Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales High Court (Technology and Construction Court) Decisions
You are here: BAILII >> Databases >> England and Wales High Court (Technology and Construction Court) Decisions >> Innovate Pharmaceuticals Ltd v University of Portsmouth Higher Education Corporation [2024] EWHC 35 (TCC) (12 January 2024)
URL: http://www.bailii.org/ew/cases/EWHC/TCC/2024/35.html
Cite as: [2024] EWHC 35 (TCC)
[New search] [Printable PDF version] [Help]
Neutral Citation Number: [2024] EWHC 35 (TCC)
Case No: HT-2021-000478
IN THE HIGH COURT OF JUSTICE
BUSINESS AND PROPERTY COURTS OF ENGLAND AND WALES
TECHNOLOGY AND CONSTRUCTION COURT (KBD)
Royal Courts of Justice
Rolls Building
London, EC4A 1NL
Date: 12/01/2024
Before :
MR ROGER TER HAAR KC
Sitting as a Deputy High Court Judge
- - - - - - - - - - - - - - - - - - - - -
Between:
|
|
INNOVATE PHARMACEUTICALS LIMITED |
Claimant |
|
|
- and - |
|
|
|
UNIVERSITY OF PORTSMOUTH HIGHER EDUCATION CORPORATION |
Defendant |
- - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - -
Thomas Roe KC and Katharine Bailey (instructed by JMW Solicitors LLP) for the Claimant
Clare Dixon KC, Nicholas Zweck and William Birch (instructed by Eversheds Sutherland (International) LLP) for the Defendant.
Hearing dates: 4, 5, 9, 10,11,12, 16, 17, 18, 19 October, 29, 30 November 2023
- - - - - - - - - - - - - - - - - - - - -
Approved Judgment
This judgment was handed down remotely at 10.30am on 12 January 2024 by circulation to the parties or their representatives by e-mail and by release to the National Archives.
.............................
Mr Roger ter Haar QC :
1. In this action the Claimant (“Innovate”) claims damages from the Defendant (“UoP”) arising out of a Research Agreement between those parties. The claim is highly unusual turning as it does upon an academic research paper published in a well-respected scientific journal which paper is alleged to have been infected by errors which were said to be at least careless, but for reasons which are important to limitation of liability clauses in the contract, are also said to have been the product of dishonesty.
The Claimant
2. Innovate was incorporated on 9 September 2014. Since February 2017 Innovate has been owned by:
(1) Simon Cohen, a podiatrist with a history of working for pharmaceutical companies and a full-time employee of Innovate;
(2) Dr Michael James Stuart, a Doctor and the sole director of Innovate; and
(3) Jan Cohen, a lawyer and Mr Simon Cohen’s brother. Where in this judgment I refer to “Mr Cohen”, I am referring to Mr Simon Cohen rather than Mr Jan Cohen.
3. Innovate holds the patent to a formulation of liquid aspirin known as IP1867B or Glioprin. The formulation is made up of 2.5% aspirin, 1% saccharin and 96.5% triacetin, which is a commonly used excipient in cosmetic and pharmaceutical formulations as a solvent. In this judgment, IP1867B is referred to as “the Drug”.
4. Innovate was incorporated by the shareholders as the vehicle through which they would seek to develop and commercially exploit the Drug. I set out in somewhat more detail below the evidence, which I accept, as to the development of the Drug.
The University and the Brain Tumour Centre of Excellence
5. The University of Portsmouth traces its roots back to the 19th century. In 2010 UoP established a “Brain Tumour Research Centre of Excellence” led by Professor Geoffrey Pilkington, Professor of Cellular and Molecular Neuro-oncology. By the time of the events with which this Court is concerned, Dr Richard Hill was the Group Leader of the Novel Therapeutics Unit at the Centre.
6. The Centre was funded, in part, by, and derived its name from, a charity called Brain Tumour Research (“BTR”). BTR was co-founded by Sue Farrington-Smith MBE when she lost her niece to brain cancer. Professor Pilkington’s evidence was that in the early 2000s Ms Farrington-Smith reached out to him as an individual with a high profile in the area of brain cancer and they developed a close working relationship. He encouraged her and the charity she founded to establish a number of research centres, dedicated to finding treatment for brain tumours. In 2010 the Brain Tumour Research Centre of Excellence was the first of these centres to receive an endowment. Imperial College London, Queen Mary University of London and the University of Plymouth were subsequently granted endowments and have BTR Centres of Excellence.
7. Professor Pilkington’s evidence was that BTR aspired to provide each Centre of Excellence with funding of £1 million a year, but during the time that he was Head of the Centre at Portsmouth the UoP did not receive this level of funding: BTR’s ability to fund the Centres was dependent on its fundraising and was inherently uncertain. The UoP team applied for grant funding from various bodies and charities to support the Centre’s work and supplement the BTR endowment. Some of the Centre’s costs were funded by the UoP. A small amount of funding also came from industry collaborations.
Types of testing
8. This section of this judgment and that which follows are principally based upon the evidence of the Defendant’s liability expert, Professor Martin Bushell, but I do not understand any part of the contents of these two sections to be controversial.
9. This case concerns the reporting of testing of the Drug. Three types of studies are used to test new or repurposed drugs: in vitro studies, in vivo studies and clinical trials or studies.
10. In vitro studies are carried out on biological cells that have been removed from their normal biological context (e.g. through a biopsy), and can be considered to be “test-tube experiments”.
11. In vivo studies are carried out on biological cells in their normal biological context, so are tests on whole, living organisms or cells, usually animals - in vivo studies can therefore be considered to be “animal testing”.
12. Studies involving humans are usually referred to as clinical trials or studies rather than as in vivo studies.
13. There was put in evidence before me a document entitled “Guidelines for phase 1 clinical trials 2012 edition” [1] which says under the heading “Developing a new medicine”:
The pharmaceutical industry is the main sponsor of medicines research in the UK. Sponsors have to demonstrate the safety, quality and efficacy of a potential new medicine - called an investigational medicinal product (IMP) - through a series of rigorous trials in humans in order to obtain a licence, so that doctors can give the medicine to patients.
However, before an IMP can be given to humans, sponsors must first test it thoroughly in animals. The main aim of these pre-clinical studies are:
· to find out the effects of the IMP on body systems (pharmacodynamics)
· to study the blood levels of the IMP, and how it is absorbed, distributed, metabolised and eliminated after dosing (pharmacokinetics)
· to find out if a range of doses of the IMP, up to many times higher than those intended for use in humans, are toxic to animals and if so, to identify the target organs and the margin of safety in terms of (a) the no-observed-adverse-effect dose level (NOAEL) relative to body weight and (b) IMP exposure - the concentration of IMP in the bloodstream over 24 hours (toxicokinetics), and
· to make a formulation of the IMP, such as a capsule or injection, suitable for early studies in humans.
After the pre-clinical studies, there are four phases of trials in humans, which in practice often overlap. Phases 1 to 3 are done before a licence is granted and Phase 4 is done after authorisation to market the drug. The phases are different in terms of the number and types of subject studied, and the questions asked. The numbers in the table are indicative only and can vary.
|
Phase |
Number and type of subject |
Questions |
|
1 |
50-200 healthy subjects (usually) or patients who are not expected to benefit from the IMP |
· Is the IMP safe in humans? · What does the body do to the IMP? (pharmacokinetics) · What does the IMP do to the body? (pharmacodynamics) · Might the IMP work in patients? |
|
2 |
100-400 patients with the target disease |
· Is the IMP safe in patients? · Does the IMP seem to work in patients? (efficacy) |
|
3 |
1000-5000 patients with the target disease |
· Is the IMP really safe in patients? · Does the IMP really work in patients? |
|
4 |
many thousands or millions patients with the target disease |
· Just how safe is the new medicine? (pharmacovigilance) Does the medicine work in the real world? (real world data collected to demonstrate value) · How does the new medicine compare with similar medic [sic] |
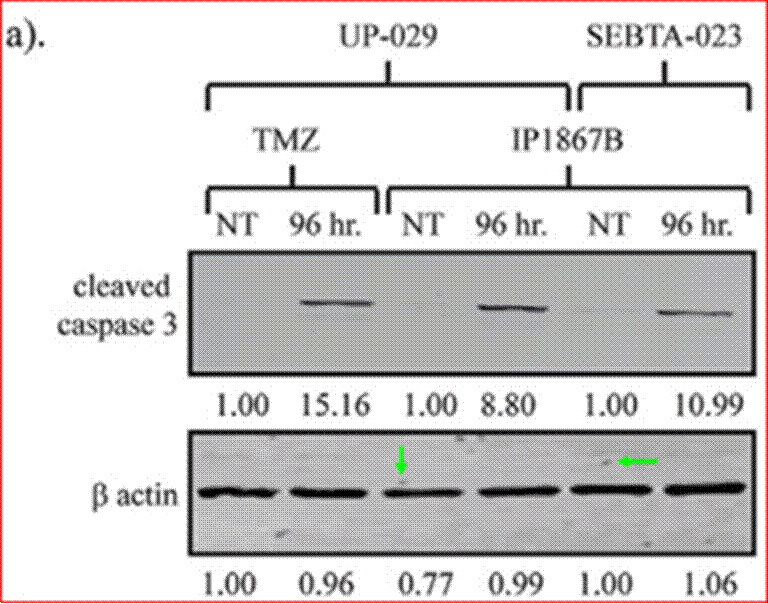
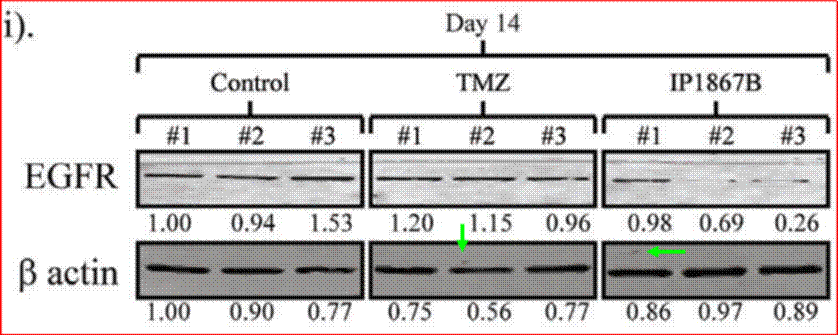
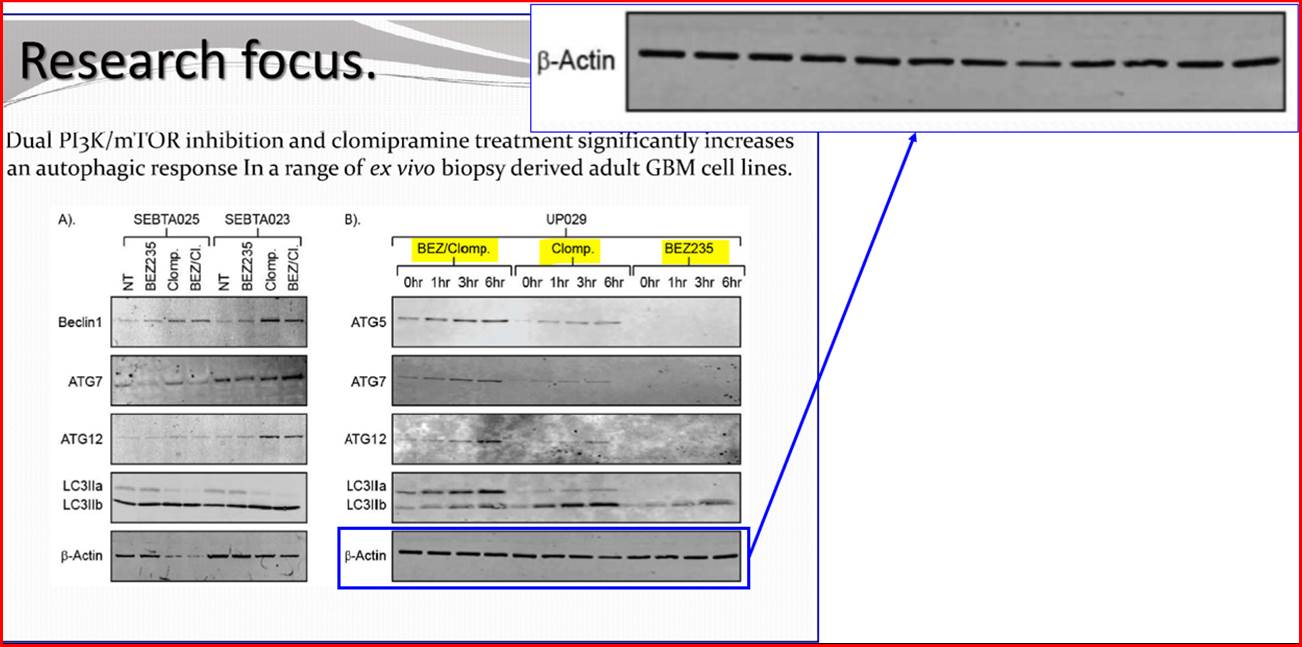
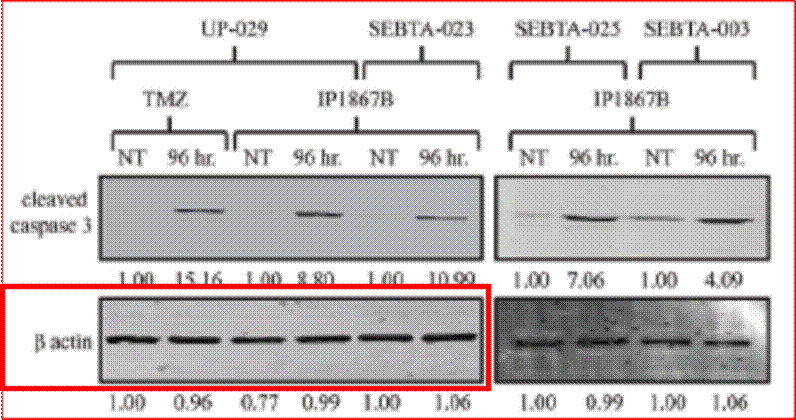
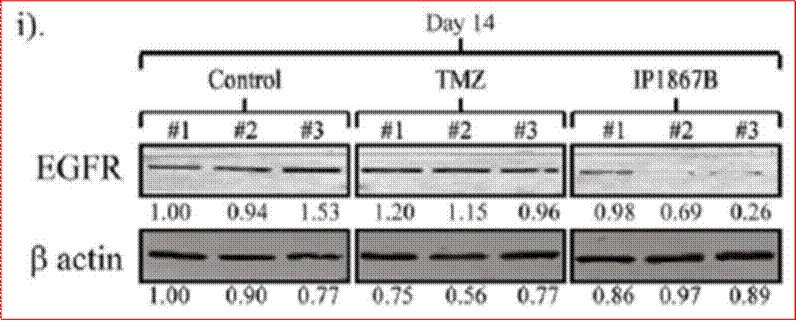
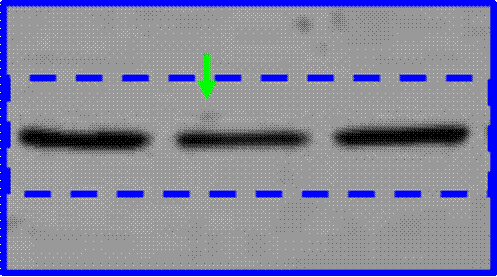
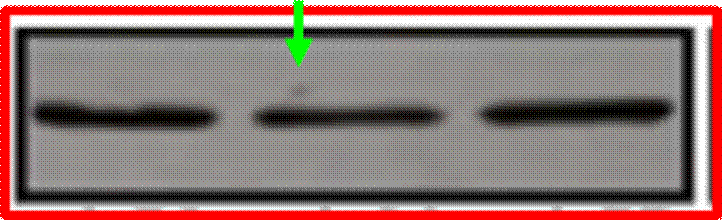
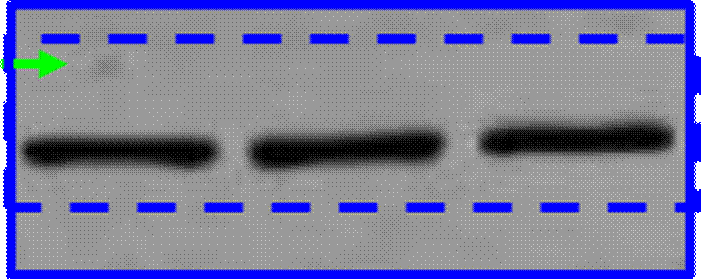
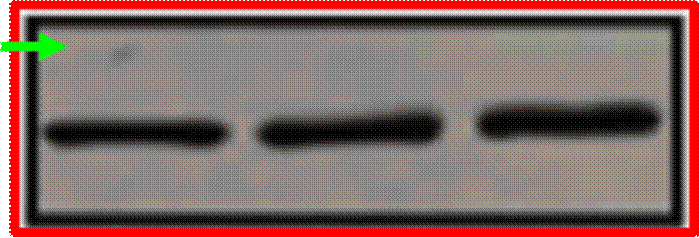
Phases are also often subdivided. For instance, small-scale, exploratory efficacy studies in a limited number of patients may be referred to as ‘Phase 2a’. In contrast, slightly larger trials that test the efficacy of a compound at different doses (‘dose-range finding’ studies) might be designated ‘Phase 2b’.
Key Scientific Terms that feature in the Proceedings
Glioblastoma Multiforme or “GBM”
14. The most common form of brain tumour is a Glioma. Gliomas can be grouped according to how quickly they are likely to grow, with grade 1 being the slowest and grade 4 the fastest. Grade 4 gliomas are also known as Glioblastoma Multiforme (“GBM”). Treatment for the disease may involve surgery, radiotherapy and/or chemotherapy. Temozolomide (“TMZ”) is a chemotherapeutic commonly used in the treatment of GBM. Although treatment is effective in some situations, the clinical prognosis for GBM sufferers is generally poor and additional treatments are required.
15. In order for a drug to be effective in the treatment of GBM, it would need to be established that:
(1) it could penetrate the barrier between the blood and the brain (in which the GBM is situated), which barrier is known as the blood-brain barrier;
(2) (a) it could shrink GBM and do so in a manner comparable with or better than existing GBM treatments, and, (b) if it could shrink GBM, the mechanisms that allowed it to do so would need to be understood;
(3) it was relatively well tolerated by bodily cells other than those contained in the GBM; and
(4) it does not give rise to unacceptable side effects.
IGFR and EGFR
16. The terms IGFR (also IGF1 and IGF1R) and EGFR are of significance in this case.
17. Cells, including cancer cells, have receptors on their surface. Protein molecules can interact with those receptors, the effect of which interactions can be to cause the cell to behave in a certain way.
18. Epidermal Growth Factor Receptor (“EGFR”) stimulates tumour cell growth when signalled to do so by the Epidermal Growth Factor protein.
19. If the effectiveness of EGFR is reduced, then tumour cell growth will probably be reduced because the Epidermal Growth Factor protein has a diminished capacity to stimulate tumour cell growth. A known way of suppressing cancer growth or survival is therefore to block or reduce the effectiveness of EGFR.
20. Treatments which inhibit EGFR are known as EGFR Inhibitors. There are a number of such treatments available including Gefitinib and AZD3759 (both of which cross the blood-brain barrier), also Erlotinib (marketed as Tarceva, a generic/off-patent drug), Lapatinib and Cetuximab.
21. Over time tumour cells can develop resistance to EGFR Inhibitors. This resistance can arise as a result of a protein called Insulin-like Growth Factor 1 (“IGF1”). In a similar way to which the Epidermal Growth Factor protein can interact with a tumour cell’s EGFR to stimulate tumour growth, the IGF1 protein can instigate cellular responses when it interacts with a tumour cell’s Insulin-like Growth Factor Receptor 1 (“IGF1R”), which is another type of cell surface receptor. The operation of IGF1R is known to cause tumour cells to develop resistance to EGFR Inhibitors.
22. Therefore, a drug which reduces the operation of IGF1R could enable more effective cancer treatment because:
(1) a reduction in the operation of IGF1R would reduce the tumour cell’s resistance to EGFR Inhibitors;
(2) a reduction in resistance to EGFR Inhibitors may allow the EGFR Inhibitors to have the intended effect of blocking or reducing the effectiveness of EGFR; and
(3) this would reduce the stimulus given to the tumour cells to grow.
23. A number of known EGFR Inhibitors were used in the research by UoP with which this case is concerned, either in conjunction with, or as a comparator against, the Drug. These EGFR Inhibitors included Gefitinib and AZD3759. UoP’s research also involved the use of the Drug either in conjunction with, or as a comparator against, TMZ.
Gene expression, “transcription” and “translation”
24. At its most basic, an organism’s genetic material is contained in chromosome molecules, which can be found within the nuclei of the organism’s cells. That genetic material can be understood as a set of instructions for the development, functioning, growth and reproduction of the organism (at a molecular level).
25. A chromosome is made up of a very long DNA molecule. Specific sections of that DNA molecule are known as genes. A chromosome therefore consists of a single, very long DNA molecule, along which thousands of genes are encoded.
26. Genes operate by encoding proteins; these proteins then dictate cell function. A particular gene can therefore best be thought of as containing a set of instructions for the creation of a particular protein, which protein (once created) in turn dictates a particular cell function. The overall process of a protein being made from its corresponding DNA is referred to as “gene expression”. Measuring the presence of a particular protein can therefore be used to indicate the level of a particular gene expression.
27. There are two main stages to gene expression: “transcription”, followed by “translation”:
(1) The “transcription” process involves a segment of DNA being copied or “transcribed” into a messenger RNA (“mRNA”) molecule. mRNA molecules act as intermediaries between genes and the proteins that the gene encodes. The term transcription therefore refers to the process of a gene creating or “transcribing” mRNA molecules.
(2) The “translation” process involves the mRNA molecules (that were created or “transcribed” during the transcription process described above) being decoded to form specific functional protein molecules. Those protein molecules in turn dictate particular cell functions.
28. Measuring the level of particular mRNAs can therefore determine how many copies of the gene have been made in the transcription process. So, one way of measuring the level of a particular gene in a cell would be to measure the level of that gene’s mRNA. mRNA can be measured with a technique called quantitative polymerase chain reaction (“RT-qPCR”).
29. From a terminology perspective, a reference to “EGFR”, without further context, may be to either the EGFR gene (i.e. the particular part of the DNA or mRNA molecule that contains the instructions for encoding EGFR proteins), or to the EGFR protein itself.
The blood-brain barrier (“BBB”)
30. The vasculature (blood supply) delivers a variety of things to our bodily tissues, including oxygen, nutrients, antibodies and immune cells. This permeability comes with the risk that pathogens may also transfer from infected blood to tissues, or from one infected site via blood to another site in the body. The central nervous system, including the brain, has a tighter, less permeable, vasculature that is referred to as the blood-brain barrier (“BBB”). This precludes the transfer of antibodies and immune cells to the brain, but also pathogens such that infections of the central nervous system are very rare.
31. These characteristics of the BBB pose problems for the treatment of many disorders as not all therapies can cross the BBB. Drugs that are lipid (fat) with an atomic mass less than 400 Daltons can, however, pass across the BBB. This applies to aspirin, which is very lipid soluble and has an atomic mass of 180 Daltons.
Brain Tumour Treatments and Drug Repurposing
32. My findings in this section of this judgment are based upon the evidence in Professor Pilkington’s first witness statement.
33. Brain tumours are very diverse and every patient is different so a range of treatment options is needed. Unfortunately, because of the lack of funding for brain tumour research and the difficulties of treating tumours in the brain, the options are very limited. Surgery is often the main treatment, but sometimes a tumour may be too difficult to reach or the risk of damaging the brain may be too high. As already mentioned above, brain tumours can also be treated with radiotherapy and chemotherapy. The chemotherapy options for brain tumours are very limited in comparison with other cancers because of the need to cross the BBB.
34. As this case illustrates, there has been and is considerable interest in using repurposed drugs in brain tumour treatment. Drug repurposing involves identifying new therapeutic uses for medicines that are outside the existing licence for the medicine. It is a relatively quick and cheap way of making new treatments available to patients and clinicians. Developing brand new drugs (that is, new chemical compounds) can take many years and huge amounts of money, largely because of bottlenecks in the therapeutic development process. Agents which have been approved for other uses have already been tested in humans and there is already information available on dosage and toxicity. Short-cuts in the drug development process may therefore be available. However, in order to get an existing drug licensed for a new therapeutic use, a significant amount of work still has to be done in order to demonstrate efficacy and safety to the relevant regulatory bodies, including pre-clinical studies and clinical (i.e. human) trials. It is difficult to get funding for clinical trials for repurposed drugs because such trials are expensive and no-one appears to have a commercial interest in these drugs (i.e. they are no longer under patent so will not bring in substantial monies to the drug companies). If clinical trials funded by one party were a success, there would be nothing to stop other parties from developing and marketing the repurposed drug for the new therapeutic use. There is therefore no commercial gain for funding such clinical trials in the first place.
35. A considerable amount of work has been done on the possibility of repurposing a drug called Clomipramine as a treatment for brain cancer. Clomipramine is a tricyclic anti-depressant. There is a lot of anecdotal evidence that brain tumour patients who are prescribed Clomipramine have improved survival rates. It has also been shown that Clomipramine has a protective effect; there was a study which showed that patients who had taken the drug long-term had a reduced incidence of brain tumours and certain other types of cancer. Therapeutically, on the basis of the anecdotal evidence relating to Clomipramine, some clinicians will prescribe the drug “off-label” as an adjunct to other brain tumour treatments like immunotherapy and radiotherapy. The problem with using Clomipramine in combination with other therapies is that it is impossible to isolate and assess the impact the drug is having separate to the impact of the other treatments. Professor Pilkington was very keen to do more work on Clomipramine, to progress understanding of whether it was an effective agent in the treatment of brain tumours in isolation, so that it could obtain regulatory approval for use in that field.
36. Reformulated drugs are those repurposed drugs which have been altered in some way or combined with other agents. These may offer a greater funding possibility for pharmaceutical companies, but clinical trials in this area are infrequent and, when they do take place, usually designed in such a way that the data from them is negated. The repurposed drug is not adopted as the “gold standard” treatment (that is the treatment which is widely accepted as being the best available method for treating a disease) but is instead tacked on as a further treatment for patients who have already received the gold standard treatment (given the impact that denying the gold standard treatment to a patient may have on their clinical outcome). The data received from the trial is therefore compromised.
The Development of the Drug
37. By the above definitions, the Drug appears to me to be a reformulated drug (I asked the two liability experts about this, and they both agreed that this was correct) [2]. I return below to the characteristics of aspirin.
38. The following passage from Mr Cohen’s first witness statement, which I accept as accurate (subject to the claim that the Drug is unique, which is a matter discussed in the liability expert evidence, and to the claim that the Drug is valuable, which is discussed by the quantum experts), describes the background to, and the process of, development of the Drug:
4. Innovate has created the world’s first (and only) stable, liquid form of aspirin. The drug is known as IP1867B (the “Drug”). The Drug was created by myself and the two other shareholders of Innovate, Michael James Stuart (“Jimmy”) and my brother Jan Cohen (“Jan”). We incorporated Innovate on 9 September 2014 and since then have been developing and testing the potential uses of the Drug and exploring the potential benefits which a liquid form of aspirin could offer to the treatment of patients. Our ultimate aim is to see the drug (if and to the extent that it proves to be useful) being used in clinical practice worldwide.
5. Prior to us forming Innovate, Jimmy and I were neighbours and had children of a similar age. We became friends and had a mutual interest in Pharmaceuticals. Jimmy is the Clinical Director at Manchester University Hospital and I am a qualified podiatrist and have a long history of working for numerous pharmaceutical companies (such as Pfizer, Merck and Ferring between 1990 and 2014) and acting as a liaison between them and the NHS.
6. In or around 2009/2010 Jimmy and I were discussing what could be done in the field of repurposing existing ‘off-patent’ drugs [3]. Jimmy said that one drug which could be repurposed, if it could be made, was a liquid form of aspirin. Jimmy thought that would be easy but I was less convinced. I started looking into it. It turned out that it was a real problem: aspirin hates water and is very resistant to being solubilised and rapidly degrades. Therefore, despite aspirin being around for years, no one had ever managed to make a liquid form.
7. We therefore got in touch with a company called Croda PLC. Croda were the world number one excipient foundation technicians and agreed to work with us to develop liquid aspirin. The process was incredibly complex involving lots of trial and error. It took four years in total but we finally found a combination that was able to solubilise aspirin and keep it stable enough to be used (the Drug).
8. Once we had established that we had managed to develop a stable form of liquid aspirin, we knew we had something unique and valuable. We wanted to explore the treatment possibilities for the Drug and how it could help patients. This is when we decided to seek patent protection in order to give ourselves control over the commercial exploitation of the Drug. This in itself was a detailed and expensive process which Jan carried out with the assistance of our patent lawyer Mewburn Ellis. The patent was registered in the UK on 15 November 2016 and we also have patent protection in 38 European countries, Argentina, the United States of America, China, and India.
39. Before turning to set out the history of the testing of the Drug, I will set out the agreed expert evidence as to the nature of aspirin.
The Nature of the Drug
40. Section D (paragraphs 34 to 42) of Professor Bushell’s first report discusses the attributes of the Drug. Those paragraphs are agreed by the Claimant’s expert, Professor Susan Short.
41. Professor Bushell says at paragraphs 34 to 41:
34. IP1867B is a liquid formulation containing the drug Acetylsalicylic acid (more commonly known as aspirin). Only 2.5% of IP1867B is aspirin; the formulation also contains saccharin (i.e. artificial sweetener) (1%) and triacetin (96.5%) which act as excipients. [4] Triacetin is a molecule derived from glycerol that is often used as an excipient in cosmetic and pharmaceutical formulations as a solvent. A number of studies have considered triacetin’s potential to assist delivery of chemotherapeutics across the BBB, including for the treatment of gliomas.
35. Aspirin is an anti-inflammatory agent that is used to provide paid relief in multiple settings. It has approved drug status. This means that data on aspirin has been reviewed by the relevant agencies and it has been determined to provide benefits that outweigh its known and potential risks for the intended population. Aspirin has been reported to have anti-cancer effects in the treatment and/or prevention of some cancer types. One complication of its use, however, is that aspirin can cause irritation, inflammation and sometimes haemorrhage of the stomach or duodenum through repeated use. Due to the formulation of IP1867B, it has been suggested that it has a reduced impact on stomach and duodenal inflammation over regular aspirin. The only evidence to support these claims regarding gastric inflammation that I am aware of is of a preliminary nature, namely a small study conducted by Liverpool University in or around March 2017 (see paragraph 42.2 below) and data showing a lack of particulate nature in IP1867B.
36. Prior to commencement of the Research Programme, there was nothing in the peer-reviewed published literature regarding IP1867B. Moreover, from the documents I have reviewed for the purpose of preparing this Report, investigation of IP1867B prior to the initiation of the Pilot Study and Research Programme appears to have been minor. Subsequent research involving IP1867B is summarised at paragraph 42 below and is limited as explained. As such, although IP1867B contains an approved drug, the formulation cannot be considered a drug for medicinal use as it has not been tested sufficiently and approved for such use.
37. In addition, from the above, it cannot be said that IP1867B has “proven efficacy against cancer types, including Glioblastoma, and greater efficacy than normal (tablet) Aspirin in its ability to penetrate the blood brain barrier in order to medicate the brain”. [5] I understand from Eversheds that when Innovate was asked to explain this claim in the Proceedings, it referred to the research carried out by the University under the Research Programme in respect of the alleged efficacy against certain cancer types, and to a study carried out by Professor Teeling in respect of the alleged ability to penetrate the BBB (see paragraph 42.1 below regarding Professor Teeling’s study). A claim that IP1867B has “proven efficacy against certain cancer types” suggests that the formulation has been tested much more extensively than it has been, including in human trials. Professor Teeling’s study did not compare the ability of IP1867B to cross the BBB with that of regular aspirin. In summary, the research that I have seen does not establish that IP1867B has “proven efficacy against certain cancer types, including Glioblastoma” or “greater efficacy than normal (tablet) Aspirin in its ability to penetrate the blood brain barrier”. I understand that Innovate has also said in the Proceedings that before the commencement of the Research Programme, IP1867B was known to have “anti-inflammatory actions mediated via IL6, STAT3 and NF-kB and anti-tumorigenesis actions mediation via HDAC”. For the avoidance of doubt the research that I have seen does not establish that IP1867B has these properties either.
38. I understand from the documents I have reviewed that Innovate holds patents for IP1867B in the UK and the USA, as well as a number of other countries. I note that the US patent was filed on 22 December 2015 and was granted on 28 September 2021. I have not considered that patent in detail, but it appears that Innovate used some of the data generated from the Pilot Study in both that patent application and its UK patent application.
39. From a brief search of a public patent database (lens.org), I note that there are several other patents in the USA, not held by Innovate, that relate to other liquid forms of aspirin. These include, for example, dimethyl isosorbide (a liquid formulation of aspirin, patent filed in 1979) and aspirin modified coconut oil suspensions (patent filed in 1955). These are only two examples of liquid aspirin from nearly 77,000 results returned when searching for “liquid aspirin” related patents.
40. While the specific formulation of IP1867B is said by Innovate to be unique, other liquid formulations of aspirin have been described, and even tested in clinical trials. For example, the bioavailability and brain analgesic effects of a glycinated formulation of aspirin have been reported. A glucose aspirin has also been investigated. The formulations of aspirin in these studies were chemical ‘conjugates’, where glycine or glucose had been chemically linked to aspirin. These are quite different to IP1867B which is a mixture of aspirin with two excipients (no chemical linkage to another compound is involved). However a formulation called PL-2200 or PC-Aspirin, which was developed by PLX Pharma and is marketed as Vazalore, is more similar to IP1867B. In the case of PL-2200 the lipid is phosphatidylcholine - based and not triacetin, but my understanding is that the principle is the same (triacetin is a type of lipid formed from glycerol and fatty acids). Aspirin is very soluble in lipids and so in both cases a particle-free solution would result. There are a number of published studies involving PL-2200 (including in relation to potential anti-cancer applications), it is FDA- approved and is available through US pharmacies and online.
41. As far as I am aware, comparative studies of IP1867B and other forms of liquid aspirin have not been undertaken, and so it is not possible to say whether IP1867B would perform better or worse than these other forms. I expect that any pharmaceutical company contemplating an investment in IP1867B would want to see the results of such a study before investing, since the results would materially impact on the commercial rationale of the investment.
42. Professor Bushell then discusses at paragraphs 42.1 to 42.7 what studies had been carried out into the Drug.
43. In her second report at page C1/127, Professor Short says of Section D of Professor Bushell’s report:
Section II Part D describes prior knowledge on the drug IP1867B (‘the drug’) and sets these against certain claims by Innovate. These are agreed including point 37 regarding the proven efficacy of this agent against certain cancer types. I have not carried out an equivalent review of literature to Professor Bushell. I do not consider this to be relevant to the main argument and I do not refute his summary.
44. At the conclusion of their evidence, I asked both liability experts for their assessment of how novel liquid aspirin is. The evidence was as follows:
THE JUDGE: That may then lead into the next question which is this. After I have finished hearing your evidence I am going to have other experts who are going to talk to me about the drug market but I want your assistance leading into that on this. It is an idea how novel liquid aspirin was. As I understand it, we all know aspirin has been around for a long time and in its powdered or tablet form, we are very familiar. As I understand it, the two excipients, triacetin and the other one which I cannot remember, are also very well known. There is in Professor Bushell’s report some discussion of other tests being carried out really with much the same idea, I think so, what I would like to know is whether you are able to help as to if this drug moved forward it is going to be dramatically different from whatever else seems to be on the market or coming up on the market. Do you have any idea, and you may not have?
PROFESSOR SHORT: Yes, it is very difficult to give a global view of horizon scanning specifically for high-grade gliomas. There have been two new big drug trials in the last new year actually, which have brought new drugs on the market for specific high-grade gliomas. But what I would say is that this sort of repurposing of an aspirin compound, that would suggest quite a different mechanism of action as an anti-tumour drug that anything else that I know about that is out there so I think potentially this could be a novel mechanism, a novel approach to glioma treatment.
THE JUDGE: If it worked it could be quite something?
PROFESSOR SHORT: Yes. It could be, yes, it could well [be] differentiated from the other treatment approaches which are out there, which are immunotherapies and conventional cytotoxics sort of approaches, I would say.
PROFESSOR BUSHELL: One thing, if I could add to that, well, let’s see what you think about this. We have heard a lot about the mechanism of action of this drug in this context, okay, and it is well known that the mechanism of action of this drug in this context, okay, and it is well known that the mechanism of action of aspirin in normal contexts in the other way that we use aspirin is via COX-1, COX-2 these particular proteins that it functions via, and so far as in the Cancer Letters Paper and through all of the preliminary data that we have seen, there is no understanding about how this drug is exerting its effect in this setting. And for a pharmaceutical company to take that forward, or anybody to take that forward, they would really need to have a very clear understanding of the mechanism of action of how this is working. The other point I would well like to put.
THE JUDGE: Interrupting for a moment, I see Professor Short nodding in agreement.
PROFESSOR SHORT: Yes. I just mumbled the start of a sentence really at the end of what I was saying before that there are some indications of potential mechanism, but I think that nobody would be able to say that the cytotoxic mechanism, what really is happening when you are giving this drug to cells even in a dish, has been confirmed. I do not think it has.
THE JUDGE: Professor Bushell, I interrupted you because I saw Professor Short nodding.
PROFESSOR BUSHELL: Thank you. The one last point I would like to put is that in my report I did point out I think it was a compound called PL-220 or something along those lines.
THE JUDGE: 2200 I think.
PROFESSOR BUSHELL: That is on the market over in America and is used quite a lot over there for a number of indications. I think one other aspect that any pharmaceutical company would be interested in taking this forward would need to see is a head-to-head with those other compounds. In these studies which we have seen presented over the last few days, and I am sure you have seen even more over the last few weeks there has been no indication that this was actually taking place. So I think that is a real key aspect to this which has not occurred.
The Pilot Study
45. In 2015 Innovate and UoP agreed that UoP would carry out a Pilot Study. This commenced in or around November 2015. The pilot study involved taking half a dozen cell lines and half a dozen different GBMs. The tumours grown in the petri dishes had been proved to be resistant to existing drugs/treatments.
46. The Drug would then be dropped onto the tumours and it would then be checked regularly to see whether the tumour cells had grown, reduced or died. The Drug was tested against another drug called Vincristine and untreated cell lines as a control.
47. The Pilot Study was conducted principally by Dr. Hill. Professor Bushell, with whom Professor Short agrees, opines in paragraph 61 of his first report:
The results of the Pilot Study appeared to show some potential for IP1867B to be used as treatment for GBM as it appeared, in vitro, to kill tumour cells but was less toxic to non-cancerous brain cells.
48. This was promising. It is Mr Cohen’s evidence that after the results of the Pilot Study were known, Dr Hill and Professor Pilkington “sold us very hard” on the need to do a full study. I doubt if much salesmanship was necessary: the Pilot Study was clearly only the start and, if Innovate were to bring the Drug to market as a drug treating brain tumour, it was obvious further testing was necessary. Mr Cohen says (at paragraph 20 of his first witness statement) that what had been done thus far “was only in a petri dish. Lots of things work in a petri dish but it needs to be tested in real animals and humans. Dr Hill told me again about the BBB system and said that UoP had to be able to test and prove whether the Drug penetrated the BBB. He said that if the Drug doesn’t get past the BBB, it is pointless for brain tumours”. I have no doubt something like this was said. I also have no doubt that this told Mr Cohen nothing he did not already know.
The Research Programme: the agreement of the Contract
49. Discussions then took place between Innovate and UoP about a Research Programme.
50. UoP did not have facilities to carry out in vivo testing, but was expert at in vitro testing. Dr. Stuart’s evidence was that a “major attraction of using UoP for the Research Programme was that we were led to believe they had a model to test the Drug’s ability to cross the BBB” [6]. At this stage, there was no question of clinical trials.
51. Whilst the Pilot Study was carried out by UoP without charge, it was understood that there would now be a Research Programme for which UoP would be paid, partly by Innovate and partly by a charity called Headcase. Headcase was interested in the possibilities of Clomipramine, to which I have referred above. In a footnote to paragraph 4(i) of the Amended Particulars of Claim [7], Innovate said “The study also involved another drug, called Clomipramine, in which the Claimant had no interest and which is not relevant”. (This is relied upon by UoP in its written Closing Submissions as being relevant to the damages to be awarded for repeating the testing programme).
52. There was a question as to who would carry out the in vivo testing. Initially it was thought that this could be carried out by the University of Bergen. I have received some evidence as to why this did not happen, but I do not need to make any findings in that regard. It was agreed that Imperial College London (“ICL”) would carry out the in vivo study in mice (“the mouse study”).
53. It was also understood that as part of the Research Programme there would be investigations into Clomipramine as well as the Drug.
54. An Agreement was entered into on 7 July 2016 [8].
55. There are issues as to the scope of what UoP was contracted to do, particularly as to whether it included studies aimed at assessing whether the Drug was capable of passing the BBB.
56. There were some negotiations as to the terms of the Agreement between Innovate and UoP, which are relevant in respect of the application of the Unfair Contract Terms Act 1977 to a limitation of liability clause.
57. There are issues as to the construction of that limitation of liability clause: a central issue is whether or not Innovate can claim more than £1 million and loss of profits on the basis that Dr Hill was dishonest.
The Terms of the Research Agreement
58. The Research Agreement is in various versions in the trial bundle. The Parties agreed before me to use the version at D5/154 to 167.
59. The Agreement was between UoP on the one hand, and Innovate and Headcase on the other. Innovate and Headcase are referred to together in the Agreement as “the Funders”. Innovate itself is referred to as “the Company” and Headcase as “the Charity”.
60. The “Contract Period” is defined in Clause 1.2 as:
“…the period from the Effective Date to Research Programme end date, which is estimated to be 30 June 2017”.
61. The “Effective Date” is defined as “1 July 2016”.
62. “Results” is defined as meaning:
“… any inventions, designs, information, know-how, specifications, formulae, data, processes, methods, techniques, and other technology that are conceived, or developed by the University, its staff or agents, alone or jointly with others, during the course of the Research Programme and the Intellectual Property Rights therein.”
63. The “Principal Investigator” is defined as:
“… Dr Richard Hill in the School of Pharmacy and Biomedical Sciences, Faculty of Science at the University or of such other member of staff as the Funders and the University shall mutually agree.”
64. Clause 2 provides:
Research Programme
2.1 The Research Programme shall be supported by the Funders for Contract Period in the first instance. Any extension shall be arranged in accordance with clause 8.
2.2 The Principal Investigator shall keep the Funders regularly updated on the progress of the Research Programme by providing regular reports not less than four times a year (every three months); meeting with representatives of the Funders at times and places mutually agreed upon to discuss the progress and results, as well as ongoing plans, or changes therein, of the Research Programme. The Principal Investigator shall also hold regular conference calls/meetings with the Funders as and when important data is obtained ….
65. Clause 5 provides:
Publications
5.1 The Funders recognise that by charity law under the University policy, the results of the Research Programme should be publishable and agree that the Principal Investigator and any staff engaged in the Research Programme shall be permitted (including but not limited to) to presenting at symposia, national, or regional professional meetings, and to publish in journals, theses or dissertations, methods and results of the Research Programme, provided, however that (i) the Funders shall have been furnished copies of any proposed publication or presentation at least 30 days in advance of the submission of such proposed publication or presentation to a journal, editor, or other third party, and (ii) with respect to Clomipramine under the Research Programme, the Charity shall have 30 days, after receipt of said copies, to request a delay to such proposed presentation or proposed publication because there is a patentable or commercially sensitive subject matter which needs protection. In the event that the Charity makes such a request, the said Principal Investigator shall refrain from making such publication or presentation for a maximum 6 months from the date of receipt of such request for delay in order for the University to file UK and/or other patent application(s) directed to the patentable or commercially sensitive subject matter contained in the proposed publication or presentation and (iii) with respect of IP1867B under the Research Programme, the Company shall have 30 days, after receipt of said copies, to request a delay to such proposed presentation or proposed publication because there is a patentable or commercially sensitive subject matter which needs protection. In the event that the Company makes such a request, the said Principal Investigator shall refrain from making such publication or presentation for a maximum 6 months from the receipt of such request for delay in order for the Company to file UK and/or other matter contained in the proposed publication or presentation. In the event that the University receives no such request for delay it shall be entitled to proceed with the publication without amendment …..
66. Clause 6 provides:
Intellectual Property
6.1 For the avoidance of doubt all Background Intellectual Property used in connection with the Research Programme shall remain the property of the party introducing the same. By operation of this Agreement, no Party transfers to another Party, any patent right, trademark right, copyright or other proprietary right that are owned or controlled by a Party prior to the execution of the Agreement.
6.2 Clomipramine: All rights to Results relating to Clomipramine under the Research Programme shall belong to the University in the first instance. The University grants to the Charity the perpetual non-exclusive right to use the Results for any non-for-profit (non-commercial) purpose, including research, education and publication, (the latter in accordance with the terms set out at clause 5).
6.3 In the event that the results relating to Clomipramine provide a commercial opportunity, the Charity and the University shall use their reasonable endeavours to agree terms for the mutual benefit of the Parties in exploiting such opportunity, including technology transfer and intellectual property rights management and revenue sharing.
6.4 IP1867B: All rights in IP1867B under the Research Programme (IP1867B related studies) and any Resulting Intellectual Property therein which is or may be patentable or otherwise protectable, shall belong to the Company. The Company grants the University the perpetual, irrevocable non-exclusive right to use the Results for any not-for-profit (non-commercial) purpose including research, education and publication, (the latter in accordance with the terms set out at clause 5 and clause 7).
67. Clause 11.1 provides:
Liabilities
11.1 Whilst the University will use all reasonable skill and care to ensure the accuracy of the work performed and any information given, the University makes no warranty, express or implied, as to accuracy and will not be held responsible for any consequences arising out of any inaccuracies or omissions unless such inaccuracies or omissions are the result of negligence on the part of the University or its agents.
11.2 Subject to clause 11.1 above, the Parties agree and declare that the obligations of the University and its agents shall cease upon delivery of the reports and that no liability whatsoever either direct or indirect shall rest upon them for the effects of any product or process that may be produced or adopted by a Funder or any other party, notwithstanding that the formulation of such product or process may be based upon the findings of the Research Programme.
11.3 No Party shall be liable to another Party for any death or injury unless it is caused by the negligence of that Party or its agents, nor shall it be liable to the other for any other loss or damage whatsoever unless it is caused by its wilful default or that of its agents.
11.4 Except as provided in clause 11.5 the University is not liable to the Funders because of any representation (unless fraudulent), or any warranty (express or implied), condition or other term, or any duty at common law, non-observance or non-performance of this Agreement, for:
any loss of profits, business, contracts, opportunity, goodwill, revenues, anticipated savings, expenses, costs or other similar loss; and/or
any indirect, special or consequential damages or losses (whether for loss of profits or otherwise).
11.5 The liability of a Party to another howsoever arising (including negligence) in respect of or attributable to any breach, non-observance or non-performance of this Agreement or any error or omission (except in the case of death or personal injury or fraudulent misrepresentation) shall be limited to £1 million.
11.6 If any sub-clause of this clause 11 is held by a court of competent jurisdiction to be invalid or unenforceable under any applicable statute or rule of law then it shall be deemed to be omitted and replaced with a similar or equivalent term which is valid and enforceable.
68. Schedule 1 is lengthy, and central to construction of the scope of the Research Agreement. I refer to it in more detail below.
The ICL In-Vivo Trial
69. As set out above, it was understood that ICL would carry out the mouse study.
70. An important part of this study was to compare the Drug’s effectiveness not only against no treatment being carried out, but also against the “gold standard” chemotherapy drug, TMZ.
71. At ICL, the person in charge of the work was Professor Amin Hajitou. In a study with mice, the mice must be exposed to the relevant cancer cells. Then either the mice are exposed to no further treatment to see how the cancerous cells develop without any drug intervening, or they are given the drug being tested (or a comparator drug also being tested). In some cases the drug being tested can be given orally (either mixed with their food or through a feeding tube). If that is not possible or desirable, then the drug can be injected intraperitoneally or possibly into the mouse’s stomach/diaphragm.
72. In this study, the cancerous tumours were implanted into the mice at the end of October 2016. Treatment was to start on 7 November 2016 intraperitoneally. As it turned out some of the mice were injected in the diaphragm, which had the effect of delivering an excessive dose. These mice died.
73. In the event, the ICL in vivo study was not a success. However, once again Professor Short agrees with Professor Bushell about the study. Professor Bushell says at paragraphs 62 to 65 of his first report:
The Research Programme: The Imperial Study
62. As noted above, this in vivo study was undertaken by Imperial College in late 2016. To assess the effectiveness of IP1867B in an animal model a number of immunocompromised mice were implanted with human GBM cells and then treated with TMZ or IP1867B or formed a “control” group. The Imperial Study was a small-scale piece of research, with a small number of groups and a small number of animals in each group. It was not much more than a pilot study in extent.
63. From the documents I have reviewed it appears that Hill and colleagues did not observe and were not directly involved with the Imperial work; they were reliant on the team at Imperial to tell them what was happening and to provide data at the conclusion of the study. It is apparent that Imperial encountered some issues/problems: for example, some of the mice in the IP1867B group died on administration. At the conclusion of the Imperial Study, after all the mice had been culled, Imperial confirmed that following administration problems the mice in various groups had been redistributed.
64. Dr Hill appears to have struggled to get data from Imperial as quickly as he wanted; there was frustration on his and Innovate’s part about this as Innovate wanted to use the data for an application to the EMA for orphan drug status for which the deadline was imminent. By 5 December 2016 however, Hill had obtained survival, weight and tumour volume data for the three groups. From his analysis of this data, Hill concluded (with several caveats regarding the size and limitations of the study) that IP1867B reduced tumour size in the GBM model used (and consequently that IP1867B crossed the BBB), that TMZ was not significantly better at reducing tumour size than IP1867B, and that IP1867B had less side-effects than TMZ. This analysis was shared with Innovate for the purposes of its application to the EMA in December 2016.
65. In February 2017, Hill received samples from the mice in the Imperial Study, which were the subject of further analysis as explained at paragraph 69.2 below.
The Orphan Drug Application and its aftermath
74. In March 2017 Innovate made an “Orphan drug” application (“ODA”) for the Drug to the European Medicine Agency. An orphan drug is a potential treatment for a rare medical condition. Where there is only a small population of patients affected by a condition, it is usually not profitable to develop a drug for its treatment. A drug with orphan designation is given financial benefits and advantages, for example approval is more likely to be given for a clinical trial and clinical trials can be undertaken with a smaller patient cohort.
75. The application was unsuccessful.
76. It is Professor Pilkington’s evidence that following the failure of the ODA there were further efforts to move the Drug towards a clinical trial. On his evidence those efforts were to involve a collaboration between BTR, Innovate, UoP and ICL. The idea was that there would be a better chance of getting the Drug into clinical trials if there was work between a multi-faceted team, including a charity, a commercial company and two sets of laboratories, working on in vivo and in vitro models, with clinicians to oversee the trials.
77. Professor Pilkington also said (in paragraph 52 of his witness statement):
….Innovate wanted BTR to fund a clinical trial but I could never see that happening. As I say above, clinical trials are very expensive and BTR did not have a lot of money. They were struggling to provide adequate funding for the Centre and I understood they were finding it difficult to raise funding from the public and other bodies. As I note above, this was an underfunded area of research and at this time BTR were lobbying heavily to increase government funding for research into brain tumours. If things had progressed Innovate would have needed significant investment from a pharmaceutical company or venture capitalists. Although Simon Cohen would often say he expected Innovate to receive large amounts of money to undertake further research and run a clinical trial, this never seemed to happen. Certainly I never saw any evidence of this.
78. Mr Simon Cohen provided a second witness statement in answer to Professor Pilkington’s statement taking issue with a number of points made by Professor Pilkington, but not with that part of his evidence.
79. What is clear is that at this stage there was a distance to go before the Drug could enter clinical trials: the attempt to take advantage of the ODA route had failed, and the ICL in vivo study had proved disappointing.
80. Innovate decided not to proceed further with ICL: I have heard evidence as to why this was, but it does not seem to me necessary for me to make any findings in that regard.
81. In the event, Innovate decided to carry out an in vivo study with Nottingham Trent University (“NTU”). However the NTU study also failed because of deficiencies in the way the study was carried out.
The Ongoing Work done by UoP
82. Whilst there were problems with the in vivo studies, UoP’s in vitro work continued.
83. As I have already said, there is a dispute between the parties as to whether UoP was contractually bound to carry out studies to see whether the Drug could pass the BBB. However, as a matter of fact, Dr Hill’s first witness statement records the work that was done.
84. In that statement he said:
48. I was continuing to work with Liquid Aspirin at this time. A lot of the work in this period involved doing re-runs of the various experiments done to date, to confirm the results and expand the data set. It was a good project for the students I was overseeing to work on because there were some really clear results from which interesting conclusions could be drawn. We did more experiments than those set out in the schedule to the Research Agreement.
49. I prepared two reports on the Liquid Aspirin research (the Pilot Study Report which is discussed above, and a Mechanism Report which summarised our findings regarding the mechanism by which Liquid Aspirin appeared to kill tumour cells) and a third report which referenced the Liquid Aspirin and the Clomipramine research. I sent these to Professor Pilkington on 19 June 2017 (the scheduled end date for the Research Programme was 30 June 2017). I do not know how Innovate used the reports I prepared. The Mechanism Report included a brief summary of the Imperial work, noting its limitations and that the deadline for the study had been moved forward two months at Innovate’s request regarding the ODA - I think I included this to highlight the effort Imperial had made to accommodate and support the project. I also noted that we were still working with the samples obtained from the Imperial study, particularly addressing the IL6/STAT3 and EGFR-related findings. Following the submission of the reports, I considered that the work under the Research Agreement that I was responsible for had been completed.
85. In paragraph 49 of his first witness statement, set out in the passage cited above, Dr Hill refers to three reports, which he suggests were the work product flowing out of the Research Agreement. Those reports were as follows:
(1) The Pilot Study Report [9];
(2) The Interim Report; [10] and
(3) The Mechanism Report. [11]
86. Dr Hill also stated in the last sentence of that paragraph that “following the submission of the reports, I considered that the work under the Research Agreement that I was responsible for had been completed”. He was cross-examined about this sentence. [12] The cross-examination concluded: [13]
MR ROE: Just looking at that last sentence of your witness statement on paragraph 49, Dr Hill, can I suggest that in fact the reason that you express yourself there in the way that you do, you “considered that the work under the Research Agreement that I was responsible for had been completed” is because you are keen to distance yourself personally from the events that follows?
A. No, my Lord. I, again, going back to what we had done in the research agreement, we had done the things that were requested in that research agreement.
Q. Had you done the blood-brain-barrier work?
A. No, my Lord.
87. Under the Agreement the period of research was to expire in June 2017. The questioning referred to above raises the issue as to whether the Research Programme came to an end at or about that time. I consider that issue, which turns in part upon construction of the Agreement, below.
88. Whether UoP’s work under the Agreement between itself and Innovate was complete or not, Dr Hill’s work continued. He confirms in paragraph 50 of his first witness statement that several students whose post-graduate work he was overseeing continued to undertake experiments involving the Drug using the samples that remained. In that paragraph he identifies the students and the work they carried out. A large part of that work involved the creation and recording of “western blots”.
Western blots
89. A large part of the evidence in this case concerns the reporting of data in what has been referred to as the “Cancer Letters Paper”. Much of that evidence concerns “western blots”. Dr Hill’s statement contained the following explanation of what was done:
52. Both Patrick Murray and Marcus Reay [14] did a lot of work with the mouse samples we had obtained from Imperial, looking to see if the same responses we had seen in the human cell lines treated with Liquid Aspirin were observed in the mice samples. This included running western blots with the samples. Western blotting is a scientific method used to separate, identify and analyse targeted proteins from complex samples that can contain thousands of other proteins. The process provides information about the quantity, molecular weight and modifications of proteins and is a useful experiment to complement transcriptional/gene expression data. The normal process for producing a western blot (and the one followed by the students whose work I oversaw) is as follows:
52.1 The first step in the process is to prepare the sample. This involves isolating the proteins from their source, usually by a technique called lysis. Lysis breaks down the cell membrane to detach the proteins from the non-soluble parts of the cell;
52.2 The disintegrated sample is then prepared for protein separation by a method called gel electrophoresis. Electrical currents travel through the sample and cause the proteins to separate according to their molecular weight;
52.3 The separated proteins within the reducing gel are then transferred onto a membrane;
52.4 Following this, the membrane is blocked (using BSA or milk) and the membrane is incubated in a specific antibody solution which binds to the target protein(s). Blocking minimises non-specific anti-body binding;
52.5 After a period of incubation, the sample is washed so that any unbound antibodies are removed;
52.6 A secondary antibody (which produces colour or light) is then applied to enable visualisation. After this, the membrane is washed;
52.7 Finally, the membrane is exposed for imaging (using a LICOR imager) and digitally scanned. The digital file is then stored on the central computer.
53. After the digital file is created, the relevant student then transfers it to another computer or USB for storage, labelling and presenting. The data files from the scanner are very large and so to use or present them in any form, the data needs to be cropped and converted for use with Microsoft Powerpoint (which was the typical format we presented western blot data when reporting on it within the team). The tagging/linking of particular data to particular experiments relies on the student conducting the test to correctly label and match the experiment to the data as it is transferred and presented. I did not often review the raw data itself, but I did once that data was presented in Powerpoint format and labelled by the relevant student. In summary, it is quite a difficult and complicated experiment to run. I think my students ran dozens, possibly hundreds, of western blots using the Liquid Aspirin data.
90. Professor Short appends to her first report the following concerning western blots:
How and why a western blot is performed
A western blot is a very commonly used assay (laboratory test) to measure the level of a specific protein, usually derived from experiments where a large number of cells are treated in a certain way, for example by exposing them to a drug, and then lysed (broken apart) to break up the cell contents and allow the cellular proteins to break away from where they usually bind in the cell so they can be measured.
The basic principle is that a mixture of proteins are embedded at specific locations (wells) in a gel and an electric current is run through the gel. The proteins move through the gel towards the (+ve) electrode. The distance they move depends on their size, so a group of proteins of different sizes become separated in the gel. The exact position where a protein ends up in the gel allows determination of a specific protein of that size. The amount of each protein can be assessed by adding a stain (often an antibody that binds to the specific protein) to the gel to make a specific protein visible. This means that the presence and relative amount of protein can be estimated. Different proteins can be stained in the same gel by exposing the gel to different antibodies that pick up different proteins.
In most experiments the mix of proteins that are added to the gel come from a collection of cells that have been treated in a certain way. The protein mixes from these cells are loaded separately (usually in different well positions across the top of the gel) and all the samples are run together, so a comparison of how the level of a certain protein changes with different treatments can be achieved by comparing staining across the gel.
Because there may be some variability in how much cell material and therefore total protein is loaded into the gel each time, a standard protein (often actin) whose levels are not expected to change across different experimental conditions is added as a comparator. This allows standardisation of the amount of other proteins in comparison to the actin, which is known as a loading control.
How a western blot result is presented
The results of this test are usually presented as a digital picture of the relevant gel with the stained proteins shown as black marks of different sizes/intensities at a certain position in the gel. These should all be at the same horizontal level if they are all the same protein. The relative amount of the protein is shown by the intensity of the stain. This can be compared by eye, but often this is augmented by data from a digital camera which reports the density of the stain and compares it to the standard protein and/or to another protein level in the gel.
In most experiments, the relevant conditions or treatments are represented by different samples (usually 6-12), labelled along the top of the gel and they are all run and measured together. Each gel therefore contains a comparison of protein levels taken from the same cells but after different treatments. The original gel should be photographed and recorded whole, with no break, insertion or deletion of stained material. However, a whole western blot is quite large and is mainly empty space as only one protein is stained at a time. Therefore, to make space constraints work most publications do not show the whole gel but only the relevant portions that show the relevant stained protein(s) that have been cropped (digitally) from the original. Importantly in most journals, authors are also asked to provide digital copies of the whole, uncropped gels to ensure that these match the cropped gel pictures that are shown in the article.
Common problems with presentation of western blots
Because these are digital pictures, western blot images can be relatively easily manipulated by software that is designed to make or enhance digital photographs such as ‘Photoshop’. This software can be used to cut stained examples from the picture of one gel and insert them into the picture of another gel, even if the samples did not originate from the same experiment. This also means that western blots or parts of blots can be easily swapped between experiments, flipped (mirrored, for example to make them appear original when they are not), missed and re-presented. This is a common problem in publications and various algorithms have been designed to pick up duplications (within and between articles), and to pick up re-use, flipping etc by recognising details in individual digital pictures that should be unique but appear more than once labelled differently. These are the problems that can be picked up by Pubpeer etc which are used to question the veracity of data.
91. At the beginning of his evidence in chief, Dr. Hill (with my permission) described the process of obtaining samples and creating western blots. [15] That process was also described to me in a joint session by the liability experts at the outset of their evidence. [16]
92. From the totality of the evidence to which I have referred, it is clear that there are number of points in the process at which errors not only can occur, but, as it appears to me, are likely to occur from time to time especially if the process is being carried out by inexperienced students. The errors can include unintentionally reversing the gel which is used to record the effect on proteins; unintentionally reversing the membrane when it is placed in an imaging machine; and unintentionally “flipping” the image when using a computer to transfer the images to a proposed publication. What I have to consider below on Innovate’s case is whether errors which Dr Hill is accused of making were or could have been accidental, or whether the proper conclusion is that those “errors” were not errors, but rather deliberate (and dishonest) manipulations of raw data.
93. An important point to note in the evidence given by Professor Short in her first report set out above is this passage:
Because there may be some variability in how much cell material and therefore total protein is loaded into the gel each time, a standard protein (often actin) whose levels are not expected to change across different experimental conditions is added as a comparator. This allows standardisation of the amount of other proteins in comparison to the actin, which is known as a loading control.
94. In the criticisms made of Dr Hill’s use of data, most concern the recording by him of levels of “β actins”, that is to say the recording of the levels of the control proteins used in the tests being reported upon by Dr Hill.
95. Before proceeding to consider the detailed allegations made by Innovate against UoP, it seems to me important to consider the legal framework which renders the issue of dishonesty relevant.
Construction of Clauses 11.4 and 11.5 of the Research Agreement
96. I have set out the terms of Clause 11 at paragraph 67 above.
97. Clause 11.1 imposes an express obligation upon UoP which has the effect of imposing upon UoP an obligation to exercise all reasonable skill and care in carrying out the Research Programme: there are issues as to the extent of the work for which UoP is responsible under Clause 11.1, including whether there was an obligation upon UoP to carry out research to establish whether the Drug could pass through the BBB, and whether the issue of the Cancer Letters paper was within UoP’s contractual responsibility. I consider those issues below. At this point I am concerned with the effect of the exclusions in Clause 11.
98. As set out above, Clause 11.4 provides:
11.4 Except as provided in clause 11.5 the University is not liable to the Funders because of any representation (unless fraudulent), or any warranty (express or implied), condition or other term, or any duty at common law, non-observance or non-performance of this Agreement, for:
any loss of profits, business, contracts, opportunity, goodwill, revenues, anticipated savings, expenses, costs or other similar loss; and/or
any indirect, special or consequential damages or losses (whether for loss of profits or otherwise).
And Clause 11.5 provides:
11.5 The liability of a Party to another howsoever arising (including negligence) in respect of or attributable to any breach, non-observance or non-performance of this Agreement or any error or omission (except in the case of death or personal injury or fraudulent misrepresentation) shall be limited to £1 million.
99. I have been referred to authority on the construction of exclusion and limitation clauses, particularly in the context of clauses excluding or limiting liability for fraud.
100. It is accepted on behalf of UoP in paragraph 12 of its written Closing Submissions that a party cannot contract out of his own fraud in inducing the making of a contract (see HIH Casualty & General Ins v Chase Manhattan Bank [2003] UKHL 6; [2003] 2 Lloyd’s Rep 61 at paragraph [16]). However, UoP submits, that is not the position with regard to the performance of a contract, especially where the fraud relates to that of an agent or employee of the contracting party.
101. In that respect, UoP referred to the decision of Gross J. in Frans Maas (UK) Ltd [2004] EWHC 1502; [2004] 2 Lloyd’s Rep. 251. In order to understand that decision, it is necessary to set out a relatively extensive passage from the judgment. The clause considered by the learned judge provided as follows:
27(A) Subject to clause 2(B) and 11(B) above and sub-clause (D) below the Company's liability howsoever arising and notwithstanding that the cause of the loss or damage be unexplained shall not exceed…. [the various limits set out in sub-clauses (A) to (C)]
(D) By special arrangement agreed in writing, the Company may accept liability in excess of the limits set out in Sub-Clauses (A) to (C) above upon the Customer agreeing to pay the Company's additional charges for accepting such increased liability. Details of the Company's additional charges will be provided upon request.
28(A) Any claim by the Customer against the Company arising in respect of any service provided for the Customer or which the Company has undertaken to provide shall be made in writing and notified to the Company within 14 days of the date upon which the Customer became or should have become aware of any event or occurrence alleged to give rise to such claim and any claim not made and notified as aforesaid shall be deemed to be waived and absolutely barred except where the Customer can show that it was impossible for him to comply with this Time Limit and that he has made the claim as soon as it was reasonably possible for him to do so.
(B) Notwithstanding the provisions of Sub-Paragraph (A) above the Company shall in any event be discharged of all liability whatsoever howsoever arising in respect of any service provided for the Customer or which the Company has undertaken to provide unless suit be brought and written notice thereof given to the Company within nine months from the date of the event or occurrence alleged to give rise to a cause of action against the Company."
102. The judgment discusses the effect of that Clause at paragraphs [128] to [152]:
129. Authority: To begin with, perhaps somewhat surprisingly, there does not appear to be any authority directly in point on the BIFA terms. That said, some but necessarily limited guidance can be obtained from the authorities principally relied on by counsel and to which I now turn.
130. Securicor is, with respect, an important authority, on striking facts, for the proposition that, at least in commercial cases (now subject only to the impact, if any, of UCTA), words, even in exclusion clauses, mean what they say and the parties will be held to the bargain into which they have entered. Further, it is a matter of construction rather than law as to whether liability for deliberate acts will be excluded, though of course the wording must be clear. Still further, as already observed when summarising Ms. Hilliard's submissions, the fact that a clause covers negligence does not entail that it does not cover deliberate acts. In that case, the House of Lords held that the general wording was clear and that the defendants were not liable for the act of their employee in deliberately starting a fire which, in the event, burnt down the plaintiffs' factory. As is apparent from the speeches (see, especially at pp. 846, 851 and 852), the reasoning had regard to the apportionment of risk agreed by the parties, coupled with their ability to insure against the risks and losses in question. It must, however, be reiterated that authority cannot be pressed too far and it is inherent in Securicor that each contract turns on its own construction.
131. Ailsa Craig establishes that limitation clauses are not regarded by the courts with the "same hostility" (Lord Wilberforce, at p.966) as exclusion clauses. As Lord Fraser explained, at p.970:
" ….these principles [i.e., those applicable to exclusion and indemnity clauses] are not applicable in their full rigour when considering the effect of clauses merely limiting liability. Such clauses will of course be read contra proferentem and must be clearly expressed , but there is no reason why they should be judged by the specially exacting standards which are applied to exclusion and indemnity clauses. The reason for imposing such standards on these clauses is the inherent improbability that the other party to a contract including such a clause intended to release the proferens from a liability that would otherwise fall upon him. But there is no such high degree of improbability that he would agree to a limitation of the liability of the proferens, especially when ….the potential losses that might be caused by the negligence of the proferens or its servants are so great in proportion to the sums that can reasonably be charged for the services contracted for…."
Again, such guidance is not to be adopted unthinkingly and certain caveats must be noted. First, the passage was dealing with negligence rather than wilful default. Secondly, as observed by Lord Hoffmann in HIH, at [63], it must be doubted whether Lord Fraser was introducing one "mechanistic rule …to mitigate the rigour of another"; all turned on the language of the clause in question and the context. I keep these cautions very much in mind.
132. HIH concerned a trial of preliminary issues in the setting of the "film finance" litigation. The context is far removed from that of the present case, a point, as it seems to me, of no little importance and to which I must return.
133. Before doing so, however, it is convenient to set out a number of propositions for which HIH is undoubtedly authority. First, fraud is a thing apart; as expressed by Lord Bingham, at [15]:
"…This is not a mere slogan. It reflects an old legal rule that fraud unravels all…It also reflects the practical basis of commercial intercourse. Once fraud is proved, 'it vitiates judgments, contracts and all transactions whatsoever'…Parties entering into a commercial contract will no doubt recognize and accept the risk of errors and omissions in the preceding negotiations, even negligent errors and omissions. But each party will assume the honesty and good faith of the other; absent such an assumption they would not deal. What is true of the principal is true of the agent, not least in a situation where, as here, the agent, if not the sire of the transaction, plays the role of a very active midwife."
Secondly, the law, on public policy grounds, does not permit a contracting party to exclude liability for its own fraud in inducing a contract: see, Lord Bingham, at [16] and Lord Hoffmann, at [76]. Thirdly, the House of Lords left unresolved the question of whether there was a similar rule in respect of the agent of a party, acting as such, in the making of a contract: Lord Bingham at [16]; Lord Hoffmann, at [76] - [81]. Where, however, it was sought to exclude the ordinary consequences of such fraud or dishonesty on the part of the agent, that intention was to be expressed in clear and unmistakeable terms on the face of the contract: Lord Bingham, at [16].
134. As foreshadowed, I must, however, now return to the context. HIH was dealing with fraud in the making of a contract, rather than the question of excluding liability for the fraud of an agent in the performance of a contract. As Lord Hobhouse expressed it, at [98]:
" I would add, for the sake of completeness that the present case is not concerned with a situation of the dishonest conduct of a servant or agent in the course of the performance of a wholly valid contract, say a contract of carriage, and an exemption of, say, the theft of the goods in transit. There questions of construction may well arise…."
See too, Lord Hoffmann, to like effect, at [76].
135. In my respectful view, the reasoning for this difference in approach is at once apparent. Parties do not contemplate fraud in the making of a contract; as observed by Lord Bingham, there would be no deal. But it is another thing altogether to say that parties do not contemplate the risk of deliberate wrongdoing at some point in the performance of a valid contract. That is a matter for construction of the contractual provisions and risk allocation, whether by way of insurance or otherwise.
….
139. What of the wording in context? Here, as it seems to me, the arguments are essentially one way:
i) The risk of employee wilful default is a real, foreseeable, commonplace risk.
ii) Cl. 27(A) is a limitation clause.
iii) Both the nature of the clause and the commercial background against which it is intended to operate, suggest that the parties intended cl. 27(A) to provide an uncomplicated safety net for FM; if, however, cl. 27(A) does not extend to the commonplace risk in question, then there would be a significant hole in that safety net. These considerations lend powerful support to the proposition that the parties intended the wording to mean what it said; "howsoever arising" meant just that - cl. 27(A) scooped up FM's liability, "howsoever arising", including employee wilful default.
iv) Nothing in the authorities to which I have referred tells against such an approach. To the contrary, this approach to cl. 27(A) is consistent with both Securicor and Ailsa Craig; commercial contracts are not to be artificially construed and liability even for deliberate wrongdoing can be excluded, a fortiori limited, provided appropriate wording is used. While a suggested limitation of liability for employee wilful default does require close scrutiny, HIH underlines that, so far as concerns deliberate wrongdoing in the course of performance of an admittedly valid contract, the matter is one of construction.
v) I add this; in practical terms, one or other party was to or would be well advised to insure against the risk of employee wilful default; the party directly at risk was SEUK; all other things being equal, it was likely to be better placed than FM to do so. The above construction of cl. 27(A) would mean that the parties had addressed this risk and left it to SEUK to obtain insurance (for losses above the limit). As a matter of fact, giving me some comfort if irrelevant to the construction of the BIFA terms, I was told by Mr. Jacobs, that SEUK did so; the present is (primarily at least) a subrogated claim.
….
141. In summary, provisionally at least, I am persuaded that the language of cl. 27(A) of the BIFA terms, read in context, was clear and was not only capable of extending to employee wilful default but was intended by the parties to do so. Neither the language of the clause nor its context points towards straining the clause so as to read into it some restriction which it is not naturally there. The words "howsoever arising", to which the parties have agreed, mean what they say.
….
147. What of the decision in Granville and SEUK's submission that it told against cl. 27(A) extending to wilful default? I am unpersuaded; to explain why, I must turn to Granville itself.
148. The decision in Granville was that the time bar provision, now found in cl. 28(B) of BIFA, satisfied the requirements of reasonableness under UCTA; in due course I shall return to this decision when dealing with UCTA. In differing from the Judge at first instance and in reaching this conclusion, Tuckey LJ said this at [15]:
" I think it is an inescapable conclusion from what he said that the Judge did think that the clause applied to a claim for fraud and to a claim which had been fraudulently concealed by the conduct of the freight forwarder….I do not think such a construction was justified. The clause is obviously designed to meet ordinary contractual claims …which a freight forwarder would expect to have to face in the ordinary course of his business. As Lord Justice Rix put it in HIH Casualty at p.512:
Parties to a contract plainly look to performance rather than non performance or misperformance, but they also contemplate the latter. It seems to me however that fraud is a thing apart. Parties contract with one another in the expectation of honest dealing.
The majority decision of the House of Lords in HIH Casualty…does not cast doubt on these principles."
149. With respect, I do not think that this passage affords Mr. Jacobs the assistance which he sought to derive from it. First, it is quite understandable that a contractual time bar provision should be inapplicable when a claim has been fraudulently concealed (cf., s.32 of the Limitation Act 1980); that can have no bearing on the true construction of cl. 27(A). Secondly, when considering the discussion in Granville as a whole, to my mind the references to "fraud" are references to fraud by the freight forwarder; they are not references to theft by the freight forwarder's employees for which the forwarder is vicariously liable. Such a construction is itself a noteworthy inroad into cl. 28 of the BIFA terms (contrast, in a different area of the law, The Captain Gregos [1990] 1 Lloyd's Rep 310), but, again sheds no light on the true construction of cl. 27(A). Thirdly, even if the time bar provisions of BIFA are inapplicable to claims involving employee dishonesty for which the freight forwarder is vicariously liable, it does not follow that cl. 27(A), a limitation clause, is similarly restricted. While it is true that the section in the BIFA terms, "Liability and Limitation" must be read as a whole, there is nothing necessarily remarkable in the finding that the scope of clauses within that section differs; it is one thing to exclude or bar a claim or liability but it is or may be another to limit liability in respect of any such claim. With respect, therefore, I do not find in Granville the answer to the question as to the true construction of cl.27(A) of the BIFA terms.
….
151. Where does cl. 27(A) stop? SEUK's final submission was that if wrong on the true construction of cl. 27(A), then, as a matter of construction, there was no stopping place short of cl. 27(A) extending to the personal fraud or wilful default of FM itself. I disagree. The short answer, as it seems to me, is that when it comes to personal fraud of a party, then, whether as a matter of public policy or construction (it is unnecessary to resolve which), fraud is indeed a thing apart. It is in this context, with respect, that the observations of Tuckey LJ in Granville at [15], set out above, are particularly apposite. So far as concerns construction, even with regard to the invocation of a limitation clause in the course of the performance of an otherwise valid contract, the parties do not contemplate that one of them may take advantage of personal fraud. The various considerations already discussed (as to risk allocation, clarity of language and context) which point to cl. 27(A) extending to FM's vicarious liability for wilful default, suggest a different conclusion where personal fraud on FM's part is concerned.
152. (4) Overall conclusion: For the reasons given, I am satisfied that cl. 27(A) of the BIFA terms entitles FM to limit its liability in respect of SEUK's claims in this case, whether advanced on the basis of employee wilful default or negligence. The commercial ramifications do not of course end with the resolution of the legal issues; subsequent to the theft, on the material available to me, SEUK decided to terminate its relationship with Maas - in the light of the facts as found in these proceedings, unsurprisingly if I may say so. But that is neither here nor there on the issue of limitation of liability. Reverting to the matters with which I must deal, I shall be grateful for assistance from counsel in determining the precise limitation figure.
103. That passage is useful in setting out well established principles:
(1) Exclusion clauses mean what they say;
(2) It is a matter of construction rather than law as to whether liability for deliberate acts will be excluded;
(3) Limitation clauses are not regarded by the courts with the same hostility as exclusion and indemnity clauses;
(4) A contracting party cannot exclude liability for its own fraud in inducing a contract;
(5) As to whether a clause excludes liability for fraud in performance of a valid contract is a matter of construction of the commercial provisions and risk allocation;
(6) An exclusion or limitation clause is more likely to be construed as effective if it is excluding the liability for fraud of an agent or employee rather than the fraud of the contracting party itself;
(7) The words “howsoever arising” are capable of effecting an exclusion of liability for wilful default.
104. Reference was also made to the judgment of Joanna Smith J. in Pinewood Technologies Asia Pacific Ltd v Pinewood Technologies Plc [2023] EWHC 2506 (TCC) at paragraph [82]:
b. However, a vital part of the setting in which parties contract is a framework of rights and obligations established by the common law. In construing an exclusion clause, the court will start from the presumption that in the absence of clear words the parties did not intend to derogate from those normal rights and obligations. (Modern Engineering (Bristol) Ltd v Gilbert Ash (Northern) Ltd [1974] AC 689 per Lord Diplock at page 717H; Triple Point at [108]-[110]).
c. The more valuable the right, the clearer the language of the exclusion clause will need to be if it is to be given effect (Triple Point at [110]).
d. However, "[i]n commercial contracts negotiated between business-men capable of looking after their own interests and of deciding how risks inherent in the performance of various kinds of contract can be most economically borne…it is…wrong to place a strained construction upon words in an exclusion clause which are clear and fairly susceptible of one meaning only…" (Photo Production Ltd v Securicor Transport Ltd [1980] AC 827 per Lord Diplock at page 851 and Fujitsu Services Ltd v IBM United Kingdom Ltd [2014] 1 CLC 353 per Carr J at [49]).
e. Notwithstanding (a)-(d) above, an exclusion clause will not normally be interpreted as extending to a situation which would defeat the main object of the contract or create a commercial absurdity, notwithstanding the literal meaning of the words used. This is a context in which it is open to the court to strain to avoid a particular construction, rather than one which requires ambiguity on a fair reading before the principle comes into play, because it is inherently unlikely that the parties intended that the clause should have so wide an ambit as in effect to deprive one party's stipulations of all contractual force such that the contract becomes 'a mere declaration of intent' (Kudos Catering (UK) Ltd v Manchester Central Convention Complex Ltd [2013] EWCA Civ 38, per Tomlinson LJ at [19] citing from the speech of Lord Wilberforce in Suisse Atlantique Societe d'Armement Maritime SA v NV Rotterdamsche Kolen Centrale [1967] 1 AC 361 at pages 431-432; AstraZeneca UK v Albemarle International [2011] 2 CLC 252, per Flaux J at [313]; and CNM Estates (Tolworth Tower) Ltd v VeCREF I Sarl [2020] 2 CLC 243, per Foxton J at [33]).
f. However, even in this context, where language is fairly susceptible of one meaning only, that meaning must be attributed to it unless "the meaning is repugnant to the contract" (see Kudos at [20]). This is a principle which "should be seen as one of last resort and there is authority that it applies only in cases where the effect of the clause is to relieve one party from all liability for breach of any of the obligations which he has purported to undertake: see Great North Eastern Rly Ltd v Avon Insurance plc [2001] EWCA Civ 780, [2001] Ll Rep IR 793. Only in such a case could it be said that the contract amounted to nothing more than a mere declaration of intent" (Transocean Drilling UK Ltd v Providence Resources plc (The GSF Arctic III) [2016] EWCA Civ 372, per Moore-Bick LJ at [27]).
105. With those authorities in mind, I approach the construction of Clauses 11.4 and 11.5.
106. Clause 11.4 provides, as is often the case, for the exclusion of liability for loss of profits as well as “opportunity, goodwill, revenues …”. It also provides for the exclusion of liability of other indirect damages or losses, but for the purposes of this judgment I do not need to explore the width of that exclusion: it is sufficient that Clause 11.4 expressly excludes liability for loss of profits and the other heads of loss I have cited at the beginning of this paragraph, which are words sufficiently clear in their meaning to encompass the claim for “Diminution in Value of the Patent in the Drug” pleaded at paragraph 20B of the Amended Particulars of Claim.
107. Clause 11.4 is subject to two carve outs: firstly, “except as provided in clause 11.5” and, secondly, “because of any representation (unless fraudulent)”.
108. The reference to Clause 11.5 is a little clumsy, but it seems to me to have the effect that where the cause of action is in respect of death, personal injury or fraudulent misrepresentation, the exclusion in Clause 11.4 of liability for loss of profits etc. will not apply.
109. This would align Clause 11.5 with Clause 11.3 which expressly preserves liability for death or injury caused by negligence and Clause 11.4 which preserves liability for representation if fraudulent.
110. On this construction, both Clauses 11.4 and 11.5 preserve liability for fraudulent representation on the part of UoP or those for whom UoP is responsible.
111. However, it is important to note that the carve out in Clause 11.4 in respect of what is fraudulent is limited to representation. As a matter of construction the word “fraudulent” applies only to representation not to the words which follow (“or any warranty (express or implied), condition or other term, or any duty at common law, or under the express terms of this Agreement”).
112. This is an important point. In paragraph 2 of its written Closing Submissions, Innovate submitted:
Innovate submit that the evidence supports their case that:
a. The University and/or their agents (i.e., Dr Hill or his students) acted in breach of contract and/or negligently in several respects; and
b. Those breaches were committed dishonestly, so (for reasons explained in more detail in due course) there can be no question of liability for them being limited by the contract.
113. Later, at paragraph 53 of those submissions it is said:
The effect of dishonesty
The University accepts that they cannot rely on the limitation clauses in the contract (see clauses 11.4 and 11.5 [D5/159]) if the Court finds that the conduct in respect of which they are liable involved dishonesty (see their Opening Note at para 196). They are right about this. It is trite that no exemption clause can protect a person from liability for his or her own fraud (Chitty on Contracts, 35th Edn., 18-067). This reflects the old legal rule: fraus omnia corrumpit. It is also settled that if a principal wishes to exclude liability for fraud on the part of an agent acting as such, then that exclusion would have to be expressed in clear and unmistakable terms on the face of the contract so as to leave the other party in no doubt that fraud was covered (HIH Casualty and General Insurance Ltd v Chase Manhattan Bank [2023] UKHL 6, [2003] 1 CLC 358, per Lord Bingham of Cornhill, with whom the majority agreed, at para 16). The contract in this case contains no such unmistakable exclusion of liability on the part of the principal (i.e., the University) for the fraudulent act of the agent (i.e., Dr Hill, or whoever else it was who flipped the images etc).
114. These passages make it clear that, in Innovate’s submission, neither Clause 11.4 nor Clause 11.5 operates to exclude or limit liability if the relevant breach of contract was committed fraudulently.
115. Whilst it may be that UoP’s Opening Submissions may have conveyed a different message, it is clear from paragraph 16 of UoP’s written Closing Submissions and from Ms Dixon KC’s oral closing submissions on behalf of UoP that it is not now its position that UoP cannot rely on the limitation clauses in the Agreement if the Court finds that the conduct in respect of which they are liable involved dishonesty.
116. I reject Innovate’s submission: in my judgment the exclusion of liability in Clause 11.4 in respect of liability for loss of profits is applicable to all claims except where the claim is based upon a fraudulent representation, that is to say a claim in the tort of deceit.
117. Thus loss of profits caused by a breach of contract not involving a representation is excluded even if that breach was committed fraudulently.
118. Even if that were not so, the limitation of liability in Clause 11.5 will apply to any claim (whether for loss of profits or otherwise) unless the relevant cause of action is in respect of death or personal injury or for fraudulent misrepresentation.
119. In this respect I generally accept the following submission in paragraph 16 of UoP’s written Closing Submissions:
It is common ground that the limitation of liability clauses apply to exclude liability for loss of profits and limit liability to £1million “save insofar as such loss was consequent upon a fraudulent misrepresentation”. As set out above, Innovate is not running a case based on fraudulent misrepresentation (or even deceit). It has suffered no loss “because of” or in reliance upon any particular “fraudulent” representation(s). Fraudulent misrepresentation is a cause of action relating specifically to the inducement of entry into an agreement by a false statement of facts. This being so, even if (contrary to the submissions set out below) the Court finds that the nature of the University’s breach of the Contract was dishonest, the University is still, in principle, able to rely upon the limitations on its liability set out in Clauses 11.4 and 11.5.
120. I would qualify my acceptance as follows:
(1) In my judgment a claim in respect of fraudulent misrepresentation is not limited to a case where the innocent party was induced into entering into an agreement;
(2) However, the cause of action is for the losses suffered by the innocent party acting to its detriment in reliance upon the false representation;
(3) I accept Innovate’s submission that in this case fraud on the part of an agent or employee of UoP would suffice: it is not necessary for Innovate to establish fraud on the part of UoP itself.
121. However, as to point (3), the case has been very much pursued upon the basis that any dishonesty was on the part of Dr Hill, and it is upon that case that the decision in this case turns. For the avoidance of doubt, I do not consider that there is evidence sufficient to base a finding of dishonesty on the part of any agent or employee of UoP other than Dr Hill.
122. As I set out below, the original Particulars of Claim set out to plead a claim based upon fraudulent misrepresentation as a separate cause of action in deceit. That case did not appear to me to be actively pursued, and this was confirmed by Mr Roe KC in his oral closing submissions [17]:
THE JUDGE: I think I am right that if the university is correct in its construction of 11.4 and 5 that the only relevant carve-out is a claim for fraudulent misrepresentation, you do not suggest that you have satisfied all the limbs that would be necessary to prove a claim in deceit. In other words, you have not got a fall-back …. position that says, “In any event I have a sound claim in deceit”, that is what you have effectively abandoned?
MR. ROE: Yes.
123. This concession by Mr Roe reflected the fact that the case set out in the Amended Particulars of Claim and now pursued does not plead the necessary elements of a claim based upon misrepresentation since it does not plead or establish losses flowing from any actions taken (or not taken) in reliance upon a misrepresentation.
124. I consider below, and reject, the suggestion that Clauses 11.4 and/or 11.5 should not be given effect by reason of the Unfair Contract Terms Act 1977.
125. Notwithstanding these conclusions, which appear to me to be fatal to the claim in paragraph 20B of the Amended Particulars of Claim and to any recovery by Innovate of any sum in excess of £1,000,000, given the importance that the allegations of fraud had in the conduct of the trial before me, it seems to me appropriate for me to set out my conclusions in respect of those allegations.
Clauses 11.4 and 11.5 of the Research Agreement: Unfair Contract Terms Act
126. Paragraph 23 of the Re-Amended Defence pleads as follows [18]:
Clauses 11.1, 11.4 and 11.5 of the Contract were reasonable given:
a. That Jan Cohen was a solicitor and negotiated (including by making substantive changes to the draft Contract) the draft Contract on behalf of the Claimant;
b. That the Research Programme could have been done elsewhere;
c. The limited amount of funding being provided by the Claimant and HCT:
i. in any event,
ii. in comparison to the cost of the research being done, and
iii. in comparison to the amount which it would have cost had the Claimant and HCT gone to a commercial provider;
d. The uncertainty in the outcome of the Research Programme;
e. The Defendant’s lack of knowledge as to the ways the Claimant intended to commercially exploit the results derived from the Research Programme;
f. The nature of the Defendant, being a body dealing with public funds;
g. That such clauses were common in the market; and
h. In relation to Clause 11.5, that all parties to the contract had the benefit of the clause.
127. Paragraph 22 of the Reply to the Re-Amended Defence responded as follows [19]:
As to paragraph 23:
It is denied that the Defendant lacked knowledge as to the ways in which the Claimant intended to exploit the results of the Research Programme commercially. The Defendant was aware, from numerous emails and messages, of the Claimant’s intention to exploit the Drug commercially. Further, Dr Hill and Professor Pilkington were invited to attend at least one meeting with the Claimant during which both Dr Hill and Professor Pilkington contributed to discussions addressing (among other things) the importance of ensuring high-quality in vivo studies to maximise access to both clinical trials and commercial opportunities. Professor Pilkington told the Claimant that he had been contacted by venture capitalists and would provide the Claimant with their contact details.
Paragraph 23 is otherwise not admitted.
128. In paragraphs 18 to 21 of its written Closing Submissions, UoP submits:
18. The University pleaded positively that Clauses 11.4 and 11.5 were reasonable within the meaning of section 11 and (Schedule 2) of UCTA 1977; a plea which Innovate did not deny but rather put the University to proof on.
19. There has, since the enactment of UCTA 1977, been a judicial awareness that a finding of unreasonableness should not lightly be reached given the interference with freedom of contract and the parties’ allocation of risk.
20. Although ‘reasonableness’ if of course case-specific, some general guidance can be gleaned from the authorities: see, for example, Overseas Medical Supplies Ltd v Orient Transport Services Ltd [1999] 1 All ER (Comm) 981 which, at [10], set out seven factors beyond those listed in Schedule 2 UCTA which may be relevant. Also useful are the four matters set out by Lord Griffiths in Smith v Eric S Bush [1990] 1 AC 831 at page 858.
21. In this case, the Court is asked to note, in particular, the following in support of the University’s contention as to “reasonableness”:
a. In terms of bargaining power, there was no inequality between the parties or none which was skewed against Innovate. In particular and as became common ground by the end of trial (insofar as it was not beforehand):
i. Jan Cohen, who negotiated the Contract for Innovate, is legally qualified, and
ii. Innovate did not simply accept the terms presented to it but actively negotiated aspects of it and made a number of suggested changes to the terms including to clause 11.2.
b. Given the work that the University was doing the sum it was being paid was very low. At the time the University thought they were being paid £50,000 for £80,000 of work. It is apparent from the quotations Innovate has obtained to repeat the Research that (on its case) to obtain the Research from a commercial provider would cost considerably more.
129. Innovate’s position on this issue was expressly pithily by Mr Roe in his oral closing submissions [20]:
THE JUDGE: While we are on clause 11, UCTA. The position in relation to UCTA is the defence pre-empts an UCTA position and says that the clause is reasonable for the following reasons. The reply takes issue to some extent factually about [the] circumstances [in] which the clause was entered into or the contract was entered into but otherwise puts the university to proof of the reasonableness of the clause. Miss Dixon in her closing submissions addresses UCTA; you do not.
MR. ROE: We do not and ought to have done, my Lord. That is our omission. My learned friend makes some factual observations about the way in which the contract was developed, negotiated and who negotiated it, and I cannot say much about that. But the ultimate question under UCTA is whether it is reasonable and … on the premise [that], contrary to my case, [the] effect of clause 11 is that the university has excluded its liability for lying about the context of its research to the world, the question whether that is a reasonable clause answers itself in my submission. Plainly not.
130. I have to consider the application of the 1977 Act upon two clauses. Neither is a blanket exclusion of all liability - clause 11.4 restricts the range of losses for which UoP can be held liable, and clause 11.5 limits the amount of UoP’s liability.
131. In approaching this issue, it is important to keep in mind my conclusion as a matter of construction that these clauses distinguish between liability for deceit and liability for breach of contract.
132. Innovate’s submission appears to me to amount to an argument that because these two clauses limit recovery for a breach of contract committed dishonestly each of those clauses is unreasonable.
133. The 1977 Act does not allow a court to strike down a clause for some purposes and not for other purposes. Accordingly, each of these clauses is either effective or ineffective in respect of all breaches of contract whether deliberately committed or not.
134. Thus, if Innovate’s argument be right, both clauses would fall away in their entirety.
135. I do not accept that argument. The circumstances of this case, if Innovate’s allegations of fraud are well-founded, are highly unusual and very unlikely indeed to have been in the contemplation of the parties when the Agreement was concluded. I would be slow to apply the 1977 Act to strike down these clauses because of the occurrence of events outside that contemplation.
136. In my judgment, for the reasons pleaded by UoP and put forward by UoP in its closing submissions, both these clauses were reasonable. The amount of the claim in this case (arguably in excess of £100 million) compared to the amount payable to UoP under the Agreement underlines the commercial reality, perhaps necessity, of the two clauses.
Clause 18
137. Clause 18 of the Agreement provides:
Business Ethics
The Parties recognise a mutual commitment to an ethical business and anti-corruption culture, and each Party hereby agrees: (i) to uphold the highest standards of business ethics in the performance of its responsibilities hereunder and adhere to the general principles of honesty, fairness and integrity in all its dealings; and (ii) not to accept from, give to or offer to any other Party (or its Affiliates) or to other contractors or suppliers or any other associated persons anything of material value which may be regarded as an improper inducement; and (iii) to comply with all applicable laws, regulations, codes and sanctions relating to anti-bribery and anti-corruption, including but not limited to the Bribery Act 2010; and (iv) to embrace, support and enact a set of core principles in the areas of human rights …..
138. Innovate rely upon this provision, but in the circumstances of this case it adds little to Clause 11, because remedies for a breach of Clause 18 would also be the subject of the exclusion/limitation provisions in clauses 11.4 and 11.5.
The August 2018 Representation
139. The documentary evidence, particularly text messages between Dr Hill on the one hand, and Dr Stuart and Mr Cohen on the other, shows that there was continuing contact between them.
140. In the Particulars of Claim as originally filed, the case turned upon a representation alleged to have been made by Dr Hill in August 2018. That representation still formally forms part of Innovate’s case, but was not actively pursued by Innovate in its written and oral submissions.
141. The original pleading alleged in paragraph 7:
By early 2018 work under the Contract had progressed to analysis of the specific mechanism by which IP1867B shrank brain tumours.
142. This paragraph was omitted from the Amended Particulars of Claim.
143. Paragraph 8 of the original pleading alleged as follows:
On 10 August 2018, by telephone and via the medium of SMS text messages Dr Hill told James Stuart, a director of the Claimant, that he had made a breakthrough in his research into the mechanism by which IP1867B operated. He stated that IP1867B had the effect of suppressing the insulin-like growth factor 1 receptor (IGF1R) which, in turn, suppressed resistance to epidermal growth factor receptor (EGFR).
Specifically, in text messages to Mr Stuart on 10 August 2018, Dr Hill stated:
“Igfr1 confers resistance.
IP18167B knocks out Igfr1.”
And:
“So IP1867B stops resistance to EGFR inhibitors by downregulating IgFR1.”
Dr Hill repeated the above claim in subsequent text messages and telephone conversations passing between himself, Mr Stuart, Mr Simon Cohen, a major shareholder in the Claimant, and Mr Colin Speirs, director and Head Fundraiser of the HCT. Indeed for a period of over 12 months, Dr Hill did nothing to qualify or correct the representations he had made. All the directors of the Claimant believed that the representations were true, and all their discussions with Dr Hill subsequently were conducted on the understanding that they were. That belief continued for over one year.
144. The pleading then continued at paragraphs 9 and 10:
9. In fact the representations were false. There was (in August 2018) and remains to date no worthwhile evidence to support the truth or accuracy of the proposition stated by Dr Hill to the Claimant.
10. Moreover, at the time he made the representations, Dr Hill knew that they were false; or else was reckless as to whether they were true or false, in the sense that he cannot have believed them to be true.
145. The Amended Particulars of Claim contain a revised paragraph 8 as follows:
On 10 August 2018, by telephone and via the medium of SMS text messages Dr Hill told James Stuart, a director of the Claimant, that he had made a breakthrough in his research into the mechanism by which the Drug IP1867B operated. He stated that the Drug IP1867B had the effect of suppressing the insulin-like growth factor 1 receptor (IGF1R) which, in turn, suppressed resistance to EGFR inhibitors epidermal growth factor receptor (EGFR).
Specifically, in text messages to Dr Mr Stuart on 10 August 2018, Dr Hill stated:
“Igfr1 confers resistance.
IP18167B knocks out Igfr1.”
And:
“So IP1867B stops resistance to EGFR inhibitors by downregulating IgFR1.”
Dr Hill repeated the above claim in subsequent text messages and telephone conversations passing between himself, Dr Mr Stuart, Mr Simon Cohen, a major shareholder in the Claimant, and Mr Colin Speirs, director and Head Fundraiser of the HCT and in an email on 11 August 2018 to Dr Stuart and Mr Cohen and in an email on 16 August 2018 to Mr Cohen. The email of 11 August 2018 had attached to it a draft of a paper for publication, the published version of which is referred to below.
Indeed for a period of over 12 months, Dr Hill did nothing to qualify or correct the representations he had made. All the directors of the Claimant believed that the representations were true, and all their discussions with Dr Hill subsequently were conducted on the understanding that they were. That belief continued for over one year.
146. Paragraphs 9 and 10 in the existing form were deleted, and instead it was and is now pleaded:
9. In the premises, the above representations to the Claimant to the effect that the Drug suppressed resistance to EGFR inhibitors were a representation that the data obtained from the Research Programme showed such to be the case.
10. This was untrue. Whether or not the Drug does suppress resistance to EGFR inhibitors (a question that is not yet answered), the data from the Research Programme did not show such to be the case.
147. Thus the current pleading, like the original Particulars of Claim, retains a claim that representations were made in August 2018 and that those representations were false, albeit false now in a different way from what had been alleged previously. Importantly, the pleading continues to allege that Dr Hill made representations in August 2018 that he knew were false or was reckless as to whether they were true or false. As I have said, that case has effectively been abandoned, but the case having been made, it seems to me right that I should set out my conclusions on it.
148. The representations made in the text messages are a matter of record, as are the two emails referred to below.
149. The first email sent on 11 August 2018 [21] said:
Please find attached the MS Word file of the paper. Please please please do not let UoP or BTR know you have seen anything at all regarding this at the moment.
It is still a draft and does not have the discussion written yet rather it’s just rough ideas I have been putting down before shaping it. Please also be aware that I still have not been told to continue working with you/Innovate and in fact the framework agreement between UoP and BTR hasn’t been signed and all of us (except Geoff) at the BTR centre have been notified that our jobs (mine included) are significantly at risk due to the reduced BTR centre funding for this upcoming year. Please also be very aware that information is strictly confidential outside of BTR circles.
However, what you do have here is a very detailed, complete results section outlining the key data for the paper. As I put the figures together and you/Innovate have seen about 1/3 of the total data/figures I have, you should be extremely excited about the findings. I am sure that the IGF1R and IGF1 sections will take a bit of reading as it tends to be complicated.
I do not like rushing things to you guys although I promised Simon that I would send him something by today and I wanted to keep my word regarding this.
I also want to emphasise that when I am back from my holiday, I will send you guys a revised version of this which will include most if not all of the figures, I simply have not been able to get them from the computers in the centre, format and process them and then construct the figures. I am sure you all know how long these things take.
What I hope you guys get from this is a very clear view of the paper, the exact direction it is going in and the fact that it’s 75% written and we’ve about 90% of the data (the in vivo sample processing … being the problem), the full paper is very VERY close to being sent to you and submitted.
I also know in [its] current format, you really can’t show it to anyone, indeed nor should you, however, you will be able to explain and clearly state key mechanisms that IP1867B is triggering and, importantly, how it is mediating an anti-HGG response.
I have my phone on me and I will be taking my computer to Portugal.
150. As the amended pleading alleged, attached to that email was a draft paper. As the email said, the draft was clearly very much a draft.
151. There was some slight dispute about what exactly was said by Dr Hill to Dr Stuart and Mr Simon Cohen. I have no doubt at all that what was said reflected what was written, namely that Dr Hill was very excited about what the Research Programme was revealing about the potential of the Drug in the attempt to control or cure “hGGs” (high-grade gliomas), i.e. Glioblastomas.
152. As I have said, Innovate’s case no longer relies in any significant way on the August 2018 representations. If the case had done so, it would have faced very considerable difficulties:
(1) It is essential to Innovate’s case that the representations were made on behalf of UoP: however Dr Hill made it clear in the email I have quoted above (and in text messages) that UoP was not to know of what he was sending Innovate;
(2) It was clear from the email and the paper that this was a draft;
(3) An internal email between Dr Stuart and the Cohens dated 11 August 2018 [22] makes it clear that at that stage Innovate was well aware that the work to show whether the Drug passed effectively through the BBB was not completed (“We need the BBB work to be completed at UoP asap”);
(4) The Abstract in the draft report recorded “These data provide a clear rationale for further investigation of IP1867B in combination with a number of anti-EGFR agents currently being evaluated in the clinic”, thus recording that this work was only a step along a journey.
153. Further, and of vital importance to Innovate’s case as then put, would have been the question whether Dr Hill believed the representations made.
154. The original allegation was that “there was (in August 2018) and remains to date no worthwhile evidence to support the truth or accuracy of the proposition stated by Dr Hill to the Claimant”. That case has gone, and is difficult to square with the case now put forward by Innovate that the Drug has good, and highly valuable, prospects of commercial success which are at least in part supported by the data gathered during the Research Programme.
155. In any event, having heard Dr Hill, I reject the case pleaded against him in paragraphs 9 and 10 of the original Particulars of Claim. Dr Hill is in my judgment a great enthusiast and I accept his evidence, which is abundantly supported by the contemporaneous documentary evidence (emails and texts), that in August 2018 he genuinely believed that there were very positive indications as to the efficacy of the Drug particularly in the treatment of GBMs.
156. Accordingly I reject the case as originally pleaded. In my judgment as at August 2018 Dr Hill did believe at the least that the data obtained showed indications that the Drug suppressed resistance to EGFR inhibitors, subject to the need for further testing.
157. However, Innovate’s case does not now centre on the August 2018 representations, but rather upon the contents of a publication in an academic journal, “Cancer Letters”.
The Cancer Letters Paper
158. As set out above, in August 2018 Innovate had received a draft report.
159. There were problems, reflected in the otherwise surprising requests by Dr Hill in the email above that the sending of the draft report should not be known to UoP or BTR, both of whom, it might be thought, were fully entitled to share with Innovate the results of the Research Programme.
160. The problems were a result of BTR’s decision to significantly reduce its funding of the brain tumour research at UoP. This meant that by the summer of 2018 it was the well-justified worry of UoP’s staff, including Dr Hill, that they would be made redundant.
161. In the event these anxieties were well justified: Dr Hill was made redundant, albeit he was very soon re-employed.
162. In its written Closing Submissions, Innovate sets out what it contends is the history of events between August 2018 and the publication of the Paper:
34. Dr Hill was under great pressure at work due to the retraction of funds from BTR, which jeopardised his job at the University (and the jobs of many of his colleagues). He admitted in cross-examination that the notification of BTR’s funding withdrawal was ‘perhaps the fourth or fifth most stressful time’ for him [Transcript, Day 5, p. 611, lines 19-20]. Professor Pilkington’s evidence chimed with this: he said this ‘was not an easy time for folks to work’ [Transcript, Day 3, p. 359, lines 6-7].
35. Dr Hill’s employment was in fact terminated by the University in November 2018, when he was made redundant. He was subsequently re-hired into a new, restructured position. Dr Hill would have known that he maximised his chances of retaining his job if he produced exciting scientific work promptly.
36. In addition to keeping BTR/the University happy, Dr Hill was candid during cross-examination about his creeping anxiety that he would fail to keep Innovate happy and so lose their business without notice; he said: ‘One nagging worries [sic] was always it going - him [i.e. Mr Cohen] going working with another group and them running off and doing something and just becoming, just being able to produce work and generate exciting findings faster than us’ [Transcript, Day 5, p. 608, lines 6-9].
37. Dr Hill was also under personal pressure at the time: his texts with Mr Cohen show that, at times, he was overwhelmed by this - see, for example: on 30 August 2017: ‘Between this and my youngest’s illness I’m just about at breaking point […]’ [D39/266]; on 8 October 2018: ‘[…] Between trying to find a job, students here, grant and finishing papers here I’m kinda stressed. That doesn’t even cover the home situation’ [D39/284]; and on 21 October 2018 ‘[…] Wife threw massive meltdown about the hours i[‘m] working’ [D39/291].
38. The full suite of text message exchanges between Dr Hill and Innovate’s Mr Cohen also quite clearly shows Innovate chasing Dr Hill to produce the paper from August 2018 until publication in May 2019 [D39/251-319].
163. As a record of the pressures upon Dr Hill, those submissions (except for the allegation in the last sentence of paragraph 35, which is highly contentious) accord with the flow of text messages between Dr Hill and Dr Stuart (to an extent) and (even more so) Mr Cohen which show that Dr Hill kept them up to date with his worries and the vicissitudes caused by the funding problems which Dr Hill’s department was facing.
164. Not only from the text messages and oral evidence, but also from the email record, it is clear that, whereas for the Research Programme Innovate had the moral and financial support of two charities (BTR and Headcase), Innovate were now thinking hard about finding alternative funding.
165. It was obvious that a first step was for Innovate to find out what UoP said was the state of the Research Programme and what UoP’s proposals were for continuing co-operation.
166. On 14 August 2018 Mr Cohen wrote to Mr Hembury at UoP [23]:
Dear Guy
Thanks for discussing this with me this morning.
Our aim is to raise significant finance over the next three months and have already advanced this with some initial meetings having taken place. In order to focus our future efforts we will chose three or four key areas to present to investors. We hoped that Glioblastoma would be one of these areas and specifically our work with Portsmouth. Having held a joint call with Portsmouth and BTR we had reached agreement on completion of outstanding work and [were therefore] hopeful that we could now move forward unencumbered.
We would like to use a portion of the funds to move the Glioblastoma research forward with Portsmouth but require at least an initial conversation with Portsmouth and Geoff to allow us to do this. We need to know:
When will the current paper be finished (at least in draft)?
When can we see it?
When is the BBB work being carried out?
What further work needs to be carried out on this and other agents.
What is needed in order to carry this out?
How do we ensure that Innovates investment in Portsmouth will result in clear communication and focused resources.
We need to move this forward quickly as we are currently completing the IM and investor pack.
167. This email received a reply the following day [24]:
Right, I have spoken to Geoff [a reference to Professor Pilkington] and conveyed our conversation and your requests. He said that the work on the paper is ongoing but not due out imminently, and the BBB work is also ongoing. But he tells me that he has a number of other important things going on that need to take priority on his time and as such he can’t progress this right now. I asked when he thinks he may be in a position to pick this up and we agreed I would contact him in the first week of October to see where we are. Sorry this is not what you hoped for, but that is where things are for the time being.
168. It is clear that by this stage Mr Cohen was becoming very concerned as to whether and when Innovate would receive usable results from the Research Programme. On 31 August 2018 he wrote to Professor Sherria Hoskins, the Dean of the Faculty of Science at UoP. His email said [25]:
I understand you are currently on annual leave and your team has informed me that you will be unavailable until December.
Following our call in July with yourself and Ashley from BTR we believed that we had agreed that the current work would now be completed and that would include the BBB work. Also most crucially Dr Hill would be able to let us have a draft of the paper for publication.
We spoke with Geoff following our call and all this seemed to be fine with him. He told us the BBB work would be done within the next couple of weeks and we should speak to Dr Hill with regards to the paper and the results. We then spoke with Dr Hill who filled us in with the final results which turned out to be significantly more important than we had realised and indicated a major breakthrough in the treatment of Glioma. He told us that Geoff had asked him to complete a draft of the paper and that he was working on it in order to look at publication in a major journal.
Since then however we have been unable to progress anything. Geoff is no longer reachable and has informed us through Guy that he will not be able to discuss anything until at least October. Dr Hill has told us that he has completed a draft but is unable to release it without permission from Portsmouth and that he has not been able to get that permission to release it or complete any more work on it.
As we discussed this has already been very significantly delayed and if this is truly a breakthrough it is vital that we are able to publish and let the research world know the fantastic work that has been done by Dr Hill and Portsmouth.
Working with BTR and Portsmouth to ensure we maximise the PR value of these discoveries will I’m sure be of great value to all of us. Most importantly it will allow us to move this into patients and actually improve outcomes for them.
In addition we are in the process of raising significant funding to enable us to accelerate further research and would like the opportunity of working with Dr Hill and the team to boost their funding to allow work to be done on combination therapies, building on the mechanistic discoveries.
I understand that it is a very busy time for you but if we could at least have permission for the paper to be released and for us to work with your PR team then I believe we will be able to move forward at pace.
169. On 3 September 2018 Professor Hoskins responded [26]:
Hi Simon
I am really confused by your interpretation of the meeting.
In fact we are waiting for BTR to agree a framework agreement with you so that we know the basis on which to work with you, in essence we can work with you based on their agreement with you.
So there will be no new work with you until you and BTR have finalised that agreement. I will await news on that front.
Regarding our existing work with you, what I thought you and I had agreed was that you were going to send me the contract/agreement re that work so I could determine what we have completed and what we have yet to complete, since you indicated that our work for you was not complete. You have not sent me anything. I can also ask for agreements from our contracts team. This will enable me to ensure we complete the agreement as agreed.
You refer to a publication that is part of that work, but I cannot imagine that it is a part of our contracted work for you. We don’t publish results by contract or on demand because we simply don’t know in advance if they will be worthy of that. Geoff and his team will decide the best time to publish any papers, when it is ready and when its publication fits with other planned publications. What and when we will publish is a matter of academic judgment. A paper does not require my permission to be ‘released’ as you say. Richard and Geoff will make the decision on when their papers are submitted for publication as all academics do.
There can be no publicity around the work until the paper has been accepted for peer review publication since only then will the results have been deemed credible by the academic community. UoP (and indeed any University) do not enter into PR around scientific results until they have been peer reviewed and published.
170. On the same day Mr Cohen responded [27]:
Thank you for your prompt response I know you have just returned from annual leave.
I am sorry if my email was in any way confusing. I will try and clarify.
We have not presently asked for any future work to be carried out. Especially under the BTR umbrella. We will have significant investment funds and were looking to decide which teams to work with.
Past work has been completed apart from the BBB work promised by Geoff. As you have copies of that agreement could you please request them so that you can view the original work plans.
The main issue is communication. The publication of the paper was due to the excitement generated by Dr Hill’s work and both Dr Hill and Geoff were keen to publish. Two years ago Geoff gave a presentation in Poland which led to a very large amount of interest from the academic world and media.
We therefore expected that as this was a possible breakthrough in the treatment of Glioma that the paper would be published.
After our telephone meeting with yourself and BTR we spoke with Geoff who told us that he had asked Dr Hill for a draft as soon as possible. This was in order to move to publication in a major journal.
This data seems to be a breakthrough and in order to allow us to move treatment forward a publication would be necessary.
The issue is that there seems to be confusion within the department and Geoff has ceased all communication with us.
So for clarification. There seems to be a draft. The paper is a breakthrough in Glioma. Geoff has told us he wishes to publish. The team cannot release the draft to us or BTR until Geoff gives permission. We can not speak to Geoff and so are again in limbo.
In effect all we were seeking was for someone to speak to us and let us know what is happening with the original work. If Geoff for whatever reason is unable or unwilling to speak with us then can we speak directly with Dr Hill or another member of the team? In this way we can move things forward without having to constantly chase you or your team.
171. These email exchanges clearly show Innovate’s frustration with UoP. It seems to me that that frustration had some justification since the internal problems at the BTR unit were causing a degree of paralysis.
172. It was also clear that for its commercial purposes Innovate was very anxious to have the publication in a peer-reviewed journal of the results about which Dr Hill had spoken and communicated so enthusiastically. Further Mr Cohen understood that as yet the BBB work had not been completed.
173. Dr Hill was also keen to produce a published paper on the work, although he was hindered not only by the problems at UoP but also by a search by Dr Hill for another job and by family concerns on his part. Despite these problems Dr Hill worked at the paper, often working late into the evening to do so as is shown by his text messages and emails.
174. Eventually on 7 May 2019 a paper was submitted to a respected academic journal, Cancer Letters (“the Paper”). This was published after peer review and some corrections made by Dr Hill. [28]
175. The title of the Paper was:
IP1867B suppresses the insulin-like growth factor 1 receptor (IGF1R) ablating epidermal growth factor inhibitor resistance in adult high grade gliomas.
176. The Abstract at the beginning of the Paper summarised its conclusions as follows:
High grade gliomas (HGGs) are aggressive primary brain tumours with local invasive growth and poor clinical prognosis. Clinical outcome is compounded by resistance to standard and novel therapeutics. We have evaluated reformulated aspirin (IP1867B) alone and in combination with conventional and novel anti-aHGG agents. We show that recent biopsy-derived aHGG models were highly resistant to conventional therapeutics although show sensitivity to IP1867B, a reformulated “liquid” aspirin. IP1867B treatment mediated a potent suppression of the IL6/STAT3 and NF-κB pathways and observed a significant reduction in EGFR transcription and protein expression. We observed the loss of the insulin-like growth factor 1 and insulin-like growth factor 1 receptor expression at both the transcript and protein level post IP1867B treatment. This increased sensitivity to EGFR inhibitors. In vivo, IP1867B was very well tolerated, had little-to-no gastric lesions versus aspirin and, directed a significant reduction of tumour burden with suppression of EGFR, IGF1 and IGFR1. With EGFR inhibitors, we noted a potent synergistic response in aHGG cells. These data provide a rationale for further investigation of IP1867B with a number of anti-EGFR agents currently being evaluated in the clinic.
177. There were 11 named authors, but there was no dispute before me that the principal author was Dr Hill.
178. Embedded in the text are a number of graphic figures illustrating and recording the data derived from the work done under the supervision of Professor Pilkington and Dr Hill.
179. As will be set out below there are a number of concerning elements in the figures (and also, on Innovate’s case, in the text, although to a less significant extent). It is Innovate’s case that the elements which it identifies show both on an example by example case, and when taken together, that Dr Hill was guilty of manipulating the data contained in the Paper.
180. The paper was published on or about 26 May 2019.
181. In August 2019 a website called “Pubpeer” launched a fierce attack upon the Paper and the data within it.
182. On 22 August 2019 Dr Hill contacted Professor Schwab of Cancer Letters regarding two errors in the Article [29]. Professor Schwab responded that there should be a Corrigendum which he asked Dr Hill to prepare [30].
183. Dr Hill submitted a Corrigendum in October 2019:
In the article, we, the authors, discovered that a single microscopy panel was inadvertently placed in Figure 1f, using the SEBTA-023 panel twice instead of the SEBTA-003 representative image. We discovered that an actin western blot loading control data associated with Figure 3a was also incorrectly placed in Figure 5i. We retrieved the original actin western blot linked to Figure 5i and corrected this error.
Neither correction alter the conclusions of the original paper; however, we sincerely apologize for any confusion that this may have caused.
184. In March 2021 Cancer Letters published a Retraction Notice:
This article has been retracted: please see Elsevier Policy on Article Withdrawal.
This article has been retracted at the request of the Editor-in-Chief due to concerns regarding the legitimacy of images and data presented in the paper. Though a corrigendum …. was previously published to address some of these concerns, this corrigendum has also been found to contain errors and therefore cannot stand. Specific concerns are listed below.
The Editor and Publisher received a letter from the University of Portsmouth alerting us to an investigation into alleged research misconduct. The University concluded their investigation with external experts and determined that misconduct did take place in relation to the research involved in this paper.
Upon our separate investigation, it has been determined that the paper headline relies on showing that there was considerable reduction of IGF1R, IL6R and EGFR post treatment in all cell lines. During review, it was determined that this cannot be concluded from the presented data. For example, in SEBTA-003 the EGFR levels go up and there is no difference in IGFR1. It is apparent from Fig. 4d that in the SEBTA-003 cell line the EGFR level does not go down, which is stated in the Results section on page 32, it is rather going up. The data for IGFR1 are inconclusive and there are concerns regarding the blot. The general implications would be that the effects of the drug IP1867B does not seem to be the same for all tested cell lines, and this should have been discussed in detail by the authors. Additionally, in subsequent experiments (Fig 4g and h) the SEBTA-003 cell line (no reduction of EGFR, rather increased expression) and the other 3 cell lines (reduction of EGFR) show similar responses. This is particularly evident in Fig. 4g: Two cell lines are compared, SEBTA-003 (increased EGFR expression), both behave similarly after exposure to drugs.
The corrigendum … issue is with respect to the Supplemental Figure 6i EGFR, particularly panel IP1867B. The Corrigendum states that the left part is a cut out of the very right part. If so, the bands for IP1867B should show the same staining pattern - but they do not. Also, in the Corrigendum, there are incorrect mentions between day 14 in the Figure and day 19 in the Figure legend.
All authors were informed of the retraction in advance….. The corresponding author, Dr Hill, did not agree to the retraction.
185. It is Innovate’s case that the errors in the Paper which led to its eventual retraction rendered the results coming from the Research Programme useless from its commercial point of view. As a result of that, Innovate says that it must obtain the results of a fresh set of tests, which will delay the date when it can enjoy the benefits of its patent.
186. That case requires me to look with care at each of the deficiencies alleged in the Paper.
187. Before doing so I should refer to the disciplinary process launched by UoP against Dr Hill.
The Disciplinary Proceedings
188. UoP has a “Procedure for the Investigation of Allegations in Research”. By paragraph 23 of that Procedure “research misconduct” comprises any breach of the UK Research Integrity Office’s Code of Practice or of “accepted procedures that seriously deviate from those commonly expected within the academic and scientific communities for proposing, conducting or reporting research.”
189. On or around 21 January 2020 a disciplinary panel convened by the UoP, comprising Dr Mernagh, Head of the UoP’s School of Biological Sciences, Professor Allan, Professor of Cell Biology at the University of Manchester, and Dr Mahadevan, Fellow in Biochemistry at Trinity College, Oxford, found Dr Hill guilty of “research misconduct”.
190. It is Innovate’s pleaded case at paragraph 18 of the Re-Amended Particulars of Claim that:
Although the Defendant’s disciplinary panel did not expressly categorise Dr Hill’s research misconduct, it is reasonable in the premises to infer that they found him guilty of conduct contrary to paragraph 23(iii), which refers to “Fraud by the manipulation of data or findings with an intention to deceive, including the fabrication of data and the falsification of data”.
191. UoP called as witnesses Professor Allan, who was on the disciplinary panel, and Mr Parry, the University Secretary for the purpose of the disciplinary procedure.
192. I did not find this evidence particularly helpful. Mr. Parry’s evidence concerned the administrative aspects of the procedure.
193. Professor Allan was, as set out above, a member of the disciplinary panel. In her witness statement she says at paragraphs 38 and 39:
38. Based on the papers I am shown by the University’s solicitors, the Panel met again on 21 January 2020 to discuss its analysis of the Portsmouth Articles and to reach a conclusion … I recall the Panel talked at length about whether or not the issues we had identified in the Portsmouth Articles had been a result of deliberate conduct by Dr Hill, or whether it had been a result of carelessness. I do not recall there having been any discussion in relation to dishonesty or data fabrication as none of the issues identified in the Portsmouth Articles indicated that there had been either dishonesty or data fabrication.
39. I recall that the Panel ultimately did not reach any conclusion as to whether or not the issues in the Portsmouth Articles had been the result of deliberate or careless conduct by Dr Hill because the Panel was unable to reach a clear decision on this issue, but the Panel agreed that some of the errors constituted misconduct in research. It was therefore not necessary for the Panel to go any further than the making the finding of misconduct in research. I do not recall there being any finding of dishonesty which, as mentioned above, I do not recall being discussed by the Panel.
194. It is of course for me to decide whether Dr Hill was or was not dishonest. The panel came to no conclusion on that issue one way or the other.
195. However, the investigation carried out in the disciplinary process is important for two reasons:
(1) Part of the reasoning for the retraction of the Article by Cancer Letters was the fact that UoP had determined that research misconduct had taken place (see paragraph 184 above); and
(2) The disciplinary process frames the pleaded case put forward by Innovate in the Amended Particulars of Claim which having set out the allegations in paragraph 15 to which I now turn, pleaded:
Full details of these matters are in the possession of the Defendant, from whose subsequent disciplinary investigation into Dr Hill’s conduct in connection with the Cancer Letters Paper and other publications the above description has been taken.
UoP’s Liability under Clause 11.1
196. Before setting out my assessment of the two liability experts and my conclusions in respect of the individual allegations in paragraph 15 of the Amended Particulars of Claim, I set out my conclusions on the scope of UoP’s contractual responsibility under Clause 11.1 of the Research Agreement.
197. As set out below (and, to an extent, accepted by UoP), the reporting of data in the Cancer Letters Paper contained errors.
198. The question arises as to whether UoP is contractually liable for those errors.
199. UoP suggests that the provision of the Cancer Letters paper stands outside the contractual scope of UoP’s work.
200. In my judgment both parties understood the production of the Cancer Letters paper to be part of the discharge by UoP of its contractual obligations.
201. As set out above, Clause 11.1 of the Research Agreement provides:
Whilst the University will use all reasonable skill and care to ensure the accuracy of the work performed and any information given, the University makes no warranty, express or implied, as to accuracy and will not be held responsible for any consequences arising out of any inaccuracies or omissions are the result of negligence on the part of the University or its agents.
202. The conclusion to which I have come is that the UoP did not use all reasonable skill and care to ensure the accuracy of the work performed (which included the work of preparing the paper) or in the giving of information (including the information given in the paper).
203. I set out below my reasoning in coming to the above conclusion. I deal below separately with the case that the errors were the result not simply of failures to exercise reasonable skill and care but were the result of dishonesty.
The Liability Experts
204. As referred to above, I heard evidence from Professor Susan Short on behalf of Innovate and from Professor Martin Bushell on behalf of UoP.
205. Professor Short is a Professor and consultant specialising in clinical neuro-oncology. Her area of clinical expertise is in the management of neuro-oncological patients with radiotherapy and chemotherapy. She is the author of many publications and several book chapters on the biology of glioma and treatment of adult brain tumours including recent consensus statements on behalf of what she describes as the European and global neuro-oncology community.
206. Her first report relied upon a separate report prepared by Dr Elisabeth Bik, who I understand to be an expert in reading western blot data. Professor Short’s use of Dr Bik’s report was understandable, but inappropriate in the context of litigation where the introduction of expert evidence is subject to permission from this Court. In reaching my conclusions I have not relied upon Dr Bik’s views.
207. Professor Bushell is a Professor of Cancer Biology at the University of Glasgow. He has considerable experience in carrying out testing of the type at issue in this case and in recording the results of such testing.
208. In my judgment Professor Bushell probably has more direct experience of the sort of testing and reporting at issue in this case, but Professor Short has sufficient experience to be able to assist the Court, as she and Professor Bushell both did.
209. When they were in joint session it appeared to me that the differences between them were more matters of nuance than deep divides.
210. This is not one of those cases which can be simply resolved by preferring one expert on liability to the other.
211. I have already referred above to the evidence of both experts where they were in agreement.
212. Where they differed was principally in respect of whether Dr Hill was guilty of dishonesty. Ultimately this is a matter for me, but it is worthy of note how restrained and careful Professor Short was when setting out her views, even when coming to the conclusion that Dr Hill was guilty of dishonesty.
213. In reaching the conclusions below, I have considered both experts’ views below in respect of each individual allegation.
214. At this stage I should record my reasons for admitting a third report from Professor Bushell, a matter which was discussed at the beginning of day 6 of the trial: in this third report Professor Bushell gave evidence about the way in which the record of blots could be flipped when being processed using a computer. The greater part of his evidence in this report was not the subject of challenge, but there was a challenge to paragraphs 42 to 45 of that report.
215. The view I took then is that what was contained in those paragraphs was a minor increase in the expert material before me and was of a nature that could be dealt with by Mr Roe KC and those beside and behind him. In the event it seemed to me that that view was vindicated.
The Alleged Misrepresentations
216. Paragraph 15 of the Amended Particulars of Claim states “the Cancer Letters Paper contained serious misrepresentations about the results of the Research Programme”, and then sets out allegations in sub-paragraphs (a) to (h).
217. I consider each of the allegations separately and then look at the total picture to assess whether Dr Hill was dishonest, dishonesty being the central issue on liability in this case. In the process I consider first whether the evidence discloses breaches of contract on the part of UoP. As will be seen, given the evidence before me there can be no real decision other than that the reporting of the data in the Cancer Letter paper contained errors.
(a) In the paper as originally published, the claim that “[r]epresentative microscopy images of each aHGG cell line [of a number that were tested] 24h post IP1867B exposure revealed widespread cell death […]” was said to be supported by figure 1f, but on close examination figure 1f used the same image to show the purported results from both the SEBTA-023 and SEBTA-003 cell lines. This was corrected in the corrigendum.
218. As UoP submits in paragraph 83 of its written Closing Submissions, it is common ground that two images in Figure 1(f) are duplicated. They are the images for the cell lines SEBTA-023 and SEBTA-003 when treated by the Drug (i.e. the bottom two images in the right hand column).
219. This error was acknowledged and corrected in the Corrigendum.
220. In my judgment, this error should not have been in the Paper as originally published and evidences a breach of Clause 11.1 of the Research Agreement.
221. However, the error was corrected, and in itself was unlikely on its own to have been the cause of loss on the part of Innovate.
(b) The same photograph of a beta-actin control blot was cropped, presented and labelled differently so as to appear looking different in each case except under close analysis, in two figures purportedly showing quite different things:
(i) figure 3a, purportedly showing “[r]epresentative immunoblots for caspase 3 cleavage following IP1867B […] exposure” of certain cell cultures, in support of the claim that “IP1867B directs significant cell death and suppression of key inflammatory networks in aHGGs”;
and
(ii) figure 5i, purportedly showing a “[r]epresentative immunoblot for EGFR following in vivo treatment [i.e. treatment of mice with brain tumours] with each therapeutic at day 19”, in support of the claim that “IP1867B IP treatment induces significant reduction of intercranial tumours”.
The blots used in figure 5i, in respect of the way Dr Hill and his co-authors labelled them (with reference to “in vivo treatment” and “#1”, “#2” etc., presumably identifying mice), did not derive from samples taken from mice at all.
222. This allegation was accepted by both parties in oral closing submissions as the most serious of all the allegations made by Innovate.
223. In the course of his oral Opening Submissions Mr Roe KC handed up to the Court a black file of demonstratives. The allegations surrounding paragraph 15(b) of the Amended Particulars of Claim are set out at pages 8 to 15 of that file.
224. The submission of that file led to an objection by UoP that the case was being expanded outside the scope of the existing pleadings. This objection led to a ruling on my part ([2023] EWHC 2525 (TCC)) that the case illustrated fell within the existing pleadings so that no amendment was necessary. However Innovate had formulated an amendment which “as a matter of good order” I directed should be made. In the event the pleading is now in the following terms:
(b) The same photograph of a beta-actin control blot (the mirror-image of which had appeared as the beta-actin control blot for tests on BEZ2325 and/or clomipramine in Dr Hill’s presentation of 25 April 2016 entitled “Understanding the mechanisms of chemotherapy resistance in cancer”) was cropped, presented and labelled differently so as to appear looking different in each case except under close analysis, in two figures purportedly showing quite different things:
(i) figure 3a, purportedly showing “[r]epresentative immunoblots for caspase 3 cleavage following IP1867B […] exposure” of certain cell cultures, in support of the claim that “IP1867B directs significant cell death and suppression of key inflammatory networks in aHGGs”;
and
(ii) figure 5i, purportedly showing a “[r]epresentative immunoblot for EGFR following in vivo treatment [i.e. treatment of mice with brain tumours] with each therapeutic at day 19”, in support of the claim that “IP1867B IP treatment induces significant reduction of intercranial tumours”.
The blots used in figure 5i, in respect of the way Dr Hill and his co-authors labelled them (with reference to “in vivo treatment” and “#1”, “#2” etc., presumably identifying mice), did not derive from samples taken from mice at all.
225. Paragraphs 10 to 13 of Innovate’s written Closing Submissions helpfully set out its case as to paragraph 15(b) of the Amended Particulars of Claim: those paragraphs are repeated in the next four paragraphs of this judgment.
226. Pages 8 to 9 of the Demonstrative illustrate paragraph 15(b) of the APOC [A/4/24-25]: that the same photograph of a beta actin control blot was cropped, presented, and labelled differently so as to appear, looking in each case except under close analysis, in two figures in the Cancer Letters Paper as showing quite different things (figs 3(a) [D36/292] and 5(i) [D36/294-295]) (‘the paragraph 15(b) allegation’).
|
Figure 3(a) Cancer Letters Paper
|
Figure 5(i) Cancer Letters Paper
|
227. Pages 10 to 15 of the Demonstrative further illustrate the paragraph 15(b) allegation by showing that the beta actin control blot images used in figs 3(a) and 5(i) of the Cancer Letters Paper are also found elsewhere in the disclosure—namely at [D2/399], which is a slide in Dr Hill’s presentation of 25 April 2016 entitled ‘Understanding the mechanisms of chemotherapy resistance in cancer’ [D2/379-408].
228. [D2/399] shows (amongst other things) a twelve-lane beta actin blot produced in a BEZ235 and clomipramine in vitro experiment. These pages of the Demonstrative explain that if that twelve-lane beta actin control blot is flipped along a vertical axis and then cropped selectively, one can see that it is the same image which appears in fig 3(a) (six lanes) and fig 5(i) (nine lanes) of the Cancer Letters Paper.
|
D2/399
| |
|
D2/399 (flipped)
|
Figure 3(a)
|
|
|
|
|
D2/399 (flipped)
|
Figure 5(i)
|
|
|
|
|
|
|
|
|
|
229. In UoP’s written Closing Submissions, it analyses paragraph 15(b) as containing four allegations.
230. The first, which UoP considers at paragraphs 78 to 80 of its Submissions, is an allegation that in Figure 5(i) what appeared to be the result of analysis of samples taken from the in vivo mouse samples was not based upon mouse samples at all.
231. I accept UoP’s submission that this allegation was not made out and falls away.
232. That leaves three other allegations which UoP summarised as follows at paragraphs 108 to 110 of its submissions:
Figure 3(a) and 5(i): (a) Same beta actin loading control blot used but labelled differently and (b) when it was corrected in the Corrigendum the quantitation data was not corrected
108. This allegation appeared at ¶15(b) of the RAPOC. It appears that, following the evidence, Innovate’s case on dishonesty realistically rests on this allegation alone.
109. This allegation can now be broken down as follows:
a. First, when corrected in the Corrigendum, the quantitation data for the corrected Figure 5(i) was incorrect (“Allegation 1”);
b. Second, that the control blot was duplicated in the Article but appeared with different labelling in Figure 5(i) (“Allegation 2”);
c. Third, that the duplicated control blot in Figure 3(a) and 5(i) was itself derived from a presentation on a different drug dated 25 April 2016; (“Allegation 3”).
110. Allegation 1 is denied as being a breach of Contract (negligent or dishonest). It is accepted that Allegations 2 and 3 comprise a breach of Clause 11.1 of the Contract but denied that the nature of that breach was dishonest.
233. Thus it is accepted that in at least two respects the allegations in paragraph 15(b) of the Amended Particulars of Claim are made out as being breaches of Clause 11.1 of the Research Agreement.
234. As to what is described as allegation 1 (as to quantitation data), I accept that that allegation falls away for the reasons given in paragraphs 111 to 114 of UoP’s Submissions.
235. I return to consider allegations 2 and 3 (accepted as being breaches of clause 11.1) when considering the fraud case below.
I Full copies of blots were provided (or purportedly provided) separately through a weblink to “supplementary data”. The cropped EFGR blot in figure 5i (see above) did not match the full EGFR blot contained in that data.
236. There is no issue but that this error constituted a breach of the Contract (see paragraph 107 of UoP’s written Closing Submissions).
237. I consider below whether this error was the result of fraud rather than lack of exercise of reasonable care and skill.
(d) The beta-actin control blot shown in figure 3b in the Cancer Letters Paper was a copy of a photograph of a blot that had been published in a different paper authored by Dr Hill, relating to a different set of experiments on a different drug (“DIVERSet JAG Compounds Inhibit Topoisomerase II and Are Effective Against Adult and Pediatric High-Grade Gliomas”, in Translational Oncology”, 30 July 2019).
238. The essence of this allegation is that there was a discrepancy between two published versions of the same data blot. This was the subject of submissions in the written and oral closing submissions and in further submissions which I permitted both parties to serve after the oral submissions had been concluded, mainly on the issue as to whether it was the Cancer Letters paper or a paper in a different journal which contained the correct data.
239. The problem for Innovate is that the version which is in the Paper appears to be the correct version, or at least may have been the correct version.
240. On that basis the publication of this figure was not a breach of Clause 11.1, or has not been shown to be a breach of Clause 11.1.
241. However, I do need to consider whether the inconsistent publication of what is at heart the same data is evidence of dishonesty: I return to this issue below.
(e) The description of the results said to have been shown by the Research Programme did not match the underlying data:
(i) The claim on page 32 that “[w]e […] noted that there was a considerable reduction on IGF1R, IL6R and EGFR protein expression post-IB1867B treatment in our aHGG cells” was false because for SEBTA-003 EGFR levels went up and IGFR1 remained the same.
(ii) Claims on page 34 and page 36 about what the data showed were likewise false when compared to the data.
242. The allegations at paragraphs 15(e), 15(f), 15(g) and 15(h) all concern the text of the Paper rather than the data set out in the figures.
243. UoP’s written Closing Submissions make the following submissions:
39. As set out in the Defendant’s opening, Professor Short and Professor Bushell agree that the data contained in Figure 1, Figure 2, Figure 3 and Figure 5 (except Figure 5(i)) was accurately described by the text in the Article. This position was not challenged during the course of the trial.
40. In other words, it is really only at Figure 4 where Dr Hill is alleged to have chosen to use wording in the Article which was not faithful to the figures it described.
41. It was unsurprising therefore that Professor Bushell stated in cross-examination: “If we were to use as many words as required to perfectly summarise all of the data in this manuscript and then summarise that data into the different sections and also into the title, I do not think that that would change those outputs of this manuscript, i.e. if we were to take all of the data into account and perfectly summarise it, I think we would still come out with the same conclusions of this paper”. That aspect of Professor Bushell’s evidence was not challenged in cross examination.
42. The University denies that any of the Substantive Allegations comprise a breach of the Contract. Dr Hill was entitled to summarise the data as he did. The fact that another author might have summarised it differently does not render Dr Hill’s work a breach of the obligation of reasonable care and skill particularly where these issues were not identified (or were identified but did not concern) the peer reviewers.
244. Innovate deals with these allegations in paragraph 50 of its written Closing Submissions:
When understood in that context, Innovate submit that the other pleaded allegations arose due to such an extreme lack of care on the part of the author of the paper that the author can be inferred to have been indifferent as to whether the relevant statements were true or not. That these remaining allegations arose because of conduct of that nature is more probable than an innocent explanation, given that conscious dishonesty in the same paper is the most probable explanation for the image manipulation allegations:
….
c. The paragraph 15(e), (f) and (g) allegations (paragraph 15(e)-(g)APOC [A/4/26] - namely, that the description of the results did not match the underlying data - also demonstrates an extreme lack of care so as to be reckless as to the truth of the representations. Professor Bushell’s evidence on this allegation is illogical: he said that any discrepancy because the words used and the data it described was due to the Cancer Letters-imposed word limit [Transcript, Day 7, p. 1075, lines 5-22]. Of course, the word limit had no bearing on the accuracy of what the authors chose to say about the data.
d. The paragraph 15(h) allegation (paragraph 15(h) APOC [A/4/26]) - namely, that the text at paragraph 3.4 of the Cancer Letters Paper claimed 6 mice had been analysed, but the data showed only 3 mice had been analysed - also demonstrates at least an extreme lack of care so as to be reckless as to the truth of the representations. However, Professor Short gave evidence under cross-examination that constituted a cogent and plausible explanation as to why this could have been done deliberately …
245. I return below to the allegation of fraud.
246. Insofar as the text of the Paper is attacked in a number of ways, it seems to me to be a legitimate criticism of the text that in the respects alleged it does not accurately reflect the data coming out of the Research Programme. I also accept that a careful writing of the text would have accurately reflected that data.
247. However, the text was written for a specialist audience, who could be expected to read the text together with the data represented in the Figures, and, indeed, quite probably to have been minded to place that data in its thinking ahead of the descriptive text.
248. At the end of the day, Innovate’s Closing Submissions suggest that the criticisms of the text are not at the forefront of its case, an approach which appears to me realistic: the important issue on causation is whether the problems in the Paper caused the eventual retraction of that Paper. There is little evidence that it was the description of the results in the text which lead to the retraction of the Paper.
249. On the other hand, Innovate relies upon the overenthusiastic description of the results of the Research Programme as being an important part of its case as to dishonesty, a matter to which I return below.
250. For completeness I will now deal with the allegations at paragraphs 15(f) to (h) of the pleading, which I reject for the following additional reasons to what I have set out at paragraphs 241 to 247 above.
(f) The blots in figures 4d and 4g showed quite different behaviours for total EGFR in the UP-029 and SEBTA-003 cell lines, but this important fact was not mentioned in the text.
251. UoP set out its submissions on this allegation in paragraphs 52 to 58 of its Closing Submissions.
252. At paragraph 57 UoP submitted:
Once again, it is fair to say that there is scope for reasonable disagreement about whether or not the Article ought to have made explicit the difference in EGFR behaviours in figures 4(d) and 4(g) (even though those differences were already apparent on the face of the figures). Whatever side one comes down on in relation to that issue, it does not comprise a breach of the Contract and the allegation that Dr Hill made a dishonest decision not to describe that difference in the text is unsustainable.
253. I accept that submission.
(g) The two sets of blots in figure 4g purported to show that, even though they were supposedly from the same experiment done at the same time, the samples had been added in different orders. The likelihood is that one or both were not actually blots generated by the Research Programme.
254. UoP set out its submissions on this allegation at paragraphs 87 to 91 of its Closing Submissions. At paragraphs 89 to 91 it submitted:
89. Professor Bushell explained in his report that “When running western blots, samples are often loaded to gels in different orders between different cell lines. This might occur, for example, if the cells grow at different rates and get processed at different times. This in no way indicates that they are not from the same experiment, nor does it diminish the value of the results in any way. The material and methods set out in the Cancer Letters Paper do not claim that these western blots were run at the same time; there is no evidence that they were run at the same time, nor is there any reason why they should have been. I therefore do not understand the basis for this criticism.”
90. Professor Short agreed with this in Short 2 at line 561.
91. The allegation was not pursued in cross examination of either Dr Hill or Professor Bushell. Effectively, therefore, this allegation has fallen away.
255. I accept those submissions.
(h) The text at paragraph 3.4 claimed that 6 mice had been analysed, but the data showed that 3 mice had been analysed.
256. At paragraphs 66 to 71 of UoP’s Closing Submissions they submit as follows:
66. In summary, it is alleged that Dr Hill was aware that only 3 mice had been tested and consequently the reference in the Article to 6 mice was a dishonest breach of contract.
67. It is common ground that Dr Hill was not involved in conducting the in vivo experiments at ICL. Nevertheless, it is apparent from the underlying documents that the ICL study proceeded as follows:
a. Each of the treatment groups contained five mice;
b. By 4 November 2016 the mice had been implanted and, from imaging, ICL could see that they were starting to develop tumours and so planned to commence treatment with the Drug;
c. However, on 9 November 2016, it was reported that 3 of the mice treated with the Drug had died due to complications with the dosing. As a consequence, 1 mouse was substituted out of the control group and into the group treated with the Drug meaning that there were then 3 mice in that group;
d. On 21 November 2016, Professor Hajitou provided an update on the research. In relation to the mice injected with the Drug, he stated that they: “don’t seem to lose weight at all, they are gaining weight, which could be a positive sign of tumour growth control under liquid aspirin treatment”. Dr Hill described these results as being “super exciting”;
e. On 25 November 2016 similar encouraging results were reported by Professor Hajitou who stated the “anti-tumour effect of Liquid Aspirin is clear and might be similar or even better than TMZ, but we will have to analyse the imaging data to confirm”;
f. However, in a call on 28 November 2016 Professor Hajitou informed Innovate, Dr Hill and Professor Pilkington that all the mice had been culled.
68. To a certain extent, the facts speak for themselves (even before turning to the Liability Experts). There was apparent scope for confusion about the numbers of mice that had been tested and it is clear that Dr Hill was in fact confused. The contemporaneous evidence shows that he was not alone in that confusion, and that Geoff Pilkington and Simon Cohen were also confused. In cross-examination, Simon Cohen recalled that, at least at one stage, he had found the mice substitution issue confusing “Yes, I did, but Jimmy had said in the previous e-mail that he said there was two mice had died, not three, so it was confusing”. The exchange with Simon Cohen regarding the confusion about the number of mice in the ICL dataset is worth reading in full.
69. Both Liability Experts also thought that the position was confusing:
a. Professor Bushell confirmed that “The information provided by Imperial is confusing and easy to misinterpret - Imperial confirmed that, due to the initial three deaths, there remained three mice in the IP1867B treatment group, and I can see how this could be interpreted as there having been six mice in each treatment group initially, but in fact there were five”; and
b. Professor Short agreed in Short 2 at line 568 the point about ICL’s description: “The description of the in vivo data, as stated by Prof Bushell are confusing”. Professor Short also explained in cross-examination that the reference to six mice “is a typographical error although I must say six is a fairly standard number to use in these circumstances. That might explain the typographical error”. She also accepted in cross-examination that the error could have been made because “of the sheer confusing nature of what happened at ICL”.
70. Further, it was apparent from other aspects of the Article that only 3 mice had been treated with the Drug. Figure 5(g-h) states in the accompanying text “n=3” and Figure 5(i) refers to there being 3 mice.
71. Given the experts’ agreement as to the explicable and understandable nature of the error, and given that Dr Hill was not alone in misunderstanding the data provided by ICL (and he held that misunderstanding throughout), it is denied that this error was a breach of Clause 11.1 and/or dishonest in nature. It is unsurprising therefore that it was not put to Dr Hill in cross-examination.
257. I accept UoP’s submissions set out above, particularly in respect of the effect of the expert evidence.
Conclusions in respect of the Cancer Letters Paper and UoP’s liability under Clause 11.1
258. For the above reasons, the reporting of data in the Cancer Letters Paper contained a number of errors in respect of the matters alleged at paragraphs 15(b) and (c) of the Amended Particulars of Claim. I have also noted problems with the Paper in respect of the allegations at paragraphs (a) and (d) of the Amended Particulars of Claim.
259. For the reasons set out above, in my judgment UoP is liable under Clause 11.1 of the Research Agreement for the errors which I have held to have existed as pleaded in paragraphs 15(b) and (c).
The Allegations of Dishonesty
260. I have set out above my conclusion that the case pleaded by Innovate does not set out a claim for fraudulent misrepresentation because there is no claim for losses caused by reliance upon the alleged misrepresentations.
261. However, in case that conclusion is successfully challenged, and in recognition of the centrality of the allegations of dishonesty in these proceedings, I set out my conclusions on those allegations.
262. I have already noted above that Innovate’s written Closing Submissions suggest that UoP might be liable for dishonesty on the part of students working on this Research Programme. As I have said above, no case of dishonesty on the part of any students has been developed thus far.
263. The case as developed to date has been all about alleged dishonesty on the part of Dr Hill personally.
264. In closing, Innovate has also raised the possibility that it can establish a case of dishonesty based upon recklessness.
265. As I have recorded above, Innovate made it clear in its oral closing submissions that no case in deceit is being put forward. However, insofar as a case of dishonesty is still at the heart of Innovate’s case, the authorities in respect of the tort of deceit are relevant as setting out what is required to establish a case of dishonesty based upon recklessness:
(1) No action based on fraud can be supported by mere proof of negligence: per Lord Buckmaster in Donoghue v Stevenson [1932] 1 AC 562 at page 570;
(2) Gross and culpable negligence is not enough: per Millett LJ in Armitage v Nurse [1998] 1 Ch. 241 at page 250;
(3) Where “blind-eye” or “shut-eye” knowledge is alleged, what must be shown is knowledge that the statement may not be true and a deliberate decision to fail to inform oneself: per Gloster J. in Petromec Inc v Petroleo Brasiliero SA [2006] EWHC 1443 (Comm); [2007] 1 Lloyd’s Rep. 629, at paragraph [94];
(4) It is a general principle of law that a conscious decision not to enquire into the existence of a fact is in many cases treated as equivalent to knowledge of that fact: per Lord Hoffmann in OBG Ltd v Allan [2007] UKHL 21; [2008] 1 AC 1 at paragraph [41];
(5) The general principle as to what must be established to prove dishonesty was stated by Lindley LJ as long ago as 1891 in the case of Angus v Clifford [1891] 2 Ch 449 at page 466:
…the matter to be inquired into is, fraud or carelessness. If it is fraud, it is actionable, if it is not fraud, but merely carelessness - it is not. The passages about knowledge - knowingly making it, and making a statement without believing its truth, are based upon the supposition that the matter was really before the mind of the person making the statement, and, if the evidence is that he never really intended to mislead, that he did not see the effect, or dream that the effect of what he was saying could mislead, and that that particular part of what he was saying was not present to his mind, that I should say is proof of carelessness rather than of fraud.
266. UoP relies upon a more modern expression of the same principle in the judgment of Lord Hughes in Ivey v Genting Casinos (UK) Ltd [2017] UKSC 67; [2018] AC 391 at paragraph [74]:
When dishonesty is in question the fact-finding tribunal must first ascertain (subjectively) the actual state of the individual’s knowledge or belief as to the facts. The reasonableness or otherwise of his belief is a matter of evidence (often in practice determinative) going to whether he held the belief, but it is not an additional requirement that his belief must be reasonable; the question is whether it is genuinely held. When once his actual state of mind as to knowledge or belief as to facts is established, the question whether his conduct was honest or dishonest is to be determined by the fact-finder by applying the (objective) standards of ordinary decent people. There is no requirement that the defendant must appreciate that what he has done is, by those standards, dishonest.
267. In paragraph 25 of its written Closing Submissions, UoP quotes the following question put to Dr Hill in cross-examination as summarising Innovate’s case:
The trouble is, Dr Hill, that perhaps because of the stress you were under or some other reason we do not know about, when it came to those specific examples, you decided, when you were putting the paper together, instead of going properly to the right image and taking the time to find it and put it into the paper, simply to take an image that was suitable from somewhere that fitted the thrust of the data you were working with, stick it in and hope no one would notice. That is the reality of what happened, was it not?
268. UoP continues at paragraph 26:
So Innovate’s case on dishonesty, in Ivey terms, is that:
a. In putting the Article and/or Corrigendum together, Dr Hill subjectively knew that he was using images or words that were not correct or accurate (having intentionally decided for some reason to use them); and
b. That conduct was objectively dishonest.
269. That appears to me accurately to set out Innovate’s case, save that it does not reflect Innovate’s alternative case that if Dr Hill did not intentionally fabricate parts of the data, he was guilty of recklessness. That case was made clear in paragraphs 51 and 52 of Innovate’s Closing Submissions:
51. The pleaded allegations of breach in this case occurred because of Dr Hill’s cavalier attitude. He was not always concerned with faithfully depicting the genuine (i.e. original) experimental results; instead, when writing, compiling and correcting the Cancer Letters Paper, the entire paper was infected by dishonesty because Dr Hill either knew that the relevant statements in the paper were false or did not care either way.
52. Applying the test in Ivey v Genting Casinos (UK) Ltd [2017] UKSC 67, the Court can also be satisfied that by the standards of ordinary decent people, Dr Hill was behaving dishonestly. That is supported by the views of the Pubpeer community, the conclusions of the University’s own investigations (it is telling that the University did not call Dr Mernagh or Professor Guille to give evidence), and the decision taken by Cancer Letters in 2021 to retract the original article and its corrigendum. As Professor Allan said in cross-examination, ‘any scientist who is behaving properly […] would […] ensure that what goes into the paper is a faithful depiction of what was photographed at the time that the experiment was photographed in the laboratory …
270. An important issue for consideration is whether Innovate has established that Dr Hill had a motive for dishonesty. In that respect there is an issue between the Parties as to whether motive is relevant, and, if so, what conclusions should be drawn by the Court as to any motive Dr Hill had to be dishonest.
271. It is Innovate’s case that the facts speak for themselves, and that on the facts of this case motive is irrelevant.
272. UoP has referred me to a number of authorities which it says are relevant.
273. In Three Rivers DC v Bank of England [2001] UKHL 16; [2003] 2 AC 1 Lord Millett said at paragraph [182]:
It is not unfair to observe that, in the absence of some financial or other incentive, a charge of dishonesty against professional men and public officials is possible but inherently improbable.
274. In Fiona Trust and Holding Corpn v Privalov [2010] EWHC 3199 (Comm), Andrew Smith J. stated at paragraph [1438]:
… [it] is well established that ‘cogent evidence is required to justify a finding of fraud or other discreditable conduct’: per Moore-Bick LJ in Jafari-Fini v Skillglass Ltd [2007] EWCA Civ 261 at [73]. This principle reflects the court’s conventional perception that it is generally not likely that people will engage in such conduct: ‘where a claimant seeks to prove a case of dishonesty, its inherent improbability means that, even on the civil burden of proof, the evidence needed to prove it must be all the stronger’, per Rix LJ in Markel International Insurance Company Ltd v Higgins [2009] EWCA Civ 790 at [50]. The question remains one of the balance of probability, although typically, as Ungoed-Thomas J put it in In re Dellow’s Will Trusts [1964] 1 WLR 451, 455 (cited by Lord Nicholls in In re H [1996] AC 563, 586H),
‘The more serious the allegation the more cogent the evidence required to overcome the unlikelihood of what is alleged and thus to prove it’.
Associated with the seriousness of the allegation is the seriousness of the consequences, or potential consequences, of the proof of the allegation because of the improbability that a person will risk such consequences: see R (N) v Mental Health Review Tribunal (Northern Region) [2005] EWCA Civ 1605; [2006] QB 468, para 62, cited in In re D (Secretary of State for Northern Ireland intervening), [2008] UKHL 33; [2008] 1 WLR 1499, para 27, per Lord Carswell.
275. In Bank St Petersburg PJSC v Arkhangelsky [2020] EWCA Civ 408; [2020] 4 WLR 55 Males J. stated at paragraph [117]:
In general it is legitimate and conventional, and a fair starting point, that fraud and dishonesty are inherently improbable, such that cogent evidence is required for their proof. But that is because, other things being equal, people do not usually act dishonestly, and it can be no more than a starting point. Ultimately, the only question is whether it has been proved that the occurrence of the fact in issue, in this case dishonesty in the realisation of the assets, was more probable than not.
276. The importance of establishing a motive, given that, as Males LJ observed, “people do not usually act dishonestly” was considered by Mann J in Mortgage Agency Services Number One Ltd (t/a Britannia Commercial Lending) v Cripps Harries LLP [2016] EWHC 2483 (Ch). The Judge stated:
88. Of particular relevance to a case of fraud such as the present is the question of motive. By and large dishonest people are dishonest for a reason. They tend not be dishonest wilfully or just for fun. Establishing a motive for deceit, or conspiracy, is not a legal requirement, but if a motive cannot be detected or plausibly suggested then wrongful intention (to tell a deliberate lie in order to deceive) is less likely. The less likely the motive, the less likely the intention to deceive, or to conspire unlawfully. In many, if not most, fraud cases this would not be a particularly live point. The defendant is often a person who would be a direct beneficiary of the fraud, and a plausible motive is, to that extent, relatively easily propounded. The present case is, however, different.
And:
474. When attention turned to CHH the claimant donned its fraud detection goggles, turned the sensitivity up to High and attributed a dishonest motive to every interesting feature in the landscape (in very delayed proceedings). That led to a large number of accusations of dishonesty being made. Some allegations came close to being allegations which should not have been made or sustained (though I acknowledge that the claimant did exercise sufficient judgment to abandon some allegations after the close of evidence). Whilst motive is not a necessary ingredient in the claim, as I have frequently said, it is obviously important and I doubt if sufficient attention was paid to the realities of that part of the case, especially once the defendant's evidence was complete.
277. In this case Innovate has sought to establish that Dr Hill had a motive to be dishonest. I have already set out at paragraph 162 above Innovate’s submissions in paragraphs 34 to 38 of its written Opening Submissions, which I have accepted were factually accurate. Those submissions were in a section of Innovate’s Submissions in which Innovate has set out its case on motive.
278. Innovate’s primary case is that it does not need to prove motive. It submits at paragraph 32 of its written Closing Submissions:
Innovate’s submission is that, based on primary findings of fact the Court is entitled to and asked to make (as set out above), dishonesty is the most probable explanation for the occurrence of the paragraph 15(b) and (d) allegations. The University has advanced no probable alternative explanation, so this is not a case involving more than one plausible explanation for the breaches of contract alleged. In that context, the Court need not move on to consider more extraneous factors, such as motive.
279. Of course, this case cannot be decided by reference to a determination on the issue of motive alone, but I accept on the basis of the authorities I have referred to above that consideration of motive is an important matter and is an appropriate starting point in this case.
280. Without prejudice to the submission in paragraph 32 of its Submissions, which I have set out above, Innovate does set out its case as to motive. After setting out the factual matters in paragraphs 34 to 38, at paragraphs 39 and following it sets out its case in that regard. Perhaps the most important points are in paragraphs 41 and 42:
41. During examination-in-chief, Professor Short provided a helpful response to the Court’s question as to why a scientist would choose to misrepresent data. In Innovate’s respectful submission, Professor Short’s explanations are entirely consistent with plausible motives for deliberately manipulating data in this case ….:
‘I think there are several different reasons why that might be the case. So you may have run an experiment that did not work quite as well as you thought it would. And you may, you know, be under time pressure to produce some data, and we have seen that actually running a western blot is time and it is a sort of an energy intensive technical process. It would be much easier obviously to take an image that already existed and use that.’
42. Finally, the fact that the paragraph 15(b) allegation concerns the manipulation of a ‘control’ blot that tends to look very similar across experiments (if done properly) in fact makes deliberate conduct (i.e., dishonesty) more probable. That is because it is understandable, as a matter of human psychology, that a scientist under immense time and personal pressure would cut corners by manipulating the same beta actin western blot image (the work of a few seconds) to look different (cropped, presented (i.e. flipped) and labelled differently) in separate figures, instead of going to the effort of finding, in a not uncomplicated electronic filing system, the ‘original’ beta actin western blot image (i.e. a beta actin image derived from the same experiment from which the particular protein of interest image is taken) and cross-referencing that with a laboratory book to ensure it is accurate. Because the image is the ‘control’ and does not illustrate the ‘protein of interest’, one can see why a scientist might be able to persuade him- or herself that, in that limited sense, manipulating this image is less culpable. It is, of course, still dishonest.
281. This case as to motive (particularly that at paragraph 42 of Innovate’s Closing Submissions) was not put to Dr Hill. This reflects Innovate’s primary case, which was clearly put, that the totality of mistakes was capable of only one conclusion, that Dr Hill was, on the balance of probabilities, guilty of dishonesty. This case was developed fully not only in Innovate’s oral closing submissions but also at paragraphs 8 to 32 of the written Closing Submissions. The points made in those paragraphs are helpfully summarised at paragraphs 30 to 32:
30. Professor Bushell agreed that a total of five or six mistakes must have been made by Dr Hill (or someone else) for the paragraph 15(b) allegation to have occurred …. He also accepted that for the paragraph 15(b) allegation to have occurred, Dr Hill (or someone else) ‘would have had to have made a number of mistakes’ ….
31. In summary, therefore, Innovate respectfully suggests:
a. The claim that paragraph 15(b) or (d) allegations could have occurred as a result of accidental (and imperceptible) flipping on Adobe Illustrator is not plausible.
b. Dr Hill’s evidence regarding the apparently built-in susceptibility of Adobe Illustrator to flip images imperceptibly was most probably made up on the spot following Innovate’s very late discovery - argument in respect of which he had listened to earlier that morning - that the image at [D2/399] was the same image used in figs 3(a) and 5(i) of the Cancer Letters paper (albeit cropped, presented (flipped), and labelled differently).
c. Further, and in any event, the number of ‘mistakes’ required [to] be made for the 15(b) and (d) allegations to occur makes an innocent, non-deliberate explanation improbable.
d. The University nowhere advanced a plausible alternative explanation for how the paragraph 15(b) allegation could otherwise have occurred. Dr Hill and Professor Bushell’s previous evidence regarding mislabelling was hardly mentioned by either witness at trial.
e. Accordingly, the more probable explanation for these allegations is that they are the result of deliberate and dishonest conduct by Dr Hill (or someone else for whom the University is liable, e.g., one of Dr Hill’s students).
32. Innovate’s submission is that, based on primary findings of fact the Court is entitled and asked to make (as set out above), dishonesty is the most probable explanation for the occurrence of the paragraph 15(b) and (d) allegations. The University has advanced no probable alternative explanation, so this is not a case involving more than one plausible explanation for the breaches of contract alleged. In that context, the Court need not move on to consider more extraneous factors, such as motive.
282. As those submissions rightly emphasise, the allegations of dishonesty require the Court to consider the evidence as a whole. Thus, for example, even if an error was corrected pre-publication or by way of the corrigendum, the fact of the original error may indicate at least recklessness. As a second important example, in respect of the paragraph 15(d) allegation, as to which I received additional written submissions after oral submissions had been concluded, I have concluded that the correct data were or may well have been in the published Cancer Letters paper. On that basis I have held that the case that there was a breach of the Agreement as alleged in paragraph 15(d) is not made out. However publication of the inconsistent but associated data in another journal is a matter which I need to consider in respect of the issue of dishonesty.
283. Innovate in its pleaded case raises a plea of “propensity” (see paragraph 47(b) of the Amended Reply). In the end, Innovate’s oral and written closing submissions make nothing of this as a separate legal theory, but rely upon what is said to be the weight of the evidence as establishing dishonesty.
284. The most powerful points in support of Innovate’s case that Dr Hill (or someone else) was dishonest are:
(1) The number of mistakes;
(2) The publication in more than one journal of erroneous data, and the duplication of data between the Paper and the presentation (D2/399), so that it can be said that errors were not just confined to the Cancer Letters paper;
(3) The lack of adequate explanation for some of the errors individually, but for the number of errors.
285. After anxious consideration, I decline to find that Dr Hill was dishonest:
(1) I had the benefit of seeing Dr Hill in the witness box. He did not appear to me to be a dishonest man. Rather he struck me as an enthusiast (as I have said above) who believed in the power of science to do good in the cure of cancer and who was passionate about his specialist field;
(2) Contrary to Innovate’s submissions, I do regard motive as being important for the reasons submitted by UoP and reflected in the authorities cited. I do not accept that Dr Hill had any motive to be dishonest - and, if he had such a motive it might have been expected to be reflected in the results of the experiments rather than in respect of the controls;
(3) I accept that there were numerous mistakes, each explicable on an individual basis as being an accidental error. However the number of mistakes seems to me to be at least consistent with a high level of carelessness which would be explicable by the personal and professional pressures which Dr Hill was under;
(4) It is to be noted that the Disciplinary Panel did not make a finding of dishonesty, although it is fair to say that this Court has more information than did that Panel (for example in respect of the D2/399 presentation relevant to paragraph 15(b) and in respect of the paragraph 15(d) allegation) and that on the information which it did have the members of the Panel did express concerns in their deliberations;
(5) I found the evidence of Professor Bushell persuasive, and formed the impression that Professor Short only reached conclusions as to dishonesty on a somewhat hesitant basis, primarily, but not exclusively, on the basis of the number of mistakes.
286. For these reasons, Innovate’s case based upon dishonesty on the part of Dr Hill fails: there is no evidence upon which I could conclude that any other person for whom UoP is responsible was dishonest.
Causation of Loss
287. The consequence of my conclusions on the construction of Clauses 11.4 and 11.5 and, in the alternative rejecting the allegations of dishonesty on the evidence, mean that the claim for loss of profits pleaded in paragraph 20B of the Amended Particulars of Claim fails, and that in paragraph 20A is limited to £1 million.
288. At the heart of Innovate’s case is the submission that because of the retraction of the Cancer Letters paper, the research carried out by UoP was commercially worthless and will have to be carried out afresh.
289. In closing submissions, both oral and written (including in particular submissions after the oral closing submissions) UoP has argued that I should look at the causative relationship between the individual allegations of breach of contract and the retraction of the paper: see in particular paragraphs 2 to 4 of UoP’s Further Written Submissions.
290. Those submissions were made in response to Innovate’s submissions at paragraphs 5 to 8 of its Further Written Submissions.
291. I accept Innovate’s submissions in those Further Written Submissions, and in particular paragraph 8 where it is said:
….It is irrelevant whether each pleaded allegation was noted expressly in the Retraction Notice or not; for the point is that the Retraction Notice destroyed the commercial credibility of the Cancer Letters Paper and thereby the commercial credibility of the work of which it was a write-up. The Court will have well in mind that the first of the ‘[s]pecific concerns’ that the editors of Cancer Letters referred to explain the retraction - before they described the results of their own ‘separate investigation’ - was that:
‘The Editor and Publisher received a letter from the University of Portsmouth alerting us to an investigation into alleged research misconduct. The University concluded their investigation with external experts and determined that misconduct did take place in relation to the research involved in this paper.’
As regards the commercial credibility of the University’s work on the Drug, there was simply no way back from that. Understood in that context, the table [in paragraph 161 of Professor Bushell’s first report] is misleading in so far as it seeks to emphasise that many of Innovate’s pleaded allegations of falsehoods were ‘Mentioned in Investigation Report’ but not ‘Mentioned’ - i.e. not mentioned expressly, in ‘Retraction Notice’. That is really neither here nor there.
292. Thus, subject to the impact of Clauses 11.4 and 11.5, in my judgment UoP is liable for the financial consequences of the destructive power of the retraction upon the commercial value of the work done by Dr Hill and his team.
Innovate’s Claim for the Cost of Repeating the Work
293. Paragraph 20(A) of the Amended Particulars of Claim pleads as follows:
(i) The work performed under the Contract is valueless, and has served only to diminish the Claimant’s reputation and standing in the pharmaceutical industry. The Claimant’s direct association with the Drug is well known, and its participation in the research conducted by Hill under the Contract is expressly acknowledged in the Cancer Letters publication. The Claimant can place no reliance whatsoever on the integrity and authenticity of any of the work done; nor will anyone else. Of necessity therefore, all of the work has to be repeated;
(ii) The costs associated with the work comprised in Schedule 1 to the Contract will inevitably be significantly higher than the contract price of £50,000 agreed (in conjunction with a charity) in 2016 (which did not reflect either the cost of the Defendant of carrying out the Research Programme or the market price of doing so, the contract price being merely a relatively modest contribution to the funds of the Defendant’s Brain Tumour Research Centre, the majority of which were at the time of the Contract being provided by BTR …. There is no prospect of the Claimant once again collaborating with a charity, and no prospect of grant funding, which is available only for new research (not repeat research). The Claimant has no option other than to incur the costs which will be charged by commercial providers of the laboratory and research services necessary to cover the Contract work. The Claimant’s current best estimate of the reasonable cost of repeating the Research Programme is US $ 4m, this being the sum the Claimant was quoted on 10 June 2022 by way of “Indicative Pricing Proposal” by Labcorp Drug Development (formerly Covance Preclinical Oncology) of Ann Arbor, Michigan, USA. The Claimant reserves the right to seek permission to re-amend this statement of case so as to update permission to re-amend this statement of case so as to update this figure in the event that it is no longer accurate at the time of trial.
294. For the reasons set out above, I am satisfied that the work carried out by UoP was commercially valueless, at least unless validated by further testing, and that accordingly Innovate is in principle entitled to recover the costs of further testing up to the £1 million limit.
295. There are some issues which I need to determine as to the scope of that testing.
296. First, it is argued that Innovate cannot recover the costs of testing to assess the efficacy of Clomipramine (see in particular paragraph 180 of UoP’s Closing Submissions).
297. This argument arises out of the structure of the Agreement under which HCT and Innovate came together to procure testing of two different drugs in respect of which each was separately interested. This was reflected in clauses 6.1 and 6.2 of the Agreement under which the benefits of the research into Clomipramine were to be shared between UoP and HCT.
298. In my judgment the Agreement shows that Innovate has no commercial interest protected or promoted by the Agreement which would justify it claiming the costs of further testing to establish the properties of Clomipramine, as was reflected and confirmed in the footnote to paragraph 4(i) of the Amended Particulars of Claim to which I have referred at paragraph 51 above.
299. Accordingly Innovate cannot recover any costs of retesting in respect of Clomipramine.
300. A second point is whether Innovate can recover the costs of testing in respect of the efficacy of treating tumours other than GBMs with the Drug.
301. This raises a point which would also be relevant in respect of the claim under paragraph 20B of the Amended Particulars of Claim.
302. Schedule 1 of the Agreement opens as follows:
Principal Investigator: Dr Richard Hill
Co-Investigator: Professor Geoff Pilkington
Collaborators: Imperial College and University of Oxford
The funding that these two respective groups will provide to the Therapeutics unit with the Neuro-oncology centre is up to 12 months with two very specific objectives
1) Conduct a range of in vitro studies to address the mechanism of action of two key, independent re-purposed drugs against GBM
2) Conduct a detailed in vivo study using a clinically relevant GBM model to evaluate two independent re-purposed drugs …
303. Paragraphs 2 and 3 of Schedule 1 make clear that their focus is upon GBM tumours.
304. Paragraph 4 of Schedule 1 provides:
Evaluate the effectiveness of IP1867B and clomipramine to treat cancers with frequent brain metastasis (lung, breast and melanoma). Conducted at the University of Portsmouth.
While the research that we have conducted to date and proposed to this point are focused on primary brain tumours (principally GBM), one of the most devastating clinical developments of lung cancer, breast cancer and melanoma is brain metastasis. Within the Brain Tumour Research Unit, we have obtained biopsies from these major cancers that have metastasised to the brain. We are, therefore, able to evaluate if IP1867B and clomipramine (in addition to having a potent effect against primary GBM cells) could significantly reduce cell viability of these lines. Preliminary studies have already instigated (for IP1867B) and shown some promising data. Consequently, it is imperative to address this in more detail to elucidate if there are conserved mechanisms that IP1867B activates/suppresses within primary GBMs compared to these secondary metastatic cancers. This could be a critical series of studies as currently all treatments against melanoma, breast and lung cancer have extremely poor characteristics to cross the BBB and target these satellite tumours.
To this end we will conduct synergy studies (similarly to the work we have already conducted for the pilot study) and extend any promising results following our studies described in objective #2 with these important ex vivo metastatic biopsy cell lines.
305. In my judgment, Schedule 1 is clear that the primary object of the research to be carried out is to assess the impact of the Drug (and Clomipramine) on GBM tumours. This might produce useful data in respect of other metastasising tumours, but that would be an incidental product of the primary objectives of the Research Programme.
306. It follows that Innovate cannot recover additional costs to investigate the impact of the Drug on tumours other than GBM tumours.
307. A third argument is set out at paragraphs 184 to 190 of UoP’s Closing Submissions:
184. Importantly, the Contract made it clear that the Research Programme as contemplated in Schedule 1 was not set in stone. Clause 2.2 provided (with added emphasis) that the Research Programme was subject to change:
“The Principal Investigator shall keep the Funders regularly updated on the progress of the Research Programme by providing regular reports not less than four times a year (every 3 months); meeting with representatives of the Funders at times and places mutually agreed upon to discuss the progress and results, as well as ongoing plans, or changes therein, of the Research Programme. The Principal Investigator shall also hold regular conference calls/meetings with the Funders as and when important data is obtained.”
185. In the University’s submission, this last point is critical in understanding the nature of the University’s obligations under Schedule 1. In the University’s submission, it is clear from clause 2.2 that the Research Programme as planned in the Schedule was subject to change depending on progress and results, and that plans with respect to the Research Programme as it related to the Drug could be changed by the Principal Investigator, Dr Hill in consultation with Innovate. That stands to reason when one considers the following particular characteristics of the Contract:
a. As a contract for a programme of scientific research, it necessarily had to have built into it a large measure of flexibility to adapt as the research progressed.
b. That flexibility was all the more important as the funding under the Contract was meagre in total, and strictly limited to £25,000 in respect of the Drug component of the research (and even then Innovate only paid half).
186. Support for the University’s interpretation can be found in the additions to the research programme that were in fact carried out: the study of IP1867B as an adjunct therapy to the EGFR inhibitors Gefitinib and AZD3759 was an adjustment to the Research Programme made by Dr Hill in consultation with Innovate and Innovate have made no complaint about that additional work.
187. In the light of the foregoing, the University’s primary submission is that, by the time of the completion of the work comprising the Research Programme which underlay the publication of the Article, the scope of the Research Programme under the Contract had evolved pursuant to clause 2.2 of the Agreement. By that point, the Research Programme under the Contract equated to the research that had actually been done (the “Completed Research”).
188. It follows that, in order to put Innovate back into the position that it would have been but for the alleged breaches of the Contract, the repeat research programme only has to be of a scope and scale approximating that of the Completed Research which underlies the Cancer Letters Paper. To go beyond that would be to award Innovate damages which go beyond compensation for its contractual loss. In the University’s submission, the correct exercise in assessing the repeat research quote relied upon by Innovate is therefore to ask whether it is of a scope and of a scale approximating to that of the Completed Research which underlies the Cancer Letters Paper, not to look to the anticipated Research Programme as set out in Schedule 1 of the Contract as executed on 7 July 2016.
189. The logic of that analysis is consistent with the pleaded case on breach as run by Innovate at trial. Innovate’s case at trial focussed exclusively on the errors which Dr Hill was said to have made in the Article. It was not suggested in cross-examination of Dr Hill or Professor Pilkington (for example) that Dr Hill had erred in his conduct by failing to undertake or complete aspects of the Research Programme as set out in Schedule 1. That stands to reason: first, Mr Cohen (and to a lesser extent Dr Stuart) were in constant communication with Dr Hill as to the progress of the Research Programme. Secondly, the research undertaken was necessarily curtailed by the pressure from Innovate to complete the Research Programme and publish the Article.
190. In the event that the Court does not accept the University’s primary position, the University submits that, at the very least, the scope and scale of any proposed repeat research programme must be referable to and limited to the obligations set out in Schedule 1 to the Contract. Again, it would offend the compensatory principle for Innovate to be awarded damages in respect of a repeat research programme which went beyond the scope of the work contemplated in Schedule 1.
308. I have already held above that the publication of the Cancer Letters paper was part of the execution by UoP of its contractual obligations under the Agreement, and in doing so I have necessarily rejected UoP’s argument above that UoP’s obligations had come to an end before that publication.
309. I also reject the argument that in some way the scope and scale of retesting for which UoP is responsible is limited: in practical terms this would exclude the costs of work to establish if the Drug could pass the BBB (see paragraph 228 of UoP’s Closing Submissions). On the contrary, from the outset it had been clear that discovering if the Drug could pass through the BBB, and do so effectively, was central to the entire research programme (see paragraph 48 above - it is also clear from paragraphs 1 and 4 of Schedule 1). In respect of this aspect of the case, I accept Innovate’s submissions at paragraphs 74 to 78 of its Closing Submissions.
310. However, there are two separate, but connected points: the first is developed in paragraphs 229 and 230 of UoP’s Closing Submissions:
Metabolism Services
229. All bar one of these services (the exception being Quote No 782248) comprises “Mouse Pharmacokinetic” Studies. Mr Cohen explains in Cohen3 that these are required to establish that the Drug crosses the BBB. [31]
230. It was common ground between the Liability Experts that studies of this nature did not form part of the Completed Research. When the experts were questioned together in concert, they agreed that following the Completed Research, and before any clinical trials could be contemplated, Innovate would require (i) a further in vivo study, and after that (ii) a toxicity study in other organs and (iii) a PKPD (pharmacokinetic and pharmacodynamic) study. [32] Accordingly, on the University’s primary case, to the extent the Labcorp programme extends to pharmacokinetic studies, compensation should not be granted for it.
311. I have set out at paragraph 13 above a table which explains the four phases of clinical testing. UoP is correct that pharmacokinetic studies form part of Phase 1 of the clinical studies. It is also correct that UoP was not in any way responsible for clinical studies. Accordingly, I accept UoP’s submission that the cost of pharmacokinetic studies fall outside the scope of recoverable retesting costs (there was a separate dispute going to the paragraph 20B claim as to whether Phase 1 studies would be necessary at all, but that is a distinct and separate point).
312. The second point in respect of BBB testing is contained in paragraph 231 of UoP’s Closing Submissions:
CDX Preclinical Oncology Model Services
Mr Cohen explains in Cohen3 that these are in vivo studies regarding the effect of the Drug on the BBB. [33] However, the Schedule provided only for in vitro studies on the BBB. [34]
313. Insofar as this argument depends upon the argument that there can be no recovery in respect of testing to investigate whether the Drug efficaciously passes the BBB, I reject it for the reasons given above.
314. With those considerations in mind, I turn now to the case as to quantum put forward by Innovate. I am very grateful for the extremely helpful analysis of the evidence contained in UoP’s Closing Submissions.
315. Paragraph 191 of those Submissions contains a helpful table of the quotations before this Court:
|
Date |
Provider/Source |
Amount |
Party |
Reference |
|
24.4.20 |
Correspondence |
£719,060 |
Claimant |
E1/3 |
|
14.9.20 |
Covance (now LabCorp) |
£0.75-1.25m |
Claimant |
D37/651 |
|
4.11.20 |
Covance (now LabCorp) |
USD $3.5m |
Claimant |
D37/653 |
|
11.1.21 |
Correspondence |
Over £2.4m |
Claimant |
E1/37 |
|
20.9.21 |
Further Information |
£719,000-£4m |
Claimant |
A/65 |
|
10.5.22 |
LabCorp Drug Development (formerly Covance) |
USD $4m |
Claimant |
D38/524 |
|
20.10.22 |
Reaction Biology |
€193,317/£165,966 |
Defendant |
D38/717 |
|
2.11.22 |
Pharmidex |
£270,000 |
Defendant |
D38/219 |
|
18.8.23 |
Labcorp |
£1,752,316 - £1,786,509 |
Claimant |
DD3/406 |
|
20.9.23 |
Reaction Biology |
£219,319 |
Defendant |
DD3/444 |
|
27.9.23 |
Pharmidex |
£555,000 |
Defendant |
DD3/459 |
316. In the event it is the August 2023 Labcorp quotation upon which Innovate relies.
317. In support of its case, Innovate has adduced a third witness statement from Mr Cohen.
318. I find his evidence persuasive that the only realistic quotation for the costs of retesting is the August 2023 quotation (more accurately, quotations). In that respect I accept the submissions of Innovate in paragraphs 79 and 80 of its Closing Submissions.
319. However, it is necessary to look a little more closely at Labcorp’s quotations.
320. Again, UoP has set out a useful table (at paragraph 193 of its Closing Submissions) which sets out Labcorp’s quotations:
|
Quote no. |
Summary |
Referability to the Research Programme |
Price |
|
Executive summary | |||
|
- |
Labcorp Programme covers both IP1867B and Clomipramine |
Innovate say Contract covered Clomipramine |
- |
|
Bioanalytical services | |||
|
782549 |
Bioanalytical Services Non-GLP Method Development and Qualification for the Determination of Aspirin and Clomipramine in Mouse Serum and Mouse Brain Homogenate by LC-MS/MS |
Unexplained by Innovate |
£13,674 |
|
Toxicology services | |||
|
782542 |
HBVP cell line In vitro cell assay (PGE2 level, qPCCR-EGFR, Cytokines, CRIPR/SiRnA-ACFR. Proliferation assay ic 50, Cell cycle, Apoptosis, Endothelial TEER. Reporting and archiving 1 year) |
Unexplained by Innovate. Appears to cover wide range of in vivo testing, including BBB work |
£372,035 |
|
782543 |
HBME cell line As per Quote 782542 HBVP cell line |
As above for 782542
|
£372,035 |
|
782544 |
GBM cell line As per Quote 782542 above |
As above for 782542 |
£372,035 |
|
Metabolism services | |||
|
782093 |
Mouse Pharmacokinetic Study Central Nervous System Penetration Comparison - Option 1 Radiolabel (ICR mice) |
Said to be for the purpose of BBB work. [35]
|
£17,589 |
|
782093 782549 |
Mouse Pharmacokinetic Study Central Nervous System Penetration Comparison, Option 2 Non-Radiobelled 2LC-MS/MS (ICR mice) |
As above
|
£36,401 |
|
782248 |
Estimate for 1mCl of acetylsalicyclic acid [ring 14-C] |
As above
|
£39,644 |
|
782094 |
Mouse Pharmacokinetic Study Peripheral PK Comparison - Option 1 Radiolabel (ICR mice) |
As above
|
£16,491 |
|
782094 782549 |
Mouse Pharmacokinetic Study Peripheral PK Comparison - Option 2 Non-Radiolabel LC-MS/MS (ICR mice) |
As above
|
£31,872 |
|
CDX Preclinical Oncology Model Services | |||
|
782923 |
Sample generation for pharmacokinetic evaluation following treatment of client-provided test agents against intracranial implanted U87MG-luc human glioblastoma model in female nude mice (IV route) |
Unexplained by Innovate other than to say that this is in vivo work. [36]
|
£90,117 |
|
782924 |
As above for 782923 |
As above
|
£90,753 |
|
782228 |
Efficacy evaluation of client- provided test agents against intracranial implanted U87MG-luc human glioblastoma model in female nude mice |
As above
|
£90,204 |
|
782229 |
As above for 782228 |
As above
|
£90,204 |
|
782230 |
Efficacy evaluation of client- provided test agents against the BT474 human breast tumor model in female NSG mice |
Said to be referable to item 4 in Schedule 1 [37]
|
£61,719 |
|
782231 |
Efficacy evaluation of client- provided test agents against a human lung tumor model in female mice |
As above
|
£61,719 |
|
782232 |
Efficacy evaluation of client- provided test agents against a human melanoma tumor model in female mice |
As above
|
£61,719 |
321. In respect of those quotations, I find as follows (subject to the Clomipramine issue to which I revert below):
(1) 782549: appears to be a legitimate claim, which I accept;
(2) 782542, 782543, 782544: the main point of objection appears to be the inclusion of BBB testing, a point I have resolved against UoP above;
(3) 782093, 782549, 782248, 782094: these appear to relate to Metabolism services, in respect of which I have accepted UoP’s submission that these should be excluded;
(4) 782923, 782924, 782228, 782229: these all appear to be allowable costs of retesting;
(5) 782230, 782231, 782232: these relate to non-GBM testing, which I have held is not allowable.
322. This makes a total of £1,491,077, as follows:
|
782549 |
£13,674 | |
|
782542 |
£372,035 | |
|
782543 |
£372,035 | |
|
782544 |
£372,035 | |
|
782923 |
£90,117 | |
|
782924 |
£90,753 | |
|
782228 |
£90,204 | |
|
782229 |
£90,204 | |
|
£1,491,057 |
323. There is a separate issue as to the cost of testing for Clomipramine. I have no figures to allow me to assess this other than on a very broad brush basis. UoP suggests that the deduction should be a division in half, but it seems to me unlikely that testing for the Drug alone would cost half of testing for the Drug and Clomipramine together.
324. In my judgment some deduction should be made, but I would limit it to 10%, thus reducing the amount recoverable to £1,341,951.30.
325. However, this exceeds the Clause 11.5 limit.
326. Accordingly there will be judgment in respect of this head of claim in the sum of £1,000,000, the limit under Clause 11.5.
The Diminution in Value of the Patent: The Amount Claimed
327. Paragraph 20(B) of the Amended Particulars of Claim pleads:
(i) For the reasons stated above, the Claimant is in no position to exploit its patent in the Drug and license the product until the Research Programme has been repeated (as above) to a reputable standard.
(ii) The Claimant, which cannot afford to pay for the repetition of the Research Programme, intends (if this claim succeeds) to pay for it using the damages awarded under paragraph 20(A) above - in so far as they are available for that purpose: some of the money will have to go on unrecovered legal expenses such as the cost of after-the-event insurance - and such other funds as the Claimant is able to raise.
(iii) The Claimant will then be able to approach pharmaceutical companies with a credible and reliable set of research results concerning the properties and potential uses of the Drug, and will in that respect be in the position it would have been in by 2018 if the Defendant had performed its obligations under the Contract.
(iv) This will still leave the Claimant with a loss because the unexpired period of patent exclusivity that the Claimant will be able to offer a potential licensee of the Drug, and thus the price the Claimant will be able to secure, will be lower than it would have been if the Defendant had performed its obligations under the Contract.
(v) The Claimant claims damages to reflect this loss. Relevant factors in the assessment of these damages are likely to include the Court’s assessment of:
(a) the price the Claimant would have been likely to secure for a licence to exploit the Drug if a properly conducted Research Programme by the Defendant had simply confirmed what was already known about the Drug’s properties;
(b) the likelihood and extent to which a properly conducted Research Programme would have credibly shown the Drug to have the properties claimed for it in the Cancer Letters Paper or other properties; and
(c) the likely date on which the Claimant will become able to offer to license the Drug ….
328. At the date of that pleading, Innovate did not have the benefit of the necessary expert evidence to quantify its claim. That evidence is now to hand.
329. The central plank of Innovate’s case is that as a result of the publication of the Cancer Letters paper, and the consequent need to carry out a fresh research programme, the date at which it can exploit its patent has been delayed.
330. This involves an evaluation of what the effect of that delay has been upon the value of the patent for the Drug.
331. The amounts involved are very large: in a schedule handed up to me in oral closing submissions the amount claimed is in excess of £100 million.
332. Because of my conclusions set out above, this claim is excluded by Clause 11.4 and, even were it not so excluded, the recoverable amount would be limited to £1 million, but that limit has already been exhausted by the amount I have found due in respect of the retesting claim.
333. I have considered whether to extend this judgment by reaching provisional views as to the amount which would be due were my conclusions above to be held to be wrong. In the event, I limit myself to the following comments:
(1) In respect of the claim for non-GBM losses, for the reasons given above it seems to me that UoP’s contractual obligations did not extend to testing for the effect of the Drug upon non-GBM tumours and/or any losses which Innovate suffered in that respect were too remote to be recoverable;
(2) In addition to point (1) above, in closing Innovate puts its probability of success in respect of use of the Drug for treatment of non-GBM cancers at 5%. There is powerful guidance in the authorities that a chance as low as that would not sound in damages: see Browning v Bachers [2002] EWCA Civ 753; Feakins v Burstow [2006] PNLR 6; Boyle v Thompsons Solicitors [2012] EWHC 36 (QB); Thomas v Albutt [2015] EWHC 2187 (Ch);
(3) In respect of the use of the Drug for the treatment of GBM tumours, it seems to me that it would be difficult to assess damages in the absence of expert evidence from someone experienced in taking drugs from early identification as useful medication to full approval and commercial exploitation;
(4) In the absence of such evidence, it seems to me that Innovate’s suggestion in closing submissions of a probability of success of 27.5% and a projection that the Drug would go to market by 2030 were far too optimistic: however without a more careful analysis of the evidence, I do not regard it as appropriate to say more than that.
Conclusion
334. There was also a small claim for recovery of the amount paid by Innovate to UoP, but that has not been pursued.
335. In the result, there will be judgment for Innovate for damages in the sum of £1 million.
[1] D17/237
[2] Transcript day 7 page 1091 line 23 to page 1092 line 4
[3] I.e. drugs where the initial patent protection has expired.
[4] An excipient is an inactive substance that acts as carrier for an active compound (which in the case of IP1867B is aspirin). Reasons for adding excipients include to aid the manufacturing process, to enhance stability, to aid delivery, or for bioavailability (the extent to which an active ingredient is absorbed from a medicine and becomes available in the body). Excipients are not necessarily inert as they may react with other ingredients in a formulation, and they may cause adverse reactions in patients.
[5] Paragraph 2(a) of the Amended Particulars.
[6] Witness Statement, paragraph 19.
[7] A/4/18
[8] D5/154
[9] D1/696
[10] D12/152
[11] D17/623
[12] Day 5 pages to 609
[13] Day 5 page 608 line 25 to 609 line 12
[14] 2 of Dr Hill’s students
[15] Transcript day 4, pages 507 to 527
[16] Transcript day 6 pages 782 to 821
[17] Transcript 29 November 2023 page 72
[18] A/tab 6/205
[19] A/tab 7/240
[20] Transcript 29 November 2023 page 71
[21] D29/321ff
[22] D29/346
[23] D29/371
[24] D29/425
[25] D30/351
[26] D30/352
[27] D30/356
[28] There are a number of copies of the paper in the bundle. In the event the copy at D36/288 was generally used at the trial.
[29] D35/481
[30] D35/487
[31] Cohen3 at ¶35 [B/13/226]
[32] Day 7/1087 line 2 - 1088 line 9
[33] Cohen3 at ¶
[34] See Schedule section 3.
[35] Cohen3, ¶35.1 [B/13/226]
[36] Cohen3, ¶33 [B/13/225]
[37] Cohen3 at ¶36 [B/13/226]