Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales Court of Appeal (Civil Division) Decisions
You are here: BAILII >> Databases >> England and Wales Court of Appeal (Civil Division) Decisions >> L'Oreal (UK) Ltd & Anor v Liqwd Inc & Anor [2019] EWCA Civ 1943 (18 November 2019)
URL: http://www.bailii.org/ew/cases/EWCA/Civ/2019/1943.html
Cite as: [2019] EWCA Civ 1943
[New search] [Printable PDF version] [Help]
ON APPEAL FROM THE BUSINESS AND PROPERTY COURTS INTELLECTUAL PROPERTY LIST (CHANCERY DIVISION) PATENTS COURT
MR JUSTICE BIRSS
Strand, London, WC2A 2LL |
||
B e f o r e :
LORD JUSTICE McCOMBE
and
LORD JUSTICE ARNOLD
____________________
| (1) L'OREAL (U.K.) LIMITED (2) L'OREAL SA |
Appellants |
|
| - and - |
||
| (1) LIQWD INC (2) OLAPLEX, LLC |
Respondents |
____________________
Iain Purvis QC and Katherine Moggridge (instructed by Hogan Lovells LLP) for the Respondents
Hearing dates: 5-6 November 2019
____________________
Crown Copyright ©
- These are appeals from two decisions of Birss J in patent infringement proceedings brought by the Respondents ("Olaplex") against the Appellants ("L'Oréal"). First, for the reasons given in his judgment dated 11 June 2018 ("the Main Judgment") he held that claims 1-10 of Olaplex's UK Patent No. 2 525 793 ("the Patent") were invalid; but that an unconditional amendment to claim 11 applied for by Olaplex was allowable and that, as amended, claim 11 was valid and had been infringed by L'Oréal. Secondly, he dismissed an application by L'Oréal to adduce further evidence after the Main Judgment had been handed down for the reasons given in his judgment dated 19 July 2018 ("the Second Judgment").
- L'Oréal appeal against the Main Judgment on two grounds. First, they contend that the judge should have refused Olaplex permission to amend claim 11 because the amendment extended the protection conferred by the Patent. There is no dispute that granted claim 11 is not entitled to the priority claimed in the Patent, and that as a result the granted claim is invalid because of intervening use by Olaplex. Thus the amendment is necessary to validate the claim. Secondly, L'Oréal contend that the judge should have held that claim 11 was obvious over Korean Patent Application No PAT 2003-0003970 ("Kim").
- L'Oréal appeal against the Second Judgment on the ground that the judge should have admitted the new evidence since, L'Oréal contend, it demonstrates that Olaplex's International Patent Application No. WO 2015/017768 ("WO 768") is entitled to priority from US Patent Application No 61/093,239 ("US 239"), with the result that WO 768 deprives claim 11 of the Patent of novelty.
- The judge found in the Main Judgment at [27]-[29] that the Patent is directed to a skilled team responsible for developing and producing hair-care products, the principal member of which would be a chemist/formulator with at least an undergraduate degree in chemistry or a related field and a few years' experience in developing and testing hair care products.
- The key elements of the skilled team's common general knowledge relevant to the appeal are set out in the following passages in the Main Judgment:
- The patented invention is based on the discovery that maleic acid prevents or reduces damage to hair during bleaching i.e. hair lightening. The judge summarised the disclosure of the Patent at [62]-[84] of the Main Judgment. It is not necessary to repeat that exercise for present purposes. I shall refer to certain passages from the specification below.
- Claim 11 as amended is in the following terms:
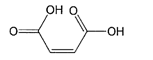
- The chemical formula in the claim is the formula for maleic acid. It is common ground that it makes no difference to the issues arising in this case that the claim sets out the formula rather than the name of the compound. For convenience, I shall follow the example of the parties and the judge of proceeding as if the claim said "maleic acid".
- Claim 11 is to the use of an active agent simultaneously with a bleaching agent for the stated purpose. It was common ground at trial that it was therefore the state of the active agent in that use that mattered. It was also common ground at trial (although not prior to trial) that the reference to maleic acid embraces both maleic acid itself and the anions it forms in aqueous solution (depending on pH), namely hydrogen maleate and maleate ions. The judge held that, read in context and with the common general knowledge, "a treatment to provide bleached hair" referred to hair lightening and did not include an oxidation dyeing process. There is no challenge to that conclusion.
- Although the Patent contains six pages of definitions and about 10 pages describing the constituent elements of formulations for implementing the claimed invention, there is no definition of the term "simple salt" nor any passage throwing any light on what the inventors meant by this term. Nor is it a term commonly used in hair care.
- The judge held at [102] that the adjective "simple" would be understood by the skilled team in one of two ways suggested by L'Oréal's main expert Dr Hefford which the judge summarised at [98] as follows:
- On its face, granted claim 11 presents two alternatives for the active agent: (1) maleic acid or (2) a simple salt of maleic acid. The amendment deletes the second of these alternatives. Prima facie, therefore, the amendment narrows the protection conferred by the Patent, it does not extend it. L'Oréal nevertheless contend that the effect of the amendment is to broaden the claim to include things which were not covered when both alternatives were in the claim, namely maleate ions in combination with non-simple cations.
- The correctness of this contention depends partly on the meaning of the word "or" and partly on the meaning of the term "a simple salt thereof" in the context of the granted claim. The judge held at [102] that, in context, "a simple salt thereof" meant "a simple salt of maleic acid in solid form". On that basis, he held that the deletion of the words "or a simple salt thereof" had the effect of narrowing the scope of the claim and not extending the protection conferred by it. L'Oréal contend that he was wrong to construe "a simple salt thereof" as meaning a simple salt in solid form.
- As counsel for Olaplex submitted, the starting point in considering this issue is to ask why, as a matter of construction, the formula in the granted claim was accepted by both parties' experts and both parties to cover hydrogen maleate and maleate ions as well as maleic acid.
