Freely Available British and Irish Public Legal Information
[Home] [Databases] [World Law] [Multidatabase Search] [Help] [Feedback]
England and Wales High Court (Patents Court) Decisions
You are here: BAILII >> Databases >> England and Wales High Court (Patents Court) Decisions >> Samsung Bioepis UK Ltd v Janssen Biotech Inc [2024] EWHC 1984 (Pat) (30 July 2024)
URL: http://www.bailii.org/ew/cases/EWHC/Patents/2024/1984.html
Cite as: [2024] EWHC 1984 (Pat)
[New search] [Printable PDF version] [Help]
Neutral Citation Number: [2024] EWHC 1984 (Pat)
Case No: HP-2023-000020
IN THE HIGH COURT OF JUSTICE
BUSINESS AND PROPERTY COURTS OF ENGLAND AND WALES
INTELLECTUAL PROPERTY LIST (ChD)
PATENTS COURT
The Rolls Building
7 Rolls Buildings
Fetter Lane
London EC4A 1NL
30 July 2024
Before:
MR. JUSTICE MEADE
- - - - - - - - - - - - - - - - - - - - -
Between:
|
|
SAMSUNG BIOEPIS UK LIMITED
|
Claimant |
|
|
- and –
|
|
|
|
JANSSEN BIOTECH, INC.
|
Defendant |
- - - - - - - - - - - - - - - - - - - - -
- - - - - - - - - - - - - - - - - - - - -
MR TOM MITCHESON KC AND DR GEOFFREY PRITCHARD
(instructed by Simmons & Simmons LLP) for the Claimant
MR THOMAS HINCHLIFFE KC and MS KATHRYN PICKARD
(instructed by Carpmaels & Ransford LLP) for the Defendant
Hearing dates: 24th to 26th June and 1st and 2nd July 2024
- - - - - - - - - - - - - - - - - - - - -
APPROVED JUDGMENT
Remote hand-down: This judgment will be handed down remotely by circulation to the parties or their representatives by email and release to The National Archives. A copy of the judgment in final form as handed down should be available on The National Archives website shortly thereafter but can otherwise be obtained on request by email to the Judicial Office (press.enquiries@judiciary.uk).
Mr Justice Meade:
Samsung’s expert, Prof Bloom.. 8
Janssen’s expert, Prof Michetti 11
Samsung’s fact witness, Prof Ochsenkühn. 14
Inflammatory Bowel Disease (“IBD”) 17
The Aetiology and Pathogenesis of IBD.. 18
First-line induction therapies. 22
First-line maintenance therapies. 23
Next line treatment in UC and CD.. 24
Higher risk UC and CD (including acute severe disease) 26
Therapy-refractory and therapy-intolerant patients. 26
Clinical Trials for Biologics in IBD.. 27
Ustekinumab clinical trials. 29
Commentary on the clinical trials. 30
Issue i): Distinction between UC and CD.. 31
Issues ii) and iii): Immunology and Genetic Risk Factors. 31
Issues iv) and vi): Treatments for UC and CD, failed treatments. 34
Corticosteroids, prednisolone (parties’ numbering, number 1) 36
5-ASAs - mesalazine (number 2) 36
Immunosuppressive agents - Methotrexate and Ciclosporin A (numbers 3 & 4) 37
Apilimod mesylate (number 8) 37
Biologics (numbers 5-7 and 9-14) 38
Claim interpretation Issues/Construction. 44
“For use in a method of treating”. 45
“Corticosteroid-free clinical remission”. 46
“Remission” and “Moderately to severely active ulcerative colitis”. 46
“At least 44 weeks after week 0”. 46
Disclosure of the Ochsenkühn Abstract 52
Presentation and Disclosure of Ochsenkühn Poster 53
Ochsenkühn Abstract - assessment of the strength of the evidence for efficacy. 56
Ochsenkühn Poster - assessment of the strength of the evidence for efficacy. 58
Anticipation by Ochsenkühn Poster 58
Obviousness over the Ochsenkühn work. 60
Obviousness over Sands Slides. 60
10% delta not especially good. 63
Dropping out of remission during the maintenance phase. 65
Different from Crohn’s Disease based on UNITI 65
Effect of publishing clinical trials data. 68
Introduction
1. In this action, the Claimant (“Samsung”) seeks to revoke European Patent (UK) No. 3 883 606 (the “Patent”) owned by the Defendant (“Janssen”).
2. The Patent is entitled “Safe and effective method of treating ulcerative colitis with anti-IL12/IL23 antibody” and has claims to the antibody ustekinumab for use in the treatment of ulcerative colitis (“UC”) according to particular regimes. The parties agreed that for the purposes of these proceedings the relevant priority date was 20 November 2018 (the “Priority Date”). An earlier priority date of 24 September 2018 was not defended by Janssen and an important consequence was that the prior art referred to below as the Sands Slides entered the picture.
3. Janssen, a wholly-owned subsidiary of Johnson & Johnson, is a biotechnology company based in the US. It has sold ustekinumab with great success as Stelara® - a multibillion dollar blockbuster - for a number of important indications including various forms of psoriasis and Crohn’s disease (“CD”) as well as UC.
4. Samsung is a UK biopharmaceutical company with a particular interest in the development of biosimilars.
5. Originally there were multiple companies with an interest in biosimilar ustekinumab products attacking the Patent, but all apart from Samsung have settled with Janssen. Part of the commercial picture is the possibility that those companies, or Samsung, might sell an ustekinumab biosimilar under a “skinny label” not covering UC. Janssen said and says that that would or might still be a (contributory) infringement. The picture is a complex one and I do not need to go into it in any detail in this judgment. Samsung would like to sell a full label product if the Patent is invalid and it is accepted that that would infringe the Patent if valid. At the moment however Samsung is only progressing a skinny label.
6. The SPC for ustekinumab expired on 19 July 2024 and for that reason among others this trial was directed to be heard on an expedited basis by my Order of 18 October 2023. Given that this trial was expedited I have prepared this judgment as quickly as possible, but for reasons that it is unnecessary to go into, Janssen does not intend to seek any interim injunction against generics who only launch with skinny labels and that means that the 19 July date was not a cliff-edge.
The Issues
7. The issues are:
a) The identity of the skilled person;
b) The scope of the CGK;
c) Three points on claim construction relating to claim 1:
i) “for use in a method of treating…”
ii) “moderately to severely active ulcerative colitis (UC)”
iii) “wherein the subject is in corticosteroid-free clinical remission at least 44 weeks after week 0”;
d) Whether a poster entitled “TU1713: Clinical Outcomes with Ustekinumab as rescue treatment in therapy-refractory or -intolerant ulcerative colitis: real world experience in a large single center cohort”, by Ochsenkühn et al. (the “Ochsenkühn Poster”) was presented at the Digestive Disease Week conference in Washington DC in June 2018 and therefore is prior art;
e) Anticipation and obviousness over the Ochsenkühn Poster;
f) Obviousness over:
i) an abstract for a poster presentation entitled “P759 Ustekinumab as rescue treatment in therapy-refractory or -intolerant ulcerative colitis” by Ochsenkühn et al., published in the Journal of Crohn’s and Colitis on 16th January 2018 (the “Ochsenkühn Abstract”);
ii) Janssen’s protocol for the Phase III clinical trial that established the efficacy of ustekinumab as a treatment for UC (the “UNIFI Protocol”), published on clinicaltrials.gov on 1st November 2018;
iii) a set of slides entitled “Safety and efficacy of Ustekinumab Induction Therapy in Patients with Moderate to Severe UC: Results from the Phase 3 UNIFI Study” (the “Sands Slides”), which were presented by Bruce E Sands and others at the 2018 American College of Gastroenterology conference in October 2018 and which report on the initial results of the first study in the UNIFI trial (the induction study); and
g) three sufficiency/lack of technical contribution squeezes. These essentially fell away before trial and I need say no more about them.
Overview
8. It will help to make reading this judgment easier if I provide an overview. It is necessarily quite heavily simplified and is not to be read in isolation: my actual reasoning appears below.
9. At the Priority Date, ustekinumab was known, as CGK, to be an effective treatment for conditions including CD. The clinical trial which had shown it to be effective for CD was called UNITI, published in the New England Journal of Medicine in 2016, by Feagan et al (“Feagan 2016”).
10. The Patent claims ustekinumab for use, according to quite a detailed dosing regimen, in treating UC, a condition related to CD. UC and CD are both forms of inflammatory bowel disease (“IBD”).
11. Also at the Priority Date, it was CGK that ustekinumab was in a phase III trial for use in UC. That clinical trial was called UNIFI.
12. In this area of medicine, drugs of this general kind (antibodies, biologics) were given in two phases, an induction phase lasting a couple of months (sometimes a little longer), and then a maintenance phase. Whether a drug was effective was assessed, in clinical trials, at the end of the induction phase and then again at the end of the maintenance phase, together lasting a year. Whether a drug was effective was assessed both in terms of whether it treated the condition in question, and whether following its use patients were also being given steroids, it being desirable that they should not be, because of side effects.
13. As I have identified above, Samsung says that the Patent is invalid over the prior art, of which there are effectively three pieces. Two are by Prof Ochsenkühn and colleagues, and the other is the “Sands Slides”. Samsung also relies on the trial protocol for UNIFI, but not on its own, and for material purposes it was CGK.
14. Because the UNIFI trial protocol is CGK and contains the dosing regimen that was going to be used, those aspects of the claims of the Patent do not enter the picture. The central issue is over whether the prior art either discloses to the anticipation standard, or renders obvious, that ustekinumab was or would be effective to treat UC, including in particular so that patients were effectively treated and not on steroids at the end of the maintenance phase of a clinical trial. The state of not being on steroids and having been successfully treated is called corticosteroid free clinical remission (“CSFCR” or “CFCR”).
15. The two pieces of Ochsenkühn prior art concern retrospective assessment of a small number of patients with UC. The work was not a clinical trial, it was unblinded, and there was no comparison with a placebo.
16. The Sands Slides give the results of UNIFI at the end of the induction phase. There is a dispute about how positive the results would have been seen to be.
17. Samsung’s cases over the different prior art citations have some common aspects. In particular, Samsung says that there was a well-established pattern of biologics, once shown effective for one of CD or UC then to be used for the other, and that ustekinumab was known to work by blocking what is referred to as the IL-23 pathway (explained further below), known to be implicated in UC.
18. Janssen challenges all that. It says that there was a very complex picture concerning past UC/CD treatments (including but not limited to biologics), how and whether they worked, and whether any confidence could be gained from the IL-23 knowledge. Janssen says that although it was CGK that IL-23 was involved in UC, it was also known that the UC pathways were much more complex and materially different from CD, and there was no adequate reason to suppose that blocking IL-23 on its own would be good enough.
19. The dispute over the CGK concerning CD/UC/IL-23 and so on feeds into a dispute over the skilled person: would they be someone who knew about such matters, and if so then in how much detail?
20. The anticipation case based on the Ochsenkühn Poster depends on the disputed claim interpretation points, on whether the poster was made public as Samsung alleges, and whether, if presented, there is a disclosure to the necessary standard of the efficacy of ustekinumab for UC.
21. Below I conclude that:
a) The Ochsenkühn Poster was made available to the public as alleged but Samsung is wrong about claim interpretation and anyway the data given do not demonstrate to the necessary standard that ustekinumab was effective for UC as claimed by the Patent. So there is no anticipation.
b) More broadly, although the results of the Ochsenkühn work superficially look impressive, the methodology and results have many problems which mean that they do not give enough confidence about treatment of UC to render the Patent obvious.
c) I also reject the obviousness case over the Ochsenkühn prior art because the evidence in support of the attack turned out to mosaic it illegitimately with the Sands Slides (the converse was not true - the evidence supporting the attack from the Sands Slides did not depend on the Ochsenkühn results; Janssen tried to say that it did, but I reject that because the oral evidence it pointed to at T2/196 and 253 established no such thing).
d) The Sands Slides contain positive results for the induction phase which the skilled person would think gave strong optimism for positive results at the end of the maintenance phase (both for a treatment effect and having patients not on steroids) and that renders the Patent obvious.
e) Because the Sands Slides demonstrate that ustekinumab was effective in UC, albeit only at the end of the induction phase with the maintenance phase still to come, it is something of a sideshow how the skilled person would view the likelihood of success prior to seeing the slides, based only on the IL-23 theory and the performance of past treatments. The question over the Sands Slides is how the skilled person would consider the prospects of success at the end of the maintenance phase given success at the end of the induction phase.
f) Nonetheless, I consider the IL-23 theory and the past treatments below and conclude that while they provided a reasonable hypothesis for using ustekinumab in UC it was not one that would give the necessary expectation of success prior to having some reliable clinical results.
g) Relatedly, I consider that the skilled person would have a greater understanding of the relevant pathways and so on than Samsung said, and Janssen has the better of such argument as there was on that point, but that it makes no great difference: whichever side is right about the level of knowledge of the skilled person, the IL-23 hypothesis would be reasonable but no more than that, and the view of the skilled person after seeing the Sands Slides would be one of very considerable optimism.
The Witnesses
22. Each side called one expert in the field of gastroenterology. Samsung’s expert was Professor Stuart Bloom and Janssen’s expert was Professor Pierre Michetti.
23. Samsung also called one fact witness, Professor Thomas Ochsenkühn.
Samsung’s expert, Prof Bloom
24. Prof Bloom is a Consultant Physician and Gastroenterologist at the University College London Hospitals (UCLH) NHS Foundation Trust and Honorary Senior Lecturer at University College London. He received his Doctorate of Medicine from the University of Oxford in 1994.
25. His current clinical practice covers acute general medicine and general gastroenterology with a specialist interest in IBD. His practice includes both NHS and private work. He sees approximately 25 patients per week, approximately 30% of those have IBD and of those approximately 50% have UC and 50% have CD.
26. Prof Bloom is a clinical supervisor to 10 gastroenterology trainees and has acted as clinical supervisor to four PhD students over the past eight years.
27. He has undertaken research alongside his clinical work, including translational research in collaboration with Professor Tony Segal in the Rayne Institute (King’s College London) exploring CD results from a failure to clear bacterial antigens due to a disorder of macrophage function. His current field of research relates to clinical trials in IBD.
28. Prof Bloom has 34 years of experience being involved in clinical trials. He has held many roles related to this, including GI Specialty Lead for the Central and East London Comprehensive Clinical Research Network (2008-2015) and Chair of the Gastroenterology section of the National Institute for Health and Care Research Comprehensive Clinical Research Network (2008-2013).
29. Between 2006 and 2009 Prof Bloom was chair of the IBD section of the British Society of Gastroenterology. Between 2007 and 2010 he was one of the UK representatives to the European Crohn’s and Colitis Organisation. In December 2010 he was made chair of the IBD National Registry Programme Board by the British Society of Gastroenterology.
30. I found Prof Bloom to be a model expert witness. He was extremely well qualified and understood the area fully and in depth (including in relation to the more detailed pathway/immunology issues that Janssen relied on). He was very clear, concise and fair in his answers.
31. Janssen criticised Prof Bloom in the following respects:
a) That he envisaged the skilled person purely as a clinician using the drugs in question or participating in clinical trials, not as someone developing new treatments for UC. I do not agree with this; he certainly was commenting on the likelihood that ustekinumab would work to treat UC and he did so with a detailed knowledge of the relevant pathways, past drugs, and the relevant clinical trials.
b) Similarly, that he “downplay[ed] almost entirely any consideration of the underlying molecular immunology and mechanisms of actions of the various drugs”, and was overly simplistic on these subjects. I do not accept that he went anything like that far. There was a difference of degree between him and Prof Michetti (and between the parties) in this respect but it was no more than that and he was able to help me understand how the skilled person would reason with various possible degrees of knowledge or understanding. In any event, this could not be a personal criticism of Prof Bloom, just a point about how he saw matters.
c) Again similarly, that he focused only on successful drugs and not unsuccessful ones. The same applies, mutatis mutandis, to this as to the previous point: it was a question of degree, I was adequately assisted to understand matters, and it is not a personal criticism.
d) That a particular paragraph of his first report (6.5) tracked in its wording the justification that Janssen gave to the FDA for its proposed clinical trial approach, that what was said was wrong, and that Prof Bloom had not used enough care. I find that the solicitors preparing the text of his report had initially chosen the words used, and those had probably come from a Janssen document in some way, although it is not clear how. I also find, however, that Prof Bloom took responsibility for the words in that he read and approved them with care during the preparation of his evidence, that if they did come from Janssen he did not know that, and that not only did he believe what was said, but Prof Michetti agreed with it in the end and it was a correct statement of the viewpoint of the skilled person.
e) That Prof Bloom gave his evidence and prepared his reports in the knowledge that ustekinumab has been proved to be effective for UC, so that there was a risk of hindsight. Prof Bloom acknowledged this. He explained that he had tried to meet the risk by consciously reflecting on the risk in the preparation of his evidence (which I accept, and in the circumstances I find that there was little more he could have done). I do not think that Prof Bloom’s evidence suffered from hindsight.
f) That hindsight had also led Prof Bloom to exercise confirmation bias in various ways, giving weight more, or only, to things that supported obviousness. I do not agree with this. I will not go into any more detail here and I address the points said to show this when I come to the merits, below. Essentially I think Prof Bloom was right on the points in question.
g) That his evidence on the Ochsenkühn prior art was heavily affected by mosaicking in the Sands Slides. I agree with this. Two answers at T2/196 and 243-244 made this explicitly clear, especially the latter. Counsel for Samsung submitted that Prof Bloom had explained in his written evidence that he had given his views on Ochsenkühn prior to the Sands Slides by way of sequential unmasking, and that that process had not been challenged. I accept that Prof Bloom started off on that track, but it was clear from his oral evidence, by which time he had of course had to factor in Prof Michetti’s written evidence, that he was in the end relying on the Sands Slides very heavily (he accepted that his conclusions from the Ochsenkühn art would be “much weaker” without the Sands Slides).
32. I therefore reject the criticisms of Prof Bloom other than the last one, which is not a reflection on his independence or integrity, just a recognition of reasoning which was logical from a scientific perspective, but not a sound basis for an obviousness attack in law.
Janssen’s expert, Prof Michetti
33. Prof Michetti was awarded his Swiss Diploma of Physicians in 1983 from the School of Medicine LU and attained his MD Thesis at the same School of Medicine in 1988.
34. In 1989 he moved to America for a second research postdoc fellowship at the Children’s Hospital, Harvard Medical School. He joined a lab focussing on the role of mucosal IgA antibodies in the protection against mucosal infections such as Shigella, Salmonella and HIV.
35. Prof Michetti returned to Switzerland in 1991. He led a research lab in the gastroenterology division of the Centre Hospitalier Universitaire Vaudois (CHUV), Lausanne. The aim of the lab was developing a mucosal IgA-driven vaccine against Helicobacter pylori. He also completed a clinical gastroenterology fellowship at this time, achieving a Board Certification from the Swiss Medical Association, FMH, in Internal Medicine with a subspeciality in Gastroenterology in 1997.
36. Prof Michetti is, and has been since 2009, Chief Physician and CEO of Gastroentérologie Beaulieu SA, Lausanne, a medical centre in Switzerland. The centre focuses on gastroenterology, digestive and inflammatory bowel disease.
37. He is also Staff Physician of the Division of Gastroenterology and Hepatology at CHUV, Lausanne and Clinique La Source, Lausanne.
38. In 2023 Prof Michetti became an Honorary Professor at Lausanne University School of Biology and Medicine in Switzerland, where he gives lectures on IBD.
39. Alongside his clinical work, he has maintained an interest in research relating to the underlying molecular biology of gut immunology and monoclonal antibodies, having published numerous articles and reviews, editorials and book chapters.
40. Samsung advanced a number of attacks against Prof Michetti, ultimately arguing that his evidence should be treated with caution. Their criticisms fell into two categories; errors in instruction and errors in approach.
41. Samsung submitted that the errors in instruction were (simplified for brevity):
a) Prof Michetti was not instructed to consider the CGK from the perspective of the UK skilled person. I agree that CGK may be local and in a couple of minor respects that was relevant in assessing the past treatments relied on, but in general this was a field where knowledge was international.
b) Prof Michetti was not asked to consider the CGK from the perspective of the relevant addressee of the Patent. I disagree with this for reasons explained in dealing with the skilled addressee.
c) Prof Michetti was shown the Patent before giving his views on any of the prior art. This is true and would have been better avoided but Prof Michetti already and inevitably knew about the successful use of ustekinumab for UC so the practical significance is minimal.
d) Prof Michetti was not instructed to approach the claims using the construction adopted by both parties in the case (that CSFCR does not require the patient to have been on steroids at the outset). This is also true but I do not think it can materially have affected what he thought or said about the CGK or obviousness.
e) Prof Michetti had dealings with ustekinumab and/or Janssen around the Priority Date in that he chaired a round table of clinicians discussing the use of Stelara in 2017 and acted as an advocate for the registration of Stelara for UC by the Swiss authorities in 2019. There was a suggestion in his oral testimony that he had also given a separate lecture on the use of ustekinumab in 2017 or 2018 but no further information was found. Counsel for Janssen conceded during closings that it would have been better if this information had been declared in Prof Michetti’s expert report. I agree with this. Prof Michetti also said that he had read the guidance for expert witnesses in MedImmune v Novartis [2011] EWHC 1669 (Pat) so he should not have made these omissions. One result is that even now I am not entirely clear about his relationship with Janssen.
42. The errors in approach which were said to undermine Prof Michetti’s credibility were:
a) Prof Michetti emphasised every difference that could be found between treatments for CD and UC to support his argument that just because something was successful in CD did not mean it would be successful in UC, but several of these differences were not reflected in clinical practice in 2018 and would not have been CGK. For example, he tried to rely on different doses of adalimumab in CD/UC. Prof Michetti later accepted that the same dose was used in 2018. These were very minor points however.
b) Prof Michetti had given interviews where he had expressed optimism about the transfer of drugs from CD to UC, for example stating that “[m]ost drugs are developed for both diseases. A small minority are specific to one of the two”, but this did not marry up with his written evidence. Similarly, he had commented positively on the off-label use of ustekinumab. The Professor was clear that he had not agreed to the freestanding off-label use of ustekinumab, but only in the much more limited context of situations with patients who were already prescribed ustekinumab for psoriasis and who also had CD/UC, when a dermatologist would take primary responsibility for the prescription. I accept this, but nonetheless Prof Michetti’s comments in these settings were much more positive about drugs, including ustekinumab, being successfully used in conditions related to those for which they were authorised, including UC, than his written evidence had acknowledged. He should have mentioned this kind of work and dealt with it in his own written evidence. This point is quite closely related to point (e) in relation to his instructions and as with that point, I feel I still do not have the full picture.
c) Prof Michetti gave a wrong explanation about whether he had a particular point on the Sands Slides in mind when he wrote his first report. In his oral evidence he said for the first time that the Sands Slides’ apparently successful results were undermined by Figure 3 of the UNITI paper, Feagan 2016, (the scientific details are not relevant to the present purpose of assessing Prof Michetti as a witness). He also said that he had had the point in mind when he wrote his first report. It was then pointed out to Prof Michetti that it was impossible that he had the point in mind when he gave his first report, which he accepted, partly because it was inconsistent with his own second report. Counsel for Samsung submitted that Prof Michetti had been lying when he first said he had realised the point at the earlier stage, alternatively that he had lost objectivity and started to act as an advocate for Janssen. Counsel for Janssen did not seek to argue that Prof Michetti had been right in claiming to have thought of the point at the time of his first report and accepted that the oral evidence was “unfortunate”, but said it was understandable in the tension of giving oral evidence. Counsel for Janssen also submitted that it was to Prof Michetti’s credit that he recognised the error straight away. I think it is a substantial overstatement to accuse Prof Michetti of lying, and I do not think he was. Nor do I think he was or is dishonest. He was badly carried away by the occasion, though. I think that offering evidence in Janssen’s favour (that the point in question was one that occurred to him when doing his first report, it being plain that a point thought of only much later would be a lot less likely to occur to the skilled person) when he did not have a basis for it and when even a little bit of careful thought would have allowed him to see that, was indeed a symptom of his acting as an advocate. I do not think his swiftly accepting that he was wrong would ameliorate this, and I do not think he faced up to it all that quickly, anyway.
43. The combined effect of his not recognising and setting out his past dealings with Janssen and his historical views around off-label use, along with the significant point about the Sands Slides and when he thought of it, lead to me conclude that Prof Michetti’s evidence lacked care and rigour to an appreciable degree, and that his independence was materially undermined. Although there are one or two instances concerning the past IBD treatments where the overall evidence and the documents lead me to accept Prof Michetti’s evidence in preference to that of Prof Bloom, on the main issues in the case and especially the prospects of success of ustekinumab in UC, I find that Prof Bloom was the much more reliable guide.
Samsung’s fact witness, Prof Ochsenkühn
44. Prof Ochsenkühn currently holds many roles. He has been the Head and Director of the IBD Centre in Munich since June 2016, he has been the Head and Director of the Department of Gastroenterology and Hepatology of the Isarklinikum, Munich since October 2012, he has been the Head and Scientific Director of the Synesis Research Center since March 2013 and he is the Founder and Head of the European Crohn’s and Colitis Foundation (ECCS).
45. Prof Ochsenkühn gave evidence primarily relating to his presentation of the Ochsenkühn Poster at the Digestive Diseases Week conference held in Washington in June 2018. It was not suggested that he was anything but honest or that he lacked independence. It was submitted that his evidence was all about his usual practice (what he “would” have done) and not at all about his actual recollection. I disagree; he was talking both about his usual approach and about his recollection. His recollection is limited in some respects but generally reliable on the bigger things. It was submitted that one reason for his limited recollection is that he is and was so busy. I agree that that may be a factor but it does not lead me to conclude that he has no recollection.
46. Prof Ochsenkühn has given written evidence on the same events in other proceedings, where he described matters in somewhat different, and briefer, terms. I do not think anything turned on that. Overall I thought he was a very good, fair witness.
The Skilled Person
47. The parties agreed that the relevant legal principles may be taken from my judgment in Alcon v Aspire [2021] EWHC 1026 (Pat) at [31], drawing as I did on the judgment of Birss J (as he then was) in Illumina v Latvia [2021] EWHC 57 (Pat).
48. On the application to this case, the parties were agreed that the skilled person would be a clinician: a gastroenterologist with practical experience treating IBD, including UC and CD, and also research knowledge and experience relating to clinical trials for the development of new treatments for such diseases.
49. The fundamental dispute was over how much knowledge and understanding that person would have in relation to the detailed immunology and mechanisms of IBD and the ways in which drugs affected them. Janssen posited relatively deep knowledge and understanding; Samsung said the skilled person would have just enough knowledge of those matters to be able to design and assess the expected outcome of a clinical trial for UC or CD and to carry out the work reported in the Patent (a clinical trial). There was some reference in argument to the possibility that a clinician might call on a separate and more specialist person to provide details of molecular immunology, but I will continue to refer to the “skilled person” rather than “skilled team” for convenience.
50. To decide this dispute, I will apply the principles from Alcon and Illumina.
51. First, therefore, I must identify the problem to be solved. In my view it is the provision of a treatment for moderate to severe UC that provides long term CSFCR. It is relevant to bear in mind that this is done by finding a new use for a known drug but I reject as artificial Samsung’s argument that the skilled person was someone only interested in new uses for ustekinumab.
52. Second, in what real world “established field” was that problem located? It was not as broad as clinical practitioners in IBD who would just use drugs to treat patients; the parties agreed that research into new treatments was also a feature of the skilled person. The field was drug development and clinical trial design in IBD. That still does not really answer the question between the parties, though: how much knowledge of immunology and mechanisms would the skilled person have?
53. To answer that question, I think it is relevant to consider the Patent itself, the witnesses who gave evidence to me (to the extent they were representative of those in the field), and the literature in the field.
54. The Patent comments on immunology and mechanisms in some detail at [0002] to [0005] with references to specialist literature (referring to IL-12 and IL-23, the p40 subunit, the role of T-helper 1 (“Th1”) and Th17 cells and more). A doctor could of course just go and treat patients according to claim 1 without seeking to understand that, but that is not the question. Rather, the Patent expects the skilled person to be able to follow what is said, and they would in my view need and want to do that to assess the viability and reliability of what is proposed.
55. Both witnesses who gave evidence to me were capable of understanding matters at the sort of level of detail argued for by Janssen, and had opined on it, albeit that Prof Bloom said that that level of detail was not necessary to his conclusions. In my view they are representative of the sort of people with a largely clinical focus working on drug development (at least on new indications for existing drugs) and clinical studies in this field.
56. The literature in the field included much contemporary work written by and directed to people interested in addressing the problem of the Patent and which went into the sort of detail Prof Michetti had provided. That included not just journals but e.g. Janssen’s discussions with the FDA.
57. I think it is telling that there was not really literature at the sort of level that Samsung argues for, which would be along the lines of “ustekinumab works on IL-12 and IL-23 and that is why it works for Crohn’s disease” and no more. Indeed, Samsung accepted that the diagram at paragraph 73 below, was CGK, and it goes into quite a lot of detail, more than I think Samsung’s argument on the skilled person envisaged. Furthermore, Samsung positively relied on some points of detail, such as the common p40 subunit of IL-12 and IL-23.
58. For these reasons I agree with Janssen’s approach to the skilled person. The skilled person would be a clinician as described above with a good knowledge of the mechanisms and immunology behind biologics for CD and UC, at the sort of level described by Prof Michetti. I agree with Janssen that Samsung’s argument is a “Goldilocks” one designed to give the skilled person just enough CGK to find the alleged invention obvious (the IL-12/IL-23 point on its own) but not so much that they could start to have doubts (because of greater complexity, other pathways, differences in cytokines, situations where blocking IL-12/IL-23 was not or might not be enough rationale).
59. However, I should make it clear at this stage, as I have already touched on in the Overview above, that I do not think the identity of the skilled person has the importance that Janssen attached to it in this way. Prof Bloom was well able to deal with matters from the perspective of Janssen’s skilled person so the argument does not impact the cogency of his evidence. And Prof Bloom was not remotely saying that the skilled person as he envisaged them would have no doubt about the efficacy of ustekinumab in the long term use in the UNIFI trial. He agreed that there was some uncertainty but still good prospects of success. When the additional mechanism and immunology points of Prof Michetti were factored in, he (Prof Bloom) said much the same. So analysis at a deeper level does not ultimately help Janssen and while I have rejected Samsung’s Goldilocks point, I also reject what I perceived as an attempt by Janssen to increase apparent complexity wherever possible so as to blunt the apparent prospects of success (neither of these comments is directed at either expert personally).
Agreed CGK
60. The parties prepared an extremely good statement of the agreed CGK, for which I am very grateful. What follows is edited down from that to focus on the most important matters. I have removed material for brevity and not because it was not CGK.
Inflammatory Bowel Disease (“IBD”)
61. The term IBD is used to encompass a number of diseases, the two major diseases being CD and UC. Some cases cannot easily be classified as one or other and are commonly referred to as indeterminate colitis or IBD unclassified (“IBDU”).
62. As a systemic disorder, IBD manifests itself primarily in the GI tract (i.e., the passageway of the digestive system that leads from the mouth to the anus) but can affect a number of other organ systems of the human body. These are commonly termed “extraintestinal manifestations” and can affect the liver, skin, eyes and the joints.
63. UC and CD share some symptoms in common, such as chronic diarrhoea, abdominal pain, rectal bleeding, fatigue and various extra-intestinal manifestations that profoundly impact the quality of life in individuals with IBD. These symptoms can vary markedly over time, and the two diseases are characterised by a ‘flaring’ and ‘remitting’ pattern.
64. UC is characterized by mucosal inflammation starting in the rectum and extending proximally in a continuous fashion. It can affect variable amounts of the colon (the large intestine) but does not affect other parts of the GI tract.
65. CD is characterized by chronic inflammation that can affect any part of the gut, from mouth to anus, but more frequently the distal small intestine (the right lower quadrant of the small intestine) and the colon. Unlike with UC, the inflammation associated with CD demonstrates patchy lesions (sometimes called ‘skip lesions’).
66. Both UC and CD are found worldwide, with a higher incidence in Western countries and sometimes with a geographical gradient (for instance, higher prevalence in northern than southern Europe). UC affects men and women equally whereas CD is slightly more common in women. Both diseases have a peak onset age in adolescence and young adults with a smaller peak in older adults aged 40-60.
The Aetiology and Pathogenesis of IBD
67. The aetiology and pathogenesis of UC and CD were not completely understood in November 2018, but both genetic and environmental factors were known to play a role.
68. IBD was generally believed to result from an inappropriate immune response, in genetically susceptible individuals, to antigens derived from microorganisms in the GI tract. Environmental factors were also believed to play a part.
69. Many theories of IBD pathogenesis proposed a defective epithelial barrier leading to the presence of matter in the intestinal lamina propria (a thin layer of connective tissue forming part of the mucous membrane of the GI tract) that would normally be kept out of this space by a functioning epithelial barrier. This in turn was thought to lead to activation of cells of the immune system resulting in a dysregulated inflammatory response. This immune response involved the production of proinflammatory cytokines.
70. Cytokines are proteins that mediate signalling and communication between immune cells (which includes interleukins (IL), interferons and chemokines). Cytokines that upregulate the inflammatory response are known as pro-inflammatory cytokines and those that dampen it as anti-inflammatory cytokines. The role of cytokines has been studied in some detail, including looking at the effects of impairing pro-inflammatory cytokines (e.g., TNFα, IL-6, IL-12, IL-13, IL-18, IL-23, IL-33 and IL-36) or augmenting anti-inflammatory cytokines (e.g., IL-2, IL-10, IL-11, IL-22, and IFNβ). These studies were initially based on mouse models of disease and led to subsequent cytokine-focused therapies that have been the subject of clinical trials in human subjects. A number of cytokine-focused therapies have been approved, as discussed further below.
71. Historically, there had been a theory that there were fundamental differences between the inflammatory mechanisms in CD and UC, with CD being characterised by a Th1 response (associated with the pro-inflammatory cytokines IL-12, TNF-α, and IFN-γ), and UC being characterised by an atypical T-helper cell type 2 (“Th2”) response, associated with increased expression of IL-5 and IL-13, but not the other characteristic Th2 cytokine IL-4.
72. However, by November 2018 support for the Th1 vs. Th2 paradigm had been called into question by two developments in the field: (1) inhibition of TNF-α, a Th1 associated cytokine, was found to be effective in treating both CD and UC; (2) another T-cell subset Th17 (which was characterised by the secretion of IL-17 cytokines) was discovered and found to play a key role in inflammatory diseases. By November 2018, it was known that the underlying signalling pathways involved in both UC and CD were complex.
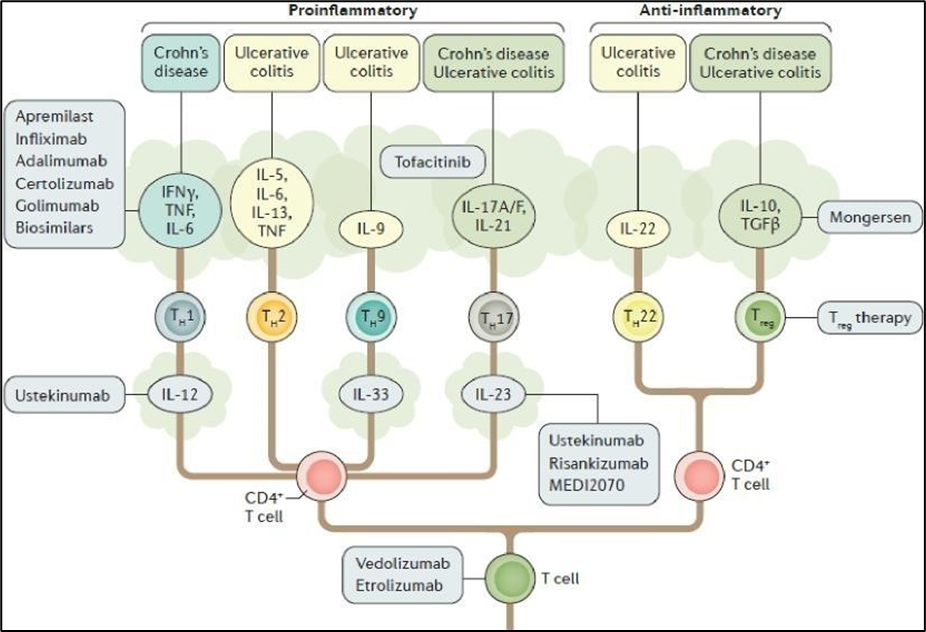 |
Figure from Neurath, M. F. (2017) Current and emerging therapeutic targets for IBD, Nature Reviews Gastroenterology & Hepatology, 14, 269 - 278 (“Neurath 2017”). This was mistakenly referred to in the agreed Statement of CGK as coming from Moschen 2019, reference given below.
Assessment of IBD
Symptoms
74. UC is a chronic disease affecting the colonic mucosa that most commonly presents with blood in the stool and diarrhoea. Symptoms can include urgency of defaecation, tenesmus (which is the feeling that you need to pass stools, even though your bowels are already empty), faecal incontinence, fatigue, increased frequency of bowel movements, mucus discharge, nocturnal defecations, and abdominal discomfort (cramps), although abdominal pain tends to be less of a hallmark feature than in CD. Fevers and weight loss can also be present in severe disease.
75. UC is classified by the extent of colonic involvement, namely proctitis, left-sided colitis and extensive colitis. Clinical presentation can vary depending on disease extent.
76. The clinical presentation of CD depends on disease location, severity of inflammation and disease behaviour.
77. The most common symptoms of CD are abdominal pain and diarrhoea. Weight loss, fatigue, anorexia and fever are also common symptoms. If CD is present in the colon, rectal bleeding or bloody diarrhoea might be the major symptoms.
Diagnosis
78. In both UC and CD, symptoms do not necessarily correlate with the degree of inflammation or with objective assessment of disease activity by endoscopy. For CD, persistent subclinical inflammation that may occur during clinical remission can lead to complications and progressive bowel damage. Diagnosis of UC and CD in November 2018 was therefore based on a combination of symptoms, endoscopy with biopsies, imaging and the exclusion of differential diagnoses.
79. A careful review of a patient’s medical history and travel history is important to exclude other causes of intestinal inflammation. For example, as noted above, non-steroidal anti-inflammatory drugs were known in 2018 to cause IBD symptoms, and infectious causes of intestinal inflammation need to be ruled out.
a) C-reactive protein (“CRP”) - a widely used serum indicator of inflammation in UC and CD.
b) Faecal biomarkers - including faecal calprotectin (“FC”) and lactoferrin (“LF”). FC is released into the faeces when neutrophils gather at the site of any gastro-intestinal tract inflammation. FC had been identified as a mucosal marker correlating more strongly with colonic disease and therefore was seen as particularly useful in UC as a surrogate for mucosal healing.
81. Endoscopy with biopsies is desirable to establish the diagnosis of UC or CD, but may not be possible if IBD affects areas of the GI tract not accessible to endoscopic biopsy.
82. Histological analysis of biopsies taken via endoscopy is recommended to differentiate between UC and CD and because the macroscopic appearance shown by endoscopy often underestimates the histological extent of inflammation.
83. In November 2018 some form of imaging (e.g. x-ray, CT scan or MRI scan) would also be performed in the diagnosis of UC and CD.
84. The skilled person would have also known that endoscopic/mucosal healing was used as another measure of disease activity, in particular for UC where the inflammation is limited to the superficial mucosal layer. Mucosal healing is difficult to ascertain in CD, owing to the patchy, transmural nature of inflammation that makes diagnosis unreliable.
Disease Activity
85. Following diagnosis, determining disease severity and activity was (and is) important to inform the choice of treatment. For UC and CD disease severity was, as now, typically classified as in remission or quiescent, mild, moderate, or severe. In November 2018, endoscopy and histology were important factors in assessing disease severity - especially in UC, which is a mucosal disease.
86. Numerous UC- and CD-specific disease activity indices existed in November 2018. These indices assign numerical values to the presence/absence or severity of different symptoms and assessments, to produce an overall score identifying the disease severity for that patient.
88. A variant that excludes the endoscopic component, referred to as the “partial Mayo score”, was also commonly used. The Mayo score has a range from 0-12 and the thresholds for classifying disease activity as mild, moderate and severe are 3-5, 6-10 and ≥10 respectively. The threshold for achieving clinical remission was a Mayo score of ≤ 2 with no subscore > 1, which is known as the global definition i.e., for countries outside the US (there is a separate US definition for clinical remission).
89. In clinical practice, the most commonly used CD disease activity index in November 2018 was the Harvey-Bradshaw index. This is a simpler index than the Crohn’s Disease Activity Index (“CDAI”).
Treatment of IBD
90. Both UC and CD are characterised by periods of clinical remission alternating with periods of recurrence.
91. There were multiple therapies available in 2018 for the treatment of UC and CD. This included anti-inflammatory and immunosuppressive agents that had been in use since the 1950s, as well as relatively newer classes of inhibitors, such as targeted biologic therapies that had rapidly gained acceptance since they were first approved in 1998.
92. The skilled person would consider implementing a staged treatment, as discussed further below, with successive agents or the use of certain combinations that were known to be advantageous. The skilled person’s approach would have been guided by various factors, including disease severity, prior drug response(s), cost and patient preferences.
93. In November 2018, one such approach was the “step-up” approach. The step-up approach to treatment started with the least potent suitable medication, with more potent drugs being used if the initial treatment proved insufficient. The primary aim of treatment was to induce and maintain remission without ongoing use of corticosteroids, which as discussed below is associated with significant side effects. The long-term goals were preventing disability, surgery and colorectal cancer. Treatment targets included resolution of clinical symptoms and endoscopic healing.
94. Treatment of UC and CD as at November 2018 involved an induction and maintenance regimen.
First-line induction therapies
Corticosteroids
96. Corticosteroids are used as anti-inflammatory agents owing to their ability to broadly attenuate recruitment of immune cells to inflamed tissue, induce apoptosis of T cells, and strongly diminish the production of the 'initial phase' cytokines IL-1 beta and TNFα, and the 'immunomodulatory' cytokines IL-2, IL-3, IL-4, IL-5, IL-10, IL-12 and IFN-gamma, as well as of IL-6, IL-8 and the growth factor GM-CSF. Consequently, it was well known that corticosteroids, and in particular those with higher glucocorticoid activity such as prednisolone and methylprednisolone, induce a rapid response and resolution of symptoms in both UC and CD. Corticosteroids typically act within 3-5 days in most patients when used intravenously, and as early as 2 weeks when administered orally. This is what makes them a leading choice not only as an induction therapy but also for managing flares that arise due to the waxing and waning nature of these diseases.
97. In November 2018 corticosteroids were used for induction therapy for moderate to severe UC and CD but were not indicated as maintenance therapy.
5-aminosalicylates (5-ASAs)
First-line maintenance therapies
UC
100. UC patients who responded to 5-ASA drugs in induction therapy i.e. achieved symptomatic remission within around 8 weeks, would continue on the same medication as maintenance therapy (usually at a lower dose).
101. If remission was achieved using corticosteroids, 5-ASAs could be considered for maintenance in patients with a mild flare who were recently diagnosed or were naïve to 5-ASA.
102. However, UC patients who required two or more courses of steroids in a year despite the use of 5-ASA or who were unable to effectively taper off steroids, would receive step-up therapy and start treatment with a class of drugs called immunomodulators or immunosuppressive agents. This included thiopurines, specifically azathioprine and 6-mercaptopurine.
CD
105. If it was not possible to maintain clinical remission in CD patients with immunomodulators, the skilled person would consider the next-line treatments as set out below.
Next line treatment in UC and CD
107. First-line biologic treatment for UC and CD was anti-TNF therapy, with either infliximab or adalimumab.
108. TNFα is a pro-inflammatory cytokine produced in immune and non-immune cells in the inflamed gut of IBD patients, including in macrophages, T cells, dendritic cells, fibroblasts and fat cells. Monoclonal antibodies targeting TNFα revolutionised the treatment of CD and UC following the approvals of infliximab (Remicade®) for treating CD in 1998 and then UC in 2006. Infliximab was the first anti-TNF to be approved for treatment of an IBD disease.
109. Golimumab was another anti-TNF drug, which was approved for UC only by November 2018.
110. The antibodies infliximab, adalimumab and golimumab bind to soluble and transmembrane forms of TNFα, neutralising the biological activity of TNFα by preventing it from binding to cellular receptors involved in inflammation.
111. Most IBD patients will respond at least initially to biologics. Non-responders are classified as primary non-responders (i.e. they have adequate drug levels and no antibodies to the drug but they do not respond adequately to the initial dose, potentially because these patients do not respond to the particular mechanism of action of the drug) or secondary non-responders (i.e. they respond initially but then lose response over time due to the development of antibodies to the drug).
112. If a patient had a primary non-response to an anti-TNF antibody, they would usually be switched to a different class of biologic, such as vedolizumab. If a patient had a secondary loss of response to an anti-TNF antibody, the patient might be switched to another anti-TNF antibody, with golimumab being an option for UC patients.
113. Vedolizumab binds to a different target, namely α4ß7 integrin, a protein on the surface of lymphocytes targeted for the GI tract. This interaction forms part of the process by which lymphocytes exit the bloodstream and enter the intestinal tissues leading to inflammation. Vedolizumab had been approved for the treatment of moderate to severe UC and CD by November 2018.
114. By November 2018 a third class of biologic was available for the treatment of CD only - ustekinumab. Ustekinumab binds to the p40 subunit found in both IL-12 and IL-23, which prevents IL-12 and IL-23 from interacting with their receptors, blocking their action and consequently reducing inflammation in the gut of patients with CD.
115. A JAK kinase inhibitor, tofacitinib, received approval in July 2018 for UC. Tofacitinib inhibits the activity of the Janus kinase family of enzymes which have a role in activating the body’s immune response involved in gut inflammation. Tofacitinib blocks the signalling pathways triggered by multiple pro-inflammatory cytokines at once and it was known to act downstream of the IL-12 and IL-23 receptors.
Higher risk UC and CD (including acute severe disease)
116. There were three different lines of treatment of moderate to severe UC (after 5-ASA failure) or CD, namely either a ‘step-up’, ‘top-down’ approach, or ‘accelerated step-up’ which would involve the following:
a) Step-up treatment, as discussed above.
b) In the top-down approach, treatment begins with early combined immunosuppression (e.g., azathioprine plus infliximab) which would be followed by de‑escalation following a response to treatment.
c) An accelerated step-up would omit the initial course of steroids alone, i.e., treatment commences immediately with a combination of steroids and an immunomodulator/immunosuppressive agent, and then followed by treatment with a TNFα inhibitor (again, preferably in combination with an immunomodulator/immunosuppressive agent and/or steroid).
117. Surgical intervention was available, usually as a last resort in case other treatments did not work in either UC or CD. For UC, and in cases of CD that involves inflammation of the colon, colectomy was routinely available as an option for recurring inflammation.
118. Patients with acute severe UC, defined by Truelove and Witts criteria, would be admitted to hospital and treated with intravenous corticosteroids to which approximately 70% would respond. If a patient did not respond to i.v. corticosteroids within the first 3 days, rescue therapy with either ciclosporin or infliximab would be used to try to avoid colectomy as the option of last resort. If there was no response to one of these drugs over the short term, colectomy would be performed.
Therapy-refractory and therapy-intolerant patients
120. For both UC and CD there were a subset of patients at the severe end of the disease spectrum who were therapy refractory, that is they continued to suffer from persistent acute symptomatic disease despite treatment. In addition, there was a subset of patients who responded to IBD treatments but suffered side effects too severe to continue on that treatment, termed therapy-intolerant patients.
Ustekinumab
121. Ustekinumab had received European approval for use in the treatment of CD in November 2016, with NICE approval following in July 2017. In November 2018 ustekinumab was used in moderate to severe CD patients.
122. In addition, the skilled person was aware through conferences, marketing, and reading drug information, that ustekinumab had been previously approved for use in treating plaque psoriasis (in 2009) and psoriatic arthritis (in 2013).
Clinical Trials for Biologics in IBD
124. The safety and efficacy of drugs is determined based on RCTs, where patients are randomly assigned to two (or more) groups to test a drug - one (or more) group(s) receive(s) the drug being evaluated, while the other receives a placebo. RCTs may be ‘blinded’, where patients (and study investigators) do not know which study group they are in. The administration of the placebo generates identical procedures in all the study arms, which preserves the blinding. Since blinding reduces bias in study analysis, IBD clinical trials were usually double-blinded.
125. Clinical trials for biologics in UC and CD included an induction phase and a maintenance phase.
126. The induction phase tended to last between 6–12 weeks while the maintenance phase would typically last around 1 year.
127. It would have been well-known that in IBD treatment, a key goal would have been achieving long-term clinical remission. The skilled person would know that achieving remission after about one year of treatment is a good proxy for assessing this outcome. This is because if clinical remission is achieved after a year of treatment, then this indicates that the therapeutic has had a real impact in altering the disease pattern and thereby treating it.
128. The success of a trial in terms of supporting an application for regulatory approval would be determined by whether a statistically significant difference was found in the active arm versus the placebo arm with regard to one or more primary endpoints. Primary endpoints would be defined in the trial protocol and agreed in advance with the relevant regulatory bodies. The primary endpoint for the induction phase of biologic trials in UC and CD tended to be clinical remission or clinical response. For the maintenance phase, typically the primary end point would be clinical remission.
129. Clinical trials in UC and CD used disease activity indices to define the endpoints of clinical remission and clinical response. For UC trials, this included the use of the Mayo score described above.
130. Other UC disease activity indices included:
a) The Lichtiger Index (also called the Modified Truelove and Witts Severity Index); and
b) The Simple Clinical Colitis Activity Index.
131. For CD trials, disease activity indices typically used the CDAI.
132. The SES-CD score would have been well known to the skilled person and was a widely used index for endoscopic assessment of disease activity in CD.
133. Clinical response is a less stringent endpoint compared to clinical remission meaning that a greater proportion of patients in a clinical trial would be expected to meet the endpoint of clinical response.
134. Each clinical trial would have one or more secondary endpoints. Secondary endpoints may provide supportive information about a therapy’s effect on the primary endpoint or demonstrate additional effects on the disease. To the extent that clinical response or clinical remission was not the primary endpoint of the study, this would tend to be included as a secondary endpoint for both UC and CD trials. Other established secondary endpoints included:
a) Corticosteroid-free clinical remission (“CFCR”) (as a secondary endpoint in the maintenance phase). CFCR mirrors the aim of the skilled person in treating IBD patients. The aim would be to get a patient with UC or CD off corticosteroids as soon as possible in light of the wide-ranging and serious side effects associated with long-term steroid exposure.
Concomitant use of corticosteroids at a stable dose through the induction stage of a clinical trial was permitted, and about one-third to one-half of all study participants entering a clinical trial in 2018 would be receiving corticosteroids at the induction baseline.
In both clinical practice and clinical trials, it was appreciated that during or after tapering off steroids, the patient might experience a disease “flare”, essentially a worsening of their symptoms, which may require the dose of corticosteroids to be increased for a period of time, before reducing it again.
b) Endoscopic healing (as a secondary endpoint in the induction and/or maintenance phase of UC trials). Endoscopic healing is characterised by a Mayo endoscopic subscore of ≤1. Endoscopic healing was known to be an objective and stringent measure of treatment efficacy given the requirement for such improvement in the endoscopic appearance of the mucosa. However, endoscopic healing was not routinely used as an endpoint in CD clinical trials.
c) Assessment of quality of life/patient-related outcome measures (as a secondary endpoint in the maintenance phase). UC and CD clinical trials would often include an assessment of improvement in quality of life for the patient.
135. Clinical trials in UC and CD would often include an analysis of the response in the biologic failure cohort of patients and analysis of changes in biomarkers. These analyses may form part of the secondary endpoints of a trial. Some clinical trials would also include an analysis of sustained response i.e. achievement of an efficacy criteria such as clinical remission in the induction phase and in the maintenance phase.
136. In case a patient had been previously treated with another biologic, a ‘wash out’ period was usually required, since biologics such as monoclonal antibodies have a long half-life in circulation and so there was a need for the effects of the previous biologic to have worn off before the drug under evaluation was administered. The skilled person would also know that patients who had failed prior treatments were a population that was considered difficult to treat.
Ustekinumab clinical trials
137. The UNITI trials were the phase III RCTs that led to the approval of ustekinumab in CD. As at November 2018 the skilled person would have been aware, at a high level, of the design of this study including the duration of the induction and maintenance phases, the dosing regimen tested in those phases and the endpoints investigated including clinical remission, clinical response and CFCR. The skilled person would have also known where to look up any missing details, for example, in the label, and/or the key paper in NEJM (Feagan 2016). The skilled person would have been aware of the ongoing UNIFI trial in UC and that details would be contained on CT.gov.
Commentary on the clinical trials
138. Although not set out in the parties’ agreed document, the following were accepted by Janssen during trial to be CGK (or that they would be found by routine, obvious means):
a) That the theory supporting UNIFI was based on ustekinumab blocking IL-23 (this is set out in the Sands Slides anyway).
b) That there was no phase II trial of ustekinumab for UC. Janssen went straight to phase III.
139. On the other hand, I note that it was not argued that the Sands Slides were CGK.
Disputed CGK
140. The parties identified six disputed areas which are set out below:
i) The extent of the skilled person’s knowledge of CD and UC including whether they were considered to be distinct diseases, the percentage of patients with IBDU and the degree to which disease activity waxed and waned over time.
ii) The underlying immunology of UC and CD and the extent to which this was known by the skilled person.
iii) The genetic risk factors for CD and UC and the extent to which the skilled person would place weight on them in developing new treatments for UC.
iv) The treatments for UC and CD, including the extent to which it was known that any individual treatment was efficacious to treat both CD and UC, their approved indications and knowledge of differential drug responses in UC and CD.
v) The extent of knowledge of the underlying IBD clinical trials (i.e. knowledge of the detail reported in underlying papers, endpoints tested, results, placebo rates, and the extent to which various UC and CD disease activity indices were used etc).
vi) Knowledge of failures of proposed treatments for UC and/or CD and the difficulty of achieving CFCR (including which drugs had not achieved it).
141. A seventh issue, about the extent to which concomitant steroids were understood to prevent formation of ADAs, fell away.
Issue i): Distinction between UC and CD
142. In closing submissions Counsel for Samsung agreed that UC and CD are distinct diseases (subject to Prof Bloom’s explanation that neither CD nor UC is ‘one disease’ as they each contain a number of sub-types) but that there are cases of UC which can be almost indistinguishable from cases of CD (IBDU). Counsel for Samsung agreed that the diseases wax and wane.
143. The parties agreed in closings that the dispute regarding the percentage of patients with IBDU did not go anywhere and I do not need to determine it; it was a small minority of patients (under 10%) and that is sufficient for the purposes of this judgment.
Issues ii) and iii): Immunology and Genetic Risk Factors
144. Issues ii) and iii) were central issues in dispute. It was Samsung’s case, based on the evidence (both written and oral) of Prof Bloom, that it was CGK that IL-23 was implicated in both CD and UC, and that the genetic association between IL-23 and both diseases was thought to be a strong one. They also submitted that it was CGK that it was likely that blocking IL-23 signalling would be beneficial in UC, as it was in CD. Janssen agreed that IL-23 was implicated in both diseases, and that there was a genetic association between IL-23 and both diseases, but they argued that it did not follow that it was likely that blocking IL-23 signalling would be beneficial in UC as it was in CD.
145. During cross-examination, Prof Michetti stated that “an association does not prove, causality does not prove that acting on IL-23 will work based on genetic association”. I accept this and I believe Prof Bloom did too.
146. In its closing skeleton, Janssen pointed to Prof Bloom’s admission that there are a significant number of genes that are classified as being either CD or UC specific and that some genetic loci associated with both diseases have opposite effects in each, such as the NOD2 gene which has protective effect in UC but is a risk in CD. Prof Bloom ultimately accepted during cross-examination that the mere fact that one gene is associated with a disease does not tell you much about the effect of it.
147. Samsung responded to this by stating that the evidence for the role of IL-12/23 pathways in both CD and UC was not limited to genetic studies, but was also supported by basic science, pathogenesis and clinical research.
148. Janssen argued that IL-23R was not the only relevant gene implicated in UC, and that it was known that the risk associated with IL-23R was greater with CD than with UC. Prof Michetti agreed during cross-examination that IL-23 was an important target in UC; but he did not agree that it was known that blocking IL-23 worked to treat UC.
149. By closing submissions, it was common ground between the parties that IL-12 is a cytokine implicated in CD but not in UC.
150. There was also a dispute regarding the state of the CGK relating to the inflammatory mechanisms of CD and UC. Samsung’s case was that the inflammatory mechanisms (once the diseases were established) were the same in UC and CD. Janssen pointed to Fig. 3 of the Agreed Statement of CGK (see figure from Neurath 2017 in paragraph 73 above) as showing the complexity of the inflammatory cascade in UC and/or CD, which involved many cytokines other than IL-12 and IL-23, and it submitted that the skilled person would know this, which I accept (not least because Figure 3 is from the Agreed CGK). During cross-examination on this topic, Prof Bloom stated that the cytokines up-regulated in UC and CD are not identical, and are not even identical within the same diseases due to sub-types likely having different cytokine profiles. However, Prof Michetti agreed during cross-examination that it would be CGK that the same cytokines were highly expressed in the intestinal mucosa of both CD and UC patients, and that the inflammatory mechanisms were the same once the diseases were established (this is the statement in Janssen’s FDA dialogue that I have referred to above).
151. Counsel for Samsung took Prof Michetti to an interview by Bruce E. Sands et al, entitled “Inhibition of Interleukin-12 and/or -23 for the Treatment of Inflammatory Bowel Disease”, published in Volume 12, Issue 12 of Gastroenterology & Hepatology in December 2016. Prof Michetti agreed that the following sentences would be CGK:
…In addition, findings from genetic studies have implicated IL-12 and -23 in susceptibility to IBD. More importantly, there is also a polymorphism of the IL-23 receptor that is highly protective for IBD, suggesting that by blocking IL-23 signalling it is possible to decrease the risk of developing Crohn’s disease or ulcerative colitis. Thus, blocking IL-23 downregulates aspects of the immune system that are thought to be important in causing these diseases.
152. During cross-examination, Prof Bloom described how IFNγ was a cytokine produced in the Th1 pathway, and so likely to be seen as a good target for treating CD; but it was actually shown not to work in that condition. Janssen pointed to this as an example of why the skilled person would have known that targeting a particular cytokine would not necessarily result in effective treatment. In his oral evidence Prof Michetti pointed to JAK inhibitors to illustrate this point, as JAK pathways were implicated in both diseases but JAK inhibitors, especially tofacitinib, had different effects in UC and CD. I return to this below.
153. Janssen also argued that cross-talk between inflammatory pathways meant that blocking one could lead to compensatory pathways emerging, so that a hoped-for clinical effect was not then seen in practice. Prof Bloom accepted this as a possibility.
154. My conclusion on this issue is that the idea of blocking IL-23 so as to treat UC was a widely known and reputable theory, but that the field also recognised that the situation was complex, that there were unknowns, that what worked or did not work in UC or CD might behave differently in the other of them, and that blocking IL-23 might not treat UC, for which one possible reason (but not the only one) could be the development of compensatory pathways. I think the overall appreciation was well and fairly identified in a passage of cross-examination of Prof Michetti based on Moschen, A. R., et al. (2019) IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting, Nature Reviews Gastroenterology & Hepatology, 16(3), 185–196 (published online on November 19, 2018) (“Moschen 2019”) which was a review article published slightly post-priority but which I am satisfied in this respect reflects CGK at the Priority Date):
21 Q. Yes. Then the very end of this section, the last sentence,
22 they say: "Although too early for speculation regarding
23 comparable effectiveness between Crohn's disease and
24 ulcerative colitis, it seems plausible that blockade of
25 IL-12-IL-23 will prove efficacious for ulcerative colitis";
2 yes?
3 A. Yes, it is exactly, speculative.
4 Q. No, it is what seems plausible. It is not just speculation,
5 professor. They have referred to various pieces of evidence
6 and, in their view, it is "plausible that blockade of
7 IL-12-IL-23 will prove efficacious for ulcerative colitis";
8 yes?
9 A. If you cut a sentence in two, you lose the meaning. It is
10 says also: "... too early for speculation regarding
11 comparable effectiveness", so they really are at the stage of
12 speculation, that the comparable reason will apply.
13 Q. Yes, they are not saying you can tell it is going to be
14 equally effective for Crohn's and UC, but they are saying it
15 will still be efficacious for UC?
16 A. It seems plausible, they say.
17 Q. Yes, and you would agree?
18 A. I agree from the plausibility, yes, certainly.
19 Q. That is what other people in the field would think also?
20 A. Yes. I was not asked to participate, but I probably would
21 have considered participating in the ulcerative colitis study
22 with ustekinumab in 2017 or 2018. It did not take place in
23 Switzerland for the reason I alluded to, we have too many
24 healthcare systems. It is impossible to participate, very
25 difficult to participate in international trials. But yes, it
2 was possible and it was an expectation.
155. I should make it clear that I do not think that Prof Michetti was using “expectation” in the sense that patent lawyers do when they contrast hope and expectation (see below). He was reflecting the sense that the IL-23 theory justified enrolling patients in a trial and was more than speculation but considerably less than a certainty.
156. I received submissions on various other detailed points on this topic (for example Samsung relied on the relevant genetic links being in the protein coding portion of the gene in question, thus invoking a point of fine detail which on its own argument the skilled person would not know about or be interested in) but it is unnecessary and would be disproportionate to deal with every single one in this judgment: I have explained my overall conclusion and the main points.
Issues iv) and vi): Treatments for UC and CD, failed treatments
157. These issues were also a major battle ground. The parties provided helpful tables with their closing skeletons summarising their positions in relation to each of the treatments for UC and/or CD discussed at trial. Annex 1 to this judgment is a table which I have prepared, based on the parties’ tables and on their closing written and oral submissions, setting out basic information about each of the 14 drugs referred to and identifying where the disputes regarding CGK arose, and what turned out to be agreed. I do not intend to repeat in the body of this judgment the material which my table identifies as having been agreed CGK save where I expressly mention it as part of my reasoning, but I have borne it all in mind. I have used the numbering for each treatment which the parties adopted by agreement, but because I have organised things differently, the numbering does not run sequentially in this judgment.
158. The main reason why these aspects of disputed CGK matter is that the parties relied on them as informing or affecting whether the skilled person would have greater or less optimism about ustekinumab succeeding in treating UC. Thus Samsung argued that, for example, the prior success of biologics for both UC and CD would increase optimism about ustekinumab succeeding in UC given its having been proved in CD (by UNITI). Similarly but conversely, Janssen argued that apilimod mesylate, a small molecule to inhibit IL-12 and IL-23 production had failed, and that that would reduce optimism.
159. Because of the forensic purpose of deploying this sort of information, the parties looked at details about the drugs in question through a very particular lens which the skilled person would not, in general and in my view, have deployed. They would not have routinely gathered or had at their fingertips every detail about every IBD drug lest it in future might prove to have some relevance to the prospects of success of ustekinumab in UC. Likewise, the parties tended to try to squeeze out every drop of information about every drug and every clinical trial, going well beyond the level of information that would be CGK. Janssen was more guilty of this than Samsung, but both offended.
160. I also heard a lot of submissions, mainly directed from Janssen, about whether the information about these drugs showed that they behaved differently in CD and in UC. Such differences, Janssen argued, would reduce the confidence of success of ustekinumab in UC based on its success in CD in the UNITI trials.
161. I will also say, before I plunge into the detail, that my clear overall conclusion is that the individual points about these drugs and their use in IBD, whether separately or in aggregate, do not move the dial in relation to the ultimate issues I have to decide. Whether Samsung or Janssen is right about some, more, or even all of them, the skilled person’s overall view from the CGK would be that there was an appreciable chance that ustekinumab would work in UC; that there was a theoretical basis for thinking that there was such a chance; that the drug nonetheless needed actually to be tested for UC; that it was being tested in UNIFI; and that the result could go either way. So based on the CGK alone there was a hope but not an expectation of success (that is just another way of saying that the Patent is not obvious over the CGK alone, which is not even alleged). If, say, Janssen were right that it was CGK that apilimod mesylate had failed in UC (which is not my finding) the skilled person’s reaction would just be that that was a minor piece of the picture which still left them with a realistic hope that had a theory to support it, and that testing was needed. Likewise, whether or not, for example, there were different rates of onset of action for a biologic in UC and CD, that would not change the overall picture any more than it would if the rates of onset were identical.
162. Although the CGK should be determined neutrally and without reference to the non-CGK prior art, which is what I have done, it will help the reader to understand my reasoning if I foreshadow that these disputes about minor matters of emphasis about other IBD drugs pass into insignificance when validity over the prior art comes to be considered. With the Sands Slides, the skilled person would know (I conclude below) that ustekinumab has succeeded for UC in the induction phase and with that key, solid fact in mind they would have no interest in whether some other biologic behaved a little differently in UC and CD. With the Ochsenkühn prior art the skilled person would think (I conclude below) that there was some generally positive but ultimately inconclusive clinical evidence which was consistent with ustekinumab working for UC, which was a hope, but did not allow any strong conclusion or positive expectation of success to be formulated. That would not be enough for obviousness whatever the precise position with e.g. apilimod mesylate, or the higher dose of adalimumab, or whatever other point of detail.
163. The one exception to what I have just said is that I think the skilled person, on reading the Sands Slides, might well think to ask themself whether there was any instance in which a biologic had failed in the maintenance phase having succeeded in the induction phase, in IBD. I find that there was no such instance in the CGK. I accept Samsung’s submission to that effect, and it supports but is not necessary to my conclusion about obviousness over the Sands Slides.
164. I will also make one more general point which is that I accept Janssen’s submission that in principle the skilled person would be interested in failures as well as successes, if they were CGK and were informative and probative. However, I do not think there were any failures which provided compelling evidence about ustekinumab’s prospects for UC, for the reasons given above and in what follows.
Corticosteroids, prednisolone (parties’ numbering, number 1)
165. I refer to the agreed CGK at paragraphs 95 to 98 above. During cross-examination Counsel for Janssen stated that these drugs were said to “damp down the flames but … do not put out the fire.” Prof Bloom agreed with this description.
166. Such disagreement as there was about the CGK on corticosteroids seems to me to have been relevant only, if at all, to the issue about ADAs, which was disputed CGK issue 7, which fell away. They were used in UC and in CD for rapid action where needed and were also used alongside biologics. It was not however suggested that this allowed any conclusion to be drawn one way or another about whether ustekinumab would be effective in UC.
5-ASAs - mesalazine (number 2)
167. These were anti-inflammatory agents. I refer to the agreed CGK at paragraphs 99 to 103. Their effective use in UC was CGK but the parties disagreed about whether it was CGK that they were effective in CD. The position is a messy one but my finding is that the CGK was that while guidelines deprecated their use in CD, they were very widely prescribed for it. The evidence as to efficacy was inconclusive. This means that there was a possibility but no more than that of a difference in efficacy between the conditions, but it could shed no light on what might happen with agents targeting IL-23.
Immunosuppressive agents - Methotrexate and Ciclosporin A (numbers 3 & 4)
168. As the agreed CGK indicates at paragraph 104, methotrexate was known to be effective for CD but the position in relation to UC was disputed. I find that clinical trials close to the Priority Date had cast serious doubt on it and guidelines were against it. However, there was still some data to support the notion that it might work in some cases, perhaps more serious ones. The position is therefore similar to the 5-ASAs but with the evidence for the disputed indication being still weaker. I find that overall the CGK was that the jury was out on efficacy in UC but with the expectation that it was more likely ineffective. So again there was a possibility of a difference in the conditions but I hold that this CGK would not have been seen as persuasive as to what would happen with an agent targeting IL-23.
169. Whatever the position with 5-ASAs and Methotrexate, it was agreed CGK that Ciclosporin-A was effective in UC but not CD. So the former drugs are a bit beside the point when there was this clear instance of a drug with different effectiveness in UC and CD. But again, I hold that the CGK did not support using it to draw an inference about targeting IL-23, or other classes of drugs generally.
Apilimod mesylate (number 8)
170. On the evidence, I hold that this drug was not CGK in the UK. Janssen did not really contest this. So it is irrelevant to what I have to decide.
171. There was a phase II clinical trial in which it failed in CD, and Janssen relied on this because the drug is intended to inhibit production of IL-12 and IL-23, although it is a small molecule and not a biologic.
172. Prof Bloom said that it was not possible to draw conclusions from apilimod mesylate because it was given orally, was reversible and was “too slow” and so would not have been seen as something from which an extrapolation to ustekinumab could be made. I accept this evidence and it is another reason why the skilled person’s thinking would not have been affected by this drug. Janssen said this was Samsung trying to have its cake and eat it. I do not agree. It is just what the skilled person would think (if, contrary to my main conclusion, the drug was CGK in the first place).
Biologics (numbers 5-7 and 9-14)
173. With biologics one enters the arena where at least the drug type is similar to ustekinumab. However, there are many different ways to look at the information available.
174. The agreed CGK is at paragraphs 106 to 115 above.
175. Samsung’s main case was that there were three biologics that had been successful in UC and CD: the anti-TNF inhibitors infliximab and adalimumab and the anti-α4/β7 integrin vedolizumab (numbers 5, 7 and 11). Thus, it said, there was a pattern of success and biologics being taken from one kind of IBD to the other.
176. I agree with this and that it meant the CGK was that it was possible to have a biologic which would treat UC and CD. But this must be very significantly tempered by the fact that the three drugs did not work via IL-12/IL-23. So general statements about “biologics” cannot be overdone.
177. Janssen said that there were other biologics which failed for UC and/or CD, and that there were in any case differences in the behaviour of the successful biologics in UC and CD. I will deal with the differences among the successful biologics and then the alleged failures.
Infliximab (number 5)
178. Janssen said that it was CGK that dose optimisation was more commonly required in UC patients than in CD patients. This was indeed one of the findings in one of the trials but it was such a minor detail that I do not think it was CGK. In any event the underlying reason was not understood and at most it is a very minor indication that there are possible differences in effects of biologics between UC and CD. It would not nearly be enough to conclude that ustekinumab would not work in UC having succeeded in CD.
Adalimumab (number 7)
179. Janssen said that there were differences in dosing between UC and CD for this drug.
180. The position is complex, but my conclusion is that although by the Priority Date the same (higher) dose was used for both conditions, at an earlier stage and still reflected in the label and CGK, there was a lower minimal effective dose for UC. This is just another minor difference in performance, though.
Vedolizumab (number 9)
181. Janssen pointed out that the agreed CGK was that this drug worked by preventing lymphocytes entering the GI tract and causing inflammation. Thus, it was in different class from the other biologics. I agree with this and it is another facet of not being able to reason freely that whatever applied to past biologics would necessarily apply to ustekinumab. They worked in different ways.
182. It was common ground that vedolizumab worked quicker in UC than CD. This is another, and probably the clearest, instance of a biologic behaving slightly differently in the two conditions.
Fontolizumab (number 6)
183. This drug failed in CD. It targets the IFNγ cytokine which is implicated in the IL-12 pathway for CD. It was untested in UC.
184. On balance I find that the failure in CD was CGK. Prof Michetti said so, and Prof Bloom said the skilled person may have known the reasons. I have said above that the skilled person would in principle be interested in failures.
185. However, the failure in CD when efficacy in UC was untested is completely unhelpful to what I have to decide. Some drugs just fail. The most that Janssen could really say was that this was an example where a drug was thought to have a mechanistic rationale but failed in the clinic. That that is possible was never in dispute.
Secukinumab (number 9)
186. This was a drug that not only failed but made IBD worse. It was an anti-IL17A. Janssen relied on it as another example of a drug which had a scientific rationale but failed when tested. I refer to my comments on fontolizumab.
Golimumab (number 10)
187. This was another anti-TNF, like infliximab and adalimumab. I find that the CGK was that it was approved for UC (this was not in dispute) and was expected to work in CD. The basis for the expectation in CD was off-label use so less solid, but I hold that it was CGK nonetheless.
188. I agree with Janssen that the fact that the drug worked for both conditions does not take the matter any further: the other anti-TNFs had already shown that could be achieved and golimumab does not make it any easier (or more difficult) to reason from anti-TNFs to ustekinumab.
189. There was contemporaneous evidence that golimumab did not achieve CSFCR in UC in trials. Janssen said that this differentiated it from infliximab and adalimumab. However, I do not think that it was CGK that golimumab could not achieve CSFCR, and the trial in question had only looked at patients on steroids at baseline, so it was a tough endpoint. So I do not think it was CGK that there was a relative success/failure difference in the anti-TNF class in this respect, and even if there had been it would not have been informative about ustekinumab.
Abrilumab (number 12)
190. This was an anti-α4/β7 integrin like vedolizumab. At the Priority Date there were some clinical trials going on with it but no papers published (at least none before me). I do not consider that Janssen has shown it was CGK. Janssen suggested it was effective in UC but not CD, but Prof Bloom said it was possible that the drug needed to be given for longer. The evidence about relative efficacy is, I find, too tenuous to reach any conclusion even if the trials had been CGK, which they were not.
Tofacitinib (number 14)
191. This drug is a JAK (Janus Kinase) inhibitor. It is common ground that it was licensed for UC but not CD.
192. However, Prof Bloom said that but for unacceptable levels of toxicity, tofacitinib appeared effective in CD. Samsung submitted that Prof Michetti accepted that there was hope that there may be some efficacy in CD, albeit at higher doses, but with side effects. This was all quite tenuous, and I find that the CGK was that tofacitinib was probably ineffective in CD, and certainly appreciably less effective than in UC.
193. I therefore find that this was an example of a drug from a particular class of biologics succeeding in one IBD indication and failing in another. It would be an illustration for the skilled person that the whole situation was complex and that it could not be taken for granted that a biologic which succeeded in one form of IBD would necessarily succeed in the other. It might fail. It is something of a counterbalance to the three biologics which succeeded in both indications, albeit that none of them was from the same class as ustekinumab.
Issue v): clinical trials
194. Knowledge of the specific clinical trials for ustekinumab is dealt with above. The overall approach to clinical trials is covered in the agreed CGK section above at paragraphs 123 to 139. There were some minor points on clinical trial methodology more generally which were not specifically agreed but which were not materially in dispute (alternatively I find them to have been the CGK):
a) The Mayo score was predominantly used in IBD clinical studies. The other known scoring systems were regarded as less good and/or less objective and in particular the Lichtiger score was known to be subjective and generally not used in clinical studies.
b) Endpoints should be set in advance to avoid the risk of researchers looking around in data retrospectively to find something positive when the primary endpoint had been missed.
c) CSFCR was an important (usually secondary) endpoint because it indicated that the drug under trial was controlling the disease and not steroids. CSFCR was not always achieved even by otherwise successful drugs (see above).
The EP’606 Specification
195. The Patent is entitled “Safe and effective method of treating ulcerative colitis with anti-IL-12/IL-23 antibody”. The parties agreed that for the purposes of these proceedings the relevant priority date was 20 November 2018.
196. The Patent says that it relates to a safe and effective treatment for moderate to severe UC by intravenous or subcutaneous administration of ustekinumab, an anti-IL-12/IL-23p40 antibody.
197. The background is set out at [0002] to [0009] and includes references to literature. Relevant information from the background section includes:
a) IBDs, including UC, are chronic relapsing disorders [0002];
b) “The involvement of the IL-12/23 pathway in the pathogenesis of IBD is well established” [0003];
c) “Genome-wide association studies have implicated genetic loci in humans in the IL-12/23 pathway that are associated with increased susceptibility to UC” [0003];
d) “Multiple lines of evidence suggest that inflammatory bowel disease (UC and Crohn’s disease) is mediated by Th1 or Th17 cells with strong contribution from the proinflammatory cytokines, IL-12 and IL-23.” [0005];
e) “Ustekinumab (STELARA®) is a fully human immunoglobulin G1 mAb to human IL-12/23p40 that prevents IL-12 and IL-23 bioactivity by inhibiting their interaction with their cell surface IL-12Rβ1 receptor protein” [0005];
f) Paragraphs [0006] to [0009] mention the clinical studies on ustekinumab for Crohn’s disease, the UNIFI trial, the Ochsenkühn Abstract and the lack of studies with ustekinumab for UC. [0006] to [0009] state:
[0006] The efficacy and safety of intravenous (IV) ustekinumab as induction therapy in Crohn's disease have been evaluated in clinical studies CRD3001 and CRD3002. In study CRD3001, subjects with demonstrated prior failure or intolerance to one or more TNF antagonists were evaluated, and in CRD3002 subjects with history of inadequate response to or intolerance of corticosteroids or immunomodulators, but without a history of an inadequate response or intolerance to TNF antagonists were evaluated. In these studies, two IV doses were evaluated: a 130 mg IV fixed dose (-2 mg/kg on a mg/kg basis) was chosen for the low-dose group, while body-weight range based doses approximating ~6 mg/kg IV (weight ≤55 kg: ustekinumab 260 mg; weight >55 and ≤85 kg: ustekinumab 390 mg; weight >85 kg: ustekinumab: 520 mg) were chosen as the high-dose group. In both studies, ustekinumab demonstrated clinically significant efficacy compared with placebo and was well-tolerated with a favorable safety profile.
[0007] Clinical trial NCT02407236 (13 August 2018) outlines a study design to evaluate ustekinumab induction and maintenance therapy in participants with moderately to severely active ulcerative colitis (UNIFI).
[0008] Ochsenkühn (2018) Journal of Crohn's and Colitis 12(1):s485 describes ustekinumab as rescue treatment in therapy-refractory or -intolerant ulcerative colitis.
[0009] Prior to the present invention, no studies had been conducted with ustekinumab for UC. there is a need in the art for improved methods of treating UC, particularly moderately to severely active UC, in subjects who had previously failed or were intolerant of a biologic therapy or other conventional therapy, or subjects who had demonstrated corticosteroid dependence.
198. The Detailed Description of the Invention is at [0032] to [0538]. The Patent provides the results of the UNIFI Phase III trials in its two examples - Example 1 starts at [0169] and provides the details of the IV administered induction study, and Example 2 starts at [0209] and provides the details of the subcutaneous administered maintenance study.
199. Fig.1 of the Patent is described as a diagrammatic representation of the UNIFI study design and contains the following abbreviations: W8 = Week 8, W16 = Week 16 and LTE = Long-term Extension.
200. Fig.1 shows that the induction stage runs from week 0 to week 8 and the maintenance stage runs from a ‘new’ week 0 to week 44.
201. [0212] explains that only patients who demonstrated a clinical response during the induction study were given a place in the maintenance study. [0214] describes the primary and secondary endpoints of the maintenance study. It states that the primary endpoint was clinical remission at week 44. The secondary endpoints included maintenance of clinical response through week 44 and CSFCR at Week 44.
202. In the Results section [0221] states:
Applying both global and US-specific definitions of clinical remission, the proportions of subjects achieving corticosteroid-free remission for at least 90 days prior to Week 44 was significantly greater (p<0.01) in the ustekinumab q8w and q12w groups compared with that in the placebo group.
203. Table 6 at [0311] is entitled “Summary of Key Efficacy Measures in UNIFI-M (week 44; 52 weeks from initiation of the induction dose)”. It shows that 42% of patients who were receiving 90mg ustekinumab every eight weeks achieved CSFCR at week 44 (with a p value of <0.001) whilst only 23% of those in the placebo group achieved CSFCR at week 44. The table also shows that 38% of patients who received 90mg ustekinumab every 12 weeks achieved CSFCR (with a p value of < 0.05).
204. Claim 1 of the Patent is as follows:
1. An anti-IL-12/IL-23p40 antibody for use in a method of treating moderately to severely active ulcerative colitis (UC) in a human subject in need thereof,
wherein the antibody comprises a heavy chain variable region of the amino acid sequence of SEQ ID NO .7 and a light chain variable region of the amino acid sequence of SEQ ID NO:8, wherein the method comprises:
a. intravenously administering to the subject the antibody in a first pharmaceutical composition at week 0 of the treatment at a dosage of 260 mg for subjects with body weight ≥35 kg and ≤55 kg, 390 mg for subjects with body weight >55 kg and ≤85 kg, and 520 mg for subjects with body weight >85 kg, and
b. subcutaneously administering to the subject the antibody in a second pharmaceutical composition at a dosage of 90 mg per administration, at week 8 of the treatment, and in a maintenance dose every 8 weeks or every 12 weeks after the treatment at week 8, wherein the subject is in corticosteroid-free clinical remission at least 44 weeks after week 0.
Claim interpretation Issues/Construction
205. The claim interpretation issues basically go to the attack of anticipation by the Ochsenkühn Poster. They do not matter to obviousness.
Legal Principles
206. The principles of claim interpretation are well established. See Saab Seaeye v Atlas Elektronik [2017] EWCA Civ 2175, adjusting slightly the principles set out in Virgin Atlantic v Premium [2009] EWCA Civ 1062 in the light of Actavis v Lilly [2017] UKSC 48.
207. Relying on the judgment of Arnold LJ in Sycurio v PCI-PAL [2024] EWCA Civ 606 at [3] and [4], Samsung’s written opening submissions said (or at least I read them to say) that if the meaning of claim language is clear on its face then it is not legitimate to go to the specification so as ultimately to arrive at a different meaning.
208. I disagree with this, and in his oral opening submissions Counsel for Samsung backed away from it.
209. In my view it is not appropriate to look at the claims alone, using the specification only if the claims appear ambiguous: see point [5](iv) in Virgin/Saab. Arnold LJ in Sycurio was not saying that it was, as is clear from [3] where he said “Thirdly, the claim must [my emphasis] be interpreted in the light of the description and drawings.” All he was saying was that the claim language is a powerful consideration; that there is a limit to the degree to which the specification can extend or cut it down; and that (at [4]) the claims will usually but not always be interpreted to cover something said to be an embodiment of the invention, emphasising that the claim language may be strong enough to achieve such a result if it is sufficiently clear.
210. The unfortunate potential result of Samsung’s initial submission can be seen in this case in relation to the “week 0” point, which I address below. If the claim had to be addressed all on its own the skilled person would no doubt conclude that “week 0” means the same thing throughout the claim. But in fact, as discussed below, “week 0” is used in two ways in the specification, to refer to week 0 of the induction phase and week 0 of the maintenance phase. In my view it would offend common sense for the skilled person to try to understand the claim without this potentially very important context (that does not mean that either side’s contention about the ultimate meaning is right or wrong - this is a point about what would go into their thinking).
211. There were a number of disputed issues of claim interpretation. The parties referred to them differently and the issues overlap. I will deal with each one identified by either party. In some instances I think that the dispute was apparent rather than actual, but I will nonetheless address them all.
“For use in a method of treating”
212. Janssen argued that this requires that the therapeutic effect is actually achieved, and I agree. It also argued that the effect need not be achieved in all patients. I agree with that, too. These were points on which I do not think the parties disagreed.
213. Janssen identified a possible difference between the parties because Prof Bloom had said that he envisaged that what was required was that the relevant therapeutic effect was achieved in “a sizeable proportion of patients”. Janssen said that requirement was stricter - a statistically significant proportion of patients compared to placebo.
214. I agree that the therapeutic effect has to be a real one and that it has to be caused by the treatment. So if all that happened when the drug was given was that no more patients got better than they would on placebo, the claim is not satisfied.
215. I do not however agree that any particular measure of statistical significance is required by claim 1 since none is stated (by contrast with claim 10).
“Corticosteroid-free clinical remission”
216. Samsung submitted that this language did not require the patient to have been on steroids prior to the treatment beginning and weaned off them, only to not be on steroids at the relevant time following treatment.
217. That is the natural meaning of the words. Prof Michetti agreed with it, and the evidence was that there were relevant patients who would be in need of biologic treatment but who had never been on steroids.
218. This makes claim 1 an easier target for the prior art attacks in relation to expectation of success.
“Remission” and “Moderately to severely active ulcerative colitis”
219. Janssen submitted that both these had to be assessed by the Mayo score. Samsung argued that the claim is not so specific, that other scoring methods were known, that those other methods are recognised in the Patent (at e.g. [0047] and [0025]), and that in dependent claims the Patent does specify the Mayo score specifically.
220. In my view Samsung is right about this. Janssen responded by itself referring to [0047], but that cannot help it given that the same paragraph also refers to other methods, as Samsung said. Janssen had no answer to the point about dependent claims and it also seems to me perfectly rational that the patentee would not want to tie itself to any particular scoring system, so as to have a practical way to prove infringement if a competitor used a different method, for example. I agree that it was CGK that the Mayo score was what was usually used in formal clinical trials, but that does not necessarily mean that that is the scope of the claims and it cannot override the other points to which I have referred.
“At least 44 weeks after week 0”
221. Following an Order that I made at a case management hearing in October 2023, the parties were required to plead out their positions on this claim feature, which is the one of greatest debate.
222. The parties’ contentions were as follows:
a) Samsung said that the feature requires that CSFCR must be present by 44 weeks after week 0 and that it was not a requirement that CSFCR still be present at 44 weeks (in other words it would be good enough for the claim if there was remission at e.g. 12 weeks and the patient then relapsed).
b) Samsung said that “week 0” meant the same thing throughout the claim and referred always to the start of the induction phase.
c) Janssen said that the feature required that CSFCR be achieved at 44 weeks after week 0.
d) Janssen also said that week 0 for the assessment of CSFCR was the start of the maintenance phase, and that week 0 in sub-paragraph a. of the claim means the start of the induction phase, i.e. week 0 is used in two different ways in the claim.
223. The EPO examiner of the Patent thought that the claim feature required CSFCR to be present for a period of 44 weeks from the induction dose. Neither party before me contended that that was correct and I respectfully disagree with it.
224. On any view this feature is not well or clearly drafted, but it is not submitted by either side that it is so unclear that it cannot be given a meaning or that the difficulty of interpretation makes it invalid, and I think that despite its suboptimal phrasing it can be understood well enough.
225. I think the skilled person would observe the actual protocol used in the Patent (seen in Example 2) and graphically at Fig 1. They would see that Week 0 was used in two different ways, but that the aggregate effect of the numbering was that ultimate clinical success was assessed at one year (8 + 44 = 52) from the start. They would know this - assessing results at one year - was a typical approach in the field and the way that UNIFI was set up. That is not to say that they would think the claim was exactly limited to the UNIFI Protocol and indeed they would know that in some ways it was less specific, but they would see the UNIFI Protocol as important context. Once they had this context I do not think they would find the claim hard to understand or find it odd that “week 0” was used in two different ways.
226. Samsung’s points were mostly minor textual ones and could not really cope with this contextual view. For example, it said that if the claim had had the scope contended for by Janssen then it could have been written better. I agree, and such is often the case, but it attracts less weight than Janssen’s practical approach.
227. Samsung did have one more practical point, which is that the skilled person would think that it was desirable to get patients into CSFCR sooner rather than later. Janssen did not dispute this as a clinical matter. However, I do not think it helps Samsung or overcomes the other points in Janssen’s favour. The claim does not deprecate earlier achievement of a treatment effect, or exclude from the definition of success any patients solely on the basis of their achieving remission earlier. A patient who achieved CSFCR early and maintained it at week 44 (whatever that might mean) would still satisfy the requirement. The claim definition is just not really about earlier success; it is about providing a relatively simple metric to determine whether long-term treatment has been achieved.
228. I do not overlook the claim language itself, of course. Neither side’s interpretation fits all that well with the rather odd “at least 44 weeks”, but that is a reflection of the poor drafting: it is not as if the words have a clear ordinary meaning on their own. However, I think Janssen’s approach is the better fit, since it focuses on a time point - as reflected in “at” - and calls for assessment after a significant minimum time - as in “at least”. Certainly I do not think the wording has any flavour of “prior to” or “by”, the approach for which Samsung contends.
229. Finally, I think it is possible that a better interpretation would be that CSFCR has to be observed (to the standard and with the frequency required) at 44 weeks or at some time thereafter. This would preserve the focus on long term treatment while implying that there would be infringement if the end point was measured at exactly the standard 52 week (total) time point, or was only looked for and found later (when the advantage of the Patent would still be achieved). It would also give more meaning to “at least”. However, neither side argued for it and in practical terms for the decision at this trial, it does not matter because the Ochsenkühn Poster does not meet either meaning.
Validity
Anticipation - the law
230. There was no dispute about the general standard for anticipation: clear and unmistakable directions. The suitability of ustekinumab for treating UC is a functional technical feature of the claim, and Samsung accepted that it is not an anticipation of such a claim merely to assert a treatment effect. Nor, Samsung accepted (based on T239/16), is a clinical trial protocol an anticipation of such a claim, although it may render it obvious (depending on the evidence: see below). The treatment effect actually has to be demonstrated by the prior art.
Obviousness - the law
231. Again, there was no dispute about the generally applicable principles: see Actavis v ICOS [2019] UKSC 15 at [52] - [73].
232. Actavis v ICOS explains that obviousness is a multifactorial question, and usually requires e.g. expectation of success to be balanced against the motivation to find a solution, the cost and resources required to carry out a trial or experiment, the other avenues of research available and so on. In the present case the parties focused almost exclusively on prospects of success. I asked both sides about the potential significance of other factors, specifically motivation, and cost or burden of taking matters forward. Although Janssen accepted that there was a material motivation (which I am sure is correct, given that the existing biologics all failed in some patients), both sides said these factors were not major ones and that assessing the cost and effort of taking matters forward could not meaningfully be factored in because clinicians were not able to use ustekinumab for UC at the Priority Date because it was not licensed. I find it a little unsatisfying to assess what is a reasonable expectation of success without attempting to balance in other matters more fully, but since that is how it was argued I will proceed on the parties’ basis (I do agree that Janssen could get no assistance from the fact that doing the sort of clinical trial in the Patent would be a big effort, for reasons explained by Arnold J as he then was in Hospira v Genentech [2015] EWHC 1796 (Pat) at [120]). In the end it does not matter because I find that the expectation of success from the Sands Slides was very strong and the expectation from the Ochsenkühn prior art was lacking: efficacy in UC remained just a hope (see Hospira at [116] about the distinction between a hope and an expectation, the former not being good enough for obviousness).
233. Samsung relied on T96/20, which is summarised in the Case Law of the Boards of Appeal of the European Patent Office, 2022 Ed as saying that:
In T96/20 The board considered that the announcement of a detailed safety and efficacy clinical trial protocol for a particular therapeutic and disease provided the skilled person with a reasonable expectation of the success of this particular therapeutic, unless there was evidence to the contrary in the state of the art.
234. And in T239/16 at 6.5 the Board said:
The board considers that the mere fact that an active agent selected from the group of bisphosphonates is being tested in a clinical study for the treatment of osteoporosis (as disclosed in document (55)) leads to an expectation of success, due to the fact that clinical studies are based on data obtained by preclinical testing both in vitro and in animals and require authority approval which takes ethical considerations into account. This means in the present case that the skilled person would expect all study arms to treat osteoporosis effectively, unless he was dissuaded from this by the prior art
235. I do not think this can be a presumption that is applicable in all circumstances or a general rule to be applied blindly. It depends on the facts. It may be relevant in an individual case that there is a clinical trial ongoing, especially if it is a major one, in phase III. The skilled person would be likely to assume that those sponsoring and undertaking the trial had reasons based on earlier work, or analysis of the mechanisms at work, for having an expectation of success. However, I think that greater importance would usually be attached by the skilled person to the concrete evidence about prospects of success that they could understand and analyse themselves. Just basing their expectation on the assumption that the trial had an underlying justification about which they would not know the details, would be potentially shaky for the skilled person. I also think a court should be careful about (effectively) delegating the decision on obviousness, even in the name of a rebuttable presumption, to the committees that approved a clinical trial: see Sandoz v Bayer [2024] EWCA Civ 562 (I did not hear argument specifically on this authority). I can see that in the case of a phase III trial the skilled person would specifically be able to infer, in most cases, that a phase II study had shown some efficacy and this might be a significant factual matter, but in the present case, in fact, the skilled person would know that there was not a phase II trial of ustekinumab in UC. This illustrates that it must all depend on the specific facts.
The Ochsenkühn Prior Art
236. The Ochsenkühn Abstract and Poster describe results from the same study, a retrospective analysis of patients with UC treated with ustekinumab as a “rescue therapy” (a last line of treatment following failure of other treatments).
237. The patients in the study started treatment between 2016 and 2017, and Prof Ochsenkühn gave evidence that the observation period continued into 2018.
238. Prof Ochsenkühn later published a paper, in 2020, Ochsenkühn et al. “Clinical outcomes with ustekinumab as rescue treatment in therapy-refractory or therapy-intolerant ulcerative colitis” United European Gastroenterology Journal 2020; 8(1): 91-98, (“Ochsenkühn 2020”) reporting on the same study. Ochsenkühn 2020 is post-priority and not itself relevant to validity but it forms part of the factual events in the context of which Prof Ochsenkühn’s evidence must be assessed and Janssen argued that it shed light on what he had done earlier in the work.
239. The Ochsenkühn Abstract was prepared for the 13th Congress of the European Crohn’s and Colitis Organization (ECCO) and was published on 16 January 2018.
240. The Ochsenkühn Poster was said by Samsung (and Prof Ochsenkühn) to have been presented at the Digestive Diseases Week Conference in Washington DC between 2 and 5 June 2018 (“DDW 2018”). Janssen disputes that the Ochsenkühn Poster relied on by Samsung was in fact the poster presented at DDW 2018. I address this issue below. There is no dispute that whatever was presented at DDW 2018 became part of the state of the art; it was open to read for those attending the conference, with no obligation of confidence or the like.
241. The Abstract and Poster have a number of differences, but it was agreed that the Poster presents more data than shown in the Abstract. The Poster provides data regarding the condition of patients nine months after the start of ustekinumab treatment, whereas the Abstract only reports on six months, and the Poster’s data reports on 19 patients whilst the Abstract reports on 17 patients. The Poster explicitly states that at three, six and nine months, all bar one of the patients in remission were free of steroids, although eight of them started with steroids.
242. It was Samsung’s case that if publication of the Poster was proven, they did not need to rely on the Abstract.
243. Counsel for Janssen put to Prof Ochsenkühn a table showing differences in the documents published by Prof Ochsenkühn between 2018 and 2020 relating to the retrospective analysis study (see the table reproduced below). The differences included that the number of patients increased from 17 to 19, the number of dropouts increased from three to five, the number of patients who had a colectomy varied between two and three, and the definition of remission (CAI) decreased from ≤5 to ≤4 in the 2020 paper.
244. Prof Ochsenkühn explained that these differences arose due to the nature of the observation carried out, the nature of the disease and because the authors were not primarily trying to create a paper that would one day be published in a high-ranking journal, but instead to get patients access to a drug that was likely to be successful for UC, so they were not concerned by adding patients to the study.
245. These differences do look odd at first sight. For example, the number of colectomy patients being three then dropping to two, then going up again does not at first sight seem to make sense. However, Prof Ochsenkühn explained that a patient would be counted in the colectomy number when he or she had been referred for surgery (and was therefore included when the number was reported as three) but not counted in the event they did not actually undergo a colectomy, but only a less drastic procedure (so the number was reduced to two). I accept this.
246. Likewise, other numbers for remission could change, as to the totals or for individual patients, because they were based on assessing patients’ case files. So, for example, patients’ files were reassessed for Ochsenkühn 2020. I accept this part of Prof Ochsenkühn’s explanation as well.
247. This all emphasises the subjective nature of what was done and potentially affects any assessment of the significance of the results, to which I will return, but it leads me to reject the apparent oddities with the data over time as undermining in any way Prof Ochsenkühn’s evidence about what was in fact done.
Disclosure of the Ochsenkühn Abstract
248. The aim is described in the ‘Background’ section as being “[t]o assess the clinical outcomes achieved with ustekinumab as rescue treatment in therapy-refractory or -intolerant UC.”
249. The ‘Methods’, including the primary outcome and the definition of clinical remission, are described as follows:
A retrospective data analysis was performed in 17 UC patients of our tertiary referral center who received ustekinumab between 2016 and 2017 as rescue therapy. All patients were intolerant or refractory to purine-analogues, TNF-antibody therapy, and anti-integrin vedolizumab. To all patients ustekinumab was provided as a rescue treatment after colectomy had been offered to them as only other option. The primary outcome was achievement of clinical remission at 3 and 6 months. Clinical remission was defined as score of ≤5 points in the modified Truelove and Witts colitis activity index (CAI).
250. The ‘Results’ are described as follows:
A total of 17 UC patients were treated with ustekinumab. All patients (17/17) previously had been steroid-refractory or -dependant and had recently failed all of the following drugs: purine-analogues, anti-TNF-antibodies and anti-integrin- antibodies. Of those, 41% (7/17) had failed infliximab and either golimumab or adalimumab, and 29% (5/17) had also failed i.v. ciclosporine. At the start of the rescue therapy, 65% of patients (11/17) had moderately or severely active disease and 35% (6/17) were in remission, but had intolerable side effects under TNF- or integrin blocking treatment, which had to be stopped. Therefore, the CAI at the start of the therapy ranged between 1 and 11 with a median of 8. All patients received ustekinumab as approved for Crohn's disease (6 mg/kg body weight as an infusion and 90 mg ustekinumab as s.c. injection every 8 weeks). Median follow-up was 27 weeks (range: 15-40). In two patients therapy was stopped due to refractory disease at months 6 and 24 and in 1 patient, therapy was stopped due to drowsiness at week 4. All 3 patients underwent colectomy. Median CAI at 4 weeks was 5 points (range 1-8). Median CAI at 3 months was 4.5 points (range 0-9). Median CAI at 6 months was 2 points (range 0-7). Including the three drop-outs, clinical remission was achieved in 65% (11/17) at 1, 3, and 6 months, whereas only 35% (6/17) of patients were in remission at the start of the study.
251. The ‘Conclusions’ are:
Ustekinumab was effective as rescue medication in therapy-refractory or -intolerant UC in a large IBD referral centre. It seems possible that large ongoing trials will confirm our findings and ustekinumab could become a new therapeutic option for refractory UC.
Presentation and Disclosure of Ochsenkühn Poster
252. The Ochsenkühn Poster can be found at Annex 2 to this judgment. Samsung alleged that the Poster was presented at the DDW Conference in Washington DC between 2 and 5 June 2018.
253. As mentioned above, it was agreed that the Poster presents more data than the Abstract. It provides a graph tracking the data of individual patients, it gives data for two further patients (19 in total), and it contains data regarding the condition of patients nine months after beginning ustekinumab treatment (three months more than in the Abstract). The most significant disclosure is about the use of steroids. The Poster states:
At 3, 6 and 9 months all but one of these patients in remission were free of steroids, although 8 of them started with steroids.
254. As I have mentioned, there was a dispute between the parties as to whether or not the Ochsenkühn Poster presented at DDW 2018 was identical (in terms of content) to the form relied upon by Samsung.
255. Janssen does not dispute that Prof Ochsenkühn attended DDW 2018 and presented a poster, but it submitted that the burden of proving that the Ochsenkühn Poster relied upon by Samsung was the same as the version made available to the public at that conference lies on Samsung and that Samsung had not met it.
256. Prof Ochsenkühn proved his attendance at DDW 2018 by means of various documents, such as a boarding pass, and also by some “selfies” which he took to show his wife. The authenticity of the photos is not disputed. In one, Prof Ochsenkühn appears in front of a poster but his body blocks part of the right-hand side of it. This was referred to at trial as “the Selfie”.
257. Importantly, the wording upon which Samsung relies regarding steroid use at three, six and nine months cannot be seen in the Selfie.
258. Prof Ochsenkühn acknowledged in his witness statement that there are some formatting differences between the Ochsenkühn Poster relied on by Samsung and the poster shown in the Selfie. He said that the differences were immaterial and had been made by the poster printing agency he used to print the poster, as recommended by the conference organisers in Washington.
259. It was put to Prof Ochsenkühn by Counsel for Janssen that at the time of preparing his witness statement, he had no specific recollection of the DDW 2018 conference. He explained that once he started thinking about the conference, he remembered the “big picture” of the conference and since many conferences work in the same way, it was easy for him essentially to jog his memory about what he did in 2018.
260. Prof Ochsenkühn explained that the version of the Ochsenkühn Poster relied upon by Samsung is the version he sent to Samsung’s solicitors, a PDF he saved as the final version of the poster he intended to present at the conference. Prof Ochsenkühn identified in his written evidence various PDF versions of the Ochsenkühn Poster. In his oral evidence he said that PowerPoint versions had also existed but that he did not circulate them, so that changes could not be made.
261. When asked in oral evidence how it was that the printers had been able to edit the PDF so as to make the formatting changes referred to above, he said that he probably sent the PowerPoint version to the printers. He had not mentioned this in his witness statement and no PowerPoint version has been disclosed.
262. The various differences between the Ochsenkühn Poster as relied upon by Samsung and the version in the Selfie were put to Prof Ochsenkühn. These differences include the layout, alignment and the inclusion of a box around the graph. Prof Ochsenkühn explained that he had checked that all of the most important things were present on the Poster and that everything was present. He stated that “the content [of the Poster] is 100% the same as it is in this PDF that was provided here”.
263. Prof Ochsenkühn’s account fits extremely well with the facts that are not disputed, or not capable of serious dispute:
a) At the time of DDW 2018 Prof Ochsenkühn had nine month data including in relation to steroid status. It was not put to him otherwise and it is proved by the PDF (at TO-4). It also fits with the chronology from the Ochsenkühn Abstract onwards.
b) Prof Ochsenkühn presented a poster at DDW 2018 which included nine month results of at least some kind. That can be seen in the Selfie.
c) Although there are differences as to formatting, alignment and presentation (the border) there is no inconsistency as to contents between the Selfie poster version and the Ochsenkühn Poster as relied on by Samsung. Everything that can be seen in the former appears in the latter.
264. As a matter of inference from the basic facts, Prof Ochsenkühn’s account is also inherently highly credible:
a) The nine month data was a key part of what he wanted to present.
b) It is inherently credible that he would carefully check it when he got the printed version for display.
c) It is inherently not credible that the printing service would intentionally change the contents (as opposed to the format).
d) It is inherently not credible that if the contents were changed, Prof Ochsenkühn would not notice.
265. I also rely on the fact that Janssen has not put forward any sensible explanation for what might be on the right-hand side of the poster in the Selfie other than the equivalent part of the Ochsenkühn Poster PDF version. There is clearly something there - what else could it be? It is inherently most improbable that there is something there different from what Prof Ochsenkühn intended and that he did not notice and does not remember now.
266. I find that Prof Ochsenkühn’s recall of DDW 2018 is good, and reliable. It was not put to him that he is biased in Samsung’s favour (although he has given evidence for it on the same point in other proceedings) or that he lacked independence generally. I consider that he was telling the truth. His account is also supported by and consistent with the primary facts that are not disputed or capable of dispute, and is inherently credible for all the reasons given above. Almost the only wrinkle in his account is that he did not mention the PowerPoint means by which the printers may have been able to make changes, but this is trivial in the overall context and is not a fact inconsistent with his account (it is clear that the printers did make changes, no other explanation having been suggested) only, at most, a failure of recollection of the finer detail.
267. I should also mention that Janssen submitted that Prof Ochsenkühn said that he was confident the steroid results were in the Poster because he checked against the Abstract, and that was a serious flaw in his evidence given that the steroid data is not in the Abstract. I do not think that is a fair interpretation of his evidence as a whole, although there is one particular answer that reads that way. I think he meant that anything that was important in the Abstract went into the Poster, not that the Poster only contained the same as the Abstract. Elsewhere he quite clearly said that he knew the steroid data was in the Poster because it was important, and I accept that. On a related point, there was some argument about the fact that a later Abstract did not contain the steroid data and over whether that constituted a pattern of some kind, but it was not really explored with Prof Ochsenkühn and it would not affect my conclusion anyway.
268. I find for Samsung on this factual issue. The information in the Ochsenkühn Poster as pleaded was made part of the state of the art at DDW 2018, in a trivially different format from the pleaded version.
Ochsenkühn Abstract - assessment of the strength of the evidence for efficacy
269. Janssen submitted that there are a number of limitations with the analysis reported in the Abstract which would lead the skilled person to not place much, if any, weight on it at the Priority Date. These boiled down to:
a) The skilled person would not consider a retrospective analysis to be particularly reliable. The experts agreed that retrospective analysis is a less reliable way of assessing patients than forward-looking studies. Janssen also argued that this allows for re-assessment of patients (which Prof Ochsenkühn confirmed did happen), which is a concern as it allows the data to fit to conclusions.
b) The data being analysed is from a single centre study which was unblinded, with no control group, no placebo and a small number of patients (17).
c) The authors used the modified Truelove and Witts index (CAI) which Janssen submits is a less preferred index because it is based on subjective factors. Prof Bloom agreed that factors such as “general patient well-being” are difficult to assess retrospectively. Janssen also submitted that normally remission would be measured as ≤ 3, but the authors measure it as ≤ 5.
d) It is unclear which patient cohort is being treated as the entry criteria for the study are not identified and it is unclear what the authors mean when they state that 65% of patients at the start of therapy had “moderately or severely active disease”. The CAI score of patients at the start of the study is said to range from 1 to 11 with a median of 8, which the skilled person would understand as only mild to moderate disease. Counsel for Janssen pointed to a letter written to the editors of the journal in which Ochsenkühn 2020 was published, where this criticism was made.
e) The reference to ‘rescue treatment’ is confusing because that term is usually used in the context of acute severe UC.
f) There is no suggestion that a wash out period was implemented to remove the effect of any previous treatments.
g) There is no information about whether any of the patients were on steroids or were in CFCR.
h) The skilled person would treat the reported results with caution, such as the remission rates which Janssen say are unrealistically high (higher than any previous biologic drug), there is rapid response at one month and the remission rate is apparently stable.
270. When the issues with the Abstract were put to Prof Bloom in cross-examination, he stated that “you could not make any reliable conclusion from that study alone; I absolutely agree.” Prof Michetti for his part said that the Ochsenkühn work made a “1% or 2% difference”; he was not trying to be qualitative, just giving a flavour of the strength of the evidence. So I think that while their ways of expressing it were different, the experts agreed in substance.
271. I have also referred above to the fact that it emerged that Prof Bloom’s assessment of whether or not ustekinumab was shown by the Ochsenkühn prior art to be effective for UC depended on combining it with the Sands Slides. He agreed that the conclusions from the Ochsenkühn studies were “very much weaker” without the Sands Slides, saying that the latter were “a really important part of persuading the skilled person and affecting his or her expectations”.
272. My conclusion is that the Ochsenkühn Abstract does not show that ustekinumab is actually effective in treating UC and nor does it provide a real expectation that such was the case. It just supports a modest degree of increase (relative to the CGK) in the hope that such might be the case. I appreciate that the first sentence of the Conclusions asserts efficacy but it has to be seen in the context of the very next sentence which walks matters back very considerably, and in the context of the evidence as a whole.
273. Additionally, the Ochsenkühn Abstract does not allow any clear conclusion to be drawn about the severity of UC in the patients in the study. It is very unclear and entirely possible that some of them had only modest disease severity.
Ochsenkühn Poster - assessment of the strength of the evidence for efficacy
274. The Poster has three months more data than the Abstract and contains steroid information but all the methodological limitations are unchanged. I do not think the extra data made any material difference to the experts’ views as I have explained them in the previous section. So my overall conclusion is the same.
Anticipation by Ochsenkühn Poster
275. Since I hold that the Ochsenkühn Poster does not demonstrate efficacy in UC, anticipation does not arise. However, I will make some factual findings about the details in case I am wrong on that.
276. On Samsung’s construction, claim 1 of the Patent would be anticipated if at any time before 44 weeks after the first IV dose, a patient is in corticosteroid-free clinical remission. I have rejected that construction, but again I will make factual findings in case I am wrong on that, too.
277. The Ochsenkühn Poster discloses that 14 of 19 patients were in remission at 9 months (39 weeks) after the first IV dose, and all but one of those patients were free of steroids i.e. 13 of 19 patients were in CSFCR.
278. In opening submissions, Counsel for Samsung explained how the graph in the Ochsenkühn Poster (reproduced below) shows that at least one patient went from moderate to severe UC to CSFCR at week 39, and submitted that was sufficient for the purposes of anticipation. The analysis, by reference to the graph below, is as follows:
a) Patients with a CAI score from 10 to 12 had moderate to severe UC. It can be seen from the graph that three patients started with moderate to severe UC, one with a score of 10, one with a score of 11 and one with a score of 12.
b) If one follows the entries in the graph for those patients, one sees that at month nine (39 weeks) they have scores of 5, 3 and 1. The Poster described clinical remission as those with a score of ≤5 points (although it became clear during the evidence that this was a higher cut off point than usual, with the more common remission definition being a score ≤3).
c) Knowing that all but one of those in remission at 39 weeks (i.e. all but one of those with scores below the black dotted line on the graph at month 9) were also free of steroids (i.e. in CSFCR), having two patients who went from moderate to severe UC to remission, for example the patients who started with CAI scores of 11 and 12 and ended at 1 and 3, is enough, because it means at least one of them was in CSFCR (even if the other was the one patient who was in remission but was not free of steroids).
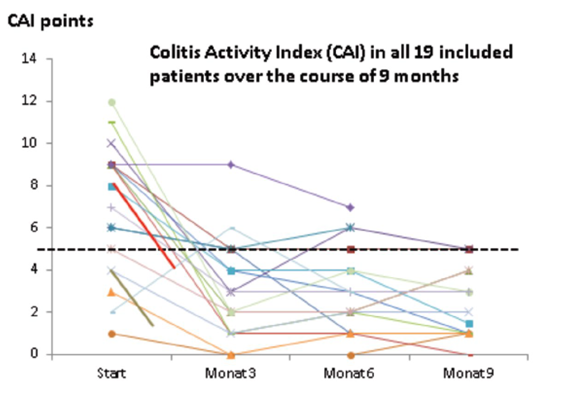
279. I agree that there was one such person having the profile that Samsung alleges. However, the fact that a patient was given ustekinumab after which their condition improved in that way does not necessarily imply, or disclose, that the drug was effective in that patient (let alone generally). The patient could easily have been someone who improved by chance, or because of the placebo effect. The single patient does not imply that ustekinumab was in fact effective, when the work as a whole fails to show that.
Obviousness over the Ochsenkühn work
280. As I have explained above, obviousness all turns on whether there was a reasonable expectation of success. I have set out the reasons above why there was not one; there was only a hope, and that applies to the Abstract and to the Poster.
Obviousness over Sands Slides
281. The Sands Slides, as is mentioned above, report on the induction phase of the UNIFI trial.
282. The Sands Slides come from an impeccable source and would be taken very seriously by the skilled person.
283. Slide 2 points out that ustekinumab is approved for moderate to severe psoriasis, psoriatic arthritis and Crohn’s disease. The skilled person would know this already. The slide also gives the IL-12/IL-23 rationale for ustekinumab but again the skilled person would already know this.
284. Slide 3 explains that the induction study was to evaluate IV ustekinumab in patients with moderately to severely active UC who had an inadequate response to, or were unable to tolerate:
a) One or more biologics (i.e. one or more TNF blockers and/or vedolizumab) or
a) Corticosteroids and/or immunomodulators.
285. The study design is described in slide 4:
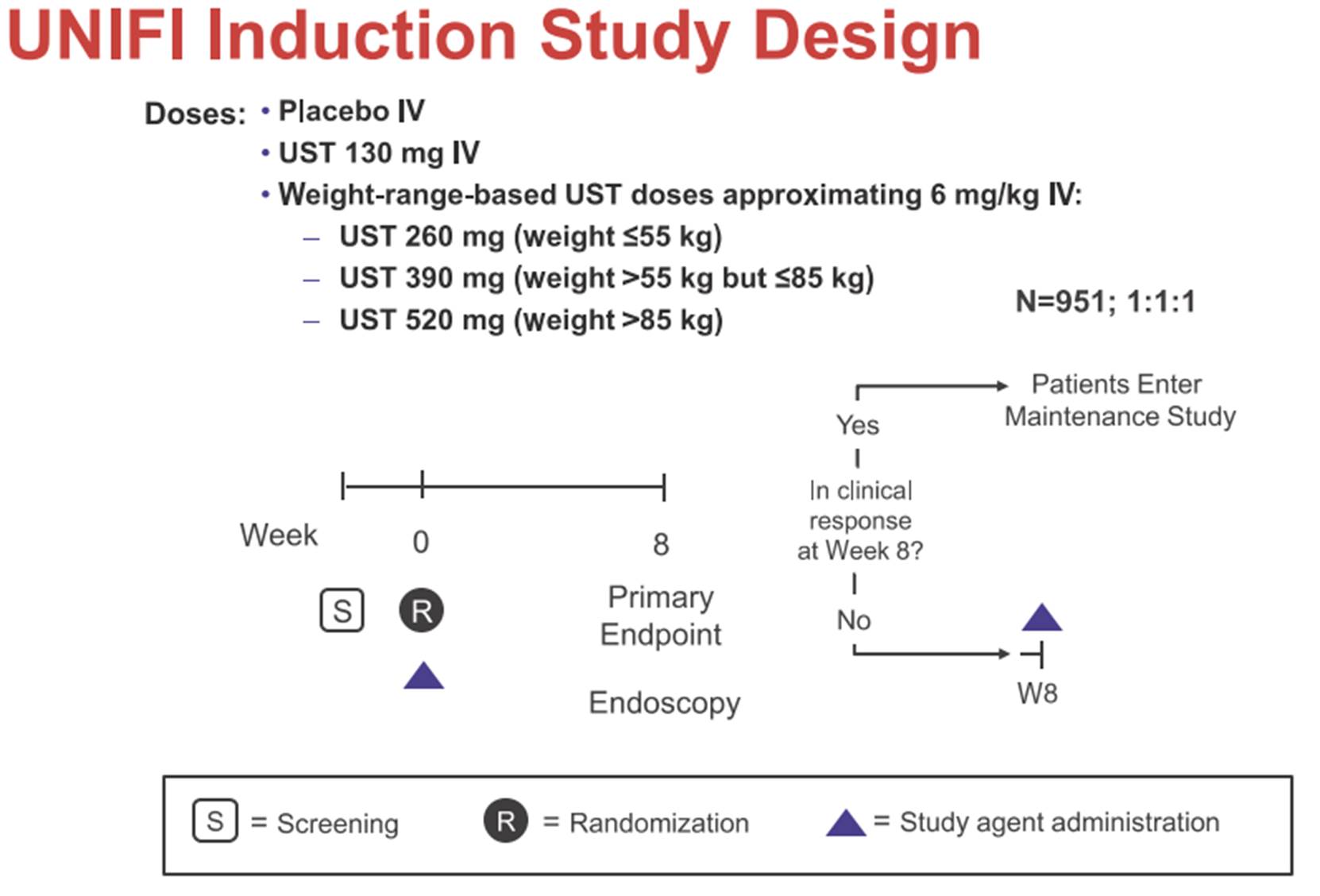
286. Points to note are that:
a) Patients received either placebo, a fixed 130mg dose of ustekinumab, or a larger weight-based dose.
b) If patients had a clinical response at week 8 then they would enter the maintenance phase.
c) If patients did not have a clinical response at week 8 then they received another dose of ustekinumab and were reassessed 8 weeks later, at which time they could again enter the maintenance phase, or otherwise dropped out.
d) The doses studied were the same as in UNITI-1 and UNITI-2 in CD.
287. The endpoints are described at slide 5. The primary endpoint was clinical remission at week 8, which was defined on the Mayo score. Four other metrics are explained (endoscopic healing at week 8, clinical response at week 8, change from baseline in IBDQ, a quality of life scale, at week 8, and mucosal healing at week 8); they are somewhat complex and I will not set out the detail here, although I revert to some points about them below.
288. Slide 8 gives the primary endpoint result:
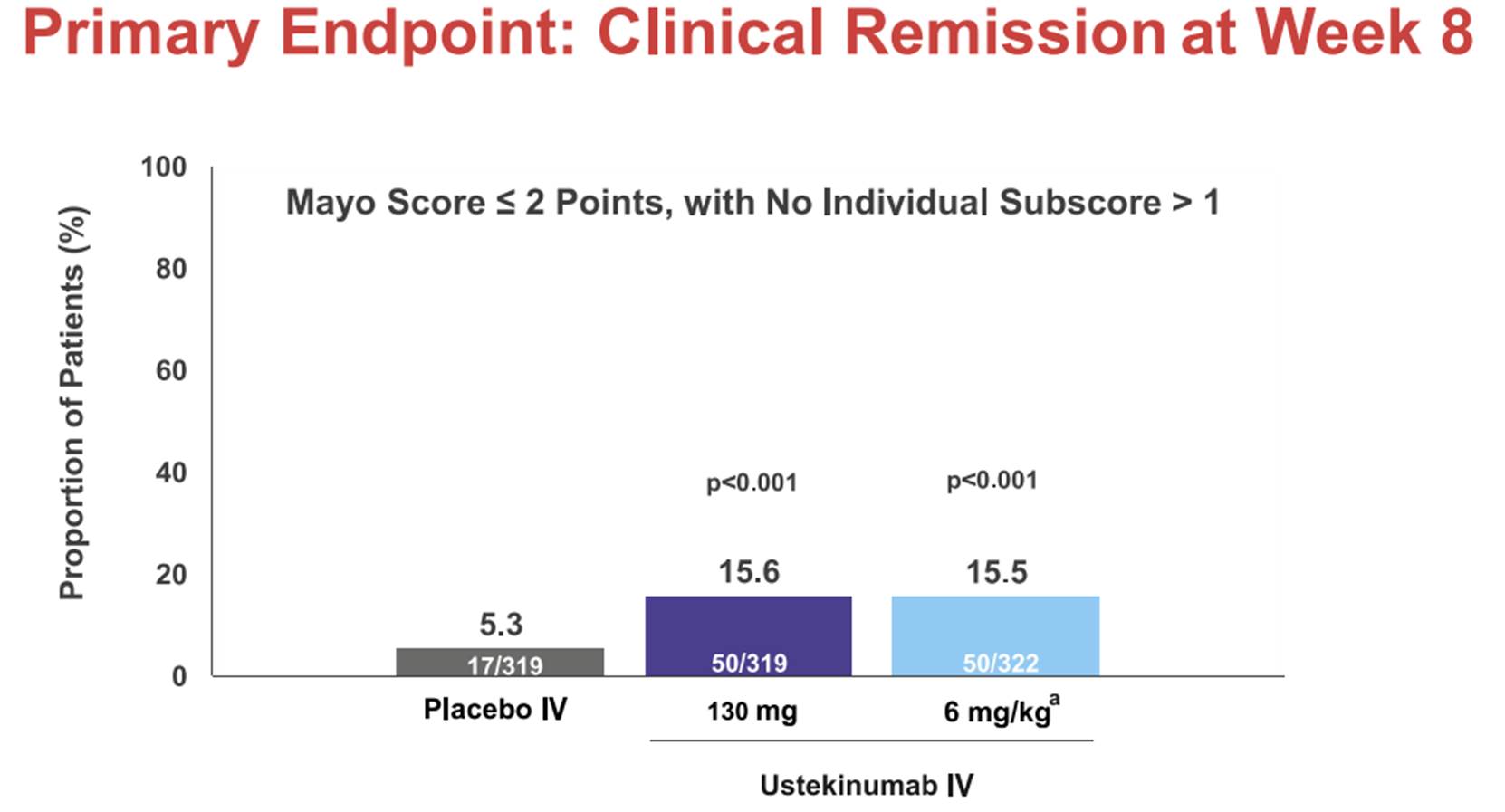
289. The results are statistically highly significant and therefore Janssen does not dispute they show a real effect for ustekinumab. The delta between placebo and either dose of ustekinumab is about 10%. There is no dose response shown, in that the effect of the drug is the same for the bigger dose (referred to as “6mg/kg”, a somewhat misleading label, but properly explained in footnote a.) and the smaller (fixed 130mg).
290. Slide 9 gives remission figures split out into the biologic failures and the no biologic failures. As would be expected, the percentage in remission is higher in the latter group, although the delta with placebo is much the same.
291. Results for endoscopic healing and clinical response at week 8 follow on slide 10, where there is a dose response for the latter but not the former. Either 51.3% (fixed) or 61.8% (6mg/kg) responded in the treatment groups. IBDQ results are given at slide 11. Mucosal healing is at slide 12 (no dose response), and partial Mayo score and Fecal calprotectin at slide 13 (dose response in each). All these results are likewise highly statistically significant.
292. The conclusions are at slide 15:

293. Samsung argued essentially that the 8 week results would have been seen as good and encouraging and, especially coming on top of the IL-12/IL-23 rationale, would give the skilled person a strong expectation of success at the end of the maintenance phase. This is a powerful argument. The key point about the Sands Slides is that they prove that ustekinumab does have an effect in treating UC; that is what the results show. The results also come from a very well regarded source and are statistically significant.
294. Janssen sought to undermine this in a number of ways. The main ones were:
a) On the basis that the 10% delta in the primary endpoint at week 8, while real, was not especially good;
b) On the basis that some of the patients in the 15% for whom ustekinumab had worked (leading to remission) at 8 weeks would drop out of remission in the maintenance phase; and
c) On the basis of there being no dose response at week 8 and, in particular, that there was a dose response at week 8 in the UNITI trial (for CD) showing that there was something unusual about use of ustekinumab in UC or at least that it behaved differently in UC and Crohn’s.
295. I will address each of these, although they overlap and interact.
10% delta not especially good
296. I accept that the skilled person would not think that 10% as such was very good. Each expert said this, in slightly different terms. But there is more to it than that.
297. First, this was a tough-to-treat population in that some patients in the study were double-failures on the other biologics. I accept Prof Bloom’s evidence on this.
298. Second, the 10% is only those patients who were actually in remission as soon as 8 weeks. There would be others who were already in clinical response by then (as seen in slide 10) or who would enter clinical response as a result of the further dose given then, and who would continue to improve and be in remission by the end of the maintenance phase.
299. Third, the 10% delta is quite similar to many other comparable drugs/situations, as shown in the following Annex to Prof Bloom’s evidence:
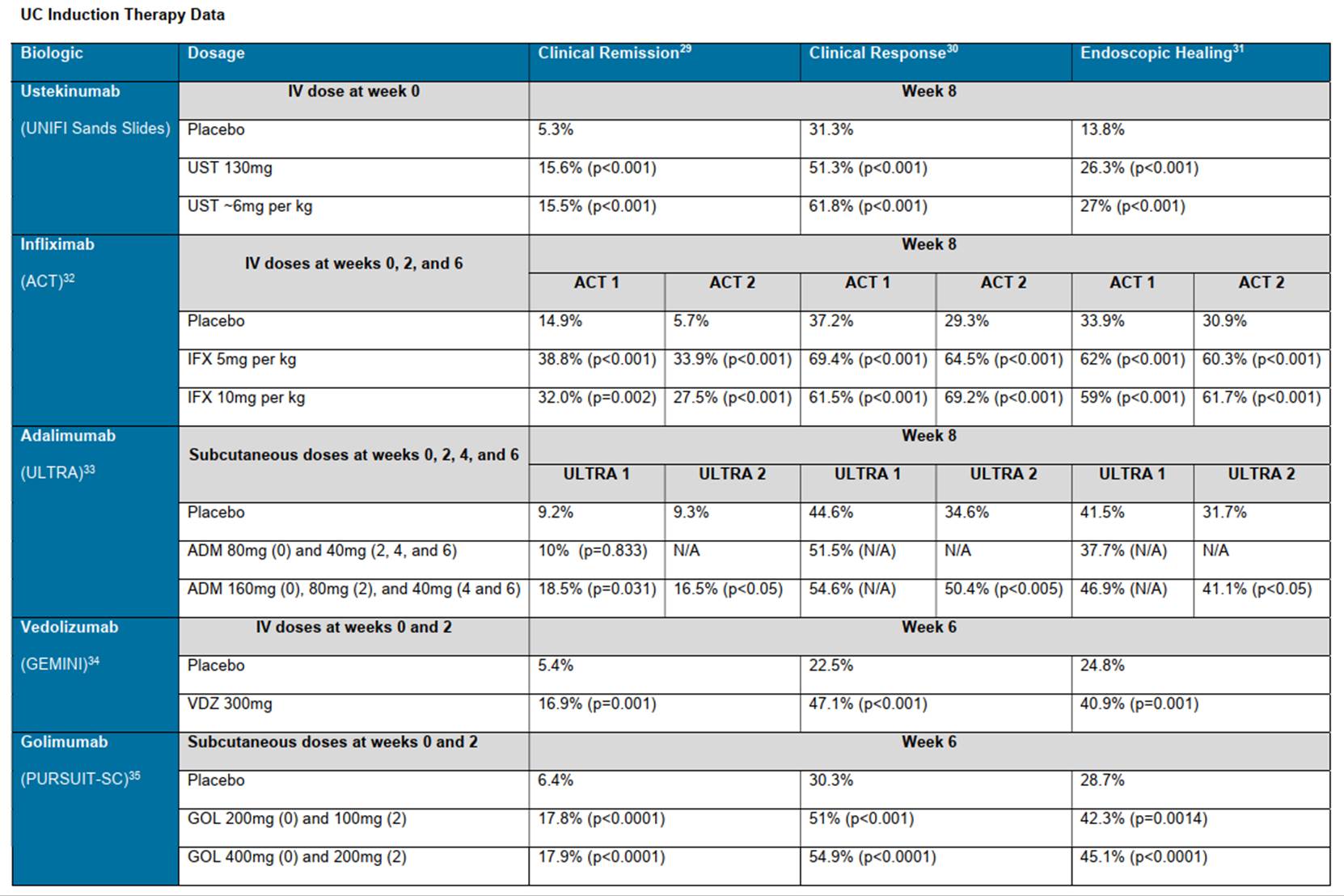
300. A notable exception is infliximab, but I accept Samsung’s answer, on the basis of Prof Bloom’s evidence, that infliximab had the advantage of being first in time and therefore being used in a population of biologic-naïve patients. Janssen submitted that ustekinumab was the second worst, better only than adalimumab but I do not accept that such a detailed ranking is the right way to look at matters: apart from infliximab, the others are all very similar.
301. Once these factors are taken into account, I think the skilled person would have been very encouraged by the 10% delta. In addition, other results are very good such as the endoscopic healing, a very difficult result to achieve in that short time.
Dropping out of remission during the maintenance phase
302. I accept Janssen’s argument that this (patients in remission at 8 weeks dropping out) in fact would happen, as it did in UNITI. Prof Michetti said that the 15% in remission at 8 weeks might be reduced by about 1/3 (on the basis of the UNITI figures), leaving only 10% in remission at the end of the maintenance phase. The answer to this lies in the first and second points I have addressed above in relation to the 10% delta: more patients would go into remission during the maintenance phase and in less challenging patient populations there would be more going into remission throughout.
303. I do not think Prof Michetti’s numbers are unreasonable (1/3 drop out, 10% left), if the point occurred to the skilled person (which I deal with below), but they are beside the point against these countervailing factors.
Different from Crohn’s Disease based on UNITI
304. The first question is whether the skilled person would compare UNIFI with UNITI, to consider similarities and differences, at all. Prof Bloom said they would, and Prof Michetti initially said they would not, but later positively relied on a comparison.
305. In my view the skilled person would make a comparison. It is only logical that they should do so given that the underlying thesis for the UNIFI trial arising from the CGK (and which Samsung relies on) was commonality of pathways and disease characteristics between UC and CD. Once results for ustekinumab in UC came in it would be foolish not to see to what extent it supported or refuted the thesis.
306. That does not mean that the skilled person would look at everything at every possible level of detail, however. I think they would be looking to make a broad conclusion about similarity. I also think they would appreciate that UNITI-1 was the trial of patients who had previously failed on biologic treatment and would see that that was important when deciding what comparison to make. They would be interested to see the delta between placebo and ustekinumab.
307. In Prof Michetti’s written evidence he focused on the overall remission level at week 8, and made the high level point that the skilled person would be looking for a benchmark of 30% remission. I reject this: the skilled person would consider that they should think about the delta between placebo and treatment and the 30% figure does not stand scrutiny in the light of the fairly typical deltas that I have identified above and did not have any other sound foundation. However, the “headline” nature of the 30% is more indicative, in my view, of the comparison that the skilled person would make between UNIFI and UNITI. It is also in line with the level of detail at which Prof Bloom first approached it.
308. Lest I am wrong about that, I will go on to consider the reaction of the skilled person to the detailed points that Janssen now makes.
309. First, dose response. Although I have held that the skilled person would compare UNITI and UNIFI at the level of overall achievement of the primary endpoint, I can see that to work out the delta between placebo and treatment they would necessarily have to look at the figures which do in fact show that there is and is not a dose response in UNITI and UNIFI respectively. I just do not think they would dig that deep and appreciate the point about dose response, in large measure because neither expert thought to do so at first. But even if they did, my view is that it would tell them that while UC and CD do not react identically to treatment with ustekinumab, they do react similarly and, critically, remission at the end of the induction phase is achieved to an appreciable degree in both. Samsung’s case and Prof Bloom’s evidence were not based on an assumption that the diseases are identical, only that they are similar enough that a treatment which succeeded in CD might well work for UC. The Sands Slides confirm this as having actually happened.
310. Second, patients dropping out of remission after week 8. In my view the skilled person probably would not actively think about this, especially once they had seen that the delta from placebo was comparable in CD and in UC. If they did think about it they would appreciate that there would be patients who would drop out of remission but that there would be many others who would be responding at week 8 (or week 16) and then enter the maintenance phase and reach remission. I do not think they would try to quantify these, but I do think, based on the evidence of Prof Bloom, that they would tend to think that dropping out of remission was relatively unlikely. If they did go into the numbers I think they would see in UNITI a quite large pool of patients in response at week 8 who went on to remission. In UNIFI they would see quite a large number of patients in response who could go into remission during the maintenance phase and this would reassure them about overall remission rates at the end of maintenance, albeit that in the end it would have to be tested.
CSFCR
311. In my view, if the skilled person thought (as I have found) that ustekinumab would be an effective treatment for UC at the end of the maintenance phase then they would also think that there would be more patients in CSFCR, compared with a placebo. There are two reasons for this.
312. The first is that in the UNIFI population, about half the patients were not on steroids at the outset. If ustekinumab was an effective treatment then at the end of the maintenance phase, those patients not on steroids to begin with and successfully treated by the drug (i.e. over and above the placebo effect) would also be in CSFCR. This is just a consequence of the fact that claim 1 does not require withdrawal from steroids for CSFCR, merely that patients treated with ustekinumab are not on steroids.
313. The second is that I accept Prof Bloom’s evidence that if the treatment were successful during the maintenance phase, as he would say was entirely predictable from the Sands Slides, and as I accept, then it would also be expected that that would enable some material number of patients who started on steroids to be taken off them. This makes sense: an extra, effective mechanism of treatment making one of the initial treatments no longer necessary.
Analysis
314. In my view, for all the reasons given above the skilled person would conclude that the Sands Slides show that ustekinumab worked for UC in the induction phase. The skilled person would have a high degree of confidence, albeit not a certainty, that it would work in the maintenance phase, too, there being no positive reason to doubt it and given that there was no track record of drugs in this field succeeding in the induction phase and then failing in maintenance phase. The skilled person would likewise positively and optimistically expect an increase compared to placebo in CSFCR, for the two reasons given above.
Three other points
315. There are three other points to cover, which I do out of completeness, two applicable to the obviousness arguments generally and one specific to the Sands Slides.
Secondary evidence
316. The first point is that Janssen relied as secondary evidence of non-obviousness on the reaction of the FDA to what Janssen proposed.
317. In December 2015 the FDA dealt with a request by Janssen, based on the similarities between CD and UC, to be allowed to do the trial which became UNIFI on the basis of a single trial instead of the normal two, and with a p value (the measure of statistical significance) of less than or equal to 0.05.
318. The FDA’s response (simplifying slightly) was that if just one trial was done then a more stringent p value of 0.001 would normally be required and that it was not prepared to excuse Janssen from that on the basis of the similarity between CD and UC.
319. Janssen said that this reflected “considerable scepticism” on the part of the FDA “that ustekinumab could be used for UC just because it worked in CD”. I do not agree. The FDA was saying that the similarity between the conditions was not so strong that it would permit Janssen to work to a more than usually lenient standard. It was not expressing scepticism about whether or not Janssen would succeed according to the normal standard.
No phase II
320. The second point arises from the fact that, as I have mentioned, there was no phase II trial of ustekinumab in UC. Janssen went straight to phase III. This potentially cuts both ways: it means that the skilled person would know there was no efficacy data, but it might indicate a strong degree of confidence on the part of Janssen and the regulators, although I have said in addressing the law on obviousness that that is a second-order matter.
321. I have borne this point in mind but I do not think it goes anywhere. On the Sands Slides the skilled person would think the absence of phase II data was irrelevant because they had phase III data to look at. On the Ochsenkühn prior art neither side said that this point had any force; Samsung might have been better off if the skilled person could have inferred from the existence of a CGK phase III trial that there had been phase II results, but they could not, because the skilled person would know there was no phase II for ustekinumab in UC.
Effect of publishing clinical trials data
322. The third point is more general. I have held Janssen’s Patent to be obvious over its own clinical trial results. Patentees can have limited room for manoeuvre when it comes to when they have to file for clinical approval, making trial protocols public, and filing a patent application. It would be a concern if the system made it hard for patentees in general to conduct clinical trials and at least have the chance to try to obtain a valid patent over a second medical use.
323. However, what happened in this case is that Janssen filed a US patent application in September 2018, which was before the Sands Slides. Its problem has arisen because it later put in claims which were not entitled to that Priority Date (owing to the addition of the CSFCR feature of the claims). So the problem is a case-specific one, not a general one.
Conclusions
324. My conclusions are:
a) The Patent is invalid for obviousness over the Sands Slides.
b) The anticipation and obviousness attacks over the Ochsenkühn Abstract and Ochsenkühn Poster fail.
325. I will hear Counsel as to the form of Order if it cannot be agreed. I direct that time for seeking permission to appeal shall not run until after the hearing on the form of Order (or the making of such Order if it is agreed). I draw attention to paragraph 19.1 of the Patents Court Guide, which says that a hearing on the form of Order should take place within 28 days of hand down. In the present case, however, owing to the vacation, the form of Order hearing will be in September.
ANNEX 1
|
|
|
Target |
Trial |
Efficacious in CD |
Efficacious in CD - CGK? |
Efficacious in UC |
Efficacious in UC - CGK? |
|
Corticosteroids | |||||||
|
1 |
Prednisolone
|
|
|
Yes
|
Yes |
Yes |
Yes
|
|
Anti-inflammatory agents | |||||||
|
2 |
5-ASAs-mesalazine
|
|
|
Disputed |
Disputed |
Yes |
Yes |
|
Immunosuppressive agents | |||||||
|
3 |
Methotrexate
|
|
METEOR (UC) MERIT-UC |
Yes |
Yes |
Disputed
|
Disputed |
|
4 |
Ciclosporin A |
|
|
No |
Yes |
Yes |
Samsung: CGK for acute UC only Janssen: Yes
|
|
Other Small Molecule | |||||||
|
8 |
Apilimod mesylate |
IL-12 / IL-23 |
|
No |
Samsung: Drug not CGK (not used in UK) Janssen: Yes
|
Samsung: No Janssen: Not tried |
Samsung: Drug not CGK (not used in UK) Janssen: Yes
|
|
Biologics | |||||||
|
5 |
Infliximab
|
TNF |
ACCENT 1 (CD) ACT 1 (UC) ACT 2 (UC) |
Yes |
Yes |
Yes |
Yes. |
|
6 |
Fontolizumab
|
IFNγ |
Phase II trials (CD) |
No |
Samsung: Drug not CGK Janssen: Yes |
Untested |
N/A |
|
7 |
Adalimumab
|
TNF |
CLASSIC I (CD) CLASSIC II (CD) ULTRA I (UC) ULTRA II (UC) |
Yes |
Yes |
Yes |
Yes |
|
9 |
Secukinumab
|
IL-17A |
Phase II Trial (CD) |
Samsung: Made CD worse Janssen: No
|
Samsung: Complete failure does not assist skilled person Janssen: Yes |
Untested |
N/A |
|
10 |
Golimumab
|
TNF |
PURSUIT (UC) |
Untested
|
N/A |
Yes |
Yes. Extent details of study were CGK is disputed. |
|
11 |
Vedolizumab
|
α4/β7 |
GEMINI I (UC) GEMINI II (CD) |
Yes |
Yes |
Yes |
Yes |
|
12 |
Abrilumab
|
α4/β7 |
|
No |
Samsung: Drug not CGK Janssen: Yes |
Samsung: No Janssen: Yes |
Samsung: Drug not CGK Janssen: Yes |
|
13 |
Ustekinumab
|
IL-12 / IL-23 |
UNITI (CD) UNIFI (UC) |
Yes |
Yes |
Disputed |
Disputed |
|
14 |
Tofacitinib
|
JAK |
OCTAVE (UC) Phase IIb (CD) |
Samsung: Untested Janssen: No |
See judgment for details. |
Yes |
Yes |
ANNEX 2