Baigent to speak to Mr Calman. In response to an inquiry whether he would be able to share a copy of any article before publication "for fact-checking", Mr Calman responded (in an email at 12.54):
- Mr Calman then spoke on the telephone to Professor Baigent, on 6 February 2019, at around 2pm. Mr Calman took notes of the call.
- After the interview, Mr Calman had some email exchanges with Professor Baigent:
- Mr Calman was cross-examined by Ms Page KC regarding his understanding of the statistical data from The Lancet 2019 Study, and the point being made by the First Claimant in her article, "Statins in the over 75s". Mr Calman confirmed that he did not have expertise in statistical analysis. Ms Page KC suggested to Mr Calman that, at the time he published the Articles, he had not understood the First Claimant's criticism of The Lancet 2019 Study. Mr Calman's answer was:
- Ms Page KC also suggested to Mr Calman that, if the First Claimant's statistical analysis of The Lancet 2019 Study was correct, this called into question the reliability of the well-publicised claim, apparently made by Professor Baigent at a press‑conference to launch The Lancet 2019 Study, that if everyone aged over 75 took statins, up to 8,000 deaths per year could be prevented. Ms Page KC suggested that, if this was right, the First Claimant was performing a public interest in drawing attention to the fact that The Lancet 2019 Study did not support such a conclusion. Mr Calman answered: "Well, she did draw attention to it. She said it publicly". Ms Page KC persisted with the point: "Do you agree that, if she was right, she was performing a public service; she was writing something in the public interest?" Mr Calman replied: "I believe that is why she does what she does, because she believes in it". Mr Calman quibbled whether the First Claimant was right to challenge the lack of foundation for the claim that 8,000 deaths would be avoided, but ultimately accepted that, if she was correct, then she had acted in the public interest by drawing attention to that fact.
- Later in his cross-examination, but relevant to his belief as to the state of mind of the two Claimants, Mr Calman was asked whether the comment, made to him by Professor Samani, that the vice was publishing material with a "grain of truth mixed with speculation and opinion" was applicable to the two Claimants. Mr Calman agreed, and added:
- It is convenient here to state some conclusions about The Lancet 2019 Study and the First Claimant's article "Statins in the over 75s". On the evidence I have, it appears to me that the First Claimant did potentially have a valid point that The Lancet 2019 Study did not support the claim, attributed to Professor Baigent, as to the number of deaths that could be avoided in the over 75s with statin treatment. Statistically, the data does not appear to support such a conclusion, for the reasons explained by the First Claimant in her article. But of more importance to the exercise with which I am concerned, Mr Calman never got an answer from Professor Baigent (or any of the other experts) why the First Claimant's analysis on this point was wrong, and he lacked a knowledge of statistical analysis to reach his own conclusion.
- On 7 February 2019, Ms Simmons emailed Mr Calman. She had identified another expert, Professor Rory Collins, as a potential contributor for the Articles:
- On 11 February 2019, the First Claimant published a further article on her Blog, titled "Why cholesterol can't cause heart disease". Mr Calman received an email copy as a result of his subscription. The text of the article is set out in Annex 3(C).
- The following day, 12 February 2019, Mr Calman forwarded this article to Professor Baigent:
- X responded:
- Around 40 minutes later, X sent some further thoughts by email to Mr Calman:
- Mr Calman agreed with Ms Page KC that he had never asked the First Claimant any of the questions suggested by X. Mr Calman said that the right-to-reply email that was eventually sent to the First Claimant (see [189] below) covered the material that the Defendants intended to publish.
- Professor Baigent also responded to Mr Calman, by email, the same day:
- Professor Collins emailed Mr Calman, at 13.50 on 12 February 2019, offering dates for a meeting. He attached a copy of the Joint Editorial:
- On 12 February 2019, Professor Baigent emailed Mr Calman (copied to Professor Collins):
- At 16.23 on 12 February 2019, Mr Calman emailed a freelance journalist requesting some research:
- On 14 February 2019, Mr Calman spoke to Professor Collins. Mr Calman noted that Professor Collins emphasised that there were multiple studies that demonstrated that high cholesterol was a cause of cardiovascular disease and that there was a positive association between concentrations of LDL-C and rates of coronary artery disease in different populations. There had been, he said, an extraordinary improvement in vascular mortality trends in the US and UK in men and women. He acknowledged that this was partly due to reduction in smoking, but partly reflected better control of blood pressure and blood cholesterol. Mr Calman noted Professor Collins' observation that claims that blood LDL-C was not causally related to cardiovascular disease were "flat earthism" and "in the same realm as claiming that smoking does not cause lung cancer".
- On 15 February 2019, Mr Calman spoke to Professor Sever. Mr Calman said that Professor Sever had told him that all major trials had shown that statins reduced cholesterol, reduce the risk of heart attacks and strokes, and reduce deaths. He said that he did not think that there was another area of medicine where a drug treatment had been shown to be so conclusively beneficial, yet the false claims based on bad science made by a small number of individuals had a hugely disastrous effect on public perceptions, and that meant that hundreds of thousands of people were being exposed to needless risks.
- At 13.15 on 15 February 2019, Mr Calman emailed Professor Collins a link to an article published in the BMJ on 12 June 2016 - "Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review" (the 2016 Ravnskov Study - see further [210] below), with the message:
- Between 14.02 and 19.07, Mr Calman ("BC") had an exchange of messages with X on WhatsApp:
- Perhaps reflecting the concerns that he had expressed to X about the causes of elevated LDL-C, on 18 February 2019 Mr Calman exchanged various emails with his experts:
- On 23 February 2019, Mr Calman emailed Ms Fox and Professors Collins, Baigent, Samani, Sever and Smeeth:
- On 24 February 2019, at 17.58, Mr Calman emailed to X a first draft of what he had written:
- X responded at 19.12, providing some comments on Mr Calman's draft article. X identified some parts that s/he said were incorrect and provided other comments, expressed to be his/her opinion. In relation to the suggestion, attributed to Professor Collins in the draft, that the statin deniers were similar to Andrew Wakefield, X perceptibly questioned: "Does he liken the deniers to AW - ie think they are fabricating the evidence - or liken the effect of what they say to the consequences of the fake data put out by AW??".
- On 26 February 2019, at 08.44, Mr Calman emailed Ms Fox and Professors Collins, Baigent, Samani, Sever and Smeeth:
- Professor Baigent responded to Mr Calman in an email, at 09.06. Although he understood Mr Calman's desire to do so "from a journalistic standpoint", Professor Baigent was opposed to use of a single patient as an example as such evidence would be 'anecdotal' and would play "right into the hands of our opponents". Professor Sever, emailed to say that he had put out a message to his colleagues and hoped to get a response. He followed up with an email disagreeing with Professor's Baigent's objections.
- Mr Calman responded to Professor Baigent at 09.24 (copied to the other recipients):
- At 17.59, on 26 February 2019, Mr Calman emailed the first draft of the main article to Ms Fox and Professors Baigent, Collins, Samani, Sever and Smeeth. Mr Calman asked that each of them send back separately any amendments. He also sent the draft to X. The draft article indicates that, at this stage, Mr Calman believed that the Second Claimant was not a practising GP.
- Professors Samani and Sever and Ms Fox responded quickly to say that they were happy with the article (with some minor amendments). X also emailed that evening to say that s/he was happy. The following morning, at 09.05, Professor Baigent emailed some suggestions "to ensure accuracy" but otherwise he was content. During 27 February 2019, further work was done on the draft in discussion between Mr Calman and Professors Baigent and Collins.
- Mr Calman states that Mr Verity saw the Article on Thursday 28 February or Friday 1 March 2019.
- On Thursday 28 February 2019, at 16.53, Mr Calman sent right-to-reply emails to the two Claimants and Dr Malhotra (see Section F(8) below).
- As noted already ([48] above). there is very little contemporaneous evidence of the extent of Mr Calman's research into the Claimants' public statements, or claims, about statins prior to publication of the Articles. Mr Calman did not keep a record of what he had considered. All that exists, now, to shed light on what he did have, prior to publication, are (1) the contemporaneous email traffic; and (2) the Articles (whether in draft or as published).
- What Mr Calman says in his witness statement about his research prior to publication is the following:
- He included, in a schedule to his witness statement, "examples from the publications of the Claimants from which the claims I attributed to them in the Articles were sourced". I have identified, and quoted from, the statements of the Claimants identified by Mr Calman in his Schedule in Annex 4 to this judgment.
- Several issues arise from Mr Calman's evidence on the research he says that he carried out prior to publication and the Schedule of material that he assembled subsequently for his witness statement.
- More generally, there is a very obvious (and significant) variance between the identification of the various claims of the Claimants, as now relied upon by Mr Calman in the schedule to his witness statement (as set out in Annex 4), what was put to the Claimants for their comment prior to publication (see Section F(8) below) and what was subsequently published in the Articles. The most reliable evidence of the claims of the Claimants that Mr Calman had in mind prior to publication are those that he put to the Claimants in his right-to-reply emails, and then published in the Articles. The reformulation of the Claimants' claims in Annex 4, and the extent to which they vary from the presentation of those claims in the right-to-reply emails and the Articles gives rise to an obvious risk that there has been an element of reverse engineering in the identification of the Claimants' claims and the material identified in Annex 4.
- In their closing submissions, the Claimants submitted that there was limited evidence corroborating Mr Calman's evidence that he had considered all the material identified in Annex 4.
- Although Mr Calman was taken to various further documents in re-examination, this process did not satisfy me that he had an actual recollection of having seen these documents prior to publication of the Articles. That is not a criticism of him. No-one could be expected to have had an independent recollection of events that happened so long ago.
- Human memory is notoriously fallible. It is now well recognised that contemporary documents are likely to be a more reliable guide when the Court is required to resolve issues of fact: see Gestmin SGPS SA -v- Credit Suisse (UK) Ltd [2013] EWHC 3560 (Comm). My task is to test Mr Calman's recollection of what he had seen and read prior to publication against the contemporaneous documents and known or probable facts, to the extent that it is possible to do so.
- In my judgment there is force in the Claimants' submissions that Mr Calman, although trying to assist the Court with his genuine recollection, faces an impossible task in attempting to separate what he had read prior to publication of the Articles and what he had read following the complaint received from the Claimants in August 2019. Only evidence in the former category is relevant when considering the public interest defence. In cross-examination, Mr Calman stated:
- Consistent with this evidence, the documents disclosed by the Defendants and identified in Annex 4 were all printed (or saved) some time after publication of the Articles. Mr Calman has no contemporaneous notes, or other record, to establish what he had read prior to publication. It is perhaps a highly technical point, but the Claimants do rely upon the fact that, in their disclosure statement, signed by Mr Calman, the Defendants did not identify any of the documents relied upon in Annex 4, as having previously been in the Defendants' possession at any time prior to publication of the Articles.
- In the absence of a contemporaneous record, or other clear evidence supporting Mr Calman's recollection, I cannot accept that Mr Calman's recollection of what he had seen at the time of publication is reliable. There is too great a risk of him, honestly, but mistakenly, confusing that material that he found and read, following complaint, in August 2019, with that which he had previously seen in February 2019. I do not doubt Mr Calman's evidence that he did read other material published by the Claimants - beyond that I have identified (see [111] above) - prior to publication of the Articles, but the problem is now identifying what that was. Ultimately, it is for the Defendants to establish the material that was considered before publication, and I do not consider that they have discharged that evidential burden in respect of the material identified in Annex 4 (save that I have identified (see [111] above)).
- Later in this judgment (Section G(1)), I deal with the law relating to public interest defences under the Defamation Act 2013. As noted there (see [276]-[277] below), the absence of contemporaneous documents recording the material that was available to a defendant and considered prior to publication is likely, as it has done in this case, to harm the prospects of success of any public interest defence because, at trial, the defendant may fail to satisfy the Court of key evidence relied upon to support the defence.
- On 27 February 2019, arrangements were made for Mr Calman to interview the case study patient, anonymised and referred to as "Colin". He had been identified the day before. Colin had his first heart attack in 2009 and was prescribed statins. He had stopped taking his medication, in 2013, without informing his GP. Earlier in the week, he had had a second heart attack. The interview, which was recorded, took place at Hammersmith Hospital.
- The interview, which was attended by Colin's doctor for the early part, included the following:
- Mr Calman asked Colin about his decision to stop taking his medication:
- Mr Calman then asked Colin whether he had ever heard of Aseem Malhotra. Colin thought that the name "rang a bell".
- Mr Calman asked whether, if he were to have a conversation of a younger version of himself, "Do they really work, these drugs, or aren't doctors just trying to push drugs that make money for big pharma on us, and we don't really need them if we eat well and exercise, we don't really need them?":
- Mr Calman asked:
- When cross-examined, Ms Page KC suggested to Mr Calman that, in the interview, he had suggested to Colin that he had stopped taking statins "because [he] had heard they weren't so great". Mr Calman accepted that. Asked by Ms Page KC whether, in summary, Colin had told him that he was a man who had made his own decisions, Mr Calman answered:
- Ms Page KC asked Mr Calman why he had withheld from readers of The Mail on Sunday that Colin had said that he was someone who made his own decisions. Mr Calman replied that he did not remember thinking that he had withheld that information.
- Ms Page KC did not ask any further questions about the interview with Colin, or how it was represented in the Articles. In re-examination, Ms Evans KC took Mr Calman to the exchange when Mr Calman had suggested that there was "no smoke without fire" (see [123] above), and asked Mr Calman what he had concluded, based on his overall conversation with Colin, about what he was saying. Mr Calman answered:
- At this distance, it would be impossible for Mr Calman to have an independent recollection of his interview with Colin in 2019. He was only shown some sections of the transcript of Colin's interview when he gave evidence. I can accept that Mr Calman honestly believed the summary of what he believed Colin had told him that he gave in evidence, but an analysis of the transcript, however, demonstrates that the interview provided very little (if any) support for the suggestion that Colin had given up statins because of misinformation he had seen or heard in the media.
- Right at the beginning, Colin had rejected Mr Calman's initial suggestion that he had given up statins because "had heard they weren't so great", answering that, in combination, his medication left him feeling lethargic and there had been problems with his digestion. He spoke to some other people, who had "gone through a heart attack", who told him that they had given up taking their medication, without adverse effect, and he made his own decision to stop the medication. When pressed by Mr Calman, whether he had heard anything negative about statins, Colin confirmed that he had heard people on the radio, who could "perpetuate quite a lot of untruths" and that there might be people who believed them, but he said he was "probably not one of those" and liked to make his own decisions.
- Asked by Mr Calman whether there was something specific - perhaps something that he had heard on the radio - which had prompted him to give up taking his medication, Colin gave an answer that suggested, if there was a trigger, it was that he had successfully completed a 2,000-mile hike and he had questioned whether he needed the medication. To the extent that he had reservations about the medical advice he was being given, it was limited to a concern that doctors might get paid to prescribe particular medication. The question about what advice Colin would give to a younger version of himself was both leading and premised on facts that Colin had previously not accepted (see [122] above). It only elicited a response from Colin that suggested that he now recognised that he should have kept taking his medication after his first heart attack.
- Mr Calman's follow-up questions (see [123] above) suggested to Colin that he had heard "myths" or "bad things" about the medication (he had suggested neither), and asked whether those had affected his judgment. Colin's answer that there was "some impact", was immediately qualified - there was "so much out there when you speak to people" - and accompanied by him repeating that he made up his own mind.
- From Mr Calman's point of view, the highpoint of the interview was Colin's suggestion that "if you hear it so many times, that subconscious little monkey at the back of your head keeps telling you, 'Oh, you've heard this, must be some truth in it.'" It was Mr Calman who used the phrase "no smoke without fire", but Colin's response showed that he was not suggesting that false information about statins had been the major (even a significant) factor in his decision to stop taking his medication. At best, his answers could be read as raising a question whether the information he had received from those whose advice he sought may have been influenced by media information about statins.
- The representation of the interview with Colin, and importantly what he had said, presented in the Editorial was misleading in the following respects (references in square brackets to paragraphs in the Editorial):
- It may well be that there existed people who had given up taking statins because of media coverage (indeed, that was the thrust of the LSHTM Paper - see Section F(7) below). On a fair review of what he told Mr Calman, Colin was not identifying himself as one of them.
- On 18 February 2019, Mr Calman sent an email to the then Health Secretary, Matt Hancock. The approach made to Mr Hancock is important, so I shall set out the message in full:
- Dame Sally was, in fact, on annual leave. In her place, the head of her office responded, on 19 February 2019, advising Mr Calman that his email had been referred to the press office at the Department of Health. Later that day, Syeda Hasnain, the Chief Communications Officer at the Department of Health ("Ms Hasnain") responded inviting Mr Calman to contact her: "I'm sure we can help". Mr Calman then spoke to Ms Hasnain on the telephone.
- In his witness statement, Mr Calman said that he could not recall a time as a health journalist when the Department of Health had been "so proactive in liaising with [him] about a story. They were very keen and very engaged and Syeda Hasnain said to me that this was exactly the type of thing that Matt Hancock wanted to be involved with". Although Mr Calman did not make a note of this call, he said this in his witness statement:
- When cross-examined, Mr Calman became more certain about what he had said during the conversation:
- In the follow up email to Ms Hasnain, following their call, Mr Calman forwarded the email he had sent to Mr Hancock (see [134] above), copied to (amongst others) Sarah Wilson, the Media & Campaigns Officer at the Department of Health, with the following message:
- On 22 February 2019, Ms Wilson responded to Mr Calman's email to Ms Hasnain, checking on the deadline for the provision of a statement for publication. Mr Calman responded that the deadline was 25 February 2019, and added:
- A statement was not provided by the deadline. Ms Wilson emailed Mr Calman, at 16.21 on 27 February 2019: "Really sorry this is in clearance - am chasing for you now! Will get over to you ASAP". Mr Calman replied, at 16.23: "Fab, thanks. Would be keen [to] brief you in more detail about the piece once we have the quote..." There is no indication that Ms Wilson ever spoke to Mr Calman to get a proper understanding of what he intended to publish. Her understanding of the context in which any statement Mr Hancock provided was therefore limited to what had been set out in Mr Calman's emails (and possibly what she had been told by Ms Hasnain).
- At 09.25 on 28 February 2019, Ms Wilson emailed a statement from Mr Hancock to Mr Calman for publication ("the Hancock Statement"):
- Mr Calman was evidently delighted with the Hancock Statement, responding by email, at 09.51: "Thanks all. This is EXACTLY what we're after". Ms Page KC asked Mr Calman whether, when he saw the Hancock Statement, with its references to "spreading reckless and ignorant misinformation" and "pernicious lies", he thought that Mr Hancock had failed to realise that his quote was going to be used in an article naming individuals. Mr Calman answered: "I had no reason to think that, no". The cross-examination continued:
- Although slightly out of chronological order, it is convenient in this section of the judgment to deal with the post-publication response - or fall out - from the Hancock Statement as it appeared in the Articles.
- At 23:40 on 3 March 2019 - the day of publication of the Articles - Dr Malhotra sent a text message to Mr Hancock:
- On 5 March 2019, during an interview with TalkRadio, Dr Malhotra said:
- Later that day, Mr Calman had a series of email exchanges with Ms Wilson at the Department of Health:
- In his witness statement Mr Calman recognised the importance of the Hancock Statement, noting that "the comments of the Secretary of State are probably what elevated the News Article in the paper to page 2". As to his communications with Ms Wilson following Dr Malhotra questioning whether Mr Hancock had been aware how his statement would be used in the Articles, in his evidence Mr Calman said:
- Commenting in his witness statement, on Ms Wilson's email at 19.41 (see [146(5)] above) specifically and the Department of Health response more generally, Mr Calman said:
- In cross-examination, Ms Page KC suggested to Mr Calman that his recollection of what had been discussed with Ms Hasnain was unreliable. If he had been clear that the intention was to name individuals, that would have been the obvious response to have given to Ms Wilson, in the email exchanges on 5 March 2019 (see [146] above), to Dr Malhotra's claims that Mr Hancock had not understood that the article would link his statement to individuals. Mr Calman did not accept that:
- I asked Mr Calman whether he thought that Ms Wilson would have appreciated that the individuals were not only going to be named, but were going to be the main focus of one of the inside articles. Mr Calman answered:
- Ms Page KC challenged this answer by reference to Mr Calman's rather more equivocal recollection in his witness statement (see the underlined passages in [136] above). Mr Calman agreed that his oral evidence was more certain than his witness statement regarding his recollection of what was discussed with Ms Hasnain, and added:
- Following publication, Dr Malhotra lodged a complaint with the Mail on Sunday. On 20 April 2019, Mr Wellington emailed a draft response to Dr Malhotra's complaint to Mr Calman asking him to review it. It contained the following, regarding the Hancock Statement:
- Perhaps unsurprisingly, given his earlier communications with Mr Hancock (see [144] above), Dr Malhotra was not satisfied with the response he received. On 20 April 2019, Dr Malhotra sent a text message to Mr Hancock, which posted a screen shot of the paragraph from the email from Mr Wellington, that I have quoted above together, with the comment: "Oh dear Matt! Really?" Mr Hancock responded: "Sorry Aseem, as I said I had no idea they'd link it. All the best".
- As there is a factual dispute as to what Mr Calman told Ms Hasnain about the Articles, I need to state my findings on the evidence.
- I do not accept Mr Calman's evidence, given during cross-examination, about what he told Ms Hasnain during his telephone call. I consider that he has misremembered the detail, and critically whether he communicated to Ms Hasnain (successfully or at all) that the Articles - to which any statement from Mr Hancock would be added - were going to feature prominently three individuals who were going to be denounced as "statin deniers" whose "deadly propaganda" was putting the lives of patients at risk. If Mr Calman mentioned names at all during this call, it is likely that they were cited only as examples. I am satisfied that no-one at the Department of Health realised, from Mr Calman's communications, the real nature, scope and intended targets of the Articles that the Defendants intended to publish.
- I have already noted the notorious fallibility of human memory (see [113] above). As before, I shall test Mr Calman's recollection against the contemporaneous documents and known or probable facts to the extent that it is possible to do so. The initial communication sent by Mr Calman to Mr Hancock (and the Department of Health) did not mention any individuals (see [134] above). As a description of what the articles were likely to contain, the email gave a materially misleading impression (itself likely to have been reinforced by the email of 22 February 2019 - see [139] above). As Mr Calman accepted in his evidence, by the time he approached Mr Hancock for a statement, the three targets of the Articles were firmly in his sight, as was what was likely to be said about them. The intention, as it had been practically from the outset, was to identify them as three prominent "statin deniers". The failure to mention them at all in the original emails is odd.
- The Court only has the evidence of Mr Calman about what was discussed in his telephone call with Ms Hasnain on 19 February 2019. The Defendants have not provided evidence from anyone at the Department of Health who dealt with the Hancock Statement. But the subsequent documentary evidence provides some indications of what was, and what was not, likely to have been discussed.
- In his oral evidence, Mr Calman became firmer than he had been in his witness statement that he had told Ms Hasnain the identities of those who were to be named in the articles. He pointed to his follow up email as providing confirmation of this (see [138] above). I am not persuaded that they provide the support that Mr Calman believes they do. The first three paragraphs of the email continue largely in the same vein as the original email; the key focus was to be concern over "fake news about statins", and the consequences in terms of harm to patients who might be persuaded to stop taking statins. In only one sentence, in the closing paragraphs, were individuals mentioned: the Second Claimant and Dr Malhotra. In the context of the emails sent by Mr Calman, this gave the impression that they were simply being identified as 'home grown' examples of 'widely-quoted' statin deniers rather than the key targets of the Articles. The follow up email of 22 February 2019 returned again to describing the focus of the article as having general rather than specific targets.
- It is important, when assessing the email of 19 February 2019 to consider the conversation that had immediately preceded it. If Mr Calman had mentioned, clearly, in the phone call with Ms Hasnain, that there were to be three named targets in the article, then the terms of this email would have been materially different. If the email was recapping names that Mr Calman had already mentioned to Ms Hasnain, it could have been expected to have said so, and it would not have omitted the First Claimant. I do not accept Mr Calman's evidence that he mentioned the First Claimant's name in his phone call with Ms Hasnain.
- In my judgment, it is also highly likely that, had Mr Calman effectively communicated to Ms Hasnain, the Chief Communications Officer at the Department of Health, that his article was likely to be targeting three individuals, she would have been much more cautious as to the terms of any statement that Mr Hancock might provide for publication. On 27 February 2019, Mr Calman had offered to Ms Wilson that he would "brief [her] in more detail about the piece". It is likely, had Ms Hasnain or Ms Wilson fully understood the nature of the articles that Mr Calman was about to publish, that they would either have asked many more questions of him, as to precisely what was to be alleged against the individuals, or ensured that any statement issued by Mr Hancock would have made clear that he was referring to the general issue of misleading information about statins and not any individuals. An article singling out individuals would have carried an obvious risk - which a person in the position of Ms Hasnain and/or Ms Wilson would have immediately appreciated and well understood - of the Minister being seen to endorse serious allegations against those individuals in circumstances where Ms Hasnain, Ms Wilson and Mr Hancock had no real information about those allegations or any knowledge of the evidence relied upon to support them.
- I cannot - and need not - make precise findings of what was said in the telephone call between Mr Calman and Ms Hasnain, but I am satisfied on the evidence that, from what was said, even if names of any individuals were mentioned, Ms Hasnain simply did not appreciate that Mr Calman was intending to publish articles that were going to feature prominently three individuals who were to be alleged to be "statin deniers" promoting "deadly propaganda" that was putting patients' health at risk. The relevant staff at the Department of Health believed that the article to be published would be dealing with the general problem of misinformation about statins. It would have been highly reckless for the Hancock Statement to have been issued for publication in the terms in which it had been drafted had there been a proper understanding of the true nature and scope of the articles that the Defendants intended to publish and the allegations they would make. Mr Calman, equally, could not have believed, based on his communications with the Department of Health, that he had effectively communicated that the Articles were going to be a very serious attack on three individuals and that the statement he was seeking was going to be used in that context.
- The subsequent events support this conclusion.
- Finally, on the issue of the Hancock Statement, I should deal with the Defendants' response to Dr Malhotra's complaint. This issue has a potential bearing on the public interest defence of the continued online publication of the Articles. I am unimpressed by Mr Wellington's defence of the treatment of the Hancock Statement in the email of 20 April 2019 sent to Dr Malhotra. Mr Calman knew, not only from Mr Hancock's message to Dr Malhotra but importantly also from Ms Wilson, that the Hancock Statement had not been intended to refer to individuals. In these circumstances, to describe Mr Hancock as "standing by his statement in full" was misleading, and should have been recognised by Mr Calman (at least) to have been misleading (as he was inclined to accept when he gave evidence).
- When Mr Wellington gave evidence, he was unable to recall whether he had seen all the emails that had passed between Mr Calman and the Department of Health when he was dealing with the post-publication complaint from Dr Malhotra. The documentary evidence shows that Mr Calman had sent him Ms Wilson's email on 5 March 2019 (see [146(5)] above). Ms Page KC asked Mr Wellington whether he thought a reader of the Articles would have appreciated that, in his statement, Mr Hancock had not been singling out any individuals for criticism. Mr Wellington answered: "Well, he wasn't singling out any individual. If he had been, we would have quoted him as saying so". Although not an answer to Ms Page's question, that answer did demonstrate that Mr Wellington appreciated that the Mr Hancock had not intended to single out any individuals as responsible for their "pernicious lies". Asked whether, if the Articles had misrepresented Mr Hancock's position as to the allegations he was making against the Claimants and/or Dr Malhotra, they should have been amended, Mr Wellington said that he could not answer the question because it was hypothetical, but added that he did not think that there had been any misrepresentation.
- In the published Articles, the Hancock Statement was clearly regarded as editorially powerful and important. Mr Calman was delighted when he saw its terms (see [142] above) and ultimately it provided the headline for, and key parts of, the News Article. As Mr Calman frankly recognised, editorially, it was likely to have been the single most important factor in propelling the News Article onto page two of the print edition of the newspaper.
- The treatment of the Hancock Statement by the Defendants in the Articles has been a focus of sustained criticism by the Claimants. I have dealt below with whether the print Articles are, by law, to be read as a single publication on the issue of meaning (see [511]-[513] below), but as a matter of practical reality, nobody who was involved in the publication of these Articles in the print edition could have believed (or intended) that their readers would have read only the News Article. One of the principal objects of the News Article was to promote, stimulate interest in, and draw readers to, the Main Article and the Editorial in the Health Section, which was given a clear signpost in the News Article. In Online Publication 1, the text of the three Articles was presented to readers on a single webpage.
- In my judgment, the use of the Hancock Statement in the Articles gave readers a completely misleading impression of what Matt Hancock had said. Mr Calman knew that; and, indeed, he was uncomfortable with the way the Hancock Statement was presented in the News Article. I am surprised that, when giving evidence, and with the benefit of the very substantial period since publication of the Articles for reflection, Mr Wellington could not see how the presentation of the Hancock Statement was (or could be) misleading.
- The Hancock Statement was a comment by Mr Hancock, as Health Secretary, on the general issue of misinformation about statins and the risks that such misinformation posed. Neither Mr Calman, nor anyone at the First Defendant involved in publication of the Articles could have failed to appreciate that. As I have found, neither Mr Hancock nor his office were aware that his statement was going to be used in an article that was going to make serious allegations against three, named, individuals.
- The treatment of the Hancock Statement, in the News Article (headline, [1], [2] and [5]), would have given readers the clear impression that Mr Hancock, as Health Secretary, apparently in full knowledge of the allegations contained in the Article, had "thrown his weight behind a Mail on Sunday campaign" and made a "passionate" public statement denouncing the Claimants (and Dr Malhotra) for their "pernicious lies" (a particularly memorable phrase that was repeated in the Main Article ([15]). As the Defendants knew, he had done no such thing.
- In answer to questions from Ms Page KC, Mr Calman's evidence was that he did not believe, nor intend to suggest in the Articles, that the Claimants were lying (i.e. publishing information they knew to be false) in their public statements. At the end of his evidence, I sought clarification from Mr Calman as to his evidence on this important point, and particularly how it related to the treatment of the Hancock Statement in the Articles:
- In that answer, Mr Calman was referring to his discussions with Mr Adams about the News Article that was sent to him on 1 March 2019 (see [229] below).
- The sensitivity to, and clear awareness of, the issue of the gravity of the allegation that it was intended to level against the Claimants in the Articles is important (and I will return to it in considering both Mr Calman's understanding of the meaning of the Articles - see Section F(10) - and more widely the public interest defence), but there remained an unresolved - and obvious - tension in the News Article (particularly). Mr Calman's suggested change to "wrongly claiming" was adopted (see [4] in the News Article), but the use of the Hancock Statement risked powerfully and unequivocally contributing to the message that the "statin deniers" (i.e. the Claimants and Dr Malhotra) were indeed "pernicious liars".
- This was a fundamental problem with the Articles and their presentation. Mr Calman was clear in his evidence that he did not believe that the Claimants were liars (see e.g. the passage quoted in [79] above). Mr Calman, apparently, did not wish to brand the Claimants "liars" in the Articles; he said he "wanted people to know that what they said was wrong". As to their motivation, in respect of the First Claimant, Mr Calman said: "I believe that is why she does what she does, because she believes in it". Questioned about the Second Claimant's blog entry on 3 February 2019 (see [73] above), Mr Calman accepted that the Second Claimant believed that it was in the public interest that he put forward his statistical analysis of The Lancet 2019 Study.
- For his part, when cross-examined, Mr Verity confirmed "we didn't think that they were liars". However, when probed about the meaning of the word "liar" he initially said that it was "someone who habitually tells lies. I think these are people who were, for whatever reason, publishing and broadcasting lies". In fairness, Mr Verity then explained, in answer to further questions:
- Nevertheless, in his evidence, Mr Calman said that he would not consider "changing" the Hancock Statement, and its reference to "pernicious lies". That missed the point. It was not, so much, that the Hancock Statement needed "changing"; it was how, if it was going to be included at all, it was going to be treated in the Articles. Mr Calman was obviously very clearly aware of the danger of calling the Claimants "liars", because he did not believe that they were. But it was very difficult to include the Hancock Statement (particularly given the way it was actually deployed in the published Articles) without it conveying, very clearly, and in a particularly memorable phrase, that the Claimants were being denounced by the Health Secretary (and the newspaper) for their "pernicious lies". In part, that was a product of the fact that the Defendants, in the treatment of the Hancock Statement, had effectively converted what was known by them to be a general statement from the Health Secretary into a very specific attack on three individuals. To avoid alleging that the Claimants (and Dr Malhotra) were, indeed, "pernicious liars", the Defendants needed to make very clear that Mr Hancock was not singling out any individuals for criticism. The Articles did not do so. On the contrary, they made it appear that Mr Hancock had done just that.
- In my judgment, the Hancock Statement, as its treatment in the Articles demonstrates, was editorially too powerful to resist. As noted, it propelled the News Article to page 2 in the newspaper, in which it is featured prominently and dominated the headline. But ultimately, in my judgment, the way in which the Hancock Statement was used was seriously misleading and gave an entirely false impression of whether Mr Hancock had criticised the three individuals (he had not). Although not acting alone in the editorial process, Mr Calman is responsible for this. He knew that Mr Hancock had never provided his Statement for the purposes, or context, in which it was ultimately used by the Defendants in the Articles. He was uncomfortable with its treatment, but ultimately, he permitted the Articles to appear in the terms in which they were published. That was a serious error.
- On 28 June 2016, The BMJ published a study: "Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data" ("the LSHTM Paper"). The Abstract summarised the study:
- It is important to note the following further matters about the LSHTM Paper:
- In support of its defence of qualified privilege under s.6 Defamation Act 2013 (see [27]‑[28] above), the Defendants have relied upon the following facts to establish the necessary elements of the defence (see Section G(3) below):
- The Defendants have provided no witness evidence relating to the peer-review process of the LSHTM Paper in The BMJ. Nevertheless, based on the documents submitted at the trial, the Defendants' case is as follows.
- In relation to evidence that, before publication, a review of the scientific merit of the LSHTM Paper was carried out by the editor of The BMJ, the Defendants rely upon the documents to demonstrate the following:
- In relation to evidence that, before publication, a review of the scientific merit of the LSHTM Paper was carried out by one or more persons with expertise in the scientific matter concerned, the Defendants rely upon the documents to show that the "reviewers" identified in Dr Weber's email of 15 April 2016, as demonstrated by their questions/comments, caried out a review of the scientific merit of the LSHTM Paper.
- As to proof that the "reviewers" had expertise in the scientific matter concerned, the Defendants rely on the evidence that they were asked to carry out a peer-review for The BMJ and that each of them was either a medical doctor or had a PhD (or, in the case of Ms Schaffer, was a PhD candidate in pharmacoepidemiology). Moreover, the Defendants rely on evidence that two of the external peer reviewers had written published papers in scientific journals analysing the effect of media coverage on the use of statins:
- As to whether the review process was "independent", the Defendants rely on:
- The Claimants have not provided any evidence from anyone involved in The BMJ's decision to publish the LSHTM Paper. Broadly, the Claimants' response on the factual case advanced by the Defendants on the publication of extracts from the LSHTM Paper is that they have failed to adduce evidence that is capable of proving the required elements of the defence under s.6 Defamation Act 2013. The Claimants complain that the Defendants have not adduced any witness evidence to prove these facts, i.e., from someone at The BMJ who was involved in the pre-publication review of the LSHTM Paper. The evidence available to the Court indicates that, at the time of publication of the LSHTM Paper, the Editor of The BMJ was Dr Fiona Godlee. The documentary evidence upon which the Defendants have relied has been taken from The BMJ website. It does not support an inference (a) that The BMJ was, in 2016, a journal "with more than one editor" in the sense contemplated in s.6(8), i.e., that it had more than one editor who had responsibility for deciding to publish material in the journal; or (b), if it did, that any person other than Dr Godlee was an editor who was in fact responsible for deciding to publish the LSHTM Paper; or (c) if any person fitted this bill, that the individual in question had also carried out the review of the statement's scientific or academic merit before it was published. In these circumstances, so far as concerns s.6(8), the Defendants have failed to discharge the burden of proof which rests on them in relation to each of the requisite factual elements.
- Further, the Claimants argue that the review of the statement's scientific or academic merit must also be carried out by "one or more persons" other than the editor of the journal, with "expertise in the scientific or academic matter concerned", that is the scientific or academic matter to which the Court has found the statement relates for the purposes of s.6(2). The Claimants do not dispute that persons other than the editor of The BMJ, whoever that person was, were involved in the process of review of the LSHTM Paper before it was published. However, they suggest that it is less clear what is the "scientific or academic matter" to which the LSHTM Paper relates for the purposes of s.6(2) - including how widely or narrowly that "matter" is to be construed - or whether any of those other persons, and if so who, had "expertise" or the necessary degree and extent of expertise in the matter concerned. The Claimants contend that the Defendants must satisfy the Court on these points too. In particular, they argue that the Defendants have failed to identify who, if anyone, among the persons who were involved in the pre-publication review of the LSHTM Paper had expertise in that matter in question, and on what basis.
- Right-to-reply emails were sent to the Claimants and Dr Malhotra on Thursday 28 February 2019 from 16.53. In each email, the stipulated deadline for response was midday the following day. Although Mr Calman stated in his evidence that he had given them 24 hours to respond, the period was shorter than that.
- In his evidence, Mr Calman explained his approach to the right-to-reply process:
- The email to the First Claimant was in the following terms:
- The email to the Second Claimant was in the following terms:
- I would note the following about the right-to-reply emails that were sent to the Claimants.
- Given the advanced stage of preparation that the Articles had reached, there are also three surprising omissions from the right-to-reply emails. First, no suggestion was made in the emails that the Claimants might potentially be criticised in the Articles as having a financial motive behind their "misleading" public statements on statins. Second, the Claimants were not informed of the terms of the Hancock Statement - which was to be presented as the Health Secretary condemning them for their "pernicious lies". Third, no reference was made to Colin's case study, or any indication that the Articles would suggest that there was a "special place in hell" for "statin deniers" like the Claimants. The direct and immediate result of this was that the Claimants had no opportunity to respond to these allegations. More indirectly, it deprived the Claimants of a real understanding of the enormity of what was about to be published about them.
- In his evidence, Mr Calman stated that he had not put allegations of "dishonesty or venal motives to the Claimants" because he did not consider that he was "levelling this sort of allegation against them". As for the Hancock Statement, Mr Calman's evidence was that he could not put every single element of an article to a subject in advance of publication and that he had to make a judgment as to the important points on which to seek comment. I will return to these points when I come to assess the public interest defence. I accept that there was, perhaps, little that the Claimants could have provided by way of meaningful response to the case study, but, had they been told that they faced being denounced by the Health Secretary for their "pernicious lies", they would have been able to advance a case in defence of their honesty.
- Looked at in the round, and compared with the Articles that were published subsequently, the right-to-reply emails failed to disclose the full nature and extent of the attack on the Claimants that was about to be published in the Articles. That had a direct impact on what the Claimants said in their responses and, ultimately, what was available to Mr Calman to include in the Articles as the Claimants' defence of themselves.
- The Second Claimant responded swiftly, by email at 18.35, which advised Mr Calman that he was a salaried GP employed by two NHS trusts. As to other points raised by Mr Calman, the Second Claimant's response included the following (with the original questions posed by Mr Calman in bold and paragraph numbers added in square brackets):
- The Second Claimant sent a further email to Mr Calman at 08.50 on 1 March 2019 (emphasis in the original):
- The First Claimant responded by email at 08.51 on 1 March 2019 (copied to Mr Verity) and sent a corrected version at 08.58. In her email she said (with the original questions posed by Mr Calman in bold, footnotes included from the original and paragraph numbers added in square brackets):
- Following receipt, in an email to a colleague, Jo Macfarlane, at 11.51, Mr Calman described the First Claimant's response as "unhinged... and even copied in Ted lol" (a reference to Mr Verity). When cross-examined, Ms Page KC suggested that this was indicative of Mr Calman having "written off" the First Claimant's response. Mr Calman clearly regretted expressing himself in this way. He described it as "puerile" and "letting off steam in a stressful situation", but rejected the suggestion that he had "written off" the response. I will return to this point below, when I consider the public interest defence.
- Mr Calman did not respond to either Claimant's emails, in particular he did not provide any examples of (a) the First Claimant's alleged reliance upon observational studies to substantiate her claims about statins and cholesterol in articles and blog posts which contradicted findings of authoritative clinical trials; and/or (b) instances where the Second Claimant had taken "a grain of truth, mixed with speculation and opinion, which made it difficult for the public to know who to trust".
- It is common ground that, in the right-to-reply emails, Mr Calman had been mistaken in attributing authorship of the 2013 BMJ Articles to the Claimants. That surprising error had perhaps an unfortunate further consequence. The right-to-reply email to each Claimant linked the LSHTM Paper's conclusions about the consequences of misinformation about the rate of side-effects of statins to the 2013 BMJ Articles. Both Claimants pointed out that they were not authors of the 2013 BMJ Articles, but Mr Calman did not reply to either of them to clarify what would nevertheless be alleged against them regarding their alleged claims that doctors were "hushing up" the side effects of statins. When published, the Editorial wrongly suggested ([17]) that the Claimants had been invited to comment on the LSHTM Paper (they had not, except on the mistaken premise I have explained), and then wrongly attributed their responses on different points. The Second Claimant's claim that he was a victim of "reprehensible bullying" was actually made in answer to the claim that the toll of misinformation about statins, in terms of death and disability, "could be far worse" than the MMR scandal (see [3] in the Second Claimant's response). The First Claimant's suggestion of "bullying" was made in response to a different point, and was taken out of context (see [9] in the First Claimant's response).
- The documents/material to which the Claimants provided links in their respective responses to Mr Calman were available at trial, and during his evidence Mr Calman was asked about some of them. As they represented each Claimant's response to the allegations against her/him, it is important to identify the key documents upon which they relied. I shall consider them in chronological order.
- On 18 December 2002, the Journal of the American Medical Association (JAMA) published the result of a clinical trial: "Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT)" (referred to in paragraph [8] of the Second Claimant's response) ("the Pravastatin Study"). The following "Abstract" from the paper summarises the study and its conclusions:
- In November 2010, the First Claimant published a Blogpost "Cholesterol & heart disease - there is a relationship, but it's not what you think" (relied upon by the First Claimant in paragraph [8] of her response to Mr Calman). This was the First Claimant's analysis of statistical data from the World Health Organisation of 192 countries correlating cholesterol levels and deaths from cardiovascular disease. Her conclusion was that higher cholesterol levels were associated with fewer cardiovascular disease deaths and lower cholesterol levels were associated with greater numbers of deaths from cardiovascular disease.
- An article from the Cholesterol Treatment Trialists' (CTT) Collaborators was published in The Lancet on 17 May 2012: "The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials" (relied upon by the First Claimant in paragraph [1] of her response to Mr Calman). The summary stated:
- On 13 June 2014, The Guardian published an article: "Professor who sparked statins row says government should intervene" (relied upon by the First Claimant in paragraph [10] of her response to Mr Calman), which included the following:
- On 15 August 2014, the BMJ published the "Report of the Independent Panel Considering the Retraction of Two BMJ papers" ("the BMJ Review Report") (relied upon by the First Claimant in paragraph [11], and the Second Claimant in paragraph [16] of their respective responses to Mr Calman). A panel, chaired by Iona Heath, Past President of the Royal College of General Practitioners and former Chair of the BMJ's ethics committee, had been commissioned by the BMJ to investigate the publication of the 2013 BMJ Articles. The background was set out in the BMJ Review Report, as follows (with footnotes omitted):
- The published correction continued (again with footnotes omitted)
- Under the heading "Summary of conclusions and recommendations", the Panel stated:
- On 24 September 2015, The BMJ published a study: "The effect of statins on average survival in randomised trials, an analysis of end point postponement" (relied upon by the First Claimant in paragraph [5] and the Second Claimant in paragraph [4] of their respective responses to Mr Calman) ("the Kristensen Study"). The following "Abstract" from the paper summarises the study and its conclusions:
- On 12 June 2016, The BMJ published a study: "Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review" (Ravnskov & others, including the Second Claimant) ("the 2016 Ravnskov Study") (relied upon by the Second Claimant in paragraph [13] of his response to Mr Calman). The following "Abstract" from the paper summarises the study and its conclusions:
- On 13 June 2016, the Second Claimant appeared on BBC Radio 4's Today programme, together with Professor Pearson (who disagreed with the Second Claimant) to talk about the 2016 Ravnskov Study. During the course of the interview, the Second Claimant stated:
- The First Claimant's Blogpost of 12 September 2016 was her published critique of The Lancet Review (see [62] above) (relied upon by the First Claimant in paragraph [1] of her response to Mr Calman). The First Claimant complained that her request of the CTT to be provided with the underlying raw data was rejected, in May 2012, on the grounds that the CTT Collaboration was "not able to provide participant level data to third parties". Broadly, the Blogpost argued that whilst statins were proven to lower cholesterol, it was not adequately demonstrated that lowering cholesterol reduced heart disease. The second issue raised in the Blogpost concerned side-effects. The First Claimant complained that the data regarding side-effects had been "withheld" by the CTT. She quoted an article which reported that: "Of the 491 patients who entered phase A... muscle symptoms occurred in 209 of 491 (42.6%) while taking atorvastatin but not while taking placebo". She also cited the patient information leaflet for Lipitor which cautioned that the "common side effects" of the medication "may affect up to 1 in 10 people". The article concluded:
- The following article, titled "Statins: we need an independent review", written by the BMJ's Editor in Chief, Dr Fiona Godlee, was published in The BMJ on 15 September 2016 (relied upon by the First Claimant in paragraph [3] of her response to Mr Calman). It was published in response to The Lancet Review (see [62] above):
- On 18 September 2016, The Sunday Times published an article "Statins expert in row over level of risk to patients" (relied upon by the First Claimant in paragraph [12] of her response to Mr Calman) which included the following:
- On 11 October 2018, the Expert Review of Clinical Pharmacology published a paper "LDL-C does not cause cardiovascular disease: a comprehensive review of the current literature" (Ravnskov & others, including the Second Claimant) (referred to in paragraph [12] of the Second Claimant's response) ("the 2018 Ravnskov Study"). The following "Abstract" from the paper summarises the study and its conclusions:
- Mr Calman did not send the full response of either Claimant to the right-to-reply email to any of the Professors for their comments/observations. At 18.55 on 28 February 2019, he sent an email to Professors Baigent, Smeeth and Sever:
- At 19.42, Mr Calman sent the Second Claimant's email to X. X did not reply by email.
- The Professors responded to Mr Calman's email as follows:
- At 16.46 on 1 March 2019, Mr Calman sent a revised draft of the Main Article - which now included material from the Claimants' right-to-reply responses - to Professors Baigent and Collins, with the message:
- The draft of the article included the following paragraphs:
- Professor Collins, who had not seen the detailed response of either Claimant, was swift to express disappointment with Mr Calman's revised draft. He emailed, at 17.46 on 1 March 2019, copied to Professors Baigent, Sever, Smeeth and Samani:
- Mr Calman replied at 17.55 (only to Professors Collins and Baigent):
- Mr Calman attached to his email page proofs of the Main Article and the Editorial. The draft of the Main Article now included the Text Box, then headlined "Revealed: The 'Experts' who say don't take your statins", the text of which then read:
- Mr Calman could not recall whether he and Professor Collins had spoken on the telephone (as he had suggested in his email at 17.55). He said that the two of them had spoken at some point, but that he had not made any notes.
- Professor Smeeth (who had also not seen the detailed responses provided by the Claimants) then added his voice criticising the revised draft article, in an email to Mr Calman (copied to the other Professors) at 18.03:
- At 18.34, Professor Sever (who was also ignorant of the detailed responses sent by the Claimants) emailed Mr Calman:
- At 18.47, in an apparent effort to appease them, Mr Calman sent further amended page proofs of the Articles to Professors Collins and Baigent. The most significant changes were to the Text Box, which now read:
- Professor Collins remained unsatisfied with Mr Calman's efforts to address his concerns. There were further discussions and amendments were made to the text, many of which led to points that had been made by the Claimants, and originally included by Mr Calman in the draft Articles, being removed.
- At 20.18, on 1 March 2019, Mr Adams emailed a draft of the News Article to Mr Calman. In that draft, Mr Adams had quoted sections of the Hancock Statement but also included the following sentence:
- As I have already noted, this exchange is important in shedding light on the concern Mr Calman had about the gravity of the charges being levelled in the Articles (see [172]-[173] above); a concern it appears that was also shared by Mr Adams.
- In an early part of his cross-examination, Ms Page KC suggested to Mr Calman that, by granting to the Professors the opportunity to review the Articles prior to publication (see [74] above), he had surrendered, or fettered, his editorial judgement as a journalist. Mr Calman rejected that. He accepted that, when they saw the "finished article", which included several elements of the Claimants' right-to-reply responses, the Professors were "horrified" and were "kicking off". Describing the process of editing the Articles prior to publication, Mr Calman said:
- Mr Calman accepted that he felt under pressure by the Professors to make changes to the Articles, but denied that he was under "heavy pressure". The exchanges between them demonstrate the difficult position in which Mr Calman found himself. He described the situation as "delicate and tricky". Having promised to give the Professors the opportunity to review the key Articles prior to publication, he had to do so. As a journalist, and no doubt with the benefit of legal advice, Mr Calman knew that it was important to achieve balance in the Articles. The Professors, being neither journalists nor media lawyers, could not be expected to understand that important point (and it appears they did not). Apart from his email to Professors Collins and Baigent on 1 March 2019 (see [222] above), there is limited support for Mr Calman's claim that he had made clear to the Professors that he was in charge of the process. The emails show that they wished the Articles to convey their evidence and arguments, almost to the exclusion of all others, and they lobbied Mr Calman to make changes to his Articles. He found himself in the, no doubt, uncomfortable position of being pressured to make changes to the text, which he probably would not otherwise have made, to appease them. The changes he made shifted the balance of the Article materially to the disadvantage of the Claimants and, perhaps most importantly, added emphasis to the suggestion that the Claimants' actions were driven by financial motives (a point Mr Calman had not put to either Claimant for comment).
- In cross-examination, Ms Page KC asked Mr Calman whether he had considered the risk that he was being "used" by the Professors; in other words, the risk that they had an agenda of their own to serve in terms of what appeared in the Articles. Mr Calman said he had not. Given what Mr Calman knew (or should have discovered) about the underlying dispute, and the wider statin debate (see further [434]-[445] below), it was rather naïve of Mr Calman not to have perceived this obvious risk and taken steps to protect his editorial independence from any undue influence. I recognise however, that the dynamic of the working relationship he had established with the Professors (and their expectations) made that very difficult to achieve. I do not accept that Mr Calman surrendered editorial control to the Professors. They were not given 'copy approval', in the sense of being able to exercise a 'veto' over what was to be published. The relationship dynamic between Mr Calman and the Professors was more complicated. Although they were not able to dictate what Mr Calman included in the Articles, they nevertheless had (and Mr Calman allowed them to have) a very significant (and in my judgment, undue) influence over the editorial process and the terms in which the Articles were ultimately published.
- My overall assessment, having considered the totality of the evidence of the editorial process, is that it was fundamentally unbalanced, and had been from the outset. Privileged access was given to the Professors with whom Mr Calman had been working closely. They had been assured, at the beginning of the process, that the draft of the Article would be shared with them before publication. The Claimants (and Dr Malhotra) were, by comparison, treated as outsiders to the process, and, from the start, had been identified and treated as the 'targets' of the Articles; to be exposed as proponents of 'fake health news' about statins. In that respect, although perhaps unguarded, Mr Calman's reference to publishing a "big takedown" revealed succinctly (and accurately) what was envisaged from the outset. Confirmed by Mr Verity, Mr Calman's motivation was to publish a "punchy", "very polemical, strong piece", that would give readers "the truth" about statins.
- Full drafts of the Main Article were willingly shared by Mr Calman with the Professors, who were given a (comparatively) generous period within which to respond. In contrast, no draft article was provided to the Claimants to enable them fully to understand the range and gravity of allegations they faced. They were, instead, given only a précis of some of the allegations that were to be made against them, and were given less than 24 hours to respond.
- In the context of the public interest defence, perhaps the most serious omission of Mr Calman was his treatment of the Claimants' right-to-reply responses. He did not provide them to the Professors (and X, in respect of the First Claimant's response), with whom he was and had been working so closely, and obtain their thoughts. On any view, the response of each Claimant was a genuine, serious and substantive effort to engage with what they understood to be the criticisms they faced. Before the Claimants could be branded "statin deniers" whose "deadly propaganda" and "pernicious lies" were putting patients at risk, Mr Calman needed to have at least clear in his own mind why the Claimants' reliance on the various studies and materials they had identified in their responses was either misplaced, could be discounted, or was, itself, evidence of their misrepresentation of the underlying scientific data. Save in respect of one study, there is no evidence that he did so.
- In his evidence, Mr Calman said this:
- One immediate consequence of giving the Claimants such limited time to provide their responses was that it similarly curtailed the time Mr Calman had both to consider them and to incorporate appropriate references to them in the Articles. There is also no indication, in any of the contemporaneous documents, that Mr Calman even set about grappling, substantively, with most of the points made by the Claimants in their right‑to-reply responses. It is likely that timing had an important bearing on this. Mr Calman had been working on the Articles since early February. They were practically complete before Mr Calman began the right-to-reply process (a draft had been sent to X on 24 February 2019).
- Mr Calman's effort to obtain the input of his experts on the Claimants' responses was limited to (a) forwarding only the Second Claimant's email to X (upon which he got no response other than, perhaps, his/her observations on the Kristensen Study); and (b) his request to three of the experts that they consider the same Kristensen Study which was only one of the significant number of items upon which the Second Claimant had relied. Beyond the responses sent in answer to those specific inquiries, Mr Calman did not obtain their input on any other aspect of the Claimants' responses. There is no further trace, in the evidence, of Mr Calman having "explored the credence" of what the Claimants had said in their right-to-reply responses.
- The point about the distinction between outcomes of death and disability or other significant impairment caused by a coronary incident was, of course, a valid one to make about the Kristensen Study (it was expressly noted in the Abstract), but the Claimants' reliance on it did not, itself, show that either of them was guilty of spreading misinformation. More importantly, it was probably a good example of how the "statin debate" was significantly more complicated than the Articles made it appear.
- Contrary to his suggestion, Mr Calman's treatment of the single Kristensen Study does not demonstrate that he had considered (in the same detail or at all) the other material relied upon by the Claimants. More importantly, Mr Calman does not say that he did specifically consider these other materials.
- On the evidence, I find as a fact that Mr Calman did not consider the material relied upon by the Claimants in their right-to-reply responses in any real detail, in all likelihood because of the limited time he had to do so. Certainly, in his evidence, Mr Calman did not explain why (a) the Claimants' reliance upon the various studies and trials was flawed or could otherwise be rejected; and (b) why there was no need to include more extensive reference to the materials relied upon by the Claimants in the final published Articles "to explore the credence of what they [had said]". Mr Calman's failure to share the Claimants' right-to-reply responses with his own experts, and obtain their input, is an astonishing failure.
- Indeed, in the remaining part of this part of his evidence, Mr Calman gives the very clear impression that individual studies, cited by the Claimants, had neither relevance nor value because, in his assessment, the meta-analyses relied upon by the Professors were qualitatively more important and provided the definitive answer: "the indisputable scientific fact" (Main Article [3]). That was a mistake. In my judgment, Mr Calman simply dismissed the various studies relied upon by the Claimants in their right-to-reply responses. Save for his single inquiry about the Kristensen Study (see [216] above), he had, indeed, "written off" the Claimants' responses without properly considering them. That was another error, the direct effect of which was to lead to a serious lack of balance in the published Articles (see further the analysis of what Mr Calman presented to readers in the Articles [418]-[456] below).
- Mr Calman's argument that the existing length of the Articles, and that there was a lack of "space" to include any further points that the Claimants had raised in their defence, limited his ability to include more of the Claimants' right-to-reply responses, is one that I reject, without hesitation. It is also contrary to the evidence he gave about the need to ensure that "space is given to explore the credence of what they say" (see [188] above). Although the presentation of an article is, in the first instance, a matter for the publisher, in this case, the argument that space was limited is somewhat undermined by the fact that nearly half the available page space, on pages 48 and 49 in the print edition, was taken up with three very large photographs of the two Claimants and Dr Malhotra. But more importantly, any newspaper has complete control over the "space" that it allocates to any article it decides to publish. In respect of online publication, that space is unlimited. If a newspaper is going to tackle a controversial topic - even more if it is going publish very serious allegations against individuals - and intends to rely upon a public interest defence, it must allocate enough "space" to do justice to the matters that need to be covered, which includes, where important, the key elements of the responses of the individuals criticised.
- For someone who said that he was anxious to "explore the credence" of what the Claimants had said in response, and had immediately available at his disposal a team of highly qualified, fully engaged, experts with whom he had been working closely, the self-denying abstention to utilise this resource is incomprehensible. For someone who recognised his own limitations in understanding the material relied upon by the Claimants, it is remarkable. Mr Calman asked his experts about only one of the numerous studies upon which the Claimants had relied in their right-to-reply responses. It was fundamental to the fairness of the process - and what he reasonably could be expected to do as a journalist - that Mr Calman understood, and fully considered, the materials relied upon by the Claimants. Where they disclosed important points, appropriate references needed to be included in the Articles. The failure properly to consider these materials rendered the right-to-reply process hollow and superficial. In the result, the final Articles, when published, failed to incorporate many of the points that should have been included from the Claimants' responses to achieve fairness and proper balance (see further Section H below).
- Ms Page KC, on behalf of the Claimants submitted that the right-to-reply process was "last-minute, rushed, sloppy and superficial" and was Mr Calman "going through the motions". In my judgment, there is much force in that submission. If Mr Calman's objective was "to explore the credence" of what the Claimants had said in their own defence, as he claimed (see [188] above), he failed to achieve it.
- When considering a public interest defence in a defamation claim, it is important for the Court to concentrate, not on the objective single natural and ordinary meaning that the law finds the publication to bear (which is assessed by the application of well‑established rules - see [380] below), but the meaning that the publisher subjectively perceived the publication to bear (or the Court is satisfied s/he should have perceived it to bear) at the time of publication: see further [281] below. The exercises are quite different, legally and evidentially. In this judgment, I have kept separate these distinct exercises.
- As principal author of the Articles, Mr Calman held the pen. He chose what to include by way of detail in the Articles, and what to ignore. Save perhaps for the headlines, and subject to the Second Defendant's editorial process, he chose the language to express himself. The law recognises, both generally as an important dimension of the right of freedom of expression under Article 10 and specifically as part of the public interest defence, that due weight must be given to editorial judgement in relation to the way that articles are presented. However, that principle is not without limit. As with any author, and particularly because he was a professional journalist, Mr Calman would have understood that the choices he made would have probably the single most important impact on the overall meaning that the Articles would be understood to bear by readers. If an author downplays, omits or impeaches the response of the subject of an allegation, the effect is to bolster the case against the accused and/or to signal to readers that the responses are to be dismissed, not treated as credible or (worse, in a case like this) that this is further evidence of their dishonesty. Ultimately, these important choices bear not only on the meaning that the journalist can reasonably be expected to perceive the publication to bear, but also on whether, ultimately, it is reasonable to believe that the publication is in the public interest.
- I explore below the way that Mr Calman chose to present the allegations against the Claimants in the Articles, including what he included from the responses of the Claimants (see [417]-[457] below). These choices also had an important impact on the overall impact of the Articles and ultimately the meaning that he could expect readers would take from them. In his approach prior to publication, Mr Calman did not simply adopt a conventional exercise of presenting the case against the Claimants and then incorporating, in a fair and straightforward way, their response. In several important instances, Mr Calman presented an extremely abbreviated summary of what the Claimants had said in their responses, and then immediately presented his experts' rebuttal of their position. The effect, as Mr Calman could not have failed to have appreciated, was to signal clearly to readers that the Claimants were wrong and, worse, that this demonstrated further dissembling on their part. In some instances, Mr Calman did not set out the Claimants' responses at all, sometimes because he had not put the relevant allegation to either Claimant for response.
- There are five distinct elements of the key message that I am satisfied Mr Calman either did perceive or, as a reasonable journalist, he should have perceived as meanings that would obviously be conveyed by the Articles.
- The most significant dispute between the parties is meaning (4); that the Claimants were not just wrong (i.e. that they were fools), but that they were liars (i.e. that they were knaves). When cross-examined on this point, Mr Calman disagreed that the Articles alleged that the Claimants were dishonest. He said that he thought that the Articles were very clear that no allegation of dishonesty was being made. He pointed to the use of the word "misinformation", as supporting his point.
- I do not accept Mr Calman's evidence on this point. He was very clearly aware of the distinction between an allegation that the Claimants were "wrong" and an allegation that they were "liars" (see [173]-[174] above). It was not only the Hancock Statement that brought this issue to the fore, the analogy between the 'statin deniers' and Andrew Wakefield and the MMR "scare" (taken as the headline for the Main Article and raised by Professor Collins in [16]) squarely raised the level of the Claimants' culpability. Indeed, this was a point expressly raised with Mr Calman by X after s/he had seen an early draft of the Articles (see [98] above).
- Mr Calman is an intelligent person. He had worked closely on these Articles for some time. As an experienced journalist, he would have understood the importance of being very clear as to the meaning he intended to convey, and he is responsible (with others) for the language ultimately used in the Articles and how that was likely to be understood in the context of their whole presentation. This is not an unimportant or trivial detail of a story, the significance of which only became apparent later. This was fundamental to the core allegation being made against the Claimants. As a result, Mr Calman could not have failed to have appreciated that the Articles very definitely went beyond calling the Claimants honest, but hopeless (perhaps even reckless), fools who were simply wrong in what they claimed about statins; the Articles, very clearly, alleged that they were dishonest. If Mr Calman had wanted to avoid making this allegation, the complete control he had over the choice of language used in the Articles and their ultimate presentation, meant that he could have done so. He did not.
- I have already noted the significant contribution that the misrepresentation of the Hancock Statement made to the impact of the Articles (see [169]-[176] above). In the News Article, and the Main Article, the (mis)use of the Hancock Statement made probably what was singularly the most important contribution to the overall statement that the Claimants were liars. But the presentation of these articles, as a whole, reinforced this message. The "devastating investigation" exposed the "fake news" by "unequivocal science". The "'fake claims' about proven medicines", amounted to "deadly propaganda", a "wanton spread of medical misinformation", and a "vastly overstated" case by the "statin deniers". The science showed that "none of which is right" and was contradicted by "researchers who have devoted their lives to understanding how to treat heart disease" and who had produced "the highest quality scientific evidence on the subject". The "pernicious lies" of the 'statin deniers' "needlessly risked lives", and damage to public health, that was "worse than the MMR scare".
- There were also clear, if more subtle, signals given to readers by Mr Calman in the Main Article to reinforce the message as to who should be believed: who should be trusted and distrusted. The First Claimant is described as a "prolific denier... who admits she is an academic not a medical doctor" [27]. The Second Claimant is said to have "admitted" that a study upon which he relied was "observational" [35] and to have "quote[d] anecdotes" and "unreliable" data as to side effects [57]. Dr Malhotra (condemned in similar terms as the Claimants) has been "far from silent" and has made "extravagant claims" [51]. As a group, the 'statin deniers' have made "incendiary" and "inflammatory" accusations, including (false) claims of conflicts of interest against and deliberate withholding of data by scientists presented in the Main Article as "devoted" researchers [60]-[61], and confirmed by Professor Collins to be a (yet another) "lie" that "just seems to be repeated" [63]. In the Editorial, the Claimants' claim that they were being bullied is dismissed as "self-pitying" [17].
- The Defendants can - and do - point to the sentence in [16] of the Main Article: "The suggestion is that the statin deniers are simply wrong rather than dishonest". It was the single part of the Articles to which Mr Verity pointed when questioned on the point (see [174] above). And Mr Calman changed the words "falsely" to "wrongly" in [4] of the News Article, which also referred to "inaccurate" claims by the 'statin deniers'.
- The problem with the former argument is that the sentence relied upon in [16] is immediately followed by a rebuttal by Professor Collins (who Mr Calman presented to readers as an honest and trustworthy expert) who drew an analogy between the behaviour of the statin deniers and Andrew Wakefield, who Mr Calman told readers, had "fabricated evidence to support his idea that the [MMR] jab triggered autism". In context, and as Mr Calman would (or should) have appreciated, the effect was immediately to undermine (and therefore to discount) the suggestion that the Claimants were simply misguided. In overall presentation, it reinforced the message that they were dishonest.
- The choice of words in [4] of the News Article could not have been regarded by Mr Calman as being remotely sufficient to displace the other language used in the articles that suggested dishonesty. But, more importantly, if this was the limit of what he intended to allege against the Claimants, he failed to ensure that the language used in the rest of the article was consistent with this meaning. In short, whilst Mr Calman clearly appreciated the need to ensure that the wording of the Articles was calibrated properly to reflect the meaning he said he intended to convey, he failed to ensure that it did.
- Up to this point, I have concentrated mainly upon the meaning that Mr Calman understood the News and Main Articles to convey to readers. Read alongside the Editorial, as they would have been in the print edition, and were presented in Online Publication 1, the effect was obvious and unmistakeable. Mr Calman roundly denounced the Claimants on a premise he made clear to readers. Places in hell are not usually reserved for people who honestly get things wrong. Indeed, in paragraph [12] of the Editorial, Mr Calman acknowledged that, in science, there was an important and valuable role to be played by those who honestly challenged medical or scientific orthodoxy, an example is given of Barry Marshall. Yet, Mr Calman immediately contrasted such honest scientists with the "trio" of dishonest 'statin deniers': "make no mistake: the statin deniers are no Barry Marshalls". The charge that the Claimants were "peddling" a "particularly insidious type of fake news apparently from a credible source, but laced with misinformation" ([15]) is not consistent with Mr Calman merely castigating them for their carelessness in making erroneous claims about statins. In the Editorial, Mr Calman condemned the Claimants as deserving a "special place in hell" on the grounds that they were deliberately spreading dangerous lies, which had potentially devastating effects on the lives of people, like Colin, who stopped taking statins as a result.
- Meaning (5) represented speculation as to the Claimants' possible financial motivation for spreading false information about statins. As well as adding a further distinct venal element to the allegations against the Claimants, it also reinforced the message that the Claimants had published information that they knew to be false. In most instances, it would be unusual to ascribe a motive to those who are simply alleged to have been careless or reckless. Motive is ordinarily ascribed only to people who have acted deliberately, as an explanation (whether in whole or in part) for why they have acted in the way alleged. Again, this is something that I am satisfied Mr Calman well understood. It was an angle of the story that Mr Calman had in mind right from an early stage, believing that the Claimants and Dr Malhotra "all make money for saying statins don't work" (see [90] above).
- In the end, the desire for a hard-hitting "polemical" piece appears to have carried the day, with the consequences I have found for the meaning Mr Calman either did foresee the Articles to bear or should have done.
- The Articles were published on 3 March 2019.
- On 5 April 2019, the First Claimant lodged a complaint about the Articles with the Independent Press Standards Organisation ("IPSO"). The complaint was dismissed, on 2 May 2019, on the grounds that the First Claimant's complaint "[did] not raise a possible breach of the Editors' Code".
- On 19 July 2019, letters of claim were sent by the Claimants' solicitors complaining about the Articles. On 1 November 2019, substantive responses to the letters of claim were sent by the Defendants' solicitors. They included reliance on potential defences of qualified privilege under s.15 Defamation Act 1996 and s.6 Defamation Act 2013.
- On 26 November 2019, the Claimants' solicitors noted the reliance upon the statutory defence of qualified privilege under s.15 Defamation Act 1996. Whilst contending that the Defendants were not entitled to rely upon that defence, the Claimants' solicitors sought publication of the following by way of explanation or contradiction:
- On 8 January 2020, the Defendants' solicitors responded:
- On 10 January 2020, the Claimants' solicitors responded to the Defendants' proposal:
- In a further letter of 20 March 2020, the Defendants denied "that there was any misrepresentation to the effect that Mr Hancock had referred to 'statin deniers' and 'doctors' or that he had not directed his remarks at [the Claimants]..." In consequence, the Defendants refused to publish the revised statement, offered by the Claimants in the letter of 10 January 2020.
- Claim Forms, which had been issued on 26 February 2020, were then served on the Defendants on 27 May 2020.
- Section 4 of the Defamation Act 2013 provides (as far as material):
- When considering a defence under s.4, there are three questions to be addressed: Economou -v- de Freitas [2019] EMLR 7 [87]:
- The first issue is an objective question for the Court, not a matter of the subjective judgment of a journalist or editor: Doyle -v- Smith [2019] EMLR 15 [64]. The wording "form part of" means that the Court must have regard to the entire publication. Lord Hoffmann's observations in Jameel -v- Wall Street Journal Europe Sprl [2007] 1 AC 359 [51] remain important:
- Matters of public interest are of potentially wide compass save that they exclude purely personal or private matters. They specifically include, for example, "the public conduct of a prominent public figure and, in particular, statements she had made or caused to be made publicly...": Riley -v- Murray [2023] EMLR 3 [73]. As Lord Bingham explained in Reynolds -v- Times Newspapers Ltd [2001] 2 AC 127, 176-177 (in a passage cited by Lord Phillips in Flood -v- Times Newspapers Ltd [2012] 2 AC 273 [33]):
- The second issue concerns the defendant's actual state of mind at the time of publication: Doyle [75]. The defendant must prove that s/he did believe that publication was in the public interest. A failure to do so will mean the defence will fail: see Doyle [76]; Turley -v- Unite the Union [2019] EWHC 3547 [149].