- As the judge explained, what is literally shown in the formula is the complete molecule of maleic acid including its two H protons. The skilled team would know from their common general knowledge, however, that, when the free acid is dissolved in water, the protons are likely to dissociate. First a hydrogen maleate ion is formed and then a maleate ion. The reactions and the balance of the species in solution depend on pH. As noted above, the claim is to the use of the active agent. Furthermore, the specific example of the invention taught in the Patent (Example 3) involves creating an aqueous solution by mixing free acid with water to form an acidic solution. The example also discloses mixing the solution with a bleaching agent which would be likely to raise the pH. The skilled team would appreciate that varying the conditions (even increasing the quantity of water) would affect the pH of the solution.
- In those circumstances it is not surprising that both experts agreed that the maleic acid molecule shown in granted claim 11 would not have been understood by the skilled team to mean simply the diprotonated acid. Rather it would have been understood to cover both of the unprotonated species, that is to say, hydrogen maleate ions and maleate ions. Moreover, this was accepted by both parties in closing submissions.
- If the formula in the unamended claim would be understood as meaning any of the three species, then it covers the use of any formulation comprising a solution in which the maleate ion is the active agent. This inevitably includes any solution created by dissolving a maleate salt in water, since the effect of dissolution of a salt of maleic acid is to dissociate maleate ions from the cations to which they are ionically bound in the salt. It makes no difference whether the maleate salt is simple or complex – the maleate ions are always released into solution. This is basic chemistry and was confirmed by the expert evidence.
- As counsel for Olaplex submitted, this is fatal to the argument on extension of protection. All solutions made by dissolving a maleate salt contain maleate ions and were therefore always covered by the maleic acid formula in the unamended claim. Deletion of "or a simple salt thereof" does not change this. Thus L'Oréal have not identified anything now covered by the amended claim which was not already covered by the granted claim.
- Counsel for L'Oréal attempted to avoid this conclusion by arguing that the words "[maleic acid] or a simple salt thereof" were a composite phrase which denoted a single class of compounds. I do not accept this argument. First, as a matter of language, the phrase denotes two alternatives: the word "or" is plainly disjunctive, not conjunctive. Secondly, as a matter of chemistry, it denotes two different types of compound: an acid and a salt. Thirdly, counsel was unable to point to any expert evidence that showed that the skilled team would for some technical reason interpret the composite phrase as denoting a single class of compounds.
- Counsel for L'Oréal also submitted that the words "or a simple salt thereof" had been inserted into the claim by Olaplex in order to limit the claim, and therefore should be interpreted as having that effect. I do not accept this either. First, there is no reason to think that that was in fact Olaplex's intention. It would be very odd for Olaplex to try to limit the claim by adding what appears to be an alternative option rather than by adding a limiting feature. Secondly, even if that were Olaplex's intention, there is nothing in the specification of the Patent to indicate to the skilled reader that was the intention. Accordingly, the skilled team would discern the patentee's intention from the words used in the claim read in the context of the specification. Read in that way, the skilled reader would think that the words "or a simple salt thereof" were an alternative to the use of maleic acid. It follows that it does not matter precisely what was meant by "a simple salt thereof" in that context.
- Nevertheless, for completeness, I will go on to consider whether the judge was correct in his construction of the term "a simple salt thereof". In my view the starting point here is to recognise that maleic acid is itself a solid at ambient temperatures (its melting point is 135oC). Thus claim 11 on its face contemplates the use of a substance that is solid at ambient temperatures before one gets to "or a simple salt thereof". Thus it would not be surprising to find that it also permitted the use of another solid.
- Furthermore, both experts agreed that "salt" was a technical term which had a clear meaning to a skilled reader. It is a solid in which anions and cations are ionically bound together in a crystal lattice. It is true that, as the experts also agreed, it is common to speak of a "salt solution", meaning a solution of a salt in water, and that in such a solution the ions are dissociated from one another and randomly distributed. Nevertheless, a salt solution is not a salt.
- L'Oréal contend that the words "a simple salt" in the claim should not be understood in the ordinary chemical sense, but should be taken to refer to solutions made by dissolving simple maleate salts. As counsel for Olaplex submitted, there are four major problems with this construction, in addition to the fact that it is not the ordinary technical meaning of the words.
- First, in such solutions, the key characteristics which define a salt have been lost. Indeed, tellingly, it is not possible to know from examining a solution of maleate ions and cations whether it was the result of dissolving a salt or not. Precisely the same solution could be, and commonly is, made by mixing an acid and a base in water. Counsel for L'Oréal was forced to accept that, on L'Oréal's construction, the term "a simple salt thereof" covered solutions made from an acid and a base. This deprives the word "salt" of any real meaning.
- Secondly, the skilled team would have no reason to give the phrase such an odd meaning, because they would understand that solutions of maleate ions and cations were covered by the formula anyway - it would be pointless duplication. Given that the claim presents "a simple salt thereof" as an alternative to the formula, the judge rightly held that the skilled team would seek to give it a meaning which was at least arguably not covered by the formula.
- Thirdly, although L'Oréal appeared to be contending in their skeleton argument for the appeal that the claim was restricted to aqueous solutions, counsel for L'Oréal eventually accepted that this was not correct. The specification is perfectly clear that solvents other than water are contemplated. Thus the specification states at page 13 lines 3-4 that the formulations "typically contain one or more cosmetically acceptable excipients". After listing many possible excipients, including "oils" and "liquid vehicles, carriers", it goes on at page 13 lines 14-16 (emphasis added):
- The significance of this point is that, as counsel for L'Oréal accepted, the dissociation constants of maleic acid may be different in different solvents. This in turn will affect the extent to which maleate ions are formed by dissolving maleic acid in the solvent.
- Fourthly, and perhaps most importantly, counsel for L'Oréal submitted that the specification did not contemplate the use of solid active agent and there was no expert evidence that it did. Both parts of this submission are incorrect, however. As the judge noted, the specification specifically describes at page 29 lines 10-12 a kit for treating hair in which the active agent is "provided as a dry powder in a sealed package and the excipient provided in a vial or other container". Olaplex's expert Professor Haddleton drew attention to this in his first report, saying:
- Counsel for L'Oréal pointed out that the passage at page 29 lines 10-12 of the Patent teaches that the dry powder can be mixed with an excipient before being applied to the hair, and submitted that it contemplated the use of a solution. The excipients listed in the specification are not required to be aqueous, however. Indeed, as noted above, they include obviously non-aqueous substances such as oils, in which the salt would not be expected to dissolve, as Prof Haddleton confirmed in cross-examination. Although Prof Haddleton did not use the word either in his report or in his oral evidence, it appears that what he was referring to in the first sentence of paragraph 55 was the formation of a suspension. Certainly, no other possibility was suggested to us by counsel for L'Oréal.
- Counsel for L'Oréal argued that, if a salt was applied to the hair, it would need to dissolve in order for the maleate ions to have the claimed effect. In support of this he relied upon a sentence from paragraph 4.32 of Dr Hefford's first report that was not challenged in cross-examination: "To put the idea of the Liqwd Patent into practice requires the salt of maleic acid to be in solution (in Example 3 it is in water)". I am not convinced that that sentence bears the weight put upon it, since it is not clear that the witness was saying that it was scientifically impossible for the invention to be put into practice unless the salt was in solution. (If it was scientifically impossible, then one would expect L'Oréal to have attacked the validity of claim 11 on the ground of insufficiency, since the claim is not limited to the use of solutions.) The witness may simply have meant that, in practice, that is how he would expect it to be done. In any event, as counsel for Olaplex pointed out, the invention can be put into practice by placing the active agent into a wet environment, since it is applied simultaneously with a bleaching formulation (either by pre-mixing or by applying them at the same time) which may be aqueous.
- Finally, I should address a major theme of counsel for L'Oréal's submissions, namely that the construction advanced by Olaplex at trial and accepted by the judge was not the construction Olaplex had pleaded in their statements of case served pursuant to a consent order dated 3 November 2017 requiring them (among other things) to set out their case as to the meaning of the term "simple salt". There is no dispute that Olaplex's statements of case pleaded that the meaning of "simple salt" was a salt containing a particular type of counter ion (the judge called this construction "the non-functional counter ion" construction: see the Main Judgment at [99]). Nor is there any dispute that the construction advanced by Olaplex at trial and accepted by the judge was not advanced in either of Prof Haddleton's reports or in Olaplex's skeleton argument. Counsel for L'Oréal submitted that the construction was first advanced in Olaplex's written closing submissions. Counsel for Olaplex disputed this, and submitted that he had advanced it in his oral opening submissions (at T1/24/11-26/6). In my judgment counsel for Olaplex is correct. It is fair to say that he did not use the word "solid" in that passage, referring instead to "a compound in which cations and [an]ions are [ion]ically bound together", "a crystal lattice" and "the salt in an undissolved form", but the sense is clear and it is evident from his questions that that was how the judge understood it.
- More importantly, counsel for L'Oréal submitted that it was not open to Olaplex to advance the construction accepted by the judge without applying to amend their statements of case. I do not accept this submission for two reasons. First, counsel for L'Oréal did not raise this objection at trial. Counsel for L'Oréal submitted that he had raised the objection in his oral closing submissions, but the transcript (at T5/630/6-632/11) shows that he did not and that the point he actually made was that the construction "did not occur to them until recently", which showed that it was not a good one. Not having taken the objection at trial, it is too late for counsel for L'Oréal to take it now.
- Secondly, the construction of the claim is a question of law and the court is not bound to accept either party's construction. Counsel for L'Oréal's answer to this point was that Olaplex's new construction required L'Oréal to have the opportunity to adduce expert evidence directed to it, for example as to the feasibility of applying solid salts to the hair. But if well founded, the time for that submission was at trial when Olaplex introduced the new construction. I would add that I am not convinced that the submission is well founded, since it seems to me that the relevant technical considerations were sufficiently addressed in the expert evidence before the court.
- Counsel for Olaplex accepted that Olaplex could, and preferably should, have articulated their final construction rather earlier than they did. As he pointed out, however, it is, regrettably, not uncommon in patent cases for points on claim construction only to emerge at trial. In the present case I am in no doubt that the construction ultimately advanced by Olaplex, and accepted by the judge, was the correct one.
- The judge described the disclosure of Kim in some detail in the Main Judgment at [228]-[240]. In brief summary, Kim discloses the use of maleic acid and derivatives thereof (including maleate ions) to reduce or prevent hair damage due to oxidative dyeing. There is no description in Kim of a hair lightening formulation. Thus the only difference between Kim and claim 11 of the Patent is that, whereas Kim is concerning with oxidative dyeing, claim 11 is concerned with bleaching i.e. hair lightening.
- For the purposes of the appeal, three passages in Kim are particularly important. The first is at page 2 lines 13-29 of the agreed translation:
- The second passage is at page 3 line 30 – page 4 line 3:
- As the judge explained at [235]:
- The third passage is at page 7 lines 7-14, after a table showing lower tensile strength measurements of 50 hairs for four examples in accordance with the invention compared to a comparative example:
- There is no dispute as to the law concerning obviousness, which was recently reviewed by the Supreme Court in Actavis Group PTC EHF v ICOS Corp [2019] UKSC 15, [2019] Bus LR 1318. Nor is there any dispute that obviousness involves a multi-factorial evaluation and therefore this Court is not justified in intervening in the absence of an error of law or principle on the part of the judge: see Actavis v ICOS at [78]-[81] (Lord Hodge). This is particularly so given that the decision was one of an experienced specialist patents judge after hearing oral evidence from experts in the field.
- The judge concluded that claim 11 was not obvious over Kim for the following reasons:
- L'Oréal contend that the judge erred in law or principle in that he misconstrued Kim, and thus this Court can and should reconsider the matter. L'Oréal case is clearly and succinctly summarised in paragraph 96 of their skeleton argument for the appeal:
- Kim is undoubtedly close prior art, and L'Oréal had a very respectable case that claim 11 was obvious in the light of it. The problem for L'Oréal on this appeal is that the judge squarely addressed himself to that case, and gave a number of reasons for rejecting it. I accept that, if L'Oréal could show that the judge's starting point was an incorrect reading of Kim, then that might well undermine his conclusion. As his reasons show, however, the question is not a matter simply of the interpretation of Kim, but of how the skilled team would react to its teaching.
- L'Oréal rely upon the first and third of the passages I have cited above as showing that Kim discloses a method of reducing the oxidative damage to hair in oxidative dyeing. The problem with this argument is that, first, that is not what Kim actually says, and secondly and perhaps more importantly, what Kim says in the second passage quoted is inconsistent with that interpretation. As the judge explained at [245], the thiol-disulphide mechanism proposed by Kim in that passage is concerned with reduction, not oxidation. It therefore has no relevance to the damage caused by bleaching (hair lightening).
- When faced with this difficulty during the course of argument, counsel for L'Oréal's response was to attack the judge's finding that the skilled team would regard the mechanism proposed by Kim as scientifically credible as being one that was not open to the judge on the evidence. This is not one of L'Oréal's grounds of appeal, however, nor is it a submission made in L'Oréal's skeleton argument. In any event, the submission is a hopeless one given that, as counsel for Olaplex pointed out, L'Oréal's own expert Dr Hefford said in paragraph 7.8 of his first report that "the mechanism would make scientific sense to the skilled addressee". Only in his third report served the day before trial did Dr Hefford attempt to row back from this. We were shown the transcript of Dr Hefford's cross-examination on this point, and I can well understand why the judge stated that he was not convinced by Dr Hefford's qualification to what he had said previously. At all events, there was certainly material which entitled the judge to make the finding that he did.
- Furthermore, as the judge noted at [250], the mechanism proposed by Kim makes sense of the fact that Kim says at page 3 lines 22-24 that direct dyes such as henna, which do not involve oxidation at all, may be used instead of an oxidative dye. Thus the authors of Kim evidently perceived their invention as applicable to dyes generally, not to oxidative processes generally.
- Counsel for L'Oréal's response to this point (again, not one advanced in L'Oréal's grounds of appeal or skeleton argument) was that the relevant passage in Kim was proposing the use of a direct dye in addition to an oxidation dye. This is a bizarre suggestion, and I do not accept it.
- Although the arguments on Kim ranged more widely, the central points are those I have dealt with above. In my judgment no basis has been shown by L'Oréal for interfering with the judge's conclusion.
- WO 768 is a novelty-only citation pursuant to section 2(3) of the Patents Act 1977 if, but only if, the relevant subject matter is entitled to an earlier priority date than amended claim 11 of the Patent. The subject matter relied upon by L'Oréal is Example 8 of WO 768. There is no dispute that this falls within amended claim 11. L'Oréal contend that Example 8 is entitled to priority from US 239, which is earlier in date than the priority document from which the Patent claims priority (the judge set out all the relevant dates in the Main Judgment at [188].) The subject matter in US 239 relied upon by L'Oréal is Example 4. Thus the key issue is whether Example 8 of WO 768 is entitled to priority from Example 4 of US 239.
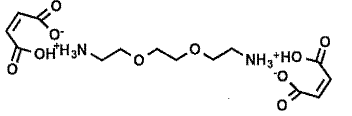
- On the face of the two Examples, they are different. Both disclose a highlighting formulation mixed with a second formulation containing a maleic acid derivative in water. In Example 8 of WO 768 the maleic acid derivative is "2,2'-(ethane-1.2-diylbis(oxy))bis(ethan-1-amine) di-maleate", which has the following structure:
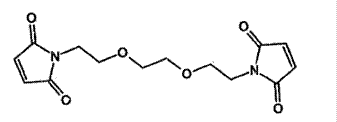
- In US 239, however, the maleic acid derivative is a "bismaleimide cross-linking agent" with the following structure:
- The synthesis of the bismaleimide cross-linking agent is described in Example 1 of US 239.
- In their expert evidence and opening skeleton argument the case advanced by L'Oréal was that Example 8 of WO 768 was entitled to priority from Example 4 of US 239 because, to put it shortly, the bismaleimide cross-linking agent would hydrolyse in storage into the corresponding di-acid which in turn would hydrolyse to the di-maleate. The judge described this is a "thin basis" for the claim to priority and rejected it on the expert evidence. There is no challenge by L'Oréal to this conclusion.
- During the cross-examination of Olaplex's expert Professor Haddleton, however, junior counsel for L'Oréal put a completely different point to the witness which had not been foreshadowed in any statement of case, evidence, skeleton argument or letter. In short, this was that the proton NMR spectrum of the product of Example 1 reproduced in Figure 1 of US 239 showed that the product described as the bismaleimide was in fact the di-maleate. This was a point which had occurred to junior counsel for L'Oréal during his preparation for trial and had been considered by L'Oréal's second expert Professor Law on the day before trial (although it was discussed further between junior counsel and Prof Law on the first and second days of the trial). Even though L'Oréal served a further expert report from Prof Law in the evening of the second day of trial, this point was not mentioned in it.
- The judge held that this point could and should have been notified to Olaplex prior to Prof Haddleton being called as a witness, and that springing the point upon the witness without prior warning, and thus without giving Olaplex the chance to adduce other evidence to rebut this new case, amounted to procedural unfairness which justified excluding the issue from consideration. Again, there is no challenge by L'Oréal to this conclusion.
- For good measure, the judge also held that, based on the evidence before him, L'Oréal had not established that Example 8 of WO 768 was entitled to priority from Example 4 of US 239 anyway. The basis for this conclusion was that, although the judge found that Prof Haddleton's evidence established on the balance of probabilities that the product shown in Figure 1 of US 239 was indeed the di-maleate, the evidence did not establish that the di-maleate was the inevitable result of following Example 1 of US 239. Again, there is no challenge by L'Oréal to this conclusion.
- The Main Judgment was handed down on 11 June 2018. As is usual in the Patents Court, argument over the consequential orders that should be made was adjourned, in the event until 19 July 2018. On 3 July 2018 L'Oréal issued an application seeking permission to adduce further evidence on the issue of whether Example 8 of WO 768 was entitled to priority from Example 4 of US 239, and hence deprived amended claim 11 of the Patent of novelty. The evidence in question, comprising a second Notice of Experiments and a third report of Prof Law, was served by L'Oréal on 5 July 2018. The application was heard on 9 July 2018. On 19 July 2018 the judge handed down the Second Judgment and dismissed the application.
- The two key points to note about this chronology are that the application was made roughly three weeks after the Main Judgment was handed down dismissing both of the cases advanced by L'Oréal on this issue at trial, but nevertheless before the order consequential upon the Main Judgment was sealed.
- In a nutshell, the further experimental and expert evidence which L'Oréal seek to adduce is directed to proving that the inevitable result of carrying out Example 1 of US 239 is the di-maleate and not the bismaleimide. The evidence sets out a repetition which Prof Law carried out and his opinion that the same result would always be obtained.
- It was common ground before the judge, and before us, that the applicable principles were those stated by the Supreme Court in In Re L (Children) (Preliminary Finding: Power to Reverse) [2013] UKSC 8, [2013] 1 WLR 634. In that case the Supreme Court confirmed that a judge had the power to reverse his or her decision at any time before the order was sealed and disapproved dicta in In Re Barrell Enterprises [1973] 1 WLR 19 to the effect that exceptional circumstances were required. As Baroness Hale explained at [27]:
- Before us counsel for Olaplex placed some reliance upon the line of cases culminating in Generics (UK) Ltd v Warner-Lambert Co LLC [2018] UKSC 56, [2019] Bus LR 360 in which courts have rejected applications made by patentees to amend the claims of patents after trial on the ground of abuse of process. It should be noted that this line of authority does not depend on whether or not the order has been sealed at the time the application is made – in many of the cases it had not been. These authorities are not directly applicable to the present situation. Nevertheless a key factor in such cases is often that the application will, if granted, necessitate a second trial: see in particular Nikken Kosakusho Works v Pioneer Trading Co [2005] EWCA Civ 906, [2006] FSR 4 at [13]-[22] (Jacob LJ), [33] (Laws LJ) and [34] (Waller LJ). That factor is also present here.
- The judge concluded that it would not be in accordance with the overriding objective to admit the further evidence. His reasons for reaching that conclusion are set out in detail in the Second Judgment at [47]-[67]. While those reasons should be read in full, I think they can be summarised as follows:
- The judge's decision is a case management decision applying principles which are not in dispute. It follows that L'Oréal face a high hurdle in attempting to show that he exceeded the boundaries of his discretion. L'Oréal contend that the judge erred in three principal respects:
- In my judgment none of these criticisms has any substance. The first criticism is completely untenable. Plainly there has to be something about the circumstances which justifies re-opening the issue, otherwise there would be no basis for acceding to the application. Moreover, the judge's approach is wholly in accordance with CPR rule 1.1. As counsel for Olaplex rightly submitted, the overriding objective is not simply about reaching the (allegedly) correct decision on the merits: see the discussion in Nikken v Pioneer. Contrary to the submission of counsel for L'Oréal, the judge was not re-introducing the test of exceptional circumstances from Re Barrell.
- Turning to the second criticism, the judge was entirely correct to attach considerable weight to the fact that the new evidence did not amount to a knock-out blow, but rather raised issues which would require a second trial to resolve.
- As for the third criticism, the judge was again entirely correct to attach weight to the fact that L'Oréal were trying to re-fight an issue on which they had lost at trial having taken the tactical decision to try to establish their case through cross-examination of Olaplex's expert rather than seeking an adjournment to adduce further evidence of their own. In this regard, I note that counsel for L'Oréal repeatedly submitted that the issue raised by the new evidence had not yet been determined. This is not correct. As discussed above, it was determined by the judge adversely to L'Oréal on the evidence before him, albeit by way of an alternative ground for his decision that L'Oréal had not established that claim 11 lacked novelty over Example 8 of WO 768. That evidence did not include the new evidence precisely because L'Oréal did not seek an adjournment.
- Counsel for L'Oréal also submitted that the judge had been wrong not to attach weight to the fact that (as the judge was prepared to assume) Olaplex had known about the error in the description of Example 1 for some time. This criticism has no more substance than the first three. The judge expressly found that there had been "no failure of disclosure or a lack of candour by Olaplex in relation to this point at trial" (Second Judgment at [62]). There is no ground of appeal challenging that conclusion, and in any event it was one that was plainly open to the judge. As counsel accepted, the relevant individual(s) might have forgotten about the matter or not appreciated its relevance. Even if they remembered it and appreciated its relevance, counsel was unable to identify any reason why they were obliged to disclose it.
- For the reasons given above, I would dismiss both appeals.
- I agree. I also agree with the judgment of Davis LJ, which I have read in draft.
- I also agree. I add a few observations of my own, if only because of the evident importance of this case to the parties.
- As a matter of language, it seems somewhat counter-intuitive that deletion of a disjunctive proposition ("or a simple salt thereof") can operate not to narrow the claim but to enlarge it. However, I do accept that that cannot of itself be a determinative point: one has also to consider the science of the matter.
- At trial it was (ultimately) common ground, and the judge found, that "maleic acid" included both the molecule and the ions, maleate and hydrogen maleate, which it forms in aqueous solution. On that basis, construing the disjunctive words as extending to simple salts in solid form on the face of it makes sense: particularly where the patent had in terms stated that the active agent may be provided as a dry powder. Mr Turner protested that, even if it could be provided as a dry powder, nevertheless it had to be applied simultaneously with the bleaching agent; and both experts had contemplated that that would in practice be done in (normally aqueous) solution in order for the desired effect to be achieved. However, I accept the submission of Mr Purvis that a patentee, with an eye to the future, may well have wished to extend its protection to cover simple salts in solid form: and the wording of the patent is consistent with that.
- It is true that Olaplex only formulated its ultimate position on this point very late in the day: inevitably, therefore, exposing it to the kinds of forensic criticism which Mr Turner forcefully deployed. But Olaplex was not precluded on the pleadings from so arguing: and at all events the judge was entitled to proceed to decide this point as he did, without unfairness to L'Oréal arising.
- As to obviousness over Kim, the repeated references in Kim to oxidation lent, in my view, considerable prima facie support to L'Oréal's position. Nevertheless, as I understand it, the approach required in this context is one of multi-factorial evaluation, as it is styled. That being so, the circumstances in which the appellate court can interfere are limited. Mr Turner appreciated this, of course. But he said that this court could interfere: because the reasoning of Birss J was, he said, flawed by his misinterpretation of the Kim patent application and that was a matter of law.
- I understand the argument but do not accept it. The undeniable fact is that Kim was entirely focused on dyeing: bleaching, as such, does not feature at all. True it is that there are repeated references to oxidation in the dyeing process (although not, I note, exclusively so). But even then the mechanism advanced in Kim is focused solely on reduction, not oxidation. That is flat against L'Oréal's argument: and the judge found as a fact (as he was entitled to, on the evidence) that the mechanism there advanced was scientifically credible.
- Overall, there is in my opinion no sufficient basis for this court, applying conventional principles, to interfere with the conclusion of the trial judge on obviousness over Kim.
- As to the fresh evidence appeal, here too I can see no proper basis for this court interfering with the judge's decision: a decision, indeed, which constituted an exercise of judicial discretion.
- L'Oréal had, at trial, identified the potential issue here. It did not, for doubtless understandable tactical reasons, seek an adjournment to investigate the matter further: instead, it pursued it in cross-examination. The trial having proceeded, and L'Oréal having in the result lost on the interpretation and obviousness over Kim issues, L'Oréal could not readily be permitted to then reopen this self-same point in reliance on proposed further evidence. Given that, and given the judge's finding that there had been no want of candour or want of disclosure (by reference to the pleaded issues) on the part of Olaplex, the decision of the judge not to permit the proposed fresh evidence to be adduced at that particular stage is unassailable.
- Mr Turner submitted that the paramount consideration of justice is that the court should reach the right result. But he was in no position to assert that the proposed fresh evidence of Professor Law would inevitably bring about a conclusion in favour of L'Oréal. In any event, fresh evidence applications (and it is established that patent cases are in this respect to be treated no differently from other civil cases) cannot be decided solely by reference to arguments that the proposed fresh evidence might well lead to a different outcome. If that were the test, then many fresh evidence applications would succeed without more. The truth is that a more wide-ranging approach, by reference to the overriding objective and established general principles relating to fresh evidence applications, is needed. There is no requirement of exceptionality as such; but a number of factors will have to be addressed. These may include, among others, the importance of finality in litigation, the reasons advanced for not adducing the proposed fresh evidence earlier, whether a further trial will be needed, fairness to other parties concerned, whether a party has acted to his detriment in the interim and so on. Ultimately, however, all such decisions are fact and circumstance specific.
- I am in respectful agreement with the reasoning and conclusion of Arnold LJ. I too would dismiss both appeals.
Lord Justice Arnold:
Introduction
The skilled team, the common general knowledge and the Patent
"Hair and hair structure
…
33. … Melanin is responsible for the hair's natural colour. …
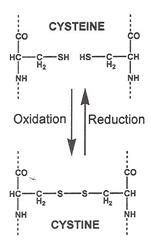
35. Keratin proteins are the major contributor to hair strength at a molecular level. Keratin has a high level of cysteine residues that result in disulphide crosslinking throughout the hair. These crosslinks are formed by the two cysteine side chains which have thiol (-SH) groups reacting to form cystine (also known as a cysteine bridge), which has a disulphide (-S-S-) bond between the two chains. Going from the thiols to the disulphide is an oxidation reaction while going from the disulphide to the thiols is a reduction reaction:
…
37. One of the most common ways to bleach hair is by the destructive oxidation of the chromophores in melanin, by applying a bleaching mixture. The chromophores are the groups of atoms in the melanin molecules responsible for giving the colour.
38. … one of the issues in the case involves considering two methods of changing the colour of hair. I will call one well known method 'hair lightening' because it changes the colour of hair by oxidation but does not involve hair dye. The other well known method is a process of dyeing hair using oxidation dyes. There are other methods of changing hair colour involving dyes which are not oxidation dyes.
Hair lightening
39. The mixture used for hair lightening principally comprises an oxidising agent such as hydrogen peroxide and a further material such as a persulfate. … The mixture is applied at an alkaline pH. This is a very common way of changing the colour of hair. It involves no dye at all. The colour change comes entirely from the process of bleaching or oxidation. …
42. The aggressive chemistry used in bleaching causes damage. …
43. In terms of chemical mechanisms, an aspect of damage by oxidising agents was believed to be due to the conversion of the disulphide bond (S-S) to cysteic acid groups (SO3).
Oxidation dyes
44. Oxidation dyes are used in the majority of hair dye treatments in the US and Europe. The process uses intermediate colouring agents which require the intervention of an oxidation agent (usually hydrogen peroxide) to react with them in order to produce permanent coloured compounds through oxidative condensation. The chemical processes involved are complex.
45. … the skilled person knew, as a matter of common general knowledge, that oxidative damage was something which could occur in oxidation dye systems, especially with repeated dyeing. It was less severe than the damage caused in hair lightening owing to the less aggressively oxidising formulations used in dyeing as compared to those used in hair lightening. So it was known that oxidation could be a cause of damage but looking at the matter the other way round, it was not the case that the skilled person necessarily would assume that any damage seen must have been caused by oxidation rather than having some other cause.
…
Trying to treat or prevent hair damage
48. The damage to hair caused by oxidation was known and those in the art had to deal with it. Professionals tried not to bleach hair too often but that was not always possible. For example actresses in film and television might have to undergo treatments which involved oxidation of hair very frequently.
…
Chemistry
52. When an acid reacts with a base the result is a salt plus water. In solid form salts are crystalline ionic compounds made up at least one cation (positively charged ion) and at least one anion (negatively charged ion). When the crystals are dissolved in water to make an aqueous solution the crystal lattice is lost and the solution is a mixture of separate cations and anions.
53. Maleic acid … is a diprotic acid, i.e. it has two protons which could dissociate. When one comes off the result is a proton and a hydrogen maleate ion in solution. When the second proton comes off the hydrogen maleate the result is two protons and a maleate ion in solution. Maleic acid has a pKa1 of 1.94 and pKa2 of 6.22. Therefore at low pH (e.g. pH 3 or 3.5) the majority ionic species is hydrogen maleate and there will be some undissociated maleic acid; whereas at high pH (e.g. pH 8 or above) both protons will dissociate and the predominant species is maleate ion. In the context of hair care, before the priority date the skilled team would only have been aware of maleic acid's potential use as a chelating agent or pH buffer/modifier."
Claim 11
"The use of an active agent which is
or a simple salt thereofsimultaneously with a bleaching agent to reduce or prevent hair damage due to a treatment to provide bleached hair."
Interpretation of claim 11
"The opposite of a simple salt could be a double salt such as NaKCl2 which as a solid would have a different lattice from either NaCl or KCl. Or the opposite could be a complex salt in the sense of a salt in which one of the ions is a complex such as the hexaminecobalt ion made up of a cobalt atom and six amine elements in hexaminecobalt (III) chloride."
The judge considered that "simple" would most probably be understood in the second way. Again, there is no challenge to that conclusion
Extension of protection by the amendment
"The formulations typically contain at least two cosmetically acceptable excipients. In some forms the formulations contain the active agent, water, and optionally a preservative and/or fragrance."
Similar language is found repeatedly in the discussion of "sprays" at page 18 line 27 – page 19 line 9. Furthermore, at page 17 lines 27-31, the specification discusses "diluents" that may be included in the formulations, saying (emphasis added):
"Diluent, as used herein, refers to a substance(s) that dilutes the active agent. Water is the preferred diluent. … Alcohols, such as ethyl alcohol and isopropyl alcohol, may be used … ".
At page 21 lines 21-23 the specification discusses liquid active agent formulations which:
"may contain any suitable concentration of active agent in a suitable carrier, typically a diluent, such as described above."
"53. The Patent does not use the term 'simple salt' other than in the claims and at page 11 lines 7-8. It does, however, make the following relevant disclosures:
…
(c) in kits for treating hair, the active agent 'may be provided as a dry powder in a sealed package and the excipient provided in a vial or other container' (page 29 lines 9 – 13);
…
54. What the skilled person would take from these disclosures is that the term 'active agent' (i.e. maleic acid or a simple salt of it) is used where it is combined into something akin to a product. Notably in the kit described in the Patent, it is to be provided as a dry powder for dilution into a liquid excipient.
55. The skilled team would therefore consider a simple salt to be a form of maleic acid that makes it possible to easily dissolve or deal with the active agent in the mixture. …"
In the last sentence I have quoted the witness referred to dissolving or dealing with the active agent in a mixture. He was not specific as to what he meant by "deal with", but it does not appear that he was asked to explain this.
Obviousness over Kim
Kim
"Conventional hair dye compositions include a first agent containing one or more dye precursors, which typically form a dye on being oxidised, and one or more couplers; and a second agent which is a diluted hydrogen peroxide solution, which is mixed with the first agent before application to the hair. The purpose of using these hair dyes is to dye the hair as completely as possible while achieving a long-lasting hair dyeing effect.
However, the hair dyes currently available on the market have the drawback that the hair is damaged and becomes rough or loses its lustre after dyeing, because in order to increase the dyeing effect on the hair, the first agent has high alkalinity and the second agent uses hydrogen peroxide.
Problem the Invention is intended to Solve
Accordingly, the present inventors, upon thorough research of oxidation hair dye compositions that can reduce hair damage after dyeing, discovered that hair damage after dyeing could be further reduced by using a maleic acid derivative in combination with a hair dye composition containing an oxidation hair dye precursor …"
"These maleic acid derivatives protect the hair after dyeing according to the following mechanism: The thiol groups that are present in the hair bring about damage to the hair accompanied by a reduced tensile strength or breaking strength compared to the original hair through thiol-disulphide bond interchange reactions during a dyeing process. The thiol groups in the hair can undergo an addition reaction with a substance that has an α,β-unsaturated carbonyl group, such as maleic acid. Thus, the addition reaction of the thiol groups with maleic acid can lower the possibility of a reaction among the thiols within the hair, resulting in reduction of hair damage."
"Although this mechanism involves the disulphide bridges in the hair, an important point in the context of this case is that this concept of thiol-disulphide interchange causing damage to the hair involves a process of reduction rather than oxidation. When reduction breaks a disulphide bridge the result is two thiols, in effect [-S-S-] is turned into [-SH HS-]. By contrast when oxidation breaks a disulphide bridge the result is two group known as cysteic acid groups or sulphoxides, in effect [-S-S-] is turned into [-SO3- -O3S-]."
"The degree of damage to hair is often evaluated by hair tensile strength. Lowered tensile strength indicates severe hair damage due to changes in the protein structure within the hair.
Effect of the Invention
This invention relates to a novel hair dye composition, more specifically, to a hair dye composition which contains an oxidation dye precursor and further a maleic acid derivative, thus allowing to reduce the rate of the reduction in hair tensile strength due to dyeing and accordingly reduce hair damage."
The law
The judgment
"245. The question in the end is whether it would be obvious to the skilled person to apply the idea disclosed in Kim of using an additive such as maleic acid to reduce or prevent hair damage due to a hair lightening treatment involving bleaching without dyeing.
246. Despite the elaboration given to this issue at trial, in my judgment this question turns on a short and simple point. The skilled person knows as part of their common general knowledge that a hair lightening treatment involving bleaching without dyeing is a highly oxidative environment. The mixtures used generally include hydrogen peroxide and persulfate. The damage caused by that sort of treatment was known to be damage caused by that oxidative environment. For the skilled person to think it was worth using any of the additives disclosed in Kim, they have to believe that those additives might have an effect in a system in which the damage the additive is there to deal with is caused by oxidation.
247. In my judgment it would not be obvious to the skilled person that the maleic acid derivatives in Kim might (let alone would) have a protective effect against damage caused by oxidation. That is for the following reasons. First and foremost the thiol-disulphide mechanism actually proposed by Kim is concerned with reduction not oxidation. The skilled person would regard it as scientifically credible. Dr Hefford agreed that the mechanism would be viewed as credible although in cross-examination (but not in his report) he suggested that while it was credible as a phenomenon, it was not as an explanation for a reduction in tensile strength. I was not convinced by that qualification. As a matter of common general knowledge, both oxidation and reduction were known to damage disulphide bonds and were known to reduce the tensile strength of hair. Since reduction was known to cause damage, the idea that an additive might act to reduce that damage by interacting with thiol groups to prevent it is credible. There was then a point on the availability of thiol groups but I am not satisfied the skilled person's thinking would go so far as to delve into the likely number and availability of thiol groups so as to lead to doubts about Kim's mechanism. That degree of insight and thought is a hallmark of inventiveness (or hindsight).
248. Second the Kim document goes out of its way to propose a mechanism. It does not simply present data and leave the reader to infer how it is working. On the face of it the inventors of Kim have done the tests they say they have done and perhaps done more tests too.
249. Third, although oxidative dye compositions do use oxidising agents, they are known to have a much less aggressively oxidising effect than the hair lightening treatments which involved bleaching without dyeing. The latter had two aggressive oxidisers – hydrogen peroxide and persulfate. The former had hydrogen peroxide alone. Olaplex overstate the case sometimes when seeking to downplay the significance of the hydrogen peroxide in an oxidative dyeing composition. While its function was in part to oxidise the dye precursors, it is clear that the skilled person would, as a matter of common general knowledge, understand that the hydrogen peroxide would often act by bleaching the hair as well. That would not always happen to an appreciable extent but it often would. That effect of the hydrogen peroxide was understood to be the cause of hair damage with repeated use of oxidative dyeing treatments. However Kim does not say anything which purports to link the hydrogen peroxide in the oxidative dye formulations described with the damage mitigated by the maleic acid derivatives. That would be contrary to the mechanism Kim proposes.
250. Fourth, although Kim does make clear that the proposal relates to oxidative dyes and a system with oxidative dye precursors, it does also expressly contemplate a system with direct dyes and therefore no hydrogen peroxide at all. Albeit that no results are presented for direct dyes, that suggestion is inconsistent with the effect being one associated with oxidative damage.
251. For a skilled person to think that maleic acid would work to prevent damage in a pure bleaching system with no dye would involve that person thinking they knew better than Kim. It is not the law that the skilled person is bound to follow whatever mechanism is proposed in a prior teaching nor is it the law that it is necessarily inventive to go against or beyond such a teaching. It always depends on the facts of the particular case. I accept this is an empirical art and that the skilled person would be interested in the data in Kim ….
252. The problem for L'Oréal is that the skilled person is aware that chemical reduction can cause damage to hair and so there is no reason for an uninventive skilled person to disbelieve Kim. For a skilled person to go ahead and test maleic acid in a hair lightening formulation involving hydrogen peroxide, persulfate and no dye would be an act of invention. …"
The appeal
"Had the learned judge construed Kim correctly and identified that it disclosed that maleic acid was effective in treating damage caused to keratin by the presence of hydrogen peroxide he would have held it was obvious to apply that teaching to bleaching without dyeing because the mechanism of damage was known to be the same."
The application to adduce further evidence
The underlying issue
L'Oréal's case at trial and the judge's conclusions
The application
The further evidence
Applicable principles
"Thus one can see the Court of Appeal [in later cases] struggling to reconcile the apparent statement of principle in Barrell [1973] 1 WLR 19, coupled with the very proper desire to discourage the parties from applying for the judge to reconsider, with the desire to do justice in the particular circumstances of the case. This court is not bound by Barrell or by any of the previous cases to hold that there is any such limitation upon the acknowledged jurisdiction of the judge to revisit his own decision at any time up until his resulting order is perfected. I would agree with Clarke LJ in Stewart v Engel [2000] 1 WLR 2268, 2282 that his overriding objective must be to deal with the case justly. A relevant factor must be whether any party has acted upon the decision to his detriment, especially in a case where it is expected that they may do so before the order is formally drawn up. On the other hand, in In re Blenheim Leisure (Restaurants) Ltd, Neuberger J gave some examples of cases where it might be just to revisit the earlier decision. But these are only examples. A carefully considered change of mind can be sufficient. Every case is going to depend upon its particular circumstances."
The judge's reasoning
i) It would not be right for the outcome of the present application to be determined by the case management decision to exclude the issue at trial, rather it should be considered afresh.
ii) Olaplex had not acted to their detriment in reliance upon the Main Judgment, and that was a significant point in L'Oréal's favour.
iii) He would assume, in the absence of evidence to the contrary, that Olaplex had known for some time that the product of Example 1 of US 239 was in fact the di-maleate. Nevertheless, there had been no failure of disclosure or lack of candour by Olaplex. Moreover, the prosecution file for WO 768 is public. L'Oréal had been able to scrutinise it throughout the litigation, and had done so.
iv) If L'Oréal had thought about the case they now wished to advance before trial, they would have been able to adduce the experimental and expert evidence which is now sought to be admitted at trial. The reason why L'Oréal did not do was because they did not think of the point until just before trial. Moreover, when L'Oréal thought of the point, they did not seek an adjournment to allow it properly to be addressed by the parties, in particular by adducing evidence on the question of inevitability. On the contrary, L'Oréal took a tactical decision to try to make the point good exclusively through the cross-examination of Prof Haddleton. Thus the present application was an attempt by L'Oréal to extricate themselves from the consequences of their own prior omissions and decisions.
v) If the further evidence was admitted, it would necessitate witnessed repetitions of the new experiments, possibly experiments in reply by Olaplex, expert evidence in reply from Olaplex, cross-examination of the experts and argument at a second trial lasting two-three days (not including judicial pre-reading and judgment writing). The issues to be considered at the second trial would be (a) the inevitability of the result of following Example 1 of US 239, (b) how the skilled team would respond to Example 1 if they were not considering the possibility that the product was misdescribed, (c) whether Example 1 of US 239 was a clear and unambiguous disclosure of the di-maleate even if that was the inevitable result of following it and (d) whether Olaplex could avoid anticipation of claim 11 by a further amendment to disclaim the active agent in Example 8 of WO 768. All of these issues were properly arguable.
The appeal
i) He misapplied the test in Re L by saying that "[f]or such an order to be in accordance with the overriding objective there must be something about the circumstances to justify that course given its inevitable consequences in terms of cost and trouble to the parties of a further trial but also the allocation of the court's resources to these litigants as well as others" (Second Judgment at [59]).
ii) He should not have placed reliance upon the fact that the new evidence did not make "all the difference between success and failure on the issue of priority" (Second Judgment at [66]).
iii) He was wrong to attach weight to the fact that L'Oréal could have sought an adjournment during the trial, but decided not to (Second Judgment at [66]).
Conclusion
Lord Justice McCombe:
Lord Justice Davis: