Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales Court of Appeal (Civil Division) Decisions
You are here: BAILII >> Databases >> England and Wales Court of Appeal (Civil Division) Decisions >> Sandoz Ltd v Bristol-Myers Squibb Holdings Ireland Unlimited Company (Re Patent - Plausibility when determining validity) [2023] EWCA Civ 472 (04 May 2023)
URL: http://www.bailii.org/ew/cases/EWCA/Civ/2023/472.html
Cite as: [2023] EWCA Civ 472
[New search] [Contents list] [Printable PDF version] [Help]
ON APPEAL FROM THE HIGH COURT OF JUSTICE, BUSINESS AND PROPERTY COURTS OF ENGLAND AND WALES, INTELLECTUAL PROPERTY LIST (ChD), PATENTS COURT
Mr Justice Meade
Strand, London, WC2A 2LL |
||
B e f o r e :
LORD JUSTICE NUGEE
and
LORD JUSTICE WARBY
____________________
| SANDOZ LIMITED |
Claimant/ Respondent |
|
| - and - |
||
| BRISTOL-MYERS SQUIBB HOLDINGS IRELAND UNLIMITED COMPANY |
Defendant/ Appellant |
|
And Between : |
||
| TEVA PHARMACEUTICAL INDUSTRIES LIMITED |
Claimant/ Respondent |
|
| - and - |
||
| BRISTOL-MYERS SQUIBB HOLDINGS IRELAND UNLIMITED COMPANY |
Defendant/ Part 20 Claimant/ Appellant |
|
| - and - |
||
| TEVA UK LIMITED |
Part 20 Defendant/ Respondent |
____________________
Michael Tappin KC, Stuart Baran and Alice Hart (instructed by Bristows LLP) for Sandoz
Justin Turner KC and Thomas Lunt (instructed by Pinsent Masons LLP) for Teva
Hearing dates : 19-20 April 2023
____________________
Crown Copyright ©
- This appeal requires this Court once again to consider the concept of plausibility when determining the validity of a patent. This is a concept which is not mentioned in either the European Patent Convention or in the provisions of the Patents Act 1977 which give effect to the EPC, yet over a period of nearly 30 years it has come increasingly to the fore in discussions of validity, resulting very recently in a decision of the Enlarged Board of Appeal of the European Patent Office in Case G 2/21 (not yet reported, 23 March 2023). This is the first occasion on which the courts of this country have had to consider that decision. It is also a case in which the question as to the role of plausibility is posed very starkly, because it concerns a claim to a single chemical compound per se.
- The Defendant ("BMS") was the proprietor of European Patent (UK) No. 1 427 415 entitled "Lactam-containing compounds and derivatives thereof as factor Xa inhibitors" ("the Patent"), which expired on 16 September 2022, and is the proprietor of UK Supplementary Protection Certificate No. SPC/GB11/042 ("the SPC") based on the Patent, which expires on 19 May 2026. The claims of the Patent relate to a compound called apixaban, marketed by BMS under the trade mark Eliquis pursuant to a marketing authorisation granted on 20 May 2011, which is used to treat thromboembolic disorders. The Claimants ("Sandoz" and "Teva") contend that the Patent is invalid, and therefore the SPC is invalid. There is no challenge to the claimed priority date of 21 September 2001.
- Apixaban's use in therapy depends on its activity as a factor Xa inhibitor. It is not in dispute that apixaban has subsequently proven to be a potent factor Xa inhibitor and a useful therapeutic for thromboembolic disorders, but the Claimants contend that the Patent is invalid because the specification did not make it plausible that apixaban would have any useful factor Xa inhibitory activity. It is common ground that, if and to the extent that plausibility is required, it should be tested by reference to the application for the Patent, published as WO 03/026652 ("the Application"), because if plausibility arose from something that was only in the Patent and not in the Application, the Patent would be invalid for added matter.
- The Claimants contend that, due to lack of plausibility, the claimed invention made no technical contribution to the art and was therefore both lacking an inventive step and insufficiently disclosed. It is common ground that it makes no difference to the outcome whether the issue is viewed as one of inventive step or one of sufficiency.
- The judge held that the Patent was invalid. BMS appeals with permission granted by Lewison LJ. The scope of the issues on the appeal is rather narrower than it was before the judge since the Claimants do not pursue a secondary ground of attack on the validity of the Patent and BMS does not pursue some of the strands of the case on plausibility it advanced before the judge.
- Article 52(1) EPC provides that European patents "shall be granted for any inventions" provided that (among other things) they "involve an inventive step". Article 56 provides an invention "shall be considered as involving an inventive step if, having regard to the state of the art, it is not obvious to a person skilled in the art". Article 83 requires that an application for a European patent "shall disclose the invention in a manner sufficiently clear and complete for it to be carried out by a person skilled in the art". Article 100 provides the grant of a European patent may be opposed on grounds that include lack of patentability under Article 56 and failure to comply with Article 83. Article 138(1) provides that a European patent may be revoked with effect for a Contracting State by the courts of that State on grounds that again include lack of patentability under Article 56 and failure to comply with Article 83. Sections 1(1)(a), 3, 14(3) and 72(1) of the 1977 Act give effect in the United Kingdom to Articles 52(1), 56, 83 and 138(1) EPC. None of those provisions mentions the criterion of plausibility. It has been developed through the case law initially of the Boards of Appeal of the EPO and latterly of the courts of the Contracting States including the UK.
- Since 1995 there have been many decisions of the Boards of Appeal in which the concept of plausibility (or credibility) has been invoked. It is neither feasible nor necessary to review all of them. It is sufficient for present purposes to mention five of the key cases. Before doing so it may help to explain that a recurrent issue in such cases is whether the patent applicant or proprietor can rely upon "post-published" evidence (i.e. evidence post-dating the filing of the application for the patent) as either demonstrating or supporting a technical effect asserted in the application.
- In T 939/92 Agrevo/Triazoles [1996] EPOR 171 the patent application claimed chemical compounds consisting of a class of triazole derivatives defined by reference to a Markush formula. The specification asserted that all these compounds had herbicidal activity, but it only contained test results for some of the compounds. The application was refused by the Examining Division, and the applicant appealed. The main issue on the appeal was whether the claims complied with the requirement for an inventive step in accordance with Article 56 EPC. In its decision the Board of Appeal began its consideration of this issue by observing:
- Having referred to the problem-and-solution approach adopted by the Boards of Appeal to the assessment of inventive step, the Board said at [2.5]:
- Although the Board was not convinced that, in the absence of any technically useful properties, the claimed compounds could be regarded as being a technical invention at all, it nevertheless considered whether the person skilled in the art would have considered the claimed compounds to be a solution to that problem. The applicant argued that, even on the basis of known starting compounds and known synthetic methods, the skilled person would have faced an unlimited number of possibilities for solving this problem, and that a particular selection from that unlimited number was inventive, even if it was arbitrary, unless there was a direct pointer to the preparation of these particular compounds in the prior art. The Board rejected this argument for the following reasons:
- The Board then proceeded to consider the position on the basis of the asserted herbicidal activity of the claimed compounds. As the Board explained at 2.6:
- The Board concluded that it was not credible that substantially all the claimed compounds possessed herbicidal activity for the following reasons:
- In T 609/02 Salk Institute/AP-1 complex (unreported, 27 October 2004) the patent claimed a method for identifying compounds useful for treating abnormal cells. The Opposition Division held that claims 1-5 and 7 were valid, but claim 6 was not. The patentee appealed and filed a new claim 6 to the use of a steroid hormone or analogue identified by the method of claims 1 to 5 which failed to stimulate transcriptional activation of certain receptor genes for the preparation of a pharmaceutical for the treatment of AP-1 stimulated tumour formation and other conditions. The Board of Appeal noted at [5] that the patent specification provided "no evidence at all relating to the invention of claim 6: no steroid hormone is identified as binding to the hormone receptor in such a way that the so-formed complex will disrupt AP-1 stimulated transcription and at the same time fail to promote steroid hormone regulated transcription; no data of any kind are presented indicating that such an [sic] hormone (if it were identified) could have an impact on any of the listed specific diseases". The Board explained at [6] that the patentee relied upon post-published evidence showing that steroid hormones of the kind specified to carry out the use of claim 6 were later identified and found to have an effect on AP-1 stimulated transcription.
- The Board rejected the patentee's argument that the post-published evidence demonstrated that the claimed invention was sufficiently disclosed for the following reasons:
- The Board went on in [9] to explain that this did not require proof of efficacy to be provided in the application:
- The Board also explained at [10] why, in general, in vitro tests were useful in relation to sufficiency of disclosure:
- In T 1329/04 Johns Hopkins/Growth differentiation factor-9 [2006] EPOR 8 the application claimed a polynucleotide of a particular sequence ID encoding a polypeptide having a particular sequence ID identified as "growth differentiation factor-9" (GDF-9) which was asserted to be a member of the transforming growth factor-ß (TGF-ß) family, and hence to have activity as a growth differentiation factor. The Examining Division refused the application, and the applicant appealed.
- The Board of Appeal held that, starting from prior art document (3), the problem to be solved could be defined as isolating a further member of the TGF-ß family. The solution provided was the claimed polynucleotide encoding the claimed polypeptide. The question was whether this solution plausibly solved the problem i.e. whether or not it was plausible that the claimed molecule constituted a further member of the TGF-ß family. The Board held that it did not for the following reasons:
- The Board went on to hold that the applicant was not assisted by post-published evidence establishing that GDF-9 was indeed a growth differentiation factor for reasons it expressed at [12] as follows:
- In T 578/06 Ipsen/Pancreatic cells (unreported, 29 June 2011) the claimed invention was the use of somatostatin or a somatostatin agonist in the preparation of a pharmaceutical formulation for the treatment of a human patient in receipt of transplanted isolated pancreatic islet cells whereby the functional life of those cells was extended. The Examining Division refused the application for lack of inventive step, but the Board of Appeal allowed the applicant's appeal. The Board agreed with the examining division that the problem to be solved was the provision of an alternative means to those disclosed by prior art document (10) for prolonging the functional survival of transplanted pancreatic islet cells in human patients. The examining division held that it was not credible that the problem had been solved because the application contained no experimental data. The Board disagreed because the specification contained a section which, as the Board put it at [11], "deals, albeit in a theoretical manner, with syngeneic islet transplantation in rats and human ß-islet xenografts in non-immunocompetent mice and which discloses an experimental methodology to test the ability of somatostatin receptor binding compounds to extend the functional life of transplanted pancreatic islet cells".
- The Board explained that experimental data was not required to demonstrate plausibility:
- In T 488/16 Bristol-Myers Squibb/Dasatinib [2019] EPOR 24 the patent as granted claimed a broad class of compounds. In proceedings before the Opposition Division BMS's main request was that the patent should be maintained as granted, while its second auxiliary request was that the patent should be maintained with claim 1 limited to a single chemical compound called dasatinib or salts thereof. Dasatinib is a 2,5-disubstituted thiazole. The Opposition Division rejected the main request on the ground of insufficiency and the second auxiliary request on the ground of lack of inventive step. On appeal BMS only sought maintenance of the patent on the basis of its second auxiliary request. It asserted that dasatinib had protein tyrosine kinase (PTK) inhibitory activity and therefore could be used to treat disorders associated with PTK, particularly cancer. It argued that dasatinib showed a clear improvement in PTK inhibitory activity compared to the compounds disclosed in prior art document (7), which disclosed 2,4-disubstituted thiazoles as a novel class of Src inhibitor, Src being a PTK implicated as a potential target for breast cancer therapy.
- BMS relied upon post-published evidence contained in document (9) in support of this argument, but the Board of Appeal held that this evidence could not be relied upon for the following reasons:
- The Board disagreed with the patentee's argument based on the PTK inhibitory activity of dasatinib for the reasons it summarised at [5.5]:
- The Board went on to explain why it followed that the claimed invention lacked an inventive step:
- There have also been a number of decisions of the Patents Court, Court of Appeal and House of Lords or Supreme Court considering plausibility. For present purposes it suffices to mention the following cases.