- The Code of Practice of the Independent Press Standards Organisation ("IPSO") contains the following provision in respect of demonstrating the public interest:
- As already noted, human memory can be unreliable (see [113] above). In the context of public interest defences, particularly where the trial process, carrying out an assessment of the journalistic process, takes place many years after the event, contemporaneous documents are likely to be of critical importance. The lack of such documents recording, and evidencing, the decision-making process prior to publication, can harm the prospects of success of a public interest defence. That is because, without records of what steps the journalist took prior to publication, and what documents s/he had and considered, it is unrealistic to expect him/her to have a reliable recollection of the detail of the steps taken and information that had been obtained pre-publication. If litigation follows, and the journalist is involved in evidence gathering process, there is a further risk (absent contemporaneous records) that s/he will innocently muddle up what s/he had prior to publication with what is discovered after publication. Only material falling in the former category is relevant to and probative of a public interest defence.
- In Lachaux -v- Independent Print Ltd [2022] EMLR 2, I noted the absence of contemporaneous documents "addressing the question whether publishing the Articles in the terms they appeared, with the information that was available to the Defendants, was in the public interest" [117] and the "lax and, frankly, amateurish approach to the recording of decisions of potentially critical importance" [119] and said:
- If the defendant establishes that s/he did believe that publication was in the public interest, the final question is whether, judged objectively, that belief was reasonable. In most cases involving s.4, the focus tends to be on this final question.
- In assessing the reasonableness of a defendant's belief that publication was in the public interest, the focus is on the things the defendant said or knew or did, or failed to do, up to the time of publication. Events that post-date publication are unlikely to have any real bearing on the issue: Economou [2017] EMLR 7 [139]; GKR Karate -v- Yorkshire Post Newspapers Ltd [2000] 1 WLR 2571 [21], [25]. "Could whoever published the defamation, given whatever they knew (and did not know) and whatever they had done (and had not done) to guard so far as possible against the publication of untrue defamatory material, properly have considered the publication in question to be in the public interest?": Flood [113], endorsed in Serafin [60].
- When determining the reasonableness of a defendant's belief that publication was in the public interest, the Court must "make such allowance for editorial judgement as it considers appropriate": s.4(4). In Banks -v- Cadwalladr [2022] 1 WLR 5236, Steyn J summarised the principles that emerge from the authorities on this point:
- An issue that arises frequently when considering a public interest defence is the approach the Court should adopt to the meaning of the publication. It is well-established that the public interest defence is not to be assessed simply by reference to the single natural and ordinary meaning of the publication: see the observations of Steyn J in Banks -v- Cadwalladr [122] and Riley -v- Sivier [2023] EMLR 6 [132] and Eady J in Lowe -v- Associated Newspapers Ltd [2007] QB 580 [13]. In Riley -v- Murray [2023] EMLR 3, Warby LJ explained:
- The Court must have regard to all the circumstances of the case: s.4(2). Although s.4(6) has abolished the old Reynolds defence (Reynolds -v- Times Newspapers Ltd [2001] 2 AC 127), the rationale for the statutory defence was not materially different and the common law principles remained relevant to the interpretation of the statutory defence: Economou [76]; Serafin -v- Malkiewicz [2020] 1 WLR 2455 [68]. The ten so-called Reynolds factors - although not to be regarded as any sort of checklist - "may well be relevant to whether the defendant's belief was reasonable within the meaning of subsection 1(b)": Serafin [69]. In Hijazi -v- Yaxley-Lennon [2021] EMLR 7 [24], I noted:
- A factor, identified by Lord Nicholls, which remains relevant to the assessment of any public interest defence, is the extent to which the defendant attempted to verify the allegations it intends to make. Save in cases of neutral reportage (under s.4(3)), efforts to verify are usually regarded "as an important factor in the assessment of the reasonableness of a defendant's belief that publication was in the public interest": Riley -v- Sivier [130(v)]. In Lachaux, I explained:
- In a section of her skeleton argument headed: "Balance of ECHR Articles 8 and 10 and the limits of acceptable criticism in relation to political/societal speech and individuals engaging in public debate", Ms Evans KC submitted that the special importance of expression in political and societal spheres is well recognised and that, correspondingly, the limits of acceptable criticism are particularly wide in relation to those who participate themselves in debate in a public forum: see Riley -v- Murray [71]-[74] and Banks (at first instance) [124]-[133].
- Ms Page KC relied upon Packham -v- Wightman [2023] EMLR 18 on the issue of whether a publisher is required to have an open mind to benefit from a public interest defence. Saini J rejected the defendants' s.4 defence. He noted ([161]):
- It is a decision for later the extent to which the Articles made, or consisted of, allegations of fact against the Claimants or expressions of opinion. Conventionally, the more obvious it is that the publication contains criticism of individuals, the more likely that (at least in part) that criticism will be found to be an expression of opinion. It is not capable of real dispute that the Articles contained serious criticism of the Claimants, and some of that criticism may well be found to be an expression of opinion. What impact does that have on the public interest defence? s.4(5) Defamation Act provides "for the avoidance of doubt" that the public interest defence "may be relied upon irrespective of whether the statement complained of is a statement of fact or a statement of opinion." I am not aware of any case where s.4(5) has been considered and it is not immediately apparent to me the purpose of this subsection. Unhelpfully, the Explanatory Notes simply state: "Subsection (5) makes clear for the avoidance of doubt that the defence provided by this section may be relied on irrespective of whether the statement complained of is one of fact or opinion." Beyond repetition of the words of the subsection, that achieves little by way of explanation.
- In her closing submissions, Ms Evans KC submitted that the inclusion of statements of opinion within the public interest defence has the following consequences:
- I will explore Ms Evans KC's further submissions as to what this means, practically, in this case when I come on to consider the public interest defence later in the judgment. It is important that I concentrate on the particular facts of this case when deciding whether the public interest defence is made out. I will limit myself, at this stage, to some broad conclusions as to Ms Evans KC's key points.
- I am not convinced that the fact that a publication, sought to be defended under s.4, is or contains an expression of opinion leads to a significant change in the overall approach to the assessment of the public interest defence.
- Ms Evans KC argues that the public interest defence can accommodate the denunciation of the Claimants, in forthright terms. I shall come on to consider whether, in this case, the Defendants are able to establish a public interest defence. But where, as here, the publication includes serious criticisms against identified individuals in relation to the accuracy, honesty and cogency of what they have published, in the context of a continuing debate in the scientific community, the requirements of a public interest defence are likely to be met only if the commentator honestly and fairly represents the debate and the contribution made by those criticised. The defence of honest opinion is available to publishers who cannot, or do not, satisfy these requirements. The public interest defence, although flexible enough to take in all relevant circumstances, cannot be expected, and nor is it required, to absorb also the law of honest opinion. The defences protect quite different aspects of freedom of expression. Reflecting this, Parliament legislated separately for each defence, albeit by s.4(5), it has been careful not strictly to compartmentalise the defences.
- Finally, in relation to a continuing publication (for example on a website), if a defendant seeks to rely upon a s.4 defence, then s/he will have to establish the ongoing elements of the defence. That will include demonstration that the defendant believed that the continued publication was in the public interest and that the belief remained reasonable, which may require consideration of any significant change in circumstances since the original publication: Lachaux -v- Independent Print Ltd [2022] EMLR 2 [159], applied by Steyn J in Banks -v- Cadwalladr [134]-[135].
- s.15 Defamation Act 1996 provides (so far as material and as amended):
- The relevant paragraphs of Schedule 1 of the Act (as amended by the Defamation Act 2013) provide:
- I note that Paragraph 12 in Part II of the Schedule to the Defamation Act 1952 provided qualified privilege (subject to explanation or contradiction) for:
- In relation to whether the relevant publication was of public interest and for the public benefit within the terms of s.15(3), in their Reply, the Claimants indicated that, if the Court were to find that the relevant statement was "used fairly and accurately", then they admitted that its publication was "to that extent in the public interest and for the public benefit".
- The Claimants have raised a preliminary point that the qualified privilege defence is simply unavailable for the Defendants. Ms Page KC has argued that if the words, in respect of which a claim for privilege is maintained, are not defamatory of the claimant, then a defence of privilege is "irrelevant and illegitimate" and should be struck out. She makes the same submission in relation to a privilege defence under s.6 Defamation Act 2013. No authority has been provided in support of this submission. By analogy, the Claimants rely upon Broadcasting Corporation of NZ -v- Crush [1988] 2 NZLR 234, 237 - in which the Court observed that proving the truth of a non-defamatory meaning would be a "pointless exercise" - and the unreported decision of Michael Davies J in Maxwell -v- Bower (10 April 1990) (cited in §32-037 Gatley on Libel and Slander (13th edition, Sweet & Maxwell, 2022)). Ms Page KC submits that the specific paragraphs complained of in Curistan and Tsikata were defamatory of the relevant claimant.
- I do not accept this argument. A qualified privilege defence protects the occasion of publication. The meaning of the privileged statement has nothing to do with the subsistence of the privilege. Subject only to being defeated by proof of malice, the privilege will protect the publication in whatever meaning it is objectively found to bear. The principle that a defendant cannot advance a defence of truth (or honest opinion) to a non-defamatory statement is founded on principle and case management. As a matter of principle, a defendant has no need of a truth (or honest opinion) defence in respect of non-defamatory statement, and it is a waste of the resources of the court and the parties to litigate factual disputes that would arise under these unnecessary defences; that it is why it is a "pointless exercise".
- The Hancock Statement did not, of course, refer to the Claimants. Read alone, therefore, it cannot be defamatory of the Claimants. But that is not an end of the privilege defence. The Claimants complain of the publication of those parts of the Hancock Statement that were included in the Articles as part of the publication as a whole. They cannot be divorced from the context in which they appeared. It is central to the Claimants' wider arguments that, in context and presentation, the Hancock Statement did refer to the Claimants, and added significantly to the overall defamatory message of the Articles. The meaning the Hancock Statement bears, in isolation, is not relevant to the issue of whether its publication in the Articles was privileged on the basis contended by the Defendants. I therefore reject the Claimants' preliminary argument on this point both in relation to s.15 Reporting Privilege and s.6 Defamation Act 2013.
- A report may fall within more than one of the categories of statutory privilege: Alsaifi ‑v- Trinity Mirror [2017] EWHC 1444 (QB) [73].
- In Qadir -v- Associated Newspapers Ltd [2013] EMLR 15 [48], Tugendhat J held that "extract" could include a "summary", in the sense that the extract need not be a word for word citation from the relevant document. This interpretation has been questioned by the authors of Duncan & Neill on Defamation (§17.39, 5th edition, Butterworths, 2020), on the grounds that Parliament provided for privilege to attach to fair and accurate "copies of or extracts from" some types of publication (e.g. paragraphs 5, 7 and 9 of the Schedule) but extended privilege to cover also a "summary" for other types of publication. This does potentially raise an interesting question of statutory interpretation. As originally enacted, Schedule 1 to the Defamation Act 1996 did not include any reference to a "summary" in any of the categories of privilege. The current wording of Paragraph 9 of Schedule 1 - which now does include the word "summary" - was introduced by amendment in s.7(4) Defamation Act 2013. So, at the date when Tugendhat J made his decision in Qadir the argument advanced by the authors of Duncan & Neill was not available.
- Ms Page KC has also sought to challenge the decision in Qadir on the basis that Tugendhat J explicitly relied upon the Oxford English Dictionary definition to hold "extract" could include "summary" and did not require a verbatim rendering of some part of a longer text. The relevant edition of dictionary is not specified in the judgment. In the 2nd edition, published in 1989, while one of the definitions of "extract" is stated to be "a summary; an outline", the dictionary indicates that this is an obsolete usage, last current between 1549 and 1681. The modern definition is given as, "a passage copied out of a book, manuscript, etc.; an excerpt, quotation". A similar definition is provided in the New Shorter Oxford English Dictionary, published in 1993.
- The issues, as they have crystallised in this case, mean that I do not need to resolve this issue (see Section H below). It is best, therefore, that I express no view and leave the matter to be resolved in a case where the issue arises directly for decision.
- What is fair and accurate is to be judged by comparing the words complained of with the document from which the words complained of are said by the defendant to be an extract. Where the complaint is of unfairness arising out of the omission to publish information extraneous to that document, such as another document or comments of the complainant, then that issue is to be decided under s.15(3) (public interest and public benefit) or s.15(1) (malice): Qadir [68].
- Fairness, in this context means fairness in terms of overall presentation: the report need not be verbatim or accurate in every detail. It is a question of substance, not form. Minor inaccuracies will not displace the privilege: Alsaifi -v- Trinity Mirror [74]; Alsaifi -v- Amunwa [2017] 4 WLR 172 [63]. A report can be selective and concentrate on one particular aspect as long as it reports fairly and accurately the impression as whole Curistan -v- Times Newspapers Ltd [2009] QB 231 [26] per Arden LJ. Fairness is to be tested by reference to the impact on the claimant's reputation. A report that contains a substantial or material misstatement of fact, that is prejudicial to the claimant's reputation, will not be privileged: Alsaifi -v- Amunwa [63]; Curistan [27].
- Reporting privilege will be lost if the quality of fairness required for reporting privilege is lost by intermingling extraneous material with the material for which privilege is claimed: Curistan [36]. Laws LJ expressed his conclusion on the issue of intermingling as follows:
- Pursuant to s.15(2), privilege under Part 2 of Schedule 1 is lost if the claimant shows that the defendant refused or neglected a request to publish, in a suitable manner, a reasonable letter or statement by way of explanation or contradiction. "Reasonable", in this context, means objectively reasonable as to content; but in addition, it must mean objectively reasonable as a statement for publication in the medium proposed: Onwude -v- Dyer [2020] EWHC 3577 (QB) [128]. In Chakravarti -v- Advertiser Newspapers Ltd (1998) 93 CLR 519 [161], Kirby J explained the principles in relation to a substantially similar provision in a South Australian statute (see [147]):
- As s.15(1) recognises, the privilege provided under the section is qualified; it can be defeated by proof of malice. No definition is provided in the section, so Parliament is taken to have intended to use the common law definition of malice for purposes of the section (see Section G(5) below).
- In support of their submissions on the proper interpretation of Paragraphs 7 and 9 of Schedule 1 to the 1996 Act, Ms Page KC and Mr Busuttil have carried out an extensive review of the history of reporting privilege from s.3 Parliamentary Papers Act 1840 and s.4 Law of Libel Amendment Act 1888, the Report of the Committee on the Law of Defamation 1948 (Cmnd 7536) ("the Porter Committee") and the following Defamation Act 1952 and the Government's consultation and subsequent passage of the bill that became Defamation Act 1996.
- Interesting though the fruits of this research are, I do need to bear in mind the fundamental principles to statutory interpretation: the object is to identify the meaning borne by the relevant Act of Parliament in its statutory context. The words which Parliament has chosen to enact are the expression of the purpose of the legislation and are the primary source by which meaning is ascertained. In R (O) -v- Secretary of State for the Home Department [2023] AC 255 Lord Hodge explained:
- I do not consider that the words in Paragraphs 7 and 9 of Schedule 1 to the 1996 Act are in any way ambiguous or unclear, or productive of absurdity. They are plain ordinary words. It is the Court's duty to give effect to their meaning.
- The Claimants can and do rely upon Blackshaw -v- Lord [1984] QB 1, in which the Court of Appeal specifically considered Paragraph 12 of the Schedule to the 1952 Act (see [294] above).
- The chairman of the Public Accounts Committee of the House of Commons gave a press conference concerning the Committee's investigations of mismanagement in the Department of Energy ("DoE"). The chairman stated that a senior official in the Department had been reprimanded. Mr Lord, a journalist, telephoned the DoE's press office to elicit the name of the official, Mr Blackshaw. The press officer, Mr Smith, denied naming Mr Blackshaw or that any member of the DoE had been dismissed but stated that Mr Blackshaw had transferred to another department before leaving the civil service for personal reasons. Mr Smith accepted that Mr Lord would have inevitably concluded from the information supplied to him that the official in question was Mr Blackshaw, but this was an inference or conclusion by Mr Lord not something Mr Smith had said. Mr Lord wrote an article implying that Mr Blackshaw had been forced to resign for incompetence. Mr Blackshaw sued for libel.
- The trial judge, held that, subject to the issue of fairness and accuracy, Mr Lord's article attracted privilege under paragraph 12 of the Schedule to the 1952 Act. However, the jury found that the article was not a fair and accurate report of what Mr Lord had been told by the press officer and awarded £45,000 damages. The defendants appealed. The plaintiff cross-appealed from the judge's finding that the article attracted privilege under paragraph 12. The appeal was dismissed on the basis that it was properly open to the jury to find against the defendants on fairness and accuracy. That conclusion meant that the cross-appeal did not arise for determination. However, each member of the Court of Appeal (Stephenson, Dunn and Fox LJJ) gave a judgment on the point. Each said that he would, if necessary, have allowed the plaintiff's cross-appeal. Stephenson LJ's is the majority judgment as Dunn LJ expressly associated himself with Stephenson LJ's conclusion and reasons (at 32E).
- Stephenson LJ held that what Mr Smith said to Mr Lord was not matter issued for the information of the public by or on behalf of the DoE: 24F-G. He addressed the significance of the statutory words "issued" and "notice" and distinguished in this context between information volunteered and information extracted by questioning (24H-25C):
- Stephenson LJ did not rule out that statements made by press officers in response to journalists' enquiries might fall within the scope of the section. However, he also made clear (a) that not every statement made to a journalist by a press officer of a government department would constitute a notice or "matter of the same kind" issued for the information of the public by or on behalf of that department, and, further, (b) that anything that in an article amounting to assumption, inference or speculation on the part of a journalist who was provided with such a notice or other equivalent matter was certainly not privileged (24C-E):
- Stephenson LJ also made the point that a statement made by a press officer would not be protected by privilege under paragraph 12 if s/he was not making the statement on behalf of their government department, but instead communicating unofficial information of his or her own (24E-F):
- Dunn LJ concurred with these conclusions (at 37H-38B):
- Further, Dunn LJ said that the statement by the press officer could not be described as "matter issued for the information of the public by or on behalf of a government department", so as to attract the privilege (38D-H). He, like Stephenson LJ, expressly adopted the following words of Jordan CJ in Campbell -v- Associated Newspapers Ltd (1948) 48 SR (NSW) 301, 303 considering a similar Australian provision:
- The distinction between, "mere gossip" or matter that is not an official notice or report and "an official notice or report" was also considered in Ferrymead Tavern Ltd -v- Christchurch Press Ltd [1999] NZAR 529. In that case, the plaintiffs carried on the management of a public house and restaurant called the Ferrymead Tavern. They sued over the publication of a newspaper article which stated that a "'free for all' with 'nasty racial overtones' at the Ferrymead Tavern was broken up by police". It was not in dispute that the reference to the Ferrymead Tavern was incorrect; there had been no such incident at the Tavern. The story had been based on incorrect information obtained from the police by one of the defendant's journalists after he had telephoned the police communications room in Christchurch. The senior communications officer had described the supposed incident to the reporter in a telephone call.
- The defendant relied on a defence of qualified privilege, under s.16(2) and Schedule 2, paragraph 15 of the New Zealand Defamation Act 1992 [19], and applied for summary judgment. The application was granted, and the claim dismissed. The Court was satisfied that the newspaper report was a fair and accurate report of the statement obtained from police (p.534). On the issue of whether the statement was of a "sufficiently official nature" to satisfy the requirement that it was "issued for the information of the public" - described as the "status requirement" (p.533) - relying upon Blackshaw -v- Lord and Campbell -v- Associated Newspapers Ltd, the Judge held that the conversation with the communications officer met this condition (pp.535‑6):
- Ms Page KC does not dispute that a statement issued in response to a reporter's enquiries may attract statutory privilege; Blackshaw -v- Lord establishes as much (24C). But she argues that the reasoning in Ferrymead Tavern should not be followed. She submits that the Judge did not consider the point that the statement was provided only to one reporter at one newspaper rather than to the press at large. Further, the analysis is inconsistent with two later Australian decisions (see [323]-[324] below).
- In Australia, s.28 Defamation Act 2005 provides (so far as material):
- In Belbin -v- Lower Murray Urban & Rural Water Corp [2012] VSC 535, the Minister for Water published a letter addressed to customers of an irrigation trust, explaining the action he had undertaken in closing down the trust. The question was whether the letter was "issued...for the information of the public". Kaye J held:
- In Chetwynd -v- Armidale Dumaresq Council [2010] NSWSC 690, the plaintiff sued on defamatory material comprised in an agenda set out in a notice of meeting for a local council. The agenda was sent to members of the council, council staff, and some members of the public and media. James J held that the agenda did not fall within s.28(4)(d), because paragraph (d) required that the purpose of informing the public had to be a principal purpose of issuing of the document. Even though some copies of the agenda were sent to the media and members of the public, the principal purpose of issuing it was not to inform the public, but members of the council and council staff, so paragraph (d) was not satisfied: [253].
- Based on these authorities, Ms Page KC submitted that there is a distinction to be drawn for the purposes of interpreting paragraph 12 in the 1952 Act - and by extension paragraph 9 of Schedule 1 to the 1996 Act - between an announcement made or handed to the press in general "for publication in their respective newspapers" and one made or handed only to one newspaper group, for its readers only.
- s.6 Defamation Act 2013 provides (so far as material):
- The Explanatory Notes to the Act state, in relation to s.6:
- The ambit of dispute under s.6 has been narrowed by admissions made by the Claimants. The Claimants admit:
- The following aspects of s.6 would appear to be uncontroversial:
- s.6 Defamation Act 2013 has no analogue in any earlier English statute or any Commonwealth or other common law jurisdiction statute. The provision has not been considered since it was enacted. Ms Page KC submitted that, considering this "blank canvas", the section is ambiguous and obscure in meaning rendering it permissible for the Court to consider the legislative history of the section including statements made in Parliament by the promoters of the relevant Bill as an aid to determining the proper meaning of the section and its constituent parts, in accordance with Pepper -v- Hart.
- The terms "independent review" and "expertise" in s.6(3) are not defined in the Act. As to the requirement that the review be "independent", the authors of Gatley suggest (§17-026 footnote 184):
- The Claimants submit that the Court should adopt this approach. By interpreting "independent" in this way - as one that requires a value judgement and a setting of standards by the Court - they contend that the Court can purposively give effect to Parliament's intention that the peer-review process must be proved to have been carried out in a demonstrably responsible and rigorous fashion if privilege is to attach. Accordingly, they submit that there needs to be proved to be something genuinely and recognisably "independent" about the "review" - going beyond the appointment one or more 'independent' persons with expertise in the scientific or academic matter concerned - if the s.6(3) condition is to be found satisfied.
- However, the Explanatory Notes state that the requirements in s.6(3) are intended to reflect the core aspects of a responsible peer-review process. The authors of Blackstone's Guide to The Defamation Act 2013 (§§7.41-7.42, OUP, 2013) state:
- In my judgment, the interpretation offered by the editors of the Blackstone's Guide is to be preferred. The requirement of independence in s.6(3) is to be interpreted as independent from the author(s) of the paper being reviewed. That was the obvious primary objective. A requirement that the review also be independent from the journal reviewing it for publication would (a) represent a significant departure from the peer‑review process adopted by most journals; and (b) mean that the wording of s.6(3) was internally inconsistent. By inclusion of the editor of the journal in s.6(3)(a), the section treats him/her as being part of the independent review. If Parliament had intended that the review should be carried out by people who were also independent from the journal itself, one would have expected to see the word "independent" transposed from its place in s.6(3) and appear instead in s.6(3)(b) so as to apply to the further person(s) additionally required to carry out a review.
- As to the interpretation of "expertise", the authors of Gatley suggest (§17-026, footnote 185):
- As to the interpretation of s.6(5), the Claimants' primary submission is that the subsection does not, and was not intended by Parliament to, confer a privilege on the press and media in general, nor indeed on any third party who was not directly involved in and responsible for the publication of the peer-reviewed statement or assessment which it might be contended is privileged by virtue of s.6(1) or s.6(4) of the Act. Ms Page KC argues that s.6 does not, and was not intended by Parliament to, affect or expand the privileges available in law to anyone other than publishers of scientific and academic journals. She contends that s.7 of the 2013 Act was designed to cater for general privileges available to everyone, not s.6.
- The Claimants argue that s.6(5) must be read in the light of that overall purpose and to be construed purposively. To give true and proper effect to Parliament's intention, they contend s.6(5) should be interpreted as if it has provided (with additions shown underlined):
- Relying upon R (O) -v- SSHD (see [309] above); R (Quintavelle) -v- Secretary of State for Health [2003] 2 AC 687 [8]; and R (Fylde Coast Farms Ltd) -v- Fylde Borough Council [2021] 1 WLR 2794 [6], the Claimants have argued that, within the permissible bounds of interpretation, the Court's task is to give effect to Parliament's purpose. To do so, the relevant provisions should not be read in isolation but should be read in the context of the statute as a whole. Even where the words used in the relevant section might at first sight to have an apparently clear and unambiguous meaning, the Court must resolve differences of interpretation by setting the particular provision in its context as part of the relevant statutory framework.
- Applying these principles, Ms Page KC advances the following arguments:
- In support of these arguments, the Claimants have relied upon various pre-legislative materials and make the following submissions:
- I have considered the legislative materials gathered by the Claimants and submitted with their trial skeleton argument. In addition to the points raised by the Claimants that I have identified in the previous paragraph, I have noted the following:
- Ms Evans KC, on behalf of the Defendants, rejects the Claimants' interpretation of s.6 and has made brief submissions the thrust of which is that there is nothing anomalous in s.6(5) granting a general reporting privilege. She has relied upon the commentary provided in the key defamation textbooks:
- One point of potential difficulty identified by the authors of the Blackstone's Guide relates to the viability of the reporting privilege under s.6(5) (or for that matter the privilege under s.6(4)) if the underlying publication of the statement in the scientific or academic journal is found to be malicious. They observe:
- Having considered the terms of s.6, and the legislative materials set out above, I reject the Claimants' interpretation of s.6(5). In my judgment, the legislative intent is clear. In s.6, Parliament created a new category of qualified privilege for statements published in a scientific or academic journal if the conditions in s.6(2) and s.6(3) are met. s.6(4) extends a privilege to "any assessment of the statement's scientific or academic merit" if the conditions in (a) and (b) are satisfied the assessment is published in the same journal. s.6(5) extends a qualified privilege to a "fair and accurate copy of, extract from or summary" of the statement or assessment. There is no indication in the terms of the section that the privilege under s.6(5) is limited in the way contended by the Claimants. In this respect, I agree with the authors of the key textbooks on the point.
- Internally, this interpretation does not produce a result that is absurd. If Parliament had wanted to limit the privilege provided by s.6(5) in the way suggested by the Claimants, it could have said so. The wording that the Claimants suggest the Court should read into s.6(5) (see [337] above) was included in s.6(4). I am not persuaded that Parliament omitted those words from s.6(5) by oversight or mistake. In my judgment, the broad construction of s.6(5) - that it gives a general reporting privilege for statements or assessments privileged by the forgoing sub-sections of the Act - far from being inconsistent, is entirely consistent with the legislative materials prior to enactment.
- The starting point is that, to qualify for any reporting privilege under s.6(5), the publisher must establish that the statement or assessment is privileged under the section. The privilege under s.6(5) is therefore strictly circumscribed. Whilst, therefore, the privilege under s.6(5) is available to everyone, it can only apply to instances where the underlying privilege in the statement or assessment has been established. Granting such a reporting privilege is not inconsistent with statements, made by those promoting the bill, that the law of defamation should be properly calibrated to hold the balance between freedom of expression and proper protection of reputation (see e.g. the Lord Chancellor's statement [341(4)] above). Nor would it make the defence "too widely available". I am not persuaded that it would be "absurd" for Parliament to grant a reporting privilege under s.6(5) in circumstances where someone relying upon it might be required to establish the underlying privilege for the statement or assessment under ss.6(1)-6(3) and/or s.6(4). The burden to prove the necessary facts to establish a privilege defence has always rested on the person relying upon the defence.
- On the contrary, what might deserve the adjective "absurd" would be for Parliament to have provided a new defence of qualified privilege for statements published in peer‑reviewed scientific journals, but then to limit that privilege only to the original publisher(s); thereby leaving any wider discussion of these statements without any protection. As is clear from statements made by promoters of the bill, the Government intended s.6 to protect and encourage "robust and open scientific debate"; an open debate where all people "should be at liberty to debate a subject without fear or favour". Statements published in peer-reviewed academic or scientific journals do not generally represent such a debate, they inform, contribute to, or stimulate that debate. The safeguards provided in the section, before privilege attaches to the relevant journal publication, were regarded by the Government as sufficient to ensure that the relevant publication would be "responsible" (i.e. deserving of protection). In other words, having established the academic or scientific value of the statement, its publication to the wider public should be privileged, and thereafter available to contribute to important public debate.
- The public interest in the publication of the statement contained in a peer-reviewed scientific or academic journal would be lost (or substantially impaired) if a corresponding reporting privilege was not also granted. Few people obtain information about science or academia by directly consulting specialist journals. Most citizens receive this information through the main-stream or other media. The "persistent and impartial" research that led to identification of serious issues with Thalidomide would, arguably, not have been as successful in reaching a wider audience without the important role played by the media in highlighting and bringing to public attention the scientific evidence, and thereby provoking and contributing to an important public debate. The Joint Committee highlighted concerns that publication of research suggesting a particular product was unsafe might lead to a defamation claim from the manufacturer. But that risk applies not only to the original publisher of the scientific paper, but to anyone who republished or repeated its findings. Although the common law of qualified privilege might yet provide a defence, the 80% of doctors who reported feeling inhibited in discussing medical treatments would get no protection under s.6(5) if it bore the Claimants' interpretation. It is therefore not surprising that Paragraph 47 of the Explanatory Notes uses the word "extends to". It is describing, in simple terms, what s.6(5) was intended to achieve; i.e. a general reporting privilege. It needed no further explanation or fanfare. Indeed, to achieve its purpose, the lack of an accompanying reporting privilege in the section as a whole would have been a striking omission.
- I do not consider that any particular significance can be attached to Parliament having decided to provide the reporting privilege in s.6, rather than in s.7. No doubt, the same outcome could have been achieved by amending Schedule 1 to the Defamation Act 1996 in suitable terms. One explanation might be that s.6(5) is a type of reporting privilege that is dependent upon proof that another publication is privileged by statute, which would make it anomalous when compared to the reporting privileges granted by Paragraphs 1 to 8 of Part 1 of Schedule 1 of the Defamation Act 1996 (which is where the provision would have been required to be inserted; the privilege not being subject to explanation or contradiction).
- The point made about malice by the authors of Blackstone's Guide has a potential bearing on this point. On the facts of this case, the point on malice in relation to the underlying publication of the statement in a scientific or academic journal does not arise, but the comparison they draw between with the statements that are privileged under Schedule 1 to the Defamation Act 1996 might perhaps shed some light on why Parliament chose to provide the reporting privilege in s.6 itself rather than amending Schedule 1 to provide for such a privilege. Although I do recognise the force of what is said in the penultimate sentence of the quotation from Blackstone's Guide, the legislative choice to provide the reporting in privilege in s.6 tends to militate against this conclusion. s.6(5) is very clearly premised on the underlying privilege being established - in reality, not in theory - as a pre-requisite of the corresponding reporting privilege. But this is speculative. I cannot - and need not - reach a conclusion as to why Parliament chose to accommodate the reporting privilege in s.6, but I am satisfied that its absence from s.7 does not support the construction of s.6 urged by the Claimants. The interesting point on malice will have to be resolved on an occasion on which it calls for determination. I shall say no more about it.
- To cater for the event that the Court ruled against their submissions on the scope of the reporting privilege provided by s.6(5), the Claimants also made submissions as to the interpretation of ss.6(1) to 6(3). They contend that it is incumbent upon the Court to interpret the section, specifically s.6(3), in a way that requires a defendant to prove that the peer-reviewed process has been carried out in a suitably rigorous way, lest the bar for the privilege be set too low. Relying upon what was said in paragraph 45 of the Explanatory Notes to the Act (set out in [327] above) and paragraph 48 of the Joint Committee Report (see [341(1)] above), the Claimants argue that it is only if the peer-review process can be proved to have been a "proper" one, that is adequately robust and exacting, that a defendant seeking to rely upon the privilege should be relieved of the burden of establishing a Reynolds - or now a s.4 public interest - defence or some other defence, i.e., truth or honest opinion.
- In answer to the Defendants' qualified privilege defences, the Claimants have alleged that any qualified privilege that they establish is defeated by proof of malice on the part of Mr Calman (see [32]-[34] above).
- Malice can be established if the claimant proves that the defendant published a statement that s/he either knew was false, or was reckless (in the sense of complete indifference) as to its truth or falsity. A plea of malice is tantamount to an allegation of dishonesty: Alexander –v- Arts Council of Wales [2001] 1 WLR 1840 [18]; it is a serious allegation to make against anyone and should not be lightly made: Webster -v- British Gas Services Ltd [2003] EWHC 1188 (QB) [28]; Sube -v- News Group Newspapers Ltd [2018] EWHC 1234 (QB) [73]. It is that state of mind that justifies depriving a defendant of a defence of qualified privilege or makes it just to allow recovery for the publication of a falsehood causing special damage (or having a tendency to cause pecuniary damage).
- In Three Rivers DC -v- Bank of England [2003] 2 AC 1 [161], Lord Hobhouse explained the high bar for proving dishonesty (which applies equally to malice):
- The classic exposition of malice is from the speech of Lord Diplock in Horrocks -v- Lowe [1975] AC 135, 149-150. In view of the malice plea advanced on behalf of the Claimants, and because the shorthand that is often used (for convenience) in discussing the principles that govern malice can on occasions remove some of their important nuance, it is worth setting out the passage in full:
- The only decision, in which s.15 has been considered previously, is Qadir. The claimant brought a claim for libel over two articles published in The Mail on Sunday. The first article purported to include a report of allegations made in Particulars of Claim from civil proceedings in which Mr Qadir was the defendant. A journalist had obtained a copy of the Particulars of Claim from the records of the Court under CPR 5.4C(1). The qualified privilege defence was advanced, pursuant to s.15 Defamation Act 1996 and Paragraph 5 of Schedule 1, in respect of the parts of the article which the defendant contended were "a fair and accurate copy of an extract from any register or other document required by law to be open for public inspection". The claimant denied that the report was privileged but, if it was, such privilege was defeated by malice.
- The issues to be resolved at the preliminary issues trial identified by the Judge included (for present purposes) ([45]):
- The Judge held that the answer to the first issue was in the affirmative ([76]), but that the publication was not for the public benefit, because the report did not identify that the allegations had been disputed by the claimant:
- Having dismissed the qualified privilege defence, the issue of malice therefore did not strictly arise for determination: [177]. Nevertheless, Tugendhat J went on to set out his conclusions on the issue because it had been fully argued: [178]. An issue to be resolved was the nature of the malice that had to be established for the purposes of defeating a statutory reporting privilege. The claimant had not alleged malice based on an alleged dominant improper motive; it was alleged on the basis of recklessness (in the sense of wilful blindness to the truth): see [179]-[180]. Naturally, the focus of the argument was on the speech of Lord Diplock in Horrocks -v- Lowe (see [181]-[188]). In [189]-[190], the Judge referred to and relied upon two decisions of Lord Esher (quoted by Lord Diplock) from Clark -v- Molyneux (1877-78) LR 3 QBD 237, 246-247 and later in Royal Aquarium and Summer and Winter Garden Society Ltd -v- Parkinson [1892] 1 QB 431, 454-455.
- The passage from Clark was as follows:
- In Royal Aquarium, Lord Esher MR said:
- Tugendhat J held that the privilege under s.15 would be lost if the defendant was shown to have "misused the occasion", in other words "using it for a purpose other than that for which it was accorded", but that "misuse, or abuse, in this context require proof that the purpose, reason or motive of the defendant must be his dominant one before malice can be proved... In these discussions of malice the courts appear to use the words purpose, motive and reason interchangeably": [191]-[192].
- Importantly, in my judgment, Tugendhat J held Lord Diplock's formulation of malice from Horrocks -v- Lowe was not "directly applicable in the present case": [193]. As he explained ([194]), that was because:
- Tugendhat J's conclusion was ([212]):
- In an earlier decision in the same case ([2012] EWHC 2064 (QB)), Tugendhat J adjourned consideration of the application to amend the malice plea (and the defendant's application to strike out the existing plea) to the trial of preliminary issues of qualified privilege and malice. I note that the Judge observed [17]:
- It is common ground between the parties, that this is the first decision on the issue of malice in the context of s.15(1) Defamation Act 1996 since Qadir, and the first at all in relation to s.6 Defamation Act 2013, although (for my part) I would not think that the Court's approach to malice would differ as between different types of what I have called 'reporting privilege'. In my judgment, it is important to give due weight to statutory qualified privilege, particularly where the statute grants a 'reporting privilege', because of the importance of Article 10. Like Tugendhat J in Qadir, I am satisfied that the rationale for granting a reporting privilege is the importance attached to dissemination of the protected information. As Lord Esher observed, in the underlined passage from Clark, the privilege is granted for some reason. When determining the scope of the privilege, and the type of malice that could defeat it, the Court must have this purpose well in mind.
- In his speech in Horrocks -v- Lowe, Lord Diplock did not draw a distinction between common law and statutory qualified privilege. In the final paragraph that I have quoted in [355] above, he is clearly there discussing common law qualified privilege, because he mentions "duty" and "interest", which are the hallmark of common law qualified privilege, and held that, to establish malice, these must have played no significant part of the defendant's motives for publication. Statutory qualified privilege is premised differently. Where a qualified privilege is granted by statute, unlike common law privilege, there is no question of the defendant having to establish a sufficient duty or interest before s/he can avail him/herself of the privilege: "Provided the statutory conditions are met, the report is privileged": Curistan [25] per Arden LJ.
- In my view, some caution needs to be adopted in mapping across concepts that, whilst readily applicable to common law qualified privilege, do not find a ready analogue in instances of statutory privilege, particularly 'reporting privilege'. Consistent with the underlying Article 10 rationale, it is difficult to talk about a 'reporting privilege' being 'misused' or for a defendant's motives in publication having any substantial bearing on the issue. Under s.15(3) Defamation Act 1996, a defendant's privilege defence would fail in respect of any publication that was not of public interest and not for the public benefit. If a defendant surmounts that hurdle, and demonstrates that his/her publication is a fair and accurate report/copy/extract/summary (as applicable) in one of the Paragraphs of Schedule 1, the question is what state of mind should amount to malice so as to defeat such privilege? Whilst I can see that privilege could be rightly denied where the defendant knew what he was publishing, under a reporting privilege, was false (or was reckless to a degree that s/he is treated as having this knowledge), the law does not require a positive belief in the truth of what is being reported. In many (if not most) reporting privilege cases, the defendant simply will not know whether the facts being reported are true or false. The reporting privilege exists to protect the publication of the relevant facts, true or false.
- Take as an example a journalist, A, who discovers evidence that, B, the sole director of a company that runs several local care homes was convicted, 2 years ago, of fraud and assaulting a resident of a care home where s/he worked. A publishes details of B's previous convictions in a local newspaper article together with an editorial calling for the Care Quality Commission to investigate whether B is a fit and proper person to be involved in the management of a care home. B brings a claim for libel. At trial the Court finds that the article is a fair and accurate report of the criminal proceedings in which B was convicted, and that the publication is found to be of public interest for the public benefit. Absent proof of knowledge of falsity (or equivalent recklessness), what is the test for malice that should be applied? Suppose A is a shareholder of a company that operates care homes in competition with B's company, and the Court finds that A hoped and intended that B and the company would suffer a loss of business as a result of publishing the article about the convictions, or if A is found to have a long-standing animosity towards B as a result of some private dispute between them, and published the article out of spite towards B. Should the reporting privilege be defeated by malice? I am inclined to think that it should not.
- Lord Diplock gave, as an example of malice, where a defendant's dominant motive may have been to obtain "some private advantage unconnected with the duty or the interest which constitutes the reason for the privilege". The Claimants' pleas of malice in this case were clearly drafted with this passage in mind. In the care home article example I have given, the journalist's shareholding in a rival business might be argued to be such a "private advantage", but it is difficult to see why its existence should defeat the otherwise viable reporting privilege. Equally, why should the journalist be deprived of the reporting privilege for his/her private spite towards B? The value of the information communicated to the public in each instance remains the same. Article 10 protects not only the right to impart information but, importantly also the right to receive it. Arguably, the primary object of a statutory reporting privilege is to the promote and protect the public's interest in receiving the information not the reporter's interest in imparting it. The latter is protected in order to promote the former. That is perhaps the key distinction from common law qualified privilege, which necessarily concentrates on the duty or interest upon which the defendant seeks to base his/her privilege defence.
- The Claimants' case of malice against the Defendants (see [32]-[34] above) is, in part, based upon an alleged dominant improper motive on the part of Mr Calman. But that motive is not "spite" or similar; it is a form of alleged "private advantage" through bolstering the credibility of the allegations made against the Claimants through reports of the (ex hypothesi) privileged information. For the reasons I have explained, and consistent with proper respect for freedom of expression, this type of malice needs to be approached with care. In Huda -v- Wells [2017] EWHC 2553 (QB) I said this:
- In my judgment, Lord Nicholls' reasoning in Cheng (albeit in the context of honest opinion), explains why a cautious approach is needed to defining the circumstances in which (if ever) a proved "dominant improper purpose" can defeat a statutory reporting privilege. Cheng was a seminal decision which, although delivered in Court of Final Appeal of Hong Kong, led to the removal of "dominant improper motive" malice from the law of honest opinion, first under the common law, and later in s.3(5) of the Defamation Act 2013.
- Lord Nicholls explained the differences between the defences of honest opinion and qualified privilege as follows (and in doing so, it is clear that he is referring to the common law privilege defence, rather than a statutory privilege defence):
- The rationale for statutory reporting privilege much more naturally fits under Lord Nicholls' definition of the honest opinion defence than conventional duty/interest common law qualified privilege. As Tugendhat J noted in Qadir, statutory reporting privilege is also not based on any notion of the performance of a duty or protection of an interest. The justification for the privilege is the high importance that is attached to the public interest in ensuring that fair and accurate reports of defined categories of information of public interest should be freely available to be disseminated to the public (or sections of the public) for the public benefit. In that respect, the rationale for statutory reporting privilege is a much closer analogue to honest opinion.
- Earlier in his judgment, before considering Horrock -v- Lowe, Lord Nicholls explained why 'spite' malice should not defeat an otherwise viable defence of honest opinion:
- Then, after an extensive review of common law authority on malice in the context of honest opinion (fair comment), Lord Nicholls set out his conclusion:
- Whilst honesty of belief is necessarily the touchstone for the honest opinion defence, that element is not (and cannot be) a pre-requisite of a statutory reporting privilege (as noted above ([368] above); often the reporter is in no position to know whether the facts are true or false, and it is not necessary to discharge the function of the privilege for him/her to know whether the allegations are true or false. In my judgment, once that important premise is recognised, the reasoning that compels the rejection of "dominant improper purpose" malice as a basis for defeating an honest opinion defence strongly supports a similar conclusion for statutory reporting privilege. Insofar as the Claimant's malice plea depends upon proof of an alleged (improper) dominant motive of providing an "endorsement" of the allegations made against the Claimants in the Articles, I would hold that this was not a proper basis upon which an otherwise viable defence of statutory reporting privilege could be defeated. Proof of some private motivation - whether it be an attempt to secure some "private advantage" for the reporter, some disadvantage for the claimant or even spite, and even if this is the dominant or sole motivation - for the publication of what has otherwise been held to be a fair and accurate report of information, of public interest, and published for the benefit of information, is insufficient to defeat a statutory reporting privilege.
- In the (perhaps unusual and rare) case where the reporter knows that the facts in an otherwise fair and accurate report are nevertheless false (or is reckless to a degree that the law treats him/her as having such knowledge), then s/he would be malicious if they were presented to the readers as true, or without suitable caveat. But then, rather like in Qadir, a journalist who published such a report might well fail to establish the objective elements of the defence - including the public benefit requirement of s.15(3) - in the first place, in which case the Court would not need to consider the issue of malice.
- Finally, in the context of malice, I should note one, uncontroversial, proposition. In cases of a continuing publication, malice may be proved at some point after original publication so as to defeat the privilege from that point: Qadir [234].
- The law relating to the determination of natural and ordinary meaning, and whether a publication is, or contains, an expression of opinion or statement of fact is well‑established. The principles, subsequently approved by the Court of Appeal, are set out in Koutsogiannis -v- The Random House Group Limited [2020] 4 WLR 25 [11]‑[17]. The Court should be astute not to be too rigid in its approach to determining the issues of meaning and fact/opinion; it should be flexible and holistic: British Chiropractic Association -v- Singh [2011] 1 WLR 133 [32]; Sube -v- News Group Newspapers Ltd [2018] EWHC 1234 (QB) [33]; Peck -v- Williams Trade Supplies Ltd [2020] EWHC 966 (QB) [11(ii)]; and Riley -v- Heybroek [2020] EWHC 1259 (QB) [49]. Although the particular context is everything, the more clearly a publication indicates that it is based on some extraneous material, the more likely it is to strike the reader as an expression of opinion: Triplark -v- Northwood Hall [2019] EWHC 3494 (QB) [17].
- In relation to an innuendo meaning, relying upon knowledge of particular extrinsic facts, the principles are the same. The issue is: "Assuming the knowledge is proved, the question for the Court is what meaning would have been conveyed to a reasonable person having that special knowledge": Sheikh -v- Associated Newspapers Ltd [2019] EWHC 2947 (QB) [16]; Baturina -v- Times Newspapers Ltd [2011] 1 WLR 1526 [56].
- So far as concerns articles that appear in print editions of newspapers, the principles regarding the circumstances in which separate articles are treated as a single publication are not controversial:
- In Dee, the Claimant had complained of an article published on the front page, but not of a further article, published on page 20 of a supplement in the same edition of the newspaper. The front-page article contained the words "Full story: S20". In the context of a summary judgment application, the Defendant argued that the Court should rule that the two articles had to be read together for the purposes of determining the single natural and ordinary meaning. Sharp J held that they should. Important factors in Dee were that the subject matter was the same in both articles and the front-page article contained a reference to the second article.
- It is necessary to keep in mind that there are two distinct concepts. The first, which Dee exemplifies: what is to be treated as the publication for the purposes of determining the single natural and ordinary meaning. The second; what is treated as admissible context for the publication for the purposes of determining that meaning: see Riley -v- Murray [2020] EMLR 20 [16]. In Monroe -v- Hopkins [2017] 4 WLR 68 [38], Warby J noted, relying upon Dee [29]: "The test is not the same as but is influenced by the test for whether two publications are to be treated as one for the purposes of defamation."
- Those basic principles also apply to online publication (Ashley -v- Times Newspapers Ltd [2021] EWHC 2082 (QB) [48]), but there can be further considerations, due to the ability in online publications to provide hyperlinks to extrinsic material.
- The issue of what is to be treated as a single publication (or admissible context on the issue of meaning) in the context of online publication has been considered in several decisions: Monroe [34]-[40]; Falter -v- Atzmon [2018] EWHC 1728 (QB) [12]-[16]; Poulter -v- Times Newspapers Ltd [2018] EWHC 3900 [21]-[24]; and Greenstein -v- Campaign Against Antisemitism [2019] EWHC 281 (QB) [17]. Of potential relevance to the specific issues in this case, I said this in Poulter [24]:
- In Wildcat Haven Enterprises CIC -v- Wightman [2020] SLT 473 (a context case), Lord Clark, in the Court of Session, reviewed several of these authorities and articulated the test in the following terms [26]:
- Finally, on the issue of meaning, I must consider the Court of Appeal's decision in Curistan. The decision has received little consideration since it was made and has, so far as I am aware, never been applied in a decided case. As I noted in the PIT Judgment [7], it has been described as "iconoclastic".
- The appeal concerned the claimant's appeal from a ruling by Gray J that certain passages in an article, published in The Sunday Times, attracted qualified privilege under the s.15 Defamation Act 1996, as a report of certain statements made by Peter Robinson MP in the House of Commons, and the defendant's cross-appeal that the judge should have found that the article bore a Chase level 2 meaning instead of one at Chase level 1. The decision addresses the question of how the court should approach for the purposes of determining meaning a "hybrid" defamatory publication; one consisting partly of privileged and partly of non-privileged material. The Court of Appeal dismissed the appeal and allowed the cross-appeal. It decided that Gray J had been right to find that the passages in question were protected by privilege but that he had failed properly to reflect that conclusion in his ruling on meaning. In consequence, the Court held that the article bore a Chase level 2 meaning: [76].
- The Court was unanimous in the result of the appeal, but each member of the panel (Lord Phillips CJ and Laws and Arden LJJ) gave a judgment giving his or her own reasons. Laws LJ agreed with Arden LJ's conclusions but did not endorse her reasoning: [78]. Lord Phillips CJ, agreed with Arden and Laws LJJ's conclusions, and endorsed Laws LJ's reasoning: [93]. He disagreed with two aspects of Arden LJ's reasoning (see [95] and [99]), and otherwise did not associate himself with Arden LJ's reasons. Under these circumstances, the starting point in the analysis of the decision is Laws LJ's judgment, given that it appears that Lord Phillips CJ expressed agreement with it.
- Laws LJ considered the well-established defamation principles, as they affect the natural and ordinary meaning of a publication, of the repetition and single meaning rules: [80]-[81], [83]. On the key issue of principle, Laws LJ said:
- So far as Arden LJ's judgment is concerned, while her reasoning is quite different from Laws LJ in certain respects, her conclusions on the applicable principles are indistinguishable from his: see [21], [22(v)], [58]-[64] & [69]-[70]. The same applies to Lord Phillips CJ's judgment. On the approach to determining meaning, he said:
- Laws LJ's decision that, when considering the natural and ordinary meaning of a publication, the repetition rule is to be disapplied to those parts of the publication that are protected by privilege, has perhaps been described as "iconoclastic" because it runs contrary to fundamental principles of the common law of defamation - principally the single meaning rule - and conflates rules that govern meaning with substantive privilege defences (which have nothing to do with meaning). Put shortly, in holding that a publication may bear two different natural and ordinary meanings, depending on whether parts of it are privileged or not, Curistan conflicts with well-established principles of the common law of defamation.
- The orthodoxy is that the single natural and ordinary meaning of a publication is to be determined by considering the publication as a whole. That single meaning is ascertained by application of well-established principles (see [380] above). Historically, that meaning was unaffected by the fate of any substantive defence raised in answer to the claim. To that extent the natural and ordinary meaning remains a constant. "[T]here is no defensible way in which the courts can adjust the meaning of meaning so as to include things which no sensible reading of the words could embrace": Berezovsky -v- Forbes [2001] EMLR 45 [14] per Sedley LJ.
- So far as regards the treatment of privilege, in this respect at least, defamation law aligns with real life. And, as a matter of logic, how the hypothetical ordinary reasonable reader understands the meaning of a publication cannot be affected by the fate of a defence that is determined after the event. A person reading an article, which contains parts that may potentially be protected by qualified privilege, in deciding what the article means (and the extent to which it damages the putative claimant's reputation), is not going to resolve first which parts of the article s/he should regard as protected by privilege and then treat them only as "context" for the remaining (unprivileged) parts of the article. Such a process is unreal. Even if the reader had the necessary skills (including a fairly sophisticated knowledge of the law of qualified privilege) to identify the potentially privileged parts of the article, his/her assessment could only be provisional. Whether or not the parts of the article were actually privileged could only be ascertained once the defence had been resolved (either by agreement or following a trial), for reasons explained in the PIT Judgment.
- With respect, the Court in Curistan may not have properly recognised that the determination of the natural and ordinary meaning, important though it is, is simply one stage in the resolution of a defamation claim. The single natural and ordinary meaning plays two key roles in the law of defamation:
- Were it not for the Curistan decision, I would have approached the decisions to be made in this case on a wholly different basis. The Court would have found the natural and ordinary meaning, using the established principles, without reference to the qualified privilege (or any other) defences. If the Court were subsequently to find that any part(s) of the Articles were protected by qualified privilege, it would be at that stage that the Court would determine the impact on that finding on the case overall, including on any substantive defences and the issue of damages (if it arose). (See [556]-[558] below for the application of Curistan to a specific issue in this case, and how it would have differed had Curistan not been applied).
- As a first instance Judge, I am, however, bound to follow the Curistan decision, as a matter of precedent. Even so, that task is not straightforward.
- With respect to the decision, and the judgments of three experienced Judges, I do not understand the effect of treating privileged parts of a publication as "context" for determining the meaning of the non-privileged parts. It is clear, from [22(v)(1)], [84(ii)] and [102], that the privileged parts are not to be removed entirely from consideration. "Whilst they cannot be relied upon as words complained of, for the purposes of meaning, they remain as context for the non-privileged parts of the publication": Alsaifi -v- Trinity Mirror plc [2018] EWHC 1954 (QB) [32(i)]. But if they remain to be read as "context", what impact does that have on the determination of the natural and ordinary meaning of the publication? The Court already determines the natural ordinary meaning having proper regard for the context in which the words complained of appeared. Is the Court supposed to adopt a different approach to determining meaning if the "context" is protected by privilege? Whilst Curistan would suggest the answer to this question is clearly "yes" - because the meaning was found to be different if the privilege claim was upheld - there is no explanation of how the Court is to adjust the normal rules of determining meaning to take account of privileged passages.
- I can readily see that much will depend on the terms of the particular publication. In Curistan, the non-privileged parts of the article were effectively adding further details to the privileged parts. In a Buchannan-type case, the non-privileged parts may expressly adopt what is said in the privileged parts. Arguably, this case is different again. Here the Hancock Statement was presented as comment upon the subject matter of (and perhaps the allegations made in) the non-privileged parts. Ultimately, if parts of the Articles are found to be privileged, I shall have to do the best I can to apply the principles in Curistan.
- At the conclusion of the trial, the parties submitted an agreed list of issues (save for one point of contention between them). I have set out this agreed list of issues in Annex 5 to this judgment.
- This formidable list of issues - by far the most complicated I have ever encountered - has, subject to the one point in (23), been agreed by the parties. The parties are represented by highly experienced solicitors and counsel. Had I been approaching this case, independently, I might well have chosen to resolve the issues differently. Nevertheless, mindful that my task, as Judge, is to resolve the issues that the parties put before the Court, I shall (subject to issue (30) which I have declined to resolve for the reasons explained) seek to abide faithfully to this agreed position.
- As a reminder (see [6] and [8] above):
- The Claimants do not raise any substantial challenge to this element, acknowledging on the authorities that it is of wide ambit. They quibble with the Defendants' formulation of the public interest.
- I am quite satisfied that the Defendants have established this element of the public interest defence. The Articles were quite obviously on a matter of public interest. The contrary I regard as unarguable. In my view, this point should have been admitted by the Claimants at the outset, allowing the Court to concentrate on the real matters in dispute. I do not consider that it is necessary (or desirable) to define precisely what was the matter of public interest, and were it not for the fact that the parties have agreed that I should determine this issue, I would not have done so. The articulation and definition of the precise "matter of public interest" tends to generate (as it does in most cases and has done in this one) a sterile and unproductive dispute that serves only to waste the parties' costs and the Court's resources. Cases in which there is a real dispute over whether the relevant publication was or formed part of a statement on a matter of public interest should be very few. I have yet to see one in the ten years that the Defamation Act 2013 has been in force. The only thing my defining the matter of public interest will produce is a potential appeal point.
- With that important caveat (and reluctantly), and insofar as it is necessary to identify the matter of public interest to which the Articles were directed it was the accuracy/reliability of the Claimants' public statements made in the context of the statin debate.
- In his evidence, Mr Calman stated clearly that he considered that publication of the Articles was in the public interest. He was not substantially challenged on this evidence in cross-examination by the Claimants. The Claimants point to the absence of any contemporary documents recording why, and on what basis, Mr Calman reached this decision, but ultimately they have not asked me to reject his evidence on this point.
- I am satisfied that the Defendants have established this element of the public interest defence. At the time of publication, Mr Calman did believe that publication of the Articles was in the public interest.
- As with most public interest defences, this is the significant area of dispute.
- As noted already (see [286]-[290] above), the Defendants' public interest defence has some element of novelty, because it is the first time that it has been applied to a publication that has significant elements of criticism and is defended as an expression of opinion.
- Based upon her submissions of the proper approach to be adopted in s.4 opinion cases, Ms Evans KC articulated what she contends Mr Calman should be expected to show by way of inquiries and checks where the publication is a statement of opinion.
- Ms Evans KC then suggested that the task for the Court, in assessing these issues, was "straightforward", and limited to consideration of (i) the Claimants' public claims; (ii) the existence of the scientific evidence; (iii) the consequences of the Claimants' public claims for public health; (iv) the right-to-reply process; and (v) the treatment of the Hancock Statement. Ms Evans submitted that, assuming that Mr Calman had accurately attributed the Claimants' public claims to them, whether (a) Mr Calman was correct to reach the view that the published meta-analyses, scientific studies and trial data did give overwhelming evidential support to mainstream medical and scientific opinion; and (b) (on the basis of this evaluation) determine whether the Claimants' challenges to mainstream medical opinion are irresponsible or reckless or (on the Claimants' meaning) dishonest "is a matter of contention and evaluative opinion which is not capable of verification".
- The Claimants contend that Mr Calman's belief that publication was in the public interest was not reasonable. Ms Page KC argues, in summary:
- At the outset, I reject the Defendants' suggested limited parameters of the consideration and determination of the public interest defence. The authorities are clear. For the purposes of the public interest defence, a belief is reasonable only if it is one arrived at after conducting such research and checks as it is reasonable to expect of the particular defendant in all the circumstances of the case. Whilst there is an important area for editorial judgement in what is reported in any article, it is not in the public interest for a publisher to misstate (or ignore) the evidence it has available. That remains the case even if the underlying material or evidence is complex. The public interest is not served by a superficial treatment of a complex area. If a publisher lacks the knowledge or ability to understand a topic, then it should consider carefully whether it will be able to publish an article that it would be reasonable to publish in the public interest. That is particularly so where, as here, readers were invited to "decide for yourself who YOU should entrust your health to", based on the evidence presented to them in the articles (Main Article [18]). If readers were given a misleading or incomplete account of the evidence, they would be deciding the issue on a false (or incomplete) basis.
- Before turning to consider the detail, in overview the following appear to me to be the important circumstances of this case:
- I am conscious of Mr Verity's evidence that the Defendants' intention was not to publish an article that simply addressed 'Are statins good or not?'. Freedom of expression means that it is open to a publisher to decide to publish an article that comes down firmly on one side of a debate and to pronounce "the truth", as Mr Verity described it. It was equally open to the Defendants to have published such a "polemical" article, without naming individuals. A publisher has a freedom to choose the manner of its expression. But with that choice can come certain consequences, particularly in relation to the defences that may be available to defend any defamatory imputations conveyed by the publication. The decision to focus upon, and name, the Claimants (and other 'statin deniers') meant that publication of the Articles engaged the legitimate reputational interests of the Claimants.
- The Main Article presented to readers four key elements of "fake news" regarding statins (presented as sub-headings in the Main Article): (1) having high cholesterol is harmless; (2) statins don't stop you from dying; (3) doctors are hushing up side-effects; and (4) experts paid by drugs giants. A further topic - not identified with a separate heading in the Main Article - was the claim that the Claimants misrepresented the quality of the scientific research, relying on observational studies rather than randomised double-blind clinical research. In sections (a) to (e) of this part of the judgment, I shall analyse what Mr Calman had, by way of evidence and the Claimants' responses, in respect of each of these areas, and how that was presented to readers in the Articles. In section (f), I shall draw together my conclusions.
- The Main Article dealt with the Claimants' claims in [24]-[30].
- Against the First Claimant, the Main Article cited her claim that "high cholesterol is not even associated with high heart disease, let alone a cause" ([27]). Although, in his evidence, Mr Calman has identified several other occasions when the First Claimant is alleged to have made this claim, it is clear from the documentary evidence that primary material upon which he relied were the First Claimant's two blog entries from earlier that month: "Statins in the over 75s", published on 4 February 2019 (see [69]-[70] and Annex 3(A)); and "Why cholesterol can't cause heart disease", published on 11 February 2019 (see [82] and Annex 3(C)). Both of the First Claimant's blog entries were themselves commentary on data drawn from The Lancet 2019 Study.
- This claim, together with the claim that her stance on statins and the link between cholesterol and heart disease amounted to "misinformation", was put to the First Claimant by Mr Calman in the email of 28 February 2019. In her response to Mr Calman, the First Claimant relied upon her analysis of statistical data from the World Health Organisation of 192 countries correlating cholesterol levels and deaths from cardiovascular disease, published on her blog in 2010 (see [203] above). Her conclusion was that higher cholesterol levels were associated with fewer cardiovascular disease deaths, and lower cholesterol levels were associated with a greater number of deaths from cardiovascular disease. The First Claimant denied the charge that she was guilty of publishing "misinformation", relying upon her WHO study. She countered that an example of misinformation was the claim, made by Professor Baigent, at the press conference to launch The Lancet 2019 Study, that up to 8,000 deaths per year could be prevented if all over 75s took statins. To support that, she relied upon her statistical analysis of the data in The Lancet 2019 Study to demonstrate that no benefit could be claimed statin therapy in the over 75s (see [69]‑[70] above).
- Against the Second Claimant, the Main Article cited two claims made by him: (1) "The way to avoid heart disease... has nothing to do with lowering cholesterol" (published on the cover of A Statin Nation) (Main Article [25]); and (2) "We found that if your bad cholesterol was higher, you were no more likely to die of heart disease or strokes" (which may have been taken from the Second Claimant's appearance on BBC Radio 4's Today programme (see [211] above)) (Main Article [26]).
- Only the first of these two claims was put to the Second Claimant in email of 28 February 2019, together with the allegation that his stance on statins and the link between cholesterol and heart disease amounted to "misinformation". In his response, in relation to the first claim, the Second Claimant relied upon the Pravastatin Study (see [202] above), and stated that he did believe that people were being conned about statins (see response paragraph [8]). In relation to the claim of "misinformation", the Second Claimant referred Mr Calman to the 2016 Ravnskov Study (see [210] above), which included in its findings: "High LDL-C is inversely associated with mortality in most people over 60 years. This finding is inconsistent with the cholesterol hypothesis (i.e. that cholesterol, particularly LDL-C, is inherently atherogenic)."
- The Main Article did not include any reference to any of the material relied upon by the Claimants in their responses on this point. Their claims were simply dismissed in the Main Article [10] and [28]. The material that Mr Calman had received from the Claimants required him first to engage with it, and second to represent it fairly in the Articles, even if, ultimately, he intended to suggest that the view of his experts was to be preferred. As I have explained (see [237]-[246] above), I do not accept that Mr Calman properly considered the material the Claimants had identified, and relied upon, in their right-to-reply responses. That is likely to have been a significant factor in his failure to include appropriate reference to their responses in the Articles.
- The substance of the Claimants' response was that the theory that reduction of LDL-C reduced the risk of cardiovascular incidents required explanation in the light of (a) the negative correlation between levels of LDL-C and cardiovascular incidents/deaths (and the various so-called paradoxes); and (b) the Pravastatin and two Ravnskov Studies.
- It was, of course, open to Mr Calman to suggest to readers that, in his view, and in the opinion of his experts, The Lancet 2019 Study was more authoritative than the materials relied upon by the Claimants, but he did not do so. Instead, he presented simply the Claimants' claims, without their justification, and quoted Professor Collins' conclusion that they were wrong ([28]) together with scientific material to support his conclusion ([29]-[30]). This presentation critically deprived readers of the opportunity to understand (and make their own assessment of) the Claimants' claims (and, importantly, their motivation) because the Articles did not explain the basis on which the Claimants had sought to justify their claims. As a result, the Claimants' genuine efforts to engage with (and refute) the criticisms of them, were simply ignored in the Articles and their claims dismissed as wrong. As a result, it may well have appeared to readers that the Claimants simply had no justification for the claims that they had made regarding levels of cholesterol and cardiovascular disease.
- The Main Article dealt with this topic in [38]-[45].
- It is not apparent that this allegation was being levelled clearly at the First Claimant. The focus of this section of the Main Article was the Second Claimant. The First Claimant appeared in the final two paragraphs [44]-[45].
- In his right-to-reply email, Mr Calman did not put this specific allegation to either Claimant.
- Paragraph [38] reported the Second Claimant's reliance upon the Kristensen Study (see his right-to-reply response [4]-[6]). This was advanced by the Second Claimant in response to the allegation that the potential consequences of the claims he had made about statins "could be far worse" than the "infamous MMR vaccine scandal". Paragraphs [39]-[42] presented readers with the rebuttal of reliance upon the Kristensen Study by three of Mr Calman's experts. Paragraph [43] reported the Second Claimant's reliance upon the 2016 and 2018 Ravnskov Studies (see his right-to-reply response [12]-[13]). This was advanced by the Second Claimant in response to the allegation that his stance on statins and the link between cholesterol and heart disease amounted to misinformation. Paragraphs [44]-[45], which would have appeared to readers to be the First Claimant's response to the charge that she had published "fake news" that statins did not stop people dying, were actually her (edited) response to the allegation that her stance on statins and the link between cholesterol and heart disease amounted to misinformation (see her right-to-reply response [16]-[17]). I would note here that in the introductory section of her right-to-reply response, dealing with the second paragraph of Mr Calman's email, the First Claimant also relied upon the Kristensen Study (see response [5]).
- From the material Mr Calman did have, on this point, the key point of dispute between the Claimants and Mr Calman's experts was the Claimants' reliance on the Kristensen Study. I have already noted that there was a legitimate dispute about the conclusions that could be extrapolated from the Kristensen Study, the limits of the study and what contribution it made to the statin debate (see [240] above). Looked at strictly in isolation, the representation of the dispute over the Kristensen study in the Main Article is a fair attempt to summarise the issue in an article in a mainstream newspaper. I have already set out my conclusions on what Mr Calman recognised as the central messages that the Articles were communicating to readers about the Claimants (see Section F(10) above).
- The Main Article dealt with this topic in [46]-[59].
- The treatment of the issue of side-effects, and the LSHTM Paper, in the right-to-reply process was negatively affected by Mr Calman's error in attributing to the Claimants authorship of the 2013 BMJ Articles (see [200] above). In consequence, the specific allegation that public statements of the Claimants had been found in the LSHTM Paper to have contributed to "rising numbers [of people] stopping statins" (Main Article [48]) was never put to the Claimants for their comment/response. Indeed, this allegation was only inserted into the Main Article after Mr Calman received the right-to-reply responses, perhaps because Mr Calman needed to establish a link between the LSHTM Paper and the Claimants after his discovery that neither had been an author of the 2013 BMJ Articles. Nevertheless, the First Claimant did provide a response about side effects (right-to-reply response [12]-[13]), albeit Mr Calman did not include either of the points she made in the published Articles (see [445] below).
- Central to this section of the Main Article were (1) the publication of the 2013 BMJ Articles (to which Dr Malhotra had contributed), which published a claim that 20% of patients on statins experienced "unacceptable" side effects; and (2) the LSHTM Paper.
- In relation to the 2013 BMJ Articles, Mr Calman knew (not least from the Claimants' right-to-reply responses) that there was a great deal more to the dispute than the summary presented in [46]-[49] of the Main Article. Indeed, both Claimants relied heavily on what had happened in this dispute as important context (and, in part, as an explanation) for the allegations of 'fake news' that they were facing.
- Mr Calman had been specifically referred, by both Claimants, to the BMJ Review Report, published on 15 August 2014. The background was summarised in the Report itself (see [206] above). Very shortly after publication of the 2013 BMJ Articles, Professor Collins had complained to Dr Fiona Godlee, the editor of The BMJ, that the 2013 BMJ Articles had misrepresented the evidence regarding statins and could cause public harm. Professor Collins rejected an offer, made by Dr Godlee, to submit an article for publication in The BMJ presenting evidence of the benefits and harms of statins. The dispute continued into 2014 leading, on 28 April 2014, to Professor Collins sending a letter, marked 'not for publication', in which he called for retraction of the 2013 BMJ Articles by the BMJ. The authors of the BMJ Review Report noted:
- On 15 May 2014, The BMJ published corrections for the 2013 BMJ Articles, withdrawing the statement that side effects of statins occur in about 18-20% of patients in which it was stated:
- The conclusion of the BMJ Review Report was that the 2013 BMJ Articles did not "meet any of the criteria for retraction", and importantly, that the error as to the precise figure for those experiencing side-effects from statins "did not compromise the principal arguments being made in either of the [2013 BMJ Articles]" adding:
- Ostensibly, this was an independent review of the publication of the 2013 BMJ Articles. Certainly, Mr Calman did not suggest that there was any reason he had to doubt the integrity of the review or its conclusions. Objectively judged, and borrowing Lord Nicholls' description, this was "an investigation which command[ed] respect", at least so far as acknowledging its existence and conclusions in the 'statin debate'.
- And Dr Godlee had made a further contribution to the statin debate, of which Mr Calman was aware following receipt of the First Claimant's right-to-reply response. After the publication of The Lancet Review (see [62] above; regarded by Mr Calman as a key document), in the edition of The BMJ published on 15 September 2016, Dr Godlee had called for an independent review of statins (see [213] above). Her article, whilst describing the benefits of statins for secondary prevention or in people at high risk of cardiovascular disease as "undisputed", suggested that proposals to offer statins to people at lower risk "remained controversial" and that there was evidence that the side effects of statins were "much more common than the trials suggest". She also referred to a suggestion, made on a BMJ blog, that reported side‑effects of statins "are too prevalent and recurrent in people who desperately want to stay on statins" and that "rather than discount a widely observed phenomenon, we should ask why there is such a mismatch with reporting in the trials." Dr Godlee asked, could this mismatch "be due to exclusion of people who experienced side effects during 'run-in periods' before randomisation?".
- In a pointed reference to the dispute with Professor Collins over the 2013 BMJ Articles, Dr Godlee added (with links removed):
- Against that full background - to which Mr Calman had been specifically referred and was readily available to him - the Main Article's summary of the dispute over the 2013 BMJ Articles in [46]-[50] was seriously misleading. Not only did it misrepresent the terms on which the 2013 BMJ Articles were withdrawn, it incorrectly stated the corrected figure for those reporting side-effects as 9% (whereas the actual figure was 17.4%). The Second Claimant had made the point about the figure being 17.4% in an article published on his Blog upon which Mr Calman had relied: see Annex 4(B)(2)(v). The 9% figure was the estimated number of people who had discontinued statin use as a result of their reported experience of side-effects (see [207] above). No doubt, there remained valid criticisms that could be made of the conclusions that could be drawn from the 2013 BMJ Articles, and the reliability and resilience of the 17.4% figure (several of which were articulated in Main Article), including that it was an observational study, but, in the Articles, Mr Calman failed fairly to represent the information and evidence he had about the dispute over the 2013 BMJ Articles. At its most basic, the materials supplied to Mr Calman by the Claimants showed that those expressing concerns about the side-effects of statins went beyond a "noisy group of sceptics" and, importantly, included a respected medical journal.
- Of wider significance, the public stance of the editor of the BMJ was perhaps the clearest indication that there was a significant, genuine and public debate as to statins between the two leading medical journals published in the UK. The Main Article referred to a "noisy group of sceptics" [8] and a "tiny minority of statin deniers" [10] (both of which would have been understood to have included the Claimants among their number). Mr Calman's failure to acknowledge in the Articles that some of the criticisms being advanced by the Claimants were shared and being echoed publicly by the editor of The BMJ, particularly when both Claimants had placed express reliance on this fact in their right-to-reply responses, was a serious omission which again contributed significantly to the lack of overall balance. As I have already noted, it also had a significant impact as to the essential message that would have been obvious that the Articles were conveying to readers about the Claimants.
- In addition, the very public dispute between Professor Collins and The BMJ should have also given Mr Calman pause to consider whether the experts with whom he was working, and upon whom he was so reliant, had reason to want to attack the Claimants.
- Dr Godlee had openly criticised Professor Collins for attempting to "shut down" public debate on statins. I can reach no conclusion, in this judgment, as to whether her criticism was well-founded or not, not least because Professor Collins has had no opportunity to respond to the charge, and the nature of the exercise upon which I am engaged also means that it is not relevant to resolve this issue (see [40]-[41] above). What is important, however, was, as Mr Calman must have realised, there had been a very public row between Professor Collins and Dr Godlee which had culminated in her alleging that Professor Collins was attempting to silence critics. That fact was relevant, at least, to Mr Calman's assessment of whether there was any substance to the complaint, made by both Claimants in their right-to-reply responses, that a similar effort was being made to silence them too, and whether there was a risk that the experts with whom he was working, and upon whom he was so reliant, were using the Articles as a platform to discredit the Claimants (and others); whether there was a risk that they had "an axe to grind". The Second Claimant had expressly drawn Mr Calman's attention to the stance of the BMJ on this point in his right-to-reply response ([16]-[20]) (see also the First Claimant's response [11]). Mr Calman's summary of the Claimants' objections that they were being similarly bullied omitted reference to Professor Collins and did not adequately convey the substance of the Claimants' complaint and the basis for it (Main Article [50]). In the Editorial ([17]), he summarily dismissed their complaints as "self-pitying".
- Neither of the matters the First Claimant had raised in her right-to-reply response was included in the Articles. Yet, her point about patient information leaflets was one that was simple, readily able to be comprehended by readers, and was capable of at least raising a question as to whether the incidence of side-effects from statins was "vastly overstated" (or non-existent), as suggested in the Main Article ([52]-[59], and headline) "Side-effects are down to worry, not statins". If the patient information form for a commonly prescribed statin stated that up to 10% of those taking the statin medication might experience a range of specified side-effects, how did that square with Professor Baigent's published claim that the rate of side-effects was less than 1% ([54])? There is no indication that Mr Calman ever resolved that issue himself, with Professor Baigent (or anyone else), and he included no reference in the Articles to the patient information form upon which the First Claimant had relied.
- Mr Calman knew from Dr Godlee's criticisms (see [439] above) that there was a persistent and widespread concern - going beyond the "noisy group of sceptics" - that there was a greater incidence of side-effects from statins than Professor Collins and his colleagues in the CTT were prepared to accept. The issue of side-effects was not straightforward, as it potentially impacted the various age groups differently (as The Lancet 2019 Study had expressly acknowledged). The First Claimant had made the point in her Blog Article "Statins in the over 75s". The Lancet 2019 Study data raised questions as to whether there was evidence of benefit among patients older than 75 years who did not already have evidence of occlusive vascular disease. For this specific group, if there was "less direct evidence" of the benefits of statins then, the incidence of side-effects became a potentially more important factor. This was another aspect that made the statin debate more complicated.
- Finally, in relation to the First Claimant's "Statins in the over 75s" Article, Mr Calman never satisfactorily resolved whether her claim that there was no support in The Lancet 2019 Study for Professor Baigent's claim that some 8,000 deaths would be prevented if the over 75s regularly took statins (see [77]-[80] above). He frankly accepted that he lacked expertise in analysing statistics to reach a view whether the First Claimant's analysis and conclusion as to what the data from The Lancet 2019 Study showed was correct (or even plausible). Mr Calman did not apparently challenge Professor Baigent with the First Claimant's specific challenge to the accuracy of the claim he had made and whether it was supported by The Lancet 2019 Study, nor include in the Articles the First Claimant's claim that Professor Baigent, in making this specific claim, was guilty of spreading misinformation about the benefits of statins.
- The Main Article dealt with this topic in [60]-[67].
- Mr Calman did put the allegation, contained in paragraph [60], squarely to the Second Claimant in his right-to-reply email. However, the Second Claimant's response went further than Mr Calman included in the Main Article. Mr Calman had suggested to the Second Claimant that he had been told by the CTT that they did not have data on the side-effects of statins, and so the Second Claimant's repeated claim that the CTT was withholding the data "amounts to a lie". The Second Claimant responded that he had never had any communication from the CTT. Of more significance, the Second Claimant also asked Mr Calman, if the CTT did not hold the relevant data, how had they managed to publish papers on the side-effects of statins? The Second Claimant referred Mr Calman to The Lancet Review as one example (see [62] above). Quoting from the Review, the Second Claimant asked: "So, they have written a paper outlining all of the issues of adverse effects and serious adverse effects - and yet they do not have the data. So, how did they manage that?". There is no evidence that Mr Calman ever resolved this point prior to publication. He did not include the point made by the Second Claimant in the Articles.
- Although Mr Calman had not included, in his right-to-reply email to her, the same point about false claims of withholding data, the First Claimant nevertheless referred to her own efforts to gain access to trial data. She also provided details of other complaints about the CTT refusing to do so (see response [1]-[3]). In particular, the First Claimant referred to Dr Godlee's article, published in The BMJ on 15 September 2016: "Statins: we need an independent review" (see [213] above). In her article, Dr Godlee, the editor of The BMJ, had called for "independent third party scrutiny of the statins trial data" which she said "remains an essential next step" in the resolution of an "increasingly bitter and unproductive dispute [over statins]". Quoting another researcher in the area, and in an obvious reference to CTT, she added "sharing the individual patient level data from the statin trials would send 'a strong message that no single person or group should have exclusive access to data' that are so important for public health". Pointedly, she questioned the independence of the CTT and suggested that they had a "vested interest in the debate being shut down".
- Again, I make clear, I am not adjudicating upon whether Dr Godlee's criticisms of the CTT were valid or not. That is not the process on which I am engaged (and I refer again to [40]-[41] above). The issue is what Mr Calman knew about the wider statin dispute and whether, in the public interest, he should have included more of the material relied upon by the Claimants in their defence of serious charges against them in the Articles.
- In the Main Article ([62]-[63]), Mr Calman quoted Professor Collins giving an explanation about the non-availability of data on adverse events. Importantly, he included Professor Collins' allegation that those claiming that the CTT was withholding data on side effects were "lying". In context, the way in which this was presented in the Articles - and the absence of any reference to the point raised by the Second Claimant - would have made it appear to readers that Professor Collins had given the definitive answer on trial data on side-effects: there was no data to share. As Mr Calman knew, there was again more to this issue than he made it appear in the Articles.
- In my judgment, on this issue as well, it was clear to Mr Calman that there had existed a significant public controversy about the availability of trial data held by CTT, again not limited to "a noisy group of sceptics", that had achieved the prominence of an editorial published in one of the UK's major medical journals. He should have included more information about this wider dispute in the Articles because it was an essential part of the Claimants' rebuttal of the charge that they were guilty of spreading misinformation. At its most basic, consideration of whether they were guilty of spreading misinformation, required at least some consideration of whether (by the same reasoning) The BMJ was similarly guilty. Inclusion of this material would not only have been important for proper balance, it would also have had a material contribution to whether the Articles were alleging that the Claimants were honest, but mistaken fools, or dishonest knaves.
- As noted already, this was not a topic that was given a separate heading in the Main Article, but it was an allegation that was made (see [31] Main Article) and it was one which Mr Calman had specifically put to the Claimants in his right-to-reply email for their comment and response.
- In the published Articles, the Second Claimant was quoted by Mr Calman as having "admitted" that he had relied on an observational study (see [35] Main Article). In cross-examination, Mr Calman seemed to regret the choice of word "admitted". More generally, Mr Calman did not fairly represent what the Second Claimant had said in answer to this point in his right-to-reply response. The First Claimant specifically asked Mr Calman for an example of when she was alleged to have relied upon observational studies, but Mr Calman did not respond.
- In the foregoing paragraphs, I have analysed the information that Mr Calman had prior to publication, including the important right-to-reply responses of the Claimants. My conclusion is that in respect of the four key charges of "fake news" levelled at the Claimants, the Articles did not properly or fairly reflect the totality of the material that Mr Calman had. In significant respects, some of this material was misrepresented. Largely this was a product of the limited time that Mr Calman left himself to consider the responses that the Claimants provided, but this is no excuse. Mr Calman's attitude also had a bearing on how he approached this important task. Mr Calman referred to the First Claimant's response as "unhinged", less than 3 hours after he had received it. Whilst this was an unguarded remark, and is now regretted, taken with the other evidence it does accurately encapsulate Mr Calman's attitude to the right-to-reply responses of the Claimants. He did not consider the Claimants' responses, or engage with them, in any depth or detail. He dismissed them. The direct result of this was that readers were deprived of important context and balance. They were misinformed about the statin debate, and the Articles failed fairly to present fully what the Claimants had said in their own defence. This was a fundamental failure on Mr Calman's part, and, in my judgment, it was not reasonable to believe that it was in the public interest to publish such serious allegations against the Claimants in the Articles with this editorial foundation.
- Although I readily accept that the issue of alleged misinformation regarding statins was one of significant public interest, it does not serve the public interest for a widely read newspaper to misrepresent important facts relating to the statin debate and to deny readers important details of the material upon which the Claimants had relied in their own defence of serious charges of wrongdoing. There is perhaps a palpable irony in the fact that the Defendants, in Articles that so roundly denounced those alleged to be the purveyors of misinformation, so seriously misinformed their own readers.
- I have made my findings regarding Mr Calman's understanding of the meaning of the Articles (see [250] and generally Section F(10) above). As I noted there, the choices made by Mr Calman in the editorial process, the language he used, and how he presented the material he had in the Articles (examined in this section of the judgment) had a significant impact on the meaning of the Articles that Mr Calman did understand (or reasonably could have been expected to have understood) the Articles to bear. Based on the facts I have found in the earlier paragraphs of this section of the judgment, the material that the Defendants had prior to publication, and the way that this material was presented to readers, it was not reasonable to believe that it was in the public interest to publish an article that contained the very serious allegations I have found that Mr Calman either did perceive or, as a reasonable journalist, he should have done. Most seriously for the Defendants' public interest defence, I have found that Mr Calman did not believe that the Claimants were dishonest, yet this was the core allegation that the Articles made against them, as Mr Calman must have known (or a reasonable journalist in his position would inevitably have realised). Although perhaps obvious, it is not reasonable to believe that it is in the public interest to publish claims that a journalist or publisher does not believe to be true.
- Finally, and for completeness, it was not reasonable to believe that it was in the public interest to publish:
- I accept that The Lancet 2019 Study was a significant milestone in the 'statin debate'. It may well have justified an attack on the reliability of many of the Claimants' claims. But it did not lay to rest some of the questions that the Claimants (and others) had raised, and it certainly did not demonstrate that the Claimants were "liars".
- Mr Calman set out to publish a "polemical" "takedown" of the Claimants. He achieved that, but, in my judgment, his belief that publication of the Articles was in the public interest was not reasonable. Therefore, my conclusion is that the public interest defence for the print publication of the Articles fails, and it will be dismissed.
- The Defendants have established this element of the public interest defence in relation to Online Publication 1. I repeat paragraphs [404]-[406] above.
- The Defendants have established this element of the public interest defence in relation to Online Publication 1. I repeat paragraphs [407]-[408] above.
- The Defendants have failed to establish this element of the public interest defence in relation to Online Publication 1. The underlying journalistic enterprise that went into the publication of the Articles is indivisible. There may be cases in which the decision as to a public interest defence for individually published articles is different from the decision when they are assessed collectively (for example if different personnel were involved in the research, preparation and publication of individual articles, and the belief of different people that publication was in the public interest is relied upon to support the defence) but that is not this case. Mr Calman wrote two of the Articles and he accepts responsibility for the third. The Defendants have not advanced an argument that, for example, even if the public interest defence fails for the Main Article and the Editorial, it should succeed for the News Article.
- In my judgment, the public interest defence for the original online publication of the Articles stands or falls with the public interest defence relied upon for the original publication of the Articles in the print edition. Therefore, for the reasons set out in paragraphs [409]-[460], the public interest defence fails for the original publication of Online Publication 1.
- As the public interest defence for the original publication of Online Publication 1 has failed, this issue does not arise for determination.
- As the public interest defence for the original publication of Online Publication 1 has failed, this issue does not arise for determination.
- Relying upon the reasons set out in paragraphs [464]-[467], the public interest defence for the original and continuing publication of Online Publication 2 fails.
- Relying upon the reasons set out in paragraphs [464]-[467], the public interest defence for the original and continuing publication of Online Publication 3 fails.
- My decision on (at least) issue (15) means it is not necessary for me to resolve this issue.
- The Defendants have proved this element of the defence. I am satisfied that the Hancock Statement was a notice or other matter issued for the information of the public on behalf of the Secretary of State for Health and Social Care ("Health Secretary") discharging his functions as a Minister of the Crown on behalf of the government.
- The Hancock Statement sent by Ms Wilson to Mr Calman on 28 February 2019 was an 'on the record' statement issued on behalf of the Health Secretary. Ms Wilson knew and intended that it would be published in a national newspaper as a statement made by Health Secretary. In that respect it was an official statement made. It was not gossip and it was not an 'off-the-cuff' remark. It was a statement that was intended to be made available for the information of the public.
- I reject the Claimants' argument that to qualify under Schedule 1, paragraph 9, that the relevant notice or other matter must be issued generally to the press for reporting and that issuing it only to one newspaper is not sufficient to qualify as issuing the notice or other matter "for the information of the public". That would be to take an unduly restrictive approach to the purpose underlying the reporting privilege.
- My decision on issue (14) means that this issue does not arise.
- The Defendants have proved this element. Looked at in isolation, the references to the Hancock Statement in the Print Publication were an extract from or summary of a notice or other matter issued for the information of the public on behalf of the Health Secretary discharging his functions as a Minister of the Crown on behalf of the government.
- My decision on issue (14) means that this issue does not arise.
- The Defendants have failed to establish this element of the defence. The references to the Hancock Statement in the Print Publication were not fair or accurate. The background to the Hancock Statement is set out in paragraphs [134]-[165] above and my findings, relevant to this issue and generally, are set out in paragraphs [166]‑[176]. Had it not been for the parties' agreement as to the issues I was asked to decide, I make clear that, based on these findings, I would have also found that the publication of the extracts from (or summaries of) the Hancock Statement in the Articles was not for the public benefit under s.15(3) - because they fundamentally misrepresented to readers what Mr Hancock had said.
- In consequence, the qualified privilege defence in respect of these parts of the Print Publication fails and is dismissed.
- My decision on issue (19) means that this issue does not arise.
- My decision on issue (19) means that this issue does not arise.
- My findings on the fairness and accuracy of the references to the Hancock Statement in the Print Publications apply equally to the Online Publications in which they appeared (see [477] above).
- In consequence, the qualified privilege defence in respect of these parts of the Online Publications fails and is dismissed.
- Although this issue is not agreed by the Defendants, my decision on issue (22) means that it does not arise for decision.
- My decision on issue (22) means that this issue does not arise.
- As preliminary points, the Claimants contend:
- Nevertheless, the parties have agreed that these points should not be determined by the Court, with the Claimants' position on these points being reserved for the purposes of any appeal. I make no comment as to whether the Court of Appeal would be willing to deal with any such points on appeal, if they arose. Ordinarily, parties are expected to submit all points that are contentious between them for determination at first instance. Nevertheless, I will attempt to resolve the issues that the parties ask me to determine.
- For the reasons set out in Section G(3) above, I have rejected the Claimants' argument as to the statutory construction of s.6(5). If the Defendants can establish the necessary elements of the defence, they are entitled to rely on s.6(5).
- On the evidence, I conclude that The BMJ was a journal with more than one editor when it published the LSHTM Paper. The documentary evidence, none of which was challenged by the Claimants, demonstrates that Dr Fiona Godlee was the overall editor, and Dr Wim Weber was the European Editor. Others also held the title of editor, e.g. Professor Elizabeth Loder: see [181(1)].
- On the evidence, the editor who was responsible for deciding to publish the LSHTM Paper was Dr Weber. Although Dr Godlee, by dint of her position, is likely to have had ultimate editorial control, there is no direct evidence available to suggest that she took any decisions regarding publication of the LSHTM paper.
- I am satisfied that the Defendants have demonstrated that there was an independent review of the LSHTM Paper's scientific or academic merit.
- The paper had been the subject of The BMJ's peer review process, which identified some questions from the manuscript committee, of which Dr Weber was a member ([179(2)] above). Dr Weber and the members of the manuscript committee who reviewed the LSHTM Paper were independent of the authors of the paper, and had signed declarations to that effect ([179(6)] and [184] above) (Insofar as the Defendants have not produced signed declarations for every member of the manuscript committee I would draw the inference either (a) that all members had signed such declarations; or (b) that the practice of declaring conflicts was well understood and followed). It is clear on the evidence that they carried out an assessment of the scientific or academic merit of the LSHTM Paper prior to publication.
- I do not consider that there is any substance to the Claimants' point that there is any real issue as to whether those reviewing the LSHTM Paper had sufficient expertise in "scientific or academic matter" for the purposes of s.6(3)(b). It will be an unusual case where it can be seriously suggested that a reputable scientific or medical journal has sent a paper or study to be peer-reviewed by people who do not have expertise in the relevant scientific of academic matter. Perhaps more unusual still, for people who did not have the necessary expertise nevertheless to purport to carry out the review. If a claimant wants to raise such a challenge, s/he will need to do so and then produce evidence of why ostensibly qualified people do not actually possess the necessary expertise. Here, I am satisfied that those who peer-reviewed the LSHTM Paper did have the necessary expertise.
- I am satisfied that the independent review of the LSHTM Paper's scientific or academic merit was carried out by the manuscript committee which included Dr Weber who was the editor responsible for deciding the publish it in The BMJ.
- Given that the Claimants have adduced no evidence to challenge the expertise of the manuscript committee, I do not consider that it is necessary to address this issue and I decline to do so. The Defendants have demonstrated that the requirements of s.6(3)(b) have been met. All that attempting to define the scientific or academic matter with which the LSHTM Paper was concerned would produce is a potential appeal point. I should resolve only those matters that are necessary in order to resolve the issues in dispute.
- The independent review of the LSHTM Paper's scientific or academic merit was carried out by the manuscript committee. Based on the evidence relied upon by the Defendants (set out in [183] above), I am satisfied that at least these two members of the manuscript committee demonstrably had expertise in the scientific or academic matter presented in the LSHTM Paper. It is likely that other members also had such expertise, but it is not necessary to go further than this for the purposes of resolving this issue.
- I am satisfied that the parts of the Articles highlighted in green in Annex 1 and marked with dotted underlining in Annex 2 are an extract from or summary of the LSHTM Paper.
- The LSHTM Paper was quoted and relied upon in the Articles ([6] News Article; [14] and [48] Main Article; and [17] Editorial). The summary of the LSHTM Paper (albeit very abbreviated) in paragraph [6] of the News Article, paragraph [14] in the Main Article, and paragraph [17] in the Editorial was fair and accurate.
- In paragraph [48] of the Main Article, the Defendants do not seek to defend the words "which included public statements from Dr Kendrick and Dr Harcombe" as being part of the report of the LSHTM Paper. The fact that the Defendants do not seek to defend the excised words as privileged, no doubt, results from a recognition that they cannot be. The LSHTM made no finding that the media coverage during the study period included public statements from the Claimants. As I have noted (see [432] above), the additional words referring to the Claimants' public statements were simply added by Mr Calman shortly before publication.
- In my judgment, it is not open to the Defendants to 'blue pencil' what is sought to be defended as a privileged report in this way. Paragraph [48] - in its description of the LSHTM Paper - was obviously presented to readers as a summary of the LSHTM Paper, including the words that the Defendants have removed. No reader would have excised those words from the summary that was presented to him/her. S/he would not have known from what was presented in paragraph [48] that the LSHTM Paper had not included this information.
- In the alternative, if it is permissible to excise the words that the Defendants have removed from this paragraph from the scope of the claimed privilege defence, the defence of the balance of the paragraph nevertheless fails because the words that were added by Mr Calman renders the extract/summary sought to be defended neither fair nor accurate. Either way, the privilege defence for paragraph [48] fails.
- I have upheld the Defendants' privilege defence in relation to paragraph [6] of the News Article, paragraph [14] in the Main Article, and paragraph [17] in the Editorial.
- In relation to those publications, the Claimants' plea of malice fails. For the reasons explained in section G(4) above, I have rejected the Claimants' submission that malice, in the form of a proved (improper) dominant motive of providing an "endorsement" of allegations made against the Claimants in the Article is capable of defeating a statutory reporting privilege. In any event, on the evidence, the Claimants have not demonstrated that Mr Calman had such a motive. Such a motive was never put to Mr Calman when he was cross-examined and there is nothing in the other evidence (beyond supposition) to support that he had such a motive.
- As I have noted, the authorities make it plain that malice is a serious allegation, akin to dishonesty. It is not to be made in litigation as an expedient. In this judgment, although I have criticised some of the decisions Mr Calman made, I am quite sure that he approached his task honestly. As perhaps the Claimants should readily appreciate, there is a material difference between being mistaken and being dishonest. Mr Calman is not dishonest, and given the malice plea he has had to face, it is right that I say so clearly. The malice claim in relation to the Print Publication is dismissed.
- My decision in relation to issue (32) (see [496] above) also determines this issue.
- I have already found (issue (33)) that the summaries of the LSHTM Paper (albeit very abbreviated) in paragraph [6] of the News Article, paragraph [14] in the Main Article, and paragraph [17] in the Editorial were fair and accurate, but the summary of the LSHTM Paper in paragraph [48] of the Main Article was not fair or accurate (see [497]‑[500] above).
- Applied to the Online Publications:
- The malice plea in relation to the Online Publications fails for the same reason as the Print Publication (see [502]-[503] above). In consequence, the malice claim in relation to the Online Publications 2 and 3 is dismissed.
- This specific point was not explored in the cross-examination of any witness. I do not consider that a person would be malicious to continue to publish Online Publications 2 and 3 after receipt of the letter of 31 January 2020.
- Before turning to the remaining issues, I should repeat that the exercise of determining the objective single natural and ordinary meaning of the Articles, and whether the Articles are or contain allegations of fact and/or expressions of opinion is wholly different and distinct from the assessment of Mr Calman's subjective assessment of the meanings that the Articles bore (see [247] above). I have necessarily had to deal with many issues, in great detail, in the earlier part of this judgment. I put all of that to one side. Save for necessarily identifying those passages of the Articles that I have found to be privileged (or are agreed by the parties to be privilege) for the purposes of Curistan, the resolution of these other issues has no bearing on the objective assessment of the natural and ordinary meaning of the Articles and fact/opinion. In that respect, my task in the next part of the judgment is to apply the well-established principles as set out in G(5) ([380]-[387] above).
- In doing so, I have stepped back, and read the Articles as an ordinary reader would have done. I did this, of course, back in 2022, before the hearing that led to the PIT Judgment. Having read the Articles I captured broadly the messages that they conveyed to me. I then looked at the parties' respective meanings, and considered the impact of Curistan on any of the meanings I had identified. It has taken me some time to finalise this judgment (longer than I would have hoped), but one benefit is that it has allowed me to return to consider afresh the Articles on the issue of meaning, divorced from the detailed consideration of the other issues that had to be resolved.
- In my judgment, the News Article, Main Article and the Editorial, as they appeared in the Print Publication, are to be treated as forming a single publication for the purposes of determining the issues of the natural and ordinary meaning and fact/opinion.
- There can be no doubt that the hypothetical ordinary reasonable reader would have read the Main Article and the Editorial together, as they were presented to readers as a single package. A reader that read only the Editorial or the Main Article would not be reasonable. The issue is whether the hypothetical ordinary reasonable reader who read the News Article would also read the Main Article and the Editorial. In my judgment, s/he would have done. The Articles appeared in a single print edition of the newspaper. They were therefore practically made available to all readers. The Articles shared the same common theme and subject matter, and were presented and intended to be read together.
- The clearest demonstration of that is that readers were specifically directed to the Main Article and Editorial by a large bold directional graphic with the text "'EXPERTS' BEHIND SCARE STORIES - AND WHY THEY'RE WRONG Pages 47-50" which appeared at the foot of the News Article. As Dee makes clear, the fact that some readers would not have read the Main Article and Editorial, notwithstanding the direction/invitation to do so, is not material to the decision on whether the notional hypothetical ordinary reasonable reader would have done. In my judgment this is a very clear case of the law treating the three Articles being treated as a single publication.
- As I shall go on to identify, and explain, in resolving issue (43) the Articles in the Print Publication contain both defamatory allegations of fact and defamatory statements of opinion concerning the Claimants.
- The result of my findings and the parties' agreement on the issue of privilege mean that Curistan applies to the part of paragraph [6] (shown in green in Annex 1 and dotted underlining in Annex 2) and paragraph [7] of the News Article; paragraph [14] of the Main Article; and the part of paragraph [17] (shown in green in Annex 1 and dotted underlining in Annex 2) in the Editorial.
- Treating the News Article, the Main Article and the Editorial as a single publication for these purposes, in my judgment the single natural and ordinary meaning that the Articles bears is:
- Meanings (1) to (3) are allegations of fact. Meaning (4) is an expression of opinion.
- The Articles, particularly the Editorial, contained significant and plainly recognisable expressions of opinion, including deductions and conclusions. In particular, the language used to condemn the Claimants would readily have been understood as an expression of opinion. I have sought to capture these elements in meaning (4).
- Nevertheless, the premise of this opinion - the base factual allegations made against the Claimants that form meanings (1) to (3) - would also have been obvious to the hypothetical ordinary reasonable reader. A reader would not need to assemble the allegations for him/herself; they were clearly identified and presented. The Articles alleged that the Claimants had repeatedly made a series of statements. I am satisfied that the Defendants' articulation of those statements - in their pleaded meaning - is a fair summary. These claims are therefore identified in Paragraph (1)(a)-(d). Readers would have had no difficulty understanding that these were the statements that the Claimants had made. Next, equally unmistakeable is the allegation that these statements were false; this was the "fake health news" for which the Claimants were responsible. Finally, I am satisfied that the hypothetical ordinary reader would have understood that the Articles, read as a whole, were alleging that the Claimants knew that the statements that they had made were false. In other words, that they were dishonest.
- Meaning (2) raises an issue of the correct Chase level for this allegation. There can be no real dispute that the Articles raise the issue of the Claimants' motivation, directly in the Text Box and indirectly in the presentation of this issue in the Articles. The question is what would the ordinary reasonable reader conclude was being alleged. In my judgment, the use of the question in the final paragraph of the Text Box, draws the reader back from concluding that this is the Claimants' motive. Rather, the effect overall is to suggest that there are strong grounds to suspect that this is. I have used "strong" because the question comes belatedly at the end of the material that would otherwise have suggested that the Claimants did have this motivation. Nevertheless, the reader would not have failed to notice that Mr Calman did raise the question. Overall, the effect is that the meaning conveyed is that there were strong grounds to suspect that this was the Claimants' motive for their false statements about statins.
- Meaning (3) is hardly contentious. The Articles were obviously telling readers that the consequence of the Claimants' false statements was that a very large number of people had given up taking statins and, as a result, they were at risk of harm (including death) from stroke and heart attack. This was a core allegation of the Articles.
- Meaning (3) is the single area where Curistan has had any impact. Doing the best I can to apply Curistan, I have not included in the meaning a specific figure for the number of people that the Articles claimed had given up taking statins as a result of the false information ("scare stories") about statins. The Articles do contain the specific figure of 200,000 people who gave up statins in a six-month period (News Article [6]; Main Article [14]), but this comes from the LSHTM Paper, and I have found these paragraphs to be protected by reporting privilege. Having regard to (a) the message conveyed by the balance of the Articles; (b) appropriate use of the privileged paragraphs as "context"; and (c) the example of Colin, I have concluded that the meaning that the ordinary reasonable reader would have understood, in respect of the harm caused by the Claimants' false statements was that they had caused a very large number of people not to take prescribed statin medication who were thereby exposed to a serious risk of a heart attack or stroke causing illness, disability or death.
- As between the parties, it appears that the only real issue of contention, so far, is the allegation of dishonesty in meaning (1). This features as part of the Claimants' pleaded meaning, whereas the Defendants contend that, in publishing the false statements, the Articles were alleging that the Claimants were "reckless" and "irresponsible", not that they were "dishonest".
- In determining meaning, the most important aspect is how the Articles, in what they said, and how they were presented would have struck the ordinary reasonable reader. It is not only what an article says, but also a matter of how, overall, it is presented. That includes headlines, which can have a significant effect on shaping a reader's understanding of the message that is being conveyed, captions and logos. Another important aspect, where (as here) readers are being presented with a dispute, can be whether the article signals to readers which side of the dispute is to be believed or trusted. There are many ways that a dispute can be reported. How that is done will usually have a significant effect on the overall meaning.
- In my judgment, the Articles very clearly conveyed the allegation that the Claimants' statements were lies; i.e. known by them to be false. The factors that contribute to that meaning are:
- These five strands, in my judgment, would have left no doubt in the ordinary reasonable reader's mind that the Claimants were being branded dishonest for the lies that they "peddled" about statins.
- I have not included, in the meaning I have found, that part of the Second Claimant's meaning that contended that the Articles bore a meaning that he was "unfit to be licensed to practise medicine" (see [11(2)] and [14(2)] above). This is neither stated, nor implied, by the Articles, not even in the Editorial where the brunt of the criticisms of the Claimants is to be found. It is an extrapolation - or deduction - that, in my judgment, goes beyond the natural and ordinary meaning of the Articles.
- This issue does not arise for decision given my determination of issue (41).
- As I shall go on to identify, and explain, in resolving issue (46), Online Publication 1 contains both defamatory allegations of fact and defamatory statements of opinion concerning the Claimants.
- Online Publication 1 is substantially in the same terms as the Print Publication. Save for one point, no party has suggested that the small changes in layout, photographs, captions etc. makes any real difference to the decisions over natural and ordinary meaning and fact/opinion.
- The only point of significant difference between Online Publication 1 and the Print Publication, in terms of content, was that absence of the Text Box from Online Publication 1. That does have a real impact on the issues to be determined. In terms of presentation, the order in which the articles would have been read in Online Publication 1 would have been different. Readers were presented with the Main Article, then the News Article and finally the Editorial. This has the potential to have a bearing on the issue of meaning.
- In my judgment the single natural and ordinary meaning that Online Publication 1 bears is:
- Meanings (1) and (2) are allegations of fact. Meaning (3) is an expression of opinion. The absence of meaning (2) from the Print Publication reflects the absence of the Text Box.
- Online Publication 1, particularly the Editorial, contained significant and plainly recognisable expressions of opinion, including deductions and conclusions. In particular, the language used to condemn the Claimants would readily have been understood as an expression of opinion. I have sought to capture these elements in meaning (3).
- Nevertheless, the premise of this opinion - the base factual allegations made against the Claimants that form meanings (1) and (2) - would also have been obvious to the hypothetical ordinary reasonable reader. I repeat and adopt the analysis in [519] above.
- In respect of meaning (2), I repeat and adopt the analysis in [521]-[522] above.
- The issue of the element of dishonesty in meaning (1) is common to the Print Publication and Online Publication 1 (see [523] above). I repeat and adopt the analysis in [524], and [525(1)-(3)], [525(5)] and [527]. In respect of Online Publication 1, these four strands, in my judgment, would have left no doubt in the ordinary reasonable reader's mind that the Claimants were being branded dishonest for the lies that they "peddled" about statins.
- In resolving this issue, it is important to concentrate on how the link to Online Publication 1 was presented to readers in Online Publication 2. It appeared like this:
- The link to Online Publication 1 was advertised to readers of Online Publication 2 in between paragraphs [6] and [7] of the text of the News Article, under the heading "RELATED ARTICLES". The left hand-link was trailed with the words "The deadly propaganda of the statin deniers: The drugs DO..." and the right-hand link referred to an unrelated article apparently about the consequences of "Going vegan".
- Applying the test in Dee, and particularly having regard to the principles in Poulter -v- Times Newspapers Ltd (see [386] above), I am not satisfied that the ordinary reasonable reader would have clicked through to read Online Publication 1.
- Whether the hypothetical ordinary reasonable reader would follow a hyperlink, such as to make it right to treat the hyperlinked material as part of the same publication, is context specific and depends very much on how the link is presented. Here, there was no exhortation to follow the link in order to know more. There was not, in contrast with the Print Publication, the same direction to read a separate article (see [513] above). Indeed, there was no more by way of encouragement to the reader to follow the link to Online Publication 1 than there was to follow the link to find out more about why "Going vegan sent me off my trolley". The link to Online Publication 1 was not embedded in the text of Online Publication 2 - the link was separated from the text of the article by two sets of tramlines, and a heading "Related Articles". I accept that a more curious or more interested reader might have followed the offered hyperlink to Online Publication 1, but the presentation of the link in the context of Online Publication 2 did not direct or encourage him/her to do so. Such a matter of personal choice, which varies reader by reader, very clearly indicates that it is not something that should be attributed to the notional ordinary reasonable reader.
- In consequence, for the purposes of determining the natural and ordinary meaning of Online Publication 2, it stands alone as a single publication and is not to be read together with Online Publication 1.
- Issue (47) has not been resolved in the Claimants favour, so issues (48) and (49) do not arise. The parties are agreed that if issue (47) is resolved in favour of the Defendants, Online Publication 2 should be excluded from further consideration. It is common ground that, if considered on its own, Online Publication 2 does not refer to the Claimants and so is not defamatory of either of them.
- In respect of those readers who did click on the hyperlink in Online Publication 2, and who also read Online Publication 1, the resulting publication is effectively of Online Publication 1 and Online Publication 2 bears, by innuendo, the same meaning, that I have found by way of natural and ordinary meaning for Online Publication 1.
- In consequence, and for ease of reference, Online Publication 2 bears the following innuendo meaning for those readers who followed the hyperlink and read Online Publication 1:
- Meanings (1) and (2) are allegations of fact. Meaning (3) is an expression of opinion.
- I repeat and adopt the analysis in [533]-[537] above.
- Resolution of issues (51) and (52) require the Court to consider only Online Publication 3 (the online publication of the Editorial). That requires me to put out of my mind the News Article and the Main Article. I have therefore started this exercise afresh.
- As I shall go on to identify, and explain, in resolving issue (52), Online Publication 3 contains both a defamatory allegation of fact and a defamatory expression of opinion concerning the Claimants.
- In my judgment, the natural and ordinary meaning of Online Publication 3 is:
- Meanings (1) is an allegation of fact. Meaning (2) is an expression of opinion.
- There is no doubt that, in Online Publication 3, readers were presented with robustly expressed opinion. Nevertheless, Online Publication 3 does contain a narrative of facts - primarily the case study of Colin - which is used as an example of a wider phenomenon of the significant health risks when people give up statins as a result of false allegations that statins do not work. The health risks of stopping taking prescribed statins and having a heart attack, like Colin, are spelled out in [12]: "illness, disability and death".
- The next factual allegation that comes across clearly is that doctors, including the Claimants have been making public claims that "statins don't work" (headline and [11]).
- There is also conveyed, more indirectly, but still made, that the 'statin deniers', including the Claimants, have been making other false claims that have undermined public confidence in statins. That emerges, particularly from the reference to whipping up controversy and peddling conspiracy theories in [14], and the reference to "insidious... fake news" in [16]. The reader is given no more information about this in the article. These parts of Online Publication 3 also contribute to the expression of opinion that would be recognised by readers (and forms meaning (2)), but there is also a clear (if rather unspecific) factual allegation underpinning it.
- Finally, in relation to the element of the meaning conveying dishonesty, or knowledge of falsity, in my judgment that emerges clearly from the comparison drawn (in [12]) between honest scientists, like Barry Marshall, who bravely (and, in context, honestly) stood up to and challenged medical orthodoxy, and the dishonest 'statin deniers' who "whip up controversy, peddle conspiracy theories, or sell diet books" and "peddle... a particularly insidious type of fake news, apparently from a respectable, credible source, but laced with misinformation". Readers were warned to "make no mistake" that the Claimants "are no Barry Marshalls". In context, the repeated use of the word "peddle" and the word "laced" (both active verbs) would also have contributed powerfully to the meaning that the Claimants were dishonest. No ordinary reasonable reader could think that someone, who had honestly published information about statins that turned out to be wrong, merited a "special place in hell". If the wrongdoing alleged against the Claimants was limited to castigating them for carelessly, even recklessly publishing false public statements about statins, then the terms of Online Publication 3 would have been materially different. As it is, the terms of Online Publication, and its strident and forceful condemnation, reinforces the message that the Claimants are dishonest knaves, not misguided fools.
- Having regard to the fact that I have upheld a reporting privilege for those parts of Online Publication 3 which are an extract from (or summary of) the LSHTM Paper, and doing the best I can to apply Curistan, I have not included in the meaning a specific figure for the number of people that the Articles claimed had given up taking statins as a result of the false information about statins. Online Publication 3 does contain a reference to "thousands of patients" having quit their statin medication ([18]) but I have found this reference to be protected by privilege. As there are no other indications in Online Publication as to the number of people who, like Colin, had quit statins, I have reflected this in the terms of meaning (1), and referred only to "people" having stopped taking statins as a result of the false information.
- Had it not been for Curistan, I would instead have found meaning (1) in the following terms (with the change shown in bold):
- Were it not for Curistan, I would have held that the effect of upholding the privilege in respect of the LSHTM Paper was not to affect the natural and ordinary meaning of the publication, but it would have relieved the Defendants, in any substantive defence where the point was relevant, of the need to prove, as a fact, that the effect of false information about statins was to lead to thousands of people to stop taking the medication. I have used shorthand, because the point I am making is illustrative.
- The finding that the Defendants did not believe that the Claimants were liars, or had published information that they knew to be false (see [173]-[174] above), and by necessary implication that he did not believe them to be "pernicious liars" has a very significant impact on the honest opinion defences advanced by (and, following this judgment, available to) the Defendants.
- In each of my findings as to the extent to which the statement complained of expressed opinion (see [516(4)] and [517], for the Print Publication of the Articles; [532(3)] and [533] for Online Publication 1; and [550(2)] and [551] for Online Publication 3), I have found (a) that the expressed opinion was consequent upon the defamatory allegations of fact made against the Claimants; and (b) (in respect of the Print Publication of the Articles and Online Publication 1) that each Claimant was "rightly to be condemned as a pernicious liar". As such, I find that Mr Calman did not hold the opinions that I have found were expressed in the relevant publications.
- For the reasons given in this judgment, the preliminary issue (identified in [36] above) are resolved as follows:
- The judgment is being handed down remotely. The parties will have a period of time to reflect on the judgment and are invited to agree consequential directions. If necessary, a hearing will be fixed at which any dispute as to the consequential directions can be resolved ("the Consequentials Hearing").
- Nevertheless, it is quite clear that, following this decision (and subject only to a successful appeal), the current defences of truth and honest opinion as advanced in the Defences cannot be maintained by the Defendants. The parties will need to provide a timetable for amendments to their statements of case to bring them into line with the decisions made on the preliminary issues. I formally adjourn any applications for permission to appeal and extend the time for appealing until 21 days after any application for permission to appeal has been resolved.
|
Section |
|
Paragraphs |
|
A. |
Parties |
[2]–[5] |
|
B. |
The Articles |
[6]–[8] |
|
C. |
Statements of Case |
[9]–[34] |
|
(1) |
Particulars of Claim |
[10]–[15] |
|
(2) |
Defence |
[16]–[30] |
|
(3) |
Reply |
[31]–[34] |
|
D. |
Order for the trial of preliminary issues |
[35]–[41] |
|
E. |
Evidence |
[42]–[47] |
|
F. |
The facts |
[48]–[269] |
|
(1) |
Mr Calman and the Claimants |
[50]–[54] |
|
(2) |
The genesis of the Articles |
[55]–[65] |
|
(3) |
Preparation for the Articles |
[66]–[105] |
|
(4) |
Mr Calman's research on the Claimants' claims | |
|
(5) |
The interview with Colin | |
|
(6) |
The Hancock Statement | |
|
(7) |
The LSHTM Paper | |
|
(8) |
Pre-Publication approaches to the Claimants and their responses | |
|
(9) |
Finalisation of the Articles for publication | |
|
(10) |
Mr Calman's understanding of the meaning of the Articles | |
|
(11) |
Post-publication response of the Claimants and request that the Defendants publish a statement by way of explanation or contradiction | |
|
G. |
Legal principles | |
|
(1) |
Public Interest | |
|
(2) |
s.15 Reporting privilege | |
|
(3) |
s.6 Privileged report of peer-reviewed scientific or academic journal | |
|
(4) |
Malice | |
|
(5) |
Natural and ordinary meaning and fact/opinion | |
|
H. |
Determination of the issues | |
|
I. |
Conclusion and next steps | |
|
The Articles as they appeared in the print edition |
| |
|
The text of the Articles |
| |
|
Text of various documents referred to in the judgment |
| |
|
Public claims made by the Claimants relied upon by Mr Calman |
| |
|
Agreed List of Issues to be determined following trial |
|
A: Parties
B: The Articles
(1) a news article, which was published in the print edition of The Mail on Sunday on page 2 of the newspaper, under the headline: "Statin deniers are putting patients at risk, says Minister" ("the News Article");
(2) a main article, which was published in a "Health, Wealth & Holidays" section of the newspaper on pages 47-50 of the newspaper, under the headlines: "Deadly propaganda of the STATIN DENIERS" (p.47), "'It's worse than the MMR scare' (pp.48-49) "'Side effects are down to worry, not statins'" (p.50) ("the Main Article"); and "REVEALED: TRUTH ABOUT THE THREE 'EXPERTS' WHO SAY DON'T TAKE STATINS" (p.48) ("the Text Box"); and
(3) an editorial, by Mr Calman which was published on page 50 of the newspaper, under the headline: "There is a special place in hell for the doctors who claim statins don't work" ("the Editorial").
C: Statements of Case
(1) Particulars of Claim
(1) in respect of each Claimant:
"(i) that s/he is a pernicious liar, who, for a venal as opposed to any proper, sincere motive, knowingly and deliberately disseminates to the public false information about statins in blatant contradiction of indisputable scientific facts; and
(ii) that by that conduct, s/he is needlessly:
(a) putting many thousands, if not millions, of people in Britain (like Colin Worthing) at a greater risk of a deadly or debilitating heart attack or stroke by misleading them into the false belief that statins do not work and/or have intolerable side effects, and thereby leading them to refuse or to abandon the treatment that has been definitively proven by medical science to benefit health in critical ways including by saving lives while causing insignificant side effects in the process; and
(b) contributing to a public health catastrophe with potential consequences in terms of preventable death and serious disability far graver than is resulting from the infamous MMR vaccine scandal involving disgraced paediatrician Andrew Wakefield - to whom [the relevant Claimant] it to be likened - who fabricated evidence to support his idea that the vaccine triggered autism in infants, leading to a decline in vaccination uptake and the resurgence of measles".
(2) and, additionally, in respect of the Second Claimant:
"(iii) that, in those circumstances, he is unfit to be licensed to practise medicine".
(1) in respect of each Claimant:
"(i) that for a venal as opposed to any proper, sincere motive s/he knowingly and deliberately disseminates to the public false information about statins; and
(ii) that by that damnable conduct, s/he is putting many thousands of people (like Colin Worthing) at a greater risk of a deadly or debilitating heart attack by misleading them into the false belief that statins don't really work and/or that drug companies are just trying to make money by getting everyone on statins, and thereby leading them to refuse or to abandon the treatment that has been definitively proven to reduce the risk of heart attack in people who have a ten per cent or greater chance of having a heart attack over ten years; and
(2) and, additionally, in respect of the Second Claimant:
"(iii) that, in those circumstances, he is unfit to be licensed to practise medicine".
(2) Defence
(1) in the print edition of the newspaper, (a) the News Article, and (b) the Main Article and the Editorial (defined as "the Health Section") would be read separately: "the two parts were not sufficiently closely connected as to be regarded as a single publication. A reader who read only the self-contained News Article and not the Health Section (or vice versa) would not be an unreasonable reader";
(2) in consequence, the News Article, read alone as a distinct publication, did not refer to the Claimants as they were not identified in it; alternatively,
(3) if the three print Articles are sufficiently connected to be regarded as a single publication, then it is admitted that they referred to the Claimants;
(5) Mr Calman denies that he was the author of the News Article, but admits that he caused the News Article to be published.
(1) honest opinion, pursuant to s.3 Defamation Act 2013;
(2) truth, pursuant to s.2 Defamation Act 2013;
(3) statutory qualified privilege, pursuant to s.15 Defamation Act 1996 ("s.15 reporting privilege");
(4) statutory qualified privilege, pursuant to s.6(5) Defamation Act 2013 ("privileged report of peer-reviewed scientific or academic journal"); and
(5) publication on a matter of public interest, pursuant to s.4 Defamation Act 2013 ("public interest").
"The Defendants admit that the First Defendant published, and the Second Defendant caused to be published, the articles complained of by the Claimants in The Mail on Sunday on 3 March 2019 and online on Mail Online and on a continuing basis... The Defendants deny that the articles bear the defamatory natural and ordinary meanings, or in the case of the [online publication of the News Article], the innuendo meaning, ascribed to them by the Claimants... The Defendants admit that the articles are defamatory at common law, save in respect of [the print and online versions of] the News Article... when read alone. No admissions are made as to whether the articles have caused or are likely to cause serious harm to the Claimants' reputations".
(a) Honest opinion
"(1) The Claimants are (each) among those who have published claims denying the health benefits of statins; specifically they have claimed that (a) cholesterol is not a cause of heart disease, (b) lowering cholesterol with statins produces a negligible benefit for patients prescribed statins, (c) the medical establishment (including pharmaceutical companies, charities, doctors, researchers and universities) is covering up the true extent of the side effects of statins, and (d) the medical establishment has conspired to silence the Claimants and other 'statin deniers', to cover up the truth (as presented by the Claimants and others), and to ensure as many people as possible take statins, in order to boost profits for the statin industry.
(2) The Claimants' (respective) behaviour in making such claims is irresponsible, reckless and deserves to be condemned, in light of:
(i) the overwhelming preponderance of expert medical opinion that statins prevent and/or lower the risk of heart disease and strokes and that they are safe (opinion for which the Claimants give no due regard); and
(ii) the likelihood of the Claimants' 'scare stories' fuelling confusion in patients or potential patients and thereby putting their health at risk by alarming them into ceasing or not starting to take statins.
(3) The Claimants' (respective) behaviour in (1) above is an example of medical misinformation or 'fake health news', the potential harmful consequences for public health of which far outweigh that of the infamous MMR vaccine scandal.
(4) (In relation to the Health Section in the print publication only) The Claimants' resolute persistence in making these claims, in the face of the strength of the expert evidence in support of statins, begs the question why they are doing it, whether they have too much to lose commercially or in terms of their high public profile to stop making their claims, and/or do profit and/or stand to profit by their stance".
(b) Truth
"(1) The Claimants were each reckless, irresponsible and condemnable in repeatedly making the following public claims, that (a) cholesterol is not a cause of heart disease, (b) lowering cholesterol with statins produces only a negligible benefit for patients prescribed statins, (c) the medical establishment (including pharmaceutical companies, charities, doctors, researchers and universities) is covering up the true extent of the side effects of statins, and (d) the medical establishment has conspired to silence the Claimants and other 'statin deniers', to cover up the truth (as presented by the Claimants and others), and to ensure as many people as possible take statins, in order to boost profits for the statin industry.
(2) The Claimants were each reckless, irresponsible and condemnable in this regard because (as they were or must have been aware);
(i) their claims were false and misleading;
(ii) the claims gave no due regard to the overwhelming preponderance of expert medical opinion, based on the highest quality evidence, that high LDL cholesterol causes an increased risk of [cardiovascular disease], statins prevent and/or lower the risk of heart disease and strokes and that they are safe; and
(iii) the likelihood of the Claimants' claims fuelling confusion in patients or potential patients and thereby putting their health at risk by alarming them into ceasing or not starting to take statins.
(3) (In relation to the Health Section [i.e. the Main Article and the Editorial] only) The Claimants' resolute persistence in making these claims, in the face of the strength of the expert evidence in support of statins, creates a reasonable suspicion that they have too much to lose commercially or in terms of their high public profile to stop making their claims, and/or do profit and/or stand to profit by their stance".
(c) s.15 reporting privilege
(1) the parts highlighted in red in Annex 1 (and shown underlined in Annex 2) [1], are protected by qualified privilege as an extract and/or summary of a statement by the Secretary of State for Health and Social Care ("the Hancock Statement") pursuant to s.15 Defamation Act 1996, paragraph 7 and/or paragraph 9(1)(a) and/or (b) of Part II of Schedule 1; and
(2) the parts highlighted in blue in Annex 1 (and shown with double underlining in Annex 2) [2], are protected by qualified privilege as an extract and/or summary of a statement by Dr Matt Kearney ("the Kearney Statement") pursuant to s.15 Defamation Act 1996, paragraph 9(1)(b) of Part II of Schedule 1.
(The relevant parts of the Defamation Act 1996 are set out in [292]-[293] below).
(d) s.6 Privileged report of peer-reviewed scientific or academic journal
"(1) In June 2016 an interrupted time series analysis by Professors Anthony Matthews, Ben Goldacre and Liam Smeeth from the London School of Hygiene and Tropical Medicine ("LSHTM") and others entitled 'Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data' ("the LSHTM paper"), published in the [British Medical Journal ("BMJ"), 28 June 2016], evaluated the effects of the media debate about statins in the UK in 2014 (in which claims published in the BMJ that 20% of patients taking statins suffered adverse effects, and which were subsequently withdrawn as being incorrect, prominently featured) on prescribing for both the primary and secondary prevention of cardiovascular disease and the consequences for public health. Analyses of the Clinical Practice Research Datalink in 2014-15 indicated that:
(i) statin therapy had been started by only about 60% of patients who recently had a first cardiovascular event and by only about 25% of patients in whom a 10-year cardiovascular risk of 20% or more had been recorded by their general practitioner within the past month;
(ii) there was a proportional increase of about 10% in patients stopping statin therapy for secondary and primary prevention (as well as reductions in the numbers of patients who had their cardiovascular risk assessed to determine their eligibility for statin therapy;
(iii) the researchers estimated that more than 200,000 UK patients had stopped taking their statin therapy in the six months after the media coverage and that (depending on what proportion resume treatment) this would result in between about 2,000 and 6,000 cardiovascular events occurring during the subsequent decade that would otherwise have been avoided. The researchers also found that their findings were consistent with other studies that found statin cessation rates were affected by negative news stories...
(2) The LSHTM paper was a statement that related to a scientific or academic matter, namely an interrupted time series analysis of how a period of intense media coverage of controversy over the risk:benefit balance of statins had affected their use using prescribing data from UK primary care records.
(3) Before the LSHTM paper was published in the BMJ an independent review of the statement's scientific and/or academic merit was carried out by (i) Dr Wim Weber, the European research editor of the BMJ, and/or the member or members of the BMJ's manuscript committee responsible for the decision to publish the LSHTM paper, and (ii) external peer reviewers, each of whom had scientific and/or academic expertise in the matter concerned for the purposes of section 6(3)(b). Insofar as is necessary, the Defendants will contend that each member of the BMJ's manuscript committee also had scientific and/or academic expertise in the matter concerned for the purposes of section 6(3)(b). Further, Dr Weber and/or each member of the manuscript committee responsible for the decision to publish the LSHTM paper was or were the editor or editors for the purposes of section 6(3)(a) (as construed with section 6(8))
(4) In the premises, the words [highlighted in green in Annex and shown dotted‑underlined in Annex 2] were (i) a fair and accurate extract or summary of a peer-reviewed statement which related to a scientific and/or academic matter and were published in a scientific and/or academic journal within the meaning of sections 6(2) and 6(3) of the 2013 Act, and (ii) were published on an occasion of qualified privilege pursuant to section 6(5) of the 2013 Act".
(e) Public interest
(3) Reply
"The Claimants aver that the publications complained of consist of defamatory statements of fact concerning them and deny that they are 'statements of opinion' within the meaning of s.3(2) of the Defamation Act 2013... The Claimants deny that the publications bear the meanings ascribed to them by the Defendants... The Defendants by their Defence downplay and seek to sanitise the true defamatory effect of what they published, which, in truth, so far as concerned the Claimants, consists of an unwarranted hatchet-job or, to use the Second Defendant's own word, 'takedown'... In particular, the Defendants seem to wish to shirk responsibility for accusing the Claimants entirely unjustifiably of dishonest and venally motivated conduct, and further accusing them of causing very many people, by that conduct, to be [at] a greater risk of a heart attack or a stroke...
So far as the Defendants' defences are concerned.
A. The Claimants deny that any of the publications complained of is honest opinion under s.3 of the Defamation Act 2013. It is denied that any of the conditions in s.3(2)-(4) is met in relation to any of the publications complained of. Further, if contrary to the foregoing, any of the publications complained of is found to be a statement of opinion, the Claimants will show (a) that the Second Defendant, for whose conduct in publishing the articles complained of the First Defendant is vicariously liable, did not hold any defamatory opinion about the Claimants that the publications may be found to bear; or, alternatively, (b) that the First Defendant knew or ought to have known that the Second Defendant did not hold any such opinion about the Claimants.
B. The Claimants deny that any of the publications complained of is substantially true under s.2 of the Defamation Act 2013...
C. In particular, the Claimants respond to the Defendants' defences of honest opinion and truth by contending that an evaluation of the relevant scientific evidence relating to cholesterol, CVD and statins, primarily in the form of published scientific studies, demonstrates that the science on these issues is not in fact cut and dried as appears from both the articles complained of and the Defence (and could not reasonably be considered to be cut and dried), and that all the published statements that the Claimants have made on these topics - which are not the same as the ones that the Defendants have imputed to them in the publications - they have made honestly and reasonably on an evidence-based basis ...
D. The Claimants deny that statutory reporting qualified privilege under s.15 of the Defamation Act 1996 attaches to any of the passages in the publications complained of to which the Defendants contend it attaches, including by virtue of the fact that the publication of each such passage was and continues to be made by the Defendants with malice...
E. The Claimants deny that peer-reviewed scientific statement privilege under s.6(5) of the Defamation Act 2013 attaches to any of the passages in the publications complained of to which the Defendants contend it attaches, including by reference to the fact that the publication of each such passage was and continues to be made by the Defendants with malice...
F. The Claimants deny that the publications complained of (or any of them) is a publication on a matter of public interest under s.4 of the Defamation Act 2013. It is denied that the Defendants believed or believed reasonably that publication of any of those publications was in the public interest..."
(1) in respect of the extracts from the Hancock Statement, that:
"(a) the Second Defendant, and through him the First Defendant, misused or abused the occasion in respect of which privilege is claimed, for a purpose other than that for which the privilege is accorded by the law, namely for the purpose of obtaining the private, professional advantages that were gained by unwarrantedly and spuriously investing his and the Mail on Sunday's defamatory remarks concerning the claimants and Dr Malhotra with the endorsement of the Secretary of State for Health and Social Care and thereby making his story seem significantly more credible than it was and more impactful; and
(b) this was the Second Defendant's, and through him the First Defendant's, dominant purpose in and motive for publishing these statements; and
(c) the Defendants published or caused to be published the representation embodied centrally in the statements that Matt Hancock MP had himself made trenchant defamatory remarks concerning the Claimants and Dr Malhotra and/or had associated himself with and endorsed the Defendants' own defamatory claims concerning those individuals in the articles complained of, in the knowledge (through the Second Defendant) that that representation was false and that Mr Hancock had not himself made any remarks regarding those individuals of that kind (or at all) or associated himself with or endorsed the Defendant's defamatory claims about them; or
(d) the Defendants published or caused to be published the aforesaid false representation recklessly (through the Second Defendant), not caring whether it was true or false."
(2) in respect of the extracts from the LSHTM Paper, that:
"(a) the Second Defendant, and through him the First Defendant, misused or abused the occasion in respect of which privilege is claimed, for a purpose other than that for which the privilege is accorded by the law, namely for the purpose of obtaining the private, professional advantages that were to be gained by unwarrantedly and spuriously investing his and the Mail on Sunday's defamatory claims about the Claimants and Dr Malhotra with the authority of the LSHTM, a well-known and reputable UK university college specialising in public health, and of an expert scientific study published by the LSHTM, thereby making those claims and his story seem significantly more credible than they were and more impactful; and
(b) this was the Second Defendant's, and through him the First Defendant's dominant purpose and motive for publishing these statements; and
(c) the Defendants published or caused to be published the representation embodied centrally in the statements that the LSHTM paper and its findings relate to 'scare stories' and 'fake news' about statins, specifically alleged statements made publicly by the Claimants said to match that description and Dr Malhotra's 2013 BMJ article, in the knowledge (through the Second Defendant) that the representation was false and that the LSHTM paper and its findings, which relate only to an alleged 'period of intense media coverage of controversy over the risk:benefit balance of statins' following the publication of Dr Malhotra's 2013 BMJ article, have nothing to do with the Claimants or anything that either has said or done or Dr Malhotra or anything that he has said or done including his 2013 BMJ article; or
(d) the Defendants published or caused to be published the aforesaid false representation recklessly (through the Second Defendant), not caring whether it was true or false"
Particulars of both pleas of malice were given in the Reply.
(1) the Defendants, through Mr Calman, had misused the reporting privilege relied upon to obtain a private advantage in terms of bolstering the credibility of the allegations made in the Articles, and this was the dominant purpose for publishing the privileged statement; and/or
(2) the relevant report was published recklessly not caring whether the report given was true or false.
D: Order for the trial of preliminary issues
[4] Early in the proceedings, the parties tried to find a way of advancing the case in stages, as is now common in defamation claims. Obvious candidates for early resolution in most claims are the issues of meaning and whether the publication complained of was or contained an allegation of fact or expression of opinion. Resolution of these issues in most defamation claims, even prior to service of a Defence, can offer substantial benefits in terms of case management, see Bokova -v- Associated Newspapers Ltd [2019] QB 861 [10] and Morgan -v- Associated Newspapers Ltd [2018] EWHC 1850 [10].
[6] The complicating factor is the Court of Appeal's decision in Curistan -v- Times Newspapers Ltd [2009] QB 231. That decision, which predated the Defamation Act 2013, is authority for the position that the court must resolve the extent to which the publication complained of is protected by privilege before the court can determine meaning. In other words, when performing the test of deciding what is the meaning, the natural ordinary meaning of an article, the court must first remove from its consideration such parts of that article as the court finds is protected by qualified privilege.
[7] There has been some criticism of that decision. The authors of Gatley suggest, at para.30.8:
"The full consequences of this iconoclastic approach to the determination of meaning remain to be seen".
With the benefit of reflection, on what was an ex tempore judgment, I think the final sentence of [6] misstates the decision. The effect of the Court of Appeal decision is that any privileged words can and should be considered as "context" when determining the meaning of the non-privileged parts (see [399] below). Nevertheless, the point remains: the Court cannot finally determine the natural and ordinary meaning of an article that includes parts in respect of which there is an unresolved privilege claim. I will return to consider the Curistan decision in more detail later in the judgment (see [388]-[400] below).
"1. The following issues will be tried separately from, and in advance of, any other issues in the proceedings (at a trial referred to below as Trial 1):
Statutory qualified privilege - section 15 Defamation Act 1996
1.1 Whether the statements complained of at paragraphs 5 ('Print Publication'), 7 ('Online Publication 1') and/or 10 ('Online Publication 2') of the Particulars of Claim attract qualified privilege under s.15(1) and (3) Defamation Act 1996 ('the 1996 Act') and Schedule 1, Part I, paragraph 7 thereto if and insofar as any of those articles consists of an extract from matter published by or on the authority of the UK government, that matter being, according to the Defendants, the statement published by or on behalf of Matt Hancock MP...
1.2 Whether the statements complained of at paragraphs 5 (Print Publication), 7 (Online Publication 1) and/or 10 (Online Publication 2) of the Particulars of Claim attract qualified privilege under s.15(1), (2) and (3) 1996 Act and Schedule 1, Part II, paragraph 9 thereto if and insofar as any of those articles consists of an extract from or summary of a notice or other matter issued for the information of the public by or on behalf of the UK government, that notice or other matter being, according to the Defendants, the statement issued by or on behalf of Matt Hancock MP...
1.3 Whether the statements complained of at paragraphs 5 (Print Publication), 7 (Online Publication 1) and/or 10 (Online Publication 2) of the Particulars of Claim attract qualified privilege under s.15(1), (2) and (3) 1996 Act and Schedule 1, Part II, paragraph 9 thereto if and insofar as any of those articles consists of an extract from or summary of a notice or other matter issued for the information of the public by or on behalf of the UK government, that notice or other matter being, according to the Defendants, the statement issued by or on behalf of Dr Matt Kearney...
Statutory qualified privilege - section 6 Defamation Act 2013
1.4 Whether the statements complained of at paragraphs 5 (Print Publication), 7 (Online Publication 1), 10 (Online Publication 2) and/or paragraph 16 ('Online Publication 3') of the Particulars of Claim attract privilege s.6(5) and (6) Defamation Act 2013 ('the 2013 Act') if and insofar as any of the articles in question consists of an extract from and/or summary of a statement whose publication is privileged by virtue of s.6 2013 Act (pursuant to sections 6(1) to (3) thereof)...
Statutory qualified privilege - malice
1.5 If and insofar as the publication of any part of any of the statements complained of would otherwise be held to be privileged by virtue of s.15 1996 Act or s.6(5) 2013 Act, whether that publication was or is made (insofar as it continues) with malice...
Publication on a matter of public interest - section 4 of the 2013 Act
1.6 Whether the Print Publication, Online Publication 1, Online Publication 2 and/or Online Publication 3 of the Particulars of Claim were publications on a matter of public interest pursuant to s.4 2013 Act...
Meaning issues
1.7 Whether the News Article and the Health Section in the Print Publication are sufficiently closely connected to be regarded as a single publication...
1.8 Whether Online Publication 2 and Online Publication 1 are sufficiently closely connected by the hyperlink in Online Publication 2 to be regarded as a single publication ... (i.e. the ordinary reasonable reader of Online Publication 2 should be taken to have clicked on the hyperlink in that publication to Online Publication 1 and to have read what s/he found there).
1.9 The natural and ordinary defamatory meaning of each of the statements complained of in the Particulars of Claim in relation to each of the Claimants.
1.10 In respect of Online Publication 2, whether it conveys by innuendo to readers who click on the hyperlink it contains to Online Publication 1, and read Online Publication 1, the meaning pleaded [in [11] above].
1.11 In respect of each of the statements complained of in the Particulars of Claim, whether they were statements of fact, or whether they were or contained statements of opinion.
Honest opinion - section 3(5) of the 2013 Act
1.12 If and insofar as the statements complained of were or contained statements of opinion, whether the Defendants held the opinion pursuant to section 3(5) of the 2013 Act...
2. The issues of honest opinion (save as identified in paragraphs 1.11 and 1.12 above), truth, serious harm and (if applicable) remedies are stayed until the conclusion of Trial 1 when they will be reviewed".
(1) The Court is not adjudicating upon the merits of the debate about statins. In other words, in this judgment, the Court is not going to determine who is "right" in the statin debate. Properly reflecting the parameters of the Court's task in this trial, I have not heard any expert evidence about statins. Such evidence would have been essential if the Court were being required to adjudicate on who was "right" in the statin debate. Although I shall have to consider a significant number of scientific studies into statins in the course of this judgment, this is because it is the existence of these studies (and what they said) that is relevant to the determination of the public interest defence (and one of the privilege defences). In this judgment, the Court is not going to resolve any apparent conflict between scientific studies as to the benefits (or risks) of statins. That is outside the scope of the issues to be decided in this litigation at this stage.
(2) In consequence, the Court is not deciding any issues as to whether the published studies or claims of the Claimants (or any of the other experts whose work is considered in the course of this judgment) were accurate or inaccurate; reliable or unreliable; honest or dishonest. That issue is also outside the scope of this judgment, and I simply have not had the evidence presented to me that would enable me fairly to adjudicate upon those issues. The Claimants have not given evidence, and nor have the various experts that were assisting Mr Calman with the Articles (because none of them has any relevant evidence to give on the issues that the Court must decide).
(3) In resolving the public interest defence, and reflecting the principles of law that are set out in Section G(1) below, the key issue that the Court is resolving is whether the Defendants (and for these purposes, that is Mr Calman) reasonably believed that it was in the public interest to publish the Articles. To resolve that issue, the Court must assess the journalism that led to publication of the Articles; what inquiries were made, what did Mr Calman know, what information did he receive, what opportunity did he give to the Claimants to comment and respond to allegations to be made against them and how ultimately did he present all of this material in his Articles?
E: Evidence
(1) Mr Calman;
(2) John Wellington, Managing Editor of The Mail on Sunday until his retirement in October 2020 ("Mr Wellington");
(3) Edward Verity, now Editor of The Daily Mail but, at the time of publication of the Articles, Editor of The Mail on Sunday ("Mr Verity"); and
(4) Stephen Adams, Medical Editor of The Mail on Sunday ("Mr Adams").
F: The facts
"During February 2019 I spoke to each of [the] experts [identified in [67] below]... on the telephone. By that time I had worked out what I believed was the thrust of the Claimants' arguments so I framed my questions to them by reference to these arguments. I ended each conversation by asking 'why do you think they do this?' I also read various research papers, articles and other materials about the evidence base for statin therapy. A key document I read was The Lancet Review..."
(1) Mr Calman and the Claimants
"I am not a single issue journalist: every week we write about a huge range of topics. Therefore, whilst I am broadly aware of lots of pockets of information - in this case her general view on statins - it is usually only once an article is underway that I will devote the time to look into people or topics in detail and start amassing information on them in order to work out what the article is going to say precisely. That is what I then started doing in preparation for this article".
"He thinks the mainstream advice around cholesterol is a conspiracy or 'con' as he says to trick people into taking statins. He may have even come up with the term 'statination' which you hear bandied about and which I understand to mean a mission by drugs companies and some in the medical establishment to get everyone on statins".
"I regarded Dr Kendrick, at least in respect of his media persona, as a contrarian. In many ways his contrarianism has defined his professional media identity. I accept that alongside his work in the media, he works as a GP where I believe it would be more difficult for him to be so out of step with the orthodoxy, as GPs must abide by the types of guidelines Dr Kendrick criticises (e.g. those relating to the prescription of statins to certain groups) to continue to work in the NHS".
It is perhaps a small detail, but Mr Calman did not realise that the Second Claimant was a practising GP until very shortly before publication of the Articles following receipt of the Second Claimant's right-to-reply response (see final bullet point in the email sent to the Second Claimant by Mr Calman on 28 February 2019, [190] below).
(2) The genesis of the Articles
"... [these] articles both cited an observational study to claim that 20% of patients on statins experience 'unacceptable' side-effects based on a misinterpretation of observational data (aside from being inaccurate, this figure was a far higher proportion than the large-scale randomised controlled trials had showed). After that there was a huge amount of public interest in and publicity about the risks and benefits of statins, which flared when the National Institute for Health and Care Excellence ("NICE") published new draft guidance on the use of statins in February 2014, which proposed lowering the threshold for offering statin treatment (from a 20% risk of developing cardiovascular disease over 10 years to a 10% risk over 10 years... This guidance was adopted later in the year. When the figures from the [2013 BMJ Articles] were repeated in the context of public discussion of NICE's draft guidance, this prompted widespread questioning of the risk-benefit of statins. The BMJ article and study were corrected by the BMJ and the authors over six months later in May 2014. This fallout from the two [2013 BMJ Articles] was widely reported and the subject of research, most significantly the [LSHTM Paper] on 28 June 2016".
"... [Mr Calman] he explained that there was a group of people on the internet and in newsletters and on the radio who were saying things about statins that were misleading and that this was having, you know, a bad influence in the real world... So, you know, if people are being put off taking statins, which could actually save their lives, because of misinformation, that's a very real concern. So, in that conversation, Barney and I agreed that what we needed to do was a piece where we spoke to the - you know, the world's best experts and presented our readers with the truth, and so the balance of the article was dictated by that, because the motivation wasn't to do a piece saying -- you know, which we could have done - 'Are statins good or not'; that wasn't the motivation. The motivation was to do a very polemical, strong piece saying, 'Statins are good for you and you shouldn't take notice - you know, if you hear chatter or read things on the internet, you shouldn't let that put you off taking them'. That was the point of the article, and that's where the balance came from."
"This Review is intended to help clinicians, patients, and the public make informed decisions about statin therapy for the prevention of heart attacks and strokes. It explains how the evidence that is available from randomised controlled trials yields reliable information about both the efficacy and safety of statin therapy. In addition, it discusses how claims that statins commonly cause adverse effects reflect a failure to recognise the limitations of other sources of evidence about the effects of treatment. Large-scale evidence from randomised trials shows that statin therapy reduces the risk of major vascular events (i.e., coronary deaths or myocardial infarctions, strokes, and coronary revascularisation procedures) by about one-quarter for each mmol/L reduction in LDL cholesterol during each year (after the first) that it continues to be taken. The absolute benefits of statin therapy depend on an individual's absolute risk of occlusive vascular events and the absolute reduction in LDL cholesterol that is achieved. For example, lowering LDL cholesterol by 2 mmol/L (77 mg/dL) with an effective low-cost statin regimen (e.g., atorvastatin 40 mg daily, costing about £2 per month) for 5 years in 10,000 patients would typically prevent major vascular events from occurring in about 1,000 patients (i.e., 10% absolute benefit) with pre-existing occlusive vascular disease (secondary prevention) and in 500 patients (i.e., 5% absolute benefit) who are at increased risk but have not yet had a vascular event (primary prevention). Statin therapy has been shown to reduce vascular disease risk during each year it continues to be taken, so larger absolute benefits would accrue with more prolonged therapy, and these benefits persist long term. The only serious adverse events that have been shown to be caused by long-term statin therapy—i.e., adverse effects of the statin—are myopathy (defined as muscle pain or weakness combined with large increases in blood concentrations of creatine kinase), new-onset diabetes mellitus, and, probably, haemorrhagic stroke. Typically, treatment of 10,000 patients for 5 years with an effective regimen (e.g., atorvastatin 40 mg daily) would cause about 5 cases of myopathy (one of which might progress, if the statin therapy is not stopped, to the more severe condition of rhabdomyolysis), 50–100 new cases of diabetes, and 5‑10 haemorrhagic strokes. However, any adverse impact of these side-effects on major vascular events has already been taken into account in the estimates of the absolute benefits. Statin therapy may cause symptomatic adverse events (e.g., muscle pain or weakness) in up to about 50–100 patients (i.e., 0·5–1·0% absolute harm) per 10,000 treated for 5 years. However, placebo-controlled randomised trials have shown definitively that almost all of the symptomatic adverse events that are attributed to statin therapy in routine practice are not actually caused by it (i.e., they represent misattribution). The large-scale evidence available from randomised trials also indicates that it is unlikely that large absolute excesses in other serious adverse events still await discovery. Consequently, any further findings that emerge about the effects of statin therapy would not be expected to alter materially the balance of benefits and harms. It is, therefore, of concern that exaggerated claims about side-effect rates with statin therapy may be responsible for its under-use among individuals at increased risk of cardiovascular events. For, whereas the rare cases of myopathy and any muscle‑related symptoms that are attributed to statin therapy generally resolve rapidly when treatment is stopped, the heart attacks or strokes that may occur if statin therapy is stopped unnecessarily can be devastating".
(1) Evidence from large population studies, combined with studies in animals, genetic research and randomised controlled trials had confirmed a causal link between higher levels of low-density lipoprotein cholesterol (LDL-C) in blood and higher risks of cardiovascular disease.
(2) The meta-analyses indicated that for each 1 millimoles per litre ("mmol/L") reduction in LDL-C the risk of coronary deaths and heart attacks, ischaemic strokes (strokes due to blood clots) and coronary revascularisation procedures is reduced by about 25% during each year (after the first) that statin treatment continues to be taken.
(3) Larger reductions in LDL-C produce larger reductions in the risks of these major vascular events, so that using a statin regimen now available, such as atorvastatin 40mg daily, to reduce LDL-C by 2 mmol/L would approximately halve a patient's risk of heart attacks and strokes during each year treatment is continued.
(4) The proportional reductions in LDL-C achieved with statins were not materially affected by the starting LDL-C concentration and were similar among different types of patient.
(5) Lowering LDL-C by 2 mmol/L for five years in 10,000 patients would typically prevent major vascular events in 1,000 people with a high risk of heart attacks and stroke, such as patients with pre-existing cardiovascular disease and in 500 people who are at lower risk, for example people at increased risk who have not yet had a vascular event.
(6) The authors were warning that the benefits of statin therapy have been underestimated, and the harms exaggerated because of a failure to acknowledge both the amount of evidence from the randomised controlled trials and the limitations of observational studies.
(7) The randomised controlled trials can determine cause and effect. In randomised trials people differ only randomly from each other so that outcome can be inferred to be causal. Blinding the trial meant unbiased ascertainment. Meta‑analyses of large, randomised trials on large numbers of people with different characteristics meant the evidence was widely generalisable. By contrast observational studies compare the health outcomes of large numbers of people who have been given particular treatment by their doctors and people who have not been given the treatment. Although, observational studies may be able to detect large increases in health outcomes that might not have been expected, based on clinical trial data, they are not able to produce reliable evidence about the effects of drug treatments when the health outcomes are common or the effects are modest.
(8) There is an important need for greater public recognition of the limitations of observational studies and anecdotal reports as a source of reliable information about the benefits and a better understanding of the strengths of randomised controlled trials with masked assignment of treatment and systematic assessment of adverse health outcomes.
(9) The public health implications of the failure to recognise the benefits of statins and of the exaggerated claims that have been made about the rates of side-effects are very serious.
"Background Statin therapy has been shown to reduce major vascular events and vascular mortality in a wide range of individuals, but there is uncertainty about its efficacy and safety among older people. We undertook a meta-analysis of data from all large statin trials to compare the effects of statin therapy at different ages.
Methods In this meta-analysis, randomised trials of statin therapy were eligible if they aimed to recruit at least 1000 participants with a scheduled treatment duration of at least 2 years. We analysed individual participant data from 22 trials (n=134,537) and detailed summary data from one trial (n=12,705) of statin therapy versus control, plus individual participant data from five trials of more intensive versus less intensive statin therapy (n=39,612). We subdivided participants into six age groups (55 years or younger, 56–60 years, 61–65 years, 66–70 years, 71–75 years, and older than 75 years). We estimated effects on major vascular events (i.e., major coronary events, strokes, and coronary revascularisations), cause-specific mortality, and cancer incidence as the rate ratio (RR) per 1·0 mmol/L reduction in LDL cholesterol. We compared proportional risk reductions in different age subgroups by use of standard χ2 tests for heterogeneity when there were two groups, or trend when there were more than two groups.
Findings 14,483 (8%) of 186,854 participants in the 28 trials were older than 75 years at randomisation, and the median follow-up duration was 4·9 years. Overall, statin therapy or a more intensive statin regimen produced a 21% (RR 0·79, 95% CI 0·77–0·81) proportional reduction in major vascular events per 1·0 mmol/L reduction in LDL cholesterol. We observed a significant reduction in major vascular events in all age groups. Although proportional reductions in major vascular events diminished slightly with age, this trend was not statistically significant (ptrend=0·06). Overall, statin or more intensive therapy yielded a 24% (RR 0·76, 95% CI 0·73–0·79) proportional reduction in major coronary events per 1·0 mmol/L reduction in LDL cholesterol, and with increasing age, we observed a trend towards smaller proportional risk reductions in major coronary events (ptrend=0·009). We observed a 25% (RR 0·75, 95% CI 0·73‑0·78) proportional reduction in the risk of coronary revascularisation procedures with statin therapy or a more intensive statin regimen per 1·0 mmol/L lower LDL cholesterol, which did not differ significantly across age groups (ptrend=0·6). Similarly, the proportional reductions in stroke of any type (RR 0·84, 95% CI 0·80–0·89) did not differ significantly across age groups (ptrend=0·7). After exclusion of four trials which enrolled only patients with heart failure or undergoing renal dialysis (among whom statin therapy has not been shown to be effective), the trend to smaller proportional risk reductions with increasing age persisted for major coronary events (ptrend=0·01), and remained non-significant for major vascular events (ptrend=0·3). The proportional reduction in major vascular events was similar, irrespective of age, among patients with pre-existing vascular disease (ptrend=0·2), but appeared smaller among older than among younger individuals not known to have vascular disease (ptrend=0·05). We found a 12% (RR 0·88, 95% CI 0·85–0·91) proportional reduction in vascular mortality per 1·0 mmol/L reduction in LDL cholesterol, with a trend towards smaller proportional reductions with older age (ptrend=0·004), but this trend did not persist after exclusion of the heart failure or dialysis trials (ptrend=0·2). Statin therapy had no effect at any age on non-vascular mortality, cancer death, or cancer incidence.
Interpretation Statin therapy produces significant reductions in major vascular events irrespective of age, but there is less direct evidence of benefit among patients older than 75 years who do not already have evidence of occlusive vascular disease. This limitation is now being addressed by further trials".
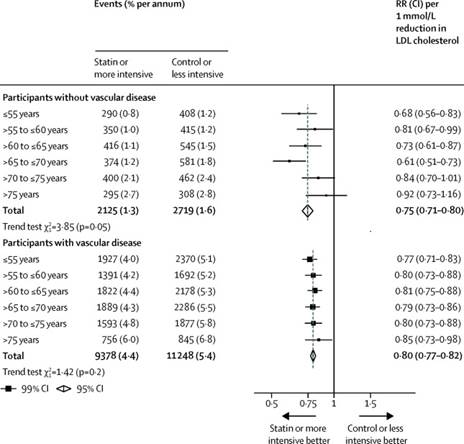
(2) as Figure 5 - described as "Effects on vascular death per mmol/L reduction in LDL cholesterol, subdivided by age at randomisation":
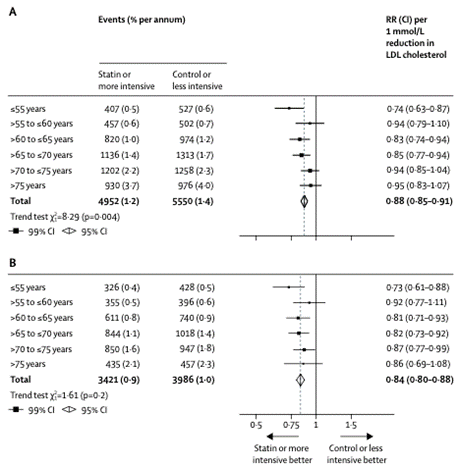
(3) Preparation for the Articles
(1) Professor Colin Baigent, the deputy director of the University of Oxford's Clinical Trial Service Unit & Epidemiological Studies Unit ("CTSU") and a professor of epidemiology ("Professor Baigent"). He specialised in cardiovascular epidemiology, and the design, conduct and interpretation of large-scale randomised trials in cardiovascular disease. He was co-author of The Lancet Review (see [62] above).
(2) Professor Sir Rory Collins, the director of CTSU, British Heart Foundation Professor of Medicine and Epidemiology, Head of the Nuffield Department of Population Health and principal investigator and chief executive of a large-scale biomedical research database, known as UK Biobank ("Professor Collins"). Professor Collins specialised in the establishment of large-scale epidemiological studies of the causes, prevention and treatment of cardiovascular disease. He was the lead author of The Lancet Review. In 2014, Professor Collins had criticised the authors and editor of the BMJ for not withdrawing the 2013 BMJ Articles promptly (see [56] above and [206]-[208] below). He had claimed that the impact on public health of this misinformation was worse than the MMR vaccine scandal.
(3) Professor Peter Sever, Professor of Clinical Pharmacology and Therapeutics at Imperial College London, Honorary Consultant Physician at the Imperial Healthcare NHS Trust, Co-Director of the international Centre for Circulatory Health and an author of scientific studies on statins ("Professor Sever"). He was one of the authors of The Lancet Review.
(4) Professor Liam Smeeth, Professor of Clinical Epidemiology at the London School of Hygiene and Tropical Medicine and a GP ("Professor Smeeth"). He was one of the authors of the LSHTM study and The Lancet Review.
(5) Professor Nilesh Samani, cardiologist and President of the British Heart Foundation ("Professor Samani").
"... the Mail on Sunday recently launched a campaign to fight fake health news. Having read your comments last week in the news, I can see this is a subject you are interested in! I wonder if we could have a chat. We really want to get behind a concerted push to highlight statins conspiracy theories for just what they are, and give readers the medical evidence and the facts..."
"Can you take a good [look] at this - we're planning a big takedown of statin deniers. Zoe is one of them".
Forwarded with the email was the First Claimant's blog article "Statins in the over 75s" (see [69]-[70] above).
"We can share the finished article with you prior to publication - and we will be doing so with all expert commentators. We want to get this 100% right".
(1) At 17.23, he emailed Professor Baigent a copy of "Statins in the over 75s" (see [69]-[70] above):
"... Just in case you've not seen, this is the sort of thing we are up against. I don't want to turn it into a 'he said, she said', but this is a good example of misinterpretation of study results".
Mr Calman has never explained what, he believed, the First Claimant had misinterpreted.
(2) At 17.29, Professor Baigent responded following up two specific issues from the telephone conversation (a) the relationship between low cholesterol and increased risk of death; and (b) mortality reduction in a particular study.
(3) In a separate email, responding to Mr Calman's forwarding of "Statins in the over 75s", Professor Baigent said:
"Thanks for sending this, Barney. I will read through (I was already aware of her views via Twitter) and then we can talk about it in due course".
To that email, Mr Calman responded, at 17.37:
"I don't think we need to pay it much heed - bizarrely, for someone who claims researchers have vested interest, she's currently flogging a diet book!!! But it's worth just keeping in mind the kind of thing being said".
"I think it's fair to say that, as a journalist, my area of expertise, even as someone who does health journalism day in, day out, that complex -- I mean, obviously, I understand about risk ratios and I know what I'm looking at in these tables, but, you know, we would look to experts to tell us, you know, what does this all, you know, mean in practice, am I getting this exactly right, et cetera, and I completely agree that Zoe and anyone is more than within their rights to write their critique of the statistical methods used by the CTT, and they could do so and write to the Lancet also and have their concerns published, as happens, and, you know, there would be an opportunity, at that point, for the authors to respond and say, you know, 'This is our response to your critique'. This is how science publications work".
"I think it sums up the - very well summed up the reason that these type of stories - that these kind of ideas catch hold; you know, that it's not about - it's not as simple as one false thing, or - it's certainly not about lying to people, or anything like that. It's about these mixtures of truth and speculation and opinion to build a picture that's wrong and misleading, and that reason does sum up. I still think it's a very good summary of the problem and why these stories are so believable and persuasive. 'Persuasive' I think is the word".
"... He will give the more forcible quotes about the statin denialists (I think he once said that Malhotra was like Andrew Wakefield, just responsible for more deaths), but he often gets criticised for receiving industry funding. He is a serious academic/researcher however, and always happy to talk about statins... Worth chasing up Professor Sir Nilesh Samani from the [British Heart Foundation] as he would give you a quote too".
"Hi Colin - below is Zoe Harcombe's latest newsletter. I've been away since Friday so will sit down and see where we are this far. Anyone reading this kind of stuff is likely to be very convinced. I would say. I have to admit, I've not read Malcolm Kendrick's book. But I'm guessing it's the same argument".
Mr Calman also forwarded the article to X:
"... I'd love to get your take on this. Bit of background, Zoe [has] turned to studying nutrition, first giving diet advice from a lay perspective while selling diet plans - the Harcombe Diet - and now, having done a PhD, as an academic with a special interest in statistics. In January, she published her latest diet book. She's one of the statin deniers I'm looking into right now and a very clever one. We are planning to publish next week or the week after. Her argument seems convincing. But is she right? I suspect not..."
"Of course she's not right. Zoe Harcombe cherry picks as much as the other side. I am afraid I came across her (a lot) when [redacted for source protection]. He shares your views - she's a clever flawed protagonist. However, I do not have the time or inclination to take on a well known blogger who has devoted her life to this.
You might be better off asking Rory Collins in Oxford for help - he's as outspoken as she is, knows the data backwards and is much more heavyweight academic. Only problem is she will cry 'big pharma'!
Alternatively see if [the British Heart Foundation] can field someone? It really should be something they are prepared to stand up to..."
"Simple first questions to ask her. Is she 'cherry picking' her papers as much as she accuses others of doing?
How does she explain the benefits of statins, ezetimibe and PCSK 9 inhibitors (all of which act solely to reduce LDL levels in various ways) in reducing the risk of a heart attack if LDL is inversely related to heart disease?
What about the evidence that people with genetically very high levels of LDL cholesterol (Familial Hyperlipidaemia) have a dramatically increased risk of heart attacks which can even occur when they are young?
Etc. Etc. The tit for tat could be endless.
Haven't had a chance to reanalyse the data she is quoting below - but at first sight I'm pretty sure she could be challenged. The correct way as a scientist to challenge data is to do so by writing to the Lancet with these points and allowing the authors of the paper to respond. Has she done this. If the answer was unsatisfactory, she can then reply to that - it's called healthy scientific debate and we all engage in it. Instead she put out her theory in the 'discredited' press....!
The problem with all this is that there is no one cause of heart disease (it is polygenetic and polyfactorial), it appears to be related to a genetic susceptibility and lifetime exposure to risk factors - of which LDL is one. And statins are not a universal panacea - they are merely one of the few and best weapons we have at the moment.
Hope that's a help ... I really am not interested in fighting idealogues online - or in print - and others should be better placed to do this".
"Thanks. Later today I will send you a note explaining why Harcombe's reasoning is deeply flawed.
I am copying my colleague Professor Sir Rory Collins, with whom I have worked on the CTT (statins) meta-analysis for many years. Rory will be able to give you a great deal of information, and it would be very important for you to speak to him at an early stage in preparing your article".
"... By the way, did you see this very recent editorial from the Editors of a very large number of major cardiovascular journals? It would seem to be directly relevant to what I understand are the issues that you are investigating".
"You may find it helpful to look at the attached Lancet paper [see [62] above], which was led by Rory Collins, as this addresses most of the issues raised by Zoe Harcombe. In particular, the argument that cholesterol is a cause of cardiovascular disease is set out on page 2540 in detail.
A general comment is that the interpretation of epidemiological data requires an awareness of the potential for studies to yield results that - if taken at face value - are misleading, and sometimes even yield the opposite of the truth. As I mentioned when we spoke, and is set out in detail in the attached paper (see, for example pp.2538-9), observational studies are particularly prone to yielding unreliable findings. In any standard course on epidemiology, students are taught ways in which hidden biases provide explanations for why the data appear to be revealing something startling. An understanding of such biases is essential when trying to interpret the findings of studies relating to cholesterol".
"...The three we have so far are:
Dr Malcolm Kendrick
Dr Aseem Malhotra
Zoe Harcombe
The main focus is their business - all make money from saying statins don't work.
All have books, journalistic careers, diet plans etc. Do they have companies that publish accounts, how many books have their (sic) sold, do they have books forthcoming, what are the dates of all their book publications, how much do they charge for speaking at gigs etc, do they sell anything else or endorse any other products via their websites, do they live in lavish houses, hobnob with celebrities, etc.
The idea is that they accuse research scientists who publish work on statins as having serious conflicts of interests, because they work for organisations that receive drug company funding.
However, could the same be said of the statins deniers? Do their livelihoods rely on their argument, which, if disproved, would mean they would be out of the job?".
"Hi Rory, thanks so much for your time yesterday. I'm interested in this study. Malhotra was invited on TV etc. to publicise the findings. It's a bit difficult for me to understand, but am I right in thinking they managed to find a total of 9 observational studies in total that recorded [cardiovascular] deaths, and that in those, low cholesterol was associated with a higher risk of death, while higher cholesterol was associated with a lower risk of death?"
There is no record of Mr Calman receiving a response to this question from Professor Collins.
BC: "Hi... as u know I'm doing this deep dive into statins deniers. One of the things they argue is that eating [saturated] fats has no relevance to LDL levels in the blood is this right? Would you give me a simple biochemical type explanation as to how we know it does? NHS and Heart UK both list eating too much sat fat as key factor [in] raising LDL".
X: "As does the AHA - whereas the original data actually derived from studies done back in the 1950s to 1970s and often confounded the intake of different fats with changes in carbohydrate contents which may have muddied the waters. Also much of the data relied on stuff like the debateable Ancel Keys epidemiological studied (sic) as well as 'force feeding' small groups of people (often 10-20 medical students or patients) for a few weeks with controlled diets. Most of the studies found that high intakes of saturated fats were associated with higher levels of LDL that (sic) diets high in poly- or monosaturated fats. (The LDL increases were actually noted to be increases in LDL particle mass and may actually have represented an increase in big fluffy (less atherogenic) LDL. The data is really old and the metabolic pathways were variable - sometimes different individuals had different responses... I can get you some abstracts (or you can search google scholar for some) but getting full papers is hard online".
BC: "Are you able to sum up how we know it does?"
X: "I've never focused overly on low saturated fat diets for this reason. But I do focus on high mono/polyunsaturated fat diets [redacted] And the data on genetic LDL and [coronary heart disease] as well as the data on drugs which act on LDL like stating [sc. statins] and PCSK9I are more persuasive".
BC: "So Kendrick is right?"
X: "I've never actually read him, mea culpa"
BC: "The top bit of advice from everyone is too much sat fat is partly behind raised cholesterol. He said this is rubbish and not supported by evidence"
X: "But I wouldn't like to have to defend the argument that high intake of saturated fat raises LDL cholesterol. Or that it necessarily worse than a high carb diet..."
BC: "Ok"
X: "I object to the naysayers against statins"
BC: "So it's bad advice being given by NHS, etc.? "
X: "And I endorse the view that the diet should focus on 'healthy oils'"
BC: "But no oils are unhealthy?"
X: "And lower carbs"
BC: "Hmmmm"
X: "If you are eating high sat fats, your cholesterol levels - and LDL levels - will be higher than if you are eating high 'healthy' fats"
BC: "Rory Collins et al are likening him to Wakefield"
X: "Or carbs"
BC: "But he's right about the first thing in his book"
X: "But the studies showing this were not at the scale or duration to see if this translated into CHD risk. And if you read [redacted] you will see that I was never in favour of low fat diets!!"
BC: "This is specifically sat fat"
X: "Have you challenged the BHF or Rory to prove that sat fat raises LDL levels (ideally without relying exclusively on Ancel Keys) and moreover to show that it leads to CHD?"
BC: "No. This is supposed to be about how statins deniers are a danger to the public"
X: "I think much of the power of Kendrick - and Harcombe - comes from some laziness on the part of the medical profession. BUT that does NOT mean that statins don't work. I would equally challenge Kendrick and Co to show that they don't work in the face of all the evidence that they do!!!"
BC: "But it doesn't look good for the medical prof. If you are giving non evidence based advice. The NHS is giving fake news in this case".
X: "It may be - as I often explain to patients - that they work through reducing the likely hood (sic) of rupture of cholesterol - rich plaques rather than simply lowering the concentration of LDL. There is some support for that in the fact that Coronary artery calcium scores continue to rise even when on a statin (? Question of degree of LDL lowering) although the risk of plaque rupture, MI and stents etc. falls. I do honestly think some of the advice given by the NHS on diet may be out of date or lazy. It still doesn't mean that statins don't work! Or that they are dangerous".
BC: "Ok"
X: "There would be room for a balanced discussion or the arguments for and against sat fat in diet - but the evidence that having a lower saturated fat diet which is also low in refined carbs and high in monosat fats is strong. And at the end of the day, that is what the NHS is promoting. The emphasis may be perceived to be wrong - it's not so much about lowering the sat and carb as raising the good oils without overeating!! Good luck. When are you publishing?"
BC: "So maybe we have to admit they're right about this".
BC: "Not for a few weeks"
X: "I always think it's wise to admit where there are legitimate questions - it makes the real arguments more powerful. But I would still give Rory et al the right to argue their case...!"
(1) at 11.13, he emailed Professors Collins, Sever and Baigent:
"Thanks all so much for your help with this article so far. One area I'm still unsure how to approach is the one about what CAUSES high cholesterol... Can you tell me: what according to the best evidence, raises LDL and how, and is it true that 'eating too much saturated fat' is one of those causes? If so, how does it happen? I'm not concerned if this is, in fact, shaky science. We all know that the original advice to avoid eating foods that contain cholesterol was debunked. It would be fine to say this is one area that these people may have a point, and might even add some needed balance. However if it is true to say excess saturated fat in the diet causes raised LDL, then we should say how we know in detail".
(2) at 11.49, Professor Baigent responded:
"The evidence that higher saturated fat consumption leads to an increase in blood LDL cholesterol comes from so-called 'metabolic ward studies', which are randomised trials done under strict conditions whereby different study arms are allocated to different diets. One of our colleagues, Professor Robert Clarke... conducted a meta-analysis of such trials, which helps to provide an overall summary of their findings - see link https://www.bmj.com/content/314/7074/112.long. Being randomised to deduced consumption of saturated fat caused a reduction in LDL cholesterol (and this reduction was much larger than the reduction achieved by reducing dietary cholesterol). Hence, by implication, increasing saturated fat consumption in the population will cause an increase in the population's average LDL cholesterol concentration.
The mechanism by which saturated fatty acids increase LDL cholesterol is complex, and it may be that it would be best to speak to Robert Clarke about this. He will be able to direct you to an up-to-date source of information". (emphasis in original)
(3) at 11.50 Professor Collins responded:
"I'm copying in my colleague Robert Clarke who has done analyses of randomised ward-based trials of the effects of various diets on LDL‑cholesterol levels which show that increased consumption of saturated fats does increase LDL cholesterol levels. As to the separate question as to why increased intake of saturated fats increases LDL cholesterol levels, the simple explanation is that the liver makes LDL-cholesterol from saturated fats. (Most of the cholesterol in the body is made in the liver rather than, as you note, obtained from eating cholesterol itself.)..." (emphasis in original)
(4) at 12:24, Mr Calman forwarded Professor Collins response to X:
"FYI. Obviously this is confidential... but I wanted to share".
(5) at 12:29, Professor Sever responded to Professor Collins email:
"Dear all, To convey a simple message to the public, should we say that the level of cholesterol in the blood is determined by the persons genes (on average about 60%) and their diet, and the saturated fat in the diet is a major contributor to the latter. Does anyone disagree?"
(6) at 12.39, Professor Sever suggested that Mr Calman should contact Tom Sanders as a person who would be "good on diet and cholesterol".
(7) at 12:57, Robert Clarke provided Professor Sanders email address and also sent Mr Calman a copy of the paper to which Professor Collins had referred.
(8) at 13.32, X responded to Mr Calman:
"I would be interested to see Robert Clarke's summary - and to see if he is cherry picking! As you say, doesn't change the message about the risks of LDL and statins but does change people's perception of dietary advice".
(9) at 13.41, Mr Calman forwarded Mr Clarke's email and paper to X (without comment).
(10) at 21.11, X emailed Mr Calman:
"Interesting. Clarkes paper is a v old study - and looking at the data it is heavily skewed by the Minnesota Coronary Experiment (MCE) (see Franz 1989 - incidentally co author Ancel Keys) which include over 4,800 subjects (all the others he included in this meta analysis had around 10-20 subjects!) This MCE study showed a fall in total cholesterol but I don't think it analysed LDL. It was also conducted at a time when the saturated fats they used were high in trans fats - which DO raise cholesterol.
Incidentally the study was reanalysed in 2016 using some unpublished data and confirmed that the unsaturated fat diet lowered cholesterol compared to the saturated fat diet. It also seemed to suggest a higher mortality on a v high linoleic acid (unsaturated) diet compared to the high sat fat diet - but there were some significant inconsistencies in the study reporting and a huge number of subjects lost to follow up, which may make this analysis useless Do Kendrick and Harcombe rely on this?"
"Dear all, thank you for your help so far. Everything is shaping up nicely. What would make this piece absolutely staller (sic) is if we could find a case study of someone who had stopped taking statins and suffered a heart attack or stroke. I know it's a big ask, but it will really bring this entire argument to life. Between you, has anyone come into contact with such a story? Or been contacted by a relative of someone who died, and had stopped taking statins? The piece is set to run next week, so it's slightly urgent. Let me know your thoughts".
"Prior to me sending this back to the Profs, I wanted to show the first draft of the statins deniers article to you [redacted] cardiology-related and someone who isn't involved in the row. You always give a really balanced view. It's long and windy, and it'll come down by at least 1,000 words, but I'd still appreciate hugely you view (sic) and any comments or suggestions. I think we're getting a comment from Matt Hancock to run in it also. And the sat fat bit is still to sort out!"
"Dear all, thank you again for all your input into this article so far. I wanted to readdress the issue of finding a case study.
One of the key factors in your collective argument is that criticism of statins discourages use amongst high risk patients, and this is a public health threat. Since putting calls out we have been inundated by stories of people who have stopped taking statins and felt far healthier. We've had two quite dramatic stories of patients who have been taken off statins by their doctors because of developing serious liver problems, and then died. The families themselves both naturally question whether statins caused the problems. What we haven't had is a single story which backs your thesis, and obviously I'm concerned.
I think it makes us look rather weak to use a very historic story about Clinton. What I do not want this piece to be is simply another exercise in singing to the choir and I fear without a real life example, we may be veering towards it all just seeming like scary theories and doctors saying 'because I said so'.
What has struck me is that the reason Kendrick, Malhotra, Harcombe and their ilk have really struck a chord is because they are great, emotive communicators. What we're offering is a chance for you all to be that too, and we are planning to devote an unprecedented amount of space to this.
Have any of you hear a real life example of someone who has suffered a heart attack or stroke because they declined/quit statins because they thought they didn't really work anyway, or similar? I really want us to do everything we can to make this work. Please do ALL let me know asap today your thoughts about how to move forward".
When cross-examined, Mr Calman accepted that the word "inundated" may have been "a bit over the top" and that he had been trying to "rally" the experts to provide case studies.
"Thanks Colin - I understand your view but you're not right on this.
Our piece is primarily looking at the evidence - but alongside this a case study brings home to readers that all this really does happen to/affect real people like them.
It would run as a few hundred words, alongside the many thousands of words looking at the science.
From an objective perspective, having been looking at this story over the past fortnight, I'd wager anyone reading our piece will agree you win hands down in terms of facts.
Where you are falling short, and have been for some time, is being able to get your message across in a way that is persuasive. Please do trust our experience in communication here, we put out Britain's most read and trusted newspaper health content and have done for decades".
Mr Calman continued to work on identifying the case study that ultimately became "Colin" (see Section F(5) below).
(4) Mr Calman's research on the Claimants' claims
"I read, watched and listened to various materials written and/or published by the Claimants and Dr Malhotra to identify their main claims about statins. From this review, I identified the following as being the most significant challenges made by the Claimants to mainstream scientific opinion as to the benefits and risks of statins:
(i) that LDL-C or bad cholesterol does not play a role in the causation of [cardiovascular disease] and that low LDL-C may be inversely associated with mortality;
(ii) that statins don't work and offer no or negligible benefits even to patients at high risk, for example those who have already suffered a heart attack, because preventing [cardiovascular disease] has nothing to do with lowering LDL-C;
(iii) that the incidents of side effects was being hushed up and that the true rate was about 20 per cent; and
(iv) that the researchers who had conducted the biggest statins studies, in a conspiracy with Big Pharma, had allowed their judgement to be influenced by their financial interests and the evidence they produced was biased and untrustworthy".
(1) The use of the word "examples" suggests that there may be further material which was considered by Mr Calman prior to publication but which he has not identified. That is not a helpful way to approach an important aspect in a case involving a public interest defence. One would usually expect to find a full list of the documentary material relevant to the issues in the claim, and upon which a party relied, in his/her disclosure list, even if the party no longer has a copy of the relevant document (CPR 31.10(4)(b)).
(2) Some of the materials identified by Mr Calman, if he had read them prior to publication, raised questions as to whether he was being fair to the Claimants in the way that he characterised them - and the overall 'statin debate' - in the Articles, and whether he has been cherry-picking the material upon which he has relied. By way of example only, from the Sunday Express article of 2 March 2014 (see Annex 4, Section (B)(2)(i)), Mr Calman has purported to identify only statements attributed to the Second Claimant. The article contains a great deal more by way of context and further information that would potentially have had a bearing on the Articles.
a) The Second Claimant was only one of the "leading doctors" who were identified in the article as warning about the potential side-effects of statins. The only direct quote attributed to the Second Claimant in the article was the suggestion that pushing someone off a cliff might stop him/her dying from heart disease. The article was, in fact, dominated by quotes from Dr Malhotra. It is not right, therefore, for Mr Calman to suggest that the Second Claimant had claimed in the article that one in four people were likely to be at risk of terrible side effects or that independent studies showed side effects in at least 20 percent. The first claim was attributed generally to "leading doctors" and the second was attributed to Dr Malhotra in the article.
b) The "health chief", identified in the headline was Dr Kailash Chand, deputy chairman of the British Medical Association, who said he had personally experienced "awful" muscle pains while taking statins. He was reported as having decided to stop taking them and "things started to improve within two or three weeks". Dr Chand was quoted as having warned that "giving the drugs to low-risk patients was 'a commercialisation device' and not in their interests".
c) Dr Fiona Godlee, the editor in chief of the BMJ was quoted as saying: "The decision to increase use of statins is based on trial data only a few chosen people have seen. We need to demand greater transparency about the research on these drugs. Why aren't we looking at changes in lifestyle that reduce heart disease risk instead of medicalising vast numbers of people?"
(1) Concerning the First Claimant, there is corroborative contemporaneous evidence demonstrating that Mr Calman had seen and read the following documents, prior to publication of the Articles:
a) the two Articles published by the First Claimant on her Blog in February 2019, whilst Mr Calman was working on the Articles: "Statins in the over 75s" (see [69]-[70] above) and "Why cholesterol can't cause heart disease" (see [82] above);
b) a further article by the First Claimant, dated 6 October 2014, from which Mr Calman agreed in evidence "it may well be" he got the phrase "statin pushers" used in the Main Article ([61]); and
c) the First Claimant's response to Mr Calman's right of reply email (see [197] below).
(2) Concerning the Second Claimant, there is corroborative contemporaneous evidence demonstrating that Mr Calman had seen and read the following documents, prior to publication of the Articles:
a) the 2016 Ravnskov Study (see 92[93] above and [210] below);
b) the interview with the Second Claimant on BBC Radio 4's Today programme (see [211] below);
c) the cover, and part of Chapter 14 of A Statin Nation; the former appearing to be the source of the quote in paragraph [25] of the Main Article and the caption to the photograph of the Second Claimant and a quote from the latter appearing in an earlier draft of the Articles;
d) an article published on the Second Claimant's blog, "Response to the Lancet paper", on 3 February 2019 (see [73] above); and
e) the Second Claimant's responses to Mr Calman's right-to-reply email (see [195]-[196] below).
"When I was asked to prepare all the documents that I relied on when the complaint was made, I then started going back through all of the blogs and pulling them off, reading them and trying to work out which ones I had been reading, which ones were relevant, and contributed to the four broad areas that we talked about in the article...
... When I came to preparing all of the information, I want back over the tweets, the historic tweets, and picked out things that I thought looked familiar... To the best of my memory, they were the things that I'd looked at during the preparation."
(5) The interview with Colin
Mr Calman: I believe that you were on statins?
Colin: I was
Mr Calman: But then you stopped taking them because you had heard they weren't so great.
Colin: I wouldn't say I heard that they [weren't] so great, but my experience was I was... on quite a lot of medication, and I couldn't narrow it down to one or the other and I was feeling lethargic... [The] digestion was changing and everything else. I also spoke with a lot of other people who had gone through a heart attack ... who had gone through similar things and they said 'Oh, I stopped taking my medication a long time ago.' So I asked them whether they had any detrimental effects, and they said they hadn't. I'm not one to actually believe everything I read. I'd sooner make my own choice.
Mr Calman: Did you ever read anything negative about them?
Colin: Yeah, it's out there all the time... And you hear a lot of things on the media. I listen to quite a lot of radio, you get people coming on there speaking about it. And you know yourself when people come on, what they believe is the truth, even though it might not be, and so they can perpetuate quite a lot of untruths going out there when, you know, people believe what they hear. They take that away, and they thing it's fact then. I'm probably not one of those. I'd like to make my own decisions...
Mr Calman: ... was there a something that triggered that moment, was there a conversation or something you heard on the radio or what was it that really triggered that moment where you said, 'right, I'm just going to stop taking these'?
Colin: I think one thing, after I did the huge hike - well over 2,000 miles unsupported. It felt quite good after that when I got back and I stated to ask myself the question... How much benefit is there in this. Is there a question of - this could be a huge myth with doctors - how much money do they get paid for giving out medicine?
Mr Calman: Where had you heard that...?
Colin: I've heard it... Doctors, you know, they have certain... levels that if they can sell, it's in their interest to. And there's a lot of medication involved in this, after a heart attack or anything along those lines.
...
Mr Calman: So, you said that... you had heard from doctors. Do you remember was it a doctor on the radio or TV that you had heard that from?
Colin: No, ... these are people in the business going out, maybe to a dinner party or something like that, and you say 'what do you do?' 'I'm a doctor'. Whenever I got an opportunity to chat about medication, I used to, to try and learn more. As I say, I never believe anything I hear - when it's radios, when it's hearsay, because you don't really know, but when you're actually speaking to somebody face to face".
Mr Calman: Because he's a cardiologist who speaks a lot on radio, telly, everywhere, ... on how statins are --- that doctors are just making money off them, and things that you've been saying that make you feel terrible. You don't remember specifically?
Colin: No, I mean, I might have heard of him on a radio station, I don't know.
Mr Calman: Or Malcolm Kendrick?
Colin: Again, that name rings a bell-ish. But that would probably be from radio that I just listened to. But I listen to LBC, so if they had been on that
Mr Calman: What would you say to your younger self who said those kind of things that you thought?
Colin: First of all, I think the key phrase is 'It's not going to happen to me. Regardless of what I do to myself'. You know... you just fell as though, 'no, I'll be all right'. Giving the advice back to myself, I'd say, 'Regardless of whether anybody's making money from it or not, whether that's the truth or not, you've got to put your health first'. And... now looking back, I'd say I was a fool to stop taking them. Especially... if you push all the stress to one side, regardless of that, ... I should have been back to the doctors, and making appointments and looking after myself.
Mr Calman: You didn't even tell anyone that you'd stopped taking them?
Colin: Well, I had tried to tell the doctor but I couldn't get an appointment... They have a chit system, that if you need a repeat prescription... I'd left a message on there saying 'If you need to contact me about this please do, I'm stopping my medication.' The system doesn't help, unfortunately.
Colin went on to suggest that he was rather stubborn and had his "own views". He had some early problems with his medication, but that had been resolved. He had been taking six or seven tablets a day and he had a concern about the cost.
Mr Calman: ... Just going back, do you think... all of these kind of myths or... bad things about medication... they were really affecting your judgment, the things that you had heard and all of that
Colin had not actually mentioned in the interview hearing "myths" or "bad things" about the medication, but, after a pause, he answered:
Colin: They would have obviously had some impact, because there's so much out there when you speak to people that, I said at the beginning, I like to make my own mind up. Regardless of that, if you hear it so many times, that subconscious little monkey at the back of your head keeps telling you, 'Oh, you've heard this, must be some truth in it.'
Mr Calman: No smoke without fire?
Colin: Absolutely. And then, when you speak to so many people - I have to say the majority of people I spoke to who had had heart attacks have stopped medication. And some of that might come down to the fact of the age of people having heart attacks, and that's the other thing I was surprised at. When I went for the self-care after the first heart attack, I thought I'd be one of the youngest. I was in the middle of the road. As I say, you know, it's that youth element again. 'I'll be all right after this, I'll be fine... I've had my scare in life now'. And I think it was the age of the people who had had heart attacked, was the most eye-opening element to that.
...
Mr Calman: So, this is going to be a really important piece... We've got some of the experts who came up with proof that statins work, and we've got them talking about how they know the drugs work and help people, and we're really trying to build a very big, persuasive case
Colin: I understand that, yeah.
Mr Calman: For people like yourself, who have heard all this stuff and really think 'There must be some truth in it, there must be'... So, we're saying there isn't.
Colin: Well, I won't be making the same mistake twice, I'm telling you now...
Mr Calman: No. And I think what the important thing is that you're the perfect example of people that we want to try and target with this piece and so it's a really big deal that you've agreed to talk.
The remainder of the interview was taken up with discussion of whether Colin would be happy for a photograph of him to be used in the article.
"Yes, but I would say, having interviewed victims of misinformation, that it's one of the things that people say. People say, 'I do my own research' or 'I make my own mind up', which kind of means, 'I'm not going to listen to what doctors say necessarily because I don't believe them", and it's part of that thought process. I think he says, 'I make my own decisions' twice, but, clearly, he was believing things that he'd heard, vague perceptions, that, essentially, the drugs didn't work, and decided -- and, interestingly, it is something I didn't pick up, that he did this in late 2013, which is the period of debate, but he decided to stop taking statins when he really needed to and then he got ill again".
"... He was saying that been influenced by things that he had read or heard. Specifically, he was -- I think he says later he's a big radio listener, and that's what he was saying the afternoon after he'd had a heart attack, in his hospital bed, in a hospital gown. So, bearing in mind I'm not going to be pushing this guy. But I think he very clearly said to me that the things he heard in the media, it's all around, 'You start to believe it, no smoke ...' I didn't use 'the monkey' analogy. I used 'no smoke without fire', to which he said 'Absolutely, you begin to believe these things'.
(1) Colin had not stated that he had stopped taking statins because he had "heard they don't really work" ([2]). At best, that can only have been Mr Calman's interpretation of what Colin had said by way of explanation for why he had ceased taking his medication. As I have explained, there was little in what Colin had said that could have supported such an interpretation. Certainly, attributing this, unequivocally, as Colin's reason for giving up his medication simply did not fairly represent what Colin had said. He provided several other reasons why he had done so.
(2) The quotations, attributed to Colin in [4] and [10], are both inaccurate. Colin did not say this in his interview. At best, these 'quotations' represent Mr Calman's inaccurate synthesis of the interview. They materially misrepresented the totality of what Colin had said.
(6) The Hancock Statement
"Having spoken to a colleague of yours by phone, I was told just to pop an email through to your office. I hope this finds you well.
We're getting in touch after a joint editorial published in the European Society for Cardiology's European Heart Journal, signed by the Editors of 30 of the world's most respected peer reviewed medical journals, claimed that recommendations to take heart-attack preventing statins are often 'rejected' by patients due to 'widely disseminated incorrect information that vastly overstates the risks of these drug.'
Following this, on the 3rd of March The Mail on Sunday is set to publish an in-depth investigation into the proliferation of 'fake news' on statins - and debunk the myths that surround the medication.
Our article will carry evidence that refutes the most commonly circulated false claims, including: the idea that statins 'don't work', 'do more harm than good' and that side effects 'are hushed up', by doctors and researchers who have a conflict of interest as they are 'paid' by drug companies.
We do this with input from the world's leading researchers including Professor Sir Rory Collins, Professor Colin Baigent and Professor Robert Clarke of Oxford University, British Heart Foundation President Professor Sir Nilesh Samani, Professor Peter Sever at Imperial College London, and others.
Recent evidence from the London School of Hygiene and Tropical Medicine suggests that public controversy over statins caused by such misinformation prompted an estimated 200,000 people in the UK to stop taking the pills in one six-month period.
The authors of the study also claimed there could be 2,000 extra heart attacks or strokes over the following 10 years as a consequence.
While recognising the importance of public debate The Mail on Sunday - which carries the only health section on Fleet Street that's checked and approved by doctors - believes this is hugely worrying, and plans to set the record straight as part of our campaign to Fight Fake Health News.
We feel it's important to include a comment from the Health Secretary, giving your view on the problem of fake news about statins, which is undeniably damaging to public health. The comment would take a prominent place in the article.
Please do let me know how we might make this happen - 10 minutes on the phone this week would be ideal. Many thanks and do also let me know if you need any further information".
A similar email was sent, a few minutes later, to Dame Sally Davies, the then Chief Medical Officer for England. Although by this stage, Mr Calman knew that the articles that he would later publish would prominently feature the two Claimants and Dr Malhotra, he did not mention them in the emails to Mr Hancock or Dame Sally.
"... by this point in my preparation of the Health Section Article each of the Claimants and Dr Malhotra were firmly in my mind as potential subjects of the reporting. I have no reason to doubt that I mentioned both of the Claimants' names (as well as Dr Malhotra's) to Syeda Hasnain when we spoke, though I cannot say definitively and do not have a note of that call. We spoke for some time. I believe I would likely have said: 'you probably know the people involved, people like Aseem Malhotra, Malcolm Kendrick and Zoe Harcombe'. I see no reason why I would have left either of the Claimants (or Dr Malhotra) out in that conversation.
I certainly believe that I was clear that the piece would focus on individuals. It's also supported by the fact I referred to specific individuals in a follow up email sent to Syeda Hasnain at 16.19 ... after our call... I note that I did not name Dr Harcombe in that follow-up email. That was likely because she is not a GMC registered doctor..." (emphasis added)
"I made it very clear that this is going to name individuals. I name the individuals we are going to name and in [the follow up email]... I don't think I could have been clearer... To my memory I mentioned all three to Syeda".
Ms Page KC asked Mr Calman whether he had a positive memory of mentioning all three names in his conversation with Ms Hasnain, and he responded: "I am fairly sure that I did mention all the names". (See further [149]-[151] below).
"Hi Syeda, thanks for your time today. I'm glad you think this is a worthwhile piece - as you can see from the attached editorial, the spread of fake news about statins is something many of the world's leading cardiologists are deeply concerned about.
A press release on the LSHTM study is here [link provided]. They call it 'a period of intense debate about statins' but essentially, what they're talking about is fake news. Prof Liam Smeeth, who led the study, is on record as saying so and will be talking in our forthcoming article.
In terms of how much this is a problem within UK patient population, to quote them: 'Scaling their findings up to the UK population, the researchers estimated that, assuming the intense media coverage was the cause of the observed changes, it could have resulted in more than 200,000 patients across the UK stopping statin therapy in the six months following the exposure period... they estimated there would be at least 2,000 cardiovascular events over the next 10 years, which would not have occurred if these patients had continued taking statins.'
And the fake news is very much home grown too: at least two of the widely quoted statins deniers, Dr Malcolm Kendrick and Dr Aseem Malhotra, are GMC registered and see patients in the UK right now. Below is the letter to Matt Hancock. Thanks again for looking at this. If we could get a statement by the end of the week echoing these concerns and encouraging patients to follow evidence based advice, it'd be great".
I would note that Mr Calman's description of the LSHTM Paper, in the second paragraph, was misleading, but if Ms Hasnain had followed the link, she would have seen for herself the parameters of the LSHTM Paper, and its concentration on the 6‑month period following publication of the 2013 BMJ Articles.
"I really feel the main thing this now needs is the political input - we have the world's leading clinicians, as outlined in my email, talking about how they feel misinformation on statins is potentially a bigger threat than the MMR scandal, evidence that people who have had heart attacks have stopped taking statins after reading doctors in the media claiming they don't need them, and evidence that this might have resulted in more heart attacks, stokes, and deaths.
These incredible doctors have devoted their lives to protecting public health.
And yet their message is being lost amid huge amounts of misinformation and falsehoods than are not back with a shred of credible evidence.
If the Secretary of State gives them his backing, it will send a strong message".
Again, this email gave no indication that the Articles were going to concentrate on three individual "statin deniers".
"Apologies for the delay, below is Matt's quote for your piece...
Matt Hancock, Secretary of State for Health and Social Care said:
'Medical evidence shows that statins save lives. Needlessly risking people's health by spreading reckless and ignorant misinformation claiming otherwise is completely unjustifiable. These kind of pernicious lies have no place in our NHS and I welcome the Mail on Sunday's work to shine a light on the scale of the problem.
'As part of our Long Term Plan for the NHS we want to save thousands more lives from preventable conditions like heart disease and strokes. Medicines like statins can and do play a huge role in keeping people at risk of cardiovascular disease healthy - I strongly urge anyone who is prescribed them to listen to the advice of their doctors and nurses.'
Ms Page KC: "Did you really believe that if Mr Hancock had known you were going to name individuals, let alone individuals... he would have given you a quote without any strings attached containing this sort of language?"
Mr Calman: "That was the official quote from the Secretary of State for Health and Social Care, and I took it as read and I was transparent about our project in emails and conversation with the department and that was what he provided. That was what he provided".
Ms Page KC: "Did it not occur to you that you might have put Mr Hancock in jeopardy because you were -- he was entrusting you with some statements that would be highly defamatory of ... GMC‑registered doctors if anyone was named?
Mr Calman: "As I said, this was the comment from a government minister. It is not for me to question the comment coming from the government minister and go back and say, 'Is he sure?'"
Ms Page KC: "Well, when we see your reaction to it... you say: 'Thanks all. This is EXACTLY what we're after.' It was just what you wanted, wasn't it?
Mr Calman: "Yes, I think I was clear in our conversation that sometimes you get very anodyne comment from the government on issues, especially controversial ones. You know, we get them all the time. And I think we'd had a conversation about, you know, 'I think the best thing would be' -- obviously, you know, a punchy comment would be what we were after, and I think she said, 'Don't worry, that's exactly what we're going to give you'".
"Matt, can I confirm that you WERE NOT told by the Mail On Sunday that the statin article was specifically attacking myself and Zoe Harcombe? Will be good to know. I'm speaking to Tom Watson about it today... And there will be an official robust response".
Two minutes later, Mr Hancock responded:
"Yes, I had no idea they'd link it"
"... Now I must mention, and I think this is really important and crucial, two points. One is, I have had a read through the article and I must say that it is inaccurate, it is distorted, it is defamatory and it's misleading and I will be writing to the Mail on Sunday and also contacting the Independent Press Standards Organisation, IPSO, to call for it to be at least majorly corrected if not retracted and on the Matt Hancock issue, what's interesting is I actually met Matt Hancock last week, I was invited to meet him. To discuss how we can help curb the epidemic of type 2 diabetes in this country and he has been, you know, I've had a very good rapport with him, he's been supportive of my work. He realises that we have a big problem here and there are certainly lifestyle issues that we can implement that will help patients reverse a disease and reduce the need for medications. Matt actually messaged me today to tell me that he had no idea that I was linked to this article. So there are some serious question marks about the journalism involved in this particular piece in the Mail on Sunday, but I am very happy to talk about the evidence around statins and what I advise my patients..."
(1) At 14.14, Mr Calman emailed Ms Wilson, forwarding a copy of his email of 19 February 2019 to Ms Hasnain (see [138] above):
"Thanks so much for working with the MOS on our investigation last week. We've heard the TalkRadio broadcast, which is defamatory. Our correspondence, below, is now with the managing editor of The Mail on Sunday, as it's now become a serious matter. I look forward to your response".
(2) Ms Wilson responded, at 14.36:
"Thanks Barney. We are aware of this issue - I will come back to you shortly".
(3) Mr Calman replied, at 14.44:
"Thanks Sara, we're keen to deal with this swiftly as obviously this claim is now being widely circulated online.
(4) Not having received a response, Mr Calman followed up with a further email at 18.18, copied to (amongst others) Ms Hasnain and Mr Wellington, the then Managing Editor of the Mail on Sunday:
"We're surprised Matt Hancock would make such a specific and strongly worded comment on an investigation into doctors making false claims without asking who the doctors are. It seems shambolic that he would not be provided with the facts. It now leads to the question why the Secretary of State for Health was privately messaging Dr Malhotra this morning, and we welcome his clarification on the matter".
"As I said on the phone, there has been no suggestion from us that Matt Hancock wants to retract his statement and he remains supportive of your campaign. This was a general comment warning about clinicians spreading misinformation it was not intended to single out any one individual. We are still very happy for you to reference his support for your campaign in any follow up coverage and to refer to his statement. However I'm sure you'll appreciate that we cannot comment on Mr Hancock's and Dr Malhotra private correspondence".
Mr Calman forwarded Ms Wilson's email to Mr Wellington at 19.53 with the message "see below".
"... I considered that Matt Hancock had a very clear idea of what and who he was commenting on. I found it contradictory that we could get such a straight response from Matt Hancock and then hear something seemingly different on the radio from Dr Malhotra".
After referring to his email exchanges with Ms Wilson, he added:
"I called Sarah Wilson at the DHSC. She said that there was no indication that Matt Hancock would like to withdraw the statement. They confirmed that the statement was about clinicians (the identities of which they knew) but the statement was not meant to be about one particular clinician. She said they would provide me with a written response shortly".
Mr Calman confirmed, in his evidence, that he did not take a note of this call and the Defendants have not relied upon any evidence from the relevant individuals at the Department of Health clarifying what they understood about the article prior to providing the Hancock Statement.
"Whilst Matt Hancock said that he was not intending to single out 'any one individual', he was commenting on the issue as a whole on which the DHSC had been briefed as to the claims, and some of the individuals responsible, which the article would address. He did not give a caveat, as many do under such circumstances, such as 'I cannot comment on an individual case'.
By this point, i.e. on 5 March, I would expect that Matt Hancock would have seen our coverage, seen the way we had used his statements, and seen the way we had presented them alongside references to the Claimants. In spite of this, through his officials he said that he did not want to retract the statements, that he remained supportive, and that we could continue to refer to the statements in follow-up coverage.
There was no suggestion from the DHSC that they wanted or that there was any need for a correction.
I note that the Claimants contend that when I said to the DHSC that the correspondence was with the Managing Editor of [The Mail on Sunday], this was apparently some sort of implicit threat to complain about the matter or 'expose' Matt Hancock for not telling the truth in his message to Dr Malhotra. In my email, I said I had involved the Managing Editor because that is what I had done. In the interview Dr Malhotra said our report was 'misleading, distorted, inaccurate, and defamatory'. These are serious allegations and, had such a complaint be (sic) made - not that one was at the time - then it would have been the Managing Editor who responded. It may well have been that he would have needed to speak with the DHSC, and so it was important to let them know straight away. I also knew that Ms Hasnain did know that we were investigating Dr Malhotra, as we had named him in our correspondence, and so I was seeking a clarification from the DHSC".
"I don't see it that way, no. I think it's really clear that I was - I was very clear - I made it very clear that we were going to name individuals in the piece. I followed that up with an email that named the individuals that I felt were, you know, the most important that they knew and, when the radio show happened, I reforwarded that email".
"Well, going back to it, I mean, I did say about - I say about the - I mean, talking - I was talking about the statins deniers and I think their ultimate response was that he was talking about clinicians, so here was real clarity, I think, from our conversations, and then from the email which named the individuals, that we were talking about the deniers, individuals, naming individuals. So, yeah".
"I believe I would likely have said, 'You probably know'. That's how I would have put it. The reason that I would have mentioned Zoe Harcombe is because of her involvement with - I think the reason that I would have mentioned Zoe Harcombe would be because of the involvement with the non-governmental - the all-party parliamentary groups and suchlike. She has spoken at those kinds of things".
"Regarding Matt Hancock, prior to publication we contacted the Department of Health and Social Care and said we intended to publish an article stating that statins deniers were circulating claims that statins are not effective and that side effects are begin hushed up by scientists who are being paid by the drug companies. We said we intended to rely on evidence from the London School of Hygiene and Tropical Medicine suggesting that public controversy and misinformation over statins has caused 200,000 UK patients to stop taking the drug and, as a result, there could be 2,000 extra heart attacks or strokes over the following ten years. We indicated that you could be named in the article. Matt Hancock provided us with the statement. Following publication, and your statement about your private correspondence with Mr Hancock, we checked with the DHSC who confirmed that Mr Hancock was standing by his statement in full".
Mr Calman accepted when giving his evidence that, considering the contents of Ms Wilson's email of 5 March 2019 (see [146(5)] above), the final sentence of Mr Wellington's email "may not have been put perfectly". The email was subsequently sent, in the same terms as the draft, in response to Dr Malhotra.
(1) There is the direct and immediate evidence from Mr Hancock, in response to Dr Malhotra's message to him, that he was unaware that the Articles would attack individuals (see [144] above). The Defendants do not suggest that Mr Hancock was not telling the truth when he provided this answer. They have not obtained any evidence from him. Had Mr Hancock's staff been aware that the Articles would target individuals, it is overwhelmingly likely that they would have advised him of this important fact, and they would have warned him of the potential consequences of issuing a statement, in the terms that the Hancock Statement was issued, to be published with the Articles. The risk that Mr Hancock would be seen to be endorsing a very serious attack on the three individuals would have been obvious to any competent media or communications adviser, and one that they would have wanted to avoid.
(2) Of greater significance are the immediate responses, sent by Mr Calman during the afternoon following the TalkRadio broadcast on 5 March 2019. As explored by Ms Page KC in cross-examination, if Mr Calman had effectively communicated to Ms Hasnain, during his telephone call with her, that the Articles were going to feature prominently the three targets as "statin deniers", the obvious answer to Ms Wilson was to protest strongly that what Mr Hancock had said to Dr Malhotra was flat contrary to what had been discussed with Ms Hasnain in the telephone call. The wording of the email of 18.18, only provides limited support for Mr Calman's recollection of the telephone call with Ms Hasnain. Even then, had the call been as described by Mr Calman, the emails sent that afternoon would have been in materially different terms. The final sentence of the email at 18.18 is oddly incongruous, and might even be read as some sort of threat. On Mr Calman's version of the telephone call, Mr Hancock's message to Dr Malhotra either demonstrated that he was lying or that his staff had completely failed to brief him properly (and had recklessly allowed a statement to go out in his name without his being fully appraised of the important context in which it was likely to appear). Yet, neither of these angles - which were journalistically potentially of interest and significance - was pursued.
(3) The final email from Ms Wilson, on 5 March 2019 (see [146(5)] above, stated clearly that Mr Hancock had provided a "general comment" and was not intended to single out any individual. That effectively confirmed to the Defendants what Mr Hancock had privately told Dr Malhotra. The absence of a note of the conversation between Mr Calman and Ms Wilson, that preceded this email, is odd, as is the failure to respond to the email. If the telephone call between Mr Calman and Ms Hasnain had been as he had described, Ms Wilson's explanation of Mr Hancock's message to Dr Malhotra was potentially very important; and the obvious retort to Ms Wilson's email was that the position adopted on behalf of the Minister was untenable in light of the conversation with Ms Hasnain. No such message was sent.
(4) I do not regard as significant (to the question of what was discussed in the telephone call) the fact that neither the Department of Health nor Mr Hancock sought to take the matter further or issued any public statement in clarification of the reporting of the Hancock Statement in the Articles. On the facts as I have found, this was an embarrassing mistake for the Department of Health to have made. The Hancock Statement had been provided for publication without a proper understanding of the context it was going to appear. That might be thought to be a basic - even elemental - failure in professional media communications. As I have noted already, the Defendants have not provided any evidence from the relevant staff at the Department of Health. It is not necessary for me to make findings as to what went through the minds of those concerned with the Department of Health's response, and specifically why no public statement was issued confirming what Mr Hancock had stated privately to Dr Malhotra (the substance of which had also been confirmed to Mr Calman in Ms Wilson's email of 5 March 2019). One possible explanation is that it was decided that to do anything more risked making a bad situation worse. Many factors may have influenced that decision, and it is not necessary for the purposes of this judgment to consider the issue further. The adequacy or appropriateness of the Department of Health's response is not a matter I need to resolve in this trial.
Q: You told Ms Page in answer to some of her questions that you did not think that the article suggested to readers that the claimants were dishonest and telling lies.
A: No, I don't think it did. I wanted it not to say that.
Q: Okay. Can I ask you then to look at the article..., page 2 in the newspaper article, paragraph 5. This is the news article:
"The health secretary said these kinds of pernicious lies have no place in our NHS and I welcome the Mail on Sunday's work to shine a light on the scale of the problem".
In light of your answer about not wanting to suggest that the two individuals were liars, did you feel uncomfortable about the inclusion of that quote?
A: At the time, I just took it as read, and I didn't question that -- I did have an exchange with Stephen Adams about the issue. I think he'd interpreted that in an earlier draft of the piece and said something along the lines of false claims, and I emailed him, and you'll see those emails, saying, "We are not suggesting they're false; it's that they're wrong", or something like -- I don't have it in front of me. So, within that, that was in my mind, and having spoken to this -- having, you know, been in contact with the legal team, that was what I felt was the due diligence in that respect, and that was really what was utmost in my mind, not changing his statement. I wouldn't consider changing his statement".
"The point is, and it says this very clearly in the article, it is not that they're deliberately telling falsehoods or they're liars, it is that they're simply mistaken, and they're mistaken with very grave consequences. You know we call them 'statin deniers', we don't call them 'statin liars'... I do not believe, and have never believed, and it was never part of my original discussion with Barney or part of the story that these people were saying these things knowing them to be false. I mean, why would they do that? That just isn't the story"
(7) The LSHTM Paper
"Objective: To quantify how a period of intense media coverage of controversy over the risk:benefit balance of statins affected their use.
Design: Interrupted time series analysis of prospectively collected electronic data from primary care.
Setting: Clinical Practice Research Datalink (CPRD) in the United Kingdom.
Participants: Patients newly eligible for or currently taking statins for primary and secondary cardiovascular disease prevention in each month in January 2011‑March 2015.
Main outcome measures: Adjusted odds ratios for starting/stopping taking statins after the media coverage (October 2013-March 2014).
Results: There was no evidence that the period of high media coverage was associated with changes in statin initiation among patients with a high recorded risk score for cardiovascular disease (primary prevention) or a recent cardiovascular event (secondary prevention) (odds ratio 0.99 (95% confidence interval 0.87 to 1.13; P=0.92) and 1.04 (0.92 to 1.18; P=0.54), respectively), though there was a decrease in the overall proportion of patients with a recorded risk score. Patients already taking statins were more likely to stop taking them for both primary and secondary prevention after the high media coverage period (1.11 (1.05 to 1.18; P<0.001) and 1.12 (1.04 to 1.21; P=0.003), respectively). Stratified analyses showed that older patients and those with a longer continuous prescription were more likely to stop taking statins after the media coverage. In post hoc analyses, the increased rates of cessation were no longer observed after six months.
Conclusions: A period of intense public discussion over the risks:benefit balance of statins, covered widely in the media, was followed by a transient rise in the proportion of people who stopped taking statins. This research highlights the potential for widely covered health stories in the lay media to impact on healthcare related behaviour".
(1) The "exposure period" for the study was media coverage between October 2013 and March 2014. The start date was chosen by the researchers to coincide with publication of the 2013 BMJ Articles. The end date was chosen using a Google trend analytics for the term "statin side effects". The resulting trial period represented a period of "widespread coverage of the debate over statin side effects across most national media outlets in the UK".
(2) The study did not involve identification of the particular impact of any individual media report on people who ceased to take statins. The analysis was of overall trends of initiation and cessation of statin therapy. In consequence, the authors noted that it was not possible in the study to confirm a "causal link between the media coverage and observed changes in the likelihood of stopping taking statins". In other words, the study had not established the link between any media coverage, and any individual's decision to cease taking statins. Colin, perhaps, stands as an example of how difficult it would be to determine the issue of causality between media reports and a person's decision to stop taking statin medication, but it was quite clear from the LSHTM Paper that its study did not investigate individual decision-making.
(3) On an assumption of causality, the authors estimated:
"... that across the UK there was an excess of 218,971 patients who stopped taking a statin in the six months after the media coverage"
and
"... that increases in statin cessation due to the period of media coverage of side-effects could result in at least 2,173 excess cardiovascular events over 10 years, depending on the proportion of 'stoppers' who re-started later. Our calculations were based on several assumptions and approximations and clearly could not take account of future chances in statin use and perceptions of other developments in prevention of cardiovascular disease. Varying assumptions also lead to substantial changes in the outcome, meaning these estimations should be interpreted with caution. We also cannot know from our data the extent to which patients were appropriately informed about the risk:benefit balance of statins and whether those who have stopped would have been aware and accepting of the consequent increases in risk of cardiovascular disease. Patients can vary widely in the choices that they make about long term preventative drug treatment, and some choose not to take drugs that will extend their life. Finally we did not attempt to take into account any possible benefits of stopping treatment with statins, which might have offset the increase in risk."
(4) The conclusion was:
"Controversy over the risks and benefits of statins reported in both the medical and popular press was followed by a transient increase in patients stopping treatment prescribed for primary and secondary prevention... This research highlights the potential for widely covered health stories in the media to have an effect on real world behaviour related to health-care and could be used to inform future interactions between clinicians, researchers, the academic press, and the wider media."
(1) On 2 February 2016, the authors of the LSHTM Paper submitted the manuscript of the paper for consideration for publication in The BMJ.
"Thank you for sending us your paper. We sent it for external peer review and discussed it at our manuscript committee meeting. We recognise its potential importance and relevance to general medical readers, but I am afraid that we have not yet been able to reach a final decision on it because several important aspects of the work still need clarifying.
We hope very much that you will be willing and able to revise your paper as explained below in the report from the manuscript meeting, so that we will be in a better position to understand your study and decide whether the BMJ is the right journal for it".
Under the heading: "Report from the BMJ's manuscript committee meeting", the email contained the following:
"These comments are an attempt to summarise the discussions at the manuscript meeting. They are not an exact transcript.
Members of the committee were: Elizabeth Loder (Chair), Gary Collins (Statistics advisor), Rubin Minhas, George Roeggia, Alison Tonks, Tiago Villanueva, Wim Weber"
(3) The report contained "Additional Questions" about the paper from the following identified "reviewers": (1) Halava Heli, described as "MD, clinical instructor" at the University of Turku, Finland; (2) Andrea Schaffer, described as "PhD candidate (Pharmacoepidemiology)" at the Centre for Big Data Research in Health, University of New South Wales; (3) Hans Wouters, described as "Postdoctoral Researcher" at the University of Groningen; (4) Cunyet Kocas, described as "MD" at the Cardiology Institute of Istanbul University; and (5) Florence van Hunsel, described as "Coordinator Signal detection" at the Netherlands Pharmacovigilance Centre Lareb . Each of these reviewers declared that s/he (a) had not in the past five years, been employed by an organisation that may in any way gain or lose financially from publication of the paper; and (b) did not hold any stocks or shares in an organisation that may in any way gain or lose financially from publication of the paper.
(4) On 11 May 2016, the authors of the LSHTM Paper submitted their comments in response to the observations of the manuscript committee.
(5) On 22 May 2016, Dr Webber confirmed that the paper had been accepted for publication in The BMJ.
"BMJ is committed to ensuring the independence and integrity of our content, products, and services. We strive, therefore, to be transparent about any interests that our users, customers, and partners might want to know about. ... Above all we want transparency about any personal or organisational interests that might be seen as a conflict of interest in relation to the task a person is being asked to do for BMJ...
3. ... If we ... commission [a] peer review report from you, we will ask you to declare your interests at the first approach in case there are conflicts of interest that preclude you from accepting the invitation.
4. ... If a full declaration was not made at the time and a conflict of interest comes to light after the event, BMJ reserves the right to retract any content affected by this conflict. BMJ may also seek to terminate contracts or employment affected in this way, and may choose not to work with the individual in the future.
6. ... Where material is peer reviewed, requests for declarations will be sent to the peer reviewers, and editors will send any author declaration of interest statements to the peer reviewer".
(2) Dr Weber, who wrote the letter of acceptance to the authors, was the editor responsible for deciding to publish the LSHTM Paper. Alternatively, the manuscript committee were the editors who were responsible for deciding to publish the LSHTM Paper.
(3) It is evident from the report of the manuscript meeting, sent by Dr Weber and which contained the committee's detailed comments on the manuscript, that Dr Weber and the other members of the manuscript committee carried out a review of the LSHTM Paper's scientific merit.
(1) In his observations, Cuneyt Kocas stated that he was the published author of a 2015 study, published in the International Journal of Cardiology, which used "nearly the same method" to study the impact of media coverage on statin adherence. In their response, the authors of the LSHTM Paper stated that they had updated the introduction and discussion to include reference to this "relevant paper".
(2) In her observations, Florence van Hunsel stated that she had written a report on the impact of the media on cessation of statins in the Netherlands in the British Journal of Clinical Pharmacology in 2009. When published, the LSHTM Paper acknowledged this study.
(1) The BMJ's published policy on declarations of interest (see [179(6)] above);
(2) the statements from each of the reviewers that they had read and understood this policy and his/her declaration that s/he had no competing interests; and
(8) Pre-publication approaches to the Claimants and their responses
"I was certainly not just going through the motions or ticking a box by sending these right of replies and including responses. I think if you are going to debunk something then you need to ensure that space is given to explore the credence of what they say. I was genuinely looking forward to their responses and hearing what they had to say. Sometimes people come back with information that stops or postpones the publishing of a story, but that didn't happen here".
"Dear Zoe - The Mail on Sunday plans to publish an article this weekend on growing concerns about claims you and a number of other individuals have publicly made about statins, the role of cholesterol in heart disease, and the allegations that researchers into the drugs are financially conflicted due to payments made to the organisations they work for, and so the evidence they provide about the effectiveness of these medications, and their side effects, are in some way untrustworthy.
Over the past 30 years, more than 200,000 patients have been put through the most rigorous forms of clinical trials to produce definitive proof the tablets lower heart attack risk by up to 50 per cent, and a stroke by 30 per cent, and reduce the risk of death - from any cause.
In January, the editors-in-chief of all 30 major heart health medical journals - each a leading cardiologist - signed a joint open letter, warning: 'Lives are at stake [due to the] wanton spread of medical misinformation. It is high time that this stopped.'
A 2016 analysis from the London School of Hygiene and Tropical Medicine, which tracks outbreaks and public health concerns, found fake news about statins may have prompted 200,000 patients in Britain alone to quit the drug over a single six- month period following an article you wrote for the BMJ which claimed, incorrectly, that 20 per cent of statins patients quit the drug because of side effects.
They estimate that up to 2,000 heart attack and strokes could be a result of this. We would like to offer you the opportunity to respond to this and the following:
* You recently blogged: 'High cholesterol is not even associated with high heart disease, let alone a cause of it.' This contradicts the aforementioned clinical trials and encourages patients to ignore medical advice to take potentially life-saving medication.
* In a 2014 newsletter you call cardiologists, researchers and bodies involved in statin research 'statin pushers' - echoing the term drug pusher, or someone encourages others to take harmful drugs and who makes money supplying those drugs. You appear to justify this by listing those who have received remuneration or funding in some form or another from pharmaceutical companies.
* It has been alleged that the potential consequences of this, and other claims you have made about statins and cholesterol, far outweigh that of the infamous MMR vaccine scandal with one researcher saying: 'In terms of death and disability that could have been prevented, this could be far worse.'
* In our article, one leading cardiologist states that comments you make in interviews and in writing about cholesterol and statins sound convincing but that in reality 'they contain a grain of truth, mixed with speculation and opinion, which makes is very difficult for the public to know who to trust.'
* You often quote observational studies as proof of your claims about statins and cholesterol in articles and blog posts which contradict findings of authoritative clinical trials, which you do not mention. This is misleading.
* Your stance on statins and the link between cholesterol and heart disease amounts to misinformation... "
"Dear Dr Kendrick - The Mail on Sunday plans to publish an article this weekend on growing concerns about claims you and a number of other individuals have publicly made about statins, the role of cholesterol in heart disease, and the allegations that researchers into the drugs are financially conflicted due to payments made to the organisations they work for, and so the evidence they provide about the effectiveness of these medications, and their side effects, are in some way untrustworthy.
Over the past 30 years, more than 200,000 patients have been put through the most rigorous forms of clinical trials to produce definitive proof the tablets lower heart attack risk by up to 50 per cent, and a stroke by 30 per cent, and reduce the risk of death - from any cause.
In January, the editors-in-chief of all 30 major heart health medical journals - each a leading cardiologist - signed a joint open letter, warning: 'Lives are at stake [due to the] wanton spread of medical misinformation. It is high time that this stopped.'
A 2016 analysis from the London School of Hygiene and Tropical Medicine, which tracks outbreaks and public health concerns, found fake news about statins may have prompted 200,000 patients in Britain alone to quit the drug over a single six- month period following an article you wrote for the BMJ which claimed, incorrectly, that 20 per cent of statins patients quit the drug because of side effects.
They estimate that up for 2,000 heart attack and strokes could be a result of this. We would like to offer you the opportunity to respond to this and the following:
* In your latest book, A Statin Nation, you state: 'People are being conned. The way to avoid heart disease... has nothing to do with lowering cholesterol.' This is despite clinical trial evidence to the contrary, and despite no evidence that there is a con, which would imply that those who claim that lowering cholesterol can help lower the risk of heart disease know this is untrue, and are deliberately misleading the public.
* It has been alleged that the potential consequences of claims you have made about statins and cholesterol, far outweigh that of the infamous MMR vaccine scandal with one researcher saying: 'In terms of death and disability that could have been prevented, this could be far worse.'
* In our article, one leading cardiologist states that the facts you and others often cite about cholesterol and statins sound convincing but that in reality 'they contain a grain of truth, mixed with speculation and opinion, which makes is very difficult for the public to know who to trust.'
* You often quote observational studies as proof of your claims about statins and cholesterol in articles and in media appearances which contradict findings of authoritative clinical trials, which you do not mention. This is misleading.
* In a recent blog you wrote: 'Professor Sir Rory Collins and Professor Colin Baigent made a pact with the dev... sorry ... they made a pact with the pharmaceutical industry to take hold of all the data on statins. They will not let anyone else see the data they hold. Including all the data on side-effects. It is kept completely secret.' Also: 'A fact that needs to be emphasised is that the CTT will not let anyone else see the data they hold. Including all the data on adverse events [side-effects] and serious adverse events.' It is a version of similar claims you have made numerous times over the years. However the CTT have stated numerous times that they did not originally request the data on all adverse events so did not have it. They also point out that the said data must be requested from the individual research organisation which carries out the trials, and is not in their gift to provide. They say you know this, as they have told you this, so to repeat the claim amounts to a lie.
* Your stance on statins and the link between cholesterol and heart disease amounts to misinformation.
* There is no evidence you work in NHS practice, or as a GP in private practice".
(1) In evidence, Mr Calman said he was solely responsible for what was contained in the right-to-reply emails. He did not seek either advice or input from anyone else.
(2) Although Mr Calman had willingly shared drafts of the Articles with the Professors with whom he had been working closely, he did not provide the drafts to the Claimants (or Dr Malhotra). As a result, the opportunity that the Claimants had to defend themselves about what was about to be published against them was dependent entirely on what Mr Calman included in the emails sent to them.
(3) Mr Calman did not provide references - or links - to the materials upon which he was relying or asking the Claimants to comment. Indeed, given that the Articles were going to concentrate on the alleged claims of the Claimants regarding statins (that it would be claimed amounted to misinformation), apart from the reference to the Second Claimant's book, "A Statin Nation", Mr Calman's failure to identify the specific claims upon which he intended to rely is particularly striking. As he was giving them less than 24 hours to respond, that was neither helpful nor likely to provide the Claimants with the best opportunity to engage with a process that Mr Calman suggested was the best way to "explore the credence of what they had to say". As it happens, no doubt due to their familiarity with the subject matter, both Claimants were (with one exception) well able to identify for themselves the material upon which Mr Calman had relied and the materials upon which they wished to rely in response. In contrast to Mr Calman's approach, both Claimants provided links to the material upon which they relied.
(4) Mr Calman did not identify, by name, those who were criticising the Claimants, instead describing the critics as "one researcher" and "one leading cardiologist". The decision not to name the Professors was not explained (or explored) in evidence. It may not be the most important point, but one consequence is that it impaired the Claimants' ability to respond fully to the criticism and it might potentially have deprived Mr Calman of information relevant to his decision to publish (although the Second Claimant, in his response (see [15]), correctly surmised the identity of his accusers). Sometimes, identifying the person making an allegation can lead to the respondent being able to provide information or evidence which undermines the credibility of his/her accuser and/or demonstrates that s/he has "an axe to grind".
"* You often quote observational studies as proof of your claims about statins and cholesterol in articles and in media appearances which contradict findings of authoritative clinical trials, which you do not mention. This is misleading.
[1] Do I not mention that the studies I quote are observational, or that I do not mention the findings of authoritative clinical trials? Which of these is a problem, and why.
[2] I would add that the proof of the link between smoking and lung cancer was based on observational studies. Does this mean that smoking does not cause lung cancer? Or is that not their argument. Whilst observational studies are not generally considered as robust as randomised clinical trials, they have value. Equally, most epidemiologists would agree that, whilst observational studies (demonstrating association) cannot prove causality (unless the hazard ratios are very high) a lack of association does disprove causation. So it can be fully valid to rely on observational studies where there is no association, or the observation is in direct contradiction to the hypothesis.
* It has been alleged that the potential consequences of claims you have made about statins and cholesterol, far outweigh that of the infamous MMR vaccine scandal with one researcher saying: 'In terms of death and disability that could have been prevented, this could be far worse.'
[3] If I am wrong, then this statement could, perhaps be true, although it does represent a form of reprehensible bullying - accusing someone of causing many thousands of deaths. This is an accusation that Rory Collins has repeatedly made. He attacked the BMJ for publishing an article suggesting statins may have a high incidence of adverse effects. You may wish to see the e-mail exchange between Rory Collins and Fiona Godlee on this site https://journals.bmj.com/sites/default/files/BMJ/statins/SP13_Emails_between_Rory_Collins_and_Fiona_Godlee.pdf
[4] I would also like to point you to a study published in the BMJ open Kristensen ML, et al. https://bmjopen.bmj.com/content/bmjopen/5/9/e007118.full.pdf
[5] The main findings of this study - not refuted by anyone were 6 studies for primary prevention and 5 for secondary prevention with a follow-up between 2.0 and 6.1 years were identified. Death was postponed between − 5 and 19 days in primary prevention trials and between −10 and 27 days in secondary prevention trials. The median postponement of death for primary and secondary prevention trials were 3.2 and 4.1 days, respectively.
[6] What this study found was that if you took a statin for five years, the increase in life expectancy would be (on average) 3.5 days. That is around 0.75 days per year of statin treatment. That is the important outcome. The figures quoted by Collins and Baigent and the Oxford CTT group are relative risk reductions, and these figures are entirely meaningless unless you know the absolute risk. Equally, to state lives can be saved is meaningless. No-ones life can be saved. The best we can achieve is to increase life expectancy. That is what matters. I covered much of this in my book Doctoring Data, which I would recommend you read, as it outlines the ways that data are presented to look as beneficial as possible.
* In our article, one leading cardiologist states that the facts you and others often cite about cholesterol and statins sound convincing but that in reality 'they contain a grain of truth, mixed with speculation and opinion, which makes is very difficult for the public to know who to trust.'
[7] I cannot answer this, what does a grain of truth mean? What is a grain of truth mixed with speculation and opinion. Specific and concrete examples would be required before I could provide any meaningful answer.
*In your latest book, A Statin Nation, you state: 'People are being conned. The way to avoid heart disease... has nothing to do with lowering cholesterol.' This is despite clinical trial evidence to the contrary, and despite no evidence that there is a con, which would imply that those who claim that lowering cholesterol can help lower the risk of heart disease know this is untrue, and are deliberately misleading the public.
[8] Yes, I believe that people are being conned, and I believe the public are being deliberately misled. That is why I called my first book The Great Cholesterol Con. I would point out that there has been one major placebo controlled double blind statin study done. ALLHAT-LLT, which was funded by the National Institutes of Health in the US. The conclusions of the study, published in 2002, were that:
CONCLUSIONS:
Pravastatin did not reduce either all-cause mortality or CHD significantly when compared with usual care in older participants with well-controlled hypertension and moderately elevated LDL-C https://www.ncbi.nlm.nih.gov/pubmed/12479764
[9] All of the industry funded studies were positive. This is either a remarkable coincidence - or something else. A con perhaps?
* In a recent blog you wrote: 'Professor Sir Rory Collins and Professor Colin Baigent made a pact with the dev... sorry ... they made a pact with the pharmaceutical industry to take hold of all the data on statins. They will not let anyone else see the data they hold. Including all the data on side-effects. It is kept completely secret.' Also: 'A fact that needs to be emphasised is that the CTT will not let anyone else see the data they hold. Including all the data on adverse events [side-effects] and serious adverse events.' It is a version of similar claims you have made numerous times over the years. However the CTT have stated numerous times that they did not originally request the data on all adverse events so did not have it. They also point out that the said data must be requested from the individual research organisation which carries out the trials, and is not in their gift to provide. They say you know this, as they have told you this, so to repeat the claim amounts to a lie.
[10] You could perhaps ask them to point you to any letter or any other form of communication that the CTT have had with me. I will let you know the answer, they have never communicated directly with me, at any time. So, for them to say that they have told me anything is, to be fully accurate, a lie. They claim do not hold the data, yet they have managed to publish major papers on statin adverse effects? For instance this one. Interpretation of the evidence for the efficacy and safety of statin therapy.
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(16)31357-5/fulltext [The Lancet Review see [62] above]
Which contains sections such as these
'The only serious adverse events that have been shown to be caused by long-term statin therapy—ie, adverse effects of the statin—are myopathy (defined as muscle pain or weakness combined with large increases in blood concentrations of creatine kinase), new-onset diabetes mellitus, and, probably, haemorrhagic stroke. Typically, treatment of 10 000 patients for 5 years with an effective regimen (eg, atorvastatin 40 mg daily) would cause about 5 cases of myopathy (one of which might progress, if the statin therapy is not stopped, to the more severe condition of rhabdomyolysis), 50–100 new cases of diabetes, and 5–10 haemorrhagic strokes. However, any adverse impact of these side-effects on major vascular events has already been taken into account in the estimates of the absolute benefits. Statin therapy may cause symptomatic adverse events (eg, muscle pain or weakness) in up to about 50–100 patients (ie, 0·5–1·0% absolute harm) per 10 000 treated for 5 years.'
[11] So, they have written a paper outlining all the issues of adverse effects and serious adverse effects - and yet they do not have the data. So, how did they manage that?
*Your stance on statins and the link between cholesterol and heart disease amounts to misinformation.
[12] Perhaps you would like to read [the 2018 Ravnskov Study - see [215] below] (which I co-authored) 'LDL-C does not cause cardiovascular disease: a comprehensive review of the current literature.' https://www.tandfonline.com/doi/pdf/10.1080/17512433.2018.1519391?needAccess=true Which was THE most downloaded paper published by Taylor and Francis in the last year.
[13] Or [the 2016 Ravnskov Study] 'Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review' Published in the BMJ open in 2016 [see [210] below]
'High LDL-C is inversely associated with mortality in most people over 60 years. This finding is inconsistent with the cholesterol hypothesis (ie, that cholesterol, particularly LDL-C, is inherently atherogenic). Since elderly people with high LDL-C live as long or longer than those with low LDL-C, our analysis provides reason to question the validity of the cholesterol hypothesis. Moreover, our study provides the rationale for a re‑evaluation of guidelines recommending pharmacological reduction of LDL‑C in the elderly as a component of cardiovascular disease prevention strategies.'
Which was the most read paper in the journal for five months in a row.
[14] All I see from your e-mail are ad-hominem attacks on me. I see no facts at all. I hope that I have given you sufficient information".
"I would also point you to this paragraph [of Mr Calman's email]
A 2016 analysis from the London School of Hygiene and Tropical Medicine, which tracks outbreaks and public health concerns, found fake news about statins may have prompted 200,000 patients in Britain alone to quit the drug over a single six‑month period following an article you wrote for the BMJ which claimed, incorrectly, that 20 per cent of statins patients quit the drug because of side effects.
[15] I did not write that article. I suggest you check your facts a little more closely before putting any article out there.
[16] Listen, we all know where this attack is coming from. The CTT and Professor Rory Collins and Baigent et al. They attacked Aseem Malhotra and Professor Abramson, then the BMJ, for publishing articles by Aseem and Abramson suggesting statins caused adverse effects in around 20% of people. Collins attacks were severe, and the BMJ was required to hold an investigation, in which Collins attacks on these papers were judged to be unfounded. The entire review can be seen here
https://www.bmj.com/content/bmj/349/bmj.g5176.full.pdf [the BMJ Review Report, see [206] below]
[17] I would strongly suggest that you read it in full. It is, in a restrained manner, damning of Rory Collins and the CTT. Here are a couple of sections from that report
[18] All cause mortality — A recent editorial by Vinay Prasad in Annals of Internal Medicine illustrates a fundamental problem that has consistently concerned the panel. Prasad compared two meta-analyses of statins in primary prevention that differed in their statistical conclusions by less than half a percentage point and yet reached opposite conclusions—namely that that 'statins reduce . . . total mortality' or conversely that 'data ... showed no reduction in mortality associated with treatment with statins'. Unfortunately, patients and clinicians have to make decisions in the grey area between these two diametrically opposed conclusions. The panel supports Prasad's contention that "The Cholesterol Treatment Trialists' study has a robust set of de-identified individual-patient data, which can improve our understanding, and those data should be made widely available.
[19] The conclusions of the BMJ report, which are carefully written are worth considering:
The panel was unanimous in its decision that the two articles do not meet any of the criteria for retraction. The error did not compromise the principal arguments being made in either of the articles. These arguments involve interpretations of available evidence and were deemed to be within the range of reasonable opinion among those who are debating the appropriate use of statins. In making this assessment, the panel is not expressing an opinion about the merits of these arguments, as that work was beyond the scope of the panel.
[20] The panel did have one final comment. It became clear to the panel that the fact that the trial data upon which this controversy is based are held by the investigators and not available for independent assessment by others may contribute to some of the uncertainty about risks and benefits. Different investigators may come to different conclusions with the same data. In fact, a particularly germane example occurred recently in which two experienced Cochrane groups were charged with evaluating a particular intervention and, despite being given the same instructions, data, and resources, did not arrive at identical results or conclusions. The panel strongly believes that the current debates on the appropriate use of statins would be elevated and usefully informed by making available the individual patient level data that underpin the relevant studies
Your truly
Dr Malcolm Kendrick
P.S. employed to work in the NHS as a doctor - which is a fact".
"The Mail on Sunday plans to publish an article this weekend on growing concerns about claims you and a number of other individuals have publicly made about statins, the role of cholesterol in heart disease, and the allegations that researchers into the drugs are financially conflicted due to payments made to the organisations they work for, and so the evidence they provide about the effectiveness of these medications, and their side effects, are in some way untrustworthy.
Over the past 30 years, more than 200,000 patients have been put through the most rigorous forms of clinical trials to produce definitive proof the tablets lower heart attack risk by up to 50 per cent, and a stroke by 30 per cent, and reduce the risk of death - from any cause.
[1] ZH comment - these are relative risk numbers, not absolute risk. Following the 2012 CTSU publication about statins [4], I wrote to the research team asking for access to the raw data so that I could examine absolute risk (among other things). I was refused access to the data. The exact reply was: "The CTT Collaboration holds data on a very strict basis and is not able to provide participant level data to third parties". [5]
[2] Other doctors, academics and researchers have asked for the CTSU/CTT Collaboration data to be shared - including that on serious adverse effects - and it has been consistently refused as "commercially sensitive/confidential".
[3] The Editor in Chief of the BMJ, Dr Fiona Godlee, has asked for an independent review into the withholding of statin trial data arguing that "no single person or group should have exclusive access to data". [6]
[4] In the absence of access to the CTSU data, we must rely on the independent assessment of all evidence for the efficacy of statins presented in the form of numbers needed to treat (an absolute measure). This reports that, for primary prevention (the majority), for those taking statins for 5 years, no lives will be saved and between 2 and 15 times as many people will be harmed (muscle damage, type 2 diabetes) as helped (avoiding an event). [7]
[5] Lives are, of course, never saved. We are all going to die. The measure of interest is - by how much might a life be extended by a particular intervention or drug? This was independently assessed in a non industry‑funded paper (Kristensen et al 2015). This paper reported that if someone took a statin for five years (and endured any side effects for that time): "The median postponement of death for primary and secondary prevention trials were 3.2 and 4.1 days, respectively". [8]
[6] In January, the editors-in-chief of all 30 major heart health medical journals - each a leading cardiologist - signed a joint open letter, warning: 'Lives are at stake [due to the] wanton spread of medical misinformation. It is high time that this stopped.'
A 2016 analysis from the London School of Hygiene and Tropical Medicine, which tracks outbreaks and public health concerns, found fake news about statins may have prompted 200,000 patients in Britain alone to quit the drug over a single six-month period following an article you wrote for the BMJ which claimed, incorrectly, that 20 per cent of statins patients quit the drug because of side effects.
They estimate that up to 2,000 heart attack and strokes could be a result of this. We would like to offer you the opportunity to respond to this and the following:
[6] ZH comment - I have not written any articles in the BMJ (or any other journal) about statins.
* You recently blogged: 'High cholesterol is not even associated with high heart disease, let alone a cause of it.' This contradicts the aforementioned clinical trials and encourages patients to ignore medical advice to take potentially life-saving (ZH comment - no lives will be saved - see above) medication.
[7] ZH comment - this is a fact. High cholesterol is associated with low mortality - from cardiovascular disease (CVD) and deaths from all causes - for all 192 counties in the world.
[8] In 2010, I examined the World Health Organisation data for 192 countries for mean cholesterol levels and deaths from CVD and all-cause mortality for men and women. The relationships turned out to be inverse, for men and women, CVD and all deaths, as shown by these graphs [9] [graphs included].
* In a 2014 newsletter you call cardiologists, researchers and bodies involved in statin research 'statin pushers' - echoing the term drug pusher, or someone encourages others to take harmful drugs and who makes money supplying those drugs. You appear to justify this by listing those who have received remuneration or funding in some form or another from pharmaceutical companies.
[9] ZH - comment. It would be naïve not to think that sums such as £268 MILLION (payments from pharmaceutical companies to CTSU by 2014) and $149 BILLION (what one statin alone has earned one company) (see below) encourage recipients to aggressively encourage people to take those drugs. Just a couple of weeks ago, the Mail on Sunday front page story argued that climate change chief, John Gummer, should quit over his green business conflicts, which amounted to £600,000. The same Mail on Sunday is now trying to bully little me into silence for blogging about vastly greater sums of conflicts of interest! [10]
* It has been alleged that the potential consequences of this, and other claims you have made about statins and cholesterol, far outweigh that of the infamous MMR vaccine scandal with one researcher saying: 'In terms of death and disability that could have been prevented, this could be far worse.'
[10] ZH comment - any attempt to connect any topic to the MMR vaccine scandal is a deliberate and nasty attempt to smear. Rory Collins heads up the CTSU/CTT Collaboration - the organisation that refuses access to data that could improve patient care. Rory Collins made this association in a deliberate and nasty attempt to smear anyone who even wants to discuss cholesterol and/or statins. [11] He is trying to bully doctors and researchers into silence and the Mail on Sunday is collaborating with this bullying.
[11] Collins tried (and failed) to have two papers withdrawn, which referenced another paper that quantified statin side effects. [12] During the investigation into Collins' demands for retractions it emerged that, as of 2014, the CTSU had received £268 MILLION from the pharmaceutical industry (perhaps the Mail on Sunday could find out how high this figure is now?) [13] That's 268 MILLION reasons to silence debate. The Mail on Sunday should be investigating this Goliath conflict of interest, not a couple of 'Davids' who are brave enough to ask questions.
[12] Collins claims that not more than 1 in 50 people will suffer side effects from statins. [14] The same Collins has a patent that identifies patients at increased risk of myopathy (muscular pain). "The test, branded as Statin–Smart, is sold online for $99 (£76) on a website that claims 29% of statin users will suffer muscle pain, weakness or cramps. The marketing material also claims that 58% of patients on statins stop taking them within a year, mostly because of muscle pain". That's misinformation.
[13] The patient leaflet must be honest, by law, about side effects. The patient leaflet for Lipitor lists common side effects, which may affect up to 1 in 10 people, as: nasal inflammation; nose bleeds; increase in blood sugar levels (google "statins diabetes lawsuit"); gastric problems; joint pain; muscle pain; back pain; abnormal liver function. [15] This is the harm that Collins wants silenced. Lipitor, by the way, has earned Pfizer, one of the CTSU funders, $149 BILLION by 2016. [16]
* In our article, one leading cardiologist states that comments you make in interviews and in writing about cholesterol and statins sound convincing but that in reality 'they contain a grain of truth, mixed with speculation and opinion, which makes is very difficult for the public to know who to trust.'
[14] ZH comment - whom to trust - the public should trust those who are not earning £MILLIONS and $BILLIONS, while refusing to share invaluable data. Data that could improve patient care.
* You often quote observational studies as proof of your claims about statins and cholesterol in articles and blog posts which contradict findings of authoritative clinical trials, which you do not mention. This is misleading.
[15] ZH - give me an example?
* Your stance on statins and the link between cholesterol and heart disease amounts to misinformation.
[16] ZH comment - This is a false accusation and I will take further action if you make false accusations. I have examined the entire data provided by the World Health Organisation and found that higher cholesterol is associated with higher deaths, from CVD and all- causes, in men and women, for all 192 countries in the world.
[17] My PhD was an examination of the diet (cholesterol) heart hypothesis. Cholesterol is mentioned 612 times. I have studied this topic at the highest level for several years and I am entitled, if not obliged, to share what I have found.
[18] I'll tell you what misinformation is - on 1st February 2019, a paper was published in The Lancet by the CTSU/CTT Collaboration. [17] A press conference was held to launch the paper. The lead CTSU author, Colin Baigent, was quoted as saying: "Only a third of the 5.5 million over 75s in the UK take statins and up to 8000 deaths per year could be prevented it all took them". This is false. It relies upon evidence in the over 75s for both deaths and primary prevention and neither was found. Figure 5 of that paper confirmed that the Rate Ratio for deaths in the over 75s was not statistically significant. Figure 4 of that paper confirmed that the Rate Ratio for primary prevention was not statistically significant. I have written to the authors (through The Lancet) asking why they have claimed something that they did not find. I have received no reply. The public should also have been warned that the patient leaflet for statins cautions against people over 70 years old taking them.
[19] Another example of misinformation (in addition to that above on side effects) is the continual use of relative risk numbers to scare people into taking statins. You reiterated this misinformation above "lower heart attack risk by up to 50 percent, and a stroke 30 per cent". The absolute difference, even for secondary prevention, for those who manage to take statins for 5 years, is 1 in 39 for a heart attack and 1 in 125 for a stroke. Meanwhile 1 in 50 were harmed by developing type 2 diabetes and 1 in 10 were harmed by muscle damage. [18]
[20] Withheld information is also an issue here. The information being withheld by those running the trials for the pharmaceutical industry is the scandal. Patients are being harmed by not knowing true benefits and true side effects.
[21] The Mail (on Sunday) has always been a bastion for debate - you're asking the wrong questions of the wrong people on this one.
* If you wish for any comments to be included in our article please send them to us by midday this Friday.
[22] ZH comment - Not least as someone who has freely assisted the Mail/Mail on Sunday with many articles and quotations, I expect to be treated fairly, with balance and not quoted out of context".
"Context: Studies have demonstrated that statins administered to individuals with risk factors for coronary heart disease (CHD) reduce CHD events. However, many of these studies were too small to assess all-cause mortality or outcomes in important subgroups.
Objective: To determine whether pravastatin compared with usual care reduces all-cause mortality in older, moderately hypercholesterolemic, hypertensive participants with at least 1 additional CHD risk factor.
Design and setting: Multicenter (513 primarily community-based North American clinical centers), randomized, nonblinded trial conducted from 1994 through March 2002 in a subset of participants from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT).
Participants: Ambulatory persons (n = 10 355), aged 55 years or older, with low‑density lipoprotein cholesterol (LDL-C) of 120 to 189 mg/dL (100 to 129 mg/dL if known CHD) and triglycerides lower than 350 mg/dL, were randomized to pravastatin (n = 5170) or to usual care (n = 5185). Baseline mean total cholesterol was 224 mg/dL; LDL-C, 146 mg/dL; high-density lipoprotein cholesterol, 48 mg/dL; and triglycerides, 152 mg/dL. Mean age was 66 years, 49% were women, 38% black and 23% Hispanic, 14% had a history of CHD, and 35% had type 2 diabetes.
Intervention: Pravastatin, 40 mg/d, vs usual care.
Main outcome measures: The primary outcome was all-cause mortality, with follow-up for up to 8 years. Secondary outcomes included nonfatal myocardial infarction or fatal CHD (CHD events) combined, cause-specific mortality, and cancer.
Results: Mean follow-up was 4.8 years. During the trial, 32% of usual care participants with and 29% without CHD started taking lipid-lowering drugs. At year 4, total cholesterol levels were reduced by 17% with pravastatin vs 8% with usual care; among the random sample who had LDL-C levels assessed, levels were reduced by 28% with pravastatin vs 11% with usual care. All-cause mortality was similar for the 2 groups (relative risk [RR], 0.99; 95% confidence interval [CI], 0.89-1.11; P =.88), with 6-year mortality rates of 14.9% for pravastatin vs 15.3% with usual care. CHD event rates were not significantly different between the groups (RR, 0.91; 95% CI, 0.79-1.04; P =.16), with 6-year CHD event rates of 9.3% for pravastatin and 10.4% for usual care.
Conclusions: Pravastatin did not reduce either all-cause mortality or CHD significantly when compared with usual care in older participants with well‑controlled hypertension and moderately elevated LDL-C. The results may be due to the modest differential in total cholesterol (9.6%) and LDL-C (16.7%) between pravastatin and usual care compared with prior statin trials supporting cardiovascular disease prevention".
"Statins reduce LDL cholesterol and prevent vascular events, but their net effects in people at low risk of vascular events remain uncertain".
The interpretation, noted:
"In individuals with a 5-year risk of major vascular events lower than 10%, each 1 mmol/L reduction in LDL cholesterol produced an absolute reduction in major vascular events of about 11 per 1000 over 5 years. This benefit greatly exceeds any known hazards of statin therapy. Under present guidelines, such individuals would not typically be regarded as suitable for LDL-lowering statin therapy. The present report suggests, therefore, that these guidelines might need to be reconsidered".
"Prof Sir Rory Collins says he has little confidence in British Medical Journal's inquiry into papers on side-effects of drugs.
The Oxford professor who triggered a public row over statins says the Department of Health and other authorities should intervene to ensure the public gets accurate information on the risks and benefits of the potentially life-saving drugs.
Prof Sir Rory Collins said he had little confidence in an inquiry convened by the British Medical Journal to decide whether two papers it published last year that made an error on the extent of side-effects should be completely withdrawn.
The papers published by the BMJ were by John Abramson, a clinician working at Harvard medical school, and Aseem Malhotra, a cardiologist in the UK. Abramson said statins in low-risk patients did not reduce mortality. Both authors said that in the low-risk group the side-effects meant they sometimes did more harm than good.
The authors have retracted statements on the frequency of side-effects but Collins said that as long as the papers were in circulation, they would wrongly undermine confidence in the drugs, and he did not believe the inquiry was truly independent. He said: 'I don't think it is appropriate for the British Medical Journal to investigate itself,' and called on the General Medical Council, the Academy of Medical Sciences or the Department of Health to investigate.
He said when the BMJ 'gets things wrong, it doesn't correct them properly; when it's shown it gets things wrong, it doesn't make that clear - for example blaming the peer reviewers when it wasn't the peer reviewers' fault - and they shouldn't be in a position where they are investigating themselves. That wouldn't be happening in any other sphere.'
Cholesterol-lowering statins are life-savers, helping prevent heart attacks and strokes in people who have already had one and so are at high risk of another. But the battle now raging is over the use of the drugs in healthy people at low risk.
Draft guidance from the National Institute for Health and Care Excellence (Nice) has recommended that everybody with a risk as low as 10% over 10 years (rather than 20% as now) should be eligible for statins from their GP. About 7 million middle-aged people are now taking a daily statin and the regulator's proposed guidance could extend that to 5 million more.
This week, one of the two BMJ authors and seven other doctors, including the president of the Royal College of Physicians, Sir Richard Thompson, and a former chair of the Royal College of GPs, Dr Clare Gerada, wrote to Nice and the health secretary, Jeremy Hunt, asking for the guidance to be delayed. The letter questioned the benefits and side-effects in low-risk people and claimed the true picture was distorted because drug companies had not put trial data into the public domain..."
"In October 2013 the BMJ published two articles in the same issue: an Analysis article by Abramson et al arguing that cholesterol lowering guidelines should not be widened to include statin therapy for low risk individuals (five year risk <10%) and an Observations article by Malhotra suggesting that saturated fat is not the main cause of cardiovascular disease. The Abramson et al article questioned the balance of risk and benefit presented in the recently updated Cochrane review and the 2012 Cholesterol Treatment Trialists' (CTT) Collaboration meta-analysis (on which the updates to the 2013 Cochrane review are largely based). Both articles quoted an article by Zhang et al to claim that the rate of side effects with statins was around 20%. This was an error. In fact, Zhang et al referred to 'statin-related clinical events that may be interpreted as adverse reactions by patients or their clinicians'. As Zhang et al themselves pointed out in a rapid response, 'implicit in this definition is the recognition that the causative association between each identified event and statin use was unknown.'
This error of interpretation was first suggested in a rapid response from Takhar immediately after publication of the Abramson et al article and subsequently clarified by Zhang et al themselves in a letter published in June 2014. Numerous rapid responses were posted, reflecting a vigorous debate on the merits and limitations of statins for those at low risk of cardiovascular disease.
On 30 October 2013, a few days after publication, Professor Sir Rory Collins, professor of medicine and epidemiology at the Clinical Trial Service Unit at Oxford University and an author on the meta-analysis by the Cholesterol Treatment Trialists' (CTT) Collaboration published in the Lancet in 2012, sent an email to the editor of the BMJ, Dr Fiona Godlee, stating that the BMJ seemed to have taken a stand against statins and that there was a danger that misrepresentation of the evidence in the BMJ could cause harm. He discussed this in person with Fiona Godlee in December 2013 and talked her through a set of slides (later submitted to the panel with additional annotations, SP16a). At that meeting Fiona Godlee invited Rory Collins to write an article presenting evidence on the benefits and harms of statins: 'Although your article would be a response to the two articles, and to Abramson et al in particular, it would be helpful if you could use the opportunity to set your piece in the wider context of the evidence on the benefits and harms of statins.' (See SP13, email 2 December). Following this discussion Rory Collins submitted a number of written, but not-for-publication, criticisms to Fiona Godlee, focussed mainly on the Abramson et al paper, and was again invited to write an article in response. At the time of this report he had not yet done so in the form of a submitted article.
In another letter to Fiona Godlee, marked 'not for publication' and dated 28 April 2014, Rory Collins called for retraction of both papers, writing: 'What the BMJ needs to do is withdraw these seriously damaging claims explicitly and unreservedly with a clear explanation of why they are so wrong and what is likely be correct, and to demonstrate that it is serious about rectifying the damage that it has caused by retracting both of these papers.' He emphasised the seriousness of his concerns, describing: 'the need to rectify the harm that has been caused –perhaps resulting in large numbers of unnecessary deaths, heart attacks and strokes among patients at elevated risk - by misleading doctors and the public with gross over-estimates of the rates of side-effects with statins.' (SP20)
On 15 May 2014, corrections were posted for both articles, withdrawing the statement that side effects of statins occur in about 18-20% of patients. 'The authors withdraw this statement. Although it was based on statements in the referenced observational study by Zhang and colleagues, that 'the rate of reported statin-related events to statins was nearly 18%', the article did not reflect necessary caveats and did not take sufficient account of the uncontrolled nature of the study.'..."
"Zhang et al observed that the rate of statin related events found in their study (18%) was 'substantially higher than the 5% to 10% usually described in randomized, placebo-controlled, clinical trials.' Two caveats must be considered. As Zhang et al point out, the rate of statin related events reported in their study was uncontrolled and therefore may be inflated because events attributed to statins might have occurred in a placebo group as well. In addition, although Zhang et al do not make this point, the 5-10% rate quoted by Zhang et al as having been observed in randomised trials was, in many cases, similar in both active and placebo groups.
The exact rate of statin related adverse events in people at low risk of cardiovascular disease remains uncertain. Clinical trials may underestimate the frequency of statin related adverse events because of patient selection, exclusion of older patients and those with comorbid conditions or potential drug interactions, under-representation of women, and selection bias created by willingness to participate in a clinical trial. In addition, when compared with the full clinical study reports, published accounts of clinical trials in medical journals report only a minority of adverse events. Access to the full data from the trials of statins would help to determine the comparative rates of serious adverse events in statin and control groups but probably would not help to determine the frequency of less than serious adverse events.
The authors also mistakenly reported that Zhang et al found that '18% of statin treated patients had discontinued therapy (at least temporarily) because of statin related events.' The correct interpretation of the data, as confirmed to The BMJ by Zhang et al, is as follows. Based on review of structured electronic medical record categories and automated review of unstructured narratives from follow-up visits of 107,835 patients over eight years, 18,778 of all study patients (17.4%) had a statin related event documented during the study. Among those who experienced a statin related event, only 59.2% had statin therapy discontinued at least temporarily. However, because of possible miscategorisation resulting from the limited options in the electronic medical record for recording reasons for discontinuation of statin therapy, Zhang et al concluded that 'as many as 87%' of these discontinuations could have been due to statin-related events. This equates to up to 9% of the study population having possibly discontinued statin therapy as a consequence of statin related events, rather than the 18% cited.
The primary finding of Abramson and colleague's article—that the Cholesterol Treatment Trialists' data failed to show that statins reduced the overall risk of mortality among people with <20% risk of cardiovascular disease over the next 10 years—was not challenged in the process of communication about this correction."
"The parties to this latest controversy over the role of statin medication in the primary prevention of cardiovascular disease have different professional backgrounds and experience, which results in different perspectives, interpretations and judgments. Unbiased groups of scientific investigators analysing the same data can reach very different conclusions.
The panel were unanimous in their decision that the two papers do not meet any of the criteria for retraction. The error did not compromise the principal arguments being made in either of the papers. These arguments involve interpretations of available evidence and were deemed to be within the range of reasonable opinion among those who are debating the appropriate use of statins. In making this assessment, the panel is not expressing an opinion about the merits of these arguments, as that work was beyond the scope of the panel.
The panel did have one final comment. It became very clear to the panel that the fact that the trial data upon which this controversy is based are held by the investigators and not available for independent assessment by others may contribute to some of the uncertainty about risks and benefits. Different investigators may come to different conclusions with the same data. In fact, a particularly germane example occurred recently in which two experienced Cochrane groups were charged with evaluating a particular intervention and, despite being given the same instructions, data and resources, did not arrive at identical results or conclusions. The panel strongly believes that the current debates on the appropriate use of statins would be elevated and usefully informed by making available the individual patient-level data that underpin the relevant studies".
"Objective: To estimate the average postponement of death in statin trials.
Setting: A systematic literature review of all statin trials that presented all-cause survival curves for treated and untreated.
Intervention: Statin treatment compared to placebo.
Primary outcome measures: The average postponement of death as represented by the area between the survival curves.
Results: 6 studies for primary prevention and 5 for secondary prevention with a follow-up between 2.0 and 6.1 years were identified. Death was postponed between -5 and 19 days in primary prevention trials and between -10 and 27 days in secondary prevention trials. The median postponement of death for primary and secondary prevention trials were 3.2 and 4.1 days, respectively.
Conclusion: Statin treatment results in surprisingly small average gain in overall survival within the trials' running time. For patients whose life expectancy is limited or who have adverse effects of treatment, withholding statin treatment therapy should be considered".
A box, titled "Strengths and limitations of this study", noted:
"• This is the first study ever to systematically evaluate statin trials using average postponement of death as the primary outcome.
• We have only estimated the survival gain achieved within the trials' running time, whereas in real life, treatment is often continued much longer
• We have only focused on all-cause mortality. Other outcomes may also be relevant, for example non-fatal cardiovascular end points".
"Objective: It is well known that total cholesterol becomes less of a risk factor or not at all for all-cause and cardiovascular (CV) mortality with increasing age, but as little is known as to whether low-density lipoprotein cholesterol (LDL-C), one component of total cholesterol, is associated with mortality in the elderly, we decided to investigate this issue.
Setting, participants and outcome measures: We sought PubMed for cohort studies, where LDL-C had been investigated as a risk factor for all-cause and/or CV mortality in individuals ≥60 years from the general population.
Results: We identified 19 cohort studies including 30 cohorts with a total of 68,094 elderly people, where all-cause mortality was recorded in 28 cohorts and CV mortality in 9 cohorts. Inverse association between all-cause mortality and LDL-C was seen in 16 cohorts (in 14 with statistical significance) representing 92% of the number of participants, where this association was recorded. In the rest, no association was found. In two cohorts, CV mortality was highest in the lowest LDL-C quartile and with statistical significance; in seven cohorts, no association was found.
Conclusions: High LDL-C is inversely associated with mortality in most people over 60 years. This finding is inconsistent with the cholesterol hypothesis (i.e., that cholesterol, particularly LDL-C, is inherently atherogenic). Since elderly people with high LDL-C live as long or longer than those with low LDL-C, our analysis provides reason to question the validity of the cholesterol hypothesis. Moreover, our study provides the rationale for a re-evaluation of guidelines recommending pharmacological reduction of LDL-C in the elderly as a component of cardiovascular disease prevention strategies".
"... we decided to look at like not total cholesterol sorry this all terminology gets very arcane very quickly. We looked the part of cholesterol called bad cholesterol in popular parlance which is LDL cholesterol and because this hadn't been done before we also looked in the elderly because there had been some idea that as people became older that cholesterol or LDL cholesterol was actually not a risk factor or attenuated. So we then decided to try and find all the work that had been done looking at the levels of LDL in groups of people and looking at over 68,000 people we found that essentially after the age of 60 ... if you have a higher LDL bad cholesterol level you will actually live longer and there is no increased risk of cardiovascular disease. That's essentially what we found.... What is going on is that LDL and/or cholesterol is not actually a cause of cardiovascular disease."
"The drug companies must declare known side-effects in patient information leaflets, or they risk jail. Sadly, drug-funded CTSU drug-promoters can claim whatever they like in a medical journal, with the breathtaking arrogance of those who literally cannot be challenged because they decide they won't share anything that could be challenged, and they have no risk of repercussions, let alone jail. What is more, the editor of that journal supports this outrage unequivocally and has the audacity to assert that further debate on statins should not be allowed to continue.
This is precisely why the debate must and will continue - whether the drug pushers and data hiders and their conspirators like it or not.
"Statins are back in the news. A review published in the Lancet last week, covered in our news story [link provided], presents what its authors clearly consider to be a definitive account of the evidence on statins that should, they say, bring an end to a dangerous debate.
Not everyone agrees. Though the benefits of statins for secondary prevention or in people at high risk of cardiovascular disease are undisputed, proposals to offer them to large numbers of people at lower risk remain controversial, much to the frustration of the statin trialists who authored the Lancet review. Commenting in The BMJ this week, Harlan Krumholz agrees on the strong case for the overall benefits of statins, but he wants more acknowledgment of the trials' limitations [link to article]. These include the lack of good evidence in elderly people, the variation in how adverse event data were collected, and the ageing of the trials themselves.
In a BMJ blog Richard Lehman says that adverse effects are much more common than the trials suggest [link provided]. 'Muscle pain and fatigability are not a figment of misattribution and public misinformation,' he says. 'They are too prevalent and recurrent in people who desperately want to stay on statins. Rather than discount a widely observed phenomenon, we should ask why there is such a mismatch with reporting in the trials.' Could this mismatch be due to exclusion of people who experienced side effects during 'run-in periods' before randomisation?
At a more fundamental level, who should decide when such questions are too dangerous to ask? Certainly not those who have a vested interest in the debate being shut down. Rory Collins, head of the Cholesterol Treatment Trialists' (CTT) Collaboration, continues to call for the retraction of two [2013 BMJ Articles] that disputed the use of statins in low risk people [link provided]. His call comes despite an independent expert panel set up by The BMJ and, subsequently, the Committee on Publication Ethics (COPE) concluding that The BMJ had acted appropriately in its handling of the papers. This week we publish documents (www.bmj.com/content/bmj/suppl/2016/11/09/bmj.i4992.DC1/copedocuments.pdf) that serve to correct Richard Horton's comments in the Lancet [link provided], in which he wrongly stated that COPE had 'declined to act' on Collins's concerns. (See also my rapid response www.bmj.com/content/351/bmj.h3908/rr-8)
Independent third party scrutiny of the statins trial data remains an essential next step if this increasingly bitter and unproductive dispute is to be resolved. I have now written to England's chief medical officer, Sally Davies, asking her to call for and fund an independent review of the evidence on statins. As Krumholz concludes, sharing the individual patient level data from the statins trials would send 'a strong message that no single person or group should have exclusive access to data' that are so important for public health.
... For more of The BMJ's content relating to the statins debate go to bmj.com/campaign/statins-open-debate".
"A leading Oxford medical researcher who says statins are safe is at loggerheads with a company that makes 'misleading claims about the drugs' side effects to sell a diagnostic kit he invented.
More than 6m people take statins - drugs which reduce cholesterol and save an estimated 7,000 lives a year - but there is a fierce debate about the benefits and side effects.
Sir Rory Collins, a professor of medicine and epidemiology at Oxford University, led a review into statins, published in The Lancet earlier this month, which found that not more than one in 50 people will suffer side effects.
Collins, who believes millions more Britons could benefit by taking statins, is also co-inventor of a test that indicates susceptibility to muscle pain from them.
In 2009, he and three co-inventors filed a patent for a genetic marker that identifies patients at increased risk of myopathy (muscular pain). The patent says that the incidence of myopathy is around one in 10,000 patients per year on a standard statin dose.
The test, branded Statin-Smart, is sold online for $99 (£76) on a website that claims 29% of statin users will suffer muscle pain, weakness or cramps. The marketing material also claims that 58% of patients on statins stop taking them within a year, mostly because of muscle pain.
Oxford University said Collins had raised his concerns 'several times' about 'misleading' marketing claims made by Boston Heart Diagnostics, the American company granted the exclusive licence for Collins' patent by the university.
Royalties from the licensing of the patent can be used to fund university research, but Collins and his co-inventors had waived personal fees...
Peter Weissberg, medical director at the British Heart Foundation, described the Lancet review as a 'masterclass in how evidence should be interpreted'.
Experts at a briefing organised by the respected Science Media Centre described it as an 'excellent' review and warned of the damage that could be done by 'uninformed scare stories' on side effects.
However, other medical experts have said they are dubious about the 'vanishingly small' level of side effects found in the trials.
Trish Greenhalgh, professor of primary health care sciences at Oxford University said: 'The authors did not highlight the huge biases that are going to happen when you exclude some people with side effects from the trials. The jury is still out.'"
"Introduction: For half a century, a high level of total cholesterol (TC) or low-density lipoprotein cholesterol (LDL-C) has been considered to be the major cause of atherosclerosis and cardiovascular disease (CVD), and statin treatment has been widely promoted for cardiovascular prevention. However, there is an increasing understanding that the mechanisms are more complicated and that statin treatment, in particular when used as primary prevention, is of doubtful benefit.
Areas covered: The authors of three large reviews recently published by statin advocates have attempted to validate the current dogma. This article delineates the serious errors in these three reviews as well as other obvious falsifications of the cholesterol hypothesis.
Expert commentary: Our search for falsifications of the cholesterol hypothesis confirms that it is unable to satisfy any of the Bradford Hill criteria for causality and that the conclusions of the authors of the three reviews are based on misleading statistics, exclusion of unsuccessful trials and by ignoring numerous contradictory observations".
(9) Finalisation of the Articles for publication
"... I have received a response from Kendrick to the key points in our article, which I put to him as our duty to offer right of reply. Within that he points to this study [link to the Kristensen Study - see [209] above].
I assume this is evidence that statins don't add anything much to lifespan.
Can you give me a steer".
(1) Professor Sever, at 21.12 on 28 Feb 2019, said:
"One for Rory and Colin.
Most patients would fear the profound disability associated with a severe stroke and the incapacitating consequences of coronary heart disease, and heart attacks leading to heart failure., the risks of which are reduced with statins.
An analysis based on extended life days in my view is less important than improvement in quality of like (sic)".
(2) Professor Baigent, at 08.28 on 1 March 2019, said:
"Although there are various serious technical issues with this paper, such as the fact that they included less (sic) than half of the trials, and some of those that were included were in populations (eg, those with heart failure) who are now known not to benefit from statins, the 2 main problems with this type of analysis are:
1. Statins extend healthy life; that is, they avoid both disabling events like heart attacks and strokes and death from vascular disease. Therefore, simply focusing attention on extra duration of life in isolation is to ignore the fact that these drugs reduce disability (as Peter Sever has already pointed out in his email to you), which is of particular importance in older people.
2. Trials of a few years duration can only assess the average extra duration of life that is accrues over those few years. Statins are life-long drugs, and the extension of life over a lifetime will be very much greater. Taken together with the ability of statins to prevent non-fatal heart attacks (which may lead to disabling chronic heart failure) and strokes, the benefits of statins when taken for decades are very substantial. The benefits are especially large in those who already have cardiovascular disease, but they will also be appreciable in those who are at increased risk of disease (eg, because of risk factors such as raised blood pressure, diabetes, or cigarette smoking).
I hope that helps".
"Below is the version of our piece. We can't add anything, but we do still have time to make amends if there is anything glaringly inaccurate. Obviously things have had to [be] cut to fit, although it is running over four pages. I've not shared with you the case study/comment piece from me, as I feel your big interest is this bit. We've had responses from Kendrick and Harcombe, which I have tried to address throughout for balance. Malhotra declined to comment."
"... WHEN approached by The Mail on Sunday last week, Dr Kendrick pointed to a 2015 study as proof of his much-made claim that the benefits of statins are negligible, and don't extend lifespan. It showed that 'if you took a statin for five years, the increase in life expectancy would be (on average) 3.5 days. That is around 0.75 days per year of statin treatment'.
Prof Baigent says: 'The 2015 study Kendrick mentions only looked at life extension over the trial period of a few years. Statins are lifelong drugs, and the extension of life over a lifetime will be very much greater. And the important thing is statins extend healthy life. They avoid both disabling events like heart attacks and strokes. Simply focusing attention on extra duration of life is to ignore the fact that these drugs reduce disability.'
...
Prof Samani says: 'Heart attacks used to kill men in their 50s and even 40s, but thanks in part to drug therapies people are living longer, healthier lives.'
However, Dr Kendrick says: 'My paper [which linked high LDL with a longer life] was the most read paper in the BMJ Open website for five months in a row and provides the rationale for a re-evaluation of cholesterol lowering guidelines.'
Dr Harcombe said: 'I have examined the entire data provided by the World Health Organisation and found that higher cholesterol is associated with lower deaths, from [heart disease] and all-causes, in men and women, for all 192 countries in the world.
'My PhD was an examination of the diet (cholesterol) heart hypothesis. I have studied this topic at the highest level for several years and I am entitled, if not obliged, to share what I have found.'
...
In October 2013, the British Medical Journal published an article by Dr Aseem Malhotra in which he claimed a study had proved 20 per cent of statins patients were forced to stop taking them due to muscle pains, stomach upsets, sleep and memory problems and erectile dysfunction.
Thanks to Dr Malhotra's article, the figure was reported worldwide but within months was revealed to be a wrong. The researchers admitted the true quit rate was nine per cent. And it was unclear how many of those had genuinely suffered side effects.
Dr Malhotra's piece, and the study that inspired it, were 'non-scientific and simply not true,' says Prof Smeeth, who led a 2016 investigation by experts at the school into the rising numbers stopping statins as a result of the articles, and the debate - which included public statements from Dr Kendrick and Dr Harcombe - which followed.
The editor of the British Medical Journal, Fiona Godlee, corrected the articles and was even forced to appear on BBC News admitting the mistake.
Dr Kendrick claimed the change, asked for by Prof Collins, indicated 'anyone who dares to criticise statins... is subjected to vitriolic attacks and a demand for silence.' And in a 2014 blog post titled 'It's not about statins - it's about censorship' Dr Harcombe claimed £116 million had 'been awarded to Colin Baigent and Rory Collins' by drug companies, implying that was the real reason they sought the correction...
...
Responding to our investigation, Dr Kendrick said: 'I believe people are being conned, deliberately misled. All of the industry-funded studies were positive. This is either a remarkable coincidence - or something else.'
Dr Harcombe said: 'Rory Collins is trying to bully doctors and researchers into silence. It would be naïve not to think that sums such as £268 million from pharmaceutical companies to CTSU [University of Oxford's research body, under which Prof Collins works] encourage recipients to aggressively encourage people to take those drugs. That's 268 million reasons to silence debate.'..."
Amongst the changes Mr Calman made following the Claimants' right-to-reply responses were the insertion of the words in bold.
"Oh dear, what a shame. It is extremely disappointing how the balance of the article has been completely changed at the last minute by the influence of the unbalanced inclusion of quotes from Harcombe and Kendrick.
In particular, the repeated allegation in the MoS that I have taken large sums of money from industry without making it clear - or allowing me to do so (by contrast with the extensive quotes from Harcombe and Kendrick) - that I personally (and staff in our Clinical Trial Service Unit as a whole) have had a policy for over 30 years of not taking any money either directly or indirectly (i.e. through the University) from industry ... no honoraria for talks, no consultancies for advice, no personal payments at all.
By contrast, you have removed any mention of the financial interests of the statin deniers that are served by their false claims, and the loss of your previous ending (which would have helped readers to understand your conclusions) is instead replaced by yet another emotive and unsubstantiated slur against me.
I have added a few comments on the draft, but it really does need a major revision back to somewhere closer to what it had been (otherwise all of your hard work will have been wasted). I realise that it is late in the day ... but then you have only just sent us this massively revised text ... and that this will be an unwelcome suggestion, but I would urge you (particularly given how much work you had put into trying to get the evidence straight) to hold back publishing the article until you can get the balance right and the messages clear".
Professor Collins was particularly concerned by the final paragraph of the draft article, which he said was "very disappointing" and ended the article with a repeat of the allegation of potential conflict of interest.
"Rory, I'm not keen to share this more widely than with you and Colin, hence why I'm not copying in all those on your last email. Take a look. We must include their comments, or else the piece will become so unbalanced we could be open to a complaint. That would mean the article could end up completely decimated by lawyers, amended by them, or worse just pulled. The section on their own financial interests remains if you take a look at the spread. Take a look and then lets have a chat on the phone about what you feel needs to change, specifically.
"Aseem Malhotra's website says he is an 'award-winning NHs cardiologist' and 'honorary consultant' at Frimley Health NHs Foundation Trust in surrey and the Lister Hospital in Stevenage, Essex. However when The Mail on Sunday contacted Frimley, they said he did not work there but 'did a few clinics here a few years back'.
His secretary at the Lister explained that Dr Malhotra, 41, was a locum - shift‑worker - cardiologist who saw patients there only on a Wednesday afternoon.
Appointments are available at a private practice in Harley Street, London, where he charges £500 for a first consultation and £300 for a follow-up. His diet book, The Pioppi Diet, was branded one of the 'top five worst celeb diets to avoid in 2018' by the British Dietetic Association.
Dr Malcolm Kendrick, 60, is author of five books (the best-read has sold a modest 22,000 copies) and works as a GP for two NHS trusts, East Cheshire and Central Cheshire Integrated Care Partnership.
For £50 a year, you can become a member of The Zoe Harcombe Diet & Health Club.
The Cambridge maths graduate has a PhD in public health nutrition but has never worked as a medical doctor".
Although the potential financial motivation of the two Claimants (and Dr Malhotra) had been in Mr Calman's mind for some time, "as a main focus" in the Articles (see [90] above), it had not featured in the right-to-reply emails that were sent to the two Claimants as an allegation that would be made in the Articles.
"This is really quite shocking to read.
It really does seem a shame given that you have clearly done a great job getting to grips with the science to let the article be marred by what amount to spurious mudslinging.
At the very least, I would hope it can be made clear that while drug companies have indeed funded trials undertaken by CTSU, neither Colin, Rory or anyone else working for CTSU has received any personal payments from drug companies - this has been a clear, longstanding policy of the CTSU and one that I have admired from afar for many years.
Major randomised trials of drugs that are sufficiently large to provide the rigorous evidence we need are hugely expensive: to be blunt they are frequently way beyond the means of public funders or charities. Drug companies have to foot the bill or the trials would not happen and we would not have the research evidence to guide treatment.
I do hope the article can at least be amended to make these facts clear".
"You did not send me the initial draft so I cannot comment.
But the current version apparently shows a major shift in the balance of the article to an unwarranted focus on the bad science promoted by Hendricks (sic) and Harcombe.
I thought the whole point of this article was to promote the good science and redress the balance.
I entirely endorse Rory's comments on the totally unfounded accusations that the reports of the Oxford CTSU were biased and influenced by industry supported funding of the unit.
The integrity of the Oxford researchers is beyond reproach. They are held in the highest esteem by scientists world wide and the insinuations of the likes of Malhotra and Kendrick have gone a long way to undermine public confidence in high quality good science. The comparison with Wakefield and the MMR vaccine is all too obvious.
The way the article now reads seems to give credibility to some of their assertions.
I totally agree that an earlier version (which I have not seen) described by Rory would be far more appropriate and send an undiluted and vitally important message to the public.
"REVEALED: Vested interests of the 'experts' who say don't take statins
SO who are these highly-influential sceptics defying years of robust research on statins? Malhotra's website says he is an 'honorary consultant' at both Frimley Health NHS Foundation Trust and the Lister Hospital Stevenage.
However when The Mail on Sunday contacted Frimley, they claimed he did not currently work there and his secretary at the Lister explained he was a locum - shift-worker - cardiologist who only saw patients there on a Wednesday afternoon. He charges £500 for a first consultation, and £300 for a follow-up at a Harley Street clinic.
41-year-old Malhotra's most recent book, The Pioppi Diet, was branded one of the 'top five worst celeb diets to avoid in 2018' by the British Dietetic Association, yet he urges blog readers to choose it over statins.
Kendrick, meanwhile, is author of five books on the statins debate, the best-read of which has sold a modest 22,000 copies. The 60-year-old doctor is employed by East Cheshire NHS Trust and Central Cheshire Integrated Care Partnership.
And for £50 a year, you can become a member of The Zoe Harcombe Diet & Health Club. The Cambridge maths graduate has a PhD in public health nutrition, and regularly blogs about cholesterol and heart disease. All three owe some, if not a large part of their status to their stance as statins deniers - and have profited from it. Despite such strong evidence to counter their claims, they are resolute. Have they just got too much to lose if their arguments are disproved?"
"However, some medics dispute the benefits [statins] bring - with a few even falsely claiming they cause serious and widespread damage to health..."
Mr Calman responded, by email at 20.22 on 1 March 2019:
"Looks good! I'd say 'wrongly claiming' - trying to avoid anything that could look like we say they're deliberately lying".
Mr Adams replied, at 20.38:
"Fine I'll amend.
PS on the main aren't you being a little tough on Malhotra? His beef is with side effects and yes he got his facts wrong and over-egged the pudding, but he's never to my knowledge denied they work.
I speak as [someone I know] takes statins and she has suffered badly from muscle pain/fatigue while on the wrong ones; it took her an age to get ones that don't have that effect on her, so I suppose I do think there's something in the side effect argument. My tuppence worth".
"... they wanted to make changes to it because they felt that a certain balance had been ticked (sic), and my job was to try and work out where to draw the line, in the middle of that to make sure that we gave - that it was - it was after the right to replies came in and I inserted lots of the current comments from the Claimants and I think it startled them and, you know it was about finding a balance and, you know, I think I made it very clear to them who was in charge of that situation ultimately and explained to them in very, very simple terms that we must include comment from them or it would not be fair".
"On receipt of Dr Kendrick's right of reply... I forwarded it on to X and asked the experts to comment on the [Kristensen Study - see [209] above], which found that if you took a statin for five years, the increase in life expectancy would be on average 3.5 days (3.2 and 4.1 days for primary and secondary prevention respectively). I had already discussed this line of argument, that statins don't prevent death, with Professor Baigent. Professor Sever responded that most patients fear the profound disability associated with a severe stroke and incapacitating consequences of coronary heart disease, and heart attacks leading to heart failure, the risk of which are (sic) reduced with statins. An analysis based on extended life days was in his view less important than safeguarding quality of life. Professor Baigent stated that the two main problems with this study were that (a) statins extend healthy life by avoiding disabling events in isolation was to ignore the fact that these drugs reduced disability, which was of particular importance in older people, and (b) trials with a short exposure period of a few years' duration (as this was) can only assess the extra duration of life that accrues over those few years.
I know that the Claimants say that I did not have regard to any of the materials referred to me by the Claimants in their replies, but I think my answer above demonstrates clearly that I did. In addition to this, the Claimants referring to specific stand-alone studies that they believe support their stance comes back to the overarching point about one study or one review being not nearly as comparable as meta-analyses that combine the results of multiple studies. In any event, I did include a reference to the Kristensen paper that he referred to in his response and a summary of what Professor Baigent and Professor Sever said in response. Due to the length of the article, which was already substantial, it would not have been possible to enter into a back and forth over a single point such as this. Ultimately, the Kristensen study examined 11 studies, between two and six years in duration. My cardiologist source X suggested it was remarkable that, even given such a small selection of studies and timeframe, that a survival benefit was seen. Again, we could have focused endlessly on one aspect such as this, however, there was not space and more to the point, the Kristensen study did not refute the totality of evidence".
I do not accept several aspects of this evidence.
(10) Mr Calman's understanding of the meaning of the Articles
(1) In cross-examination, Mr Calman accepted that he intended the Articles to allege that the Claimants' public statements had caused people at high risk of heart disease to refuse or abandon statin medication.
(2) Mr Calman understood (and intended) that the Articles went on to allege that the consequence of people refusing or abandoning prescribed statin medication was that they were thereby at significant risk of adverse health outcomes including stroke and heart attack (including death); "illness, disability and death" [12] Editorial. This meaning emerges from the headline and paragraphs [1], [2], [6] of the News Article; the headlines and paragraphs [5], [14]-[16] of the Main Article; and the headline, the case study of Colin, and paragraphs [1], [12], [17] of the Editorial.
(3) Mr Calman understood (and intended) that the Articles also alleged that public statements made by the Claimants about statins were false; they represented "fake health news". This meaning emerges from the repeated used of the graphic "Fight fake health news", paragraphs [3]-[5], [7] and the graphic "'Experts' behind the scare stories - and why they're wrong" in the News Article, headlines and paragraphs [12]-[15], and the four sections identifying the "fake news" of the 'statin deniers' in paragraphs [19]-[67] of the Main Article, and paragraphs [A], [J], [K] of the Text Box.
(4) I am satisfied that, at the very least, Mr Calman recognised that the Articles could be read as alleging that the Claimants knew that their statements about statins were false. This meaning emerges, most significantly and in my judgment inescapably, from the allegation that the Claimants (and other 'statin deniers') were promoting "pernicious lies" (i.e. deliberate falsehoods that had serious consequences). If Mr Calman did not intend to make that allegation, he failed in the presentation of the Articles and his use of language to make that clear, if he had wanted to avoid readers understanding this obvious meaning, he could and should have done so. My conclusion is that, although he had some reticence, in the end Mr Calman was willing for the Articles to be read as alleging that the Claimants were liars.
(5) Finally, I am satisfied that Mr Calman knew that, in the Articles, he was suggesting that the Claimants might have a financial motive to publish information about statins that they knew to be false. The financial interests of the Claimants and Dr Malhotra were set out in the Text Box. Reference is made to the financial motivation in paragraphs [13] and [17] of the Editorial, and the specific question is asked in the final paragraph of the Text Box: "have they just got too much to lose if their arguments are disproved?".
(11) Post-publication response of the Claimants and request that the Defendants publish a statement by way of explanation or contradiction
"Correction & Clarification
In The Mail on Sunday for 3 March 2019 we gave extensive coverage to the subject of statins, both on page 2 of the newspaper and by way of a special report in our Health Section at pages 47 to 50. Our coverage profiled Dr Malcolm Kendrick and Dr Zoë Harcombe PhD, whom we described as 'statin deniers'. Our article was headlined 'Statin deniers are putting patients at risk, says Minister' and our special report was entitled 'Deadly propaganda of the statin deniers'.
In the course of this coverage we also referred to a statement that had been made to The Mail on Sunday by the Health Secretary, Matt Hancock, on the subject of statins. We attributed to Mr Hancock that he had accused such 'statin deniers' and 'doctors' of needlessly putting the lives of patients at risk by spreading reckless and ignorant misinformation, and that he had described some of the statements about statins which we had assigned to Dr Kendrick and Dr Harcombe as 'pernicious lies'.
We wish to clarify that Mr Hancock's remarks to The Mail on Sunday were not in fact directed by him towards either of Dr Kendrick or Dr Harcombe. Contrary to how we reported his words, Mr Hancock had made no reference to 'statin deniers' or to 'doctors'. Moreover, his reference to 'pernicious lies' had not been directed by him to any statements issued by either of Dr Kendrick or Dr Harcombe on the subject of statins, contrary to how we reported the matter.
We are happy to correct the misleading impression given otherwise in our coverage, by way of addressing one aspect of the legal complaints we have received from Dr Kendrick and Dr Harcombe about our coverage".
"Without prejudice to our client's contentions as to the applicability of paragraph 7 of Schedule 1 to the Defamation Act 1996 to the statements in question, our client is willing to publish the following statement in accordance with your request for a statement by way of explanation and contradiction:
A health report on March 3 2019 about statins quoted Health Minister Matt Hancock on the 'pernicious lies' of clinicians 'spreading reckless and ignorant misinformation' to deny the drugs' benefits. Our report also accused Dr Malcolm Kendrick and Dr Zoe Harcombe of being 'statins deniers' - allegations they deny. Following publication, we were informed by the Department of Health that Mr Hancock's remarks were not intended to single out any individuals.
If your clients agree, our client proposes to publish this statement in the clarifications and corrections column in the next issue of the Mail on Sunday.
A statement will also be published online below the relevant article. Given that it will appear as a footnote to the article, some changes to the above wording are required as follows:
The above report quoted Health Minister Matt Hancock on the 'pernicious lies' of clinicians 'spreading reckless and ignorant misinformation' to deny the drugs' benefits. Our report also accused Dr Malcolm Kendrick and Dr Zoe Harcombe of being 'statins deniers' - allegations they deny. Following publication, we were informed by the Department of Health that Mr Hancock's remarks were not intended to single out any individuals.
Please confirm that your clients agree to the publication of the above statements".
"Your proposed wording is not suitable as a statement by way of explanation and contradiction and is not acceptable to our clients. They are entitled to have made clear what misrepresentation is being corrected. The misrepresentation was that Mr Hancock, in his highly critical remarks to the Mail Online, had referred to 'statin deniers' and 'doctors'. It needs also to be made clear why this is a correction relating to our clients, specifically, because they were two individuals repeatedly identified in the coverage as the 'statin deniers' and the 'doctors'. Your proposed statement is drafted so as completely to obscure what is being corrected and why.
We invite your client to publish this alternative:
Dr Malcolm Kendrick and Dr Zoe Harcombe PhD
In our special health report on March 3 2019 about statins, in which we described Dr Malcolm Kendrick and Dr Zoe Harcombe as 'statin deniers', we attributed to Health Secretary Matt Hancock that he had accused such 'doctors' and 'statin deniers' of needlessly risking patients' lives by 'spreading reckless and ignorant misinformation' and of circulating 'pernicious lies', conduct which Dr Kendrick and Dr Harcombe deny. Mr Hancock did not use the term 'doctors' or 'statin deniers' and it has been confirmed to us by the Department of Health that Mr Hancock's remarks had not been intended to single out any individuals. We wish to correct the impression that we gave to the contrary.
This will need to appear in the hard copy, where it should be positioned as the first item in the clarifications and corrections column and online at the [website addresses given] at the top of the articles..."
G: Legal Principles
(1) Public Interest
4. Publication on matter of public interest
(1) It is a defence to an action for defamation for the defendant to show that—
(a) the statement complained of was, or formed part of, a statement on a matter of public interest; and
(b) the defendant reasonably believed that publishing the statement complained of was in the public interest.
(2) Subject to subsections (3) and (4), in determining whether the defendant has shown the matters mentioned in subsection (1), the court must have regard to all the circumstances of the case.
(3) ...
(4) In determining whether it was reasonable for the defendant to believe that publishing the statement complained of was in the public interest, the court must make such allowance for editorial judgement as it considers appropriate.
(5) For the avoidance of doubt, the defence under this section may be relied upon irrespective of whether the statement complained of is a statement of fact or a statement of opinion.
(6) The common law defence known as the Reynolds defence is abolished.
(1) was the statement complained of, or did it form part of, a statement on a matter of public interest? If so,
(2) did the defendant believe that publishing the statement complained of was in the public interest? If so,
(3) was that belief reasonable?
"If the article as a whole concerned a matter of public interest, the next question is whether the inclusion of the defamatory statement was justifiable. The fact that the material was of public interest does not allow the newspaper to drag in damaging allegations which serve no public purpose. They must be part of the story. And the more serious the allegation, the more important it is that it should make a real contribution to the public interest element in the article".
"By ['public interest'] we mean matters relating to the public life of the community and those who take part in it, including within the expression public life activities such as the conduct of government and political life, elections ... and public administration, but we use the expression more widely than that, to embrace matters such as (for instance) the governance of public bodies, institutions and companies which give rise to a public interest in disclosure, but excluding matters which are personal and private, such that there is no public interest in their disclosure".
"Editors invoking the public interest will need to demonstrate that they reasonably believed publication - or journalistic activity taken with a view to publication - would both serve, and be proportionate to, the public interest and explain how they reached that decision at the time".
[122] ... as the burden of establishing a public interest defence under s.4 Defamation Act 2013 lies upon the defendant, a defendant seeking to prove that s/he reasonably believed that publishing the statement complained of was in the public interest is likely to find that the prospects of success are enhanced by being able to produce contemporaneous records of the decision(s) taken. Leggatt J's observations about memory of beliefs being particularly susceptible to decay over time (Gestmin [18]) are likely to be equally relevant in publication cases in which a defendant's belief that publication was in the public interest is an issue to be resolved.
[123] Defendants seeking to rely upon such a belief - whether in support of a s.4 defence or otherwise - would be well advised to ensure that they are able to demonstrate that they reasonably believed that publication would be in the public interest and how, and with whom, that was established at the time. My confidence that the Court is not setting unrealistic targets is also somewhat fortified by the fact that the current Codes of both IPSO and IMPRESS both impose substantially the same requirements as the old PCC Code of Practice (see [25] above). The IMPRESS Code, particularly, contains a very clear explanation why it is good practice to retain contemporaneous documents that record important decisions about the public interest justification for publication. As an explanation of the importance of contemporaneous documents, it can hardly be bettered.
[124] The Court may ultimately disagree with the journalist's assessment that it was in the public interest to publish, but contemporaneous documents will at least assist the journalist in being able to demonstrate his/her thought processes at the time. In relevant cases, evidence of that process is also likely to be relevant to, even if not necessarily determinative of, the Court's assessment of editorial judgment under s.4(4).
(As the Second Defendant is a member of IPSO, the IMPRESS Code has no direct relevance in this case, but its statement of the importance of contemporaneous documents in demonstrating a "audit trail" succinctly explains the point and the effect it has on the cogency of the evidence relied upon to support a public interest defence.)
[112] The importance of giving respect, within reason, to editorial judgment is relevant when considering the tone and content of the material and the nature and degree of the steps taken by way of verification prior to publication: Serafin, Lord Wilson, [60], Flood, Lord Mance, [137], Jameel [2007] 1 AC 359, Lord Hoffmann, [51].
[113] It is important to consider the process and the publication in the round. As Lord Mance noted in Flood, in Bonnick -v- Morris [2003] 1 AC 300 the journalist had fallen short both in not making further inquiries about the anonymous source and in not including the claimant's explanation, but the Privy Council was "prepared to overlook some respects in which the journalist's conduct could legitimately be criticised" in reaching an overall judgment as to the availability of the public interest defence ([130]). Lord Mance continued at [131]:
"The need to look at the position in the round was also identified by Lord Bingham in Jameel [34] when he disclaimed too close a focus on particular ingredients which have (or have not) been included in a composite story. He said:
'This may, in some instances, be a valid point. But consideration should be given to the thrust of the article which the publisher has published. If the thrust of the article is true, and the public interest condition is satisfied, the inclusion of an inaccurate fact may not have the same appearance of irresponsibility as it might if the whole thrust of the article is untrue.'"
[114] Journalistic freedom covers possible recourse to a degree of exaggeration or even provocation. It is well established that this is something the court must tolerate: Yeo -v- Times Newspapers Ltd [2017] EMLR 1. It is not for the court to substitute its views for those of journalists as to what techniques of reporting should be adopted.
[82] ... In Bonnick, Lord Nicholls said at [24] that a journalist should not be penalised for making a wrong decision on a question of meaning on which people might reasonably take different views. But he went on at [25] to say that this "should not be pressed too far".
"In the normal course a responsible journalist can be expected to perceive the meaning an ordinary reasonable reader is likely to give to his article. Moreover, even if the words are highly susceptible of another meaning, a responsible journalist will not disregard a defamatory meaning which is obviously one possible meaning of the article".
In Banks Steyn J, DBE applied these observations in the context of the s.4 defence. At [123] she summarised her analysis in this way:
"A defamatory meaning should not be ignored by a journalist if it is 'obviously one possible meaning' ([25]) or 'glaringly obvious' ([27]); to do so would not be reasonable. But if that threshold is not reached, the reasonable belief of a journalist who did not perceive the more damaging meaning falls to be assessed by reference to the less damaging meaning".
[83] It may be that these principles do not transpose directly into a situation such as the present, but I do not think the defendant can reasonably argue for any more generous test. She has never done so. She has not addressed the issue. The defendant's argument has always been that her conduct should be assessed exclusively by reference to what she reasonably believed the GAT to mean, and that on that footing it was reasonable for her to believe that it was in the public interest to publish the Factual Allegation and the Opinion. That, in my view, is simplistic and wrong. When assessing the reasonableness of a belief that it is the public interest to denounce a person as dangerous and stupid for what they have said in a public statement, it must be relevant that the statement has an obvious alternative and lesser meaning which is not worthy of such denunciation. Here, the Judge was entitled to conclude that the defendant ought reasonably to have appreciated that the GAT could also be interpreted as conveying the hypocrisy meaning and that it was therefore unreasonable for her to believe that presenting the position unambiguously, as she did, was in the public interest.
[84] This approach seems to me consistent with one strand of the authorities to date, which is that "a belief [is] reasonable for the purposes of s.4 only if it is one arrived after conducting such enquiries and checks as it is reasonable to expect of the particular defendant in all the circumstances of the case": Economou [2017] EMLR 4 [241], approved [2019] EMLR 7 [101] and endorsed by the Supreme Court as "no doubt helpful" in Serafin [67].
[85] I see no inconsistency with my judgment in Yeo, aspects of which are relied on by the defendant. In the passages relied on, at [175] and [179], I said that in a case such as that one "it will be 'fair' to present readers with factual conclusions honestly and reasonably drawn by journalists who were themselves witnesses to the key events; it is permissible to summarise, and to be selective; ... fairness does not require the publisher to present the reader with all the factual material that could support a competing assessment ... it is not incumbent on the responsible journalist to lay out for the reader all the pros and cons relevant to a particular conclusion". Yeo was very different from this case. It was a decision on the application of the Reynolds defence to newspaper reports of an undercover journalistic investigation of a leading politician. But I also said (at [175]) that "if the evidential picture is misrepresented or presented in a wholly unbalanced way, that may well be unfair". Here, the Judge found that the evidential picture had been unreasonably misrepresented.
"... At the stage of the assessment as to what information the defendant had and what inquiries s/he made, Lord Nicholls' third to fifth factors are likely to remain valid in many cases:
'3. The source of the information. Some informants have no direct knowledge of the events. Some may have their own axes to grind, or are being paid for their stories. 4. The steps taken to verify the information. 5. The status of the information. The allegation may have already been the subject of an investigation which commands respect.'"
[134] It is also clear from [Economou [110]], as endorsed by Lord Wilson in Serafin [69], that providing they are not treated as any sort of 'checklist', the Reynolds factors will remain potentially relevant when assessing whether a defendant's belief that publication was in the public interest was objectively reasonable. Lord Wilson traced the legislative history of s.4 through the post-Reynolds authorities in [57] to [59], and observed in [60]:
"In [Flood -v- Times Newspapers Ltd [2012] 2 AC 273] ..., the defendant published an article taken to mean that there were reasonable grounds to suspect that the claimant, a police officer, had corruptly taken bribes. The allegation was false. This court held that the defendant nevertheless had a valid defence of public interest. Lord Phillips of Worth Matravers, the President of the court, said at [26] that in that case analysis of the defence required particular reference to two questions, namely public interest and verification; at [27] that it was misleading to describe the defence as privilege; at [78], building on what Lord Hoffmann had said in the Jameel case at [62], that the defence normally arose only if the publisher had taken reasonable steps to satisfy himself that the allegation was true; and at [79] that verification involved both a subjective and an objective element in that the journalist had to believe in the truth of the allegation but it also had to be reasonable for him to have held the belief. Lord Brown at [113] chose to encapsulate the defence in a single question. 'Could', he asked, 'whoever published the defamation, given whatever they knew (and did not know) and whatever they had done (and had not done) to guard so far as possible against the publication of untrue defamatory material, properly have considered the publication in question to be in the public interest?'. Lord Mance at [137], echoing what Lord Nicholls had said in the Reynolds case at p.205, stressed the importance of giving respect, within reason, to editorial judgement in relation not only to the steps to be taken by way of verification prior to publication but also to what it would be in the public interest to publish; and at [138] Lord Mance explained that the public interest defence had been developed under the influence of the principles laid down in the European Court of Human Rights."
[135] As Lord Wilson noted ([66]), the Explanatory Notes to the Defamation Act 2013 stated that the intention behind s.4 was to: "reflect the common law as recently set out in the Flood case and in particular the subjective and objective elements of the requirement now both contained in subsection 1(b)".
[136] In [60], Lord Wilson referred to Lord Brown's question from Flood. To similar effect, in Economou [2017] EMLR 4 [241], Warby J held:
"I would consider a belief to be reasonable for the purposes of section 4 only if it is one arrived at after conducting such inquiries and checks as it is reasonable to expect of the particular defendant in all the circumstances of the case".
This statement was approved by Sharp LJ in Economou [101] and by the Supreme Court in Serafin [67]. See also Warby J's observations as to reasonableness of belief in Economou [239].
[138] In Flood, Lord Phillips explained why neutral reportage justified a journalist being relieved from the normal obligation to verify:
[77] ... Reportage is a special, and relatively rare, form of Reynolds privilege. It arises where it is not the content of a reported allegation that is of public interest, but the fact that the allegation has been made. It protects the publisher if he has taken proper steps to verify the making of the allegation and provided that he does not adopt it. Jameel ‑v- Wall Street Journal Europe Sprl [2007] 1 AC 359 was analogous to reportage because it was the fact that there were names of substantial Saudi Arabian companies on the black list that was of public interest, rather than the possibility that there might be good reason for the particular names to be listed. Just as in the case of reportage, the publishers did not need to verify the aspect of the publication that was defamatory.
[78] The position is quite different where the public interest in the allegation that is reported lies in its content. In such a case the public interest in learning of the allegation lies in the fact that it is, or may be, true. It is in this situation that the responsible journalist must give consideration to the likelihood that the allegation is true. Reynolds privilege absolves the publisher from the need to justify his defamatory publication, but the privilege will normally only be earned where the publisher has taken reasonable steps to satisfy himself that the allegation is true before he publishes it. Lord Hoffmann put his finger on this distinction in Jameel [62] when he said
"In most cases the Reynolds defence will not get off the ground unless the journalist honestly and reasonably believed that the statement was true, but there are cases ('reportage') in which the public interest lies simply in the fact that the statement was made, when it may be clear that the publisher does not subscribe to any belief in its truth."
[79] Thus verification involves both a subjective and an objective element. The responsible journalist must satisfy himself that the allegation that he publishes is true. And his belief in its truth must be the result of a reasonable investigation and must be a reasonable belief to hold. What then does the responsible journalist have to verify in a case such as this, and what does he have to do to discharge that obligation? If this were a Chase level 1 case he would have to satisfy himself, on reasonable grounds, that the claimant had in fact been guilty of corruption. His defence would not "get off the ground" unless he reasonably believed in the claimant's guilt. This is not, however, a Chase level 1 case...
[139] In Axel Springer AG -v- Germany [2012] EMLR 15 [82], the Grand Court held, under the heading "Limits on freedom of expression":
"However, art.10(2) of the Convention states that freedom of expression carries with it 'duties and responsibilities', which also apply to the media even with respect to matters of serious public concern. These duties and responsibilities are liable to assume significance when there is a question of attacking the reputation of a named individual and infringing the 'rights of others'. Thus, special grounds are required before the media can be dispensed from their ordinary obligation to verify factual statements that are defamatory of private individuals. Whether such grounds exist depends in particular on the nature and degree of the defamation in question and the extent to which the media can reasonably regard their sources as reliable with respect to the allegations (see Pedersen -v- Denmark (2006) 42 EHRR 24 [78], and Tønsbergs Blad AS and Haukom -v- Norway (2007) 46 EHRR 40 [89])."
[140] Similarly, from Times Newspapers Ltd -v- United Kingdom [2009] EMLR 14 ("Loutchansky"):
[41] The Court observes that the most careful of scrutiny under art.10 is required where measures or sanctions imposed on the press are capable of discouraging the participation of the press in debates on matters of legitimate public concern (Bladet Tromsø -v- Norway (2000) 29 EHRR 125 [64]). The Court further recalls that particularly strong reasons must be provided for any measure limiting access to information which the public has the right to receive (see Timpul Info-Magazin and Anghel -v- Moldova [2007] ECHR 976 [31]).
[42] However, the Court reiterates that art.10 does not guarantee a wholly unrestricted freedom of expression to the press, even with respect to press coverage of matters of serious public concern. When exercising its right to freedom of expression, the press must act in a manner consistent with its duties and responsibilities, as required by art.10(2). These duties and responsibilities assume particular significance when, as in the present case, information imparted by the press is likely to have a serious impact on the reputation and rights of private individuals. Furthermore, the protection afforded by art.10 to journalists is subject to the proviso that they act in good faith in order to provide accurate and reliable information in accordance with responsible journalism (Fressoz -v- France (2001) 31 EHRR 2 [54] and Bladet Tromsø [65]).
"The Court further reiterates that a general requirement for journalists systematically and formally to distance themselves from the content of a quotation that might insult or provoke others or damage their reputation is not reconcilable with the press's role of providing information on current events, opinions and ideas, and that 'punishment of a journalist for assisting in the dissemination of statements made by another person in an interview would seriously hamper the contribution of the press to discussion of matters of public interest and should not be envisaged unless there are particularly strong reasons for doing so'."
"... having an agenda does not, in and of itself, disqualify a person including citizen journalists such as D1 and D2 from being able to benefit from a public interest defence. Indeed, in general terms many publications and professional journalists approach stories with what might be called an agenda. However, the agenda adopted by D1 and D2 meant that they approached what might be facts suggesting (at the very highest) that questions might be asked about the accuracy of the fundraising statements, as proving fraud and dishonesty on the part of Mr Packham."
(1) Expressions of opinion are likely to be a strong indicator that the statement complained of is or formed part of a statement on a matter of public interest under s.4(1)(a).
(2) It is likely to be a weighty factor in all the circumstances to which the Court must have regard under s.4(2), given that opinion is a particularly strong defence: "The question of whether a statement is factual or opinion can be important. Opinions are easier to defend than factual assertions, as should be the case, given the importance of the free exchange of ideas in a democracy": Triplark [14] per Warby J.
(3) Attempts to verify a statement of opinion will not be required or possible. All the previous authorities on Reynolds' and s.4 defences have been cases where the allegation complained of was a statement of fact, not opinion. The judgments in those cases as to the 'usual' or 'normal' practice of verification, so that the defendant can say that s/he believes the allegation is true, based on verification, is not applicable to a s.4 case where what is sought to be protected is the expression of an opinion. An opinion cannot be verified.
(4) The subjective element of s.4(1)(b) - that the defendant reasonably believed that publication was in the public interest - readily accommodates the subjective test for honesty in s.3(5): that the defendant honestly held the opinion expressed.
(5) The practical and flexible approach to meaning built into Bonnick at least arguably accommodates opinion. Although there would be a tension in the application of these principles since the Bonnick concept of a range of glaringly obvious (alternative) defamatory meanings does not sit comfortably with the subjective nature of honestly holding 'the opinion'.
(6) The margin of editorial judgement must be greater where an opinion is in play, for the reason that freedom to express opinion is given the strongest protection under both domestic and ECHR law.
(1) I do not accept Ms Evans KC's first point. A commentator, for example, can express the most vitriolic criticism of a person's private sexual relationship. The key to determining whether the statement is on a matter of public interest is not how obvious it is that the behaviour is being criticised, but whether the behaviour itself is a matter of public interest.
(2) As to the remaining points, the starting point is that it is clear, from the statute itself, that the honest opinion defence, under s.3, is not being absorbed into the public interest defence in s.4. The law, now on a statutory footing, continues to give generous protection to the honest expression of opinions, subject to the (now statutory) requirements of the defence. Key, for the present argument, is the requirement that the publication must indicate, whether in general or specific terms, the basis of the opinion. The statutory honest opinion defence, like its common law predecessor, provides no shelter either for defamatory bare opinions expressed without premise, or defamatory opinions expressed on facts which are false. If a newspaper publishes an article denouncing someone as unfit to hold public office (expression of opinion) on the basis that s/he is guilty of some discreditable acts (allegation of fact), the focus of any s.4 defence is going to be on whether the publisher reasonably believed it was in the public interest to publish details of the alleged discreditable acts. If the Court finds that it did, then that will protect the publication of the facts relating to the discreditable acts (under s.4) and the expression of opinion based upon them (under s.3(7)(a)). The Court cannot approach the task by looking only at the question whether it was in the public interest honestly to express the opinion. In conclusion, I do not accept that s.4 provides an expansive public interest defence for the expression of opinions, which is subject only to a requirement of subjective honesty.
(3) Whatever the nature of the publication (whether allegation of fact and/or expression of opinion) the test under s.4(1)(b) remains whether the defendant reasonably believed that publishing the statement complained of was in the public interest.
(2) s.15 Reporting privilege
"(1) The publication of any report or other statement mentioned in Schedule 1 to this Act is privileged unless the publication is shown to be made with malice, subject as follows.
(2) In defamation proceedings in respect of the publication of a report or other statement mentioned in Part II of that Schedule, there is no defence under this section if the plaintiff shows that the defendant—
(a) was requested by him to publish in a suitable manner a reasonable letter or statement by way of explanation or contradiction, and
(b) refused or neglected to do so.
For this purpose 'in a suitable manner' means in the same manner as the publication complained of or in a manner that is adequate and reasonable in the circumstances.
(3) This section does not apply to the publication to the public, or a section of the public, of matter which is not of public interest and the publication of which is not for the public benefit..."
"Part 1 - Statements have qualified privilege without explanation or contradiction
...
7. A fair and accurate copy of or extract from matter published by or on the authority of a government or legislature anywhere in the world...
Part 2 - Statements privileged subject to explanation or contradiction
9. ... A fair and accurate copy of or extract from or summary of a notice or other matter issued for the information of the public by or on behalf of - (a) a legislature or government anywhere in the world; (b) an authority anywhere in the world performing governmental functions..."
"A copy or fair and accurate report or summary of any notice or other matter issued for the information of the public by or on behalf of any government department, officer of state, local authority or chief officer of police".
[87] Finally I add these short comments about embellishment and adoption. It is plain that there will be no qualified privilege in an account of Parliamentary speech if the publisher has so embellished the material that it cannot be said to be a fair and accurate report. So much, I think, is shown by this passage from Lord Denning's speech in Dingle -v- Associated Newspapers Ltd [1964] AC 371, 411:
"But if it [sc. the publisher] adds its own spice and prints a story to the same effect as the parliamentary paper, and garnishes and embellishes it with circumstantial detail, it goes beyond the privilege and becomes subject to the general law. None of its story on that occasion is privileged. It has 'put the meat on the bones' and must answer for the whole joint".
[88] Some care is I think needed in considering the concept of adoption, discussed by Arden LJ at paragraphs [37]–[40]. In a sense the publisher who embellishes Parliamentary speech may be said to have adopted it: by "putting the meat on the bones" he has made the allegation his own. But I think it is misleading to characterise such a case as one of adoption. Rather than adopting what was said, the publisher has produced a critically different text. Since what he has produced cannot be said to be a fair and accurate report of Parliamentary speech, the law gives him no shield of qualified privilege. That is the whole analysis of the case; no recourse to any such idea as adoption is required.
"The objects of providing [an opportunity to request publication of a letter or statement by way of explanation or contradiction] emerge clearly enough from a consideration of the section. The point of affording a special privilege to publishers of newspaper, radio or television reports concerning proceedings of public meetings of the specified kind is to recognise their particular role in a society which enjoys a high measure of freedom of communication. That role extends today to providing reports to the public, although the matters reported might later turn out to be inaccurate, unfair or defamatory of the persons mentioned. It is the public or official character of the specified meetings which, it has been considered, will ensure, at least in most cases, appropriate restraint against the reporting of irresponsible or groundless accusations. This purpose is made doubly clear by the closing words of proviso (c). Those words withdraw the protection otherwise applicable if the matter is 'not of public concern and the publication ... is not for the public benefit'. The emphasis upon the public character of the meetings and the criterion of public concern and public benefit help to explain the true purpose of proviso (b). It is to enhance the information given to the public on a particular matter. It is also to recognise that, in the nature of the particular meetings specified, inaccurate, unfair or defamatory statements may be made which can then be published under qualified privilege. Fairness requires the balancing of that right with a provision, to those complaining about its exercise, of the opportunity to place a contradictory statement or explanation before the public. The request would have to be reasonably contemporaneous with the publication. It would not ordinarily be reasonable to expect publication of a letter or statement years or perhaps even months later. The criterion of the public's interest must be kept in mind in giving meaning to the section, including proviso (b).
... By the terms of the proviso, any such letter or statement must be 'reasonable'. It was urged that this meant reasonable from the point of view of the person complaining or tendering the statement. However, in my view, 'reasonable', in this context, means objectively reasonable for the purpose for which the statutory facility has been provided. This is to allow already published facts to be contradicted or explained by those claiming to be hurt by the report of them. The reference to 'reasonable' is intended to control such matters as the length of the letter or statement, the terms in which it is expressed and the avoidance of gratuitous defamation of third parties. I do not regard the word 'reasonable' as affording an editorial veto to the publication of a letter which is strongly expressed or contains disputable propositions or arguable inaccuracies. After all, it is always open to the publisher to have the last word. It is not uncommon, where letters of complaint are published, for an editorial note to be added expressing the publisher's reply. The sting of defamation often causes emotion and anger. That is the context in which Parliament has made provision for a letter or statement in reply to be given its statutory status. Publishers of newspapers who have considerable power to harm reputations should not be overly tender about complaints and expressions of anger when appearing in a letter or statement to which proviso (b) applies.
... The proviso makes no express mention of editorial amendment, abbreviation or alteration. But neither does it expressly state that the letter or statement must be 'accurate'. In the real world, if some aspect of a letter or statement were thought to take it outside the bounds of reasonableness, it would be sensible for there to be negotiation between the publisher and the person complaining. A failure or refusal to enter into such negotiation might, in a particular case, confirm an opinion that, on the whole, the letter or statement tendered was 'reasonable'. Otherwise, all of the cards are stacked in favour of the publisher and against the person complaining. The purpose of the proviso is to afford the complainant a statutory means to secure the publication of a relevant contradiction or explanation. The purpose of the publication envisaged by the proviso is not to afford the complainant, or anyone else, the opportunity to insult the publisher, to extract an apology or to defame others. The ultimate purpose is to contribute to equalising the power to communicate with the audience which has already heard or seen matter considered to be defamatory where it is desired by the person affected to offer to the public other material in contradiction or explanation..."
I consider this accurately to state the principles that apply to consideration of s.15(2). In Henry -v- BBC [2005] EWHC 2787 (QB) [91(iii)], Gray J expressed the view (albeit strictly obiter) that, if the publisher has objections to the terms of the claimant's proposed statement, then s/he should raise them with the claimant and try to reach agreement on the points in issue. For the reasons given by Kirby J, a publisher who is asked to publish any statement or letter by way of explanation or contradiction would be well advised to engage constructively with the putative claimant on the terms of the statement or letter to be published.
[29] The courts in conducting statutory interpretation are "seeking the meaning of the words which Parliament used": Black-Clawson International Ltd ‑v- Papierwerke Waldhof-Aschaffenburg AG [1975] AC 591, 613 per Lord Reid of Drem. More recently, Lord Nicholls of Birkenhead stated:
"Statutory interpretation is an exercise which requires the court to identify the meaning borne by the words in question in the particular context".
(R -v- Secretary of State for the Environment, Transport and the Regions, Ex parte Spath Holme Ltd [2001] AC 349, 396). Words and passages in a statute derive their meaning from their context. A phrase or passage must be read in the context of the section as a whole and in the wider context of a relevant group of sections. Other provisions in a statute and the statute as a whole may provide the relevant context. They are the words which Parliament has chosen to enact as an expression of the purpose of the legislation and are therefore the primary source by which meaning is ascertained. There is an important constitutional reason for having regard primarily to the statutory context as Lord Nicholls explained in Spath Holme, 397:
"Citizens, with the assistance of their advisers, are intended to be able to understand parliamentary enactments, so that they can regulate their conduct accordingly. They should be able to rely upon what they read in an Act of Parliament".
[30] External aids to interpretation therefore must play a secondary role. Explanatory Notes, prepared under the authority of Parliament, may cast light on the meaning of particular statutory provisions. Other sources, such as Law Commission reports, reports of Royal Commissions and advisory committees, and Government White Papers may disclose the background to a statute and assist the court to identify not only the mischief which it addresses but also the purpose of the legislation, thereby assisting a purposive interpretation of a particular statutory provision. The context disclosed by such materials is relevant to assist the court to ascertain the meaning of the statute, whether or not there is ambiguity and uncertainty, and indeed may reveal ambiguity or uncertainty: Bennion, Bailey and Norbury on Statutory Interpretation, 8th ed (2020), para 11.2. But none of these external aids displace the meanings conveyed by the words of a statute that, after consideration of that context, are clear and unambiguous and which do not produce absurdity...
[31] Statutory interpretation involves an objective assessment of the meaning which a reasonable legislature as a body would be seeking to convey in using the statutory words which are being considered. Lord Nicholls, again in Spath Holme, 396, in an important passage stated:
"The task of the court is often said to be to ascertain the intention of Parliament expressed in the language under consideration. This is correct and may be helpful, so long as it is remembered that the 'intention of Parliament' is an objective concept, not subjective. The phrase is a shorthand reference to the intention which the court reasonably imputes to Parliament in respect of the language used. It is not the subjective intention of the minister or other persons who promoted the legislation. Nor is it the subjective intention of the draftsman, or of individual members or even of a majority of individual members of either House ... Thus, when courts say that such-and-such a meaning 'cannot be what Parliament intended', they are saying only that the words under consideration cannot reasonably be taken as used by Parliament with that meaning".
"The judge approached the words of the paragraph 'in not a strictly literal sense but in a fairly liberal way,' to include information painfully extracted by journalists, like a tooth, from an official of a government department acting in the course of his employment, as well as formal statements released to the press by the government department.
That seems to me to pay too little attention to the word 'issued' and to the language's indication that the matter issued must be of the same kind as a notice. It would unduly restrict the words to confine them to written hand-outs, including photographs, sketches or other pictorial representations... but it is right to confine them to official notices and the like, such as, for example, the police message broadcast on television in Boston -v- WS Bagshaw & Sons [1966] 1 WLR 1126, the only reported case on the paragraph: 'statements of a genuinely official nature formally issued for the information of the public,' in the words accepted by Jordan CJ considering a statutory provision in similar terms in Campbell -v- Associated Newspapers Ltd (1948) 48 SRNSW 301, 303".
"It may be right to include in the paragraph's ambit the kind of answers to telephoned interrogatories which Mr Lord, quite properly in the discharge of his duty to his newspaper, administered to Mr Smith. To exclude them in every case might unduly restrict the freedom of the press and I did not understand Mr Eady to submit the contrary. But information which is put out on the initiative of a government department falls more easily within the paragraph than information pulled out of the mouth of an unwilling officer of the department, and I accept Mr Eady's argument that not every statement of fact made to a journalist by a press officer of a government department is privileged, and what is certainly outside the privilege is assumption, inference, speculation on the part of the journalist. That is not authorised; that is not official".
"If the assumption, inference, speculation were the press officer's, it would not be within the paragraph; Mr. Smith was not speaking on behalf of his department if he told Mr. Lord the reprimanded official was or must have been the plaintiff ... A fortiori the reporter's own assumption, inference, speculation could not be attributed to the press officer's department. That would be to accord to investigative journalism the protection provided for reporting of official information".
"Whether the phrase 'must be Blackshaw' resulted from the mental process of Mr Lord (as the jury must have found) or that of Mr Smith (as Mr Lord alleged) or was a joint effort between them, it would, it seems to me, be a misuse of words to describe the result as 'matter issued for the information of the public'. One thing is clear, namely that Mr Smith was not prepared to take responsibility on behalf of the Ministry for giving the name; he was not giving it or purporting to give it on behalf of his department. Not every piece of information given by a spokesman acting in the course of his employment is necessarily information given on behalf of his department".
"The notice or report must be of a genuinely official nature, and must be issued in such circumstances that it may fairly be regarded as issued for the information of the public. It is not, of course, for this court to assume to lay down rules for what is, and what is not, proper to be made the subject of a governmental or police notice or report. I see no reason for doubting that an authoritative announcement of an official character made or handed to members of the press for publication in their respective newspapers would, or at least could, constitute a notice or report issued for the information of the public, and if published in the form in which it was supplied would be published with the consent of the department, etc., supplying it. On the other hand, if the matter so supplied was such as to admit of a reasonable inference that it was mere gossip and not an official notice or report, or that an official report so supplied was not published in substantially the form in which it was issued, it would be competent to the tribunal of fact to find that the defence had not been made out..."
"Although the information in the statement was supplied in answer to an inquiry from the reporter, it was supplied willingly by the police. Further, it was supplied from the communications room run by the police. It may be inferred the police communications room exists to provide information held by the police to the public. The information is no doubt edited and controlled by the police. One means of disseminating the information to the public is via the media.
[The journalist] rang the police communications room specifically to obtain information about incidents the police were involved in over the weekend. The information was imparted by a person in authority, Senior Sergeant Freeman. The senior sergeant was on duty at the time as the communications senior in the communications room. There is no suggestion that he was not authorised to pass on the information he did to the reporter. In those circumstances, in my view the evidence establishes that the information came from a formal source within the police. The circumstances in which the reporter obtained the information does not defeat the claim to statutory privilege".
"(1) It is a defence to the publication of defamatory matter if the defendant proves that the matter was contained in--
(a) a public document or a fair copy of a public document, or
(b) a fair summary of, or a fair extract from, a public document.
...
(4) In this section,
'public document' means --
...
(d) any document issued by the government (including a local government) of a country, or by an officer, employee or agency of the government, for the information of the public ..."
(1) The letter had not been "issued" by the Minister. Not every document published by a government is per se "issued" by a government: [89]. It was contrary to the ordinary meaning of the word "issued" to characterise disseminating the letter as "issuing it". The letter did not contain the requisite official character or quality which is common to the other documents defined in s.28(4) (such as reports or papers published by a Parliamentary body; records of the proceeding of a Parliamentary body; judgments, orders or determinations of courts and tribunals; records or other documents open for inspection by the public): [91], [93].
(2) Even if the Minister did "issue" the letter, he did not do so "for the information of the public", but, for the information of individual customers: [120]. The phrase "the public" clearly means the people or the community as a whole, as distinct from a closed confined class of the community: [114].
(3) s.6 Privileged report of peer-reviewed scientific or academic journal
"(1) The publication of a statement in a scientific or academic journal (whether published in electronic form or otherwise) is privileged if the following conditions are met.
(2) The first condition is that the statement relates to a scientific or academic matter.
(3) The second condition is that before the statement was published in the journal an independent review of the statement's scientific or academic merit was carried out by—
(a) the editor of the journal, and
(b) one or more persons with expertise in the scientific or academic matter concerned.
(4) Where the publication of a statement in a scientific or academic journal is privileged by virtue of subsection (1), the publication in the same journal of any assessment of the statement's scientific or academic merit is also privileged if—
(a) the assessment was written by one or more of the persons who carried out the independent review of the statement; and
(b) the assessment was written in the course of that review.
(5) Where the publication of a statement or assessment is privileged by virtue of this section, the publication of a fair and accurate copy of, extract from or summary of the statement or assessment is also privileged.
(6) A publication is not privileged by virtue of this section if it is shown to be made with malice.
...
(8) The reference in subsection (3)(a) to 'the editor of the journal' is to be read, in the case of a journal with more than one editor, as a reference to the editor or editors who were responsible for deciding to publish the statement concerned".
"44. This section creates a new defence of qualified privilege relating to peer‑reviewed material in scientific or academic journals (whether published in electronic form or otherwise). The term 'scientific journal' would include medical and engineering journals.
45. Subsections (1) to (3) provide for the defence to apply where two conditions are met. These are condition 1: that the statement relates to a scientific or academic matter; and condition 2: that before the statement was published in the journal an independent review of the statement's scientific or academic merit was carried out by the editor of the journal and one or more persons with expertise in the scientific or academic matter concerned. The requirements in condition 2 are intended to reflect the core aspects of a responsible peer-review process. Subsection (8) provides that the reference to 'the editor of the journal' is to be read, in the case of a journal with more than one editor, as a reference to the editor or editors who were responsible for deciding to publish the statement concerned. This may be relevant where a board of editors is responsible for decision-making.
46. Subsection (4) extends the protection offered by the defence to publications in the same journal of any assessment of the scientific or academic merit of a peer-reviewed statement, provided the assessment was written by one or more of the persons who carried out the independent review of the statement, and the assessment was written in the course of that review. This is intended to ensure that the privilege is available not only to the author of the peer-reviewed statement, but also to those who have conducted the independent review who will need to assess, for example, the papers originally submitted by the author and may need to comment.
47. Subsection (5) provides that the privilege given by the section to peer‑reviewed statements and related assessments also extends to the publication of a fair and accurate copy of, extract from or summary of the statement or assessment concerned.
48. By subsection (6) the privilege given by the section is lost if the publication is shown to be made with malice. This reflects the condition attaching to other forms of qualified privilege..."
(1) that BMJ Open, the journal in which the LSHTM Paper was published, is "a scientific or academic journal" for the purposes of s.6(1); and
(2) that "the first condition" in s.6(2) is satisfied; the LSHTM Paper relates to "a scientific or academic matter".
(1) To establish any privilege defence under the section, the required facts must be proved by the defendant (or admitted).
(2) Under s.6(3), a defendant must demonstrate that, prior to publication:
a) there has been a review of the statement's scientific or academic merit; and
b) the review was (i) independent; and (ii) carried out by the editor of the journal and one or more persons with expertise in the scientific or academic matter concerned.
(Under s.6(8), in the case of a journal with more than one editor, the statement must have been reviewed by the editor or editors who were responsible for deciding to publish the relevant statement.)
(3) The reporting privilege under s.6(5) requires proof (a) that the underlying statement or assessment was protected by privilege; and (b) that the publication is a fair and accurate copy of, extract from or summary.
(4) A publication is not privileged under the section if the claimant shows that it was made with malice. Malice in this context bears its usual meaning in relation to a defence of qualified privilege (see Section G(5) below).
"What conditions must be satisfied for the review to be 'independent' are not defined. Independent of the authors of the paper, or something more rigorous? It is submitted that the latter interpretation is to be preferred if the adjective is to have any meaning, for if it were the former, the adjective need not have been included. Accordingly, scientific and academic journals wishing to take advantage of the defence would be well advised to develop a clear peer-review policy that articulates the basis on which they carry out the process of peer-review and in particular how they ensure that the process is objectively fair, reliable and free from conflicts of interest".
"Given that one of the persons who is required to carry out an independent review is the editor of the journal, could the other reviewer(s) also be a member(s) of the journal's staff? It is suggested that what the section is aimed at is an expert review which is independent of the authors of the paper being reviewed...
It was stated in the parliamentary debates that the process of peer review was well understood in the scientific and academic community and that the provisions of the draft section had been shared with the editors of all the key journals who confirmed that the conditions attached were appropriate to ensure that only material subject to a responsible peer-review process would be protected (HL Grand Committee, 15 January 2012, GC 238). The courts will have little difficulty in resolving these questions if the Bill's sponsors were correct in their assessment of the common understanding of what the process entails and its accurate transposition into legislative terms".
"It is not made clear what degree of expertise is required or how that will be judged. For example, if the statement complained of features in an article about the law of libel, will a person who has written on the law of tort but not defamation be possessed of the necessary expertise? In an appropriate case, would the court permit the claimant to introduce expert evidence for the purpose of challenging the alleged expertise of the reviewers?"
"(5) Where the publication of a statement or assessment is privileged by virtue of this section, the publication in the same journal of a fair and accurate copy of, extract from or summary of the statement is also privileged".
(1) s.6 of the 2013 Act appears in the Act as a self-contained provision on a discrete topic, in accordance with the overall scheme of the Act. The provision describes, primarily in ss.6(1) to (3), the conditions of a privilege, subject to proof of malice, for "[t]he publication of a statement in a scientific or academic journal (whether published in electronic form or otherwise)". The heading of the section indicates that it is concerned with "Peer-reviewed statement[s] in scientific or academic journal etc". It is argued that "etc", in this context, refers to ss.6(4) and 6(5) which provide provisions ancillary to the primary privilege for the publication of peer-reviewed statements in scientific or academic journals. Within the overall structure of the 2013 Act, it is s.7 - not s.6 - which is concerned with general privileges from suit in defamation which anyone and everyone may take advantage of, including of course the press and the media, and which embodies Parliament's wishes as to extensions to privileges of that kind. It is submitted that in these circumstances one would not naturally expect to find a new, general privilege for anyone in s.6 as opposed to s.7, and that, as a starting point, this is a strong indicator that this is not what Parliament intended to achieve by enacting s.6(5).
(2) The ancillary privilege described in s.6(4) for "the publication in the same journal of any assessment of the statement's scientific or academic merit" is stated in terms to be parasitic upon the primary privilege provided for in ss.6(1)-(3) for the publication of a peer-reviewed statement in a scientific or academic journal. The s.6(4) privilege arises only "[w]here the publication of a statement in [the same] scientific or academic journal is privileged by virtue of subsection (1)" (emphasis added) and where the assessment in question was produced as part of the peer-review process relating to that statement, namely where "(a) the assessment was written by one or more of the persons who carried out the independent review of the statement; and (b) the assessment was written in the course of that review".
(3) The second ancillary privilege provided for in s.6, described in s.6(5) as being for "the publication of a fair and accurate copy of, extract from or summary of the statement or assessment" is also parasitic upon and ancillary to (a) the primary privilege provided for in ss.6(1)-(3), and (b), where what is in issue is an "assessment" published in the same journal, the privilege provided for in s.6(4) as well. It is submitted that this observation is a pointer that s.6(5), like s.6(4) (although it does not say so in terms) is and is intended to be concerned exclusively with fair and accurate copies of, extracts from, or summaries of a statement or assessment, published in the same journal. Section 6, considered as a whole, including ss.6(4) and (5), seems to be focussed exclusively on the publication of statements relating to scientific or academic matters in scientific and academic journals following a process of peer-review overseen in relation to a particular statement or assessment by the same editors and reviewers. Against this background, it is submitted that the omission of the words "in the same journal" from s.6(5) appears objectively much more likely to be the product of a wish on the part of the Parliamentary draftsman to avoid repetition and surplusage than of an intention to confer (without fanfare) the privilege described in that subsection on everyone and anyone.
(4) It would be curious, it is argued, if s.6(5) were held to confer a privilege on a newspaper, or some other stranger to the peer-review process, in circumstances where that would require that publisher to satisfy the conditions for a privilege under s.6(1) and, where relevant, the further conditions in s.6(4), when that third party will invariably (a) have had no involvement in the publication of the ex hypothesi peer-reviewed statement or in the process of peer-review which preceded it, and (b) have no direct knowledge of how the peer-review process was carried out, who was involved in it, or how the statement in question came to be published. In whatever way s.6(5) is to be interpreted, there can be no question other than that these are matters which a person who wishes to rely on s.6(5) is required to prove. In this context, the idea that s.6(5) contemplates or intends that a person with no direct knowledge of or access to evidence about the peer-review and publication process should be expected to satisfy these requirements is one that "borders on the absurd", Ms Page KC submits.
(5) The fifth point is based upon the wording of Paragraph 47 of the Explanatory Notes (see [327] above). It is argued that the use of the phrase "also extends to" in this way reinforces the view that what Parliament intended by s.6(5) was a further ancillary privilege for the publisher of the scientific or academic journal in which the peer-reviewed statement and any assessment of it had been published if it decided also to publish in the same journal a copy, etc. of the statement or assessment, not for anyone one else. If what had been intended was a fresh, free-standing, general privilege for everyone and anyone, one would expect that at the very least this would have been spelled out in the Explanatory Note.
(6) While s.6(5) may not, in express terms, state that it is confined to the publication of a fair and accurate copy, etc. in the same journal, equally it does not say in terms that it extends to the publication of such matter by anyone and everyone. Again, one would have expected Parliament to have stated this explicitly in clear and unambiguous terms if it were the case, especially in the light of s.7 and the provisions affecting the scope of the general privileges in Schedule 1 to the Defamation Act 1996 that it contains.
(1) The proposals that were eventually enacted as s.6 and s.7(9)[20] of the 2013 Act were regarded by the Government as forming two limbs of a policy to increase the protection for scientific and academic speech. However, the two innovations were treated by Parliament as separate matters, the former as a new, sui generis privilege for peer-reviewed statements and the latter as a new, general reporting privilege. For example, Lord Hunt of Chesterton's proposed Amendment to Clause 6 (which became s.6) was debated at a different sitting in the House of Lords (on a different day) from Amendments 41-42 in relation to the s.7(9) privilege for reports of scientific and academic conferences. Furthermore, while what became s.7(9) had formed part of the Government's Draft Defamation Bill from the outset, Clause 6 only came into the Bill at the point it entered Parliament, on the recommendation of the Joint Committee on Defamation. In short, from the Government's point of view, while Clause 7 was always concerned with possible extensions to general privileges for everyone and anyone - most importantly for the press and the media - Clause 6 was wholly and exclusively concerned, consistent with its heading, with "Peer-reviewed statement[s] in scientific or academic journal[s] etc", nothing else.
(2) Although the Joint Committee on Defamation, was instrumental in the introduction into the Bill of Clause 6, it made no recommendation for a general privilege for the press for the publication of copies of, extracts from or summaries of material they had come across in peer-reviewed journals. It is not even something that was discussed as a possibility. The Joint Committee's focus was exclusively on the need to protect from suit in defamation scientists and academics publishing in serious scientific and academic journals, the hallmark of which was that they operated a proper system of peer-review. The key paragraph in its Report on this point is [48]:
Let us be clear: right from the start, I wanted to provide protection for genuine academic and scientific debate. I have to say to my noble friend Lord Phillips that 'academic and scientific' is a term that is generally understood — it does not mean the Beano. People know one when they see one. Within that, there is also the important context that we are looking for genuine peer review, which, again, is understood. I worry, as I think the noble Lord, Lord Bew, does — I will also be interested in the response from the noble Lord, Lord Hunt, to the specific questions — that we must not push the envelope too far on this, otherwise we will run into some of the problems that the noble Lord, Lord Browne, raised. We are right to be cautious.
As I say, the issue featured prominently in our discussions with the scientific community. We also held discussions with the editors of all the key journals to ensure that appropriate conditions were attached, so that the clause applied only where responsible peer-review process was used. We shared the relevant aspect of the clause with those editors to confirm that this was achieved.
Amendment 31 would extend the defence to peer-reviewed material on,
'a website edited and controlled by a chartered professional or learned body'.
We are concerned that this would make the defence too widely available. We believe that it is important to ensure that only bona fide publications with appropriate procedures are given the protection of the new defence. That is why we have focused the clause on scientific and academic journals, where there is a well established process for peer review. I can confirm that the existing clause would cover peer-reviewed material that was published by such a journal in an electronic form. However, a potentially wide range of bodies may fall within the categories proposed by the noble Lord, and we are concerned that this would extend the defence into areas where peer review is not a common practice. That may lead to the defence being available in instances where it is more likely that the peer-review process will not have been applied sufficiently robustly". (emphasis added)
Ms Page KC argues that it would be extraordinary if Lord McNally had expressed himself in this way, particularly in the underlined passage, without making it clear that Clause 6(5) if enacted would make provision for a general privilege for the press and everyone else, if that is what the Government intended.
(4) Save in relation to Lord Hunt's Amendment 31 (which was withdrawn) and Amendment 35 (which was not moved), Clause 6 was not the subject of significant Parliamentary discussion and debate. It was enacted in the same terms as it entered the House of Commons, with only one exception: the insertion into Clause (which became s.6(1)) of the phrase "(whether published in electronic form or otherwise)". The relevant Explanatory Notes were also unchanged. Nothing was said substantively about Clause 6(5) during the Parliamentary debate. The possibility that the sub-clause might confer a further general privilege on the world at large was not acknowledged.
(5) While it was generally the Government's purpose in enacting the Defamation Act 2013 to re-balance protection of reputation and freedom of expression in favour of freedom of expression, the Government was distinctly cautious in legislating in the specific context of Clause 6 and was concerned to ensure that the privilege for peer-reviewed publications was kept within proper bounds. It is suggested that this is unsurprising given that the privilege was an entirely novel one. There were also concerns around the clarity and certainty of the language being used and the definition of "peer-review" - as there also were around the term "conference" in Clause 7(9)) - which Parliament decided to leave to the courts to interpret. None of this appears consistent with the idea that Parliament was intending by Clause 6(5) to create a new, general privilege for anyone and everyone, as opposed to an ancillary privilege for the publisher of a peer‑reviewed paper, which had overseen the peer-review process itself, and which might wish to publish an abstract or summary of its peer-reviewed paper, whether on the home page of the journal's website or as part of an editor's 'choice' or 'round-up' in addition to the paper itself, or otherwise.
[48] The draft Bill goes some way towards tackling this problem by extending qualified privilege to include fair and accurate reports of what is said at a 'scientific or academic conference'. We welcome this development, provided the conference is reputable. However, our inquiry revealed unanimous support for extending protection of qualified privilege to peer-reviewed articles published in scientific or academic journals, as recommended in 1975 by the Faulks Committee when the law of defamation was last reviewed comprehensively. Peer-reviewed articles are arguably the main platform for scientific and academic debate, and more reliable in their quality than conferences. Such articles may, in principle, be protected by other types of legal privilege, including qualified privilege and the so-called Reynolds defence, but the Reynolds defence in particular is often time consuming and costly to make out. In our view a proper peer review process should lead to the publication being treated as responsible and should have special protection in the public interest without the burden of having to prove 'responsibility' in every individual case. Scientists and academics must not be left in fear of being sued simply for doing their job. We recommend that a provision is added to the draft Bill extending qualified privilege to peer-reviewed articles in scientific or academic journals..."
(2) On 29 February 2012, the Ministry of Justice published the Government's response to the Report of the Joint Committee (Cmnd. 8295). On the issue of peer-reviewed papers it included (at paragraph 48):
"The draft Bill already provides for qualified privilege to be extended to fair and accurate reports of academic and scientific conferences. This was supported by the majority of consultation responses, and we will retain provisions on this in the substantive Bill. We are sympathetic to the need to provide clear protection for peer-reviewed articles published in scientific and academic journals and will consider further whether this can best be achieved through qualified privilege or other means, and how key elements of the peer-review process can be defined to ensure that the scope of any provision is clear".
(3) The Defamation Bill was introduced to the House of Commons and received its first reading on 10 May 2012. The Bill included a new clause 6 ("Peer-reviewed statement in scientific or academic journal etc".), which had not been in the Draft Defamation Bill presented with the Consultation Paper in March 2011. Clause 6 of the Bill, as presented to Parliament, was in substantially the same terms of section 6 of the Act as it was ultimately enacted. The only change was the addition of the words "(whether published in electronic form or otherwise)" into s.6(1).
"I am confident that everybody in this Chamber agrees that freedom of expression is the cornerstone of our democracy. In an open society, people should be at liberty to debate a subject without fear or favour, whether the matter be political, scientific, academic or anything else. That is how power is held to account, abuses of authority are uncovered and truth is advanced. But freedom of speech does not mean that people should be able to ride roughshod over the reputations of others without regard to the facts. Life and career can be destroyed by false allegations that go unanswered. The issue for our defamation laws is ultimately one of striking the right balance between protection of freedom of expression on the one hand and protection of reputation on the other.
I share the mounting concern of recent years that our defamation laws are becoming out of date, costly and over-complicated, and that they are at risk of damaging freedom of speech without affording proper protection. No one can be satisfied with a situation where the threat of lengthy and costly proceedings has sometimes been used to frustrate robust scientific and academic debate, to impede responsible investigative journalism and to undermine the good work undertaken by many non-governmental organisations...
In a further important step forward for the protection of scientists and academics, clause 6 creates a defence of qualified privilege for peer-reviewed material in scientific and academic journals, as recommended by the Joint Committee on the draft Bill. The clause defines key elements of the peer-review process to ensure that publications with appropriate procedures will now be given the protection of this new defence".
Towards the end of the debate (cols. 262-263), the Parliamentary Under‑Secretary of State for Justice, Jonathan Djanogly, said:
"... we believe that extending the clause 6 protection is important in order to help encourage robust and open scientific and academic debate, and I, too, acknowledge the principled stand and ongoing participation of Dr Simon Singh in this area. In drafting the clause, we have given careful consideration to defining key elements of the peer-review process to ensure that the scope of the provisions is clear and appropriate, and we are satisfied that it is".
The reference to Dr Simon Singh was likely to have been prompted by the defamation case brought against him by the British Chiropractic Association (see British Chiropractic Association -v- Singh [2011] 1 WLR 133).
(5) At the Public Bill committee debate on 26 June 2012, Mr Djanogly said the following regarding Clause 6 (cols. 137-140):
"It is nice to see that there is clear consensus on support for the clause, which creates a new defence of qualified privilege for peer-reviewed material in scientific or academic journals. A core concern underlying our commitment to reforming the law is to protect scientific and academic debate from the threat of unjustified libel proceedings. Clause 6 is one of a number of measures in the Bill that are intended to encourage open and robust scientific and academic debate. As hon. Members have said, it responds to a recommendation made by the Joint Committee on the draft Bill. Subsections (1) to (3) provide for the defence to apply where certain key elements that relate to the peer review process are met. Those are that the statement concerned 'relates to a scientific or academic matter' and that before it was published 'an independent review of the statement's scientific or academic merit was carried out by the editor of the journal, and one or more persons with expertise' in the matter concerned.
Subsection (8) clarifies that where a journal has more than one editor, the reference is to the editor or editors who were responsible for the decision to publish the statement concerned. Those requirements stem from discussions we have had with editors of major journals, and they are intended to reflect the core aspects of a responsible peer-review process to ensure that only publications with appropriate procedures are given the protection of the new defence.
Subsection (4) ensures that the protection offered by the defence is available not only to the author of the peer-reviewed statement but to those who have conducted the independent review of its scientific or academic merit. We consider it fair that reviewers participating in the process, who may need to assess the papers submitted by the author and comment on them, and whose assessments are published in the journal, should also be protected.
Subsection (5) extends qualified privilege to fair and accurate copies, extracts and summaries of the peer-reviewed statement. Subsections (6) and (7) contain provisions that apply to qualified privilege in other contexts. They establish that privilege is lost if publication is shown to be made with malice, and that a person who publishes material in a scientific or academic journal is not prevented from relying on other forms of privilege such as that conferred by clause 7(9) on fair and accurate reports of proceedings at a scientific or academic conference. The hon. Member for Stoke-on-Trent South asked whether the peer review defence would have been available in the Simon Singh case, and the answer is no. Simon Singh might, however, have been able to benefit from the other changes we are making on issues such as serious harm and honest opinion".
Simon Singh would not have benefited from the new statutory defence because the publication that led to the libel claim against him was neither a peer‑reviewed statement in a scientific or academic journal and nor was it a fair and accurate copy of, extract from or summary of such a statement.
(6) On 12 September 2012, the then Lord Chancellor, Chris Grayling, said the following during the Bill's third reading (HC Vol. 550, cols. 366-369):
"The Bill reflects our view that the law is out of kilter, and that our defamation regime is out of date, costly and over-complicated. It needs urgent reform so as to offer more effective protection for freedom of speech and to stop the threat of long and costly libel proceedings being used to stifle responsible investigative reporting and scientific and academic debate...
In addition to [several] general measures, the Bill takes specific steps to encourage robust scientific and academic debate by creating a new defence against libel for peer-reviewed material in scientific and academic journals, and by extending qualified privilege to reports of scientific and academic conferences. Given the work that my right hon. Friend the Minister for Universities and Science is doing to promote science in this country, the more we can send messages that we value scientific research in this country, the better".
(7) On 15 January 2013, the third day of Committee Stage in the House of Lords, peers considered an amendment to Clause 6(1), proposed by Lord Hunt, to insert, after "journal", the words "or on a website edited and controlled by a chartered professional or learned body (a 'recognised website')". At the end of the debate, Lord McNally, Minister of State at the Ministry of Justice, asked Lord Hunt to withdraw the amendment, because it would "make the defence too widely available". Lord Hunt did withdraw his amendment. This is the context of the Lord McNally's speech, relied upon by the Claimants (see [340(3)] above).
(1) In Gatley, the authors state (§17-026):
"Where the publication of the statement or assessment is privileged under the section then, by virtue of s.6(5), 'the publication of a fair an accurate copy of, extract from, or summary of the statement or assessment is also privileged'. A newspaper or other report fairly and accurately summarising, or providing an extract from, the statement would consequently be privileged".
(2) The authors of Duncan & Neill on Defamation (§18-04) and Blackstone's Guide (§§7.35-7.39) agree.
"A difficult question may conceivably arise, as to whether publication of a fair and accurate copy of, extract from or summary of the statement or assessment could be privileged under subsection (5), if privilege for publication of the statement or assessment is defeated by malice. Publication of the copy, extract or summary is, by subsection (5), privileged only where publication of the statement or assessment is itself privileged, and by subsection (6), that is not so where publication of the statement or assessment was malicious...
In the case of privilege for fair and accurate copies, extracts and summaries under Schedule to the Defamation Act 1996, malice on the part of the person who made the underlying statement is irrelevant. But it is not a condition of that privilege that publication of the underlying statement should have been privileged.
This situation would have some similarity to that provided for, in relation to the defence of honest opinion, by s.3(6) of the Act. The court may consider that ... persons who fairly, accurately and honestly report peer-reviewed statements or assessments from scientific and academic journals should not have to concern themselves with the motives of those involved in the publication in the journal. This could perhaps be achieved by construing the requirement that the underlying statement must be privileged, as meaning privileged in principle, subject to malice".
(4) Malice
"The law quite rightly requires that questions of dishonesty be approached more rigorously than other questions of fault. The burden of proof remains the civil burden—the balance of probabilities—but the assessment of the evidence has to take account of the seriousness of the allegations and, if that be the case, any unlikelihood that the person accused of dishonesty would have acted in that way. Dishonesty is not to be inferred from evidence which is equally consistent with mere negligence."
"The public interest that the law should provide an effective means whereby a man can vindicate his reputation against calumny has nevertheless to be accommodated to the competing public interest in permitting men to communicate frankly and freely with one another about matters in respect of which the law recognises that they have a duty to perform or an interest to protect in doing so. What is published in good faith on matters of these kinds is published on a privileged occasion. It is not actionable even though it be defamatory and turns out to be untrue. With some exceptions which are irrelevant to the instant appeal, the privilege is not absolute but qualified. It is lost if the occasion which gives rise to it is misused. For in all cases of qualified privilege there is some special reason of public policy why the law accords immunity from suit - the existence of some public or private duty, whether legal or moral, on the part of the maker of the defamatory statement which justifies his communicating it or of some interest of his own which he is entitled to protect by doing so. If he uses the occasion for some other reason he loses the protection of the privilege.
So, the motive with which the defendant on a privileged occasion made a statement defamatory of the plaintiff becomes crucial. The protection might, however, be illusory if the onus lay on him to prove that he was actuated solely by a sense of the relevant duty or a desire to protect the relevant interest. So he is entitled to be protected by the privilege unless some other dominant and improper motive on his part is proved. 'Express malice' is the term of art descriptive of such a motive. Broadly speaking, it means malice in the popular sense of a desire to injure the person who is defamed and this is generally the motive which the plaintiff sets out to prove. But to destroy the privilege the desire to injure must be the dominant motive for the defamatory publication; knowledge that it will have that effect is not enough if the defendant is nevertheless acting in accordance with a sense of duty or in bona fide protection of his own legitimate interests.
The motive with which a person published defamatory matter can only be inferred from what he did or said or knew. If it be proved that he did not believe that what he published was true this is generally conclusive evidence of express malice, for no sense of duty or desire to protect his own legitimate interests can justify a man in telling deliberate and injurious falsehoods about another, save in the exceptional case where a person may be under a duty to pass on, without endorsing, defamatory reports made by some other person.
Apart from those exceptional cases, what is required on the part of the defamer to entitle him to the protection of the privilege is positive belief in the truth of what he published or, as it is generally though tautologously termed, 'honest belief.' If he publishes untrue defamatory matter recklessly, without considering or caring whether it be true or not, he is in this, as in other branches of the law, treated as if he knew it to be false. But indifference to the truth of what he publishes is not to be equated with carelessness, impulsiveness or irrationality in arriving at a positive belief that it is true. The freedom of speech protected by the law of qualified privilege may be availed of by all sorts and conditions of men. In affording to them immunity from suit if they have acted in good faith in compliance with a legal or moral duty or in protection of a legitimate interest the law must take them as it finds them. In ordinary life it is rare indeed for people to form their beliefs by a process of logical deduction from facts ascertained by a rigorous search for all available evidence and a judicious assessment of its probative value. In greater or in less degree according to their temperaments, their training, their intelligence, they are swayed by prejudice, rely on intuition instead of reasoning, leap to conclusions on inadequate evidence and fail to recognise the cogency of material which might cast doubt on the validity of the conclusions they reach. But despite the imperfection of the mental process by which the belief is arrived at it may still be 'honest,' that is, a positive belief that the conclusions they have reached are true. The law demands no more.
Even a positive belief in the truth of what is published on a privileged occasion - which is presumed unless the contrary is proved - may not be sufficient to negative express malice if it can be proved that the defendant misused the occasion for some purpose other than that for which the privilege is accorded by the law. The commonest case is where the dominant motive which actuates the defendant is not a desire to perform the relevant duty or to protect the relevant interest, but to give vent to his personal spite or ill will towards the person he defames. If this be proved, then even positive belief in the truth of what is published will not enable the defamer to avail himself of the protection of the privilege to which he would otherwise have been entitled. There may be instances of improper motives which destroy the privilege apart from personal spite. A defendant's dominant motive may have been to obtain some private advantage unconnected with the duty or the interest which constitutes the reason for the privilege. If so, he loses the benefit of the privilege despite his positive belief that what he said or wrote was true.
Judges and juries should, however, be very slow to draw the inference that a defendant was so far actuated by improper motives as to deprive him of the protection of the privilege unless they are satisfied that he did not believe that what he said or wrote was true or that he was indifferent to its truth or falsity. The motives with which human beings act are mixed. They find it difficult to hate the sin but love the sinner. Qualified privilege would be illusory, and the public interest that it is meant to serve defeated, if the protection which it affords were lost merely because a person, although acting in compliance with a duty or in protection of a legitimate interest, disliked the person whom he defamed or was indignant at what he believed to be that person's conduct and welcomed the opportunity of exposing it. It is only where his desire to comply with the relevant duty or to protect the relevant interest plays no significant part in his motives for publishing what he believes to be true that 'express malice' can properly be found."
(1) are the words complained of a fair and accurate extract of the Particulars of Claim?
(2) if so, was the publication by the defendant to the public of the words complained of the publication of matter which was of public concern (now public interest) and for the public benefit, as provided by s.15(3)?
[99] In my judgment s.15 reflects the need to have regard both to the public interest in freedom of expression and to the public interest that an individual's right to his reputation be not interfered with otherwise than for a legitimate aim and when it is proportionate to do so. Parliament has itself carried out the balancing exercise in part. Where a defendant has filed an admission, a non-party has no right to obtain the claim form or particulars of claim, so Sch.1 para.5 will not apply at all. Where a defendant has filed a defence or an acknowledgement of service, then that will necessarily state or imply that the defendant disputes the claim.
[100] In my judgment it follows that, as a general rule (that is one to which there may be exceptions) it will not be for the public benefit to publish any defamatory allegations made in a claim form or particulars of claim available to the public from the court under CPR r.5.4C without at the same time publishing the fact that the defendant has denied, or is disputing, the allegations, as the case may be. The effect of s.15(3) is to give the court trying a defamation action the power and duty to consider a balancing exercise on the particular facts of the case. In effect in that, and in the predecessor legislation, Parliament has required the court to carry out a balancing exercise similar to the one which has now become familiar under the HRA, namely art.10 and art.8 (see Re S (A Child) (Identification: Restrictions on Publication) [2005] 1 AC 593, Lord Steyn at [17]).
[101] I find it hard to envisage any circumstances in which there would be a public benefit in publishing defamatory extracts from a claim form or particulars of claim without there being included in the publication a statement that the allegations are disputed, or, if it be the case, denied. It is the very fact that the allegations are disputed or denied that provides the condition, set out in para.5 itself, which gives rise to the legal requirement that the court make the document available to the public. In the present case I see no public interest in ANL publishing a defamatory extract from the ... particulars of claim which omitted a statement that the claim is disputed."
"If the occasion is privileged it is so for some reason, and the defendant is only entitled to the protection of the privilege if he uses the occasion for that reason. He is not entitled to the protection if he uses the occasion for some indirect and wrong motive. If he uses the occasion to gratify his anger or his malice, he uses the occasion not for the reason which makes the occasion privileged, but for an indirect and wrong motive. If the indirect and wrong motive suggested to take the defamatory matter out of the privilege is malice, then there are certain tests of malice. Malice does not mean malice in law, a term in pleading, but actual malice, that which is popularly called malice. If a man is proved to have stated that which he knew to be false, no one need inquire further." (emphasis added)
"Therefore, though what is said amounts to a slander, it is privileged, provided the person who utters it is acting bonâ fide, in the sense that he is using the privileged occasion for the proper purpose and is not abusing it. It is sometimes said that he must be acting bonâ fide and not maliciously; but I do not think that that way of expressing the rule is quite exhaustive or correct. I think the question is whether he is using the occasion honestly or abusing it. If a person on such an occasion states what he knows to be untrue, no one ever doubted that he would be abusing the occasion. The jury here appear to have thought that the defendant said what was false knowing it to be false. I cannot agree with that view of the case. If the case depended on a finding to that effect, I should be very loth to find it. But there is a state of mind, short of deliberate falsehood, by reason of which a person may properly be held by a jury to have abused the occasion, and in that sense to have spoken maliciously".
"The statutory privileges... relied upon by [the defendant] are plainly accorded for occasions where the publisher need not be acting out of any duty or interest, and where the publisher is not required to have any belief in the truth or falsehood of the document of which he is publishing an extract... Lord Diplock referred in passing at 150 to 'the exceptional case where a person may be under a duty to pass on, without endorsing, defamatory reports made by some other person'. But he did not refer to the occasions which are the norm, not the exception, in statutory privilege, where the publisher (being under not duty) chooses to pass on, without endorsing, defamatory statements in which he has no belief. In statutory privileges it is the public interest that provides the basis of the legislative purpose, not the interests of the publisher, still less any duty of the publisher"
"If the tests in the Schedule to the 1996 Act (fairness and accuracy) and in s.15(3) (public concern and public benefit) were satisfied in this case, contrary to what I have decided above, then malice could be established in principle by proof that ANL knew that the claim was disputed but knowingly published the false statement that it was not disputed, or if ANL knew that the form in which it reported the extract from the particulars of claim ... was misleading or unfair."
"... The place and meaning of malice in the law of qualified privilege could, it seems to me, be the subject of further argument in the light of Article 10..."
"This species of malice may still have a legitimate role in malicious falsehood claims (particularly trade libel) but it has a dubious justification when advanced in answer to a well-founded plea of qualified privilege. It has been expressly excluded as a basis for proving malice in answer to a fair comment/honest opinion defence: Albert Cheng –v- Paul [2001] EMLR 777. In 2002, Eady J noted that he could not recall an instance of 'dominant intention' malice having been proved and described this form of malice as an 'endangered species' in relation to qualified privilege: Lillie & Reed –v- Newcastle City Council [2002] EWHC 1600 (QB) [1093]. I am not aware of any such case in the 15 years since."
(In passing I would note that the period has now grown to 22 years).
[56] ... The rationale of the defence of qualified privilege is the law's recognition that there are circumstances when there is a need, in the public interest, for a particular recipient to receive frank and uninhibited communication of particular information from a particular source: see Reynolds -v- Times Newspapers Ltd [2001] 2 AC 127, 195. Traditionally, these occasions have been described in terms of persons having a duty to perform or an interest to protect in providing the information. If, adopting the traditional formulation for convenience, a person's dominant motive is not to perform this duty or protect this interest, he is outside the ambit of the defence. For instance, if a former employer includes defamatory statements in an employment reference with the dominant purpose of injuring the former employee, the former employer is misusing the privileged occasion and this will vitiate his defence of qualified privilege.
[57] The rationale of the defence of fair comment is different, and is different in a material respect. It is not based on any notion of performance of a duty or protection of an interest. As already noted, its basis is the high importance of protecting and promoting the freedom of comment by everyone at all times on matters of public interest, irrespective of their particular motives. In the nature of things the instances of misuse of privilege highlighted by Lord Diplock (for example, "some private advantage unconnected with the duty or interest which constitutes the reason for the privilege") are not necessarily applicable to fair comment..."
[49] ... Take the case of a politician or a journalist who genuinely believes that a minister is untrustworthy and not fit to hold ministerial office. Facts exist from which an honest person could form that view. The politician or journalist states his view, with the intention of injuring the minister. His reason for doing so was a private grudge, derived from a past insult, actual or supposed. I am far from persuaded that the law should give the minister a remedy. The spiteful publication of a defamatory statement of fact attracts no remedy if the statement is proved to be true. Why should the position be different for the spiteful publication of a defamatory, genuinely held comment based on true fact?
[79] ... To summarise, in my view a comment which falls within the objective limits of the defence of fair comment can lose its immunity only by proof that the defendant did not genuinely hold the view he expressed. Honesty of belief is the touchstone. Actuation by spite, animosity, intent to injure, intent to arouse controversy or other motivation, whatever it may be, even if it is the dominant or sole motive, does not of itself defeat the defence. However, proof of such motivation may be evidence, sometimes compelling evidence, from which lack of genuine belief in the view expressed may be inferred. Proof of motivation may also be relevant on other issues in the action, such as damages.
(5) Natural and ordinary meaning and whether fact/opinion
(1) Where a single article is published, for the purposes of deciding the single natural and ordinary meaning of the publication, the ordinary reasonable reader is taken to have read the entire article (including headlines etc.): Charleston -v- News Group Newspapers Ltd [1995] 2 AC 65, 72F-73D. This principle applies even if the article continues over several pages: Dee -v- Telegraph Media Group Ltd [2010] EMLR 20 [27].
(2) Where several articles are printed in a single edition of a newspaper, the Court must decide whether they were sufficiently closely connected as to be regarded as a single publication. If they are, it does not matter that some readers may have read only some, but not all, of the articles: Dee [29]-[30].
(3) The manner of the presentation of multiple articles may nevertheless be relevant to the assessment of the natural and ordinary meaning, "since [that] is affected by the mode of publication (that is, the relative prominence or emphasis given to what is published) as well as by context": Dee [30]; Charleston p.74D, per Lord Nicholls.
"... where the [claimant] submits that more than one publication is to be taken into account to found the defamatory imputation, I conclude that the test to be applied is whether, having regard to all of the circumstances, it is to be inferred that hypothetical ordinary reasonable reader of the material complained of will also have read, or have in mind, the other material which is relied upon as context. For that to be possible, there must be a sufficient nexus, connection or association between the publications, which could include a reference or hyperlink or the publications being part of for example, a Twitter conversation, or a series or sequence of material."
(i) The report of what was said in Parliament is subject to qualified privilege. This necessarily involves a disapplication of, or an exception to, the repetition rule as regards that part of the publication. If the rule were applied, the privilege would be nullified. The privilege allows the publisher to rely on the fact that he is reporting what another has said. That other is a legislator speaking in Parliament. The very purpose of the privilege is to facilitate what s/he has said. It can only be done if the repetition rule is set aside.
(ii) The meaning of the publisher's own comments is to be ascertained separately from the meaning of the report of Parliamentary speech. This necessarily involves a disapplication of, or an exception to, the single meaning rule. So much follows from proposition (i): once it is accepted that those parts of the publication consisting in the report of Parliamentary speech, being covered by qualified privilege, must be understood without reference to the repetition rule, the publisher's own comments must necessarily be interpreted according to their own terms and no special rule applies. Accordingly the relation between the report and the comments is that the first sets the context for the second; no more.
...
Embellishment and Adoption
[87] Finally I add these short comments about embellishment and adoption. It is plain that there will be no qualified privilege in an account of Parliamentary speech if the publisher has so embellished the material that it cannot be said to be a fair and accurate report. So much, I think, is shown by this passage from Lord Denning's speech in Dingle -v- Associated Newspapers Ltd [1964] AC 371, 411:
"But if it [sc. the publisher] adds its own spice and prints a story to the same effect as the parliamentary paper, and garnishes and embellishes it with circumstantial detail, it goes beyond the privilege and becomes subject to the general law. None of its story on that occasion is privileged. It has 'put the meat on the bones' and must answer for the whole joint."
[88] Some care is I think needed in considering the concept of adoption, discussed by Arden LJ at paragraphs 37 - 40. In a sense the publisher who embellishes Parliamentary speech may be said to have adopted it: by "putting the meat on the bones" he has made the allegation his own. But I think it is misleading to characterise such a case as one of adoption. Rather than adopting what was said, the publisher has produced a critically different text. Since what he has produced cannot be said to be a fair and accurate report of Parliamentary speech, the law gives him no shield of qualified privilege. That is the whole analysis of the case; no recourse to any such idea as adoption is required.
[89] In Buchanan -v- Jennings [2005] 1 AC 115 a Member of Parliament effectively re-stated outside Parliament what he had earlier stated inside it. The first statement was absolutely privileged. It could not sensibly be suggested (and was not) that the later utterance was somehow a fair and accurate report of the earlier. Thus the species of qualified privilege which arises in this case did not arise there. Again, no recourse to adoption is needed for the case's analysis.
(1) First, the single natural and ordinary meaning governs the parameters of any defence of truth and (to a lesser extent) any defence of honest opinion. If upheld, the effect of any defence is not (retrospectively) to adjust the single meaning but to relieve the defendant for liability for the reputational harm caused by its publication.
(2) Second, together with the extent of publication, it governs the assessment of the objective harm caused to the reputation of the claimant by the relevant publication, which is a key element in the assessment of any award of damages. That objective harm remains the same regardless of whether the publication (or any part of it) is protected by privilege (or any other defence). If some, but not all, of a defamatory publication is found to be privileged, the damages may be reduced: Dingle -v- Associated Newspapers Ltd [1964] AC 371, 394 (per Lord Radcliffe), 410 (per Lord Denning), 414 (per Lord Morris)
H: Determination of the issues
(1) the "Print Publication" consists of the publication, in the hardcopy of The Mail on Sunday of the News Article, the Main Article (including the Text Box) and the Editorial;
(2) "Online Publication 1" is a single continuous page on Mail Online, on which the three Articles were published together - Main Article (minus the Text Box), News Article and Editorial;
(3) "Online Publication 2" is a separate page on Mail Online, on which the News Article was published, but with a hyperlink to the Main Article; and
(4) "Online Publication 3" is a further separate page on Mail Online, on which the Editorial alone was published.
(A) Publication on a matter of public interest under s.4 Defamation Act 2013
Print Publication
(1) In relation to the publication of the Articles in the hardcopy of the newspaper ("the Print Publication"), have the Defendants shown that the Print Publication was, or formed part of, a statement on a matter of public interest, and if so, what is that matter of public interest?
(2) If so, have the Defendants shown that Mr Calman believed that publishing the Print Publication was in the public interest?
(3) If so, have the Defendants shown that Barney Calman reasonably believed that publishing the Print Publication was in the public interest?
(1) Mr Calman carried out such checks and made such inquiries as it was reasonable to expect of a journalist in his position, investigating and writing about this subject matter (i.e. the rival contentions as to the existence, reliability, strength, conclusiveness etc., of scientific evidence drawn from the studies into statin use).
(2) This would include Mr Calman satisfying himself of the accuracy of certain facts, namely:
a) that the claims he used in the articles were accurately attributed to the Claimants;
b) that (outline) results of the scientific research which Mr Calman used for the Articles were accurately summarised (e.g. the LSHTM Paper); and
c) the quotes from experts, the Hancock Statement, and joint editorial (referred to in Main Article [13]) were accurately summarised and attributed in the article.
(3) However, "the secondary evaluative/comparative inferences themselves, i.e. with what reliability/confidence/solidity are the rival scientific opinions of established experts and the Claimants held ('definitive'/'indisputable' etc.) could not be verified".
"There was no reasonable basis for such a belief. Rather, objectively speaking, Mr Calman could only have reasonably believed that publication was contrary to the public interest. Mr Calman's 'investigation' and the journalism that it produced were not of a quality or standard such as to warrant protection under s.4, making all due allowances for editorial judgment, especially in circumstances where the material in question was being published to the [newspaper's] extremely large readership. This was not an open-minded investigation in the conventional sense of having no fixed conclusion (see Packham)... but a quest for material to support as strongly as possible Mr Calman's takedown, where the ultimate outcome - the 'utter discrediting' of the Claimants, as Ms Page KC put it to Mr Calman in cross-examination... was predetermined"
(1) This was a controversial subject, upon which it was obvious to Mr Calman that there were competing views. There was a longstanding, and ongoing, public dispute about the benefits of statins, particularly when measured against potential side-effects; the 'statin debate'. Mr Calman had, himself, described it as a "row" (see [97] above). As stated in the BMJ Review Report (see [206] above): "Unbiased groups of scientific investigators analysing the same data can reach very different conclusions". As Mr Calman noted in the Editorial ([12]), and similarly accepted in his evidence, "Debate should - must - be at the heart of science", and at an early stage of his research, Mr Calman had been specifically advised by X, in the context of the statin debate, "that there is no one cause of heart disease, ... statins are not a universal panacea - they are merely one of the few and best weapons we have at the moment", and that it was "wise to admit where there are legitimate questions" (see [85] and [94] above).
(2) There were two main sides to the statin debate, as Mr Calman well understood. On the side of "scientific consensus" or orthodoxy, were included the experts with whom he had worked closely and relied upon in preparing the Articles. Mr Calman expressly recognised that his experts were on one side of "the row" (see [97] above). On the side of the critics, were the Claimants, but importantly others. On 12 February 2019, X had warned Mr Calman that the First Claimant "cherry picks as much as the other side", that Professor Collins was "as outspoken as she is" and the "tit for tat could be endless" (see [84]-[85] above).
(3) Put neutrally, the Claimants, in their published works and other public statements, had raised questions about whether the medical orthodoxy on statins was correct. In doing so, they had identified and relied upon several scientific studies published in peer-reviewed journals and (for the First Claimant) had drawn upon her own research and (for the Second Claimant) his experience as a GP. Although the weight to be attached to any particular scientific study or trial might be a matter of debate, it has not been suggested that any of the papers upon which they relied (save in respect of some of the conclusions of the 2013 BMJ Articles) are not serious contributions to scientific learning and debate on statins published by reputable academic journals.
(4) A proper understanding, assessment and presentation of the claims and counterclaims made in the statin debate required an understanding of a large number of scientific papers that had been published on the topic which, in turn, required familiarity with the terminology and concepts used as well as statistical methods and analysis.
(5) Arising from Mr Calman's close working relationship he had with the experts whilst preparing the Articles, and the privileged status he had granted them to review the main articles prior to publication, there was an obvious risk that they might seek to exercise a disproportionate influence over the editorial decision-making and ultimate terms in which the Articles were published. Put shortly, as they represented one side of the debate (or "row", as he described it to X) there was a clear risk that they had "an axe to grind"; a risk that they would want to see the Claimants (and others) publicly discredited. This risk was not fanciful, as Mr Calman must have appreciated. From the material he had prior to publication, it would have been apparent that there was an ongoing public dispute about statins of which his experts represented only one side. That should have been apparent to Mr Calman at least from the terms of the BMJ Review Report (to which both Claimants had specifically directed to Mr Calman's attention) following Professor Collins' demands, in 2013 and 2014, that the 2013 BMJ Articles should be withdrawn and that the journal should publish a statement that the claims about side-effects of statins were "wrong".
(6) Given that Mr Calman was inviting readers to make up their own minds, it was imperative that he gave the Claimants a fair and proper opportunity to respond to the points upon which he intended to criticise them in the Articles and that, in the published Articles, he faithfully represented what the Claimants had said in their own defence, and any other matters of which Mr Calman was aware that could fairly be regarded as having a bearing on the merits of the statin debate as they were to be presented to readers.
(a) Claim 1: Having high cholesterol is harmless
(b) Claim 2: Statins don't stop you from dying
(c) Claim 3: Doctors are hushing up side-effects
"[Professor Collins] emphasised the seriousness of his concerns, describing: 'the need to rectify the harm that has been caused –perhaps resulting in large numbers of unnecessary deaths, heart attacks and strokes among patients at elevated risk - by misleading doctors and the public with gross over-estimates of the rates of side-effects with statins.'"
"The authors withdraw this statement. Although it was based on statements in the referenced observational study by Zhang and colleagues, that 'the rate of reported statin-related events to statins was nearly 18%', the article did not reflect necessary caveats and did not take sufficient account of the uncontrolled nature of the study."
The corrections were highlighted in an editorial by Dr Godlee published on the same date.
"These arguments involve interpretations of available evidence and were deemed to be within the range of reasonable opinion among those who are debating the appropriate use of statins. In making this assessment, the panel is not expressing an opinion about the merits of these arguments, as that work was beyond the scope of the panel."
"At a more fundamental level, who should decide when such questions are too dangerous to ask? Certainly not those who have a vested interest in the debate being shut down. Rory Collins, head of the Cholesterol Treatment Trialists' (CTT) Collaboration, continues to call for the retraction of two [2013 BMJ Articles] that disputed the use of statins in low risk people. His call comes despite an independent expert panel set up by The BMJ and, subsequently, the Committee on Publication Ethics (COPE) concluding that The BMJ had acted appropriately in its handling of the papers. This week we publish documents that serve to correct Richard Horton's comments in the Lancet, in which he wrongly stated that COPE had 'declined to act' on Collins's concerns.
Independent third party scrutiny of the statins trial data remains an essential next step if this increasingly bitter and unproductive dispute is to be resolved. I have now written to England's chief medical officer, Sally Davies, asking her to call for and fund an independent review of the evidence on statins. As Krumholz concludes, sharing the individual patient level data from the statins trials would send 'a strong message that no single person or group should have exclusive access to data' that are so important for public health."
(d) Claim 4: Experts paid by drugs giants
(e) The allegation that Claimants misrepresented the quality of the scientific research
(f) Conclusions on the public interest defence
(1) as a key part of the Articles, the case study of Colin which materially misrepresented what he had said;
(2) the Hancock Statement in a way that made it appear that the Health Secretary had endorsed the claims made against the Claimants in the Articles and had denounced the Claimants as "pernicious liars"; and/or
(3) a claim that the LSHTM Paper had found that the publications considered in its study included public statements of the Claimants when Mr Calman knew that it had not.
Online Publication 1: initial publication
(4) In relation to Online Publication 1, have the Defendants shown that Online Publication 1 was, or formed part of, a statement on a matter of public interest, and if so, what is that matter of public interest?
(5) If so, have the Defendants shown that Mr Calman believed that publishing Online Publication 1 was in the public interest?
(6) If so, have the Defendants shown that Mr Calman reasonably believed that publishing Online Publication 1 was in the public interest?
Online Publication 1: continuing publication
(7) If so, have the Defendants shown that Mr Calman believed, and believed reasonably, following receipt of Ms Wilson's email, dated 5 March 2019 (19:41), that continuing publication of Online Publication 1 was in the public interest?
(10) If so, have the Defendants shown that Mr Calman believed, and believed reasonably, following their receipt of Carter-Ruck's letter to RPC, dated 31 January 2020 (annexing a list of factual misrepresentations in the articles complained of), that continuing publication of Online Publication 1 was in the public interest?
Online Publication 2
(12) Same issues as in respect of Online Publication 1 mutatis mutandis. In relation to the continuing publication of Online Publication 2, Online Publication 2 also contains references to the Hancock Statement which may be found to have been published maliciously after 5 March 2019 and references to the LSHTM Paper which may be found to have been published maliciously after 31 January 2020.
Online Publication 3
(13) Same issues as in respect of Online Publication 1 mutatis mutandis. In relation to the continuing publication of Online Publication 3, Online Publication 3 also contains references to the LSHTM Paper which may be found to have been published maliciously after 31 January 2020.
B: Statutory qualified privilege
(14) Is the Hancock Statement matter published by or on the authority of a government?
(15) Further or alternatively, is the Hancock Statement a notice or other matter issued for the information of the public on behalf of a government or an authority performing governmental functions?
Print Publication
(16) If the answer to (14) above is yes, are any of the references to the Hancock Statement in the Print Publication (as highlighted in red in the copies of the Articles in Annex 1, and underlined in Annex 2 to the judgment) an extract from matter published by or on the authority of a government?
(17) Further or alternatively, if the answer to (15) above is yes, are any of the references to the Hancock Statement in the Print Publication an extract from or summary of a notice or other matter issued for the information of the public on behalf of a government or an authority performing governmental functions?
(18) If the answer to (16) above is yes, are any of the relevant references to the Hancock Statement in the Print Publication a fair and accurate extract from matter published by or on the authority of a government?
(19) Further or alternatively, if the answer to (17) above is yes, are any of the relevant references to the Hancock Statement in the Print Publication a fair and accurate extract from or summary of a notice or other matter issued for the information of the public on behalf of a government or an authority performing governmental functions?
(20) If and insofar as any of the references to the Hancock Statement in the Print Publication satisfies the conditions in (19) above, have the Claimants shown that the Defendants were (a) requested by them to publish in a suitable manner a reasonable letter by way of explanation or contradiction and (b) refused or neglected to do so, so as to mean that there is no defence under s.15 of the 1996 Act in relation to the publication of that reference?
(21) If so, have the Claimants shown that any reference to the Hancock Statement in the Print Publication which satisfies the conditions above was made with malice?
Online Publications
(22) Paragraphs (16)-(21) above again in relation to the references to the Hancock Statement in Online Publications 1 and 2 (as highlighted in red in the copies of the Articles in Annex 1, and dotted underlining in Annex 2 to the judgment). (There are none in Online Publication 3.)
(23) If and insofar as the conditions detailed in (22) above are satisfied in relation to any of the references to the Hancock Statement in the Online Publications, did that publication become, by 5 March 2019 (date of receipt by the Defendants of the Sarah Wilson email of 5 March 2019 (19:41), the publication to the public, or a section of the public, of matter which is not of public interest or the publication of matter which is not for the public benefit, so as to preclude s.15 of the 1996 Act from applying to it from that date onwards.
(24) If and insofar as the answer to (23) above is no, have the Claimants shown that the publication of any reference to the Hancock Statement in the Online Publications which satisfies the conditions above was being made with malice (as relevant): after 5 March 2019 (date of receipt by the Defendants of the Sarah Wilson email of 5 March 2019 (19:41))
Does privilege under s.6, Defamation Act 2013 (Peer-reviewed statement in scientific or academic journal etc), specifically s.6(5) of the 2013 Act, attach to any of the references in the articles complained of to the LSHTM Paper of 28 June 2016?
(1) that privilege under s.6 of the 2013 Act may only properly be held to attach to the statement complained of as a whole, not to particular words within it; and
(2) that Curistan was wrongly decided.
(25) Does the privilege defence under s.6(5) apply at all to the Defendants and the references to the LSHTM Paper in the articles complained of (i.e., as a matter of statutory construction)?
(26) If so, was the BMJ a journal with more than one editor when it published the LSHTM Paper?
(27) If so, who was the editor/were the editors who were responsible for deciding to publish the LSHTM Paper?
(28) Before the LSHTM Paper was published in the BMJ, was an independent review of the LSHTM Paper's scientific or academic merit carried out?
(29) If so, depending on the answers to (27) & (28) above, was that independent review of the LSHTM Paper's scientific or academic merit carried out by the editor or editors of the BMJ who were responsible for deciding to publish the LSHTM Paper?
(30) If so, (for the purposes of s.6(3)(b)) what was the scientific or academic matter with which the LSHTM Paper was concerned?
(31) Depending on the answer to (30), was the independent review of the LSHTM Paper's scientific or academic merit carried out by one or more persons with expertise in the scientific or academic matter concerned?
Print Publication
(32) If so, are any of the references to the LSHTM Paper in the Print Publication (highlighted in green in the copies of the Articles in Annex 1, and dotted underlined in Annex 2 to the judgment) an extract from or summary of the LSHTM Paper?
(33) If so, are any of those references to the LSHTM Paper in the Print Publication a fair and accurate extract from or summary of the LSHTM Paper?
(34) If so, have the Claimants shown that the publication of those references to the LSHTM Paper in the Print Publication was made with malice?
Online Publications
(35) Are any of the references to the LSHTM Paper in Online Publication 1, Online Publication 2 or Online Publication 3 an extract from or summary of the LSHTM Paper?
(36) If so, are any of those references to the LSHTM Paper in the Online Publications a fair and accurate extract from or summary of the LSHTM Paper?
(1) The summary of the LSHTM in paragraph [48] of the Main Article as it appeared in Online Publication 1 was not fair or accurate.
(2) Otherwise, the other summaries of the LSHTM Paper that appeared in Online Publications 2 and 3 were fair and accurate.
(37) If so, have the Claimants shown that the initial publication of those references to the LSHTM Paper in the Online Publications was made with malice?
(39) If not, have the Claimants shown that the publication of those references after 31 January 2020 (date of receipt by the Defendants of a Carter-Ruck letter to RPC of that date, annexing a list of factual misrepresentations contained in the articles complained of) was made with malice?
C: Meaning and fact/opinion, including the application of Curistan
Print Publication
(41) In relation to the Print Publication, are the articles complained of to be treated for the purposes of determining the natural and ordinary meaning and fact / opinion as forming a single publication which ordinary reasonable readers would have read together or as falling into two distinct parts, namely (1) the article appearing on p.2 of the newspaper and (2) the articles appearing on pp.47-50 of the newspaper, which ordinary reasonable readers would have read separately?
(42) Is the Print Publication or does it contain a statement of defamatory fact concerning the Claimants or either of them or is it or does it contain a statement of defamatory opinion concerning the Claimants or either of them?
(43) If the issue in (41) above is resolved in favour of the Claimants, what is the natural and ordinary defamatory meaning of the Print Publication considered as a single publication, taking due account of the principles in Curistan and the court's findings on the statutory privilege issues as relevant?
(1) each Claimant had repeatedly made the following public statements, knowing each statement was false:
a) that cholesterol is not a cause of heart disease;
b) that lowering cholesterol with statins produces only a negligible benefit for patients prescribed statins;
c) that the medical establishment (including pharmaceutical companies, charities, doctors, researchers and universities) has covered up the true extent of the side effects of statins; and
d) that the medical establishment has conspired to silence the Claimants to ensure as many people as possible take statins, in order to boost profits for the statin industry
(2) there were strong grounds to suspect that each Claimant had made these knowingly false statements motivated by the hope that s/he would benefit from doing so either financially or from enhanced status; and
(3) the direct effect of the publication of these knowingly false statements by the Claimant was (a) to cause a very large number of people not to take prescribed statin medication; and (b) thereby to expose them to a serious risk of a heart attack or stroke causing illness, disability or death; and
(1) The very prominent, and very memorable, denunciation by the Health Secretary that the claims were "pernicious lies". Mr Hancock's statement was used as the headline of the News Article, reflecting its importance, and introduced readers right from the start to the 'statin deniers' who were the focus of the Articles. Paragraphs [1] and [2] continue to focus on Mr Hancock's statement, and clearly convey to readers that he is condemning those who "peddle myths" about statins, which is "needlessly risking people's health by spreading reckless and ignorant misinformation". A reader might, at this early point, be left undecided as to whether the 'statin deniers' are reckless fools, or dishonest knaves, but a clear steer is given in paragraph [5] where Mr Hancock denounces the "pernicious lies". Although the adjective "pernicious" would be readily understood to be describing the consequences of the lies, it is a particularly memorable and powerful phrase. In terms of impact on a reader, in my judgment it cannot be underestimated, and it was repeated in [15] of the Main Article.
(2) The next aspect of the Articles that is likely to have had an immediate and lasting impact on a reader's understanding of this aspect of the meaning is the clear comparison that was being made with the MMR vaccine scandal. The headline, "It's worse than the MMR scare", dominated the Main Article, across two pages. For readers who were not already familiar with the scandal, a summary was given in [16] of the Main Article. Andrew Wakefield, described as "disgraced", had "fabricated" (false) evidence to support his theory linking the MMR vaccine and autism. This paragraph is the only part of the Articles that raised whether, perhaps, the 'statin deniers' were "simply wrong rather than dishonest". For any reader who had not already identified the crux of what was being alleged against the Claimants, that will have crystallised it. But any notion that the 'statin deniers' are "simply wrong rather than dishonest" was apparently quashed immediately and (it would have seemed) authoritatively by Professor Collins who stated that "in terms of death and disability that could have been prevented, this could be far worse than we saw with MMR". If a reader had stopped to analyse carefully what Professor Collins was quoted as saying, s/he may have thought that it was not actually an answer to whether the 'statin deniers' were "simply wrong rather than dishonest". Indeed, Professor Collins' remark was actually directed at the consequences of the MMR scare, rather than whether they were the product of simple error or dishonesty. But, as the authorities governing the determination of meaning make clear, readers do not analyse text like this. An ordinary reader will simply have read the Articles through once. Doing that, the presentation in [16] is likely to have been memorable only for drawing a direct comparison between the MMR scandal - which was an unmistakable example of dishonest manufacture of false claims - and the false claims of the 'statin deniers', and Professor Collins' rejection that they were "simply wrong rather than dishonest".
(3) Another, and potent, influence on the meaning of dishonesty is the forthright terms in which the Claimants were condemned in the Articles, and particularly in the Editorial. The Claimants were denounced as the purveyors of "Deadly propaganda" in the headline to the Main Article. Again, if a reader (who had not already been influenced by the description "pernicious lies") analysed that term carefully, and in isolation, s/he might conclude that it was equivocal as to whether the "propaganda" was known by the Claimants to be false. But again, this is not the test. The immediate, and likely lasting, impact of "propaganda" on the reader, particularly when used in a headline, is that the conduct is deliberate, not mistaken. The Editorial is unequivocal. The Claimants deserved a "special place in hell" for "peddling" a "particularly insidious type of fake news" about statins. No ordinary reasonable reader could think that someone who had, honestly published information about statins that turned out to be wrong merited a "special place in hell". If the wrongdoing alleged against the Claimants was limited to castigating them for carelessly, even recklessly publishing false public statements about statins, then the terms of the Editorial would have been materially different. As it is, the terms of the Editorial, and its strident and forceful condemnation, reinforces the message that the Claimants are dishonest knaves, not misguided fools.
(4) The dishonesty meaning is supported (in readers' minds) by the speculation as to the Claimants' motive. Motive is not usually ascribed to careless or reckless acts. Motive is more usually associated with deliberate acts. The Text Box directly (and several paragraphs of the Articles) by insinuation, raised in the minds of readers the question whether the Claimants "have... just got too much to lose if their arguments are disproved?". The suggestion is that the Claimants' motivation is financial and possibly to maintain status. The First Claimant, readers were told, charged £50 a year for membership of her "Diet and Health Club" (Text Box [H]) and has sold 120,000 copies of her diet book (Editorial [17]). The Second Claimant had published five books relating to statins (Text Box [F]). The Claimants (and Dr Malhotra): "all... owe some, if not a large part of their status to their stance as statin deniers and have profited from it" (Text Box [I]). Again, I accept, that if s/he paused to think deeply about the questions of "profit", "status" and the motivation of the Claimants raised in the Articles, a reader might conclude that it was possible to be motivated by money and status, and yet still be honest, but mistaken. However, this is the sort of analysis that would either be the product of significant analysis, or someone at the reader's elbow challenging them that there might be a charge other than dishonesty that was being alleged against the Claimants. Neither is the proper test of the natural and ordinary meaning. Put shortly, the Articles alleged that the Claimants had a venal motive for their lies. This was one of the aspects that made them so deserving of contempt, and a "special place in hell".
(5) Finally, the overall presentation of the Claimants' statements in the Articles does nothing to detract from the dishonesty meaning. If the Claimants had been presented as honest, but mistaken critics of statins, that would have led to the Articles bearing a different meaning. But they were not. In the four instances of "Fake News", the Claimants' statements were presented, and then discredited by the 'trustworthy' experts. No independent support for the Claimants' statements was provided for the readers to consider and take into account (and which might have caused them to conclude that the Claimants were honest, but mistaken, rather than dishonest). Perhaps most significantly on this issue, in the Editorial ([12]), readers saw the comparison drawn between honest scientists, like Barry Marshall, who bravely (and, in context, honestly) challenged, and stood up to, medical orthodoxy, and the dishonest 'statin deniers' who "whip up controversy, peddle conspiracy theories, or sell diet books" and "peddle... a particularly insidious type of fake news, apparently from a respectable, credible source, but laced with misinformation". Readers were warned to "make no mistake" that the Claimants "are no Barry Marshalls". In context, the repeated use of the word "peddle" and the word "laced" (both active verbs) would also have contributed powerfully to the meaning that the Claimants were dishonest.
(44) If the issue in (41) above is resolved in favour of the Defendants, what is the natural and ordinary defamatory meaning of the articles appearing on pp.47-50 of the newspaper, taking due account of the principles in Curistan and the court's findings on the statutory privilege issues as relevant? (It is not in issue that if the article appearing on p.2 of the newspaper is considered on its own it does not refer to the Claimants and so is not defamatory of either of them.)
Online Publications
(45) Is Online Publication 1 or does it contain a statement of defamatory fact concerning the Claimants or either of them or is it or does it contain a statement of defamatory opinion concerning the Claimants or either of them?
(46) What is the natural and ordinary defamatory meaning of Online Publication 1, taking due account of the principles in Curistan and the court's findings on the privilege issues as relevant?
(1) each Claimant had repeatedly made the following public statements, knowing each statement was false:
a) that cholesterol is not a cause of heart disease;
b) that lowering cholesterol with statins produces only a negligible benefit for patients prescribed statins;
c) that the medical establishment (including pharmaceutical companies, charities, doctors, researchers and universities) has covered up the true extent of the side effects of statins; and
d) that the medical establishment has conspired to silence the Claimants to ensure as many people as possible take statins, in order to boost profits for the statin industry
(2) the direct effect of the publication of these knowingly false statements by the Claimant was (a) to cause a very large number of people not to take prescribed statin medication; and (b) thereby to expose them to a serious risk of a heart attack or stroke causing illness, disability or death; and
(47) In relation to Online Publication 2, are Online Publication 2 and Online Publication 1 to be treated for the purposes of determining fact/opinion and the natural and ordinary meaning as forming a single publication which ordinary reasonable readers would have read together, owing to the hyperlink from Online Publication 2 to Online Publication 1, or as separate publications which ordinary reasonable readers would have only read separately?
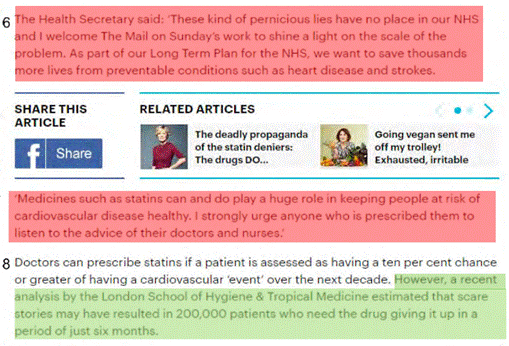
Although not relevant for the issue under consideration, the red and green shading indicates, on the same basis as set out in Annex 1, those parts of Online Publication 2 in respect of which the Defendants advance a privilege defence.
(48) If (47) is resolved in favour of the Claimants, is Online Publication 2 or does it contain a statement of defamatory fact concerning the Claimants or either of them or is it or does it contain a statement of defamatory opinion concerning the Claimants or either of them?
(49) Further, what is the natural and ordinary defamatory meaning of Online Publication 2 considered together with Online Publication 1 as a single publication, taking due account of the principles in Curistan and the court's findings on the privilege issues as relevant?
(50) Further or alternatively, in relation to Online Publication 2, assuming that at least one reader of Online Publication 2 would have clicked on the hyperlink to Online Publication 1 and read what s/he found there, what is the meaning that Online Publication 2 would have conveyed to readers of this kind by innuendo, taking due account of the principles in Curistan and the court's findings on the privilege issues as relevant?
(1) each Claimant had repeatedly made the following public statements, knowing each statement was false:
a) that cholesterol is not a cause of heart disease;
b) that lowering cholesterol with statins produces only a negligible benefit for patients prescribed statins;
c) that the medical establishment (including pharmaceutical companies, charities, doctors, researchers and universities) has covered up the true extent of the side effects of statins; and
d) that the medical establishment has conspired to silence the Claimants to ensure as many people as possible take statins, in order to boost profits for the statin industry
(2) the direct effect of the publication of these knowingly false statements by the Claimant was (a) to cause a very large number of people not to take prescribed statin medication; and (b) thereby to expose them to a serious risk of a heart attack or stroke causing illness, disability or death; and
(3) in consequence, each Claimant was rightly to be condemned as a pernicious liar, for whom there was a special place in hell, whose lies, deadly propaganda, insidious fake news, scare stories, and crackpot conspiracy theories, had recklessly caused a very large number of people, like Colin, for whom the proven benefits of taking statins were demonstrated by indisputable scientific evidence, to stop taking them risking needless deaths and causing harm on a scale that was worse than the infamous MMR vaccine scandal.
(51) Is Online Publication 3 or does it contain a statement of defamatory fact concerning the Claimants or either of them or is it or does it contain a statement of defamatory opinion concerning the Claimants or either of them?
(52) What is the natural and ordinary defamatory meaning of Online Article 3, taking due account of the principles in Curistan and the court's findings on the privilege issues as relevant?
(1) each Claimant had made false public statements, knowing that they were false, the effect of which was to cause doubt as to whether statins were effective (or otherwise undermine public confidence in statins) and thereby led to people, like Colin, to stop taking prescribed statin medication which exposed them to a serious risk of a heart attack resulting in illness, disability or death; and
"(1) each Claimant had made false public statements, knowing that they were false, the effect of which was to cause doubt as to whether statins were effective (or otherwise undermine public confidence in statins) and thereby led thousands of patients, like Colin, to stop taking prescribed statin medication which exposed them to a serious risk of a heart attack resulting in illness, disability or death"
D: The issue under s.3(5) DA 1996 (if it arises): did the Second Defendant 'not hold the opinion'?
(53) If and insofar as the Court finds that any of the articles complained of is or contains a statement of defamatory opinion concerning the Claimants or either of them, did Mr Calman hold or not hold that opinion?
I: Conclusion and next steps
(1) Issues 1.1 and 1.2 - none of the reports of the Hancock Statement, included in the Articles, is privileged.
(2) Issue 1.3 - this has been resolved by agreement - see [37] above.
(3) Issue 1.4 - save for [48] of the Main Article, which is not privileged, the parts of the Articles that reported the LSHTM Paper are privileged.
(4) Issue 1.5 - insofar as it arises, the Claimants' allegation of malice fails.
(5) Issue 1.6 - the public interest defence fails and is dismissed for all publications.
(6) Issue 1.7 - the News Article and the Health Section (the Main Article and the Editorial) in the Print Publication are to be regarded as a single publication.
(7) Issue 1.8 - Online Publication 2 and Online Publication 1 are not to be regarded as a single publication.
(8) Issue 1.9 - the natural and ordinary defamatory meaning of the relevant publications is set out in [516], [532] and [550] above.
(9) Issue 1.10 - Online Publication 2 bears the innuendo meaning set out in [545] above.
(10) Issue 1.11 - the decision on fact opinion in respect of each publication is set out in [517], [533], [546] and [551] above.
(11) Issue 1.12 - Mr Calman did not hold the opinions that the Court has found the publications to bear.
Annex 1 - The Articles as they appeared in the print edition





Annex 2 - The text of the Articles
In this Annex:
· Paragraph numbers, and other identifying information, have been added in square brackets.
· Underlining indicates that publication of the relevant section is defended by the Defendant on the basis of a reporting privilege attaching to the Hancock Statement (see judgment [25(1)]).
· Double underlining indicates that publication of the relevant section is defended by the Defendant on the basis of a reporting privilege attaching to the Kearney Statement (see judgment [25(2)]).
· Dotted underlining indicates that publication of the relevant section is defended by the Defendant on the basis that it is a statement in a scientific or academic journal (see judgment [27])
(A) The News Article
[p.2]
[Headline]
Statin deniers are putting patients at risk, says Minister
By Barney Calman
and Stephen Adams
[1] DOCTORS who cast doubt on the effectiveness of statins are 'needlessly' risking lives, the Health Secretary warns today.
[2] In a passionate intervention, he has thrown his weight behind a Mail on Sunday campaign to fight 'fake' claims about proven medicines. Condemning those who peddle myths about the daily pills, Mr Hancock said: 'Medical evidence shows that statins save lives. 'Needlessly risking people's health by spreading reckless and ignorant misinformation claiming otherwise is completely unjustified'.
[3] About eight million Britons take statins, which can substantially reduce the risk of having a heart attack or stroke by lowering cholesterol - a fatty substance that contributes to the blocking of arteries - in the blood.
[4] However, some medics dispute the benefits and a few wrongly assert they can cause serious and widespread damage to health. Inaccurate claims by 'statin deniers' include that high cholesterol is not linked to an increase risk of cardiovascular disease. These rely on small, observational studies, rather than 'gold standard' randomised controlled trials over many years that show statins do cut death from heart attacks.
[Logo of The Mail on Sunday in a circle with the words] FIGHT FAKE HEALTH NEWS
[5] The Health Secretary said: 'These kind of pernicious lies have no place in our NHS and I welcome The Mail on Sunday's work to shine a light on the scale of the problem. As part of our Long Term Plan for the NHS, we want to save thousands more lives from preventable conditions such as heart disease and strokes. 'Medicines such as statins can and do play a huge role in keeping people at risk of cardiovascular disease healthy. I strongly urge anyone who is prescribed them to listen to the advice of their doctors and nurses.'
[6] Doctors can prescribe statins if a patient is assessed as having a ten per cent chance or greater of having a cardiovascular 'event' over the next decade. However, a recent analysis by the London School of Hygiene & Tropical Medicine estimated that scare stories may have resulted in 200,000 patients who need the drug giving it up in a period of just six months.
[7] Dr Matt Kearney, NHS England's National Clinical Director for Cardiovascular Disease Prevention, said: 'There is overwhelming evidence that statins prevent heart attacks and strokes and that they are safe. But if patients get worried by false, alarmist or misleading reports they see on social media and in the press and, as a result, decide not to take these life-saving medicines, they can end up making the wrong decision about how to stay well, and cause themselves real harm'.
[8] The Mail on Sunday campaign comes amid growing concern about the erosion of trust in mainstream evidence-based medicines caused by 'fake news' on the internet. On Friday, NHS England Chief Executive Simon Stevens warned that 'vaccine deniers' who went online to spout 'fake messages' were making it harder for doctors to 'win the public argument' on vaccination.
[9] He told a meeting at the Nuffield Trust think-tank: 'The vaccination deniers are getting some traction. Although nine in ten parents support vaccination, half of them say they have seen fake messages about vaccination on social media.'
[text in a block at the foot of the News Article]
'EXPERTS' BEHIND SCARE STORIES - AND WHY THEY'RE WRONG Pages 47-50
(B) The Main Article
[p.47]
[Intro]
The science is unequivocal: statins DO protect you from heart attacks. But, as this devastating investigation reveals, thousands refuse them because of the... [Headline] Deadly propaganda of the STATIN DENIERS
[image of tablets formed in the shape of a heart]
[Logo of The Mail on Sunday in a circle with the words] FIGHT FAKE HEALTH NEWS
SPECIAL REPORT
By Barney Calman
HEALTH EDITOR
[1] DO YOU want to suffer a heart attack? How about a stroke? The answer will, without doubt, be a resolute 'Not on your life'.
[2] No one does. That's why some eight million Britons take a cholesterol lowering statin pill every day - doctors prescribe them to anyone with a ten per cent or greater risk of a major cardiac event within ten years.
[3] Statins reduce those risks. This is an indisputable scientific fact.
[4] Over the past 30 years, more than 200,000 patients have been put through the most rigorous forms of clinical trials to produce definitive proof that the tablets lower the risk of heart attack by up to 50 per cent, stroke by 30 percent, and the risk of death - from any cause.
[5] Yet millions of middle-aged men and women who would benefit from taking statins don't. Research... TURN TO PAGE 48
[Headline over pages 48 and 49]
'It's worse than the MMR scare'
[Logo of The Mail on Sunday in a circle with the words] FIGHT FAKE HEALTH NEWS
FROM PAGE 47
... suggests, astonishingly, that many who refuse the pills or abandon them have already had a heart attack. Disturbingly, this could well be because they have been led to believe, wrongly, that the drugs don't work.
[6] The debate on the role of cholesterol in heart disease began decades ago when scientists were looking for major causes of the condition and drugs to halt it. In early studies, high cholesterol levels in heart-attack patients initially led to the belief that consuming cholesterol-rich foods such as eggs and prawns was to blame, which was later found not to be the case.
[7] Then, saturated fat became the culprit, but there was little good evidence to prove this. Today, it is thought to be only small (sic) part of the equation.
[8] But as the science has evolved, so too has a distrust of prevailing medical advice among a small but noisy group of sceptics who publish blogs, newsletters and books, and are invited to share their opinions on television and radio. Their arguments hinge around similar themes: that contrary to what most doctors say, cholesterol is not a cause of heart disease and so lowering it with statins offers 'negligible' benefit.
[9] They say the real side effects of statins are being hushed up, and there is a conspiracy between pharmaceutical companies, heart charities, doctors, our most respected universities and the researchers who work in them, to silence them, cover up 'the truth' and ensure as many people as possible take the medication to boost profits.
[10] None of it is right. But this tiny minority of statin deniers can sound very convincing.
[11] Professor Sir Nilesh Samani, cardiologist and Medical Director of the British Heart Foundation, explains: 'The stories contain a grain of truth, mixed with speculation and opinion.
[12] 'It makes it very difficult for the public to know what the facts are, or whom to trust. Of course, we should debate the evidence, but all they have done is confuse people about the benefits of statins, which means those who really need them might stop taking them'.
[13] In January, the editors-in-chief of all 30 major heart-health medical journals - each a leading cardiologist - signed a joint open letter, warning: 'Lives are at stake due to the wanton spread of medical misinformation. It is high time that this stopped'.
[14] A 2016 analysis from the London School of Hygiene and Tropical Medicine, which tracks outbreaks and public-health concerns, found fake news about statins may have prompted 200,000 patients in Britain alone to quit the drug over a single six-month period - and thousands of heart attacks and strokes may occur as a result.
[15] Last night Health Secretary Matt Hancock voiced his concern about the 'pernicious lies' being circulated and added: 'Statins play a huge role in keeping people at risk of cardiovascular disease healthy. Risking people's health by spreading reckless and ignorant misinformation claiming otherwise is completely unjustifiable'.
[16] Professor Sir Rory Collins, the British scientist behind pivotal research into statins, says the potential consequences far outweigh that of the infamous MMR vaccine scandal, in which disgraced paediatrician Andrew Wakefield fabricated evidence to support his idea that the jab triggered autism in infants, leading to a decline in vaccination uptake and the resurgence of measles. The suggestion is that the statin deniers are simply wrong rather than dishonest, but Prof Collins says: 'In terms of death and disability that could have been prevented, this could be far worse than we saw with MMR'.
[17] So what of the claims themselves - how do they stand up to scrutiny. To find out, this newspaper examined the most commonly circulated fake news on statins.
[18] And to test it, we enlisted the help of researchers who have devoted their lives to understanding how to treat heart disease, and who have produced the highest quality scientific evidence on the subject. Read on... and decide for yourself who YOU should entrust your health to.
FAKE NEWS: HAVING HIGH CHOLESTEROL IS HARMLESS
[19] FIRST, a few facts about what is known about heart disease. As we age, our heart attack risk increases and one of the key factors is levels of cholesterol - a type of waxy fat, mostly produced by the liver - in the blood. The terms 'bad cholesterol' and 'good cholesterol' are often used, but today doctors are mainly concerned with (LDL), 'balls' of fat and protein which carry cholesterol from the liver into the bloodstream.
[20] A combination of genetic predisposition, poor diet and lifestyle factors can mean that the body produces too much LDL, which contributes to a process known as atherosclerosis.
[21] This begins when the walls of the arteries, called the endothelium, become inflamed. High blood pressure, raised blood sugar levels, smoking, genetic predisposition and LDL itself all contribute to this process.
[22] Once the endothelium is damaged, LDL begins to penetrate and build up within the artery walls where it hardens and forms deposits called plaques. The endothelium can then rupture in places, exposing the fatty plaque contents to the blood, which forms a clot to seal the split as part of the body's natural healing mechanism.
[23] It is the narrowings caused by this process that hinders the circulation and can block blood flow to the heart or brain - with potentially catastrophic consequences.
[24] But statins deniers still insist LDL is not a problem.
[25] One of the key figures, Dr Malcolm Kendrick, a GP from Cheshire, warns in his latest book: 'People are being conned. The way to avoid heart disease... has nothing to do with lowering cholesterol'.
[26] In 2016 he appeared on BBC News promoting research he had published by the British Medical Journal. He said: 'We found that if your bad cholesterol was higher you were no more likely to die of heart disease or strokes'.
[27] Another prolific denier, Dr Zoë Harcombe - who admits she is an academic, not a medical doctor - author of one of the bestselling diet books of 2019, recently blogged: 'High cholesterol is not even associated with high heart disease, let alone a cause'.
[28] So are they right? No says Prof Collins, who points to one unquestionable area of proof against this argument: genetics.
[29] One inherited condition, familial hypercholesterolaemia, causes sky-high cholesterol levels, even in children. Left untreated, it can kill sufferers in their 20s or 30s.
[30] But with high-doses of statins many can and do survive into old age. In recent years further DNA faults have been identified that also cause modest LDL increases, and raised heart risk. Those with genetically low LDL levels have a very low risk of heart disease.
[31] Another thing deniers seem to rely on is the perception that all scientific studies are equally authoritative, when in fact they aren't. There are observational studies, where researchers follow volunteers over time in order to observe the relationship between a certain risk factor or treatment and their chances of developing an illness.
[32] This kind of research can reveal a link, or association, for example, that among people with a high LDL level, there are more cases of heart disease than in those with a lower LDL level.
[33] But such studies cannot determine cause and effect: it is impossible to know whether it is the LDL or other things in that person's life causing the heart problems.
[34] The most reliable data about the effects of lowering LDL comes from randomised double blind clinical trials. In these, patients are randomly split into groups. Some receive a dummy 'placebo' pill. No one, not even the researchers, know which is which - and all patients are otherwise treated in exactly the same way. This proves is the drug works, and no other factor could be of influence.
[35] Dr Kendrick admitted that his study was observational but said: 'The proof of the link between smoking and lung cancer was based on observational studies. They have value'.
[36] And this may be the case. But Professor Colin Baigent, an epidemiologist at the University of Oxford, who is also involved in major statins trials, explains that it is misleading to claim observational studies on statins refute what has been proven in clinical trials, and it's not comparing like for like. He said: 'We know statins work because there have been numerous, very large randomised clinical trials that prove that lowering LDL with these drugs reduces the risk of heart attacks and strokes'.
[37] Prof Collins says some observational studies have found low LDL is associated with higher death rates in older people, but of course, have not proved cause and effect. 'It's known that in older patients, other illnesses such as cancer can cause low LDL,' he says. Statins trials also show benefits in the elderly.
FAKE NEWS: STATINS DON'T STOP YOU FROM DYING
[38] WHEN approached by The Mail on Sunday last week, Dr Kendrick pointed to a 2015 study as proof of his much-made claim that the benefits of statins are negligible, and don't extend lifespan. It showed that 'if you took a statin for five years, the increase in life expectancy would be (on average) 3.5 days. That is around 0.75 days per year of statin treatment'.
[39] Prof Baigent says: 'The 2015 study Kendrick mentions only looked at life extension over the trial period of a few years. Statins are lifelong drugs, and the extension of life over a lifetime will be very much greater. And the important thing is statins extend healthy life. They avoid both disabling events like heart attacks and strokes. Simply focusing attention on extra duration of life is to ignore the fact that these drugs reduce disability'.
[40] Professor Liam Smeeth of the London School of Hygiene and Tropical Medicine (LSHTM) explains: 'What matters is your heart attack risk when treatment starts. If you have a 30 per cent risk then statins could reduce that risk by at least a quarter.'
[41] For anyone in any doubt, UK heart disease and stroke deaths plummeted by two-thirds between 1980 and 2013, partly due to fewer smokers and better emergency care, but also because of wider statin use.
[42] Prof Samani says: 'Heart attacks used to kill men in their 50s and even 40s, but thanks in part to drug therapies people are living longer, healthier lives'.
[43] However, Dr Kendrick says: 'My paper (which linked high LDL with a longer life) was the most read paper in the BMJ Open website for five months in a row and provides the rationale for re-evaluation of cholesterol-lowering guidelines'.
[44] Dr Harcombe said: "I have examined the entire data provided by the World Health Organisation and found that higher cholesterol is associated with lower deaths, from heart disease and all-causes, in men and women, for all 192 countries in the world.
[45] 'My PhD was an examination of the diet (cholesterol) heart hypothesis. I have studied this topic at the highest level for several years and I am entitled, if not obliged, to share what I have found'.
FAKE NEWS: DOCTORS ARE HUSHING UP SIDE EFFECTS
[46] IN OCTOBER 2013, the British Medical Journal published an article by Dr Aseem Malhotra in which he claimed a study had proved 20 per cent of statins patients were forced to stop taking them due to muscle pains, stomach upsets, sleep and memory problems and erectile dysfunction. This was far higher than had previously been reported.
[47] Thanks to Dr Malhotra's article, the figures were repeated worldwide but within months was revealed to be wrong. The researchers admitted the true quit rate was nine per cent. And it was unclear how many of those had genuinely suffered side effects.
[48] Dr Malhotra's piece, and the study that inspired it, were 'non-scientific and simply not true' says Prof Smeeth, who led a 2016 investigation by experts at the school into the rising numbers stopping statins as a result of the articles, and the debate - which included public statements from Dr Kendrick and Dr Harcombe - which followed.
[49] The editor of the British Medical Journal, Fiona Godlee, corrected the articles and was even forced to appear on BBC News admitting the mistake.
[50] Dr Kendrick claimed the change indicated 'anyone who dares to criticise statins... is subjected to vitriolic attacks and a demand for silence'. This was echoed by Dr Harcombe.
[51] And yet, Dr Malhotra, who is described on his website as 'a world-leading expert in the prevention, diagnosis and treatment of heart disease', has been far from silent. Indeed, he has since made more extravagant claims, stating 75 percent of people prescribed a statin quit within a year - two-thirds because of side effects. At a medical event in 2017 he claimed: 'Side effects of these drugs have not been properly investigated. Patients are guinea pigs and they don't even know it'.
[52] There's no doubt some people feel rotten on statins. But the deniers are vastly overstating their case.
[53] Clinical trials show three serious side effects: on average, over a year of treatment, in every 10,000 patients on statins, one suffers myopathy, a potentially serious muscle weakening condition, ten to 20 develop diabetes, with those who are pre-diabetic being 'pushed over the edge', and one or two may suffer a brain bleed or haemorrhagic stroke.
[54] Prof Baigent explains: 'In clinical trials we see these adverse effects in far less than one per cent of patients'.
[55] And Professor Peter Sever, an expert in pharmacology at Imperial College London who conducted one of the largest-ever studies into statins, suggests that the claims and counter-claims have left some patients incredibly ... TURN TO PAGE 50
[pages 48-49 were illustrated with three large photographs of the Claimants and Dr Malhotra with the following captions:]
DENIER ONE
'Patients are being conned into taking statins'
Dr Malcolm Kendrick
DENIER TWO
'High cholesterol is not linked to heart disease'
Dr Zoë Harcombe
DENIER THREE
'People are being used as guinea pigs'
Dr Aseem Malhotra
[between the photographs was the Text Box with the headline:]
REVEALED: TRUTH ABOUT THE THREE 'EXPERTS' WHO SAY DON'T TAKE STATINS
[A] SO WHO are these highly influential sceptics defying years of robust research on statins?
[B] Dr Aseem Malhotra's website says he is an 'honorary consultant' at both Frimley Health NHS Foundation Trust in Surrey and the Lister Hospital in Stevenage.
[C] However, when The Mail on Sunday contacted Frinley, they said he did not currently work there. At the Lister, his secretary claimed he was a locum - shift-worker - cardiologist who only saw patients there on a Wednesday afternoon.
[D] Dr Malhotra, 41, offers consultations at a Harley Street clinic, charging £500 for a first consultation, and £300 for a follow-up.
[E] His most recent book, The Pioppi Diet, was branded one of the 'top five worst celeb diets to avoid in 2018' by the British Dietetic Association, yet he urges blog readers to choose it over statins.
[F] Dr Malcolm Kendrick, meanwhile, is author of five books on the statins debate, the best‑read of which has sold a modest 22,000 copies.
[G] The 60-year-old doctor is employed by East Cheshire NHS Trust and Central Cheshire Integrated Care Partnership.
[H] And for £50 a year, you can become a member of the Zoë Harcombe Diet & Health Club. The Cambridge maths graduate has a PhD in public health nutrition, and regularly blogs about cholesterol and heart disease.
[I] All three owe some, if not a large part of their status to their stance as statin deniers - and have profited from it.
[J] Despite such strong evidence to counter their claims, they are resolute.
[K] Have they just got too much to lose if their arguments are disproved?
[at the foot of the page a box with the text:] HELP US FIGHT FAKE HEALTH NEWS Have you fallen victim to fake health news. Tell us at FightFakeNews@mailonsunday.co.uk]
[on page 50 in a box at the foot of the page]
[headline] 'Side effects are down to worry, not statins'
FROM PAGE 49
... anxious about taking the medication. In his trial, 20 per cent of patients in both placebo and statin groups, reported muscle pains, weakness and stomach problems - suggesting many of the problems were not caused by the drug after all.
[56] The trial ended early because the evidence of the benefits of statins became so great it was deemed unethical not to offer them to everyone. Some volunteers chose to take the statin, while some did not. Among those who did, there was a 40 per cent increase in side-effect claims.
[57] Prof Sever says: 'We believe this is because people now worry so much about these side effects that all ill health is blamed on them.' He says, Dr Malhotra and Dr Kendrick 'quote anecdotes and evidence from observational studies, which are unreliable indicators of true side-effect rates'.
[58] Genuine side effects can be a problem but are manageable.
[59] Prof Samani says: 'When patients suffer side effects we take it seriously but these symptoms can be caused by many other things, so it can be difficult to tell the cause. We may temporarily stop statins for a while to see if the problems go away. If they don't we know it's not the statins. If they do, we can try the same or another statin'.
FAKE NEWS: EXPERTS PAID BY DRUG GIANTS
[60] PERHAPS the most incendiary of all accusations levelled by the deniers is that researchers who have conducted the biggest statin studies are paid by pharmaceutical companies trying to cash in on their drugs. In a recent blog Dr Kendrick wrote: 'Professor Sir Rory Collins and Professor Colin Baigent made a pact with the dev... sorry... they made a pact with the pharmaceutical industry to take hold of all the data on statins. They will not let anyone else see the data they hold. Including all the data on side effects'. Responding to our investigation, Dr Kendrick added: 'I believe people are being conned, deliberately misled. All industry-funded sturdies were positive. This is either a remarkable coincidence or something else.
[61] In a newsletter Dr Harcombe calls cardiologists, researchers and bodies involved in heart research 'statin pushers' - echoing the term drug pusher. Her inflammatory accusation, shared by the other deniers, is that statin researchers have received payments of around £286 million from drug manufacturers. She added: 'It would be naïve not to think that sums such as £268 million from pharmaceutical companies encourage recipients to aggressively encourage people to take those drugs'.
[62] But Prof Collins says there is a simple explanation for the 'hidden data' - they did not have it. 'In 1995 we began gathering results from research groups who conducted major trials on statins which we added to our own data.
[63] 'We requested information on heart attacks and strokes, deaths from all causes and serious adverse events like cancer. We did not seek data on any other side effects and have publicly stated this. The lie just seems to be repeated, that we held data on adverse events and have not made if available'.
[64] To put an end to this, Prof Collins and Prof Baigent have now requested every single adverse event in all the major studies and plan to publish the first analyses of these data later this year.
[65] Of conflicts of interest, Prof Baigent added: 'We have a long-standing policy of not accepting any personal payment from the pharma industry. Grants have been provided to the University of Oxford from drug companies but our salaries don't depend on that money.
[66] 'Our trial of niacin, another drug to treat high levels of fat in the blood, led to a billion dollar a year drug being withdrawn from the European market, and warnings being added in the US. The results fall where they will'.
[67] Prof Samani adds: 'I'm a cardiologist, not involved in trials. I look after patients with heart conditions. I'd just like to see fewer people get ill and die'. Dr Malhotra declined to comment.
(C) The Editorial
[p.50]
There is a special place in hell for the doctors who claim statins don't work
By Barney Calman
HEALTH EDITOR
[1] STATISTICS are one thing. But it's hard to argue against the dangers of stopping taking statins when they're staring you in the face.
[2] Last week, I met 49-year-old Colin Worthing as he recovered in his hospital bed following a heart attack in the early hours of Tuesday. He had been prescribed cholesterol-lowering tablets ten years ago but quit them - having 'heard they don't work'.
[3] Colin suffered his first heart attack in 2009, with little warning. 'It was a shock as I'd felt well otherwise,' he said. 'Later I was told I had high blood pressure and high cholesterol. My mother has heart problems, so I think it runs in my family.
[4] He was prescribed statins and blood pressure-lowering medication. 'I took them to start with, but I felt lethargic. I was always hearing on the radio that statins didn't really work, and drug companies were just trying to make money by getting us all on tablets. You do start to think there's no smoke without fire'.
[5] In 2013, he decided to stop all medication. 'I wrote to my GP saying I no longer needed my repeat prescription, and never heard any more,' he says.
[6] Over the next five years he felt well, 'although I suppose I was stressed with work, and I did put on quite a bit of weight'.
[7] And then, at about 1am on Tuesday, he woke feeling clammy, with a familiar tightness in his chest. 'I knew it was a heart attack, and called 999'.
[8] Colin was rushed to hospital where he had surgery to insert a stent which will keep blood flowing through his cardiac arteries while he awaits a full heart bypass operation. His consultant at Hammersmith Hospital, London, Dr Rahsa Al-Lamee, said: 'We regularly see patients who, like Colin, have stopped taking statins because they believe the myth that they don't do any good. In fact, he's one of the lucky ones. He's alive.
[9] 'There will be numerous reasons his heart disease progressed so far, but one of the factors will be because he stopped taking statins'.
[10] Colin added: 'I was a fool to stop taking the medication. Who cares whether or not someone is making money from statins. If I had carried on taking them, I might not be where I am now'.
[11] To paraphrase Donald Tusk, there is a special place in hell for the statins deniers who continue to fuel public confusion and a vague perception that the drugs, as Colin said, 'don't really work'.
[12] OK, I don't actually believe in hell. Or Donald Tusk, much, for that matter. But they need to realise that the ultimate fall out from high-risk patients, such as Colin, stopping proven treatment will be illness, disability and death. Debate should - must - be at the heart of science. Just because someone has been awarded the title professor doesn't make them right. And some of our greatest medical discoveries have come from so‑called mavericks who ignored the orthodoxies. For decades, doctors believed stomach ulcers were caused by stress and there was no cure. It was only when Australian researcher Barry Marshall willingly swallowed H Pilori bacteria in an attempt to prove his thesis, that it was the infection that caused the ulcer, that the truth emerged - and a simply course of antibiotics is now offered as a solution. 'Everyone was against me, but I knew I was right,' he famously said.
[13] But what he didn't do was whop up controversy, peddle conspiracy theories, or sell diet books. For we should make no mistake: the statins deniers are no Barry Marshalls.
[14] The trio mentioned in our piece aren't the only ones. There is Dr John Abramson at Harvard, author of the misleading '20 per cent side effect' BMJ study; Joseph Mercola, a discredited anti-medicine campaigner who claims to have millions of website views a day; Dr Uffe Ravnskov in Denmark, founder of The International Network of Cholesterol Skeptics, and others.
[15] It is a particularly insidious type of fake news they peddle, apparently from a respectable, credible source, but laced with misinformation. They seem now even to have the ear of policy-makers.
[16] Just last week Dr Harcombe and Dr Malhotra were invited to brief deputy Labour leader Tom Watson.
[17] Invited to comment on the study which suggests thousands of patients have quit medication due to statin confusion, and of these, many will have heart attacks, Dr Kendrick claimed it was he who was the victim, as such a claim amounted to 'reprehensible bullying'. Dr Harcombe, who has sold 120,000 copies of her diet book was similarly self-pitying: 'You're trying to bully me into silence for blogging about conflicts of interest.' Don't doubt that our investigation will be giving them fuel for their crackpot conspiracy theories for months to come.
[18] The truth is, the benefit of statins are (sic) crystal clear if you are at risk. Your doctor know it's not just about the level of LDL - the cholesterol they're most interested in - but also age, family history and other factors, such as lifestyle, diabetes or high blood pressure.
[19] If you have only a slightly high LDL, they will simply ask you to follow a heart-healthy diet and lifestyle. But if you have a ten per cent or greater chance of a heart attack over the next ten years, they will offer you the chance of taking a statin to help reduce that risk.
[20] No one will force you to take the pills. Whether you take their advice is up to you. But if you do, it might just save your life.
Annex 3: Text of various documents referred to in the judgment
(A) The First Claimant's Blog entry of 4 February 2019 titled "Statins in the over 75s"
(see judgment [69]-[70])
"Executive summary
- A study was published in The Lancet, which was reported worldwide as 'giving statins to people over the age of 75 could save thousands of lives.'
- This claim came from a press conference to launch the Lancet Paper, where a lead author, Colin Baigent, was quoted as saying: 'Only a third of the 5.5 million over 75s in the UK take statins and up to 8,000 deaths per year could be prevented if all took them.'
- This is false. It relies upon evidence in the over 75s for both deaths and primary prevention (people who do not already have heart disease) and neither was found.
- Figure 5 in The Lancet paper confirmed that the Rate Ratio (RR) for vascular deaths for over 75s was not statistically significant. Nor was it for those aged 70-75 for that matter. Even with the attempt to achieve a significant result, by excluding trials that failed to show benefit of statins, the RR for vascular deaths for over 75s was not statistically significant.
- Figure 4 in The Lancet paper confirmed that the RR for major vascular events for over 75s without vascular disease was not statistically significant. Nor was it for those aged 70-75 for that matter.
- This article was reported worldwide as 'statins can save thousands of lives in the over 75s'. No statistical significance was achieved for deaths or primary prevention in this age group. The falsehoods need to be corrected.
Introduction
The Cholesterol Treatment Trialists' (CTT) Collaboration has been quiet for a while, so a major publication in The Lancet (the favoured journal for CTT publications) was perhaps overdue. One duly appeared on 1st February 2019. It was entitled "Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials" (Ref 1).
It attracted much media attention on both sides of the pond. CNN reported "After years of uncertainty, study finds statins can benefit all ages, including those over 75" (Ref 2). The UK Times reported "Giving statins to all older people could save 8,000 lives every year" (Ref 3). The story took up the front page of the UK Express newspaper "Statins really do save lives" (Ref 4).
The CTSU/CTT
As I shared in this post (Ref 5), "the CTSU is the Oxford Clinical Trial Service Unit. Where this ends and the CTT (Cholesterol Treatment Trialists) Collaboration begins or where CTT ends and where Sir Professor Rory Collins and Colin Baigent and co. begin or end, I know not. One gets the impression that the web between the parties is not intended to be clear".
It is important to read that post for context to this note. That post went through the attacks made by Collins on authors of two articles published in the BMJ and attacks on the BMJ itself. Before this happened (in the summer of 2014), I had already identified approximately £115 million of funding from drug companies to the CTSU. In the disclosures that were required as part of the full BMJ independent investigation, it emerged that the full extent of the funding from pharma to the CTSU was beyond £268 million. That was back in 2014; it can only have increased since then.
The CTSU is also the group that, despite requests from journalists and the BMJ, has refused to share information from their statin trials, so that it can be independently examined. The CTSU has also refused to share data about serious adverse events, so we have the outrageous situation that data exist that could inform prescription practices and the holders of these data refuse to share them.
The lives saved claim
The claim about lives saved was remarkably consistent across the media reports:
"But scientists said up to 8,000 lives could be saved annually in the UK alone if everyone over the age of 75 received statin therapy". (The Express)
"Everyone over the age of 75 should be considered for cholesterol-lowering statins, experts have urged, after an analysis found up to 8,000 lives a year could be saved". (The Times)
"Researchers said up to 8,000 deaths a year could be prevented if GPs simply prescribed drugs costing pennies a day". (The Telegraph) (Ref 6).
"Up to 8,000 pensioners a year are needlessly dying of heart disease because they are not being given statins, leading experts warn". (The Daily Mail) (Ref 7). The 8,000 lives saved claim came from a press conference, which was held on January 30th to launch the paper. The press conference was reported in a BMJ article, which quoted Colin Baigent as saying "Only a third of the 5.5 million over 75s in the UK take statins and up to 8000 deaths per year could be prevented if all took them" (Ref 8).
The problem with the 8,000 lives saved/deaths prevented claim is that it cannot be supported from evidence in the paper.
The study
The study published on 1st February was a meta-analysis of trials for which the CTSU holds data. The objective of the study was set out in the introduction: "We aimed to do a meta-analysis of data from all large statin trials to compare the effects of statin therapy at different ages and explore the effects of statin therapy among older individuals". The specific age of interest in the abstract of the paper and throughout the paper and in media coverage was the over 75 age group.
The paper reported that, from the available data on 186,854 people, 14,483 people were over the age of 75. Of these 14,483 people, 55% had a history of heart disease of some kind (so 45% didn't). The average total cholesterol for the over 75s was 5.1 mmol/l and the average LDL cholesterol for this group was 3.2 mmol/l. The abstract (summary) of the paper reported that "Overall, statin therapy or a more intensive statin regimen produced a 21% (RR 0·79, 95% CI 0·77–0·81) proportional reduction in major vascular events per 1·0 mmol/L reduction in LDL cholesterol" (Ref 9).
There are five Figures in the paper. The only one that looked at deaths was Figure 5. It reported that the rate ratio (RR) for statins (achieving a 1 mmol/l reduction in LDL) vs. the control group in the over 75s was 0.95 (95% CI 0.83–1.07). That includes the line of no effect (1.0) and thus could have happened by chance and thus was not statistically significant. Interestingly the result for the age group 70-75 was also not statistically significant. Neither of these can be reported as a finding therefore.
The CTT personnel even re-calculated the RRs after leaving out four trials where "statin therapy had not been shown to be effective". Yes - really - ponder on that for a second. Even leaving out four trials that didn't show support for statins, they still could not achieve a statistically significant result in the over 75 age group.
All the reports in the media that "up to 8,000 lives a year could be saved", with reference to over 75s (or over 70s without the fudge) are false.
The evidence for cholesterol lowering in the elderly
I knew to doubt this claim as soon as I saw it because I worked on a paper published in 2016 with Dr Uffe Ravnskov as the lead author (Ref 10). The paper was called "Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review". I spent several weeks doing the data extraction with Uffe and cross checking each other's tables. I withdrew my name from the paper before publication because the correct (PRISMA) systematic review/meta-analysis methodology was not followed. This did not impact the results and, having seen the data first hand, the conclusion is robust. The conclusion was: "High LDL-C is inversely associated with mortality in most people over 60 years". i.e. high LDL‑cholesterol is associated with lower deaths in most people over 60.
I also knew to doubt the "lives saved" claim because statin patient leaflets caution against statin use in the over 70s. Here's an extract from the Lipitor leaflet (the most prescribed statin) (Ref 11):
[figure shown of "Warnings and precautions: Talk to your doctor, pharmacist of nurse before taking Lipitor - ... if you are older than 70 years"]
What about events?
Even if the death claims are lies, what about the claimed reduction in events? The newspapers reported that, "for every 10,000 people aged 78, who take statins, but have no history of cardiovascular problems, 80 heart attacks or strokes would be prevented every year".
This came from the press release (Ref 12) and the Lancet paper itself. Both identically reported "In the primary prevention setting...". [i.e. in individuals with no known history of vascular disease], "Reducing those risks by a fifth with a 1.0 mmol/L LDL cholesterol reduction would prevent first major vascular events from occurring each year in 50 individuals aged 63 years and 80 individuals aged 78 years per 10,000 people treated".
This is also false.
- Figure 1 reported effects on major vascular events by age. This is the Figure that gave the top level claim in the abstract of the paper as shared in "The Study" section above: "Overall, statin therapy or a more intensive statin regimen produced a 21% (RR 0·79, 95% CI 0·77–0·81) proportional reduction in major vascular events per 1·0 mmol/L reduction in LDL cholesterol".
- Figure 2 reported major vascular events by age and by type of trial (heart failure trials vs. dialysis trials vs. other trials).
- Figure 3 reported major vascular events by age and by type of event (coronary event, stroke etc).
- Figure 4 reported major vascular events by age and by previous vascular disease. There was no statistically significant difference between the statin group and the control group in people over 75 (or in the 70-75 age group). Benefit in those who have "no history of cardiovascular problems" cannot be claimed, therefore.
Serious Adverse Effects
Notwithstanding that no benefit can be claimed for those without previous vascular disease - and hence there will not be 80 fewer events per 10,000 78-year olds given statins - there will be serious adverse events in those people if they take statins.
The Number Needed to Treat (NNT) is the important measure to examine. The web site for this is www.thennt.com Frustratingly, these numbers seem to change every time I look at the page (I suspect that there is intense pressure from drug companies for these figures to be 're-visited'). Currently, the NNT numbers advise that 1 in 10 people who take statins without known heart disease will be harmed by muscle damage and 1 in 50 will be harmed by developing (type 2) diabetes (Ref 13). So, for every 10,000 people given statins, 1,000 are likely to develop muscle damage and 200 are likely to develop (type 2) diabetes. For no benefit in heart disease.
The Daily Mail article reported Colin Baigent as having said that "a number of misleading studies - which he branded 'fake news' - had created confusion over the effectiveness and side-effects of statins among doctors and patients".
The only fake news that I can find is coming from Baigent himself.
Until the next time
All the best - Zoë
References
Ref 1: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31942-1/fulltext
Ref 2: https://edition.cnn.com/2019/01/31/health/statins-elderly-cholesterol-study/index.html
Ref 4: https://www.express.co.uk/life-style/health/1081016/statins-save-lives-study-cholesterol-elderly
Ref 5: http://www.zoeharcombe.com/2014/08/ctsu-funding-from-drug-companies/
Ref 6: https://www.telegraph.co.uk/news/2019/01/31/75s-should-offered-statins-ageism-failing-patients/
Ref 7: https://www.dailymail.co.uk/news/article-6655239/Thousands-pensioners-dying- ageism-denies-statins-aged-75.html
Ref 8: https://www.bmj.com/content/364/bmj.l522.full
Ref 9: An email group of academics/medics, of which I'm a member, can't work out what this means. Did they only include people who achieved a 1.0 mmol/l reduction in LDL (which is large, from a starting point of 3.2 mmol/l) in the comparisons with the controls? Did they 'adjust' actual benefit to what they estimated it would had there been a reduction of 1.0 mmol/l? Who knows?
Ref 10: https://bmjopen.bmj.com/content/6/6/e010401
Ref 11: https://www.medicines.org.uk/emc/product/1059/pil
Ref 12: https://www.eurekalert.org/pub_releases/2019-01/tl-tls013019.php
Ref 13: http://www.thennt.com/nnt/statins-for-heart-disease-prevention-without-prior-heart-disease-2/
(B) The Second Claimant's Blog entry of 3 February 2019 titled "Response to the Lancet paper"
(see judgment [73])
A number of people have asked for my views on the Lancet Paper 'Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomized controlled trials.'
It was reported in various major newspapers.
The Times reported the study thus: "Everyone over the age of 75 should be considered for cholesterol-lowering statins, experts have urged, after an analysis found up to 8,000 lives a year could be saved."1
The Telegraph had this to say. "Researchers said up to 8,000 deaths a year could be prevented if GPs simply prescribed drugs costing pennies a day."
This comes hot on the heels of a concerted effort to silence statin critics around the world by a coalition of 'experts'. I suspect the coordinated timing is more than a coincidence.
'The editors of more than two dozen cardiology-related scientific journals around the world published an editorial Monday to "sound the alarm that human lives are at stake" because of medical misinformation.
These physicians describe regularly encountering patients hesitant to take potentially lifesaving medications or adhere to other prescribed treatments because of something they read online. Or heard from friends. Or saw on television.
"There is a flood of bad information on the internet and social media that is hurting human beings," said Dr. Joseph Hill, the architect of the essay and editor-in-chief of the American Heart Association journal Circulation. "It's not just an annoyance, this actually puts people in harm's way."
The primary example illustrated in the editorial is the use of statins, a cholesterol-lowering medicine that can reduce heart attack and stroke risk in certain people. But doctors say too many of their patients shun taking statins because of bad information they picked up - often from politicians, celebrities and others who lack medical expertise.'2
Essentially, they feel that certain issues, such as prescribing statins, are so vitally important that critics should be silenced. Perhaps all these editors should try reading this:
'Congress shall make no law respecting an establishment of religion or prohibiting the free exercise thereof; or abridging the freedom of speech, or of the press; or the right of the people peaceably to assemble, and to petition the Government for a redress of grievances.'
Yes, the US founding fathers knew the first thing tyrannies always wish to do is remove freedom of speech. From that, all else follows. If they don't get that message, they should all be forced to read 1984 by George Orwell.
"Freedom is the freedom to say that two plus two make four. If that is granted, all else follows."
Getting back to the Lancet paper. What do I think of it? The first thing to note is 'who done it.' Well, of course, it was the Cholesterol Treatment Triallists Collaboration (CTT) from Oxford. Run by Professor Sir Rory Collins and Professor Colin Baigent. They do almost all these meta-analyses on statins, because they hold all the data. So, no-one else can really do them.
The CTT is in this hallowed position because they made a pact with the dev... sorry ... they made a pact with the pharmaceutical industry to take hold of all the data on statins from all the pharmaceutical companies that manufacture statins and collate the data.
The CTT are very closely associated with the Oxford Clinical Trials Service Unit (CTSU) which is run by, and has employed, most of those in the CTT. Collins and Baigent etc. The CTSU is a clinical trials unit which, last time I looked, had obtained nearly £300 million in funding from the pharmaceutical industry for running clinical trials on various cholesterol lowering medications.
A fact that needs to be emphasised is that the CTT will not let anyone else see the data they hold. Including all the data on adverse events [side-effects] and serious adverse events. It is kept completely secret. I have the e-mail exchange between an Australian journalist and Professor Colin Baigent where the journalist attempts to find out if it is true that the CTT will not let anyone else see the safety data.
It starts quite well and the tone is amiable. Eventually Professor Colin Baigent clams up and refuses to answer any further questions. I have promised said journalist to keep this exchange under wraps, but almost every day I am tempted to publish it. It is toe-squirming.
Anyway, my point here is that the CTT is a horribly conflicted organisation, and has been paid, directly, or indirectly, a great deal of money by the pharmaceutical industry. Here are the conflicts of interest of those involved in writing the Lancet paper:
Conflicts of interest of statement from the Lancet paper: Commercial organisations in bold.
RO'C, EB, IF, CW, and JS have nothing to disclose. JF reports personal fees from Amgen, Bayer, Pfizer, Boehringer Ingelheim, Sanofi, and AstraZeneca, outside the submitted work; and non‑financial support from Amgen, Bayer, and Pfizer, outside the submitted work. BM reports grants from the Medical Research Council, British Heart Foundation, and the National Institute for Health Research Oxford Biomedical Research Centre during the conduct of the study, and grants from Merck outside the submitted work. CR report grants from the Medical Research Council and British Heart Foundation during the conduct of the study; and grants from Merck, outside the submitted work. JE reports grants from the Medical Research Council and the British Heart Foundation during the conduct of the study, and a grant from Boehringer Ingelheim outside the submitted work. LB reports grants from the Medical Research Council and the British Heart Foundation during the conduct of the study. MK is an employee of a company that has received study grants and consulting fees from manufacturers of PCSK9 inhibitors and treatments for lipid disorders, outside the submitted work. AT reports personal fees from Amgen and Sanofi, outside the submitted work. PR reports a research grant from AstraZeneca during the conduct of the study; and research grants from Novartis, Pfizer, and Kowa, outside the submitted work. CP reports a grant from Merck, outside the submitted work; and personal fees from Merck, Pfizer, Sanofi, Amgen, and Daiichi-Sankyo, outside the submitted work. EL reports grants from AstraZeneca, Bayer, Boehringer Ingelheim, Amgen, and Merck, outside the submitted work; and personal fees from Bayer, Amgen, Novartis, and Sanofi, outside the submitted work. WK reports grants and non-financial support from Roche, Beckmann, Singulex, and Abbott, outside the submitted work; and personal fees from AstraZeneca, Novartis, Pfizer, The Medicines Company, GlaxoSmithKline, Dalcor, Sanofi, Berlin-Chemie, Kowa, and Amgen, outside the submitted work. AG reports personal fees from Aegerion Pharmaceuticals, Arisaph Pharmaceuticals, DuPont, Esperion Therapeutics, Kowa, Merck, Roche, Vatera Capital, ISIS Pharmaceuticals, Weill Cornell Medicine, and Amgen, outside the submitted work. SY reports a grant from AstraZeneca, outside the submitted work. RC reports support from the Nuffield Department of Population Health, during the conduct of the study; grants from the British Heart Foundation, Cancer Research UK, Medical Research Council, Merck, National Institute for Health Research, and the Wellcome Trust, outside the submitted work; personal fees from the British Heart Foundation and UK Biobank, outside the submitted work; other support from Pfizer to the Nuffield Department of Population Health (prize for independent research); and a patent for a statin-related myopathy genetic test licensed to University of Oxford from Boston Heart Diagnostics (RC has waived any personal reward). CB reports grants from the Medical Research Council and British Heart Foundation, during the conduct of the study; and grants from Pfizer, Merck, Novartis, and Boehringer Ingelheim, outside the submitted work. AK reports grants from Abbott and Mylan, outside the submitted work; and personal fees from Abbott, Amgen, AstraZeneca, Mylan, and Pfizer, outside the submitted work. LB reports grants from UK Medical Research Council and the British Heart Foundation during the conduct of the study.
As to the study itself. I wrote this as a 'rapid response' to an article Colin Baigent wrote in the BMJ about the study. It may be published, it may not be.
'I would like to ask Colin Baigent one question on this study - at this time. He claims that the Lancet study was a meta-analysis of twenty-eight RCTs. The study was called. 'Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials.'
However, in the Appendix to the Lancet paper it is made clear that five of the studies are a comparison of high dose vs. low dose statins. PROVE-IT, A to Z, TNT, IDEAL and SEARCH. They cannot be used to test the hypothesis that statins are beneficial in the over 75s vs. placebo, as they were not done to answer this question.
Also, in nine of the RCTs used in the meta-analysis there were 0% participants over the age of 75 at the start of the study. These were 4S, WOSCOPS, CARE, Post CABG, AFCAPS/TexCaps, ALERT, LIPID, ASPEN and MEGA.
Which means that five of the studies could not address the question of statins vs placebo in the over 75s, and nine of the studies had no participants over the age of 75, which leaves fourteen studies that would be relevant to the issue of prescribing statins in the over 75s.
My question is, why did you call this study 'Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials.'
Yes, they claimed to have done a meta-analysis of twenty-eight studies, yet they could only use data from fourteen to make their claims. The largest of which was the Heart Protection Study (HPS), carried out by, guess who, Rory Collins from the CTSU and CTT.
As for the actual data, it is the usual obfuscation, skirting as close to the direct lie as possible without crossing that line. I am just going to look at one issue. The main claim was that "statin therapy or a more intensive statin regimen produced a 21% (RR 0·79, 95% CI 0·77–0·81) proportional reduction in major vascular events per 1·0 mmol/L reduction in LDL cholesterol."
A 21% reduction in major vascular events. That sounds terribly impressive. However, if you have read my book Doctoring Data you will know that what is most important here is not what is said, it is what is not said.
Do you see any mention of overall mortality here? No, you don't. Which means that it did not change. Also, you may note this wording 'reduction in major vascular events.' What is a major vascular event? Well, it is mainly a non-fatal heart attack or a non-fatal stroke. There are other CV events, but they are much less common.
Note again, no mention of fatal CV events. If there had been a reduction here, it would have been trumpeted from the rooftops. Which means that we have no reduction in mortality and no reduction in fatal CV events. Of course, it is worth preventing non-fatal heart attacks and strokes, as these can be extremely damaging and harmful things.
However, there is something worth mentioning here that I have not really covered before. There are heart attacks and heart attacks, and strokes and strokes. A heart attack (MI) can be a crushing near‑death event, leaving the heart severely weakened and liable to trigger into a fatal heart arrythmia at any time. The patient can be left a cardiac cripple.
Alternatively, a heart attack can be diagnosed by a marginal rise in cardiac enzymes with no symptoms at all, and no residual problems. Yet, both of these events, so completely different in their impact, will be listed as a non-fatal heart attack, with precisely the same weighting.
Equally, a stroke can leave the person virtually paralysed down one side, incontinent, unable to speak, eat, or move. Or, it can be a half hour strange sensation with slight facial weakness that fully resolves. Again, both these events will be listed with precisely the same weighting.
That is a problem in itself, in that these trials list events of completely different severity as being equivalent. It also leads into another problem, who is going to make the diagnosis of a mild heart attack or stroke - and on what grounds?
It will most likely be a doctor, and that doctor will have prior knowledge of whether or not the patient was on a statin - or placebo. Yes, I know, clinical trials are supposed to be double-blinded, which means that neither the participant, nor the investigator, should know who is taking the drug, or the placebo.
However, in reality, they both know full well.
I was at a meeting a while back where one of the investigators for the PCKS-9 drug Repatha was talking about the study. At one point he mentioned that a trial participant had told him that he knew he was not taking the cholesterol lowering agent. When questioned how he knew this, the participant said - because my cholesterol level is the same as it always was.
He still wanted to continue on the trial, because he thought we was doing a 'good' thing and helping to move medicine forward - and suchlike. I feel it may be considered churlish to point out that the only thing he was helping to move forward was the profit margins for Amgen.
The reality is that when you have a medication that has a significant effect, e.g. lowering cholesterol by 40%, this is a very difficult to thing to hide from the patient, or the doctor. They can see the figures on a computer screen in front of them. And when you are on a clinical trial, and you enter hospital, the doctors have to be told you are in a clinical trial, and what it is.
So, these double blinded studies on statins are not effectively, or even remotely, double-blinded. Which means that bias in clinical decision making is now an option. Was it a heart attack, or not? Well, they are on a statin - so probably not. Or, they are taking a placebo, so it probably is. Bias, the very thing you are trying to remove has crept straight back in the side door.
Another issue with an event is that there are many different sorts of clinical event. Death would be one - obviously. Breaking your leg another. Kidney failure would also count as one, as would a severe rash, or emergency admission to a hospital for almost any reason.
So, when a study states, as this one does 'reduction in major vascular events' my mind, as it is now trained to do, thinks to itself: "What about other events, what happened to them? Were they also reduced, did they say the same, or did they go up?"
Because if you reduce major vascular events, but other serious events go up, then you have achieved exactly and precisely nothing. This is a variation on pushing people off cliffs to stop them dying of heart attacks.
Results: 'Pushing one hundred people prevented all trial participants from suffering a fatal heart attack. We therefore recommend pushing everyone off a cliff to reduce the incidence of heart attacks in the general population.' A.N. Idiot et al.
Statins reduce major vascular events. [A major non-fatal vascular event could also be called as Serious Adverse Event (SAE)]. But do they reduce all serious adverse events (SEAs). If not, you are simply replacing a major vascular event with something equally nasty.
Which leads on to the next question, do we know from the statin trials if statins do reduce SAEs in total? The answer is that we do not know this, for sure, because the CTT has these data, and refuses to let anyone else see them. However, some data has not been censored by big brother. The Cochrane collaboration (before they started the sad slide to bias and corruption) looked at this issue - way back in 2003.
They got as much data as they could from the five major primary prevention statin trials at the time. Here was their conclusion on Serious Adverse Events:
'In the two trials where serious adverse events are reported, the 1.8% absolute reduction in myocardial infarction and stroke should be reflected by a similar absolute reduction in total serious adverse events; myocardial infarction and stroke are, by definition, serious adverse events. However, this is not the case; serious adverse events are similar in the statin group, 44.2%, and the control group, 43.9%.
This is consistent with the possibility that unrecognized serious adverse events are increased by statin therapy and that the magnitude of the increase is similar to the magnitude of the reduction in cardiovascular serious adverse events in these populations. This hypothesis needs to be tested by analysis of total serious adverse event data in both past and future statin trials. Serious adverse event data is available to trial authors, drug companies and drug regulators. The other measure of overall impact, total mortality, is available in all five trials and is not reduced by statin therapy.' 3
What does this mean in reality? Well, gathering it all together. Statins (in the over 75s) do not reduce mortality. They do not prevent fatal Mis and strokes. Whilst they reduce serious cardiac events, previously published results demonstrate they do not reduce total serious adverse events.
Which means that they are, wait for it, absolutely and completely useless.
Two plus two does equal four. Always bear that fact in mind.
1: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31942-1/fulltext
2: https://www.heart.org/en/news/2019/01/28/medical-experts-sound-the-alarm-on-medical-misinformation
3: https://www.ti.ubc.ca/pages/letter48.htm
(C) The First Claimant's Blog entry of 11 February 2019 titled "Why cholesterol can't cause heart disease"
(see judgment [82])
[omitting some tables and diagrams]
The more you examine the cholesterol-heart hypothesis, the more it doesn't make sense. This week's note has been inspired by three things that I read last week. The first was the Appendix to last week's statin paper in the Lancet (Ref 1) [The Lancet 2019 Study (see judgment [64])]. The second was an article "How to beat Heart Disease" in the UK Mail on Sunday newspaper (Ref 2) and the third was Dr Malcolm Kendrick's latest book "A Statin Nation" (Ref 3).
Before I get on to Malcolm's book, I have had the pleasure and privilege of spending many hours listening to Malcolm talking about heart disease. The logic with which he approaches the subject is second to none. I have come across no-one worldwide who has thought about this topic for as long and as openly as Malcolm has. He comes up with a hypothesis himself and then he tries to disprove it. If he can disprove it (and he usually can), he goes back to square one to consider a different hypothesis. The trouble with the cholesterol hypothesis, is that it can so easily be shown not to hold and yet those who believe in it won't let it go.
One of the most memorable things that Malcolm said about heart disease was this: We know that being male and getting older are risk factors for heart disease. But until we can explain why being male or why getting older increases the risk of heart disease, we don't understand heart disease. It is not enough to say that older people are more at risk of heart disease - we need to explain why.
The age paradox
The Mail on Sunday (MoS) article thinks it knows why older people die of heart disease. I share this not because the media is an accredited source of medical information, but because millions more people will read a newspaper than an academic paper and it is important to be aware of beliefs - where they come from and how they get perpetuated. The MoS tells us: "As you get older... the effect of your diet and lifestyle accumulates over time leading to a build-up of cholesterol and plaque in the arteries. Four out of five people who die of heart disease are over 65."
But I had just been looking at the Appendix for last week's Lancet paper and there was a very interesting table (Webtable 3). The full title for Webtable 3 was: "Mean plasma lipid concentrations at baseline and mean difference in plasma lipid concentrations at 1 year in participants in all studies, by category of age."
I captured only the left hand side of Webtable 3 I the image below, to show baseline cholesterol levels for the 186,854 people who were included in the Lancet statins study. The participants are grouped by age range. The top part of the table includes the 147,242 people in the statin vs. placebo trials. The bottom part of the table includes the 39,612 people in the lower vs. higher dose statin trials (those people were on some level of statin, hence the lower cholesterol levels overall).
As you can see, the total cholesterol falls steadily from the youngest to the oldest age groups in both the top and bottom sections of the table. As you can see, the LCL-Cholesterol falls steadily from the youngest to the oldest age groups in both the top and bottom sections of the table. As you can see, HDL-cholesterol rises steadily from the youngest to the oldest age groups in both the top and bottom sections of the table
[Webtable 3: Mean plasma lipid concentrations at baseline and mean difference in plasma]
I used the main Lancet paper to extract the incidence of major vascular events (Figure 1) - given as a percentage per annum (pa). I then plotted the LDL-Cholesterol per age group against the incident rate per age group - whether the people were on statins (red line) or in the control group (green line), as shown in the diagram below. As the event rates ranged from 2.6% to 5%, this worked well using the same vertical axis - where LDL-Cholesterol ranged from 3.8 to 3.3 mmol/l.
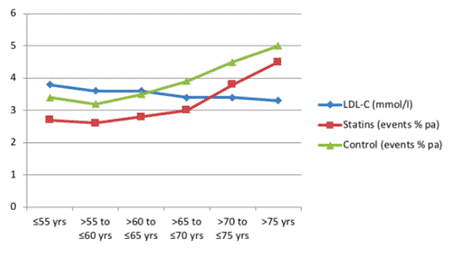
As you can see, and as you would expect, the cardiovascular incident rate goes up with age. As you can see, and the opposite of what the cholesterol hypothesis would tell us to expect, LDL‑Cholesterol goes down with age and goes down as the incident rate goes up.
So higher LDL-Cholesterol is not even associated with older age, or higher incidence of major vascular events, so how can LDL-Cholesterol cause heart disease?
The Mail on Sunday further reported "It's your ratio of LDL to HDL rather than overall cholesterol that matters". Well, LDL-Cholesterol decreases with age and HDL-Cholesterol increases with age, so the over 75s have the absolute best cholesterol profile of all the age groups. Alas they have approximately double the incidence of major vascular events. As Malcolm would say "oops!"
That's a definite age 'paradox', which renders the cholesterol-heart hypothesis useless across men and women as an explanation of why older people get heart disease more than younger people.
The gender paradox
The gender paradox has long been known - women have higher average cholesterol levels and less heart disease than men. To provide a reference for this is surprisingly difficult, as data for average cholesterol levels are conspicuous in their absence. It has become common to report raised cholesterol levels, which are subjective judgments against a made-up cholesterol target, rather than actual average figures.
The most recent European cardiovascular Disease (CVD) Statistics document (2017) provides information on cholesterol levels and disease by incidence by gender (Ref 4). P155 reported that "In Central and Eastern European and in Western Asian countries, raised blood cholesterol tended to be more common in females than in males." To compare these higher cholesterol levels with heart disease: Table 1.5 (p.28-31) reported that 177 per 100,000 men died from heart disease vs. 87 per 100,000 women (Ref 5). That's gender paradox.
The many other paradoxes
* The Japanese paradox
Malcolm summarised numerous paradoxes in Chapter 13 of "A Statin Nation". In this chapter he explored whether raised cholesterol (LDL) caused CVD. To illustrate the Japanese paradox, he reported that, over the past 50 years, the average cholesterol level has rising in Japan from 3.9 mmol/l to 5.2 mmol/l. Deaths from heart disease have fallen by 60% and rates of stroke have fallen seven-fold in parallel. A 25% rise in cholesterol levels has thus accompanied a six-fold drop in death from CVD (Ref 6)
* Other paradoxes (from Malcolm's chapter)
The French paradox is well known - the French have the lowest cardiovascular disease (CVD) rate in Europe and higher than average cholesterol levels (and the highest saturated fat consumption in Europe, by the way). Russia has over 10 times the French death rate from heart disease, despite having substantially lower cholesterol levels than France. Switzerland has one of the lowest death rates from heart disease in Europe with one of the highest cholesterol levels. Malcolm reported the finding of an Austrian study: "In men, across the entire age range... and in women from the age of 50 onward only, low cholesterol was significantly associated with all-cause mortality" (Ref 7), i.e. people with low cholesterol were more likely to die from anything - not just heart disease.
I was delighted to see that Malcolm included some of my work in his chapter. Back in 2010, I used the World Health Organisation data for all 192 where average (mean) cholesterol and death rates from CVD and all-causes were available. I showed that there was a relationship for men and women, with average cholesterol levels and CVD deaths and deaths from any cause - but that the relationship was inverse in every case (Ref 8). Lower cholesterol was associated with higher deaths and higher cholesterol was associated with lower deaths. This was for total cholesterol - LDL‑Cholesterol data were not available, but LDL-Cholesterol is the major part of total cholesterol and thus it would be inconceivable that the associations were anything other than inverses for LDL‑Cholesterol and deaths. The charts are as follows [set out].
The starting point to be able to claim that A causes B is for there to be a clear and consistent relationship between A and B. An inverse relationship exists between total cholesterol and LDL‑Cholesterol and vascular incidents with age. An inverse relationship exists between total cholesterol and heart disease with gender. Inverse relationships exist between cholesterol and deaths from heart disease and deaths from any cause for males and females for all 192 countries in the world. Unsurprisingly numerous country paradoxes can be found among global country data.
The 'cholesterol causes heart disease' hypothesis falls at the most basic level. If there is a clear and consistent relationship, it's the opposite of what is claimed.
Summary
- The cholesterol-heart hypothesis has mutated many times over the years, but a central theme - that cholesterol causes heart disease - has prevailed. It used to be claimed that total cholesterol cause heart disease. More typically, we are now told that LDL-Cholesterol causes heart disease (that's the cholesterol in a low density lipoprotein - one of the taxis in the blood stream). Sometimes the hypothesis is strained even further and we are told that the ratio of LDL-Cholesterol (that's the ratio of cholesterol in one lipoprotein/'taxi' to the cholesterol in another) causes heart disease.
- Before it can be claimed that cholesterol causes heart disease, cholesterol and heart disease must have a clear and consistent relationship.
- There is much evidence to show that, if there is such a relationship, it's inverse, i.e. high cholesterol is associated with lower heart disease and vice versa.
- An interesting table was found in the Appendix to the Lancet Statins for the over 75s paper. This showed that - before any intervention took place - total cholesterol and LDL-Cholesterol were lower, the higher the age group. However, vascular events were higher, the higher the age group. That confirms the inverse relationship.
- The gender paradox has undermined the cholesterol-heart hypothesis for 50% of the population from the outset, as women have higher cholesterol levels and lower heart disease on average. That confirms the inverse relationship.
- Other 'paradoxes' have been documented for specific countries. An examination of 192 countries, which I did in 2010, showed that the higher the total cholesterol, the lower the deaths from heart disease and the lower the deaths from any cause. This held for males and females. That confirms the inverse relationship.
- To continue to believe that cholesterol causes heart disease is to disregard evidence to the contrary.
References
Ref 1: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31942-1/fulltext
Ref 3: https//www.amazon.co.uk/Statin-Nation-Damaging-Post-health/dp/1786068257
Ref 4: http://www.ehnheart.org/images/CVD-statistics-report-August-2017.pdf
Ref 5: Heart disease was taken as IHD - Ischemic Heart Disease, the most recent data were from 2014 and the deaths were age standardised
Ref 6: https://www.ncbi.nlm.nih.gov/pubmed/18174657
Ref 7: http://health-heart.org/eve-not-adam.pdf
Annex 4: Public claims made by the Claimants relied upon by Mr Calman
(see judgment [108])
(A) Claims (i) that LDL-C (or bad cholesterol) does not play a role in the causation of cardiovascular disease (and that low LDL-C) may be inversely associated with mortality and (ii) that statins 'don't work' and offer no or negligible benefits even to patients at high risk (e.g. those who already survived a heart attack) as preventing cardiovascular disease has nothing to do with lowering LDL-C
(1) First Claimant
(i) "Replacing, not lowering, cholesterol would be more accurate": blog, 26 May 2014, which Mr Calman summarised:
"Dr Harcombe claimed that:
'... pure (not-played-with) data from the World Health Organisation for 192 countries' shows that the 'true relationship between cholesterol and deaths' is that 'the higher the cholesterol, the lower the deaths from CVD' or from any cause for men and women. This was under a section headed 'Lowering cholesterol - General consensus vs. evidence'.
This data is 'just about as good as data gets and it shows the exact opposite of what we are being told'.
'It has become so well known that "lowering cholesterol is a good thing" that we are forgetting to challenge the entire and immense industry that has followed from this (drugs and spreads). Lowering cholesterol is a bad thing... made even worse if the human being being tampered with is of child bearing age/has parent-like aspirations. It's also very bad for any human who wants to live longer. But, what the heck, so long as the cholesterol industry is worth billions - who cares?!'.
In response to a reader asking about cholesterol levels for a friend prescribed statins, Dr Harcombe stated: 'In my view, the cholesterol test is worse than useless'; that statins 'kill you one cell at a time' and that 'even if your friend has already had a heart attack, he has a 98.2/100 chance of statins doing nothing for him anyway (http://drmalcomkendrick.org/2014/12/01/what-is-t/)".
(ii) "Worried about cholesterol and/or statins": blog, 24 March 2015, which Mr Calman summarised:
"Dr Harcombe claimed that:
The cholesterol test is a 'scam' and that her 'top tip is: don't have a cholesterol test and then you'll have one fewer thing to worry about'.
'HIGH cholesterol is associated with LOW deaths and LOW cholesterol is associated with HIGH deaths for men and women, CVD deaths and all-cause mortality.'
'If you are in the highest risk group possible (men of a certain age who have already had a heart attack), for every 100 of these men given statins for five years, 1.8 men will live, on average, an extra 6 months and 98.2 will gain no benefit'.
'The intelligent thinking in the world of cholesterol is that any "benefit" of statins for this small, very high risk group, results from anti-inflammatory properties of statins; the cholesterol lowering being a serious price to pay'.
Adverse effects 'are substantially understated and those who mention side effects are viciously attacked'.
'As a result of the fall-out from this attack on two doctors who dared to mention statin side-effects, it was revealed that Professor Rory Collins's CTSU team, working on statin (and other) studies has received c.£268 MILLION from pharmaceutical companies that make statins'.
In response to a reader worried about a high LDL cholesterol reading and that she may be prescribed statins Dr Harcombe stated: 'I can't advise anyone about what to do. I can only say what I would do. 1) I would not have a cholesterol test but you've already done that 2) if prescribed statins I wouldn't take them. I've heard or other people use a term called 'devious compliance' when under pressure from the medical profession (which should be a crime in itself). They cash the prescription, but don't take them'".
(iii) "The Lancet Statin Study": blog, 12 September 2016 [following The Lancet Review (see [62] in the judgment)], which Mr Calman summarised:
"Dr Harcombe claimed that:
'The first thing you need to know about the CTSU is that the spat that the CTSU leader, Rory Collins, kicked off with the BMJ in 2014 led to him having to declare the previously well-hidden pharmaceutical funding enjoyed by the CTSU. This added up to £268m'.
'I accept the second part (statins lower cholesterol); I do not accept that lowering cholesterol lowers heart disease. Statins may lower heart disease (by a tiny amount, which is outweighed by the harm they cause), but the non-industry-funded research that I have done shows that lowering cholesterol has no impact on heart disease and that some methods of lowering cholesterol increase heart disease. The intelligent view is that - any tiny benefit that statins may confer is due to their anti-inflammatory properties - the cholesterol lowering properties of statins are serious side effects'.
'If the data (on benefits or side effects) were robust, there would be no concern about sharing them. Given that the data has been deliberately withheld since the CTT group was set up in 1994, we must assume that it would not withstand scrutiny'.
'The claimed benefits for statins should be ignored because what really matters is not the number of events/deaths, but for what period of time statins may delay an event/death. The most recent and independent review, prior to this CTT pharmaceutical funded review, concluded that for a follow-up period of 2-6.1 years, the average postponement of death for primary (no previous cardiovascular event) and secondary (already had an event) prevention trials were 3.2 and 4.1 days i.e. take statins for 2-6.1 years and maybe buy yourself a couple of days - meanwhile, risk suffering from side effects for the entire time'.
'Sadly, drug funded CTSU drug-promoters can claim whatever they like in a medical journal, with the breathtaking arrogance of those who literally cannot be challenged because they decide they won't share anything that could be challenged, and they have no risk of repercussions, let alone jail. What is more, the editor of that journal supports this outrage unequivocally and has the audacity to assert that further debate on statins should not be allowed to continue. This is precisely why the debate must and will continue - whether the drug pushers and drug hiders and their conspirators like it or not'.
(iv) "Statins in the over 75s": blog, 4 February 2019 [set out in full in Appendix 3(A)], which Mr Calman summarised:
"I knew to doubt this claim as soon as I saw it because I worked on a paper published in 2016 with Dr Uffe Ravnskov as the lead author (Ref 10). The paper was called "Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review". I spent several weeks doing the data extraction with Uffe and cross checking each other's tables. I withdrew my name from the paper before publication because the correct (PRISMA) systematic review/meta-analysis methodology was not followed. This did not impact the results and, having seen the data first hand, the conclusion is robust. The conclusion was: "High LDL-C is inversely associated with mortality in most people over 60 years". i.e. high LDL- cholesterol is associated with lower deaths in most people over 60".
(v) "Why cholesterol can't cause heart disease": blog, 11 February 2019, which Mr Calman summarised:
"Dr Harcombe claimed that:
'There is much evidence to show that, if there is such a relationship [between cholesterol and CVD], it's inverse. i.e. high cholesterol is associated with lower heart disease and vice versa'.
'An examination of 192 countries, which I did in 2010, showed that the higher the total cholesterol, the lower the deaths from heart disease and the lower the deaths from any cause.'
(2) Second Claimant
(i) "Is cholesterol a cause of heart disease": Interview with Dr Kendrick on BBC Radio 4's Today programme on 13 June 2016 (see [211] above), which Mr Calman summarised:
"This interview was to publicise a review co-authored by Dr Kendrick entitled 'Lack of an association or an inverse relationship between low‑density-lipoprotein cholesterol and mortality in the elderly: a systematic review' by Ravnskov et al on 12 June 2016 on BMJ Open [see [210] above]. The study claimed to provide the 'basis... for a re‑evaluation of the guidelines for cardiovascular prevention, in particular because the benefits from statin treatment have been exaggerated'.)
Dr Kendrick claimed that:
The authors of the study 'decided to try and find all the work that had been done looking at levels of LDL' in the elderly 'because this hadn't been done before' and 'looking at over 68,000 people we found that essentially after the age of 60... if you have a higher LDL bad cholesterol level, you will actually live longer and there is no increased risk of CVD'.
He had 'been working on this for years and years, writing articles for years and years, and 10 years ago wrote a book called "The Great Cholesterol Con". What is going on is that LDL and/or cholesterol is not actually a cause of CVD'.
'If you look at the data in more detail it's clear that... if you take a statin for 5 years and you are in a high risk group you may expect to live an approximately 5 days longer. Whether you think that is worth it or not the effect is real, although very smally. However, my contention would be that it has nothing to do with the LDL lowering, that statins work through other mechanisms and that would fit with the fact that many other medications have lowered cholesterol and/or LDL cholesterol and have had no impact on overall morality or CVD.'
It is 'completely incorrect' to state that most of the long-term studies with statins have shown a reduction of CVD and total death and an increase in life span of several years".
(ii) "Bad cholesterol helps you live longer": Article in The Times, 13 June 2016, which Mr Calman summarised:
"Dr Kendrick made claims to like effect to BBC interviews, and claimed that: 'The diet/heart cholesterol hypothesis [has been called] the greatest scam in the history of medicine. It seems that is right".'
(iii) "High cholesterol 'does not cause heart disease' new research finds, so treating with statins 'a waste of time'": Article in The Daily Telegraph, 13 June 2016, which Mr Calman summarised:
"Dr Kendrick made claims to like effect to BBC interviews, and claimed that: 'cholesterol does not cause heart disease in the elderly and trying to reduce it with drugs like statins is a waste of time... The guidelines for the prevention of CVD and atherosclerosis should be re-evaluated because the benefits of statin treatment have been exaggerated... The findings would cause controversy but were robust and thoroughly reviewed".'
(iv) "A Statin Nation": published on 27 December 2018, from which Mr Calman identified:
"Back cover: 'people are being conned. It is clear that statins are not all that is claimed for them... Commonly-viewed as a wonder-drug, in reality, as the book shows, they increase life expectancy by a negligible amount, while adverse effects are all too often concealed. The way to avoid heart disease, and strokes, is simple - but it has nothing to do with lowering cholesterol levels.'"
(B) Claims that the incidence of adverse effects caused by statins was being hushed up and the true rate was about 20 per cent
(1) First Claimant
(i) Mr Calman has identified several Tweets of the First Claimant posted on 12-13 February 2014, following an interview with Dr Malhotra that was broadcast on BBC Radio 4's Today programme on 12 February 2014. In square brackets I have noted the number of 'likes' and/or Retweets and or replies the relevant Tweet received (as recorded in the evidence):
"Scandalous reporting of side effects Go @DrAseemMalhotra On Radio 4 now" [1 like]
"@DrAseemMalhotra's opponent on @BBCRadio4today was Mark Baker. Past links = Roche and Pfizer. NICE supposed to be independent" [4 Retweets, 2 replies]
"This was yesterday's news [link to article in Independent] Today's news is give everyone statins. Spot the connection!" [12 Retweets, 1 like]
"Thank goodness at least one sane and non-conflicted person was quoted @DrAseemMalhotra. Good Jon Abramson quote too [link to Guardian article] [2 Retweets, 1 like]
"NICE & anyone half persuaded to listen to the outrageous headlines today should read this [link to the First Claimant's Blog article "How statin drugs really lower cholesterol and kill you one cell at a time" [10 Retweets, 2 likes]
"An even better @drbiffa post on Mr BHF [link which Mr Calman says states that "chances of significant side effects including diabetes, muscle pain, fatigue, liver damage or kidney damage are about 20 per cent" [no likes, Retweets or replies]
"How great to see this counter to yesterday's profit-motivated scare‑tactics [link to Daily Mail article, written by Dr Malhotra, under the headline: 'As go-ahead's given for one in four adults to be offered heart drug, one doctor says this mass pill-popping is folly' in which he stated: 'reliable data from the real world, published recently in the British Medical Journal and backed up by anecdotal evidence from my experience as a cardiac physician, suggests that the real figure for serious side-effects associated with statin use is closer to one in five] Well done @draseemmalhotra" [6 Retweets, 1 like, 1 reply]
"[Link to same Daily Mail article] This needs conflicts declared. @DrAseemMalhotra none. Baigent = Astra Zeneca, Merck, GSK, & J&J (google him and Merck)" [no likes, Retweets or replies]
"[Link to same Daily Mail article] Big Colin Baigent study funded by Merck [link to Lancet article in 2011]. No such funding for @DrAseemMalhotra" [no likes, Retweets or replies]
(ii) Mr Calman also identified further Tweets of the First Claimant posted between 16-19 May 2014. The context was that, on 15 May 2014, the BMJ (and authors of the relevant papers) withdrew the figures that suggested that 20% of patients prescribed statins suffered adverse side‑effects and announced an inquiry by an independent panel:
"This is so serious - barely days after Australian dissent was silenced, UK freedom/truth is under attack" [This was a reply Tweet, with 1 like]
"This is so serious [link to article on BBC news website] The CTT won't release Serious Adverse Effect data so how can we know [link to article on www.healthinsightuk.org 'SOS sanity over statins CTT the house of statin secrets']" [9 Retweets, 2 likes, 10 replies]
"This is the official BMJ article [link given]. The BMJ was alerted by Rory Collins - the top dog who refuses to share the SAE info" [1 Retweet]
"Will this panel have the power to force CTT to release the SAE data on statins that they refuse to disclose?" [3 Retweets, 1 like]
"@DrAseemMalhotra standing up to pharma funded bullies [link to article in the Independent under the headline: 'Statins row critics are biased says doctor who warned of drugs side-effects'] [16 Retweets, 9 likes and 2 replies]
"Collins seems to have forgotten to have declared over £100 million in drug company money. He must be on statins! [link to Blog post 'It's not about statins, it's about censorship']" [19 Retweets, 7 likes, 3 replies]
(iii) Mr Calman identified the First Claimant's Blog post on, 19 May 2014, "It's not about statins, it's about censorship" in which he said that the First Claimant had claimed, in reference to the BMJ's withdrawal of the figure of 18-20% of people taking statins experiencing side-effects:
'In the UK, in the same week, same drug, we also experience censorship. You [Professor Collins] head the CTT. Why will the CTT not release Serious Adverse Effect data (and raw data generally) from clinical trials so that researchers, doctors and patients can fully understand the side effects of statins? How can you claim that statin side effects are negligible when you won't share the data?'
(iv) Mr Calman also identified the First Claimant's Blog post on, 19 June 2014, "Doctor's tell NICE you're not independent and you're not evidence based". He relied upon the following claims by the First Claimant that the statements in the BMJ October 203 articles that statin therapy has an 18-20% risk of causing side effects was from 'a peer reviewed paper that estimated the side effects of statins to be approximately 20%'; that it was an 'assault on science to suggest otherwise; that 'it's an absolute disgrace that a Sir/Professor/Lord‑high‑executioner can make demands [that the BMJ retract the October 2013 articles] in this way from the worst position of conflict I have ever seen'; that 'the data has been manipulated [by "drug company funded 'researchers' conducting trials"] to ensure whatever side effects were reported in the statin victims, the placebo arm looks the same'; and 'it is just incomprehensible that NICE don't seem bothered that they have 8 out of 12 fat cats [on their guideline development group] dealing with biased pharma controlled data'.
(v) Mr Calman also identified the First Claimant's Blog post on, 4 August 2014, "CTSU Funding from drug companies". He relied upon the following claims by the First Claimant: that 'the CTSU is the Oxford Clinical Trial Service Unit. Where this ends and the CTT (Cholesterol Treatment Trialists) Collaboration begins or where the CTT ends and where Sir Professor Rory Collins and Colin Baigent and co. begin or end, I know not. One gets the impression that the web between the parties is not intended to be clear'; 'this is the group that holds data on statin side effects, which it won't share with doctors and patients who would like to know about these statin side effects. Pause to digest that for a second. There is clinical trial information about what the most prescribed drugs in the US and UK could do to humans and researchers refuse to share it'; that the statements in the BMJ October 2013 articles that statin therapy has an 18-20% risk of causing side effects was a 'peer review article finding about statin side effects' and not a misrepresentation of that study; that Professor Collins' attack on the BMJ for publishing the articles was an 'attempt at censorship'; and that '[she] interprets this as - we receive £268 million from trial sponsors and it is not in their interests for side effect data to be shared. And if some patient loving cardiologist, with no such conflicts of interest, dares to suggest that statins have side effects (when we hold that information and won't tell him) we'll bypass the BMJ process and demand a retraction.'
(2) Second Claimant
(i) "Health expert slams statins: Millions face terrible side effects as drug is planned for 1 in 4; Don't hand out statins like sweets say experts"; Article in the Sunday Express on 2 March 2014. Mr Calman stated that, in the Article, the Second Claimant had claimed that (1) one in four Britons will soon be at risk of terrible side effects from statins if the current NICE guideline was introduced; (2) that NICE should not rely on evidence from drug company sponsored trials, which have been shown to play down the risk of side effects including diabetes, impotence, cataracts, muscle pains, mental impairment, fatigue and liver dysfunction; (3) '[he] can stop people dying from heart disease by pushing them off the edge of a cliff. They might not like the end result. Statins might alter what is written on your death certificate, but they are extremely unlikely to change the date'; and (4) 'independent studies show [side effects] in at least 20 per cent'.
(ii) "You are killing patients": Second Claimant's blog, 4 June 2014, from which Mr Calman identified:
'Professor Collins has attacked the BMJ for publishing articles about statins which claim that they have significant side effects' and that he and Professor Magdi Yacoub who is also 'pressing the "you're killing patients" button with great enthusiasm' are engaging in 'the tactics of the playground bully' by 'claiming that the alarm caused by [the BMJ's articles] was probably killing more people than had been harmed as a result of the paper on the MMR vaccine by Andrew Wakefield'.
(iii) Press release, dated 10 June 2014, "Leading Doctors Reject Statin Guidance from the National Institute for Health and Care Excellence" referencing a letter to NICE dated 10 June 2014, of which the Second Claimant was one of 9 signatories. The press release included:
'[The signatories] call on the Cholesterol Treatment Trialists Collaboration who have commercial agreements with the pharmaceutical industry to release all data on statins which is currently being concealed for review by independent researchers to help explain major discrepancies in several industry sponsored studies of statin adverse events.'
(iv) Interview with the Second Claimant on BBC Breakfast on 11 June 2014 in which he claimed:
'... we're putting another five or six million people on medication, which I believe causes quite a lot of side effects, for the rest of their lives'
Asked about the data on the incidence and degree of side effects, the Second Claimant stated:
'... when you do clinical studies, you're doing them in a very artificial population... Unfortunately, we're told, well that's anecdotal data,, and you know anecdotes don't equal data, but the reality on the ground is that you do see an awful lot of people with significant side effects. And is it worth it? Because when we're looking at a risk of 10%, the actual benefits, the benefit of say extending your life, is vanishingly small, if it exists at all... pain, difficulty getting up, memory loss, stomach pains, irritation, mood changes are quite common things. If you don't ask for them patients won't tell you these things but the reality is that they are common.'
(v) "A humiliating climb down - or a Machiavellian move?": Second Claimant's blog, 16 February 2015 (in response to publication of the Report of the Independent Panel (see [206] judgment), from which Mr Calman noted that the Second Claimant had said that Sir Rory Collins had "ruthlessly attacked anyone who dares make any criticism of them... he tried to get the BMJ to retract two articles claiming that statins had side effects of around 18-20%. He stated that these articles were irresponsible, worse that both articles [published in the 2013 BMJ Articles] quoted a paper which stated that 17.4% of people suffered adverse effects. So yes, a pedant would say that the 18-20% figure was wrong - although not very wrong. Certainly not worthy of instant retraction and apology... What is certain, and must be reiterated, is that Rory Collins has consistently refused to allow anyone to see the side effect data, or any other data, that the CTT may, or may not, hold".
(vi) In an article in The Scotsman, 4 May 2017, published following publication of a study in The Lancet, on 2 May 2017, "Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm", the Second Defendant stated:
'... a "nocebo" effect? All in the mind? No, of course not... I have spoken to many other GPs who have reported seeing side-effects in many patients taking statins. I suppose if you are trying to push statins as hard as possible, and you built your academic reputation on running trials on statins, you will naturally want to push them as hard as possible... But this latest report pushes things to a completely ridiculous point... The reality is that, unless you have had a previous heart attack, statins have no effect on overall mortality. To put that another way: they don't save lives. The don't even prevent heart attacks or strokes in women with no previous history of heart disease... The statistic you really want to know about statins is the following. If you have had a heart attack, or stroke, and take a statin for five years, you will increase your life expectancy by 4.2 days. Balance that against a 20 per cent chance of having side-effects, some of which are very unpleasant and long-lasting, and you can see why I'm no fan of statins.'
The Second Defendant published The Scotsman article on his Blog.
(vii) In chapter 11 of A Statin Nation, published in 2018, the Second Defendant stated:
'Stripping aside all the horrible dead science speak, I shall translate: "You only think you are having an adverse effect of taking a statin, the reality is that you have been fooled into thinking this. You are not. So stop whinging, you pathetic worm. By the way anyone who criticises statins should probably be thrown in jail... I put it to you that writing off patient-reported symptoms of muscle pain and weakness as the nocebo effect is medicine at its very very worst. Paternalistic, dismissive, it flies in the face of the evidence..."
(viii) "Response to the Lancet Paper", responding to The Lancet 2019 Study (see [64] judgment): Second Claimant's blog, 3 February 2019 (see [73] judgment), the Second Defendant stated:
'I can stop people dying from heart disease by pushing them off the edge of a cliff. They might not like the end result... Statins might alter what is written on your death certificate but they are extremely unlikely to change the date... What does this mean in reality? Well, gathering it all together. Statins (in the over 75s) do not reduce mortality. They do not prevent fatal [heart attacks] and strokes. Whilst they reduce serious cardiac events, previously published results demonstrate they do not reduce total serious adverse events. Which means that they are, wait for it, absolutely and completely useless...'
(C) Claims that the researchers who have conducted the biggest statin studies, in a conspiracy with the pharmaceutical companies, had allowed their judgment to be influenced by their financial interests and the evidence they produced, notably the evidence from the RCTs which was biased and untrustworthy
(1) First Claimant
(i) "Statins: just say no. Sensible reasons why they are stupid medicine", an article published in Health Insight on 17 February 2014. Mr Calman cites this as instance where the First Claimant claimed that it was an "absolute disgrace" and "unforgiveable" that the NICE guidance development groups' interests "are not declared in the report even though NICE claims to be a body producing evidence based guidance... created by independent and unbiased advisory committees"; that "the Chair... used to be a HEART UK trustee, a body which calls itself the cholesterol charity but it is actually the mouthpiece of the statin and polyunsaturated spread manufacturers. No doubt Mr HEART UK resigned as a trustee before heading up the group looking at statin guidelines in the UK, but his hand has been clearly declared, in addition to his previous leadership at HEART UK, declares interests in Amgen; Genzyme Corporation; Merck, Sharp & Dohme; Pfizer and Sanofi‑Aventis; and the guideline development group were not "a truly independent and unbiased bunch".
(ii) "SOS: Sanity over Statins - Sssssh Side effects, pharma's fingers and a cunning plan", an article published in Health Insight on 4 March 2014. Mr Calman cites this as instance where the First Claimant stated, of the statement by the CTT that the agreement with the academic investigators of the trials that trial data will not be released to third parties "was necessary in order that analyses of the totality of the available trial data could be conducted by the CTT Collaboration":
'Pull the other one. Such an agreement is necessary to protect the interests of the companies who stand to lose billions if unfavourable data is released. That's why observers are present. That's why the drug company web runs through CTT, NICE and the bodies that are supposed to be acting in the public interest. And who is acting in the interests of doctors who do not wish to inflict harm? Or the patients who do not wish harm inflicted upon themselves?'
(iii) "NICE has ended any debate about its independence": First Claimant's blog, 14 July 2014:
'The NICE approach to conflict is an utter disgrace and the Department of Health needs to intervene urgently to establish a medical advisory body that is genuinely independent and evidence‑based. Not one that is staffing guideline development groups with conflicted majorities and then publishing the views of these drug/surgery representatives as policy for practitioners to be ordered to adhere to.'
(iv) "And underhand stunt revealed a pharma funded web": First Claimant's blog, 6 October 2014. Mr Calman identified and relied upon the following claims by the First Claimant:
· The survey by the British Cardiovascular Society ("BCS") in 2014 was 'not an open and genuine survey - honestly interested in evidence based medicine [but] a loaded and leading survey... with a clear and underhand end in mind'.
· 'The Joint British Societies - when declarations are fully made - reads like a pretty comprehensive representation of the statin making pharmaceutical industry. Sure enough, this is the Joint British Societies view on lipid lowering therapy: "Intensive statin therapy is recommended..."'.
· Representatives from the Joint British Societies ("JBS"), and various named academics and doctors claimed no declaration of interest, and yet their research showed that they had received money from pharmaceutical companies.
· The First Claimant had 'discover[ed] a web of organisations, charities and affiliates with pharmaceutical funding, shared addresses and what seems to be an extensive network of statin pushers. Why? 1) It gives the appearance of a number of "respectable' organisations/charities all believing in the same thing. In this case that "intensive statin therapy" should be enforced with a 'lower is better" approach and 2) it creates a "once removed" structure, so that the pretty much universally conflicted members of the Joint British Societies group seem to think that it's OK that they put their names to a "lower is better" policy as members of the JBS and then "forget" that they are funded by companies who will benefit from this exact policy'.
· The First Claimant 'given [her] abhorrence towards conflicts of interest [the reader] won't be surprised to know that [she] has received no such payments from any organisation and never will [she] sell [her] soul in this way. It's just disgraceful that this position is not shared by people in positions of power and influence.'
(v) Mr Calman has identified several Tweets of the First Claimant posted on 11 September 2016, following publication of The Lancet Review (see [62] in the judgment). In square brackets I have noted the number of 'likes' and/or Retweets and or replies the relevant Tweet received (as recorded in the evidence):
"Just looking at the mother of all statin propaganda [link to The Lancet Review] NB CTSU never share data (ensures truth can't be uncovered)" [9 Retweets, 3 replies, 8 likes]
"Drug funded team claim statin side effects 0.5-1% [Link to The Lancet Review]. How come the [patient information leaflet] has effects for 1 in 10? [link to example of patient information leaflet]" [3 Retweets, 7 likes]
"Remember the recent conclusions of non drug funded study [Link to a study published in The BMJ on 24 September 2015, "The effect of statins on average survival in randomised trials, an analysis of end point postponement" (see [209] in judgment)] Take statins for 2-6 years & maybe gain 3-4 days (+ SAEs)" [19 Retweets, 2 Quote Tweets, 21 likes, 2 replies]
(2) Second Claimant
(i) "Statin fan funded by drug firm"; Article in the Scottish Express on 30 March 2014. Mr Calman identified the following quote attributed to the Second Claimant: 'Professor Collins may claim to receive no funding directly from industry. However, his personal and professional standing is underpinned by his research unit which relies on industry funding.'
(ii) "You are killing patients": Second Claimant's blog, 4 June 2014, from which Mr Calman identified claims by the Second Claimant:
· Anyone who dares to criticise statins "is subjected to vitriolic attacks and a demand for silence" by Professor Collins.
· The survey by the British Cardiovascular Society ("BCS") "is a very unsubtle variation of the 'You're killing my patients' tactic which is regularly used to silence any who dares criticise current medical opinion".
· "The BCS are funded and supported by the pharmaceutical industry. Any industry that is not, currently, that bothered about statins - as the patents have run out. But it is an industry that remains extremely interested in the whole idea of lowering cholesterol. Which remains THE multi-multi-billion dollar market. Any attack on statins threatens the foundations of this market, one that has been painstakingly constructed over the last thirty years".
· "The following attack by the BCS can be considered, to all intents and purposes, an attack by the pharmaceutical industry on anyone who dares to suggest that drugs lowering cholesterol may not be such a brilliant idea. So when you see the headlines in the newspapers damning and rubbishing Aseem Malhotra, John Abramson, and me (and a few others), you know exactly where this attack originated and why".
(iii) "Medical censorship in the twenty first century": Second Claimant's blog, 11 September 2016, posted following publication of The Lancet Review (see [62] in the judgment), from which Mr Calman identified claims by the Second Claimant:
· The editorial in The Lancet was "the most frightening thing you will read this year, possibly this decade and maybe the entire century as it is a direct attack on human freedoms' and that 'what [the editor] is saying is that any who questions current accepted medical dogma should be very tightly controlled, and probable should not be allowed to publish anything at all".
· "... the entire editorial is an exercise in trying to silence any dissent with what some might view as threats and bullying..."
· The editorial's conclusion that 'the debate about statins, as for MMR, has important implications for journals. Some research papers are more high risk to public health than others. Those papers deserve extra vigilance. They should be subjected to rigorous and extensive challenge during peer review. The risk of publication should be explicitly discussed and evaluated. If publication is agreed, it should be managed with exquisite care', "is basically censorship".
(iv) "It's official statins do not have any side effects": Second Claimant's blog, 8 May 2017, posted following publication of a study in The Lancet, on 2 May 2017, "Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial-Lipid-Lowering Arm", from which Mr Calman identified claims by the Second Claimant:
· "... you could argue that the nocebo effect can only account for 0.26% of adverse effects. Therefore, the other 29.74% (30% in the Statin USAGE study - 0.26% nocebo effects) represents the true rate of adverse effects. You could argue that randomised controlled clinical trials do not reflect the experience of taking medication in the real-world environment. You could say that you believe one of these studies, but not both..."
· "... on the other hand you could move sideways a bit, and wonder why researchers suddenly decided to 'data dredge' a twenty-year old study - not set up to look at adverse effects as a primary end-point - to prove that statins do not have any adverse effects. You could then look at who funded that research and you could ask yourself why would a company currently being sued in the US for not highlighting the adverse effects of statins, decided to use a study to prove that statins do not have adverse effects".
· "Alternatively, you could ask people who have taken statins, whether they suffered adverse effects, and try to match the number who claim that they do, with the one in ten thousand figure of Professor Peter Sever. And good luck with that. It is hard, I find, not to think that 'he who pays the piper calls the tune'".
(v) "Cholesterol lowering the end of the beginning": Second Claimant's blog, 22 May 2017, posted following publication of the same study in The Lancet, on 2 May 2017, from which Mr Calman identified claims by the Second Claimant:
· "... it turns out that the lead author... was provided with financial support for the 'Foundation of Circulatory Health'. Looking at their accounts, the Foundation of Circulatory Health seems to be funded largely (almost entirely?) by the pharmaceutical industry. Companies which include, guess who, Pfizer, who funded the initial ASCOT study and who also funded the recent Lancet Nocebo paper".
· "... digging further it then turned out that Peter Sever and Neil Poulter (key authors on the 'nocebo' paper) are also directors of the Foundation for Circulatory Health, which Funded ... work on the Nocebo paper - supported by Pfizer. Well, who'd a thunk? [Well, me actually]".
· "Neil Poulter is a very well-known researcher in [cardiovascular] medicine, well known to those who keep track of such things. His name turns up all over the place. Here was his declaration of interest statement in the Lancet paper: Poulter disclosed receiving ad hoc payments to appear on advisory boards/deliver lectures for 'all the major pharmaceutical companies that produce major agents in hypertension and [cardiovascular] medicine' and receiving grant income from Pfizer and Servier. Perhaps he just forgot that he had received money from all the major pharmaceutical companies that produce major agents in hypertension and [cardiovascular] medicine. Must be hard to keep track of what you have previously disclosed. Is there a time limit on conflicts of interest?"
(vi) "Response to the Lancet Paper": Second Claimant's blog, 3 February 2019 (see [73] judgment), posted following publication of The Lancet 2019 Study (see [64] in judgment) from which Mr Calman identified claims by the Second Claimant:
· "... this [paper] comes hot on the heels of a concerted effort to silence statin critics around the world by a coalition of 'experts'" and that he "suspect[s] the coordinated timing [of this paper and the Joint Editorial (see [58] in the judgment)] is more than a coincidence".
· The CTT "do almost all these meta-analyses on statins, because they hold all the data. So, no-one else can really do them".
· "... the CTT is in this hallowed position because they made a pact with the dev... sorry... they made a pact with the pharmaceutical industry to take hold of all the data on statins from all the pharmaceutical companies that manufacture statins and collated the data".
· "... a fact that needs to be emphasised is that the CTT will not let anyone else see the data they hold. Including all the data on adverse events [side-effects] and serious adverse events. It is kept completely secret".
· "... anyway, my point is that the CTT is a horribly conflicted organisation, and has been paid, directly, or indirectly, a great deal of money by the pharmaceutical industry".
· "... as for the actual data [in the CTT meta-analysis], it is the usual obfuscation, skirting as close to the direct lie as possible without crossing that line".
· "... what does this mean in reality? Well, gathering it all together. Statins (in the over 75s) do not reduce mortality. They do not prevent fatal [heart attacks] and strokes. Whilst they reduce serious cardiac events, previously published results demonstrate they do not reduce total serious adverse events. Which means that they are, wait for it, absolutely and completely useless".
·
Annex 5: Agreed List of Issues to be determined following trial
(see judgment [401]-[403])
(The parties are not agreed as to Issue (23) (see below for the rival contentions). Paragraphs shown struck through, indicate that the parties are agreed that this issue need not be determined)
A: Publication on matter of public interest under s.4 Defamation Act 2013
Print Publication
(1) In relation to the publication of the Articles in the hardcopy of the newspaper ("the Print Publication"), have the Defendants shown that the Print Publication was, or formed part of, a statement on a matter of public interest, and if so, what is that matter of public interest?
(2) If so, have the Defendants shown that Mr Calman believed that publishing the Print Publication was in the public interest?
(3) If so, have the Defendants shown that Barney Calman reasonably believed that publishing the Print Publication was in the public interest?
Online Publication 1: initial publication
(4) In relation to Online Publication 1, have the Defendants shown that Online Publication 1 was, or formed part of, a statement on a matter of public interest, and if so, what is that matter of public interest?
(5) If so, have the Defendants shown that Mr Calman believed that publishing Online Publication 1 was in the public interest?
(6) If so, have the Defendants shown that Mr Calman reasonably believed that publishing Online Publication 1 was in the public interest?
Online Publication 1: continuing publication
(7) If so, have the Defendants shown that Mr Calman believed, and believed reasonably, following receipt of Ms Wilson's email, dated 5 March 2019 (19:41), that continuing publication of Online Publication 1 was in the public interest?
(8) If so, have the Defendants shown that Mr Calman believed, and believed reasonably, following receipt of Carter-Ruck's letters to RPC, dated 19 July 2019 (the letters of claim), that continuing publication of Online Publication 1 was in the public interest?
(9) If so, have the Defendants shown that Mr Calman believed, and believed reasonably, following their receipt of Carter-Ruck's letter to RPC, dated 26 November 2019, that continuing publication of Online Publication 1 was in the public interest?
(10) If so, have the Defendants shown that Mr Calman believed, and believed reasonably, following their receipt of Carter-Ruck's letter to RPC, dated 31 January 2020 (annexing a list of factual misrepresentations in the articles complained of), that continuing publication of Online Publication 1 was in the public interest?
(11) If so, have the Defendants shown that Mr Calman believed, and believed reasonably, following receipt of the Claimants' Reply, dated 18 December 2020, that continuing publication of Online Publication 1 was in the public interest?
Online Publication 2
(12) Same issues as in respect of Online Publication 1 mutatis mutandis. In relation to the continuing publication of Online Publication 2, Online Publication 2 also contains references to the Hancock Statement which may be found to have been published maliciously after 5 March 2019 and references to the LSHTM Paper which may be found to have been published maliciously after 31 January 2020.
Online Publication 3
(13) Same issues as in respect of Online Publication 1 mutatis mutandis. In relation to the continuing publication of Online Publication 3, Online Publication 3 also contains references to the LSHTM Paper which may be found to have been published maliciously after 31 January 2020.
B: Statutory qualified privilege
Before the question of the application of Curistan may be considered, issues of privilege must be addressed.
Accordingly, does qualified privilege under s.15 Defamation Act 1996 Sch. 1, Part I, §7 or Part II, §9(a) or (b) attach to any of the references to the Hancock Statement in the articles complained of?
General
(For the avoidance of doubt, (1) the points (a) that qualified privilege under s.15 of the 1996 Act may only properly be held to attach to the statement complained of as a whole not to particular words within it, and (b) that Curistan was wrongly decided - will not be pursued, but the Claimants' position is reserved for the purposes of any appeal; (2) the Claimants have indicated that they agree to the Court proceeding on the basis that the references to the Kearney Statement in the Articles are privileged under s.15 of the 1996 Act; and (3) in relation to the references to the Hancock Statement in the original publications, the Claimants admit that the issues of public interest and public benefit under s.15(3) stand or fall with the issue of fairness and accuracy).
(14) Is the Hancock Statement matter published by or on the authority of a government?
(15) Further or alternatively, is the Hancock Statement a notice or other matter issued for the information of the public on behalf of a government or an authority performing governmental functions?
Print Publication
(16) If the answer to (14) above is yes, are any of the references to the Hancock Statement in the Print Publication (highlighted in red in the copies of the Articles in Annex 1, and underlined in Annex 2 to the judgment) an extract from matter published by or on the authority of a government?
(17) Further or alternatively, if the answer to (15) above is yes, are any of the references to the Hancock Statement in the Print Publication an extract from or summary of a notice or other matter issued for the information of the public on behalf of a government or an authority performing governmental functions?
(18) If the answer to (16) above is yes, are any of the relevant references to the Hancock Statement in the Print Publication a fair and accurate extract from matter published by or on the authority of a government?
(19) Further or alternatively, if the answer to (17) above is yes, are any of the relevant references to the Hancock Statement in the Print Publication a fair and accurate extract from or summary of a notice or other matter issued for the information of the public on behalf of a government or an authority performing governmental functions?
(20) If and insofar as any of the references to the Hancock Statement in the Print Publication satisfies the conditions in (19) above, have the Claimants shown that the Defendants were (a) requested by them to publish in a suitable manner a reasonable letter by way of explanation or contradiction and (b) refused or neglected to do so, so as to mean that there is no defence under s.15 of the 1996 Act in relation to the publication of that reference?
(21) If so, have the Claimants shown that any reference to the Hancock Statement in the Print Publication which satisfies the conditions above was made with malice?
Online Publications
(22) Paragraphs (16)-(21) above again in relation to the references to the Hancock Statement in Online Publications 1 and 2 (highlighted in red in the copies of the Articles in Annex 1, and underlined in Annex 2 to the judgment). (There are none in Online Publication 3.)
(23) If and insofar as the conditions detailed in (22) above are satisfied in relation to any of the references to the Hancock Statement in the Online Publications, did that publication become, by 5 March 2019 (date of receipt by the Defendants of the Sarah Wilson email of 5 March 2019 (19:41), the publication to the public, or a section of the public, of matter which is not of public interest or the publication of matter which is not for the public benefit, so as to preclude s.15 of the 1996 Act from applying to it from that date onwards. (23) is not agreed by the Defendants.
The Defendants' position is that (23) only arises on the Claimants' pleaded case if and insofar as the references were not fair and accurate extracts from or summaries of the Hancock Statement. The Claimants' position is that any public interest or public benefit that there may have been in the original publication of the references to the Hancock Statement may have become lost owing to significant changes of circumstance.
(24) If and insofar as the answer to (23) above is no, have the Claimants shown that the publication of any reference to the Hancock Statement in the Online Publications which satisfies the conditions above was being made with malice (as relevant): after 5 March 2019 (date of receipt by the Defendants of the Sarah Wilson email of 5 March 2019 (19:41))
Does privilege under s.6, Defamation Act 2013 (Peer-reviewed statement in scientific or academic journal etc), specifically s.6(5) of the 2013 Act, attach to any of the references in the articles complained of to the LSHTM Paper of 28 June 2016?
General
For the avoidance of doubt, the points (a) that privilege under s.6 of the 2013 Act may only properly be held to attach to the statement complained of as a whole not to particular words within it, and (b) that Curistan was wrongly decided - will not be pursued, but the Claimants' position is reserved for the purposes of any appeal.
(25) Does the privilege defence under s.6(5) apply at all to the Defendants and the references to the LSHTM Paper in the articles complained of (i.e., as a matter of statutory construction)?
(26) If so, was the BMJ a journal with more than one editor when it published the LSHTM Paper?
(27) If so, who was the editor/were the editors who were responsible for deciding to publish the LSHTM Paper?
(28) Before the LSHTM Paper was published in the BMJ, was an independent review of the LSHTM Paper's scientific or academic merit carried out?
(29) If so, depending on the answers to (27) & (28) above, was that independent review of the LSHTM Paper's scientific or academic merit carried out by the editor or editors of the BMJ who were responsible for deciding to publish the LSHTM Paper?
(30) If so, (for the purposes of s.6(3)(b)) what was the scientific or academic matter with which the LSHTM Paper was concerned?
(31) Depending on the answer to (30), was the independent review of the LSHTM Paper's scientific or academic merit carried out by one or more persons with expertise in the scientific or academic matter concerned?
Print Publication
(32) If so, are any of the references to the LSHTM Paper in the Print Publication (highlighted in green in the copies of the Articles in Annex 1, and dotted underlined in Annex 2 to the judgment) an extract from or summary of the LSHTM Paper?
(33) If so, are any of those references to the LSHTM Paper in the Print Publication a fair and accurate extract from or summary of the LSHTM Paper?
(34) If so, have the Claimants shown that the publication of those references to the LSHTM Paper in the Print Publication was made with malice?
Online Publications
(35) Are any of the references to the LSHTM Paper in Online Publication 1, Online Publication 2 or Online Publication 3 an extract from or summary of the LSHTM Paper?
(36) If so, are any of those references to the LSHTM Paper in the Online Publications a fair and accurate extract from or summary of the LSHTM Paper?
(37) If so, have the Claimants shown that the initial publication of those references to the LSHTM Paper in the Online Publications was made with malice?
(38) If not, have the Claimants shown that the publication of those references after 19 July 2019 (date of receipt by the Defendants of the Letters of Claim) was made with malice?
(39) If not, have the Claimants shown that the publication of those references after 31 January 2020 (date of receipt by the Defendants of a Carter-Ruck letter to RPC of that date, annexing a list of factual misrepresentations contained in the articles complained of) was made with malice?
(40) If not, have the Claimants shown that the publication of those references after 18 December 2020 (date of receipt by the Defendants of the Claimants' Reply statement of case for the abortive preliminary issues trial) was made with malice?
C: Meaning and fact/opinion, including the application of Curistan
Print Publication
(41) In relation to the Print Publication, are the articles complained of to be treated for the purposes of determining the natural and ordinary meaning and fact / opinion as forming a single publication which ordinary reasonable readers would have read together or as falling into two distinct parts, namely (1) the article appearing on p.2 of the newspaper and (2) the articles appearing on pp.47-50 of the newspaper, which ordinary reasonable readers would have read separately?
(42) Is the Print Publication or does it contain a statement of defamatory fact concerning the Claimants or either of them or is it or does it contain a statement of defamatory opinion concerning the Claimants or either of them?
(43) If the issue in (41) above is resolved in favour of the Claimants, what is the natural and ordinary defamatory meaning of the Print Publication considered as a single publication, taking due account of the principles in Curistan and the court's findings on the statutory privilege issues as relevant?
(44) If the issue in (41) above is resolved in favour of the Defendants, what is the natural and ordinary defamatory meaning of the articles appearing on pp.47-50 of the newspaper, taking due account of the principles in Curistan and the court's findings on the statutory privilege issues as relevant? (It is not in issue that if the article appearing on p.2 of the newspaper is considered on its own it does not refer to the Claimants and so is not defamatory of either of them.)
Online Publications
(45) Is Online Publication 1 or does it contain a statement of defamatory fact concerning the Claimants or either of them or is it or does it contain a statement of defamatory opinion concerning the Claimants or either of them?
(46) What is the natural and ordinary defamatory meaning of Online Publication 1, taking due account of the principles in Curistan and the court's findings on the privilege issues as relevant? (POC §9; Defence §§8.1, 10, 19, 25; Amended Reply §§10, 19-20, 25)
(47) In relation to Online Publication 2, are Online Publication 2 and Online Publication 1 to be treated for the purposes of determining fact/opinion and the natural and ordinary meaning as forming a single publication which ordinary reasonable readers would have read together, owing to the hyperlink from Online Publication 2 to Online Publication 1, or as separate publications which ordinary reasonable readers would have only read separately? (POC §11, Defence §12; Amended Reply §§11.3, 12)
(48) If (47) is resolved in favour of the Claimants, is Online Publication 2 or does it contain a statement of defamatory fact concerning the Claimants or either of them or is it or does it contain a statement of defamatory opinion concerning the Claimants or either of them?
(49) Further, what is the natural and ordinary defamatory meaning of Online Publication 2 considered together with Online Publication 1 as a single publication, taking due account of the principles in Curistan and the court's findings on the privilege issues as relevant?
(Conversely, if (47) is resolved in favour of the Defendants, Online Publication 2 should be discounted from further consideration: it is not in dispute that if Online Publication 2 is considered on its own it does not refer to the Claimants and so is not defamatory of either of them.)
(50) Further or alternatively, in relation to Online Publication 2, assuming that at least one reader of Online Publication 2 would have clicked on the hyperlink to Online Publication 1 and read what s/he found there, what is the meaning that Online Publication 2 would have conveyed to readers of this kind by innuendo, taking due account of the principles in Curistan and the court's findings on the privilege issues as relevant?
(51) Is Online Publication 3 or does it contain a statement of defamatory fact concerning the Claimants or either of them or is it or does it contain a statement of defamatory opinion concerning the Claimants or either of them?
(52) What is the natural and ordinary defamatory meaning of Online Article 3, taking due account of the principles in Curistan and the court's findings on the privilege issues as relevant?
D: The issue under s.3(5) DA 1996 (if it arises): did the Second Defendant 'not hold the opinion'?
Finally, the question whether the Claimants can show that Barney Calman 'did not hold the opinion' for the purposes of s.3(5) of the Defamation Act 2013. This issue only arises if and insofar as the Court finds that the articles complained of are or contain a statement of defamatory opinion concerning the Claimants or either of them.
(53) If and insofar as the Court finds that any of the articles complained of is or contains a statement of defamatory opinion concerning the Claimants or either of them, did Mr Calman hold or not hold that opinion?
[1] News Article: Headline and [1], [2], and [5]; and Main Article: [15] (and equivalent parts of the online publication)
[2] News Article: [7] (and equivalent part of the online publication)
[3] News Article: [6]; Main Article: [14] and [48] and Editorial [17] (and equivalent parts of the online publication)
[4] The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta- analysis of individual data from 27 randomised trials, published by The Lancet 17 May 2012 https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(12)60367-5/fulltext - see [204] below.
[5] A blogpost by the First Claimant The Lancet Statin Study dated 12 September 2016 raising her concerns regarding The Lancet Review (see [62] above) http://www.zoeharcombe.com/2016/09/the-lancet-statin-study/ - see [212] below.
[6] Statins: we need an independent review, written by Dr Fiona Godlee, Editor in Chief of the BMJ, and published in the BMJ on 15 September 2016 https://www.bmj.com/content/354/bmj.i4992.full.print - see [213] below.
[8] The effect of statins on average survival in randomised trials, an analysis of end point postponement, published in The BMJ on 24 September 2015 https://bmjopen.bmj.com/content/5/9/e007118.abstract - see [209] below.
[9] A blogpost by the First Claimant Cholesterol & heart disease - there is a relationship, but it's not what you think dated 23 November 2010 http://www.zoeharcombe.com/2010/11/cholesterol-heart-disease-there-is-a- relationship-but-its-not-what-you-think/ - see [203] below.
[10] https://www.dailymail.co.uk/news/article-6661513/Climate-Change-chief-John- Gummer-faces-calls-quit-payments-green-businesses.html
[11] Professor who sparked statins row says government should intervene, published in The Guardian on 13 June 2014 https://www.theguardian.com/society/2014/jun/13/professor-statins-row-government-intervene - see [205] below.
[12] Report of the Independent Panel Considering the Retraction of Two MBJ Papers published by the BMJ on 15 August 2014 https://www.bmj.com/content/independent-statins-review-panel-report-0 - see [206] below.
[13] https://www.bmj.com/content/independent-statins-review-panel-report-0 (See SP21) and http://www.zoeharcombe.com/2014/08/ctsu-funding-from-drug-companies/
[14] Statins expert in row over level of risk to patients published in The Sunday Times on 18 September 2016 https://www.thetimes.co.uk/article/statins-expert-in-row-over-level-of-risk-to-patients-gmd30wqvj - see [214] below.
[16] https://www.fool.com/investing/2017/03/13/the-19-best-selling-prescription- drugs-of-all-time.aspx
[17] https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31942-1/fulltext: the Lancet 2019 Study (see [64] above)
[19] The section provides (in similar terms to paragraph 12 of the Schedule Defamation Act 1952) that a defamatory publication will be protected by qualified privilege where it is: "A copy or a fair and accurate report or summary of a statement, notice, or other matter issued for the information of the public by or on behalf of the Government or any department or departmental officer, or any local authority or officer of the authority".
[20] s.7(9) inserted a new Paragraph 14A to Schedule 1, Part II of the Defamation Act 1996 to provide a qualified privilege (subject to explanation or contradiction) for a fair and accurate "(a) report of proceedings of a scientific or academic conference held anywhere in the world, or (b) copy of, extract from or summary of matter published by such a conference".