- In Conor Medsystems Inc v Angiotech Pharmaceuticals Inc [2008] UKHL 49, [2008] RPC 28 claim 12 was to a taxol-coated stent "for treating or preventing restenosis", which the House of Lords construed as meaning that it would prevent or treat restenosis. The specification included the results of tests carried out in various potential anti-angiogenics using a CAM assay in which taxol performed best. The specification theorised that preventing angiogenesis would prevent restenosis, but offered no proof of this. The issue was whether claim 12 was obvious. In holding that it was not, Lord Hoffmann said:
- Having reviewed Agrevo and Johns Hopkins, Lord Hoffmann went on:
- In Generics (UK) Ltd v Yeda Research & Development Co Ltd [2013] EWCA Civ 925, [2014] RPC 4 the patent concerned an improved composition of a synthetic mixture of polypeptides known as copolymer-1. Mylan attacked the validity of the patent on grounds which included lack of inventive step due to lack of technical contribution. In this context Floyd LJ said at [39]:
- Having reviewed Agrevo, Johns Hopkins and Conor v Angiotech Floyd LJ went on:
- In Idenix Pharmaceuticals Inc v Gilead Sciences Inc [2016] EWCA Civ 1089 claim 1 of the patent was to a broad class of chemical compounds defined by reference to a Markush formula. There were also various narrower claims. It was common ground that the validity of the claims, and in particular inventive step, should be assessed on the basis that they were claims to compounds with anti-Flaviviridae activity. At first instance I held that the claims were invalid as lacking an inventive step because the disclosure of the application did not make it plausible that substantially all of the compounds claimed had such activity. This conclusion was upheld by the Court of Appeal. Having reviewed Generics v Yeda, Human Genome Sciences, Inc v Eli Lilly & Co [2011] UKSC 51, [2012] RPC 6 and Warner-Lambert Company LLC v Generics (UK) Ltd [2016] EWCA Civ 1006, [2017] RPC 1, Kitchin LJ (as he then was) concluded at [114]:
- In Warner-Lambert Co LLC v Generics (UK) Ltd [2018] UKSC 56, [2019] Bus LR 360 the patent contained second medical use claims in Swiss form of a known pharmaceutical, pregabalin. Claim 1 claimed the use of pregabalin to treat pain. Claim 3 claimed the use of pregabalin to treat neuropathic pain, and there were subsidiary claims directed to specific types of neuropathic pain. There were also claims directed to inflammatory pain. The specification contained data from animal models supporting the claim to efficacy against inflammatory pain, but neither experimental data nor theoretical reasoning supporting the claim to efficacy against neuropathic pain. At first instance I held that the claims directed to inflammatory pain were valid, and that conclusion was not challenged on appeal. So far as neuropathic pain was concerned, I held that the specification made it plausible that pregabalin was efficacious to treat peripheral neuropathic pain, but not central neuropathic pain. Since claim 3 covered both types of neuropathic pain and Warner-Lambert had not applied, even conditionally, to amend claim 3 down to peripheral neuropathic pain, it followed that claim 3 was invalid on the ground of insufficiency. On the other hand, claims 10, 11 and 12, which were directed to specific types of peripheral neuropathic pain, were valid. A subsequent application by Warner-Lambert to amend claim 3 was summarily dismissed as an abuse of process. The Court of Appeal and Supreme Court upheld my conclusions as to the construction of claim 3 and as to abuse of process. The Court of Appeal upheld my conclusions as to plausibility and hence sufficiency. The majority of the Supreme Court (Lord Reed, Lord Sumption and Lord Briggs) held, for the reasons given by Lord Sumption, that the disclosure in the specification did not make it plausible that pregabalin was efficacious to treat any kind of neuropathic pain. Accordingly, the Supreme Court not only dismissed Warner-Lambert's appeal, but also allowed Actavis' and Mylan's cross-appeal as to the validity of claims 10, 11 and 12.
- Lord Sumption began his judgment by explaining the legal problems presented by second medical use patents, particularly those in Swiss form. Having briefly explained the main claims of the patent and summarised the course of the proceedings, he turned to consider sufficiency and plausibility. He began this part of his judgment by saying at [17]:
- At [19]-[20] Lord Sumption noted that the problem with interpreting the requirement of sufficiency in the context of a second medical use claim as merely requiring the disclosure of the new purpose was that "it would enable a patent to be obtained on a wholly speculative basis". He went on at [22]:
- At [23] Lord Sumption noted that the concept of plausibility had originated in the case law of the Boards of Appeal of the EPO "as a response to over-broad claims, in particular claims to whole classes of chemical compounds supported by a description which fails to show which compounds can be expected to work". He went on:
- Lord Sumption proceeded to review Johns Hopkins (citing [12]) and BMS/Dasatinib (citing [4.9]) in [24], Biogen Inc Medeva plc [1997] RPC 1 in [25], the case law of the Boards of Appeal concerning the interpretation of claims to new uses of old products in [26], Re Prendergast's Application [2000] RPC 446 in [27] and Salk (citing [9] and [10]) at [28]-[29]. At [30] he explained that Warner-Lambert had argued that later decisions of the Boards of Appeal showed that "the Salk principle applies only where the therapeutic effect suggested in the patent is inherently implausible". Having reviewed the cases relied upon by Warner-Lambert, including Ipsen, he concluded at [35]:
- Lord Sumption disagreed with the Court of Appeal's statement of the effect of the plausibility test, saying at [36]:
- Lord Sumption summarised the position at [37] as follows (emphases and line breaks added):
- Two further points should be noted. First, Lord Sumption rejected Warner-Lambert's argument that the courts below were wrong to reject later published data as relevant for the reasons he explained at [40]:
- Secondly, Lord Sumption disagreed with the Court of Appeal that the plausibility of the claims directed to peripheral neuropathic pain was supported by the fact that the skilled team would be encouraged by the data in the patent to carry out simple tests (the Bennett and Chung tests), which were themselves identified in the patent, to confirm the suitability of pregabalin for that purpose. As he explained at [53]:
- Lord Hodge and Lord Mance disagreed because they accepted Warner-Lambert's argument that cases such as Ipsen showed that a lower standard of plausibility was to be applied than that articulated by Lord Sumption. As Lord Mance put it at [195]:
- Although plausibility has subsequently been considered in two decisions of this Court, namely FibroGen Inc v Akebia Therapeutics Inc [2021] EWCA Civ 1279 and Illumina Cambridge Ltd v Latvia MGI Tech SIA [2021] EWCA Civ 1924, it is not necessary to review those cases for present purposes.
- In G 2/21 the Enlarged Board of Appeal considered three questions about the circumstances in which it was permissible to rely on post-published evidence of a technical effect in support of inventive step. The first question asked whether such evidence had to be disregarded on the ground that proof of the effect rested exclusively on the post-published evidence. The Enlarged Board's answer was that evidence submitted by a patent applicant or proprietor to prove a technical effect relied on in support of inventive step may not be disregarded solely on the ground that such evidence had not been made public before the filing date of the patent and was filed after that date.
- Although the referring Board of Appeal only asked its second and third questions on the premise that the answer to the first question was yes, the Enlarged Board considered them anyway. In its decision to refer (T 116/18 Sumitomo/Insecticide compositions, unreported, 11 October 2021) the referring Board had identified what it regarded as two divergent lines of Board of Appeal case law. The first line, represented by decisions such as Johns Hopkins and BMS/Dasatinib, it labelled "ab initio plausibility". The second line, represented by decisions such as Ipsen, it labelled "ab initio implausibility". The distinction it saw between these two lines was that, in the first, post-published evidence could be taken into account if, based on the information in the application and the skilled person's common general knowledge, the skilled person would have considered the technical effect plausible. In the second line, post-published evidence could be taken into account if, based on the information in the application and the skilled person's common general knowledge, the skilled person would not have considered the technical effect implausible.
- The Enlarged Board began its consideration of these questions by observing at [58] that it considered "the conceptional notion inherent in the term 'plausibility', which is often used as a generic catchword, as not being a distinct condition of patentability and patent validity, but a criterion for the reliance on a purported technical effect". This observation chimes with what Lord Sumption said in Warner-Lambert at [23] and [36].
- From [60] onwards, the Enlarged Board embarked on an analysis of the "jurisprudence regarding the reliance on a technical effect for inventive step". It began with some "general considerations", referring among other cases to Ipsen. At [66]-[68] it considered cases in the "ab initio plausibility" or "type I" line of case law identified by the referring Board, including Johns Hopkins and BMS/Dasatinib. In [69] it considered cases in the "ab initio implausibility" or "type II" line. It expressed its "intermediate conclusion" as follows:
- In other words, the Enlarged Board regarded the two lines of case law as being reconcilable. In each case, the core question being addressed was what the technical teaching of the application was to the skilled person with the common general knowledge in mind at the filing date, and whether the technical effect relied upon by the patent applicant or proprietor was derivable from the application.
- Although the reference was only concerned with inventive step, at [73]-[76] the Enlarged Board considered the case law of the Boards of Appeal regarding sufficiency, in particular in the context of second medical use claims. It expressed its "intermediate conclusion" on those cases at [77] as follows:
- In my view it is tolerably clear that the Enlarged Board's reference to "a claimed therapeutic effect" means a therapeutic effect which is asserted as the basis for a second medical use claim.
- At [78]-[85] the Enlarged Board turned to consider decisions of courts of EPC contracting states "with regard to the reliance on technical effect for inventive step". In particular, at [84]-[85] it considered judgments of the UK courts, including Warner-Lambert and the judgment under appeal in the present case. It expressed its "intermediate conclusion" as follows:
- In other words, the Enlarged Board interpreted the decisions of the national courts as approaching matters in a similar manner to the Board of Appeal decisions which it had encapsulated in [71]-[72].
- At [88]-[95] the EBA set out its "concluding considerations", including the following:
- It is clear from these observations as well as the Enlarged Board's earlier reasoning that the fundamental consideration when a court or tribunal is considering whether a claimed invention involves an inventive step is whether the technical effect asserted by the patent applicant or proprietor is derivable by the skilled person from the application as filed read with the common general knowledge. It is perhaps worth adding that this passage (and in particular the last sentence of [93]) confirms that the parties in this case were correct to agree that the issues of inventive step and sufficiency should be assessed by reference to the Application and not the Patent.
- As the judge recorded at [12], by the end of the trial there was no dispute that the Patent was addressed to a skilled team comprising (i) a medicinal chemist and (ii) a biochemist or pharmacologist with relevant experience in industry.
- The parties provided the judge with a document setting out agreed common general knowledge which the judge appended to his judgment (note that in the final version of the judgment handed down by the judge, the paragraph numbering in this document has gone awry and runs from 123 to 291 instead of 1 to 169). There were a number of disputes as to common general knowledge which the judge considered and resolved at [79]-[111]. Given the narrower scope of the issues on the appeal, I can take the agreed common general knowledge and the judge's findings on the disputed matters as read. It is only necessary to explain three points.
- First, factor Xa is an enzyme involved in the "coagulation cascade" of enzymatic reactions in the body. The inhibition of factor Xa is associated with reductions in blood coagulation, i.e., it prevents or at least reduces the formation of potentially fatal blood clots. At the priority date, considerable work was being undertaken by most major pharmaceutical companies to identify novel factor Xa inhibitors.
- Secondly, the first step in testing a potential factor Xa inhibitor would be to carry out in vitro chromogenic enzyme inhibition assays to assess the ability of the compound to inhibit factor Xa (and other serine proteases for selectivity). By completing the assay with a number of concentrations of the candidate inhibitor, a concentration-response curve can be produced, and parameters of potency (IC50 and Ki) can be determined for each compound tested. These assays are simple to set up (commercial kits were available for factor Xa, and other enzymes, in 2001), quick to run, and easy to control. IC50 is the concentration of inhibitor required to reduce the enzymatic activity to half of the uninhibited value. The lower the IC50, the less of the compound is required to produce 50% inhibition, and therefore the more potent the compound is at inhibiting enzyme activity in the assay. Ki is the dissociation equilibrium constant of the enzyme-inhibitor complex and is used to describe the binding affinity that an inhibitor has for an enzyme. Again, the lower Ki, the more potent the compound is. Ki is considered a more accurate measure of potency, since the Ki of an enzyme-inhibitor complex is a constant and accounts for any changes in substrate concentration.
- Thirdly, the judge found at [81]-[85] that the pharmacologist member of the skilled team would consider that, for a factor Xa inhibitor to be potentially useful in treating thromboembolic disorders, it would need to have Ki/IC50 values in the nanomolar range and that 1-10 µM was known not to be a good enough level of potency.
- The closest prior art in the present case is International Patent Application No. WO 00/39131 entitled "Nitrogen containing heterobicycles as factor Xa inhibitors" ("WO 131") published on 6 July 2000. WO 131 was filed by Du Pont Pharmaceuticals Company, whose business was later acquired by BMS. It runs to 326 pages of double-spaced type.
- WO 131 identifies the "field of the invention" at page 1 lines 5-9 as relating generally to "nitrogen containing heterobicycles, which are inhibitors of trypsin-like serine protease enzymes, especially factor Xa, pharmaceutical compositions containing the same, and methods of using the same as anticoagulant agents for treatment and prevention of thromboembolic disorders". The "background of the invention" at page 1 line 11 – page 2 line 24 discusses certain items of prior art. The "summary of the invention" at page 3 lines 1-21 identifies five objects of the invention, the first of which is "to provide novel nitrogen containing heterobicycles that are useful as factor Xa inhibitors or pharmaceutically acceptable salts or prodrugs thereof". This section concludes by stating that these and other objects "have been achieved by the inventors' discovery that the presently claimed bicyclic compounds, or pharmaceutically acceptable salt or prodrug forms thereof, are effective factor Xa inhibitors".
- WO 131 then sets out at page 3 line 23ff a "detailed description of preferred embodiments" which describes 13 embodiments of the invention. The first is a novel compound selected from a group defined by a series of Markush formulae. The second "preferred" embodiment is a novel compound selected from a group defined by a series of slightly more narrowly defined Markush formulae. The third "more preferred" embodiment is a novel compound selected from a group defined by a series of slightly still more narrowly defined Markush formulae. The fourth "even more preferred" embodiment is a novel compound wherein one of the substituents in the Markush formulae is selected from 14 possibilities. The fifth "still more preferred" embodiment is a novel compound selected from a group defined by narrower ranges of various other substituents in the Markush formulae. The sixth "further preferred" embodiment is a novel compound selected from a group defined by narrower ranges of two of the substituents in claim 6. The seventh "even further preferred" embodiment is a novel compound selected from a long list of specific compounds. The eighth and ninth embodiments are novel compounds selected from groups defined by two more sets of Markush formulae. Embodiments 10-13 are respectively pharmaceutical compositions comprising a therapeutically effective amount of a claimed compound, a method of treating or preventing a thromboembolic disorder by administering a therapeutically effective amount of a claimed compound, novel compounds for use in therapy and use of the claimed compounds for the treatment of thromboembolic disorders in Swiss form.
- WO 131 then sets out some definitions (page 57 line 21 – page 62 line 30), describes methods of synthesising the claimed compounds (page 63 line 1 – page 96 line 8) and gives 109 examples of the synthesis of particular compounds (page 96 line 10 – page 206 line 2). This is followed by two tables listing 1456 and 104 examples of combinations of three of the substituents in the Markush formulae (page 207 line 1 – page 262 line 4). The number of compounds represented by these tables is significantly higher.
- Under the heading "utility" WO 131 states at page 263 lines 2-3 that the compounds of the invention "are useful as anticoagulants for the treatment or prevention of thromboembolic disorders in mammals". It goes on to say at page 263 line 11ff that the effectiveness of the compounds as factor Xa inhibitors "was determined" by means of a chromogenic assay which it describes. It then says at page 264 lines 3-10:
- At page 264 line 32ff WO 131 states that "[s]ome compounds of the present invention were shown to be direct acting inhibitors of the serine protease thrombin" by another assay which it describes. It goes on to say at page 265 lines 13-16 that using this methodology "some compounds of this invention were evaluated and found to exhibit a Ki of less than 10 µM, thereby confirming the utility of the compounds of the present invention as effective thrombin inhibitors". Finally, WO 131 sets out some guidance as to "dosage and formulation" (page 268 line 1 – page 272 line 33).
- The remainder of the document consists of the claims. Claims 1-13 correspond to embodiments 1-13.
- It is common ground that:
- The Application runs to 438 pages of double-spaced type. Unsurprisingly, there are considerable similarities in both content and style between the Application and WO 131.
- The Application identifies the "field of the invention" at page 1 lines 5-12 as relating generally to "lactam-containing compounds and derivatives thereof, which are inhibitors of trypsin-like serine protease enzymes, especially factor Xa, pharmaceutical compositions containing the same, and methods of using the same as anticoagulant agents for the treatment and prevention of thromboembolic disorders". The "background of the invention" at page 1 line 14 – page 6 line 35 discusses certain items of prior art, including WO 131, and explains (in more detail than WO 131) why it is desirable to discover new factor Xa inhibitors. The "summary of the invention" at page 7 lines 1 – page 8 line 5 begins by stating that "the present invention provides novel lactam-containing compounds and derivatives thereof that are useful as factor Xa inhibitors or pharmaceutically acceptable salts or prodrugs thereof". This section concludes by stating that these and other objects "have been achieved by the inventors' discovery that lactam-containing compounds of Formula I …, or pharmaceutically acceptable salt or prodrug forms thereof, are effective factor Xa inhibitors".
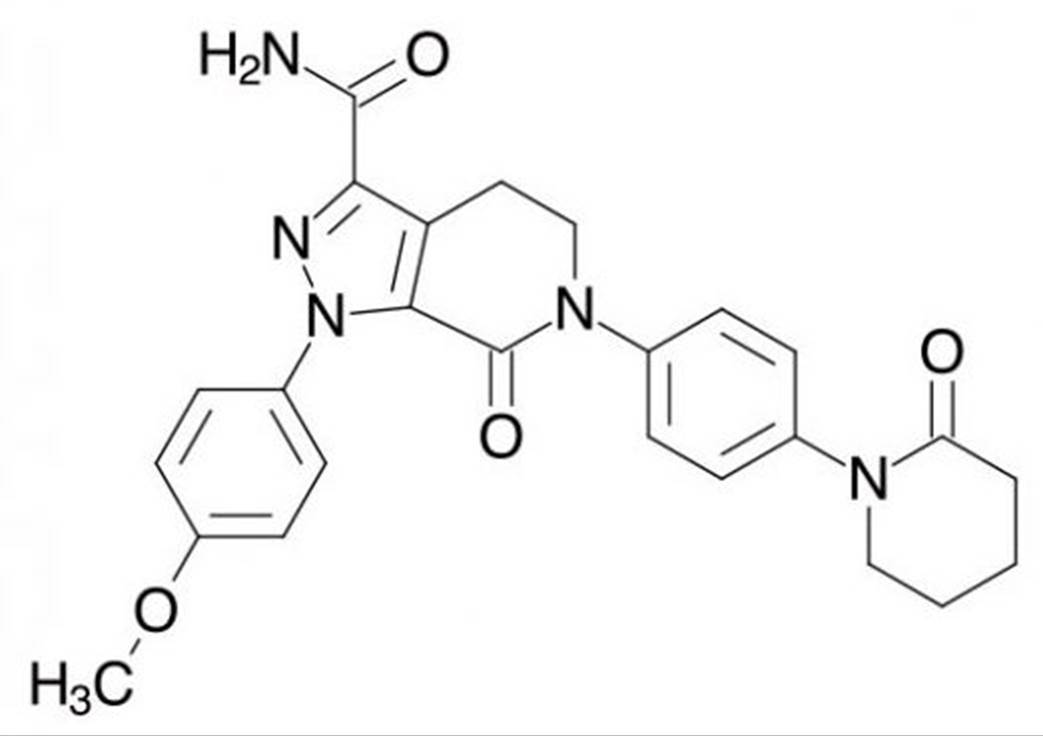
- The Application then sets out at page 8 line 7ff a "detailed description of preferred embodiments" which describes 15 numbered embodiments and a further 20 unnumbered embodiments of the invention. The first embodiment is a novel compound of Formula I, which is a broad Markush formula, while the second "preferred" embodiment is a novel compound of Formula II, which is another broad Markush formula. Embodiments 3-15 are all described as "preferred". It is not necessary to mention all of these. Embodiment 8 is a novel compound selected from a list of 74 compounds, one of which is apixaban. Embodiment 15 is a novel compound selected from a list of another 124 compounds. There is no embodiment directed specifically to apixaban.
- The Application then sets out some definitions (page 134 line 22 – page 143 line 14) and describes methods of synthesising the claimed compounds (page 143 line 16 – page 168 line 12).
- Under the heading "utility" the Application states at page 168 lines 15-18 that the compounds of the invention "are inhibitors of factor Xa and are useful as anticoagulants for the treatment or prevention of thromboembolic disorders in mammals (i.e., factor Xa-associated disorders)". It goes on to say at page 169 line 22ff that the effectiveness of the compounds as factor Xa inhibitors "was determined" by means of the same chromogenic assay as in WO 131. It then says at page 170 lines 21-32:
- At page 171 line 31ff the Application states that "[s]ome compounds of the present invention were shown to be direct acting inhibitors of the serine protease thrombin" by the same thrombin inhibition assay as in WO 131. It goes on to say at page 172 lines 17-21 that using this methodology "some compounds of this invention were evaluated and found to exhibit a Ki of less than 10 µM, thereby confirming the utility of the compounds of the present invention as effective thrombin inhibitors".
- The Application sets out some guidance as to dosage and formulation at page 172 line 22 – page 188 line 25.
- The Application gives 140 examples of the synthesis of particular compounds at page 188 line 27 – page 298 line 7, although characterising data are only reported for 110. Example 18 (page 220 line 1 – page 222 line 30) is apixaban. The product of the final reaction was purified by chromatography to yield 3.5 g of solid. A first recrystallisation yielded 2.5 g of apixaban. A second recrystallisation afforded an additional 0.57 g, giving a total yield of 3.07 g (68%). Characterising proton NMR data are reported.
- Finally, the Application then sets out five tables listing respectively 174, 203, 6293, 29 and 928 examples of combinations of two of the substituents in the Markush formulae (page 298 line 13 – page 315 line 154). Again, the number of compounds represented by these tables is significantly higher.
- The remainder of the document consists of the claims. Claims 1-15 correspond to embodiments 1-15. There is no claim directed specifically to apixaban. Claims 16-20 are to pharmaceutical compositions comprising a therapeutically effective amount of a claimed compound, use of a claimed compound for treating a thromboembolic disorder, or a selected thromboembolic disorder, in Swiss form and a claimed compound for use in therapy.
- Claim 1 of the Patent is as follows:
- Claim 7 is as follows:
- BMS applied at trial conditionally to amend claim 7 to insert either the words "that is a factor Xa inhibitor" or the words "that is an effective factor Xa inhibitor". The judge held at [248]-[256] that the first of these amendments was formally allowable, but refused the amendment on the ground that it did not cure the invalidity of claim 7.
- On the appeal BMS did not rely upon the amendment. Moreover, even though claim 1 is a broader claim than claim 7, BMS focussed its arguments on claim 1 and did not suggest that, if it failed on claim 1, it could nevertheless succeed on claim 7.
- Prior to trial BMS was ordered to, and did, serve a statement of its case on plausibility which it subsequently amended in minor respects. It is not necessary for present purposes to set this out, but for reasons that will appear it is important to note that there was no averment that the skilled team reading the Application with the benefit of their common general knowledge would interpret the Application as disclosing (whether explicitly or implicitly) that apixaban (or any compounds of the invention) had been tested and found to have nanomolar Kis.
- The judge considered the applicable legal principles at [24]-[45]. For present purposes, it is only necessary to set out the following part of his analysis:
- As this passage indicates, at trial BMS relied upon a number of arguments as supporting its case on plausibility, including (i) a contention that the structure of apixaban made it plausible that it would be an effective factor Xa inhibitor and (ii) a so-called "frequency of use" analysis. As mentioned above, however, BMS did not pursue some of these arguments on the appeal. In particular, BMS did not pursue the arguments based on structure and frequency of use. Before this Court BMS only relied on two passages in the Application, read in the context of the Application as whole, namely: (i) the passage at page 170 lines 21-32 (set out in paragraph 71 above), in particular the last sentence at page 170 lines 28-32; and (ii) Example 18, in particular the statements that I have highlighted in paragraph 74 above. It is therefore only necessary to set out the judge's assessment of those two passages and his overall evaluation.
- His assessment of the passage at page 170 lines 21-32 was as follows:
- His assessment of Example 18 was as follows:
- His overall evaluation was as follows:
- Finally, so far as relevant to the appeal, he returned to the question of the tests which could be carried out:
- Practice Direction 52C paragraph 5 requires grounds of appeal to "identify as concisely as possible the respects in which the judgment of the court below is wrong" and states that "[t]he reasons why the decision under appeal is wrong … must not be included in the grounds of appeal and must be confined to the skeleton argument" (emphases added). As is too often the case, BMS's Grounds of Appeal did not succinctly identify the respects in which BMS contended that the judgment was wrong. Rather, it was a discursive document running to 22 paragraphs organised under three headings setting out the reasons why BMS said the decision was wrong. To make matters worse, the Claimants detected from BMS's skeleton argument that BMS was advancing an argument not foreshadowed in the Grounds of Appeal. In order to regularise the position I directed BMS to amend its Grounds. In response BMS filed Amended Grounds of Appeal which did not appear to advance that argument, but instead added four more discursive paragraphs under a new heading. In the course of oral argument, however, it emerged that BMS was indeed advancing the contention which the Claimants had detected.
- With the benefit of the oral argument, it seems to me that BMS's grounds of appeal can be summarised as follows:
- Before turning to consider these grounds, it is important to note that BMS does not contend that the invention claimed in claim 1 of the Patent involved an inventive step and was sufficiently disclosed in the Application on the basis that the technical problem solved, and the technical contribution made, by the invention was merely the identification of a new chemical compound. On the contrary, BMS contends that the claimed invention involves an inventive step and was sufficiently disclosed because apixaban is an effective factor Xa inhibitor, as has subsequently been confirmed.
- These grounds can be considered together since they are essentially alternative submissions. The starting point here is that it is common ground that the majority judgment in Warner-Lambert is binding on this Court at least in cases involving claims to second medical uses, and it is not suggested by BMS that G 2/21 would justify this Court in departing from Warner-Lambert in such a case. Equally, however, it is common ground that there is a factual difference between Warner-Lambert and the present case since claim 1 of the Patent is a claim to a single chemical compound. The question is whether that factual difference means that the present case is legally distinguishable from Warner-Lambert, as BMS argues.
- In my judgment the answer to that question is no. It is true that, as Lord Sumption noted at [23], the concept of plausibility originated as a response to over-broad claims, in particular claims to whole classes of compounds, as in Agrevo. Idenix is an example of its application in that context by the courts of this country. It is also true that, as Lord Sumption noted at [19]-[20], that the concept was also found to be of utility in addressing one of the problems with second medical use claims. Nevertheless the concept was applied by the Board of Appeal to a claim to single compound in BMS/Dasatinib, which was one of the cases relied upon by Lord Sumption (and one of the cases reviewed by the Enlarged Board in G 2/21). As the Claimants point out, the present case is strikingly similar to BMS/Dasatinib. Moreover, BMS/Dasatinib does not stand on its own, because the claim in Johns Hopkins, which was another of the cases relied upon by Lord Sumption and reviewed by the Enlarged Board, was effectively a claim to a specific molecule. The concept has also been applied by this Court in Generics v Yeda to a claim to what was in substance a single product, albeit a product comprising a mixture of polypeptides. Furthermore, the underlying principles are applicable as much to claims to single chemical compounds as to claims to classes of compounds and second medical use claims. The fundamental principle is that the scope of the patent monopoly must be justified by the patentee's technical contribution to the art. This remains so whether the scope of the claim is broad or narrow. Thus when considering inventive step it is necessary to consider what technical problem the claimed invention solves. If it is not plausible that the invention solves any technical problem then the patentee has made no technical contribution and the invention does not involve an inventive step. Equally, when considering insufficiency it is necessary to consider whether the specification sufficiently discloses the claimed invention. If it is not plausible that the invention solves any technical problem then the patentee has made no technical contribution and the specification does not disclose any invention. It follows that, in order for a claim to a single chemical compound to be patentable, the application must make it plausible, when read in the light of the skilled person's common general knowledge, that the compound has the utility asserted for it. Moreover, it makes no difference whether the claim incorporates the use of the compound as a technical feature or whether the claim is simply to the compound per se and the assertion of utility is only to be found in the specification. This is because, as explained above, there is no invention in merely identifying a new chemical compound; invention can only lie in identifying its utility.
- Given that the present case cannot be distinguished from Warner-Lambert, it follows that the criterion of plausibility must be applied when determining whether the claimed invention involves an inventive step and is sufficiently disclosed. I therefore reject ground 1. I would add that I do not understand how it is possible to determine whether a claimed invention is speculative other than by assessing whether it is plausible. They are two sides of the same coin.
- It also follows that the standard of plausibility which should be applied is the standard adopted by the majority in Warner-Lambert, not the standard espoused by the minority or some other "less strict" standard. It is fair to say that the standard adopted by the majority corresponds to the "ab initio plausibility" test identified in Sumitomo, while the standard espoused by the minority corresponds to the "ab initio implausibility" test. As discussed above, the Enlarged Board has taken the view in G 2/21 that the two approaches can be reconciled. I am bound to say that it seems to me that the divergence of opinion in the Supreme Court shows that the two approaches do not necessarily produce the same outcome. It also appears to me, however, that the harmonised approach adopted by the Enlarged Board, while eschewing the language of "ab initio plausibility" and "ab initio implausibility", is as a matter of substance much closer to the former than to the latter. Be that as it may, as I have already noted, it is not suggested by BMS that G 2/21 justifies this Court in departing from Warner-Lambert. I therefore reject ground 2.
- Given that the standard of plausibility to be applied is that explained by Lord Sumption, it also follows that, as he explained at [53], it is not sufficient for the application to encourage the skilled person to carry out simple tests identified in the specification to confirm the efficacy of the claimed product even if carrying out such tests would indeed show that the product is likely to be efficacious. As Lord Sumption said at [40], subsequent data cannot be a substitute for sufficient disclosure in the specification. Although BMS again relied upon BMS/Dasatinib, I agree with the judge that this does not support BMS's argument for the reasons he gave at [44]. I would add that in my view Lord Sumption's analysis is confirmed, if confirmation is needed, by the Enlarged Board's insistence in G 2/21 on focussing on the technical teaching of the specification read with the common general knowledge. I therefore reject ground 3.
- Finally, there is nothing in Lord Sumption's speech to support BMS's contention that the judge should have stood back at the end of his evaluation and considered whether the claimed invention fulfilled the "patent bargain". Nor is there is any reason to think that, if the judge had done so, he would have come to a different conclusion. Fulfilling the patent bargain requires sufficient disclosure in the specification. I therefore reject ground 4.
- I conclude that the judge made no error of law or principle in his approach to the assessment of plausibility. He applied Warner-Lambert, and he was correct to do so.
- The Claimants object that this ground raises a new case which was not advanced below, and argue that BMS should not be given permission to advance the new case on appeal because it would have affected the evidence given at trial. Counsel for BMS denied that the case was a new one. He did not argue that, if it was new, BMS should nevertheless be permitted to advance it on appeal. The judge's view, as he made clear when BMS applied to him for permission to appeal, was that BMS had not advanced any case that the Application impliedly disclosed that apixaban had a nanomolar Ki at trial. This Court should be slow to question the judge's view given that he was best placed to know what case BMS ran at trial, but in any event I consider he was plainly correct and that this is a new case. As noted above, BMS's statement of case on plausibility did not aver that the skilled team reading the Application with the benefit of their common general knowledge would interpret the Application as disclosing (whether explicitly or implicitly) that apixaban (or any compounds of the invention) had been tested and found to have nanomolar Kis. Nor did BMS adduce any evidence to that effect, put that proposition to any of the Claimants' witnesses or advance such a case in submissions. Instead, the case which BMS advanced at trial was that the Application impliedly disclosed that apixaban had been tested and found to have a Ki of <10 µM.
- I would add that this ground cannot assist BMS anyway. If the judge was correct that the Application did not impliedly disclose that apixaban had been tested and found to have a Ki of <10 µM, as I consider that he was for the reasons explained below, it necessarily follows that it did not impliedly disclose that apixaban had been tested and found to have a nanomolar Ki.
- Ground 6 amounts to a bald assertion that the judge was wrong in his evaluation of plausibility. As BMS's own submissions to the judge correctly recognised, however, plausibility involves a multi-factorial evaluation. It follows that this Court is only justified in intervening if the judge has made an error of law or principle: compare Actavis Group PTC EHF v ICOS Corp [2019] UKSC 15, [2019] Bus LR 1318 at [78]-[81] (Lord Hodge) and see Re Sprintroom Ltd [2019] EWCA Civ 932, [2019] BCC 1031 at [72]-[78] (McCombe, Leggatt and Rose LJJ). Counsel for BMS sought to get round this difficulty by arguing that the limited matters which BMS now relied upon merely involved interpretation of the Application and thus could be reviewed by this Court. I accept this point so far as the passage at page 170 line 28-32 is concerned, but counsel for BMS made no serious attempt to argue that the judge had misinterpreted that passage. Rather, he concentrated his submissions on Example 18, and in particular the statements I have highlighted in paragraph 74 above. He stressed that much more of apixaban was made than was reported in any other Example and that a second recrystallisation step was performed unlike in any other Example.
- There is no dispute as to what Example 18 says, however, or what the highlighted statements mean. The issue is what the skilled team would think the patentee's reason was for making 3 g of apixaban given that no explanation is given in the Application. Counsel for BMS argued that the skilled team would infer that this was because early results had been favourable and the patentee wanted to take work on the compound forwards. The problem with this argument is that the judge made a finding, based on the expert evidence, that the skilled team reading Example 18 would think that, although that was a possible explanation, there were other possible reasons why the patentee had made such a large quantity of apixaban. Counsel for BMS did not submit that the judge's finding as to the existence of other possible reasons which would occur to the skilled reader based on their common general knowledge and their reading of the Application was not open to him on the evidence. It follows that the skilled team would not draw the inference for which BMS contend. I would add that BMS's argument presupposes that the patentee had carried out a prior synthesis of apixaban to that reported in Example 18, whereas there is no hint of that in the Application.
- Counsel for BMS also argued that the judge had not taken into account the second recrystallisation performed in Example 18. It is true that the judge did not mention this, but it does not assist BMS. It is clear from Example 18 that the extra step of recrystallisation was performed in order to increase the yield. Thus this adds nothing of substance to the disclosure that a much greater quantity of apixaban was made than of any other compound.
- It follows that the judge made no error in his assessment of the significance of Example 18. Indeed, I agree with it.
- Counsel for BMS's final argument was that the judge had failed to consider the combined effect of the passage at page 170 lines 28-32 and Example 18. This argument goes nowhere for two reasons. First, the judge explicitly considered the cumulative effect of the points relied upon by BMS and held that the whole was no greater than the sum of the parts.
- Secondly, as the judge rightly held, there is nothing in the Application to link the assay results briefly summarised at page 170 lines 28-32 with apixaban. Apixaban may have been one of the compounds tested, but it may not. One does not know because, for whatever reason, the Application does not identify which compounds have been tested, nor does it reveal the actual results which have been obtained. Thus the Application does not disclose either expressly or impliedly that apixaban has been tested and found to have Ki of <10 µM (let alone nanomolar Ki). In the absence of any theory based on e.g. its structure or any data in the specification, there is simply nothing in the Application to support the assertion that apixaban is a factor Xa inhibitor, let alone a factor Xa inhibitor of sufficient potency to be useful in therapy. The assertion is not plausible because the Application gives the skilled team no reason for thinking that there is a reasonable prospect that the assertion will prove to be true. It is therefore speculative. It follows that the invention claimed in claim 1 of the Patent made no technical contribution to the art. It is irrelevant that BMS subsequently proved that the assertion was well founded and limited the claim to apixaban.
- For the reasons given above I would dismiss this appeal. It follows that it is unnecessary to consider Teva's contention, raised by a respondent's notice, that claim 1 is too broad even if the Application makes it plausible that apixaban is an effective factor Xa inhibitor, and is therefore likely to be useful for treating thromboembolic disorders, because claim 1 claims all uses of apixaban.
- I agree.
- I also agree.
Lord Justice Arnold:
Introduction
The law
Case law of the Boards of Appeal prior to G 2/21
"2.4.2 … it has for long been a generally accepted legal principle that the extent of the patent monopoly should correspond to and be justified by the technical contribution to the art …. Now, whereas in both the above decisions this general legal principle was applied in relation to the extent of the patent protection that was justified by reference to the requirements of Articles 83 and 84 EPC, the same legal principle also governs the decision that is required to be made under Article 56 EPC, for everything falling within a valid claim has to be inventive. If this is not the case, the claim must be amended so as to exclude the obvious subject-matter in order to justify the monopoly."
"… if the claimed compounds were to be assumed not to have any technically useful property, then it could be postulated that the technical problem which is solved by the claimed compounds (or, in other words, the technical result achieved by them, on the basis of which the question of inventive step has to be decided), would be the minimalist one in such a situation, namely the mere provision of further (or alternative) chemical compounds as such, regardless of their likely useful properties."
"2.5.3 … The answer to the question as to what a person skilled in the art would have done depends on the result he wished to obtain, as explained in point 2.4.2 above. If this result is only to be seen in obtaining further chemical compounds, then all known chemical compounds are equally suitable as the starting point for structural modification, and no inventive skill needs to be exercised in selecting, for instance, the compound of formula XIV of [prior art citation] D3 for this purpose. Consequently, all structurally similar chemical compounds, irrespective of their number, that a skilled person would expect, in the light of the cited prior art, to be capable of being synthesised, are equally suitable candidates for solving such a hypothetical 'technical problem' to the skilled person, and would therefore all be equally 'suggested' to the skilled person. It follows from these considerations that a mere arbitrary choice from this host of possible solutions of such a 'technical problem' cannot involve an inventive step ... In other words, the Board holds that, in view of the underlying general legal principle set out in point 2.4.2 above, the selection of such compounds, in order to be patentable, must not be arbitrary but must be justified by a hitherto unknown technical effect which is caused by those structural features which distinguish the claimed compounds from the numerous other compounds. …
2.5.4 It follows directly from these considerations that a technical effect which justifies the selection of the claimed compounds must be one which can be fairly assumed to be produced by substantially all the selected compounds. …"
"… the Board holds that, contrary to the appellant's submission, the assessment of the technical contribution to the art must take account of the actual technical reason for providing the very compounds now being claimed, as distinct from the host of other theoretically possible modified chemical compounds. In this respect, the description … asserts that all claimed compounds do have herbicidal activity. Herbicidally active chemical compounds which are structurally similar to the claimed ones, since they are also triazole derivatives, are known from D3, D7 and D8 …. Any one of these documents may therefore serve as the 'closest state of the art' in the present case.
In view of this state of the art the technical problem which the present patent application asserts to solve is the provision of further (alternative) chemical compounds with herbicidal activity.
However, in the light of the Board's finding in point 2.4.3 above, this technical problem could only be taken into account if it could be accepted as having been solved, that is, if, in deciding the issue under Article 56 EPC, it would be credible that substantially all claimed compounds possessed this activity (see also point 2.5.4 above). Accordingly, the Board has examined whether this requirement is fulfilled."
"2.6.2 In the present case, the appellant's submission that the test results contained in the description show that some of the claimed compounds are indeed herbicidally active cannot be regarded as sufficient evidence to lead to the inference that substantially all the claimed compounds possess this activity. The reason for this is that there is no proven common general knowledge to show that the type of substituent that may be present in the claimed compounds would be irrelevant to the existence of the alleged herbicidal activity. On the contrary, the Board accepts the appellant's own submission that the structural differences between the compounds disclosed, for example, in D3, D7 and D8 on the one hand, and the claimed compounds on the other hand, are such that a person skilled in the art would have been unable to predict on the basis of his common general knowledge that the claimed compounds would have herbicidal activity …., and that it can therefore be accepted as undisputed common general knowledge that even small structural modifications may cause major differences in biological activity. Nevertheless, it is also well accepted that the properties of chemical compounds do indeed largely depend on their chemical structure, and that a skilled person would therefore normally expect that the properties of two compounds would become the more similar the more similar their chemical structures became …. In view of all the above considerations, the Board finds that reasonable predictions of relations between chemical structure and biological activity are in principle possible, but that there is a limit beyond which no such prediction can be validly made.
…
2.6.5 In the tests which are reported on pages 37 to 40 of the description, a great number of compounds was used. However, in all these compounds R1 was always either unsubstituted phenyl or 2-pyrimidinyl optionally substituted by methyl groups and R3 was always phenyl substituted by halogen atoms or methyl groups. Thus, despite the number of tested compounds, these test results do not support the alleged herbicidal activity of compounds in which, for example, the phenyl ring in position R3 may be substituted by absolutely anything, having regard to the common general knowledge relied on by the appellant himself, namely that the influence of structural modifications on the desired herbicidal activity is unpredictable.
2.6.6 Such an allegation is likewise not supported by the content of documents D3, D7 and D8, which all disclose classes of herbicidally active compounds with limited substitution possibilities ….
2.6.7 The appellant had been informed about the insufficiency of the evidence submitted by him in the present case, and had also been given ample opportunity either to restrict his claims to such a group of compounds for which the Board was prepared to accept the credibility of their alleged herbicidal activity …, or to provide further evidence, either by test results or by other means, that in the present case the kind of substitution of the phenyl ring R3 is not relevant to the herbicidal activity. Despite these clear and helpful leads, which the Board was not obliged to afford, neither appropriate amendments nor further evidence were forthcoming.
2.7 For these reasons, and on the basis of what evidence there is in the case, the Board is not satisfied that substantially all compounds now being claimed are likely to be herbicidally active. Since, as set out above in points 2.4.2, 2.5.4 and 2.6, only those of the claimed chemical compounds could possibly involve an inventive step which could be accepted as solutions of the technical problem of providing further herbicidally active compounds, the subject-matter of the main request extends to compounds which are not inventive and therefore does not meet the requirement of Article 56 EPC."
"8. … Sufficiency of disclosure must be satisfied at the effective date of the patent, ie on the basis of the information in the patent application together with the common general knowledge then available to the skilled person. Acknowledging sufficiency of disclosure on the basis of relevant technical information produced only after this date would lead to granting a patent for a technical teaching which was achieved, and, thus, for an invention which was made, at a date later than the effective date of the patent. The general principle that the extent of monopoly conferred by a patent should correspond to, and be justified by, the technical contribution to the art, has to be kept in mind ….
9. Where a therapeutic application is claimed … in the form of the use of a substance or composition for the manufacture of a medicament for a defined therapeutic application, attaining the claimed therapeutic effect is a functional technical feature of the claim …. As a consequence, under article 83 EPC, unless this is already known to the skilled person at the priority date, the application must disclose the suitability of the product to be manufactured for the claimed therapeutic application."
"The patent system takes account of the intrinsic difficulties for a compound to be officially certified as a drug by not requiring an absolute proof that the compound is approved as a drug before it may be claimed as such. The Boards of Appeal have accepted that for a sufficient disclosure of a therapeutic application, it is not always necessary that results of applying the claimed composition in clinical trials, or at least to animals are reported. Yet, this does not mean that a simple verbal statement in a patent specification that compound X may be used to treat disease Y is enough to ensure sufficiency of disclosure in relation to a claim to a pharmaceutical. It is required that the patent provides some information in the form of, for example, experimental tests, to the avail that the claimed compound has a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se. Showing a pharmaceutical effect in vitro may be sufficient if for the skilled person this observed effect directly and unambiguously reflects such a therapeutic application … or … if there is a 'clear and accepted established relationship' between the shown physiological activities and the disease …."
"… in vitro tests cannot be performed unless the 'protagonists' of the test are available. This means that the skilled person is made aware of the structure of the active ingredient proposed for the pharmaceutical composition as well as, in technical terms, of a definite link between the ingredient and the mechanism allegedly involved in the disease state. The presence of a cause/effect relationship is, thus, made plausible. For how[ever] incomplete the data might be, they nonetheless go one step further towards disclosing the invention without leaving an undue burden to the reader."
"8. … members of the TGF-ß superfamily share sequence homology. In the part of the application as filed describing the prior art related to the invention …, it is disclosed that subgroups in the family had been defined according to the percentage of homology between members, the members of a given subgroup being from 70% to 90% homologous. Here, GDF-9 is very far from fulfilling this criteria [sic] as its sequence is stated to be significantly divergent from those of other family members …, the maximal percentage of homology which was observed being 34% with the bone morphogenetic protein, BMP-4. This implies that GDF-9 cannot be attributed to any subgroup and, thus, must at best be considered as the first member of a yet unidentified subgroup. This finding and that in point 7 lead to the conclusion that, contrary to GDF-1 in document (3), GDF-9 cannot be clearly and unambiguously identified as a member of the TGF-ß superfamily by only using a 'structural approach'.
9. Of course, the situation could most probably be looked at differently if it had been demonstrated in the application as filed that GDF-9 played a role similar to that of the transforming factor-Beta (as was the case for all of the factors which initially served to define the superfamily). Yet, there is no evidence at all in this respect. In fact, the application only discloses that expression of GDF-9 is localised in ovarian tissues, which per se is useful but insufficient information in relation to any function the molecule might have.
10. As already pointed out above (cf. point 8), in the application …, it is admitted that '..., the sequence of GDF-9 is significantly diverged from those of other family members'. Yet, functions of members of the TGF-ß superfamily previously isolated from ovarian follicular fluid (inhibins) or shown to inhibit ovarian cancer (MIS) are recited, and tentatively and presumptively attributed to GDF-9. Further putative roles are also suggested for GDF-9 which cover some of the effects observed with TGF-ß …. At oral proceedings, it was argued that speculations of this kind should be permitted because of the 'first to file approach' of the European patent system which forced the applicant to cover any and all subject-matter connected with its invention. The board is unable to endorse this reasoning. On the contrary, in a first-to-file system the (earlier) filing date of the application, not the date at which the invention was made determines to whom of several persons having made an invention independently of each other, the right to a European patent belongs …. Hence, it is particularly important in such a system that the application allows to conclude that the invention had been made, i.e. that a problem had indeed been solved, not merely put forward at the filing date of the application. Therefore, the issue here is rather how much weight can be given to speculations in the application in the framework of assessing inventive step, which assessment requires that facts be established before starting the relevant reasoning. In the board's judgment, enumerating any and all putative functions of a given compound is not the same as providing technical evidence as regard a specific one.
11. Accordingly, as a significant structural feature fails to be identical in TGF-9 and the members of the TGF-ß superfamily, and no functional characterisation of TGF-9 is forthcoming in the application, it is concluded that the application does not sufficiently identify this factor as a member of this family i.e. that there is not enough evidence in the application to make at least plausible that a solution was found to the problem which was purportedly solved."
"This cannot be regarded as supportive of an evidence [sic] which would have been given in the application as filed since there was not any. The said post-published documents are indeed the first disclosures going beyond speculation. For this reason, the post-published evidence may not be considered at all. Indeed, to do otherwise would imply that the recognition of a claimed subject-matter as a solution to a particular problem could vary as time went by. Here, for example, had the issue been examined before the publication date of the earliest relevant post-published document, GDF-9 would not have been seen as a plausible solution to the problem of finding a new member of the TGF-ß superfamily and inventive step would have had to be denied whereas, when examined thereafter, GDF-9 would have to be acknowledged as one such member. This approach would be in contradiction with the principle that inventive step, as all other criteria for patentability, must be ascertained as from the effective date of the patent. The definition of an invention as being a contribution to the art, i.e. as solving a technical problem and not merely putting forward one, requires that it is at least made plausible by the disclosure in the application that its teaching solves indeed the problem it purports to solve. Therefore, even if supplementary post-published evidence may in the proper circumstances also be taken into consideration, it may not serve as the sole basis to establish that the application solves indeed the problem it purports to solve."
"12. The examining division based its negative decision on the fact that neither the application as filed nor post-published documents 'illustrated' the use of somatostatin by way of experimental data showing the claimed effect. In relation to the latter, the examining division considered that other tests were needed which the applicant had not been able to carry out. The board notes that neither in its decision nor during the prosecution of the application has the examining division produced arguments which could discredit the plausibility of the claimed invention. Also the board sees no reasons to doubt the usefulness of somatostatin to attain the claimed effect.
13. The board notes that the EPC requires no experimental proof for patentability and considers that the disclosure of experimental data or results in the application as filed and/or post-published evidence is not always required to establish that the claimed subject-matter solves the objective technical problem. This is in particular true in the absence of any formulated substantiated doubt as is the case here.
14. The boards of appeal have indeed dealt with cases where, in the context of the assessment of inventive step, there could only be an invention if the application made it at least plausible that its teaching did indeed solve the problem it purported to solve and in which to establish plausibility the disclosure of experimental results in a patent application, or, under certain circumstances, by post-published evidence, was considered necessary ….
15. The board re-emphasises in this context however that this case law considers the establishment of plausibility only relevant when examining inventive step if the case at hand allows the substantiation of doubts about the suitability of the claimed invention to solve the technical problem addressed and when it is thus far from straightforward that the claimed invention solves the formulated problem. This is all the more clear from decisions where an inventive step was in fact denied because the formulated problem was not considered to have been solved. …"
" 4.2 It is established jurisprudence of the boards of appeal that the assessment of inventive step is to be made at the effective date of the patent on the basis of the information in the patent together with the common general knowledge then available to the skilled person. Post-published evidence in support that the claimed subject-matter solves the technical problem the patent in suit purports to solve may be taken into consideration, if it is already plausible from the disclosure of the patent that the problem is indeed solved ….
Thus, for post-published evidence to be taken into account, it is necessary to establish whether or not the asserted activity has been made sufficiently plausible for dasatinib at the effective date of the patent in suit. Basis for this assessment is the application as filed and the common general knowledge of the person skilled in the art at the filing date.
4.3 The application is directed to an extremely broadly defined group of compounds of the following generic formula I … The application also discloses 580 compounds falling within the scope of general formula I, including dasatinib (see Example 455).
…
4.5 On p.50, ln.4 to p.53, ln.18, the application refers to assays 'which can be employed in ascertaining the degree of activity of a compound ("test compound") as PTK inhibitor' (see p.49, lnn.29–30). The assays are generically described and refer to the 'protein kinase of interest' and the 'test compound' or 'compounds of interest' to be assayed. No further details are provided in this respect. Nor are any results, for example IC or Ki values, provided. Indeed, there is no evidence at all in the application as filed that shows that any of the compounds falling within the scope of Formula I, let alone dasatinib, is active as an inhibitor for any of the specific protein tyrosine kinases, except a mere assertion on p.50, lnn.1–2 with reads that 'Compounds described in the following Examples have been tested in one or more of these assays and have shown activity.' No further information is provided. No individual values or range of values are given. No information as to whether the observed 'activity' is suitable for the intended use, i. e. the treatment of a number of diseases and disorders, is provided. In the board's judgement, a mere verbal statement that 'compounds have been found active' in the absence of any verifiable technical evidence is not sufficient to render it credible that the technical problem the application purports to solve, namely providing PTK inhibitors to treat disorders or diseases associated therewith, is indeed solved, in particular in the present case, where the invention is directed to a very broadly defined class of compounds encompassing millions of structurally rather different candidates with unknown properties, where even the examples show a broad structural variation and where it is inherently unlikely for any skilled person that all of the compounds of the invention or at least a substantial amount of them will exhibit the alleged PTK inhibitory activity.
In the present case, there is also no evidence on file showing that, at the date of filing, the skilled person was in the possession of common general knowledge which, even in the absence of data, made it plausible that the compounds of the invention, in particular dasatinib, could be expected to show PTK inhibitory activity. …
…
4.6.2 … In the board's opinion, the skilled reader can be expected to react in a way common to all persons skilled in the art, which means that any acceptance as to whether or not a particular assertion is correct must be based on verifiable facts, be it information provided in the patent application or available to the skilled person as common general knowledge. In the present case, no such verifiable facts exist. The situation is further aggravated taking into account that, contrary to the appellant's view, the skilled person is not in a position to readily verify the assertion on page 50 in the absence of any detailed information as to the conditions under which the assays are to be carried out. …
…
4.8 The appellant also argued that the EPC does not require experimental proof. A summary statement as provided on p.50, lnn.1–2 was sufficient to meet the low plausibility threshold, which was satisfied in the absence of any substantiated doubts. No absolute proof was required and there was no legal basis to provide any raw data. As the threshold test had been met, the post-published evidence which merely confirmed the PTK inhibitory activity of dasatinib should be taken into account.
4.9 The board agrees with the appellant insofar as it is not always required to include experimental data or results in an application …. It is however a conditio sine qua non that it is shown that the technical problem underlying the invention was at least plausibly solved at the filing date. If, as in the present case, the nature of the invention is such that it relies on a technical effect, which is neither self-evident nor predictable or based on a conclusive theoretical concept, at least some technical evidence is required to show that a technical problem has indeed been solved. In the board's judgement, it is not acceptable to draw up a generic formula, which covers millions of compounds, vaguely indicate an 'activity' against PTKs and leave it to the imagination of the skilled reader or to future investigations to establish which compound inhibits which kinase and is therefore suitable to treat the respective diseases associated therewith. In this context, the board notes that it has been acknowledged by the appellant that the skilled person would not expect that each compound would be active against all kinases. The board would also like to emphasise that in the present case the issue is not the absence of any in vivo data or clinical data, but rather the absence of any verifiable data with regard to the asserted technical effect."
"The patent in suit does not contain any evidence that the problem as formulated by the appellant has been successfully solved. There is no evidence at all that any compound of the examples, let alone dasatinib, had been tested for Src inhibitory activity and is thus useful for the treatment associated therewith, in particular cancer. Furthermore, the post-published Document (9), on which the appellant relied as evidence for the PTK inhibitory activity of dasatinib, cannot be taken into consideration, for the reasons set out in detail in Point 4 above. … The board therefore concurs with the Opposition Division and the respondents that the effect on which the appellant relied (i. e. any PTK inhibitory activity) cannot be taken into account in formulating the technical problem."
"5.6 It follows from the above that the problem to be solved has to be defined in a less ambitious way, namely as the provision of a further chemical compound.
5.7 According to the jurisprudence of the boards of appeal, a chemical compound is not patentable merely because it potentially enriches chemistry and [has] structural [uniqueness], since originality has no intrinsic value or significance for the assessment of inventive step as long as it does not manifest itself in a valuable property in the widest sense, an effect or an increase in the potency of an effect …. In other words, the mere provision of a chemical compound capable of being synthesised, which was not contested, and not showing any effect does not require inventive ingenuity. The structural uniqueness of dasatinib alone cannot therefore support an inventive step.
5.8 The appellant's additional arguments in favour of an inventive step were focused on the PTK inhibitory activity of dasatinib …. They are, however, not pertinent in a situation where this effect could not be acknowledged and the problem to be solved was merely the provision of [a] further chemical compound."
UK case law
"28. The question was whether [the fact that a taxol-coated stent would prevent or treat restenosis] was obvious and not whether it was obvious that taxol (among many other products) might have this effect. It is hard to see how the notion that something is worth trying or might have some effect can be described as an invention in respect of which anyone would be entitled to a monopoly. …
29. It is true that a patent will not be granted for an idea which is mere speculation, unsupported by anything disclosed in the specification. …
31. … There is also a line of authority in the EPO in which claims to broad classes of chemical compounds alleged to have some common technical effect have been rejected under Art.56 (obviousness) when there was nothing to show that they would all have that technical effect. …"
"36. These cases are in my opinion far from the facts of this case. The specification did claim that a taxol coated stent would prevent restenosis and Conor did not suggest that this claim was not plausible. That would have been inconsistent with the evidence of its experts that taxol was just the thing to try. It is therefore not surprising that implausibility was neither pleaded nor argued. ….
37. The Court of Appeal upheld the judgment of Pumfrey J. on the ground that the patent contained no 'disclosure' saying that taxol was specially suitable for preventing restenosis. Again, I agree that the description, though offering a theory (its anti-angiogenic properties) as to why taxol would prevent restenosis, did not offer any evidence that this would turn out to be true. If it had not turned out to be true, the patent would have been insufficient. But there is in my opinion no reason as a matter of principle why, if a specification passes the threshold test of disclosing enough to make the invention plausible, the question of obviousness should be subject to a different test according to the amount of evidence which the patentee presents to justify a conclusion that his patent will work."
"As with any consideration of obviousness, the technical results or effects must be shared by everything falling within the claim under attack. This follows from the fundamental principle of patent law, which underpins many of the grounds of objection to validity, that the extent of the monopoly conferred by a patent must be justified by the technical contribution to the art. If some of the products covered by a claim demonstrate a particular property, but others do not, then the technical problem cannot be formulated by reference to that property. Either the products which do not exhibit the property must be excised from the claim by amendment, or the problem must be formulated by reference to some other, perhaps more mundane, technical contribution common to the whole claim."
"47. In Dr Reddy's Laboratories (UK) Ltd v Eli Lilly & Co Ltd [2009] EWCA Civ 1362, [2010] R.P.C. 222, the Court of Appeal was concerned with the rules which apply where a patent is sought for a compound or class of compounds which are a selection from a broader class disclosed by a prior document. Jacob LJ summarised the approach of the EPO to that question at [50] as being:
'Has the patentee made a novel non-obvious technical advance and provided sufficient justification for it to be credible? This is the basis of all the reasoning—see eg [2.4.2] of AgrEvo. A 'selection' which makes a real technical advance in the art is patentable."
48. Later he explained the basis of the rule against 'arbitrary' selection as being found in the guiding principle 'is there a real technical advance?'
49. I would summarise the position thus far in the following way:
(i) Article 56 of the EPC is in part based on the underlying principle that the scope of the patent monopoly must be justified by the patentee's contribution to the art;
(ii) If the alleged contribution is a technical effect which is not common to substantially everything covered by a claim, it cannot be used to formulate the question for the purposes of judging obviousness;
(iii) In such circumstances the claim must either be restricted to the subject matter which makes good the technical contribution, or a different technical solution common to the whole claim must be found;
(iv) A selection from the prior art which is purely arbitrary and cannot be justified by some useful technical property is likely to be held to be obvious because it does not make a real technical advance;
(v) A technical effect which is not rendered plausible by the patent specification may not be taken into account in assessing inventive step;
(vi) Later evidence may be adduced to support a technical effect made plausible by the specification;
(vii) Provided the technical effect is made plausible, no further proof of the existence of the effect is to be demanded of the specification before judging obviousness by reference to the technical effect propounded."
"In my judgment the same approach should be adopted in considering obviousness and whether a technical effect is plausible in the light of the teaching in the specification and the common general knowledge. There must be a real reason for supposing that the claimed invention will indeed have the promised technical effect."
"Elementary as it is, it is worth reminding oneself at the outset of the juridical basis on which patents are granted, sometimes called the 'patent bargain'. The inventor obtains a monopoly in return for disclosing the invention and dedicating it to the public for use after the monopoly has expired. The point was succinctly made by Lord Mansfield in Liardet v Johnson (Morning Post, 23 February 1778, No 1667, p2, col 4), quoted in Hulme, 'On the History of Patent Law' (1902) 18 LQR 280, 285:
'The condition of giving encouragement is this: that you must specify upon record your invention in such a way as shall teach an artist, when your term is out, to make it—and to make it as well by your directions: for then at the end of the term, the public shall have benefit of it. The inventor has the benefit during the term, and the public have the benefit after …'
The principle remains the foundation of modern patent law, and is recognised in the case law of both the United Kingdom and the European Patent Office. In Exxon/Fuel Oils (T-409/91) [1994] OJ EPO 653, paras 3.3 and 3.4, the EPO Technical Board of Appeal observed that it was
'the general legal principle that the extent of the patent monopoly, as defined by the claims should correspond to the technical contribution to the article in order for it to be supported, or justified. … This means that the definitions in the claims should essentially correspond to the scope of the invention as disclosed in the description. … Although the requirements of articles 83 and 84 are directed to different parts of the patent application, since article 83 relates to the disclosure of the invention, whilst article 84 deals with the definition of the invention by the claims, the underlying purpose of the requirement of support by the description, in so far as its substantive aspect is concerned, and of the requirement of sufficient disclosure is the same, namely to ensure that the patent monopoly should be justified by the actual technical contribution to the art'.
The principal conditions of validity, novelty, inventive step, industrial application and sufficiency are all, in one way or another, directed to satisfying the principle thus expressed."
"The Court of Appeal's reference to 'armchair inventors' suggests that what they meant by speculative claiming was claiming by persons who had done nothing new or inventive at all but had simply sought to patent abstract possibilities. That may well be a particular risk in the case of patents for new uses of known compounds, especially when they are commercially successful in their existing use. In reality, however, speculative claiming of this kind is simply one of a number of ways in which a patentee may attempt to claim a monopoly more extensive than anything which is justified by his contribution to the art. Other ways in which this can happen include claiming a monopoly wider than the disclosure in the patent can support. An over-broad claim will not necessarily be speculative. The inventor may really have invented something corresponding to the full breadth of the claim. Research may subsequently demonstrate this. But the claim will still exceed his contribution to the art if that contribution is not sufficiently disclosed in the patent."
"The Technical Board of Appeal treats the condition of sufficiency under EPC article 83 as satisfied if it is possible to work the invention across the scope of the claim from the information in the specification, interpreted in the light of common general knowledge at the priority date. It addresses the broader question whether the disclosed contribution to the art is commensurate with the monopoly claimed under EPC article 56 , in the context of inventive step. In that context, its case law requires the formulation of a problem which the claims of the patent could be said to solve: see Agrevo/Triazole sulphonamides (T-939/92) [1996] EPOR 171. It imports a requirement that the patent should disclose not just what the invention is and how to replicate it, but some reason for expecting that it will work. Plausibility was the standard to which the patentee was expected to demonstrate this."
"All of these judgments deal with highly fact-specific issues arising from objections or potential objections on the ground of insufficiency. When reading them, it is important not to miss the wood for the trees. The fundamental principle which they illustrate is that the patentee cannot claim a monopoly of a new use for an existing compound unless he not only makes but discloses a contribution to the art. None of them casts doubt on the proposition that the disclosure in the patent must demonstrate in the light of the common general knowledge at the priority date that the claimed therapeutic effect is plausible. On the contrary, they affirm it … "
"The principle is that the specification must disclose some reason for supposing that the implied assertion of efficacy in the claim is true. Plausibility is not a distinct condition of validity with a life of its own, but a standard against which that must be demonstrated. Its adoption is a mitigation of the principle in favour of patentability. It reflects the practical difficulty of demonstrating therapeutic efficacy to any higher standard at the stage when the patent application must in practice be made. The test is relatively undemanding.But it cannot be deprived of all meaning or reduced … to little more than a test of good faith."
"Plausibility is not a term of art, and its content is inevitably influenced by the legal context. In the present context, the following points should be made.
First, the proposition that a product is efficacious for the treatment of a given condition must be plausible.
Second, it is not made plausible by a bare assertion to that effect, and the disclosure of a mere possibility that it will work is no better than a bare assertion. ….
But, third, the claimed therapeutic effect may well be rendered plausible by a specification showing that something was worth trying for a reason, ie not just because there was an abstract possibility that it would work but because reasonable scientific grounds were disclosed for expecting that it might well work. The disclosure of those grounds marks the difference between a speculation and a contribution to the art. This is in substance what the Technical Board of Appeal has held in the context of article 56, when addressing the sufficiency of disclosure made in support of claims extending beyond the teaching of the patent. In my opinion, there is no reason to apply a lower standard of plausibility when the sufficiency of disclosure arises in the context of EPC articles 83 and 84 and their analogues in section 14 of the Patents Act. In both contexts, the test has the same purpose.
Fourth, although the disclosure need not definitively prove the assertion that the product works for the designated purpose, there must be something that would cause the skilled person to think that there was a reasonable prospect that the assertion would prove to be true.
Fifth, that reasonable prospect must be based on what the TBA in Salk (para 9) called 'a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se.'
Sixth, in Salk, this point was made in the context of experimental data. But the effect on the disease process need not necessarily be demonstrated by experimental data. It can be demonstrated by a priori reasoning. For example, and it is no more than an example, the specification may point to some property of the product which would lead the skilled person to expect that it might well produce the claimed therapeutic effect; or to some unifying principle that relates the product or the proposed use to something else which would suggest as much to the skilled person.
Seventh, sufficiency is a characteristic of the disclosure, and these matters must appear from the patent. The disclosure may be supplemented or explained by the common general knowledge of the skilled person. But it is not enough that the patentee can prove that the product can reasonably be expected to work in the designated use, if the skilled person would not derive this from the teaching of the patent."
"This submission also is contrary to the legal basis of this particular head of insufficiency. We know that pregabalin works for the treatment of both peripheral and central neuropathic pain, because like any other medicament on the market, it underwent demanding clinical trials after the priority date, the results of which were made public. On that basis it received marketing authorisation for all neuropathic pain. This is always the case for a commercially valuable medicament, and no other kind will be worth litigating about. The question is not whether it works but whether the contribution to the art consisting in the discovery that it can be expected to work has been sufficiently disclosed in the patent. The inherent difficulty of demonstrating this before clinical trials is taken into account in the modest standard (ie plausibility) which is applied to test it. … This does not mean that subsequent data is never admissible in a dispute about sufficiency, but the purpose for which it is admitted is strictly limited. Where the asserted therapeutic effect is plausible in the light of the disclosure in the patent, subsequent data may sometimes be admissible either to confirm that or else to refute a challenger's contention that it does not actually work… But it cannot be a substitute for sufficient disclosure in the specification."
"In classical insufficiency cases, where the question is whether the disclosure in the patent enables the skilled person to perform the invention, the skilled person may be assumed to supplement the disclosure by carrying out simple tests. In cases like this one, where the invention is novel but the objection of insufficiency is that the claim exceeds the disclosed contribution to the art, the role of hypothetical 'simple tests' is necessarily more limited. As the … Board of Appeal observed in Johns Hopkins … para 12, the specification can be said to contribute to the art if it solves a problem, but not if it merely poses one. Or as Lord Hoffmann observed in [Conor v Angiotech], the notion that something is 'worth trying' cannot be enough without more to justify a monopoly. The specification in the present case says nothing about neuropathic pain of any kind. It says nothing about central sensitisation, which is said to provide a link between neuropathic and inflammatory pain. The mere fact that the skilled team, faced with an apparent discrepancy between the breadth of the claims and the absence of supporting data in the specification, would be encouraged to fill the gap by carrying out tests of its own, serves only to confirm the absence of any disclosed contribution to the art."
"… I consider that it puts the test too high to suggest that 'the specification must disclose some reason for supposing that the implied assertion of efficacy in the claim is true' (Lord Sumption JSC's judgment, para 36). That amounts on its face to, or certainly risks being read as, a requirement that the plausibility of the claim must appear to be established prima facie through scientifically cogent reasoning or experimental evidence set out in the specification. Admittedly, Lord Sumption JSC goes on in para 36 to suggest that the test is 'relatively undemanding'. But he continues in para 37 to say that it is sufficient if the specification 'would cause the skilled person to think that there was a reasonable prospect that the assertion would prove to be true', and then that '[the] reasonable prospect must be based on what the [Board of Appeal] in Salk (T-609/02), at para 9, called "a direct effect on a metabolic mechanism specifically involved in the disease, this mechanism being either known from the prior art or demonstrated in the patent per se?"'. It also explains that, in so far as no experimental data is produced, it can be, per Lord Sumption JSC, at para 37:
'demonstrated by a priori reasoning. For example, …, the specification may point to some property of the product which would lead the skilled person to expect that it might well produce the claimed therapeutic effect; or to some unifying principle that relates the product or the proposed use to something else which would suggest as much to the skilled person.'
Despite the use of phrases such as 'reasonable prospect' and 'might well produce', there is a real risk that the test as described by Lord Sumption JSC would amount to, or be understood as, involving a requirement to establish a prima facie case on the material contained in the specification. In my opinion, the authorities analysed above do not put the standard so high. They certainly reject speculative or wide-ranging unsubstantiated claims. But they accept as sufficient a tailored claim which appears scientifically possible, even though it cannot be said to be even prima facie established, without for example testing or assays according to the state of the art. Only if a person skilled in the art would have significant doubts about the workability of the invention would it, in such a case, fail for insufficiency of disclosure."
G 2/21
"70. The Enlarged Board takes note of the classification done by the referring board in respect of the case law of the boards of appeal concerning the relevance of post-published evidence to prove an asserted technical effect for acknowledgement of inventive step ...
71. However, when analysing the case law in more detail and irrespective of the conceptual terminologies for what questions 2 and 3 refer to as two distinct plausibility approaches, the Enlarged Board understands from the case law of the boards of appeal as common ground that the core issue rests with the question of what the skilled person, with the common general knowledge in mind, understands at the filing date from the application as originally filed as the technical teaching of the claimed invention.
72. Applying this understanding to the aforementioned decisions, not in reviewing them but in an attempt to test the Enlarged Board's understanding, the Enlarged Board is satisfied that the outcome in each particular case would not have been different from the actual finding of the respective board of appeal. Irrespective of the use of the terminological notion of plausibility, the cited decisions appear to show that the particular board of appeal focussed on the question whether or not the technical effect relied upon by the patent applicant or proprietor was derivable for the person skilled in the art from the technical teaching of the application documents."
"The reasoned findings of the boards of appeal in the decisions referred to above make clear that the scope of reliance on post published evidence is much narrower under sufficiency of disclosure (Article 83 EPC) compared to the situation under inventive step (Article 56 EPC). In order to meet the requirement that the disclosure of the invention be sufficiently clear and complete for it to be carried out by the person skilled in the art, the proof of a claimed therapeutic effect has to be provided in the application as filed, in particular if, in the absence of experimental data in the application as filed, it would not be credible to the skilled person that the therapeutic effect is achieved. A lack in this respect cannot be remedied by post-published evidence."
"86. Like the EPC, none of the legal systems of the EPC Contracting States provide for an explicit patentability requirement for what the referring decision discusses and addresses with what is referred to in questions 2 and 3 under the term 'plausibility'.
87. Notwithstanding the fact that the aforementioned decisions were taken on the decisive facts of the case in hand and the particular submissions made by the parties to those proceedings, the Enlarged Board recognises a certain degree of common ground that the courts of the EPC Contracting States, when confronted with the examination of an asserted technical effect in the assessment of inventive step and with the question whether a patent proprietor may rely on post-published evidence to confirm that technical effect, ponder on the technical teaching of the claimed subject-matter that the person skilled in the art, with the common general knowledge in mind, understands from the patent application."
"92. The term 'plausibility' that is found in the case law of the boards of appeal and relied upon by the referring board in questions 2 and 3 of the referral and the reasons for it, does not amount to a distinctive legal concept or a specific patent law requirement under the EPC, in particular under Article 56 and 83 EPC. It rather describes a generic catchword seized in the jurisprudence of the boards of appeal, by some national courts and by users of the European patent system.
93. The relevant standard for the reliance on a purported technical effect when assessing whether or not the claimed subject-matter involves an inventive step concerns the question of what the skilled person, with the common general knowledge in mind, would understand at the filing date from the application as originally filed as the technical teaching of the claimed invention. The technical effect relied upon, even at a later stage, needs to be encompassed by that technical teaching and to embody the same invention, because such an effect does not change the nature of the claimed invention.
94. Hence, a patent applicant or proprietor may rely upon a technical effect for inventive step if the skilled person, having the common general knowledge in mind, and based on the application as originally filed, would consider said effect as being encompassed by the technical teaching and embodied by the same originally disclosed invention.
95. The Enlarged Board is aware of the abstractness of some of the aforementioned criteria. However, apart from the fact that the Enlarged Board, in its function assigned to it under Article 112(1) EPC, is not called to decide on a specific case, it is the pertinent circumstances of each case which provide the basis on which a board of appeal or other deciding body is required to judge, and the actual outcome may well to some extent be influenced by the technical field of the claimed invention. Irrespective of the actual circumstances of a particular case, the guiding principles set out above should allow the competent board of appeal or other deciding body to take a decision on whether or not post-published evidence may or may not be relied upon in support of an asserted technical effect when assessing whether or not the claimed subject-matter involves an inventive step."
The skilled team
Common general knowledge
WO 131
"Using the methodology described above, a number of compounds of the present invention were found to exhibit a Ki of <10 µM, thereby confirming the utility of the compounds of the present invention as effective Xa inhibitors.
Compounds tested in the above assay are considered to be active if they exhibit a Ki of <10 µM. Preferred compounds of the present invention have Ki's of <1 µM. More preferred compounds of the present invention have Ki's of <0.1 µM. Even more preferred compounds of the present invention have Ki's of <0.01µM. Still more preferred compounds of the present invention have Ki's of <0.001 µM."
i) apixaban is embraced within the first, second, third, fourth, eighth and ninth embodiments of WO 131;
ii) there is no individualised disclosure in WO 131 of apixaban;
iii) nor does WO 131 make it obvious that apixaban would be likely to be efficacious as a factor Xa inhibitor.
The Application
"Compounds tested in the above assay are considered to be active if they exhibit a Ki of <10 µM. Preferred compounds of the present invention have Ki's of <1 µM. More preferred compounds of the present invention have Ki's of <0.1 µM. Even more preferred compounds of the present invention have Ki's of <0.01µM. Still more preferred compounds of the present invention have Ki's of <0.001 µM. Using the methodology described above, a number of compounds of the present invention were found to exhibit a Ki of <10 µM, thereby confirming the utility of the compounds of the present invention as effective Xa inhibitors."
The claims of the Patent
"A compound, which is represented by formula (1):
or a pharmaceutically acceptable salt thereof."
The compound depicted in the formula is apixaban. Thus, whereas the narrowest claim in the Application was claim 8 covering 74 compounds, claim 1 of the Patent has been restricted to just apixaban.
"A compound of claim 1 or 2 for use in treating a thromboembolic disorder."
The reference to claim 2 can be ignored.
BMS's case on plausibility
The judge's judgment
"37. Sensing that BMS might be arguing that Warner-Lambert is confined to second medical use claims, Counsel for the Claimants pointed out in closing that while that was the context of the case, Lord Sumption's analysis of plausibility was not limited to it, and that a number of key cases that he considered (Agrevo at [23], Johns Hopkins at [24], and BMS/Dasatinib also at [24]) were not about second medical use patents. I agree with this but I do not think that BMS took such a stance in the end, in any event.
38. For its part, BMS stressed in opening the relatively low standard for plausibility identified by Lord Sumption at [37], third point: 'not just … an abstract possibility that it would work but because reasonable scientific grounds were disclosed for expecting that it might well work'.
39. In closing, BMS sharpened its argument and developed a more detailed analysis of Warner-Lambert in connection with the significance of a patent specification not containing efficacy/activity data.
40. The first part of this contention was that there is no requirement as such that a patent must contain efficacy data because plausibility can be established by a theory, in particular a theory based on the structure of a compound (or class of compounds). I agree with this, and in itself I do not think the Claimants disputed it. When I come to the facts I will therefore have to assess whether there is a theoretical basis for the plausibility of apixaban arising from structure.
41. The second part of the contention was that Lord Sumption had left open the possibility that tests not done by the patentee but which might be done by the reader of a specification, could be relevant to plausibility. This submission turned on Lord Sumption's statement in [53] that in the sort of case where insufficiency is said to arise from exceeding the technical contribution, the 'role of hypothetical "simple tests" is necessarily more limited'.
42. Counsel for BMS argued that this meant that although Lord Sumption thought the Court of Appeal had gone too far in its reliance on the possibility of doing the Bennett and Chung tests once 'encouraged' by the specification, such tests could have a role. The purpose of this submission was to create a legal basis for the argument that the reader of [the Application] would see something of potential value by working out what the patentee was likely to have done and, encouraged by that but having no data, would themselves test apixaban.
43. I disagree with BMS's argument on this point. Lord Sumption clearly rejected the encouragement-plus-later-tests argument in [53], and all that he meant by simple tests having a more limited role was a reference back to the case law of the EPO on post-filed data that he had identified in [40]. That he was rejecting a role for tests which had not been done for inclusion in a patent specification is clear from [53] itself in his reference to Johns Hopkins to the effect that setting a problem is not a contribution and that the notion of 'worth trying' does not without more justify a monopoly.
44. BMS sought to reinforce its argument on this front by reference to BMS/Dasatinib T0488/16, at 4.6.2. BMS argued that that case showed that one of the factors that the TBA considered in assessing plausibility was the availability of tests. In fact, what the Board referred to was the lack of availability of any CGK tests for verifying the assertion in question, and its statement was that that 'further aggravated' the lack of plausibility arising from the specification. In complete isolation from any context I can see how BMS might argue that it could be inferred that tests could theoretically have a role, but in reality that is plainly not what the Board was saying. I note that BMS/Dasatinib was referred to by Lord Sumption at [24] and although he referred to a different paragraph in the decision (4.9) he was dealing with the issue of post-filed data, so this too is a reason to reject BMS's reliance on the decision.
45. In my view my analysis of plausibility should be firmly guided by the points in [37] of Warner-Lambert and by the principle laid out by that case that a contribution by the patentee that is in the specification is needed. The latter is important because, as I hope will become clear when I address the facts, in very large measure, if not entirely, BMS's case for plausibility arises not from anything in [the Application] but from matters which it contends were CGK. CGK is not BMS's contribution."
"136. In my view, the only statement of work actually done is that 'a number of compounds' were tested and had a Ki of 10 µM or less. The statements about lower Kis for preferred/more preferred/still more preferred compounds are aspirational targets, and the statement that the utility of 'the compounds of the present invention' was confirmed is an assertion that an inference can be drawn from the tests that were done. I understood that BMS accepted this.
…
158. Although its written submissions relied on the very general statements in e.g. the abstract of [the Application], in oral submissions Counsel for BMS accepted that they were not themselves good enough for plausibility and I agree with that, since at most they are bare assertions of utility. So the focus fell on the sentence at page 170 lines 28-32.
159. Counsel for BMS submitted that although it was not explicitly stated which compounds were tested, the skilled reader would assume that all the synthesised compounds, or at least the vast bulk of them, had been tested. The basis for this was said to be that [the Application] described the invention as being about lactams, that the patentee could only have tested compounds that were actually made, and that there was no point making them unless they were going to be tested.
160. Counsel for BMS did however accept that the skilled reader would infer that not all the compounds tested would have been successful; some might have failed. I agree with this.
161. In my view BMS seeks to read far too much into the sentence. On its own it would not be understood as standing with any reliability for anything more than it says, which is that some unidentified compounds had been tested with activities at the level indicated, and that utility for some broader class (i.e. broader than just the ones tested) could, in the patentee's opinion, be inferred. What that broader class might be cannot be worked out, both because of the lack of detail and because of the inherent ambiguity in the expression "compounds of the present invention" in this sort of specification where many different Markush formulae are given.
162. Further, there is no way from this sentence alone to draw any sort of inference about any individual compound, be it apixaban or any other. There is simply no information, and given Counsel for BMS's acceptance that some compounds might also have failed, there is no way for the reader to know of any particular compound whether it was good or bad.
163. To be fair, I do not think that BMS really seriously maintained a case that the disclosure on page 170 was enough on its own. It therefore sought to tie it to apixaban by means to which I will now turn."
"164. As I have already said, apixaban is Example 18 in [the Application] and at page 222 line 25 it is identified that 3.07g was ultimately made. …
165. In addition, I find that that was the most of any compound reported to have been made in [the Application], by some distance.
166. There is no explicit disclosure of why the patentee made that amount. BMS said that the reader would infer that it was because early results had been favourable and the patentee wanted to take work on the compound forwards. The evidence of the DMPK experts … was that this was possible, with the further work intended being, possibly, second species pharmacokinetics or early toxicology.
167. The Claimants responded that there were other possible reasons, such as making apixaban as an intermediate on the way to making something else (although Dr Redshaw [the Claimants' medicinal chemistry expert] could not make any concrete suggestion) or as a thrombin inhibitor, which seems possible given the teaching of [the Application] on that topic, if not especially likely.
168. In cross-examination Dr Camp [BMS's medicinal chemistry expert] was taken to a 2003 publication by Scott Sheehan of Lilly ('A four component coupling strategy for the synthesis of D-phenylglycinamide-derived non-covalent factor Xa inhibitors') where a similar large amount was made of a compound which was not successful. He accepted on the basis of it that the amount of a compound made could not be taken as an indicator of success in every case; one possibility was just that 'the chemistry worked better'.
169. There was, Dr Camp accepted, no evidence in any of the CGK review articles of the authors selecting compounds for review or inclusion based on the amount made.
170. In her oral evidence, Dr Redshaw maintained her overall position that judgments could not be made about a compound's qualities from the amounts made.
171. The 3g point is not completely without relevance. It is a point which, unlike other aspects of BMS's case, is relatively free of hindsight, in the sense that it sets apixaban apart from the other exemplified compounds based on information in [the Application] itself that I think the skilled reader would notice.
172. However, in its substance it is a very weak point. Lacking any data, one does not know why the patentee made such a quantity and reasons other than factor Xa inhibitory activity are real possibilities. And I do not see how the point can go any further than that the patentee thought that apixaban was promising. A bare assertion to that effect in [the Application] (bare in the sense of lacking data or reasoning) would not have been any use in establishing plausibility, as is clear from the second point in [37] in Warner-Lambert. But [the Application] does not even contain such an assertion."
"216. Taking all the above matters together, I conclude that [the Application] does not make it plausible that apixaban would have factor Xa binding of the level of 10 µM as referred to on page 170, or any useful degree of binding. The fundamental problem is that identified by the Claimants: there is simply no reference to apixaban there to allow an inference that it was one of the compounds for which useful results had been achieved. The frequency of use analysis suffers from the problems identified above and while the reader of [the Application] would infer that work of some kind had been done on lactams with quite a number made, there is no way to connect any particular compound to any degree of activity. Apixaban had been made in quantity but that does not mean anything for activity, and the structural arguments fail on the facts.
217. So BMS's points fail individually and their whole is no greater than the sum of their parts. Since there is no plausibility of any meaningful factor Xa binding the Patent is invalid, since all the applications for apixaban depend on factor Xa binding. …"
"222. I accept BMS's contentions that it would not have been difficult or burdensome to test apixaban for its factor Xa inhibitory activity, and that if such tests were done a very good level of activity would have been found. ….
223. However, the fact that I accept BMS's factual contentions about testing does not help it. In the absence of making some showing of plausibility based on one of the other matters relied on (the teaching on page 170, the 3g point, frequency of use, structure), the ability to test cannot get BMS any further than the patentee in Warner-Lambert. It provides (at a maximum) the sort of encouragement-plus-ability-to-test that the Supreme Court rejected, as I set out above. I say 'at a maximum' because my analysis above means there is not even any encouragement concretely referable to apixaban."
Grounds of appeal
(1) The judge erred in law because, in the case of a claim to a single chemical compound, there is no requirement that the specification makes it plausible that the compound is useful. It is sufficient that the specification discloses the structure of the compound and a method of synthesis and contains an assertion of potential utility for the compound, provided that that assertion is not manifestly speculative or wrong.
(2) The judge erred in law because he applied the standard of plausibility laid down by the majority in Warner-Lambert when he should either have applied the standard advocated by the minority or applied the standard laid down by the majority less strictly.
(3) The judge erred in law because he wrongly held that it was not enough for the specification to encourage the skilled person to test the efficacy of the claimed compound and to identify simple tests which the skilled person could carry out for that purpose and which, if carried out, would confirm that the compound was likely to have the efficacy claimed for it.
(4) The judge erred in law or principle because he failed to stand back and consider whether the claimed invention fulfilled the "patent bargain".
(5) The judge erred in principle because he should have held that the Application contained an implicit disclosure that apixaban had a nanomolar Ki against factor Xa or (which comes to the same thing given the judge's finding as to common general knowledge) had a Ki which made it suitable for therapeutic use.
(6) The judge was wrong to hold that page 170 line 28-32 and Example 18 taken together did not make it plausible that apixaban was an effective factor Xa inhibitor.
Grounds 1-4
Ground 5
Ground 6
Conclusion
Lord Justice Nugee:
Lord Justice Warby: